Abstract
Objective
The aims of this study were to investigate trends in bone mineral density (BMD) loss and related factors in early postmenopausal women in Japan, identify risk factors for future osteoporosis, and predict osteoporosis before it occurs.
Methods
The study population consisted of women who were 50 to 54 years old at the time of the survey in 2002 or 2006. The study included a questionnaire and physical measurement findings (BMD, height, body weight [WT], body mass index [BMI], and handgrip strength). One hundred sixty-seven women continued to participate in the study and had BMD measurements at the 9- or 10-year follow-up of the Japanese Population-based Osteoporosis study. Statistical analyses were performed using Pearson correlation to examine each factor of physical measurement and BMD for lumbar spine (LS) and femoral neck (FN). The receiver operating characteristic curve of this data was also predictive of osteoporosis in 2011 for 2002 data; BMD at the age of 50 to 54 years was then used to predict the likelihood of being diagnosed with osteoporosis 9 and 10 years later.
Results
At the baseline in 2002 and 2006, WT, BMI, height, and handgrip strength were positively correlated with BMD. The optimal cutoff values for BMD in 2006 to predict osteoporosis in 2016 were LS less than 0.834 g/cm2 and FN less than 0.702 g/cm2. These data were also predictive of osteoporosis in 2011 for 2002 data; applying this to the 2002 data, LS/FN had a sensitivity of 92%/100%, a specificity of 87%/81%, a positive predictive value of 55%/48%, and a negative predictive value of 98%/100%. The larger WT and BMI also resulted in a greater decrease in BMD of FN after 9 or 10 years.
Conclusions
We have identified a cutoff value for BMD to predict future osteoporosis in menopausal women and found a negative correlation between WT and BMI in menopausal women and changes in BMD of the FN over the next 10 years.
Key Words: Body mass index (BMI), Bone mineral density (BMD), Menopause, Osteoporosis
Osteoporosis is a condition with an increased risk of fragility fractures. When fractures of the lumbar spine (LS) or femoral neck (FN) occur because of osteoporosis, there is a simultaneous decline in physical function, activities of daily living, and quality of life.1 Bone mineral density (BMD) is one of the most important indicators for screening the risk of osteoporosis and fragility fractures. In Japan, the first step in diagnosing osteoporosis is to check for fragility fractures. A proximal femoral or vertebral fracture is considered osteoporosis, whereas a fragility fracture other than a proximal femoral or vertebral fracture is considered osteoporosis if the BMD is less than 80% of the young adult mean (YAM). In the absence of fragility fractures, osteoporosis is present if BMD is less than 70% of YAM or −2.5 SD. Depending on the site being measured, there is a large discrepancy between YAM70% and −2.5 SD. It is difficult to detect the risk of osteoporosis or fragility fracture by oneself in advance because it is often asymptomatic, even when BMD decreases. Decreased BMD and aging are both risk factors for osteoporosis, and the number of osteoporosis patients is expected to rise in Japan, where the population is aging rapidly. According to previously published population-based cohort studies in Japan, the estimated number of patients with LS osteoporosis in the Japanese population 40 years and older was approximately 6.4 million (800,000 men and 5.6 million women), and the estimated number of patients with FN osteoporosis was approximately 10.7 million (2.6 million men and 8.1 million women).2,3 However, in Japan, the rate of osteoporosis screening is low, and many osteoporosis patients are given a diagnosis after a fracture. It has been shown that fragility fractures due to osteoporosis directly impact life expectancy, but being osteoporotic and having low BMD also increase the risk of death.4–7
To date, various studies have been conducted to elucidate the causes of osteoporosis and the factors that control it, resulting in identifying therapeutic targets and the development of therapeutic agents. However, in Japan, which has become a super-aged society ahead of the rest of the world, it is extremely important to prevent osteoporosis in the future, treat osteoporosis, and search for factors that cause BMD loss.
BMD in women does not fluctuate significantly from their 20s to late 40s, and healthy women without underlying diseases are not at a high risk of osteoporosis. However, a rapid decrease in estrogen after menopause leads to a significant decrease in BMD, and the rapid decrease is said to last for about 10 years.8,9 Therefore, even if BMD is not low enough to meet the diagnostic criteria for osteoporosis during the perimenopausal period but does begin to decline, there is a high possibility that osteoporosis will be diagnosed in the future because of a further decline in BMD after menopause. In addition, even if BMD is not low in the perimenopausal period, if there are factors that increase BMD loss, there is a high possibility that osteoporosis will be diagnosed in the future because of postmenopausal BMD loss. Therefore, if we can identify women at a high risk of osteoporosis at the time of perimenopause when BMD is thought to begin to decline, we can intervene at an earlier stage by raising awareness and providing lifestyle guidance and thus prevent osteoporosis in the future.
FRAX is a predictive tool for fragility fractures associated with osteoporosis.10 FRAX uses an algorithm based on risk factors obtained from a meta-analysis of cohort studies worldwide to identify individuals at a high risk of fracture and provide an index for therapeutic intervention. In Japan, FRAX was incorporated into the revised “Guidelines for the Prevention and Treatment of Osteoporosis” in 2011. However, more than 90% of Japanese women older than 75 years have a 15% FRAX fracture probability, so although FRAX is added to the criteria for initiating treatment, the target population is limited to those younger than 75 years.11
Therefore, we focused on the data collected in the Japanese Population-based Osteoporosis (JPOS) cohort study, an epidemiological study that started in 1996 to prevent osteoporosis in women in Japan, that captures the characteristics of Japanese women.12 The reason for focusing on these data is that multiple follow-up studies have been conducted on the same case since 1996. One of the main objectives of the JPOS study was to establish a cutoff value of BMD for the diagnosis of osteoporosis in Japanese women. In 2001, Iki et al13 published the cutoff values of BMD by measurement site in Japanese women. The cutoff values set from the World Health Organization criteria and the data obtained in the JPOS study showed no difference to a fourfold to fivefold difference depending on the site. According to the data obtained in this study, which is more characteristic of Japanese women, the decline in BMD began to become more pronounced in their 50s.
