Abstract
Aims
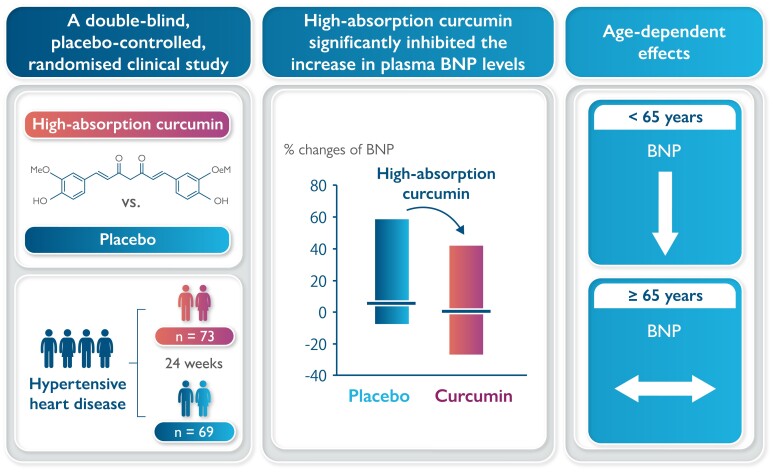
Hypertension is a strong risk factor for heart failure with preserved ejection fraction. Curcumin has p300-specific histone acetyltransferase inhibitory activity, suppresses cardiomyocyte hypertrophy and fibrosis, and significantly reduces myocardial brain natriuretic peptide (BNP) expression without altering blood pressure in a rat model of hypertensive heart disease. This double-blind, placebo-controlled, randomized study, for the first time, aimed to examine the efficacy of a high-absorption curcumin for the prevention of hypertensive heart disease in humans.
Methods and results
Patients exhibiting initial signs of hypertensive heart disease with left ventricular ejection fraction ≥60% and stable blood pressure <140/90 mmHg orally took a double-blinded capsule (either a 90 mg curcumin capsule or placebo) twice daily for 24 weeks. The primary endpoint was per cent changes in left ventricular diastolic function (E/E′) from baseline to 6 months after administration. The secondary endpoint was the per cent change in plasma BNP levels. The E/E′ ratio per cent change from baseline to 6 months after administration was similar between the placebo (n = 69) and the curcumin (n = 73) groups. The per cent change in plasma BNP levels was significantly lower in the curcumin group than in the placebo group. In patients <65 years, BNP per cent changes were significantly lower in the curcumin group than in the placebo group, but similar between groups in ≥65 years (<65 vs. ≥65 years: P for interaction = 0.011).
Conclusions
A high-absorption curcumin agent did not affect the E/E′ ratio, rather it significantly inhibited the increase in plasma BNP levels in patients with initial signs of hypertensive heart disease.
Keywords: Curcumin, Hypertension, Cardiac hypertrophy, Diastolic dysfunction, B-type natriuretic peptide, Prevention
Graphical Abstract
Graphical abstract.
Introduction
The first-line drugs used in pharmacotherapy of heart failure with reduced ejection fraction (EF) are angiotensin-converting enzyme inhibitors (ACE-Is), angiotensin receptor-neprilysin inhibitors, β-receptor blocking agents, mineralocorticoid receptor antagonists, and SGLT2 inhibitors.1,2 Previously, pharmacotherapy for heart failure targeted extracellular neuronal/humoral factors activated in patients with heart failure. The signalling pathways of cardiomyocytes finally reach a common pathway in the nucleus, but no treatment technique targeting such an area has been developed. This study showed that cooperation between p300, which possesses endogenous Histone Acetyltransferase (HAT) activity, and GATA transcription factors (the p300/GATA pathway) is particularly important for regulating gene expression in myeloid cells in patients with heart failure.3,4 Analyses of genetically modified animals have shown that p300-HAT activation is a target for heart failure treatment. Curcumin, a polyphenol compound derived from the natural herb turmeric, possesses bioactive effects including anti-inflammatory and antioxidant effects. This compound is a p300-specific HAT inhibitor.5 Curcumin inhibits the onset of systolic dysfunction attributable to compensatory hypertrophy of the left ventricle (LV) in rat models of hypertensive heart failure and myocardial infarction.6 Furthermore, curcumin administration before the onset of cardiac hypertrophy in rat models of hypertensive heart failure significantly reduced the increase in myocardial cell diameter, perivascular fibrosis, and activation of hypertrophic-responsive transcription genes, as well as an increase in plasma brain natriuretic peptide (BNP) levels and the LV mass index. Such positive effects were achieved without influencing blood pressure.7 Therefore, curcumin might be effective in preventing the development of heart failure with preserved EF (HFpEF). Curcumin has an additive effect on the ACE-1 inhibitor (enalapril), commonly used as a standard treatment for heart failure in clinical practice.8 The absorption efficiency of natural curcumin in aqueous solutions is extremely low because it forms very large granules. We enhanced the absorption efficiency of curcumin in the intestine using a drug delivery system and developed a high-absorption curcumin drug with the aim of clinical application. In the high-absorption curcumin agent, the particle size of curcumin became extremely small after extreme miniaturization and surface processing. Such characteristics enabled stable dispersion in aqueous solutions, resulting in a ∼27-fold increase in the absorption of curcumin in the intestine.9 Even at extremely low doses, this high-absorption curcumin agent improved cardiac function in rats with heart failure following myocardial infarction.10
PARAGON-HF11 and EMPEROR-Preserved12 trials failed to show significant differences in cardiovascular death rates and hospitalization rates in patients with an LV EF ≥ 62.5%13 or those with an LVEF ≥ 65%.14 Therefore, no effective drug treatment has been found for patients with HFpEF and normal LVEF. Hypertension constitutes a major risk factor for HFpEF.15 Beta-blockers have been reported to be relatively less effective in reducing the LV mass index among anti-hypertensive drugs.16 Although there may be differences depending on the type of anti-hypertensive drug, control of blood pressure is widely accepted as essential to preventing the future development of HFpEF. Curcumin suppresses cardiomyocyte hypertrophy and fibrosis and significantly reduces myocardial BNP expression without altering blood pressure in a rat model of hypertensive heart disease. However, there have been no clinical trials examining the effects of curcumin on cardiac hypertrophy/heart failure or its biomarkers in humans. We hypothesized that oral administration of high-absorption curcumin in addition to blood pressure control may be a strategy to prevent future development of HFpEF in patients with a history of hypertension and initial signs of hypertensive heart disease. This double-blind, placebo-controlled, randomized study aimed to determine whether a high-absorption curcumin agent possesses beneficial effects in patients who exhibit initial sings of hypertensive heart disease and have normal EF (≥60%) and stable blood pressure (<140/90 mmHg).
