Keywords: arterial stiffness; 3,3-dimethyl-1-butanol; endothelial function; high-fat diet; physical function
Abstract
Consumption of a Western-style diet (WD; high fat, high sugar, low fiber) is associated with impaired vascular function and increased risk of cardiovascular diseases (CVD), which could be mediated partly by increased circulating concentrations of the gut microbiome-derived metabolite trimethylamine N-oxide (TMAO). We investigated if suppression of TMAO with 3,3-dimethyl-1-butanol (DMB; inhibitor of microbial TMA lyase) in mice could prevent: 1) WD-induced vascular endothelial dysfunction and aortic stiffening and 2) WD-induced reductions in endurance exercise tolerance and increases in frailty, as both are linked to WD, vascular dysfunction, and increased CVD risk. C57BL/6N mice were fed standard chow or WD (41% fat, ∼25% sugar, 4% fiber) for 5 mo beginning at ∼2 mo of age. Within each diet, mice randomly received (n = 11–13/group) normal drinking water (control) or 1% DMB in drinking water for the last 8 wk (from 5 to 7 mo of age). Plasma TMAO was increased in WD-fed mice but suppressed by DMB. WD induced endothelial dysfunction, assessed as carotid artery endothelium-dependent dilation to acetylcholine, and progressive increases in aortic stiffness (measured serially in vivo as pulse wave velocity), both of which were fully prevented by supplementation with DMB. Endurance exercise tolerance, assessed as time to fatigue on a rotarod test, was impaired in WD-fed mice but partially recovered by DMB. Lastly, WD-induced increases in frailty (31-point index) were prevented by DMB. Our findings indicate DMB or other TMAO-lowering therapies may be promising for mitigating the adverse effects of WD on physiological function, and thereby reducing risk of chronic diseases.
NEW & NOTEWORTHY We provide novel evidence that increased circulating concentrations of the gut microbiome-derived metabolite trimethylamine N-oxide (TMAO) contribute to vascular dysfunction associated with consumption of a Western-style diet and that this dysfunction can be prevented by suppressing TMAO with DMB, thereby supporting translation of this compound to humans. Furthermore, to our knowledge, we present the first evidence of the role of TMAO in mediating impairments in endurance exercise tolerance and increased frailty in any context.
INTRODUCTION
Consumption of a “Western-style” diet (WD) that is high in fat and sugar, and low in fiber and nutrient density increases risk for cardiovascular diseases (CVD) (1–3), which remain the leading cause of death in the developed world (4). Although the mechanisms by which WD so greatly exacerbates risk of CVD are multifaceted, a primary means is through the development of vascular dysfunction (5–8), characterized by vascular endothelial dysfunction and stiffening of the large elastic arteries, both of which are independent risk factors for CVD (9, 10). Vascular dysfunction with WD is largely driven by excess superoxide-related oxidative stress and subsequent reductions in bioavailability of the vasodilatory molecule nitric oxide (NO) (5, 6, 11).
The upstream mechanisms by which consumption of a WD induces vascular dysfunction are incompletely understood, but the gut microbiome is likely involved because 1) the gut microbiome is a strong influencer of host physiology through the production and release of metabolites that enter systemic circulation (12–14) and 2) WD consumption induces adverse changes in gut microbiome composition and the profile of metabolites it produces (15–19). A well-established gut microbiome-derived metabolite implicated in the development of age-related vascular dysfunction (20, 21) and CVD (22–24) is trimethylamine N-oxide (TMAO). Indeed, circulating concentrations of TMAO are typically higher in individuals with Westernized dietary patterns (25, 26); however, the role of TMAO in mediating WD-induced vascular dysfunction has not been explored.
Microbial production of TMAO can be suppressed with 3,3-dimethyl-1-butanol (DMB) (27). Accordingly, the primary purpose of the present study was to investigate the potential therapeutic effects of supplementation with DMB for mitigating WD-associated vascular dysfunction in mice. We hypothesized that WD feeding would impair endothelial function and increase aortic stiffness, but that DMB supplementation would mitigate these impairments back towards levels observed in mice fed standard rodent chow (i.e., a “non”-WD). We expected that improvements in vascular function with DMB would be mediated by preserved NO bioavailability and suppression of superoxide-associated oxidative stress.
As a secondary exploratory aim, we investigated whether DMB supplementation could attenuate WD-associated reductions in endurance during whole body dynamic exercise (i.e., endurance exercise tolerance) and increases in frailty. Vascular endothelial dysfunction is linked to lower endurance exercise tolerance (28–30) and is also thought to be an early predictor of frailty (31, 32), a clinical syndrome characterized by impairments in multiple domains of physiological function and vulnerability to adverse health outcomes. Indeed, endurance exercise tolerance is reduced (33, 34) and the prevalence of clinical frailty is ∼60% greater (33, 35–37) in individuals who habitually consume a WD compared with those who adhere to healthier dietary patterns. In turn, both reduced exercise tolerance (38, 39) and accelerated development of frailty (40–42) exacerbate risk for CVD. For this exploratory aim, we hypothesized that supplementation with DMB in WD-fed mice would restore endurance exercise capacity and attenuate frailty towards levels observed in standard chow-fed mice.
MATERIALS AND METHODS
Animals
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Colorado Boulder (Protocol No. 2539) and adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
A total of 50 C57Bl/6N male mice were purchased for this study from Charles River at 8 wk of age. Mice were housed in a conventional facility on a 12-h light/dark cycle. Mice were given ad libitum access to drinking water and fed either standard rodent chow diet (SC; n = 26; Envigo, Cat. No. 7917: https://insights.envigo.com/hubfs/resources/data-sheets/7017-datasheet-0915.pdf; Envigo, Madison, WI) or a customized WD (n = 24; Envigo Teklad TD.96132: https://insights.envigo.com/hubfs/resources/data-sheets/96132.pdf; Envigo). WD chow was designed to approximately match the 75th percentiles of US diets for total fat and sugar intake based on NHANES data (43, 44). Macronutrient content of the two diets is provided in Table 1. Per the manufacturers, content of TMAO precursors choline and l-carnitine were comparable between the two diets, although the manner in which these were provided did differ (see Table 1 caption) and choline from phosphatidylcholine in the ingredients added to the WD to increase fat content were not accounted for. Mice were multihoused until the beginning of the intervention (∼5 mo of age), at which point they were single-housed (at least 1 wk before baseline testing) to avoid mouse-to-mouse effects on the gut microbiome (mice are coprophagic) and to ensure accurate quantification of water and food intake.
Table 1.
Macro- and select micronutrient content of diets
| Standard Chow Envigo Cat. No. 7917 |
Western Diet TD.96132 |
|
|---|---|---|
| Energy density, kcal/g | 3.0 | 4.5 |
| Total fat, % kcal | 14 | 41 |
| Fatty acid profile, % total fat | ||
| Saturated fats | 21 | 41 |
| Trans fats | 0 | 17 |
| Monounsaturated fats | 29 | 35 |
| Polyunsaturated fats | 50 | 7 |
| Protein, % kcal | 24 | 19 |
| Carbohydrate, % kcal | 62 | 41 |
| Fiber, % kg | 18 | 4 |
| Choline, mg/kg | ∼1,890 | ∼1,660 |
To achieve higher fat content of the WD, hydrogenated vegetable shortening and beef tallow (both 100 g/kg) were added. The following were added to the WD to increase sugar content and made up all kcal from carbohydrates: sucrose (183 g/kg), cornstarch (160 g/kg), and maltodextrin (120 g/kg). The majority of fiber in both diets was insoluble fiber. Approximately 772 mg/kg of choline in the SC was provided as supplemental choline chloride; the remainder was in other ingredients. Choline content of the WD was provided in a vitamin mix (choline from phosphatidylcholine in added fat ingredients not accounted for). Both diets contained very low amounts of l-carnitine from fishmeal (SC) or casein (WD). Both diets were irradiated. SC, standard chow; WD, Western diet.
DMB Supplementation and Overall Study Design
At ∼5 mo of age (i.e., after ∼3 mo in our facility and on the diets), mice were randomly assigned (n = 11–13/treatment group/diet) to continue receiving normal drinking water (control; groups: SC-C or WD-C) or drinking water supplemented with 1% (vol/vol) DMB (groups: SC-DMB or WD-DMB; Sigma-Aldrich, Corp., St. Louis, MO), as previously described (20, 27), both administered in volumetric water bottles that allowed for precise quantification of water intake. Water bottles were shaken and replenished as needed 3 times per week; they were completely refreshed every 2 wk. Body weight, water intake, and food consumption were measured at least 2–3 times per week throughout the intervention. All mice survived the full intervention and were studied.
Functional in vivo (noninvasive) testing to serially assess aortic pulse wave velocity (aPWV), tail cuff blood pressure, exercise endurance, and grip strength was conducted over ∼10 days immediately before and after 8 wk of the intervention. aPWV was also assessed after 4 wk of the intervention.
Mice were euthanized for terminal measures and collection of tissues after 8 wk of the intervention. After first assessing frailty index, mice were euthanized by exsanguination via cardiac puncture while anesthetized under inhaled isoflurane (>5%), immediately followed by removal of the carotid arteries and heart. Heparinized blood was separated by centrifugation and plasma was stored at −80°C for later analysis of TMAO and related metabolites and cholesterol. The carotid arteries were immediately used for isolated vessel experiments. The carotid arteries were used, rather than other arteries, because our laboratory has established carotid artery endothelium-dependent dilation (EDD) to acetylcholine is a surrogate for brachial artery flow-mediated dilation in humans (20, 45), which is an independent risk factor for CVD (46). The thoracic aorta was excised, cleaned of perivascular adipose and other surrounding tissue, segmented into rings 1.0–1.5 mm in length, and frozen appropriately for later assessment of intrinsic mechanical stiffness. The quadriceps were isolated and flash frozen to investigate potential mechanisms of differences in exercise tolerance. Other key internal organs and skeletal muscles were dissected and weighed.
Detailed descriptions of all procedures are provided below. All investigators were blinded to diet and treatment group for data collection and plasma and biochemical analyses.
Plasma TMAO and Related Metabolites
Circulating concentrations of TMAO and its dietary precursors choline, l-carnitine, and betaine were quantified in heparinized plasma by isocratic ultraperformance liquid chromatography tandem mass spectrometry (LC-MS) using a stable isotope dilution method against internal standards, as previously described (20, 47, 48).
Plasma Cholesterol
Total circulating concentrations of high-density lipoprotein (HDL) cholesterol and combined low- and very-low-density lipoprotein (LDL/VLDL) cholesterol were determined in heparinized plasma by colorimetric commercially available kit (Abcam, Cambridge, United Kingdom; Cat. No. ab65390), according to manufacturer’s instructions. Using this kit, LDL/VLDL cholesterol were first separated from HDL cholesterol using a precipitation buffer and cholesterol esterase was added to hydrolyze cholesteryl ester into free cholesterol, i.e., the assay measured total cholesterol.
Vascular Endothelial Function
Immediately after euthanasia, the carotid arteries were excised, cleaned free of surrounding tissue, and cannulated onto glass pipette tips in pressure myograph chambers (DMT, Inc.; Aarhus, Denmark) containing buffered physiological saline solution. Arteries were maintained at 37°C, pressurized to ∼50 mmHg, allowed to equilibrate to these conditions for 45 min before the beginning of experiments, and preconstricted with 20 µM phenylephrine (Sigma-Aldrich Corp.) before all dose responses. Endothelium-dependent dilation (EDD) was assessed by measuring the increase in luminal diameter in response to incremental doses of acetylcholine (ACh; 1 × 10−9 to 1 × 10−4 M; Sigma-Aldrich Corp.). To determine the contribution of NO to EDD, dose responses to ACh were repeated on one artery following 30-min incubation with the NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 100 µM; Sigma-Aldrich Corp.). To determine the extent to which excessive superoxide suppresses EDD, dose responses to ACh were repeated on the other artery following 60-min incubation with the superoxide dismutase mimetic 4-hydroxy-2,2,6,6,-tetramethylpiperidin-1-oxyl (TEMPOL; 1 mM; Sigma-Aldrich, Corp.). To assess smooth muscle sensitivity to NO, endothelium-independent dilation (EID) to increasing doses of the exogenous NO donor sodium nitroprusside (SNP; 1 × 10−10 to 1 × 10−4 M; Sigma-Aldrich, Corp.) was determined in both arteries. At the end of dose responses, maximal dilation was confirmed by 20-min incubation in Ca2+-depleted physiological saline solution. To account for differences in vessel diameter, all dose response data are presented as % dilation between the preconstricted diameter before each dose response and the maximal diameter. Peak EDD was determined as the largest diameter achieved during the dose response, at any dose of ACh.
Aortic Stiffness
In vivo aortic stiffness was assessed serially by the aortic pulse wave velocity (aPWV) at baseline and after 4 and 8 wk of the intervention, as previously described (5, 21). Briefly, mice were anesthetized (2% isoflurane with oxygen adjusted to maintain heart rate between 400–500 beats/min) and placed supine on a warming pad. Doppler ultrasound probes (Indus Instruments, Webster, TX) were placed over the transverse aortic arch and abdominal aorta. Time delay between the R-wave of the electrocardiogram and the foot of the Doppler signal was determined for each site, and aPWV was calculated as the physical distance between the probes divided by the difference in time delay between sites.
