Abstract
Endogenous Cushing’s syndrome (CS) is associated with morbidities (diabetes, hypertension, clotting disorders) and shortens life because of infections, pulmonary thromboembolism, and cardiovascular disease. Its clinical presentation is immensely variable, and diagnosis and treatment are often delayed. Thus, there are many opportunities for basic and clinical research leading to better tests, faster diagnosis, and optimized medical treatments. This review focuses on CS caused by excessive adrenocorticotropin (ACTH) production. It describes current concepts of the regulation of ACTH synthesis and secretion by normal corticotropes and mechanisms by which dysregulation occurs in corticotrope (termed “Cushing’s disease”) and noncorticotrope (so-called ectopic) ACTH-producing tumors. ACTH causes adrenal gland synthesis and pulsatile release of cortisol; the excess ACTH in these forms of CS leads to the hypercortisolism of endogenous CS. Again, the differences between healthy individuals and those with CS are highlighted. The clinical presentations and their use in the interpretation of CS screening tests are described. The tests used for screening and differential diagnosis of CS are presented, along with their relationship to cortisol dynamics, pathophysiology, and negative glucocorticoid feedback regulation in the two forms of ACTH-dependent CS. Finally, several gaps in current understanding are highlighted in the hope of stimulating additional research into this challenging disorder.
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS
Adrenocorticotropin (ACTH)-dependent Cushing’s syndrome (CS) is caused by excessive ACTH production by a corticotrope [termed “Cushing’s disease” (CD)] or a nonpituitary (termed “ectopic”) tumor, which leads to excessive cortisol production.
In contrast to the normal stimulation of pituitary ACTH production by corticotropin-releasing hormone (CRH) and vasopressin, other drivers of tumoral ACTH production have been identified recently, and the role of CRH is unclear.
The normal glucocorticoid-induced suppression of ACTH is reduced in ACTH-dependent Cushing’s syndrome (CS), especially with ectopic ACTH production.
These patients have elevated cortisol (and ACTH) levels after sleep onset, in contrast with the normal nadir at that time.
Current diagnostic tests take advantage of the differences between the healthy and CS pattern and amount of cortisol production, the reduced response to negative feedback and the differential responses of CD and ectopic tumors to glucocorticoids, CRH, and vasopressin analogs.
There is a need for improved understanding of basic physiology and its derangements in ACTH-dependent CS so as to reduce the time to an accurate diagnosis and develop new therapeutic agents.
Pathologic cortisol production, the hallmark biochemical characteristic of endogenous Cushing’s syndrome (CS), is associated with a variety of comorbidities that may cause death or disability (eg, cardiovascular disease, infection, thrombotic events, or diabetes) (1). As a result, early diagnosis and treatment are critical. However, all too often, diagnosis is delayed: a recent literature analysis showed a mean latency of up to 38 months (2). This delay is caused by challenges in establishing the diagnosis and cause of CS.
Endogenous CS is caused by (1) excessive corticotropin (ACTH) production or (2) independent autonomous cortisol secretion by 1 or both adrenal glands, termed “ACTH-independent CS.” Excess ACTH may be produced by a pituitary corticotrope tumor [termed “Cushing’s disease” (CD)] or a nonpituitary tumor [termed “ectopic ACTH secretion” (EAS)], while autonomous adrenal secretion results from several pathologic conditions, including solitary adrenal adenomas or carcinoma, or bilateral and multiple adrenal lesions that may be macro- or micronodular, with or without surrounding hyperplasia (3). These adrenal disorders are generally easy to recognize because high cortisol levels suppress ACTH secretion from normal pituitary corticotropes (Fig. 1). As a result, a low or undetectable morning plasma ACTH level usually leads to this diagnosis, as there is usually minimal overlap with ACTH-dependent CS or values in subjects without CS (3). Subsequent imaging usually identifies the abnormal site(s). By contrast, the clinical and biochemical presentation of the ACTH-dependent forms of CS overlap with those of normal individuals who may have some physical features consistent with hypercortisolism, or individuals with physiologic, nontumoral hypercortisolism (described in the following discussion).
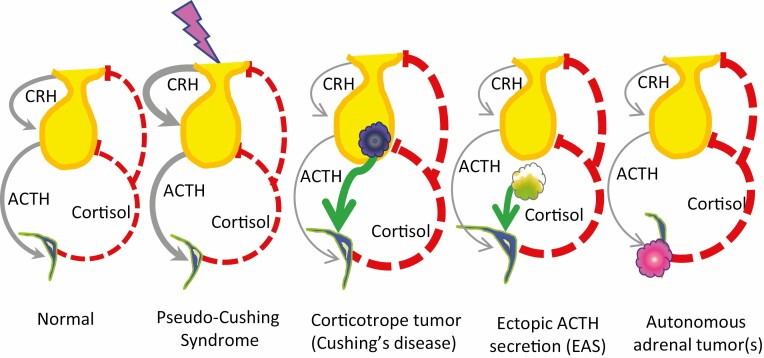
Figure 1.
The hypothalamic-pituitary-adrenal axis of healthy people, those with pseudo-Cushing’s syndrome (CS), and those with the 3 main causes of CS. In healthy people, corticotropin-releasing hormone (CRH) is secreted from the hypothalamus, travels to the pituitary gland, and stimulates ACTH production and secretion. ACTH stimulates the adrenal gland to make cortisol. Cortisol negative feedback (shown by dashed red lines) reduces ACTH production by action on the hypothalamus (to decrease CRH) and the corticotrope (to directly inhibit proopiomelanocortin production). In addition, cortisol negative feedback inhibits corticotrope secretion of ACTH. Stress is thought to stimulate CRH production, which increases ACTH secretion, resulting in excess cortisol production. However, the excessive CRH and ACTH can be turned off by the excess cortisol, bringing the person back into balance. Unregulated, excessive cortisol production is the hallmark of CS, as shown by thicker lines. Another common feature is that the pathologic hypercortisolism of CS acts via negative feedback to suppress normal CRH and ACTH, so that these lines are now quite thin. Pituitary corticotrope tumors and nonpituitary (ectopic) tumors make excessive ACTH, which drives cortisol production. Primary adrenal tumors, which may be unilateral or bilateral, benign or malignant, make cortisol autonomously.
This review focusses on optimal strategies to identify ACTH-dependent CS. A deep understanding of normal physiology and pathophysiology guides and assists in the choice and interpretation of diagnostic tests. Thus, this review first addresses foundational concepts: transcription and repression of proopiomelanocortin (Pomc/POMC; the prohormone of ACTH), ACTH and cortisol pulses and rhythms, and how these lead to dysregulation seen in CD and EAS. The current recommended tests are evaluated with these concepts in mind. This review is intended to be useful to clinicians who want to focus on test rationale only or to understand how the tests harness physiology, as well as for scientists who are interested in how the physiology and molecular biology of the corticotrope could be exploited in clinical and basic investigations. Finally, gaps in our understanding of physiology will be described with the aim of stimulating future basic and clinical research.
Molecular and Tissue Regulators of ACTH Synthesis and Secretion
Because ACTH is the driver of ACTH-dependent hypercortisolism, it is important to understand the current conceptual schema of its synthesis and secretion, which informs understanding of diagnostic tests (Table 1). In healthy people, the corticotrope cells of the anterior pituitary gland produce the prohormone POMC, which is cleaved to liberate ACTH and other hormones. ACTH is then packaged into dense granules and stored in vesicles until it is secreted via exocytosis.
Table 1.
Factors that influence glucocorticoid secretion (cortisol in primates and corticosterone in rodents)
| Hypothalamus |
| SCN |
| Neuronal input to the adrenal gland via the splanchnic nerve |
| PVN |
| Support of production via CRH secretion |
| Initiation of pulses via CRH and AVP |
| CRH secretion (and possibly production) is modulated by slow glucocorticoid feedback |
| Pituitary gland |
| POMC production and post-translational cleavage into ACTH, endorphins, etc |
| Glucocorticoid mediated fast feedback to terminate pulses |
| Glucocorticoid mediated slow feedback to reduce transcription of POMC and POMC regulators |
| Adrenal gland |
| Production and secretion of glucocorticoid in response to ACTH |
| Production is modulated by neuronal input from SCN |
Abbreviations: AVP, arginine vasopressin; CRH, corticotropin-releasing hormone; POMC, proopiomelanocortin; PVN, paraventricular neuron of the hypothalamus; SCN, suprachiasmatic neuron of the hypothalamus.
The putative molecular mechanisms that regulate POMC production and/or secretion in normal cells and ACTH-producing tumors often have been evaluated using the murine AtT-20 corticotrope tumor cell line, which was derived from animals exposed to atomic radiation (4). Thus, it is possible that differences exist between species or between normal and tumoral cells, so that the current theoretical construct of POMC control is flawed. Additionally, not all techniques could exclude additional partners in the process. For example, evaluation of mutated or partial promoter regions by transfection often included base pairs in addition to a putative response element (RE) or binding sites. As a result, deletion of the region or mutational change cannot exclude additional contributions from other transcription factors (TFs) and binding sites.
Stimulation of POMC Transcription
The group of Jacques Drouin established many of the foundational mechanisms of Pomc transcription and repression. Two composite regulatory elements in the Pomc/POMC promoter are important for basal transcriptional activation (Fig. 2). The first is a Tpit/Pitx RE (Tpit/PitxRE) that contains separate binding sites for each TF and requires binding of both (and recruitment of proto-oncogene tyrosine-protein kinase Src coactivators) for activation. The second regulatory element is located 68 bp upstream from the Tpit/PitxRE. It contains a Nur RE (NurRE), which binds members of the nerve growth factor-induced clone B (NGFI-B; also termed “Nur77”) TFs, and the enhancer box in cells of the nervous system (Eboxneuro), which binds heterodimers of the basic helix-loop-helix (bHLH) family (such as neurogenic differentiation factor 1, achaete-scute homolog 1, or TF PERIANTHIA). Achaete-scute homolog 1 binds to Eboxneuro and, in contrast to NeuroD, is expressed in normal adult corticotrope cells (5). It can substitute for other bHLH family members such as NeuroD during development (5), suggesting that it may be the more important postnatal bHLH regulator. However, others have found that NeuroD protein is overexpressed in corticotrope adenomas (6).
Figure 2.
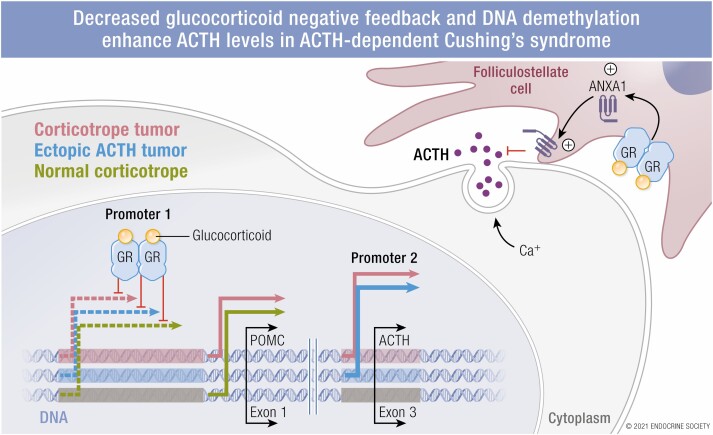
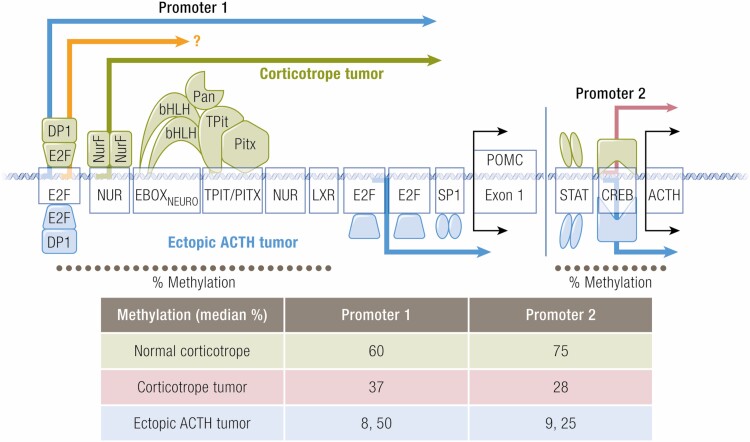
Representational schema promoter regions 1 and 2 of the human proopiomenocortin gene. Shown are the response elements, (the DNA binding regions) that are important for transcription, and some of the co-factors for each, many of which recruit additional factors to the response element complex. The symbols and arrows denote transcription as follows: green, normal corticotopes and corticotrope tumors 2) pink, corticotrope tumor (not normal cells), 3) orange, unknown whether used by normal or tumoral corticotropes 4) blue, ectopic ACTH-secreting tumors. The estimated median percentage of methylation for the two promoter regions for corticotrope and ectopic ACTH-secreting tumors, and normal tissue is shown in the table below, for the DNA regions denoted by the dotted line (estimated from reference 58). Note that individual estimates for two patients with ectopic ACTH secretion are shown.
These 2 regulatory elements (Tpit/PitxRE and NurRE) interact with each other. Poulin et al proposed that the observed protein:protein interactions between Tpit and the Eboxneuro complex is bridged by TF PERIANTHIA 1 via a U-shaped bend of the promoter; the complex is further stabilized by direct interaction of one of the Eboxneuro dimers with Tpit (7,8) (Fig. 2).
Basal POMC transcription is mediated in part by increased intracellular calcium. Corticotropes maintain action potentials via ion channels; in the absence of corticotropin-releasing hormone (CRH), they show spontaneous firing of the action potential with single spikes or bursts, which increase intracellular calcium concentrations, leading to basal POMC transcription (9).
CRH is the main secretagogue for ACTH production and secretion in humans (10). It is produced by neurons emanating from the paraventricular nucleus (PVN) of the hypothalamus and reaches the pituitary gland via secretion into portal vessels of the median eminence. Binding to its receptor on corticotrope cells results in Pomc transcription through cyclic adenosine 5′-monophosphates (cAMP)/protein kinase A and the mitogen-activated protein kinase cascades and subsequent calcium influx. CRH/cAMP-stimulated activation of protein kinase A can modulate ion channels to enhance or decrease their activity (9). These pathways ultimately converge to dephosphorylate the DNA binding domain of NGFI-B (Nur77), allowing its homo- or heterodimerization with other related Nur family members and recruitment of other cofactors (11,12). A reduction in calcium influx has the opposite effect to suppress CRH-stimulated Pomc transcription (13).
A second PVN hormone, arginine vasopressin (AVP), and certain inflammatory cytokines [eg, interleukin 1B, interleukin 6, leukemia inhibitory factor (LIF)] also stimulate Pomc transcription, particularly in conditions of stress and inflammation (14,15). AVP binds to the V1b receptor on corticotropes and activates the protein kinase C pathway.
Suppression of POMC Transcription and Secretion
Through actions mediated by the glucocorticoid receptor (GR), glucocorticoids (GCs) play the largest role in physiologic suppression of ACTH production and secretion. A detailed understanding of the sequences involved in repression provide the context by which corticotrope tumor insensitivity to GC can be understood.
After free GC (cortisol in people and corticosterone in rodents) diffuses into cells, it binds to the GR, which leads to long-term changes in transcription. GC binding can only occur when the GR is part of a multiprotein complex containing a dimer of heat shock protein 90 (HSP90) and other “chaperone” proteins (16). Heat shock proteins have many functions: they can bind their “client” protein to help prevent its aggregation, induce folding into the correct stabilized quaternary structure, and direct proteins into intracellular trafficking paths (17,18). HSP90 (named for its molecular mass of 90 kDa) plays an important role in the folding of steroid receptors and activation by their cognate ligands.