In this study, to examine risk factors for osteoporosis, we analyzed cases that participated in the survey in their early 50s, when BMD is expected to start declining, and also participated in the survey 9 to 10 years after menopause when BMD decline is expected to be significant.
METHODS
The JPOS cohort study
The JPOS cohort study, a large-scale epidemiological study using a representative sample of Japanese women, is still ongoing to clarify the actual status of osteoporosis. The JPOS study has previously defined and published age-specific reference values for BMD in Japanese women, and the World Health Organization and the Japanese Society for Bone and Mineral Research criteria for BMD are used for the diagnosis of osteoporosis.13 The JPOS study began in 1996 with a survey of 50 randomly selected women aged 15 to 79 years in seven Japanese cities, divided into five age groups and 650 women per district, for 4,550 women. The number of survey targets has not been increased since then, and follow-up surveys were conducted in 1999, 2002, 2006, 2011, and 2016 in cities where cooperation was obtained and ongoing. In the 1996 survey, the survey items included BMD measurement by dual-energy x-ray absorptiometry (DEXA) and vertebral deformity determination, general examination and bone metabolism marker measurement by a blood test, bone metabolism marker measurement by a urine test, confirmation of medical history by interview, and hearing about the life history and calcium intake. In the follow-up survey, in addition to BMD measurement by DEXA and vertebral deformity determination, body composition measurement was added from the 2011 survey, and exercise capacity tests, such as handgrip (HG) strength and 10-m walking speed, were added from the 2011 survey, and arterial stiffness test was added from the 2006 survey.
Participants
The study population consisted of 235 women aged 50 to 54 years who participated in the JPOS follow-up surveys in 2002 and 2006. Of these, 167 women whose BMD was still being measured 9 to 10 years later were also examined for changes in BMD. The number of people who could participate in the 2011 follow-up survey might have been reduced because of the earthquake that occurred in Japan in 2011. The background of the study participants is shown in the Table 1. In the interview, we asked about menopause, history of preference, hormone therapy up to the survey time, and history of fracture of the own mother at 50 years or older. Menopause was defined as amenorrhea of 1 year or more, and menstrual irregularities in the perimenopausal period were included in menstruation. Habitual smokers were counted according to whether they never smoked or had smoked, and the number of cigarettes smoked and whether or not they had quit smoking were not taken into account.
TABLE 1.
The baseline characteristics of participants in the 2002 and 2006 follow-up surveys
| 2002 survey | 2006 survey | |
|---|---|---|
| No. subjects | 137 | 98 |
| Age, mean (SD), y | 52.6 (1.47) | 52.6 (1.44) |
| Height, mean (SD), cm | 154.0 (4.65) | 154.3 (4.88) |
| Weight, mean (SD), kg | 56.2 (9.26) | 56.2 (8.80) |
| BMI, mean (SD), kg/m2 | 23.7 (3.70) | 23.6 (3.54) |
| Postmenopausal women, n (%) | 100 (73.0) | 64 (65.3) |
| Current smokers, n (%) | 10 (7.3) | 7 (7.1) |
| Habitual drinkers, n (%) | 57 (41.6) | 33 (33.7) |
| Women with a history of hormone therapy, n (%) | 22 (16.1) | 18 (18.4) |
| Women with a history of fragility fractures in their own mother at 50 years or older, n (%) | 34 (24.8) | 24 (24.5) |
BMI, body mass index; HT, height; WT, weight.
Similarly, habitual drinkers were counted based on whether they never or ever drank alcohol and not on the amount of alcohol they drank per day, how often they drank, or whether they abstained from alcohol for a period. We do not consider when, what drug, route of administration, or duration of hormone therapy ever received. The design of the JPOS study reduces the number of people surveyed each year; the most recent follow-up data for the JPOS study are from 2016, and the next most recent is from 2011. The city in which the JPOS study was being conducted and the year in which the study was conducted limit the number of cases for which data can be extracted to ages 50 to 54 years and about 10 years after that. This analysis compared the 2011 data as follow-up data with the 2002 baseline data and the 2016 data as follow-up data with the 2006 baseline data. Because of the small number of cases in the 2016 data alone, we decided to use the 2011 data, so the study population was the same as above.
All participants provided written informed consent before participating in the study. The ethics committee approved the study protocol of the Faculty of Medicine, Kindai University, which reviewed the research for scientific rationality and ethical considerations following the ethical principles of the Declaration of Helsinki.
BMD measurement
BMD (g/cm2) was measured by a certified radiological technologist using a single DEXA scanner at the LS (L2-L4) and right FN (QDR4500A; Hologic, Inc, Bedford, MA). To obtain BMD values at the LS, we excluded vertebrae with fractures or degenerative changes causing an increase of more than 1 SD in BMD than the BMD of immediately adjacent vertebrae following the International Society for Clinical Densitometry rules.14 The in vivo short-term precision (coefficient of variation [CV]) values of the BMD measurement calculated based on five measurements collected on different days from each of the five volunteers were 1.2% and 1.2% for the spine and FN, respectively.13
A DEXA was used in all seven cities, so there is no difference between the study sites. The in vivo reproducibility of a BMD, represented by CVs, was 1.04% for a BMD at the LS and 1.10% for a BMD at the FN.13 An LS phantom was scanned daily before and after study measurements for quality control purposes (0.40%, in vitro CV). No remarkable drift in the BMD value of the phantom was observed during the study period.
