Abstract
The renin-angiotensin system (RAS) within the brain is implicated in the control of fluid and electrolyte balance, autonomic functions, blood pressure, and energy expenditure. Mouse models are increasingly used to explore these mechanisms; however, sex and dose dependencies of effects elicited by chronic intracerebroventricular (ICV) angiotensin II (ANG II) infusion have not been carefully established in this species. To examine the interactions among sex, body mass, and ICV ANG II on ingestive behaviors and energy balance, young adult C57BL/6J mice of both sexes were studied in a multiplexed metabolic phenotyping system (Promethion) during chronic infusion of ANG II (0, 5, 20, or 50 ng/h). At these infusion rates, ANG II caused accelerating dose-dependent increases in drinking and total energy expenditure in male mice, but female mice exhibited a complex biphasic response with maximum responses at 5 ng/h. Body mass differences did not account for sex-dependent differences in drinking behavior or total energy expenditure. In contrast, resting metabolic rate was similarly increased by ICV ANG II in a dose-dependent manner in both sexes after correction for body mass. We conclude that chronic ICV ANG II stimulates water intake, resting, and total energy expenditure in male C57BL/6J mice following straightforward accelerating dose-dependent kinetics, but female C57BL/6J mice exhibit complex biphasic responses to ICV ANG II. Furthermore, control of resting metabolic rate by ANG II is dissociable from mechanisms controlling fluid intake and total energy expenditure. Future studies of the sex dependency of ANG II within the brain of mice must be designed to carefully consider the biphasic responses that occur in females.
Keywords: angiotensin, brain, drinking, metabolism, sex
INTRODUCTION
The renin-angiotensin system (RAS) exists both as a circulating hormone system and as a local autocrine/paracrine signaling mechanism within individual tissues of the body, including the brain (1). Within the brain, considerable evidence supports the roles for angiotensin II (ANG II) as a neurotransmitter and as a neuromodulator, and the brain RAS is strongly implicated in the control of fluid and electrolyte homeostasis, ingestive behaviors, energy balance, and blood pressure through an array of actions on autonomic, adeno- and neurohypophyseal outputs (for review, see Refs. 2–4).
Evidence supports the concept that under healthy, unchallenged conditions, the brain RAS is generally quiescent. Previously we demonstrated that chronic intracerebroventricular (ICV) infusion of ANG II type 1 receptor (AT1) antagonists or genetic deletion of Agtr1a from selected neuronal populations in mice have no major effect on energy balance or blood pressure in lean, healthy adult mice. In contrast, in response to various stimuli including deoxycorticosterone acetate (DOCA)-salt treatment or a high-fat diet, mice with these manipulations exhibit profound attenuations of energy expenditure, neuroendocrine responses, and blood pressure that depend on the specific site or cell type in which Agtr1a is manipulated or antagonized (5–11). Thus, it appears that various physiological stressors require the induction of the brain RAS to elicit their effects.
Delivery of exogenous ANG II into the brain is a common approach to study the role of the brain RAS activation in these many cardiometabolic functions. Although a wealth of literature has explored the consequences of acute ANG II injection or chronic ANG II infusion into the brain of other species (especially the rat; for review, see Refs. 12–14), substantially less work has documented the nuanced effects of exogenous ANG II delivery to the mouse brain, especially regarding the effects of chronic infusion of ANG II. Previous work has demonstrated that manipulations of the circulating RAS have differential effects on fluid homeostasis in mice versus rats (15). Furthermore, mice with genetic manipulations of the brain RAS are of increasing use to dissect angiotensinergic neurocircuitry (5, 6, 16–18). In addition, historically there has been a tendency to only study physiological endpoints in male rodents. Thus, there is a critical lack of data characterizing the dose-dependent effects of chronic delivery of ANG II into the brains of mice of both sexes.
In the current study, we explored the tacit hypothesis that adult male and female C57BL/6J mice exhibit similar dose-dependent fluid and food intake, energy expenditure, and body composition responses to chronic ICV infusion of ANG II. Resulting data demonstrate that mice exhibit behavioral and metabolic responses to chronic ICV ANG II that differ between the sexes. These findings, therefore, provide a comprehensive reference data set describing behavioral and metabolic effects of chronic ICV ANG II in C57BL/6J mice and serve to highlight the complex sex-dependent kinetics of action of ANG II within the mouse brain.
MATERIALS AND METHODS
Animals
All animal studies were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee and conform to expectations laid out in the 8th edition of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (19).
Male and female C57BL/6J mice were obtained from the Jackson laboratories (Stock No. 000664) between 6 and 8 wk of age and randomized to treatment groups on arrival. Mice were singly housed in ventilated microisolation caging on commercial racks. Mice received ad libitum access to the natural ingredient Teklad 2920x irradiated soy protein-free extruded diet (Envigo, Indianapolis, IN; 0.065 mEq Na/g, 3.13 kcal/g, 24% from protein/16% from fat/60% from carbohydrates) and reverse osmosis-filtered, chlorinated water via an automatic water system (Avidity Science, Waterford, WI). Animal rooms were maintained at 22°C–23°C (72°F–73°F) and on a 14:10 h light:dark cycle.
Intracerebroventricular Infusion
At 10.1 ± 0.3 wk of age (range 8–11 wk), mice underwent stereotaxic surgery under isoflurane anesthesia to implant an ICV cannula (Alzet, Brain Infusion Kit 3) that was connected to an osmotic minipump (Alzet, 1004) within the subcutaneous space in the upper back, as previously described (5, 6). Coordinates for the ICV cannula were: 1.1 mm lateral, 0.5 mm caudal to bregma, and 3.0 mm ventral from the surface of the skull, as previously described (6). Minipumps were loaded either with artificial cerebrospinal fluid (aCSF; Tocris 3525) or ANG II (Sigma-Aldrich, A9525) dissolved in aCSF to achieve the targeted infusion rates of 5, 20, or 50 ng/h. After surgery, animals were observed daily until completion of the study to qualitatively assess health (grooming, ingestion of food and water, body mass maintenance, etc.). At completion of the study, targeting coordinates were confirmed in a subset of mice.
Multiplexed Phenotyping
Between 5 and 8 days after implantation of the ICV pump, mice were then placed into a Promethion multiplexed phenotyping system (Sable Systems International), and remained in the system for four overnight periods (thereby representing the second week of infusion). Body masses, food intake behaviors, respirometric gas exchange, and photoelectric grid interruption data were recorded continuously and subsequently analyzed to assess ingestive behaviors, energy expenditure, and physical activity as previously described (20, 21). Meal patterning (0–1 g/meal, min 30 s/meal), fluid intake patterning (0–1 g/bout, min 10 s/bout), and sleep (no beam interruptions for >40 s) analyses were performed on data generated by the Promethion system using default cutoff values established by the manufacturer and described previously (21).
Body Composition and Tissues
On exit from the Promethion system at 11–14 days after implantation of the ICV minipump, mice were euthanized by CO2 asphyxiation. Metallic implants were removed, and the carcass was analyzed for total body composition by nuclear magnetic resonance (NMR, Bruker LF110). Tissues were then isolated and weighed. Fat-free mass (FFM) was calculated as the difference between total body mass and fat mass as determined by NMR. Total body water was estimated at 73.2% of FFM. Hydration was calculated as the ratio of total body water to total body mass.
Statistics
Analytical comparisons were made using parametric approaches. Two-tailed independent t test, analysis of variance (ANOVA), and generalized linear modeling (GLM) were used throughout, as noted in figure legends.