The structure of HSP90 is critical to its ability to fold and activate the GR. The N-terminal region contains an adenosine 5′-triphosphate–binding domain that acts as a switch to alter HSP conformation; the middle domain binds to GR and co-chaperones; and the C-terminal domain allows for dimerization and contains a tetratricopeptide repeat region that binds certain co-chaperones (19). Tetatricopeptide repeat domains consist of short helix-turn-helix repeats with grooves that form a binding scaffold for protein-protein interactions.
The current conceptualization of GR activation begins with its binding to a HSP70•multi-co-chaperone complex, leading to a partial unfolding of the receptor. There follows a sequential addition and loss of other co-chaperones to form a large multiprotein complex of HSP90 dimer•HSP70•GR•additional co-chaperones that inhibits the HSP90 N-terminal domain adenosine triphosphatase activity. With subsequent exchange of the co-chaperone 23, HSP binds ATP, which powers a conformational twisting of the HSP dimer so that the 2 N termini come in contact in a “closed” position, and HSP70 is released. Subsequent hydrolysis of ATP releases co-chaperones. Depending on the experimental approach, reports differ as to when the GR binds ligand (20). If the GR•HSP70 complex does not bind to HSP90, the improperly folded GR tends to aggregate and is subsequently degraded (21).
The proposed mechanism for nuclear translocation of the liganded GR has changed over time. The initial hypothesis envisioned that unliganded GR was bound to the HSP90 complex, which functioned as an “anchoring factor” to retain GR in the cytoplasm (22). By contrast, hormone binding was thought to uncouple the activated GR from HSP and uncover a nuclear localization signal(s), allowing GR to enter the nucleus. Evidence has accumulated over the last 20 years that the activated GR remains in the HSP90 complex and is transported into the nucleus after FK506-binding protein 4 (FKBP4) substitutes for FK506-binding protein 5 (FKBP5) in the cytoplasmic GR heterocomplex and recruits the transport protein dynein (23). As proposed by Pratt et al, this converts the heterocomplex to a transportosome, allowing for transport into the nucleus along microtubular tracks (24).
FKBP5 transcription is stimulated by dexamethasone, so that increased availability and binding of this co-chaperone to HSP90 reduces nuclear entry of the GR complex, leading to GC resistance (16).
Once in the nucleus, in a poorly understood process, the activated GR dissociates from the HSP90 complex and is then available to suppress Pomc transcription. As described in the following discussion, GR inhibits POMC transcription by direct repression though a negative GRE element (nGRE) at bp −69 to −61, by transrepression through disruption of protein:protein contacts, and by transcriptional repression of the essential TF NeuroD.
The nGRE was the first sequence shown to be repressed by GR; surprisingly, binding involves binding of a GR homodimer with subsequent monomer binding on the other side of the DNA helix (25). However, an elegant series of studies using promoter constructs containing various combinations of REs showed that the nGRE alone did not support either transactivation or repression, TpitRE was involved only in activation, and only NurRE-dependent activity was required for both Pomc activation and GC repression (26,27). Through targeted mutation in the E-box, Parvin et al demonstrated that it, and specifically NeuroD, also was essential for dexamethasone suppression: dexamethasone suppressed NeuroD expression while NeuroD overexpression reconstituted GC suppression (28). These studies excluded the possibility of GR binding to DNA and identified a mechanism involving GR protein:protein contacts facilitated by the presence of Brg-1 [further reviewed in (29)] with subsequent recruitment of histone deacetylase (HDAC) 2, decreased histone acetylation, and decreased RNA polymerase II recruitment and promoter clearance (30).
Regulation of ACTH Secretion
As described in the previous discussion, CRH is the major stimulus for ACTH production and secretion in healthy people. The increased intracellular calcium that results from CRH binding leads to exocytosis of ACTH-containing vesicles, in addition to Pomc transcription (9).
While the molecular mechanism of GR inhibition of ACTH secretion is not fully understood, it is clearly related to modulation of electrical excitability of the corticotrope. In electrophysiologic experiments using mouse corticotrope cells, spontaneous and CRH-induced burst number decreased when cells were exposed to corticosterone for 90 or 150 min before perfusion with CRH. Further evaluation showed that the large-conductance calcium- and voltage-activated potassium (BK) channels were partly responsible for this inhibition after an intermediate-term GC exposure (31). Glucocorticoids alter large-conductance calcium- and voltage-activated potassium channels by affecting messenger RNA (mRNA) splicing and increasing phosphorylation (32,33).
The previous experiments evaluated intermediate responses after exposure to dexamethasone for at least 30 min. Experimental data suggest that GCs terminate ACTH secretion and thus complete a pulse, through nongenomic effects (presumably) at the cell membrane, within a shorter time frame (termed “fast” effects). In perifusion experiments with dispersed pituitary cells from intact rats, the addition of corticosterone to a CRH-containing perifusate resulted in a 40% decrease of ACTH within 15 min, with an increase to previous levels within 5 min of corticosterone discontinuation. As a result, the administration of two 30 min CRH + corticosterone infusions interspersed by 30 min of CRH-only perifusate resulted in 2 ACTH pulses (34). In parallel, GR immunoreactivity in total cytoplasmic membranes increased during corticosterone exposure, with a 50% increase at 5 min and a peak of 3.5-fold at 30 min, following by a rapid decrease of nearly 50% 7.5 min after corticosterone withdrawal (34).
Anterior pituitary hormone secreting cells form an organized cellular architecture characterized by homotypic networks comprised of cells of the same type, as well as heterotypic networks, in which a different cell type(s) Participates. Three-dimensional imaging revealed that corticotrope cells develop cytonemes that extend from the cell body and contact the vasculature and perivascular space. The normal pituitary gland also contains folliculostellate cells, some of which have a star-like shape allowing them to touch and thereby influence hormone-producing cells. One can imagine that this 3-dimensional organization allows for stimulation and suppression and coordination of responses to incoming signals (35).
One important action of folliculostellate cells is to suppress ACTH secretion through annexin 1 (ANXA1) secretion and interaction at the corticotrope cell membrane. Glucocorticoids induce ANXA1 folliculostellate expression and translocation to cytoplasmic projections that abut the outer surface of the corticotroph cell. The importance of this paracrine effect on AtT-20 cells was shown by reduction of ACTH secretion after dexamethasone exposure and CRH stimulation only when the cells were co-cultured with a folliculostellate cell line (36).
Regulation of ACTH Pulsatility
In healthy individuals with regular sleep-wake cycles, blood cortisol levels have a marked circadian rhythm characterized by a nadir (<1.8 ug/dL, 50 nmol/L) within 1 h of sleep initiation (37), followed by a gradual increase. Peak levels, up to 10-fold higher (22-25 ug/dL, 610-695 nmol/L), are achieved within a few hours after wakening (38), followed by a decline to the next nadir (Fig. 3). In healthy people, individual cortisol bursts (pulses) are preceded by an ACTH pulse, demonstrating that cortisol secretion is clearly linked to and stimulated by ACTH (38).
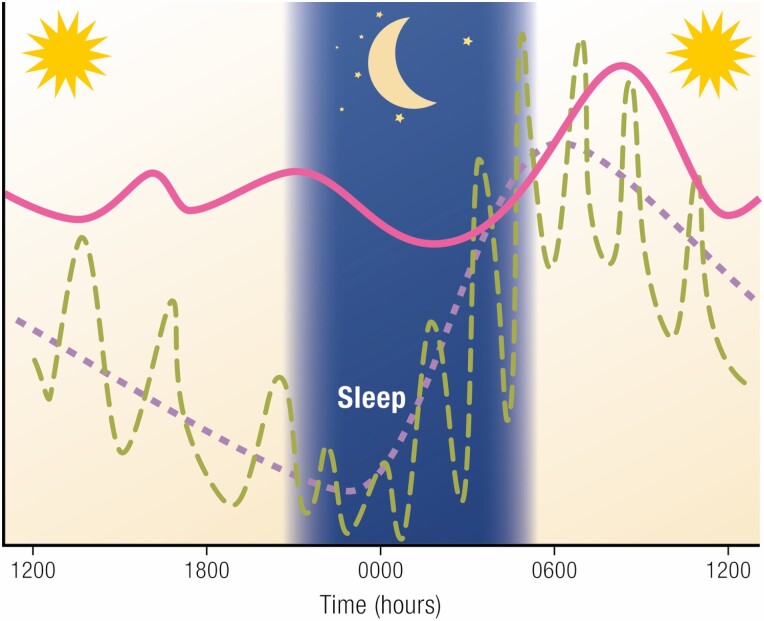
Figure 3.
Schematic representation of cortisol patterns over 24 h, showing ultradian pulses (green dashed line) and mean values (purple dashed line) in healthy individuals. The normal nadir is tightly entrained to the onset of sleep. The pink line represents the relatively invariant levels seen in Cushing’s syndrome, in which the bedtime nadir is lost.
An important characteristic of circulating cortisol is its high affinity, low-capacity binding to corticosteroid binding protein/globulin (CBG) and its low affinity high-capacity binding to albumin. As a result, about 95% of cortisol circulates as protein-bound to either CBG (~80%-90%) or albumin (~10%-15%) (39). To date, most data suggest that this relationship achieves 4 purposes: (1) providing transport of cortisol in the circulation; (2) providing a reservoir of inactive (bound) cortisol that can be accessed during febrile illness (due to reduced CBG affinity), (3) which can absorb/render inactive excess cortisol, allowing for modulation of free levels because of decreased coupling in states of acidosis, fever or inflammation, and ultimately; and (4) regulating the availability of free cortisol, currently considered the only biologically active intracellular modality (40). Since only free cortisol is biologically active, conditions that influence the bound:free ratio or that alter the total amount of measured cortisol need to be kept in mind. Unfortunately, due to the laborious technique for measuring free cortisol, data on pulsatility are available only for total blood concentrations.
Deconvolution analysis of human blood samples obtained every 10 min for 24 h showed that cortisol secretion occurred in random secretory bursts with a 2-fold variability in frequency and a nearly 7-fold increase in burst amplitude, without interval tonic secretion (41). As a result, amplitude-modulated variable pulses of cortisol underlie its diurnal rhythm. Additional sampling intervals of 2, 4, 8, and 12 min revealed relatively regular ACTH pulses with similar amplitudes across the day; additional pulses were detected by more frequent sampling so that the interinterval duration decreased from 73 + 11 min at 12-min intervals to 18 + 0.8 min when samples were obtained every 2 min. As with cortisol, the diurnal ACTH excursion was modulated by the pulse amplitude, not frequency (42).
The foregoing links cortisol and ACTH, but how does the hypothalamic-pituitary unit link to the adrenal gland? The conceptualization of CRH as a hypothalamic hormone (43) and a subsequent report that destruction of the suprachiasmatic nucleus (SCN) ablated circadian rhythms (44) led to an explosion of investigation into central and peripheral “clocks” that govern physiologic periodicity of hormone secretion and function. Because the SCN communicates with the paraventricular nucleus (PVN), which produces CRH and AVP, it was thought that these hormones might transduce a pulsatile hypothalamic input to the pituitary gland. The identification and sequencing of CRH (45) presented an opportunity to test Harris’s theory of hypothalamic hormonal and neuronal control of the anterior pituitary gland (46).
One possible explanation for the pulsatility and diurnal variability of ACTH is that the SCN sends pulses to the PVN that in turn encode ACTH pulsatility. Tested in isolation, dispersed rat pituitary cells responded immediately after CRH introduction into the perfusate chamber, with a maximal ACTH response within 1 min and continued response during a 3-min exposure to CRH; unexpectedly, secretion continued with a slow decrease to baseline for 4 min after discontinuation of CRH exposure. These data were in keeping with the hypothesis that pulses of ACTH were induced by CRH pulses.
However, CRH pulses in the median eminence of free-running rats were found to occur about 3 times each hour (47), without variation in diurnal amplitude. This did not mimic the diurnal pattern or amplitude of ACTH pulses, suggesting that CRH does not encode differences in either ACTH pulses or diurnal variation. Additionally, the observation that ACTH pulses remained intact in ewes with transection of the pituitary stalk, which could not receive hypothalamic hormonal signals, also was against the concept that ACTH pulses result from suprapituitary hormonal input (48).
An intracellular corticotrope clock system might direct the pulse pattern and diurnal variability of ACTH. However, examination of human autopsy specimens did not support this possibility: compared to other times of day, ACTH transcripts decreased at night, while period circadian regulator 1 transcripts decreased only at dawn (49).
Taken together, these data suggest that CRH plays a supportive role for POMC production and secretion but does not encode pulsatility. While secretagogues can initiate secretion, the termination of that release (which defines a pulse) appears to be controlled at the pituitary level by GC feedback. This 2-step approach allows for additional signals to modulate ACTH production and release to achieve a variety of pulse patterns that are nonsynchronous with secretagogue pulses or exposure.
Recently, the Lightman group developed predictive mathematical modelling of cortisol and ACTH pulses at varying constant CRH inputs that mimics experimental in vivo findings in the rat (50). The model assumes that cortisol in humans and corticosterone in rodents terminates pulses and considers various amounts of delay between an ACTH pulse and a subsequent cortisol/corticosterone pulse; this delay is biologically inherent because the adrenal gland does not store GCs but rather synthesizes and secretes them upon ACTH stimulation (51).
There is mounting evidence for the existence of an intraadrenal clock mechanism that contributes to the circadian rhythm. Wild-type mice and Per2/Cry1 double mutant animals that do not have circadian variation in the clock genes were used to examine the presence of an intraadrenal clock gene. Organ culture of adrenal slices showed that the mutant mice had corticosterone responses like those of the wild-type mice when stimulated with ACTH at the time of the usual corticosterone nadir. By contrast, when stimulated at the time of the usual corticosterone zenith, wild-type mice showed, as expected, a greater response, while the response of mutant mice was unchanged (52). In intact animals, autonomic input from the SCN to the adrenal gland also may modulate the diurnal response via SCN neuronal connection to the neurons in the intermediolateral column of the spinal cord, which then project via splanchnic fibers to the adrenal glands (53).
By closing the negative feedback loop, GCs play a major role in modulating ACTH pulsatility through effects on CRH as well as ACTH secretion. Dexamethasone infusion in conscious sheep, at levels sufficient to reduce cortisol concentrations, decreased hypophysial portal CRH concentrations in the basal state but not after an audiovisual stress. However, despite normal CRH levels with the audiovisual stressor, both ACTH and cortisol levels decreased, presumably due to corticotrope inhibition. However, the same dexamethasone infusion decreased CRH, AVP, ACTH, and cortisol responses to hypoglycemic stress (54). Thus, the ability of dexamethasone to attenuate hormonal responses varied according to the magnitude of the stress.
Additionally, there is an inverse dose-response relationship between the amount of GC exposure and CRH concentration. As shown in an adrenalectomized rat model, corticosteroid replacement to achieve trough physiologic levels resulted in increased CRH concentrations at baseline and after acute and chronic stress, compared to intact animals. However, in adrenalectomized animals given corticosterone replacement at peak physiologic levels, baseline CRH was normal at baseline and after chronic stress (ie, negative feedback was intact), but CRH was increased after acute stress (55). This suggests that the chronic lack of negative feedback in the underreplaced animals prevents the usual adaptation to chronic stress, that acute stress can increase CRH despite chronic exposure to high levels of corticosterone and that chronic overexposure represses CRH.
Mechanisms Leading to Hypercortisolism in ACTH-Secreting Tumors
Rarely, excessive CRH secretion, with or without concomitant tumoral ACTH production, leads to CS. All other ACTH-driven causes of CS presumably suppress hypothalamic CRH production. This leads to the question of whether hypercortisolism is driven by a lack of negative feedback effect on ACTH production or whether it is driven by excessive ACTH secretion from a non-CRH secretagogue, or both.