As aPWV is influenced by in vivo factors (e.g., endothelial function and blood pressure), we also measured intrinsic mechanical stiffness of the aorta ex vivo to isolate the structural component of aortic stiffness, as previously described (5, 21). At euthanasia, 1.0–1.5 mm rings of thoracic aorta were saved and stored frozen in physiological saline solution for later stress-strain testing. Rings were mounted in a warmed (37°C) force myograph chamber (DMT) filled with calcium-free phosphate-buffered saline. Ring diameter was increased to achieve an initial force of 1 mN and then stretched in increments of 50 µm every 3 min until failure. Force for each degree of stretch was recorded and used to construct stress-strain curves, where strain and stress were calculated as follows:
where d is diameter and di is initial diameter.
where L is one-dimensional load, H is wall thickness, and D is vessel length.
The elastic modulus of the curve was calculated as the slope of linear high-force region of the curve (i.e., last 4 points before failure).
To calculate stress and strain, aortic wall thickness and diameter were measured in sections of thoracic aorta. At the time of euthanasia, ∼1 mm sections were thaw-mounted in optimal cutting temperature (OCT) compound in liquid nitrogen-cooled 2-methylbutane and stored at −80°C. Samples were later sectioned (7 µm; Leica CM 3000; Leica Biosystems, Wetlzlar, Germany) at −22°C, plated onto untreated tissue preparation slides, and bright-field images were captured with a Nikon Eclipse TS100 photomicroscope (Nikon, Tokyo, Japan) at ×4 magnification. Images were analyzed using ImageJ software (National Institutes of Health, Bethesda, MA). For analysis of IMT, the distinction between medial and adventitial layers was determined to be the point at which the regular banding patterns (media) abruptly shift to diffuse patterning (adventitia).
Blood Pressure
Blood pressure was assessed in vivo at baseline and after 8 wk of the intervention using a CODA noninvasive tail cuff system (Kent Scientific, Torrington, CT). Mice were awake for these measurements and placed in restrainer tubes on a warm pad. Five acclimation cycles followed by 20 data collection cycles of blood pressure measurements from the tail artery were recorded. Blood pressure measurements were conducted at the same time of day on 3 consecutive days and averaged. No other tests were performed on the same day as blood pressure measurements.
Aerobic Exercise Endurance
Exercise endurance testing was conducted over 3 consecutive days before and after the intervention using a rota-rod device, as previously described (49). On day 1, a familiarization session was conducted in which mice were replaced onto a 5-lane rota-rod (Ugo Basile, Comerio, Italy) at low speed until they could stay on the rota-rod for at least 90 s. On day 2, mice performed three trials of an accelerating rota-rod test, each separated by ∼1 h. Briefly, mice were placed onto the rota-rod, which was gradually accelerated from 4 to 40 revolutions/min over 5 min. Latency to fall was recorded and speed at fall was calculated based on the current revolutions/min. Maximal running speed was considered the fastest speed attained across the three trials.
On day 3, mice performed one trial of an endurance rota-rod test. Mice with similar maximal running speeds (from the baseline accelerating rota-rod test) were run at the same time on the 5-station rota-rod, with speeds determined as the average maximal running speed of each set of five mice. Although the accelerating rota-rod test was also performed postintervention, the baseline maximal running speeds were still used to determine speeds for the postintervention endurance rota-rod test, such that absolute workload was constant pre/post intervention. Following a warm-up period (2 min at 25% maximal speed and 5 min at 50% maximal speed), mice ran at 75% of maximal running speed until falling or for up to 10 min. If still running, the speed was increased to 100% of maximal running speed until falling or for up to 20 min. Mice were allowed to fall and were placed back on the rota-rod once. Latency to the second fall was recorded, and total distance run until this time of fatigue was calculated.
Protein Quantification in Skeletal Muscle
Quadriceps muscles were lysed in RIPA buffer containing protease inhibitors in a Bullet Blender (Next Advance; Troy, NY). Total protein was quantified using a BCA assay (Pierce BCA Protein Assay Kit; Thermo Fisher, Waltham, MA; Cat. No. 23225). Protein abundance was determined using capillary electrophoresis Western detection using a WES instrument (ProteinSimple, San Jose, CA). Aliquots of quadriceps muscle lysate containing 3.2 µg of protein were separated by capillary electrophoresis. Electrophoretic separation and immunodetection were performed automatically using the default settings, except primary antibody incubation phase was extended to 150 min. Samples, blocking reagent, wash buffer, primary antibodies, species-appropriate secondary antibodies (ProteinSimple), and chemiluminescent substrate were aliquoted by the instrument. Resulting data were analyzed with the installed Compass software (ProteinSimple). Primary antibodies used were antiphosphorylated adenosine monophosphate-activated protein kinase (p-AMPK; 1:100; Cell Signaling Technology, Danvers, MA; Cat. No. 2535 s), anti-total AMPK (1:100; Cell Signaling Technology; Cat. No. 2603), anticitrate synthase (1:500; Abcam; Cat. No. ab96600), and antiglyceraldehyde 3-phosphate dehydrogenase (GAPDH, loading control; 1:200; Cell Signaling Technology, Cat. No. 2118). Prior to use, all antibodies used were validated for specificity using recombinant protein, either performed by ProteinSimple or by B.P.Z. and optimized for appropriate dilution and reproducibility in quadriceps muscle lysates by B.P.Z. Relative intensity of p-AMPK, AMPK, and citrate synthase were normalized against intensity of GAPDH signal. Electropherograms in figures are represented as pseudo blots generated using the Compass software.
Frailty Index
Frailty was assessed by the same blinded investigator (V.E.B.) for all mice using a 31-point clinical frailty index developed and validated by Whitehead et al. (50). Mice were given scores of 0 (=absent), 0.5 (=mild), or 1 (=severe) for each of the following criteria. Integumentary: alopecia, loss of fur color, dermatitis, loss of whiskers, and coat condition; physical/musculoskeletal: tumors, distended abdomen, kyphosis, tail stiffening, gait disorders, tremor, forelimb grip strength, and body condition score; vestibulocochlear/auditory: vestibular disturbance and hearing loss; ocular/nasal: cataracts, corneal opacity, eye discharge/swelling, microphthalmia, vision loss, menace reflex, and nasal discharge; digestive/urogenital: malocclusions, rectal prolapse, penile prolapse, and diarrhea; respiratory: breathing rate/depth; discomfort: mouse grimace scale and piloerection; temperature score; and body weight score.
Forearm grip strength was measured as the average force over 5–10 trials (30 s between trials) recorded at forepaw release from a custom grip strength device that includes a force transducer (0.5 kg; Imada PS Series, Northbrook, IL) attached to a trapeze grip bar 1.5 mm in diameter. After 2–3 min of acclimation to being held and being able to explore the grip strength device, each mouse was grasped by its tail, suspended just above the trapeze bar, and lowered until it successfully grasped the bar with both forepaws. A gradual horizontal tug was then applied until the mouse released its grip. Grip strength was assessed by the same investigator (V.E.B.) for all mice at all timepoints.
Hearing was assessed by making a sharp noise (ballpoint pen click) just behind the mouse’s head and watching for a response in the direction of the sound (50). Vision was assessed by holding the mouse by its tail above a counter, slowly lowering it to the counter, and watching for when it reached its paws out to land (vision loss was considered severe if the mouse did not reach out until its nose was within 1 cm of the counter) (50). Mouse grimace scale involved inspecting for signs of pain, for example, orbital tightening, nose/cheek flattening, ears back.
To determine scores for grip strength and body weight, mean and SD were determined for each measure across all healthy SC-fed mice studied within our laboratory between 2017 and 2018. A score of “0” was assigned for mice within 1 SD of this mean (grip strength ≥120 kg; body weight: 30.4–36.8 g), “0.5” was assigned for mice within 1–2 SD of this mean (grip strength: 100–119 kg; body weight: 27.2–30.3 g or 36.9–40.1 g), and a score of “1” was assigned for mice >2 SD of this mean (grip strength ≤99 kg; body weight: <27.2 g or >40.1 g).
Statistical Analyses
Based on previous studies conducted in our laboratory, we considered a difference across groups of ≥10% in peak carotid artery EDD and ≥50 cm/s in aPWV (i.e., our primary outcome variables) to be the minimum difference that would be physiologically meaningful. The pooled standard deviation from a pilot study comparing old control versus DMB-supplemented mice was 17.5% for peak EDD and 82 cm/sec for PWV, yielding effect sizes of 0.57 and 0.61, respectively. With these effect sizes, we determined that n = 9 mice per condition would be sufficient to detect differences in carotid artery EDD across groups (two-way design) and in aPWV across groups × time into the intervention with >85% statistical power using a significance level of α = 0.05. To ensure we would obtain useable data from at least n = 9 mice/group for each outcome, we aimed for n = 11 mice/group to complete the intervention. Starting group sizes for the DMB-supplemented mice were larger (n = 13) to account for any potential taste aversions to the DMB that might result in poor adherence to the intervention; however, all mice drank sufficiently and were included in analyses.
Statistical analyses were performed in Prism Version 9.2.0 (GraphPad; San Diego, CA). All data are presented as means ± SE, with individual data provided when possible. Statistical significance was set at α = 0.05. All data were checked for statistical outliers (ROUT: Q = 1%) and outliers were removed if identified.
Differences in all variables measured at end-intervention/euthanasia, with the exception of carotid artery vasodilatory dose responses, were assessed using two-way ANOVA with factors of diet (SC vs. WD) and treatment (control vs. DMB). When significant interaction or main effects were observed, pairwise differences were compared using Šidák’s multiple comparisons test. Differences in carotid artery EDD and EID across increasing doses of ACh or SNP were compared using two-way mixed ANOVA with between factor of group and repeated factor of drug dose. Differences in weekly body mass, aPWV, tail cuff systolic and diastolic blood pressures, endurance capacity (distance to fatigue), and grip strength were compared using two-way mixed ANOVA with between factor of group (SC-C, SC-DMB, WD-C, vs. WD-DMB) and repeated factor of time across the intervention. When significant main effects were observed, pairwise comparisons were made with Tukey’s post hoc test. Two-way ANOVAs were used for comparisons of vasodilatory dose response data and variables measured across the intervention because the statistical power was not sufficient to perform three-way mixed ANOVAs (to separate effects of diet and treatment). However, to confirm significant differences in repeated variables, we also compared the 8-wk/end-intervention timepoint across diets and treatments using two-way ANOVA, as described above for end-intervention variables (presented in tables or figure captions; not performed for dose responses as peak EDD and EID were already compared in this manner). Differences in peak EDD in the presence of TEMPOL were compared between control versus DMB-treated WD-fed mice using Student’s unpaired t test. The relation between peak EDD and changes in aPWV across the intervention was determined using simple linear regression and Pearson’s correlation. Lastly, to determine if differences in body mass influenced the effects of DMB on key outcomes, we performed two-way analysis of covariance (ANCOVA) with factors of diet × treatment and body mass included as a covariate for peak EDD, aPWV at 8 wk, endurance rota-rod distance-to-fatigue at 8 wk, and frailty score. P values provided in the text are pairwise comparisons unless otherwise specified.
RESULTS
Water Intake and Plasma Concentrations of TMAO and Related Metabolites
Average daily intake of control versus DMB-supplemented water is provided in Table 2. WD-fed mice in both treatment groups drank less water on average than standard chow-fed mice (effect of diet: P < 0.0001). DMB did not affect water intake in standard chow-fed mice (P = 0.38). Water intake was lower in DMB-treated versus control WD-fed mice (P = 0.047) but appeared to still be sufficient to maintain adequate hydration based on veterinary examination (no loss of skin elasticity, sunken eyes, weakness, etc.).
Table 2.