RESULTS
Body Mass and Composition
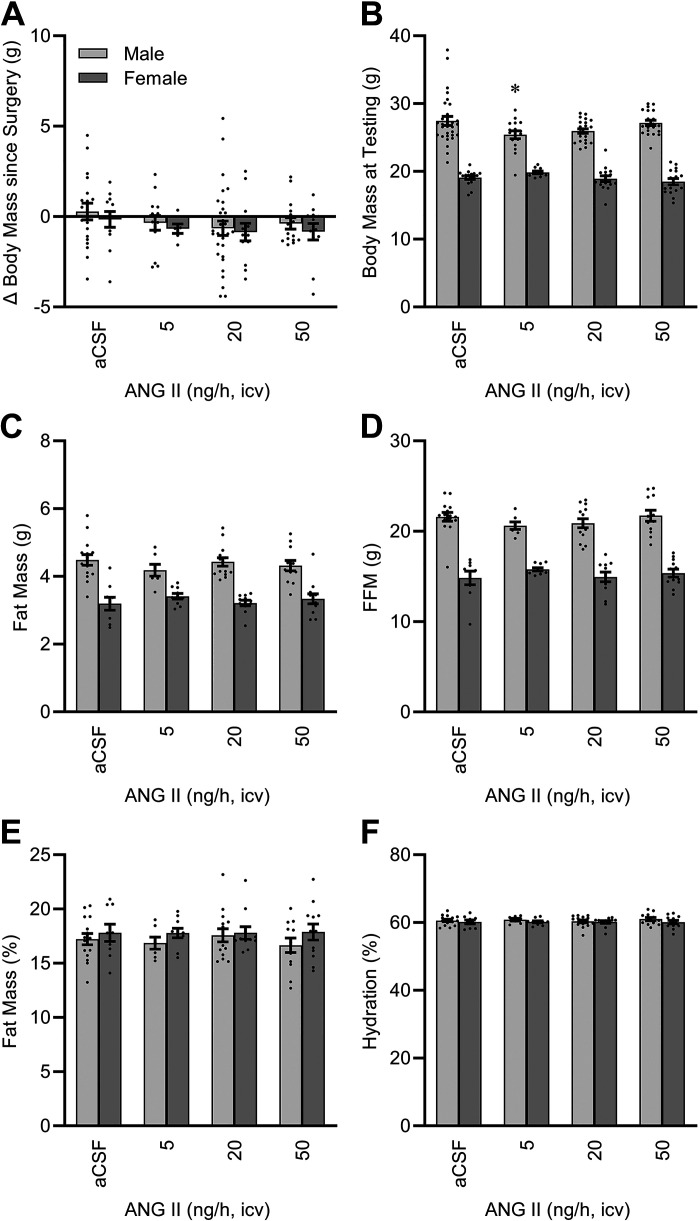
Chronic (2 wk) ICV infusion of aCSF or any of the selected doses of ANG II (5, 20, or 50 ng/h) had no effect on body mass, regardless of sex (Fig. 1A). Body masses of females were significantly lower than males, as expected (Fig. 1B). Because of differences in presurgery masses of mice randomly assigned to each treatment group, body masses varied slightly between male treatment groups during the testing week. Total body fat mass, determined by NMR, was different between sexes but was unaffected by ICV ANG II infusion (Fig. 1C). Fat-free mass (FFM) was also different between sexes but unaffected by ICV ANG II infusion (Fig. 1D). Relative to total body mass, the relative body composition (i.e., contribution of fat mass and FFM to total mass) was indistinguishable between sexes, and ANG II had no modulatory effect (Fig. 1E). Total body water was estimated at 73.2% of FFM, and total body hydration was then calculated as the ratio of total body water to total body mass. Hydration was not different between sexes and was unaffected by ANG II infusion (Fig. 1F).
Figure 1.
Body composition. A: change in body mass with 2 wk of intracerebroventricular (ICV) angiotensin II (ANG II) infusion. Two-way ANOVA: ANG II P = 0.31, sex P = 0.32, and ANG II × sex P = 0.99. B: body mass at testing, after 2 wk of ICV ANG II infusion. ANG II P = 0.39, sex P < 0.01, and ANG II × sex P = 0.03. C: fat mass after 2 wk of ICV ANG II infusion. ANG II P = 1.00, sex P < 0.01, and ANG II × sex P = 0.33. D: fat-free mass (FFM) after 2 wk of ICV ANG II infusion. ANG II P = 0.66, sex P < 0.01, and ANG II × sex P = 0.38. E: fat mass relative to total body mass after 2 wk of ICV ANG II infusion. ANG II P = 0.90, sex P = 0.11, and ANG II × sex P = 0.86. F: hydration after 2 wk of ICV ANG II infusion. ANG II P = 0.90, sex P = 0.11, and ANG II × sex P = 0.86. For A and B: aCSF n = 30 male + 17 female, 5 ng/h n = 16 male + 10 female, 20 ng/h n = 22 male + 16 female, 50 ng/h n = 20 male + 16 female. For C–F: aCSF n = 15 male + 9 female, 5 ng/h n = 7 male + 10 female, 20 ng/h n = 14 male + 11 female, 50 ng/h n = 16 male + 12 female. For all panels, *P < 0.05 vs. aCSF within males after Bonferroni multiple comparison correction. aCSF, artificial cerebrospinal fluid.
Fluid Intake Behaviors
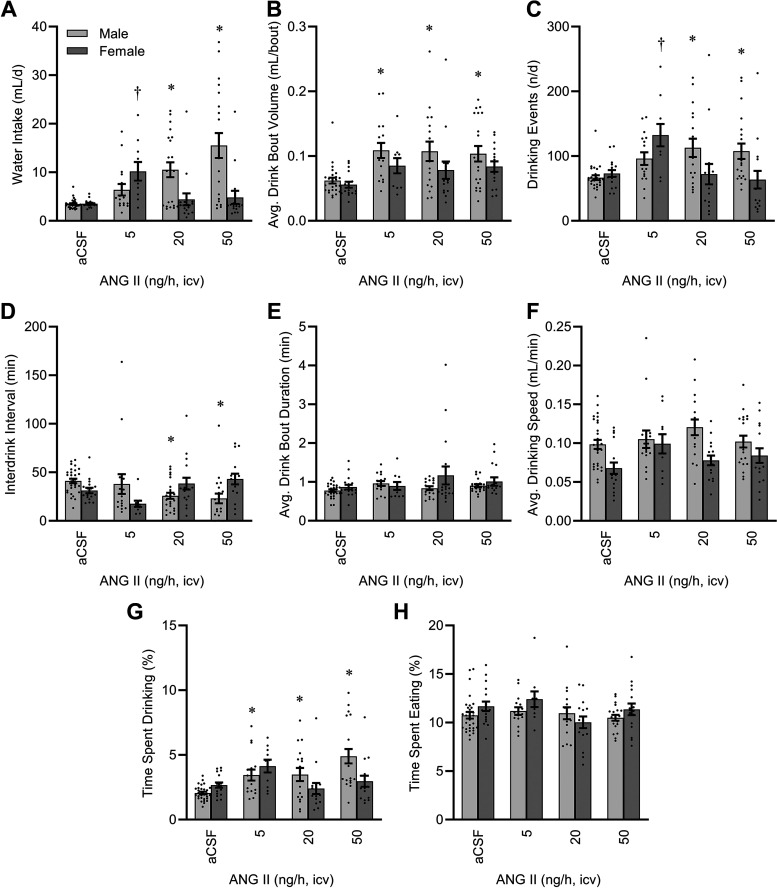
Total daily water intake was increased by ICV ANG II in a complex sex- and dose-dependent manner (Fig. 2A). Whereas males exhibited a simple dose-dependent increase in fluid intake, females exhibited a biphasic response peaking at 5 ng/h. Body mass was not a significant covariate, and therefore correction for body mass by GLM resulted in qualitatively and quantitatively similar outcomes (model P < 0.01, intercept P = 0.19, body mass P = 0.94, ANG II P < 0.01, sex P = 0.15, and ANG II × sex interaction P < 0.01) to uncorrected data. Similarly, food intake per day was also not a significant covariate, so GLM considering both body mass and food intake also resulted in similar conclusions (model P < 0.01, intercept P = 0.16, body mass P = 0.84, food intake P = 0.53, ANG II P < 0.01, sex P = 0.15, and ANG II × sex interaction P < 0.01).
Figure 2.
