Abstract
Background
People with asthma may experience exacerbations, or 'attacks', during which their symptoms worsen and additional treatment is required. Written action plans sometimes advocate a short‐term increase in the dose of inhaled corticosteroids (ICS) at the first sign of an exacerbation to reduce the severity of the attack and to prevent the need for oral steroids or hospital admission.
Objectives
To compare the clinical effectiveness and safety of increased versus stable doses of ICS as part of a patient‐initiated action plan for the home management of exacerbations in children and adults with persistent asthma.
Search methods
We searched the Cochrane Airways Group Specialised Register, which is derived from searches of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and CINAHL (Cumulative Index to Nursing and Allied Health Literature), and handsearched abstracts to 20 December 2021. We also searched major trial registries for ongoing trials.
Selection criteria
We included parallel and cross‐over randomised controlled trials (RCTs) that allocated people with persistent asthma to take a blinded inhaler in the event of an exacerbation which either increased their daily dose of ICS or kept it stable (placebo).
Data collection and analysis
Two review authors independently selected trials, assessed quality, and extracted data. We reassessed risk of bias for all studies at the result level using the revised risk of bias tool for RCTs (Risk of Bias 2), and employed the GRADE approach to assess our confidence in the synthesised effect estimates. The primary outcome was treatment failure, defined as the need for rescue oral steroids in the randomised population. Secondary outcomes were treatment failure in the subset who initiated the study inhaler (treated population), unscheduled physician visits, unscheduled acute care, emergency department or hospital visits, serious and non‐serious adverse events, and duration of exacerbation.
Main results
This review update added a new study that increased the number of people in the primary analysis from 1520 to 1774, and incorporates the most up‐to‐date methods to assess the likely impact of bias within the meta‐analyses. The updated review now includes nine RCTs (1923 participants; seven parallel and two cross‐over) conducted in Europe, North America, and Australasia and published between 1998 and 2018. Five studies evaluated adult populations (n = 1247; ≥ 15 years), and four studies evaluated child or adolescent populations (n = 676; < 15 years). All study participants had mild to moderate asthma. Studies varied in the dose of maintenance ICS, age, fold increase of ICS in the event of an exacerbation, criteria for initiating the study inhaler, and allowed medications. Approximately 50% of randomised participants initiated the study inhaler (range 23% to 100%), and the included studies reported treatment failure in a variety of ways, meaning assumptions were required to permit the combining of data.
Participants randomised to increase their ICS dose at the first signs of an exacerbation had similar odds of needing rescue oral corticosteroids to those randomised to a placebo inhaler (odds ratio (OR) 0.97, 95% confidence interval (CI) 0.76 to 1.25; 8 studies; 1774 participants; I2 = 0%; moderate quality evidence). We could draw no firm conclusions from subgroup analyses conducted to investigate the impact of age, time to treatment initiation, baseline dose, smoking history, and fold increase of ICS on the primary outcome. Results for the same outcome in the subset of participants who initiated the study inhaler were unchanged from the previous version, which provides a different point estimate with very low confidence due to heterogeneity, imprecision, and risk of bias (OR 0.84, 95% CI 0.54 to 1.30; 7 studies; 766 participants; I2 = 42%; random‐effects model). Confidence was reduced due to risk of bias and assumptions that had to be made to include study data in the intention‐to‐treat and treated‐population analyses. Sensitivity analyses that tested the impact of assumptions made for synthesis and to exclude cross‐over studies, studies at overall high risk of bias, and those with commercial funding did not change our conclusions.
Pooled effects for unscheduled physician visits, unscheduled acute care, emergency department or hospital visits, and duration of exacerbation made it very difficult to determine where the true effect may lie, and confidence was reduced by risk of bias. Point estimates for both serious and non‐serious adverse events favoured keeping ICS stable, but imprecision and risk of bias due to missing data and outcome measurement and reporting reduced our confidence in the effects (serious adverse events: OR 1.69, 95% CI 0.77 to 3.71; 2 studies; 394 participants; I² = 0%; non‐serious adverse events: OR 2.15, 95% CI 0.68 to 6.73; 2 studies; 142 participants; I² = 0%).
Authors' conclusions
Evidence from double‐blind trials of adults and children with mild to moderate asthma suggests there is unlikely to be an important reduction in the need for oral steroids from increasing a patient's ICS dose at the first sign of an exacerbation. Other clinically important benefits and potential harms of increased doses of ICS compared with keeping the dose stable cannot be ruled out due to wide confidence intervals, risk of bias in the trials, and assumptions that had to be made for synthesis. Included studies conducted between 1998 and 2018 reflect evolving clinical practice and study methods, and the data do not support thorough investigation of effect modifiers such as baseline dose, fold increase, asthma severity and timing. The review does not include recent evidence from pragmatic, unblinded studies showing benefits of larger dose increases in those with poorly controlled asthma. A systematic review is warranted to examine the differences between the blinded and unblinded trials using robust methods for assessing risk of bias to present the most complete view of the evidence for decision makers.
Plain language summary
Increasing the dose of inhaled steroids or continuing the usual dose to treat asthma attacks in adults and children
Key messages
People who follow an action plan to take an inhaler containing an increased dose inhaled corticosteroids at the start of an asthma attack instead of a stable dose are probably as likely to worsen and need oral steroids. Other benefits and harms are uncertain, but overall studies that used 'blinded inhalers' so participants and staff were unaware of who received an increased dose did not suggest a benefit for people with mild to moderate asthma. It should be noted that more favourable results for poorly controlled asthma have been found in recent studies that were not eligible for this review because blinded inhalers were not used.
What is asthma?
Asthma is a common, long‐term lung condition that causes cough, shortness of breath, and wheezing. People with asthma often experience short‐term worsening of symptoms known as exacerbations, or 'attacks', that range from mild to life‐threatening.
Why is this important for people with asthma?
Asthma attacks are frightening for people with asthma and often require urgent treatment at home or in hospital. Knowing how best to control asthma attacks at the first sign of symptoms is important to avoid the need for oral steroids or emergency treatment in hospital.
Inhaled corticosteroids are a common treatment for asthma that are taken daily to reduce the likelihood of attacks occurring. Written action plans are given to people with asthma to tell them what to do if their symptoms do worsen, and these sometimes recommend a short‐term increase in the dose of inhaled corticosteroids to get symptoms back under control.
What did we want to find out?
We looked at whether increasing the dose of inhaled corticosteroids when asthma symptoms worsen reduces the need for further treatment, and if there are any harms with doing so.
What did we do?
We looked for all studies that randomly allocated people with asthma taking a daily inhaled corticosteroid to take a blinded inhaler if their symptoms worsened. The blinded inhaler either increased their usual dose of inhaled corticosteroid or kept it the same. We were interested in whether fewer people allocated to receive an increased dose went on to have an asthma attack. We measured asthma attacks in two ways: those needing a course of oral steroids, and those needing urgent care in the emergency department or in hospital. We also looked at whether the increased inhaled corticosteroids doses led to more adverse events compared with a stable dose.
We conducted broad searches, and two researchers independently evaluated studies to judge if they should be included. We recorded information about the studies, participants, and treatment strategies. We used the latest methods for bringing the results together and assessing how much each study result could be trusted. We rated each combined result as high, moderate, low, or very low quality, depending on how confident we were that it was reliable.
What did we find?
We included nine randomised controlled trials (studies where participants are randomly assigned to one of two or more treatment groups) of people with mild to moderate asthma. Five studies looked at adults, and four looked at children.
People who were given the inhaler with an increased dose of inhaled corticosteroid were about as likely to get worse and need a course of oral corticosteroids as those who were given an inhaler with a placebo (dummy treatment) or their usual dose. We have moderate confidence in this main result, but it was much more difficult to tell whether there was a benefit of a dose increase for other types of unscheduled care (seeing a doctor or going to hospital) or for reducing the duration of the attack. The results for adverse events suggest that it may be safer to keep inhaled corticosteroids stable, but we had very low confidence in the results.
What are the limitations of the evidence?
Studies varied in the dose of inhaled corticosteroids people were taking at the start of the study, how much the dose was increased in the treatment group, when and how people were told to start the inhaler, and what other medicines they were allowed to take. Only about half the participants actually needed to take the study inhaler, and when we looked just at those people, it appeared that there might be a small benefit, but we had very low confidence because the study results varied and there was a high risk of bias.
Whilst not many people needed to go to hospital or visit the emergency department during the course of the studies, this made it difficult to tell if a short‐term increase in inhaled corticosteroids is worthwhile, and our confidence in the evidence was low or very low. Studies did not report harms consistently, and the combined results were very uncertain.
How up‐to‐date is this evidence?
The review is current to 20 December 2021, and the studies were published between 1998 and 2018.
Summary of findings
Summary of findings 1. Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children.
| Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children | ||||||
| Patient or population: adults and children with chronic asthma Setting: outpatient Intervention: increased ICS dose during exacerbations Comparison: stable ICS dose during exacerbations | ||||||
| Outcomes* | Anticipated absolute effects** (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with stable ICS | Risk with increased ICS | |||||
|
Treatment failure: need for systemic corticosteroids (ITT) 46 weeks |
184 per 1000a | 180 per 1000 (147 to 220) | OR 0.97 (0.76 to 1.25) | 1774 (8 RCTs) | ⊕⊕⊕⊝
MODERATEb,c Due to risk of bias |
|
|
Treatment failure: need for systemic corticosteroids (of those starting inhaler) 45 weeks |
337 per 1000 | 299 per 1000 (215 to 398) | OR 0.84 (0.54 to 1.30) | 766 (7 RCTs) | ⊕⊝⊝⊝
VERY LOWd,e,f,g Due to inconsistency, imprecision, and very serious risk of bias |
Analysed using random‐effects model because of heterogeneity |
|
Unscheduled physician visits 44 weeks |
147 per 1000 | 142 per 1000 (102 to 195) | OR 0.96 (0.66 to 1.41) | 931 (3 RCTs) | ⊕⊕⊝⊝
LOWd,h,i,j Due to very serious imprecision |
|
|
Unscheduled acute care, ED visit, or hospital admission 47 weeks |
23 per 1000 | 12 per 1000 (4 to 35) | POR 0.50 (0.16 to 1.56) | 704 (4 RCTs) | ⊕⊝⊝⊝
VERY LOWd,k Due to risk of bias and very serious imprecision |
|
|
Serious adverse events 48 weeks |
56 per 1000 | 91 per 1000 (44 to 181) | OR 1.69 (0.77 to 3.71) | 394 (2 RCTs) | ⊕⊝⊝⊝
VERY LOWd,l,m Due to very serious risk of bias and imprecision |
|
|
Non‐serious adverse events 43 weeks |
72 per 1000 |
144 per 1000 (50 to 345) |
OR 2.15 (0.68 to 6.73) | 142 (2 RCTs) |
⊕⊝⊝⊝ VERY LOWd,l,m Due to very serious risk of bias and imprecision |
|
|
Duration of exacerbation ‐ time to symptom recovery and lung function recovery 52 weeks |
Mean time to symptom recovery was 6.1 days Time to lung function recovery was 7 days. |
Time to symptom recovery was 0.7 days longer in the intervention group (1.06 lower to 2.46 higher). Time to lung function recovery was 0.2 days shorter (1.88 shorter to 1.48 longer). |
‐ | 207 (1 RCT) | ⊕⊕⊝⊝
LOWd,e,h Due to risk of bias and imprecision |
|
| *Follow‐up duration is calculated as a weighted average of studies in each analysis. **The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ED: emergency department; ICS: inhaled corticosteroids; ITT: intention‐to‐treat population; OR: odds ratio; POR: Peto odds ratio; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aAn approximation of Rice‐McDonald 2005 events and totals was required because cross‐over adjustment was used to permit inclusion of the study in the meta‐analysis. We used the total number of participants (18) and events for each arm (11 each), and halved both (rounding up where necessary) to include approximate absolute data and weightings for the study. bStudies carrying 13.3% of the analysis weight had an overall high risk of bias, and studies carrying a further 49.8% of the weight had some concerns. Studies with overall low risk of bias accounted for approximately a third of the weight of the analysis. Biases arose mostly in domains 2 and 3 (deviations from the intended interventions and missing data), often relating to assumptions that had to be made when there were differences between the way the study reported the outcome and how it was needed for the analysis, or uncertainty regarding the population used for the study analysis (−1 for risk of bias). cWe did not prespecify bounds for downgrading for imprecision or concluding no difference between treatments. The upper and lower limits of the confidence interval may be considered clinically important benefit or harm of the intervention, but we did not consider it sufficient to downgrade given the number of events and participants in the analysis (no downgrade for imprecision). dAll studies were well‐matched to our review question. We resolved uncertainties in the definitions of outcomes through contact with study authors. Where outcome definitions or the populations used for analysis (e.g. ITT or those taking the study inhaler) were unclear or differed from what was defined in the review protocol, this was accounted for as missing data and deviation from the intended intervention in the risk of bias assessment (no downgrade for indirectness across outcomes). eUpper and lower confidence intervals include important benefit of increased or stable ICS (−1 imprecision). fI2 = 42%, P = 0.11; clear variation noted between direction and magnitude of study results by visual inspection of the forest plot (−1 inconsistency). gStudies carrying 81.6% of the analysis weight had overall high risk of bias. Biases mostly arose in domains 2 and 3 (deviations from the intended interventions and missing data), relating to assumptions that had to be made to include imperfect data in the review analysis (e.g. where the population used for the study analysis was unclear or differed from the population defined for the review analysis). There was also risk of bias from unclear and inconsistent implementation of criteria for initiating the study inhaler in some studies (−2 for risk of bias). hSeveral studies did not appear in the analysis, but contact with study authors meant this was unlikely due to selective reporting (no downgrade for publication bias). iThree studies observed 136 events leading to very wide confidence intervals, which made the result very difficult to interpret (−2 imprecision). jNo studies in the analysis were at overall high risk of bias, although there were some concerns for a study carrying 56% of the weight (no downgrade for risk of bias). kOnly 12 events in the analysis, leading to substantial imprecision in the estimate. Two studies did not observe any events and so did not contribute to the effect estimate (−2 imprecision). A large amount of heterogeneity between the two contributing study effects warranted downgrading for heterogeneity (I2 = 62%), but was captured by imprecision and very low grading. lStudies contributing the majority of the weight in both adverse events analyses were at overall high risk of bias, primarily in domains 2 and 3 (deviations from intended interventions and missing data), and additionally in domains 4 and 5 (measurement of the outcome and selection of the reported result) for non‐serious adverse events (−2 for risk of bias). mConfidence intervals included a significant increase in adverse events on increased‐dose ICS and did not exclude the possibility of no difference against stable ICS. Very few events were included in either of the adverse event analyses (−1 imprecision).
Background
Description of the condition
Asthma is the second most prevalent chronic respiratory condition worldwide, and is estimated to affect 272 million people of all ages (Soriano 2017). According to the Global Burden of Disease Study in 2017, asthma was the second leading cause of death amongst chronic respiratory diseases (Soriano 2015). Asthma exacerbations involve short‐term mild to life‐threatening worsening of symptoms which are considered an important feature in defining the severity of the disease (GINA 2021). The frequency of exacerbations is a key parameter of asthma control. Furthermore, asthma exacerbations are associated with significant morbidity, mortality, and healthcare expenditure (Ramsahai 2019). A severe exacerbation is defined as the need for systemic corticosteroids, unscheduled healthcare visits, or hospitalisation (Reddel 2009). A mild to moderate exacerbation impacts a patient’s quality of life and prompts treatment escalation to prevent its progression (Reddel 2009). Achieving early control of asthma exacerbations is thus paramount in avoiding hospitalisation and its associated costs, as well as in improving health‐related quality of life.
Description of the intervention
The underlying mechanism of asthma exacerbations is airway inflammation, often triggered by respiratory virus infection, allergen exposure, and/or respiratory irritants (Sears 2008). This airway inflammation sets up a vicious cycle of bronchial hyper‐responsiveness and mucus hypersecretion, leading to decreased expiratory flow (Sears 2008). Acute exacerbations of asthma are a medical emergency regardless of age and can be highly dependent on seasonal variation (Ramsahai 2019).
Systemic corticosteroids have potent anti‐inflammatory properties and are the most effective drugs for suppressing the underlying inflammatory response in asthma exacerbations. Common short‐term side effects of corticosteroids include sleep disturbances, increased appetite, and mood changes. However, the cumulative impact of chronic corticosteroid use includes a significantly elevated risk of osteoporosis, hypertension, diabetes mellitus, and obesity (Volmer 2018). This provides a rationale for an alternative management strategy such as the use of inhaled corticosteroids in mild‐moderate asthma exacerbation to reduce the need for systemic corticosteroids.
How the intervention might work
Inhaled corticosteroids (ICS) can reduce the frequency and severity of respiratory exacerbations (GINA 2021). Poor day‐to‐day asthma control and type 2 airway inflammation, as measured by blood eosinophils or elevated exhaled nitric oxide, are both risk factors for acute exacerbations (Kupczyk 2014). Treatment with ICS remains the cornerstone strategy in the management of chronic asthma.
The Global Initiative for Asthma and other international respiratory societies recommend self‐management strategies to reduce the impact of acute exacerbations. A written asthma action plan includes a description of maintenance therapy and instructions for increasing therapy as required. This helps patients to recognise and response appropriately to worsening symptoms. For example, when asthma symptoms are interfering with normal daily activities, or peak expiratory flow measurement has decreased by over 20% for more than two days, this should prompt a dose increase in a maintenance inhaled corticosteroid‐containing treatment (Gibson 2004; GINA 2021).
The use of short‐acting beta agonists (SABA) helps to relieve the symptoms of asthma by bronchodilation, but does not address the underlying airway inflammation. This can potentially delay seeking medical attention and may increase adverse outcomes in acute asthma (Mcivor 1998). Recent literature data have demonstrated the increased risk of exacerbation and mortality with the overuse of SABA (Nwaru 2020). The latest Global Initiative for Asthma report thus no longer recommends reliever treatment with SABA alone (GINA 2021).
Why it is important to do this review
With the recognition that early treatment of asthma exacerbations is the best strategy for management, the use of ICS as a part of an action plan is essential. Furthermore, it is important to determine the efficacy of an increased versus a stable dose of ICS in this setting. The primary outcome for this review is treatment failure, defined as the need for rescue systemic corticosteroids. This is an update of a Cochrane Review originally published in 2010 (Quon 2010), and updated in 2016 (Kew 2016), whilst incorporating the most recent clinical trials from the literature.
Objectives
To compare the clinical effectiveness and safety of increased versus stable doses of inhaled corticosteroids as part of a patient‐initiated action plan for home management of exacerbations in children and adults with persistent asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel and cross‐over randomised controlled trials (RCTs) reported as full text, those published as abstract only, and unpublished data. We included only double‐blinded, placebo‐controlled trials (participant and administrator blinded) to avoid treatment bias with respect to activation of the asthma action plan and determination of subjective treatment outcomes such as treatment failure necessitating rescue systemic corticosteroids.
Types of participants
We included adults and children with asthma exacerbation as defined by guideline criteria such as those outlined in GINA 2015, or by a set of criteria predefined in the included studies. The diagnosis of asthma was confirmed by a physician before the time of enrolment. Participants had to have taken a stable dose of ICS for a minimum of two weeks before enrolment. We excluded studies involving participants treated with continuous daily oral corticosteroids.
Types of interventions
We included studies that compared continuing a stable daily maintenance dose versus increasing the daily dose of ICS as part of an asthma exacerbation action plan. Active or placebo step‐up therapy was to be increased at home or shortly after the onset of symptoms signalling the beginning of an exacerbation. Other co‐interventions such as long‐acting beta agonists, leukotriene modifiers, and other asthma medications were permitted, provided that the dose remained unchanged throughout the study. The only exception to this was the allowance of increased short‐acting beta agonist use during exacerbations. Specifically, inhaled short‐acting beta agonists and short courses of systemic corticosteroids were allowed as rescue medications.
Types of outcome measures
The primary and secondary outcomes in this review include all core outcomes for asthma exacerbations reported in Fuhlbrigge 2012.
Primary outcomes
Treatment failure: need for rescue systemic corticosteroids* in all randomised participants (i.e. intention‐to‐treat (ITT) analysis).
Secondary outcomes
Treatment failure: need for rescue systemic corticosteroids** in participants using the study inhaler.
Unscheduled physician visits.
Unscheduled acute care or emergency department visits or need for hospital admission.
Serious*** and non‐serious adverse events.
-
Duration of exacerbation as defined by:
recovery of lung function;
recovery of symptoms; or
beta‐2 agonist use back to baseline.
*oral, intramuscular (IM), or intravenous (IV).
**In the previous version of this review this outcome was referred to as the treated‐population analysis, and is described in some studies as such or as the per‐protocol analysis. For clarity, we refer to the outcome as the effect in the treated population.
***Serious adverse events were defined as fatality, need for hospitalisation, prolongation of hospitalisation, disability, or study withdrawal due to the adverse event. We noted in the analysis whether definitions used within the included studies differed.
Search methods for identification of studies
Electronic searches
We have detailed the search methods used in the previous version of this review in Appendix 1. The previously published version included searches up to March 2016, whilst the current update includes searches up to 20 December 2021.
For this update, we identified trials from the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Information Specialist for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED), and PsycINFO, and by handsearching of respiratory journals and meeting abstracts (see Appendix 2 for further details). We searched all records in the CAGR using the search strategy presented in Appendix 3.
The search of ClinicalTrials.gov (clinicaltrials.gov) was included in the CAGR search, and we updated additional searches of the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (who.int/ictrp/en/) for ongoing and unpublished trials. We searched all databases from their inception to the present, with no restriction on language of publication.
Searching other resources
We updated additional searches of trial registries and grey literature databases to identify articles that might not have appeared in the main electronic database searches (see Appendix 4). Historical searches for previous versions of this review included controlled-trials.com and www.clinicalstudyresults.org/, which are now covered within the WHO ICTRP and ClinicalTrials.gov. We also checked reference lists of retrieved articles and reviews and asked field experts if they knew of any relevant ongoing or unpublished trials.
Data collection and analysis
Author initials given in this section relate to the current update. Contributions for previous versions of the review are summarised in Contributions of authors.
