Abstract
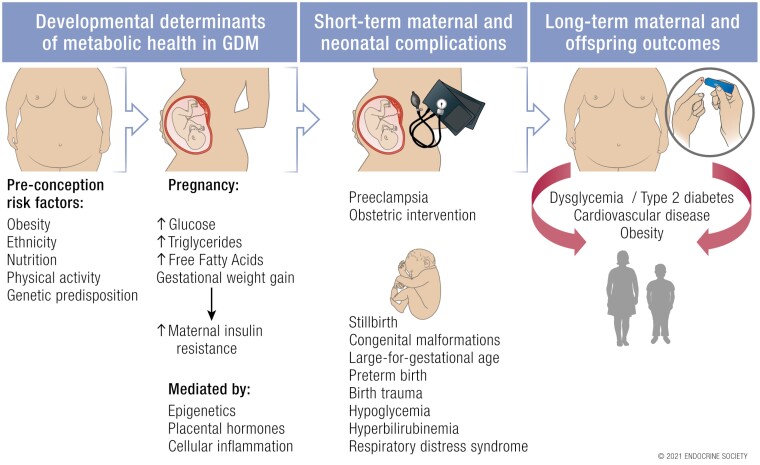
Gestational diabetes mellitus (GDM) traditionally refers to abnormal glucose tolerance with onset or first recognition during pregnancy. GDM has long been associated with obstetric and neonatal complications primarily relating to higher infant birthweight and is increasingly recognized as a risk factor for future maternal and offspring cardiometabolic disease. The prevalence of GDM continues to rise internationally due to epidemiological factors including the increase in background rates of obesity in women of reproductive age and rising maternal age and the implementation of the revised International Association of the Diabetes and Pregnancy Study Groups’ criteria and diagnostic procedures for GDM. The current lack of international consensus for the diagnosis of GDM reflects its complex historical evolution and pragmatic antenatal resource considerations given GDM is now 1 of the most common complications of pregnancy. Regardless, the contemporary clinical approach to GDM should be informed not only by its short-term complications but also by its longer term prognosis. Recent data demonstrate the effect of early in utero exposure to maternal hyperglycemia, with evidence for fetal overgrowth present prior to the traditional diagnosis of GDM from 24 weeks’ gestation, as well as the durable adverse impact of maternal hyperglycemia on child and adolescent metabolism. The major contribution of GDM to the global epidemic of intergenerational cardiometabolic disease highlights the importance of identifying GDM as an early risk factor for type 2 diabetes and cardiovascular disease, broadening the prevailing clinical approach to address longer term maternal and offspring complications following a diagnosis of GDM.
Keywords: gestational diabetes mellitus, diagnosis, pathophysiology, genetics, outcomes, management, precision medicine, biomarkers, diabetes prevention, COVID
Graphical Abstract
Graphical Abstract.
Essential Points.
Gestational diabetes mellitus (GDM) is 1 of the most common medical complications of pregnancy and is increasing in prevalence globally.
GDM is associated with obstetric and neonatal complications primarily due to increased birthweight and is a major risk factor for future type 2 diabetes, obesity, and cardiovascular disease in mother and child.
Detecting GDM is important because perinatal complications and stillbirth risk are greatly reduced by treatment.
A precision medicine approach to GDM which recognizes severity and onset of maternal hyperglycemia as well as genetic and physiologic subtypes of GDM may address the current diagnostic controversy via accurate risk stratification and individualized treatment strategies, leading to improved clinical care models and outcomes.
The traditional focus on normalization of obstetric and neonatal outcomes achieved via short-term antenatal maternal glucose management should now shift to early postnatal prevention strategies to decrease the progression from GDM to type 2 diabetes and address longer term maternal and offspring metabolic risk given the global epidemic of diabetes, obesity, and cardiovascular disease.
Diabetes in pregnancy was first described in 1824 by Bennewitz in Germany (1), with subsequent case series in the United Kingdom and United States reporting high perinatal mortality rates in women with diabetes in pregnancy (2-4). In 1909, Williams reported arguably the first diagnostic criteria for diabetes in pregnancy in the United States, proposing physiological and pathophysiological thresholds for “transient glycosuria in pregnancy” (5).
In 1964, O’Sullivan and Mahan defined specific diagnostic criteria for gestational diabetes mellitus (GDM) in the United States derived from the 100-g 3-hour oral glucose tolerance test (OGTT) undertaken in the second and third trimester of pregnancy in 752 women (6). GDM was defined as ≥2 venous whole blood glucose values greater than 2 SD above the mean glucose values for pregnancy in their initial cohort. These glucose thresholds were primarily chosen because the resulting GDM prevalence of 2% corresponded to the background population prevalence of diabetes, while the requirement of ≥2 elevated glucose values sought to minimize the risk of preanalytical error (7). These thresholds were validated by their identification of subsequent diabetes up to 8 years postpartum in an additional cohort of 1013 women. Increased perinatal mortality was also observed in women with ≥2 glucose values exceeding the proposed diagnostic criteria (6). In 1965, the World Health Organization (WHO) concurrently recommended that GDM be diagnosed by either a 50- or 100-g OGTT using the 2-hour postload glucose value, but the threshold used was the same as for diagnosing diabetes in the nonpregnant population (8). The WHO continued to diagnose GDM based on glucose thresholds for diabetes in the nonpregnant population (9,10) until its endorsement of the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) diagnostic criteria in 2013 (11).
Since 1973, the screening approach to GDM frequently adopted a 2-step procedure with the 50-g 1-hour glucose challenge test (GCT) followed by the 100-g 3-hour OGTT if the GCT was positive. This was based on data from O’Sullivan et al, which showed that a 2-step diagnostic approach to GDM using the GCT as the initial screening test and a glucose threshold of 7.9 mmol/L (143 mg/dL) was 79% sensitive and 87% specific for diagnosing GDM on the 100-g 3-h OGTT in a cohort of 752 women (12). The rationale for this approach was the efficient identification of women most at risk of GDM.
In 1979, the US National Diabetes Data Group (NDDG) published conversions of the original O’Sullivan and Mahan 100-g 3-hour OGTT diagnostic criteria for GDM, reflecting the transition from venous whole blood glucose to plasma blood glucose analysis (13). These revised criteria were subsequently adopted by the American Diabetes Association (ADA) and internationally (9,14,15). In 1982, Carpenter and Coustan recommended lowering of the NDDG diagnostic criteria, reflecting newer preanalytical enzymatic methods that were more specific for plasma glucose (7,16). They also advised lowering the GCT glucose threshold to 7.5 mmol/L (135 mg/dL) based on their study of 381 women who underwent the 100-g 3-h OGTT after screening positive on the GCT, whereby a GCT glucose threshold ≤ 7.5 mmol/L (135 mg/dL) strongly correlated with a normal OGTT (17). However, in the absence of clear evidence supporting a specific glucose threshold for the GCT, the ADA and the American College of Obstetricians and Gynecologists (ACOG) continued to recommend a screen positive GCT glucose threshold from 7.2 to 7.8 mmol/L (130-140 mg/dL) for GDM (18,19).
The ADA did however recommend the modified Carpenter and Coustan diagnostic glucose thresholds for GDM from 2000 (20), supported by the findings of the Toronto Tri-Hospital Gestational Diabetes Project (21,22). These data demonstrated a positive correlation between increasing maternal hyperglycemia even below the NDDG diagnostic criteria for GDM and risk of obstetric and neonatal complications including preeclampsia, cesarean section, and macrosomia (neonatal birthweight > 4000 g) (21,22). In addition, several large cohort studies showed that women diagnosed (but not treated) with GDM based on the Carpenter and Coustan criteria were at increased risk of perinatal complications including hypertensive disorders of pregnancy, increased birthweight, macrosomia, neonatal hypoglycemia, hyperbilirubinemia, and shoulder dystocia, compared to women diagnosed and treated as GDM by NDDG diagnostic criteria (16,23-25). From 2003 the ADA additionally endorsed the 1-step 75-g 2-hour OGTT for the diagnosis of GDM derived from the modified Carpenter and Coustan fasting, 1- and 2-hour glucose thresholds for the 100-g 3-hour OGTT, particularly for women at high-risk (26). This approach was deemed more cost-effective, albeit less validated, than the 100-g 3-hour OGTT. The use of the modified Carpenter and Coustan thresholds was associated with an almost 50% increase in prevalence of GDM (16,23).
The evolution of diagnostic criteria for GDM illustrates the historic lack of consensus for the diagnosis of GDM, with the presence or absence of disease varying dependent on expert consensus. The underlying rationale for the diagnosis of GDM also shifted over time toward identifying perinatal risk rather than future maternal diabetes risk.
Current GDM Diagnostic Criteria
The seminal Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study sought to provide an evidence base to guide risk in GDM, and its results were published in 2008 (27). This large, international, prospective, observational study evaluated the relationship between glucose levels on the 75-g 2-hour OGTT performed at 24 to 32 weeks’ gestation (mean 27.8 weeks’ gestation) in over 25 000 pregnant women with the following primary perinatal outcomes: birthweight > 90th percentile for gestational age, primary cesarean section delivery, neonatal hypoglycemia, and cord blood serum C-peptide > 90th centile. Secondary outcomes were preeclampsia, preterm delivery (defined as delivery before 37 weeks’ gestation), shoulder dystocia or birth injury, hyperbilirubinemia, and neonatal intensive care admission. The results showed a continuous positive linear relationship between maternal fasting; 1- and 2-hour plasma glucose levels obtained on the OGTT, below those that were diagnostic of diabetes outside pregnancy; and risk of primary outcomes (27). Notably, there were no specific glucose thresholds at which obstetric and neonatal complications significantly increased.
Based on these findings and supported by trials [the Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) and the Maternal-Fetal Medicine Units Network (MFMU) trial] showing benefit of treatment of more severe and “mild” degrees of maternal hyperglycemia, respectively (28,29), the IADPSG revised its diagnostic criteria for GDM. Despite the lack of a clear diagnostic glucose threshold in HAPO, the consensus of the IADPSG was to define diagnostic thresholds for the fasting, 1- and 2-hour glucose values for the 75-g 2-hour OGTT based on the average glucose values at which the odds of the primary outcomes were 1.75 times the odds of these outcomes occurring at the mean glucose levels for the HAPO cohort (30). The IADPSG consensus was also that only 1 elevated glucose level for the OGTT was required for GDM diagnosis, as each glucose threshold represented broadly comparable level of risk. Thus, the main purpose of the diagnostic criteria for GDM post-HAPO was to define the level of risk associated with increased perinatal complications.
Post-HAPO, there exist several different screening and testing approaches for the diagnosis of GDM internationally. The IADPSG and WHO recommend universal testing of all pregnant women between 24 to 28 weeks’ gestation with the 75-g 2-hour OGTT (11,30). These revised recommendations were largely endorsed by several organizations including the ADA (18), Endocrine Society (31), International Federation of Gynecology and Obstetrics (32), Australasian Diabetes in Pregnancy Association (33), Japan Diabetes Society (34), Ministry of Health of China (35), and the European Board of Gynecology and Obstetrics (36).
The National Institutes of Health did not endorse the IADPSG recommendations, citing the expected increase in prevalence of GDM, cost, and intervention in the context of a lack of evidence for an associated improvement in perinatal outcomes (37). The National Institutes of Health and ACOG continue to recommend a 2-step testing approach, with the initial screening GCT for all women and those who screen positive proceeding to the diagnostic 100-g 3-hour OGTT (19,37). This approach is also endorsed by ADA (18). However, the ACOG’s 2018 guidelines now acknowledge that individual practices and institutions may instead choose to use the IADPSG’s 1-step testing approach and diagnostic criteria if appropriate for their population (19). The UK National Institute for Health and Care Excellence (NICE) guidelines advise a selective screening approach, whereby women with risk factors for GDM are recommended to undergo a diagnostic 75-g 2-hour OGTT at 26 to 28 weeks’ gestation, with diagnostic (fasting or 2-hour) glucose thresholds higher than the IADPSG diagnostic criteria for GDM (38). Several other European bodies also currently recommend selective risk factor-based screening, with only women fulfilling specific high-risk criteria proceeding to a diagnostic OGTT, even if the IADPSG diagnostic criteria for GDM are applied (39,40). The revised IADPSG diagnostic criteria and testing approach to GDM in comparison to other international organizations are summarized in Table 1.
Table 1.
Current international testing approach to gestational diabetes mellitus
| Organization/country | Selective vs universal testing | Method of screening | Screen positive threshold (mmol/L) | Diagnostic test | Diagnostic (plasma glucose) threshold for GDM (mmol/L) |
|---|---|---|---|---|---|
| IADPSG (30) WHO (11) ADIPS (33) FIGO (32) JDS (34) EBCOG (36) Endocrine Society (31) China (35) |
Universal | One-step: 75-g 2-h OGTT | 75-g 2-hour OGTT | Fasting ≥ 5.1 1-h ≥ 10.0 2-h ≥ 8.5 One abnormal value needed for diagnosis |
|
| ADA (41) | Universal | One-step: 75-g 2-h OGTT Two-step: 50-g GCT |
≥7.2 to 7.8a | 75-g 2-hour OGTT 100-g 3-hour OGTT |
Fasting ≥ 5.1 1-h ≥ 10.0 2-h ≥ 8.5 One abnormal value needed for diagnosis Carpenter and Coustanb (17) or NDDG (13) Fasting ≥ 5.3 Fasting ≥ 5.8 1-hour ≥ 10.0 1-hour ≥ 10.6 2-hour ≥ 8.6 2-hour ≥ 9.2 3-hour ≥ 7.8 3-hour ≥ 8.0 Two abnormal values needed for diagnosis |
| ACOGc (19) | Universal | Two-step: 50-g GCT | ≥7.2 to 7.8* | 100-g OGTT | Carpenter and Coustanb (17) or NDDG (13) Fasting ≥ 5.3 Fasting ≥ 5.8 1-hour ≥ 10.0 1-hour ≥ 10.6 2-hour ≥ 8.6 2-hour ≥ 9.2 3-hour ≥ 7.8 3-hour ≥ 8.0 Two abnormal values needed for diagnosisd |
| CDA (42) | Universal | Two-step: 50-g GCT (preferred) One-step: 75-g 2-h OGTT (alternative) |
≥7.8 | 50-g GCT 75-g 2-hour OGTT |
≥11.1 mmol/Le Fasting ≥ 5.3 1-hour ≥ 10.6 2-hour ≥ 9.0 One abnormal value needed for diagnosis |
| NICE (38) | Selective | Risk factorsf | 75-g 2-hour OGTT | Fasting ≥ 7.0 2-hour ≥ 7.8 One abnormal value needed for diagnosis |
|
| CNGOF (39) | Selectiveg | First trimester fasting glucose 75-g OGTTh |
≥5.1 Fasting ≥ 5.1 1-hour ≥ 10.0 2-hour ≥ 8.5 One abnormal value needed for diagnosis |
||
| DDG/DGGG (43) | Universal | Two-step: 50-g GCT One-step: 75-g OGTT (preferred) |
≥7.5 | 50-g GCT 75-g OGTT |
≥11.1 mmol/Le Fasting ≥ 5.1 1-hour ≥ 10.0 2-hour ≥ 8.5 One abnormal value needed for diagnosis |
| DIPSI (44) | Universal | One-step: 75-g OGTT | 75-g OGTT | 2-hour ≥ 7.8i |
Abbreviations: ACOG, American College of Obstetricians and Gynecologists; ADA, American Diabetes Association; ADIPS, Australasian Diabetes in Pregnancy Association; CDA, Canadian Diabetes Association; CNGOF, Organisme professionnel des médecins exerçant la gynécologie et l'obstétrique en France; DDG, German Diabetes Association; DGGG, European Board of Gynecology and Obstetrics; DIPSI, Diabetes in Pregnancy Study Group of India; FIGO, International Federation of Gynecology and Obstetrics; GCT, glucose challenge test; IADPSG, International Association of the Diabetes and Pregnancy Study Groups; JDS, Japan Diabetes Society; NDDG, US National Diabetes Data Group; NICE, National Institute for Health and Care Excellence; OGTT, oral glucose tolerance test; WHO, World Health Organization.
aThe ADA states that the choice of a specific positive GCT screening threshold is based upon the trade-off between sensitivity and specificity (41). ACOG advises that in the absence of clear evidence that supports a specific GCT threshold value between 7.2 and 7.8 mmol/L, obstetricians and obstetric care providers may select a single consistent GCT threshold for their practice based on factors such as community prevalence rates of GDM (19).
bPlasma or serum glucose.
cACOG 2018 Clinical Practice Bulletin on GDM continues to recommend 2-step testing for GDM but states that individual practices and institutions may choose to use the IADPSG’s 1-step testing approach and diagnostic criteria if appropriate for their population (19).
dACOG 2018 Clinical Practice Bulletin on GDM acknowledges that women who have even 1 abnormal value on the 100-g 3-hour OGTT have a significantly increased risk of adverse perinatal outcomes compared to women without GDM but state that further research is needed to clarify the risk of adverse outcomes and benefits of treatment in these women (19).
eA glucose level ≥ 11.1 mmol/L following the initial screening GCT is classified as GDM, and there is no need for a subsequent 2-hour 75-g OGTT.
fBMI > 30 kg/m2, previous macrosomia (≥4500 g), previous GDM, family history of diabetes, and family origin with a high prevalence of diabetes (South Asian, Black Caribbean, Middle Eastern) (38).
gMaternal age ≥ 35 years, body mass index ≥ 25 kg/m2, family history of diabetes, previous GDM, previous macrosomia (39).
hIf first trimester fasting glucose normal (ie, < 5.1 mmol/L).
It is important to consider the increase in GDM prevalence associated with the IADPSG diagnostic criteria in the context of the rising background rates of impaired glucose tolerance, type 2 diabetes, and obesity among young adults and women of reproductive age (46,47). For example, almost 18% of HAPO study participants would have met the IADPSG diagnostic thresholds for GDM. By comparison, the rate of prediabetes in US adults aged between 20 and 44 years is >29% (48,49).
Studies in Indian, Israeli, and US cohorts have suggested that the IADPSG testing approach and intervention for GDM is cost-effective based on a combination of delaying future type 2 diabetes and preventing perinatal complications (50-53). For example, a US study found that the IADPSG diagnostic criteria would be cost-effective if associated intervention decreased the absolute incidence of preeclampsia by >0.55% and cesarean delivery by >2.7% (53). In contrast, UK health economic data show that routinely identifying GDM is not cost-effective based on perinatal outcomes (54) and that the universal WHO (IADPSG) testing approach is less cost-effective than the NICE selective screening approach (55).
Contemporary Clinical Evidence Following the Revised IADPSG GDM Diagnostic Criteria
The lack of randomized controlled trials (RCTs) evaluating outcomes in women diagnosed with GDM based on the IADPSG criteria and the clinical relevance of treating the resulting milder degrees of hyperglycemia remain controversial (56). Several retrospective studies have shown that women diagnosed with GDM by the IADPSG criteria but who were previously classified as having normal glucose tolerance were still at increased risk for obstetric and neonatal complications, including gestational hypertension, preeclampsia, cesarean delivery, macrosomia, large-for-gestational-age (LGA), shoulder dystocia, and neonatal intensive care admission, compared to women with normal glucose tolerance (57-59). For example, a 2015 retrospective study in Taiwan comparing pregnancy outcomes in women diagnosed and treated for GDM using the 2-step (GCT followed by the 100-g 3-hour OGTT) approach compared to the IADPSG 1-step approach found that the latter was associated with a reduction in gestational weight gain (GWG), birthweight, macrosomia, and LGA (60). Another retrospective study in the United Kingdom reported that women who were diagnosed with GDM based on modified IADPSG diagnostic glucose thresholds but who screened negative for GDM on 2015 NICE diagnostic criteria had a higher risk of LGA, cesarean delivery, and polyhydramnios (61). Other retrospective studies have also demonstrated higher birthweight, birthweight z-score, ponderal index, and increased rates of LGA and cesarean delivery in untreated women diagnosed with GDM based on the IADPSG criteria, compared to women with normal glucose tolerance (62,63).
The recent randomized ScreenR2GDM trial compared 1-step screening (75-g 2-hour OGTT) with 2-step screening (2 GCT thresholds ≥7.2 mmol/L and ≥7.8 mmol/L used, followed by the 100-g 3-hour OGTT) in 23 792 pregnant women in the United States (64). Despite doubling the diagnosis of GDM with the 1-step approach (16.5% vs 8.5%), there were no differences in pregnancy complications including LGA [relative risk (RR) 0.95; 97.5% CI 0.87-1.05], perinatal composite outcome (RR 1.04; 97.5% CI 0.88-1.23), gestational hypertension or preeclampsia (RR 1.00; 97.5% CI 0.93-1.08), and primary cesarean section (RR 0.98; 97.5% CI 0.93-1.02) between the different screening approaches. These findings have not resolved the diagnostic debate for GDM, with some arguing that the 1-step approach therefore demonstrates insufficient perinatal benefit for the associated increased healthcare costs (65), while others have identified potential limitations in study methodology (7,47,65,66). Despite randomization to either testing strategy, the pragmatic trial design allowed clinicians to select a preferred strategy. Consequently, one third of women randomized to the 1-step approach did not adhere to the assigned screening and were tested via the 2-step approach, compared to only 8% of women randomized to the 2-step approach. Although the study attempted to adjust for this difference using inverse probability weighting, residual provider bias cannot be excluded (47). Given this was a population level analysis of GDM screening, GDM (treatment) status differed only for the 8% of women not diagnosed with GDM based on the 2-step approach who may have otherwise been diagnosed with GDM based on the 1-step approach. Whether these women had potentially worse outcomes that may have been mitigated by treatment cannot be determined by this study. However, given the rates of pharmacotherapy were similar between the 1- and 2-step cohorts at 43% and 46%, respectively (64), this strategy detected women with essentially an equivalent risk of hyperglycemia warranting pharmacotherapy (47). This observation is consistent with other studies in UK cohorts comparing the IADPSG testing approach to the less sensitive NICE and Canadian criteria, whereby women demonstrated insulin resistance and required pharmacotherapy for control of hyperglycemia even at the most sensitive thresholds of the IADPSG diagnostic criteria (67).
More generally, the GCT fails to detect approximately 20% to 25% of women with GDM, particularly those diagnosed with GDM based on an elevated fasting glucose (68). The frequency of GDM diagnosed by the OGTT fasting glucose threshold in the HAPO study ranged from 24% to 26% in Thailand and Hong Kong to >70% in the United States (69). This highlights the variability and thus limitations of post-glucose load screening based on ethnicity. Moreover, a recent systematic review and meta-analysis of 25 studies (n = 4466 women) showed that even 1 abnormal value on the diagnostic 3-hour 100-g OGTT is associated with an increased risk of perinatal complications compared to women with a normal GCT, and this risk was similar to that of women actually diagnosed with GDM (70).
The degree of benefit of treating women with GDM defined by the IADPSG diagnostic criteria is yet to be determined. The potential benefit is inferred from the treatment of maternal hyperglycemia described in the ACHOIS and MFMU intervention trials (28,29), whereby maternal glucose levels overlapped with the thresholds recommended by the IADPSG. It is worth noting that there are differences in these 2 trials with regards to the diagnostic criteria used to define GDM and cohort characteristics (eg, women were excluded from the MFMU trial if they had an abnormal glucose screening test prior to 24 weeks’ gestation or previous GDM), and thus the generalizability of these findings in women diagnosed with GDM based on the IADPSG criteria remains contentious.
Current Classification of Hyperglycemia in Pregnancy and GDM
The WHO first defined GDM in 1965 as “hyperglycemia of diabetic levels occurring during pregnancy” (8). Thus, historically, the term “GDM” encompassed the entire spectrum of maternal hyperglycemia in pregnancy, from pregestational diabetes to hyperglycemia first detected in pregnancy. In 1979, the NDDG defined GDM as “glucose intolerance that has its onset or recognition during pregnancy” (13). This was subsequently modified in 1985 at the Second International Workshop-Conference on Gestational Diabetes as “carbohydrate intolerance resulting in hyperglycemia of variable severity with onset or first recognition during pregnancy” and remained the most widely used definition of GDM until recently (71).
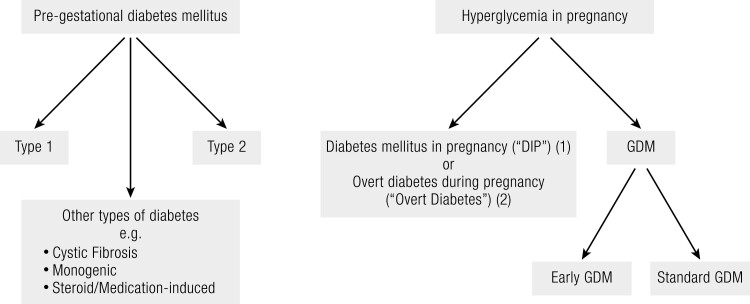
Contemporary nomenclature and diagnostic criteria now more clearly differentiate between women with pregestational diabetes and those with hyperglycemia first detected in pregnancy (30) (Fig. 1). Pregestational diabetes includes type 1 diabetes, type 2 diabetes, and other types of diabetes such as cystic fibrosis-related diabetes, steroid/medication-induced diabetes, and monogenic diabetes.