Fluid intake behavior after 2 wk of intracerebroventricular (ICV) infusion. A: total daily water intake. Two-way ANOVA: angiotensin II (ANG II) P < 0.01, sex P < 0.01, and ANG II × sex P < 0.01. B: average water intake per drinking bout. ANG II P < 0.01, sex P < 0.01, and ANG II × sex P = 0.70. C: total number of drinking bouts per day. ANG II P < 0.01, sex P = 0.20, and ANG II × sex P < 0.01. D: average interbout interval. ANG II P = 0.48, sex P = 0.87, and ANG II × sex P < 0.01. E: average duration of drinking bouts. ANG II P = 0.26, sex P = 0.11, and ANG II × sex P = 0.32. F: average speed of water intake during drinking bouts. ANG II P = 0.12, sex P < 0.01, and ANG II × sex P = 0.22. G: percentage of total time spent drinking water. ANG II P < 0.01, sex P = 0.14, and ANG II × sex P < 0.01. H: percentage of total time spent eating food. ANG II P = 0.12, sex P = 0.15, and ANG II × sex P = 0.15. For all panels, aCSF n = 30 male + 17 female, 5 ng/h n = 16 male + 10 female, 20 ng/h n = 17 male + 16 female, 50 ng/h n = 20 male + 16 female. For all panels, *P < 0.05 vs. aCSF within males, and †P < 0.05 vs. aCSF within females after Bonferroni multiple comparison correction. aCSF, artificial cerebrospinal fluid.
The increase in fluid intake in males was due to increased volume consumed per drinking bout (Fig. 2B), increased numbers of drinking bouts per day (Fig. 2C), and reduced interbout intervals (Fig. 2D). In contrast, the major increase in fluid intake by females receiving 5 ng/h infusion was primarily characterized by an increased number of drinking bouts per day (Fig. 2C). No difference in the duration of any individual drinking bout was observed in either sex (Fig. 2E). Females exhibited a significant reduction in the speed of water intake during drinking bouts regardless of ANG II infusion (Fig. 2F). Total time spent drinking was significantly increased by ANG II in both sexes, though this effect was more robust in males (Fig. 2G). In contrast to water intake, and thereby highlighting the specificity of the induction of drinking behavior by ANG II, neither sex nor ANG II infusion had any significant effect to modify the total time animals spent eating food (Fig. 2H).
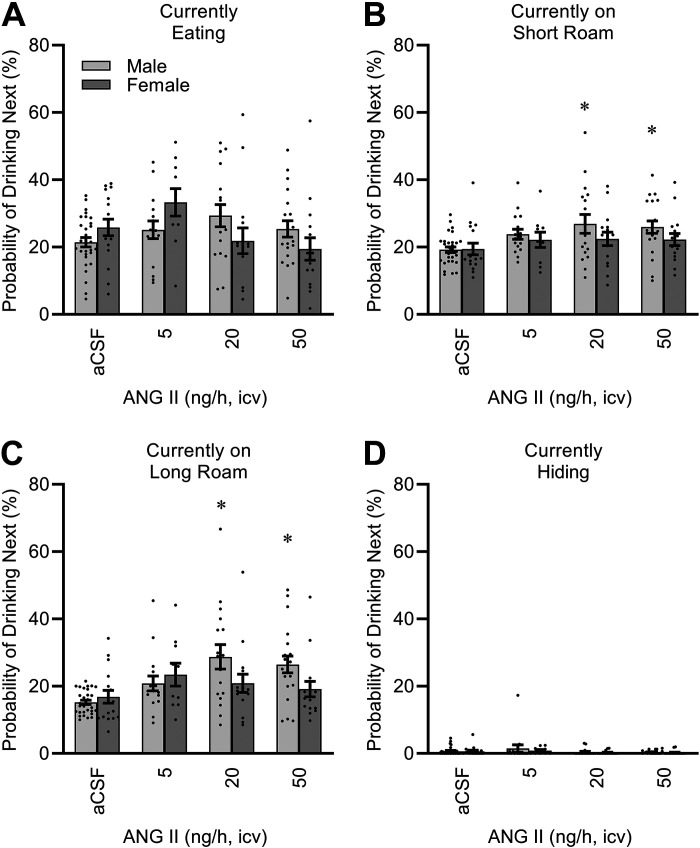
The multiplexed Promethion system provides an automated analysis of time distributions among various categories of behavior, and analysis of the transitions between these behaviors (“Ethoscan”). To further understand the effects of sex and ICV ANG II infusion on behavior, we calculated the probability of interacting with the fluid spout (i.e., drinking) next, depending on the current behavior of the mouse. As illustrated in Fig. 3, male mice infused with higher doses of ANG II exhibited an increased probability of transitioning to drinking whenever they were “roaming” the cage (i.e., not currently interacting with food, drink, or body mass hoppers for >1 s). In contrast, when males were eating or hiding, ICV ANG II did not have a statistically significant influence on the probability that they would subsequently drink fluid. Regardless of ICV ANG II infusion, female mice did not appear to exhibit major changes in likelihood to drink next when roaming the cage or hiding. Interestingly, a significant interaction between sex and ANG II modified the probability of drinking next when the mice were eating.
Figure 3.
Behavioral probability matrix. Data presented as likelihood of drinking next, after interaction with food hopper (eating; A), brief wandering around cage between 1 and 60 s (short roam; B), extended wandering around cage >60 s (long roam; C), or resting in body mass monitoring habitat (hiding; D). Eating: ANG II P = 0.13, sex P = 0.91, and ANG II × sex P = 0.02. Short roam: ANG II P < 0.01, sex P = 0.07, and ANG II × sex P = 0.52. Long roam: ANG II P < 0.01, sex P = 0.11, and ANG II × sex P = 0.04. Hiding: ANG II P = 0.21, sex P = 0.41, and ANG II × sex P = 0.92. For all panels, aCSF n = 30 male + 17 female, 5 ng/h n = 16 male + 10 female, 20 ng/h n = 17 male + 16 female, 50 ng/h n = 20 male + 16 female. For all panels, *P < 0.05 vs. aCSF within males after Bonferroni multiple comparison correction. aCSF, artificial cerebrospinal fluid; ANG II, angiotensin II.
Food Intake Behaviors
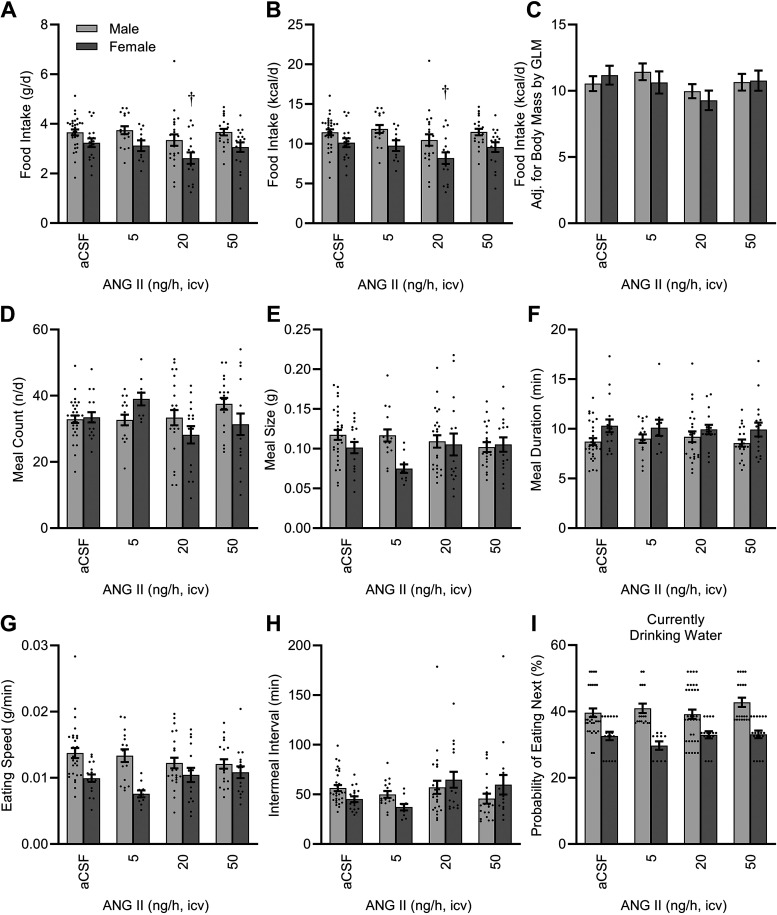
Though more obvious in females, food intake per day was reduced in mice of both sexes with ICV ANG II infusion (Fig. 4, A and B). This effect, however, was driven by the difference in body size between the groups, as correction for body mass by GLM abolished the statistically significant effect of ICV ANG II on total food intake (Fig. 4C). Despite this marginally important difference in total food consumption, infusion of ANG II had minor modulatory effects on some of the individual behaviors that contribute to total intake. For example, ICV ANG II caused sex-specific changes in the number of meals per day (Fig. 4D). Sex modified the average individual meal size (Fig. 4E), the duration of meals (Fig. 4F), and the speed of food consumption (Fig. 4G), though none of these effects were significantly influenced by ICV ANG II infusion. Finally, the average intermeal interval may be sensitive to ICV ANG II infusion, though the effect of ANG II did not formally reach statistical significance (ANG II P = 0.05, ANG II × sex interaction P = 0.06; Fig. 4H).