As discussed in the following text, a variety of mechanisms have been postulated or proven for corticotrope tumors, while relatively few have been elucidated for ectopic ACTH production.
Many theories about the etiology of CD derive from studies of the mouse AtT-20 cell (4), as there are no human CD cell lines. Ideally, data derived using this model should be validated with human tumors, but corticotroph tumors are rare and small. As a result, many of the conclusions from AtT-20 studies have not been examined further in human tissues.
Neuroendocrine tumors (NETs), most commonly of the lung, thymus, pancreas, and appendix, as well as pheochromocytoma and medullary thyroid cancer, produce ACTH ectopically (ie, not in the eutopic place, the pituitary corticotrope) (56). There are few models or cell lines with which to study POMC regulation in these tumors. Two POMC-producing and GC resistant cell lines with neuroendocrine properties, COLO320 (a colon cancer-derived line) and DMS79 (a small cell lung cancer–derived line lacking GR) have been used, in addition to primary cultures of human tumors and murine tumoral xenografts.
Ectopic ACTH Secretion
Limited but compelling studies suggest that EAS may arise from the use of alternative POMC promoter regions. If the region and its resulting transcription complex were GR insensitive, this would provide a unifying mechanism by which ACTH secretion is enhanced while simultaneously avoiding GC repression.
E2F, a group of genes that encodes a family of TFs in higher eukaryotes, binding to the POMC promoter was implicated in several EAS studies. In healthy people, the POMC promoter is heavily methylated (57,58), particularly in the distal domain E2F1 binding region (Fig. 2). In studies of ACTH-producing bronchial, thymic, and pancreatic NETs, that region was hypomethylated in compared to corresponding normal (non-ACTH expressing) tissues, corresponding to increased promoter activation (57,59,60). Activation of the upstream E2F site by E2F1 (and DP1, 1 of its usual partners), but not the usual E-box-NurRE region, stimulated POMC transcription in DMS79 cells (61,62). Interestingly, this region is mutated in the rodent Pomc promoter (compared to the human), where it is not transcriptionally active (61). (As shown in Figure 2, it is not known whether this region is actively transcribed in normal or tumoral corticotropes.)
Araki et al found that 70% of promoter activity arose from a proximal region of the POMC promoter (−42/+68) in COLO320 and DMS79 cell lines and human tumors causing EAS (6 lung, 1 liver, and 1 thymic NET) (63). This area proved to contain an specificity protein 1 or 3 binding site and 2 mirrored E2F binding sites. In vitro overexpression of E2F and DP1 upregulated POMC promoter activity. Additionally, E2F and POMC expression were correlated in the ACTH-producing pulmonary NETs. Finally, treatment of the human tumors in primary culture with R-roscovitine, which suppresses the mouse proopiomelanocortin gene in AtT-20 cells (64), reduced ACTH production and displaced E2F1 from binding sites, and a direct inhibitor of E2F1 reduced ACTH levels in tumor xenografted mice (63). Interestingly, EAS tumors may express epidermal growth factor receptor (EGFR), which can act through E2F to promote this pathway (65).
A second POMC promoter region was recently identified also by the Melmed group. Their novel approach involved 5’-rapid amplification of complimentary DNA ends using RNA obtained from normal human pituitary tissue and from ACTH-secreting pituitary and ectopic tumors (and cell lines). The expected 5’-ends from the known upstream promoter were found in all tissues. However, additional sequences from tumors mapped to the downstream section of intron 2 and part of exon 3 (bp +6657 to +7136) and contained cAMP RE-binding protein and signal transducer and activator of transcription binding sequences. Cells transfected with this an expression vector containing POMC constructs tagged to identify products from this promoter secreted tagged ACTH into the culture medium. While the classic promoter (denoted as promoter 1 in the graphical abstract) responded as expected to LIF and CRH stimulation, this new promoter showed enhanced basal activity that was downregulated with LIF and CRH exposure. Evaluation of methylation status of this region in 11 normal pituitary specimens, 3 corticotrope tumors, and 2 ectopic ACTH-secreting tumors, showed significant demethylation in 2 CD and 1 ectopic ACTH-secreting tumor and detectable demethylation in the final CD patient. In all patients, transcription from this region corresponded to the amount of its demethylation (58). Interestingly, studies of the upstream promoter showed that it was demethylated in patients with ubiquitin carboxyl-terminal hydrolase 8 (USP8) mutations, while tumors with a more aggressive phenotype tended to show demethylation in the second promoter region. Taken together, these findings suggest that demethylation may regulate activities of the 2 promoters, leading to different biologic phenotypes. In addition, the robust basal activity of the second promoter may underlie the excess production of ACTH in ectopic ACTH-secreting tumors.
Limited studies suggest that GC resistance can play a role in the pathogenesis of EAS. GR mutations in the N-terminal and DNA-binding domain accounted for complete resistance to GC action in 1 small cell lung cancer cell line (66), but normal GR mRNA was found in another study of 3 bronchial carcinoids that showed in vivo suppression to dexamethasone, 8 mg (67).
Posttranslational modifications of cofactors have been implicated in the development of ectopic ACTH-secreting tumors. One study of ACTH-secreting thymic carcinoids demonstrated upregulation of silencing mediator of retinoic acid and thyroid hormone receptors (SMRT), by increased SUMOylation of an HDAC binding site. SMRT SUMOylation was confirmed in the DMS-79 and prevented formation of the SMRT-HDAC-GR complex required for GC suppression of POMC (68).
Cushing’s Disease
Altered promoter activation
The liver X receptor (LXR) binds to the rat Pomc promoter at bp −75/−52 and appears to increase transcription of Pomc (69). The oral pan-HDAC inhibitor suberoylanilide hydroxamic acid (Vorinostat) decreased protein levels of LXRa and suppressed POMC transcription in dispersed human corticotrope tumor cells, suggesting that LXRa may play a role in tumorigenesis (70). However, since a pan-HDAC inhibitor can act at other locations, these effects need to be confirmed in mutational experiments with mutations at this site. However, interestingly, the −77 to −51 bp region of the promoter contains partially overlapping potential binding sites, including the nGRE (25), retinoid X receptor/LXR (68), and nerve growth factor 1B (NGFI-B)-responsive element (NBRE) binding regions.
As previously described for EAS tumors, a novel POMC promoter region recently identified by the Melmed group (bp +6657 to +7136) does not respond to CRH and was demethylated in 3 corticotrope tumors. The transcription observed from this region corresponded to the amount of demethylation (71). Use of this promoter would provide a mechanism by which ACTH production is sustained in the absence of hypothalamic CRH.
Recent exciting advances in the genetics of CD include the discovery of somatic and germline mutations. Somatic mutations in the USP8 gene have been reported in up to 36% to 62% of corticotroph adenomas (72-74). This mutation increases the enzyme’s ability to deubiquinate proteins, preventing their degradation and increasing intracellular concentration. While this may affect many proteins, EGFR levels are increased in these tumors (72). Melmed’s group has shown that EGFR signals through mitogen-activated protein kinase and E2F1, which binds to the POMC promoter and increases transcription in human tumor cells (75).Others have found increased EGFR protein expression in corticotrope tumors that are initially resected (47%) and more frequent expression in tumors that recurred (70%) (76). USP8 integrity was not analyzed; thus, it is possible that these represented cells carrying the mutation, or alternatively EGFR may be increased for other reasons. As speculated regarding the previously described novel promoter, EGFR-driven transcription would abrogate the need for CRH stimulation.
Autocrine regulation of POMC production
As will be discussed later, normal people decrease cortisol levels to < 1.8 ug/dL (50 nmol) after 1 mg dexamethasone because of ACTH suppression. Presumably CRH also is suppressed, leading to the question of whether CRH is available to a corticotrope tumor. While it is possible that POMC production is stimulated by other signal transducers (like EGFR), there is some evidence that CRH and vasopressin are produced by mouse corticotrope cells and human tumors (67,77). If these are secreted, it is possible that tumors autocontrol ACTH production.
Taken together, data suggest that use of alternate promoter regions enhances the ability of corticotrope tumors to escape GC negative feedback and to increase POMC production.
Mechanisms that decrease GC negative feedback
Increased cortisol inactivation leading to less intracellular ligand for GR activation would reduce negative feedback. Compared to normal pituitaries, corticotrope tumors showed increased expression of 11βHSD2 mRNA and protein and decreased mRNA or protein expression of 11βHSD1 (78).
Decreases and increases in the ratio of nuclear HSP90:GR each lead to decreased GR activity (22,79). HSP90 is overexpressed in human corticotroph adenomas compared to the normal pituitary gland (80), leading to increased binding to the GR without dissociation. Presumably this reduces GR availability for nuclear and membrane translocation and a decreased POMC suppression and fast GC negative feedback. The C-terminal HSP90 inhibitors silibinin and novobiocin cause activated GR to be released from HSP90 and partially restore GC sensitivity in human corticotrope cell lines and a nude mouse tumor model (80). By contrast, the N-terminal inhibitor 17-AAG blocks the adenosine triphosphatase activity needed for transfer of GR from HSP70 to HSP90; as a result partially folded GR is released and degraded (21).
FKBP5 transcription is stimulated by dexamethasone, so that increased availability and binding of this co-chaperone to HSP90 reduces nuclear entry of the GR complex, leading to GC resistance (16).
An inability to interact with other TFs is another possible mechanism for decreased GR sensitivity despite normal mRNA levels. The TF testicular orphan receptor 4 (TR4) is normally found primarily in the corticotrope cell cytoplasm. However, in a study of 12 corticotrope tumors, TR4 protein expression was increased, and was primarily intranuclear, in 10 of these tumors. (81). TR4 bound in the upstream POMC promoter (−854 to −637 bp) and blocked GR transrepression in AtT-20 and human corticotrope cells, probably through protein:protein interactions that prevented GR binding to DNA. (82).
A germline mutation in CABLES1 (CDK5 and ABL1 enzyme substrate 1) has been reported in 4 female patients among a cohort of 146 children and 35 adults with CD (83). These tumors were large and more aggressive than others in the group. This gene is normally strongly expressed in normal human corticotrope cells and in AtT-20 cells, where it is upregulated by GC exposure and prevents progression into the cell cycle. Conversely, its absence abrogates dexamethasone suppression of proliferation. CABLES1 protein is absent in about 50% of corticotroph adenomas, suggesting that it contributes to tumor formation and GC resistance, without a germline mutation (84).
Bilodeau et al reported reduced or mislocated HDAC2 or reduced expression of Brahma-related gene-1 in 17/36 corticotrope tumors. As previously described, Brg1 and HDAC2 are recruited to the POMC promoter by an activated GR and act as transcriptional corepressors through protein interactions with GR (30). Thus, a decrease in either factor would reduce GC sensitivity.
These mechanisms to reduce GR feedback are likely to play a role in enhanced POMC secretion but are unlikely to account for many-fold increased production in some patients with CD.
Mechanisms yet untested
O-linked-N-acetylglucosaminylation (O-GlcNAcylation), like phosphorylation, is a rapidly cycling posttranslational modification of proteins. The enzyme O-linked N-acetylglucosamine (O-GlcNAc) transferase catalyzes the addition of O-GlcNAc at a serine or threonine residue and the enzyme O-GlcNAcase catalyzes its removal. The catalytic subunit of RNA polymerase contains a domain that can undergo O-GlcNAcylation or phosphorylation (which then initiates transcription) (85). Data support the concept that recruitment of O-GlcNAc transferase prevents Pomc transcription by blocking phosphorylation (86).
Posttranslational modification of GR by phosphorylation, O-GlcNAcylation, and acylation have the potential to alter GR function. While acetylation has been shown to be important for repression of nuclear factor kappa-light-chain-enhancer of activated B cells–regulated genes (87), it has not been established as a mechanism to modulate Pomc.
Relatively little is known about the 3-dimensional architecture of corticotrope tumors and whether folliculostellate cells are present in any area. Since GCs induce ANXA1 folliculostellate expression and translocation to reduce ACTH secretion, reduction in the corticotrope:folliculostellate cell adjacency would likely lead to greater ACTH secretion.
Physiology and Fates of Cortisol in Healthy Individuals
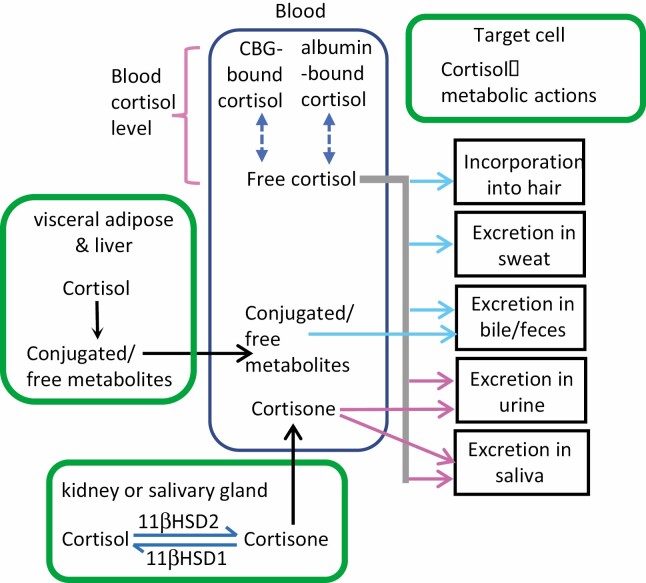
CS is defined by overproduction of cortisol. Thus, to understand the screening tests that define over-production, one must consider the fates of cortisol in healthy persons (Fig. 4).
Figure 4.
The fates of blood cortisol after secretion by the adrenal glands. Nearly all cortisol circulates in blood bound to a chaperone protein, either corticosteroid binding globulin (CBG) or albumin. The remaining unbound (free) fraction is biologically active and diffuses into target tissues, where it (1) exerts metabolic effects, (2) is metabolized (eg, renal inactivation into cortisone by 11βHSD2 or additional hepatic conversions), (3) is incorporated into growing hair, or (4) is excreted in sweat, urine, or feces. The pink arrows denote tissues in which free cortisol and/or its metabolites can be measured using commercially available assays; the blue arrows indicate research assays. Free cortisol in blood can be measured commercially or calculated using serum cortisol, albumin, and CBG values.
Urinary Free Cortisol
As implied by the low-capacity binding affinity of CBG, cortisol saturates CBG binding sites at physiologic (~16.2 ug/dL; ~450 nmol/L) levels, leading to an increase in free cortisol levels (88,89). Plasma free cortisol is filtered into the collecting duct at the juxta glomerular apparatus. Early studies (90,91) showed that only a small fraction of the free plasma cortisol was excreted in urine. At the time this was attributed to tubular reabsorption of cortisol.
More recently, the contribution of 11-beta hydroxysteroid dehydrogenase type 2 (11βHSD2) to renal cortisol metabolism has been appreciated as an additional factor explaining the low ratio of excreted: circulating free cortisol. 11βHSD2 is present in the renal cortical and medullary collecting ducts where it inactivates cortisol by conversion to cortisone (92). The reduction in cortisol levels in the collecting duct is demonstrated by a higher cortisone:cortisol ratio in urine compared to plasma. As cortisol binds avidly to the mineralocorticoid receptor and circulates well in excess of aldosterone, renal inactivation of cortisol prevents or reduces its binding to the renal mineralocorticoid receptor. From a teleologic perspective, this protects the body from sodium retention and possible hypertension.
Metabolism and Conversion
As mentioned earlier, cortisol can be inactivated to cortisone in the kidney by11βHSD2 and reactivated by 11βHSD1 in the liver and adipose. It can be further metabolized by 5α- and 5β-reductase (to 5α- and 5β-dihydrocortisol), by 3α-hydroxysteroid dehydrogenase (to 5α-and 5β-tetrahydrocortisol), and 20α- and 20β-hydroxysteroid dehydrogenases to cortols. Cortisone is metabolized by the same sets of enzymes to dihydrocortisones, tetrahydrocortisones, and cortolones (93). Steroid hormone profiling by tandem mass spectrometry is a promising technique to diagnose CS and its causes that requires additional validation and commercial availability before it comes into common use.