Mouse characteristics
| Group |
ANOVA P Values |
||||||
|---|---|---|---|---|---|---|---|
| SC-C | SC-DMB | WD-C | WD-DMB | Interaction | Diet | Treatment | |
| n | 13 | 13 | 11 | 13 | |||
| Water intake, mL/day | 3.8 ± 0.1 | 3.5 ± 0.2 | 2.6 ± 0.1* | 2.0 ± 0.1† | 0.39 | <0.0001 | 0.01 |
| Plasma concentrations of TMAO-related metabolites | |||||||
| Choline, µM | 36 ± 3 | 39 ± 4 | 93 ± 9* | 74 ± 6b | 0.07 | <0.0001 | 0.17 |
| l-carnitine, µM | 23 ± 2 | 18 ± 2 | 11 ± 1* | 9 ± 2 | 0.65 | <0.0001 | 0.06 |
| Betaine, µM | 86 ± 21 | 68 ± 14 | 46 ± 9a | 28 ± 5 | 0.98 | <0.01 | 0.19 |
| Body mass at euthanasia, g | 30.9 ± 0.9 | 30.4 ± 0.7 | 40.7 ± 2.0* | 36.8 ± 1.3b | 0.18 | <0.0001 | 0.09 |
| Mass of key organs | |||||||
| Total heart, mg | 133 ± 3 | 132 ± 3 | 156 ± 4* | 152 ± 6 | 0.69 | <0.0001 | 0.58 |
| Left ventricle, mg | 86 ± 4 | 88 ± 2 | 96 ± 3* | 97 ± 4 | 0.81 | <0.01 | 0.61 |
| Lungs, mg | 165 ± 5 | 163 ± 5 | 191 ± 6* | 189 ± 6 | 0.97 | <0.0001 | 0.69 |
| Liver, g | 1.5 ± 0.1 | 1.5 ± 0.03 | 2.7 ± 0.3* | 2.2 ± 0.2† | 0.11 | <0.0001 | 0.08 |
| Kidneys, mg | 408 ± 9 | 417 ± 19 | 449 ± 14a | 454 ± 8 | 0.87 | <0.01 | 0.59 |
| Visceral fat, g | 0.9 ± 0.1 | 0.7 ± 0.1 | 2.4 ± 0.2* | 2.0 ± 0.2 | 0.42 | <0.0001 | 0.08 |
| Spleen, mg | 72 ± 2 | 74 ± 4 | 105 ± 5* | 91 ± 2† | 0.02 | <0.0001 | 0.12 |
| Brain, mg | 397 ± 13 | 402 ± 12 | 447 ± 9* | 443 ± 9 | 0.70 | <0.001 | 0.95 |
| Skeletal muscle mass | |||||||
| Quadriceps, mg | 339 ± 17 | 349 ± 20 | 416 ± 11* | 422 ± 6 | 0.89 | <0.0001 | 0.60 |
| Gastrocnemius, mg | 270 ± 7 | 269 ± 9 | 328 ± 6* | 321 ± 4 | 0.59 | <0.0001 | 0.60 |
| Tibialis anterior, mg | 101 ± 6 | 102 ± 6 | 74 ± 5* | 74 ± 6 | 0.94 | <0.0001 | 0.91 |
| Soleus, mg | 19 ± 1 | 18 ± 2 | 21 ± 1 | 23 ± 1 | 0.22 | 0.04 | 0.57 |
| Food intake, kcal/day | 12.5 ± 0.3 | 12.6 ± 0.2 | 13.6 ± 0.3* | 12.5 ± 0.2† | <0.01 | 0.04 | 0.05 |
| Norm. food intake, kcal/day/g BW | 0.43 ± 0.01 | 0.42 ± 0.01 | 0.36 ± 0.01* | 0.36 ± 0.01* | 0.45 | <0.0001 | 0.48 |
| Artery diameters, µm | |||||||
| Carotid artery resting | 447 ± 9 | 435 ± 6 | 436 ± 10 | 430 ± 8 | 0.73 | 0.30 | 0.36 |
| Carotid artery, maximal | 453 ± 9 | 443 ± 7 | 455 ± 11 | 448 ± 8 | 0.86 | 0.36 | 0.67 |
| Aorta, resting | 646 ± 15 | 604 ± 13 | 627 ± 19 | 608 ± 21 | 0.53 | 0.67 | 0.09 |
Data are mean ± SE. Groups are standard chow-control water (SC-C), standard chow-DMB water (SC-DMB), Western diet-control water (WD-C), and Western diet-DMB water (WD-DMB). Statistics are two-way ANOVA with Šidák’s multiple comparisons tests. Symbols denote pairwise comparisons as follows: *P < 0.05 vs. SC-C; †P < 0.05 vs. WD-C; aP < 0.10 vs. SC-C; bP < 0.15 vs. WD-C. Statistical outliers (ROUT: Q = 1%) were removed as follows: two for l-carnitine (1 SC-C group, 1 SC-DMB); two for kidney and spleen masses—these data were from the same two mice in the WD-DMB group who had enlarged kidneys and spleens (the WD-DMB group average with these mice still included were 473 ± 15 mg for kidneys and 112 ± 15 mg for spleen); and one from aorta diameter (SC-DMB). BW, body weight; TMAO, trimethylamine N-oxide.
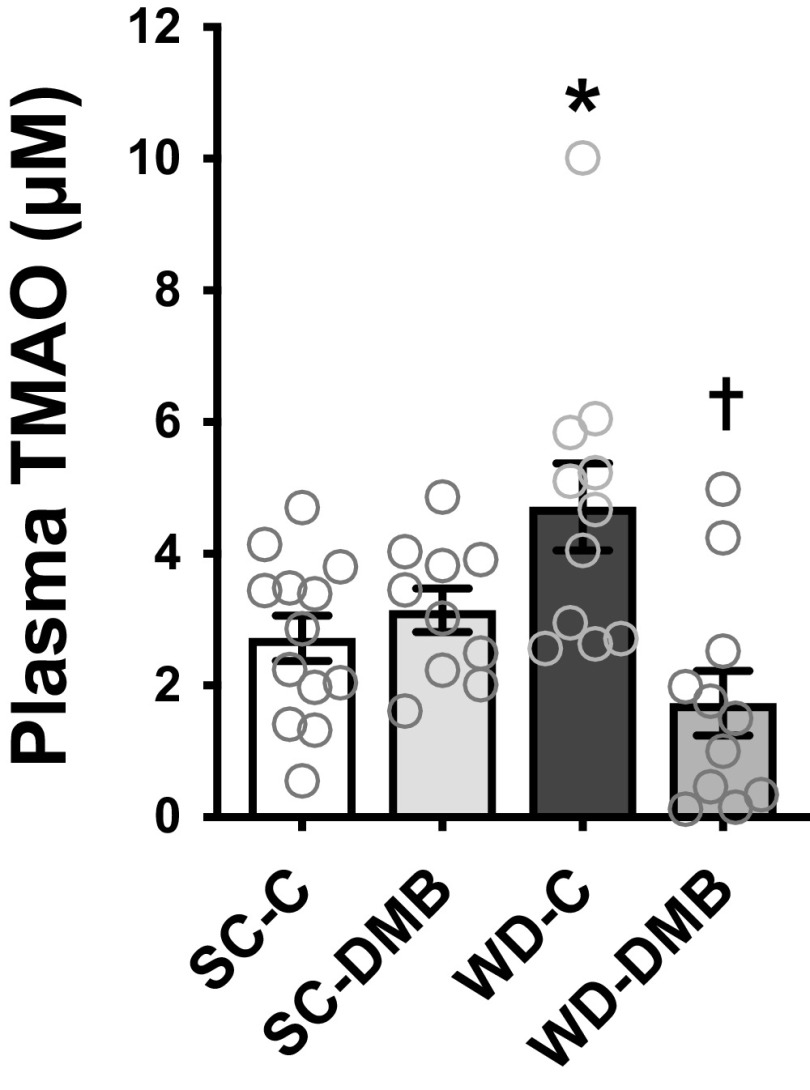
Plasma concentrations of both TMAO (Fig. 1) and dietary-based precursors of TMAO (Table 2) were measured by LC-MS. WD feeding increased plasma TMAO concentrations compared with standard chow-fed mice (SC-C vs. WD-C: P < 0.01). DMB treatment suppressed WD-associated increases in plasma TMAO (WD-C vs. WD-DMB: P < 0.0001) but did not affect plasma TMAO levels in standard chow-fed mice (SC-C vs. SC-DMB: P = 0.77). Relative to standard chow-fed mice, WD-fed mice had higher plasma concentrations of the TMAO precursor choline and lower concentrations of l-carnitine and betaine (effect of diet: P < 0.01 for all three). With the exception that DMB tended to mildly lower choline levels in WD-fed mice (P = 0.051), there were no effects of DMB treatment on circulating concentrations of these precursors (post hoc tests for all other Control vs. DMB comparisons P ≥ 0.14). As such, DMB supplementation suppressed WD-induced increases in TMAO without obviously affecting its precursors.
Figure 1.
Supplementation with 1% 3,3-dimethyl-1-butanol (DMB) prevented Western-style diet (WD)-induced increases in plasma concentrations of trimethylamine N-oxide (TMAO). Young mice (6–7 mo at euthanization) were fed either standard rodent chow (SC) or WD and given either normal drinking water (control; SC-C, WD-C) or water supplemented with 1% DMB (3,3-dimethyl-1-butanol; SC-DMB, WD-DMB) for 8–10 wk. n = 10–13 mice/group. Data are means ± SE with individual data. There were no statistical outliers. Two-way ANOVA, diet × treatment interaction: P < 0.001; diet effect: P = 0.54; treatment effect: P = 0.01. Šidák’s multiple comparisons test: *P < 0.05 vs. SC-C; †P < 0.05 vs. WD-C.
Mouse Characteristics
Body mass and mass of key internal organs and skeletal muscles at euthanasia are presented in Table 2. Body mass over the intervention is presented in Fig. 2A. Relative to standard chow-fed mice, WD-fed mice had higher body mass at the beginning of the intervention (i.e., after ∼3 mo on the diet; P < 0.001). Body mass remained stable over the 8- to 10-wk intervention in standard chow-fed mice but continued to increase in WD-fed mice (P < 0.01 baseline vs. 10 wk). There were no differences in body mass at any time point between control versus DMB-treated standard chow-fed mice (all P > 0.85), whereas DMB treatment attenuated further increases in body mass across the intervention in WD-fed mice. At the time of euthanasia, body mass was higher in WD-C mice versus SC-C mice (P < 0.001) but DMB tended to attenuate this WD-induced increase in body mass (P = 0.07 WD-DMB vs. WD-C).
Figure 2.
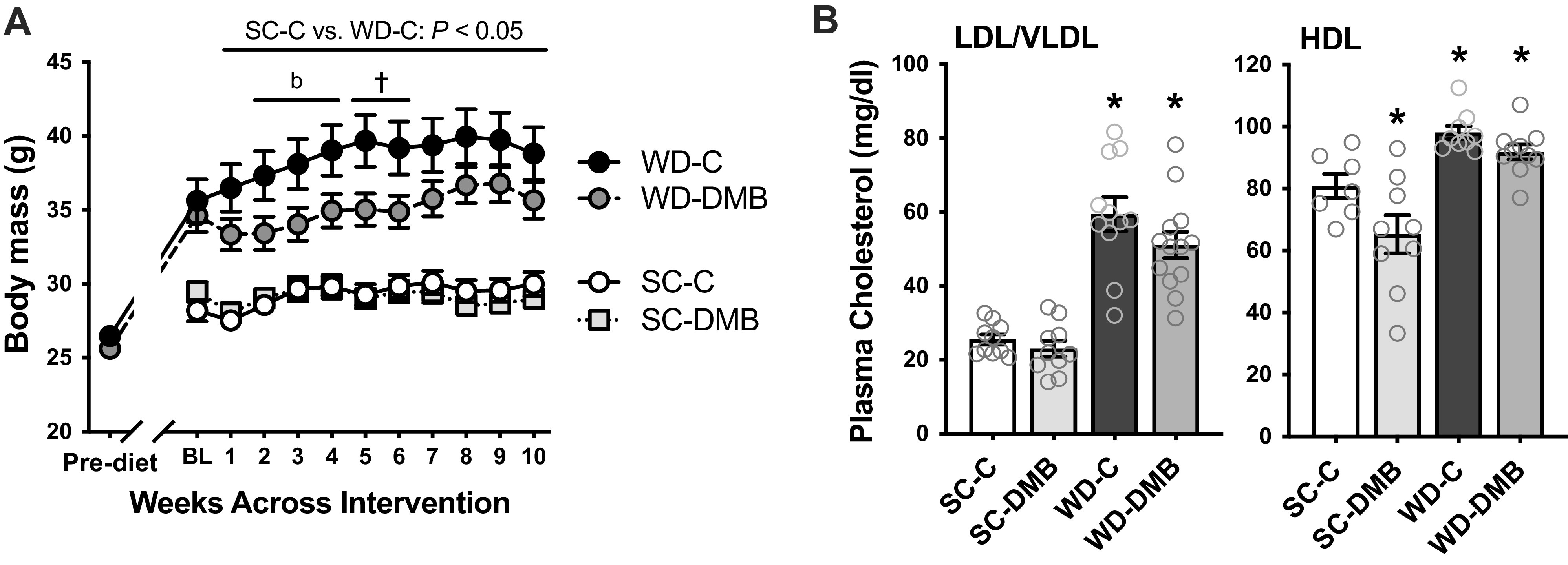
Supplementation with 3,3-dimethyl-1-butanol (DMB) attenuates Western-style diet (WD)-induced gains in body weight without affecting circulating cholesterol. A: body mass measured before initiation of the standard rodent chow (SC) diet or WD, and over the 8–10-wk control (C) vs. 1% DMB (3,3-dimethyl-1-butanol) supplementation intervention (body mass interpolated at 9 and 10 wk of intervention for mice euthanized in wk 8 or 9). B: combined low-density and very low-density lipoprotein (LDL/VLDL) cholesterol (left) and high-density lipoprotein (HDL) cholesterol (right) in heparinized plasma collected at euthanasia from SC and WD-fed control and DMB-supplemented mice. Data are means ± SE. n = 11–13 mice/group. *P < 0.05 vs. SC-C. †P < 0.05 WD-C vs. WD-DMB within time point. bP < 0.10 WD-C vs. WD-DMB. Stats are two-way mixed ANOVA, with Tukey’s post hoc test for A and Šidák’s multiple comparisons test for B.