Methods
Study design, ethics approval, and consent
This study was approved by the Institutional Review Board of the National Hospital Organization Kyoto Medical Center (approval number: 13-86). Subsequently, it was registered with the University Hospital Medical Information Network: UMIN000014232 https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&type=summary&language=J&recptno=R000016575. The study investigator or subinvestigator explained the details on the informed consent form to the patients. Sufficient time was given for the patients to understand what the study entailed, after which, their participation in the study was requested. Patients who consented to participate in the study provided their signatures for the consent form. The enrolment period was May 2015 to March 2019. This was a multicentre, double-blind, placebo-controlled, randomized study, and the observation period was from ‘the start of the study sample administration’ to ‘the end of the 6-month administration’. The study data were centrally assessed. Using a table of random numbers, the patients were randomized to receive either a high-absorption curcumin agent (Theracurmin®) or placebo. The study statisticians numbered the test meals before initiating the study. The numbered test meals, whose appearances were identical, were prepared according to the planned study participations and distributed to each study facility. After obtaining approval from the patients and upon completion of the study enrolment, the investigator assigned the test meals to each patient according to the number described on the test foods. The investigator instructed the patients to orally take a double-blinded capsule (either a placebo capsule or that containing 90 mg curcumin) twice daily for 24 weeks. If patients were receiving diuretics, inotropic agents (digitalis, etc.), vasodilators (ACE-Is, calcium channel blockers, angiotensin receptor blockers, and others), or antiarrhythmic drugs as basic underlying therapies, it was confirmed that these patients are in stable conditions for >3 months prior to the study sample administration. Changes or new additions to the underlying therapeutic agents were not made during the administration of the study sample. The primary endpoint was % changes in LV diastolic function (E/E′) from baseline to Week 24, which was evaluated using Doppler echocardiography. The secondary endpoint was the % change in plasma BNP level from baseline to Week 24. The safety outcome was the onset of adverse events.
Patients
The inclusion criteria were as follows:
Underlying disease: stable patients (i.e. blood pressure <140/90 mmHg for 3 months before initiating this study) with cardiac hypertrophy and moderate LV diastolic dysfunction attributable to hypertension.
Patients with cardiac hypertrophy and moderate LV diastolic dysfunction attributable to hypertension. This consists of those with a history of blood pressure ≥135/85 who met the following criteria on echocardiography: (i) an interventricular septum (IVS) thickness or LV posterior wall thickness (PWT) ≥11 mm, (ii) a LVEF ≥60%, and (iii) a mitral septal E/E′ ratio ≥10 and a deceleration time (DT) of ≥140 ms (i.e. differences between ‘the E/E′ values obtained during the period from 8 to 24 weeks before initiating this study’ and ‘the E/E′ values obtained just before initiating the study’ were within 20%).
Male and female patients aged ≥20 and <80 years at the time of providing informed consent.
Patients (or legally acceptable representatives) who provided informed consent.
Patients using an antiplatelet agent must undergo head magnetic resonance imaging within 5 years before providing informed consent because they may meet the exclusion criteria (3).
Patients who used multiple antithrombotic drugs experienced gastrointestinal bleeding in a previous study that used this high-absorption curcumin agent; thus, the following exclusion criteria were set.17
Patients with chronic atrial fibrillation.
Patients who used two or more antiplatelet agents or concomitant use of one antiplatelet agent and antithrombotic drugs (e.g. anticoagulants, EPA (eicosapentaenoic acid) agents, and prostacyclin agents).
Patients with a history of cerebral haemorrhage (including slight bleeding) who used antiplatelet agents.
Patients with unstable angina, acute myocardial infarction, or severe coronary artery disease (at the left main coronary trunk or three-vessel coronary artery disease). This included patients who experienced acute myocardial infarction within 3 months before providing informed consent; patients with unstable angina, vasospastic angina, or angina at rest; patients who underwent coronary artery bypass graft or percutaneous coronary intervention within 3 months before providing informed consent; and patients who were scheduled to undergo the above procedure.
Patients with severe arrhythmia (e.g. sustained ventricular tachycardia and ventricular fibrillation or bradycardia indicated for an implanted pacemaker) and those with an implanted pacemaker.
Patients with valve stenosis or severe valve regurgitation.
Patients with cardiomyopathy (e.g. hypertrophic cardiomyopathy, dilated cardiomyopathy, or arrhythmogenic right ventricular cardiomyopathy) or progressive myocarditis.
Patients with serious respiratory illnesses (e.g. chronic obstructive pulmonary disease IV).
Cardiogenic shock or systolic blood pressure <80 mmHg.
Severe hepatic dysfunction/cirrhosis (Child Classification C).
Patients with severe renal dysfunction (i.e. serum creatinine ≥3.0 mg/dL) or those on dialysis.
Patients who experienced cerebrovascular disease (e.g. cerebral bleeding, subarachnoid haemorrhage, or cerebral infarction) within 3 months before providing informed consent.
Patients with malignant tumours.
Diabetic patients with poor glycaemic control (persistently high HbA1c despite treatment, i.e. HbA1c ≥10.0).
Patients with persistent anaemia (haemoglobin ≤8.0 mg/dL).
Patients who used or injected steroids regularly.
Patients with a history of allergy against the test meals.
Lactating and pregnant women or women desiring to become pregnant within 6 months after providing informed consent.
Any patient otherwise deemed unfit as a subject by the attending physician.
Patients who regularly consumed healthy foods with curcumin.
Rationale for inclusion criteria
At the beginning of the formation of LV hypertrophy caused by hypertension, the systolic function is maintained, while the diastolic function is impaired. Redfield et al.18 defined moderate/severe diastolic dysfunction as a mitral septal E/E′ ratio ≥10, moderate diastolic dysfunction as an E-wave DT ≥140 ms, and severe diastolic dysfunction as an E-wave DT ≤140 ms. This study included patients with hypertensive cardiac hypertrophy who had LV hypertrophy and moderate LV diastolic dysfunction, although their systolic function was maintained. Specifically, moderate LV diastolic dysfunction was defined as an E-wave DT ≥140 ms and a mitral septal E/E′ ratio ≥10. Moderate systolic function was defined as an LVEF (%EF) ≥60% (i.e. normal value), a representative index used for systolic function assessment. The onset of remarkable cardiac hypertrophy is rare at the beginning of LV hypertrophy. The presence or absence of ventricular hypertrophy was determined based on the upper limit of the normal range, that is, IVS thickness or LV PWT ≥11 mm (10.5 mm). Venous blood in a fasting state was collected from the cutaneous vein of the forearm by the investigator before, 12 weeks after, and 24 weeks after administration of the test meals.
