ABSTRACT
Amiodarone, a primarily class III antiarrhythmic drug is one of the most commonly used drug in atrial fibrillation. A possible rare side effect of amiodarone treatment is to develop a diffuse parenchymal lung disorder - amiodarone pulmonary toxicity (APT). There is no pathognomonic findings to diagnose APT. A 64-year-old patient with multiple comorbidities presented in our hospital with worsening a five-month history of grade 3 mMRC dyspnea, wheezing, frequent nonproductive cough, fatigue. She has a medical history of atrial fibrillation in treatment with amiodarone 400mg/day for 2 years. Her oxygen saturation was 90% on room air, chest radiography showed disseminated lung irregular opacities with a tendency to confluence in right and left lung and chest computed tomography scan showed asymmetric centrilobular nodules and asymmetrical areas of dense ground glass opacity with few consolidation. Amiodarone pulmonary toxicity was suspected, the drug was stopped and treatment with methylprednisolone started. Worsening and progression of the disease can still be noted despite stopping amiodarone because of the long persistence and elimination of the drug, with the tendency to concentrate in tissues, such as lung. In our patient case the evolution and prognosis were good even the case illustrates neglected effects of amiodarone, potential severe one.
Keywords: amiodarone, pulmonary drug toxicity, computed tomography scan, amiodarone complications
INTRODUCTION
Interstitial lung disease (ILD) include a multitude of diffuse airway and parenchymal lung abnormalities of different entities, more then 200, due to a known exposure like medications, emissions from the environment, radiation, emissions from occupational area or idiopathic interstitial pneumonia – chronic illness or acute one, (idiopathic pulmonary fibrosis, idiopathic nonspecific interstitial pneumonia, cryptogenic organizing pneumonia, acute interstitial pneumonia). ILD can be due to a drug, a biomolecule, medicinal plant, can suggest a primary disease (sarcoidosis, amyloidosis, granulomatosis with polyangiitis, Langerhans cell histiocytosis, neurofibromatosis, Gaucher's disease) or for example can be associated with smoking (desquamative interstitial pneumonia, respiratory bronchiolitis) [1,2].
Pulmonary manifestation cases due to drugs are about 3% of all interstitial disease and about 10% are due to amiodarone. In amiodarone cases reactions correspond to a dose related cytopathic mechanism and are unpredictable in individuals. Withdrawal of the drug may not be sufficient for pulmonary toxicity to resolve [1,2].
CASE REPORT
A 64-year-old female patient, pensioner nurse, with no history of smoking, presented to our hospital 4 years ago with symptoms of worsening a five-month history of grade 3 mMRC dyspnea by a month, wheezing, frequent chronic nonproductive cough, generalized weakness and fatigue, low fever, retrosternal pain. She has a medical history of supraventricular and ventricular extrasystolic arrhythmia (since 1997), atrial fibrillation (FiA) (since 2016), chronic oral anticoagulants (since 2016), hypertension (since 1997), hypothyroidism (since 2012), type 2 diabetes with diet (since 2016), ostheopenia (since 2012) and obesity grade 1.
She was maintained on amiodarone 400 mg/day interleaved with 300 mg/day for the past 2 years due to recurrent episodes of FiA and frequent arrhythmia. Other home medications included bisoprolol, olmesartan/amlodipine, indapamide, trimetazidine, dabigatran, levothyroxine.
In her first presentation in our hospital, five months ago - compared to the current presentation, for sudden grade 4 mMRC dyspnea all symptoms, examination and tests (Figures 1A, 2, 3, and 4) weren’t full suggestive for amiodarone toxicity but for interstitial pneumonia and she followed treatment with methylprednisolone 16 mg/ day for 10 days and azithromycin 500mg/3 days/week for three months with improvement of dyspnea, chest and computed tomography.
Fig. 1.