WD feeding increased the mass of key internal organs, including the heart, lungs, liver, spleen, and brain (all P < 0.01 WD-C vs. SC-C). Kidney mass tended to be higher as well (P = 0.07). Visceral fat mass (epididymal white adipose tissue) was also higher in WD-C mice (P < 0.0001 vs. SC-C). DMB attenuated WD-associated increases in liver (WD-C vs. WD-DMB: P = 0.04) and spleen (P = 0.02) mass, but no other comparisons within WD-fed mice reached statistical significance (all P ≥ 0.13). Two mice in the WD-DMB group had enlarged spleens and were removed from statistical analyses as outliers. Importantly, DMB treatment had no effect on the mass of key organs in standard chow-fed mice (SC-C vs. SC-DMB all P > 0.73). Masses of skeletal muscles are also presented in Table 2 but discussed in Aerobic Exercise Endurance.
Average food intake across the intervention (Table 2) was higher in WD-C versus SC-C mice (P < 0.01). DMB treatment did not affect food intake in standard chow-fed mice (P = 0.83) but reduced food intake in WD-fed mice (P < 0.01). When food intake was normalized to body mass (calculated at each time point and then averaged across the intervention), there was no difference between WD-C versus WD-DMB mice (P > 0.99). As such, it appears that the lower food intake was secondary to attenuation of WD-induced gains in body mass with DMB.
As attenuation of WD-induced increases in body mass with DMB was accompanied by lower visceral fat and liver masses, we measured circulating cholesterol concentrations (Fig. 2B). Both plasma LDL/VLDL (sum of both) and HDL cholesterol were higher in WD-fed versus SC-C mice (effect of diet: P < 0.0001 for both). There was a slight reduction in plasma HDL cholesterol in SC-fed mice supplemented with DMB (P = 0.02); however, there was no effect of DMB on LDL/VLDL cholesterol within either diet (effect of treatment: P = 0.11), nor on HDL cholesterol in WD-fed mice (P = 0.44). Thus, DMB attenuated gains in body mass without noticeably affecting circulating cholesterol.
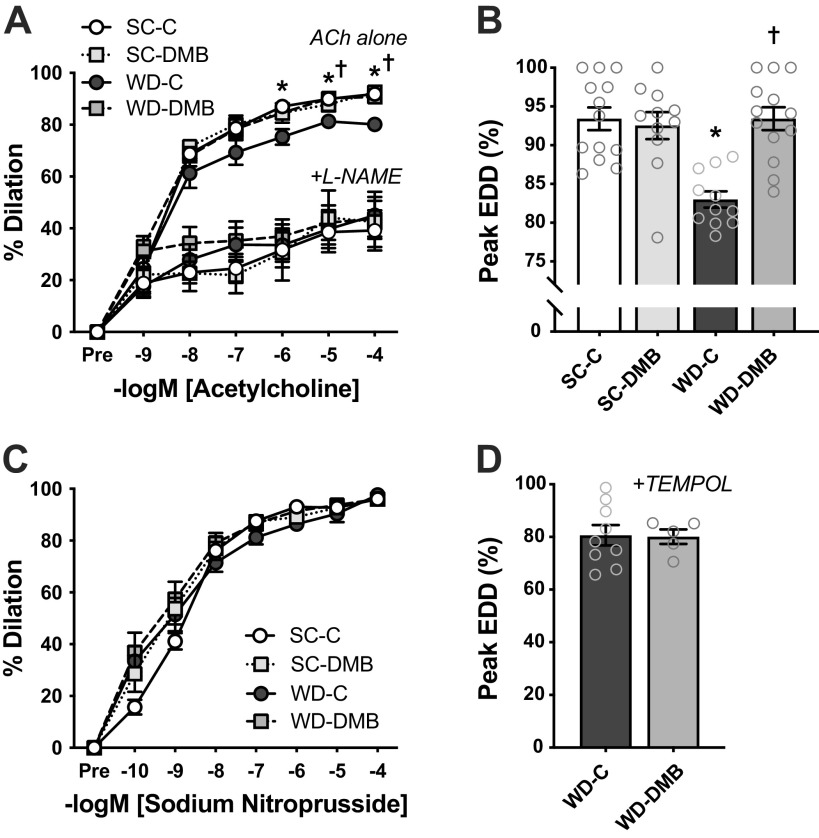
Vascular Endothelial Function
Vascular endothelial function was assessed in excised carotid arteries as EDD to increasing doses of ACh. There were no differences across groups in either resting/unstimulated or maximal carotid artery diameter (Table 2). WD consumption impaired endothelial function compared with mice fed standard chow (peak EDD, SC-C vs. WD-C: P < 0.0001), and this impaired EDD was fully prevented by DMB treatment (peak EDD, WD-C vs. WD-DMB: P < 0.0001; Fig. 3, A and B). There was no effect of DMB treatment on EDD in standard chow-fed mice (peak EDD, SC-C vs. SC-DMB: P = 0.89). Importantly, the effects of diet and DMB on peak EDD persisted when body mass was added to statistical models as a covariate (adjusted diet × treatment interaction: P < 0.001; effect of body mass: P = 0.42).
Figure 3.
Supplementation with 3,3-dimethyl-1-butanol (DMB) mitigates Western-style diet (WD)-induced endothelial dysfunction. In young mice fed either a standard chow (SC) or WD and given either normal drinking water (control; SC-C, WD-C) or water supplemented with 1% DMB (3,3-dimethyl-1-butanol; SC-DMB, WD-DMB) for 8–10 wk: ex vivo carotid artery endothelium-dependent dilation (EDD) to acetylcholine (ACh) in the absence (ACh alone) or presence of the nitric oxide (NO) synthase inhibitor l-NAME (A); peak EDD to ACh alone (highest diameter achieved during the dose response) (B); ex vivo carotid artery endothelium-independent dilation to sodium nitroprusside (C); and peak carotid artery EDD to ACh in the presence of the superoxide dismutase mimetic TEMPOL (D). n = 11–13 mice/group. Data are means ± SE. *P < 0.05 SC-C vs. SC-DMB; †P < 0.05 WD-C vs. WD-DMB. Stats are two-way mixed (group × dose) ANOVA with Tukey’s post hoc test (A and C), two-way (diet × treatment) ANOVA with Šidák’s multiple comparisons tests (B), or Student’s unpaired t test (D). TEMPOL, 4-hydroxy-2,2,6,6,-tetramethylpiperidin-1-oxyl.
To investigate the potential molecular mechanisms by which DMB preserves endothelial function during exposure to WD, we measured vascular smooth muscle sensitivity to NO, assessed by carotid artery EID to increasing doses of the NO donor SNP (Fig. 3C). There were no differences in EID across groups (peak EID, group × SNP dose: P = 0.21). To determine if DMB instead preserves EDD by enhancing bioavailability of endothelial-derived NO, we repeated ACh doses responses in the presence of the NO synthase inhibitor l-NAME (Fig. 3A). The addition of l-NAME attenuated EDD (vs. ACh alone) and abolished group differences (group × ACh dose: P > 0.99), indicating that group differences in EDD to ACh alone were due to differences in NO bioavailability.
Next, to assess whether NO bioavailability may have been altered in WD-fed mice via superoxide signaling, we repeated dose responses to ACh in the presence of the superoxide dismutase mimetic TEMPOL. The addition of TEMPOL abolished the difference in EDD between control and DMB-treated WD-fed mice (P = 0.93; Fig. 3D), indicating that DMB may have prevented WD-induced impairments in EDD by suppressing superoxide-related oxidative stress. In SC-fed mice, the addition of TEMPOL tended to reduce EDD in carotid arteries from mice supplemented with DMB (92.5 ± 1.8% with ACh alone vs. 81.8 ± 3.9% with TEMPOL; P = 0.16; P = 0.22 vs. SC-C), which may be reflective of nonspecific effects of TEMPOL on NO-independent vasodilatory pathways or interruption of normal vasomotor signaling, which can occur with antioxidants in conditions of low basal reactive oxygen species (ROS) (51).
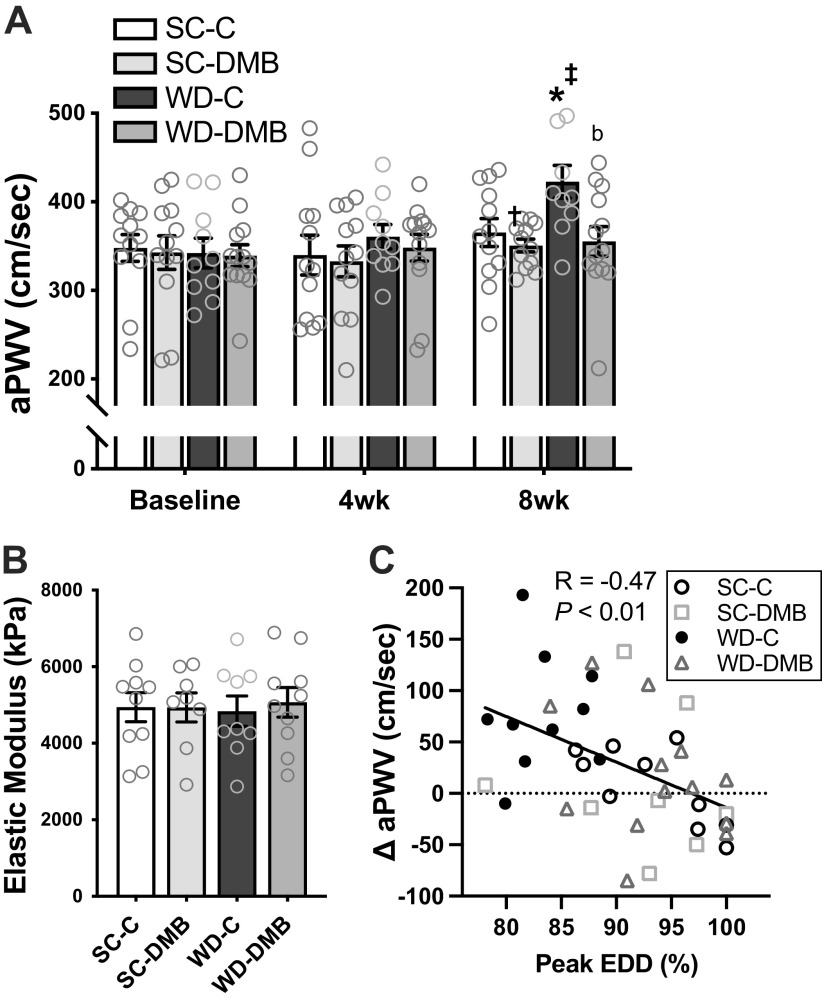
Aortic Stiffness
Aortic stiffness was measured serially in vivo by aPWV. There were no changes in aPWV across the intervention in mice fed standard chow (baseline vs. 8 wk, SC-C: P = 0.39, SC-DMB: P = 0.89). In contrast, WD-fed controls exhibited increases in aPWV across the intervention (P < 0.01 vs. baseline; at 8 wk: P = 0.05 vs. SC-C). This WD-induced aortic stiffening was fully prevented by DMB treatment (WD-DMB: baseline vs. 8 wk: P = 0.62; P = 0.01 vs. WD-C at 8 wk) such that aPWV in WD-DMB mice was not different from mice fed a standard chow at the end of the intervention (P = 0.96; Fig. 4A).
Figure 4.
3,3-Dimethyl-1-butanol (DMB) prevents Western-style diet (WD)-induced increases in aortic pulse wave velocity (aPWV) by improving the functional, but not structural, component of aortic stiffness. In young mice fed either a standard chow (SC) or WD and administered either normal drinking water (control; SC-C, WD-C) or water supplemented with 1% DMB (SC-DMB, WD-DMB) for 8–10 wk: aPWV across the 8-wk intervention (A), aortic elastic modulus, a measure of intrinsic mechanical (wall) stiffness (B), and the relation between changes in aPWV from baseline to 8 wk of the intervention vs. peak endothelium-dependent dilation (EDD) to acetylcholine in isolated carotid arteries (C). n = 11–13 mice/group. Data are means ± SE. There were no statistical outliers. Statistics for A are two-way mixed (group × time) ANOVA with Tukey’s post hoc test: *P < 0.05 vs. SC-C within time point. bP < 0.07 vs. WD-C within time point. ‡P < 0.05 vs. baseline within group. Two-way diet × treatment ANOVA at 8 wk showed similar differences (diet effect: P < 0.01; treatment effect: P = 0.04; SC-C vs. WD-C: P = 0.02; WD-C vs. WD-DMB: P < 0.001).