Rationale for the target sample size
According to a previous study that used 180 mg of a high-absorption curcumin and met the inclusion criteria for this study, changes in septal E/E′ from baseline to 6 months after initiating treatment were −1.37 ± 1.76 [mean ± standard deviation (SD), n = 15]. Furthermore, a previous study that met the inclusion criteria for this study showed that changes in septal E/E′ from baseline to 6 months after initiating treatment were −0.08 ± 3.37 (mean ± SD, n = 15) in the placebo group. Based on these data, we hypothesized the following values as a conservative estimate of changes in septal E/E′ from baseline to 6 months after initiating treatment. Specifically, the lower limit of the one-sided confidence interval (CI; confidence coefficient: 0.70) would be −1.13 in the high-absorption curcumin 180 mg group, and no such changes would be observed in the placebo group. The upper limit of the one-sided CI (confidence coefficient: 0.70) would be 2.93, which would be used as a conservative estimate of the SD for differences between the two groups. We estimated that we would need to enrol 107 patients in the high-absorption curcumin group and placebo group to have 0.80 power at a one-sided significance level of 0.05 to detect differences in mean changes. We then included 230 patients (115 patients in each group) to account for the dropout rate.
Statistical analysis
For continuous data, parametric and non-parametric data were represented as mean ± SD and median (minimum and maximum values), respectively. Comparing data between the two groups, parametric and non-parametric data were analysed using the non-paired t-test and Mann–Whitney U test, respectively. Regarding the percentage change in each parameter from baseline to 6 months after initiating treatment in the placebo group and high-absorption curcumin group, the mean difference (high-absorption curcumin group – placebo group), 95% CI, and Cohen’s d effect size were calculated. Absolute values in each parameter at baseline and 6 months after initiating treatment were compared using the paired t-test for parametric data and Wilcoxon signed-rank test for non-parametric data. Change of the values of E/E′, blood BNP level, and systolic blood pressure from baseline to 6 months were compared between the placebo and curcumin groups using the two-way analysis of variance for the interaction. All statistical analyses were performed using SPSS version 22.0 for Windows (IBM Japan, Ltd, Tokyo, Japan).
Results
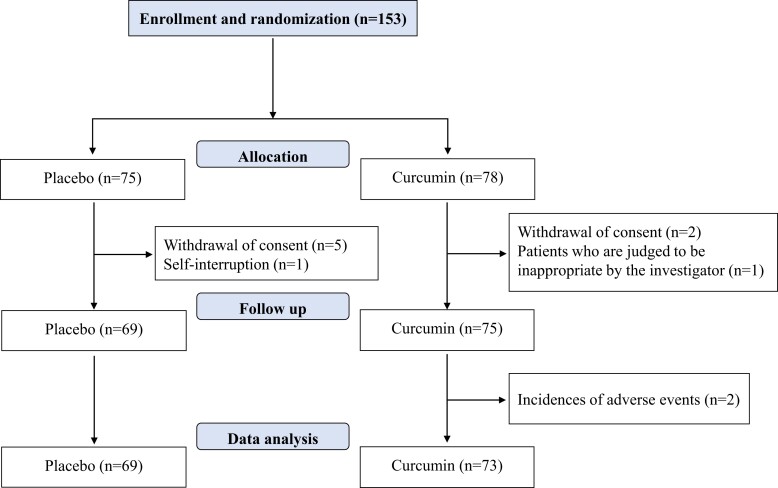
Patient enrolment for the study was concluded in March 2019. Even though the target patient sample size was not achieved, the study period was not extended. This was a result of a delay in patient enrolment and limitations of the research grant. Of the 153 patients enrolled, the following patients were excluded: six patients in the placebo group [withdrawal of consent (five patients) and discontinuation of treatment by themselves (one patient)] and three patients in the high-absorption curcumin group [discontinuation of treatment by themselves (two patients) and deemed unfit as a subject by the primary physician (one patient)]. Therefore, a total of 144 patients were included in this study. During the follow-up period, 2 patients in the high-absorption curcumin group discontinued treatment because of adverse events; therefore, 142 patients completed the follow-up period (Figure 1). Echocardiography data and plasma BNP levels were obtained 6 months after study entry, and analyses were performed. There were 69 patients in the placebo group [56 male and 13 female patients with a mean age of 66.0 years (59.5, 73.0)] and 73 patients in the high-absorption curcumin group [58 men and 15 women with a mean age of 67.0 years (59.0, 73.8)]. Table 1 shows the baseline patient characteristics of the two groups. No differences in the following parameters were observed between the two groups: sex, age, body mass index (BMI), systolic blood pressure, diastolic blood pressure, pulse, plasma BNP levels, echocardiography (e.g. left axis deviation, LV end-diastolic diameter, EF, IVS, and PWT), and Doppler echocardiography (e.g. E/A, DcT, and septal E/E′). As shown in Supplementary material online, Table S1, E/E′ did not significantly change from baseline to 6 months after administration in either the placebo or curcumin groups. Blood BNP levels were significantly increased in the placebo group (P = 0.028) but not in the curcumin group. Systolic blood pressure significantly decreased in the curcumin group (P = 0.038) but not in the placebo group (P = 0.432).
Figure 1.
Clinical trial flow chart.
Table 1.