Selection of studies
We used Cochrane’s Screen4Me workflow to help assess the search results. Screen4Me comprises three components: known assessments – a service that matches records in the search results to records that have already been screened in Cochrane Crowd and been labelled as an RCT or as Not an RCT; the RCT classifier – a machine learning model that distinguishes RCTs from non‐RCTs; and if appropriate, Cochrane Crowd – Cochrane’s citizen science platform where the Crowd help to identify and describe health evidence. For more information about Screen4Me and the evaluations that have been done, please visit the Screen4Me webpage on the Cochrane Information Specialists' portal: community.cochrane.org/organizational-info/resources/resources-groups/information-specialists-portal. In addition, more detailed information regarding evaluations of the Screen4Me components can be found in the following publications: Marshall 2018; McDonald 2017; Noel‐Storr 2018; Thomas 2017.
Two review authors (EF and KK) independently screened the titles and abstracts identified by the search using Covidence (Covidence), coding them as 'include' (eligible or potentially eligible/unclear) or 'exclude'. We retrieved the full‐text study reports/publications for all references coded as 'include' by either review author, and the same two review authors independently screened the full‐text studies for inclusion, recording the reasons for exclusion of all excluded studies. Any disagreements were resolved through discussion or by consulting one of the clinical authors (BSQ or CL) if required. We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram for Cochrane Review updates and Characteristics of excluded studies tables (Stovold 2014).
Data extraction and management
For this update, we replicated the previous data collection form for study characteristics and outcome data in Covidence (Covidence), which had been piloted previously. Two review authors (EF and KK) extracted a range of study characteristics relating to methods (e.g. study design, funding, duration, inclusion and exclusion criteria, run‐in, setting); study populations (e.g. sample size, withdrawals, age, sex, asthma severity, number who started the study inhaler); interventions (criteria for starting the study inhaler, fold increase, inhaled steroid dose, allowed and disallowed medications); and outcomes (e.g. time points, scales, definitions) from the included studies, which are provided in Supplementary file 1.
We noted in the Characteristics of included studies table if outcome data were not reported in a useable way, and how data were included in review analyses when there was a discrepancy in the way the study reported results (e.g. within a subset of the population) or the way review outcomes had been defined. Where assumptions were required to include imperfect data in a meta‐analysis, we made explicit the underlying assumptions within the notes section for each study table and in Supplementary file 1.
Any disagreements were resolved through consensus or by consulting with the clinical authors if required (BSQ and CL). One review author transferred study characteristics and risk of bias judgements into Review Manager Web (EF) RevMan Web 2022), and two review authors checked and transferred study data into the analyses (EF and KK). Cochrane Airways editorial staff performed a statistics check to double‐check that data were entered correctly by comparing data entered in the analyses with extracted data from Supplementary file 1 and study reports where necessary.
Assessment of risk of bias in included studies
Two review authors (KK and EF) independently assessed risk of bias using the Cochrane Risk of Bias 2 (RoB 2) tool (Higgins 2016; Sterne 2019), August 2019 version, for the following outcomes at latest follow‐up.
Treatment failure: need for rescue systemic corticosteroids in all randomised participants.
Treatment failure: need for rescue systemic corticosteroids in participants using the study inhaler.
Unscheduled physician visits.
Unscheduled acute care or emergency department visits or need for hospital admission.
Serious and non‐serious adverse events at last follow‐up.
Duration of exacerbation.
For all outcomes except outcome 2, the effect of interest was the effect of assignment of the intervention (ITT). Outcome 2 is defined as a conditional outcome, so the effect of interest was the effect of adhering to the intervention (in this case, starting the study inhaler, a per‐protocol effect). Any disagreements were resolved by discussion, and methodologists from the Cochrane Methods Support Unit reviewed judgements for accuracy and consistency. We assessed risk of bias according to the following domains.
Risk of bias in the randomisation process
Bias due to deviations from intended interventions
Bias due to missing outcome data
Bias in measurement of the outcome
Bias in selection of the reported result
We assessed each domain as ‘high risk of bias’, ‘some concerns’, or ‘low risk of bias’ using the responses to the signalling questions and algorithms within the RoB 2 tool. The tool algorithm was used to reach an overall risk of bias for each outcome. We quoted evidence to support our judgements, and if we disagreed with a judgement recommended by the algorithm, we included an explicit statement as to why. When information on risk of bias was related to unpublished data or correspondence with a trialist, this was noted. We managed our risk of bias assessments using the RoB 2 Excel tool (available from the Risk of bias 2 resources webpage), and a consensus‐based version has been made publicly available as Supplementary file 2.
We used the guidance set out by the RoB 2 working group on cross‐over trials and the tool extension to capture additional considerations associated with data from cross‐over studies (Higgins 2021).
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported deviations from it in the Differences between protocol and review section of the review. We updated some sections of the Methods for the most recent version of the review.
Measures of treatment effect
We analysed dichotomous data as odds ratios (ORs), and continuous data as mean differences (MDs) or standardised mean differences (SMDs). We entered data presented as a scale with a consistent direction of effect.
We undertook meta‐analyses only when this was meaningful (i.e. when treatments, participants, and the underlying clinical question were similar enough for pooling to make sense).
We narratively described skewed data reported as medians and interquartile ranges.
When multiple trial arms were reported in a single trial, we included only the relevant arms. If two comparisons (e.g. drug A versus placebo and drug B versus placebo) were combined in the same meta‐analysis, we halved the control group to avoid double‐counting.
Unit of analysis issues
We pooled the results of parallel and cross‐over studies when we were satisfied that data could be appropriately analysed to account for intercorrelation in cross‐over studies. We identified no new cross‐over studies in this update. We analysed data using participants with one or more events as the unit of analysis. For dichotomous outcomes, when it was unclear whether the number of events applied to the entire population or only to those taking the study inhaler, we used the total number randomised per group as the denominator. We performed sensitivity analyses by using the number of participants using their study inhaler at least once as the denominator to test this assumption.
If no events were reported in the control or treatment groups, we used the Peto odds ratio to avoid use of the continuity correction.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when a study was identified as abstract only). When this was not possible, and when missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by performing a sensitivity analysis.
Assessment of heterogeneity
We examined homogeneity of effect sizes between pooled studies using the I2 statistic (Higgins 2003). In the absence of heterogeneity (I2 < 25%), we used the fixed‐effect model (Greenland 1985); otherwise we applied summary estimates and reported the DerSimonian and Laird random‐effects model (DerSimonian 1986). Unless otherwise specified, we reported the fixed‐effect model, as it is better equipped than the random‐effects method to detect small effect sizes (Fields 2001).
Assessment of reporting biases
We were not able to pool more than 10 trials, therefore we did not create a funnel plot to explore possible small‐study and publication biases.
Data synthesis
For dichotomous outcomes, we pooled parallel studies using Mantel‐Haenszel (M‐H) ORs unless few events were reported, thus requiring Peto odds ratios. We obtained ORs from cross‐over studies by comparing the number of participants who needed oral corticosteroids with increased dose (but not with placebo) versus those who needed oral corticosteroids whilst taking placebo (but not whilst taking increased ICS dose). We presented ORs with 95% confidence intervals (CIs). For continuous outcomes, such as length of exacerbation, we calculated pooled statistics as MDs and reported them with 95% CIs. All primary analyses included all eligible studies irrespective of risk of bias.
Subgroup analysis and investigation of heterogeneity
We planned the following a priori subgroup analyses of the primary outcome to identify potential effect modifiers, irrespective of the presence or absence of heterogeneity.
Age group (children < 15 years versus adults ≥ 15 years).
Smoking status (smokers versus ex‐smokers or never‐smokers).
Time elapsed before initiation of treatment (< 48 hours versus ≥ 48 hours).
Maintenance ICS dose (ex‐valve) before increase (low versus moderate versus high*).
Achieved daily dose of ICS (ex‐valve) during exacerbation (low versus moderate versus high*).
Fold increase in baseline ICS dose during exacerbation (double dose versus quadruple dose).
In the previous version of the review, subgroup analyses were repeated post hoc for the secondary outcome of treatment failures only within those participants who started the study inhaler. In the current version, we conducted subgroup analyses on the primary outcome alone.
*ICS dose was classified according to Global Initiative for Asthma Guidelines (GINA 2015), as follows.
-
High dose:
Adults: > 1000 μg/d of chlorofluorocarbon‐propelled beclomethasone dipropionate (CFC‐BDP) dose or equivalent.
Children: > 400 μg/d equivalent CFC‐BDP dose.
-
Moderate dose:
Adults: > 500 μg/d to 1000 μg/d CFC‐BDP equivalent.
Children: > 200 μg/d to 400 μg/d CFC‐BDP equivalent.
-
Low dose:
Adults: 200 μg/d to 500 μg/d CFC‐BDP equivalent.
Children: 100 μg/d to 200 μg/d CFC‐BDP equivalent.
Fluticasone propionate was converted to CFC‐BDP equivalents by multiplying the ex‐valve dose by two because its reported potency in asthmatic patients is two‐fold relative to CFC‐BDP (Barnes 1993). Budesonide was converted to CFC‐BDP equivalents by multiplying the ex‐valve dose by 1.25, as reported in the Canadian Asthma Guidelines (Lemiere 2003).
Sensitivity analysis
We planned the following sensitivity analyses for the primary outcome.
Study design (removing cross‐over studies).
Methodological quality (removing studies at overall high risk of bias).
Source of study funding (removing studies funded by pharmaceutical companies).
Summary of findings and assessment of the certainty of the evidence
We created a summary of findings table using the following outcomes: treatment failure as defined by the need for rescue systemic corticosteroids (ITT analysis), treatment failure as defined by the need for rescue systemic corticosteroids in participants using the study inhaler (treated population), unscheduled physician visits, unscheduled acute care or emergency visits or hospital admissions, serious and non‐serious adverse events, and duration of exacerbations. We used the five GRADE considerations (overall risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to studies that contributed data to meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions to guide the application of GRADE methodology (Schünemann 2021), employing GRADEpro GDT software (GRADEpro GDT). We justified all decisions to down‐ or upgrade the quality of the evidence by using footnotes, and made comments to aid readers' understanding of the review where necessary.
Results
Description of studies
Results of the search
The searches for this update covered March 2016 to 20 December 2021. Three database searches during the update process identified a total of 2212 records. We identified three additional records through other sources (a trial registration for one of the included studies and a record associated with a previously excluded study) and a further 195 records through searches of trial registry platforms, grey literature databases, and reference lists of included studies. Altogether, the searches identified 2410 records. The Screen4Me process described in Selection of studies excluded 167 records from the main database search, and we identified and excluded 1153 duplicate records (1134 from the main database searches and 19 from the additional searches). We screened the remaining 1090 records, excluding 1072 on the basis of title and abstract alone. We obtained the full texts for the remaining 18 records. We identified one of these as a newly included study (Jackson 2018); one was a duplicate of an existing included study (ACTRN12605000631606); and eight studies (16 records) were newly excluded studies. Figure 1 shows the screening process for this update with the number of studies included from the previous version (Stovold 2014). Full details of searches for previous versions can be found in earlier publications of this review.
1.
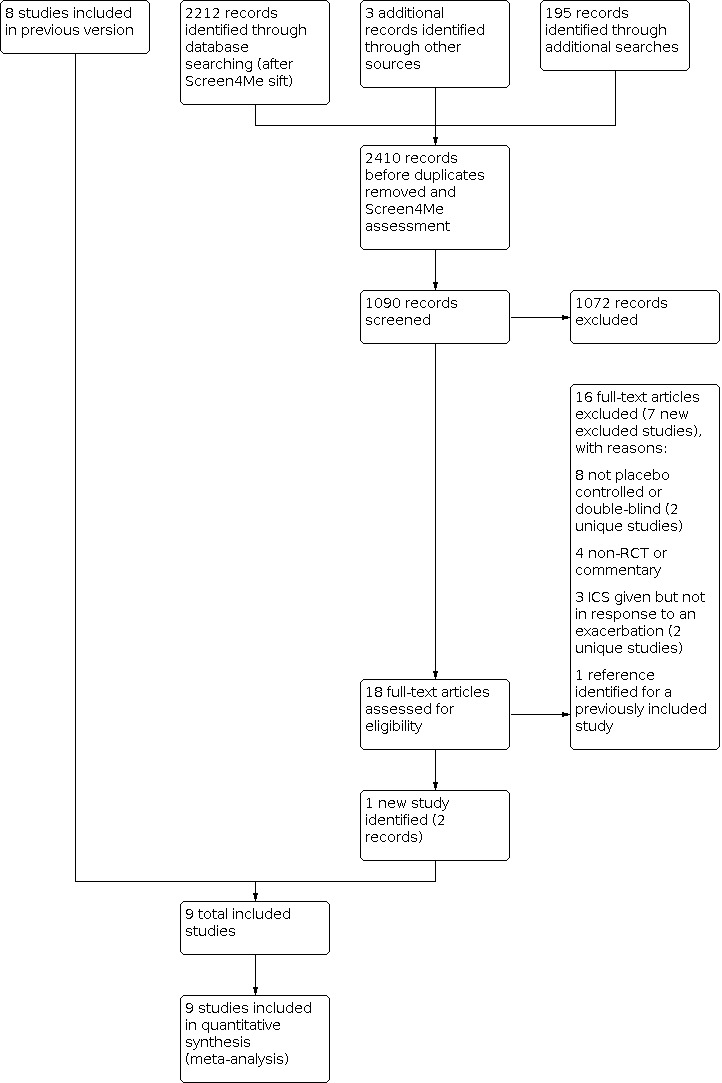
Study flow diagram.
Included studies
This review update added one more study (254 participants) to the review, for a total of nine eligible studies. A summary of key study, participant, and treatment characteristics most important to this review are summarised below; for details, see Table 2, Table 3, and Characteristics of included studies.
1. Summary of study characteristics.
| Study ID |
N randomised* |
N (%) who took study inhaler | Country (N centres) | Design | Age range | % male | Smoking status | Diagnosis by | Asthma severity (at baseline)** | Funding | Results contributed to |
| FitzGerald 2004 | 290 | 98 (34) | Canada (4) | 6‐month parallel, DB, PC | 13+ | 28 | 86% non‐smokers, 14% ex‐smokers of fewer than 10 pack‐years | Medical records |
Asthma severity: NR ICS dose (mean): 635 μg/d (budesonide) Lung function: mean FEV1 2.8 L, mean PEFR 423 L/min |
AstraZeneca |
|
| Foresi 2000 | 142 | 36 (25) | Italy (14) | 6‐month parallel, DB, PC | 18 to 65 | 47 | 70% non‐smokers, 22% ex‐smokers, and 8% smokers | Medical records |
Asthma severity: moderate ICS dose (range): 500 to 1000 μg/d Duration of asthma: 28% < 5 years, 22% 5 to 10 years, 50% > 10 years Lung function: FEV1 74%, PEFR 75% Other: 41% taking salmeterol (LABA), 17% theophylline |
Astra Farmaceutici |
|
| Garrett 1998 | 28 | 18 (64) | New Zealand (1) | 6‐month cross‐over, DB, PC | 6 to 14 | 68 | NR (paediatric trial) | NR |
Asthma severity: mild to moderate ICS dose (range): not exceeding 800 μg/d Lung function: FEV1 99% predicted, PEFR 100% predicted |
New Zealand Asthma Society |
|
| Harrison 2004 | 390 | 207 (53) | UK (1) | 1‐year parallel, DB, PC | 16+ | 33 | 61% non‐smokers, 36% ex‐smokers, and 3% smokers | Medical records |
Asthma severity: NR ICS dose (mean): 710 μg/d Lung function: FEV1 2.4 L/80%; PEF 384 L/min Other: symptom score (range 0 to 7): 0.5, 35% on LABA |
National Health Service Executive |
|
| Jackson 2018 | 254 | 168 (66) | USA (17) | 48‐week parallel, DB | 5 to 11 | 64 | 38% had tobacco smoke exposure. | Physician |
Asthma severity: mild to moderate ICS dose: NR Markers of inflammation: blood eosinophil count 346.4 cells/mm3 Other: 11.8% no previous controller therapy at enrolment (71.3% and 16.9% had Step 2 and Step 3 controller therapy, respectively). In previous year, mean 1.7 systemic glucocorticoid courses (SD 0.9); mean urgent care or ED visits 2.0 (SD 1.7); 12.2% with hospital admissions |
NHLBI |
|
| Martinez 2011 | 143 | 143 (100) | USA (5) | 44‐week parallel, DB, PC | 6 to 18 | 57 | NR | Medical records |
Asthma severity: mild ICS dose (mean): NR (≤ 160 μg daily equivalent) Lung function: mean FEV1 (pre‐BD): 101.5 (11.7) active, 100.1 (10.8) control; mean PEFR: 321.0 (113.1) active, 301.8 (125.9) control Other: 5% on LABA, recent admission, or OCS; in the previous year, 82% had taken ICS, 10% had taken a leukotriene inhibitor, 1% had taken salmeterol, and none had taken theophylline or sodium cromoglycate |
NHLBI |
|
| Oborne 2009 | 403 | 94 (23) | UK (1) | 1‐year parallel, DB, PC | 16+ | 32 | 69% never‐smokers, 21% ex‐smokers, and 10% smokers | Medical records |
Asthma severity: NR ICS dose (mean): 520 μg Lung function: FEV1 2.2 L or 82% predicted, PEFR 380 L/min |
Asthma UK |
|
| Rice‐McDonald 2005 | 22 | 18 (82) | Australia (1) | Cross‐over until exacerbation in each phase | 18+ | 41 | NR | Physician |
Asthma severity: mild and moderate ICS dose: NR Lung function: FEV1 73% predicted |
Asthma Foundation of Queensland |
|
| ACTRN12605000631606 | 251 | 187 (75) | Australia (8) | 1‐year parallel, PC | 3 to 14 | 60 | NR | Physician |
Asthma severity: NR ICS dose: minimum 125 μg fluticasone/d; 27% on 500 μg/d ICS and 9% on > 500 μg/d ICS Other: previous 12 months, 52% admitted to ED, 28% had used OCS once, 37% twice, and 35% 3 times |
Asthma Foundation of Queensland |
|
Abbreviations: DB: double‐blind, ED: emergency department, FEV1: forced expiratory volume in one second, ICS: inhaled corticosteroids, ID: identifier, LABA: long‐acting beta‐agonist, N: number, NHLBI: National Heart, Lung and Blood Institute, NR: not reported, OCS: oral corticosteroids, PC: placebo controlled, PEF: peak expiratory flow, PEFR: peak expiratory flow rate; pre‐BD: pre‐bronchodilator; SD: standard deviation.
*The number randomised to the groups relevant to this review. **See Characteristics of included studies for study inclusion and exclusion criteria.