To determine if differences in aPWV across groups were accompanied by structural changes in the arterial wall, we measured aortic intrinsic mechanical stiffness (elastic modulus). Although WD feeding increased aPWV, we observed no difference in aortic elastic modulus across groups (main effect of diet: P = 0.97; diet × water condition interaction: P = 0.76; Fig. 4B), consistent with previous findings from our laboratory that WD feeding does not influence the structural component of aortic stiffness (5). Aorta diameter and intima-media thickness were measured to calculate aortic elastic modulus. There were no differences across groups in aorta diameter (Table 2). There was a significant main effect of diet on aortic intima-media thickness (P < 0.001), but there was clearly no effect of DMB within either diet group (both P > 0.64; SC-C: 39 ± 1 µm; SC-DMB: 39 ± 1 µm; WD-C: 33 ± 2 µm; WD-DMB: 31 ± 3 µm).
The lack of group differences in intrinsic mechanical stiffness or effects of DMB on other measures of vascular structure (i.e., diameter and intima-media thickness) indicate that prevention of WD-induced aortic stiffening by DMB likely occurred due to changes in the functional modulators of aortic stiffness, e.g., blood pressure and vascular tone. We measured blood pressure noninvasively in vivo at baseline and after 8 wk of the intervention by the tail cuff method but observed no differences across groups or across the intervention (Table 3). Conversely, the change in aPWV across the intervention was significantly related to peak EDD to ACh, both among all mice (Fig. 4C; R = −0.47, P = 0.002) and WD-fed mice only (R = −0.48, P = 0.02), indicating that preserved endothelial function with DMB may have contributed to the prevention of WD-induced increases in aPWV, possibly as a result of the DMB-associated maintenance of NO bioavailability and its effect on vascular smooth muscle tone, the major functional determinant of arterial stiffness. Lastly, group differences in aPWV at 8 wk were no longer significant when models were adjusted for body mass (adjusted diet × treatment interaction: P = 0.19; effect of body mass: P = 0.49), suggesting that the preventive effects of DMB on aPWV may also have been mediated in part by attenuation of WD-induced body mass gain.
Table 3.
Blood pressure
| Group |
P Values |
|||||||
|---|---|---|---|---|---|---|---|---|
| Tail Cuff Blood Pressure, mmHg | SC-C | SC-DMB | WD-C | WD-DMB | Group × Time | Diet × Treatment | Diet | Treatment |
| Systolic | ||||||||
| Baseline | 98 ± 2 | 99 ± 1 | 99 ± 1 | 97 ± 1 | 0.34 | 0.78 | 0.79 | |
| 8 Wk | 98 ± 2 | 100 ± 2 | 95 ± 2 | 98 ± 2 | 0.91 | 0.44 | 0.30 | 0.83 |
| Diastolic | ||||||||
| Baseline | 74 ± 2 | 72 ± 2 | 71 ± 1 | 70 ± 1 | 0.58 | 0.02 | 0.95 | |
| 8 Wk | 74 ± 2 | 71 ± 2 | 69 ± 2 | 72 ± 2 | 0.27 | 0.40 | 0.88 | 0.56 |
Data are mean ± SE. n = 11–13 mice/group. Groups are standard chow-control water (SC-C), standard chow-DMB water (SC-DMB), Western diet-control water (WD-C), and Western diet-DMB water (WD-DMB). Statistics are two-way mixed (group × time) ANOVA with Tukey’s post hoc test and, within each time point, two-way diet × treatment ANOVA with Šidák’s multiple comparisons test. There were no statistical outliers and no significant pairwise comparisons (all P > 0.40).
Aerobic Exercise Endurance
Endurance exercise tolerance was assessed before and after the intervention as distance to fatigue on an endurance rota-rod test (Fig. 5A). The submaximal speeds used for the endurance rota-rod test were determined based on maximal running speed attained on an accelerating rota-rod test conducted at baseline, and speeds for the endurance test were consistent pre-/postintervention. However, the accelerating rota-rod test was still conducted at the end of the intervention to assess any differences in maximal running speed and to keep the order of tests consistent. Results from the accelerating rota-rod test are presented in Table 4.
Figure 5.
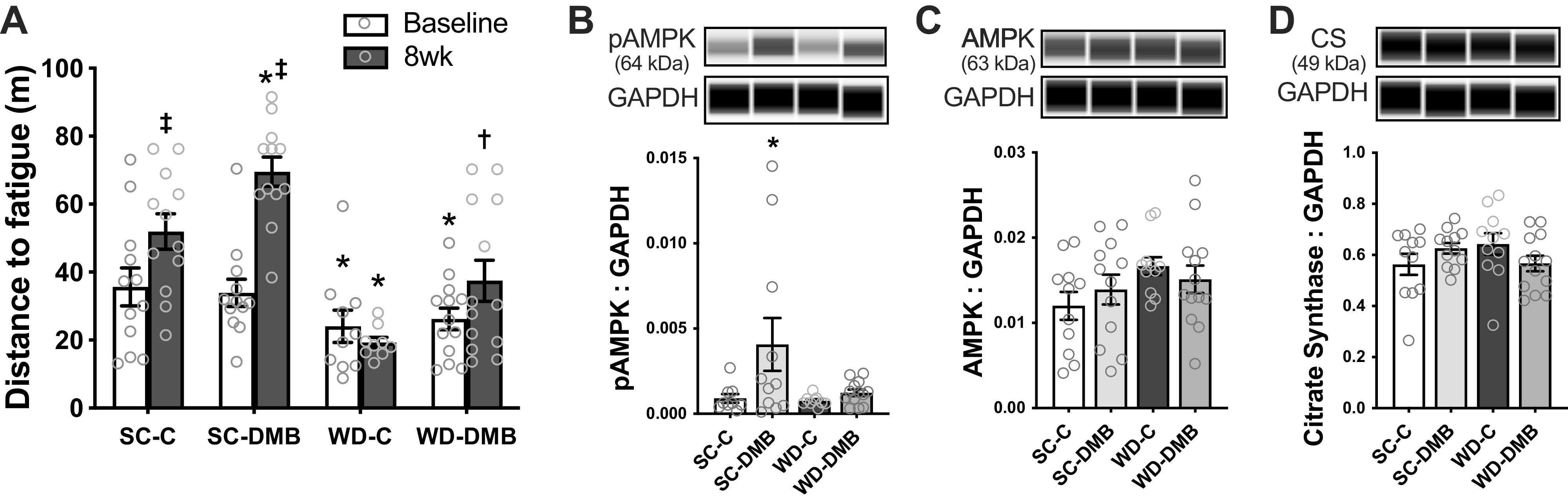
3,3-Dimethyl-1-butanol (DMB) improves aerobic exercise endurance in mice fed standard chow and Western-style diet (WD). A: endurance capacity, assessed as total distance run until fatigue on an endurance rota-rod test, measured at baseline and following 8 wk of normal drinking water (control) or supplementation with 1% 3,3-dimethyl-1-butanol (DMB) in drinking water. n = 10–13/group (one statistical outlier removed from the WD-C group). Statistics for A are two-way mixed (group × time) ANOVA with Tukey’s post hoc test. Two-way diet × treatment ANOVA at 8 wk showed similar differences (diet effect: P < 0.0001; treatment effect: P < 0.001; SC-C vs. WD-C: P = 0.0001; SC-C vs. SC-DMB and WD-C vs. WD-DMB: both P = 0.03). In the same mice postintervention, quadriceps skeletal muscle lysate abundance of phosphorylated adenosine monophosphate-activated protein kinase (pAMPK; B), total AMPK (C), and citrate synthase (D), all normalized to GAPDH with representative Western blot images generated from WES electropherograms shown above each panel. For B–D, statistics are two-way diet × treatment ANOVA with Šidák’s multiple comparisons test. n = 9–13 mice/group. Statistical outliers (ROUT: Q = 1%) were removed before further analysis for pAMPK as follows: 5 for pAMPK (2 SC-C, 1 SC-DMB that was approximately twofold higher than remaining data points, 2 WD-C). There were no statistical outliers for total AMPK or citrate synthase. All data are means ± SE, with individual data points. Symbols denote pairwise comparisons: *P < 0.05 vs. SC-C within time point. †P < 0.05 vs. WD-C within time point. ‡P < 0.05 vs. baseline within group. SC-C, standard chow-control water; SC-DMB, standard chow-DMB water; WD-C, Western diet chow-control water; WD-DMB, Western diet chow-DMB water.
Table 4.
Accelerating Rota-Rod
| Group |
P Values |
|||||||
|---|---|---|---|---|---|---|---|---|
| SC-C | SC-DMB | WD-C | WD-DMB | Group × Time | Diet × Treatment | Diet | Treatment | |
| Longest running time, s | ||||||||
| Baseline | 180 ± 14 | 184 ± 12 | 176 ± 19 | 163 ± 14 | 0.557 | 0.39 | 0.74 | |
| 8 Wk | 250 ± 20‡ | 265 ± 19‡ | 164 ± 15* | 191 ± 14 | <0.01 | 0.71 | <0.0001 | 0.24 |
| Maximal running speed, revs/min | ||||||||
| Baseline | 25 ± 2 | 26 ± 2 | 25 ± 2 | 23 ± 2 | 0.56 | 0.39 | 0.74 | |
| 8 Wk | 34 ± 2‡ | 36 ± 2‡ | 23 ± 2* | 27 ± 2 | <0.01 | 0.71 | <0.0001 | 0.24 |
Data presented are the longest time or maximal speed attained across 3 accelerating rota-rod trials, separated by ∼1 h each. Groups are: standard chow-control water (SC-C), standard chow-DMB water (SC-DMB), Western diet-control water (WD-C), and Western diet-DMB water (WD-DMB). Data are means ± SE. n = 10–13 mice/group (1 statistical outlier removed from WD-C group at 8 wk). Statistics are two-way mixed (group × time) ANOVA and, within each time point, two-way diet × treatment ANOVA. Symbols are pairwise comparisons (Tukey’s post hoc test) from the two-way group × time ANOVA as follows: *P < 0.05 vs. SC-C within time point. ‡P < 0.05 vs. baseline within group.
At baseline, there were no differences in longest run time or maximal running speed on the accelerating rota-rod test across groups (all pairwise comparisons P > 0.76); however, standard chow-fed mice could run further before fatigue on the endurance test than WD-fed mice (34.8 ± 3.4 vs. 25.3 ± 2.7 m; mice from control and DMB groups combined; Student’s unpaired t test: P = 0.03).
Maximal running speed increased across the intervention in SC-fed control mice (P < 0.0001 vs. baseline), representing either a familiarization effect and/or an increase in cardiorespiratory fitness or running economy with age over this period of young adulthood. Subsequently, endurance exercise tolerance was also increased in SC-fed control mice (P = 0.04), presumably as this test was now conducted at a lower percentage of their maximal capacity (same absolute workload). In SC-fed mice treated with DMB, maximal running speed improved comparably to controls (SC-C vs. SC-DMB at 8 wk: P > 0.99), whereas endurance exercise tolerance improved by a greater extent (P = 0.04 vs. SC-C at 8 wk), indicating a potential performance-enhancing effect of DMB. In WD-fed control mice, there was no difference in maximal running speed or endurance exercise tolerance pre- versus postintervention (both P > 0.92); i.e., the adverse effects of WD may have negated any familiarization/aging effect over the intervention. On the other hand, endurance exercise tolerance was higher postintervention in WD-fed mice treated with DMB versus controls (P = 0.03), although the pre- versus postintervention comparison within the WD-DMB group did not reach statistical significance (P = 0.23). We cannot say whether DMB mitigated some of the adverse effects induced by WD or had a direct performance-enhancing effect in these mice, but, regardless of the reason, supplementation with DMB increased exercise tolerance in WD-fed mice.
Of note, these differences in exercise tolerance were observed despite no effect of DMB treatment on skeletal muscle masses (see Table 2). Although WD-fed mice had greater quadriceps and gastrocnemius muscle mass, and lower tibialis anterior muscle mass, than standard chow-fed mice (all P < 0.01), DMB treatment did not affect these diet-based differences in skeletal muscle masses (all differences between SC-C vs. SC-DMB and WD-C vs. WD-DMB, P ≥ 0.37). Unfortunately, as skeletal muscle masses were measured after euthanasia, we could not assess potential changes in muscle mass across the intervention. When statistical models were adjusted for body mass, the effect of diet was no longer significant (P = 0.24; effect of body mass: P < 0.01); however, the effect of treatment persisted (P = 0.01), suggesting that, although WD-induced reductions in exercise tolerance may be driven by adiposity, DMB appears to have an independent performance-enhancing effect.
To explore potential mechanisms by which DMB improved endurance exercise tolerance, we measured key protein markers of increased oxidative metabolism in quadriceps skeletal muscle. Phosphorylated (i.e., activated) AMPK, a key regulator of oxidative metabolism, was higher in DMB-treated mice fed standard chow compared with controls (P = 0.03; main effect of water condition: P = 0.04), but there was no effect of DMB treatment in WD-fed mice (P = 0.90; Fig. 5B). Total AMPK tended to be higher in WD-fed mice (P = 0.09 WD-C vs. SC-C; main effect of diet: P = 0.07), but there was clearly no effect of DMB (main effect of treatment: P = 0.91; Fig. 5C). Lastly, we measured abundance of quadriceps muscle citrate synthase, a rate-limiting enzyme for oxidative metabolism and common marker of oxidative capacity (Fig. 5D). There was a significant diet × treatment interaction (P = 0.047), but no effect of DMB (main effect of treatment: P = 0.85) and no significant pairwise differences across groups (all P ≥ 0.22). As such, examination of these markers of oxidative metabolism failed to provide clear insight into the mechanisms by which DMB enhances endurance exercise tolerance.