The cutoff values for the diagnosis of osteoporosis in Japanese women were based on previous reports, with BMD corresponding to 70% of YAM and −2.5 SD in T score: 0.722 g/cm2 and 0.759 g/cm2 for LS and 0.561 g/cm2 and 0.536 g/cm2 for FN.13 Recalculating the respective YAM70% BMD to T score, the LS BMD of 0.722 g/cm2 was −2.83486, and the FN BMD of 0.561 g/cm2 was −2.26415.
Body measurements
Height (HT) and weight (WT) were measured using an automatic scale. BMI (kg/m2) was calculated as WT divided by HT squared. HG strength (kg) was measured using a digital HG strength dynamometer, and the CV was 3.62%. Participants were asked to squeeze the dynamometer with maximum effort; two attempts were made with each hand.15 The maximum value (in 0.1 kg) and the average of the values were used for this study. The HG was measured twice, once on the left and once on the right, and the one with the higher value was used.
Analysis methods
First, we used the data from 2002 and 2006 as a baseline analysis to examine the correlation between BMD and the results of physical measurements.
Next, we used the data from 2006 to 2016 as a training set and the data from 2002 to 2011 as a test set to calculate a cutoff value to predict future cases of osteoporosis. The cutoff value was determined from the receiver operating characteristic (ROC) curve to determine the number of patients who did not have osteoporosis in 2006 but would have osteoporosis in 2016. Next, we examined whether the cutoff value determined in the training set could be reproduced in the test set to predict future osteoporosis.
Similarly, we analyzed the FN BMD of the patients who were 50 to 54 years old in 2006, compared it with that in 2016, calculated the cutoff value for osteoporosis after 10 years, and applied it to the 2002 data to see whether future osteoporosis could be predicted. The T score corresponding to this cutoff value was calculated using the mean BMD values and standard deviations of young women reported in the JPOS study.13
Finally, we examined the association between baseline physical measurements at 50 to 54 years old and BMD change 10 years later. The amount of change in BMD was calculated from the difference in data. The rate of change in BMD was calculated from the percentage of decrease based on the BMD at baseline.
Statistical analysis
Heat maps were drawn using R version 3.2.2 (a language and environment for statistical computing, https://www.R-project.org/). Statistical computations were performed using GraphPad Prism version 9. Pearson correlation was used to examine each factor and the BMD of LS and FN. Regression equations were also calculated. The ROC curves for BMD at ages 50 to 54 years were used to predict the likelihood of being given a diagnosis of osteoporosis 9 and 10 years later, with LS of 0.722 g/m2 and FN of 0.536 g/m2 to predict the likelihood of being given a diagnosis of osteoporosis 9 and 10 years later. Set as the cutoff, the ROC curve with Youden's index as the maximum, “sensitivity − (1 − specificity)” was used to examine the results.16 Youden's index was calculated using the cutoff value at the BMD value at which each of the [sensitivity + (1 − specificity)] values was the largest. A P value less than 0.05 was considered statistically significant for all studies.
RESULTS
The number of participants aged 50 to 54 years in 2002 was 137, and the number of participants aged 50 to 54 years in 2006 was 98. The baseline characteristics (the means and standard deviations of age, HT, WT, and BMI) of 137 participants in the 2002 follow-up survey and 98 participants in the 2006 follow-up survey are shown in the Table, and there was no bias between the groups. The percentages for being menopausal, having smoked, having a drinking habit, having taken hormone therapy, having a birth mother older than 50 years, and having had a fracture, as confirmed by questionnaires respectively, are also shown in the Table, with no bias between groups.
To examine the correlation with BMD, we plotted all measurements and BMD and drew an approximate line. The significant correlations are shown in (Supplemental Figures 1 to 4, http://links.lww.com/MENO/A986, http://links.lww.com/MENO/A987, http://links.lww.com/MENO/A988, http://links.lww.com/MENO/A989). In the 2002 and 2006 data, LS (Fig. 1A, B) and FN (Fig. 1C, D) were positively correlated with WT (r = 0.32, P < 0.001), BMI (r = 0.25, P < 0.01), mean HG strength (r = 0.34, P < 0.001), and maximum HG strength (r = 0.34, P < 0.001) with significant differences. In 2002, it was also positively correlated with HT (r = 0.23, P < 0.01).
FIG. 1.
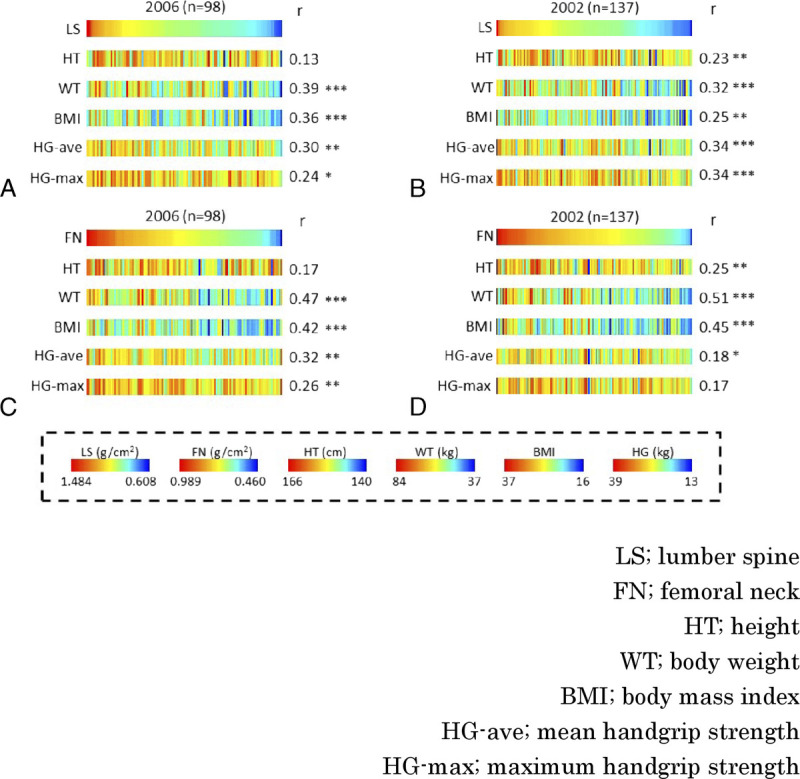
Baseline analysis. Correlations between BMD and HT, WT, BMI, HG-ave, and HG-max at BMD in women aged 50 to 54 years. (A) Correlations are shown by sorting by LS in 2006 (n = 98), (B) LS in 2002 (n = 137), (C) FN in 2006, and (D) FN in 2002. BMD, bone mineral density; r, Pearson correlation coefficient. *P < 0.05, **P < 0.01, ***P < 0.001.