Removal From Circulation into Hair and Feces
Cortisol also is incorporated into hair and is excreted in feces. Measurement of hair cortisol has shown to be useful to identify the association of mild hypercortisolism with potential comorbidities but has not yet been validated as a test to diagnose CS (50,94). While this has been interpreted as reflecting adrenal steroidogenesis, demonstration of regulated cortisol production from cultures of isolated scalp hair may reflect local synthesis as well (95).
Derangements in Physiology of Blood and Urine Cortisol in CS
Importantly, plasma concentrations of (total) cortisol >20 to 25 ug/dL saturate both CBG and 11βHSD2. This increases free plasma cortisol levels so that the filtered concentration increases, and less of the filtered concentration can be inactivated (89,96,97). As a result, urine cortisol values increase when plasma levels exceed this threshold. One might speculate that stressful activities of daily living with transient increases in cortisol may account in part for the broad “normal” range of 24-h urinary free cortisol (UFC) and that elevated UFC in CS requires sustained increases in cortisol secretion during the usually quiescent late afternoon to early morning hours. This presumption is suggested by 3 different observations. First, patients with recurrent CS have increased late night salivary (free) cortisol values before UFC values are increased, suggesting that mild changes in the nadir of cortisol are not sufficient to increase 24-h UFC (98). Second, patients with dysregulated cortisol secretion due to adrenal adenomas may have relatively normal LNSC and normal UFC (99). Third, patients with physiologic non-neoplastic hypercortisolism tend to have increased UFC out of the proportion to the late-night salivary cortisol level, which might be normal (100). This suggests that physiologic hypercortisolism occurs primarily during waking hours, with some increase in cortisol levels above those that saturate CBG capacity.
However, some patients with mild CS may not have increased UFC. This phenomenon may reflect an increased mean 24-h blood cortisol level insufficient to saturate CBG, day-to-day variability in UFC, as is known to occur in patients with established CD (98) or use of assays that measure only UFC (see following discussion).
Considering the Diagnosis of Cushing’s Syndrome
The clinical diagnosis of CS is not always straightforward. As shown in Table 2 and Figure 5, there are many clinical manifestations, which vary in severity and in their combinations at presentation. It is also important to recognize that the presence of any feature must be considered in the context of the entire patient’s history. For example, while the specificity of osteoporosis is reported to be high, the presence of osteoporosis in a postmenopausal woman is common and may not provide useful information regarding hypercortisolism. No single feature makes the diagnosis of CS; rather, it is the development and presence of multiple features that coalesce into recognition of the syndrome. Table 3 describes several reasons why clinicians fail to consider CS as a potential diagnosis:
Table 2.
Clinical features of Cushing’s syndrome
| Clinical Feature | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Increased fatigue | 100 | |
| Decreased libido | 33-100 | |
| Weight gain | 79-97 | |
| Irritability; emotional lability | 40-86 | |
| Insomnia | 69 | |
| Decreased concentration | 66 | |
| Impaired short-term memory | 83 | |
| Changes in appetite | 54 | |
| Lethargy, depression | 40-67 | 57 |
| Menstrual changes | 35-86 | 49-74 |
| Osteopenia or recent fracture | 48-83 | 91-95 |
| Headache Nugent | 47-58 | 63 |
| Backache | 39-83 | |
| Glucose intolerance/Diabetes | 45-70 | 70-83 |
| Recurrent infections | 14-25 | |
| Generalized obesity | 51-90 | 71 |
| Truncal obesity | 3-97 | 38 |
| Plethora | 78-94 | 69 |
| Round face | 88-92 | |
| Hirsutism | 64-84 | 48-80 |
| Hypertension | 74-90 | 52-83 |
| Eccymoses | 60-68 | 94 |
| Striae wider than 1 cm and purple in color | 50-64 | 61-78 |
| Weakness, especially in hips and shoulders | 56-90 | 70-93 |
| Abnormal fat distribution: centripetal, dorso-cervical, supraclavicular and temporal | 34-67 | |
| Edema | 48-66 | 83 |
| Thinness and fragility of skin | 84 | |
| Abdominal pain | 21 | |
| Acne | 21-82 | 61-90 |
| Female balding | 13-51 |
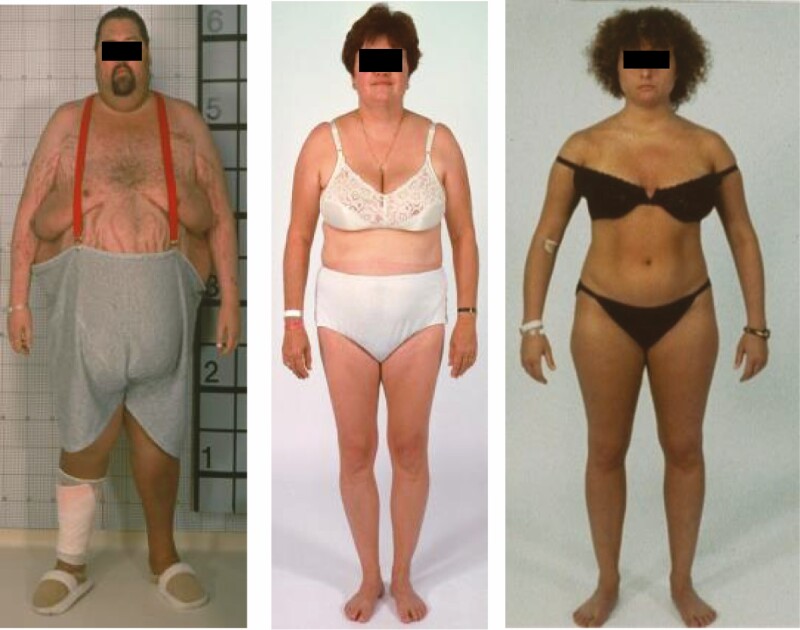
Figure 5.
Photographs of 3 patients with Cushing’s syndrome, illustrating the range of physical features. The man on the left demonstrates central obesity, striae, supraclavicular fullness, rounded face with filling of the temporal fossae, and poor skin healing (bandage). The woman in the middle has increased supraclavicular fullness, facial plethora, and a history of new hypertension and depression. The woman on the right appears normal except for some facial fullness in comparison to previous photographs. By history, she was gained weight, with increased abdominal girth, and a history of mild decreases in cognition and short-term recall (memory).
Table 3.
Reasons why clinicians fail to consider the diagnosis of Cushing’s syndrome
| Many of the features of Cushing’s syndrome are common in the general population (Table2). |
| Signs and symptoms accumulate and often worsen over time, so that the initial presentation may include only one or two features, such as weight gain and irritability. |
| Features with the greatest specificity for Cushing’s syndrome (ie, uncommon in the general population) such as wide purple striae, thrombotic phenomenon, severe hypokalemia, or fracture tend to occur late, after long exposure, or with the most severe hypercortisolism. |
| Some features (eg, fractures, amenorrhea, central serous retinopathy) are most likely to be evaluated by practitioners less likely to have experience with Cushing’s syndrome. |
What strategies can clinicians use to ensure that they consider the diagnosis of CS? Given the wide spectrum of possible symptoms, the possibility that the diagnosis is not suspected and the short timeframe for many clinical interactions, we have found that evaluation of a completed review of symptoms questionnaire before the appointment may present a constellation of signs and symptoms that lead to consideration of the diagnosis. Subsequent questioning during the patient-clinician interaction can be more targeted.
Questions are more useful when they are not framed with the possible answer. For example, if asked “Do you forget things that happened recently?” many patients will say no. However, if you ask, “Do you use a grocery list/appointment calendar/Post-It notes for X?” [when X is a known activity of the patient], one may discover that a memory aid is necessary for normal function and infer a problem with recent memory.
After the initial history is taken, a careful physical examination, including a mini-mental test, the skin (striae, acne, hirsutism/baldness, bruising), remembering 3 objects in the room (103), and muscle strength may lead to questions that characterize the temporal development and quantification of changes. It is also important to document abnormal adipose (particularly in the temporal, dorsocervical, and supraclavicular regions).
The diagnostic approach continues with formulation of the pretest probability of CS, which is based on data from the history and physical examination and influences interpretation of screening tests (106). The pretest probability increases if the patient’s signs and symptoms are not common in most people of the patient’s age and sex and accumulate over time (107). Development of mood lability is extremely common in CS and its absence is slightly against the diagnosis (103).
Choosing and Interpreting Screening Tests for Cushing’s Syndrome
The second part of the evaluation for CS involves biochemical and provocative (dynamic) testing. Before this, exposure to exogenous GCs is excluded by thorough questioning about any nondietary substances taken by mouth, including oral prescribed and over-the-counter medications, herbal preparations/tonics (which may contain GCs), injections (especially for joint or spine pain), and the use of topical (eg, skin bleaching agents), rectal, and inhaled agents. In certain settings (eg, if intentional overuse is suspected), measurement of synthetic steroids in plasma or urine may be helpful (3).
The Endocrine Society’s clinical guidelines advocate the use of at least 2 of the following screening tests: 1-mg overnight dexamethasone suppression, 24-h urine cortisol measurement, and/or late night salivary/serum cortisol measurement (3). The choice and timing of each test is critical to avoiding false-positive and false-negative results and should be individualized to each patient (Table 4). Each test is discussed next, including factors influencing its choice and the interpretation.
Table 4.
Caveats and restrictions for tests used to screen for hypercortisolism
| Test | Caveat | When can the test be used despite the caveat? | Verifying the result |
|---|---|---|---|
| All tests | May be falsely normal in a patient with mild or cyclic CS | If the pretest probability is high based on history and physical examination, repeat testing at intervals or when patient feels worst. | Review caveats for each test |
| UFC | May be falsely increased with fluid intake > 4 L/d (106) | If subjects reduce intake | Measure volume; if volume is high, ask about intake |
| May be falsely increased or decreased with incorrect collection | Measure creatinine, which should be ±15% from day to day; check volume | ||
| Will be decreased in the setting of renal impairment (107) | Use with caution when GFR is 30-50 mL/min | Only use result if value is elevated | |
| May be falsely increased with high sodium intake (108,109) | Impact is not clear, as normal ranges may reflect sodium intake; advise patient to reduce sodium when collecting | ||
| May be increased in pseudo-CS (110) | Values > 3-fold upper normal limit are more likely to be true CS | ||
| 1 mg DST | May be falsely normal in Cushing’s disease patients, presumably because of slow drug metabolism (111) | Measure dexamethasone; if in range expected of an 8 mg dose, consider Cushing’s disease | |
| May be falsely abnormal in patients with elevated CBG (2o oral estrogen) or in those with fast metabolism of dexamethasone (112) | Fewest false-positive results in patients not taking medications that interact with CYP3A4 (113) | Measure dexamethasone level—if low, increase dose to achieve correct level; measure CBG—if high, discontinue estrogen for 4-6 weeks | |
| May be falsely abnormal in renal failure (114) | |||
| Bedtime salivary cortisol | May be falsely abnormal in older men and women, and in hypertensive or diabetic patients (115) | If used in these populations, consider accepting only normal results. | |
| May be falsely abnormal in individuals with variable sleeping times (eg, shift workers) (116) | If used in this population, consider accepting only normal results. |
Abbreviations: CBG, corticosteroid-binding globulin; CS, Cushing’s syndrome; 1 mg DST, 1 mg overnight dexamethasone suppression test; salF, salivary cortisol; GFR, glomerular filtration rate; UFC, urinary free cortisol.
Urinary Free Cortisol
As previously discussed, urine cortisol excretion increases when serum cortisol levels exceed CBG capacity and are an integrated measurement of cortisol exposure over a 24-h period. Detection of hypercortisolism by this method requires normal renal function and a complete urine collection. Factors that influence the choice of this test include the following.
Assay issues
Historically, assays to evaluate excess GC production evolved as follows:
Measurement of urine 17-hydroxysteroids, using the Porter-Silber reaction.
Measurement of urinary cortisol by immunoassays using antibodies that cross-reacted primarily with cortisol and to some extent with other precursors and metabolites of cortisol.
More specific (cortisol-only) immunoassays using a purification step prior to assay.
Current use (by many commercial laboratories in the United States) of structural assays such as liquid chromatography/ tandem mass spectrometry (LC/MSMS). These do not use antibodies for analyte detection and were thought to measure only cortisol, although a recent report documented interference with 20-alpha and beta-dihydrocortisone (108).
Comparisons of LC/MSMS with immunoassay results showed cross-reactivity with cortisone, and the A ring reduced cortisol metabolites 5α- and 5β-dihydrocortisol and their glucuronide conjugates (109,110). As a result, some immunoassays give results up to 60% higher than structural assays, with a larger discrepancy at higher values, leading to large difference in the reference ranges of assays, with structural assays giving the lowest upper limit of normal.
Two studies comparing LC/MSMS and immunoassays using dichloromethane extraction showed similar diagnostic sensitivity of the 2 methods in patients with and without CS, albeit requiring different diagnostic cutoff points (111,112). However, it is possible that a direct nonextracted UFC assay that cross-reacts with other GCs would have superior sensitivity, especially for patients with mild hypercortisolism (113). Unfortunately, to our knowledge, there are no published data comparing assay techniques in this patient population.
False-negative results: settings in which false-negative results occur in a patient with CS
Incomplete collection.
This is best understood in the context of the correct ways to achieve a complete 24-h collection: (1) The patient wakes up and discards the first voided urine; all subsequent voids are collected until waking at the same time the next day, at which time the waking void is collected, or (2) the collection is started at a specific time (usually in the morning), with a urine void that is discarded. The patient then collects all urine until the same time the next day, when a void is collected.
If patients are unable to follow these instructions, under- or overcollection may occur. Provision of both written and oral instructions may prevent this problem. The volume and creatinine excretion of the UFC collection provide useful information to assess completeness of the collection: a very low volume (less than ~800 mL) may imply undercollection, while a high volume (eg, >4000 mL) may imply overcollection or water loading (see previous discussion). As creatinine reflects lean body mass, it is relatively stable from day to day in any given individual. A change of more than 15% suggests variability in the collection duration.
Renal failure.
UFC decreases progressively below a GFR of 60 mL/min, until complete anuria. There is no way to correct for this, and therefore UFC is not a good screening test in these patients (114).
Mild hypercortisolism.
CS patients with very mild hypercortisolism may have elevated mean serum cortisol levels over 24 h but may not exceed levels that saturate CBG often enough to increase UFC.
Cyclic CS.
Cyclic CS is characterized by periods of hypercortisolism alternating with normal or diminished cortisol production (115). If UFC is collected during a normal or low phase, values will not identify CS.
False-positive results: settings in which false-positive results occur in a patient without CS
Overcollection.
As previously noted, if urine is collected for more than 24 h, cortisol excretion may be increased, because the additional urine is collected in the morning, when cortisol excretion is highest. Strategies to assess for this are described in the previous discussion.
Diuresis/water load.
Older studies using nonspecific cortisol assays reported that UFC increased after a water load (116). Subsequent work by Fenske using a thin layer chromatography assay after dichloromethane extraction showed that both cortisol and cortisone excretion are increased after a water load. The enhanced excretion of cortisol presumably occurs because of decreased reabsorption in the proximal tubule, while cortisone excretion increases because of increased delivery of cortisol and enhanced conversion to cortisone by 11βHSD2 in the distal convoluted tubule. Patients should be cautioned to not drink more than 3 L while collecting the specimen.
High-sodium diet.