Figure 1.
Flowchart summarizing the contemporary nomenclature for hyperglycemia in pregnancy.
Hyperglycemia in pregnancy is now subclassified by the IADPSG into 2 separate categories, namely “overt diabetes mellitus during pregnancy” (overt diabetes) and GDM (30). Similarly, the WHO has a binary definition of hyperglycemia in pregnancy but has replaced the term “overt diabetes” with “diabetes mellitus in pregnancy” (DIP) (11). The rationale for the IADPSG recommendation for early testing in high-risk women is to diagnose DIP early in pregnancy. This is because DIP, diagnosed based on nonpregnant diabetes glucose thresholds, recognizes the increasing prevalence of undiagnosed preexisting diabetes in women of childbearing age as well as the greater risk associated with this degree of hyperglycemia (72-74). For example, a recent study in almost 5000 women in France found that DIP was associated with a 3.5-fold greater risk of hypertensive disorders in pregnancy compared to women with normal glucose tolerance, while early‐diagnosed DIP was associated with an increased risk of congenital malformation (7.7% vs 1.0% for women with normal glucose tolerance), suggesting that early hyperglycemia in pregnancy may sometimes be present at conception (75). However, DIP is not synonymous with preexisting diabetes. In Australian, women with DIP who performed an OGTT at 6 to 8 weeks postpartum, 21% had diabetes, 38% had impaired fasting glucose or impaired glucose tolerance, and 41% returned to normal glucose tolerance (76).
Regardless of the specific nomenclature used, DIP is distinct from GDM, which is defined by lower glucose thresholds on the OGTT and was historically considered to be a condition of mid to late pregnancy. The ADA has not accepted this nomenclature and defines GDM based on timing of diagnosis: women diagnosed with diabetes in the first trimester are classified as having (preexisting) type 2 diabetes, while GDM is defined as diabetes diagnosed in later pregnancy and not meeting the diagnostic criteria for type 2 diabetes (18). A summary of the current international nomenclature and diagnostic criteria for hyperglycemia in pregnancy is presented in Table 2.
Table 2.
Classification and diagnostic criteria for hyperglycemia in pregnancy
| Organization | Results | ||
|---|---|---|---|
| IADPSG/EBCOG (30,36) | |||
| GDM | 75-g 2-hour OGTT Fasting glucose 5.1-6.9 mmol/L 1-hour glucose ≥ 10.0 mmol/L 2-hour glucose 8.5-11.0 mmol/L |
||
| Overt diabetes during pregnancy | Fasting glucose ≥ 7.0 mmol/L Random glucose ≥ 11.1 mmol/La HbA1c ≥ 6.5% |
||
| WHO/FIGO/ADIPS (11,32,33) | |||
| GDM | 75-g 2-hour OGTT Fasting glucose 5.1-6.9 mmol/L 1-hour glucose ≥ 10.0 mmol/L 2-hour glucose 8.5-11.0 mmol/L |
||
| Diabetes mellitus in pregnancy | Fasting glucose ≥ 7.0 mmol/L 2-hour glucose ≥ 11.1 mmol/L post 75-g OGTT Random glucose ≥ 11.1 mmol/L in the presence of diabetes symptoms |
||
| ADA (41) | |||
| GDM | 1-step strategy: 75-g 2-h OGTT Fasting glucose ≥ 5.1 mmol/L 1-hour glucose ≥ 10.0 mmol/L 2-hour glucose ≥ 8.5 mmol/L |
2-step strategy: 50-g 1-hour GCT ≥ 7.8 mmol/L 100 g 3-hour OGTT Carpenter and Coustan (17) or Fasting glucose ≥ 5.3 mmol/L 1-hour glucose ≥ 10.0 mmol/L 2-hour glucose ≥ 8.6 mmol/L 3-hour glucose ≥ 7.8 mmol/L |
NDDG (13) Fasting glucose ≥ 5.8 mmol/L 1-h glucose ≥ 10.6 mmol/L 2-h glucose ≥ 9.2 mmol/L 3-h glucose ≥ 8.0 mmol/L |
| Type 2 diabetes mellitus | Fasting glucose ≥ 7.0 mmol/L 2-hour glucose ≥ 11.1 mmol/L post 75 g 2-hour OGTT Random glucose ≥ 11.1 mmol/L in the presence of diabetes symptoms HbA1c ≥ 6.5% |
||
75-g 2-hour OGTT: only 1 plasma glucose level needs to be elevated for the diagnosis of GDM. 100 g 3-hour OGTT: at least 2 plasma glucose levels need to be elevated for the diagnosis of GDM.
Abbreviations: ADA, American Diabetes Association; ADIPS, Australasian Diabetes in Pregnancy Association; EBCOG, European Board & College of Obstetrics and Gynaecology; FIGO, International Federation of Gynecology and Obstetrics; GCT, glucose challenge test; HbA1c, hemoglobulin A1c; IADPSG/; International Association of the Diabetes and Pregnancy Study Groups; GDM, gestational diabetes mellitus; OGTT, oral glucose tolerance test; WHO, World Health Organization.
aThe IADPSG recommends confirmation by fasting plasma glucose or HbA1c for the diagnosis of overt diabetes during pregnancy (30).
Early GDM
Most international guidelines now recommend early antenatal testing for women at high risk to identify women with DIP (11,18,30,38,39,42-44). This has resulted in increased detection of milder degrees of hyperglycemia below the threshold of DIP, referred to as GDM diagnosed prior to 24 weeks’ gestation or early GDM. Studies in women with GDM have reported that between 27% and 66% of GDM can be detected in early pregnancy depending on the population as well as the screening and diagnostic criteria used (77-81).
Recent studies evaluating the relationship between maternal glycemia and fetal growth trajectories confirm the early impact of maternal glycemia on excess fetal growth and adiposity prior to the diagnosis of standard GDM from 24 weeks’ gestation. A US multiethnic prospective cohort study of 2458 women enrolled between 8 and 13 weeks’ gestation included 107 (4.4%) women with GDM (82). GDM was associated with an increase in estimated fetal weight from 20 weeks’ gestation, which became significant at 28 weeks’ gestation. Similarly, Sovio et al showed that excessive fetal growth occurred between 20 to 28 weeks’ gestation, prior to the diagnosis of GDM, especially among women with higher body mass index [BMI (kg/m2)] (83). An Indian study also showed that excess subcutaneous abdominal adiposity was first detected at 20 weeks’ gestation, at least 4 weeks prior to the diagnosis of GDM (84). Early excess adiposity persisted despite adjustments for maternal age, BMI, GWG, fetal sex, and gestational age and remained higher at 32 weeks’ gestation (84).
Currently, there is no consensus for the preferred testing approach or diagnostic glycemic thresholds for early GDM. The IADPSG recommends diagnosing early GDM based on a fasting glucose of 5.1 mmol/L to 6.9 mmol/L (92-124 mg/dL) (30), consistent with the diagnostic fasting glucose threshold for standard GDM. The utility of a single fasting glucose measurement for early GDM diagnosis warrants consideration. First, preanalytical glucose handling variation, particularly in the setting of a single glucose measurement, is a major issue for GDM diagnostic accuracy (discussed in the following text). Second, an Israeli cohort study of 6129 women who underwent a fasting glucose test at a median of 9.5 weeks’ gestation demonstrated a positive association between first trimester fasting glucose up to 5.8 mmol/L (104.5 mg/dL) and increased risk for subsequent diagnosis of GDM, LGA, macrosomia, and cesarean section (85). Similar to the HAPO study, a clear glucose threshold was lacking, with pregnancy complications evident at fasting glucose levels <5.1 mmol/L (92 mg/dL). Third, maternal fasting glucose decreases in the first trimester, most pronounced between 6 to 10 weeks’ gestation [median decrease in glucose 0.11 mmol/L (1.98 mg/dL)] (86), while studies have consistently shown that early fasting glucose is poorly predictive of GDM at 24 to 28 weeks’ gestation (86-88), leading to potential overdiagnosis of GDM. In China, an early fasting glucose between 6.1 mmol/L to 6.9 mmol/L (110-124 mg/dL) best corresponded to later GDM diagnosis (88), but this requires further validation.
The WHO recommends the same diagnostic OGTT glucose thresholds for GDM in early pregnancy as those derived from HAPO by the IADPSG (11). However, the prognostic value of these glucose levels in early pregnancy is yet to be established. Others have proposed an hemoglobin A1c (HbA1c) risk threshold (89), based primarily on evidence that an early HbA1c ≥ 5.9% (41 mmol/mol) detected all cases of DIP and predicted adverse pregnancy outcomes in a New Zealand cohort (90). However, studies in other cohorts have found that while an elevated HbA1c in early pregnancy is highly specific, it lacks sensitivity for identifying hyperglycemia and certain perinatal complications (91,92), with no clear benefit of treating women with HbA1c 5.7% to 6.4% (39-46 mmol/mol) in early pregnancy (93,94). A summary of the various international criteria for testing of GDM in early pregnancy is presented in Table 3.
Table 3.
International criteria for testing of gestational diabetes mellitus in early pregnancy
| Organization | Early pregnancy testing | Method of testing | Diagnostic test | Criteria for diagnosing early GDM (mmol/L) |
|---|---|---|---|---|
| IADPSG (30) | Yes | Selective—women at risk of overt diabetes during pregnancya | Fasting glucoseb | ≥5.1 |
| WHO (11) | Not specifiedc | 75-g 2-hour OGTT | Fasting 5.1-6.9 or 1-hour ≥ 10.0 or 2-hour 8.5-11.0 |
|
| ADIPS (33) | Yes | Selective—women at risk of hyperglycemia in pregnancyd | 75-g 2-hour OGTT | Fasting 5.1-6.9 or 1-hour ≥ 10.0 or 2-hour 8.5-11.0 |
| ADA (41) | Yes | Selective—women with risk factors for undiagnosed type 2 diabetese | One-step: 75-g 2-hour OGTT Two-step: 50-g GCT 100-g 3-hour OGTT |
Fasting 5.1-6.9 or 1-hour ≥ 10.0 or 2-hour 8.5-11.0 ≥7.2 to 7.8 Carpenter and Coustan (17) NDDG (13) Fasting ≥ 5.3 ≥ 5.8 1-hour ≥ 10.0 ≥ 10.6 2-hour ≥ 8.6 ≥ 9.2 3-hour ≥ 7.8 ≥ 8.0 |
| ACOG (19) | Yes | Selective—women with risk factors for undiagnosed type 2 diabetes or GDMf | 75-g 2-h OGTT or 50-g GCT Confirmatory 100-g 3-hour OGTT |
Fasting ≥ 7.0 or 2-hour ≥ 11.1 ≥7.2 to 7.8 Carpenter and Coustan (17) NDDG (13) Fasting ≥ 5.3 ≥ 5.8 1-hour ≥ 10.0 ≥ 10.6 2-hour ≥ 8.6 ≥ 9.2 3-hour ≥ 7.8 ≥ 8.0 |
| EBCOG (36) | Yes | Selective—women at risk of overt diabetes during pregnancyg | 75-g 2-hour OGTT | Fasting 5.1-6.9 or 1-hour ≥ 10.0 or 2-hour 8.5-11.0 |
| DDG/DGGG (43) | Yes | Selective—women with risk factors for “manifest diabetes”h | Random glucose or Fasting glucose or 75-g 2-hour OGTT |
7.8-11.05 mmol/L followed by a second blood glucose measurement or an OGTT 5.1-6.9 Fasting 5.1-6.9 or 1-hour ≥ 10.0 or 2-hour 8.5-11.0 |
| CNGOF (39) | Yes | Selectivei | Fasting glucose | ≥5.1 |
| NICE (38) | Yes | Selectivej | 75-g 2-hour OGTT | Fasting ≥ 5.6 2-hour ≥ 7.8 |
| DIPSI (44) | Yes | Universal | 75-g 2-hour OGTTk | 2-hour ≥ 7.8 |
75-g 2-h OGTT: Only 1 abnormal glucose level needs to be elevated for the diagnosis of GDM. 100-g 3-h OGTT: 2 abnormal glucose levels need to be elevated for the diagnosis of GDM.
Abbreviations: ADA, American Diabetes Association; ACOG, American College of Obstetricians and Gynecologists; ADIPS, Australasian Diabetes in Pregnancy Association; CNGOF, Organisme professionnel des médecins exerçant la gynécologie et l'obstétrique en France; DDG, German Diabetes Association; DGGG, European Board of Gynecology and Obstetrics; DIPSI, Diabetes in Pregnancy Study Group of India; EBCOG, European Board & College of Obstetrics and Gynaecology; GCT, glucose challenge test; GDM, gestational diabetes mellitus; IADPSG, International Association of the Diabetes and Pregnancy Study Groups; NICE, National Institute for Health and Care Excellence; OGTT, oral glucose tolerance test; WHO, World Health Organization.
aHigh-risk criteria not explicitly defined.
bIADPSG does not recommend routinely performing the 75-g 2-h OGTT prior to 24 weeks’ gestation but advises that a fasting glucose ≥ 5.1 mmol/L in early pregnancy be classified as GDM (30).
cGDM diagnosed at any time in pregnancy based on an abnormal 75-g 2-h OGTT (11).
dHigh-risk criteria defined as previous hyperglycemia in pregnancy; previously elevated blood glucose level; maternal age ≥ 40 years; ethnicity: Asian, Indian subcontinent, Aboriginal, Torres Strait Islander, Pacific Islander, Maori, Middle Eastern, non-White African; family history of diabetes (first-degree relative with diabetes or sister with hyperglycemia in pregnancy); prepregnancy body mass index > 30 kg/m2; previous macrosomia (birth weight > 4500 g or > 90th percentile); polycystic ovary syndrome; and medications: corticosteroids, antipsychotics (33).
eHigh-risk criteria defined as body mass index ≥ 25 kg/m2 (≥ 23 kg/m2 in Asian Americans) plus 1 of the following: physical inactivity; previous GDM; previous macrosomia (≥ 4000 g); previous stillbirth; hypertension; high density lipoprotein cholesterol ≤ 0.90 mmol/L; fasting triglycerides ≥ 2.82 mmol/L; polycystic ovary syndrome; acanthosis nigricans; nonalcoholic steatohepatitis; morbid obesity and other conditions associated with insulin resistance; hemoglobulin A1c ≥ 5.7%; impaired glucose tolerance or impaired fasting glucose; cardiovascular disease; family history of diabetes (first-degree relative); and ethnicity: African American, American Indian, Asian American, Hispanic, Latina, or Pacific Islander ethnicity. Note that the ADA recommends testing for GDM at 24 to 28 weeks’ gestation and have no specific definition for early GDM (41).
fACOG states that the best test for early GDM screening is not clear but suggest the testing approach and diagnostic criteria used to diagnose type 2 diabetes in the nonpregnant population and thus have no specific definition for early GDM (19).
gHigh-risk criteria defined as previous GDM; overweight/obesity; family history of diabetes (first-degree relative with diabetes); previous macrosomia (>4000g or >90th percentile); polycystic ovary syndrome; ethnicity: Mediterranean, South Asian, black African, North African, Caribbean, Middle Eastern, or Hispanic (36).
hHigh-risk criteria defined as age ≥ 45 years; prepregnancy body mass index ≥ 30 kg/m2; physical inactivity; family history of diabetes; high-risk ethnicity (eg. Asians, Latin Americans); previous macrosomia ≥ 4500 g; previous GDM; hypertension; prepregnancy dyslipidemia (high-density lipoprotein cholesterol ≤ 0.90 mmol/L, fasting triglycerides ≥ 2.82 mmol/L); polycystic ovary syndrome; prediabetes in an earlier test; other clinical conditions associated with insulin resistance (eg, acanthosis nigricans); history of coronary artery disease/peripheral artery disease/cerebral vascular disease; medications associated with hyperglycemia (eg. glucocorticoids). Note that the DDG/DGGG recommends that a 75-g 2-h OGTT be the initial early test in high-risk women (defined as women with ≥2 risk factors for GDM) (43).
iHigh-risk criteria are defined as previous GDM, previous impaired glucose tolerance, and/or obesity (39).
jHigh-risk criteria defined as body mass index> 30 kg/m2; previous macrosomia (≥4500 g); previous GDM; family history of diabetes (first-degree relative with diabetes); minority ethnic family origin with a high prevalence of diabetes. The updated 2015 NICE guidelines state that women with previous GDM should undergo early self-monitoring of blood glucose or a 75-g 2-hour OGTT as soon as possible after booking (first or second trimester), and a repeat 75-g 2-hour OGTT at 24 to 28 weeks’ gestation if the initial OGTT was negative (38).
k2-hour postload glucose measured on nonfasting 75-g OGTT (44).
Despite the lack of diagnostic clarity for early GDM, increasing evidence suggests that women with early GDM represent a high-risk cohort (81). Early studies also reported worse pregnancy outcomes and increased insulin resistance in early GDM (78,95-97) but were confounded by the inclusion of women with pregestational diabetes. The first large retrospective cohort study excluding women with DIP showed that women diagnosed and treated for early GDM, especially those diagnosed in the first trimester, were more insulin resistant and at significantly greater risk for obstetric and neonatal complications compared to women diagnosed and treated for GDM from 24 weeks’ gestation (81). Other studies have since confirmed these findings (98,99). Concerningly, an increased risk of perinatal mortality and congenital abnormalities has also been reported in the offspring of women with early GDM (75,78,95,96), with some data demonstrating that 5% of women with early GDM have abnormal fetal echocardiograms (97). A recent meta-analysis of 13 cohort studies showed greater perinatal mortality among women with early GDM (RR 3.58; 95% CI 1.91-6.71) compared to women with a later diagnosis of GDM despite treatment (100).
A recent study assessing the pathophysiological characteristics of women diagnosed with GDM at a median of 16 weeks’ gestation compared to those diagnosed from 24 weeks’ gestation using IADPSG diagnostic criteria reported that women with early GDM had lower insulin sensitivity (defined by insulin-mediated glucose clearance during an OGTT), even after accounting for maternal BMI (101). Consistent with the pathophysiology of GDM, women with both early and standard GDM demonstrated impairment in pancreatic β-cell function (102). These data underscore GDM phenotypic differences, specifically based on timing of diagnosis and degree of hyperglycemia (103).
A key issue is the current lack of high-quality evidence that diagnosing and treating early GDM improves pregnancy outcomes. A recent major RCT in the United States evaluating early testing for GDM in 962 women with obesity included a subgroup analysis of women diagnosed and treated for GDM [early n = 69 (15.0%) vs standard n = 56 (12.1%)] based on the 2-step testing approach (104). The average gestational age at GDM diagnosis was similar at 24.3 ± 5.2 weeks for the early screen group compared to 27.1 ± 1.7 weeks in the routine screen group. There was no difference in pregnancy outcomes, although the primary composite perinatal outcome (macrosomia, primary cesarean delivery, gestational hypertension, preeclampsia, hyperbilirubinemia, shoulder dystocia, and neonatal hypoglycemia) was nonsignificantly higher in the early-screen group (56.9% vs 50.8%; P = 0.06). Requirement for insulin therapy was almost 4-fold higher, while gestational age at delivery was lower (36.7 vs 38.7 weeks’ gestation; P = 0.001) in women with early GDM. In a post hoc analysis of the Lifestyle in Pregnancy study (105), no difference in pregnancy outcomes was shown between women randomized to either lifestyle intervention (n = 36) or standard treatment (n = 54) in early pregnancy. Whether different glycemic targets are required reflecting physiological differences in early maternal glucose or whether additional risk factors contributing to a more insulin resistant phenotype such as maternal adiposity might also have a role remain unanswered (81). The ongoing Treatment of Booking Gestational Diabetes Mellitus study, evaluating the impact of immediate vs delayed care for gestational diabetes diagnosed at booking, will seek to determine whether or not there is benefit from treating early GDM (106).
The Impact of Preanalytical Glucose Processing Standards on the Diagnosis of GDM
Although the contemporary testing approach to GDM remains contentious, it is important to recognize that the diagnosis of GDM is based on the laboratory measurement of maternal glucose rather than a clinical diagnosis. Arguably then, a major issue in the contemporary diagnosis of GDM is optimizing preanalytical processing and measurement of maternal plasma glucose to ensure diagnostic accuracy (107,108). This includes optimization of sample handling and minimization of any analytic error. Unfortunately, stringent preanalytical processing standards are not currently routinely applied. The American Association for Clinical Chemistry (AACC) and ADA recommendations on laboratory testing in diabetes advise collection of plasma glucose in sodium fluoride tubes, with immediate placement in an ice slurry and centrifugation within 30 minutes (109). Citrate tubes are recommended as an alternative where early centrifugation is not possible. These standards are important because a major source of preanalytical glucose measurement error in sodium fluoride tubes is glycolysis by erythrocytes and leukocytes, which at room temperature lowers glucose levels prior to centrifugation at a rate of 5% to 7% per hour [~0.6 mmol/L (10 mg/dL)] (109,110). By 1 hour, this degree of glucose lowering is higher than the total analytical error threshold for glucose based on biological variation (107).
Recent studies have shown that OGTT preanalytical glucose processing variability greatly impacts the prevalence of GDM (67,111). Implementation of the AACC/ADA recommendations in a UK cohort resulted in higher mean glucose concentrations and 2.7-fold increased detection of GDM based on IADPSG criteria compared with the standard practice of storing sodium fluoride tubes at room temperature and delaying centrifugation until collection of all 3 OGTT samples (112). This increase in GDM diagnosis was entirely attributable to control of glycolysis (107). Similarly, in a large Australian multiethnic cohort (n = 12317), the rate of GDM diagnosis based on IADPSG criteria increased from 11.6% to 20.6% with early (within 10 minutes) vs delayed centrifugation (111). Mean glucose concentrations for the fasting, 1-hour, and 2-hour OGTT samples were 0.24 mmol/L (5.4%), 0.34 mmol/L (4.9%), and 0.16 mmol/L (2.3%) higher with early centrifugation, with the increase in GDM diagnosis primarily due to the resulting increase in fasting glucose levels (111). Importantly, the HAPO study, upon which the IADPSG diagnostic criteria for GDM was based, followed these AACC/ADA preanalytical glucose processing standards (111).
Incidence and Prevalence of GDM
GDM is 1 of the most common medical complications of pregnancy (73). In 2019, the International Diabetes Federation (IDF) estimated that 1 in 6 live births worldwide were complicated by GDM (113). More than 90% of cases of hyperglycemia in pregnancy occur in low- and middle-income countries (114), where the prevalence and severity of maternal and neonatal complications associated with GDM (47,113) contrast with the near-normal pregnancy outcomes of modern management of GDM in developed countries (115).
The prevalence of GDM varies widely, depending on the population, the specific screening and the diagnostic criteria utilized. A 2012 systematic review of the diagnostic criteria used to define GDM reported a worldwide prevalence of GDM of 2% to 24.5% for the WHO criteria, 3.6% to 38% for the Carpenter and Coustan criteria, 1.4 to 50% for the NDDG criteria, and 2% to 19% for the IADPSG criteria (116).
Regardless of the specific diagnostic criteria or population, the prevalence of GDM continues to rise internationally, corresponding to epidemiological factors including the background rates of type 2 diabetes and increased incidence of obesity in women of childbearing age and rising maternal age (117-124). Implementation of the revised IADPSG diagnostic criteria have further increased the proportion of women being diagnosed with GDM (69,125,126). The incidence of GDM in the original HAPO study cohort applying the IADPSG diagnostic criteria ranged from 9.3% to 25.5% depending on study site (69). Recent international prevalence data also demonstrate marked variability in the rate of GDM, ranging from 6.6% in Japan and Nepal to 45.3% of pregnancies in the United Arab Emirates (127).
Risk Factors for GDM
Several modifiable and nonmodifiable risk factors for GDM have been identified (Table 4). A history of GDM in a previous pregnancy is the strongest risk factor for GDM, with reported recurrence rates of up to 84% (128). The risk of recurrence varies greatly depending on ethnicity (128). Ethnicities at increased risk for development of type 2 diabetes, such as South and East Asians, Hispanic, Black and Native Americans, Aboriginal and Torres Strait Islanders, and Middle Easterners are also associated with an increased risk of GDM (129,130). A US study of over 123 000 women reported the prevalence of GDM using the 2000 ADA diagnostic criteria to be the highest among Filipinas (10.9%) and Asians (10.2%), followed by Hispanics (6.8%), non-Hispanic Whites (4.5%) and Black Americans (4.4%) (131). Women who have had GDM are at increased risk for subsequent type 2 diabetes, while family history of type 2 diabetes in a first-degree relative or sibling with GDM is a major risk factor for GDM (129,132-134).