Figure 4.
Food intake behavior after 2 wk of intracerebroventricular (ICV) infusion. Total daily food intake, presented as mass/time (A), and total daily food intake, presented as energy/time (B). Two-way ANOVA: ANG II P = 0.03, sex P < 0.01, and ANG II × sex P = 0.83. C: caloric intake corrected for body mass by GLM: model P < 0.01, intercept P = 0.02, body mass P = 0.01, ANG II P = 0.07, sex P = 0.82, and ANG II × sex P = 0.56. Estimated marginal means presented at covariate (body mass) = 23.57 g. D: total number of meals per day. ANG II P = 0.13, sex P = 0.46, and ANG II × sex P = 0.03. E: average meal size. ANG II P = 0.48, sex P = 0.02, and ANG II × sex P = 0.09. F: average meal duration. ANG II P = 0.92, sex P < 0.01, and ANG II × sex P = 0.85. G: average consumption speed. ANG II P = 0.51, sex P < 0.01, and ANG II × sex P = 0.07. H: average intermeal interval. ANG II P = 0.05, sex P = 0.89, and ANG II × sex P = 0.06. I: behavioral probability matrix data, demonstrating likelihood of eating next, after water intake. ANG II P < 0.01, sex P = 0.26, and ANG II × sex P = 0.41. For all panels, aCSF n = 30 male + 17 female, 5 ng/h n = 16 male + 10 female, 20 ng/h n = 17 male + 16 female, 50 ng/h n = 20 male + 16 female. For all panels, †P < 0.05 vs. aCSF within females after Bonferroni multiple comparison correction. aCSF, artificial cerebrospinal fluid; ANG II, angiotensin II; GLM, generalized linear modeling.
Food and water intake behaviors can be linked in some circumstances. Therefore, to evaluate the influence of each ingestive behavior on the other, Deming (type II) regression analysis was performed on water intake versus food intake. In male mice, water intake per day was not significantly correlated with food intake per day for any of the treatment groups (aCSF P = 0.29, 5 ng/h P = 0.11, 20 ng/h P = 0.74, 50 ng/h P = 0.55). In female mice, water intake was significantly and positively correlated with food intake in the aCSF group (i.e., [water intake] = 0.69 × [food intake] + 2.21, P < 0.01); however, water intake and food intake were not significantly correlated in any of the groups receiving ICV ANG II infusion (5 ng/h P = 0.58, 20 ng/h P = 0.28, 50 ng/h P = 0.77). Thus, regardless of sex, water and food ingestive behaviors were uncoupled during ICV ANG II infusion; ANG II caused dose-dependent changes in total daily water intake that were not associated with changes in total daily food intake. As shown above (Fig. 3), analysis of the Ethoscan behavioral probability matrix indicated that when eating, ICV ANG II caused sex-dependent increases in the probability that mice will next ingest water. In contrast, analysis of the behavioral probability matrix indicated that regardless of sex, ICV ANG II caused a progressive reduction in the probability of consuming food immediately after drinking water (Fig. 4I). Thus, ICV ANG II infusion caused an uncoupling of total daily fluid versus food intake, and this is mediated in part through nuanced behavioral changes that increase fluid intake after eating and suppress food intake after drinking.
Energy Expenditure
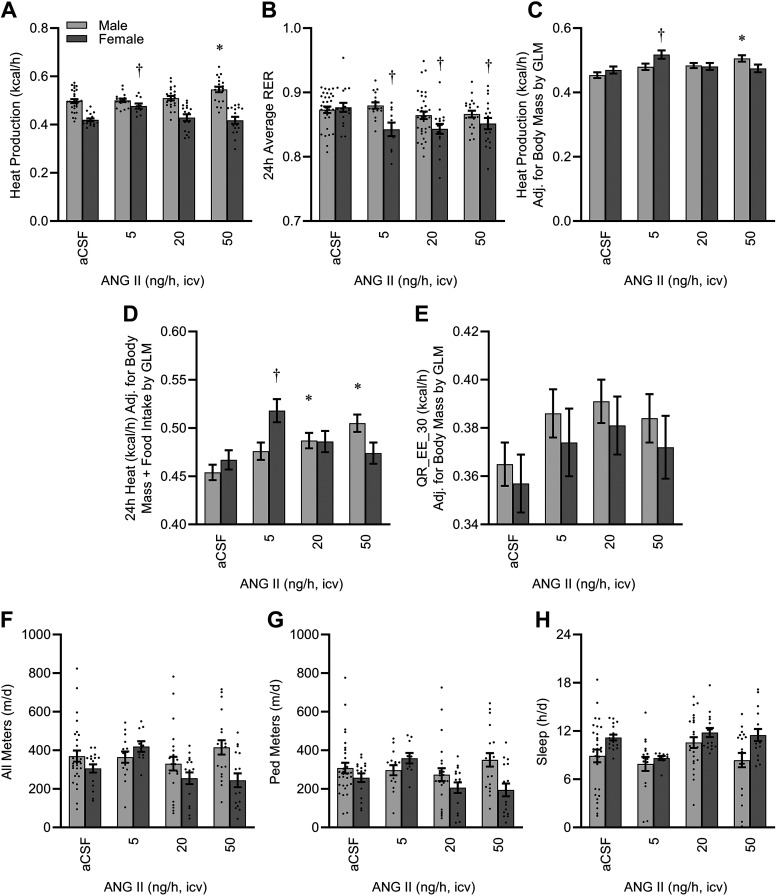
Chronic ICV infusion of ANG II caused a complex sex- and dose-dependent effect to increase aerobic metabolism (Fig. 5A). This effect was accompanied by a small but statistically significant sex-dependent effect on respiratory exchange ratio, potentially indicating a shift in fuel utilization in response to ANG II (Fig. 5B). Importantly, as energy expenditure is sensitive to body size and feeding status, we examined the effect of sex and ANG II infusion on heat production after correction for these covariates using GLM. Correction for body mass alone (Fig. 5C), or both body mass and total daily food intake (Fig. 5D), clarified that ICV ANG II had dose-dependent effects on heat production in both sexes, but that the specific effects were subject to a complex interaction between dose and sex. In addition, this approach indicates that the overall effect of sex on energy expenditure was largely due to the difference in body masses between the sexes.
Figure 5.