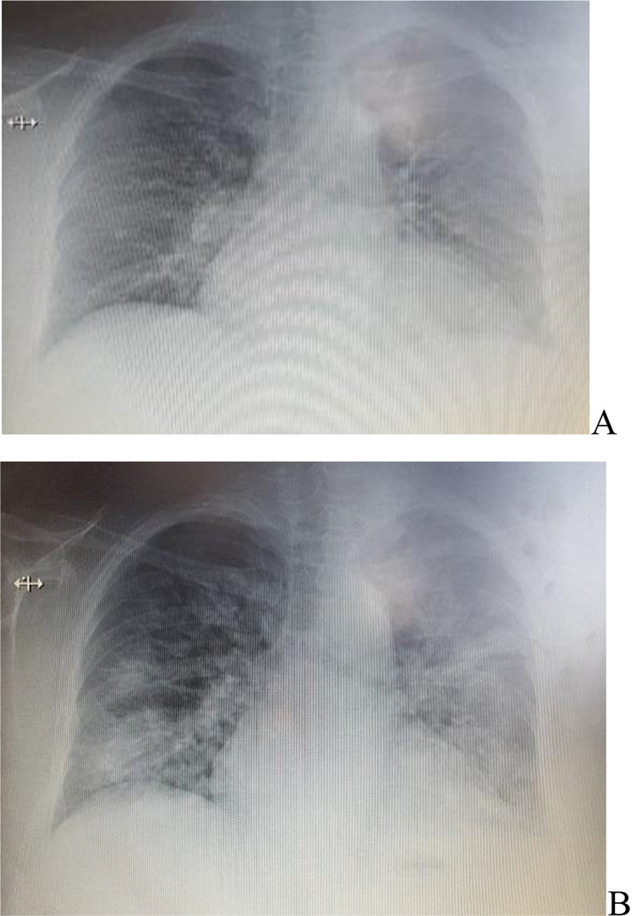
Chest X-ray. A. The first presentation in hospital – 5 months prior to current presentation - rare irregular opacities in right and left lung and interstitial pattern. B. Current presentation - Disseminated right and left lung irregular opacities with a tendency to confluence.
Fig. 2.
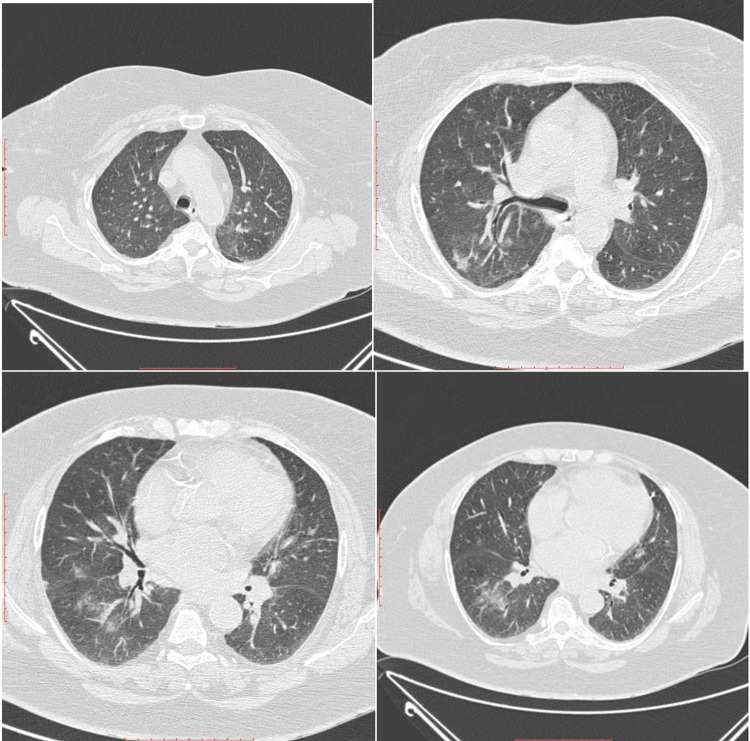
The first presentation in hospital – 5 months prior to current presentation - CT examination - areas of dense ground glass opacity in posterior segment of upper right lobe, postero-lateral medium lobe and apical of inferior right lobe segment; smaller ground glass area in apico-posterior of left upper lobe and anterior basal segments were noted.