Both observational (117) and interventional (118) studies show that UFC increases when daily sodium intake exceeds 150 mEQ (3450 mg/d sodium; ~ 9 g salt). The observational study of 370 adults found that UFC of participants with increased urine sodium levels (>150 mEq/d) were, on average, 55% greater than UFC of those with normal urine sodium levels (50-149 mEq/day). Urinary tetrahydrocortisol metabolites (α-tetrahydrocortisol, β-tetrahydrocortisol, tetrahydrocortisone, cortol, β-cortol, cortolone, and β-cortolone) also were significantly higher in the high-sodium diet group (8·4 ± 3·2 vs. 6·1 ± 2·7mg/d). Participants in the crossover interventional study consumed a diet with restricted sodium intake [about 10 mmol/d (230 mg/d)] or a liberalized sodium intake [about 200 mmol/d (4600 mg/d)]. UFC, measured by Siemens Coat-A-Count radioimmunoassay, was 2 to 4 times higher at various thresholds, during high vs low sodium intake. Patients should be counseled to avoid high salt intake when collecting the specimen.
Physiologic nonneoplastic CS (also known as pseudo-CS)
In 1976 a report of 3 Cushingoid-appearing patients with variably elevated serum and/or urine GC levels and lack of dexamethasone suppression led to an initial proposal of alcohol-induced cushingoid syndrome (119). With the recognition that the clinical and biochemical manifestations of endogenous hypercortisolism resolved with abstinence, these patients were said to have alcohol-induced pseudo-CS (119,120). In 1998 the vague “pseudo-CS” term was defined as “some or all of the clinical features that resemble true CS together with some evidence of hypercortisolism, but resolution of the underlying primary condition results in the disappearance of the Cushing’s-like state” by Newell-Price et al (37). Over the ensuing years, most authors used the term to describe patients with physical and hormonal features consistent with CS, but some did not require abnormal biochemical findings for this syndrome. In response, in 2017 Findling and Raff proposed the term “physiologic/non-neoplastic hypercortisolism” to characterize hypercortisolemic patients without a neoplastic etiology (as is seen in CS). They further divided these patients as those with or without a phenotype (ie, clinical features) consistent with CS (121). While not all of the states included under this rubric are physiologic, certainly all are non-neoplastic.
Non-neoplastic hypercortisolism associated with clinical features includes chronic alcohol dependence and withdrawal (122), poorly controlled diabetes mellitus, GC resistance syndrome (123). obstructive sleep apnea (124),obesity, physical stress (hospitalization, surgery, pain), elderly people (125,126), poorly controlled diabetes, and those with psychological stress (acute or chronic psychological stressors, depression, psychosis, or obsessive-compulsive disorder) (Table 5) (37,127).
Table 5.
Conditions associated with physiologic hypercortisolism in the absence of CS, divided into those that may be associated with physical features of hypercortisolism and those in which hypercortisolism is unlikely to be suspected based on physical features
| Physical features of CS may be present |
| Acute psychological stressors, depression, psychosis, obsessive-complusive disorder |
| Alcohol dependence/withdrawal |
| Poorly controlled diabetes mellitus |
| Severe obesity |
| Obstructive sleep apnea |
| Glucocorticoid resistance syndromes |
| End-stage renal disease |
| Older subjects |
| Physical features of hypercortisolism less likely |
| Pregnancy |
| Malnutrition/anorexia nervosa |
| Intense chronic exercise (eg marathon competitors) |
| Physical stress (hospitalization, surgery, pain) |
| Hypotension/septic shock |
Other considerations: inconvenience/convenience
For some patients, collection of and storage while collecting a 24-h urine is difficult or inconvenient, usually relating to work requirements.
Advantages of UFC
Because it relies on the unbound fraction of cortisol, UFC is not affected by issues related to increased CBG and may be chosen instead of the 1-mg dexamethasone suppression test where CBG may cause a false-positive result.
Interpretation of UFC
The Endocrine Society guidelines suggest using the upper limit of normal of the assay used for any specimen as the criterion for interpretation, since assays differ widely in their reference ranges, with immunoassays showing larger variability than LC/MSMS, as expected given the use of different antibodies. A recent large literature review of studies comparing responses of 1621 patients with proven CS and 2105 in whom CS was excluded showed a sensitivity, specificity, positive likelihood ratio, and negative likelihood (with 95% CI estimates) of 92.3 (88.8-94.8), 86.7 (77.2-92.7), 7.0 (3.9-12.4), and 0.09 (0.06-0.13), respectively (128).
Late Night (Bedtime) Salivary Cortisol
As noted earlier, the diurnal nadir of serum cortisol occurs within 1 h of sleep onset. (Given the entrainment to sleep, this test is perhaps best called “bedtime” salivary cortisol.) Patients with CS have unregulated secretion of ACTH or cortisol, so that this nighttime serum cortisol nadir increases progressively and may be lost completely (129). As it is impractical to obtain bedtime serum cortisol levels on outpatients, measurement of salivary cortisol, deemed “late-night” salivary cortisol is a convenient screening test. Factors that influence the choice of this test include the following:
Assay issues: The normal ranges of published immunoassays differ, and not all are well characterized in terms of the specificity for CS (vs normal or pseudo-CS).
-
False negative results:
i. Patients with cyclic CS will have normal results if tested during a nadir period.
-
False positive results:
i. Patients who have inconsistent sleep-wake cycles—these patients do not have normal diurnal rhythms (130). Thus, this test is not advisable for individuals with inconsistent shift work or those with large variations in sleep onset time, who risk a false-positive result. It can be relied upon for individuals with consistent sleep patterns that do not occur at night; in all cases, the instructions should call for collection at bedtime and not at a specific clock time.
ii. Travel over multiple time zones: Healthy individuals have abnormal diurnal rhythms when crossing time zones: rhythms tend to lag initially closest to the home time zone response, with gradual resolution, like shift workers (131), potentially leading to a false-positive result.
iii. Other reasons for false positive results: Salivary cortisol can be elevated by excitement (132), contamination with hydrocortisone in skin creams (133), chewing tobacco with licorice, and cigarette smoking (due to inhibition of 11βHSD 2 in salivary glands) (134). Thus, this test is not ideal for individuals whose lifestyle includes bedtime shortly after excitement or agitation. Theoretically, even 1% of blood would contaminate the specimen, but this has not been reported. To avoid the preventable and theoretical increases, patients are told to collect on a “quiet” evening, without engaging in anything exciting, to refrain from flossing or brushing teeth until after the collection, and to spit directly into the collection container or to move the pledget to and from the collection container with their mouth (ie, to not touch it with their fingers). The potential contamination with exogenous cortisol (or subnormal 11βHSD2) can be evaluated by measuring both cortisol and cortisone by tandem mass spectrometry; the cortisol level will be much higher than the cortisone level in these situations (133). Some conditions, such as older age (>60 years), diabetes, and hypertension are associated with higher bedtime salivary cortisol values, so that as many as 43% of subjects would be falsely diagnosed with CS if all 3 conditions are present (125,126). Consideration should be given to using other tests in this population.
Advantages of bedtime salivary cortisol. This is a convenient test. Samples can be collected at home, stored at room temperature, and either mailed or delivered to a laboratory.
Interpretation of bedtime salivary cortisol.
The Endocrine Society guidelines suggest using the upper limit of normal of the assay used for any specimen as the criterion for interpretation, since assays differ widely in their reference ranges. A recent large literature review of studies comparing responses of 1102 patients with proven CS and 2039 in whom CS was excluded showed a sensitivity, specificity positive likelihood ratio, and negative likelihood (with 95% confidence interval estimates) of 94.5 (91.3-96.6), 89.7 (85.9-92.6), 9.2 (6.6-12.8), and 0.06 (0.04-0.10), respectively (128).
The 1-mg Dexamethasone Suppression Test
Liddle’s 1974 reports established that dexamethasone, 0.5 mg every 6 h for 2 days, suppresses ACTH and, in turn, GCs within 9 h in healthy individuals but not in CS patients (135). When this regimen was followed by 2 mg every 6 h for 2 days, CS patients with pituitary etiologies showed at least 50% suppression of 24-h 17-hydroxycorticosteroid secretion, while those with adrenal etiologies did not. Subsequent studies have modified and simplified both the low- and high-dose regimens. Current protocols for the low dose screening test involve administration of dexamethasone, 1 mg by mouth, between 11 pm and midnight, with measurement of serum cortisol (and a dexamethasone level) between 8 and 9 am the following morning. An alternative strategy, used more commonly in the United Kingdom, consists of a 0.5 mg dose every 6 h for 8 doses, ending 6 h before cortisol measurement (often termed the 2-mg, 2-day test) (136). Factors that influence the choice of this test include the following:
-
False-positive results that inappropriately suggest CS:
i. Elevated CBG: Since total serum cortisol is the endpoint for the test, any condition that increases CBG (eg, oral estradiol-containing contraceptives, pregnancy, mitotane treatment) will increase total cortisol and may exceed the cutoff point for the test (137).
ii. Increased metabolism: Conditions that increase dexamethasone metabolism (eg, use of stimulators of CYP3A4) will decrease the biologic exposure to dexamethasone (ie, the effective dose is less than the actual dose), with less suppression of POMC/ACTH production and secretion and hence higher cortisol levels.
iii. Renal failure: In 1 study of 800 patients, 10% had an abnormal response to dexamethasone, despite having adequate levels and normal CBG (138).
-
False negative results that are inappropriately normal:
-
i. Cushing’s disease: Since CD patients have some response to GCs, they are more likely than ectopic patients to have a false-negative response. One report of CD patients noted that 8% of surgically confirmed cases had a normal response to 1-mg dexamethasone (139). To our knowledge, no studies have demonstrated the reasons for this, but the following represent potential causes:
a. Mild hypercortisolism: In 1 study, the amount of suppression correlated with the UFC; thus, it is possible that patients with mild hypercortisolism reflect more intact responsiveness to GC.
b. Decreased metabolism of dexamethasone: Conditions that decrease dexamethasone metabolism (eg, liver disease, use of inhibitors of CYP3A4) will increase the biologic exposure to dexamethasone (ie, the effective dose is higher than the actual dose), with greater suppression of POMC/ACTH production and secretion in CD patients and hence lower cortisol levels (140).
iv. Cyclic CS: Patients with cyclic CS have periods of eucortisolism interspersed with periods of hypercortisolism. If tested during a eucortisolemic phase, the results will be normal.
-
Interpretation of the 1 mg dexamethasone suppression test.
For each test, the criterion for a normal response is a cortisol of <1.8 ug/dL (50 nmol/L). A recent large literature review of studies of the 1-mg overnight test that comparing responses of 965 patients with proven CS and 1876 in whom CS was excluded showed a sensitivity, specificity positive likelihood ratio, and negative likelihood (95% CI estimates) of 99.1 (96.9-99.7), 89.2 (83.3-93.1), 9.1 (5.9-14.2), and 0.010 (0.003-0.035), respectively (128). The same report also evaluated the 2 mg, 2-day test, in 458 patients with and 269 without CS, and found its performance to be somewhat less than the overnight test. The sensitivity, specificity positive likelihood ratio, and negative likelihood (95% CI) were 94.0 (90.4-96.3), 84.9 (76.5-90.7), 6.2 (3.9-10.0), and 0.070 (0.043-0.115), respectively.
Other Potential Tests That Could Take Advantage of Cortisol Physiology and Metabolism
Mass spectrometry determination of urine cortisol and metabolites (93,141) has been shown to distinguish between CS and normal patients but requires additional study to determine appropriate interpretation criteria.
Hair cortisol has been used in research to evaluate the timing of the onset of hypercortisolism and the relationship of hypercortisolism to various comorbidities (94,142,143). This approach requires additional validation and is not currently commercially available.
Microdialysis is an exciting new collection device that allows for measurement of cortisol in interstitial fluid in a timed way over many hours (144); results correlate well with serum measurements. One advantage of this approach is that patients can be assessed as outpatients. However, this approach needs to be validated in patients suspected of having CS to determine optimal interpretation strategies.
Tests to Determine the Cause of ACTH-Dependent CS
Once CS is established, the next step is to determine whether it is ACTH-dependent or not. Because GC negative feedback on normal corticotropes is intact in all hypercortisolemic patients with CS, the measured plasma ACTH concentrations reflect tumoral secretion only. Thus, ACTH values will be low (generally <10 pg/mL) in primary adrenal (ACTH-independent) causes of CS and inappropriately normal (generally >20 pg/mL) in those with ACTH-dependent causes. Patients with intermediate values (generally 10-20 pg/mL) rarely have EAS (unless it is cyclic) and can be evaluated further with CRH stimulation testing and/or dehydroepiandrosterone sulfate measurement. Patients with ACTH-independent CS will have little or no response to CRH, as negative GC feedback suppresses the normal corticotrope response, and will have a low dehydroepiandrosterone sulfate level, which reflects chronically low ACTH stimulation of the adrenal reticularis zone (145). Recent reports highlight the potential for falsely elevated ACTH results with certain immunoassay (146), leading to an incorrect diagnosis of CS or inappropriate testing. It is important to consider the patient’s presentation in its entirety and to question any results that are discordant with the remainder of the results.
Tests that distinguish between patients with CD and EAS include the high-dose 8-mg dexamethasone suppression test, the CRH stimulation test, and desmopressin (ddAVP) stimulation test. Ideally, the sensitivity and specificity of the test should both be high, as the ratio of CD:EAS is about 7:1. Interestingly, EAS is more common in patients with hypokalemia, extremely elevated UFC (>10-fold normal) and elevated ACTH (> 3-fold normal). These biochemical features are less common in CD patients and may be seen in more aggressive macroadenomas as a reflection of high ACTH secretion leading to high UFC. The hypokalemia in these patients is attributed to saturation of renal 11βHSD2, resulting in binding of cortisol to the mineralocorticoid receptor, leading to excess potassium excretion (147).
Evaluation of Negative Feedback: The 8-mg Dexamethasone Suppression Test
As previously discussed, the chronic hypercortisolism of CS profoundly inhibits ACTH secretion from normal corticotropes. The resulting secondary adrenal insufficiency is apparent after surgical resection of ACTH- or cortisol-secreting tumor leads to remission. In this setting, the response to dexamethasone evaluates the response of the pituitary or ectopic tumor to GC negative feedback.
Clinical studies show that ACTH-secreting corticotrope tumors (CD) require higher than normal doses of GC to suppress ACTH and ACTH-secreting ectopic tumors generally are not inhibited by further increases in GC exposure (148). While data are relatively sparse (see previous discussion), it is likely that ectopic ACTH-secreting tumors resistant to GCs use at least 1 part of the POMC promoter that does not respond to GC repression, and they may not all express CRH receptors. In addition, little is known about the secretion of ACTH from these tumors, and it is possible that secretion may not be regulated by GCs. However, in 1 study 6/13 EAS patients did suppress cortisol to less than 50% of baseline, suggesting at least partial preservation of some GC-responsive mechanism(s) (149).
There are few studies correlating in vivo and in vitro responses of corticotrope tumors to dexamethasone. In 1 study, neither patients with the USP8 mutation nor those with wild-type USP8 showed a correlation between the 2 responses (150). Large corticotropinomas tend to be more resistant to negative feedback by GCs (151). As little is known about the relationship between folliculostellate cells and tumor cells, it is possible that GC-induced inhibition of secretion is disrupted, particularly as tumors enlarge.
As a result of the wide variability in dexamethasone suppression of cortisol in both EAS and CD, there is nearly complete overlap after high-dose dexamethasone, with CD patients being more sensitive overall (149).
Currently, the 8-mg dexamethasone test is usually done by measuring cortisol on the morning before and after administration of a single 8-mg dose of dexamethasone between 11 pm and midnight (148); ideally, dexamethasone concentrations are measured with cortisol on the following day to assist in interpretation if values are low.