Energy expenditure after 2 wk of intracerebroventricular (ICV) infusion. A: 24 h average heat production as estimated using the modified Weir equation. Two-way ANOVA: ANG II P = 0.02, sex P < 0.01, and ANG II × sex P < 0.01. B: 24 h average respiratory exchange ratio (RER). Two-way ANOVA: ANG II P < 0.01, sex P < 0.01, and ANG II × sex P = 0.04. C: heat production, corrected for body mass by GLM: model P < 0.01, intercept P < 0.01, body mass P < 0.01, ANG II P < 0.01, sex P = 0.683, and ANG II × sex P < 0.01. Estimated marginal means presented at covariate (body mass) = 23.57 g. D: heat production, corrected for both body mass and 24 h food intake by GLM: model P < 0.01, intercept P < 0.01, body mass P < 0.01, ANG II P < 0.01, sex P = 0.62, and ANG II × sex P < 0.01. Estimated marginal means presented at covariates (body mass) = 23.57 g and (food) = 3.36 g/day. E: average heat production during the 30-min period with lowest activity (QR_EE_30), as an estimate of resting metabolic rate, adjusted for body mass by GLM: model P < 0.01, intercept P < 0.01, body mass P < 0.01, ANG II P = 0.03, sex P = 0.41, and ANG II × sex P = 1.00. F: total spontaneous physical activity. ANG II P = 0.07, sex P = 0.01, and ANG II × sex P = 0.03. G: spontaneous physical activity with nonambulatory/fine motion (e.g., grooming) subtracted. ANG II P = 0.10, sex P = 0.03, and ANG II × sex P = 0.03. H: total sleep time as estimated by physical inactivity. ANG II P = 0.01, sex P < 0.01, ANG II × sex P = 0.48. For all panels, aCSF n = 30 male + 17 female, 5 ng/h n = 16 male + 10 female, 20 ng/h n = 17 male + 16 female, 50 ng/h n = 20 male + 16 female. For all panels, *P < 0.05 vs. aCSF within males, and †P < 0.05 vs. aCSF within females after Bonferroni multiple comparison correction. aCSF, artificial cerebrospinal fluid; ANG II, angiotensin II; GLM, generalized linear modeling.
To further understand the effect of ICV ANG II infusion on resting metabolism, we examined the effects of sex and dose on heat production during the lowest average 30-min period throughout the 24-h cycle (reported as QR_EE_30 by the Promethion output macros). In contrast to 24 h total heat production (which includes the integration of heat production during both resting and active states), QR_EE_30 was similarly stimulated by ICV ANG II regardless of sex (Fig. 5E) after accounting for differences in body mass by GLM. Total 24 h food intake was not a significant covariate in this analysis (P = 0.52) and was therefore excluded from this analysis.
Total spontaneous physical activity (All Meters, Fig. 5F) and ambulatory activity after subtraction of fine behaviors such as grooming (Ped Meters, Fig. 5G) were both sensitive to complex interactions between sex and dose of ICV ANG II infusion, apparently driven by increased motion of females receiving the low 5 ng/h infusion. Total sleep time, estimated from periods of extended inactivity, was sensitive to both ANG II and sex but no interaction was observed between these factors (Fig. 5H).
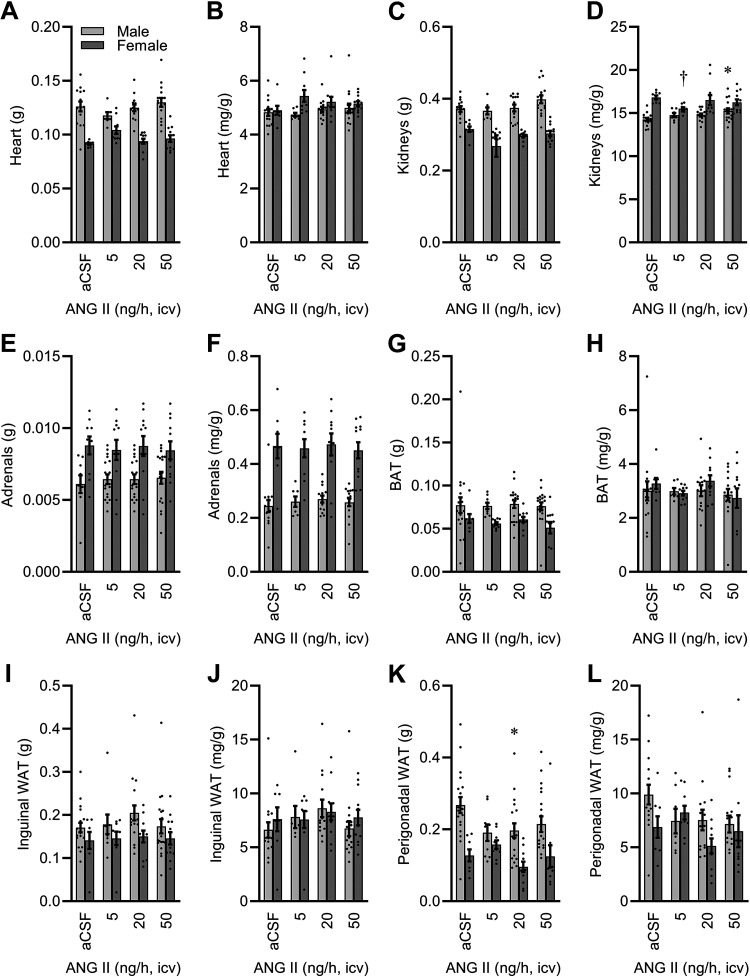
Tissue Effects
At the conclusion of the study, major cardiovascular and metabolic tissues were collected to examine the effects of ICV ANG II infusion. Heart mass was modified by ANG II in a sex-dependent manner (Fig. 6, A and B). Kidney mass was also modified by ANG II in a sex-dependent manner after correction for total body mass (Fig. 6, C and D). Adrenal mass was not modified by ICV ANG II infusion, though sex differences were noted (Fig. 6, E and F). Interscapular brown adipose tissue (BAT) pad mass differed between the sexes due to differences in body mass, and ICV ANG II infusion did not modify the mass of this fat pad (Fig. 6, G and H). Similar sex differences secondary to body mass differences, without the effect of ICV ANG II infusion, were noted for subcutaneous inguinal white adipose tissue (Fig. 6, I and J). Differences in perigonadal fat between sexes were also secondary to total body mass, however, possible effects of ICV ANG II were noted in males (Fig. 6, K and L).
Figure 6.
Tissue masses after 2 wk of intracerebroventricular (ICV) infusion. A: heart. Two-way ANOVA: ANG II P = 0.62, sex P < 0.01, and ANG II × sex P < 0.05. B: heart per body mass. ANG II P = 0.45, sex P < 0.01, and ANG II × sex P = 0.28. C: kidneys. ANG II P = 0.09, sex P < 0.01, and ANG II × sex P = 0.37. D: kidneys per body mass. ANG II P = 0.33, sex P < 0.01, and ANG II × sex P = 0.04. E: adrenals. ANG II P = 0.99, sex P < 0.01, and ANG II × sex P = 0.91. F: adrenals per body mass. ANG II P = 0.91, sex P < 0.01, and ANG II × sex P = 0.96. G: interscapular brown adipose tissue (BAT). ANG II P = 0.72, sex P < 0.01, and ANG II × sex P = 0.89. H: BAT per body mass. ANG II P = 0.40, sex P = 0.57, and ANG II × sex P = 0.73. I: inguinal white adipose tissue (WAT). ANG II P = 0.59, sex P < 0.01, and ANG II × sex P = 0.84. J: inguinal WAT per body mass. ANG II P = 0.33, sex P = 0.54, and ANG II × sex P = 0.75. K: perigonadal WAT. ANG II P = 0.19, sex P < 0.01, and ANG II × sex P = 0.22. L: perigonadal WAT per body mass. ANG II P = 0.15, sex P = 0.07, and ANG II × sex P = 0.27. For all panels, aCSF n = 19 male + 8 female, 5 ng/h n = 9 male + 10 female, 20 ng/h n = 18 male + 11 female, 50 ng/h n = 20 male + 12 female. For all panels, *P < 0.05 vs. aCSF within males, and †P < 0.05 vs. aCSF within females after Bonferroni multiple comparison correction. aCSF, artificial cerebrospinal fluid; ANG II, angiotensin II.
DISCUSSION
Collectively, these studies document dose-dependencies of chronic ICV ANG II infusion in male and female C57BL/6J mice for the modulation of ingestive behaviors and energy balance. Results from male mice illustrate simple, accelerating dose-dependent effects of ICV ANG II on drinking behaviors, and energy expenditure that largely parallel previous reports of the effects of chronic ICV ANG II infusion in rats. In contrast, results from female mice indicate increased sensitivity (i.e., leftward shifted) and complex biphasic (i.e., U-shaped) dose-response effects. These findings therefore simultaneously represent an important reference data set for the selection of relevant ICV ANG II dosing schedules in C57BL/6J mice and serve to highlight major sex differences in the biology of the brain RAS.