In addition, LS BMD decreased significantly over the 10-year period from baseline and included those without osteoporosis (<0.722 g/cm2 or <0.759 g/cm2) in 2006 but given a diagnosis of osteoporosis in 2016 (Fig. 2A). Therefore, we drew an ROC curve in 2006 to determine whether LS BMD could predict whether LS BMD would be osteoporotic (<0.722 g/cm2 or <0.759 g/cm2) 10 years later.
FIG. 2.
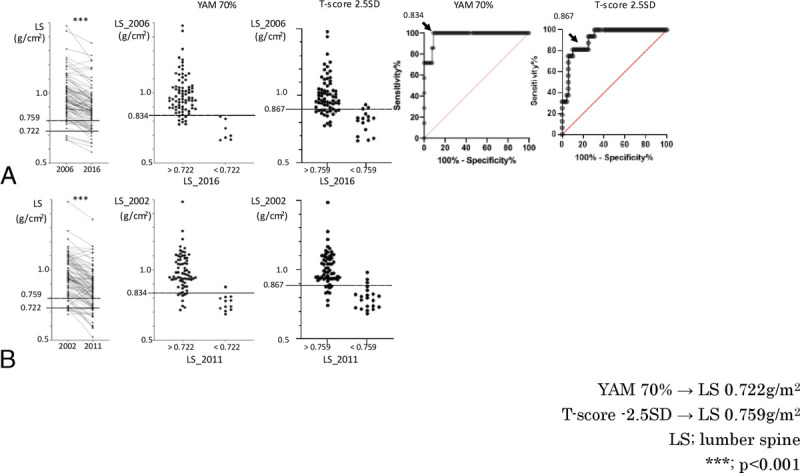
Follow-up analysis of LS BMD. (A) Analysis of 84 patients who had BMD measurements in 2006 and 2016. Left: Women 50 to 54 years old in 2006 had a significantly lower LS BMD in 2016. The line of osteoporosis (<0.722 g/cm2) is shown. Middle (Left): LS BMD in 2006 in cases without osteoporosis (>0.722) and cases with osteoporosis (<0.722) in 2016. The cutoff line for the diagnosis of osteoporosis (<0.759 g/cm2) is shown. Middle (Right): LS BMD in 2006 in cases without osteoporosis (>0.759) and cases with osteoporosis (<0.759) in 2016. Right (Left): in the ROC curve, the cutoff (arrow) of LS BMD in 2006, when the Youden Index was maximum, was less than 0.834 g/cm2. Right (Right): in the ROC curve, the cutoff (arrow) of LS BMD in 2006, when the Youden Index was maximum, was less than 0.867 g/cm2. (B) Analysis of 83 patients who had BMD measurements in 2002 and 2011. Left: LS BMD of women 50 to 54 years old in 2002 was significantly lower in 2011. Middle (Left): cutoff less than 0.834, determined by the change in 2006 to 2016, was applied to 2002. Middle (Right): cutoff less than 0.867, determined by the change in 2006 to 2016, was applied to 2002. BMD, bone mineral density; ROC, receiver operating characteristic. ***P < 0.001.
The optimal cutoff value for LS BMD in 2006, when LS BMD would be less than 0.722 g/cm2 or less than 0.759 g/cm2 in 2016, was less than 0.834 g/cm2 (T score = −1.8) or less than 0.867 g/cm2 (T score = −1.5). The optimal cutoff value for LS BMD in 2006 was less than 0.834 g/cm2 (T score = −1.8), with a sensitivity of 100%, a specificity of 91%, a positive predictive value of 50%, and a negative predictive value of 100% (<0.867 g/cm2 [T score = −1.5], with a sensitivity of 81%, a specificity of 90%, a positive predictive value of 65%, and a negative predictive value of 95%) (Fig. 2A).
Therefore, we examined whether this cutoff value (<0.834 g/cm2) was also applicable to the change from 2002 to 2011. The sensitivity was 92%, specificity was 87%, positive predictive value was 55%, and negative predictive value was 98% (<0.867 g/cm [T score = −1.5], with a sensitivity of 81%, a specificity of 81%, a positive predictive value of 77%, and a negative predictive value of 93%) for LS BMD less than 0.834 g/cm2 in 2002, and LS BMD was osteoporotic (<0.722 g/cm2) in 2011 (Fig. 2B).
When we analyzed FN BMD in the same way as LS, we found that the BMD significantly decreased in 10 years from the age of 50 to 54 years (Fig. 3A), and when we drew an ROC curve to examine whether we could predict that FN BMD would decrease to the osteoporosis level (0.561 g/cm2 or 0.536 g/cm2) in 10 years in 2006, we found that the optimal cutoff value for FN BMD in 2006 was less than 0.702 g/cm2 (T score = −0.9) or 0.676 g/cm2 (T score = −1.2), with a sensitivity of 96%, a specificity of 79%, a positive predictive value of 63%, and a negative predictive value of 98% (<0.676 g/cm2 [T score = −1.2], with a sensitivity of 100%, a specificity of 82%, a positive predictive value of 57%, and a negative predictive value of 100%). When we examined whether this cutoff value also applied to the change from 2002 to 2011, we found that if the FN BMD was 0.702 g/cm2 in 2002, the sensitivity was 100%, specificity was 81%, positive predictive value was 48%, and negative predictive value was 100% (<0.676 g/cm2 [T score = −1.2], with a sensitivity of 68%, a specificity of 82%, a positive predictive value of 26%, and a negative predictive value of 97%), and in 2011, the FN BMD was osteoporotic (<0.561 g/cm2 or <0.536 g/cm2) (Fig. 3B).