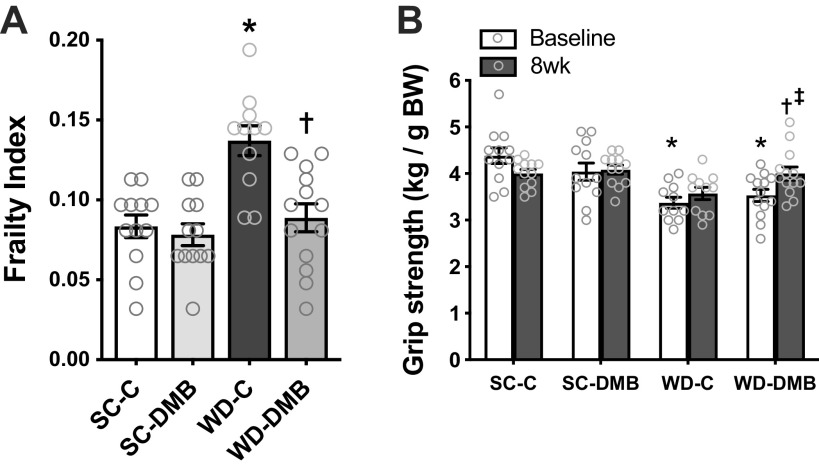
Frailty
Frailty was measured using a validated 31-item frailty index designed to assess signs of deterioration in mice and modeled after clinical assessments of frailty in patients (50) (Fig. 6A). Between control groups, WD-fed mice had a higher frailty index than standard chow-fed mice (P < 0.0001), which was driven primarily by WD mice having higher body mass/adiposity, greater loss of fur color, greater piloerection, worse coat condition (i.e., poorer grooming), higher incidence of vision and hearing loss, dermatitis, and tumors, and lower grip strength. DMB treatment prevented the WD-associated increase in frailty index (P < 0.001, WD-C vs. WD-DMB), which was driven in part by the attenuation in body mass gain, as body weight and body condition scores were lower in DMB-supplemented WD-fed mice versus controls and as the diet × treatment interaction was no longer significant when the model was adjusted for body mass (P = 0.06; effect of body mass: P = 0.08).
Figure 6.
Supplementation with 3,3-dimethyl-1-butanol (DMB) mitigates Western-style diet (WD)-induced frailty and impaired grip strength. In mice fed standard rodent chow (SC) or WD for 5 mo and given either normal drinking water (control; SC-C, WD-C) or water supplemented with 1% DMB (SC-DMB, WD-DMB) for the last 8–10 wk: scores from a clinically validated 31-point frailty index (A), measured just before euthanasia and forepaw grip strength normalized to body weight (BW; B), measured at baseline (after 3 mo of diet, but before beginning the control vs. DMB intervention) and following 8 wk of intervention. All data are means ± SE, with individual data points. n = 11–13 mice/group. There were no statistical outliers. Statistics are two-way diet × treatment ANOVA with Šidák’s multiple comparisons test (A) or two-way mixed (group × time) ANOVA with Tukey’s post hoc test (B). Symbols denote pairwise comparisons: *P < 0.05 vs. SC-C within time point; †P < 0.05 vs. WD-C within time point; ‡P < 0.05 vs. baseline within group. Comparisons of grip strength at 8 wk (B) using two-way diet × treatment ANOVA showed similar differences (diet effect: P = 0.03; treatment effect: P = 0.03; SC-C vs. WD-C: P = 0.03; WD-C vs. WD-DMB: P = 0.03).
Of these components of frailty, low grip strength is particularly associated with higher morbidity and mortality in humans (52). As such, we measured forepaw grip strength before and after the intervention (Fig. 6B). At baseline, standard chow-fed mice had higher forepaw grip strength than WD-fed mice (P < 0.0001). There were no changes in grip strength across the intervention in standard chow fed mice (SC-C: P = 0.07; SC-DMB: P > 0.99) nor control WD-fed mice (P = 0.73). However, grip strength was improved following the intervention in WD-fed mice treated with DMB (P = 0.01 vs. baseline).
DISCUSSION
Consumption of a WD is a highly prevalent, lifestyle-based risk factor for CVD and other chronic diseases (1–3). Although modifiable, public health initiatives to change dietary patterns in the United States have largely failed (53, 54). Novel interventions, especially those that could be adhered to more feasibly, are drastically needed to mitigate the adverse effects of WD on CV health. Our findings indicate that DMB could be one such promising intervention, as we observed that supplementation with DMB in WD-fed mice 1) suppressed circulating levels of the atherosclerosis-linked gut-derived metabolite TMAO; 2) attenuated WD-induced gains in body mass; 3) reversed WD-induced vascular endothelial dysfunction via enhancing NO bioavailability and reducing superoxide-associated oxidative stress; 4) prevented WD-induced in vivo aortic stiffening; 5) improved endurance exercise performance; and 6) mitigated WD-induced increases in frailty index.
Suppression of Circulating TMAO
TMAO is produced via gut microbiota-dependent conversion of phosphatidylcholine, choline, and l-carnitine in food into TMA, which then enters the circulation and is subsequently converted into TMAO in the liver (22, 27). We observed that circulating concentrations of TMAO were higher in mice fed WD compared with standard chow, which is consistent with other reports in rodents fed WD (55, 56) and in humans with Westernized dietary patterns (57). Although there were no appreciable differences in free choline and l-carnitine content of our rodent diets, phosphatidylcholine content was likely higher in the WD due to the added fat ingredients, as indicated by the higher plasma choline concentrations observed in WD-fed mice; high-fat diets are known to increase circulating choline concentrations without differences in free choline content (58). Circulating l-carnitine and betaine were slightly lower in WD-fed mice, which may have been due to lower protein content in the WD. In addition to typically having higher content of TMAO precursors, Westernized dietary patterns alter gut microbiome composition (59, 60), including the abundance of TMA-producing microbes (57, 61, 62). Thus, circulating TMAO likely increased with WD feeding due to a combination of greater dietary phosphatidylcholine content and altered gut microbiota-dependent metabolism.
Supplementation with DMB suppressed WD-induced increases in circulating TMAO, without markedly altering concentrations of choline or other precursors. DMB is nonlethal to bacteria and acts specifically as a competitive inhibitor of the microbial enzyme that converts choline into TMA (27)—TMA lyase (also known as CutC/D)—with no known effects on host/human cells. Thus, beneficial effects of DMB on physiological function are presumed to be attributable to its TMAO-lowering properties. Based on these attributes, DMB is expected to be safe for human use and shows promise for translation. However, other inhibitors of TMA lyase are also under development, which may prove to have even greater therapeutic potential (63).
Body Mass
Many of the detrimental health effects of WD are linked with WD-induced obesity. Interestingly, we observed that DMB supplementation attenuated body weight gain in WD-fed mice. Mice were fed ad libitum and food intake normalized to body mass was comparable across groups. Therefore, this difference does not appear to be due to caloric restriction. Rather, DMB may have increased metabolic efficiency or cage activity. The latter is possible as these mice had higher exercise endurance than WD-fed controls, as discussed more in the Exercise Tolerance section.
To explore whether DMB supplementation improved physiological function, at least in part, by attenuating WD-induced body weight gain, we added body mass as a covariate into statistical models for key outcomes. These adjusted models indicated that body mass may have influenced the effects of DMB on in vivo aortic stiffness and frailty, whereas the effects of DMB on preventing WD-induced impairments in endothelial function and exercise tolerance appeared to be independent of differences in body mass. Lower body mass in WD-fed mice supplemented with DMB also tended to be accompanied by lower visceral fat and liver mass. As such, we measured circulating cholesterol concentrations, with the thought that differences in circulating lipids could influence other domains of physiological function. However, there were no effects of DMB on circulating cholesterol in WD-fed mice. Thus, future studies are needed to explore the mechanistic, interactive links between DMB supplementation, body mass, and other domains of physiological function.
Vascular Function
Supplementation with DMB fully prevented WD-induced impairments in endothelial function, assessed by carotid artery EDD to ACh, and increases in arterial stiffness, assessed by aortic PWV. Our measures of carotid artery EDD and aortic PWV are surrogates of gold-standard measures for assessing vascular function in humans—brachial artery flow-mediated dilation and carotid-femoral PWV, respectively, both of which are independently predictive of CV risk (9, 10). Thus, our findings suggest that DMB could be a potential therapeutic strategy for mitigating risk of CVD with WD consumption in humans.
Impaired EDD in WD-fed mice was mediated by reduced NO-mediated dilation, consistent with previous reports from our laboratory (5, 64). DMB supplementation prevented impaired EDD with WD via preservation of NO bioavailability. Although the role of reduced NO bioavailability in mediating WD-induced vascular dysfunction is fairly established (5, 64), the upstream mechanisms have not been fully elucidated. Our findings indicate that WD-induced increases in circulating gut microbiota-derived TMAO likely contribute. In support of this, both dietary supplementation with TMAO and incubating isolated arteries from mice with TMAO impairs NO-mediated vascular function (20, 21) and suppression of the gut microbiota with broad-spectrum antibiotics, including TMA-producing bacteria, reverses vascular dysfunction in WD-fed mice by enhancing NO bioavailability (65). In addition, our results using the superoxide dismutase mimetic TEMPOL suggest that DMB prevented WD-induced impairments in EDD by suppressing superoxide-driven oxidative stress, which is consistent with our previous studies showing that increased circulating TMAO induces vascular oxidative stress in mice and humans (20, 21).
DMB supplementation prevented WD-induced in vivo aortic stiffening. Arterial stiffening can be mediated by structural changes in the arterial wall, functional changes (i.e., increased vascular smooth muscle tone), or both. To determine potential changes in the structural component, we assessed intrinsic (mechanical) wall stiffness in aortic rings but observed no differences across groups, thus eliminating this determinant of increases in stiffness. This suggests that WD feeding in young mice induces aortic stiffening via functional changes, consistent with a previous study from our laboratory (5), and thus DMB supplementation prevented aortic stiffening also via functional changes. In contrast, we have previously observed that dietary supplementation with TMAO can induce structural changes in the arteries, in particular, increased abundance of advanced glycation end-products, which can form crosslinks between structural proteins in the arterial wall and thereby increase mechanical stiffness (21). Circulating concentrations of TMAO achieved with TMAO supplementation in chow were on average approximately sixfold higher than observed with WD in the present study (TMAO chow supplementation was designed to match the upper range of circulating TMAO levels observed with aging in humans). Thus, it is possible that consuming a WD for a longer duration, e.g., across the lifespan, and/or the compounding effects of WD and aging would induce adverse structural changes in the arteries. Indeed, we observed increases in aortic intrinsic stiffness at 19 mo of age with lifelong WD feeding (initiated at 3 mo) (5). Moreover, WD is a rich dietary source of advanced glycation end-products (66). As such, the direct adverse effects of dietary components of a WD may be compounded by elevated TMAO.
In addition to vascular dysfunction, TMAO has been implicated in mediating various other adverse processes that are exacerbated by WD consumption and contribute to the development of CVD, including hypertension (21), cardiac fibrosis (67), cardiac systolic and diastolic dysfunction (55), and thrombus formation (63). Therefore, our findings contribute meaningfully to this growing body of literature and support the translation of TMAO-lowering therapies for mitigating WD-related CV risk.
Exercise Tolerance
As vascular endothelial dysfunction is linked to lower endurance exercise tolerance (28–30), as a secondary aim, we assessed time to fatigue on a rota-rod test. To our knowledge, this is the first investigation of a role of TMAO in mediating impairments in physical function.
Unexpectedly, we observed that DMB increased endurance exercise performance in standard chow-fed mice, which occurred independently of differences in circulating TMAO concentrations and may reflect a direct performance-enhancing effect of DMB. To provide initial insight into the mechanisms by which DMB improved endurance exercise performance, we measured key protein markers of oxidative metabolism in locomotor skeletal muscle. DMB increased abundance of phosphorylated AMPK in standard chow-fed mice, suggesting DMB may upregulate skeletal muscle oxidative metabolism and/or mitochondrial biogenesis. This finding is intriguing, as DMB has generally been thought to act specifically in the gut with minimal effects on host cells (27). To our knowledge, the only other report of effects of DMB that were independent of differences in circulating TMAO was a recent study by Mao et al. who showed that DMB altered select social behaviors in mice (68). Therefore, one possibility in the present study is that DMB may have increased cage activity and caused a mild exercise training adaptation, although it is important to note that there were no running wheels in cages; i.e., mice were supposedly sedentary. Mice were also single-housed to prevent mouse-to-mouse effects on the microbiome, i.e., removed from their normal social groups. Besides a slight (and likely unrelated) reduction in circulating HDL cholesterol with DMB, there were no other differences between control and DMB-supplemented standard chow-fed mice, among the outcomes that we measured.