Interpretation of the 8-mg dexamethasone suppression test
A decrease of at least 68% is considered to represent suppression, with a sensitivity of 71% for the diagnosis of CD and specificity of 100% to exclude EAS. Since some patients with ectopic ACTH syndrome show suppression (and some with CD are somewhat more resistant), use of a less conservative criterion of 50% generally increases sensitivity (88%) but decreases specificity (57%) of the test (148).
Pitfalls of the 8-mg dexamethasone suppression test
As discussed for the 1-mg test, medications that alter dexamethasone metabolism can result in a false-negative test in patients with CD; for this reason, a dexamethasone level should be obtained to evaluate whether the exposure to dexamethasone was adequate.
Evaluation of CRH Responsiveness: The Peripheral CRH Test and Inferior Petrosal Sinus Sampling
As would be expected from the in vitro studies of corticotrope tumors, patients with CD show in vivo increases in ACTH and cortisol at 15 to 45 min after administration of ovine or human CRH. In studies of ovine CRH using differing timepoints and criteria and combining those results, the sensitivity and specificity (with 95% CI) for CD using ACTH and cortisol were 91% (84%-95%) and 96% (78%-99%), respectively, for ACTH and 81% (73%-86%) and 81% (60%-93%), respectively, for cortisol (152,153). Similarly varied studies of human CRH, when combined, yielded the following sensitivity and specificity (with 95% CI): 79% (72%-85%) and 90% (71%-97%), respectively, for ACTH and 87% (81%-92%) and 90% (72%-97%), respectively, for cortisol (154,155).
The reasons for lack of response to CRH in CD are largely unknown. As in all diagnostic tests, a threshold for “no response” is arbitrarily set to achieve optimal separation between the patient populations. It is possible that some CD patients have a small response that is not sufficient to meet diagnostic criteria, perhaps because of higher sensitivity to GC feedback after a recent endogenous pulse of ACTH. Conversely, some ectopic tumors have been noted to contain CRH receptor mRNA, which might explain a response; alternatively, a spontaneous pulse not related to CRH administration might explain apparent responses in some patients with EAS, or patients with cyclic CS might regain some pituitary responsiveness during a eucortisolemic phase.
Petrosal sinus sampling involves catheterization of the petrosal sinuses that drain the pituitary gland. The procedure and its pitfalls were recently reviewed extensively (156). While basal levels of ACTH in the petrosal sinuses may not be much greater than peripheral values, levels increase after CRH administration in patients with CD so that petrosal values are almost always 3-fold greater than peripheral values (157). Since the normal corticotropes are suppressed in patients with EAS, no central-to-peripheral gradient is seen. Rarely, these patients will show a central response, probably during a eucortisolemic cyclic phase.
Evaluation of AVP Responsiveness: The Desmopressin Stimulation Test
Various vasopressin analogs were found to increase ACTH in patients with CD, but because of the risk of hypertension from binding to the V1a receptor, the tests did not achieve universal acceptance. With the advent of the vasopressin analog ddAVP, reports showed ACTH responses in up to 80% of patients with CD, which was attributed to signaling through upregulation of the pituitary V3 (V1b) receptor (158). Interestingly, normal individuals do not respond to ddVAP, despite expression of the V3 pituitary receptor. A meta-analysis of results in patients with non-neoplastic hypercortisolism, showed that 7 of 64 responded to ddAVP, for a specificity of 89% (158). Thus, patients with a positive response are more likely to have CD, but its absence cannot be excluded. Initial studies included relatively few patients with EAS. However, literature meta-analyses showed that a small number of EAS patients do respond and that their tumors contain the V2 receptor (and sometimes the V3 receptor) (158,159). This raises the possibility of signaling with cAMP and/or mitogen-activated protein kinase pathways and stimulation of nonclassic POMC promoter regions, as noted previously in this review.
It is of interest that the AVPR1B gene was found to be downregulated in 1 study of corticotrope tumors with USP8 mutation. However, there was no clinical correlation of this finding with the response to ddVAP (150).
Potential Research Questions
Many interesting research questions remain.
Validation of the hypotheses regarding alternative POMC promoters could be done using in human tumors exposed to putative secretagogues (eg, EGF) and studied with electrophysiologic experiments or with classic dose vs ACTH response studies.
Patients’ in vivo responses to CRH, DDAVP, and dexamethasone could be correlated with molecular characteristics of tumors such as their preferred promoters (see previous discussion), mutations, amount of CABLES1 protein, and their biochemical characteristics.
Use of machine learning to incorporate potassium level, age, sex, ACTH, and cortisol level and response to dexamethasone, CRH, and/or inferior petrosal sinus sampling into an algorithm for differential diagnosis.
Characterization of the presence of folliculostellate cells in corticotrope tumors; Does their presence or location correlate with classical responses to biochemical testing, or does their absence predict lack of dexamethasone suppression or CRH stimulation?
Characterization of the proteome of EAS may provide additional clues as to its pathogenesis.
Would outpatient microdialysis for cortisol and other GCs help to distinguish pseudo-CS vs CS?
The physiology of cortisol dynamics and the pituitary response to GCs (dexamethasone) in end-stage renal failure are not well understood. Further studies on cortisol and dexamethasone metabolism and half-life may aid in the evaluation of CS in these patients (160).
The potential diagnostic importance of cortisone levels in serum, saliva and hair suggests that further multicenter studies to characterize reference ranges may assist in diagnosis of CS (93,141-143,161-164).
Is there a role for development of additional secretagogues/inhibitors (eg, EGF, somatostatin) as a panel of diagnostic tests?
Does GR suppress transcription from the ultra-distal POMC promoter (bp +6657 to +7136) in corticotrope tumors?
What regulates or stimulates the secretion of ACTH in ectopic ACTH-secreting tumors? If this is different from secretion in corticotrope tumors, it might form the basis of a new test to discriminate the two causes of ACTH-dependent CS.
Can mass spectrometry profiling of urine cortisol and metabolites (93,141) be refined to distinguish between etiologies of ACTH-dependent and independent CS?
What is the role of mineralocorticoids in the hippocampus, hypothalamus, and pituitary (165) to regulate GC negative feedback, and can that be used in the diagnosis of CS?
There is much to be done to understand the biology of ACTH-producing tumors and to ultimately apply that knowledge to improve the diagnosis and differential diagnosis of CS.
Acknowledgments
Financial Support: This work was performed in and funded by the intramural program of the NIH. (1 ZIA DK075121-06)
Glossary
Abbreviations
- 11βHSD1/2
11-beta hydroxysteroid dehydrogenase type 1 or 2
- ANXA1
annexin 1
- ATP
adenosine 5′-triphosphates
- AVP
arginine vasopressin
- bHLH
basic helix-loop-helix
- CABLES1
CDK5 and ABL1 enzyme substrate 1
- cAMP
cyclic adenosine 5′-monophosphates
- CBG
corticosteroid binding protein/globulin
- CD
Cushing’s disease
- CHR
corticotropin-releasing hormone
- CS
Cushing’s syndrome
- DDAVP
desmopressin
- EAS
ectopic ACTH secretion
- Eboxneuro
enhancer box in cells of the nervous system
- EGFR
epidermal growth factor receptor
- FKBP5
FK506-binding protein 5 (also termed FKBP51)
- FKBP4
FK506-binding protein 4 (also termed FKBP52)
- GC
glucocorticoid
- GR
glucocorticoid receptor
- HDAC
histone deacetylase
- LC/MSMS
liquid chromatography with tandem mass spectrometry
- LIF
leukemia inhibitory factor
- LXR
liver X receptor
- NET
neuroendocrine tumor
- NGFI-B
nerve growth factor-induced clone B (also termed Nur77)
- nGRE
negative glucocorticoid response element
- Nur
nerve growth factor-induced clone B (also termed NGFI-B)
- NurRE
Nur response element
- O-GlcNAc
O-linked N-acetylglucosamine
- O-GlcNAcylation
O-linked-N-acetylglucosaminylation
- Pomc/POMC
proopiomelanocortin
- PVN
paraventricular neuron
- RE
response element
- SCN
suprachiasmatic neuron
- SMRT
silencing mediator of retinoic acid and thyroid hormone receptors
- TF
transcription factor
- Tpit/PitxRE
Tpit/Pitx response element
- TR4
testicular orphan receptor 4
- UFC
urinary free cortisol
- USP8
ubiquitin carboxyl-terminal hydrolase 8
Additional Information
Disclosure Summary: The author has nothing to disclose.
References
- 1. Nieman LK, Biller BM, Findling JW, et al. ; Endocrine Society . Treatment of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rubinstein G, Osswald A, Hoster E, et al. Time to diagnosis in Cushing’s syndrome: a meta-analysis based on 5367 patients. J Clin Endocrinol Metab. 2020;105(3):e12-e23. [DOI] [PubMed] [Google Scholar]
- 3. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Upton AC, Furth J. Spontaneous and radiation-induced pituitary adenomas of mice. J Natl Cancer Inst. 1955;15(4):1005-1021. [PubMed] [Google Scholar]
- 5. Poulin G, Lebel M, Chamberland M, Paradis FW, Drouin J. Specific protein-protein interaction between basic helix-loop-helix transcription factors and homeoproteins of the Pitx family. Mol Cell Biol. 2000;20(13):4826-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Batsché E, Moschopoulos P, Desroches J, Bilodeau S, Drouin J. Retinoblastoma and the related pocket protein p107 act as coactivators of NeuroD1 to enhance gene transcription. J Biol Chem. 2005;280(16):16088-16095. [DOI] [PubMed] [Google Scholar]
- 7. Ando M, Goto M, Hojo M, et al. The proneural bHLH genes Mash1, Math3 and NeuroD are required for pituitary development. J Mol Endocrinol. 2018;61(3):127-138. [DOI] [PubMed] [Google Scholar]
- 8. Seltzer J, Ashton CE, Scotton TC, Pangal D, Carmichael JD, Zada G. Gene and protein expression in pituitary corticotroph adenomas: a systematic review of the literature. Neurosurg Focus. 2015;38(2):E17. [DOI] [PubMed] [Google Scholar]
- 9. Fletcher PA, Sherman A, Stojilkovic SS. Common and diverse elements of ion channels and receptors underlying electrical activity in endocrine pituitary cells. Mol Cell Endocrinol. 2018;463:23-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gagner JP, Drouin J. Opposite regulation of pro-opiomelanocortin gene transcription by glucocorticoids and CRH. Mol Cell Endocrinol. 1985;40(1):25-32. [DOI] [PubMed] [Google Scholar]
- 11. Maira M, Martens C, Batsché E, Gauthier Y, Drouin J. Dimer-specific potentiation of NGFI-B (Nur77) transcriptional activity by the protein kinase A pathway and AF-1-dependent coactivator recruitment. Mol Cell Biol. 2003;23(3):763-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maira M, Martens C, Philips A, Drouin J. Heterodimerization between members of the Nur subfamily of orphan nuclear receptors as a novel mechanism for gene activation. Mol Cell Biol. 1999;19(11):7549-7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maira M, Couture C, Le Martelot G, Pulichino AM, Bilodeau S, Drouin J. The T-box factor Tpit recruits SRC/p160 co-activators and mediates hormone action. J Biol Chem. 2003;278(47):46523-46532. [DOI] [PubMed] [Google Scholar]
- 14. Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79(1):1-71. [DOI] [PubMed] [Google Scholar]
- 15. Lamberts SW, Verleun T, Oosterom R, de Jong F, Hackeng WH. Corticotropin-releasing factor (ovine) and vasopressin exert a synergistic effect on adrenocorticotropin release in man. J Clin Endocrinol Metab. 1984;58(2):298-303. [DOI] [PubMed] [Google Scholar]
- 16. Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood). 2003;228(2):111-133. [DOI] [PubMed] [Google Scholar]
- 17. Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18(3):306-360. [DOI] [PubMed] [Google Scholar]
- 18. Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475(7356):324-332. [DOI] [PubMed] [Google Scholar]
- 19. Pratt WB, Silverstein AM, Galigniana MD. A model for the cytoplasmic trafficking of signalling proteins involving the hsp90-binding immunophilins and p50cdc37. Cell Signal. 1999;11(12):839-851. [DOI] [PubMed] [Google Scholar]
- 20. Lorenz OR, Freiburger L, Rutz DA, et al. Modulation of the Hsp90 chaperone cycle by a stringent client protein. Mol Cell. 2014;53(6):941-953. [DOI] [PubMed] [Google Scholar]
- 21. Kirschke E, Goswami D, Southworth D, Griffin PR, Agard DA. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell. 2014;157(7):1685-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Picard D, Khursheed B, Garabedian MJ, Fortin MG, Lindquist S, Yamamoto KR. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990;348(6297):166-168. [DOI] [PubMed] [Google Scholar]
- 23. Davies TH, Ning YM, Sánchez ER. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem. 2002;277(7):4597-4600. [DOI] [PubMed] [Google Scholar]
- 24. Pratt WB, Czar MJ, Stancato LF, Owens JK. The hsp56 immunophilin component of steroid receptor heterocomplexes: could this be the elusive nuclear localization signal-binding protein? J Steroid Biochem Mol Biol. 1993;46(3):269-279. [DOI] [PubMed] [Google Scholar]
- 25. Drouin J, Sun YL, Chamberland M, et al. Novel glucocorticoid receptor complex with DNA element of the hormone-repressed POMC gene. Embo J. 1993;12(1):145-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Philips A, Maira M, Mullick A, et al. Antagonism between Nur77 and glucocorticoid receptor for control of transcription. Mol Cell Biol. 1997;17(10):5952-5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martens C, Bilodeau S, Maira M, Gauthier Y, Drouin J. Protein-protein interactions and transcriptional antagonism between the subfamily of NGFI-B/Nur77 orphan nuclear receptors and glucocorticoid receptor. Mol Endocrinol. 2005;19(4):885-897. [DOI] [PubMed] [Google Scholar]
- 28. Parvin R, Saito-Hakoda A, Shimada H, et al. Role of NeuroD1 on the negative regulation of Pomc expression by glucocorticoid. PloS One. 2017;12(4):e0175435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trotter KW, Archer TK. The BRG1 transcriptional coregulator. Nucl Recept Signal. 2008;6:e004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bilodeau S, Vallette-Kasic S, Gauthier Y, et al. Role of Brg1 and HDAC2 in GR trans-repression of the pituitary POMC gene and misexpression in Cushing disease. Genes Dev. 2006;20(20):2871-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duncan PJ, Tabak J, Ruth P, Bertram R, Shipston MJ. Glucocorticoids inhibit CRH/AVP-evoked bursting activity of male murine anterior pituitary corticotrophs. Endocrinology. 2016;157(8):3108-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tian L, Knaus HG, Shipston MJ. Glucocorticoid regulation of calcium-activated potassium channels mediated by serine/threonine protein phosphatase. J Biol Chem. 1998;273(22):13531-13536. [DOI] [PubMed] [Google Scholar]