Baseline characteristics of the participants in the placebo and curcumin groups
| Placebo | Curcumin | P-value | |||
|---|---|---|---|---|---|
| n | Data | n | Data | ||
| Sex (M/F) | 69 | 56/13 | 73 | 58/15 | 0.836 |
| Age, years old | 69 | 66.0 (59.5–73.0) | 72 | 67.0 (59.0–73.8) | 0.816 |
| BMI, kg/m2 | 68 | 26.2 (23.8–28.8) | 69 | 26.6 (24.0–29.1) | 0.605 |
| SBP, mmHg | 68 | 131.5 (119.3–141.0) | 71 | 130.0 (122.0–144.0) | 0.606 |
| DBP, mmHg | 68 | 75.0 (69.0–81.0) | 71 | 75.0 (69.0–83.0) | 0.859 |
| Pulse, b.p.m. | 68 | 73.3 ± 11.8 | 71 | 73.3 ± 13.1 | 0.999 |
| Cre, mg/dL | 65 | 0.85 (0.80–0.98) | 66 | 0.88 (0.74–1.01) | 0.980 |
| BNP, pg/mL | 64 | 10.9 (6.1–35.5) | 63 | 12.7 (6.0–27.9) | 0.809 |
| LAD, mm | 69 | 38.8 ± 5.1 | 72 | 38.6 ± 5.1 | 0.823 |
| LVDd, mm | 69 | 47.3 ± 4.3 | 72 | 47.1 ± 5.0 | 0.835 |
| EF, % | 69 | 67.0 (63.5–69.0) | 72 | 67.0 (63.3–69.8) | 0.437 |
| IVS, mm | 69 | 11.0 (11.0–11.5) | 72 | 11.0 (10.0–11.0) | 0.115 |
| PWT, mm | 69 | 11.0 (11.0–11.0) | 72 | 11.0 (11.0–11.0) | 0.575 |
| E/A | 69 | 0.81 (0.69–0.93) | 72 | 0.85 (0.73–0.99) | 0.142 |
| DcT, ms | 68 | 233.0 (208.8–267.8) | 72 | 237.0 (206.5–262.5) | 0.993 |
| E/E′ | 68 | 11.4 (10.3–13.7) | 72 | 11.7 (10.3–13.7) | 0.871 |
| Past history | |||||
| Cardiovascular disease | 69 | 46 | 73 | 56 | 0.203 |
| DM | 69 | 33 | 73 | 26 | 0.173 |
| COPD | 69 | 24 | 73 | 25 | >0.999 |
| Cancer | 69 | 16 | 73 | 11 | 0.285 |
| Medication | |||||
| RA inhibitors | 69 | 57 | 73 | 50 | 0.084 |
| Beta-blockers | 69 | 16 | 73 | 8 | 0.039 |
| Vasodilators | 69 | 7 | 73 | 3 | 0.316 |
| Statins | 69 | 40 | 73 | 43 | >0.999 |
| Any anti-DM drugs | 69 | 23 | 73 | 22 | 0.721 |
| Any antithrombotic drugs | 69 | 24 | 73 | 26 | >0.999 |
Data are presented as the mean ± SD for parametric data or as median (interquartile range) for non-parametric data. Bold values indicate P < 0.05, which means ‘significant’.
BMI, body mass index; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; Cre, creatinine; DBP, diastolic blood pressure; DcT, deceleration time; EF, ejection fraction; IVS, interventricular septum; LAD, left axis deviation; LVDd, left ventricular end-diastolic diameter; PWT, posterior LV wall thickness; SBP, systolic blood pressure.
Table 2 shows the percentage change in each parameter from baseline to 6 months after treatment initiation in the two groups. The percentage change in systolic blood pressure was significantly lower in the high-absorption curcumin group than in the placebo group. The percentage change in plasma BNP levels was significantly lower in the high-absorption curcumin group than in the placebo group. No significant difference was observed in the percentage change in septal E/E′ between the two groups.
Table 2.
Per cent changes of parameter in each group
| Placebo | Curcumin | P-value | Mean difference | 95% CI | Cohen’s d | |||
|---|---|---|---|---|---|---|---|---|
| n | % change | n | % change | |||||
| Δ BMI (%) | 62 | 0.0 (−1.7, 1.3) | 58 | 0.0 (−1.6, 1.7) | 0.823 | −0.1 | −1.2, 1.0 | 0.03 |
| Δ SBP (%) | 67 | 0.0 (−4.5, 9.9) | 68 | −3.4 (−8.1, 3.3) | 0.026 | −4.2 | −7.9, −0.4 | 0.38 |
| Δ DBP (%) | 67 | 1.1 (−7.0, 10.9) | 68 | −1.4 (−8.1, 7.4) | 0.386 | −1.6 | −6.3, 3.1 | 0.11 |
| Δ pulse (%) | 67 | −1.1 (−8.4, 6.3) | 68 | 0.0 (−10.8, 10.8) | 0.336 | 3.8 | −1.4, 8.9 | 0.25 |
| Δ Cre (%) | 64 | 0.4 (−5.9, 6.8) | 63 | 0.0 (−4.8, 5.6) | 0.630 | 1.2 | −2.5, 4.8 | 0.11 |
| Δ BNP (%) | 62 | 5.0 (−7.1, 58.5) | 60 | 0.0 (−26.8, 42.1) | 0.032 | −34.7 | −63.9, −5.5 | 0.43 |
| Δ LAD (%) | 67 | 0.0 (−7.5, 5.4) | 70 | 0.0 (−7.9, 6.1) | 0.935 | 0.0 | −3.7, 3.7 | 0.00 |
| Δ LVDd (%) | 67 | 0.0 (−2.4, 4.5) | 70 | 0.0 (−4.4, 3.9) | 0.253 | −1.0 | −3.3, 1.4 | 0.14 |
| Δ EF (%) | 67 | −1.4 (−7.2, 4.5) | 70 | −1.5 (−7.3, 3.7) | 0.814 | −0.2 | −3.3, 3.0 | 0.02 |
| Δ IVS (%) | 67 | 0.0 (−8.3, 0.0) | 69 | 0.0 (−8.3, 0.0) | 0.403 | 1.6 | −1.9, 5.1 | 0.15 |
| Δ PWT (%) | 67 | 0.0 (−8.3, 0.0) | 69 | 0.0 (−9.1, 0.0) | 0.662 | −0.5 | −3.5, 2.4 | 0.06 |
| Δ E/A (%) | 67 | −3.8 (−13.9, 11.8) | 69 | −6.1 (−16.0, 10.7) | 0.531 | −2.5 | −9.7, 4.8 | 0.11 |
| Δ DcT (%) | 66 | −1.1 (−14.8, 9.8) | 69 | −2.9 (−15.6, 20.0) | 0.490 | 6.6 | −2.8, 15.9 | 0.24 |
| ΔE/E′ (%) | 66 | −3.6 ± 21.7 | 69 | −2.7 ± 22.8 | 0.833 | 0.8 | −6.8, 8.4 | 0.04 |
Data are presented as the mean ± SD for parametric data or as median (interquartile range) for non-parametric data. Bold values indicate P < 0.05, which means ‘significant’.
Abbreviations used in this table are the same as in Table 1.