In WD-fed mice, endurance exercise tolerance was impaired in controls and DMB supplementation partially rescued this WD-induced impairment. We also measured forepaw grip strength as part of our 31-index frailty assessment and observed similar changes: WD impaired grip strength and DMB supplementation restored grip strength back to levels observed in mice fed standard chow. Contrary to what we observed in standard chow-fed mice, there was no effect of DMB supplementation on phosphorylated AMPK in WD-fed mice, or on any other markers of oxidative metabolism that we measured. It is possible this direct effect of DMB may have been masked in the setting of WD and/or that improvements in exercise tolerance in WD-fed mice were related more so to the TMAO-suppressing effects of DMB.
In addition to having no noticeable effect on markers of oxidative metabolism, DMB supplementation in WD-fed mice did not affect lower limb skeletal muscle masses nor concentrations of circulating cholesterol, and differences in endurance exercise tolerance persisted when controlling for body mass. Instead, it is possible the improvements were related to improved skeletal muscle blood flow during exercise. Skeletal muscle microvascular responsiveness to acetylcholine is impaired in high-fat diet-fed mice (69). As DMB improved conduit vessel endothelial function in the present study, it is possible DMB also improved skeletal muscle microvascular function resulting in greater oxygen delivery to the working muscle. In addition, our laboratory has shown that WD-induced impairments in physical function are accompanied by increased skeletal muscle inflammation (70). Due to technical limitations, we were not able to assess skeletal muscle inflammation in the present study, but it is possible that DMB improved endurance exercise tolerance in part by mitigating WD-induced skeletal muscle inflammation. Regardless of the mechanisms, our findings indicate that DMB and/or other TMAO-lowering therapies could be effective for mitigating WD-induced reductions in endurance exercise tolerance, which, in turn, could also help lower CVD risk.
Frailty
Although frailty is usually considered to be a syndrome of aging, it can be observed at a younger age in response to disease, for example, in childhood cancer survivors who had been treated with anthracycline chemotherapeutic drugs (71) and patients with rheumatoid arthritis (72). Of greatest interest to the current study, frailty also is associated with consumption of a WD (33, 35–37). Consistent with these reports in humans, we observed that WD feeding increased frailty index in mice. Most importantly, we found that DMB supplementation fully reversed this WD-induced frailty. Of translational relevance, we used a multicomponent frailty index that has been validated to mirror the progression of frailty across the lifespan in humans (50). Such indices may be more informative than clinical assessments where individuals are determined to be frail or not frail based on the presence (or absence) of clinical symptoms, as frailty indices capture the process of becoming frail. As such, they may allow for early identification and intervention of those at highest risk of clinical frailty as they age rather than waiting until someone is clinically frail to intervene, which may be too late to reverse deterioration. Consistent with this concept, the magnitude of increase in frailty index with WD that we observed was not as great as typically reported in old mice (50) but was fully reversible by 8 wk of DMB supplementation. Thus, initiating interventions such as TMAO-lowering therapies earlier in life in individuals who consume a WD may reduce the incidence of clinical frailty later in life. Furthermore, given the association between clinical frailty and risk for CVD (40–42), interventions like DMB that can attenuate the development of frailty may be most effective for also preventing CVD and other chronic age-related diseases.
Limitations
We observed that WD-fed mice had lower water intake than standard chow-fed mice, and that WD-fed mice supplemented with DMB had the lowest water intake of any group. Water intake in these mice was still sufficient to maintain hydration based on veterinary examination and that body weight was maintained; however, it is possible these differences in water intake could have confounded our results. That said, if WD-DMB mice were dehydrated, we would have expected this to impair physiological function and increase frailty, whereas we observed the opposite effects of DMB. If anything, we may have observed more consistent benefits of DMB across all outcomes measured if water intake had been similar across groups. We chose to supplement DMB in drinking water, as this method of administration has been established for suppressing TMAO production without nonspecific effects on host cells (27, 52).
Conclusions
We found that suppressing circulating TMAO levels via supplementation with the natural compound DMB in mice could prevent WD-induced vascular endothelial dysfunction and aortic stiffening, improve WD-induced impairments in endurance exercise tolerance, and reverse WD-induced frailty. These findings support the translation of DMB or other TMAO-lowering therapies to humans for mitigation of the adverse effects of WD on physiological function. As vascular dysfunction, exercise intolerance, and frailty all work in a feed-forward manner to exacerbate risk of CVD (31, 38, 40, 41), interventions like DMB with beneficial effects on multiple domains of physiological function may prove most effective for preventing CVD and other chronic diseases.
GRANTS
This work was supported by National Institutes of Health awards R01 HL134887 (to D.R.S) and R21 AG058931 (to K.P.D.), and the Hatch Program of the National Institute of Food and Agriculture, US Department of Agriculture (to A.P.N.). V.E.B. is supported by National Heart Lung and Blood Institute awards F32 HL140875 and K99 HL151818.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.E.B., Z.J.S., R.A.G.-R., A.P.N., K.P.D., and D.R.S. conceived and designed research; V.E.B., Z.J.S., J.J.R., N.S.V., B.P.Z., and A.P.N. performed experiments; V.E.B., N.T.G., A.G.C., and N.S.V. analyzed data; V.E.B., N.T.G., A.G.C., A.P.N., K.P.D., and D.R.S. interpreted results of experiments; V.E.B. prepared figures; V.E.B. and N.T.G. drafted manuscript; V.E.B., N.T.G., Z.J.S., A.G.C., J.J.R., N.S.V., R.A.G-R., B.P.Z., A.P.N., K.P.D., and D.R.S. edited and revised manuscript; V.E.B., N.T.G., Z.J.S., A.G.C., J.J.R., N.S.V., R.A.G-R., B.P.Z., A.P.N., K.P.D., and D.R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors sincerely thank Amanda N. Mercer, Jill Miyamoto-Ditmon, Melanie C. Zigler, Zachary Cook, Zachary Condon, Christopher J. Angiletta, and Laura E. Griffin for assistance with data collection and analysis.
Present addresses: V. E. Brunt, Division of Renal Diseases and Hypertension, Dept. of Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO; A. P. Neilson, Dept. of Food, Bioprocessing and Nutrition Sciences, Plants for Human Health Institute, North Carolina State University, Kannapolis, NC.
REFERENCES
- 1. Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr 72: 912–921, 2000. doi: 10.1093/ajcn/72.4.912. [DOI] [PubMed] [Google Scholar]
- 2. Iqbal R, Anand S, Ounpuu S, Islam S, Zhang X, Rangarajan S, Chifamba J, Al-Hinai A, Keltai M, Yusuf S; INTERHEART Study Investigators. Dietary patterns and the risk of acute myocardial infarction in 52 countries: results of the INTERHEART study. Circulation 118: 1929–1937, 2008. doi: 10.1161/CIRCULATIONAHA.107.738716. [DOI] [PubMed] [Google Scholar]
- 3. Fung TT, Rimm EB, Spiegelman D, Rifai N, Tofler GH, Willett WC, Hu FB. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr 73: 61–67, 2001. doi: 10.1093/ajcn/73.1.61. [DOI] [PubMed] [Google Scholar]
- 4. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al.. Heart Disease and Stroke Statistics-2020 Update: a report from the American Heart Association. Circulation 141: e139–e596, 2020. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 5. Gioscia-Ryan RA, Clayton ZS, Zigler MC, Richey JJ, Cuevas LM, Rossman MJ, Battson ML, Ziemba BP, Hutton DA, VanDongen NS, Seals DR. Lifelong voluntary aerobic exercise prevents age- and Western diet- induced vascular dysfunction, mitochondrial oxidative stress and inflammation in mice. J Physiol 599: 911–925, 2021. doi: 10.1113/JP280607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kramer B, França LM, Zhang Y, Paes Am de A, Gerdes AM, Carrillo-Sepulveda MA. Western diet triggers Toll-like receptor 4 signaling-induced endothelial dysfunction in female Wistar rats. Am J Physiol Heart Circ Physiol C 315: H1735–H1747, 2018. doi: 10.1152/ajpheart.00218.2018. [DOI] [PubMed] [Google Scholar]
- 7. Keogh JB, Grieger JA, Noakes M, Clifton PM. Flow-mediated dilatation is impaired by a high-saturated fat diet but not by a high-carbohydrate diet. Arterioscler Thromb Vasc Biol 25: 1274–1279, 2005. doi: 10.1161/01.ATV.0000163185.28245.a1. [DOI] [PubMed] [Google Scholar]
- 8. Donato AJ, Henson GD, Morgan RG, Enz RA, Walker AE, Lesniewski LA. TNF-α impairs endothelial function in adipose tissue resistance arteries of mice with diet-induced obesity. Am J Physiol Heart Circ Physiol 303: H672–H679, 2012. doi: 10.1152/ajpheart.00271.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y, Shechter A, Feinberg MS. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol 134: 52–58, 2009. doi: 10.1016/j.ijcard.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 10. Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37: 1236–1241, 2001. doi: 10.1161/01.HYP.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 11. Erdei N, Tóth A, Pásztor ET, Papp Z, Édes I, Koller A, Bagi Z. High-fat diet-induced reduction in nitric oxide-dependent arteriolar dilation in rats: role of xanthine oxidase-derived superoxide anion. Am J Physiol Heart Circ Physiol 291: H2107–H2115, 2006. doi: 10.1152/ajpheart.00389.2006. [DOI] [PubMed] [Google Scholar]
- 12. Agus A, Clément K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 70: 1174–1182, 2021. doi: 10.1136/gutjnl-2020-323071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blacher E, Levy M, Tatirovsky E, Elinav E. Microbiome-modulated metabolites at the interface of host immunity. J Immunol 198: 572–580, 2017. doi: 10.4049/jimmunol.1601247. [DOI] [PubMed] [Google Scholar]
- 14. Tang WHW, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res 120: 1183–1196, 2017. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagpal R, Shively CA, Appt SA, Register TC, Michalson KT, Vitolins MZ, Yadav H. Gut microbiome composition in non-human primates consuming a Western or Mediterranean diet. Front Nutr 5: 28, 2018. doi: 10.3389/fnut.2018.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature 486: 222–227, 2012. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–563, 2014. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3: 213–223, 2008. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “Western-lifestyle” inflammatory diseases. Immunity 40: 833–842, 2014. doi: 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 20. Brunt VE, Gioscia-Ryan RA, Casso AG, VanDongen NS, Ziemba BP, Sapinsley ZJ, Richey JJ, Zigler MC, Neilson AP, Davy KP, Seals DR. Trimethylamine-N-oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension 76: 101–112, 2020. doi: 10.1161/HYPERTENSIONAHA.120.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brunt VE, Casso AG, Gioscia-Ryan RA, Sapinsley ZJ, Ziemba BP, Clayton ZS, Bazzoni AE, VanDongen NS, Richey JJ, Hutton DA, Zigler MC, Neilson AP, Davy KP, Seals DR. Gut microbiome-derived metabolite trimethylamine N-oxide induces aortic stiffening and increases systolic blood pressure with aging in mice and humans. Hypertension 78: 499–511, 2021. doi: 10.1161/HYPERTENSIONAHA.120.16895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung Y-M, Wu Y, Schauer P, Smith JD, Allayee H, Tang WHW, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472: 57–63, 2011. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 368: 1575–1584, 2013. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Senthong V, Wang Z, Li XS, Fan Y, Wu Y, Tang WHW, Hazen SL. Intestinal microbiota-generated metabolite trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: the contributory role of intestinal microbiota in a COURAGE-like patient cohort. J Am Heart Assoc 5: e002816, 2016. doi: 10.1161/JAHA.115.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoo W, Zieba JK, Foegeding NJ, Torres TP, Shelton CD, Shealy NG, Byndloss AJ, Cevallos SA, Gertz E, Tiffany CR, Thomas JD, Litvak Y, Nguyen H, Olsan EE, Bennett BJ, Rathmell JC, Major AS, Bäumler AJ, Byndloss MX. High-fat diet–induced colonocyte dysfunction escalates microbiota-derived trimethylamine N-oxide. Science 373: 813–818, 2021. doi: 10.1126/science.aba3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malinowska AM, Szwengiel A, Chmurzynska A. Dietary, anthropometric, and biochemical factors influencing plasma choline, carnitine, trimethylamine, and trimethylamine-N-oxide concentrations. Int J Food Sci Nutr 68: 488–495, 2017. doi: 10.1080/09637486.2016.1256379. [DOI] [PubMed] [Google Scholar]