- 33. Tian L, Hammond MS, Florance H, Antoni FA, Shipston MJ. Alternative splicing determines sensitivity of murine calcium-activated potassium channels to glucocorticoids. J Physiol. 2001;537(Pt 1):57-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deng Q, Riquelme D, Trinh L, et al. Rapid glucocorticoid feedback inhibition of ACTH secretion involves ligand-dependent membrane association of glucocorticoid receptors. Endocrinology. 2015;156(9):3215-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moriarty GC. Adenohypophysis: ultrastructural cytochemistry. A review. J Histochem Cytochem. 1973;21(10):855-894. [DOI] [PubMed] [Google Scholar]
- 36. Tierney T, Christian HC, Morris JF, Solito E, Buckingham JC. Evidence from studies on co-cultures of TtT/GF and AtT20 cells that Annexin 1 acts as a paracrine or juxtacrine mediator of the early inhibitory effects of glucocorticoids on ACTH release. J Neuroendocrinol. 2003;15(12):1134-1143. [DOI] [PubMed] [Google Scholar]
- 37. Newell-Price J, Trainer P, Besser M, Grossman A. The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr Rev. 1998;19(5):647-672. [DOI] [PubMed] [Google Scholar]
- 38. Oster H, Challet E, Ott V, et al. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr Rev. 2017;38(1):3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lewis JG, Bagley CJ, Elder PA, Bachmann AW, Torpy DJ. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clin Chim Acta. 2005;359(1-2):189-194. [DOI] [PubMed] [Google Scholar]
- 40. Carrell RW, Read RJ. How serpins transport hormones and regulate their release. Semin Cell Dev Biol. 2017;62:133-141. [DOI] [PubMed] [Google Scholar]
- 41. Veldhuis JD, Iranmanesh A, Lizarralde G, Johnson ML. Amplitude modulation of a burstlike mode of cortisol secretion subserves the circadian glucocorticoid rhythm. Am J Physiol. 1989;257(1 Pt 1):E6-14. [DOI] [PubMed] [Google Scholar]
- 42. Iranmanesh A, Lizarralde G, Short D, Veldhuis JD. Intensive venous sampling paradigms disclose high frequency adrenocorticotropin release episodes in normal men. J Clin Endocrinol Metab. 1990;71(5):1276-1283. [DOI] [PubMed] [Google Scholar]
- 43. Guillemin R, Schally AV. On the nature of the hypothalamic substance which controls the secretion of ACTH. Acta Neuroveg (Wien). 1961;23:58-62. [DOI] [PubMed] [Google Scholar]
- 44. Abe K, Kroning J, Greer MA, Critchlow V. Effects of destruction of the suprachiasmatic nuclei on the circadian rhythms in plasma corticosterone, body temperature, feeding and plasma thyrotropin. Neuroendocrinology. 1979;29(2):119-131. [DOI] [PubMed] [Google Scholar]
- 45. Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213(4514):1394-1397. [DOI] [PubMed] [Google Scholar]
- 46. Harris GW. Neural control of the pituitary gland. II. The adenohypophysis, with special reference to the secretion of A.C.T.H. Br Med J. 1951;2(4732):627-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ixart G, Siaud P, Barbanel G, Mekaouche M, Givalois L, Assenmacher I. Circadian variations in the amplitude of corticotropin-releasing hormone 41 (CRH41) episodic release measured in vivo in male rats: correlations with diurnal fluctuations in hypothalamic and median eminence CRH41 contents. J Biol Rhythms. 1993;8(4):297-309. [DOI] [PubMed] [Google Scholar]
- 48. Engler D, Pham T, Liu JP, Fullerton MJ, Clarke IJ, Funder JW. Studies of the regulation of the hypothalamic-pituitary-adrenal axis in sheep with hypothalamic-pituitary disconnection. II. Evidence for in vivo ultradian hypersecretion of proopiomelanocortin peptides by the isolated anterior and intermediate pituitary. Endocrinology. 1990;127(4):1956-1966. [DOI] [PubMed] [Google Scholar]
- 49. Wunderer F, Kühne S, Jilg A, et al. Clock gene expression in the human pituitary gland. Endocrinology. 2013;154(6):2046-2057. [DOI] [PubMed] [Google Scholar]
- 50. Wester VL, van Rossum EF. Clinical applications of cortisol measurements in hair. Eur J Endocrinol. 2015;173(4):M1-10. [DOI] [PubMed] [Google Scholar]
- 51. Henry FJ, Bassett JR. Corticosterone storage within the adrenal cortex: evidence for a sulphate conjugate. J Endocrinol. 1985;104(3):381-386. [DOI] [PubMed] [Google Scholar]
- 52. Oster H, Damerow S, Kiessling S, et al. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4(2):163-173. [DOI] [PubMed] [Google Scholar]
- 53. Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R1128-R1135. [DOI] [PubMed] [Google Scholar]
- 54. Canny BJ, Funder JW, Clarke IJ. Glucocorticoids regulate ovine hypophysial portal levels of corticotropin-releasing factor and arginine vasopressin in a stress-specific manner. Endocrinology. 1989;125(5):2532-2539. [DOI] [PubMed] [Google Scholar]
- 55. Pinnock SB, Herbert J. Corticosterone differentially modulates expression of corticotropin releasing factor and arginine vasopressin mRNA in the hypothalamic paraventricular nucleus following either acute or repeated restraint stress. Eur J Neurosci. 2001;13(3):576-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing’s syndrome due to ectopic corticotropin secretion: twenty years’ experience at the National Institutes of Health. J Clin Endocrinol Metab. 2005;90(8):4955-4962. [DOI] [PubMed] [Google Scholar]
- 57. Newell-Price J, King P, Clark AJ. The CpG island promoter of the human proopiomelanocortin gene is methylated in nonexpressing normal tissue and tumors and represses expression. Mol Endocrinol. 2001;15(2):338-348. [DOI] [PubMed] [Google Scholar]
- 58. Araki T, Tone Y, Yamamoto M, et al. Two distinctive POMC promoters modify gene expression in Cushing disease. J Clin Endocrinol Metab. 2021;106(9):e3346-e3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ye L, Li X, Kong X, et al. Hypomethylation in the promoter region of POMC gene correlates with ectopic overexpression in thymic carcinoids. J Endocrinol. 2005;185(2):337-343. [DOI] [PubMed] [Google Scholar]
- 60. Zhang C, Jin J, Xie J, et al. The clinical features and molecular mechanisms of ACTH-secreting pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2020;105(11):3449-3458. [DOI] [PubMed] [Google Scholar]
- 61. Picon A, Bertagna X, de Keyzer Y. Analysis of the human proopiomelanocortin gene promoter in a small cell lung carcinoma cell line reveals an unusual role for E2F transcription factors. Oncogene. 1999;18(16):2627-2633. [DOI] [PubMed] [Google Scholar]
- 62. Picon A, Leblond-Francillard M, Raffin-Sanson ML, Lenne F, Bertagna X, de Keyzer Y. Functional analysis of the human pro-opiomelanocortin promoter in the small cell lung carcinoma cell line DMS-79. J Mol Endocrinol. 1995;15(2):187-194. [DOI] [PubMed] [Google Scholar]
- 63. Araki T, Liu X, Kameda H, et al. EGFR induces E2F1-mediated corticotroph tumorigenesis. J Endocr Soc. 2017;1(2):127-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu NA, Araki T, Cuevas-Ramos D, et al. Cyclin E-mediated human proopiomelanocortin regulation as a therapeutic target for Cushing disease. J Clin Endocrinol Metab. 2015;100(7):2557-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Perez-Rivas LG, Oßwald A, Knösel T, et al. Expression and mutational status of USP8 in tumors causing ectopic ACTH secretion syndrome. Endocr Relat Cancer. 2017;24(9):L73-L77. [DOI] [PubMed] [Google Scholar]
- 66. Ray DW, Littlewood AC, Clark AJ, Davis JR, White A. Human small cell lung cancer cell lines expressing the proopiomelanocortin gene have aberrant glucocorticoid receptor function. J Clin Invest. 1994;93(4):1625-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Suda T, Tozawa F, Dobashi I, et al. Corticotropin-releasing hormone, proopiomelanocortin, and glucocorticoid receptor gene expression in adrenocorticotropin-producing tumors in vitro. J Clin Invest. 1993;92(6):2790-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jiang J, Li N, Wang X, et al. Aberrant expression and modification of silencing mediator of retinoic acid and thyroid hormone receptors involved in the pathogenesis of tumoral cortisol resistance. Endocrinology. 2010;151(8):3697-3705. [DOI] [PubMed] [Google Scholar]
- 69. Matsumoto S, Hashimoto K, Yamada M, Satoh T, Hirato J, Mori M. Liver X receptor-alpha regulates proopiomelanocortin (POMC) gene transcription in the pituitary. Mol Endocrinol. 2009;23(1):47-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lu J, Chatain GP, Bugarini A, et al. Histone deacetylase inhibitor SAHA is a promising treatment of Cushing disease. J Clin Endocrinol Metab. 2017;102(8):2825-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Araki T, Liu NA, Tone Y, et al. E2F1-mediated human POMC expression in ectopic Cushing’s syndrome. Endocr Relat Cancer. 2016;23(11):857-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Perez-Rivas LG, Theodoropoulou M, Ferraù F, et al. The gene of the ubiquitin-specific protease 8 is frequently mutated in adenomas causing Cushing’s disease. J Clin Endocrinol Metab. 2015;100(7):E997-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ma ZY, Song ZJ, Chen JH, et al. Recurrent gain-of-function USP8 mutations in Cushing’s disease. Cell Res. 2015;25(3):306-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Reincke M, Sbiera S, Hayakawa A, et al. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat Genet. 2015;47(1):31-38. [DOI] [PubMed] [Google Scholar]
- 75. Fukuoka H, Cooper O, Ben-Shlomo A, et al. EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J Clin Invest. 2011;121(12):4712-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu X, Feng M, Dai C, et al. Expression of EGFR in pituitary corticotroph adenomas and its relationship with tumor behavior. Front Endocrinol (Lausanne). 2019;10:785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pecori Giraldi F, Cavagnini F. Corticotropin-releasing hormone is produced by rat corticotropes and modulates ACTH secretion in a paracrine/autocrine fashion. J Clin Invest. 1998;101(11):2478-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Korbonits M, Bujalska I, Shimojo M, et al. Expression of 11 beta-hydroxysteroid dehydrogenase isoenzymes in the human pituitary: induction of the type 2 enzyme in corticotropinomas and other pituitary tumors. J Clin Endocrinol Metab. 2001;86(6):2728-2733. [DOI] [PubMed] [Google Scholar]
- 79. Kang KI, Meng X, Devin-Leclerc J, et al. The molecular chaperone Hsp90 can negatively regulate the activity of a glucocorticosteroid-dependent promoter. Proc Natl Acad Sci U S A. 1999;96(4):1439-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Riebold M, Kozany C, Freiburger L, et al. A C-terminal HSP90 inhibitor restores glucocorticoid sensitivity and relieves a mouse allograft model of Cushing disease. Nat Med. 2015;21(3):276-280. [DOI] [PubMed] [Google Scholar]
- 81. Du L, Bergsneider M, Mirsadraei L, et al. Evidence for orphan nuclear receptor TR4 in the etiology of Cushing disease. Proc Natl Acad Sci U S A. 2013;110(21):8555-8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhang D, Du L, Heaney AP. Testicular receptor-4: novel regulator of glucocorticoid resistance. J Clin Endocrinol Metab. 2016;101(8):3123-3133. [DOI] [PubMed] [Google Scholar]
- 83. Hernández-Ramírez LC, Gam R, Valdés N, et al. Loss-of-function mutations in the CABLES1 gene are a novel cause of Cushing’s disease. Endocr Relat Cancer. 2017;24(8):379-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Roussel-Gervais A, Couture C, Langlais D, et al. The Cables1 gene in glucocorticoid regulation of pituitary corticotrope growth and Cushing disease. J Clin Endocrinol Metab. 2016;101(2):513-522. [DOI] [PubMed] [Google Scholar]
- 85. Comer FI, Hart GW. Reciprocity between O-GlcNAc and O-phosphate on the carboxyl terminal domain of RNA polymerase II. Biochemistry. 2001;40(26):7845-7852. [DOI] [PubMed] [Google Scholar]
- 86. Makita K, Takayasu S, Usutani M, et al. O-linked β-N-acetylglucosamine transferase is involved in pro-opiomelanocortin gene expression in mouse pituitary corticotroph AtT-20 cells. Neurosci Lett. 2019;711:134407. [DOI] [PubMed] [Google Scholar]
- 87. Ito K, Yamamura S, Essilfie-Quaye S, et al. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med. 2006;203(1):7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ballard PL. Delivery and transport of glucocorticoids to target cells. Monogr Endocrinol. 1979;12:25-48. [DOI] [PubMed] [Google Scholar]
- 89. Aardal E, Holm AC. Cortisol in saliva—reference ranges and relation to cortisol in serum. Eur J Clin Chem Clin Biochem. 1995;33(12):927-932. [DOI] [PubMed] [Google Scholar]
- 90. Beisel WR, Cos JJ, Horton R, Chao PY, Forsham PH. Physiology of urinary cortisol excretion. J Clin Endocrinol Metab. 1964;24:887-893. [DOI] [PubMed] [Google Scholar]
- 91. Schedl HP, Chen PS Jr, Greene G, Redd D. The renal clearance of plasma cortisol. J Clin Endocrinol Metab. 1959;19:1223-1229. [DOI] [PubMed] [Google Scholar]
- 92. Bujalska I, Shimojo M, Howie A, Stewart PM. Human 11 beta-hydroxysteroid dehydrogenase: studies on the stably transfected isoforms and localization of the type 2 isozyme within renal tissue. Steroids. 1997;62(1):77-82. [DOI] [PubMed] [Google Scholar]
- 93. Athimulam S, Grebe S, Bancos I. Steroid profiling in the diagnosis of mild and overt Cushing’s syndrome. Best Pract Res Clin Endocrinol Metab. 2021;35(1):101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Faresjö T, Strömberg S, Jones M, et al. Elevated levels of cortisol in hair precede acute myocardial infarction. Sci Rep. 2020;10(1):22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ito N, Ito T, Kromminga A, et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19(10):1332-1334. [DOI] [PubMed] [Google Scholar]
- 96. Ferrari P. The role of 11β-hydroxysteroid dehydrogenase type 2 in human hypertension. Biochim Biophys Acta. 2010;1802(12):1178-1187. [DOI] [PubMed] [Google Scholar]
- 97. Tunn S, Möllmann H, Barth J, Derendorf H, Krieg M. Simultaneous measurement of cortisol in serum and saliva after different forms of cortisol administration. Clin Chem. 1992;38(8 Pt 1):1491-1494. [PubMed] [Google Scholar]
- 98. Petersenn S, Newell-Price J, Findling JW, et al. ; Pasireotide B2305 Study Group . High variability in baseline urinary free cortisol values in patients with Cushing’s disease. Clin Endocrinol (Oxf). 2014;80(2):261-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Debono M, Newell-Price J. Subclinical hypercortisolism in adrenal incidentaloma. Curr Opin Endocrinol Diabetes Obes. 2015;22(3):185-192. [DOI] [PubMed] [Google Scholar]
- 100. Papanicolaou DA, Mullen N, Kyrou I, Nieman LK. Nighttime salivary cortisol: a useful test for the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab. 2002;87(10):4515-4521. [DOI] [PubMed] [Google Scholar]