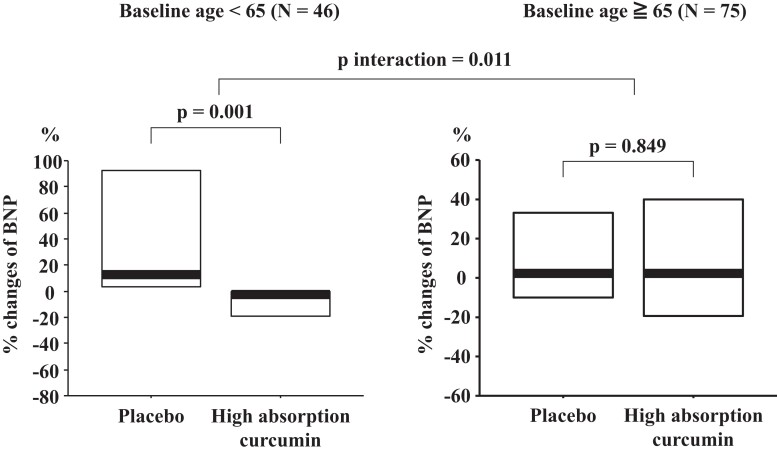
The percentage change in plasma BNP levels from baseline to 6 months after initiating treatment was stratified according to plasma BNP levels (≤18.4 and >18.4 pg/mL), systolic blood pressure (<130 and ≥130 mmHg), and BMI (<25 and ≥25 kg/m2; Table 3). After stratification, no significant difference was found in the per cent change in the plasma BNP levels between the placebo and high-absorption groups. In addition, no significant difference was noted in the effects of increased absorption of curcumin between the two groups. In patients aged <65 years, the percentage change in plasma BNP levels from baseline to 6 months after initiating treatment was significantly lower in the high-absorption curcumin group than in the placebo group; whereas, in patients aged ≥65 years, no significant difference was observed in the percentage change in plasma BNP levels between the two groups from baseline to 6 months after initiating treatment. The effects of high-absorption curcumin on BNP levels showed a significant interaction in the above two age groups (P for interaction = 0.011, Figure 2).
Table 3.
Per cent change in brain natriuretic peptide divided by baseline value in each group
| Placebo | Curcumin | P-value | P-value interaction | |||
|---|---|---|---|---|---|---|
| n | % change | n | % change | |||
| ΔBNP (%) | ||||||
| Baseline BNP ≤ 18.4 pg/mL | 37 | 8.4 (0.0, 114.2) | 38 | 0.0 (−18.2, 47.1) | 0.052 | |
| Baseline BNP > 18.4 pg/mL | 25 | 2.7 (−14.1, 29.1) | 22 | −18.5 (−41.1, 31.3) | 0.201 | 0.348 |
| Baseline SBP < 130 mmHg | 34 | 2.2 (−7.5, 46.8) | 28 | 0.0 (−26.4, 43.8) | 0.228 | |
| Baseline SBP ≥ 130 mmHg | 27 | 7.3 (−8.6, 109.9) | 30 | 0.0 (−28.7, 47.8) | 0.117 | 0.292 |
| Baseline age < 65 years old | 23 | 12.4 (0.0, 119.0) | 23 | 0.0 (−25.2, 0.0) | 0.001 | |
| Baseline age ≥ 65 years old | 39 | 2.7 (−14.4, 43.4) | 36 | 3.9 (−26.8, 51.7) | 0.849 | 0.011 |
| Baseline BMI < 25 kg/m2 | 24 | 5.0 (−5.9, 40.4) | 19 | −6.8 (−34.9, 46.6) | 0.171 | |
| Baseline BMI ≤ 25 kg/m2 | 38 | 5.0 (−9.0, 100.5) | 37 | 0.0 (−25.0, 49.0) | 0.207 | 0.588 |
Abbreviations used in this table are the same as in Table 1. Bold values indicate P < 0.05, which means ‘significant’.
Figure 2.
Per cent change in brain natriuretic peptide divided by baseline age in each group. Bold line indicates the median and lower and upper lines of the box indicate the lower and upper quartiles, respectively.
Supplementary material online, Table S2 shows the baseline data for the placebo group and high-absorption curcumin groups after stratification. In patients aged ≥65 years, plasma BNP levels were higher, and E/A was lower in the high-absorption curcumin and placebo groups. These results are consistent with those of a previous study that reported that E/A decreased with age due to the progression of LV diastolic dysfunction.19
Supplementary material online, Table S3 compares the percentage change in each parameter from baseline to 6 months after initiating treatment between the placebo group and the high-absorption curcumin group in patients aged <65 years. The per cent changes in plasma BNP levels and systolic blood pressure were significantly lower in the high-absorption curcumin group than in the placebo group. As shown in Supplementary material online, Table S4, in patients aged <65 years, E/E′ did not significantly change from baseline to 6 months after administration in both the placebo and curcumin groups. Blood BNP levels were significantly increased in the placebo group (P = 0.004) but not in the curcumin group (placebo vs. curcumin: P for interaction = 0.001). Systolic blood pressure significantly decreased in the curcumin group (P = 0.023) but not in the placebo group (placebo vs. curcumin: P for interaction = 0.029).
On the other hand, in patients aged ≥65 years, no significant difference was observed in the percentage change of each parameter between the placebo group and the high-absorption curcumin group (Supplementary material online, Table S5). E/E′, blood BNP level, and systolic blood pressure did not change from baseline to 6 months after administration in both the placebo and curcumin groups (Supplementary material online, Table S6).
Two patients in the high-absorption curcumin group discontinued the treatment because of adverse events. Specifically, one patient was hospitalized due to epididymitis, but the patient had a history of this disease; thus, this adverse event was assessed as unrelated to the study meal. The other patient discontinued treatment because of mild adverse events (soft stools and heartburn). The relationship between these adverse events and study meal (high-absorption curcumin) was unknown. However, this patient likely had diarrhoea as blood test results, including liver enzymes, renal function, and blood cells, were unremarkable.
Discussion
In this study, the subjects were patients exhibiting initial signs of hypertensive heart disease with LVEF ≥60%, stable blood pressure <140/90 mmHg, and mild LV hypertrophy with a interventricular septum (IVS) or LV posterior wall (PWd) >11 mm. This study aimed to examine whether a high-absorption curcumin agent possesses preventable effects in addition to blood pressure control. The percentage change in plasma BNP levels from baseline to 6 months after initiating treatment was significantly lower in the high-absorption curcumin group than in the placebo group. Furthermore, in patients aged <65 years, the percentage change in plasma BNP levels was considerably lower in the high-absorption curcumin group than in the placebo group, but it was similar between the two groups in patients aged ≥65 years, showing a significant interaction between patients aged <65 and ≥65 years. Blood pressure control is the first priority to prevent the future development of HFpEF in patients with hypertension. The results of this study might provide a novel strategy in addition to blood pressure control for the prevention of HFpEF development in the future. However, long-term studies are needed to examine this hypothesis.