Table 4.
Key risk factors for gestational diabetes mellitus
| Previous GDM |
| An ethnicity with a high prevalence of diabetes |
| Maternal age > 35 years |
| Family history of diabetes (first-degree relative with diabetes) |
| Obesity (BMI > 30 kg/m2) |
| Previous macrosomia (birthweight > 4500 g) |
| Polycystic ovary syndrome |
| Iatrogenic: glucocorticoids and antipsychotic medication |
Abbreviations: BMI, body mass index; GDM, gestational diabetes mellitus.
Increasing maternal age is also a risk factor for GDM (129,133-135). The prospective First and Second Trimester Evaluation of Risk trial (n = 36 056) demonstrated a continuous positive relationship between increasing maternal age and risk for adverse pregnancy outcomes, including GDM (135). Maternal age 35 to 39 years and ≥40 years was associated with an adjusted odds ratio (OR) for GDM of 1.8 (95% CI 1.5-2.1) and 2.4 (95% CI 1.9-3.1), respectively (135). Other studies in high-risk cohorts have reported a lesser risk between increasing maternal age and GDM after adjustment for other risk factors (136).
Maternal prepregnancy overweight (BMI 25-29.99 kg/m2) or obesity (BMI ≥ 30 kg/m2) are common risk factors for GDM (129,130,133,134,136,137). The risk of GDM is increased almost 3-fold (95% CI 2.1-3.4) in women with class I obesity (BMI 30-34.99 kg/m2) and 4-fold (95% CI 3.1-5.2) in women with class II obesity (BMI 35-39.99 kg/m2), compared to women with a BMI < 30 kg/m2 (138). High GWG, particularly in the first trimester, is also associated with an increased risk for GDM (131,139,140). Further, women with obesity and high GWG are 3- to 4-fold more likely to develop abnormal glucose tolerance compared to women who remained within the 1990 Institute of Medicine (IOM) recommendations for GWG (131,141). Interpregnancy weight gain is also a risk factor for GDM and perinatal complications in a subsequent pregnancy (142) and may be a potential confounder when considering the risk of GDM recurrence.
Studies have demonstrated an association between polycystic ovary syndrome and GDM, although this is significantly attenuated after adjustment for maternal BMI (143,144). Other risk factors for GDM include multiparity (133,134), twin pregnancy (145,146), previous macrosomia (123), a history of perinatal complications (134), maternal small-for-gestational-age (SGA) or LGA (134), physical inactivity (129,147,148), low-fiber high-glycemic load diets (149), greater dietary fat and lower carbohydrate intake (137), and medications such as glucocorticoids and anti-psychotic agents (150,151). Maternal pre- and early pregnancy hypertension is also associated with an increased risk of developing GDM (152,153).
Overall, noting the variation in performance and utility of clinical risk factors based on local population factors, previous GDM and family history of diabetes appear to be the strongest clinical risk factors for GDM (154-157). Ethnicity, higher maternal age, and BMI are also strong predictors for GDM (154-158).
Pathophysiology of GDM
Normal pregnancy is associated with marked changes in glycemic physiology (159,160). There is a progressive increase in insulin resistance, predominantly due to increased circulating placental hormones including growth hormone, corticotrophin-releasing hormone, human placental lactogen, prolactin, estrogen, and progesterone (161-166). Increased maternal adiposity particularly in early pregnancy also promotes insulin resistance, contributing to facilitated lipolysis by late pregnancy (167,168). The resultant increase in maternal free fatty acid (FFA) levels exacerbates maternal insulin resistance by inhibiting maternal glucose uptake and stimulating hepatic gluconeogenesis (168,169). By late pregnancy, studies have reported decreases in maternal glucose sensitivity between 40% and 80% in women with normal or increased BMI (170-172). Increased maternal insulin resistance results in higher maternal postprandial glucose levels and FFAs for maternal growth (164,167,173) and increased facilitated diffusion across the placenta, leading to greater availability of glucose for fetal growth (161,174). This progressive rise in maternal insulin resistance underpins the delayed testing approach to GDM, aiming to maximize detection of GDM when insulin resistance is at its greatest in mid- to late gestation.
In addition to increased insulin resistance and elevated postprandial glucose, adaptations in normal pregnancy include enhanced insulin secretion (160,165). Maternal glucose levels are maintained at lower levels than in healthy nonpregnant women (175,176), and euglycemia is maintained by a corresponding 200% to 250% increase in insulin secretion, most notable in early pregnancy (161,167,177). Human placental lactogen, in addition to prolactin and growth hormone, primarily regulate increased maternal β-cell insulin secretion and proliferation during pregnancy (178-180). Rodent studies have demonstrated a 3- to 4-fold increase in β-cell mass during pregnancy, mediated via hypertrophy, hyperplasia, neogenesis, and/or reduced apoptosis (181,182).
GDM is characterized by a relative insulin secretory deficit (177), in which maternal β-cell insulin secretion is unable to compensate for the progressive rise in insulin resistance during pregnancy (183). This leads to decreased glucose uptake, increased hepatic gluconeogenesis, and maternal hyperglycemia (167). It is hypothesized that this results from the failure of β-cell mass expansion (182,184). Hyperlipidemia, characterized predominantly by higher serum triglycerides, may also cause lipotoxic β-cell injury, further impairing insulin secretion (185,186). The pathogenesis of GDM therefore parallels that of type 2 diabetes, characterized by both increased insulin resistance and relative insulin deficiency arising from a reduction in β-cell function and mass (187,188).
Serial studies of the insulin secretory response in women who develop GDM suggest that the abnormal insulin secretory response is present from prepregnancy and increases in early pregnancy, prior to and independent of changes in insulin sensitivity (170,189-191). These data suggest that many women with GDM may have chronic or preexisting β-cell dysfunction, potentially mediated by circulating hormones including leptin (191).
Genetics of GDM
The genetics of GDM and glucose metabolism in pregnancy remain poorly defined. Data on epigenetic mechanisms in GDM are especially lacking and primarily limited to the potential role of DNA methylation in mediating the intrauterine effects of GDM on offspring outcomes (192,193).
Most genetic studies have focused on variants associated with type 2 diabetes and have demonstrated a similar association with GDM (194,195). A meta-analysis of 28 case-control studies (n = 23425) (196) identified 6 genetic polymorphisms at loci involved in insulin secretion [insulin-like growth factor 2 messenger RNA-binding protein 2 (IGF2BP2), melatonin receptor 1B (MTNR1B) and transcription factor 7-like 2 (TCF7L2)] (197-199), insulin resistance [insulin receptor substrate 1 (IRS1) and peroxisome proliferator-activated receptor gamma (PPARG)] (200,201), and inflammation [tumor necrosis factor alpha (TNF-α)] (202) in type 2 diabetes. Overall, only MTNR1B, TCF7L2, and IRS1 were also significantly associated with GDM, supporting the role of both impaired insulin secretion and insulin resistance in the pathogenesis of GDM as well as type 2 diabetes (196). Subgroup analysis showed the risk alleles of TCF7L2 and PPARG were significant only in Asian populations, while the association between IRS1 and TCF7L2 and GDM risk varied depending on diagnostic criteria and genotype methodology (196), highlighting the need for further large confirmatory studies.
Two genome-wide association studies (GWAS) have evaluated the genetic associations for GDM and glucose metabolism (194,203). The first, a 2-stage GWAS in Korean women, compared 468 women with GDM and 1242 normoglycemic women using 2.19 million genotyped markers before further genotyping 11 loci in 1714 women, identifying 2 loci significantly associated with GDM (203). A variant in cyclin-dependent kinase 5 regulatory subunit-associated protein 1-like 1 (CDKAL1) had the strongest association with GDM, followed by a variant near MTNR1B expressed in pancreatic β-cells (204). The IGF2BP2 variant did not reach genome-wide significance with GDM in this study. CDKAL1 was significantly associated with decreased fasting insulin concentration and homeostasis model assessment of β-cell function in women with GDM, consistent with impaired β-cell compensation. MTNR1B was associated with decreased fasting insulin concentrations in women with GDM and increased fasting glucose concentrations in both women with and without GDM (203). Variants in CDKAL1 and MTNR1B have previously been associated with type 2 diabetes risk (205,206).
A subsequent GWAS performed in a subset of the HAPO cohort (n = 4528) comprising European, Thai, Afro-Caribbean, and Hispanic women evaluated maternal metabolic traits in pregnancy (194). This study reported 5 variants associated with quantitative glycemic traits in the general population (207,208) that were also associated with glucose or C-peptide levels in pregnancy, although strength of association varied across cohorts (194). Specifically, loci in glucokinase regulator (GCKR), glucose-6-phosphatase 2 (G6PC2), proprotein convertase subtilisin/kexin type 1 (PCSK1), protein phosphatase 1, regulatory subunit 3B (PPP1R3B), and MTNR1B were associated with fasting glucose. In addition, GCKR and PPP1R3B were associated with fasting C-peptide levels, while MTNR1B was associated with 1-hour postload glucose. These loci have also previously been associated with lipid metabolism (GCKR and PPP1R3B), glycogen metabolism (PPP1R3B), and obesity-related traits (PCSK1) (209-214).
Two additional novel loci identified near hexokinase domain containing 1 (HKDC1) associated with 2-hour postload glucose, and β-site amyloid polypeptide cleaving enzyme 2 (BACE2) associated with fasting C-peptide, demonstrated limited association with glycemic traits outside of compared to in pregnancy (215). In general, however, studies evaluating associations between genetic risk scores, glycemic traits in pregnancy, and GDM have also confirmed that genetic determinants of fasting glucose and insulin, insulin secretion, and insulin sensitivity reported outside of pregnancy influence GDM risk (216). A summary of the genes associated with GDM is provided in Table 5.
Table 5.
Genes linked to gestational diabetes mellitus
| Gene symbol | Gene name | Function |
|---|---|---|
| MTNR1B | Melatonin receptor 1B | Receptor mediating the action of melatonin, including its inhibitory effect on insulin secretion |
| TCF7L2 | Transcription factor 7-like 2 | Blood glucose homeostasis |
| IRS1 | Insulin receptor substrate 1 | Receptor mediating the control of various cellular processes by insulin |
| CDKAL1 | Cyclin-dependent kinase 5 regulatory subunit-associated protein 1-like 1 | Proinsulin to insulin conversion |
| GCKR | Glucokinase regulator | Inhibits glucokinase in liver and pancreatic islet cells |
| G6PC2 | Glucose-6-phosphatase 2 | Glucose metabolism |
| PCSK1 | Proprotein convertase subtilisin/kexin type 1 | Endoprotease involved in proteolytic activation of polypeptide hormones and neuropeptides precursors including proinsulin, proglucagon-like peptide 1, and pro-opiomelanocortin |
| PPP1R3B | Protein phosphatase 1, regulatory subunit 3B | Regulates glycogen metabolism |
| HKDC1 | Hexokinase domain containing 1 | Involved in glucose homeostasis and hepatic lipid accumulation |
| BACE2 | Beta-site amyloid polypeptide cleaving enzyme 2 | Proteolytic processing of CLTRNa in pancreatic β-cells |
Genes were identified and selected from the genome-wide association studies (194,203). The name and function of each gene was determined from GeneCards (https://www.genecards.org).
aCollectrin, amino acid transport regulator is a stimulator of β-cell replication.
Maturity-onset Diabetes of the Young
Maturity-onset diabetes of the young (MODY) is the most common form of monogenic diabetes; inherited forms of diabetes characterized by defects in single genes regulating β-cell development and function (217,218). MODY consists of several autosomal dominant forms of diabetes accounting for up to 2% of all diabetes diagnoses (219). A diagnosis of MODY requires confirmatory molecular genetic testing, and thus MODY is frequently misdiagnosed as preexisting diabetes or GDM, accounting for up to 5% of GDM “cases” (220-223). A UK study reported that HNF-1α (MODY3) (52%) and glucokinase (GCK)-MODY subtype (MODY2) (32%) were most frequent in probands confirmed with MODY, followed by HNF-4α (MODY1) and HNF-1β (MODY5) (224).
Women with GCK-MODY often first present following antenatal screening for GDM, with an estimated prevalence of 1% of all GDM “cases” actually GCK-MODY (220,222). GCK-MODY is caused by mutations in the glucokinase gene, leading to a greater set point for glucose stimulated insulin release (219). Clinically, GCK-MODY is defined by mild, stable fasting hyperglycemia [fasting glucose 98-150 mg/dL (5.4-8.3 mmol/L)] and low rates of microvascular and macrovascular complications (220). It should be suspected following a positive OGTT in pregnancy if the fasting glucose is ≥5.5 mmol/L, the glucose increment from the fasting to 2-hour (75-g) OGTT is small (<4.6 mmol/L), and there is a positive family history of mild hyperglycemia or diabetes. In addition, a combination of fasting glucose ≥ 100 mg/dL (5.6 mmol/L) and BMI < 25 kg/m2 has been shown to have a sensitivity of 68% and a specificity of 99% for differentiating GCK-MODY from GDM (220). Importantly, management differs from that of GDM because the need for intensive maternal glycemic control largely depends on whether the GCK-MODY mutation is also present in the fetus (220,225,226). Maternal insulin therapy is therefore only recommended in the presence of increased fetal abdominal growth (>75th centile) measured on serial ultrasounds from 26 weeks’ gestation, as this indicates that the fetus does not have the GCK mutation (220).
Consequences of GDM
GDM is associated with excess neonatal and maternal short- and long-term morbidity, summarized in Table 6.
Table 6.
Maternal and neonatal complications of gestational diabetes mellitus
| Complications | Maternal | Neonatal |
|---|---|---|
| Short term | Preeclampsia Gestational hypertension Hydramnios Urinary tract/vaginal infections Instrumental delivery Cesarean delivery Traumatic labor/perineal tears Postpartum hemorrhage Difficulty initiating and/or maintaining breastfeeding |
Stillbirth Neonatal death Preterm birth Congenital malformations Macrosomia Cardiomyopathy Birth trauma: Shoulder dystocia Bone fracture Brachial plexus injury Hypoglycemia Hyperbilirubinemia Respiratory distress syndrome |
| Long term | Recurrence of GDM Type 2 diabetes mellitus Hypertension Ischemic heart disease Nonalcoholic fatty liver disease Dyslipidemia Chronic kidney disease |
Metabolic syndrome Hyperinsulinemia Childhood obesity Excess abdominal adiposity Higher blood pressure Possible earlier onset cardiovascular disease Possible attention-deficit hyperactivity disorder Autism spectrum disorder |
Neonatal Complications
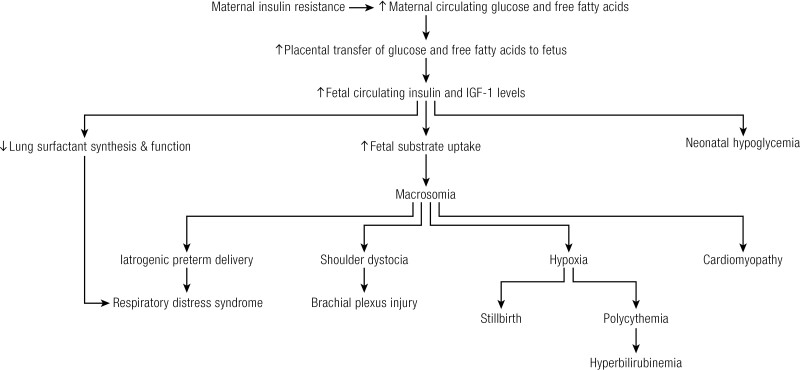
The Pedersen hypothesis describes the pathophysiology contributing to perinatal complications in GDM (229). Maternal hyperglycemia results in fetal hyperglycemia via facilitated diffusion of glucose by the glucose transporter 1 (GLUT1) (230). Fetal hyperglycemia results in fetal hyperinsulinemia, promoting fetal anabolism, excessive fetal adiposity, and accelerated growth, leading to LGA and macrosomia (231-239). Maternal hyperlipidemia also contributes to excess fetal growth (233,240). Macrosomia and LGA increase the risk of cesarean section, birth trauma, and perinatal complications including shoulder dystocia, brachial plexus injury and fracture, and perinatal asphyxia (27,132,237,238,241-243). Increased risk of perinatal asphyxia is associated with fetal death in utero, polycythemia, and hyperbilirubinemia (27,244-246). Fetal hyperinsulinemia can also increase the risk of metabolic abnormalities including neonatal hypoglycemia, hyperbilirubinemia, and respiratory distress syndrome postpartum (27,244). The risk appears to be greater among offspring of women with more severe hyperglycemia (247). Figure 2 summarizes the perinatal consequences of GDM.
Figure 2.
Perinatal consequences of gestational diabetes mellitus.
Short-term Risk
In the HAPO study, higher maternal glucose levels were associated with an increased risk of LGA, shoulder dystocia or birth injury, and neonatal hypoglycemia (27). A recent systematic review (n = 207 172) confirmed similar positive linear associations for maternal glycemia based on maternal glucose thresholds for the GCT, 75-g 2-hour OGTT, or 100-g 3-hour OGTT and risk of cesarean section, induction of labor (IOL), LGA, macrosomia, and shoulder dystocia (248). GDM has also been associated with an increased risk of preterm birth, birth trauma, neonatal respiratory distress syndrome, and hypertrophic cardiomyopathy (27,244,249). An increased risk of congenital malformations in the offspring has been reported, although whether this persists after adjustment for maternal age, BMI, ethnicity, and other contributing factors is unknown (250). A French cohort study (n = 796 346) reported a 30% higher risk of cardiac malformations in the offspring of women with GDM compared to women with normal glucose tolerance, after excluding women with likely undiagnosed pregestational diabetes (249). However, this increased risk only reached statistical significance in women treated with insulin therapy. Maternal BMI, which was not evaluated in these studies, may account for these findings (251,252). Similarly, a reported increase in perinatal mortality after 35 weeks’ gestation in the offspring of women with GDM may also be confounded by obesity (253-256). An increased risk of perinatal mortality after 37 weeks’ gestation was demonstrated in French women with GDM on dietary intervention, possibly because these women delivered later than women treated with insulin therapy (249). In contrast, the HAPO study did not demonstrate excess perinatal mortality in their untreated cohort (27).
Modern management of GDM and associated maternal risk factors is associated with near-normal birthweight in developed countries (115,257). This is important because birthweight is the major risk factor for shoulder dystocia, brachial plexus injury, neonatal hypoglycemia, and neonatal respiratory distress syndrome in the offspring of women with and without GDM (242). A retrospective cohort study of 36 241 pregnancies in the United States reported that the risk of shoulder dystocia among infants of women without GDM compared to women with GDM was 0.9% vs 1.6% if birthweight was <4000 g and 6.0% vs 10.5% if birthweight was ≥4000 g (macrosomia) (242). The risk of neonatal hypoglycemia in infants with birthweight < 4000 g was 1.2% vs 2.6% and 2.4% vs 5.3% for birthweight ≥ 4000 g, in women without GDM compared to women with GDM, respectively. Similar findings were seen for brachial plexus injury and neonatal respiratory distress syndrome. Thus, GDM confers increased risk of perinatal complications independent of birthweight.
The risk of stillbirth is also greater in women with GDM. A large US retrospective analysis examined stillbirth rates at various stages of gestation in over 4 million women, including 193 028 women with GDM. The overall risk of stillbirth from 36 to 42 weeks’ gestation was higher in women with GDM compared to women without GDM (17.1 vs 12.7 per 10 000 deliveries; RR 1.34; 95% CI 1.2-1.5) (253). This increased risk of stillbirth was also observed at each gestational week: 3.3 to 8.6 per 10 000 ongoing pregnancies in women with GDM compared to 2.1 to 6.4 per 10 000 ongoing pregnancies in women without GDM from 36 to 41 weeks’ gestation (253). For women with GDM, the relative risk of stillbirth was highest in week 37 (RR 1.84, 95% CI 1.5-2.3). Notably, the risk of stillbirth is highest in women with undiagnosed GDM. In a UK prospective case-control study (n = 1024), women with undiagnosed GDM based on a fasting glucose level ≥ 5.6mmol/L (≥100 mg/dL) had a 4-fold greater risk of late stillbirth (defined as occurring ≥28 weeks’ gestation) compared to women with fasting glucose < 5.6mmol/L (<100 mg/dL) (74). In contrast, women at risk of GDM based on NICE risk factors who were diagnosed with GDM on the OGTT had a similar risk of stillbirth to women who were not at risk of GDM. This suggests that diagnosing and managing GDM reduces the risk of stillbirth to near-normal levels (74).
Long-term Risk in the Offspring
Recent epidemiological studies suggest an increased risk of later adverse cardiometabolic sequelae in the offspring of women with GDM (227,258). A large Danish population-based cohort study (n = 2 432 000) demonstrated an association between maternal diabetes and an increased rate of early onset cardiovascular disease (CVD; ≤40 years of age) among offspring (259). GDM specifically was associated with a 19% increased risk of early onset CVD (95% CI 1.07-1.32). A longitudinal UK study provides potential mechanistic insight, finding that GDM was associated with alterations in fetal cardiac function and structure, with reduced systolic and diastolic ventricular function persisting in infancy (260). This is consistent with the association between in utero exposure to maternal hyperglycemia and fetal programming first reported in the Native American Pima population, characterized by a high prevalence of obesity, type 2 diabetes, and GDM (261).
The recent HAPO Follow Up Study (HAPO-FUS), which was not confounded by treatment of maternal glycemia, included 4832 children 10 to 14 years of age whose mothers were participants of HAPO (227). The HAPO-FUS demonstrated a durable impact of maternal glycemia with long-term offspring glucose metabolism, including at glucose levels lower than those diagnostic for GDM (227). A generally linear relationship between maternal antenatal glucose and offspring glucose levels and related outcomes was observed. Increasing maternal glucose categories were associated with a higher risk of impaired fasting glucose and impaired glucose tolerance and higher timed glucose measures and HbA1c levels and were inversely associated with insulin sensitivity and disposition index by 14 years of age, independent of maternal and childhood BMI and family history of diabetes (227). A positive association was observed between GDM defined by any criteria and glucose levels and impaired glucose tolerance in the offspring at ages 10 to 14 years and an inverse association with offspring insulin sensitivity (262). Higher frequencies of childhood obesity and measures of adiposity across increasing categories of maternal OGTT glucose levels were also noted (262). Recent evidence for increased glucose-linked hypothalamic activation in offspring aged 7 to 11 years previously exposed to maternal obesity and GDM in utero, which predicted higher subsequent BMI, represents 1 possible mechanism for this increased childhood obesity risk (263).
Maternal Complications
Short-term Risk
Women with GDM are at an increased risk of obstetric intervention including IOL, cesarean section (27-29,264,265), and complications associated with delivery including perineal lacerations and uterine rupture, predominantly relating to fetal macrosomia and polyhydramnios (266).
As demonstrated in HAPO and other studies, women with GDM also have an increased risk of gestational hypertension and preeclampsia (267-269). Consistent with the association between diabetes and microvascular disease, abnormalities in glucose metabolism affect trophoblast invasion, leading to impaired placentation and greater risk for preeclampsia (270). The mechanism likely relates to insulin resistance and inflammatory pathway activation (271,272), with in vitro studies showing that elevated glucose concentrations inhibit trophoblast invasiveness by preventing uterine plasminogen activator activity (272).
Long-term Maternal Risk Following GDM
Women diagnosed with GDM based on pre-IADPSG diagnostic criteria are at increased risk of GDM in future pregnancies, with reported recurrence rates of 30% to 84% (128). A diagnosis of GDM is also associated with up to a 20-fold greater lifetime risk of type 2 diabetes (273,274). A recent large meta-analysis and systematic review (20 studies, n = 1 332 373 including 67 956 women with GDM) showed that women with a history of GDM have a 10-fold increased risk of developing type 2 diabetes, mostly within the first 5 years post-GDM (273). HAPO-FUS demonstrated that over 50% of women whose OGTT thresholds met (untreated) IADPSG diagnostic criteria for GDM had developed impaired glucose tolerance after 14 years of follow-up (275). These data highlight the importance of a management approach to GDM that focuses on early prevention of type 2 diabetes. For example, the updated NICE guidelines now recommend diabetes prevention for all women with previous GDM (276,277).
Previous GDM is also associated with cardiovascular risk factors such as obesity, hypertension, and dyslipidemia (274,278-280). The lifetime risk of cardiovascular disease following GDM is almost 3-fold higher in women who develop type 2 diabetes and 1.5 fold higher even in women without type 2 diabetes (280). Studies also report a 26% greater risk of hypertension and a 43% greater risk of myocardial infarction or stroke in women with previous GDM compared to women without GDM (281,282). The significance of GDM as a risk factor for type 2 diabetes and cardiovascular disease has been recently recognized by international organizations including the American Heart Association (283).