2. Treatment format.
| Study ID | Maintenance ICS | Exacerbation inhaler | Study treatment details | Action plan activation | Action plan compliance |
| FitzGerald 2004 | Budesonide 100, 200, or 400 μg twice daily (mean 635 μg/d BDP) |
Additional inhaler used with the maintenance inhaler. Intervention: budesonide 100, 200, or 400 μg to double dose Control: placebo |
Home setting; intervention administered by participants; measurements, symptoms, and inhaler use recorded in an electronic diary morning and night of each day | Exacerbation defined as a combination of 2 of the following on 2 consecutive days: PEF ≤ 80% mean baseline morning value (or 2 consecutive mornings); bronchodilator ≥ 4 inhalations/day; nocturnal awakenings; total asthma symptom score ≥ 3 (combines chest tightness, breathlessness, coughing and wheezing); inability to go to school or work; or unscheduled physician visit. Electronic diary alerted participants of an exacerbation depending on the data entered; at this point, the participant alerted a study nurse or practitioner to confirm that they needed to take the intervention inhaler. Participants used study inhaler for 14 days. 3‐month surveillance period monitored participants once they were stable again. |
Monthly check‐up visit independent of exacerbation status ensured none where missed and to check compliance; all visits encouraged compliance. Compliance was > 97% for the total randomised population, 99% and 97% in the control and intervention groups, respectively, after an exacerbation. |
| Foresi 2000 | Budesonide 100 μg twice daily (200 μg) | Additional inhaler used with the maintenance inhaler. Intervention: budesonide 200 μg 4 times daily to add 800 μg Control: placebo |
Home setting; intervention administered by participants; participants kept daily record of respiratory symptoms (wheeze, cough, chest tightness, and shortness of breath), number of asthmatic exacerbations, morning and evening PEF values, and daily use of additional treatments | Exacerbation defined as a fall in PEF < 70% baseline on 2 consecutive days. Following an exacerbation, participants used study inhaler for 7 days. If PEF remains < 70% for 2 additional consecutive days, participants administered oral steroids. |
Monthly check‐up visit independent of exacerbation status assessed diaries. Compliance was between 75% and 94% in 18% of participants and > 95% in 80% of participants. 2% of participants took < 75% of their scheduled doses. |
| Garrett 1998 | Beclomethasone < 800 μg/d | Additional inhaler used with the maintenance inhaler. Intervention: beclomethasone < 800 μg/d Control: placebo |
Home setting; intervention administered by participants and parents; participants kept daily diaries of morning and evening PEFR, cough and wheeze symptom scores, daily activities, medication use, and presence of upper respiratory tract infection or other illnesses. Diary was used to calculate baseline. Each child received a 3‐zone asthma action plan: green (> 80% baseline PEFR and no other symptoms); orange (exacerbation criteria as described); and red (> 60% baseline PEFR). |
Exacerbation defined as 1 of: PEFR > 80% of baseline for 24 hours or more, woken at night with a cough or wheeze, or bronchodilator requirement doubled. Following an exacerbation, child used the study inhaler in addition to their maintenance inhaler for 3 days and was visited at home. Symptom review 1 week after exacerbation in paediatric outpatient department Parents recorded an opinion score of the effectiveness of the study inhaler on a visual analogue scale that ranged from −3 (made asthma worse) through to +3 (made asthma better). |
No details on how compliance was monitored reported. For the 2‐week period after an exacerbation, mean diary completion rate was 95%. 86% of participants who had an exacerbation followed the protocol correctly. |
| Harrison 2004 | Usual ICS dose (mean 710 μg/d BDP) |
Additional inhaler used with the maintenance inhaler. Intervention: matching ICS inhaler to double dose Control: placebo |
Home setting; intervention administered by participants; participants kept daily diaries and recorded morning peak flow and daytime symptoms scores | Exacerbation defined as fall of morning peak flow by 15% or daily symptom score increased by 1 from mean peak flow and median symptom score from run‐in. Following an exacerbation, the study inhaler was used for 14 days in addition to the maintenance inhaler. Participants continued to record morning peak flow and daytime symptoms scores for 28 days. |
The importance of following study instructions was emphasised at each visit, but no details on how compliance was monitored. |
| Jackson 2018 | Fluticasone 88 μg twice daily | Maintenance inhaler stopped and study inhaler started. Intervention: fluticasone 440 μg twice daily Control: fluticasone 88 μg twice daily |
Home setting; intervention administered by participants and parents; participants kept daily electronic diaries (completed nightly) of daily symptoms and medication use. No electronic link between inhaler and diary. Participants provided with action plan to start study inhaler even if no electronic diary alert (to prevent delays). Peak expiratory flow obtained nightly, with participants blinded to results. | Exacerbation defined by 1 of: 4 inhalations of rescue albuterol in 6 hours, 6 inhalations of rescue albuterol in 24 hours, or 1 awakening in the night due to asthma treated with albuterol. Following an exacerbation, the study inhaler was used for 7 days (maintenance inhaler stopped). |
4‐week run‐in established adherence of more than 75% to the medication and electronic diary completion. Electronic diaries were completed 73% and 72% of days by the intervention and control group, respectively. Adherence to the daily therapy with ICS was reported on 98% of the days in both groups. |
| Martinez 2011 | Beclomethasone 40 μg twice daily | Additional inhaler used with the maintenance inhaler. Intervention: beclomethasone 40 μg twice daily to double dose Control: placebo |
Home setting; intervention administered by participants and parents; participants kept daily diaries of peak flow, medications (electronic monitoring) | Exacerbations defined as 1 of: use of < 12 puffs of albuterol in 24 h (excluding preventive use before exercise), a peak expiratory flow of less than 70% of consecutive days, a peak expiratory flow of less than 50% of reference value despite relief treatment, or an emergency room visit because of worsening of asthma symptoms. Following an exacerbation, study inhaler was taken until symptoms returned to baseline. |
Run‐in period established adherence of more than 75% to the medication and diary completion. 4‐ to 8‐weekly check‐up visits independent of exacerbation status checked compliance with diaries. |
| Oborne 2009 | Usual ICS dose (mean 520 μg/d BDP) |
Additional inhaler used with the maintenance inhaler. Intervention: matching ICS inhaler to double dose Control: placebo |
Home setting; intervention administered by participants and parents; participants only recorded symptoms (including morning PEF) if their asthma deteriorated or if they developed symptoms of an upper respiratory tract infection | Exacerbations defined as 1 of: PEF fell by ≥ 15% on 2 consecutive days, or 30% on 1 day. Following an exacerbation, the study inhaler was used for 7 days in addition to the maintenance inhaler, and a daily diary of morning PEF kept. Study inhaler taken for a further 7 days if morning PEF had not returned to baseline. Participants contacted research team after using the study inhaler to submit completed diary and to obtain replacements. |
Reports that due to the pragmatic trial design they accepted variable compliance (no details about how compliance was monitored reported) |
| Rice‐McDonald 2005 | Usual fluticasone dose (range not specified) | Additional inhaler used with the maintenance inhaler. Intervention: matching ICS inhaler to double dose Control: placebo |
Home setting; intervention administered by participants and parents; participants kept daily diaries of symptoms and medication, and PEF were recorded electronically | Asthma exacerbation was defined as: nocturnal awakening for 2 out of any 3 nights due to asthma, or requiring reliever medication on 4 occasions more than baseline requirements in any 24‐hour period, or symptoms due to asthma necessitating cessation of usual activities of daily living, or decrease in PEF to less than 80% of run‐in morning pre‐bronchodilator best on 2 occasions in any 24‐hour period or on 2 days out of any 3‐day period. Following an exacerbation, the study inhaler was used for 14 days in addition to the maintenance inhaler. |
2‐ to 4‐week run‐in ensured that participants did not provide erroneous or falsified diary entries (those who did were excluded). 5 participants were excluded due to inadequate compliance. Compliance was monitored by symptom and medication diaries, downloading PEF recordings from electronic diaries, and counting completed/returned treatments packs. However, compliance data were not reported. Participants contacted fortnightly by research nurse and reviewed by a study investigator every 8 weeks. |
| ACTRN12605000631606 | Fluticasone 125 μg/d, or usual higher dose | Additional inhaler used with the maintenance inhaler. Intervention: matching fluticasone to double dose for 14 days Control: placebo |
Home setting; intervention administered by participants and parents; during exacerbations, participants kept daily diaries of symptoms and peak flow | Exacerbation confirmed by participants ringing study team at first sign of URTI or change in asthma symptoms. Following an exacerbation, the study inhaler was used in addition to the maintenance inhaler until return to baseline. Called weekly by study nurse |
Routine check‐in visits occurred every 3 months or 2 weeks after every exacerbation. No other details about compliance reported. |
Abbreviations: BDP: beclomethasone dipropionate, ICS: inhaled corticosteroids, ID: identifier, PEF(R) = peak expiratory flow (rate), URTI: upper respiratory tract infection.
Characteristics of studies
The included studies were published over a 20‐year period from 1998 to 2018, with two studies now conducted over 20 years ago (Foresi 2000; Garrett 1998), and only the newly added study published less than 10 years ago (Jackson 2018). Three studies were conducted in Europe (Foresi 2000; Harrison 2004; Oborne 2009), three in North America (FitzGerald 2004; Jackson 2018; Martinez 2011), and three in Australia and New Zealand (ACTRN12605000631606; Garrett 1998; Rice‐McDonald 2005). Two European studies conducted in the UK and two of the Australasian studies were conducted at a single centre, whilst the remaining studies were conducted at between four and 17 sites. Two of the adult studies were commercially funded (FitzGerald 2004; Foresi 2000), with the remaining studies funded by independent bodies such as research institutes and national asthma charities (see Table 2).
All studies were published as full‐text papers except ACTRN12605000631606, for which study details and results were provided by the lead investigator. The nine studies randomised a total of 1923 participants to the comparison of interest for this review. Of all randomised participants, 50.4% had an exacerbation that led to use of the study inhaler. The mean number of people randomised to treatment groups relevant to this review was 214 (range 22 to 403).
All included trials compared the efficacy of an increased dose of ICS at the onset of an exacerbation versus a control (maintenance ICS dose) as part of an asthma action plan. All other medications, mainly rescue short‐acting beta agonist inhalers, were kept equal between treatment and placebo groups and are noted in individual Characteristics of included studies tables.
Eight of the nine studies were placebo‐controlled trials, where during an exacerbation participants either used a placebo or active inhaler to increase their ICS dose in addition to their maintenance inhaler. In the remaining study (Jackson 2018), during an exacerbation, participants ceased using the maintenance inhaler and either used a control inhaler, with the same ICS dose as the maintenance inhaler, or an intervention inhaler, which increased their ICS dose. Seven of the nine studies used a parallel‐group design. Garrett 1998 used a cross‐over design, whereby children were randomised to one of two possible treatment sequences for serial exacerbations: placebo then corticosteroid, or corticosteroid then placebo. Rice‐McDonald 2005 also used a cross‐over design, with three treatment phases, one of which was not relevant to this review (oral steroid rescue). For this study, we used results from the paper showing the number of people who needed oral steroids in one, neither, or both of the two relevant phases, and analysed them to account for correlation (see 'Analysis 1.1 and 1.2' tab in Supplementary file 1).
Characteristics of participants
Details regarding the age range, gender, smoking status, and asthma severity of participants in each study are provided in Table 2.
For the subgroup analysis by age (children < 15 years versus adults ≥ 15 years), we classified four studies as having child populations (ACTRN12605000631606; Garrett 1998; Jackson 2018; Martinez 2011), and five studies as having adult populations (FitzGerald 2004; Foresi 2000; Harrison 2004; Oborne 2009; Rice‐McDonald 2005). FitzGerald 2004 had a lower age limit of 13 years; we included this study in the adult subgroup because the age range was more consistent with the adult studies, and the mean age of participants was 32 years. Similarly, Martinez 2011 included adolescents up to 18 years, and was classified as a child population because the age range was more consistent with the other child studies, and the mean age was 11 years. Mean participant age ranged from 32 to 56 (median 46.5) years in the five adult studies, and from 7.6 to 11 (median 8.1) years in the four paediatric studies (a rough mean age of 7.6 was calculated from age‐group categories reported for ACTRN12605000631606).
All studies included both male and female participants. All adult studies included more women than men (median percentage male 33%, range 28% to 47%), and all paediatric studies recruited more boys than girls (median percentage male 62%, range 57% to 67%).
Four of the nine trials reported the smoking status of study participants. Never‐smokers made up most of the study samples (61% to 86%), with ex‐smokers making up between 14% and 36%, and active smokers 10% or less of the samples. Rice‐McDonald 2005 and three of the four paediatric studies did not report smoking status. Jackson 2018 reported tobacco smoke exposure in 38% of its paediatric population.
Baseline asthma severity was mild in Martinez 2011, mild‐to‐moderate in three studies (Garrett 1998; Jackson 2018; Rice‐McDonald 2005), and moderate in Foresi 2000. The remaining studies did not explicitly state asthma severity, although they did include baseline measurements or inclusion criteria relating to asthma (ACTRN12605000631606; FitzGerald 2004; Harrison 2004; Oborne 2009). Full details regarding how severity was measured at baseline for each study are available in Table 2, including ICS dose, lung function, markers of inflammation, and other reported data. Inclusion criteria for each study are provided in Characteristics of included studies.
Treatment format
During the original protocol development for this review, it was not anticipated that this would be a complex intervention. However, as more studies have been added at each update, complexities in the designs have resulted in the creation of Table 3, which highlights differences in treatment format for each study.
Study treatment details
The ICS dose was increased five‐fold in two studies (Foresi 2000; Jackson 2018), four‐fold in Oborne 2009, and doubled in the remaining six studies. The mean ICS dose achieved during exacerbations ranged from 1000 μg/d to 2075 μg/d in CFC‐BDP equivalents in the adult studies (FitzGerald 2004; Foresi 2000; Harrison 2004; Oborne 2009), and from 160 μg/d to 500 μg/d in the paediatric studies (ACTRN12605000631606; Jackson 2018; Martinez 2011). Mean dose achieved was not reported in the paediatric study of Garrett 1998, although the maximum dose achieved was 1600 μg/d. Studies used metred dose or dry powder inhalers, but within studies the treatment or placebo inhaler provided for use during exacerbation was identical to the maintenance corticosteroid inhaler. Moreover, the additional use of a spacer was reported in Garrett 1998 and ACTRN12605000631606. More study treatment details are provided in Table 3.
Action plan activation
Criteria for an asthma exacerbation that prompted initiation of the study inhaler were predefined in all studies on the basis of a combination of peak expiratory flow rate (PEFR) worsening, increase in asthma symptoms, and/or an increase in rescue bronchodilator use relative to run‐in values. Study inhaler use was initiated by the participants (or carer) following the predefined management plan in all studies except FitzGerald 2004, which was initiated following consultation with the study team. Daily symptom or medication use diaries (or both) were kept by participants in all studies except Oborne 2009, which only recorded a daily diary after an exacerbation. Electronic diaries were used in FitzGerald 2004 and Jackson 2018. The minimum time elapsed between onset of asthma deterioration and initiation of increased ICS dose (as recommended by the action plan) varied from immediate use of the study inhaler as a rescue treatment, ACTRN12605000631606; Jackson 2018; Martinez 2011, to 24 hours after symptoms worsened, Garrett 1998; Harrison 2004; Rice‐McDonald 2005, to 48 hours, FitzGerald 2004; Foresi 2000. For Oborne 2009, elapsed time varied from 24 to 48 hours, depending on how much PEFR had dropped from baseline. More details on exacerbation criteria and action plan activation are available for each study in Table 3.
Action plan compliance
Five studies monitored compliance with symptom or study treatment recording, or both (FitzGerald 2004; Foresi 2000; Garrett 1998; Jackson 2018; Rice‐McDonald 2005). Investigators evaluated compliance by reviewing self‐reported symptom diaries, self‐reported medication diaries, PEFR recordings, and by counting tablets from returned treatment packs. Self‐reported study treatment compliance was high in three studies, ranging from a mean of 86% in Garrett 1998 to 98% in Jackson 2018, and was not reported in Rice‐McDonald 2005. More details on how studies monitored or encouraged compliance are provided in Table 3.
Outcome reporting and assumptions required for synthesis
All studies except Foresi 2000 reported data relevant to the primary outcome of treatment failure (need for oral steroids). However, in some studies it was unclear whether the reported number of exacerbations was within the full randomised population (primary outcome of the review) or the subset who met the criteria to start the study inhaler (secondary outcome), and whether it was appropriate to include the same number of events in each analysis with a different denominator. It was sometimes necessary to make assumptions about the data in order to include it in the primary or secondary treatment failure analyses, depending on how the data were reported, and we have made explicit where this was done in the Characteristics of included studies tables.
Where assumptions were required to include studies in the review analyses, we also captured the potential for introducing missing data biases into the analysis within the risk of bias assessments for those results (see Risk of bias in included studies and links to risk of bias outcome tables in the Effects of interventions). This was most notable when studies reported the number of events (e.g. treatment failures) for the subset of people who had an exacerbation and started the study inhaler (or reported a number of events or percentage without stating the population), and we included those data with the denominator for the full population for the primary ITT analysis. Doing so assumes that those who did not start the study inhaler did not have the event of interest, and the potential for bias depends on the size of the subset as a proportion of the full population.
Regarding the outcome definition for the primary outcome, generally participants were withdrawn from use of the study inhaler and started on rescue oral corticosteroids if they failed to respond adequately to an increase in ICS dose, or if their PEFR dropped to below a predefined safety cut‐off (usually 60%). Treatment failure was defined by deterioration or lack of improvement in pulmonary function or symptoms, or both. Rescue oral corticosteroids were participant‐initiated if PEFR fell below a predefined threshold of 60% at any point during the treatment period, or after discussion with a study physician based on symptom frequency and PEFR measurements. Harrison 2004 and Oborne 2009 required rescue oral corticosteroid use if a participant's asthma control deteriorated to the point that they would usually start oral corticosteroids.
Predefined secondary outcomes were reported inconsistently across studies, with no more than three studies included in any of the other secondary analyses.
Excluded studies
A further eight studies were excluded in this update in addition to the 39 studies excluded in previous versions of the review, for a total of 47 excluded studies. Reasons for exclusion are documented in the Characteristics of excluded studies section. Common reasons for exclusion across all versions of the review included the absence of a placebo control; recruitment of a population that were not taking maintenance ICS; and a design that compared the relative effectiveness of two doses of ICS as maintenance therapy rather than changing the dose in response to worsening symptoms. Two studies excluded in this update, one of which was a large and independently funded study (McKeever 2018), assessed the research question of interest but in a pragmatic and unblinded design, which did not meet the eligibility criteria for our review. Results from the blinded studies included in this review are compared and contrasted with those of McKeever 2018 and other important real‐world studies in the Discussion (Agreements and disagreements with other studies or reviews). A further large study that assessed a similar research question to our review was deemed ineligible because the inhalers were for general rescue use and as a preventative measure before exercise, and not as part of an action plan as a measure to prevent exacerbations (Papi 2022).
Risk of bias in included studies
For each outcome prespecified for risk of bias assessments, results‐level RoB 2 tables include the judgements and support for judgements for each domain and the overall risk of bias (Table 16; Table 17; Table 18; Table 19; Table 21; Table 20). For the two cross‐over trials (Garrett 1998; Rice‐McDonald 2005), the cross‐over trial‐specific risk of bias assessments and support for judgements are detailed in the overall risk of bias column of each risk of bias table. Full consensus responses to the signalling questions for each domain across all studies and results are provided in Supplementary file 2.
Risk of bias for analysis 1.1 Treatment failure: need for systemic corticosteroids (primary outcome, all randomised participants).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| ACTRN12605000631606 | Low risk of bias | "Stratified block randomisation by age (3‐5, 6‐10, 11‐14), gender, centre". "Sequential study number allocated from a list according to blocking details". "...blocking details emailed to Dept of Epidemiology and Preventative Medicine, Monash Med School, Melbourne". "Study puffer number was allocated. Pre‐numbered puffers were held at RCH Brisbane pharmacy". Imbalances noted in disease characteristics (higher proportion of patients in the intervention group than the control group had persistent asthma and atopic history), but likely to be down to chance given sample size. | Some concerns | Placebo inhalers were used to presume this was to blind participants and personnel from the study medication, but no explicit definition of masking procedures. Outcome reported as percentages and unclear whether they related to the ITT or per protocol (those who started the inhaler) population. For the purposes of including data in the review, we assumed it was the latter and then used the full population numbers to include data for the primary outcome of the review. This assumes no one who didn't take the study inhaler needed oral steroids. Most participants started the study inhaler but approx 25% of each group did not, so there is some potential for bias. | Low risk of bias | Outcome data accounted for and a much higher number of events than those who withdrew. 11 in the intervention group (8.7%) an 10 in the control group (8.1%) withdrew from the study ‐ low and balanced. Only those having an exacerbation could be included in the main analyses. | Low risk of bias | The method of measuring the outcome was appropriate. Measurment of the outcome likely to be the same between the groups. Clear instructions for what to do in the event of worsening symptoms and called weekly by study nurse ‐ all events likely to have been recorded. The outcome assessor was the study participant (who was blinded). | Some concerns | Trial registered (https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=630) but after participant enrollment was completed. No details about the analyses available. No published journal report. The numerical result is not likelyt to have been selected on the basis of the results from multiple outcome measurements or analyses. | Some concerns | Overall risk of bias from tool algorithm. |
| FitzGerald 2004 | Low risk of bias | Patients were randomised to treatment groups at visit 2 according to a blocked computer generated randomisation list for each centre. As a randomisation list was generated for each centre, this sounds like it was centrally generated. Baseline characteristics not shown for the full population, only those who had an exacerbation and started the study inhaler. Paper states that "there were no differences in patient characteristics between those who experienced an exacerbation and those who did not". | High risk of bias | Methods state 'double blind' so assume this means participants. The participants themselves delivered the intervention. For the purposes of the review, the denominator for the analysis was all randomised participants which assumes no one in the study deteriorated to the point of needing oral steroids without first meeting the criteria for initiating the study inhaler and doing so. Most people did not start the study inhaler, so there is potential for the assumption to have a substantial impact on the result. | Some concerns | Number of participants lost to follow up were larger than the number of events and only participants who started study inhaler were included in the analyses. No analysis methods that correct for bias due to missing outcome data mentioned. Patients who discontinued who did not use the study inhaler might have done so for reasons relating to their disease worsening, but the CONSORT diagram does not suggest that this was the case although the most common reason is 'other' in both groups. | Low risk of bias | Method of measuring the outcome was appropriate. Measurement of the outcome was the same between intervention groups. The study participant (who was blinded) entered details into an electronic diary with thorough pre‐defined criteria on the outcome. | Some concerns | No study protocol or trial registration record is available. Clear definition of outcome and timepoints. | High risk of bias | Used the tool algorithm. |
| Garrett 1998 | Low risk of bias | Only information about the allocation is that the children were randomised by the hospital pharmacist and the investigators were blinded to this allocation. Baseline characteristics were not provided by intervention group. | High risk of bias | Used a blinded inhaler delivered by the participant. 10/28 randomised participants not included in the analyses as they didn't have an exacerbation in both arms ‐ therefore a per protocol analysis. A large proportion of those randomised were exlcuded from the analyses. | High risk of bias | Only those with completed pairs of exacerbations were included in the final analysis ‐ 10/28 excluded from the analyses as they only had an exacerbation in one arm. No anlyses were reported to address bias from missing outcome data. Reported reason for excluding from analyses was that they had good health status (no exacerabtions). | Low risk of bias | Method for measuring the outcome was appropriate, and unlikely to differ between groups. Reported that investigators were blinded to the allocation. | Some concerns | No trial registration, study protocol or SAP (even though article mentions a study protocol). The numerical result is not likely to have been selected on the basis of the results from multiple outcome measurements or analyses. | High risk of bias | Used algoritm. Domain S = LOW RISK OF BIAS SQ1 = NI on whether the number of participants allocated to each of the two sequences equal or nearly equal. SQ2 = LOW ‐ Cross‐over data were included in the review by obtaining two‐by‐two data and applying a formula to account for inter‐correlation of matched pairs (Elbourne 2002). SQ3 = LOW ‐ Sufficient time was given for carryover effects to have disappeared before outcome assessment in the second period ‐ followed for two weeks and study tated that spirometry returned to normal within 1 week. |