Brain natriuretic peptide is a hormone secreted primarily by the LV in response to increased cardiac wall stretching and stress. Blood BNP levels increase sharply when a load is exerted on the LV. Due to these characteristics, BNP levels have been widely used as indicators for heart failure diagnosis. Blood BNP levels are higher in women than in men and they spontaneously increase with age (∼2 pg/mL/year).20 Testosterone deficiency may be associated with age- and sex-related differences in BNP levels.21 Young men with high testosterone levels tend to have lower blood BNP levels.22,23 This study showed a 5% increase in blood BNP levels from baseline to 6 months after initiating treatment in the placebo group. This increase may be attributed to the presence of hypertensive cardiac hypertrophy and spontaneous increase with age. However, the percentage change in plasma BNP levels from baseline to 6 months after initiating treatment was 0% in the high-absorption curcumin group. Curcumin has been reported to exhibit vasodilating effects through the following possible mechanisms: (i) inhibition of angiotensin-converting enzyme via nuclear factor-kappa B activation and (ii) nitric oxide production via endothelial nitric oxide synthase activation.24 The percentage change in systolic blood pressure from baseline to 6 months after initiating treatment was lower in the high-absorption curcumin group than in the placebo group (P = 0.026). Therefore, the improvement in peripheral circulation by curcumin may be associated with the inhibition of an increase in BNP levels in patients with hypertension. However, this study failed to show a significant association between the percentage change in plasma BNP levels and that of systolic blood pressure (r = 0.049, P = 0.596). In contrast, there was a significant association between the percentage change in plasma BNP levels and that of left atrial dimensions (r = 0.293, P = 0.001), suggesting that a decrease in blood BNP levels reflects a decrease in the LV end-diastolic pressure. Moreover, our previous study demonstrated that curcumin reduced phenylephrine-induced BNP mRNA levels in cultured cardiomyocyte hypertrophy.6,25 Furthermore, we found that curcumin treatment significantly decreased plasma BNP and BNP mRNA levels in the myocardium but had no effect on blood pressure in an experimental animal model of hypertensive heart failure.7 Based on the above findings, curcumin-induced suppression of blood BNP levels in this study may not be explained by only a slight decrease in systolic blood pressure. We believe that the mechanism of curcumin-induced suppression of blood BNP levels in these patients may be associated with inhibition of BNP expression in the myocardium.
In addition to the suppression of myocardial pathological growth, curcumin possesses various bioactive effects, such as anti-inflammatory (inhibiting nuclear factor-kappa B), anti-growth (antagonizing platelet-derived growth factor), and antioxidant actions (scavenging reactive oxygen species, inhibiting lipid peroxidation).17,26–28 The inhibition of the increase in BNP levels observed in this study may be associated with the suppression of inflammatory and oxidative stress in the heart due to curcumin. In fact, many patients with HFpEF have complications such as diabetes, hypertension, and obesity, which cause systemic inflammatory and oxidative stress. This study did not evaluate an inflammatory marker; however, previous studies that used the same high-absorption curcumin agent have shown that this drug reduces oxidized low-density lipoprotein.17,26
After stratification according to age, the percentage change in plasma BNP levels from baseline to 6 months after initiating treatment in patients aged <65 years was significantly lower in the high-absorption curcumin group than in the placebo group (P = 0.001). However, such a significant difference was not observed in patients aged ≥65 years. It is well known that compliance of the heart and blood vessels decreases with aging. Once age-related phenomena such as calcification and fibrosis occur in the cardiovascular system, it is difficult to completely restore them with drug interventions. This study is the first to examine the cardiac effects of high-absorption curcumin in a double-blind, randomized controlled trial. Currently, the precise mechanisms that explain the lack of curcumin-induced reduction in BNP levels in elderly patients are unclear. Further studies are needed to clarify these mechanisms.
Curcumin did not significantly affect the echocardiographic parameters. The patients in this study received anti-hypertensive drugs, and their blood pressure was stable at <140/90 mmHg. Generally, changes in blood BNP levels are more sensitive to drug interventions compared with echocardiographic parameters.29 Thus, a much longer time may be required to achieve evident changes in LV functional and structural parameters following curcumin treatment.
One patient in the high-absorption curcumin group developed mild adverse events (soft stools and heart burn). A previous study using the same high-absorption curcumin agent reported the occurrence of soft stools.17 Curcumin facilitates gall bladder contraction and bile secretion,30 which may be associated with its effects on the digestive system. Curcumin is a less soluble compound and this may be the cause of the soft stools. Further studies are required to evaluate the relationship between curcumin and soft stool.
This study has some limitations. First, the sample size of this study was small, and the follow-up period was 6 months. The endpoint was an examination index (i.e. plasma BNP levels), not death or hospitalization due to heart failure. To examine whether a high-absorption curcumin agent prevents future onset of HFpEF in patients with hypertensive heart disease, a large and long-term study is needed. Moreover, this study included patients exhibiting initial signs of hypertensive heart disease, which was diagnosed based on echocardiographic findings. Therefore, plasma BNP levels were normal or only minimally elevated in these patients. Further studies are required to evaluate whether curcumin improves the prognosis of HFpEF patients.
Conclusions
The results from this study showed that a high-absorption curcumin agent does not change E/E′, rather it significantly inhibits an increase in plasma BNP levels in patients with mild hypertensive cardiac hypertrophy/LV diastolic dysfunction. This effect was apparent in non-elderly individuals (aged 65 years). The P-values for the interactions and subgroups were not adjusted for multiple testing, and therefore, the reported age-based differences should be viewed as hypothesis-generating only. Further long-term studies are needed to clarify whether a high-absorption curcumin agent will prevent future HFpEF development in this patient population.
Supplementary Material
Acknowledgements
The authors wish to sincerely thank and acknowledge the individuals who helped to facilitate this study, including clinical research coordinators.
Contributor Information
Masafumi Funamoto, Division of Translational Research, Clinical Research Institute, National Hospital Organization Kyoto Medical Center, 1-1 Mukaihata-cho, Fukakusa, Fushimi-ku, Kyoto 612-8555, Japan; Division of Molecular Medicine, School of Pharmaceutical Sciences, University of Shizuoka, Shizuoka, Japan; Department of Pharmacology, Institute of Biomedical Sciences, Tokushima University Graduate School, Tokushima, Japan.