Fig. 3.
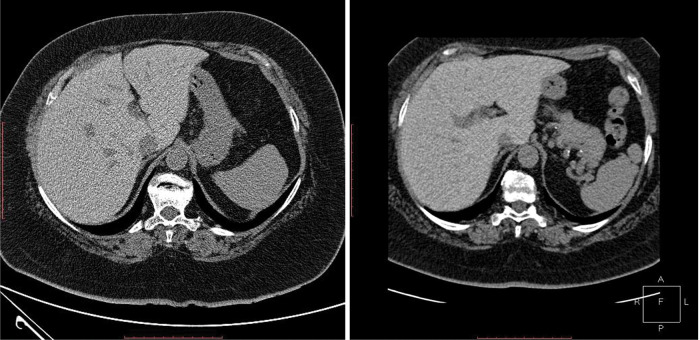
Increased hepatic attenuation at first and second CT examination.
Fig. 4.
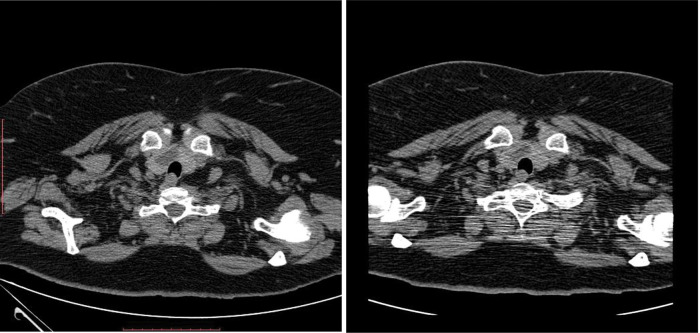
Thyroid nodule in right lobe at first and second CT examination.
In current presentation we noted a blood pressure 132/70 mmHg, a heart rate regular with 80 beats/min, with normal heart sounds, a respiratory rate of 24 breaths/min, and oxygen peripheral saturation (SpO2) 90% on room air. Lung examination was significant for decreased air entry over the both lungs and fine basal crackling. Examination of the limbs showed no peripheral edema, with minimal venous stasis changes on the lower extremities. Laboratory workup showed white blood cell (WBC) count of 12440/uL, hemoglobin level 13.7 g/dL, aspartate aminotransferase 20 U/l, alanine aminotransferase 20 U/l, total protein 63.8 g/l, creatinine 0.84 mg/dl, urea 35.2 mg/dl, erythrocyte sedimentation rate (ESR) 90mm/1h.
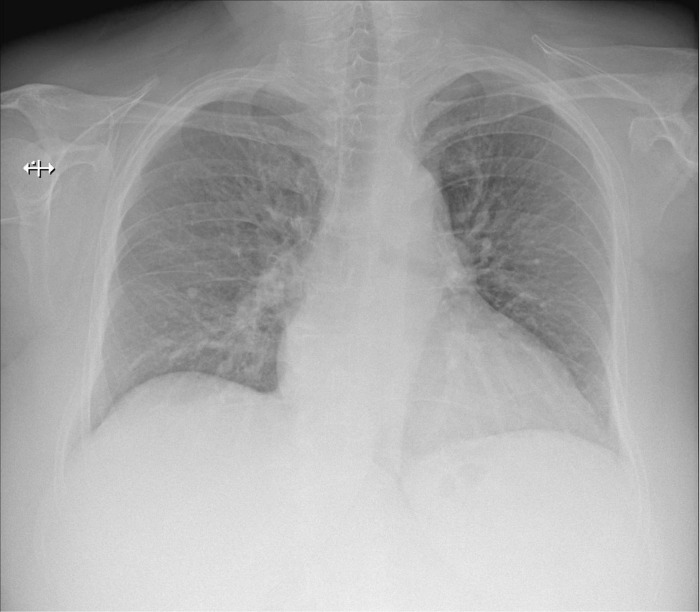
Chest radiography (X-ray) showed disseminated lung irregular opacities with a tendency to confluence, cardiomegaly (Figure 1B).