| Harrison 2004 | Low risk of bias | An independent pharmacist randomly allocated individuals using computer generated random number tables. Statistical tests for imbalances not presented but group differences at baseline do not suggest a problem with randomisation. | Low risk of bias | Study report does not explicitly state "double‐blind", but a placebo was used which implies that it was not open label, and text mentions "A range of active and placebo study inhalers was available to enable the type of inhaler and daily dose to be matched to patients’ regular inhaled corticosteroid, type of inhaler, and dose". Participants delivered the intervention themselves. Used a IIT analysis. | Low risk of bias | Nearly all data available and number withdrawn much smaller than the number of events observed. 8.9 and 10.1% withdrew from the intervention and placebo groups respectively which is relatively low and balanced, although the study does not report whether and how missing data were imputed. | Low risk of bias | Method for measuring the outcome was appropriate. Participants were given a 10‐day course of prednisolone (30 mg per day) to be taken if their asthma control deteriorated to the point that they would usually start oral corticosteroids, or if their peak flow fell by 40% from the mean run‐in value. Unlikely that the measurement of Measurement of the outcome was the same between intervention groups. Oral steroids were patient initiated and they were blinded to the contents of the study inhalers. Outcome assessors were the study participants. | Some concerns | No publicly available trial registration, study protocol or SAP (SAP is mentioned in the study report). Clear definition of outcome and timepoints. | Low risk of bias | Overall risk of bias assessed as low as the only 'some concerns' was due to no trial registration or study protocol details, but this is an old trial when making these available was not standard. |
| Jackson 2018 | Some concerns | Method of sequence generation and allocation concealment not described (in paper, supplementary materials of protocol). However, the report mentions stratification which implies the sequence was computer generated. Statistical tests for imbalances not presented but group differences at baseline do not suggest a problem with randomisation. | Low risk of bias | Participants and their carers/parents were blinded. "The primary intention‐to‐treat analysis compared the overall efficacy of each treatment strategy, regardless of whether any yellow‐zone episodes actually occurred." | Low risk of bias | Number of participants lost to follow up is similar to the number of events; however, most participants were included in the study for most of the duration [mean follow‐up among all 127 participants in each group was 42.5 weeks and 40.3 weeks, respectively (i.e. most of the full 48 week follow‐up)]. | Low risk of bias | Method of measuring the outcome was appropriate. Measurement of the outcome was the same between intervention groups. The outcome assessor was the study participant (who was blinded). However, systematic glucocorticoids were started after consultation with the trial clinician and it is unclear whether they were blind to the outcome. | Low risk of bias | Protocol dated February 2014 and trial enrolment began in August 2014. The numerical result is not likely to have been selected on the basis of the results from multiple outcome measurements or analyses. | Low risk of bias | Insufficient reporting warrants an assessment of 'some concerns' for the randomisation process, but the overall reporting and conduct of the trial suggests that rigorous procedures were followed to minimize bias. |
| Martinez 2011 | Low risk of bias | The Data Coordinating Center generated the random allocation sequence via computer software.The DCC had no contact with participants. No obvious imbalances between groups. | Low risk of bias | States double blind and placebo interventions were used. Those delivering the intervention were the participants. Number of treatment failures measured in the whole population. | Low risk of bias | Withdrawals from the study were relatively low and even between the two groups included in this review: 11.3% in the intervention group (double ICS) and 12.5% in the control group (stable ICS). However, in some groups the number of events was similar to the number of participants with missing data, therefore their outcomes could impact the estimated effect of intervention. Authors didn't correct or assess for bias from missing outcome data. Reasons for missing data were accounted for and most were not related to the ouctome of interest (only two participants across the study were lost because they were unsatisfied with asthma control, both in the rescue beclomethasone group). | Low risk of bias | Method for measuring the outcome was appropriate. Measurement of the outcome would not have differed between groups. Drugs were masked A to D to mask statisticians to treatment group. | Some concerns | Trial registered before participant enrolment began (https://clinicaltrials.gov/ct2/show/study/NCT00394329). However, registry record contains no prospective information about statistical analyses and no protocol or SAP available. The numerical result is not likely to have been selected on the basis of the results from multiple outcome measurements or analyses. | Low risk of bias | Overall risk of bias assessed as low as the only 'some concerns' was due to no study protocol or SAP available. However, it was registered and no other details from the trial for this outcome gives us reason to believe that there are some concerns regarding risk of bias. |
| Oborne 2009 | Low risk of bias | An allocation sequence of random permuted blocks of 10 was generated using a random number table by an independent pharmacist and implemented by one of the study investigators once participants were enrolled into the trial. Mentions an investigator providing the randomization schedule and concealed allocation of masked inhalers. Inhalers were identical and participants blind to the intervention. Baseline data provided for all participants and those starting the study inhaler ‐ no issues noted. | Low risk of bias | Double‐blind, placebo inhaler was used. Participants were not aware of the intervention, which they adminstered. An intention to trate analyses was used. | Low risk of bias | The number of events was less than the number of participants with missing. Thirty‐eight drop‐outs in active vs 39 dropouts in placebo vs 18 events in active vs 29 events in placebo. However, all participants contributed data for the intention‐to‐treat analysis up to the point at which they left the study. Only three were lost to follow‐up with no outcome data. | Low risk of bias | Method of measuring the outcome was appropriate. Measurement of the outcome unlikely to have differed between intervention groups. Doesn't state if outcome assessors were blineded (only investigators and participants in relation to how the inhaler looked). Assessment of the outcome did not require judgement. | Some concerns | The trial was rgeistered (http://www.isrctn.com/ISRCTN46018181). It was registered after participant enrolment began but before recruitment was completed. The registry doesn't include anything about the anlyses and a protocol/SAP isn't available. Clear definition of outcome and timepoints. | Low risk of bias | Overall risk of bias assessed as low as the only 'some concerns' was due to no study protocol or SAP available. However, it was registered before recruitment completed and no other details from the trial from this outcome gives us reason to believe that there are some concerns regarding risk of bias. |
| Rice‐McDonald 2005 | Low risk of bias | Method of sequence generation not described but "order and allocation of treatment by concealed randomisation". Does not report baseline characteristics for each intervention group. | High risk of bias | A double‐blind, double‐dummy study with order and allocation of treatment by concealed randomisation. The participants administered the intervention. An intention to treat analysis was used, though only those with data for both periods were included. Of 35 randomized participants, 13 subsequently withdrew prior to any asthma exacerbations. A large proportion of those randomised were exlcuded from the analyses. | High risk of bias | Of the 35 people ranomised, 13 withdrew from the study (and unclear in what intervention or period they were in). Also, 11 participants had exacerbationsand went straight to oral corticosteroids (no study inhaler) as their symptoms were very bad (excluded from analysis and unsure which period and intervention each were in). No analyses corrected for bias due to missing outcome data and a within‐group change was assessed (not within person differences). The reasons for withdrawal from the trial are not associated with the intervention or outcome. However, 11 participants who were randomised and had exacerbations that went straight to oral corticosteroids (no study inhaler) were not analysed and this missingness woudl depend on its true value. | Low risk of bias | Method for measuring the outcome was appropriate, and unlikely to differ between groups. Treatment failure well defined. Outcome assessors were the particpants, who were blinded. | Some concerns | No trial registry, protocol or SAP available. The numerical result is not likely to have been selected on the basis of the results from multiple outcome measurements or analyses. | High risk of bias | Algorithm used. Domain S (for crossover trials) = LOW SQ S1 = the number of participants allocated to each of the sequences was nearly equal. SQ S2 = N/A SQ S3 = a four week washout period was used which is sufficient time for any carryover effects to have disappeared before outcome assessment in the second period. |
Risk of bias for analysis 1.2 Treatment failure: need for systemic corticosteroids (of those starting inhaler).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| ACTRN12605000631606 | Low risk of bias | "Stratified block randomisation by age (3‐5, 6‐10, 11‐14), gender, centre". "Sequential study number allocated from a list according to blocking details". "...blocking details emailed to Dept of Epidemiology and Preventative Medicine, Monash Med School, Melbourne". "Study puffer number was allocated. Pre‐numbered puffers were held at RCH Brisbane pharmacy". Imbalances noted in disease characteristics (higher proportion of patients in the intervention group than the control group had persistent asthma and atopic history), but likely to be down to chance given sample size. | High risk of bias | Placebo inhalers were used to presume this was to blind participants and personnel from the study medication, but no explicit definition of masking procedures. No details about how they monitored or increased adherence to the intervention so unsure whether there were failures in implementing it. No details about methods to derive an unbiased estimate of the effect of adhering to intervention. | Low risk of bias | Have assumed that all participants who used oral steroid rescue had an exacerbation. Outcome data accounted for and a much higher number of events than those who withdrew. | Low risk of bias | The method of measuring the outcome was appropriate. Measurment of the outcome likely to be the same between the groups. Clear instructions for what to do in the event of worsening symptoms and called weekly by study nurse ‐ all events likely to have been recorded. The outcome assessor was the study participant (who was blinded). | Some concerns | Trial registered (https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=630) but after participant enrollment was completed. No details about the analyses available. No published journal report. The numerical result is not likelyt to have been selected on the basis of the results from multiple outcome measurements or analyses. | High risk of bias | Used tool algorithm. |
| FitzGerald 2004 | Low risk of bias | Patients were randomised to treatment groups at visit 2 according to a blocked computer generated randomisation list for each centre. As a randomisation list was generated for each centre, this sounds like it was centrally generated. Baseline characteristics not shown for the full population, only those who had an exacerbation and started the study inhaler. Paper states that "there were no differences in patient characteristics between those who experienced an exacerbation and those who did not". | Low risk of bias | Methods state 'double blind' so assume this means participants. The participants themselves delivered the intervention. Participants used a computerised diary to track doses. Authors state that compliance to the intervention was high for both groups. | High risk of bias | Statistical analysis used the "all patients treated" (APT) approach but most did not meet the threshold to start the study inhaler and so were not included in the analysis. In the full population, Of the 148 randomised to the control group, 115 completed the study (22% dropout), and 117/142 in the intervention group completed the study (17.6% dropout) which is higher than the % who experienced an event in each group. Patients who discontinued who did not use the study inhaler might have done so for reasons relating to their disease worsening, but the CONSORT diagram does not suggest that this was the case although the most common reason is 'other' in both groups. | Low risk of bias | Method of measuring the outcome was appropriate. Measurement of the outcome was the same between intervention groups. The study participant (who was blinded) entered details into an electronic diary with thorough pre‐defined criteria on the outcome. | Some concerns | No study protocol or trial registration record is available. Clear definition of outcome and timepoints. | High risk of bias | Used the tool algorithm. |
| Garrett 1998 | Low risk of bias | Only information about the allocation is that the children were randomised by the hospital pharmacist and the investigators were blinded to this allocation. Baseline characteristics were not provided by intervention group. | Some concerns | Used a blinded inhaler delivered by the participant. Participants' were familiarised with the intervention and recording during the run‐in period. Authors state that 95% of all the diary entries during an exacerbation period were completed and the study protocol was followed correctly in all but five exacerbations. Authors included the data from the five exacerbations where the protocol was not followed in the analysis. Five deviations from the protocol involved the study inhaler being used for an incorrect number of days, which could have affected the outcome (additional active ingredient). Authors state that the approach was appropriate as it reflects the reality of asthma treatment and adherence. In the review, cross‐over data were included by obtaining two‐by‐two data and applying a formula to account for inter‐correlation of matched pairs (Elbourne 2002). | High risk of bias | Only participants who had two matching pairs of exacerbations were analysed, which was 18 out of 28 enrolled. Those who had 1 or 3+ exacerbations during the study were not included. No anlyses were reported to address bias from missing outcome data. Reported reason for excluding from analyses was that they had good health status (no exacerabtions). Those who had 1 or 3+ exacerbations during the study were not included. | Low risk of bias | Method for measuring the outcome was appropriate, and unlikely to differ between groups. Reported that investigators were blinded to the allocation. | Some concerns | No trial registration, study protocol or SAP (even though article mentions a study protocol). The numerical result is not likely to have been selected on the basis of the results from multiple outcome measurements or analyses. | High risk of bias | Used tool alogorithm. Domain S = LOW RISK OF BIAS SQ1 = NI on whether the number of participants allocated to each of the two sequences equal or nearly equal. SQ2 = LOW ‐ Cross‐over data were included in the review by obtaining two‐by‐two data and applying a formula to account for inter‐correlation of matched pairs (Elbourne 2002). SQ3 = LOW ‐ Sufficient time was given for carryover effects to have disappeared before outcome assessment in the second period ‐ followed for two weeks and study tated that spirometry returned to normal within 1 week |
| Harrison 2004 | Low risk of bias | An independent pharmacist randomly allocated individuals using computer generated random number tables. Statistical tests for imbalances not presented but group differences at baseline do not suggest a problem with randomisation. | High risk of bias | Study report does not explicitly state "double‐blind", but a placebo was used which implies that it was not open label, and text mentions "A range of active and placebo study inhalers was available to enable the type of inhaler and daily dose to be matched to patients’ regular inhaled corticosteroid, type of inhaler, and dose". Participants delivered the intervention themselves. There is no information on how the trialists ensured adherence to the intervention. Clear criteria given to participants as part of an action plan to guide how they should measure their symptoms and at what point they should initiate the study inhaler. However, no details about how they monitored or increased adherence to the intervention so unsure whether there were failures in implementing it. Report doesn't detail methods used to derive an unbiased estimate of the effect of adhering to intervention but the trial measured treatment failure only in those participants who started the study inhaler. | Low risk of bias | 8.9 and 10.1% withdrew from the total intervention and placebo groups respectively which is relatively low and balanced, although the study does not report whether and how missing data were imputed. | Low risk of bias | Method for measuring the outcome was appropriate. Participants were given a 10‐day course of prednisolone (30 mg per day) to be taken if their asthma control deteriorated to the point that they would usually start oral corticosteroids, or if their peak flow fell by 40% from the mean run‐in value. Unlikely that the measurement of Measurement of the outcome was the same between intervention groups. Oral steroids were patient initiated and they were blinded to the contents of the study inhalers. Outcome assessors were the study participants. | Some concerns | No publicly available trial registration, study protocol or SAP (SAP is mentioned in the study report). Clear definition of outcome and timepoints. | High risk of bias | Used tool algorithm. If bias due to deviations from intended interventions was Low, would have assigned Low to overall risk of bias assessed as the only 'some concerns' was due to no trial registration or study protocol details, but this is an old trial when making these available was not standard. |
| Martinez 2011 | Low risk of bias | The Data Coordinating Center generated the random allocation sequence via computer software.The DCC had no contact with participants. No obvious imbalances between groups. | Low risk of bias | States double blind and placebo interventions were used. Those delivering the intervention were the participants. Measures were taken to ensure adherence, inc. electronic measurement, participant diaries and pump canister checks. | Low risk of bias | The same raw data were used in the ITT and per protocol anlayses for this review because the study's design implies all participants would have used their study inhaler. Withdrawals from the study were relatively low and even between the two groups included in this review: 11.3% in the intervention group (double ICS) and 12.5% in the control group (stable ICS). However, in some groups the number of events was similar to the number of participants with missing data, therefore their outcomes could impact the estimated effect of intervention. Authors didn't correct or assess for bias from missing outcome data. Reasons for missing data were accounted for and most were not related to the ouctome of interest (only two participants across the study were lost because they were unsatisfied with asthma control, both in the rescue beclomethasone group). | Low risk of bias | Method for measuring the outcome was appropriate. Measurement of the outcome would not have differed between groups. Drugs were masked A to D to mask statisticians to treatment group. | Some concerns | Trial registered before participant enrolment began (https://clinicaltrials.gov/ct2/show/study/NCT00394329). However, registry record contains no prospective information about statistical analyses and no protocol or SAP available. The numerical result is not likely to have been selected on the basis of the results from multiple outcome measurements or analyses. | Low risk of bias | Overall risk of bias assessed as low as the only 'some concerns' was due to no study protocol or SAP available. However, it was registered and no other details from the trial for this outcome gives us reason to believe that there are some concerns regarding risk of bias. |
| Oborne 2009 | Low risk of bias | An allocation sequence of random permuted blocks of 10 was generated using a random number table by an independent pharmacist and implemented by one of the study investigators once participants were enrolled into the trial. Mentions an investigator providing the randomization schedule and concealed allocation of masked inhalers. Inhalers were identical and participants blind to the intervention. Baseline data provided for all participants and those starting the study inhaler ‐ no issues noted. | High risk of bias | Placebo inhaler was used. Participants were not aware of the intervention, which they adminstered. Clear criteria give to participants as part of an action plan to guide how they should measure their symptoms and at what point they should initiate the study inhaler. However, no details about how they monitored or increased adherence to the intervention so unsure whether there were failures in implementing it. Report doesn't detail methods used to derive an unbiased estimate of the effect of adhering to intervention but the trial measured treatment failure only in those participants who started the study inhaler. | High risk of bias | The per protocol analysis based on only participants who started their study inhaler represents a relatively small, non‐randomized proportion of the full cohort, and there was an imbalance in the number of people in each group who did so. No analyses corrected for bias. Patients who discontinued who did not use the study inhaler might have done so for reasons relating to their disease worsening. The CONSORT diagram includes changes to inhaler therapy and other medical reasons for reasons for discontinuing the intervention. | Low risk of bias | Method of measuring the outcome was appropriate. Measurement of the outcome was the same between intervention groups. Investigators blinded and they were likely the assessors. | Some concerns | The trial was rgeistered (http://www.isrctn.com/ISRCTN46018181). It was registered after participant enrolment began but before recruitment was completed. The registry doesn't include anything about the anlyses and a protocol/SAP isn't available. Clear definition of outcome and timepoints. | High risk of bias | Used tool algorithm. |
| Rice‐McDonald 2005 | Low risk of bias | Method of sequence generation not described but "order and allocation of treatment by concealed randomisation". Does not report baseline characteristics for each intervention group. | Low risk of bias | A double‐blind, double‐dummy study with order and allocation of treatment by concealed randomisation. The participants administered the intervention. Compliance to the intervention was monitored by symptom and medication diaries and a four week run in tested participants compliance to the protocol as part of the inclusion criteria. Participants were contacted regularly by a nurse to judge when to initiate the study inhaler. | High risk of bias | Of 35 randomized participants, 13 subsequently withdrew prior to any asthma exacerbations. There is no evidence that the result was not biased by missing outcome data. The reasons for withdrawal from the trial are not associated with the intervention or outcome. However, 11 participants who were randomised and had exacerbations that went straight to oral corticosteroids (no study inhaler) were not analysed and this missingness woudl depend on its true value. | Low risk of bias | Method for measuring the outcome was appropriate, and unlikely to differ between groups. Treatment failure well defined. Outcome assessors were the particpants, who were blinded. | Some concerns | No trial registry, protocol or SAP available. The numerical result is not likely to have been selected on the basis of the results from multiple outcome measurements or analyses. | High risk of bias | Used tool algorithm. Domain S (for crossover trials) = LOW SQ S1 = the number of participants allocated to each of the sequences was nearly equal. SQ S2 = N/A SQ S3 = a four week washout period was used which is sufficient time for any carryover effects to have disappeared before outcome assessment in the second period. |
Risk of bias for analysis 1.3 Unscheduled physician visits.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| ACTRN12605000631606 | Low risk of bias | "Stratified block randomisation by age (3‐5, 6‐10, 11‐14), gender, centre". "Sequential study number allocated from a list according to blocking details". "...blocking details emailed to Dept of Epidemiology and Preventative Medicine, Monash Med School, Melbourne". "Study puffer number was allocated. Pre‐numbered puffers were held at RCH Brisbane pharmacy". Imbalances noted in disease characteristics (higher proportion of patients in the intervention group than the control group had persistent asthma and atopic history), but likely to be down to chance given sample size. | Some concerns | Placebo inhalers were used to presume this was to blind participants and personnel from the study medication, but no explicit definition of masking procedures. Outcome reported as percentages and unclear whether they related to the ITT or per protocol (those who started the inhaler) population. For the purposes of including data in the review, we assumed it was the latter and then used the full population numbers to include data for the primary outcome of the review. This assumes no one who didn't take the study inhaler had the outcome. Most participants started the study inhaler but approx 25% of each group did not, so there is some potential for bias. | Low risk of bias | Outcome data accounted for and a much higher number of events than those who withdrew. 11 in the intervention group (8.7%) an 10 in the control group (8.1%) withdrew from the study ‐ low and balanced. | Low risk of bias | The method of measuring the outcome was appropriate. Measurment of the outcome likely to be the same between the groups. Clear instructions for what to do in the event of worsening symptoms and called weekly by study nurse ‐ all events likely to have been recorded and unlikely to have differed between groups. No information on whether the assessors were aware of the intervention. The outcome assessment is not subjective. | Some concerns | Trial registered (https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=630) but after participant enrollment was completed. No details about the analyses available. No published journal report. The numerical result is not likelyt to have been selected on the basis of the results from multiple outcome measurements or analyses. | Some concerns | Used tool algorithm. |
| FitzGerald 2004 | Low risk of bias | Patients were randomised to treatment groups at visit 2 according to a blocked computer generated randomisation list for each centre. As a randomisation list was generated for each centre, this sounds like it was centrally generated. Baseline characteristics not shown for the full population, only those who had an exacerbation and started the study inhaler. Paper states that "there were no differences in patient characteristics between those who experienced an exacerbation and those who did not". | High risk of bias | Methods state 'double blind' so assume this means participants. The participants themselves delivered the interventin. Used an 'all patients treated' approach and only those who had exacerbations being included in the analysis (this means they were grouped according to the intervention they received and not the assigned intervention). Those excluded from the analysis in the trial should not have had a substantial impact on the result as we (the review) have access to the raw numbers for our analysis. | Some concerns | Some concernsNumber of participants lost to follow up were larger than the number of events. No analysis methods that correct for bias due to missing outcome data mentioned. About a third of participants lost to follow up was due to disease not improving and adverse effects, so it is possible that missingness in the outcome was influenced by its true value. The analysis does not account for participant characteristics that are likely to explain the relationship between missingness in the outcome and its true value but neither does it meet any of the five reasons that make it likely that missingness in the outcome depended on its true value. | Low risk of bias | Method of measuring the outcome was appropriate and the same between groups. Participants (who were blinded) reported this outcome. | Some concerns | Some concernsNo study protocol or trial registration record is available. The study participant (who was blinded) entered details into an electronic diary with pre‐define criteria criteria for the outcome. It's unlikely that the result being assessed was selected from multiple eligible analyses | High risk of bias | Used tool algorithm. |
| Harrison 2004 | Low risk of bias | An independent pharmacist randomly allocated individuals using computer generated random number tables. Statistical tests for imbalances not presented but group differences at baseline do not suggest a problem with randomisation. | Low risk of bias | Study report does not explicitly state "double‐blind", but a placebo was used which implies that it was not open label, and text mentions "A range of active and placebo study inhalers was available to enable the type of inhaler and daily dose to be matched to patients’ regular inhaled corticosteroid, type of inhaler, and dose". Participants delivered the intervention themselves. Stated an ITT analysis would be used for the primary objective, and have handled this outcome similarly. | Low risk of bias | Nearly all data available though number withdrawn similar to the number of events (31 in intervention, 28 in placebo). 8.9 and 10.1% withdrew from the intervention and placebo groups respectively which is relatively low and balanced, although the study does not report whether and how missing data were imputed. | Low risk of bias | Method for measuring the outcome was appropriate. Report doesn't mention who assessed this outcome but it's objective so would not have been influenced by the knowledge of the intervention received. | Some concerns | No trial registration, study protocol or SAP. The result being assessed was not selected from mutiple outcome measurements. It's unlikely that the result being assessed was selected from multiple eligible analyses ‐ though the trialists did use both an ITT and per protocol analysis on the outcomes. | Low risk of bias | Overall risk of bias assessed as low as the only 'some concerns' was due to no study protocol or SAP available. However, it was registered and no other details from the trial for this outcome gives us reason to believe that there are some concerns regarding risk of bias. |