Management of GDM
Benefits of Intervention on Perinatal Outcomes
Contemporary changes to the detection and management of GDM have been associated with almost comparable neonatal birthweight and adiposity outcomes to the background maternity population in developed countries (115).
The ACHOIS trial (n = 1000) was the first large RCT to evaluate whether treatment of women with GDM reduced the risk of perinatal complications (28). GDM was diagnosed based on a combination of fasting glucose < 7.8 mmol/L (140 mg/dL) and 2-hour postload glucose 7.8 to 11.0 mmol/L (140-199 mg/dL), respectively, using the 75-g 2-hour OGTT between 24 and 34 weeks’ gestation, following screening with either positive clinical risk factors or the GCT (28). ACHOIS demonstrated that a combination of dietary advice, self-monitoring of maternal glucose levels (SMBG), and insulin therapy, if required, to achieve SMBG targets [fasting glucose 3.5-5.5 mmol/L (63-99 mg/dL), preprandial glucose ≤ 5.5 mmol/L (99 mg/dL), and 2-hour postprandial glucose ≤ 7.0 mmol/L (126 mg/dL)], reduced the rate of serious perinatal complications (a composite of death, shoulder dystocia, nerve palsy, and fracture) compared to routine care (1% vs 4%; P = 0.01). In addition, such interventions were associated with a reduced incidence of macrosomia (10% vs 21%; P < 0.001), preeclampsia (12% vs 18%; P = 0.02), and improved maternal health-related quality of life (28).
In 2009, the MFMU trial (n = 958) reported that treatment of “mild” GDM was also associated with improved outcomes (29). Following a positive GCT between 24 and 30 + 6 weeks’ gestation, “mild” GDM was defined on a positive 100-g 3-hour OGTT by a fasting glucose < 5.3 mmol/L (95 mg/dL), and at least 2 postload glucose thresholds that exceeded the 2000 ADA diagnostic thresholds [1-, 2-, or 3-hour thresholds 10.0 mmol/L (180 mg/dL), 8.6 mmol/L (155 mg/dL), and 7.8 mmol/L (140 mg/dL), respectively]. Women with previous GDM were excluded from the study. Dietary intervention, SMBG, and insulin therapy, if required, to achieve a fasting glucose target < 5.3 mmol/L (95 mg/dL) and 2-hour postprandial glucose target < 6.7 mmol/L (121 mg/dL) was associated with reduced rates of macrosomia (5.9% vs 14.3%; P < 0.001), LGA (7.1% vs 14.5%; P < 0.001), shoulder dystocia (1.5% vs 4.0%; P = 0.02), cesarean section (26.9% vs 33.8%; P = 0.02), and preeclampsia and gestational hypertension (8.6% vs 13.6%; P = 0.01) compared to routine care. However, the intervention did not lead to a significant difference in the primary composite outcome of stillbirth, perinatal death, and neonatal complications (hyperbilirubinemia, hypoglycemia, hyperinsulinemia, and birth trauma) (29). Treatment targets in the MFMU trial were lower than that of the ACHOIS trial, and whether this may account for the reduction in cesarean section not shown in the ACHOIS trial is unclear. These key findings, supported by other studies (22,284), were highlighted by the IADPSG to support the lowering of the GDM diagnostic criteria and treating mild hyperglycemia (30).
A recent Cochrane review (8 RCTs; n = 1418) reported that GDM treatment, including dietary intervention and insulin therapy, reduced a composite outcome of perinatal morbidity (death, shoulder dystocia, bone fracture, and nerve palsy) by 68% compared to routine antenatal care (285). Treatment was also associated with reductions in macrosomia, LGA, and preeclampsia but an increase in IOL and neonatal intensive care admission.
Lifestyle Intervention
The main objective of GDM management is to attain maternal normoglycemia because evidence suggests that excessive fetal growth can be attenuated by maintaining near normal glucose levels (286,287). The foundation of this approach is medical nutrition therapy. Given carbohydrates are the primary determinant of maternal postprandial glucose levels, current dietary practice aims to modify carbohydrate quality (glycemic index) and distribution (32,288,289). The original nutritional approach for GDM decreased total carbohydrate intake to 33% to 40% of total energy intake (EI) and was associated with reduced postprandial glycemia and fetal overgrowth (290). More recent evidence suggests that higher carbohydrate intake and quality (lower glycemic index) between 60% and 70% EI can also limit maternal hyperglycemia (291-293). Nevertheless, there remain limited data to support a specific dietary intervention for GDM (294). A recent meta-analysis (18 RCTs; n = 1151) showed that enhancing nutritional quality (modified dietary intervention, defined as a dietary intervention different from the usual one used in the control group) after GDM diagnosis, irrespective of the specific dietary approach, improved maternal fasting and postprandial glycemia, and reduced pharmacotherapy requirements, birthweight, and macrosomia (295).
Guidelines therefore currently recommend a range of carbohydrate intake between 33% and 55% EI (32,288,289). Studies have reported improved pregnancy outcomes in GDM with both lower carbohydrate (42%E) and high‐carbohydrate (55%E) diets (296), reflected in the most recent Academy of Nutrition and Dietetics guidelines, which state that beneficial effects on pregnancy outcomes in GDM are seen with a range of carbohydrate intakes (288). The IOM guidelines recommend a carbohydrate intake of at least 175 g/day and a total daily caloric intake of 2000 to 2500 kilocalories during pregnancy (289). The ACOG recommends a lower carbohydrate diet (33-40%E) (297). However, the ADA has raised concerns over the corresponding higher maternal fat intake, fetal lipid exposure, and overgrowth resulting from lowering carbohydrate intake (298) and withdrew specific dietary guidelines for GDM in 2005 (299).
Given maternal glucose primarily supports fetal growth and brain development (300), theoretically if the maternal diet is too low in carbohydrate, the maternal-fetal glucose gradient may be compromised. Restriction of total maternal EI is associated with reduced fetal growth (301). A recent systematic review similarly showed that lower carbohydrate intake correlated with lower birthweight and greater incidence of SGA (302), with a lower carbohydrate threshold of 47% EI associated with appropriate fetal growth (302,303). Importantly, the lower carbohydrate threshold independent of energy restriction in GDM is yet to be established. Related safety concerns with lower carbohydrate diets include the potential risk of higher fetal exposure to maternal ketones (304) and micronutrient deficiency (305,306). In vitro studies have shown that ketones suppress trophoblast uptake of glucose, jeopardizing glucose transfer across the placenta (307). Clinically, a prospective US cohort study of women with preexisting diabetes, GDM, or normal glucose tolerance demonstrated an inverse correlation between higher maternal third trimester beta-hydroxybutyrate and FFAs and lower offspring intellectual development scores at 2 to 5 years of age, although total carbohydrate, EI, and maternal BMI were not reported (304).
Gestational Weight Gain
The IOM has published recommendations for weight gain during pregnancy based on prepregnancy BMI (289), but no specific recommendations for weight gain in GDM exist (286). In women with overweight or obesity, studies have suggested that weight reduction or gain ≤ 5 kg increased the risk of SGA (308). A recent systematic review based on data from almost 740 000 women demonstrated that GWG of 5 kg to 9 kg in women with class I obesity (BMI 30-34.99 kg/m2), 1 to <5 kg for class II obesity (35-39.99 kg/m2), and no GWG for women with class III obesity (BMI ≥ 40kg/m2), minimized the combined risk of LGA, SGA, and cesarean section (309).
A meta-analysis (n = 88 599) evaluating the relationship between GWG and pregnancy outcomes in GDM specifically showed that GWG greater than the IOM recommendations was associated with an increased risk of pharmacotherapy, as well as of hypertensive disorders of pregnancy, cesarean section, LGA, and macrosomia (310). GWG below the IOM recommendations was protective for LGA (RR 0.71; 95% CI 0.56-0.90) and macrosomia (RR 0.57; 95% CI 0.40-0.83) and did not increase the risk of SGA (RR 1.40; 95% CI 0.86-2.27) (289). This suggests that GWG targets in GDM may need to be lower than the current recommendations for normal pregnancy. However, from a practical perspective, only 30% of women gained less than the recommended IOM GWG targets (310).
Maternal Glucose Targets
Fasting and postprandial glucose testing with either the 1- or 2-hour postprandial glucose value is recommended in women with GDM. The 1-hour postprandial glucose approximates to the peak glucose excursion in pregnancy in women without diabetes and those with type 1 diabetes (175). Studies have shown that the 1-hour postprandial peak glucose level correlates with amniotic fluid insulin levels, reflecting fetal hyperinsulism (311) and with fetal abdominal circumference in women with type 1 diabetes (286). An RCT that compared pre- to postprandial maternal SMBG values showed that titrating insulin therapy based on the 1-hour postprandial values was associated with improved maternal glycemic control and may better attenuate the risk of neonatal complications attributed to fetal hyperinsulinemia (312).
Treatment targets based on maternal SMBG levels vary internationally (Table 7). There is some suggestion that lower glucose targets may improve pregnancy outcomes in GDM (176,313,314), but this is yet to be evaluated in adequately powered RCTs. Conversely, lower glycemic targets may be associated with an increased risk of SGA (315-317) and maternal and fetal hypoglycemia (318,319). A small study evaluating stringent glycemic targets in 180 women with GDM failed to demonstrate additional benefits, with no differences in the rates of cesarean section, birthweight, macrosomia, or SGA in the offspring of women randomized to intensive [preprandial glucose ≤ 5.0 mmol/L (90 mg/dL) and 1-hour postprandial glucose ≤ 6.7 mmol/L (121 mg/dL)] compared to standard treatment targets [preprandial glucose ≤ 5.8 mmol/L (104.5 mg/dL) and 1-hour postprandial glucose ≤ 7.8 mmol/L (140 mg/dL)] (320).
Table 7.
Recommended glycemic treatment targets in GDM
| Fasting plasma glucose (mmol/L) | Preprandial plasma glucose (mmol/L) | 1-hour post-prandial plasma glucose (mmol/L) | 2-h post-prandial plasma glucose (mmol/L) | |
|---|---|---|---|---|
| ADIPS (33) | ≤5.0 | ≤7.4 | ≤6.7 | |
| ADA (41) CDA (42) |
≤5.3 | ≤7.8 | ≤6.7 | |
| NICE (38) | <5.3 | <7.8 | <6.4 | |
| ACHOIS (37) | 3.5-5.5 | ≤5.5 | ≤7.0 | |
| MFMU (38) | <5.3 | <6.7 |
Abbreviations: ACHOIS, Australian Carbohydrate Intolerance Study in Pregnant Women Study; ADA, American Diabetes Association; ADIPS, Australasian Diabetes in Pregnancy Society; CDA, Canadian Diabetes Association; NICE, UK National Institute for Health and Care Excellence; MFMU, National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network.
Insulin Therapy
Insulin has traditionally been the preferred treatment for GDM if maternal glucose levels remain elevated on medical nutrition therapy (267). Depending on targets, approximately 50% of women with GDM are prescribed insulin therapy to maintain normoglycemia (321,322), with a combination of evening intermediate-acting insulin if fasting glucose levels are elevated and mealtime rapid-acting insulin when indicated. Additional daytime intermediate-acting insulin may also be needed to control prelunch or predinner hyperglycemia.
Decreasing insulin doses in the third trimester may simply reflect the physiological increase in maternal insulin sensitivity observed at this stage of pregnancy (176,323). However, substantial insulin dose reduction, recurrent maternal hypoglycemia, and/or slowing of fetal growth or preeclampsia may indicate underlying pathophysiological placental insufficiency (324), impacting the timing of delivery and intensity of obstetric monitoring.
Risk factors for insulin therapy include earlier diagnosis of GDM (81), the pattern and degree of elevation of the 75-g 2-hour OGTT diagnostic glucose thresholds (325), and ethnicity (325). Other risk factors including gestational age and HbA1c level at the time of GDM diagnosis, BMI, and family history of diabetes account for only 9% of the attributable risk for insulin therapy (321). A recent Australian study found that maternal age > 30 years, family history of diabetes, prepregnancy obesity, previous GDM, early diagnosis of GDM, fasting glucose ≥ 5.3 mmol/L (96 mg/dL) and HbA1c ≥ 5.5% (37 mmol/mol) at diagnosis were all independent predictors for insulin therapy (326). Insulin usage could also be estimated according to the number of predictors present, with up to 93% of women with 6 to 7 predictors using insulin therapy compared with less than 15% of women with 0 to 1 predictors (326).
Oral Pharmacotherapy
Oral pharmacotherapy options include glyburide and metformin. Oral pharmacotherapy is associated with improved cost effectiveness, compliance, and acceptability compared to insulin therapy (327). However, there are issues regarding efficacy and safety, particularly longer term, and thus insulin is generally preferred as first-line pharmacotherapy following lifestyle intervention.
Glyburide is commonly prescribed as first-line therapy for GDM in the United States (328). An early study evaluating the efficacy of glyburide vs insulin therapy in 404 women with GDM reported no differences in maternal glucose levels or neonatal outcomes between the treatment groups (329). However, subsequent studies show that approximately 20% of women treated with glyburide required additional insulin therapy to achieve adequate maternal glycemia (330). Moreover, a large retrospective US study of almost 111 000 women with GDM, in which 4982 women were treated with glyburide and 4191 women were treated with insulin, reported that glyburide was associated with an increased risk of neonatal complications including neonatal intensive care admission, respiratory distress syndrome, hypoglycemia, birth injury, and LGA compared to insulin therapy (331). Although transplacental transfer of glyburide to the fetus is highly variable, it can reach 50% to 70% of maternal plasma concentration (332), potentially causing direct stimulation of fetal insulin production (333).
The use of metformin in pregnancy continues to rise (334). However, its use remains controversial, due to the potential concerns regarding long-term metabolic programming effects of placental transfer of metformin to the fetus, with some studies suggesting similar plasma concentrations of metformin in the maternal and fetal circulation (335). A recent systematic review and meta-analysis of 28 studies (n = 3976) evaluating growth in offspring of women with GDM exposed to metformin compared to insulin therapy found that neonates exposed to metformin had lower birthweights (mean difference −107.7 g; 95% CI −182.3 to −32.7), decreased risk of LGA (OR 0.78; 95% CI 0.62-0.99), and macrosomia (OR 0.59; 95% CI 0.46-0.77) and lower ponderal indices than neonates whose mothers were treated with insulin (336). No difference in the risk of SGA was found, in contrast to outcomes in women with type 2 diabetes, with the Metformin in Women with Type 2 Diabetes RCT observing more than double the rate of SGA (95% CI 1.16-3.71) in the metformin treated cohort, in association with lower insulin doses, HbA1c, and GWG (337). Offspring of women with GDM exposed to metformin also demonstrate accelerated postnatal growth at 18 to 24 months of age (2 studies; n = 411; mean difference in weight 440 g; 95% CI 50-830), resulting in higher BMI at 5 to 9 years of age (3 studies; n = 520; BMI mean difference 0.78 kg/m2, 95% CI 0.23-1.33) (336).
The Metformin in Gestational Diabetes trial randomized 751 women to receive either metformin or insulin therapy, finding no significant difference in the composite neonatal outcome of neonatal hypoglycemia, respiratory distress syndrome, hyperbilirubinemia, low Apgar scores, birth trauma, and preterm birth (322). There was a trend toward increased preterm birth and decreased maternal GWG in women treated with metformin, while severe neonatal hypoglycemia was highest in those treated with insulin. Almost 50% of women treated with metformin required the addition of insulin therapy (322). Other studies have reported that between 14.0% and 55.8% of women treated with metformin also require insulin therapy to achieve optimal glycemic control (338,339). The Metformin in Gestational Diabetes: The Offspring Follow-Up 2-year follow-up study found that children exposed to metformin had increased subcutaneous fat localized to the arm compared with children whose mothers were treated with insulin alone (340). By 7 and 9 years of age the children exposed to metformin had similar offspring total and abdominal body fat percentage and metabolic biochemistry including fasting glucose, insulin, and lipids but were larger overall based on measures including weight, arm and waist circumference, waist-to-height ratio, and dual-energy X-ray absorptiometry fat mass and lean mass (341). These findings are consistent with a recent follow-up study of metformin therapy in pregnant women with polycystic ovary syndrome, which showed that children exposed to metformin in utero had higher BMI and rates of overweight and obesity at 4 years of age (342).
A recent Cochrane review (8 RCTs; n = 1487) evaluating the use of metformin, glyburide, and acarbose in women with GDM found that the benefits and potential harms of these therapies in comparison to each other are unclear (343). Other meta-analyses comparing glyburide, metformin, and insulin have shown that metformin was associated with lower GWG, gestational hypertension, and postprandial maternal glucose levels compared to either glyburide or insulin (344,345), but metformin was associated with an increased risk of preterm birth compared to insulin (345). Compared to metformin, glyburide was associated with a higher risk of increased birthweight, LGA, macrosomia, neonatal hypoglycemia, and increased GWG (344). More recently, a small RCT (n = 104) suggested that glyburide and metformin were comparable in terms of maternal glycemia and perinatal outcomes (346). Treatment success after second-line (oral) therapy was higher in the (first-line) metformin vs glyburide cohort (87% vs 50%; P = 0.03), suggesting that metformin may be the preferred first-line therapy. Overall, most women required either a combination of metformin and glyburide to achieve glycemic control and/or replacement of first-line oral therapy due to hypoglycemia and gastrointestinal side effects, suggesting neither agent alone is likely to be successful in most women with GDM. Combined oral pharmacotherapy had an efficacy rate of 89%, with only 11% of women required third-line therapy with insulin (346). However, the effects of dual oral therapy crossing the placenta on long-term potential fetal programming via their effects on cellular metabolism, hepatic gluconeogenesis, and insulin sensitivity (metformin) (347) and fetal hyperinsulinemia (glyburide) is unknown (348).
Obstetric Management
A recent Cochrane review consisting of only 3 small RCTs (n = 524) reported insufficient (very low certainty) evidence to evaluate the use of fetal biometry in guiding the medical management of GDM (349). Nevertheless, serial fetal growth ultrasounds, particularly assessing fetal abdominal circumference, are potentially useful in guiding the intensity of maternal glucose targets and insulin therapy (350-352). Studies have demonstrated that neonates with an estimated fetal weight ≥ 75th percentile on early third trimester ultrasound were 10-fold more likely to be LGA compared to neonates with an estimated fetal weight < 75th percentile (353). Measured fetal abdominal circumference < 90th percentile on 2 ultrasounds at 3- to 4-week intervals has also been shown to provide high reliability in excluding the risk of LGA (351). Moreover, a recent retrospective study (n = 275) found that estimated fetal weight or abdominal circumference up to the 30th percentile on third trimester ultrasound was associated with a greater risk of adverse neonatal outcomes, comparable to that observed with abdominal circumference or estimated fetal weight > 95th percentile in women with hyperglycemia in pregnancy (including GDM) (354). These findings suggest the potential utility of fetal biometry at thresholds other than defining SGA or LGA in identifying higher risk pregnancies in GDM.
The optimal timing of delivery in GDM is complex, guided by maternal glycemic control in addition to maternal and fetal factors, and has not been definitively established. Current guidelines recommend delivery by 40 + 6 weeks’ gestation in low-risk women with GDM managed with diet alone and from 39 + 0 to 39 + 6 weeks’ gestation for women with GDM well controlled with therapy (38,277,355). A recent Canadian population-based cohort study examining the week-specific risks of severe pregnancy complications in women with diabetes included 138 917 women with GDM and 2 553 243 women without diabetes over a 10-year period (356). There was no significant difference in gestational age-specific maternal mortality or morbidity (defined as ≥1 of the following in the immediate perinatal period: obstetric embolism, obstetric shock, postpartum hemorrhage with hysterectomy or other procedures to control bleeding, sepsis, thromboembolism, or uterine rupture) between iatrogenic delivery and expectant management in women with GDM. However, iatrogenic delivery was associated with an increased risk of neonatal mortality and morbidity (birth or fetal asphyxia, grade 3 or 4 intraventricular hemorrhage, neonatal convulsions, other disturbances of cerebral status of newborn, respiratory distress syndrome, birth injury, shoulder dystocia, stillbirth or neonatal death) at 36 to 37 weeks’ gestation (76.7 and 27.8 excess cases per 1000 deliveries, respectively) but a lower risk of neonatal morbidity and mortality at 38 to 40 weeks’ gestation (7.9, 27.3, and 15.9 fewer cases per 1000 deliveries, respectively) compared with expectant management, suggesting that delivery at 38, 39, or 40 weeks’ gestation may provide the best neonatal outcomes in women with GDM (356).
Longer Term Management of Women Following GDM
Up to one third of women with GDM diagnosed by pre-IADPSG criteria will have glucose levels consistent with diabetes or prediabetes on postpartum testing at 6 to 12 weeks (357). Thus, a repeat OGTT or fasting glucose as early as 6 to 12 weeks’ postpartum is recommended to confirm maternal glucose status (41,277). Only around 25% of women are tested at this time point with compliance with postpartum testing ranging between 23% and 58% (357,358). In women with GDM with overweight or obesity, a reduction in interpregnancy BMI of ≥2.0 kg/m2 reduces the risk of subsequent GDM by 74% (359). Longer term, women should perform regular cardiometabolic health assessment and optimization of lifestyle measures to reduce their greater risk of type 2 diabetes and cardiovascular disease (282,360,361). Up to 74% of women with obesity and previous GDM develop type 2 diabetes compared with <25% of women who achieve a normal BMI postpartum following GDM (362). It is unclear how relevant these studies in older women are for current clinical care given recent data that 50% of women develop type 2 diabetes within 5 to 10 years post-GDM diagnosis (273). The Diabetes Prevention Program demonstrated that lifestyle intervention and metformin therapy improved insulin sensitivity and preserved β-cell function in women with a history of previous GDM (363). Early type 2 diabetes prevention following GDM is therefore an essential component of the contemporary GDM detection and management paradigm (276).
Treatment of GDM and Long-term Offspring Outcomes
Importantly, despite a reduction in the risk of macrosomia at birth, the ACHOIS and MFMU follow-up studies did not demonstrate a beneficial impact on childhood obesity and glucose tolerance at 5 to 10 years of age in the offspring of women who received treatment for maternal hyperglycemia (364,365). Other prospective cohort studies similarly suggest that the offspring of women with treated GDM still have a greater risk of obesity, type 2 diabetes, the metabolic syndrome, and cardiovascular disease from early childhood and adolescence (258,366-380). For example, a 2017 Danish National Birth Cohort study (n = 561) reported increased adiposity, an adverse cardiometabolic profile, and earlier onset puberty among adolescent females of women with GDM (381). A prospective offspring cohort study of women with GDM who achieved good antenatal glycemic control demonstrated that offspring adiposity (adipose tissue quantity measured using magnetic resonance imaging) was similar in the GDM and normal glucose tolerance groups within 2 weeks postpartum but was 16.0% greater (95% CI 6.0-27.1; P = 0.002) by 2 months of age (382). The mechanism for this greater adiposity and rapid weight gain in early infancy is uncertain given both groups were predominantly breastfed. Consistent with the ACHOIS and MFMU follow-up studies (364,365), these data suggest that the current approach to glycemic control in GDM may not mitigate its impact on longer term infant health. Further, this pathway may be potentially mediated by excess infant adiposity, which correlates with childhood adiposity (383). Table 8 presents practical tips for managing women with GDM.
Table 8.
Practical tips for managing women with GDM
| Period | Tips |
|---|---|
| Preconception | All women should be encouraged to plan for pregnancy. Optimize modifiable risk factors prior to pregnancy (eg. BMI, diet, physical activity). Glucose assessment in high-risk women to detect undiagnosed preexisting glucose intolerance or diabetes. |
| During pregnancy | All pregnant women should be encouraged to have a nutritionally dense diet and undertake regular exercise during pregnancy unless there are obstetric contraindications. All pregnant women should be given personalized gestational weight gain targets and have their weight monitored at clinical reviews. High-risk women who have not undergone prepregnancy glucose assessment should be tested early for diabetes in pregnancy. Test all pregnant women without known diabetes/early GDM for GDM at 24 to 28 weeks’ gestation according to recommended screening and diagnostic criteria. GDM management ideally involves a multidisciplinary team with regular diabetes and obstetric assessment and includes patient education, lifestyle modification and support. Women should monitor their blood glucose levels. Pharmacotherapy, usually insulin, should be commenced if glucose levels are elevated despite lifestyle optimization. Metformin can be considered unless there are concerns with inadequate fetal growth. Timing of delivery is an individualized decision based on maternal and fetal well-being in addition to maternal glycemic control. |
| Postpartum | Early postpartum OGTT to assess glucose status. Regular long-term follow-up focused on diabetes and vascular risk factor modification and assessment to reduce subsequent risk of GDM, diabetes, and cardiovascular disease. Family lifestyle support, which includes optimizing diet, physical activity, and weight in the offspring. |
Abbreviations: GDM, gestational diabetes mellitus; OGTT, oral glucose tolerate test.