A bronchoscopy with bronchoalveolar lavage was performed and showed intense inflammatory reaction with polymorphonucleares. Gram and acid-fast stains, aerobic, anaerobic, fungal, tubercular cultures and tumor cytology were negative.
Upon review of the laboratory findings, she was noted to have grade 4 mMRC dyspnea, SpO2 of 74-84% on room air and 88-94% on 3l/min O2 flow nasal cannula. Laboratory workup showed normal ranges of WBC and decline of ESR at 68mm/1h with corticotherapy. All the time she was exhausted.
Spirometry showed values on inferior limit of normal into restrictive ventilator disorder with vital capacity (VC) 2.4L, forced vital capacity (FVC) 2.3L, forced expiratory volume in one second (FEV1) 1.9L, ratio of the forced expiratory volume in the first one second to the forced vital capacity of the lungs (FEV1/FVC) 90.3.
Pulmonary diffusing capacity of the lungs for carbon monoxide (DLCO) showed a decrease of 37% from baseline, a medium reduced diffusing capacity.
Chest computed tomography (CT) scan on day 4 of presentation showed in upper lobes asymmetric centrilobular nodules and areas of dense ground glass opacity and also asymmetrical areas of dense ground glass opacity with few consolidation (Figure 5); cardiomegaly without pericardial effusion. A diffuse increase in hepatic attenuation, with 90 Hounsfield units was observed on CT scan images, consistent with iodine deposition from amiodarone (Figure 6). Also a heterogeneous thyroid gland was noted (Figure 7).
Fig. 5.
Current presentation - CT scan - In upper lobes asymmetric centrilobular nodules and dense ground glass opacities and also another asymmetrical areas of dense ground glass opacities with few consolidation in lower lobes.
Fig. 6.
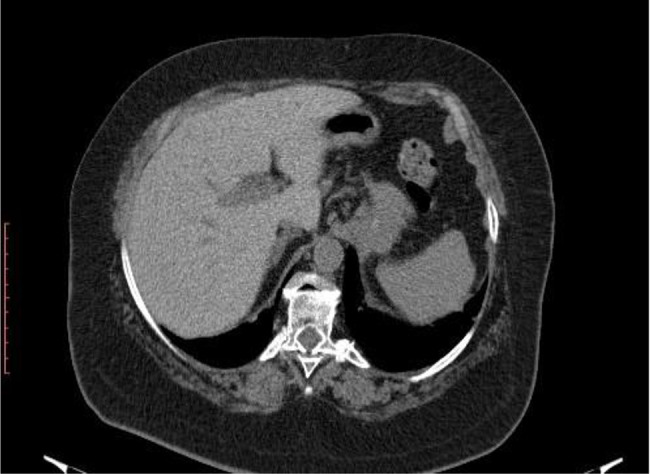
Current presentation - Diffuse increase in attenuation of liver.
Fig. 7.
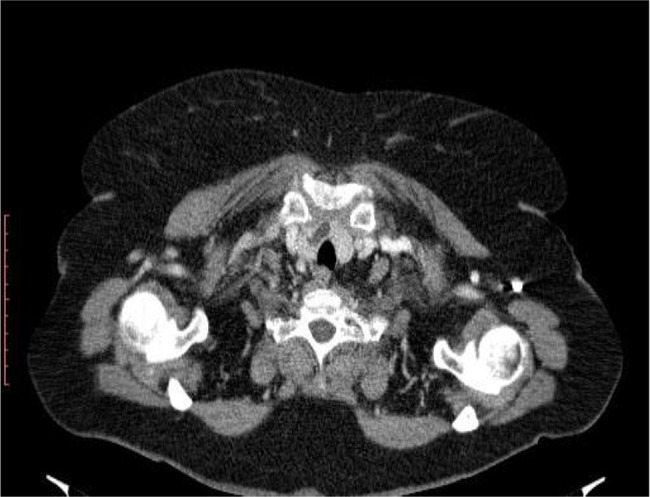
Current presentation - Lobulated heterogeneous thyroid gland.