Risk of bias for analysis 1.4 Unscheduled acute care, ED visit, or hospital admission.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| ACTRN12605000631606 | Low risk of bias | "Stratified block randomisation by age (3‐5, 6‐10, 11‐14), gender, centre". "Sequential study number allocated from a list according to blocking details". "...blocking details emailed to Dept of Epidemiology and Preventative Medicine, Monash Med School, Melbourne". "Study puffer number was allocated. Pre‐numbered puffers were held at RCH Brisbane pharmacy". Imbalances noted in disease characteristics (higher proportion of patients in the intervention group than the control group had persistent asthma and atopic history), but likely to be down to chance given sample size. | Some concerns | Placebo inhalers were used to presume this was to blind participants and personnel from the study medication, but no explicit definition of masking procedures. Outcome reported as percentages and unclear whether they related to the ITT or per protocol (those who started the inhaler) population. For the purposes of including data in the review, we assumed it was the latter and then used the full population numbers to include data for the primary outcome of the review. This assumes no one who didn't take the study inhaler had the outcome. Most participants started the study inhaler but approx 25% of each group did not, so there is some potential for bias. | High risk of bias | Outcome data accounted for but a much lower number of events than those who withdrew. There is no evidence that the result was not biased by missing outcome data. Patients who discontinued who did not use the study inhaler might have done so for reasons relating to their disease worsening. Reasons for withdrawal not presented. | Low risk of bias | The method of measuring the outcome was appropriate. Clear instructions for what to do in the event of worsening symptoms and called weekly by study nurse ‐ all events likely to have been recorded. Measurment of the outcome likely to be the same between the groups. No information on whether the assessors were aware of the intervention. The outcome assessment is not subjective. | Some concerns | Trial registered (https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=630) but after participant enrollment was completed. No details about the analyses available. No published journal report. The numerical result is not likelyt to have been selected on the basis of the results from multiple outcome measurements or analyses. | High risk of bias | Used tool algorithm. |
| Garrett 1998 | Low risk of bias | Only information about the allocation is that the children were randomised by the hospital pharmacist and the investigators were blinded to this allocation. Baseline characteristics were not provided by intervention group. | High risk of bias | Used a blinded inhaler delivered by the participant. 10/28 randomised participants not included in the analyses as they didn't have an exacerbation in both arms ‐ therefore a per protocol analysis. No hospitalisations reported in either group but there is a large proportion of those randomised were exlcuded from the analyses. | High risk of bias | Only those with completed pairs of exacerbations were included in the final analysis ‐ 10/28 excluded from the analyses as they only had an exacerbation in one arm. No mention of any children dropping out of the trial. No anlyses were reported to address bias from missing outcome data. Reported reason for excluding from analyses was that they had good health status (no exacerabtions). Those who had 1 or 3+ exacerbations during the study were not included. | Low risk of bias | The method for measuring the outcome as appropriate. Measurement of the outcome would not have differed between groups. Investigators were blinded. | Some concerns | No trial registration, study protocol or SAP (even though article mentions a study protocol). The numerical result is not likely to have been selected on the basis of the results from multiple outcome measurements or analyses. | High risk of bias | Used tool algorithm. Domain S = LOW RISK OF BIAS SQ1 = NI on whether the number of participants allocated to each of the two sequences equal or nearly equal. SQ2 = LOW ‐ Cross‐over data were included in the review by obtaining two‐by‐two data and applying a formula to account for inter‐correlation of matched pairs (Elbourne 2002). SQ3 = LOW ‐ Sufficient time was given for carryover effects to have disappeared before outcome assessment in the second period ‐ followed for two weeks and study tated that spirometry returned to normal within 1 week |
| Jackson 2018 | Some concerns | Method of sequence generation and allocation concealment not described (in paper, supplementary materials of protocol). However, the report mentions stratification which implies the sequence was computer generated. Statistical tests for imbalances not presented but group differences at baseline do not suggest a problem with randomisation. | Low risk of bias | Participants and their carers/parents were blinded. "The primary intention‐to‐treat analysis compared the overall efficacy of each treatment strategy, regardless of whether any yellow‐zone episodes actually occurred." | Low risk of bias | Number of participants lost to follow up is similar to the number of events; however, most participants were included in the study for most of the duration [mean follow‐up among all 127 participants in each group was 42.5 weeks and 40.3 weeks, respectively (i.e. most of the full 48 week follow‐up)]. | Low risk of bias | Method of measuring the outcome was appropriate. Measurement of the outcome was the shame between intervention groups. The outcome person who decided on this outcome was the study participant and carer (who were blinded). | Low risk of bias | Protocol dated February 2014 and trial enrolment began in August 2014. The numerical result is not likelyt to have been selected on the basis of the results from multiple outcome measurements or analyses. | Low risk of bias | Insufficient reporting warrants an assessment of 'some concerns' for the randomisation process, but the overall reporting and conduct of the trial suggests that rigorous procedures were followed to minimize bias. |
| Martinez 2011 | Low risk of bias | The Data Coordinating Center generated the random allocation sequence via computer software.The DCC had no contact with participants. No obvious imbalances between groups. | Low risk of bias | States double blind and placebo interventions were used. Those delivering the intervention were the participants. Report states an IIT analysis was used. | Low risk of bias | Withdrawals from the study were relatively low and even between the two groups included in this review: 11.3% in the intervention group (double ICS) and 12.5% in the control group (stable ICS). Raw numbers are available for the review's analyses. However, for this outcome there were 0 events reported so missing participant data could impact the estimated effect of intervention. No analyses on this outcome. Authors kept good accounts of reasons for missing data. | Low risk of bias | Method for measuring the outcome was appropriate and would not have differed between groups. Report doesn't mention who assessed this outcome but it's objective so would not have been influenced by the knowledge of the intervention received. | Some concerns | Trial registered before participant enrolment began (https://clinicaltrials.gov/ct2/show/study/NCT00394329). Registry record contains no prospective information about analyses and no SAP or protocol available. However, there was no analyses for SAEs. The result being assessed was not selected from mutiple outcome measurements. It's unlikely that the result being assessed was selected from multiple eligible analyses. | Low risk of bias | Overall risk of bias assessed as low as the only 'some concerns' was due to no study protocol or SAP available. However, it was registered and no other details from the trial for this outcome gives us reason to believe that there are some concerns regarding risk of bias. |
Risk of bias for analysis 1.6 Duration of exacerbation.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.6.1 days to symptom recovery | ||||||||||||
| Harrison 2004 | Low risk of bias | An independent pharmacist randomly allocated individuals using computer generated random number tables. Statistical tests for imbalances not presented but group differences at baseline do not suggest a problem with randomisation. | Low risk of bias | Study report does not explicitly state "double‐blind", but a placebo was used which implies that it was not open label, and text mentions "A range of active and placebo study inhalers was available to enable the type of inhaler and daily dose to be matched to patients’ regular inhaled corticosteroid, type of inhaler, and dose". Participants delivered the intervention themselves. Used a log rank test. | Low risk of bias | 8.9 and 10.1% withdrew from the intervention and placebo groups respectively which is relatively low and balanced. However, the study does not report whether and how missing data were imputed and the outcome can only be measured in those who had an exacerbation which was a subset of the randomised population. | Low risk of bias | Method for measuring the outcome was appropriate. Unlikely that the measurement of the outcome would have differed between the groups. Study participants were outcome assessors (so blinded). | Some concerns | No publicly available trial registration, study protocol or SAP (SAP is mentioned in the study report). Clear definition of outcome and timepoints. | Low risk of bias | Overall risk of bias assessed as low as the only 'some concerns' was due to no trial registration or study protocol details, but this is an old trial when making these available was not standard. |
| Subgroup 1.6.2 days to lung function recovery | ||||||||||||
| Harrison 2004 | Low risk of bias | An independent pharmacist randomly allocated individuals using computer generated random number tables. Statistical tests for imbalances not presented but group differences at baseline do not suggest a problem with randomisation. | Low risk of bias | Study report does not explicitly state "double‐blind", but a placebo was used which implies that it was not open label, and text mentions "A range of active and placebo study inhalers was available to enable the type of inhaler and daily dose to be matched to patients’ regular inhaled corticosteroid, type of inhaler, and dose". Participants delivered the intervention themselves. Used a log rank test. | Low risk of bias | 8.9 and 10.1% withdrew from the intervention and placebo groups respectively which is relatively low and balanced. However, the study does not report whether and how missing data were imputed and the outcome can only be measured in those who had an exacerbation which was a subset of the randomised population. | Low risk of bias | Method for measuring the outcome was appropriate. Unlikely that the measurement of the outcome would have differed between the groups. Study participants were outcome assessors (so blinded). | Some concerns | No publicly available trial registration, study protocol or SAP (SAP is mentioned in the study report). Clear definition of outcome and timepoints. | Low risk of bias | Overall risk of bias assessed as low as the only 'some concerns' was due to no trial registration or study protocol details, but this is an old trial when making these available was not standard. |
Risk of bias for analysis 1.5 Serious and non‐serious adverse events.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.5.1 Serious adverse events | ||||||||||||
| ACTRN12605000631606 | Low risk of bias | "Stratified block randomisation by age (3‐5, 6‐10, 11‐14), gender, centre". "Sequential study number allocated from a list according to blocking details". "...blocking details emailed to Dept of Epidemiology and Preventative Medicine, Monash Med School, Melbourne". "Study puffer number was allocated. Pre‐numbered puffers were held at RCH Brisbane pharmacy". Imbalances noted in disease characteristics (higher proportion of patients in the intervention group than the control group had persistent asthma and atopic history), but likely to be down to chance given sample size. | High risk of bias | Placebo inhalers were used to presume this was to blind participants and personnel from the study medication, but no explicit definition of masking procedures. Outcome reported as as counts on a bar chart so unclear whether participants or events are the unit of analysis, and whether the events were counted in the full population or only those starting the inhaler. For the purposes of including data in the review, data were included as people with full population denominators. Most participants started the study inhaler but approx 25% of each group did not, so there is some potential for bias. | High risk of bias | Number of events similar to the number that withdrew, and no reasons for withdrawal specified. No evidence that the analysis corrected for bias. Withdrawal from the study could be related to participants’ health status so it is possible that missingness in the outcome was influenced by its true value. The circumstances of the trial make it likely that missingness in the outcome depends on its true value, e.g. participants might drop out if their asthma symptioms get worse or they had an adverse reaction. | Low risk of bias | No information about the method of measuring the outcome. Measurment of the outcome likely to be the same between the groups. No information on whether the assessors were aware of the intervention. The outcome assessment is not subjective. | Some concerns | Trial registered (https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=630) but after participant enrollment was completed. No details about the analyses available. No published journal report. The numerical result is not likelyt to have been selected on the basis of the results from multiple outcome measurements or analyses. | High risk of bias | Used tool algorithm. |
| Martinez 2011 | Low risk of bias | The Data Coordinating Center generated the random allocation sequence via computer software.The DCC had no contact with participants. No obvious imbalances between groups. | Low risk of bias | States double blind and placebo interventions were used. Those delivering the intervention were the participants. Number adverse events measured in the whole population. | Low risk of bias | Withdrawals from the study were relatively low and even between the two groups included in this review: 11.3% in the intervention group (double ICS) and 12.5% in the control group (stable ICS). Raw numbers are available for the review's analyses. However, in some groups the number of events was similar to the number of participants with missing data, therefore their outcomes could impact the estimated effect of intervention. No analyses on this outcome. Authors kept good accounts of reasons for missing data and reported the SAEs. | Some concerns | Study report mentions a data and safety monitoring board which implies adverse event measuring procedures were followed. Measurement of the outcome would not have differed between groups. The outcome assessor was the study participant (who was blinded) and AEs were overseen by a safety monitoring board, though unsure if they were blinded. Assessment of the outcome may have been influenced by knowledge of intervention received. It was unlikely that assessment of the outcome was influenced by knowledge of intervention received given the small events numbers and oversight from the safety monitoring board. | Some concerns | Trial registered before participant enrolment began (https://clinicaltrials.gov/ct2/show/study/NCT00394329). However, registry record contains no prospective information about analyses and no SAP or protocol available. The numerical result is not likely to have been selected on the basis of the results from multiple outcome measurements or analyses. | Some concerns | Used tool algorithm. |
| Subgroup 1.5.2 Non‐serious adverse events | ||||||||||||
| Foresi 2000 | Low risk of bias | Described as randomized but no details to assess sequence generation or allocation concealment. Some imbalances across groups (statistical tests not presented) but may be expected for sample size and do not systematically favour one group over another. | High risk of bias | Not described fully but described as double‐blind ‐ placebo used to blind intervention in the event of an exacerbation. | High risk of bias | Per‐protocol analysis was performed on 175 out of 213 randomized patients (191 who completed minus 38 with protocol violations), and adverse events were only reported for those who had an exacerbation (minority of population). Those included in the analysis were those who had an exacerbation, for which rates differed across groups and relate to asthma severity and effectiveness of the intervention. Withdrawal rates fromthe study also likely to be related to participants’ health status, as these include adverse events and other reasons. | Low risk of bias | No information about how adverse events were measured or managed. However, all participants visited the clinic once a month to discuss progress, symtpoms and adherence, and all kept daily symptom/management diaries. Symptoms were recorded in daily dairies by the participant, who was blinded. | High risk of bias | No trial registration, study protocol or SAP. No clear definitions for non‐serious adverse events. Outcome was not analysed. | High risk of bias | Used algorithm |
| Oborne 2009 | Low risk of bias | An allocation sequence of random permuted blocks of 10 was generated using a random number table by an independent pharmacist and implemented by one of the study investigators once participants were enrolled into the trial. Mentions an investigator providing the randomization schedule and concealed allocation of masked inhalers. Inhalers were identical and participants blind to the intervention. Baseline data provided for all participants and those starting the study inhaler ‐ no issues noted. | Low risk of bias | An allocation sequence of random permuted blocks of 10 was generated using a random number table by an independent pharmacist and implemented by one of the study investigators once participants were enrolled into the trial. Mentions an investigator providing the randomization schedule and concealed allocation of masked inhalers. Inhalers were identical and participants blind to the intervention. | Some concerns | "double‐blind, placebo‐controlled trial" "Drug groups were labelled as A, B, C, and D to mask statisticians to treatment group during the first complete run‐through of data analyses." "Active and placebo inhalers were…identical apart from the presence or absence of inhaled corticosteroid, to achieve allocation concealment and blinding of investigators and pariticipants". | High risk of bias | Only 94 out of 403 participants (23%) started the study inhaler, so most of the population were not included in the analysis of adverse events No evidence that the analysis was not biased by focusing on those who had an exacerbation. Those included in the analysis were those who had an exacerbation, for which rates differed across groups and relate to asthma severity and effectiveness of the intervention. | High risk of bias | No information about how adverse events were measures or monitored. The participant's management plan only requested the daily diary of symptoms was completed if they felt their asmtha was deterioating. As there were more exacerbations in the active group we can assume more patients deteriotaed. Therefore, more participants in the active group would have been capturing other non‐series adverse events in their diaries (and possibly why the active group had 9 events and the placebo 3). | High risk of bias | The trial was registered (http://www.isrctn.com/ISRCTN46018181) after participant enrolment began but before recruitment was completed. The registry doesn't include anything about the anlyses and a protocol/SAP isn't available. No clear definitions for non‐serious adverse events. |
In general, there was low risk of bias related to the randomisation process across studies. Four studies had some concerns in only one of the RoB 2 domains, but based on the overall reporting and conduct of the trials for specific outcomes, it was suggested that rigorous procedures were followed to minimise bias, therefore for these studies, we overwrote the RoB 2 tool algorithm and judged the overall risk of bias to be low. This included no trial registration or study protocol details for Harrison 2004, Martinez 2011, and Oborne 2009, and insufficient reporting of the randomisation process for Jackson 2018.
There was an interesting difference between the risk of bias assessments for Analysis 1.1 and Analysis 1.2, which have the same outcome (treatment failure: need for systematic corticosteroids) in different populations: all randomised participants (see Table 16) and those starting the inhaler (see Table 17), respectively. For studies contributing results to treatment failure in all randomised participants, half of the studies had an overall low risk of bias and half had some concerns or high risk of bias. Two cross‐over trials had an overall high risk of bias due to bias in deviations from intended interventions and missing outcome data, as a large proportion of those randomised were excluded from the analysis, either for worsening asthmatic symptoms so they went straight to corticosteroids (did not use the study inhaler) (Rice‐McDonald 2005), or because only those who had exacerbations in both periods were included in the analysis (Garrett 1998). Whereas for studies contributing results to treatment failure in those who started the inhaler, all studies but one, Martinez 2011, were at high risk of bias overall. The domains that contributed to this were either bias due to deviations from intended interventions or missing outcome data. Common issues included that the number of participants that dropped out or were not included in the analysis was higher than the number with treatment failure events; it was unclear whether those who dropped out did so for disease worsening; and because only those who started the inhaler were included in the analysis (so we are unsure whether those who did not start a study inhaler required systematic corticosteroids).
1.1. Analysis.

Comparison 1: Increased versus stable doses of ICS, Outcome 1: Treatment failure: need for systemic corticosteroids (primary outcome, all randomised participants)
1.2. Analysis.

Comparison 1: Increased versus stable doses of ICS, Outcome 2: Treatment failure: need for systemic corticosteroids (of those starting inhaler)
For serious and non‐serious adverse events (Table 20), three studies had an overall high risk of bias, and one study had some concerns due to key issues that related to adverse event reporting. Concerns that led to these judgements were similar to those in Analysis 1.2, including that it was unclear whether the reported counts related to participants or events; it was unclear whether the reported counts related to the full randomised population or only those who took their study inhaler; and the events were similar in number or fewer than the number who dropped out, with reasons for dropping potentially relating to the participants' health.
Effects of interventions
See: Table 1
Absolute and relative effects for all primary and secondary outcomes are summarised with their GRADE ratings signifying confidence in the effect estimates in Table 1.
Primary outcome
Treatment failure: need for systemic corticosteroids (ITT analysis)
People randomised to increase their ICS dose at the first signs of an exacerbation had similar odds of requiring rescue oral corticosteroids to those randomised to take a placebo inhaler (odds ratio (OR) 0.97, 95% confidence interval (CI) 0.76 to 1.25; 8 studies; 1774 participants; I2 = 0%; Analysis 1.1). Approximately 50% of randomised participants actually required use of the study inhaler (mean 50.4%, range 23% to 100%).
We had moderate confidence in the result due to concerns relating to risk of bias and the assumptions made to include study data in the ITT and treated‐population analyses (see Table 16). Whilst we did not prespecify bounds for concluding no difference or assessing imprecision, the point estimate and width of the confidence intervals suggest there is unlikely to be a clinically important effect of increasing ICS dose to avoid the need for oral steroids. In absolute terms, 184 people out of 1000 needed oral corticosteroids in the control group over 46 weeks, compared with 180 (95% CI 147 to 220) out of 1000 for those randomised to increase their ICS dose in the event of an exacerbation.
Subgroup and sensitivity analyses
There were sufficient data to investigate five of the six expected effect modifiers on results of the primary outcome with subgroup analyses. Results did not suggest a visible or statistical difference between the subgroups investigated, but the observational nature of subgroup analysis and the small number of studies in each subgroup means the possibility of important differences cannot be ruled out:
adult versus paediatric study populations (Analysis 2.1; test for subgroup differences: Chi² = 0.66, df = 1, P = 0.41, I² = 0%);
initiation of the study inhaler within 48 hours versus after 48 hours (Analysis 2.2; test for subgroup differences: Chi² = 0.43, df = 1, P = 0.51, I² = 0%);
low versus medium versus high maintenance doses of ICS (Analysis 2.3; test for subgroup differences: Chi² = 2.79, df = 2, P = 0.25, I² = 28.3%);
low versus high exacerbation doses of ICS (Analysis 2.4; test for subgroup differences: Chi² = 0.31, df = 1, P = 0.58, I² = 0%;
doubling versus larger dose increases (Analysis 2.5; test for subgroup differences: Chi² = 0.01, df = 1, P = 0.91, I² = 0%).
2.1. Analysis.

Comparison 2: Primary outcome subgroup and sensitivity analyses, Outcome 1: Subgrouped by age
2.2. Analysis.

Comparison 2: Primary outcome subgroup and sensitivity analyses, Outcome 2: Subgrouped by time to treatment initiation
2.3. Analysis.

Comparison 2: Primary outcome subgroup and sensitivity analyses, Outcome 3: Subgrouped by maintenance ICS dose
2.4. Analysis.

Comparison 2: Primary outcome subgroup and sensitivity analyses, Outcome 4: Subgrouped by ICS dose during exacerbation
2.5. Analysis.

Comparison 2: Primary outcome subgroup and sensitivity analyses, Outcome 5: Subgrouped by ICS fold increase
We could not include Garrett 1998 in the maintenance or exacerbation ICS dose subgroup analyses because of the large dose range, which included no details about average doses on which to base a categorisation. We could not examine the impact of smoking status on the odds of requiring oral corticosteroids during an exacerbation because all studies recruited non‐smokers or ex‐smokers.
There was overlap in the studies removed in the planned sensitivity analysis, and results should be considered exploratory. The results showed minimal impacts on the synthesised result for the primary outcome:
removing the two cross‐over studies (Analysis 2.6; OR 0.96, 95% CI 0.74 to 1.24; I² = 7%);
removing the three studies at overall high risk of bias (Analysis 2.7; OR 0.93, 95% CI 0.71 to 1.21; I²= 13%);
removing the two commercially funded studies (Analysis 2.8; OR 0.93, 95% CI 0.72 to 1.21; I² = 0%).
2.6. Analysis.

Comparison 2: Primary outcome subgroup and sensitivity analyses, Outcome 6: Sensitivity analysis: parallel‐group studies only
2.7. Analysis.

Comparison 2: Primary outcome subgroup and sensitivity analyses, Outcome 7: Sensitivity analysis: removing studies at overall high risk of bias
2.8. Analysis.

Comparison 2: Primary outcome subgroup and sensitivity analyses, Outcome 8: Sensitivity analysis: independently funded studies only
Secondary outcomes
Treatment failure: need for systemic corticosteroids (treated‐population analysis)
Results within the treated population to assess the effect of increasing ICS dose in participants who needed to initiate the study inhaler remain unchanged from the previous version of this review, because the new study, Jackson 2018, only reported results for the full randomised population. The analysis is based on 766 people who had exacerbations and met the study criteria to initiate the study inhaler, rather than all 1774 in the full randomised sample. The point estimate was more in favour of increased ICS dose than the primary ITT analysis, but does not suggest that participants randomised to increase their ICS dose have lower odds of requiring oral corticosteroids than those assigned to placebo (OR 0.84, 95% CI 0.54 to 1.30; 7 studies; 766 participants; I2 = 42%; random‐effects model; Analysis 1.2).
In two studies, all randomised participants took their study inhaler, so the data were the same as those entered for the primary outcome. We had very low confidence in the result because of inconsistency between study results, imprecision in the pooled effect, and very serious risk of bias (see Table 17).
Unscheduled physician visits
The pooled effect of three parallel‐group studies that could be included in the analysis was very imprecise (OR 0.96, 95% CI 0.66 to 1.41; 3 studies; 931 participants; I2 = 0%; Analysis 1.3; unchanged from previous version of the review). Harrison 2004 and ACTRN12605000631606 reported unscheduled visits only for people who took their study inhaler, but we used the total number randomised as the denominator. Authors of the previous version of the review performed a post hoc sensitivity analysis using only those taking the study inhaler as the denominator for these two studies, which did not change the conclusions (OR 0.89, 95% CI 0.59 to 1.35).