FIG. 3.
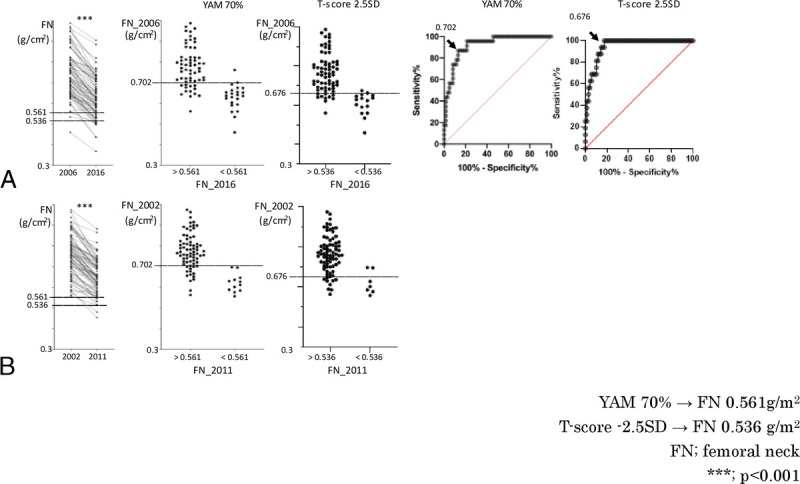
Follow-up analysis of FN BMD. (A) Left: women who were 50 to 54 years old in 2006 had a significantly lower FN BMD in 2016. This indicates a line of osteoporosis (<0.561 g/cm2). Middle (Left): FN values in 2006 in cases who were not osteoporotic (FN > 0.561) and those who were osteoporotic (FN < 0.561) in 2016. This indicates a line of osteoporosis (<0.536 g/cm2). Middle (Right): FN values in 2006 in cases who were not osteoporotic (FN > 0.536) and those who were osteoporotic (FN < 0.536) in 2016. Right (Left): in the ROC curve, the cutoff (arrow) of FN in 2006, when the Youden Index was maximum, was less than 0.702 g/cm2. Right (Right): in the ROC curve, the cutoff (arrow) of FN in 2006, when the Youden Index was maximum, was less than 0.676 g/cm2. (B) Left: FN BMD of women 50 to 54 years old in 2002 was significantly lower than that in 2011. Middle (Left): cutoff less than 0.702, determined by the change in 2006 to 2016, was fitted to 2002. Middle (Right): cutoff less than 0.676, determined by the change in 2006 to 2016, was fitted to 2002. BMD, bone mineral density; ROC, receiver operating characteristic. ***P < 0.001.
Similarly, in the analysis of participants aged 50 to 54 years in 2002, none of the baseline physical measurements significantly correlated with changes in LS BMD until 2011 (Fig. 4A). Similarly, in the analysis of participants aged 50 to 54 years in 2002, none of the baseline anthropometric results were significantly correlated with changes in LS BMD until 2011 (Fig. 4B). On the other hand, for FN BMD, the larger the WT (r = −0.44, P < 0.001) and BMI (r = −0.43, P < 0.001) in 2006, the greater the decrease over the following 10 years (Fig. 4C). This trend was replicated for WT (r = −0.36, P < 0.001) and BMI (r = −0.34, P < 0.01) in 2002 and for changes in BMD in FN over the next 9 years (Fig. 4D). Similar to Figure 1, Figure 4 also correlates with BMD changes in (Supplemental Figures 5 to 8, http://links.lww.com/MENO/A990, http://links.lww.com/MENO/A991, http://links.lww.com/MENO/A992, http://links.lww.com/MENO/A993).
FIG. 4.