A rheumatology examination to exclude a rheumatoid arthritis and secondary touch was performed because patient had accused chronic pain and deform of hand articulation for 4 years without any presentation on specialist. Physician has excluded the detection of clinical signs of rheumatoid arthritis, systemic sclerosis, Sjogren syndrome, mixed connective tissue disease and advised rheumatoid factor, anti-cyclic citrullinated peptide, p ANCA, c ANCA.
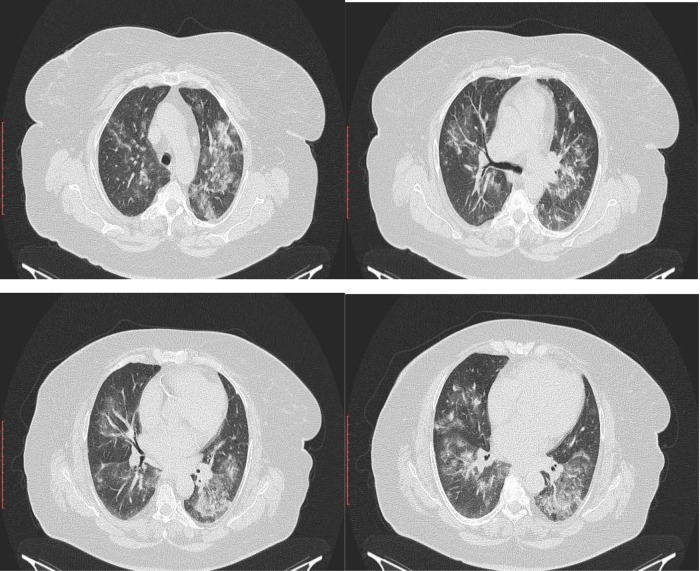
Clinical and radiological images modifications (from this current presentation - and the third one) were in severe and fulminant aggravation from one month ago, when the patient had presented again with worsening dyspnea (grade 3 mMRC) from four months history. CT images from one month ago showed right and left small areas of alveolar filling, in posterior segment and anterior segment of left upper lobe, different as location to five months ago CT examination, which showed areas of dense ground glass opacity in posterior of upper right lobe, postero-lateral of medium lobe and apical of inferior right lobe segments; in the left lung this areas were smaller and in apico-posterior and anterior basal segments; air-trapping elements and mosaic of pulmonary ventilation (Figure 2). Increased hepatic attenuation (Figure 3) and a thyroid nodule in the right lobe (Figure 4) were noted in both examinations. The chest X-ray five months ago described irregular opacities in both right and left lungs, accentuated interstitial sketch and cardiomegaly (Figure 1A), while four months later, the chest X-ray showed areas of irregular opacities in right and left lungs, with other localization, and cardiomegaly.
Five months prior to current presentation, the pulmonologist advised to stop amiodarone because all that modification could suggest involvement of amiodarone and start another antiarrhythmic agent, also to take azithromycin 500mg/3 days/week for three months but the patient and cardiologist physician decided to carry on with old treatment – amiodarone 300mg/day, bisoprolol 5mg/day, olmesartan/amlodipine 40/5 mg/day, indapamide 1.5 mg/day, trimetazidine 70mg/day, dabigatran 300mg/day and for hypothyroidism levothyroxine 75 mcg/day.
In actual presentation the patient had started on methylprednisolone 16 mg/day suit to her personal pathology for the management of amiodarone-induced thyrotoxicosis, liver and lung toxicity had to stop amiodarone, started oxygen therapy with 2-3 l/min for minimum 16h/day and then check up at a month. A week change with interruption of amiodarone and methylprednisolone therapy was major with improvement of dyspnea and oxygenation - SpO2 89-90% in room air and 96-98% with O2 therapy. Findings on chest X-ray take time to resolve and in our case in three months follow up were near normal (Figure 8). Treatment with methylprednisolone was started with low doses 16mg/day for a month then 8 mg/day 2 months and 4 mg/day for the next 3 months. After 3 months our patient was free from oxygen therapy at home, the SpO2 value normalized and dyspnea was grade 2 mMRC.