1.3. Analysis.

Comparison 1: Increased versus stable doses of ICS, Outcome 3: Unscheduled physician visits
The width of the confidence intervals makes it very difficult to determine where the true effect may lie, so our confidence in the effect estimate is low.
Unscheduled acute care or emergency department visits or need for hospital admission
The pooled effect of three studies that could be included in the analysis was very imprecise because only one study observed any events (Peto OR 0.50, 95% CI 0.16 to 1.56; 4 studies; 704 participants). Again, conclusions were unchanged when the number taking the study inhaler instead of the number randomised was used as the denominator (Peto OR 0.52, 95% CI 0.17 to 1.65; 4 studies; 505 participants).
We had very low confidence in the effect estimate due to very serious imprecision and risk of bias (see Table 19).
Serious and non‐serious adverse events
We analysed serious adverse events and non‐serious adverse events separately due to the way they were reported in the included studies, with two pairs of different studies in each analysis (no new data since the previous version of the review). Both point estimates were in favour of keeping ICS stable, but imprecision reduced our confidence in the effect estimates (serious adverse events OR 1.69, 95% CI 0.77 to 3.71; 2 studies; 394 participants; I² = 0%; non‐serious adverse events OR 2.15, 95% CI 0.68 to 6.73; 2 studies; 142 participants; I² = 0%).
We had very low confidence in either result due to imprecision and risk of bias, arising primarily from missing data, and additionally from measurement of the outcome and selection of the reported result for non‐serious adverse events (see Table 20).
Serious adverse events in Martinez 2011 included bronchitis in the increased dose group and viral meningitis in the stable daily dose group. ACTRN12605000631606 reported six occurrences of upper respiratory tract infection/otitis media/croup in the increased ICS group and low numbers of the following in one or both groups, for which no formal analyses have been conducted: ear/nose/throat surgery, fracture and orthopaedic events, chest infection/pneumonia, and death (one in double‐dose group). Three studies reporting lists of specific non‐serious side effects generally reported low occurrence (one or two people) in either group (Foresi 2000; Oborne 2009; Rice‐McDonald 2005), and Garrett 1998 and Harrison 2004 provided minimal information regarding adverse events.
Duration of exacerbation
We made no changes to the analyses of duration of exacerbation from the previous version of the review. Although three studies reported the outcome as defined by the time required for PEFR to return to baseline values (Garrett 1998; Harrison 2004; Oborne 2009), group mean and standard deviation values were only available for Harrison 2004, which did not suggest a difference between stable and increased ICS (Analysis 1.6). Mean time to symptom recovery in the placebo group was 6.1 days, and mean time to lung function recovery was 7 days. In those who took an increased dose of ICS, time to recovery was 0.7 days longer (95% CI 1.06 shorter to 2.46 longer) and 0.2 days shorter (95% CI 1.88 shorter to 1.48 longer), respectively.
1.6. Analysis.

Comparison 1: Increased versus stable doses of ICS, Outcome 6: Duration of exacerbation
We had low confidence in the estimates due to risk of bias (see Table 21) and imprecision.
Discussion
Summary of main results
This review update incorporates a large new study which increases the number of people in the primary analysis from 1520 to 1774, and uses the latest methods to investigate the impact of bias within the meta‐analyses. The precision added by the new study contributing to the review's primary outcome and the more thorough, results‐based approach to bias assessment strengthen the main conclusion, that double‐blind trials do not support increasing ICS dose at the first sign of an exacerbation to reduce the need for oral steroids.
The updated review now includes nine RCTs, seven parallel and two cross‐over, with a mix of adult and paediatric populations. Mean maintenance doses of ICS varied in adult and paediatric studies, as did use of concomitant medications, action plan activation criteria, ICS fold increases, smoking history, and patient severity. Though there is increased confidence in the bottom‐line finding for the primary outcome, the new study sheds little additional light on the secondary outcomes that were experienced infrequently in the studies (hospital attendance and resource use), which likely reflects the severity of the recruited populations. Furthermore, the reliance on aggregate data from a relatively small number of moderately sized, heterogenous studies means there remains little information to delve into the cost‐benefit profiles of the strategy for populations at different baseline doses, of different dose increases against other strategies, and how these interplay with clinical characteristics.
Results for the main oral steroid outcome within the subset of patients who initiated the study inhaler are unchanged from the previous version of the review, and subject to significant biases, both from the primary studies and the assumptions made to allow synthesis. Sensitivity analyses testing the impact of assumptions made for synthesis and to exclude cross‐over studies, studies at overall high risk of bias, and those with commercial funding did not change our conclusions.
Unfortunately, the review update does not resolve uncertainties about the safety implications of temporary ICS increases, with conclusions again limited by inconsistent reporting, serious imprecision, and risk of bias from missing data.
Overall completeness and applicability of evidence
To our knowledge, this is an update of the only systematic review and meta‐analysis in the literature examining the safety and effectiveness of increasing versus maintaining the same ICS dose at the onset of an asthma exacerbation as part of a patient‐initiated action plan. The most recent Global Initiative for Asthma Guidelines recommend at least doubling ICS dose or consider increasing ICS to a high dose as part of the asthma self‐management action plan for worsening asthma (GINA 2021). The study populations included in this review had mild to moderate asthma, therefore the results may not be applicable to those with severe asthma. The criteria for action plan activation were based on a combination of PEFR worsening, increase in asthma symptoms, and/or an increase in rescue bronchodilator use, which reflect current clinical practice.
The primary objective of some studies was to measure the need for oral steroids in those who started the study inhaler, which ignores potential differences in exacerbation frequency and intervention application between groups, and blurs a lack of need with other reasons for failing to initiate the study inhaler such as suboptimal adherence or understanding. More recent studies follow the 'intention‐to‐treat' approach to measure the effect of being allocated to a stable or increased ICS action strategy regardless of how frequently or accurately it was enacted, assuming any differences reflect those that would occur in practice. Though the ITT approach is more methodologically reliable, both angles are likely to be of interest to decision makers, and our confidence is reflected in the risk of bias assessments and GRADE ratings.
It should be noted that a recent large, National Institute for Health and Care Research‐funded study commissioned to address unanswered questions of effectiveness and safety was not eligible for inclusion because it used a pragmatic, unblinded design. The review protocol was designed to focus on the highest‐quality evidence, but exclusion of this study prevented us from investigating fully the intricacies of differences in effects and reliability for decision makers. The careful inclusion of unblinded evidence and analysis with subgroups or sensitivity analyses alongside the blinded evidence base would help to pick apart questions of effectiveness.
There may be several reasons for the overall lack of benefit from an increased ICS dose strategy on our primary and secondary outcomes. Firstly, most study participants were on maintenance ICS, which is an effective method of preventing exacerbations and specifically reduce the need for rescue oral corticosteroids. In several of the included studies, the dose of maintenance ICS was in a high range. Further increasing ICS dose with the onset of a respiratory exacerbation may therefore have little benefit given the shape of the ICS dose‐response curve (Holt 2001). In addition, although self‐reported compliance with the action plan protocol and study inhaler was high (86% in Garrett 1998 and > 97% in FitzGerald 2004), actual compliance was neither monitored nor measured objectively. Amongst the studies, there were minor differences in the timing of action plan activation after symptom onset or PEFR worsening, ranging from immediate start to 48 hours after. This detail is important to note, as a delay in initiating increased ICS may also affect clinical outcomes.
Quality of the evidence
The evidence in this review ranges from moderate to very low quality across outcomes, meaning we have variable confidence in the results. We downgraded the primary outcome only for risk of bias because studies carrying 13% of the analysis weight had overall high risk of bias, and studies carrying a further 50% of the weight had some concerns. Biases mostly arose in domains 2 and 3 (deviations from the intended interventions and missing data), often relating to assumptions made when there were differences between the way the study reported the outcome and how it was needed for the analysis, or uncertainty about the population used for the study analysis. We did not downgrade the primary outcome for imprecision because we judged that the number of events and participants reflected in the analysis provided reasonable certainty that there is unlikely to be an important benefit or harm of the intervention. However, the bounds are not sufficient to conclude no difference between the two strategies with certainty.
Confidence was reduced for several of the secondary outcomes due to risk of bias and assumptions made to permit inclusion of study data in the ITT and treated‐population analyses. The most common limitation across outcomes was the risk of bias inherent within study designs and introduced through the assumptions that were required to include studies in the analysis (e.g. to limit the impact of missing data or due to a difference between how the study reported the outcome and how it was needed to combine with other studies). Imprecision was a particular issue in the analysis of serious adverse events and resource‐use outcomes that would be expected to occur infrequently in the recruited populations, who had mild to moderate asthma (e.g. unscheduled physician visits, acute care, emergency department visits and hospital admissions).
It is notable that all GRADE ratings changed from the previous version of the review, resulting mainly from the reassessment of all studies with the revised risk of bias tool for RCTs. The tool allowed us to take a more thorough, results‐based approach to teasing out the issues of variable reporting and the impact of review assumptions and data transformations on the ITT and treated‐population analyses of treatment failure.
All studies were well‐matched to our review question, therefore no downgrades were required for indirectness of study populations, interventions, or outcomes. We resolved uncertainties in the definitions of outcomes through contact with study authors, and where outcome definitions or the populations used for analysis (e.g. ITT or those taking the study inhaler) were unclear or differed from what was defined in the review protocol, this was accounted for as missing data and deviation from the intended intervention in the risk of bias assessment (no downgrade for indirectness across outcomes).
Potential biases in the review process
A number of complexities have arisen during the life cycle of this review due to changes in practice and methodology, which have required amendments to the original protocol, which was published in 2009 (Quon 2009), and post hoc decision‐making by the study authors. The most notable evolution within the review that has the potential to introduce bias is the approach to defining the primary outcome of treatment failure and the assumptions that can be reasonably made to account for variations in study outcome reporting. The nature of differences in how study investigators defined their outcome population (ITT or treated population) and how they dealt with participants who did not initiate the study inhaler were not fully anticipated. Unclear reporting and study definitions that did not match the preferred ITT population for the meta‐analysis meant that assumptions were required to permit the inclusion of study data, and these have been made explicit throughout the Methods, Results, and in supplementary files to allow our choices to be interrogated, understood, and reanalysed as necessary.
We made other deviations from the study protocol to increase efficiency and bring the review up to date with current methods, including updating the type of software used to manage study processes and adopting the revised Cochrane risk of bias tool for RCTs (Higgins 2016; Sterne 2019). Where thresholds were used for GRADE decisions to downgrade the quality of the evidence, these have been made explicit within footnotes (e.g. for concluding no difference), and data assumptions and transformations are all provided in appendices and supplementary files.
Agreements and disagreements with other studies or reviews
This review is an update of a previously published Cochrane Review (Kew 2016), and added one more study including 254 participants to the review (Jackson 2018). The overall study findings and conclusions are consistent with those of our prior review. There are two pragmatic studies that were excluded with findings that are in disagreement with our review, but that provide insightful perspectives.
A recent, non‐blinded, randomised trial involving adults and adolescents with asthma compared a self‐management plan that included quadrupling versus not quadrupling the dose of inhaled glucocorticoids (McKeever 2018). The non‐blinded nature of the intervention was the reason for exclusion from this review. The adjusted hazard ratio for the time to a first severe asthma exacerbation, defined as treatment with systemic glucocorticoids or an unscheduled healthcare consultation for asthma, over a 12‐month period was 0.81 (95% CI 0.71 to 0.92; P = 0.002). Furthermore, the percentage of participants who used systemic glucocorticoids was lower in the quadrupling group than in the non‐quadrupling group (33% versus 40%), with a mean number of courses of 0.50 versus 0.61 (incidence rate ratio 0.82, 95% CI 0.70 to 0.96). Amongst those who reported activation of the self‐management plan, 50% of those in quadrupling group and 42% of those in the non‐quadrupling group were judged to have good adherence. In this pragmatic study, 80% of the participant recruitment was in primary care. Approximately 50% of the patients included in the trial had an exacerbation within a year, which may suggest more poorly controlled baseline. These factors may account for the observed benefit of quadrupling inhaled glucocorticoids in this study.
Cardet and colleagues published a pilot study to determine the feasibility of a pragmatic trial testing the Patient‐Activated Reliever‐Triggered ICS (PARTICS) strategy of using ICS concomitantly with rescue inhalers (Cardet 2020). The study population included mostly female (age > 40 years) African‐American and Hispanic participants who had uncontrolled asthma (mean Asthma Control Test score < 20) of varying severity (mild, moderate, severe). Although participant recruitment was feasible in the allotted 12‐week timeframe, key findings included low response rates (61% to 70%) and self‐reported adherence (62% to 88%), which led to the need for modifications to the full study protocol. These pragmatic clinical trials likely better reflect the real‐world setting, which can inform our interpretation of the results in efficacy trials.
Authors' conclusions
Implications for practice.
Evidence from double‐blind trials of adults and children with mild to moderate asthma suggests there is unlikely to be an important reduction in the need for oral steroids from increasing a patient's inhaled corticosteroids (ICS) dose at the first sign of an exacerbation. Other clinically important benefits and potential harms of increased doses of ICS compared with keeping the dose stable cannot be ruled out due to wide confidence intervals, risk of bias in the trials, and assumptions made to permit synthesis. The included studies, conducted between 1998 and 2018, reflect evolving clinical practice and study methods, and the data did not support thorough investigation of effect modifiers such as baseline dose, fold increase, asthma severity and timing. The review does not include recent evidence from pragmatic, unblinded studies that suggest a benefit of larger dose increases in those with poorly controlled asthma.
Implications for research.
A new systematic review protocol may be warranted to look at the differences between the blinded and unblinded evidence using robust methods for assessing risk of bias, in order to present and critique the full evidence base for decision makers.
Access to individual patient data in one or more of the larger, more recent trials may shed light on effect modifiers that are difficult to investigate with aggregate data across a small set of heterogeneous studies. Effectiveness in patients with lower baseline ICS dose and higher fold increases may be a reasonable focus in light of recent findings from pragmatic studies. Additional randomised controlled trials of a similar size in comparable populations are unlikely to add much certainty to what is already known from this review given the extent of existing variation between studies and the low frequency of important resource‐use outcomes in the population of interest.
It remains a priority for study investigators to report core outcomes consistently and transparently with clear descriptions of the population on which the analysis was conducted, and to provide access to raw and adjusted data to facilitate reanalysis and synthesis. Clear and structured descriptions of complex intervention components are also key in research to support synthesis for implementation.
Feedback
feedback, 27 October 2010
Summary
The abstract and document appear to mix up use of mg and mcg throughout the document. I assume the units should be mcg throughout but mg is used widely, particularly in the abstract. This could potentially lead to significant error and risk to patient safety. Could you confirm whether these are errors?
Reply
We are very grateful to the author for highlighting the typo in the review, along with others who pointed this out. We have corrected the typo and apologise for any confusion caused.
Contributors
Vanessa Chapman
What's new
| Date | Event | Description |
|---|---|---|
| 20 December 2021 | New search has been performed | New literature search run. |
| 20 December 2021 | New citation required and conclusions have changed | One new study added, methods updated to use the revised risk of bias tool for RCTs, including reapplication of GRADE for all outcomes. |
History
Protocol first published: Issue 1, 2009 Review first published: Issue 10, 2010
| Date | Event | Description |
|---|---|---|
| 8 June 2016 | New search has been performed | Three new studies including 419 additional participants were included in this review update (Martinez 2011; Rice‐McDonald 2005; ACTRN12605000631606). |
| 8 June 2016 | New citation required but conclusions have not changed | Although we included additional data in this review, the original conclusions remain unchanged. |
| 8 November 2010 | New citation required but conclusions have not changed | Feedback has triggered a new citation version. |
| 8 November 2010 | Feedback has been incorporated | We received feedback and corrected several typos by which mcg was confused with mg. |
Risk of bias
Acknowledgements
We are grateful to all authors who developed the protocol for this review (Quon 2009), and who contributed to previous review versions in 2010, Quon 2010, and 2016, Kew 2016:
Francine Ducharme helped develop the original protocol and contributed in the interpretation, write‐up, and sign‐off of the 2010 and 2016 reviews;
Michael Quinn co‐led the 2016 update including sift and study selection, data extraction, analysis, risk of bias assessment, and write‐up;
Mark FitzGerald, Catherine Lemière, and Neal Shahidi were all authors of the 2010 version of the review, and their contributions are described in Quon 2010.
We are very grateful to the study authors Vernon Chinchilli, Fernando Martinez, Claire Wainwright, Tim Harrison, and Janet Oborne, who kindly responded to requests for additional information for the previous version and the current update.
The authors and Cochrane Airways editorial team are grateful to the following peer and consumer reviewers for their time and comments: Hebatullah M Abdulazeem (Germany), Amnuayporn Apiraksakorn (Khon Kaen Hospital, Thailand), Aireen Wingert (Canada), and Nausherwan K Burki (UConn Health (University of Connecticut)).
The views and opinions expressed in this review are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, National Institute for Health and Care Research (NIHR), National Health Service (NHS), or the Department of Health and Social Care.
We thank the Cochrane Airways Review Group, namely Liz Stovold, for assistance with the literature search and ongoing support.
The Background and Methods sections of this review are based on a standard template used by the Cochrane Airways Review Group.
Appendices
Appendix 1. Search strategy for the previous version of this review
All records in the Specialised Register coded as ‘asthma’ were searched using the following terms:
(exacerbat* OR acute* or status* or severe* OR worsen* OR emergenc* OR attack* or crisis) and (dose* or dosing or dosage) and (doubl* or increas*) OR "dose response" or "drug dose") and (glucocorticoid* OR corticosteroid* OR "inhaled steroid*" OR fluticasone OR Flovent OR beclomethasone OR Becloforte OR budesonide OR Pulmicort OR flunisolide OR Aerobid OR triamcinolone OR Beclovent OR Azmacort OR Vanceril OR Becotide OR Flixotide OR Aerobec OR Mometasone OR Qvar or ciclesonide or Alvesco)
Appendix 2. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 3. Search strategy to identify relevant trials from the CAGR
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 MeSH DESCRIPTOR Adrenal Cortex Hormones Explode All
#6 ICS:TI,AB
#7 (beclomethasone* or beclometasone* OR triamcinolone* OR fluticasone* OR budesonide* OR betamethasone* OR flunisolide* OR ciclesonide* OR mometasone*)
#8 (inhal*) NEAR5 (steroid* or corticosteroid* or glucocorticoid*)
#9 #5 or #6 or #7 or #8
#10 MeSH DESCRIPTOR Dose‐Response Relationship, Drug
#11 (dose* or dosing or dosage) AND (doubl* or increas*)
#12 step‐up* OR (step* NEXT up*)
#13 dose* NEXT reponse*
#14 drug* NEXT dose*
#15 #10 or #11 or #12 or #13 or #14
#16 MeSH DESCRIPTOR Disease Progression
#17 exacerbat* OR acute* or status* or severe* OR worsen* OR emergenc* OR attack* or crisis
#18 #16 or #17
#19 #4 AND #9 AND #15 AND #18
[Note: in search line #1, MISC1 denotes the field in the record where the reference has been coded for condition, in this case, asthma]
Appendix 4. Additional searches (6 September 2021)
| Source | Terms and limits | Hits |
| WHO International Clinical Trials Registry Platform | Condition: Asthma exacerbation, date of registration from 1 March 2016 to present (any recruitment status, sponsor, location ). Condition: Asthma, Intervention: inhaled corticosteroids, date of registration from 1 March 2016 to present (any recruitment status, sponsor, location ). |
40 18 |
| AstraZeneca clinical trials | Terms: Asthma exacerbation, Phases 3 or 4, Therapeutic area: respiratory (any recruitment status, sponsor, location or product). | 14 |
| GlaxoSmithKline study register | Terms: asthma exacerbation, medical condition: asthma, Phases 3 or 4, study type: interventional (any recruitment status, phase, age, sex) | 99 |
| OpenGrey | Terms: asthma exacerbation | 20 |
| New York Academy of Medicine Grey Literature Report | Terms: asthma exacerbation | 4 |
Data and analyses
Comparison 1. Increased versus stable doses of ICS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Treatment failure: need for systemic corticosteroids (primary outcome, all randomised participants) | 8 | 1774 | Odds Ratio (IV, Fixed, 95% CI) | 0.97 [0.76, 1.25] |
| 1.2 Treatment failure: need for systemic corticosteroids (of those starting inhaler) | 7 | 766 | Odds Ratio (IV, Random, 95% CI) | 0.84 [0.54, 1.30] |
| 1.3 Unscheduled physician visits | 3 | 931 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.66, 1.41] |
| 1.4 Unscheduled acute care, ED visit, or hospital admission | 4 | 704 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.50 [0.16, 1.56] |
| 1.5 Serious and non‐serious adverse events | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 Serious adverse events | 2 | 394 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.77, 3.71] |
| 1.5.2 Non‐serious adverse events | 2 | 142 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [0.68, 6.73] |
| 1.6 Duration of exacerbation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.1 days to symptom recovery | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.2 days to lung function recovery | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
1.4. Analysis.

Comparison 1: Increased versus stable doses of ICS, Outcome 4: Unscheduled acute care, ED visit, or hospital admission