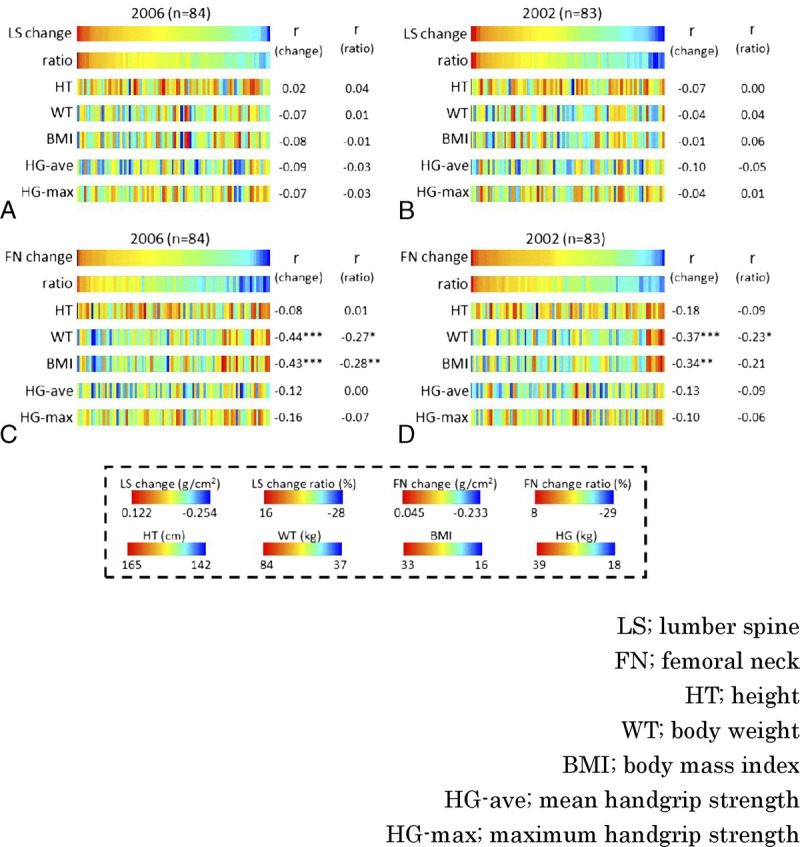
Follow-up analysis. Correlation between the amount or rate of change in BMD in women aged 50 to 54 years after 9 or 10 years and HT, WT, BMI, HG-ave, and HG-max at BMD measurement in women aged 50 to 54 years. (A) Sorted by the amount of change in LS from 2006 to 2016 (n = 84). (B) Sorted by the amount of change in LS from 2002 to 2011 (n = 83). (C) Sorted by the amount of change in FN from 2006 to 2016. (D) Sorted by the amount of change in FN from 2002 to 2011. Correlation with the amount or rate of change showed that women with heavier WT and larger BMI had a larger decrease in FN BMD. r, Pearson correlation coefficient. BMD, bone mineral density. *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
Because BMD is greatly influenced by cultural and ethnic background, it is important to analyze a homogeneous population to perform robust analyses.17 The JPOS cohort study is unique because repeated BMD measurements were performed on the same cohort of Japanese women over a long period.12 Previously, we reported a significant association between elevated levels of perimenopausal bone metabolism markers and osteopenia of the FN.18 In this study, we used more recent data and focused our analysis on cases with a baseline of 50 to 54 years. This study is unique in that we focused on the perimenopausal period, examined BMD changes over approximately 10 years, and validated the results in two populations. In this study, we used the data from 2006 to 2016 as the training set and the data from 2002 to 2011 as the test set and found that a BMD less than 0.834 g/cm2 for LS (Fig. 2) and a BMD less than 0.702 g/cm2 for FN (Fig. 3) at the age of 50 to 54 years would lead to osteoporosis 9 to 10 years later. On the basis of the high negative predictive value of the test set, it was found that if the cutoff was exceeded in the early 50s, osteoporosis would not occur after 10 years, but because the positive predictive value was about 50% for both LS and FN, if the cutoff was less than 50% in the early 50s, osteoporosis would occur after 10 years in about half of the cases. The positive predictive value for LS and FN is about 50%, so if the cutoff is less than the cutoff in the early 50s, about half of the patients will develop osteoporosis in 10 years. From these data, it can be said that BMD screening in the early 50s in Japanese women can indicate the risk of osteoporosis in the future more concretely.
When examining the association between body measurements and BMD at baseline at 50 to 54 years old, HG strength was positively correlated with BMD (Fig. 1), which is consistent with the data reported in the JPOS study, indicating that muscle strength is important for increasing BMD.19 Furthermore, HT was also positively correlated with BMD (Fig. 1). This might be why BMD and HT showed a correlation because a lack of growth hormone has been reported to cause a decrease in BMD, and short stature can be associated with a low level of growth hormone production to the degree that is not considered pathological.20,21 However, HG strength and HT at baseline did not correlate with the extent of the subsequent decline in BMD (Fig. 4).
Both WT and BMI at the age of 50 to 54 years were positively correlated with BMD (Fig. 1). Smaller WT is a risk for osteoporosis, whereas obesity has been thought to reduce the risk of osteoporosis.9,12,22,23 This has been thought to be that weight-bearing maintains bone strength because of the secretion of estrogen from fat, which acts protectively on the bone. However, surprisingly, in this study, the greater the WT and BMI, the more likely the FN BMD tended to decrease from perimenopause to 10 years (Fig. 4). In women with high WT and BMI, BMD is high in the perimenopausal period of 50 to 54 years old (Fig. 1), so even if BMD is checked in the perimenopausal period and it is similar to the average, the risk of future osteoporosis cannot be determined because it is not known whether or not it is a value that is decreasing significantly from the originally high value.
Few studies, such as the JPOS study, have measured BMD multiple times in the same patient and examined BMD changes, which might account for the difference between the previous report and our data. Recently, an epidemiological study reported that the risk of postmenopausal FN fracture increases in obese women in the early postmenopausal period up to the age of 70 years.24 These data on fracture risk seem to be consistent with the data we observed on changes in BMD. Recently, it was reported that obesity-induced low-grade systemic inflammation is accompanied by elevated tumor necrosis factor α, which induces osteoclastogenesis and bone loss through pathways such as RANKL.25,26 In animal models, the chronic inflammatory environment in the bone marrow in obesity induced by a high-fat diet leads to increased adipocytes and bone resorption.27,28 It was also shown that removing the ovaries in an obese rat model causes a large decrease in BMD.29 Adipocytes and osteoblasts are derived from a common progenitor cell, bone marrow–derived mesenchymal stem cells, and estrogen, which prevents their differentiation into adipocytes and promotes their differentiation into osteoblasts.30–32 Therefore, obesity during the postmenopausal period, when estrogen levels are low, might increase the tendency for adipocytes and osteoblasts to decrease in the bone marrow, which in turn may promote bone loss. The results of these basic studies also support our data.
A limitation of this study is that it is difficult to draw definitive conclusions because it is an analysis of a relatively small number of cases: 84 cases from 2006 to 2016 and 83 cases from 2002 to 2011. We did not consider the effect of ovariectomy or pathological loss of BMD in women younger than 50 years. There may also be a cohort effect because the studies were conducted in different years. In this study, we included cases after 2002, which is relatively recent for an epidemiological study with long-term follow-up. The rate of medical intervention for pathological BMD loss under the age of 50 years is considered high. We did not conduct enough studies to exclude the effects of such pathological BMD loss and medical interventions, and the number of cases was insufficient. A large cohort study is needed to test this, focusing on the first 10 years after perimenopause, when BMD is most likely to decline.