Precision Medicine in GDM: Physiological Heterogeneity, Subtype Classification, Risk Prediction, and Biomarker Utility
Precision medicine seeks to improve diagnostics, prognostics, prediction, and therapeutics in diabetes, including GDM, by evaluating and translating various biological axes including metabolomics, genomics, lipidomics, proteomics, technology, clinical risk factors and biomarkers, and mathematical and computer modeling into clinical practice (384). The Precision Medicine in Diabetes Initiative was launched in 2018 by the ADA, in partnership with the European Association for the Study of Diabetes, with their first consensus report published in 2020 (384).
In GDM, precision medicine represents the increasing understanding of heterogeneity within its genotype and phenotype (170,385-388) to identify and translate subclassification of GDM into more personalized clinical care (388). For example, physiologic subtypes of GDM based on the underlying mechanisms leading to maternal hyperglycemia have been recently characterized (386). Among 809 women from the Genetics of Glucose Regulation in Gestation and Growth pregnancy cohort, heterogeneity in the contribution of insulin resistance and deficiency to GDM were characterized based on validated indices of insulin sensitivity and secretory response measured during the 75-g OGTT performed between 24 and 30 weeks’ gestation (388). Compared to women with normal glucose tolerance, women with insulin resistant GDM (51% of GDM) had higher BMI and fasting glucose, hypertriglyceridemia, and hyperinsulinemia, larger infants, and almost double the risk of GDM-associated pregnancy complications. In contrast, women with predominantly insulin secretion defects had comparable BMI, fasting glucose, infant birthweight, and risk of adverse outcomes to those with normal glucose tolerance (388).
Other studies have also suggested that greater insulin resistance in GDM carries a higher risk of perinatal complications (389). A recent multicenter prospective study of 1813 women evaluating subtypes of GDM based on insulin resistance (389) found that women with GDM and high insulin resistance [n = 189 (82.9%)] had a higher BMI, systolic blood pressure, fasting glucose, and lipid levels in early pregnancy compared to women with normal glucose tolerance or those diagnoses with insulin-sensitive GDM. Insulin-sensitive women with GDM [n = 39 (17.1%)] had a significantly lower BMI than women with normal glucose tolerance but similar blood pressure, early pregnancy fasting glucose and lipid levels, and pregnancy outcomes. Despite no differences in insulin treatment and early postpartum glucose intolerance among the GDM subtypes, women with GDM and high insulin resistance had a greater than 2-fold risk of preterm birth and an almost 5-fold increased risk of neonatal hypoglycemia compared with women with normal glucose tolerance. This suggests the high insulin resistance GDM subtype has a greater risk of pregnancy complications potentially arising from the resultant fetal hyperinsulinemia (389).
The contemporary precision medicine approach to GDM also includes the increasing exploration of early pregnancy risk prediction and risk management models (390). The traditional binary clinical risk factor approach to identifying women at high risk in early pregnancy is limited by poor sensitivity and specificity, with studies showing that clinical risk factor-based screening fails to identify 10% to over 30% of women with GDM (391-396). The Pregnancy Outcome for Women with Pre-gestational Diabetes Along the Irish Atlantic Seaboard study found that the prevalence of women with GDM who had no risk factors was low, ranging from 2.7% to 5.4% (397). However, despite the absence of risk factors, these women with GDM had more pregnancy complications than those with normal glucose tolerance (397). Other studies have also reported that women without risk factors diagnosed with GDM have comparable pregnancy outcomes to women with GDM identified as high risk (393). Thus, clinical risk factors alone are not predictive of GDM risk for all women. Although some improvement in the predictive accuracy for GDM is seen in clinical risk scoring approaches (158,398), greater improvement via multivariate risk prediction and mathematical or computer models combining clinical risk factors and biomarkers have been reported in the GDM research setting (154-156,399-403).
Biomarkers are defined as a biological observation that substitutes and ideally predicts the clinically relevant endpoint (ie, GDM) (404). Biomarker discovery and application in the early detection of GDM has become a major research area. However, few biomarkers are specific enough for clinical application (405). Most novel biomarkers with potential utility for the prediction of GDM are involved in pathophysiological pathways related to insulin resistance, dyslipidemia, and type 2 diabetes (402,406) but are frequently mediated by maternal obesity (240,407). Early pregnancy risk prediction models for GDM combining clinical risk factors and biomarkers have included various measures of maternal glucose, lipids, adipokines, inflammatory markers, and pragmatic aneuploidy and preeclampsia screening markers, with model performance (area under the curve) up to 0.91 (153,154,399,402,403,408-416). Limitations to the clinical application of novel biomarkers and model performance include heterogeneity in the testing approach to GDM and cohort characteristics, potential overestimation of model performance due to overfitting of the data to the index study population, the lack of external clinical validation studies, and limited regulatory guidance for validating biomarker assays (405).
The COVID-19 Pandemic and GDM
The COVID-19 pandemic has led to dynamic changes in the testing approach and model of care for women with GDM to minimize the risk of virus transmission and because of decreased clinical capacity. Several temporary pragmatic diagnostic strategies have been suggested as an alternative to the OGTT, including measurement of fasting plasma glucose, random plasma glucose, and HbA1c (417-419). A secondary analysis of 5974 women from the HAPO study (420), reported that the UK, Canadian, and Australian COVID-19–modified diagnostic approaches reduced the frequency of GDM by 81%, 82%, and 25%, respectively. Short-term pregnancy complications in the subgroup of women now with undiagnosed GDM (“missed GDM”) were comparable to women diagnosed with GDM based on the Canadian-modified diagnostic criteria, slightly lower for the UK-modified criteria, but significantly lower for the Australasian Diabetes in Pregnancy Association–modified criteria. While all approaches recommend universal testing, the Australian approach adopts a lower fasting glucose threshold of 4.7 mmol/L to identify women who require an OGTT and does not include HbA1c measurement (420). A retrospective UK study of over 18 000 women sought to define evidence-based recommendations for pragmatic GDM testing during the COVID-19 pandemic (421), reporting that ~5% of women would be identified as GDM based on a random glucose threshold ≥ 8.5 mmol/L (153 mg/dL) at 12 weeks’ gestation and fasting glucose ≥ 5.2 to 5.4 mmol/L (94-97 mg/dL) or HbA1c ≥ 5.7% (39 mmol/mol) measured at 28 weeks’ gestation. Each test predicted some, but not all, obstetric and perinatal complications, lacking the sensitivity of the OGTT for the diagnosis of GDM but overall may provide adequate risk stratification where the OGTT is not feasible (421).
Conclusion
GDM is one of the most common complications of pregnancy and is increasing in global prevalence. Diagnosing GDM is important because perinatal complications and stillbirth risk are reduced by treatment. Despite the benefit of identifying and treating GDM, much of the current (short-term) diagnostic and management approach to GDM remains contentious. These differences confound interpretation and application of trial data, preventing a single standard international approach to GDM.
Recent data indicates near normal birthweight and maternity population outcomes in women with GDM based on modern IADPSG criteria in developed countries, demonstrating that even treatment of “milder” maternal hyperglycemia improves pregnancy outcomes. However, most cases of GDM occur in low- and middle-income countries where perinatal risks are far greater and universal 1-step testing may be more practical. There are limited RCT data to guide diagnosis and management in this setting, and further evidence is urgently needed. In developed countries including the United Kingdom, the main issue arguably does not pertain to women diagnosed with GDM but rather high-risk women who remain unscreened (associated with factors such as lower socioeconomic status and higher BMI) who are at highest risk of stillbirth (74).
The background to the various GDM diagnostic criteria is informative in demonstrating that no approach clearly separates risk groups. It is also now evident that a continuum of risk for GDM exists based on both the timing and degree of maternal hyperglycemia. This underscores the difficulty of defining absolute glucose thresholds at a single timepoint in pregnancy for the diagnosis of GDM and is confounded further by variation in glucose measurement due to preanalytical glucose processing and reproducibility issues. Thus, current diagnostic glucose thresholds for GDM must inevitably reflect compromise and consensus.
A precision medicine approach that recognizes GDM subtype and heterogeneity, enhanced by further research into the genetics of GDM and validation of novel biomarkers and new technologies such as continuous glucose monitoring may improve risk stratification, optimize clinical models of care, and facilitate more individualized and consumer-friendly detection and treatment strategies.
The recent HAPO-FUS data confirming the long-term impact of maternal hyperglycemia on maternal and offspring metabolic health (227,262) highlight an important paradigm shift. The approach to GDM should reflect an evidence base that evaluates diagnostic glucose thresholds and measurement within a framework that includes timing of detection and treatment trials with long-term clinical and health economic outcomes. For example, if the ongoing Treatment of Booking Gestational Diabetes Mellitus trial demonstrates a benefit for early GDM detection and treatment, there are implications for the prevailing diagnostic GDM glucose thresholds in later pregnancy. This is because these thresholds were derived from the risk of perinatal complications in a heterogeneous GDM cohort, which included women who would fulfill early GDM criteria.
Other important areas for research include the evaluation of dietary interventions establishing the optimal carbohydrate threshold in GDM, further clarity on the potential long-term impact of intrauterine metformin on the offspring, as well as the efficacy of preconception and early pregnancy preventive strategies targeting risk factors other than glycemia, such as maternal obesity and GWG. Improved obstetric assessment of placental function, especially in late pregnancy, to inform timing of delivery and identify women at highest risk of stillbirth in GDM is also needed.
The complications of GDM may indeed be greater based on the severity of maternal glycemia and associated vascular risk factors. Nevertheless, the traditional focus on diagnostic criteria and short-term antenatal maternal glucose management fails to address the importance of identifying “milder” (IADPSG-defined) GDM as a risk factor for future maternal and offspring diabetes and CVD risk. It should also be apparent that the increasing prevalence of GDM largely reflects the worsening metabolic health burden including prediabetes and obesity in women of childbearing age. The clinical focus for GDM must therefore urgently shift to early postnatal prevention strategies to decrease the progression from GDM to type 2 diabetes and address longer term maternal and offspring cardiometabolic risk post-GDM via a life course management approach.
Contributor Information
Arianne Sweeting, Department of Endocrinology, Royal Prince Alfred Hospital, Sydney, Australia; Faculty of Medicine and Health, University of Sydney, Sydney, Australia.
Jencia Wong, Department of Endocrinology, Royal Prince Alfred Hospital, Sydney, Australia; Faculty of Medicine and Health, University of Sydney, Sydney, Australia.
Helen R Murphy, Diabetes in Pregnancy Team, Cambridge University Hospitals, Cambridge, UK; Norwich Medical School, Bob Champion Research and Education Building, University of East Anglia, Norwich, UK; Division of Women’s Health, Kings College London, London, UK.
Glynis P Ross, Department of Endocrinology, Royal Prince Alfred Hospital, Sydney, Australia; Faculty of Medicine and Health, University of Sydney, Sydney, Australia.
Financial Support
A.S. was supported by an NHMRC Fellowship Grant (GNT1148952).
Disclosure Summary
A.S., J.W., H.M., and G.P.R. have nothing to declare.
References
- 1. Bennewitz H. De Diabete Mellito, Gravidatatis Symptomate. MD thesis, University of Berlin; 1824. [Google Scholar]
- 2. Barker DJP. Mothers Babies and Diseases in Later Life. BMJ; 1994. [Google Scholar]
- 3. Duncan J. On puerperal diabetes. Trans Obstet Soc Lond. 1882;24:256-285. [Google Scholar]
- 4. Miller HC. The effect of diabetic and prediabetic pregnancies on the fetus and newborn infant. J Pediatr. 1946;29(4):455-461. [DOI] [PubMed] [Google Scholar]
- 5. Williams J. The clinical significance of glycosuria in pregnant women. Am J Med Sci. 1909;137:1-26. [Google Scholar]
- 6. O’Sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964;13:278-285. [PubMed] [Google Scholar]
- 7. Coustan DR. Gestational diabetes. In: Harris MI, Cowie CC, Stern MP, Boyko EJ, Reiber GE, Bennett PH, eds. Diabetes in America. National Institute of Health; 1995:703-717. [Google Scholar]
- 8. World Health Organization. Technical Report Series. No 310. Diabetes Mellitus. Report of a WHO Expert Committee. 1965. [PubMed] [Google Scholar]
- 9. World Health Organization. Technical Report Series. No 646. Second Report on Diabetes Mellitus. Report of a WHO Expert Committee. 1980. [PubMed] [Google Scholar]
- 10. World Health Organization. Technical Report Series. No 727. Second Report on Diabetes Mellitus. Report of a WHO Expert Committee. 1985. [Google Scholar]
- 11. World Health Organisation. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization guideline. Diabetes Res Clin Pract. 2014;103(3):341-363. [DOI] [PubMed] [Google Scholar]
- 12. O’Sullivan JB, Mahan CM, Charles D, Dandrow RV. Screening criteria for high-risk gestational diabetic patients. Am J Obstet Gynecol. 1973;116(7):895-900. [DOI] [PubMed] [Google Scholar]
- 13. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28(12):1039-1057. [DOI] [PubMed] [Google Scholar]
- 14. American College of Obstetricians and Gynecologists. Management of Diabetes Mellitus During Pregnancy. Technical Bulletin No. 92. 1986. [Google Scholar]
- 15. American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 1986;9(4):430-431. [PubMed] [Google Scholar]
- 16. Ferrara A, Hedderson MM, Quesenberry CP, Selby JV. Prevalence of gestational diabetes mellitus detected by the National Diabetes Data Group or the Carpenter and Coustan plasma glucose thresholds. Diabetes Care. 2002;25(9):1625-1630. [DOI] [PubMed] [Google Scholar]
- 17. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144(7):768-773. [DOI] [PubMed] [Google Scholar]
- 18. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(suppl 1):S13-S27. [DOI] [PubMed] [Google Scholar]
- 19. American College of Obstetricians and Gynecologists. Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):e49-e64. [DOI] [PubMed] [Google Scholar]
- 20. American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2000;23(suppl 1):S77-S79. [PubMed] [Google Scholar]
- 21. Sermer M, Naylor CD, Farine D, et al. . The Toronto tri-hospital gestational diabetes project: a preliminary review. Diabetes Care. 1998;21(Suppl 2):B33-B42. [PubMed] [Google Scholar]
- 22. Sermer M, Naylor CD, Gare DJ, et al. . Impact of increasing carbohydrate intolerance on maternal-fetal outcomes in 3637 women without gestational diabetes: the Toronto Tri-Hospital Gestational Diabetes Project. Am J Obstet Gynecol. 1995;173(1):146-156. [DOI] [PubMed] [Google Scholar]
- 23. Berggren EK, Boggess KA, Stuebe AM, Jonsson Funk M. National Diabetes Data Group vs Carpenter-Coustan criteria to diagnose gestational diabetes. Am J Obstet Gynecol. 2011;205(3):253 e1-253.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheng YW, Block-Kurbisch I, Caughey AB. Carpenter-Coustan criteria compared with the National Diabetes Data Group thresholds for gestational diabetes mellitus. Obstet Gynecol. 2009;114(2 Pt 1):326-332. [DOI] [PubMed] [Google Scholar]
- 25. Chou CY, Lin CL, Yang CK, et al. . Pregnancy outcomes of Taiwanese women with gestational diabetes mellitus: a comparison of Carpenter-Coustan and National Diabetes Data Group criteria. J Womens Health (Larchmt). 2010;19(5):935-939. [DOI] [PubMed] [Google Scholar]
- 26. American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2003;26(suppl 1):S103-S105. [DOI] [PubMed] [Google Scholar]
- 27. Metzger BE, Lowe LP, Dyer AR, et al. ; HAPO Study Cooperative Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991-2002. [DOI] [PubMed] [Google Scholar]
- 28. Crowther CA, Hiller JE, Moss JR, et al. . Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477-2486. [DOI] [PubMed] [Google Scholar]
- 29. Landon MB, Spong CY, Thom E, et al. . A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Metzger BE, Gabbe SG, Persson B, et al. ; International Association of Diabetes and Pregnancy Study Groups Consensus Panel . International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blumer I, Hadar E, Hadden DR, et al. . Diabetes and pregnancy: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hod M, Kapur A, Sacks DA, et al. . The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(suppl 3):S173-S211. [DOI] [PubMed] [Google Scholar]
- 33. Nankervis A, McIntyre HD, Moses R, et al. . ADIPS Consensus Guidelines for the Testing and Diagnosis of Hyperglycaemia in Pregnancy in Australia and New Zealand. Modified November 2014. http://adips.org/downloads/2014ADIPSGDMGuidelinesV18.11.2014_000.pdf. Accessed June 1, 2021.
- 34. Japan Diabetes Society. Evidence-based practice guideline for the treatment for diabetes in Japan 2013. Last updated November 26, 2020. http://www.jds.or.jp/modules/en/index.php?content_id=44. Accessed June 1, 2021.
- 35. Yang HX. Diagnostic criteria for gestational diabetes mellitus. Chin Med J. 2012;125(7):1212-1213. [PubMed] [Google Scholar]
- 36. Benhalima K, Mathieu C, Damm P, et al. . A proposal for the use of uniform diagnostic criteria for gestational diabetes in Europe: an opinion paper by the European Board & College of Obstetrics and Gynaecology (EBCOG). Diabetologia. 2015;58(7):1422-1429. [DOI] [PubMed] [Google Scholar]
- 37. Vandorsten JP, Dodson WC, Espeland MA, et al. . NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. 2013;29(1):1-31. [PubMed] [Google Scholar]
- 38. National Institute for Health and Care Excellence. Diabetes in Pregnancy: Management of Diabetes and its Complications from Pre-conception to the Postnatal Period. NICE Clinical Guideline NG3. 2015. [PubMed] [Google Scholar]
- 39. Vambergue A. Expert consensus on gestational diabetes mellitus. Diabetes Metab. 2010;36(6 Pt 2):511. [DOI] [PubMed] [Google Scholar]
- 40.Associazione Medici Diabetologi and Società Italiana di Diabetologia. Italian National Health System Guidelines for the screening of gestational diabetes mellitus. May 28, 2014. http://www.standarditaliani.it/skin/www.standarditaliani.it/pdf/STANDARD_2014_May28.pdf. Accessed June 1, 2021.