1.5. Analysis.

Comparison 1: Increased versus stable doses of ICS, Outcome 5: Serious and non‐serious adverse events
Comparison 2. Primary outcome subgroup and sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Subgrouped by age | 8 | Odds Ratio (IV, Fixed, 95% CI) | 0.97 [0.76, 1.25] | |
| 2.1.1 Children | 4 | Odds Ratio (IV, Fixed, 95% CI) | 1.07 [0.76, 1.49] | |
| 2.1.2 Adults | 4 | Odds Ratio (IV, Fixed, 95% CI) | 0.87 [0.60, 1.26] | |
| 2.2 Subgrouped by time to treatment initiation | 8 | Odds Ratio (IV, Fixed, 95% CI) | 0.97 [0.76, 1.25] | |
| 2.2.1 < 48 hours | 5 | Odds Ratio (IV, Fixed, 95% CI) | 1.02 [0.77, 1.36] | |
| 2.2.2 ≥ 48 hours | 3 | Odds Ratio (IV, Fixed, 95% CI) | 0.84 [0.51, 1.39] | |
| 2.3 Subgrouped by maintenance ICS dose | 7 | Odds Ratio (IV, Fixed, 95% CI) | 0.96 [0.75, 1.24] | |
| 2.3.1 Low | 2 | Odds Ratio (IV, Fixed, 95% CI) | 1.30 [0.83, 2.02] | |
| 2.3.2 Medium | 3 | Odds Ratio (IV, Fixed, 95% CI) | 0.78 [0.52, 1.18] | |
| 2.3.3 High | 2 | Odds Ratio (IV, Fixed, 95% CI) | 0.91 [0.58, 1.42] | |
| 2.4 Subgrouped by ICS dose during exacerbation | 7 | Odds Ratio (IV, Fixed, 95% CI) | 0.96 [0.75, 1.24] | |
| 2.4.1 Low | 1 | Odds Ratio (IV, Fixed, 95% CI) | 1.17 [0.57, 2.40] | |
| 2.4.2 High | 6 | Odds Ratio (IV, Fixed, 95% CI) | 0.94 [0.72, 1.22] | |
| 2.5 Subgrouped by ICS fold increase | 8 | Odds Ratio (IV, Fixed, 95% CI) | 0.97 [0.76, 1.25] | |
| 2.5.1 Double dose | 6 | Odds Ratio (IV, Fixed, 95% CI) | 0.98 [0.72, 1.34] | |
| 2.5.2 More than double | 2 | Odds Ratio (IV, Fixed, 95% CI) | 0.96 [0.63, 1.45] | |
| 2.6 Sensitivity analysis: parallel‐group studies only | 6 | 1728 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.74, 1.24] |
| 2.7 Sensitivity analysis: removing studies at overall high risk of bias | 5 | Odds Ratio (IV, Fixed, 95% CI) | 0.93 [0.71, 1.21] | |
| 2.8 Sensitivity analysis: independently funded studies only | 6 | Odds Ratio (IV, Fixed, 95% CI) | 0.93 [0.72, 1.21] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ACTRN12605000631606.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Population 251 children were randomised; 187 participants experienced an exacerbation and contributed to the analysis. Participants were between 3 and 14 years old; 38% of children were 3 to 5 years of age; 43% between 6 and 11 years; and 19% between 12 and 14 years. 60% of participants were male. Smoking status not reported (likely all never‐smokers, as paediatric study). Inclusion criteria: informed consent obtained from parent/carer and assent from child when possible. Age between 3 and 14 years, doctor diagnosis of asthma and taking regular ICS (minimum 125 μg fluticasone/d), at least 1 exacerbation in previous 12 months requiring admission to hospital, presentation to emergency department + use of oral steroids Exclusion criteria: children with comorbidities that may affect growth; children with other respiratory illness; unable to obtain informed consent; unable to speak English Baseline asthma severity See Table 2. |
|
| Interventions |
Run‐in period: 3‐month run‐in period including 2 weeks of peak flow measurement Study period Control arm: maintenance fluticasone inhaler at child's usual dose + placebo inhaler to keep dose stable during exacerbations Study arm: maintenance fluticasone inhaler at child's usual dose + study puffer to double dose during exacerbations. Continued until back to baseline Other medications allowed: not reported |
|
| Outcomes |
Primary outcome: use of oral steroid rescue and admission to hospital Secondary outcomes: growth over 12 months; time off work for parents, school for children; time for peak flow to return to baseline |
|
| Notes |
Funding source: Asthma Foundation Queensland; RCH Foundation Brisbane; fluticasone propionate, placebo, and peak flow metres provided by GlaxoSmithKline Funder role: details about funder's role reported. Registration: ACTRN12605000631606 Ethics approval: approved by Royal Children's Hospital & Health Service District (see registration details) Consent to participate: reports parents or carers provided informed consent, and children provided assent where possible Trial reporting vs review analysis: study reports need for oral steroids as a percentage with unclear denominators (43.8% increased group, 50.2% usual group). Number of events calculated using those who started the inhaler as the denominator (93 and 94), giving 41 and 47 events for Analysis 1.2. To include in Analysis 1.1, the same number of events was used with the number of participants in the full population. |
|
FitzGerald 2004.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Population 290 participants were randomised; 98 participants experienced an exacerbation and contributed to the analysis. Participants were 13 years of age or older; mean age was 32 years; 28% were male; 86% were non‐smokers, and 14% were ex‐smokers of fewer than 10 pack‐years. Inclusion criteria Age ≥ 13 years; documentation of the diagnosis of asthma within the previous year based on FEV1 reversibility postbronchodilator, methacholine provoking a fall in FEV1 and/or diurnal PEF variability; at least 1 previous asthma exacerbation (with mean duration from recent exacerbation to visit 1 of 131 day); stable dose of ICS (< 1200 μg/d of beclomethasone or equivalent twice daily) for 1 month before visit 1 Exclusion criteria Severe or near‐fatal asthma; current smokers and ex‐smokers > 10 pack‐years; baseline use of LABA; pregnant or lactating women; women of childbearing potential not on effective birth control; exacerbation due to chronic sinusitis; hospitalisation in previous 3 months; respiratory tract infection ≤ 1 month before visit 1 Baseline asthma severity See Table 2. |
|
| Interventions |
Run‐in period 3‐ to 6‐week period whereby participants using other forms of inhalers were switched to budesonide Turbuhaler at an equivalent dose and placed on a twice‐daily dose regimen Study period Control arm: maintenance inhaler of budesonide (100, 200, or 400 μg twice daily) + placebo inhaler twice daily for exacerbations Study arm: maintenance inhaler of budesonide + inhaler with budesonide to double dose of ICS (200, 400, or 800 μg twice daily) for exacerbations Other medications allowed Terbutaline sulfate inhaler as rescue medication; theophylline; anticholinergics; nasal corticosteroids |
|
| Outcomes |
Primary outcome The proportion of participants with treatment failure as judged by the need for treatment with oral methylprednisolone or an unscheduled visit to a physician or medical emergency department due to asthma or unstable asthma after 14 days of treatment Secondary outcomes None |
|
| Notes |
Funding source: AstraZeneca Canada Inc Funder role: author with AstraZeneca affiliation involved in drafting the protocol and manuscript and not on the trial steering committee or involved in designing the trial. No other details about funder's role reported. Registration: not registered Ethics approval: approved by institutional ethics committees at each research site Consent to participate: reports all participants provided written informed consent prior to enrolment Trial reporting vs review analysis: study reports need for oral steroids only for those who took their study inhaler, which is suitable for Analysis 1.2. To include in Analysis 1.1, we used the same number of events with the full population denominators. |
|
Foresi 2000.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Population 213 participants were randomised to 3 treatment groups; 47 participants experienced an exacerbation; Groups 2 and 3 accounted for 36 exacerbations and contributed to the analysis. Participants were 18 to 65 years of age; mean age was 39 years; 47% were male; 70% were non‐smokers, 22% ex‐smokers, and 8% smokers. Inclusion criteria Age 18 to 65 years; baseline FEV1 ≥ 50% and ≤ 90% of predicted values; daily PEF variability ≥ 20% on at least 4 different days during a 2‐week period; daily requirement of inhaled beta‐2 agonist; presence of wheeze, cough, chest tightness, shortness of breath that interfered with normal daily activity during a 2‐week pre‐study observation period Exclusion criteria Treatment with a high dose of beclomethasone (> 1000 μg/d); history of seasonal asthma Baseline asthma severity See Table 2. |
|
| Interventions |
Run‐in period 4‐week pre‐study treatment period whereby participants were asked to inhale budesonide 800 μg twice daily Study period Control arm (Group 3): maintenance inhaler of budesonide 100 μg twice daily + placebo inhaler 4 times daily for exacerbations (total 200 μg per day) Study arm (Group 2): maintenance inhaler of budesonide 100 μg twice daily + budesonide 200 μg 4 times daily for exacerbations (total 1000 μg per day) Other medications allowed Inhaled beta‐2 agonist; LABA; theophylline; anticholinergics |
|
| Outcomes |
Primary outcome: not specified Secondary outcomes
|
|
| Notes |
Funding source: Astra Farmaceutici Funder role: no details about funder's role reported. Registration: not registered Ethics approval: approved by ethics committees at all clinics Consent to participate: reports all participants provided informed consent Trial reporting vs review analysis: reports the number of participants having exacerbations defined by PEF reduction and the number of days participants had exacerbations and required OCS, but not the number of participants. Cannot be included in Analysis 1.1 or Analysis 1.2 |
|
Garrett 1998.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Population 28 participants were randomised; 18 pairs of exacerbations in both cross‐over periods contributed to the analysis. Participants were 6 to 14 years old; mean age was 8.2 years; 67% were male; smoking status not reported as paediatric trial (likely all non‐smokers). Inclusion criteria Age 6 to 14 years; currently taking inhaled corticosteroid prophylaxis (not exceeding 800 μg/d) Exclusion criteria Taking oral corticosteroids, sodium cromoglycate, or LABA; any previous intensive care admission, recent inpatient care for asthma, or any change in dose of inhaled corticosteroids in the past 2 months; any concurrent illness Baseline asthma severity See Table 2. |
|
| Interventions |
Run‐in period 2‐week run‐in period during which participants were required to use beclomethasone via MDI and spacer and a salbutamol MDI. Participants previously taking budesonide were switched to beclomethasone, but the child's daily dose was not changed. Study period Sequence 1: maintenance beclomethasone inhaler (< 800 μg/d) + placebo inhaler for exacerbation 1, followed by maintenance beclomethasone inhaler + inhaler with beclomethasone to double dose of ICS for exacerbation 2 Sequence 2: maintenance beclomethasone inhaler + inhaler with beclomethasone to double dose of ICS for exacerbation 1. Maintenance beclomethasone inhaler (< 800 μg/d) + placebo inhaler for exacerbation 2 Other medications allowed Salbutamol MDI |
|
| Outcomes |
Primary outcome: not specified Secondary outcomes
|
|
| Notes |
Funding source: New Zealand Asthma Society Funder role: no details about funder's role reported Registration: not registered Ethics approval: approved by Southern Regional Health Authority ethics committee Consent to participate: reports all participants and their parent provided informed consent Trial reporting vs review analysis: study reports need for oral steroids only for those who took their study inhaler, which is suitable for Analysis 1.2. To include in Analysis 1.1, we used the same number of events with the full population denominators. |
|
Harrison 2004.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Population 390 participants were randomised; 207 experienced an exacerbation and contributed to the analysis. Participants were 16 years or older; mean age was 49 years; 33% were male; 61% were non‐smokers, 36% ex‐smokers, and 3% smokers. Inclusion criteria Age ≥ 16 years; clinical diagnosis of asthma; taking an inhaled corticosteroid (100 to 2000 μg/d) on a regular basis; previous course of oral corticosteroids or doubled dose of inhaled corticosteroid in the previous 12 months for treatment or prevention of an asthma exacerbation Exclusion criteria History of smoking > 10 pack‐years; unstable asthma during a 2‐week run‐in period Baseline asthma severity See Table 2. |
|
| Interventions |
Run‐in period 2‐week period whereby participants continued their usual dose of inhaled corticosteroid and recorded morning peak flow and daytime symptom scores to ensure asthma stability Study period Control arm: maintenance inhaled corticosteroid (100 to 2000 μg/d) + identical placebo inhaler for exacerbations Study arm: maintenance inhaled corticosteroid (100 to 2000 μg/d) + identical inhaler with corticosteroid to double dose of ICS for exacerbations Participants were to use study inhaler for 14 days in addition to usual treatment when peak flow or symptoms deteriorated. Other medications allowed Not specified |
|
| Outcomes |
Primary outcome Proportion of participants who needed prednisolone in each group Secondary outcomes
|
|
| Notes |
Funding source: NHS Executive (through National Asthma Campaign) Funder role: provided critical review of the protocol but no role in study design, data collection, data analysis, data interpretation, or writing of the report Registration: not registered Ethics approval: approved by Nottingham City Hospital ethics committee Consent to participate: reports all participants provided written informed consent Trial reporting vs review analysis: study reports need for oral steroids separately for those who took their study inhaler (suitable for Analysis 1.2) and all randomised participants (suitable for Analysis 1.1) |
|
Jackson 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Population 254 participants were randomised; 168 participants experienced an exacerbation, with 68 resulting in treatment failure. Participants were 5 to 11 years old; mean age was 8 years; 64% were male; 38% had tobacco smoke exposure. Inclusion criteria 5 to 11 years of age; doctor‐diagnosed asthma mild to moderate; persistent asthma and had had at least 1 asthma exacerbation treated with systemic glucocorticoids in the previous year Exclusion criteria Asthma too severe (> 5 exacerbations in the previous year that had been treated with systemic glucocorticoids or a history of life‐threatening asthma) Baseline asthma severity See Table 2. |
|
| Interventions |
Run‐in period 4 weeks to establish adherence of 1) more than 75% to the use of open‐label trial medication (fluticasone propionate at a dose of 44 μg per inhalation, 2 inhalations twice daily); 2) daily completion of an electronic diary; and 3) asthma control (C‐ACT score > 19) at the randomisation visit Study period Control arm: maintenance inhaler of budesonide (88 μg twice daily) + control inhaler budesonide (88 μg twice daily) for exacerbations for 7 days at the early signs of loss of asthma control Study arm: maintenance inhaler of budesonide (88 μg twice daily) + study inhaler budesonide (440 μg twice daily) for exacerbations for 7 days at the early signs of loss of asthma control Other medications allowed Albuterol sulfate 90 μg/inhalation; rescue therapy oral prednisone will be administered for the treatment of impending episodes of severe asthma when bronchodilator therapy is inadequate |
|
| Outcomes |
Primary outcome Rate of severe asthma exacerbations treated with systemic glucocorticoids during the blinded treatment period Secondary outcomes Time to first asthma exacerbation, treatment failure, area under the curve for symptom scores during yellow‐zone episodes, albuterol use during yellow‐zone episodes, unscheduled emergency department or urgent care visits for asthma, hospitalisations for asthma, total glucocorticoid exposure (inhaled glucocorticoids plus systemic glucocorticoids), and linear growth. Exploratory outcomes included peak expiratory flows and number of days of asthma control. |
|
| Notes |
Funding source: National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH) Funder role: Program Officers from NHLBI serve on the steering committee for oversight of the interests/priorities of the NIH as well as applicable regulations. GlaxoSmithKline, who donated the trial medication, was not involved in trial design, data collection or interpretation. They were given the opportunity to read the draft manuscript, but did not provide any comments. Registration:NCT02066129 Ethics approval: approved by AsthmaNet steering committee, protocol review committee, and data and safety monitoring board Consent to participate: reports parents or legal guardians provided written informed consent, and children provided assent Trial reporting vs review analysis: number of participants needing oral steroids reported for the full population (suitable for Analysis 1.1). Study reports the number of treatment failures out of total number of yellow‐zone episodes and the number of participants with at least 1 yellow‐zone episode (i.e. those who took their study inhaler; 80 and 88), but not the number of participants in that population who needed oral steroids, so data could not be included in Analysis 1.2. Also reports mean number per year (primary method of analysis in the study), which could be reported narratively with the results |
|
Martinez 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Population 288 participants were randomised to 1 of 4 groups, of which 143 contributed to this analysis (71 combined group, 72 daily group). Participants were aged between 5 and 18 years; mean age was 11.2 years; 56.6% were male; smoking status not reported, as paediatric trial (likely all non‐smokers). Inclusion criteria Children and adolescents 6 to 18 years of age, history of mild persistent asthma during the previous 2 years, qualified for interruption or discontinuation of controller treatment because their illness was well‐controlled (as defined in US National Asthma Education and Prevention Program asthma care guidelines), naive to controller treatment with a history of 1 to 2 exacerbations in the previous year, those treated for the previous 8 weeks with monotherapy other than inhaled corticosteroids, and those whose illness was controlled for the previous 8 weeks on low‐dose corticosteroids as monotherapy (≤ 160 μg daily with a beclomethasone equivalent) Exclusion criteria Pre‐bronchodilator FEV1 < 60% predicted at the first visit; admitted to hospital for asthma in the previous year; any asthma exacerbation in the previous 3 months or more than 2 in the previous year; history of life‐threatening asthma exacerbations that required intubation or mechanical ventilation, or that resulted in a hypoxic seizure Baseline asthma severity See Table 2. |
|
| Interventions |
Run‐in period 4‐week run‐in period, during which participants received twice‐daily treatment with 1 puff of beclomethasone dipropionate and rescue treatment with a placebo inhaler added to rescue albuterol every time they needed albuterol Study period Control arm: maintenance inhaler of beclomethasone 40 μg twice daily + placebo twice‐daily inhaler and albuterol as rescue for exacerbations Study arm: maintenance inhaler of beclomethasone 40 μg twice daily + 40 mg beclomethasone twice daily and albuterol as rescue for exacerbations (combined group) Other medications allowed Low‐dose ICS or other monotherapy in previous 8 weeks. ICS > 160 μg beclomethasone equivalent was not allowed (daily beclomethasone group). Definition of exacerbation: use of more than 12 puffs of albuterol in 24 hours (excluding preventive use before exercise), PEF < 70% of consecutive days, PEF < 50% of reference value despite relief treatment, emergency room visit due to worsening of asthma symptoms |
|
| Outcomes |
Primary outcome Time to first exacerbation that required treatment with prednisone Secondary outcomes Spirometry FEV1, FENO, symptom diaries and control and quality of life questionnaires, linear growth |
|
| Notes |
Funding source: grants from the National Heart, Lung and Blood Institute (NHLBI); TEVA Pharmaceutical Industries Ltd provided beclomethasone dipropionate‐HFA and placebo Funder role: the NHLBI established and managed the independent data and safety monitoring board. Reports that the authors had complete independence over the conduct, integrity, and publication of the study Registration:NCT00394329 Other study identifier(s): TREXA Ethics approval: approved by local institutional review boards Consent to participate: reports that parents or guardians provided written informed consent, and children provided verbal or written assent Trial reporting vs review analysis: study design implies that everyone took their study inhaler, so there is no difference between the all‐randomised (Analysis 1.1) and treated population (Analysis 1.2) The study used a factorial design, which had implications for the independence of treatments and subsequent analysis of results. |
|
Oborne 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Population 403 participants were randomised; 94 participants experienced an exacerbation, for a total of 121 exacerbations that contributed to the analysis. Participants were 16 years of age or older; mean age was 56 years; 32% of participants were male; 69% were never‐smokers, 21% were ex‐smokers, and 10% were smokers. Inclusion criteria: age > 16 years, stable asthma, treated with ICS (200 to 1000 μg budesonide or equivalent), taken a course of oral corticosteroid or doubled dose of ICS in the previous 12 months but not in the preceding 4 weeks Exclusion criteria: > 20 pack‐year smoking history, other clinically significant medical conditions, pregnant or lactating Baseline asthma severity See Table 2. |
|
| Interventions |
Run‐in period: 2‐week period whereby participants continued their usual dose of inhaled corticosteroid and recorded morning peak flow and daytime symptom scores to ensure asthma stability Study period Control arm: maintenance inhaled corticosteroid (200 to 1000 μg/d) + identical placebo inhaler for exacerbations Study arm: maintenance inhaled corticosteroid (200 to 1000 μg/d) + identical inhaler with corticosteroid to quadruple dose of ICS for exacerbations Participants were to use study inhaler for 14 days in addition to usual treatment when peak flow or symptoms deteriorated. Other medications allowed Not specified |
|
| Outcomes |
Primary outcome Number of participants who had exacerbations of asthma treated with oral corticosteroids (ITT analysis) Secondary outcomes Number of participants who started the study inhaler and went on to require treatment with oral corticosteroids (treated population) |
|
| Notes |
Funding source: Asthma UK Funder role: not reported Registration:ISRCTN46018181 Ethics approval: approved by Nottingham Research Ethics Committee and relevant Research and Development departments in Nottinghamshire and Derbyshire Consent to participate: reports that participants provided written informed consent Trial reporting vs review analysis: study reports the need for oral steroids separately for those who took their study inhaler (suitable for Analysis 1.2) and all randomised participants (suitable for Analysis 1.1) |
|
Rice‐McDonald 2005.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Population 22 participants were randomised; 18 experienced an exacerbation in both phases and contributed to the analysis. Participants were 18 years of age or older; mean age was 46.5 years; 40.9% were male; smoking status not reported. Inclusion criteria: consenting adults ≥ 18 years of age; physician‐diagnosed asthma; reversible airways obstruction evidenced by (i) ≥ 15% reversibility in FEV1; or (ii) ≥ 20% variability in PEF over the 2‐ to 4‐week run‐in period (% variability defined as highest PEF–lowest PEF/highest PEF 3100); assessment by investigator that ongoing treatment with ICS was appropriate; participant did not meet any exclusion criteria Exclusion criteria: mild asthma when exacerbations with PEF < 80% of best were thought to be unlikely during the course of the study; demonstration by potential volunteers of erroneous or falsified PEF entries during a 2– to 4‐week reliability check; reliability was determined by comparison of self‐recorded PEF with actual PEF as recorded on personal Vitalograph 2110 electronic PEF/FEV1 diaries (Vitalograph, Buckingham, UK); participants were unaware that the diaries recorded all PEF values; asthma requiring continuous oral steroids or immunosuppressive‐type therapies; concomitant use of LABA, theophylline, or LTRA did not exclude individuals from participating Baseline asthma severity See Table 2. |
|
| Interventions |
Run‐in period: 2‐ to 4‐week run‐in period to ensure inclusion criteria, demonstrate competence in taking ICS via spacer, and ensure that asthma was stable Study period Control phase: maintenance ICS inhaler (usual type/dose) + same number of placebo inhalations for 14 days during exacerbations Study phase: maintenance ICS inhaler (usual type/dose) + same number of ICS inhalations for 14 days during exacerbations Participants also received placebo oral steroids for 7 days during these phases and their usual SABA inhaler. Other medications allowed: concomitant use of LABA, theophylline, or LTRA was not exclusionary |
|
| Outcomes | Treatment failure rates; PEF at endpoint; adverse events. The endpoint was assessed at 7 days if no treatment failure, or at time of treatment failure in the event of failure. Outcomes were not defined as primary and secondary. |
|
| Notes |
Funding source: Asthma Foundation of Queensland Funder role: no details about funder's role reported Registration: not registered Ethics approval: approved by institutional ethics committees of the participating unit Consent to participate: reports that all participants had to give consent Trial reporting vs review analysis: study population is defined by those who took the study inhaler in order to have matched pairs for analysis, so the same data were used in Analysis 1.1 and Analysis 1.2 |
|
C‐ACT: Childhood Asthma Control Test FEF: forced expiratory flow FENO: fractional exhaled nitric oxide FEV1: forced expiratory volume in one second FVC: forced vital capacity HFA: hydrofluoroalkane ICS: inhaled corticosteroids ITT: intention‐to‐treat LABA: long‐acting beta agonist LTRA: leukotriene receptor antagonist MDI: metred dose inhaler NR: not reported OCS: oral corticosteroids PEF: peak expiratory flow PEFR: peak expiratory flow rate SABA: short‐acting beta‐agonist
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bateman 2008 | Comparison of 2 doses of ciclesonide; not placebo controlled; uncontrolled asthma at baseline |
| Boushey 2005 | Budesonide vs LTRA for mild persistent asthma |
| Brand 2011 | ICS stopped during run‐in, therefore no baseline ICS. |
| Bullard 1996 | Systemic corticosteroids vs placebo for COPD, not asthma exacerbations |
| Clearie 2010 | Stopped ICS for 2 weeks before trial. Not focused on exacerbations |
| Condemi 1999 | Low‐ vs high‐dose ICS; not placebo controlled; uncontrolled asthma at baseline |
| Connett 1993 | No use of ICS at baseline |
| Currie 2003 | Salmeterol‐fluticasone vs fluticasone for uncontrolled asthma (not exacerbations) |
| De Benedictis 2005 | Nebulised fluticasone vs budesonide; not placebo controlled; no use of ICS at baseline |
| Devidayal 1999 | Nebulised budesonide vs oral prednisone; not placebo controlled; no use of ICS at baseline |
| Farber 2016 | Not an RCT; commentary |
| FitzGerald 2000 | Use of systemic corticosteroids first; not placebo controlled |
| Gilbert 2018 | Wrong design; commentary, not original RCT |
| Greening 1994 | BDP + salmeterol vs high‐dose BDP; not placebo controlled; uncontrolled asthma at baseline |
| GSK 2005 | Not placebo controlled; uncontrolled asthma at baseline |
| Hanania 2020 | No short‐term increase in ICS dose in response to an exacerbation |
| Hedlin 1999 | Inhaled budesonide vs oral betamethasone; not placebo controlled |
| Heinig 1999 | Budesonide vs fluticasone; not placebo controlled; uncontrolled asthma at baseline |
| Karpel 2007 | Severe persistent asthma (not exacerbations); participants on OCS at baseline |
| La Rosa 1997 | Salbutamol‐flunisolide vs salbutamol; not placebo controlled |
| Lee‐Wong 2002 | Inhaled flunisolide vs systemic corticosteroids following IV corticosteroids; not placebo controlled |
| Lemanske 2010 | 3 step‐up options and no stable study arm. ICS increased, but not in response to exacerbation. |
| Leuppi 2002 | Unstable dose of ICS (dose reduction) before exacerbation |
| Levy 1996 | Fluticasone vs oral prednisolone; not placebo controlled; not all participants on ICS at baseline |
| Manjra 2000 | Nebulised fluticasone vs oral prednisolone; not placebo controlled; not all participants on ICS at baseline |
| Matz 2001 | Salmeterol‐fluticasone vs high‐dose fluticasone for stable asthma (not exacerbations) |
| McKeever 2018 | Study design; pragmatic, unblinded trial with no placebo control |
| Milani 2004 | No use of ICS at baseline |
| Nana 1998 | Inhaled budesonide vs oral prednisolone; not placebo controlled |
| NCT02995733 | Participants not blinded to exacerbation strategy |
| Nuhoglu 2001 | No use of ICS at baseline |
| O'Connor 2010 | ICS given, but not in response to exacerbation. |
| Papi 2022 | ICS given, but not in response to an exacerbation. All participants were on maintenance ICS, and the study included groups where the dose was kept stable or increased. However, the inhalers were used as a general rescue medication and preventative measure before exercise, not as part of an action plan to prevent exacerbations. |
| Pedersen 2009 | ICS given, but not in response to exacerbation. |
| Prentice 2017 | Wrong design; commentary on previous version of this review |
| Razi 2008 | 2 dosing regimens of nebulised budesonide; not placebo controlled |
| Rodrigo 1998 | No use of ICS at baseline |
| Rodrigo 2005 | Inhaled fluticasone vs IV hydrocortisone; no use of ICS at baseline |
| Schuh 2000 | Inhaled fluticasone vs oral prednisolone; not all participants on ICS at baseline |
| Schuh 2006 | Inhaled fluticasone vs oral prednisolone; not all participants on ICS at baseline |
| Sekerel 2005 | Not all participants on ICS at baseline |
| Singhi 1999 | Not all participants on ICS at baseline |
| Svedmyr 1995 | ICS started at onset of URTI but not a confirmed asthma exacerbation; no ICS use at baseline |
| Volovitz 1998 | Inhaled budesonide vs oral prednisone; not placebo controlled; no ICS use at baseline |
| Weinberger 2016 | Wrong design; commentary on evidence, not primary study |
| Wilson 1990 | Not all participants on ICS at baseline |
| Yousef 2012 | No stable ICS arm |
BDP: beclomethasone dipropionate COPD: chronic obstructive pulmonary disease ICS: inhaled corticosteroids IV: intravenous LTRA: leukotriene receptor agonists OCS: oral corticosteroids RCT: randomised controlled trial URTI: upper respiratory tract infection
Differences between protocol and review
The protocol for this review was initially written and published in 2009, and so some methods have been updated to reflect current guidance. In the current version, we reassessed all studies with the revised Cochrane risk of bias tool for randomised controlled trials (RoB 2) (Higgins 2016; Sterne 2019), and the extension for cross‐over trials. In line with guidance for the revised tool, we elaborated the methods for presenting results and investigating the impact of bias with sensitivity analyses (excluding studies at overall high risk of bias). We also updated the tools used to sift search results, extract data, and conduct analyses to online versions of Covidence and Review Manager Web (Covidence; RevMan Web 2022), and have made the underlying data available for scrutiny and reuse via Figshare (Supplementary file 1; Supplementary file 2).