CONCLUSIONS
Our study identified cutoff values of BMD at the LS and FN for predicting future osteoporosis at the time of perimenopause in Japanese women. In addition, although the results were surprising from the predictions made at the beginning of the study, we found that obesity was associated with a greater loss of BMD about 10 years after perimenopause, consistent with the results of recent basic research. Causality was difficult to prove because of the small sample size. Quality of life in osteoporosis is known to be reduced even when there is no fracture, although it is not surprising that it is reduced when there is a fracture.33–37 These results indicate that measuring WT, BMI, and BMD during the perimenopausal period and identifying high-risk groups for osteoporosis through medical examinations would likely help to improve the quality of life of elderly women.
Supplementary Material
Footnotes
Funding support: This study was supported in part by Japan Society for the Promotion of Science KAKENHI grant number 20H03948 (Grant-in-Aid for Scientific Research B for M.I.) and 18K19711 (Grant-in-Aid for Scientific Research A for M.I.).
Financial disclosure/conflicts of interest: N.M. reports AstraZeneca, Takara Bio, and Takeda Pharmaceuticals. The other authors have nothing to declare.
Supplemental digital content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s Website (www.menopause.org).
Contributor Information
Akiko Kanto, Email: a-kanto@med.kindai.ac.jp.
Kosuke Murakami, Email: kmurakami@med.kindai.ac.jp.
Junko Tamaki, Email: jtamaki@osaka-med.ac.jp.
Yuho Sato, Email: y-sato@jindai.ac.jp.
Sadanobu Kagamimori, Email: skagamimori@hb.tp1.jp.
Noriomi Matsumura, Email: noriomi@med.kindai.ac.jp.
Masayuki Iki, Email: masa@med.kindai.ac.jp.
REFERENCES
- 1.Report of a WHO Study Group . Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organ Tech Rep Ser 1994;843:1–129. doi: 10.1007/BF01622200 [DOI] [PubMed] [Google Scholar]
- 2.Yoshimura N, Muraki S, Oka H, Kawaguchi H, Nakamura K, Akune T. Cohort profile: research on Osteoarthritis/Osteoporosis Against Disability study. Int J Epidemiol 2010;39:988–995. doi: 10.1093/ije/dyp276 [DOI] [PubMed] [Google Scholar]
- 3.Yoshimura N Muraki S Oka H, et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metab 2009;27:620–628. doi: 10.1007/s00774-009-0080-8 [DOI] [PubMed] [Google Scholar]
- 4.Ensrud KE Thompson DE Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc 2000;48:241–249. doi: 10.1111/j.1532-5415.2000.tb02641.x [DOI] [PubMed] [Google Scholar]
- 5.Nguyen ND, Center JR, Eisman JA, Nguyen TV. Bone loss, weight loss, and weight fluctuation predict mortality risk in elderly men and women. J Bone Miner Res 2007;22:1147–1154. doi: 10.1359/jbmr.070412 [DOI] [PubMed] [Google Scholar]
- 6.Suzuki T, Yoshida H. Low bone mineral density at femoral neck is a predictor of increased mortality in elderly Japanese women. Osteoporos Int 2010;21:71–79. doi: 10.1007/s00198-009-0970-6 [DOI] [PubMed] [Google Scholar]
- 7.Qu X Huang X Jin F, et al. Bone mineral density and all-cause, cardiovascular and stroke mortality: a meta-analysis of prospective cohort studies. Int J Cardiol 2013;166:385–393. doi: 10.1016/j.ijcard.2011.10.114 [DOI] [PubMed] [Google Scholar]
- 8.Takao S. Natural history of osteoporosis—strategy of prevention for osteoporotic fractures. Nihon Rinsho 2004;62:225–232. [Article in Japanese] [PubMed] [Google Scholar]
- 9.Waugh EJ Lam MA Hawker GA, et al. Risk factors for low bone mass in healthy 40‐60-year-old women: a systematic review of the literature. Osteoporosis Int 2009;20:1–21. doi: 10.1007/s00198-008-0643-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 1994;4:368–381. doi: 10.1007/BF01622200 [DOI] [PubMed] [Google Scholar]
- 11.Orimo H Nakamura T Hosoi T, et al. Japanese 2011 guidelines for prevention and treatment of osteoporosis—executive summary. Arch Osteoporos 2012;7:3–20. doi: 10.1007/s11657-012-0109-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iki M Tamaki J Sato Y, et al. Cohort profile: the Japanese Population-based Osteoporosis (JPOS) cohort study. Int J Epidemiol 2015;44:405–414. doi: 10.1093/ije/dyu084 [DOI] [PubMed] [Google Scholar]
- 13.Iki M, Kagamimori S, Kagawa Y, Matsuzaki T, Yoneshima H, Marumo F. Bone mineral density of the spine, hip, and distal forearm in representative samples of the Japanese female population: Japanese Population-Based Osteoporosis (JPOS) study. Osteoporosis Int 2001;12:529–537. doi: 10.1007/s001980170073 [DOI] [PubMed] [Google Scholar]
- 14.Lewiecki EM Gordon CM Baim S, et al. International Society for Clinical Densitometry 2007 adult and pediatric official positions. Bone 2008;43:1115–1121. doi: 10.1016/j.bone.2008.08.106 [DOI] [PubMed] [Google Scholar]
- 15.Huang C Niu K Kobayashi Y, et al. An inverted J-shaped association of serum uric acid with muscle strength among Japanese adult men: a cross-sectional study. BMC Musculoskelet Disord 2013;14:258. doi: 10.1186/1471-2474-14-258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–35 [DOI] [PubMed] [Google Scholar]