- 41. American Diabetes Association. 14. Management of diabetes in pregnancy: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S183-SS92. [DOI] [PubMed] [Google Scholar]
- 42. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Clinical practice guidelines for the prevention and management of diabetes in Canada. Canadian J Diabetes. 2013;37(suppl 1):S1-S3. [DOI] [PubMed] [Google Scholar]
- 43. Kleinwechter H, Schafer-Graf U, Buhrer C, et al. . Gestational diabetes mellitus (GDM) diagnosis, therapy and follow-up care: practice Guideline of the German Diabetes Association (DDG) and the German Association for Gynaecology and Obstetrics (DGGG). Exp Clin Endocrinol Diabetes. 2014;122(7):395-405. [DOI] [PubMed] [Google Scholar]
- 44. Seshiah V, Das AK, Balaji V, et al. . Gestational diabetes mellitus—guidelines. J Assoc Physicians India. 2006;54:622-628. [PubMed] [Google Scholar]
- 45. World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Report of WHO Consultation. 1999. [Google Scholar]
- 46. Coustan DR, Lowe LP, Metzger BE, et al. ; on behalf of the International Association of Diabetes in Pregnancy Study Groups. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. Am J Obstet Gynecol. 2010;202(6):654 e1-654 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McIntyre HD, Oats JJ, Kihara AB, et al. . Update on diagnosis of hyperglycemia in pregnancy and gestational diabetes mellitus from FIGO’s Pregnancy & Non-Communicable Diseases Committee. Int J Gynaecol Obstet. 2021;154(2):189-194. [DOI] [PubMed] [Google Scholar]
- 48. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):1021-1029. [DOI] [PubMed] [Google Scholar]
- 49. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. US Department of Health and Human Services; 2020. [Google Scholar]
- 50. Lieberman N, Kalter-Leibovici O, Hod M. Global adaptation of IADPSG recommendations: a national approach. Int J Gynaecol Obstet. 2011;115(suppl 1):S45-S47. [DOI] [PubMed] [Google Scholar]
- 51. Marseille E, Lohse N, Jiwani A, et al. . The cost-effectiveness of gestational diabetes screening including prevention of type 2 diabetes: application of a new model in India and Israel. J Matern Fetal Neonatal Med. 2013;26(8):802-810. [DOI] [PubMed] [Google Scholar]
- 52. Werner EF, Pettker CM, Zuckerwise L, et al. . Screening for gestational diabetes mellitus: are the criteria proposed by the International Association of the Diabetes and Pregnancy Study Groups cost-effective? Diabetes Care. 2012;35(3):529-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mission JF, Ohno MS, Cheng YW, Caughey AB. Gestational diabetes screening with the new IADPSG guidelines: a cost-effectiveness analysis. Am J Obstet Gynecol. 2012;207(4):326 e1-326 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Farrar D, Simmonds M, Griffin S, et al. . The identification and treatment of women with hyperglycaemia in pregnancy: an analysis of individual participant data, systematic reviews, meta-analyses and an economic evaluation. Health Technol Assess. 2016;20(86):1-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jacklin PB, Maresh MJ, Patterson CC, et al. . A cost-effectiveness comparison of the NICE 2015 and WHO 2013 diagnostic criteria for women with gestational diabetes with and without risk factors. BMJ Open. 2017;7(8):e016621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cundy T, Ackermann E, Ryan EA. Gestational diabetes: new criteria may triple the prevalence but effect on outcomes is unclear. BMJ. 2014;348:g1567. [DOI] [PubMed] [Google Scholar]
- 57. O’Sullivan EP, Avalos G, O’Reilly M, et al. . Atlantic Diabetes in Pregnancy (DIP): the prevalence and outcomes of gestational diabetes mellitus using new diagnostic criteria. Diabetologia. 2011;54(7):1670-167 5. [DOI] [PubMed] [Google Scholar]
- 58. Lapolla A, Dalfra MG, Ragazzi E, et al. . New International Association of the Diabetes and Pregnancy Study Groups (IADPSG) recommendations for diagnosing gestational diabetes compared with former criteria: a retrospective study on pregnancy outcome. Diabet Med. 2011;28(9):1074-1077. [DOI] [PubMed] [Google Scholar]
- 59. Benhalima K, Hanssens M, Devlieger R, et al. . Analysis of pregnancy outcomes using the new IADPSG recommendation compared with the Carpenter and Coustan criteria in an area with a low prevalence of gestational diabetes. Int J Endocrinol. 2013;2013:248121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hung TH, Hsieh TT. The effects of implementing the International Association of Diabetes and Pregnancy Study Groups criteria for diagnosing gestational diabetes on maternal and neonatal outcomes. PLoS One. 2015;10(3):e0122261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Meek CL, Lewis HB, Patient C, et al. . Diagnosis of gestational diabetes mellitus: falling through the net. Diabetologia. 2015;58(9):2003-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Djelmis J, Pavic M, Mulliqi Kotori V, et al. . Prevalence of gestational diabetes mellitus according to IADPSG and NICE criteria. Int J Gynaecol Obstet. 2016;135(3):250-254. [DOI] [PubMed] [Google Scholar]
- 63. Ethridge JK Jr, Catalano PM, Waters TP. Perinatal outcomes associated with the diagnosis of gestational diabetes made by the international association of the diabetes and pregnancy study groups criteria. Obstet Gynecol. 2014;124(3):571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hillier TA, Pedula KL, Ogasawara KK, et al. . A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384(10):895-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Casey B. Gestational diabetes—on broadening the diagnosis. N Engl J Med. 2021;384(10):965-966. [DOI] [PubMed] [Google Scholar]
- 66. Dunne F, Lindsay R, Loeken M. This is the decade to find the solution for gestational diabetes mellitus screening and treatments. Diabet Med. 2021;38(8):e14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. O’Malley EG, Reynolds CME, O’Kelly R, et al. . The diagnosis of gestational diabetes mellitus (GDM) using a 75 g oral glucose tolerance test: a prospective observational study. Diabetes Res Clin Pract. 2020;163:108144. [DOI] [PubMed] [Google Scholar]
- 68. van Leeuwen M, Louwerse MD, Opmeer BC, et al. . Glucose challenge test for detecting gestational diabetes mellitus: a systematic review. BJOG. 2012;119(4):393-401. [DOI] [PubMed] [Google Scholar]
- 69. Sacks DA, Hadden DR, Maresh M, et al. . Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study. Diabetes Care. 2012;35(3):526-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Roeckner JT, Sanchez-Ramos L, Jijon-Knupp R, et al. . Single abnormal value on 3-hour oral glucose tolerance test during pregnancy is associated with adverse maternal and neonatal outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;215(3):287-297. [DOI] [PubMed] [Google Scholar]
- 71. Freinkel N, Metzger BE, Phelps RL, et al. . Gestational diabetes mellitus: heterogeneity of maternal age, weight, insulin secretion, HLA antigens, and islet cell antibodies and the impact of maternal metabolism on pancreatic B-cell and somatic development in the offspring. Diabetes. 1985;34(suppl 2):1-7. [DOI] [PubMed] [Google Scholar]
- 72. Schaefer UM, Songster G, Xiang A, et al. . Congenital malformations in offspring of women with hyperglycemia first detected during pregnancy. Am J Obstet Gynecol. 1997;177(5):1165-1171. [DOI] [PubMed] [Google Scholar]
- 73. Omori Y, Jovanovic L. Proposal for the reconsideration of the definition of gestational diabetes. Diabetes Care. 2005;28(10):2592-2593. [DOI] [PubMed] [Google Scholar]
- 74. Stacey T, Tennant P, McCowan L, et al. . Gestational diabetes and the risk of late stillbirth: a case-control study from England, UK. BJOG. 2019;126(8):973-982. [DOI] [PubMed] [Google Scholar]
- 75. Cosson E, Bentounes SA, Nachtergaele C, et al. . Prognosis associated with sub-types of hyperglycaemia in pregnancy. J Clin Med. 2021;10(17):3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wong T, Ross GP, Jalaludin BB, Flack JR. The clinical significance of overt diabetes in pregnancy. Diabet Med. 2013;30(4):468-474. [DOI] [PubMed] [Google Scholar]
- 77. Bartha JL, Martinez-Del-Fresno P, Comino-Delgado R. Early diagnosis of gestational diabetes mellitus and prevention of diabetes-related complications. Eur J Obstet Gynecol Reprod Biol. 2003;109(1):41-44. [DOI] [PubMed] [Google Scholar]
- 78. Bartha JL, Martinez-Del-Fresno P, Comino-Delgado R. Gestational diabetes mellitus diagnosed during early pregnancy. Am J Obstet Gynecol. 2000;182(2):346-350. [DOI] [PubMed] [Google Scholar]
- 79. Berkowitz GS, Roman SH, Lapinski RH, et al. . Maternal characteristics, neonatal outcome, and the time of diagnosis of gestational diabetes. Am J Obstet Gynecol. 1992;167(4 Pt 1):976-8282. [DOI] [PubMed] [Google Scholar]
- 80. Meyer WJ, Carbone J, Gauthier DW, et al. . Early gestational glucose screening and gestational diabetes. J Reprod Med. 1996;41(9):675-679. [PubMed] [Google Scholar]
- 81. Sweeting AN, Ross GP, Hyett J, et al. . Gestational diabetes mellitus in early pregnancy: evidence for poor pregnancy outcomes despite treatment. Diabetes Care. 2016;39(1):75-81. [DOI] [PubMed] [Google Scholar]
- 82. Li M, Hinkle SN, Grantz KL, et al. . Glycaemic status during pregnancy and longitudinal measures of fetal growth in a multi-racial US population: a prospective cohort study. Lancet Diabetes Endocrinol. 2020;8(4):292-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sovio U, Murphy HR, Smith GC. Accelerated fetal growth prior to diagnosis of gestational diabetes mellitus: a prospective cohort study of nulliparous women. Diabetes Care. 2016;39(6):982-987. [DOI] [PubMed] [Google Scholar]
- 84. Venkataraman H, Ram U, Craik S, et al. . Increased fetal adiposity prior to diagnosis of gestational diabetes in South Asians: more evidence for the “thin-fat” baby. Diabetologia. 2017;60(3):399-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Riskin-Mashiah S, Damti A, Younes G, et al. . Normal fasting plasma glucose levels during pregnancy: a hospital-based study. J Perinat Med. 2011;39(2):209-211. [DOI] [PubMed] [Google Scholar]
- 86. Mills JL, Jovanovic L, Knopp R, et al. . Physiological reduction in fasting plasma glucose concentration in the first trimester of normal pregnancy: the diabetes in early pregnancy study. Metabolism. 1998;47(9):1140-1144. [DOI] [PubMed] [Google Scholar]
- 87. Corrado F, D’Anna R, Cannata ML, et al. . Correspondence between first-trimester fasting glycaemia, and oral glucose tolerance test in gestational diabetes diagnosis. Diabetes Metab. 2012;38(5):458-61. [DOI] [PubMed] [Google Scholar]
- 88. Zhu WW, Yang HX, Wei YM, et al. . Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in china. Diabetes Care. 2013;36(3):586-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. McIntyre HD, Sacks DA, Barbour LA, et al. . Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39(1):53-54. [DOI] [PubMed] [Google Scholar]
- 90. Hughes RC, Moore MP, Gullam JE, et al. . An early pregnancy HbA1c ≤5.9% (41 mmol/mol) is optimal for detecting diabetes and identifies women at increased risk of adverse pregnancy outcomes. Diabetes Care. 2014;37(11):2953-2959. [DOI] [PubMed] [Google Scholar]
- 91. Sweeting AN, Ross GP, Hyett J, et al. . Baseline HbA1c to identify high-risk gestational diabetes: utility in early vs standard gestational diabetes. J Clin Endocrinol Metab. 2017;102(1):150-156. [DOI] [PubMed] [Google Scholar]
- 92. Immanuel J, Simmons D, Desoye G, et al. . Performance of early pregnancy HbA1c for predicting gestational diabetes mellitus and adverse pregnancy outcomes in obese European women. Diabetes Res Clin Pract. 2020;168:108378. [DOI] [PubMed] [Google Scholar]
- 93. Osmundson SS, Norton ME, El-Sayed YY, et al. . Early screening and treatment of women with prediabetes: a randomized controlled trial. Am J Perinatol. 2016;33(2):172-179. [DOI] [PubMed] [Google Scholar]
- 94. Roeder HA, Moore TR, Wolfson T, Ramos GA. Treating hyperglycemia in the first trimester: a randomized controlled trial. Am J Obstet Gynecol MFM. 2017;1(1):33-41. [DOI] [PubMed] [Google Scholar]
- 95. Hawkins JS, Lo JY, Casey BM, et al. . Diet-treated gestational diabetes mellitus: comparison of early vs routine diagnosis. Am J Obstet Gynecol. 2008;198(3):287 e1-287 e6. [DOI] [PubMed] [Google Scholar]
- 96. Most OL, Kim JH, Arslan AA, et al. . Maternal and neonatal outcomes in early glucose tolerance testing in an obstetric population in New York city. J Perinat Med. 2009;37(2):114-117. [DOI] [PubMed] [Google Scholar]
- 97. Gupta S, Dolin C, Jadhav A, et al. . Obstetrical outcomes in patients with early onset gestational diabetes. J Matern Fetal Neonatal Med. 2016;29(1):27-31. [DOI] [PubMed] [Google Scholar]
- 98. Harreiter J, Simmons D, Desoye G, et al. . IADPSG and WHO 2013 gestational diabetes mellitus criteria identify obese women with marked insulin resistance in early pregnancy. Diabetes Care. 2016;39(7):e90-e92. [DOI] [PubMed] [Google Scholar]
- 99. Egan AM, Vellinga A, Harreiter J, et al. . Epidemiology of gestational diabetes mellitus according to IADPSG/WHO 2013 criteria among obese pregnant women in Europe. Diabetologia. 2017;60(10):1913-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Immanuel J, Simmons D. Screening and treatment for early-onset gestational diabetes mellitus: a systematic review and meta-analysis. Curr Diab Rep. 2017;17(11):115. [DOI] [PubMed] [Google Scholar]
- 101. Bozkurt L, Gobl CS, Pfligl L, et al. . Pathophysiological characteristics and effects of obesity in women with early and late manifestation of gestational diabetes diagnosed by the International Association of Diabetes and Pregnancy Study Groups criteria. J Clin Endocrinol Metab. 2015;100(3):1113-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bozkurt L, Gobl CS, Hormayer AT, et al. . The impact of preconceptional obesity on trajectories of maternal lipids during gestation. Sci Rep. 2016;6:29971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sweeting AN, Ross GP, Hyett J, et al. . Gestational diabetes in the first trimester: is early testing justified? Lancet Diabetes Endocrinol. 2017;5(8):571-573. [DOI] [PubMed] [Google Scholar]
- 104. Harper LM, Jauk V, Longo S, et al. . Early gestational diabetes screening in obese women: a randomized controlled trial. Am J Obstet Gynecol. 2020;222(5):495 e1-495 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Vinter CA, Tanvig MH, Christensen MH, et al. . Lifestyle intervention in Danish obese pregnant women with early gestational diabetes mellitus according to WHO 2013 criteria does not change pregnancy outcomes: results from the LiP (Lifestyle in Pregnancy) study. Diabetes Care. 2018;41(10):2079-2085. [DOI] [PubMed] [Google Scholar]
- 106. Simmons D, Hague WM, Teede HJ, et al. . Hyperglycaemia in early pregnancy: the Treatment of Booking Gestational Diabetes Mellitus (TOBOGM) study: a randomised controlled trial. Med J Aust. 2018;209(9):405-406. [DOI] [PubMed] [Google Scholar]
- 107. Bruns DE, Metzger BE, Sacks DB. Diagnosis of gestational diabetes mellitus will be flawed until we can measure glucose. Clin Chem. 2020;66(2):265-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bogdanet D, O’Shea P, Lyons C, et al. . The oral glucose tolerance test-is it time for a change?-a literature review with an emphasis on pregnancy. J Clin Med. 2020;9(11):3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sacks DB, Arnold M, Bakris GL, et al. . Position statement executive summary: guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34(6):1419-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Chan AY, Swaminathan R, Cockram CS. Effectiveness of sodium fluoride as a preservative of glucose in blood. Clin Chem. 1989;35(2):315-317. [PubMed] [Google Scholar]
- 111. Potter JM, Hickman PE, Oakman C, et al. . Strict preanalytical oral glucose tolerance test blood sample handling is essential for diagnosing gestational diabetes mellitus. Diabetes Care. 2020;43(7):1438-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Daly N, Flynn I, Carroll C, et al. . Impact of implementing preanalytical laboratory standards on the diagnosis of gestational diabetes mellitus: a prospective observational study. Clin Chem. 2016;62(2):387-391. [DOI] [PubMed] [Google Scholar]
- 113. International Diabetes Federation. IDF Diabetes Atlas. 9th ed. 2019. [Google Scholar]
- 114. Guariguata L, Linnenkamp U, Beagley J, et al. . Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103(2):176-185. [DOI] [PubMed] [Google Scholar]
- 115. Prentice PM, Olga L, Petry CJ, et al. . Reduced size at birth and persisting reductions in adiposity in recent, compared with earlier, cohorts of infants born to mothers with gestational diabetes mellitus. Diabetologia. 2019;62(11):1977-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hartling L, Dryden DM. Screening and diagnosing gestational diabetes mellitus. Evid Rep Technol Assess (Full Rep). 2012;210:1-327. [PMC free article] [PubMed] [Google Scholar]
- 117. Bottalico JN. Recurrent gestational diabetes: risk factors, diagnosis, management, and implications. Semin Perinatol. 2007;31(3):176-184. [DOI] [PubMed] [Google Scholar]
- 118. Anna V, van der Ploeg H, P, Cheung NW, et al. . Sociodemographic correlates of the increasing trend in prevalence of gestational diabetes mellitus in a large population of women between 1995 and 2005. Diabetes Care. 2008;31(12):2288-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Chu SY, Callaghan WM, Kim SY, et al. . Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30(8):2070-2076. [DOI] [PubMed] [Google Scholar]
- 120. Torloni MR, Betran AP, Horta BL, et al. . Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194-203. [DOI] [PubMed] [Google Scholar]
- 121. Mokdad AH, Ford ES, Bowman BA, et al. . Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76-79. [DOI] [PubMed] [Google Scholar]
- 122. Mulla WR, Henry TQ, Homko CJ. Gestational diabetes screening after HAPO: has anything changed? Curr Diab Rep. 2010;10(3):224-228. [DOI] [PubMed] [Google Scholar]
- 123. Petry CJ. Gestational diabetes: risk factors and recent advances in its genetics and treatment. Br J Nutr. 2010;104(6):775-787. [DOI] [PubMed] [Google Scholar]
- 124. Hunt KJ, Schuller KL. The increasing prevalence of diabetes in pregnancy. Obstet Gynecol Clin North Am. 2007;34(2):173-199, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Agarwal MM, Dhatt GS, Shah SM. Gestational diabetes mellitus: simplifying the international association of diabetes and pregnancy diagnostic algorithm using fasting plasma glucose. Diabetes Care. 2010;33(9):2018-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Moses RG, Morris GJ, Petocz P, et al. . The impact of potential new diagnostic criteria on the prevalence of gestational diabetes mellitus in Australia. Med J Aust. 2011;194(7):338-340. [DOI] [PubMed] [Google Scholar]
- 127. Brown FM, Wyckoff J. Application of one-step IADPSG versus two-step diagnostic criteria for gestational diabetes in the real world: impact on health services, clinical care, and outcomes. Curr Diab Rep. 2017;17(10):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kim C, Berger DK, Chamany S. Recurrence of gestational diabetes mellitus: a systematic review. Diabetes Care. 2007;30(5):1314-1319. [DOI] [PubMed] [Google Scholar]
- 129. Solomon CG, Willett WC, Carey VJ, et al. . A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278(13):1078-1083. [PubMed] [Google Scholar]
- 130. Chamberlain C, McNamara B, Williams ED, et al. . Diabetes in pregnancy among indigenous women in Australia, Canada, New Zealand and the United States. Diabetes Metab Res Rev. 2013;29(4):241-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol. 2010;115(3):597-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kjos SL, Buchanan TA. Gestational diabetes mellitus. N Engl J Med. 1999;341(23):1749-1756. [DOI] [PubMed] [Google Scholar]
- 133. Di Cianni G, Volpe L, Lencioni C, et al. . Prevalence and risk factors for gestational diabetes assessed by universal screening. Diabetes Res Clin Pract. 2003;62(2):131-137. [DOI] [PubMed] [Google Scholar]
- 134. Cypryk K, Szymczak W, Czupryniak L, et al. . Gestational diabetes mellitus—an analysis of risk factors. Endokrynol Pol. 2008;59(5):393-397. [PubMed] [Google Scholar]
- 135. Cleary-Goldman J, Malone FD, Vidaver J, et al. . Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105(5 Pt 1):983-990. [DOI] [PubMed] [Google Scholar]
- 136. Yang H, Wei Y, Gao X, et al. . Risk factors for gestational diabetes mellitus in Chinese women: a prospective study of 16,286 pregnant women in China. Diabet Med. 2009;26(11):1099-1104. [DOI] [PubMed] [Google Scholar]
- 137. Morisset AS, St-Yves A, Veillette J, et al. . Prevention of gestational diabetes mellitus: a review of studies on weight management. Diabetes Metab Res Rev. 2010;26(1):17-25. [DOI] [PubMed] [Google Scholar]
- 138. Weiss JL, Malone FD, Emig D, et al. . Obesity, obstetric complications and cesarean delivery rate—a population-based screening study. Am J Obstet Gynecol. 2004;190(4):1091-1097. [DOI] [PubMed] [Google Scholar]
- 139. Gibson KS, Waters TP, Catalano PM. Maternal weight gain in women who develop gestational diabetes mellitus. Obstet Gynecol. 2012;119(3):560-565. [DOI] [PubMed] [Google Scholar]
- 140. Morisset AS, Tchernof A, Dube MC, et al. . Weight gain measures in women with gestational diabetes mellitus. J Womens Health (Larchmt). 2011;20(3):375-380. [DOI] [PubMed] [Google Scholar]
- 141. Tovar A, Must A, Bermudez OI, et al. . The impact of gestational weight gain and diet on abnormal glucose tolerance during pregnancy in Hispanic women. Matern Child Health J. 2009;13(4):520-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Teulings N, Masconi KL, Ozanne SE, et al. . Effect of interpregnancy weight change on perinatal outcomes: systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;19(1):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lo JC, Feigenbaum SL, Escobar GJ, et al. . Increased prevalence of gestational diabetes mellitus among women with diagnosed polycystic ovary syndrome: a population-based study. Diabetes Care. 2006;29(8):1915-1917. [DOI] [PubMed] [Google Scholar]
- 144. Mikola M, Hiilesmaa V, Halttunen M, et al. . Obstetric outcome in women with polycystic ovarian syndrome. Hum Reprod. 2001;16(2):226-229. [DOI] [PubMed] [Google Scholar]
- 145. Dinham GK, Henry A, Lowe SA, et al. . Twin pregnancies complicated by gestational diabetes mellitus: a single centre cohort study. Diabet Med. 2016;33(12):1659-1667. [DOI] [PubMed] [Google Scholar]
- 146. Rauh-Hain JA, Rana S, Tamez H, et al. . Risk for developing gestational diabetes in women with twin pregnancies. J Matern Fetal Neonatal Med. 2009;22(4):293-299. [DOI] [PubMed] [Google Scholar]
- 147. Chasan-Taber L, Schmidt MD, Pekow P, et al. . Physical activity and gestational diabetes mellitus among Hispanic women. J Womens Health (Larchmt). 2008;17(6):999-1008. [DOI] [PubMed] [Google Scholar]
- 148. Mottola MF. The role of exercise in the prevention and treatment of gestational diabetes mellitus. Curr Diab Rep. 2008;8(4):299-304. [DOI] [PubMed] [Google Scholar]
- 149. Zhang C, Liu S, Solomon CG, et al. . Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care. 2006;29(10):2223-2230. [DOI] [PubMed] [Google Scholar]
- 150. Kucukgoncu S, Guloksuz S, Celik K, et al. . Antipsychotic exposure in pregnancy and the risk of gestational diabetes: a systematic review and meta-analysis. Schizophr Bull. 2020;46(2):311-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Galbally M, Frayne J, Watson SJ, et al. . The association between gestational diabetes mellitus, antipsychotics and severe mental illness in pregnancy: a multicentre study. Aust N Z J Obstet Gynaecol. 2020;60(1):63-69. [DOI] [PubMed] [Google Scholar]
- 152. Hedderson MM, Ferrara A. High blood pressure before and during early pregnancy is associated with an increased risk of gestational diabetes mellitus. Diabetes Care. 2008;31(12):2362-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lao TT, Ho LF. First-trimester blood pressure and gestational diabetes in high-risk Chinese women. J Soc Gynecol Investig. 2003;10(2):94-98. [DOI] [PubMed] [Google Scholar]
- 154. Sweeting AN, Appelblom H, Ross GP, et al. . First trimester prediction of gestational diabetes mellitus: a clinical model based on maternal demographic parameters. Diabetes Res Clin Pract. 2017;127:44-50. [DOI] [PubMed] [Google Scholar]
- 155. Syngelaki A, Visser GH, Krithinakis K, et al. . First trimester screening for gestational diabetes mellitus by maternal factors and markers of inflammation. Metabolism. 2016;65(3):131-137. [DOI] [PubMed] [Google Scholar]
- 156. Nanda S, Savvidou M, Syngelaki A, et al. . Prediction of gestational diabetes mellitus by maternal factors and biomarkers at 11 to 13 weeks. Prenat Diagn. 2011;31(2):135-141. [DOI] [PubMed] [Google Scholar]
- 157. van Leeuwen M, Opmeer BC, Zweers EJ, et al. . Estimating the risk of gestational diabetes mellitus: a clinical prediction model based on patient characteristics and medical history. BJOG. 2010;117(1):69-75. [DOI] [PubMed] [Google Scholar]
- 158. Naylor CD, Sermer M, Chen E, et al. ; Toronto Trihospital Gestational Diabetes Project Investigators. Selective screening for gestational diabetes mellitus. N Engl J Med. 1997;337(22):1591-1596. [DOI] [PubMed] [Google Scholar]
- 159. Catalano PM. Trying to understand gestational diabetes. Diabet Med. 2014;31(3):273-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Catalano PM. Carbohydrate metabolism and gestational diabetes. Clin Obstet Gynecol. 1994;37(1):25-38. [DOI] [PubMed] [Google Scholar]
- 161. Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. 2007;50(4):938-948. [DOI] [PubMed] [Google Scholar]
- 162. Costrini NV, Kalkhoff RK. Relative effects of pregnancy, estradiol, and progesterone on plasma insulin and pancreatic islet insulin secretion. J Clin Invest. 1971;50(5):992-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Kalkhoff RK, Kissebah AH, Kim HJ. Carbohydrate and lipid metabolism during normal pregnancy: relationship to gestational hormone action. Semin Perinatol. 1978;2(4):291-307. [PubMed] [Google Scholar]
- 164. Leturque A, Hauguel S, Sutter Dub MT, et al. . Effects of placental lactogen and progesterone on insulin stimulated glucose metabolism in rat muscles in vitro. Diabete Metab. 1989;15(4):176-181. [PubMed] [Google Scholar]