- 101. Nugent CA, Warner HR, Dunn JT, Tyler FH. Probability theory in the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab. 1964;24:621-627. [DOI] [PubMed] [Google Scholar]
- 102. Plotz CM, Knowlton AI, Ragan C. The natural history of Cushing’s syndrome. Am J Med. 1952;13(5):597-614. [DOI] [PubMed] [Google Scholar]
- 103. Starkman MN. Neuropsychiatric findings in Cushing syndrome and exogenous glucocorticoid administration. Endocrinol Metab Clin North Am. 2013;42(3):477-488. [DOI] [PubMed] [Google Scholar]
- 104. Alwani RA, Schmit Jongbloed LW, de Jong FH, van der Lely AJ, de Herder WW, Feelders RA. Differentiating between Cushing’s disease and pseudo-Cushing’s syndrome: comparison of four tests. Eur J Endocrinol. 2014;170(4):477-486. [DOI] [PubMed] [Google Scholar]
- 105. Pecori Giraldi F, Pivonello R, Ambrogio AG, et al. The dexamethasone-suppressed corticotropin-releasing hormone stimulation test and the desmopressin test to distinguish Cushing’s syndrome from pseudo-Cushing’s states. Clin Endocrinol (Oxf). 2007;66(2):251-257. [DOI] [PubMed] [Google Scholar]
- 106. Elamin MB, Murad MH, Mullan R, et al. Accuracy of diagnostic tests for Cushing’s syndrome: a systematic review and metaanalyses. J Clin Endocrinol Metab. 2008;93(5):1553-1562. [DOI] [PubMed] [Google Scholar]
- 107. Nieman LK. Diagnosis of Cushing’s syndrome in the modern era. Endocrinol Metab Clin North Am. 2018;47(2):259-273. [DOI] [PubMed] [Google Scholar]
- 108. Israelsson M, Brattsand R, Brattsand G. 20α- and 20β-dihydrocortisone may interfere in LC-MS/MS determination of cortisol in saliva and urine. Ann Clin Biochem. 2018;55(3):341-347. [DOI] [PubMed] [Google Scholar]
- 109. Taylor RL, Machacek D, Singh RJ. Validation of a high-throughput liquid chromatography-tandem mass spectrometry method for urinary cortisol and cortisone. Clin Chem. 2002;48(9):1511-1519. [PubMed] [Google Scholar]
- 110. Wood L, Ducroq DH, Fraser HL, et al. Measurement of urinary free cortisol by tandem mass spectrometry and comparison with results obtained by gas chromatography-mass spectrometry and two commercial immunoassays. Ann Clin Biochem. 2008;45(Pt 4):380-388. [DOI] [PubMed] [Google Scholar]
- 111. Aranda G, Careaga M, Hanzu FA, et al. Accuracy of immunoassay and mass spectrometry urinary free cortisol in the diagnosis of Cushing’s syndrome. Pituitary. 2016;19(5):496-502. [DOI] [PubMed] [Google Scholar]
- 112. Oßwald A, Wang R, Beuschlein F, et al. Performance of LC-MS/MS and immunoassay based 24-h urine free cortisol in the diagnosis of Cushing’s syndrome. J Steroid Biochem Mol Biol. 2019;190:193-197. [DOI] [PubMed] [Google Scholar]
- 113. Raff H, Auchus RJ, Findling JW, Nieman LK. Urine free cortisol in the diagnosis of Cushing’s syndrome: is it worth doing and, if so, how? J Clin Endocrinol Metab. 2015;100(2):395-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chan KC, Lit LC, Law EL, et al. Diminished urinary free cortisol excretion in patients with moderate and severe renal impairment. Clin Chem. 2004;50(4):757-759. [DOI] [PubMed] [Google Scholar]
- 115. Meinardi JR, Wolffenbuttel BH, Dullaart RP. Cyclic Cushing’s syndrome: a clinical challenge. Eur J Endocrinol. 2007;157(3):245-254. [DOI] [PubMed] [Google Scholar]
- 116. Fenske M. Urinary free cortisol and cortisone excretion in healthy individuals: influence of water loading. Steroids. 2006;71(11-12):1014-1018. [DOI] [PubMed] [Google Scholar]
- 117. Chen AX, Haas AV, Williams GH, Vaidya A. Dietary sodium intake and cortisol measurements. Clin Endocrinol (Oxf). 2020;93(5):539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Baudrand R, Campino C, Carvajal CA, et al. High sodium intake is associated with increased glucocorticoid production, insulin resistance and metabolic syndrome. Clin Endocrinol (Oxf). 2014;80(5):677-684. [DOI] [PubMed] [Google Scholar]
- 119. Smals AG, Njo KT, Knoben JM, Ruland CM, Kloppenborg PW. Alcohol-induced Cushingoid syndrome. J R Coll Physicians Lond. 1977;12(1):36-41. [PMC free article] [PubMed] [Google Scholar]
- 120. Rees LH, Besser GM, Jeffcoate WJ, Goldie DJ, Marks V. Alcohol-induced pseudo-Cushing’s syndrome. Lancet. 1977;1(8014):726-728. [DOI] [PubMed] [Google Scholar]
- 121. Findling JW, Raff H. Diagnosis of endocrine disease: differentiation of pathologic/neoplastic hypercortisolism (Cushing’s syndrome) from physiologic/non-neoplastic hypercortisolism (formerly known as pseudo-Cushing’s syndrome). Eur J Endocrinol. 2017;176(5):R205-R216. [DOI] [PubMed] [Google Scholar]
- 122. Groote Veldman R, Meinders AE. On the mechanism of alcohol-induced pseudo-Cushing’s syndrome. Endocr Rev. 1996;17(3):262-268. [DOI] [PubMed] [Google Scholar]
- 123. Chrousos GP, Loriaux DL, Brandon D, et al. Primary cortisol resistance: a familial syndrome and an animal model. J Steroid Biochem. 1983;19(1B):567-575. [DOI] [PubMed] [Google Scholar]
- 124. Bozic J, Galic T, Supe-Domic D, et al. Morning cortisol levels and glucose metabolism parameters in moderate and severe obstructive sleep apnea patients. Endocrine. 2016;53(3):730-739. [DOI] [PubMed] [Google Scholar]
- 125. Liu H, Bravata DM, Cabaccan J, Raff H, Ryzen E. Elevated late-night salivary cortisol levels in elderly male type 2 diabetic veterans. Clin Endocrinol (Oxf). 2005;63(6):642-649. [DOI] [PubMed] [Google Scholar]
- 126. Raff H, Raff JL, Duthie EH, et al. Elevated salivary cortisol in the evening in healthy elderly men and women: correlation with bone mineral density. J Gerontol A Biol Sci Med Sci. 1999;54(9):M479-M483. [DOI] [PubMed] [Google Scholar]
- 127. Yanovski JA, Cutler GB Jr, Chrousos GP, Nieman LK. Corticotropin-releasing hormone stimulation following low-dose dexamethasone administration. A new test to distinguish Cushing’s syndrome from pseudo-Cushing’s states. JAMA. 1993;269(17):2232-2238. [PubMed] [Google Scholar]
- 128. Galm BP, Qiao N, Klibanski A, Biller BMK, Tritos NA. Accuracy of laboratory tests for the diagnosis of Cushing syndrome. J Clin Endocrinol Metab. 2020;105(6). [DOI] [PubMed] [Google Scholar]
- 129. Papanicolaou DA, Yanovski JA, Cutler GB Jr, Chrousos GP, Nieman LK. A single midnight serum cortisol measurement distinguishes Cushing’s syndrome from pseudo-Cushing states. J Clin Endocrinol Metab. 1998;83(4):1163-1167. [DOI] [PubMed] [Google Scholar]
- 130. Niu SF, Chung MH, Chu H, et al. Differences in cortisol profiles and circadian adjustment time between nurses working night shifts and regular day shifts: A prospective longitudinal study. Int J Nurs Stud. 2015;52(7):1193-1201. [DOI] [PubMed] [Google Scholar]
- 131. Paragliola RM, Corsello A, Troiani E, et al. Cortisol circadian rhythm and jet-lag syndrome: evaluation of salivary cortisol rhythm in a group of eastward travelers. Endocrine. 2021;73(2):424-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Raff H, Raff JL, Findling JW. Late-night salivary cortisol as a screening test for Cushing’s syndrome. J Clin Endocrinol Metab. 1998;83(8):2681-2686. [DOI] [PubMed] [Google Scholar]
- 133. Raff H, Singh RJ. Measurement of late-night salivary cortisol and cortisone by LC-MS/MS to assess preanalytical sample contamination with topical hydrocortisone. Clin Chem. 2012;58(5):947-948. [DOI] [PubMed] [Google Scholar]
- 134. Olff M, Meewisse ML, Kleber RJ, et al. Tobacco usage interacts with postdisaster psychopathology on circadian salivary cortisol. Int J Psychophysiol. 2006;59(3):251-258. [DOI] [PubMed] [Google Scholar]
- 135. Liddle GW. Tests of pituitary-adrenal suppressibility in the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab. 1960;20:1539-1560. [DOI] [PubMed] [Google Scholar]
- 136. Isidori AM, Kaltsas GA, Mohammed S, et al. Discriminatory value of the low-dose dexamethasone suppression test in establishing the diagnosis and differential diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab. 2003;88(11):5299-5306. [DOI] [PubMed] [Google Scholar]
- 137. Baid SK, Rubino D, Sinaii N, Ramsey S, Frank A, Nieman LK. Specificity of screening tests for Cushing’s syndrome in an overweight and obese population. J Clin Endocrinol Metab. 2009;94(10):3857-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Cardoso EM, Arregger AL, Budd D, Zucchini AE, Contreras LN. Dynamics of salivary cortisol in chronic kidney disease patients at stages 1 through 4. Clin Endocrinol (Oxf). 2016;85(2):313-319. [DOI] [PubMed] [Google Scholar]
- 139. Findling JW, Raff H, Aron DC. The low-dose dexamethasone suppression test: a reevaluation in patients with Cushing’s syndrome. J Clin Endocrinol Metab. 2004;89(3):1222-1226. [DOI] [PubMed] [Google Scholar]
- 140. Valassi E, Swearingen B, Lee H, et al. Concomitant medication use can confound interpretation of the combined dexamethasone-corticotropin releasing hormone test in Cushing’s syndrome. J Clin Endocrinol Metab. 2009;94(12):4851-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Eisenhofer G, Masjkur J, Peitzsch M, et al. Plasma steroid metabolome profiling for diagnosis and subtyping patients with Cushing syndrome. Clin Chem. 2018;64(3):586-596. [DOI] [PubMed] [Google Scholar]
- 142. Manenschijn L, Koper JW, van den Akker EL, et al. A novel tool in the diagnosis and follow-up of (cyclic) Cushing’s syndrome: measurement of long-term cortisol in scalp hair. J Clin Endocrinol Metab. 2012;97(10):E1836-E1843. [DOI] [PubMed] [Google Scholar]
- 143. Brossaud J, Charret L, De Angeli D, et al. Hair cortisol and cortisone measurements for the diagnosis of overt and mild Cushing’s syndrome. Eur J Endocrinol. 2021;184(3):445-454. [DOI] [PubMed] [Google Scholar]
- 144. Bhake RC, Leendertz JA, Linthorst AC, Lightman SL. Automated 24-hours sampling of subcutaneous tissue free cortisol in humans. J Med Eng Technol. 2013;37(3):180-184. [DOI] [PubMed] [Google Scholar]
- 145. Morio H, Terano T, Yamamoto K, et al. Serum levels of dehydroepiandrosterone sulfate in patients with asymptomatic cortisol producing adrenal adenoma: comparison with adrenal Cushing’s syndrome and non-functional adrenal tumor. Endocr J. 1996;43(4):387-396. [DOI] [PubMed] [Google Scholar]
- 146. Greene LW, Geer EB, Page-Wilson G, Findling JW, Raff H. Assay-specific spurious ACTH results lead to misdiagnosis, unnecessary testing, and surgical misadventure-a case series. J Endocr Soc. 2019;3(4):763-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Stewart PM, Walker BR, Holder G, O’Halloran D, Shackleton CH. 11 beta-Hydroxysteroid dehydrogenase activity in Cushing’s syndrome: explaining the mineralocorticoid excess state of the ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 1995;80(12):3617-3620. [DOI] [PubMed] [Google Scholar]
- 148. Dichek HL, Nieman LK, Oldfield EH, Pass HI, Malley JD, Cutler GB Jr. A comparison of the standard high dose dexamethasone suppression test and the overnight 8-mg dexamethasone suppression test for the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 1994;78(2):418-422. [DOI] [PubMed] [Google Scholar]
- 149. Aron DC, Raff H, Findling JW. Effectiveness versus efficacy: the limited value in clinical practice of high dose dexamethasone suppression testing in the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 1997;82(6):1780-1785. [DOI] [PubMed] [Google Scholar]
- 150. Sesta A, Cassarino MF, Terreni M, et al. Ubiquitin-specific protease 8 mutant corticotrope adenomas present unique secretory and molecular features and shed light on the role of ubiquitylation on ACTH processing. Neuroendocrinology. 2020;110(1-2):119-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Woo YS, Isidori AM, Wat WZ, et al. Clinical and biochemical characteristics of adrenocorticotropin-secreting macroadenomas. J Clin Endocrinol Metab. 2005;90(8):4963-4969. [DOI] [PubMed] [Google Scholar]
- 152. Nieman LK, Oldfield EH, Wesley R, Chrousos GP, Loriaux DL, Cutler GB Jr. A simplified morning ovine corticotropin-releasing hormone stimulation test for the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 1993;77(5):1308-1312. [DOI] [PubMed] [Google Scholar]
- 153. Reimondo G, Paccotti P, Minetto M, et al. The corticotrophin-releasing hormone test is the most reliable noninvasive method to differentiate pituitary from ectopic ACTH secretion in Cushing’s syndrome. Clin Endocrinol (Oxf). 2003;58(6):718-724. [DOI] [PubMed] [Google Scholar]
- 154. Newell-Price J, Morris DG, Drake WM, et al. Optimal response criteria for the human CRH test in the differential diagnosis of ACTH-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 2002;87(4):1640-1645. [DOI] [PubMed] [Google Scholar]
- 155. Ceccato F, Tizianel I, Vedolin CK, Boscaro M, Barbot M, Scaroni C. Human corticotropin-releasing hormone tests: 10 years of real-life experience in pituitary and adrenal disease. J Clin Endocrinol Metab. 2020;105(11):e3938-e3949. [DOI] [PubMed] [Google Scholar]
- 156. Perlman JE, Johnston PC, Hui F, et al. Pitfalls in performing and interpreting inferior petrosal sinus sampling: personal experience and literature review. J Clin Endocrinol Metab. 2021;106(5):e1953-e1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Oldfield EH, Doppman JL, Nieman LK, et al. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing’s syndrome. N Engl J Med. 1991;325(13):897-905. [DOI] [PubMed] [Google Scholar]
- 158. Vassiliadi DA, Tsagarakis S. Diagnosis of endocrine disease: the role of the desmopressin test in the diagnosis and follow-up of Cushing’s syndrome. Eur J Endocrinol. 2018;178(5):R201-R214. [DOI] [PubMed] [Google Scholar]
- 159. Terzolo M, Reimondo G, Alì A, et al. The limited value of the desmopressin test in the diagnostic approach to Cushing’s syndrome. Clin Endocrinol (Oxf). 2001;54(5):609-616. [DOI] [PubMed] [Google Scholar]
- 160. Raff H, Trivedi H. Circadian rhythm of salivary cortisol, plasma cortisol, and plasma ACTH in end-stage renal disease. Endocr Connect. 2013;2(1):23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Garrahy A, Forde H, O’Kelly P, et al. The diagnostic utility of late night salivary cortisol (LNSF) and cortisone (LNSE) in Cushing’s syndrome. Ir J Med Sci. 2021;190(2):615-623. [DOI] [PubMed] [Google Scholar]
- 162. Kannankeril J, Carroll T, Findling JW, et al. Prospective evaluation of late-night salivary cortisol and cortisone by EIA and LC-MS/MS in suspected Cushing syndrome. J Endocr Soc. 2020;4(10):bvaa107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ponzetto F, Settanni F, Parasiliti-Caprino M, et al. Reference ranges of late-night salivary cortisol and cortisone measured by LC-MS/MS and accuracy for the diagnosis of Cushing’s syndrome. J Endocrinol Invest. 2020;43(12):1797-1806. [DOI] [PubMed] [Google Scholar]
- 164. Ueland GÅ, Kellmann R, Jørstad Davidsen M, et al. Bedtime salivary cortisol as a screening test for Cushing syndrome in children. J Endocr Soc. 2021;5(5):bvab033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Berardelli R, Karamouzis I, D’Angelo V, et al. Role of mineralocorticoid receptors on the hypothalamus-pituitary-adrenal axis in humans. Endocrine. 2013;43(1):51-58. [DOI] [PubMed] [Google Scholar]