Fig. 8.
Chest X-ray - Slowly interstitial accentuated pulmonary sketch.
DISCUSSIONS
Amiodarone, a primarily class III antiarrhythmic - potassium channel-blocking - who can evident prolong the QRS duration and QT interval, is one of the most commonly drug used as antiarrhythmic. Amiodarone interferes with activity of beta-adrenergic receptors, calcium channels, and sodium channels - Classes II, IV and I of anti-arrhythmic drugs [3]. There are risk factors for developing amiodarone pulmonary toxicity (APT) like higher plasma concentrations of amiodarone when patients were usually taking a dose of 400 mg or more per day (but also lower doses were reported – example 200 mg per day for two years), a duration of therapy that exceed more than two months, increased patient age, preexisting lung disease, it is more frequent in man and also there may be ethnic or racial differences in the susceptibility to APT [4].
In lung amiodarone induce injuries through multiple mechanisms like direct cytotoxicity to alveolar epithelial cells with implications of foamy macrophages and hyperplasia of type II alveolar epithelial cells who are the most common features of amiodarone induced pulmonary fibrosis, also to vascular endothelial cells, fibroblasts; as well can produce an imbalance between Th1 and Th2 cells, withal grow production of tumor necrosis factor alpha (TNF-α) and transforming growth factor β (TGF-β) by macrophages from alveoli, and can produce angiotensin II-induced apoptosis of alveolar epithelial cells [5,6]. In this way the pulmonary endothelium go on reactive oxygen species (ROS) who generate endothelial dysfunction manifested by leukocyte recruitment, increased permeability of the epithelial cells, adhesion and transmigration, thrombosis, other ways who initiate and propagate inflammation. Interstitial capillary dilatation may explain the effect of the drug in increasing capillary permeability and subsequent edema formation. The edema occurs early after amiodarone administration and produce infiltration of inflammatory cells days after [3,5,7].
Lung patterns noted under the term “amiodarone pneumonitis” include nonspecific interstitial pneumonia (NSIP) cellular or fibrotic type, with alveolar filling by foamy macrophages, or organizing pneumonia, or diffuse alveolar damage, even interstitial lung fibrosis, can be a pattern of “usual interstitial pneumonia”, or any combination - depending on the patient and the stage of the disease, the possibly of the area of the lung biopsy if it’s necessary; so there is no pathognomonic finding to diagnose APT, and evaluation involves rigorous examination to exclude alternative diagnoses. At the hospital patients frequent present with dry cough, dyspnea and have opacities on chest X-ray [1] APT can lead to irreversible pulmonary fibrosis in about 30% of cases.
CT and chest X-ray detected in time, in our patient, various manifestation of the APT and also hyperdense in the liver and thyroid. CT has high accuracy than a chest radiograph, which can show multiple images such as in outpatient: areas of asymmetric centrilobular nodules and asymmetrical areas of dense ground glass opacities with few consolidations.
DLCO monitoring in our case was mild decreased meantime in literature it is a test wild studied in amiodarone induced interstitial lung disease with a sensitivity varied from 20% to 100% and specificity varied from 54% to 83% [8].
Smoking, occupational or environmental emissions were less likely to be involved in the pathogenesis of our patient’s lung toxicity as she was not found to be exposed to known agents. She had no history of past medication that can lead to toxicity and no radiation exposure, so is less likely to be a factor as she had not been exposed to other known drugs found to be a cause of interstitial lung disease. Examination for system diseases like rheumatoid arthritis, systemic lupus erythematosus, scleroderma, Sjogren syndrome and mixed connective tissue was performed.
A clinical diagnosis was made on worsening a five-month grade 3 mMRC dyspnea, nonproductive cough, lower fever, other areas of new radiographic features, improvement with withdrawal of amiodarone, exclusion of lung infection and heart failure. The onset of dry cough may occur within 6–12 months of starting amiodarone, which is also characteristic of APT. In our patient started from 3 months.