Yoichi Sunagawa, Division of Translational Research, Clinical Research Institute, National Hospital Organization Kyoto Medical Center, 1-1 Mukaihata-cho, Fukakusa, Fushimi-ku, Kyoto 612-8555, Japan; Division of Molecular Medicine, School of Pharmaceutical Sciences, University of Shizuoka, Shizuoka, Japan.
Yasufumi Katanasaka, Division of Translational Research, Clinical Research Institute, National Hospital Organization Kyoto Medical Center, 1-1 Mukaihata-cho, Fukakusa, Fushimi-ku, Kyoto 612-8555, Japan; Division of Molecular Medicine, School of Pharmaceutical Sciences, University of Shizuoka, Shizuoka, Japan.
Toru Kato, Department of Clinical Research, National Hospital Organization Tochigi Medical Center, Tochigi, Japan.
Junichi Funada, Department of Cardiology, National Hospital Organization Ehime Medical Center, Ehime, Japan.
Yoichi Ajiro, Division of Clinical Research, National Hospital Organization Yokohama Medical Center, Kanagawa, Japan.
Maki Komiyama, Division of Translational Research, Clinical Research Institute, National Hospital Organization Kyoto Medical Center, 1-1 Mukaihata-cho, Fukakusa, Fushimi-ku, Kyoto 612-8555, Japan.
Masaharu Akao, Division of Translational Research, Clinical Research Institute, National Hospital Organization Kyoto Medical Center, 1-1 Mukaihata-cho, Fukakusa, Fushimi-ku, Kyoto 612-8555, Japan.
Akihiro Yasoda, Division of Translational Research, Clinical Research Institute, National Hospital Organization Kyoto Medical Center, 1-1 Mukaihata-cho, Fukakusa, Fushimi-ku, Kyoto 612-8555, Japan.
Hajime Yamakage, Division of Translational Research, Clinical Research Institute, National Hospital Organization Kyoto Medical Center, 1-1 Mukaihata-cho, Fukakusa, Fushimi-ku, Kyoto 612-8555, Japan.
Noriko Satoh-Asahara, Division of Translational Research, Clinical Research Institute, National Hospital Organization Kyoto Medical Center, 1-1 Mukaihata-cho, Fukakusa, Fushimi-ku, Kyoto 612-8555, Japan.
Hiromichi Wada, Division of Translational Research, Clinical Research Institute, National Hospital Organization Kyoto Medical Center, 1-1 Mukaihata-cho, Fukakusa, Fushimi-ku, Kyoto 612-8555, Japan.
Yasumasa Ikeda, Department of Pharmacology, Institute of Biomedical Sciences, Tokushima University Graduate School, Tokushima, Japan.
Tatsuya Morimoto, Division of Translational Research, Clinical Research Institute, National Hospital Organization Kyoto Medical Center, 1-1 Mukaihata-cho, Fukakusa, Fushimi-ku, Kyoto 612-8555, Japan; Division of Molecular Medicine, School of Pharmaceutical Sciences, University of Shizuoka, Shizuoka, Japan.
Koji Hasegawa, Division of Translational Research, Clinical Research Institute, National Hospital Organization Kyoto Medical Center, 1-1 Mukaihata-cho, Fukakusa, Fushimi-ku, Kyoto 612-8555, Japan; Division of Molecular Medicine, School of Pharmaceutical Sciences, University of Shizuoka, Shizuoka, Japan.
Lead author biography
 Koji Hasegawa, MD, PhD, is currently, Director at Division of Translational Research in National Hospital Organization (NHO) Kyoto Medical Center and Leader of NHO Cardiovascular Clinical Research Network. He is also serving as Clinical Professor, Faculty of Medicine, at Kyoto University as well as Visiting Professor at the University of Shizuoka. He has been widely involved in the study of cardiology, covering areas ranging from prevention to intervention and from translational science to clinical medicine. Professor Koji Hasegawa is internationally active as an executive board director at the International Society of Cardiovascular Pharmacotherapy (ISCP) and a member of the Tobacco Expert Group at the World Heart Federation (WHF).
Koji Hasegawa, MD, PhD, is currently, Director at Division of Translational Research in National Hospital Organization (NHO) Kyoto Medical Center and Leader of NHO Cardiovascular Clinical Research Network. He is also serving as Clinical Professor, Faculty of Medicine, at Kyoto University as well as Visiting Professor at the University of Shizuoka. He has been widely involved in the study of cardiology, covering areas ranging from prevention to intervention and from translational science to clinical medicine. Professor Koji Hasegawa is internationally active as an executive board director at the International Society of Cardiovascular Pharmacotherapy (ISCP) and a member of the Tobacco Expert Group at the World Heart Federation (WHF).
Data Availability
The data that support the findings of this study are available from the corresponding author (K.H.) upon reasonable request.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Funding
This work was supported in part by a Grant-in-Aid for Clinical Research from the National Hospital Organization.