- 165. Ryan EA, Enns L. Role of gestational hormones in the induction of insulin resistance. J Clin Endocrinol Metab. 1988;67(2):341-347. [DOI] [PubMed] [Google Scholar]
- 166. Alvarez JJ, Montelongo A, Iglesias A, Lasuncion MA, Herrera E. Longitudinal study on lipoprotein profile, high density lipoprotein subclass, and postheparin lipases during gestation in women. J Lipid Res. 1996;37(2):299-308. [PubMed] [Google Scholar]
- 167. Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care. 2007;30(suppl 2):S112-S119. [DOI] [PubMed] [Google Scholar]
- 168. Bomba-Opon D, Wielgos M, Szymanska M, et al. . Effects of free fatty acids on the course of gestational diabetes mellitus. Neuro Endocrinol Lett. 2006;27(1-2):277-280. [PubMed] [Google Scholar]
- 169. Boden G, Chen X, Ruiz J, et al. . Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93(6):2438-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Catalano PM, Huston L, Amini SB, et al. . Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180(4):903-916. [DOI] [PubMed] [Google Scholar]
- 171. Buchanan TA, Metzger BE, Freinkel N, et al. . Insulin sensitivity and B-cell responsiveness to glucose during late pregnancy in lean and moderately obese women with normal glucose tolerance or mild gestational diabetes. Am J Obstet Gynecol. 1990;162(4):1008-1014. [DOI] [PubMed] [Google Scholar]
- 172. Sivan E, Chen X, Homko CJ, et al. . Longitudinal study of carbohydrate metabolism in healthy obese pregnant women. Diabetes Care. 1997;20(9):1470-1475. [DOI] [PubMed] [Google Scholar]
- 173. Caro JF, Dohm LG, Pories WJ, et al. . Cellular alterations in liver, skeletal muscle, and adipose tissue responsible for insulin resistance in obesity and type II diabetes. Diabetes Metab Rev. 1989;5(8):665-689. [DOI] [PubMed] [Google Scholar]
- 174. Freinkel N, Metzger BE, Nitzan M, et al. . Faciltated anabolism in late pregnancy: some novel maternal compensations for accelerated starvation. In: Malaise WJ, Pirart J, Vallence-Own J, eds. International Congress Series 312. Excerpta Medica; 1974:474-488. [Google Scholar]
- 175. Yogev Y, Ben-Haroush A, Chen R, et al. . Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol. 2004;191(3):949-953. [DOI] [PubMed] [Google Scholar]
- 176. Hernandez TL, Friedman JE, Van Pelt RE, et al. . Patterns of glycemia in normal pregnancy: should the current therapeutic targets be challenged? Diabetes Care. 2011;34(7):1660-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Kuhl C. Etiology and pathogenesis of gestational diabetes. Diabetes Care. 1998;21(suppl 2):B19-B26. [PubMed] [Google Scholar]
- 178. Parsons JA, Brelje TC, Sorenson RL. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology. 1992;130(3):1459-1466. [DOI] [PubMed] [Google Scholar]
- 179. Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res. 1997;29(6):301-307. [DOI] [PubMed] [Google Scholar]
- 180. Nielsen JH, Galsgaard ED, Moldrup A, et al. . Regulation of beta-cell mass by hormones and growth factors. Diabetes. 2001;50(suppl 1):S25-S29. [DOI] [PubMed] [Google Scholar]
- 181. Parsons JA, Bartke A, Sorenson RL. Number and size of islets of Langerhans in pregnant, human growth hormone-expressing transgenic, and pituitary dwarf mice: effect of lactogenic hormones. Endocrinology. 1995;136(5):2013-2021. [DOI] [PubMed] [Google Scholar]
- 182. Rieck S, White P, Schug J, et al. . The transcriptional response of the islet to pregnancy in mice. Mol Endocrinol. 2009;23(10):1702-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Buchanan TA, Kitzmiller JL. Metabolic interactions of diabetes and pregnancy. Annu Rev Med. 1994;45:245-260. [DOI] [PubMed] [Google Scholar]
- 184. Rieck S, Kaestner KH. Expansion of beta-cell mass in response to pregnancy. Trends Endocrinol Metab. 2010;21(3):151-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Koukkou E, Watts GF, Lowy C. Serum lipid, lipoprotein and apolipoprotein changes in gestational diabetes mellitus: a cross-sectional and prospective study. J Clin Pathol. 1996;49(8):634-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Nolan CJ. Controversies in gestational diabetes. Best Pract Res Clin Obstet Gynaecol. 2011;25(1):37-49. [DOI] [PubMed] [Google Scholar]
- 187. Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150(6):1223-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Halban PA, Polonsky KS, Bowden DW, et al. . beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care. 2014;37(6):1751-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Catalano PM, Tyzbir ED, Wolfe RR, et al. . Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol. 1993;264(1 Pt 1):E60-E67. [DOI] [PubMed] [Google Scholar]
- 190. Homko C, Sivan E, Chen X, et al. . Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus. J Clin Endocrinol Metab. 2001;86(2):568-573. [DOI] [PubMed] [Google Scholar]
- 191. Powe CE, Huston Presley LP, Locascio JJ, et al. . Augmented insulin secretory response in early pregnancy. Diabetologia. 2019;62(8):1445-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Dluski DF, Wolińska E, Skrzypczak M. Epigenetic changes in gestational diabetes mellitus. Int J Mol Sci. 2021;22(14):7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Elliott HR, Sharp GC, Relton CL, et al. . Epigenetics and gestational diabetes: a review of epigenetic epidemiology studies and their use to explore epigenetic mediation and improve prediction. Diabetologia. 2019;62(12):2171-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Hayes MG, Urbanek M, Hivert MF, et al. . Identification of HKDC1 and BACE2 as genes influencing glycemic traits during pregnancy through genome-wide association studies. Diabetes. 2013;62(9):3282-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Liu S, Liu Y, Liao S. Heterogeneous impact of type 2 diabetes mellitus-related genetic variants on gestational glycemic traits: review and future research needs. Mol Genet Genomics. 2019;294(4):811-847. [DOI] [PubMed] [Google Scholar]
- 196. Wu L, Cui L, Tam WH, et al. . Genetic variants associated with gestational diabetes mellitus: a meta-analysis and subgroup analysis. Sci Rep. 2016;6:30539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Chon SJ, Kim SY, Cho NR, et al. . Association of variants in PPARgamma(2), IGF2BP2, and KCNQ1 with a susceptibility to gestational diabetes mellitus in a Korean population. Yonsei Med J. 2013;54(2):352-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Kim JY, Cheong HS, Park BL, et al. . Melatonin receptor 1 B polymorphisms associated with the risk of gestational diabetes mellitus. BMC Med Genet. 2011;12:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Klein K, Haslinger P, Bancher-Todesca D, et al. . Transcription factor 7-like 2 gene polymorphisms and gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2012;25(9):1783-1786. [DOI] [PubMed] [Google Scholar]
- 200. Alharbi KK, Khan IA, Abotalib Z, et al. . Insulin receptor substrate-1 (IRS-1) Gly927Arg: correlation with gestational diabetes mellitus in Saudi women. Biomed Res Int. 2014;2014:146495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Tok EC, Ertunc D, Bilgin O, et al. . PPAR-gamma2 Pro12Ala polymorphism is associated with weight gain in women with gestational diabetes mellitus. Eur J Obstet Gynecol Reprod Biol. 2006;129(1):25-30. [DOI] [PubMed] [Google Scholar]
- 202. Montazeri S, Nalliah S, Radhakrishnan AK. Is there a genetic variation association in the IL-10 and TNF alpha promoter gene with gestational diabetes mellitus? Hereditas. 2010;147(2):94-102. [DOI] [PubMed] [Google Scholar]
- 203. Kwak SH, Kim SH, Cho YM, et al. . A genome-wide association study of gestational diabetes mellitus in Korean women. Diabetes. 2012;61(2):531-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Lyssenko V, Nagorny CL, Erdos MR, et al. . Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41(1):82-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, et al. . A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41(1):89-94. [DOI] [PubMed] [Google Scholar]
- 207. Saxena R, Elbers CC, Guo Y, et al. . Large-scale gene-centric meta-analysis across 39 studies identifies type 2 diabetes loci. Am J Hum Genet. 2012;90(3):410-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Manning AK, Hivert MF, Scott RA, et al. . A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet. 2012;44(6):659-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Sparso T, Andersen G, Nielsen T, et al. . The GCKR rs780094 polymorphism is associated with elevated fasting serum triacylglycerol, reduced fasting and OGTT-related insulinaemia, and reduced risk of type 2 diabetes. Diabetologia. 2008;51(1):70-75. [DOI] [PubMed] [Google Scholar]
- 210. Vaxillaire M, Cavalcanti-Proenca C, Dechaume A, et al. . The common P446L polymorphism in GCKR inversely modulates fasting glucose and triglyceride levels and reduces type 2 diabetes risk in the DESIR prospective general French population. Diabetes. 2008;57(8):2253-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Agius L. Glucokinase and molecular aspects of liver glycogen metabolism. Biochem J. 2008;414(1):1-18. [DOI] [PubMed] [Google Scholar]
- 212. Chasman DI, Pare G, Mora S, et al. . Forty-three loci associated with plasma lipoprotein size, concentration, and cholesterol content in genome-wide analysis. PLoS Genet. 2009;5(11):e1000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Seidah NG. The proprotein convertases, 20 years later. Methods Mol Biol. 2011;768:23-57. [DOI] [PubMed] [Google Scholar]
- 214. Benzinou M, Creemers JW, Choquet H, et al. . Common nonsynonymous variants in PCSK1 confer risk of obesity. Nat Genet. 2008;40(8):943-945. [DOI] [PubMed] [Google Scholar]
- 215. Hayes A, Chevalier A, D’Souza M, et al. . Early childhood obesity: association with healthcare expenditure in Australia. Obesity (Silver Spring). 2016;24(8):1752-8. [DOI] [PubMed] [Google Scholar]
- 216. Powe CE, Nodzenski M, Talbot O, et al. . Genetic determinants of glycemic traits and the risk of gestational diabetes mellitus. Diabetes. 2018;67(12):2703-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Fajans SS, Bell GI, Polonsky KS. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med. 2001;345(13):971-980. [DOI] [PubMed] [Google Scholar]
- 218. Hattersley AT, Patel KA. Precision diabetes: learning from monogenic diabetes. Diabetologia. 2017;60(5):769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Dickens LT, Naylor RN. Clinical management of women with monogenic diabetes during pregnancy. Curr Diab Rep. 2018;18(3):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Chakera AJ, Steele AM, Gloyn AL, et al. . Recognition and management of individuals with hyperglycemia because of a heterozygous glucokinase mutation. Diabetes Care. 2015;38(7):1383-1392. [DOI] [PubMed] [Google Scholar]
- 221. Urbanova J, Brunerova L, Nunes M, et al. . Identification of MODY among patients screened for gestational diabetes: a clinician’s guide. Arch Gynecol Obstet. 2020;302(2):305-314. [DOI] [PubMed] [Google Scholar]
- 222. Wang Z, Ping F, Zhang Q, et al. . Preliminary screening of mutations in the glucokinase gene of Chinese patients with gestational diabetes. J Diabetes Investig. 2018;9(1):199-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Gjesing AP, Rui G, Lauenborg J, et al. . High prevalence of diabetes-predisposing variants in MODY genes among Danish women with gestational diabetes mellitus. J Endocr Soc. 2017;1(6): 681-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Shields BM, Hicks S, Shepherd MH, et al. . Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010;53(12):2504-2508. [DOI] [PubMed] [Google Scholar]
- 225. Spyer G, Hattersley AT, Sykes JE, et al. . Influence of maternal and fetal glucokinase mutations in gestational diabetes. Am J Obstet Gynecol. 2001;185(1):240-241. [DOI] [PubMed] [Google Scholar]
- 226. Hattersley AT, Beards F, Ballantyne E, et al. . Mutations in the glucokinase gene of the fetus result in reduced birth weight. Nat Genet. 1998;19(3):268-270. [DOI] [PubMed] [Google Scholar]
- 227. Scholtens DM, Kuang A, Lowe LP, et al. . Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal glycemia and childhood glucose metabolism. Diabetes Care. 2019;42(3):381-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Saravanan P; Diabetes in Pregnancy Working Group . Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 2020;8(9):793-800. [DOI] [PubMed] [Google Scholar]
- 229. Pedersen J. Hyperglycaemia-hyperinsulinism theory and birthweight. In: The Pregnant Diabetic and Her Newborn: Problems and Management. Williams and Wilkins; 1977:211-220. [Google Scholar]
- 230. Illsley NP. Glucose transporters in the human placenta. Placenta. 2000;21(1):14-22. [DOI] [PubMed] [Google Scholar]
- 231. Pedersen J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinol (Copenh). 1954;16(4):330-342. [DOI] [PubMed] [Google Scholar]
- 232. Whitelaw A. Subcutaneous fat in newborn infants of diabetic mothers: an indication of quality of diabetic control. Lancet. 1977;1(8001):15-18. [DOI] [PubMed] [Google Scholar]
- 233. Vrijkotte TG, Krukziener N, Hutten BA, et al. . Maternal lipid profile during early pregnancy and pregnancy complications and outcomes: the ABCD study. J Clin Endocrinol Metab. 2012;97(11):3917-3925. [DOI] [PubMed] [Google Scholar]
- 234. Yang X, Hsu-Hage B, Zhang H, et al. . Women with impaired glucose tolerance during pregnancy have significantly poor pregnancy outcomes. Diabetes Care. 2002;25(9): 1619-1624. [DOI] [PubMed] [Google Scholar]
- 235. Spellacy WN, Miller S, Winegar A, et al. . Macrosomia—maternal characteristics and infant complications. Obstet Gynecol. 1985;66(2):158-161. [PubMed] [Google Scholar]
- 236. Jang HC, Cho NH, Min YK, et al. . Increased macrosomia and perinatal morbidity independent of maternal obesity and advanced age in Korean women with GDM. Diabetes Care. 1997;20(10):1582-1588. [DOI] [PubMed] [Google Scholar]
- 237. Langer O, Yogev Y, Most O, et al. . Gestational diabetes: the consequences of not treating. Am J Obstet Gynecol. 2005;192(4):989-997. [DOI] [PubMed] [Google Scholar]
- 238. Weiss PA, Haeusler M, Tamussino K, et al. . Can glucose tolerance test predict fetal hyperinsulinism? BJOG. 2000;107(12):1480-1485. [DOI] [PubMed] [Google Scholar]
- 239. Boulet SL, Alexander GR, Salihu HM, et al. . Macrosomic births in the united states: determinants, outcomes, and proposed grades of risk. Am J Obstet Gynecol. 2003;188(5):1372-1378. [DOI] [PubMed] [Google Scholar]
- 240. Ryckman KK, Spracklen CN, Smith CJ, et al. . Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis. BJOG. 2015;122(5):643-651. [DOI] [PubMed] [Google Scholar]
- 241. Jovanovic L, Pettitt DJ. Gestational diabetes mellitus. JAMA. 2001;286(20):2516-2518. [DOI] [PubMed] [Google Scholar]
- 242. Esakoff TF, Cheng YW, Sparks TN, et al. . The association between birthweight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200(6):672 e1-672 e4. [DOI] [PubMed] [Google Scholar]
- 243. Henriksen T. The macrosomic fetus: a challenge in current obstetrics. Acta Obstet Gynecol Scand. 2008;87(2):134-145. [DOI] [PubMed] [Google Scholar]
- 244. Reece EA, Leguizamon G, Wiznitzer A. Gestational diabetes: the need for a common ground. Lancet. 2009;373(9677):1789-1797. [DOI] [PubMed] [Google Scholar]
- 245. Cetin H, Yalaz M, Akisu M, et al. . Polycythaemia in infants of diabetic mothers: beta-hydroxybutyrate stimulates erythropoietic activity. J Int Med Res. 2011;39(3):815-821. [DOI] [PubMed] [Google Scholar]
- 246. Farrar D, Fairley L, Santorelli G, et al. . Association between hyperglycaemia and adverse perinatal outcomes in south Asian and white British women: analysis of data from the Born in Bradford cohort. Lancet Diabetes Endocrinol. 2015;3(10):795-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Balsells M, Corcoy R, Adelantado JM, et al. . Gestational diabetes mellitus: metabolic control during labour. Diabetes Nutr Metab. 2000;13(5):257-262. [PubMed] [Google Scholar]
- 248. Farrar D, Simmonds M, Bryant M, et al. . Hyperglycaemia and risk of adverse perinatal outcomes: systematic review and meta-analysis. BMJ. 2016;354:i4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Billionnet C, Mitanchez D, Weill A, et al. . Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia. 2017;60(4):636-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Allen VM, Armson BA. SOGC Clinical Practice Guideline: teratogenicity associated with pre-existing and gestational diabetes. J Obstet Gynaecol Can. 2007;29(11):927-934. [DOI] [PubMed] [Google Scholar]
- 251. Owens LA, O’Sullivan EP, Kirwan B, et al. . ATLANTIC DIP: the impact of obesity on pregnancy outcome in glucose-tolerant women. Diabetes Care. 2010;33(3):577-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Dennedy MC, Avalos G, O’Reilly MW, et al. . ATLANTIC-DIP: raised maternal body mass index (BMI) adversely affects maternal and fetal outcomes in glucose-tolerant women according to International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria. J Clin Endocrinol Metab. 2012;97(4):E608-E612. [DOI] [PubMed] [Google Scholar]
- 253. Rosenstein MG, Cheng YW, Snowden JM, et al. . The risk of stillbirth and infant death stratified by gestational age in women with gestational diabetes. Am J Obstet Gynecol. 2012;206(4):309 e1-309 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Mitanchez D. Foetal and neonatal complications in gestational diabetes: perinatal mortality, congenital malformations, macrosomia, shoulder dystocia, birth injuries, neonatal complications. Diabetes Metab. 2010;36(6 Pt 2):617-627. [DOI] [PubMed] [Google Scholar]
- 255. Cundy T, Gamble G, Townend K, et al. . Perinatal mortality in type 2 diabetes mellitus. Diabet Med. 2000;17(1):33-39. [DOI] [PubMed] [Google Scholar]
- 256. Hutcheon JA, Kuret V, Joseph KS, et al. . Immortal time bias in the study of stillbirth risk factors: the example of gestational diabetes. Epidemiology. 2013;24(6):787-790. [DOI] [PubMed] [Google Scholar]
- 257. Poston L, Bell R, Croker H, et al. . Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3(10):767-777. [DOI] [PubMed] [Google Scholar]
- 258. Tam WH, Ma RCW, Ozaki R, et al. . In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in Offspring. Diabetes Care. 2017;40(5):679-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Yu Y, Arah OA, Liew Z, et al. . Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: population based cohort study with 40 years of follow-up. BMJ. 2019;367:l6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Hod M. The fetal heart in gestational diabetes: long-term effects. BJOG. 2021;128(2):280. [DOI] [PubMed] [Google Scholar]
- 261. Dabelea D, Hanson RL, Lindsay RS, et al. . Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49(12):2208-2211. [DOI] [PubMed] [Google Scholar]
- 262. Lowe WL Jr., Lowe LP, Kuang A, et al. . Maternal glucose levels during pregnancy and childhood adiposity in the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study. Diabetologia. 2019;62(4):598-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Page KA, Luo S, Wang X, et al. . Children exposed to maternal obesity or gestational diabetes mellitus during early fetal development have hypothalamic alterations that predict future weight gain. Diabetes Care. 2019;42(8):1473-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Anderberg E, Kallen K, Berntorp K. The impact of gestational diabetes mellitus on pregnancy outcome comparing different cut-off criteria for abnormal glucose tolerance. Acta Obstet Gynecol Scand. 2010;89(12):1532-1537. [DOI] [PubMed] [Google Scholar]
- 265. Ju H, Rumbold AR, Willson KJ, et al. . Borderline gestational diabetes mellitus and pregnancy outcomes. BMC Pregnancy Childbirth. 2008;8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Jastrow N, Roberge S, Gauthier RJ, et al. . Effect of birth weight on adverse obstetric outcomes in vaginal birth after cesarean delivery. Obstet Gynecol. 2010;115(2 Pt 1):338-343. [DOI] [PubMed] [Google Scholar]
- 267. Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. 1998;21(suppl 2):B161-B167. [PubMed] [Google Scholar]
- 268. Hiden U, Lassance L, Tabrizi NG, et al. . Fetal insulin and IGF-II contribute to gestational diabetes mellitus (GDM)-associated up-regulation of membrane-type matrix metalloproteinase 1 (MT1-MMP) in the human feto-placental endothelium. J Clin Endocrinol Metab. 2012;97(10):3613-3621. [DOI] [PubMed] [Google Scholar]
- 269. Dunne FP, Avalos G, Durkan M, et al. . ATLANTIC DIP: pregnancy outcomes for women with type 1 and type 2 diabetes. Ir Med J. 2012;105(5 suppl):6-9. [PubMed] [Google Scholar]
- 270. Roberts JM, Redman CW. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341(8858):1447-1451. [DOI] [PubMed] [Google Scholar]
- 271. Desoye G, Hauguel-de Mouzon S. The human placenta in gestational diabetes mellitus. The Insulin and Cytokine Network. Diabetes Care. 2007;30(suppl 2):S120-S126. [DOI] [PubMed] [Google Scholar]
- 272. Belkacemi L, Lash GE, Macdonald-Goodfellow SK, et al. . Inhibition of human trophoblast invasiveness by high glucose concentrations. J Clin Endocrinol Metab. 2005;90(8): 4846-4851. [DOI] [PubMed] [Google Scholar]
- 273. Vounzoulaki E, Khunti K, Abner SC, et al. . Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Daly B, Toulis KA, Thomas N, et al. . Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: a population-based cohort study.. PLoS Med. 2018;15(1):e1002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Lowe WL Jr, Scholtens DM, Kuang A, et al. . Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019;42(3):372-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Murphy HR. 2020 NICE guideline update: good news for pregnant women with type 1 diabetes and past or current gestational diabetes. Diabet Med. 2021;38(6):e14576. [DOI] [PubMed] [Google Scholar]
- 277. National Institute for Health and Care Excellence. Diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period. NICE Guidelines NG3.2015. Last updated December 16, 2020. https://www.nice.org.uk/guidance/ng3
- 278. Carr DB, Utzschneider KM, Hull RL, et al. . Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care. 2006;29(9):2078-2083. [DOI] [PubMed] [Google Scholar]
- 279. Gunderson EP, Chiang V, Pletcher MJ, et al. . History of gestational diabetes mellitus and future risk of atherosclerosis in mid-life: the Coronary Artery Risk Development in Young Adults study. J Am Heart Assoc. 2014;3(2):e000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Retnakaran R. Glucose tolerance status in pregnancy: a window to the future risk of diabetes and cardiovascular disease in young women. Curr Diabetes Rev. 2009;5(4):239-244. [DOI] [PubMed] [Google Scholar]
- 281. Tobias DK, Hu FB, Forman JP, et al. . Increased risk of hypertension after gestational diabetes mellitus: findings from a large prospective cohort study. Diabetes Care. 2011;34(7):1582-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Tobias DK, Stuart JJ, Li S, et al. . Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US Women. JAMA Intern Med. 2017;177(12):1735-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Brown HL, Warner JJ, Gianos E, et al. . Promoting risk identification and reduction of cardiovascular disease in women through collaboration with obstetricians and gynecologists: a presidential advisory from the American Heart Association and the American College of Obstetricians and Gynecologists. Circulation. 2018;137(24):e843-ee52. [DOI] [PubMed] [Google Scholar]
- 284. Knudsen LS, Christensen IJ, Lottenburger T, et al. . Pre-analytical and biological variability in circulating interleukin 6 in healthy subjects and patients with rheumatoid arthritis. Biomarkers. 2008;13(1):59-78. [DOI] [PubMed] [Google Scholar]
- 285. Alwan N, Tuffnell DJ, West J. Treatments for gestational diabetes. Cochrane Database Syst Rev. 2009;3:CD003395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Jovanovic-Peterson L, Peterson CM, Reed GF, et al. . Maternal postprandial glucose levels and infant birth weight: the Diabetes in Early Pregnancy Study. The National Institute of Child Health and Human Development—Diabetes in Early Pregnancy Study. Am J Obstet Gynecol. 1991;164(1 Pt 1):103-111. [DOI] [PubMed] [Google Scholar]
- 287. Bain E, Crane M, Tieu J, et al. . Diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 0443;2015;4:CD01. [DOI] [PubMed] [Google Scholar]
- 288. Duarte-Gardea MO, Gonzales-Pacheco DM, Reader DM, et al. . Academy of nutrition and dietetics gestational diabetes evidence-based nutrition practice guideline. J Acad Nutr Diet. 2018;118(9):1719-1742. [DOI] [PubMed] [Google Scholar]
- 289. Rasmussen KM, Yaktine AL, eds.; Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press; 2009. [PubMed] [Google Scholar]
- 290. Jovanovic-Peterson L, Peterson CM. Dietary manipulation as a primary treatment strategy for pregnancies complicated by diabetes. J Am Coll Nutr. 1990;9(4):320-325. [DOI] [PubMed] [Google Scholar]
- 291. Hernandez TL, Van Pelt RE, Anderson MA, et al. . A higher-complex carbohydrate diet in gestational diabetes mellitus achieves glucose targets and lowers postprandial lipids: a randomized crossover study. Diabetes Care. 2014;37(5):1254-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Hernandez TL, Van Pelt RE, Anderson MA, et al. . Women with gestational diabetes mellitus randomized to a higher-complex carbohydrate/low-fat diet manifest lower adipose tissue insulin resistance, inflammation, glucose, and free fatty acids: a pilot study. Diabetes Care. 2016;39(1):39-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Asemi Z, Tabassi Z, Samimi M, et al. . Favourable effects of the Dietary Approaches to Stop Hypertension diet on glucose tolerance and lipid profiles in gestational diabetes: a randomised clinical trial. Br J Nutr. 2013;109(11):2024-2030. [DOI] [PubMed] [Google Scholar]
- 294. Han S, Middleton P, Shepherd E, et al. . Different types of dietary advice for women with gestational diabetes mellitus. Cochrane Database Syst Rev. 2017;2:CD009275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Yamamoto JM, Kellett JE, Balsells M, et al. . Gestational diabetes mellitus and diet: a systematic review and meta-analysis of randomized controlled trials examining the impact of modified dietary interventions on maternal glucose control and neonatal birth weight. Diabetes Care. 2018;41(7):1346-1361. [DOI] [PubMed] [Google Scholar]