Once the diagnosis of APT is suspected the patient should stop the drug and be treated with another therapy for FiA. Worsening and progression of the disease can still be noted despite stopping amiodarone because of long persistence and long elimination (up to 6 months) with the inclination to concentrate in tissues such as lung. Corticosteroids are the mainstay treatment of APT and we performed a low dose therapy with rigorous supervision and with good results. Amiodarone induced lung diseases usually have a good prognosis when diagnosed and treated early but also in our case the evolution and prognosis were rightful. A different approach patient case was made that could inform and improve clinical care.
We performed documents for the charge of amiodarone in this patient case adverse effects with the Arimone Y et al descriptions and models who showed clear interactions between cause and effect [9,10].
We haven’t chased other amiodarone adverse effects consultations such as neurologic, ophthalmologic with corneal microdeposits, which occur in at least 90% of patients taking amiodarone, but advised the patient to carry out these consultations [3]. No bradycardia was noticed as long as patient stayed in hospital.
CONCLUSION
It’s not easy to perform the APT diagnostic and this case illustrates neglected effects of amiodarone, potential severe one, who carry into demise in two or three years if effect persist or the intervention is too late. Mortality from APT has been reported to be close to 10%. The case showed spectacular damage from a month to a month in clinic and paraclinical state of patient with an abnormal clinical presentation after short time assessment. If the patient followed the second advice of pulmonologist maybe these complications could have been avoid and amiodarone toxicity should be considered in every extensive use of the drug, and more, if patients present with progressive onset of respiratory symptoms.
This case report needed an acute diagnosis and needed complex evaluations from a team of physicians to individualize best treatment for the patient.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor in Chief of this journal.
Conflict of interest
The author(s) declare that they have no competing interests.
REFERENCES
- 1.Camus P, Foucher P, Bonniaud P, et al. Drug-induced infiltrative lung disease. Eur Respir J. 2001;18:93–100. doi: 10.1183/09031936.01.00019901. [DOI] [PubMed] [Google Scholar]
- 2.Kubo K, Azuma A, Kanazawa M, et al. Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respir Investig. 2013;51(4):260–277. doi: 10.1016/j.resinv.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Florek JB, Girzadas D. Amiodarone. Book shelf. National Library of Medicine 2021. https://www.ncbi.nlm.nih.gov/books/NBK482154/. available at 11.07.2022.
- 4.Wolkove N, Baltzan M. Amiodarone pulmonary toxicity. Can Respir J. 2009;16(2):43–48. doi: 10.1155/2009/282540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahavadi P, Knudsen L, Venkatesan S, et al. Regulation of macroautophagy in amiodarone induced pulmonary fibrosis. J Pathol. 2015;1(4):252–263. doi: 10.1002/cjp2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2(2):103–121. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Shammari B, Khalifa M, Bakheet SA, et al. A Mechanistic Study on the Amiodarone-Induced Pulmonary Toxicity. Oxid Med Cell Longev. 2016;2016:6265853. doi: 10.1155/2016/6265853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bui A, Han S, Alexandre M, et al. Pulmonary function testing for the early detection of drug-induced lung disease: a systematic review in adults treated with drugs associated with pulmonary toxicity. Intern Med J. 2020;50(11):1311–1325. doi: 10.1111/imj.14647. [DOI] [PubMed] [Google Scholar]
- 9.Bégaud B, Evreux JC, Jouglard J, et al. Unexpected or toxic drug reaction assessment (imputation). Actualization of the method used in France. Therapie. 1985;40:111–118. [PubMed] [Google Scholar]
- 10.Arimone Y, Bidault I, Dutertre JP, et al. Réactualisation de la méthode francaise d’imputabilité des effets indésirables des medicaments. Therapie. 2011;66(6):517–525. doi: 10.2515/therapie/2011073. [DOI] [PubMed] [Google Scholar]