- 27. Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163: 1585–1595, 2015. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, Yu J, Adams V, Niebauer J, Schuler G. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation 98: 2709–2715, 1998. doi: 10.1161/01.CIR.98.24.2709. [DOI] [PubMed] [Google Scholar]
- 29. Montero D. The association of cardiorespiratory fitness with endothelial or smooth muscle vasodilator function. Eur J Prev Cardiol 22: 1200–1211, 2015. doi: 10.1177/2047487314553780. [DOI] [PubMed] [Google Scholar]
- 30. Welsch MA, Dobrosielski DA, Arce-Esquivel AA, Wood RH, Ravussin E, Rowley C, Jazwinski SM. The association between flow-mediated dilation and physical function in older men. Med Sci Sports Exerc 40: 1237–1243, 2008. doi: 10.1249/MSS.0b013e31816c5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amarasekera AT, Chang D, Schwarz P, Tan TC. Vascular endothelial dysfunction may be an early predictor of physical frailty and sarcopenia: A meta-analysis of available data from observational studies. Exp Gerontol 148: 111260, 2021. doi: 10.1016/j.exger.2021.111260. [DOI] [PubMed] [Google Scholar]
- 32. Alonso-Bouzón C, Carcaillon L, García-García FJ, Amor-Andrés MS, Assar ME, Rodríguez-Mañas L. Association between endothelial dysfunction and frailty: the Toledo Study for Healthy Aging. Age (Dordr) 36: 495–505, 2014. doi: 10.1007/s11357-013-9576-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. León-Muñoz LM, Guallar-Castillón P, López-García E, Rodríguez-Artalejo F. Mediterranean diet and risk of frailty in community-dwelling older adults. J Am Med Dir Assoc 15: 899–903, 2014. doi: 10.1016/j.jamda.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 34. Peri-Okonny PA, Baskin KK, Iwamoto G, Mitchell JH, Smith SA, Kim HK, Szweda LI, Bassel-Duby R, Fujikawa T, Castorena CM, Richardson J, Shelton JM, Ayers C, Berry JD, Malladi VS, Hu M-C, Moe OW, Scherer PE, Vongpatanasin W. High-phosphate diet induces exercise intolerance and impairs fatty acid metabolism in mice. Circulation 139: 1422–1434, 2019. doi: 10.1161/CIRCULATIONAHA.118.037550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gajic-Veljanoski O, Papaioannou A, Kennedy C, Ioannidis G, Berger C, Wong AKO, Rockwood K, Kirkland S, Raina P, Thabane L, Adachi JD; CaMos Research Group. Osteoporotic fractures and obesity affect frailty progression: a longitudinal analysis of the Canadian multicentre osteoporosis study. BMC Geriatr 18: 4, 2018. doi: 10.1186/s12877-017-0692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bollwein J, Diekmann R, Kaiser MJ, Bauer JM, Uter W, Sieber CC, Volkert D. Dietary quality is related to frailty in community-dwelling older adults. J Gerontol A Biol Sci Med Sci 68: 483–489, 2013. doi: 10.1093/gerona/gls204. [DOI] [PubMed] [Google Scholar]
- 37. Fard NRP, Amirabdollahian F, Haghighatdoost F. Dietary patterns and frailty: a systematic review and meta-analysis. Nutr Rev 77: 498–513, 2019. doi: 10.1093/nutrit/nuz007. [DOI] [PubMed] [Google Scholar]
- 38. Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 301: 2024–2035, 2009. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 39. Kokkinos P, Myers J, Faselis C, Panagiotakos DB, Doumas M, Pittaras A, Manolis A, Kokkinos JP, Karasik P, Greenberg M, Papademetriou V, Fletcher R. Exercise capacity and mortality in older men: a 20-year follow-up study. Circulation 122: 790–797, 2010. doi: 10.1161/CIRCULATIONAHA.110.938852. [DOI] [PubMed] [Google Scholar]
- 40. Kelly SG, Wu K, Tassiopoulos K, Erlandson KM, Koletar SL, Palella FJ. Frailty is an independent risk factor for mortality, cardiovascular disease, bone disease, and diabetes among aging adults with human immunodeficiency virus. Clin Infect Dis 69: 1370–1376, 2019. doi: 10.1093/cid/ciy1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stewart R. Cardiovascular disease and frailty: what are the mechanistic links? Clin Chem 65: 80–86, 2019. doi: 10.1373/clinchem.2018.287318. [DOI] [PubMed] [Google Scholar]
- 42. Aida K, Kamiya K, Hamazaki N, Matsuzawa R, Nozaki K, Ichikawa T, Nakamura T, Yamashita M, Maekawa E, Yamaoka-Tojo M, Matsunaga A, Ako J. Usefulness of the simplified frailty scale in predicting risk of readmission or mortality in elderly patients hospitalized with cardiovascular disease. Int Heart J 61: 571–578, 2020. doi: 10.1536/ihj.19-557. [DOI] [PubMed] [Google Scholar]
- 43.US Department of Agriculture, Agricultural Research Service. Nutrient intakes from food and beverages: mean amounts consumed per individual, by gender and age. What We Eat in America. National Health and Nutrition Examination Survey 2017–2018, 2020. https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/1718/Table_1_NIN_GEN_17.pdf. [Google Scholar]
- 44. Marriott BP, Olsho L, Hadden L, Connor P. Intake of added sugars and selected nutrients in the United States, National Health and Nutrition Examination Survey (NHANES) 2003-2006. Crit Rev Food Sci Nutr 50: 228–258, 2010. doi: 10.1080/10408391003626223. [DOI] [PubMed] [Google Scholar]
- 45. Rossman MJ, Gioscia-Ryan RA, Santos-Parker JR, Ziemba BP, Lubieniecki KL, Johnson LC, Poliektov NE, Bispham NZ, Woodward KA, Nagy EE, Bryan NS, Reisz JA, D’Alessandro A, Chonchol M, Sindler AL, Seals DR. Inorganic nitrite supplementation improves endothelial function with aging: translational evidence for suppression of mitochondria-derived oxidative stress. Hypertension 77: 1212–1222, 2021. doi: 10.1161/HYPERTENSIONAHA.120.16175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang Z, Levison BS, Hazen JE, Donahue L, Li X-M, Hazen SL. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem 455: 35–40, 2014. doi: 10.1016/j.ab.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boutagy NE, Neilson AP, Osterberg KL, Smithson AT, Englund TR, Davy BM, Hulver MW, Davy KP. Probiotic supplementation and trimethylamine-N-oxide production following a high-fat diet. Obesity (Silver Spring) 23: 2357–2363, 2015. doi: 10.1002/oby.21212. [DOI] [PubMed] [Google Scholar]
- 49. Justice JN, Carter CS, Beck HJ, Gioscia-Ryan RA, McQueen M, Enoka RM, Seals DR. Battery of behavioral tests in mice that models age-associated changes in human motor function. Age (Dordr) 36: 583–595, 2014. doi: 10.1007/s11357-013-9589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Whitehead JC, Hildebrand BA, Sun M, Rockwood MR, Rose RA, Rockwood K, Howlett SE. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol Ser A Biol Sci Med Sci 69: 621–632, 2014. doi: 10.1093/gerona/glt136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev 2013: 956792, 2013. doi: 10.1155/2013/956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Celis-Morales CA, Welsh P, Lyall DM, Steell L, Petermann F, Anderson J, Iliodromiti S, Sillars A, Graham N, Mackay DF, Pell JP, Gill JMR, Sattar N, Gray SR. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ 361: k1651, 2018. doi: 10.1136/bmj.k1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Institute of Medicine (US) Committee on Dietary Guidelines Implementation. Improving America’s Diet and Health From Recommendations to Action, edited by Thomas PR. Washington, DC: National Academies Press, 1991. doi: 10.17226/1452. [DOI] [PubMed]
- 54. Grooms KN, Ommerborn MJ, Pham DQ, Djousse L, Clark CR. Dietary fiber intake and cardiometabolic risks among US adults, NHANES 1999-2010. Am J Med 126: 1059–1067.e4, 2013. doi: 10.1016/j.amjmed.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen K, Zheng X, Feng M, Li D, Zhang H. Gut microbiota-dependent metabolite trimethylamine N-oxide contributes to cardiac dysfunction in Western diet-induced obese mice. Front Physiol 8: 139, 2017. doi: 10.3389/fphys.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang H, Meng J, Yu H. Trimethylamine N-oxide Supplementation abolishes the cardioprotective effects of voluntary exercise in mice fed a Western diet. Front Physiol 8: 944, 2017. doi: 10.3389/fphys.2017.00944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WHW, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19: 576–585, 2013. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rubio-Aliaga I, Roos B, de Sailer M, McLoughlin GA, Boekschoten MV, Erk M, van Bachmair E-M, Schothorst EM, van Keijer J, Coort SL, Evelo C, Gibney MJ, Daniel H, Muller M, Kleemann R, Brennan L. Alterations in hepatic one-carbon metabolism and related pathways following a high-fat dietary intervention. Physiol Genomics 43: 408–416, 2011. [Erratum in Physiol Genomics 44: 593, 2012]. doi: 10.1152/physiolgenomics.00179.2010. [DOI] [PubMed] [Google Scholar]
- 59. Telle-Hansen VH, Holven KB, Ulven SM. Impact of a healthy dietary pattern on gut microbiota and systemic inflammation in humans. Nutrients 10: 1783, 2018. doi: 10.3390/nu10111783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rinninella E, Cintoni M, Raoul P, Lopetuso LR, Scaldaferri F, Pulcini G, Miggiano GAD, Gasbarrini A, Mele MC. Food components and dietary habits: keys for a healthy gut microbiota composition. Nutrients 11: 2393, 2019. doi: 10.3390/nu11102393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHW, Krauss RM, Hazen SL. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J 40: 583–594, 2019. doi: 10.1093/eurheartj/ehy799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Romano KA, Vivas EI, Amador-Noguez D, Rey FE. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 6: e02481, 2015. doi: 10.1128/mBio.02481-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Roberts AB, Gu X, Buffa JA, Hurd AG, Wang Z, Zhu W, Gupta N, Skye SM, Cody DB, Levison BS, Barrington WT, Russell MW, Reed JM, Duzan A, Lang JM, Fu X, Li L, Myers AJ, Rachakonda S, DiDonato JA, Brown JM, Gogonea V, Lusis AJ, Garcia-Garcia JC, Hazen SL. Development of a gut microbe–targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med 24: 1407–1417, 2018. doi: 10.1038/s41591-018-0128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lesniewski LA, Zigler ML, Durrant JR, Nowlan MJ, Folian BJ, Donato AJ, Seals DR. Aging compounds western diet-associated large artery endothelial dysfunction in mice: prevention by voluntary aerobic exercise. Exp Gerontol 48: 1218–1225, 2013. doi: 10.1016/j.exger.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Battson ML, Lee DM, Jarrell DK, Hou S, Ecton KE, Weir TL, Gentile CL. Suppression of gut dysbiosis reverses western diet-induced vascular dysfunction. Am J Physiol Endocrinol Metab 49: 96–34, 2017. doi: 10.1152/ajpendo.00187.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bettiga A, Fiorio F, Marco FD, Trevisani F, Romani A, Porrini E, Salonia A, Montorsi F, Vago R. The modern Western diet rich in advanced glycation end-products (AGEs): an overview of its impact on obesity and early progression of renal pathology. Nutrients 11: 1748, 2019. doi: 10.3390/nu11081748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li Z, Wu Z, Yan J, Liu H, Liu Q, Deng Y, Ou C, Chen M. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab Invest 99: 346–357, 2019. doi: 10.1038/s41374-018-0091-y. [DOI] [PubMed] [Google Scholar]
- 68. Mao J, Zhao P, Wang Q, Chen A, Li X, Li X, Liu T, Tao Z, Wang X, Du Y, Gong M, Song L, Gao Y, Shi H. Repeated 3,3-Dimethyl-1-butanol exposure alters social dominance in adult mice. Neurosci Lett 758: 136006, 2021. doi: 10.1016/j.neulet.2021.136006. [DOI] [PubMed] [Google Scholar]
- 69. Huang J-P, Hsu S-C, Li D-E, Chen K-H, Kuo C-Y, Hung L-M. Resveratrol mitigates high-fat diet–induced vascular dysfunction by activating the Akt/eNOS/NO and Sirt1/ER pathway. J Cardiovasc Pharmacol 72: 231–241, 2018. doi: 10.1097/FJC.0000000000000621. [DOI] [PubMed] [Google Scholar]
- 70. Clayton ZS, Gioscia-Ryan RA, Justice JN, Lubieniecki KL, Hutton DA, Rossman MJ, Zigler MC, Seals DR. Lifelong physical activity attenuates age- and Western-style diet-related declines in physical function and adverse changes in skeletal muscle mass and inflammation. Exp Gerontol 157: 111632, 2022. doi: 10.1016/j.exger.2021.111632. [DOI] [PubMed] [Google Scholar]
- 71. Ness KK, Armstrong GT, Kundu M, Wilson CL, Tchkonia T, Kirkland JL. Frailty in childhood cancer survivors. Cancer 121: 1540–1547, 2015. doi: 10.1002/cncr.29211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Haider S, Grabovac I, Berner C, Lamprecht T, Fenzl K-H, Erlacher L, Quittan M, Dorner TE. Frailty in seropositive rheumatoid arthritis patients of working age: a cross-sectional study. Clin Exp Rheumatol 37: 585–592, 2019. [PubMed] [Google Scholar]