- 296. Major CA, Henry MJ, De Veciana M, et al. . The effects of carbohydrate restriction in patients with diet-controlled gestational diabetes. Obstet Gynecol. 1998;91(4):600-604. [DOI] [PubMed] [Google Scholar]
- 297. American College of Obstetrics and Gynecology. Practice Bulletin No. 137: gestational diabetes mellitus. Obstet Gynecol. 2013;122(2 Pt 1):406-416. [DOI] [PubMed] [Google Scholar]
- 298. Metzger BE, Buchanan TA, Coustan DR, et al. . Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. [DOI] [PubMed] [Google Scholar]
- 299. American Diabetes Association. Standards of medical care in diabetes-2011. Diabetes Care. 2011;34(suppl 1):S11-S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Battaglia FC, Meschia G.. An Introduction to Fetal Physiology. Academic Press; 1986. [Google Scholar]
- 301. Harding JE, Johnston BM. Nutrition and fetal growth. Reprod Fertil Dev. 1995;7(3):539-547. [DOI] [PubMed] [Google Scholar]
- 302. Sweeting A, Markovic TP, Mijatovic J, et al. . The carbohydrate threshold in pregnancy and gestational diabetes: how low can we go? Nutrients. 2021;13(8):2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Morisaki N, Nagata C, Yasuo S, et al. . Optimal protein intake during pregnancy for reducing the risk of fetal growth restriction: the Japan Environment and Children’s Study. Br J Nutr. 2018;120(12):1432-1440. [DOI] [PubMed] [Google Scholar]
- 304. Rizzo T, Metzger BE, Burns WJ, et al. . Correlations between antepartum maternal metabolism and intelligence of offspring. N Engl J Med. 1991;325(13):911-916. [DOI] [PubMed] [Google Scholar]
- 305. Shaw GM, Yang W. Women’s periconceptional lowered carbohydrate intake and NTD-affected pregnancy risk in the era of prefortification with folic acid. Birth Defects Res. 2019;111(5):248-253. [DOI] [PubMed] [Google Scholar]
- 306. Mijatovic J, Louie JCY, Buso MEC, et al. . Effects of a modestly lower carbohydrate diet in gestational diabetes: a randomized controlled trial. Am J Clin Nutr. 2020;112(2):284-292. [DOI] [PubMed] [Google Scholar]
- 307. Shubert PJ, Gordon MC, Landon MB, et al. . Ketoacids attenuate glucose uptake in human trophoblasts isolated from first-trimester chorionic villi. Am J Obstet Gynecol. 1996;175(1):56-62. [DOI] [PubMed] [Google Scholar]
- 308. Catalano PM, Mele L, Landon MB, et al. . Inadequate weight gain in overweight and obese pregnant women: what is the effect on fetal growth? Am J Obstet Gynecol. 2014;211(2):137 e1-137 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Faucher MA, Barger MK. Gestational weight gain in obese women by class of obesity and select maternal/newborn outcomes: a systematic review. Women Birth. 2015;28(3):e70-e79. [DOI] [PubMed] [Google Scholar]
- 310. Viecceli C, Remonti LR, Hirakata VN, et al. . Weight gain adequacy and pregnancy outcomes in gestational diabetes: a meta-analysis. Obes Rev. 2017;18(5):567-580. [DOI] [PubMed] [Google Scholar]
- 311. Kainer F, Weiss PA, Huttner U, et al. . Levels of amniotic fluid insulin and profiles of maternal blood glucose in pregnant women with diabetes type-I. Early Hum Dev. 1997;49(2):97-105. [DOI] [PubMed] [Google Scholar]
- 312. de Veciana M, Major CA, Morgan MA, et al. . Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med. 1995;333(19):1237-1241. [DOI] [PubMed] [Google Scholar]
- 313. Hernandez TL. Glycemic targets in pregnancies affected by diabetes: historical perspective and future directions. Curr Diab Rep. 2015;15(1):565. [DOI] [PubMed] [Google Scholar]
- 314. Abell SK, Boyle JA, Earnest A, et al. . Impact of different glycaemic treatment targets on pregnancy outcomes in gestational diabetes. Diabet Med. 2019;36(2):177-183. [DOI] [PubMed] [Google Scholar]
- 315. Garner P, Okun N, Keely E, et al. . A randomized controlled trial of strict glycemic control and tertiary level obstetric care versus routine obstetric care in the management of gestational diabetes: a pilot study. Am J Obstet Gynecol. 1997;177(1):190-195. [DOI] [PubMed] [Google Scholar]
- 316. Langer O, Levy J, Brustman L, et al. . Glycemic control in gestational diabetes mellitus--how tight is tight enough: small for gestational age versus large for gestational age? Am J Obstet Gynecol. 1989;161(3):646-653. [DOI] [PubMed] [Google Scholar]
- 317. Langer O, Rodriguez DA, Xenakis EM, et al. . Intensified versus conventional management of gestational diabetes. Am J Obstet Gynecol. 1994;170(4):1036-1046. [DOI] [PubMed] [Google Scholar]
- 318. Pregnancy outcomes in the diabetes control and complications trial. Am J Obstet Gynecol. 1996;174(4):1343-1353. [DOI] [PubMed] [Google Scholar]
- 319. Martis R, Brown J, Alsweiler J, et al. . Different intensities of glycaemic control for women with gestational diabetes mellitus. Cochrane Database Syst Rev. 2016;4:CD011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Snyder JMI, Melzter S, Nadeau J. Gestational diabetes and glycemic control: a randomized clinical trial. Am J Obstet Gynecol. 1998;178(1 Pt 2):S55. [Google Scholar]
- 321. Pertot T, Molyneaux L, Tan K, et al. . Can common clinical parameters be used to identify patients who will need insulin treatment in gestational diabetes mellitus? Diabetes Care. 2011;34(10):2214-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Rowan JA, Hague WM, Gao W, et al. . Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358(19):2003-2015. [DOI] [PubMed] [Google Scholar]
- 323. Murphy HR, Rayman G, Duffield K, et al. . Changes in the glycemic profiles of women with type 1 and type 2 diabetes during pregnancy. Diabetes Care. 2007;30(11):2785-2791. [DOI] [PubMed] [Google Scholar]
- 324. Padmanabhan S, McLean M, Cheung NW. Falling insulin requirements are associated with adverse obstetric outcomes in women with preexisting diabetes. Diabetes Care. 2014;37(10):2685-2692. [DOI] [PubMed] [Google Scholar]
- 325. Wong VW. Gestational diabetes mellitus in five ethnic groups: a comparison of their clinical characteristics. Diabet Med. 2012;29(3):366-371. [DOI] [PubMed] [Google Scholar]
- 326. Barnes RA, Wong T, Ross GP, et al. . A novel validated model for the prediction of insulin therapy initiation and adverse perinatal outcomes in women with gestational diabetes mellitus. Diabetologia. 2016;59(11):2331-2338. [DOI] [PubMed] [Google Scholar]
- 327. Ryu RJ, Hays KE, Hebert MF. Gestational diabetes mellitus management with oral hypoglycemic agents. Semin Perinatol. 2014;38(8):508-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Camelo Castillo W, Boggess K, Sturmer T, et al. . Trends in glyburide compared with insulin use for gestational diabetes treatment in the United States, 2000-2011. Obstet Gynecol. 2014;123(6):1177-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Langer O, Conway DL, Berkus MD, et al. . A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343(16):1134-1138. [DOI] [PubMed] [Google Scholar]
- 330. Kremer CJ, Duff P. Glyburide for the treatment of gestational diabetes. Am J Obstet Gynecol. 2004;190(5):1438-1439. [DOI] [PubMed] [Google Scholar]
- 331. Camelo Castillo W, Boggess K, Sturmer T, et al. . Association of adverse pregnancy outcomes with glyburide vs insulin in women with gestational diabetes. JAMA Pediatr. 2015;169(5): 452-458. [DOI] [PubMed] [Google Scholar]
- 332. Schwartz RA, Rosenn B, Aleksa K, et al. . Glyburide transport across the human placenta. Obstet Gynecol. 2015;125(3):583-588. [DOI] [PubMed] [Google Scholar]
- 333. Cheng YW, Chung JH, Block-Kurbisch I, et al. . Treatment of gestational diabetes mellitus: glyburide compared to subcutaneous insulin therapy and associated perinatal outcomes. J Matern Fetal Neonatal Med. 2012;25(4):379-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Lindsay RS, Loeken MR. Metformin use in pregnancy: promises and uncertainties. Diabetologia. 2017;60(9):1612-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Charles B, Norris R, Xiao X, et al. . Population pharmacokinetics of metformin in late pregnancy. Ther Drug Monit. 2006;28(1): 67-72. [DOI] [PubMed] [Google Scholar]
- 336. Tarry-Adkins JL, Aiken CE, Ozanne SE. Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: a systematic review and meta-analysis. PLoS Med. 2019;16(8):e1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Feig DS, Donovan LE, Zinman B, et al. . Metformin in women with type 2 diabetes in pregnancy (MiTy): a multicentre, international, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2020;8(10):834-844. [DOI] [PubMed] [Google Scholar]
- 338. Niromanesh S, Alavi A, Sharbaf FR, et al. . Metformin compared with insulin in the management of gestational diabetes mellitus: a randomized clinical trial. Diabetes Res Clin Pract. 2012;98(3):422-429. [DOI] [PubMed] [Google Scholar]
- 339. Khin MO, Gates S, Saravanan P. Predictors of metformin failure in gestational diabetes mellitus (GDM). Diabetes Metab Syndr. 2018;12(3):405-410. [DOI] [PubMed] [Google Scholar]
- 340. Rowan JA, Rush EC, Obolonkin V, et al. . Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition at 2 years of age. Diabetes Care. 2011;34(10):2279-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Rowan JA, Rush EC, Plank LD, et al. . Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6(1):e000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Hanem LGE, Stridsklev S, Juliusson PB, et al. . Metformin use in PCOS pregnancies increases the risk of offspring overweight at 4 years of age: follow-up of two RCTs. J Clin Endocrinol Metab. 2018;103(4):1612-1621. [DOI] [PubMed] [Google Scholar]
- 343. Brown J, Martis R, Hughes B, et al. . Oral anti-diabetic pharmacological therapies for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;1:CD011967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Balsells M, Garcia-Patterson A, Sola I, et al. . Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Poolsup N, Suksomboon N, Amin M. Efficacy and safety of oral antidiabetic drugs in comparison to insulin in treating gestational diabetes mellitus: a meta-analysis. PLoS One. 2014;9(10):e109985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Nachum Z, Zafran N, Salim R, et al. . Glyburide versus metformin and their combination for the treatment of gestational diabetes mellitus: a randomized controlled study. Diabetes Care. 2017;40(3):332-337. [DOI] [PubMed] [Google Scholar]
- 347. Sacco F, Calderone A, Castagnoli L, et al. . The cell-autonomous mechanisms underlying the activity of metformin as an anticancer drug. Br J Cancer. 2016;115(12):1451-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Barbour LA, Davies JK. Comment on Nachum et al. Glyburide versus metformin and their combination for the treatment of gestational diabetes mellitus: a randomized controlled study. Diabetes Care. 2017;40:332-337. Diabetes Care. 2017;40(8):e115. [DOI] [PubMed]
- 349. Rao U, de Vries B, Ross GP, et al. . Fetal biometry for guiding the medical management of women with gestational diabetes mellitus for improving maternal and perinatal health. Cochrane Database Syst Rev. 2019;9:CD012544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Schaefer-Graf UM, Kjos SL, Fauzan OH, et al. . A randomized trial evaluating a predominantly fetal growth-based strategy to guide management of gestational diabetes in Caucasian women. Diabetes Care. 2004;27(2):297-302. [DOI] [PubMed] [Google Scholar]
- 351. Schaefer-Graf UM, Wendt L, Sacks DA, et al. . How many sonograms are needed to reliably predict the absence of fetal overgrowth in gestational diabetes mellitus pregnancies? Diabetes Care. 2011;34(1):39-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Kjos SL, Schaefer-Graf UM. Modified therapy for gestational diabetes using high-risk and low-risk fetal abdominal circumference growth to select strict versus relaxed maternal glycemic targets. Diabetes Care. 2007;30(suppl 2):S200-S205. [DOI] [PubMed] [Google Scholar]
- 353. Nelson L, Wharton B, Grobman WA. Prediction of large for gestational age birth weights in diabetic mothers based on early third-trimester sonography. J Ultrasound Med. 2011;30(12):1625-1628. [DOI] [PubMed] [Google Scholar]
- 354. McLean A, Katz M, Oats J, et al. . Rethinking third trimester ultrasound measurements and risk of adverse neonatal outcomes in pregnancies complicated by hyperglycaemia: a retrospective study. Aust N Z J Obstet Gynaecol. 2021;61(3):366-372. [DOI] [PubMed] [Google Scholar]
- 355. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 560: medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121(4):908-910. [DOI] [PubMed] [Google Scholar]
- 356. Metcalfe A, Hutcheon JA, Sabr Y, et al. . Timing of delivery in women with diabetes: a population-based study. Acta Obstet Gynecol Scand. 2020;99(3):341-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Hunt KJ, Logan SL, Conway DL, et al. . Postpartum screening following GDM: how well are we doing? Curr Diab Rep. 2010;10(3):235-241. [DOI] [PubMed] [Google Scholar]
- 358. Gabbe SG, Landon MB, Warren-Boulton E, et al. . Promoting health after gestational diabetes: a National Diabetes Education Program call to action. Obstet Gynecol. 2012;119(1):171-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Ehrlich SF, Hedderson MM, Feng J, et al. . Change in body mass index between pregnancies and the risk of gestational diabetes in a second pregnancy. Obstet Gynecol. 2011;117(6):1323-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862-1868. [DOI] [PubMed] [Google Scholar]
- 361. Bellamy L, Casas JP, Hingorani AD, et al. . Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773-1779. [DOI] [PubMed] [Google Scholar]
- 362. Dornhorst A, Bailey PC, Anyaoku V, et al. . Abnormalities of glucose tolerance following gestational diabetes. Q J Med. 1990;77(284):1219-1228. [DOI] [PubMed] [Google Scholar]
- 363. Aroda VR, Christophi CA, Edelstein SL, et al. . The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab. 2015;100(4):1646-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Pirc LK, Owens JA, Crowther CA, et al. . Mild gestational diabetes in pregnancy and the adipoinsular axis in babies born to mothers in the ACHOIS randomised controlled trial. BMC Pediatr. 2007;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Landon MB, Rice MM, Varner MW, et al. . Mild gestational diabetes mellitus and long-term child health. Diabetes Care. 2015;38(3):445-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Dabelea D, Pettitt DJ. Intrauterine diabetic environment confers risks for type 2 diabetes mellitus and obesity in the offspring, in addition to genetic susceptibility. J Pediatr Endocrinol Metab. 2001;14(8):1085-1091. [DOI] [PubMed] [Google Scholar]
- 367. Pettitt DJ, Baird HR, Aleck KA, et al. . Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med. 1983;308(5):242-245. [DOI] [PubMed] [Google Scholar]
- 368. Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics. 2003;111(3):e221-e226. [DOI] [PubMed] [Google Scholar]
- 369. Plagemann A, Harder T, Kohlhoff R, et al. . Overweight and obesity in infants of mothers with long-term insulin-dependent diabetes or gestational diabetes. Int J Obes Relat Metab Disord. 1997;21(6):451-456. [DOI] [PubMed] [Google Scholar]
- 370. Pribylova H, Dvorakova L. Long-term prognosis of infants of diabetic mothers. Relationship between metabolic disorders in newborns and adult offspring. Acta Diabetol. 1996;33(1):30-34. [DOI] [PubMed] [Google Scholar]
- 371. Silverman BL, Metzger BE, Cho NH, et al. . Impaired glucose tolerance in adolescent offspring of diabetic mothers: relationship to fetal hyperinsulinism. Diabetes Care. 1995;18(5):611-617. [DOI] [PubMed] [Google Scholar]
- 372. Crume TL, Ogden L, West NA, et al. . Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54(1):87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. West NA, Crume TL, Maligie MA, et al. . Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia. 2011;54(3):504-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Hillier TA, Pedula KL, Schmidt MM, et al. . Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30(9):2287-2292. [DOI] [PubMed] [Google Scholar]
- 375. Whincup PH, Kaye SJ, Owen CG, et al. . Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300(24):2886-2897. [DOI] [PubMed] [Google Scholar]
- 376. Guerrero-Romero F, Aradillas-Garcia C, Simental-Mendia LE, et al. . Birth weight, family history of diabetes, and metabolic syndrome in children and adolescents. J Pediatr. 2010;156(5):719-723, 23 e1. [DOI] [PubMed] [Google Scholar]
- 377. Harder T, Roepke K, Diller N, et al. . Birth weight, early weight gain, and subsequent risk of type 1 diabetes: systematic review and meta-analysis. Am J Epidemiol. 2009;169(12):1428-1436. [DOI] [PubMed] [Google Scholar]
- 378. Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2010;23(3): 199-203. [DOI] [PubMed] [Google Scholar]
- 379. Ornoy A. Prenatal origin of obesity and their complications: gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol. 2011;32(2):205-212. [DOI] [PubMed] [Google Scholar]
- 380. Page KA, Romero A, Buchanan TA, et al. . Gestational diabetes mellitus, maternal obesity, and adiposity in offspring. J Pediatr. 2014;164(4):807-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Grunnet LG, Hansen S, Hjort L, et al. . Adiposity, dysmetabolic traits, and earlier onset of female puberty in adolescent offspring of women with gestational diabetes mellitus: a clinical study within the Danish National Birth Cohort. Diabetes Care. 2017;40(12):1746-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Logan KM, Emsley RJ, Jeffries S, et al. . Development of early adiposity in infants of mothers with gestational diabetes mellitus. Diabetes Care. 2016;39(6):1045-1051. [DOI] [PubMed] [Google Scholar]
- 383. Catalano PM, Farrell K, Thomas A, et al. . Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90(5):1303-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Chung WK, Erion K, Florez JC, et al. . Precision medicine in diabetes: a consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(7):1617-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Cheney C, Shragg P, Hollingsworth D. Demonstration of heterogeneity in gestational diabetes by a 400-kcal breakfast meal tolerance test. Obstet Gynecol. 1985;65(1):17-23. [PubMed] [Google Scholar]
- 386. Powe CE, Allard C, Battista MC, et al. . Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus. Diabetes Care. 2016;39(6):1052-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Catalano PM, Tyzbir ED, Roman NM, et al. . Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol. 1991;165(6 Pt 1):1667-1672. [DOI] [PubMed] [Google Scholar]
- 388. Powe CE, Hivert MF, Udler MS. Defining heterogeneity among women with gestational diabetes mellitus. Diabetes. 2020;69(10):2064-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Benhalima K, Van Crombrugge P, Moyson C, et al. . Characteristics and pregnancy outcomes across gestational diabetes mellitus subtypes based on insulin resistance. Diabetologia. 2019;62(11):2118-2128. [DOI] [PubMed] [Google Scholar]
- 390. Sweeting A, Park F, Hyett J. The first trimester: prediction and prevention of the great obstetrical syndromes. Best Pract Res Clin Obstet Gynaecol. 2015;29(2):183-193. [DOI] [PubMed] [Google Scholar]
- 391. Coustan DR, Nelson C, Carpenter MW, et al. . Maternal age and screening for gestational diabetes: a population-based study. Obstet Gynecol. 1989;73(4):557-561. [PubMed] [Google Scholar]
- 392. Lavin JP Jr. Screening of high-risk and general populations for gestational diabetes. Clinical application and cost analysis. Diabetes. 1985;34(suppl 2):24-27. [DOI] [PubMed] [Google Scholar]
- 393. Weeks JW, Major CA, de Veciana M, et al. . Gestational diabetes: does the presence of risk factors influence perinatal outcome? Am J Obstet Gynecol. 1994;171(4):1003-1007. [DOI] [PubMed] [Google Scholar]
- 394. Cosson E, Benbara A, Pharisien I, et al. . Diagnostic and prognostic performances over 9 years of a selective screening strategy for gestational diabetes mellitus in a cohort of 18,775 subjects. Diabetes Care. 2013;36(3):598-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Chevalier N, Fenichel P, Giaume V, et al. . Universal two-step screening strategy for gestational diabetes has weak relevance in French Mediterranean women: should we simplify the screening strategy for gestational diabetes in France? Diabetes Metab. 2011;37(5):419-425. [DOI] [PubMed] [Google Scholar]
- 396. Moses RG, Moses J, Davis WS. Gestational diabetes: do lean young Caucasian women need to be tested? Diabetes Care. 1998;21(11):1803-1806. [DOI] [PubMed] [Google Scholar]
- 397. Avalos GE, Owens LA, Dunne F, et al. . Applying current screening tools for gestational diabetes mellitus to a European population: is it time for change? Diabetes Care. 2013;36(10): 3040-3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Teede HJ, Harrison CL, Teh WT, et al. . Gestational diabetes: development of an early risk prediction tool to facilitate opportunities for prevention. Aust N Z J Obstet Gynaecol. 2011;51(6):499-504. [DOI] [PubMed] [Google Scholar]
- 399. Syngelaki A, Pastides A, Kotecha R, et al. . First-trimester screening for gestational diabetes mellitus based on maternal characteristics and history. Fetal Diagn Ther. 2015;38(1):14-21. [DOI] [PubMed] [Google Scholar]
- 400. Savvidou M, Nelson SM, Makgoba M, et al. . First-trimester prediction of gestational diabetes mellitus: examining the potential of combining maternal characteristics and laboratory measures. Diabetes. 2010;59(12):3017-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Lamain-de Ruiter M, Kwee A, Naaktgeboren CA, et al. . Prediction models for the risk of gestational diabetes: a systematic review. Diagn Progn Res. 2017;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Sweeting AN, Wong J, Appelblom H, et al. . A novel early pregnancy risk prediction model for gestational diabetes mellitus. Fetal Diagn Ther. 2019;45(2):76-84. [DOI] [PubMed] [Google Scholar]
- 403. Sweeting AN, Wong J, Appelblom H, et al. . A first trimester prediction model for gestational diabetes utilizing aneuploidy and pre-eclampsia screening markers. J Matern Fetal Neonatal Med. 2018;31(16):2122-2130. [DOI] [PubMed] [Google Scholar]
- 404. Aronson JK, Ferner RE. Biomarkers-a general review. Curr Protoc Pharmacol. 2017;76:9 23 19 17. [DOI] [PubMed] [Google Scholar]
- 405. Allinson JL. Clinical biomarker validation. Bioanalysis. 2018;10(12):957-968. [DOI] [PubMed] [Google Scholar]
- 406. Sattar N, Wannamethee SG, Forouhi NG. Novel biochemical risk factors for type 2 diabetes: pathogenic insights or prediction possibilities? Diabetologia. 2008;51(6):926-940. [DOI] [PubMed] [Google Scholar]
- 407. O’Malley EG, Reynolds CME, Killalea A, et al. . The use of biomarkers at the end of the second trimester to predict Gestational Diabetes Mellitus. Eur J Obstet Gynecol Reprod Biol. 2020;250:101-106. [DOI] [PubMed] [Google Scholar]
- 408. Richardson AC, Carpenter MW. Inflammatory mediators in gestational diabetes mellitus. Obstet Gynecol Clin North Am. 2007;34(2):213-224, viii. [DOI] [PubMed] [Google Scholar]
- 409. Eleftheriades M, Papastefanou I, Lambrinoudaki I, et al. . Elevated placental growth factor concentrations at 11-14 weeks of gestation to predict gestational diabetes mellitus. Metabolism. 2014;63(11):1419-1425. [DOI] [PubMed] [Google Scholar]
- 410. Lovati E, Beneventi F, Simonetta M, et al. . Gestational diabetes mellitus: including serum pregnancy-associated plasma protein-A testing in the clinical management of primiparous women? A case-control study. Diabetes Res Clin Pract. 2013;100(3):340-347. [DOI] [PubMed] [Google Scholar]
- 411. White SL, Lawlor DA, Briley AL, et al. . Early antenatal prediction of gestational diabetes in obese women: development of prediction tools for targeted intervention. PLoS One. 2016;11(12):e0167846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Rasanen JP, Snyder CK, Rao PV, et al. . Glycosylated fibronectin as a first-trimester biomarker for prediction of gestational diabetes. Obstet Gynecol. 2013;122(3):586-594. [DOI] [PubMed] [Google Scholar]
- 413. Watanabe N, Morimoto S, Fujiwara T, et al. . Prediction of gestational diabetes mellitus by soluble (pro)renin receptor during the first trimester. J Clin Endocrinol Metab. 2013;98(6):2528-2535. [DOI] [PubMed] [Google Scholar]
- 414. Theriault S, Giguere Y, Masse J, et al. . Early prediction of gestational diabetes: a practical model combining clinical and biochemical markers. Clin Chem Lab Med. 2016;54(3):509-518. [DOI] [PubMed] [Google Scholar]
- 415. Syngelaki A, Kotecha R, Pastides A, et al. . First-trimester biochemical markers of placentation in screening for gestational diabetes mellitus. Metabolism. 2015;64(11):1485-1489. [DOI] [PubMed] [Google Scholar]
- 416. Gobl CS, Bozkurt L, Rivic P, et al. . A two-step screening algorithm including fasting plasma glucose measurement and a risk estimation model is an accurate strategy for detecting gestational diabetes mellitus. Diabetologia. 2012;55(12):3173-3181. [DOI] [PubMed] [Google Scholar]
- 417. Australasian Diabetes in Pregnancy Society. Diagnostic testing for gestational diabetes mellitus (GDM) during the COVID 19 pandemic: antenatal and postnatal testing advice: a statement from the Australasian Diabetes in Pregnancy Society (ADIPS). Last updated April 7, 2020. Accessed June 12, 2021. https://www.adips.org/documents/COVID19-WITHQLDGUIDELINES0704201150ADIPSADSADEADAupdated.pdf
- 418. Royal College of Obstetricians. Guidance for maternal medicine in the evolving coronavirus Covid-19 pandemic. Published July 10, 2020. Accessed June 30, 2021. https://www.rcog.org.uk/globalassets/documents/guidelines/2020-07-10-guidance-for-maternal-medicine.pdf
- 419. Simmons D, Rudland VL, Wong V, et al. . Options for screening for gestational diabetes mellitus during the SARS-CoV-2 pandemic. Aust N Z J Obstet Gynaecol. 2020;60(5):660-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. McIntyre HD, Gibbons KS, Ma RCW, et al. . Testing for gestational diabetes during the COVID-19 pandemic. An evaluation of proposed protocols for the United Kingdom, Canada and Australia. Diabetes Res Clin Pract. 2020;167:108353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Meek CL, Lindsay RS, Scott EM, et al. . Approaches to screening for hyperglycaemia in pregnant women during and after the COVID-19 pandemic. Diabet Med. 2021;38(1):e14380. [DOI] [PMC free article] [PubMed] [Google Scholar]