- 17.Leslie WD. Clinical review: ethnic differences in bone mass-clinical implications. J Clin Endocrinol Metab 2012;97:4329–4340. doi: 10.1210/jc.2012-2863 [DOI] [PubMed] [Google Scholar]
- 18.Iki M Morita A Ikeda Y, et al. Biochemical markers of bone turnover predict bone loss in perimenopausal women but not in postmenopausal women-the Japanese Population-based Osteoporosis (JPOS) cohort study. Osteoporosis Int 2006;17:1086–1095. doi: 10.1007/s00198-005-0052-3 [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, Gong HS. Measurement and interpretation of handgrip strength for research on sarcopenia and osteoporosis. J Bone Metab 2020;27:85–96. doi: 10.11005/jbm.2020.27.2.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appelman-Dijkstra NM, Rijndorp M, Biermasz NR, Dekkers OM, Pereira AM. Effects of discontinuation of growth hormone replacement in adult GH-deficient patients: a cohort study and a systematic review of the literature. Eur J Endocrinol 2016;174:705–716. doi: 10.1530/EJE-15-1086 [DOI] [PubMed] [Google Scholar]
- 21.Henry RK. Childhood growth hormone deficiency, a diagnosis in evolution: the intersection of growth hormone history and ethics. Growth Horm IGF Res 2020;55:101358. doi: 10.1016/j.ghir.2020.101358 [DOI] [PubMed] [Google Scholar]
- 22.Lloyd JT, Alley DE, Hawkes WG, Hochberg MC, Waldstein SR, Orwig DL. Body mass index is positively associated with bone mineral density in US older adults. Arch Osteoporos 2014;9:175. doi: 10.1007/s11657-014-0175-2 [DOI] [PubMed] [Google Scholar]
- 23.Gkastaris K, Goulis DG, Potoupnis M, Anastasilakis AD, Kapetanos G. Obesity, osteoporosis, and bone metabolism. J Musculoskelet Neuronal Interact 2020;20:372–381. [PMC free article] [PubMed] [Google Scholar]
- 24.Rikkonen T, Sund R, Sirola J, Honkanen R, Poole KES, Kröger H. Obesity is associated with early hip fracture risk in postmenopausal women: a 25-year follow-up. Osteoporos Int 2021;32:769–777. doi: 10.1007/s00198-020-05665-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ootsuka T, Nakanishi A, Tsukamoto I. Increase in osteoclastogenesis in an obese Otsuka Long-Evans Tokushima fatty rat model. Mol Med Rep 2015;12:3874–3880. doi: 10.3892/mmr.2015.3811 [DOI] [PubMed] [Google Scholar]
- 26.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest 2005;115:282–290. doi: 10.1172/JCI23394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patsch JM Kiefer FW Varga P, et al. Increased bone resorption and impaired bone microarchitecture in short-term and extended high-fat diet-induced obesity. Metabolism 2011;60:243–249. doi: 10.1016/j.metabol.2009.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halade GV, El Jamali A, Williams PJ, Fajardo RJ, Fernandes G. Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Exp Gerontol 2011;46:43–52. doi: 10.1016/j.exger.2010.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radzki RP, Bieńko M, Filip R, Polak P, Michalik Wolska J. Bone losses in obese, ovariectomized rats appear to be independent from sclerostin-induced inhibition of the Wnt/β-catenin pathway. Ann Agric Environ Med 2020;27:394–400. doi: 10.26444/aaem/110527 [DOI] [PubMed] [Google Scholar]
- 30.Sekiya I, Larson BL, Vuoristo JT, Cui JG, Prockop DJ. Adipogenic differentiation of human adult stem cells from bone marrow stroma (MSCs). J Bone Miner Res 2004;19:256–264. doi: 10.1359/JBMR.0301220 [DOI] [PubMed] [Google Scholar]
- 31.Gao B Huang Q Lin YS, et al. Dose-dependent effect of estrogen suppresses the osteo-adipogenic transdifferentiation of osteoblasts via canonical Wnt signaling pathway. PLoS One 2014;9:e99137. doi: 10.1371/journal.pone.0099137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin RB, Zissimos SL. Relationships between marrow fat and bone turnover in ovariectomized and intact rats. Bone 1991;12:123–131. doi: 10.1016/8756-3282(91)90011-7 [DOI] [PubMed] [Google Scholar]
- 33.Oleksik A Lips P Dawson A, et al. Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res 2000;15:1384–1392. doi: 10.1359/jbmr.2000.15.7.1384 [DOI] [PubMed] [Google Scholar]
- 34.Dhillon V, Hurst N, Hannan J, Nuki G. Association of low general health status, measured prospectively by Euroqol EQ5D, with osteoporosis, independent of a history of prior fracture. Osteoporos Int 2005;16:483–489. doi: 10.1007/s00198-004-1705-3 [DOI] [PubMed] [Google Scholar]
- 35.Masunari N, Fujiwara S, Nakata Y, Nakashima E, Nakamura T. Historical height loss, vertebral deformity, and health-related quality of life in Hiroshima cohort study. Osteoporos Int 2007;18:1493–1499. doi: 10.1007/s00198-007-0392-2 [DOI] [PubMed] [Google Scholar]
- 36.Hagino H, Nakamura T, Fujiwara S, Oeki M, Okano T, Teshima R. Sequential change in quality of life for patients with incident clinical fractures: a prospective study. Osteoporos Int 2009;20:695–702. doi: 10.1007/s00198-008-0761-5 [DOI] [PubMed] [Google Scholar]
- 37.Wilson S, Sharp CA, Davie MW. Health-related quality of life in patients with osteoporosis in the absence of vertebral fracture: a systematic review. Osteoporos Int 2012;23:2749–2768. doi: 10.1007/s00198-012-2050-6 [DOI] [PubMed] [Google Scholar]