The effect of sex to modify the effects of the RAS has been previously noted (for review, see Ref. 22), and some progress has been made to understand the molecular mechanisms that underlie these differences, especially with regard to blood pressure control. In a series of recent studies of mice, the involvement of glutamatergic signaling within the paraventricular nucleus in blood pressure responses to peripheral ANG II infusion has been demonstrated to be greatly dependent on sex and ovarian hormones (23–25). In addition, nitric oxide (NO) signaling within the brain has been implicated in autonomic and blood pressure responses to peripheral ANG II in mice (26). Studies in rats have demonstrated that deoxycorticosterone acetate (DOCA)-salt treatment increases blood pressure through a mechanism involving angiotensin-converting enzyme (ACE) and ANG II AT1 receptors within the brain (10, 11, 27). Moreover, female rats are more resistant to DOCA-salt hypertension due to the protective actions of endogenous ANG II type 2 (AT2) receptor signaling within the brain (28). This protective effect of AT2 signaling appears to be modified by ovarian hormones (29). Similarly, endoplasmic reticulum stress within the subfornical organ has been associated with sex- and ovary-dependent differences in blood pressure responses to peripheral ANG II in rats (30). Sex differences in drinking responses to acute ICV ANG II have also been partially explored. In rats, some apparent sex differences in fluid intake behaviors in response to ANG II may be secondary to body mass differences, as water intake responses to high doses of ICV ANG II can be similar after correction for body mass, though complex changes in water and saline drinking microstructure support more nuanced sex-dependent differences in responses (31). Also supporting more nuanced mechanisms, repeated exposures to ANG II cause sex-dependent desensitization of drinking behaviors in rats (32). Thus, although some of the differences in physiological responses to ANG II between male and female rodents can be attributed to body mass and composition differences, evidence also supports important sex-dependent differences in activation and involvement of multiple molecular pathways. More work is clearly required to address sex-mediated differences in autonomic, cardiovascular, fluid and electrolyte, and energy balance control mechanisms within the brain.
Our work highlights important sex-dependent differences in ingestive behaviors in response to ANG II, particularly fluid intake behaviors, in C57BL/6J mice. Of particular interest is the relatively simple, accelerating ICV ANG II dose-dependent increase in water intake in males, which contrasted sharply with an inverted U-shaped biphasic dose dependency of this effect in females. In addition, it should be noted that correction for body mass did not impact these relationships (within or between sexes), which stands in contrast to previous studies of the sex-dependency of drinking responses to acute ICV ANG II in rats (31). These results illustrate that mice cannot be thought of simply as small rats and that dose selection for studies of the effect of chronic ICV ANG II in female mice is exceptionally complicated. Given the previous implication of AT2 receptors in buffering some female responses to ANG II (28, 29), we are left to hypothesize a potential role for AT2 signaling in this difference. As AT1 and AT2 exhibit largely similar affinities for ANG II (33), it seems likely that sex-dependent differences in expression of AT2, localization or colocalization of the receptors, or second-messenger coupling efficiencies may ultimately underlie such effects.
Although ANG II infusion did not have a robust effect on total daily caloric intake, microstructure analyses of feeding behavior provide some clues as to potential effects of ANG II within the brain on neurocircuits influencing food ingestion. In particular, ANG II caused dose- and sex-dependent effects to modulate satiety (e.g., meal number and perhaps, intermeal interval) but not satiation (e.g., meal size and duration). Complementing these results, recent studies by Rathod and Fulvio (34) demonstrate that aspects of both satiety and satiation are influenced by sex and aging in C57BL/6J mice. As our team has previously demonstrated a critical role of ANG II type AT1A receptors expressed in cells that also express the leptin receptor in the control of autonomic responses to leptin (6, 35), it follows that the satiety-inducing effects of leptin may also involve or be mediated by AT1A receptors. Thus, further study of the influence of ANG II, and its interactions with sex and aging on satiety, are warranted.
Energy expenditure effects of chronic ICV ANG II infusion complement and extend our previous studies using ICV ANG II infusion in mice. For example, we previously demonstrated that resting metabolic rate (RMR) was significantly increased in male C57BL/6J mice with 10 days of ICV ANG II infusion (6). Importantly, the values reported in that study were actually akin to basal metabolic rate (BMR), as they were determined in studies in animals at rest, in the postabsorptive phase, and in a thermoneutral environment. In the present study, RMR was estimated by examining the QR_EE_30 value (representing the lowest average 30 min heat production values from animals housed at standard room temperature with free access to food). Thus, although these two approaches measure qualitatively different functions (BMR vs. the nadir of a “normal” 24 h heat production function as an estimate of RMR), both approaches support the conclusion that ICV ANG II infusion increases energy expenditure at rest. The current study builds on our previous work by demonstrating that female mice exhibit similar accelerating dose-dependent responses in this resting energy expenditure, despite the observation that total, integrated 24 h heat production in females exhibits a complex U-shaped biphasic response to ICV ANG II. We conclude that ANG II in the brain stimulates resting energy expenditure in both sexes, whereas other (nonresting) mechanisms must explain sex differences in total energy expenditure. Such mechanisms might include differences in the thermic effect of food, the caloric cost of physical activity, etc.
In addition to having effects on resting energy expenditure, data presented here support the conclusion that ICV infusion of ANG II has effects on ambulatory activity in mice in a sex-dependent manner. Although no significant effects were noted for any specific dose within either sex after multiple comparison procedures were applied, general trends consistent with an increased ambulatory activity in males at 50 ng/h, plus a biphasic response with maximal effect at 5 ng/h in females, were noted. Interestingly, these patterns parallel the effect of ICV ANG II on drinking behaviors and on the behavioral probability matrices displayed in Fig. 3. Further, ICV ANG II infusion had effects to reduce total sleeping time in both sexes. Together with reduced interdrink intervals and increased numbers of drinking events per day, these data lead us to hypothesize that the increased ambulatory activity and decreased time spent sleeping during ICV ANG II infusion are secondary to an increased drive to procure water.
Multiple limitations of the current study must be noted. First, only C57BL/6J mice were used throughout. We chose to use this strain, as it is widely used for cardiometabolic studies, and because many transgenic animal models are maintained on this strain. As the genetic background has a strong modulatory effect on fluid and energy turnover phenotypes in mouse models (https://phenome.jax.org/), it follows that distinct dosing regimens may be required to elicit behavioral and metabolic phenotypes in other strains. Second, mice were only studied at 10–12 wk of age, which represents a relatively narrow range in early adulthood. The actions of ANG II are documented to change with advancing age in rats (36), and therefore it is likely that aging will modify the effects of ICV ANG II in mice. Third, mice were maintained on Teklad 2920x soy-free diet throughout the current study. This choice was made to avoid potential confounding effects of diet-derived phytoestrogens; however, we and others have demonstrated complex modulatory effects of diet (e.g., soy-free vs. various grain-based chows) on the development of cardiometabolic phenotypes in C57BL/6J male mice when treated with DOCA-salt treatment, which is known to elicit behavioral and hemodynamic effects through activation of the endogenous brain RAS (37). Furthermore, dietary sodium intake is a known modulator of the effects of ANG II on various physiological processes and although food intake (as the only source of sodium for animals in the current study) did not differ among groups, one may reasonably expect that the specific dose-response relationships documented here may be altered if animals were maintained on a different diet with a different sodium concentration. Thus, diet choice is also likely to further modify the effects of ICV ANG II. Fourth, we did not control or assess estrous status in the female mice in the current study. In rats, it has been demonstrated that blood pressure response to peripheral ANG II infusion is estrogen sensitive (38). Although the low variability in most endpoints studied in females in the current data set may indicate that estrous status is less important for the modulation of effects of ICV ANG II, or in species-specific responses to ANG II, future studies to evaluate this potential interaction are required. Fifth, we cannot rule out possible modulatory effects of season on data presented herein. Male mice were studied in the Promethion system in March, July, September, October, and November, whereas female mice were studied only in October and November. Although the ambient temperature was maintained between 22°C and 23°C for all animals, relative humidity (RH) did vary between individual cohorts, which resulted in modest but statistically significant effects based on sex (ANG II P = 0.39, sex P < 0.01, and ANG II × sex P = 0.03). Ambient RH values ranged from 19% to 38% for males versus 15%–23% for females. Importantly, no significant differences in RH were noted between cohorts that exhibited significant induction of drinking by ICV ANG II. For example, RH was indistinguishable for females treated with aCSF versus 5 ng/h ANG II, and RH was indistinguishable between males treated with aCSF or with 20 or 50 ng/h ANG II. Thus, although RH was not explicitly controlled and was noted to differ during analyses of males versus females, RH differences do not appear to contribute to drinking responses to ICV ANG II within either sex. Future studies to examine possible interactions between ICV ANG II infusion, RH, and season may be warranted. Sixth, because we did not biochemically assess the concentrations of ANG II within cerebrospinal fluid, we are unable to formally rule out the possibility of technical failures (ICV cannulas, osmotic minipumps, etc.) for individual animals. We contend that failed targeting is unlikely, given the large target represented by the lateral ventricle plus the reliable coordinates that we have used in many previous studies. In contrast, we have noted occasional failures of osmotic minipumps in previous studies. Thus, it is possible that some small subset of animals may have been implanted with failed pumps, but it is exceptionally unlikely that all such failures would cluster within a single treatment group, especially with the robust numbers of animals included in each group in the current study. For example, failed targeting and/or failed pumps almost certainly cannot explain the (tightly clustered) lack of effect of ICV Ang II to cause increased water intake in all n = 16 female mice infused at the 50 ng/h dose. Thus, we acknowledge the possibility that some of the complex (e.g., bimodal) dose-response relationships noted herein may be influenced by technical failures but argue that the influence of such failures is likely minimal. Finally, multiple additional endpoints relevant for whole body energy metabolism were not explored in the current study. We have previously implicated the RAS in the modulation of digestive efficiency (39) and anaerobic metabolism (40) in mice, but we did not explore the effects of ICV ANG II on these functions in the current study. Future work to establish the modulatory effects of the brain RAS (and its interaction with sex) on these endpoints are needed.