Conflict of interest: The Theravalues Corporation markets highly absorbable curcumin (Theracurmin®). There is a joint research agreement related to this study between Theravalues and the National Hospital Organization of the Kyoto Medical Center. Based on this agreement, Theravalues provided the ‘highly absorptive curcumin’ and ‘placebo’ study test samples free of charge.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 2. Maddox TM, Januzzi JL, Allen LA, Breathett K, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Lindenfeld JA, Masoudi FA, Motiwala SR, Oliveros E, Patterson JH, Walsh MN, Wasserman A, Yancy CW, Youmans QR. 2021 update to the 2017 ACC Expert Consensus Decision Pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2021;77:772–810. [DOI] [PubMed] [Google Scholar]
- 3. Yanazume T, Hasegawa K, Morimoto T, Kawamura T, Wada H, Matsumori A, Kawase Y, Hirai M, Kita T. Cardiac p300 is involved in myocyte growth with decompensated heart failure. Mol Cell Biol 2003;23:3593–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miyamoto S, Kawamura T, Morimoto T, Ono K, Wada H, Kawase Y, Matsumori A, Nishio R, Kita T, Hasegawa K. Histone acetyltransferase activity of p300 is required for the promotion of left ventricular remodeling after myocardial infarction in adult mice in vivo. Circulation 2006;113:679–690. [DOI] [PubMed] [Google Scholar]
- 5. Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol 2007;595:105–125. [DOI] [PubMed] [Google Scholar]
- 6. Morimoto T, Sunagawa Y, Kawamura T, Takaya T, Wada H, Nagasawa A, Komeda M, Fujita M, Shimatsu A, Kita T, Hasegawa K. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J Clin Invest 2008;118:868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sunagawa Y, Funamoto M, Shimizu K, Shimizu S, Sari N, Katanasaka Y, Miyazaki Y, Kakeya H, Hasegawa K, Morimoto T. Curcumin, an inhibitor of p300-HAT activity, suppresses the development of hypertension-induced left ventricular hypertrophy with preserved ejection fraction in Dahl rats. Nutrients 2021;13:2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sunagawa Y, Morimoto T, Wada H, Takaya T, Katanasaka Y, Kawamura T, Yanagi S, Marui A, Sakata R, Shimatsu A, Kimura T, Kakeya H, Fujita M, Hasegawa K. A natural p300-specific histone acetyltransferase inhibitor, curcumin, in addition to angiotensin-converting enzyme inhibitor, exerts beneficial effects on left ventricular systolic function after myocardial infarction in rats. Circ J 2011;75:2151–2159. [DOI] [PubMed] [Google Scholar]
- 9. Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fukuda H, Hashimoto T, Wada H, Katanasaka Y, Kakeya H, Fujita M, Hasegawa K, Morimoto T. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull 2011;34:660–665. [DOI] [PubMed] [Google Scholar]
- 10. Sunagawa Y, Wada H, Suzuki H, Sasaki H, Imaizumi A, Fukuda H, Hashimoto T, Katanasaka Y, Shimatsu A, Kimura T, Kakeya H, Fujita M, Hasegawa K, Morimoto T. A novel drug delivery system of oral curcumin markedly improves efficacy of treatment for heart failure after myocardial infarction in rats. Biol Pharm Bull 2012;35:139–144. [DOI] [PubMed] [Google Scholar]
- 11. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Cleland J, Düngen H-D, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609–1620. [DOI] [PubMed] [Google Scholar]
- 12. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca H-P, Choi D-J, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461. [DOI] [PubMed] [Google Scholar]
- 13. Packer M, Zannad F, Anker SD. Heart failure and a preserved ejection fraction: a side-by-side examination of the PARAGON-HF and EMPEROR-Preserved trials. Circulation 2021;144:1193–1195. [DOI] [PubMed] [Google Scholar]
- 14. Butler J, Packer M, Filippatos G, Ferreira JP, Zeller C, Schnee J, Brueckmann M, Pocock SJ, Zannad F, Anker SD. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur Heart J 2022;43:416–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kasiakogias A, Rosei EA, Camafort M, Ehret G, Faconti L, Ferreira JP, Brguljan J, Januszewicz A, Kahan T, Manolis A, Tsioufis K, Weber T, von Lueder TG, Smiseth OA, Wachtell K, Kjeldsen SE, Zannad F, Mancia G, Kreutz R. Hypertension and heart failure with preserved ejection fraction: position paper by the European Society of Hypertension. J Hypertens 2021;39:1522–1545. [DOI] [PubMed] [Google Scholar]
- 16. Fagard RH, Celis H, Thijs L, Wouters S. Regression of left ventricular mass by antihypertensive treatment: a meta-analysis of randomized comparative studies. Hypertension 2009;54:1084–1091. [DOI] [PubMed] [Google Scholar]
- 17. Funamoto M, Sunagawa Y, Katanasaka Y, Miyazaki Y, Imaizumi A, Kakeya H, Yamakage H, Satoh-Asahara N, Komiyama M, Wada H, Hasegawa K, Morimoto T. Highly absorptive curcumin reduces serum atherosclerotic low-density lipoprotein levels in patients with mild COPD. Int J Chron Obstruct Pulmon Dis 2016;11:2029–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 19. Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 2011;306:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AHB, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002;347:161–167. [DOI] [PubMed] [Google Scholar]
- 21. Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002;40:976–982. [DOI] [PubMed] [Google Scholar]
- 22. Dockery F, Bulpitt CJ, Agarwal S, Vernon C, Nihoyannopoulos P, Kemp M, Hooper J, Rajkumar C. Anti-androgens increase N-terminal pro-BNP levels in men with prostate cancer. Clin Endocrinol (Oxf) 2008;68:59–65. [DOI] [PubMed] [Google Scholar]
- 23. Saenger AK, Dalenberg DA, Bryant SC, Grebe SK, Jaffe AS. Pediatric brain natriuretic peptide concentrations vary with age and sex and appear to be modulated by testosterone. Clin Chem 2009;55:1869–1875. [DOI] [PubMed] [Google Scholar]
- 24. Li KX, Wang ZC, Machuki JO, Li MZ, Wu YJ, Niu MK, Yu KY, Lu QB, Sun HJ. Benefits of curcumin in the vasculature: a therapeutic candidate for vascular remodeling in arterial hypertension and pulmonary arterial hypertension. Front Physiol 2022;13:848867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Funamoto M, Sunagawa Y, Katanasaka Y, Shimizu K, Miyazaki Y, Sari N, Shimizu S, Mori K, Wada H, Hasegawa K, Morimoto T. Histone acetylation domains are differentially induced during development of heart failure in dahl salt-sensitive rats. Int J Mol Sci 2021;22:1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Funamoto M, Shimizu K, Sunagawa Y, Katanasaka Y, Miyazaki Y, Kakeya H, Yamakage H, Satoh-Asahara N, Wada H, Hasegawa K, Morimoto T. Effects of highly absorbable curcumin in patients with impaired glucose tolerance and non-insulin-dependent diabetes mellitus. J Diabetes Res 2019;2019:8208237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shimizu K, Funamoto M, Sunagawa Y, Shimizu S, Katanasaka Y, Miyazaki Y, Wada H, Hasegawa K, Morimoto T. Anti-inflammatory action of curcumin and its use in the treatment of lifestyle-related diseases. Eur Cardiol 2019;14:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Altinel Y, Yalçın Ş, Ercan G, Yavuz E, Erçetin C, Gülçiçek OB, Çelik A, Özkaya G, Uzun H. The efficacy of curcumin on PDGF expression and NF-kappa B pathway: TNBS-induced colitis. Ulus Travma Acil Cerrahi Derg 2020;26:663–670. [DOI] [PubMed] [Google Scholar]
- 29. Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med 2003;115:41–46. [DOI] [PubMed] [Google Scholar]
- 30. Rasyid A, Lelo A. The effect of curcumin and placebo on human gall-bladder function: an ultrasound study. Aliment Pharmacol Ther 1999;13:245–249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (K.H.) upon reasonable request.