In conclusion, chronic ICV infusion of ANG II into C57BL/6J mice caused the induction of an array of behavioral and metabolic phenotypes, but these effects are dependent on both ANG II dose and the sex of the mouse. Importantly, correction for body mass eliminated some apparent sex-based differences, whereas the sex dependency of other endpoints was independent of body size or composition. Finally, these data demonstrate the dissociation of brain ANG II-mediated control of water intake and total energy expenditure versus resting energy expenditure. Future studies to establish the molecular mechanisms underlying sex-dependent differences in fluid intake and energy expenditure responses are necessary and must be designed carefully to account for the complex interactions between sex and dose as established herein.
GRANTS
This work was supported by grants from the National Institutes of Health (HL134850, HL084207, HL144807, and DK133121), the American Heart Association (18EIA33890055, 903246, 898067, 826132, 898004), the MCW Clinical & Translational Science Institute (UL1TR001436), the Children’s Research Institute (CRI22700), the American Physiological Society Postdoctoral Fellowship program, and the Advancing a Healthier Wisconsin endowment.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.O., J.L.S., C.D.S., and J.L.G. conceived and designed research; V.O., J.J.R., K.B., M.L.R., N.M.M., M.A.O., K.-T.L., C.C.G., S.D.S., K.K.W., P.N., J.L.S., C.D.S., and J.L.G. performed experiments; V.O., J.J.R., K.B., M.L.R., N.M.M., M.A.O., K.-T.L., C.C.G., K.K.W., P.N., J.L.S., C.D.S., and J.L.G. analyzed data; V.O., C.D.S., and J.L.G. interpreted results of experiments; C.D.S. and J.L.G. prepared figures; V.O. drafted manuscript; V.O., J.J.R., K.B., M.L.R., N.M.M., M.A.O., K.-T.L., C.C.G., K.K.W., P.N., J.L.S., C.D.S., and J.L.G., edited and revised manuscript; V.O., J.J.R., K.B., M.L.R., N.M.M., M.A.O., K.-T.L., C.C.G., S.D.S., K.K.W., P.N., J.L.S., C.D.S., and J.L.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the assistance of the MCW Biomedical Resource Center and the MCW Rat Research Models Service Center.
REFERENCES
- 1. Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system–an endocrine and paracrine system. Endocrinology 144: 2179–2183, 2003. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 2. Grobe JL, Xu D, Sigmund CD. An intracellular renin-angiotensin system in neurons: fact, hypothesis, or fantasy. Physiology (Bethesda) 23: 187–193, 2008. doi: 10.1152/physiol.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Phillips MI, de Oliveira EM. Brain renin angiotensin in disease. J Mol Med (Berl) 86: 715–722, 2008. doi: 10.1007/s00109-008-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Phillips MI, Sumners C. Angiotensin II in central nervous system physiology. Regul Pept 78: 1–11, 1998. doi: 10.1016/s0167-0115(98)00122-0. [DOI] [PubMed] [Google Scholar]
- 5. Sandgren JA, Linggonegoro DW, Zhang SY, Sapouckey SA, Claflin KE, Pearson NA, Leidinger MR, Pierce GL, Santillan MK, Gibson-Corley KN, Sigmund CD, Grobe JL. Angiotensin AT1A receptors expressed in vasopressin-producing cells of the supraoptic nucleus contribute to osmotic control of vasopressin. Am J Physiol Regul Integr Comp Physiol 314: R770–R780, 2018. doi: 10.1152/ajpregu.00435.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Claflin KE, Sandgren JA, Lambertz AM, Weidemann BJ, Littlejohn NK, Burnett CM, Pearson NA, Morgan DA, Gibson-Corley KN, Rahmouni K, Grobe JL. Angiotensin AT1A receptors on leptin receptor-expressing cells control resting metabolism. J Clin Invest 127: 1414–1424, 2017. doi: 10.1172/JCI88641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hilzendeger AM, Cassell MD, Davis DR, Stauss HM, Mark AL, Grobe JL, Sigmund CD. Angiotensin type 1a receptors in the subfornical organ are required for deoxycorticosterone acetate-salt hypertension. Hypertension 61: 716–722, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grobe JL, Buehrer BA, Hilzendeger AM, Liu X, Davis DR, Xu D, Sigmund CD. Angiotensinergic signaling in the brain mediates metabolic effects of deoxycorticosterone (DOCA)-salt in C57 mice. Hypertension 57: 600–607, 2011. doi: 10.1161/HYPERTENSIONAHA.110.165829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, de Lange WJ, Li H, Sakai K, Thedens DR, Cassis LA, Rahmouni K, Mark AL, Johnson AK, Sigmund CD. The brain Renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab 12: 431–442, 2010. doi: 10.1016/j.cmet.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kubo T, Yamaguchi H, Tsujimura M, Hagiwara Y, Fukumori R. Blockade of angiotensin receptors in the anterior hypothalamic preoptic area lowers blood pressure in DOCA-salt hypertensive rats. Hypertens Res 23: 109–118, 2000. doi: 10.1291/hypres.23.109. [DOI] [PubMed] [Google Scholar]
- 11. Park CG, Leenen FH. Effects of centrally administered losartan on deoxycorticosterone-salt hypertension rats. J Korean Med Sci 16: 553–557, 2001. doi: 10.3346/jkms.2001.16.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daniels D. Diverse roles of angiotensin receptor intracellular signaling pathways in the control of water and salt intake. In: Neurobiology of Body Fluid Homeostasis: Transduction and Integration, edited by De Luca LA Jr., Menani JV, and Johnson AK.. Boca Raton, FL: CRC Press, 2014. [PubMed] [Google Scholar]
- 13. Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev 78: 583–686, 1998. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- 14. Epstein AN, Fitzsimons JT, Rolls BJ. Drinking induced by injection of angiotensin into the brain of the rat. J Physiol 210: 457–474, 1970. doi: 10.1113/jphysiol.1970.sp009220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rowland NE, Goldstein BE, Robertson KL. Role of angiotensin in body fluid homeostasis of mice: fluid intake, plasma hormones, and brain Fos. Am J Physiol Regul Integr Comp Physiol 284: R1586–R1594, 2003. doi: 10.1152/ajpregu.00730.2002. [DOI] [PubMed] [Google Scholar]
- 16. Mohammed M, Johnson DN, Wang LA, Harden SW, Sheng W, Spector EA, Elsaafien K, Bader M, Steckelings UM, Scott KA, Frazier CJ, Sumners C, Krause EG, de Kloet AD. Targeting angiotensin type 2 receptors located on pressor neurons in the nucleus of the solitary tract to relieve hypertension in mice. Cardiovasc Res 118: 883–896, 2022. doi: 10.1093/cvr/cvab085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Kloet AD, Wang L, Pitra S, Hiller H, Smith JA, Tan Y, Nguyen D, Cahill KM, Sumners C, Stern JE, Krause EG. A unique “Angiotensin-Sensitive” neuronal population coordinates neuroendocrine, cardiovascular, and behavioral responses to stress. J Neurosci 37: 3478–3490, 2017. doi: 10.1523/JNEUROSCI.3674-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, Seeley RJ, Herman JP, Woods SC, Krause EG. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J Neurosci 33: 4825–4833, 2013. doi: 10.1523/JNEUROSCI.3806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals. The National Academies Collection: reports funded by National Institutes of Health. In: Guide for the Care and Use of Laboratory Animals (8th ed.). Washington DC: National Academies Press; 2011. [Google Scholar]
- 20. Soto JE, Burnett CML, Ten Eyck P, Abel ED, Grobe JL. Comparison of the effects of high-fat diet on energy flux in mice using two multiplexed metabolic phenotyping systems. Obesity (Silver Spring) 27: 793–802, 2019. doi: 10.1002/oby.22441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reho JJ, Nakagawa P, Mouradian GC Jr, Grobe CC, Saravia FL, Burnett CML, Kwitek AE, Kirby JR, Segar JL, Hodges MR, Sigmund CD, Grobe JL. Methods for the comprehensive in vivo analysis of energy flux, fluid homeostasis, blood pressure, and ventilatory function in rodents. Front Physiol 13: 855054, 2022. doi: 10.3389/fphys.2022.855054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xue B, Johnson AK, Hay M. Sex differences in angiotensin II-induced hypertension. Braz J Med Biol Res 40: 727–734, 2007. doi: 10.1590/s0100-879x2007000500018. [DOI] [PubMed] [Google Scholar]
- 23. Wang G, Woods C, Johnson MA, Milner TA, Glass MJ. Angiotensin II infusion results in both hypertension and increased AMPA GluA1 signaling in hypothalamic paraventricular nucleus of male but not female mice. Neuroscience 485: 129–144, 2022. doi: 10.1016/j.neuroscience.2021.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ovalles AC, Contoreggi NH, Marques-Lopes J, Van Kempen TA, Iadecola C, Waters EM, Glass MJ, Milner TA. Plasma membrane affiliated AMPA GluA1 in estrogen receptor β-containing paraventricular hypothalamic neurons increases following hypertension in a mouse model of post-menopause. Neuroscience 423: 192–205, 2019. doi: 10.1016/j.neuroscience.2019.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Kempen TA, Narayan A, Waters EM, Marques-Lopes J, Iadecola C, Glass MJ, Pickel VM, Milner TA. Alterations in the subcellular distribution of NADPH oxidase p47(phox) in hypothalamic paraventricular neurons following slow-pressor angiotensin II hypertension in female mice with accelerated ovarian failure. J Comp Neurol 524: 2251–2265, 2016. doi: 10.1002/cne.23944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xue B, Singh M, Guo F, Hay M, Johnson AK. Protective actions of estrogen on angiotensin II-induced hypertension: role of central nitric oxide. Am J Physiol Heart Circ Physiol 297: H1638–H1646, 2009. doi: 10.1152/ajpheart.00502.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Itaya Y, Suzuki H, Matsukawa S, Kondo K, Saruta T. Central renin-angiotensin system and the pathogenesis of DOCA-salt hypertension in rats. Am J Physiol Heart Circ Physiol 251: H261–H268, 1986. doi: 10.1152/ajpheart.1986.251.2.H261. [DOI] [PubMed] [Google Scholar]
- 28. Dai SY, Peng W, Zhang YP, Li JD, Shen Y, Sun XF. Brain endogenous angiotensin II receptor type 2 (AT2-R) protects against DOCA/salt-induced hypertension in female rats. J Neuroinflammation 12: 47, 2015. doi: 10.1186/s12974-015-0261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dai SY, Zhang YP, Peng W, Shen Y, He JJ. Central infusion of angiotensin II type 2 receptor agonist compound 21 attenuates DOCA/NaCl-induced hypertension in female rats. Oxid Med Cell Longev 2016: 3981790, 2016. doi: 10.1155/2016/3981790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dai SY, Fan J, Shen Y, He JJ, Peng W. Endoplasmic reticulum stress in the brain subfornical organ contributes to sex differences in angiotensin-dependent hypertension in rats. Acta Physiol (Oxf) 217: 33–44, 2016. doi: 10.1111/apha.12635. [DOI] [PubMed] [Google Scholar]
- 31. Santollo J, Torregrossa AM, Daniels D. Sex differences in the drinking response to angiotensin II (AngII): effect of body weight. Horm Behav 93: 128–136, 2017. doi: 10.1016/j.yhbeh.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santollo J, Volcko KL, Daniels D. Sex differences in the behavioral desensitization of water intake observed after repeated central injections of angiotensin II. Endocrinology 159: 676–684, 2018. doi: 10.1210/en.2017-00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lenkei Z, Palkovits M, Corvol P, Llorens-Cortès C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol 18: 383–439, 1997. doi: 10.1006/frne.1997.0155. [DOI] [PubMed] [Google Scholar]
- 34. Rathod YD, Di Fulvio M. The feeding microstructure of male and female mice. PLoS One 16: e0246569, 2021. doi: 10.1371/journal.pone.0246569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morselli LL, Claflin KE, Cui H, Grobe JL. Control of energy expenditure by AgRP neurons of the arcuate nucleus: neurocircuitry, signaling pathways, and angiotensin. Curr Hypertens Rep 20: 25, 2018. doi: 10.1007/s11906-018-0824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thunhorst RL, Beltz TG, Johnson AK. Drinking and arterial blood pressure responses to ANG II in young and old rats. Am J Physiol Regul Integr Comp Physiol 299: R1135–R1141, 2010. doi: 10.1152/ajpregu.00360.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Patil CN, Ritter ML, Wackman KK, Oliveira V, Balapattabi K, Grobe CC, Brozoski DT, Reho JJ, Nakagawa P, Mouradian GC Jr, Kriegel AJ, Kwitek AE, Hodges MR, Segar JL, Sigmund CD, Grobe JL. Cardiometabolic effects of DOCA-salt in male C57BL/6J mice are variably dependent upon sodium and non-sodium components of diet. Am J Physiol Regul Integr Comp Physiol 322: R467–R485, 2022. doi: 10.1152/ajpregu.00017.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sampson AK, Hilliard LM, Moritz KM, Thomas MC, Tikellis C, Widdop RE, Denton KM. The arterial depressor response to chronic low-dose angiotensin II infusion in female rats is estrogen dependent. Am J Physiol Regul Integr Comp Physiol 302: R159–R165, 2012. doi: 10.1152/ajpregu.00256.2011. [DOI] [PubMed] [Google Scholar]
- 39. Weidemann BJ, Voong S, Morales-Santiago FI, Kahn MZ, Ni J, Littlejohn NK, Claflin KE, Burnett CM, Pearson NA, Lutter ML, Grobe JL. Dietary sodium suppresses digestive efficiency via the renin-angiotensin system. Sci Rep 5: 11123, 2015. doi: 10.1038/srep11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burnett CM, Grobe JL. Direct calorimetry identifies deficiencies in respirometry for the determination of resting metabolic rate in C57Bl/6 and FVB mice. Am J Physiol Endocrinol Physiol 305: E916–E924, 2013. doi: 10.1152/ajpendo.00387.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]