Differences between the protocol and previous versions that have been carried forward in this update include:
changing the original primary outcome of treatment failure (need for oral corticosteroids) in participants who required the study inhaler to a secondary outcome. Though of interest, this definition is a 'modified intention‐to‐treat' population or 'conditional' outcome which breaks randomisation, and its reliability and risk of bias are impacted by the absolute and relative number of participants initiating the study inhaler in each group;
assessing the primary outcome of treatment failure (need for oral corticosteroids) within all randomised participants, to capture the effect of being randomised to follow the exacerbation strategy regardless of how many participants had an exacerbation in each group during the study period;
defaulting to the use of frequency data from primary studies for exacerbations and treatment failures (number of participants as the unit of analysis) instead of event rates (events as the unit of analysis per person‐years);
defaulting to a fixed‐effect model for synthesis and performing a sensitivity analysis using a random‐effects model if there was notable heterogeneity in the meta‐analysis;
pooling parallel and cross‐over studies where the primary study reported adjusted data or suitable data from the first period, or where we had access to 2 x 2 data to adjust for period effects, which was done by applying a formula to account for intercorrelation of matched pairs (Elbourne 2002);
using Peto odds ratios instead of Mantel‐Haenzel methods where there were very few events in a meta‐analysis;
assessing magnitude of inhaled corticosteroids dose increase (two‐fold versus four‐fold) as a subgroup analysis;
performing all subgroup analyses on the intention‐to‐treat primary outcome instead of the treated‐population conditional outcome that was previously the primary outcome;
being explicit about the criteria for removing studies in planned and post hoc sensitivity analyses, and the categories for subgroups;
extending the definition of serious adverse events in the list of outcomes to include prolongation of hospitalisation or disability as the standard definition. We also noted in the analysis whether definitions used within studies differed;
extending the definition of exacerbations to include a set of criteria predefined in the included studies, because guidelines were not always cited but it was clear that a list of criteria had to be met before the study medication could be initiated.
Important note: For some studies, the number of treatment failures was only reported for those who started their study inhaler, or it was not clear how many participants were analysed and under what criteria treatment failures were counted. The only way to include these studies in the redefined primary analysis based on the full randomised population was to make assumptions about the data and use the reported number of treatment failures with the denominator for the full randomised population. This assumes that no participants who did not need their inhaler failed treatment and required oral steroids. The potential impact of making this assumption is dealt with explicitly in the review through the risk of bias assessment and reporting, and with sensitivity analyses using instead the number taking their study inhaler as the denominator.
Contributions of authors
KK: co‐lead for the 2016 and 2022 updates (sift and study selection, data extraction, analysis, risk of bias and GRADE assessment, write‐up).
EF: co‐lead for the 2022 update (sift and study selection, data extraction, analysis, risk of bias and GRADE assessment, write‐up).
BSQ: study assessment, data extraction, and write‐up of first review version (2010). Critical appraisal of 2016 and 2022 updates (clinical input for inclusion decisions, contributing to write‐up, reviewing manuscript).
CL: critical appraisal of the 2022 update (clinical input for inclusion decisions, contributing to write‐up, reviewing manuscript).
Contributions of editorial team
Sally Spencer (Co‐ordinating Editor) edited the review; advised on methodology, interpretation, and content; approved the review prior to publication.
Iain Crossingham (Contact Editor): edited the review; advised on methodology, interpretation, and content.
Rebecca Fortescue (Co‐ordinating Editor): checked the data entry prior to write‐up.
Emma Dennett (Deputy Co‐ordinating Editor): advised on methodology, interpretation and content; edited the review.
Emma Jackson (Managing Editor): co‐ordinated the editorial process; conducted peer review; edited the review and references.
Elizabeth Stovold (Information Specialist): designed the search strategy; ran the searches; edited the Search methods section of the review.
Sources of support
Internal sources
-
No internal sources of support received., Other
Not applicable
External sources
-
National Institute for Health and Care Research, UK
This project was supported by the National Institute for Health and Care Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to the Airways Group.
Declarations of interest
Kayleigh Kew: former employee of the Cochrane Central Executive Team (2020 to 2021), during which time most of the work for the update was completed, and former employee of the Cochrane Airways editorial team (2012 to 2016). No commercial or non‐commercial conflicts of interest relevant to this review.
Ella Flemyng: employee of the Cochrane Central Executive Team. No commercial or non‐commercial conflicts of interest relevant to this review.
Bradley Quon: none known
Clarus Leung: none known
These authors contributed equally to this work
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
ACTRN12605000631606 {unpublished data only}ACTRN12605000631606
- ACTRN12605000631606. Treatment of acute exacerbations of asthma in children with a doubling of the usual inhaled corticosteroid (ICS) dose [A multicentre randomised controlled trial of treatment of acute exacerbations of asthma in children with a doubling of the usual inhaled corticosteroid (ICS) dose to identify efficacy of intervention ]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=630 (first received 13 September 2015).
FitzGerald 2004 {published data only}
- FitzGerald JM, Becker A, Sears MR, Mink S, Chung K, Lee J, et al. Doubling the dose of budesonide versus maintenance treatment in asthma exacerbations. Thorax 2004;59(7):550-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Foresi 2000 {published data only}
- Foresi A, Morelli MC, Catena E. Low-dose budesonide with the addition of an increased dose during exacerbations is effective in long-term asthma control. Chest 2000;117(2):440-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Garrett 1998 {published data only}
- Garrett J, Williams S, Wong C, Holdaway D. Treatment of acute asthmatic exacerbations with an increased dose of inhaled steroid. Archives of Disease in Childhood 1998;79(1):12-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harrison 2004 {published data only}
- Harrison TW, Oborne J, Newton S, Tattersfield AE. Doubling the dose of inhaled corticosteroid to prevent asthma exacerbations: randomised controlled trial. Lancet 2004;363(9405):271-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jackson 2018 {published data only}
- Jackson DJ, Bacharier LB, Mauger DT, Boehmer S, Beigelman A, Chmiel JF, et al. Quintupling inhaled glucocorticoids to prevent childhood asthma exacerbations. New England Journal of Medicine 2018;378(10):891-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02066129. Step-up yellow zone inhaled corticosteroids to prevent exacerbations (STICS). clinicaltrials.gov/ct2/show/NCT02066129 (first received 19 February 2014).
Martinez 2011 {published data only}
- Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF, Mauger DT, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet 2011;377:650-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oborne 2009 {published data only}
- Oborne J, Mortimer K, Hubbard RB, Tattersfield AE, Harrison TW. Quadrupling the dose of inhaled corticosteroid to prevent asthma exacerbations: a randomized, double blind, placebo controlled, parallel group, clinical trial. American Journal of Respiratory and Critical Care Medicine 2009;180(7):598-602. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rice‐McDonald 2005 {published data only}
- Rice-McDonald G, Bowler S, Staines G, Mitchell C. Doubling daily inhaled corticosteroid dose is ineffective in mild to moderately severe attacks of asthma in adults. Internal Medicine Journal 2005;35(12):693-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bateman 2008 {published data only}
- Bateman ED, Cheung D, Lapa e Silva J, Göhring UM, Schäfer M, Engelstätter R. Randomized comparison of ciclesonide 160 and 640 μg/day in severe asthma. Pulmonary Pharmacology and Therapeutics 2008;21(3):489-98. [PMID: ] [DOI] [PubMed] [Google Scholar]
Boushey 2005 {published data only}
- Boushey HA, Sorkness CA, King TS, Sullivan SD, Fahy JV, Lazarus SC, et al. Daily versus as-needed corticosteroids for mild persistent asthma. New England Journal of Medicine 2005;352(15):1519-28. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brand 2011 {published data only}
- Brand PL, Luz Garcia-Garcia M, Morison A, Vermeulen JH, Weber HC, Brand PLP. Ciclesonide in wheezy preschool children with a positive asthma predictive index or atopy. Respiratory Medicine 2011;105(11):1588-95. [DOI] [PubMed] [Google Scholar]
Bullard 1996 {published data only}
- Bullard MJ, Liaw SJ, Tsai YH, Min H. Early corticosteroid use in acute exacerbations of chronic airflow obstruction. American Journal of Emergency Medicine 1996;14(2):139-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Clearie 2010 {published data only}
- Clearie KL. Airway Challenges in Different Clinical Phenotypes and Their Relationship to Markers of Disease and Treatment [PhD thesis]. Dundee, UK: University of Dundee, 2010. [Google Scholar]
Condemi 1999 {published data only}
- Condemi JJ, Goldstein S, Kalberg C, Yancey S, Emmett A, Rickard K. The addition of salmeterol to fluticasone propionate versus increasing the dose of fluticasone propionate in patients with persistent asthma. Annals of Allergy, Asthma & Immunology 1999;82(4):383-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Connett 1993 {published data only}
- Connett G, Lenney W. Prevention of viral induced asthma attacks using inhaled budesonide. Archives of Disease in Childhood 1993;68(1):85-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Currie 2003 {published data only}
- Currie GP, Bates CE, Lee DK, Jackson CM, Lipworth BJ. Effects of fluticasone plus salmeterol versus twice the dose of fluticasone in asthmatic patients. European Journal of Clinical Pharmacology 2003;59(1):11-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
De Benedictis 2005 {published data only}
- De Benedictis FM, Del Giudice MM, Vetrella M, Tressanti F, Tronci A, Testi R, et al. Nebulized fluticasone propionate vs. budesonide as adjunctive treatment in children with asthma exacerbation. Journal of Asthma 2005;42(5):331-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Devidayal 1999 {published data only}
- Devidayal, Singhi S, Kumar L, Jayshree M. Efficacy of nebulized budesonide compared to oral prednisolone in acute bronchial asthma. Acta Paediatrica 1999;88(8):835-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Farber 2016 {published data only}
- Farber HJ. POINT: is escalation of the inhaled corticosteroid dose appropriate for acute loss of asthma control in an attempt to reduce need for oral corticosteroids in children? Yes. Chest 2016;150(3):488-90. [DOI] [PubMed] [Google Scholar]
FitzGerald 2000 {published data only}
- FitzGerald JM, Shragge D, Haddon J, Jennings B, Math JLM, Bai T, et al. A randomized, controlled trial of high dose, inhaled budesonide versus oral prednisone in patients discharged from the emergency department following an acute asthma exacerbation. Canadian Respiratory Journal 2000;7(1):61-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gilbert 2018 {published data only}
- Gilbert JA. Effect of increasing glucocorticoids on asthma exacerbations. Lancet Respiratory Medicine 2018;6(5):P328. [DOI] [PubMed] [Google Scholar]
Greening 1994 {published data only}
- Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher-dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Lancet 1994;344(8917):219-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
GSK 2005 {published data only}
- GlaxoSmithKline. A multicentre, double-blind, randomised, four-week, parallel-group comparison of inhaled fluticasone propionate and inhaled beclomethasone dipropionate in patients with severe asthma controlled by high-dose inhaled corticosteroids (FLIP04). www.gsk-clinicalstudyregister.com/study/FMS30049#rs (first received 17 December 2015).
Hanania 2020 {published data only}
- Hanania N, Kerwin E, Pavord I, Kerstjens H, Pascoe S, Peachey G, et al. Step-up to high dose fluticasone furoate in combination with long-acting bronchodilator in inadequately controlled asthma: the CAPTAIN study. Journal of Allergy and Clinical Immunology 2020;145(2):AB24. [Google Scholar]
Hedlin 1999 {published data only}
- Hedlin G, Svedmyr J, Ryden AC. Systemic effects of a short course of betamethasone compared with high-dose inhaled budesonide in early childhood asthma. Acta Paediatrica 1999;88(1):48-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Heinig 1999 {published data only}
- Heinig JH, Boulet LP, Croonenborghs L, Mollers MJ. The effect of high-dose fluticasone propionate and budesonide on lung function and asthma exacerbations in patients with severe asthma. Respiratory Medicine 1999;93(9):613-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Karpel 2007 {published data only}
- Karpel JP, Nayak A, Lumry W, Craig TJ, Kerwin E, Fish JE, et al. Inhaled mometasone furoate reduces oral prednisone usage and improves lung function in severe persistent asthma. Respiratory Medicine 2007;101(3):628-37. [PMID: ] [DOI] [PubMed] [Google Scholar]
La Rosa 1997 {published data only}
- La Rosa M, Ranno C, Mandair G, Barbato A, Biraghi M. Double-blind study of inhaled salbutamol versus salbutamol plus high-dose flunisolide in exacerbation of bronchial asthma: a pilot study. Pediatric Asthma, Allergy and Immunology 1997;11(1):23-30. [EMBASE: 1997208440] [Google Scholar]
Lee‐Wong 2002 {published data only}
- Lee-Wong M, Dayrit FM, Kohli AR, Acquah S, Mayo PH. Comparison of high-dose inhaled flunisolide to systemic corticosteroids in severe adult asthma. Chest 2002;122(4):1208-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lemanske 2010 {published data only}
- Lemanske R, Mauger DT, Sorkness CA, Jackson DJ, Boehmer SJ, Martinez FD, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. New England Journal of Medicine 2010;362(11):975-85. [DOI: 10.1056/NEJMoa1001278] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leuppi 2002 {published data only}
- Leuppi JD, Downie SR, Salome CM, Jenkins CR, Woolcock AJ. A single high dose of inhaled corticosteroids: a possible treatment of asthma exacerbations. Swiss Medical Weekly 2002;132(1-2):7-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Levy 1996 {published data only}
- Levy ML, Stevenson C, Maslen T. Comparison of short courses of oral prednisolone and fluticasone propionate in the treatment of adults with acute exacerbations of asthma in primary care. Thorax 1996;51(11):1087-92. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manjra 2000 {published data only}
- Manjra AI, Price J, Lenney W, Hughes S, Barnacle H. Efficacy of nebulized fluticasone propionate compared with oral prednisolone in children with an acute exacerbation of asthma. Respiratory Medicine 2000;94(12):1206-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Matz 2001 {published data only}
- Matz J, Emmett A, Rickard K, Kalberg C. Addition of salmeterol to low-dose fluticasone versus higher-dose fluticasone: an analysis of asthma exacerbations. Journal of Allergy and Clinical Immunology 2001;107(5):783-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
McKeever 2018 {published data only}
- Chung S, Zappetti D. Can quadrupling inhaled glucocorticoid dose during early asthma exacerbation reduce the need for systemic steroids or hospital admission? Clinical Pulmonary Medicine 2019;26(3):102-4. [Google Scholar]
- Maselli DJ, Peters JI. Quadrupling inhaled glucocorticoid dose for deteriorating asthma control reduced severe exacerbations. Annals of Internal Medicine 2018;168(12):JC65. [DOI] [PubMed] [Google Scholar]
- McKeever T, Mortimer K, Bradshaw L, Haydock R, Pavord I, Higgins B, et al. Temporarily quadrupling the dose of inhaled steroid to prevent asthma exacerbations: FAST. Health Technology Assessment 2018;22(70):1-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeever T, Mortimer K, Duley L, Bradshaw L, Swinden R, Skeggs A, et al. Late breaking abstract - can a self-management plan, which includes a four-fold increase in inhaled corticosteroid dose, reduce severe asthma exacerbations: a randomised, pragmatic trial. European Respiratory Journal 2017;50(Suppl 61):PA1367. [DOI: 10.1183/1393003.congress-2017.PA1367] [DOI] [Google Scholar]
- McKeever T, Mortimer K, Wilson A, Walker S, Brightling C, Skeggs A, et al. Quadrupling inhaled glucocorticoid dose to abort asthma exacerbations. New England Journal of Medicine 2018;378(10):902-10. [DOI] [PubMed] [Google Scholar]
- Skeggs A, McKeever T, Duley L, Mitchell E, Bradshaw L, Mortimer K, et al. FourFold Asthma Study (FAST): a study protocol for a randomised controlled trial evaluating the clinical cost-effectiveness of temporarily quadrupling the dose of inhaled steroid to prevent asthma exacerbations. Trials 2016;17:Article number: 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Milani 2004 {published data only}
- Milani GK, Rosario Filho NA, Riedi CA, Figueiredo BC. Nebulized budesonide to treat acute asthma in children. Jornal de Pediatria 2004;80(2):106-12. [EMBASE: 2006186583] [PubMed] [Google Scholar]
Nana 1998 {published data only}
- Nana A, Youngchaiyud P, Charoenratanakul S, Boe J, Lofdahl C, Selroos O, et al. High-dose inhaled budesonide may substitute for oral therapy after an acute asthma attack. Journal of Asthma 1998;35(8):647-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
NCT02995733 {published data only}
- NCT02995733. Patient empowered strategy to reduce asthma morbidity in highly impacted populations; PeRson EmPowered Asthma RElief (PREPARE). clinicaltrials.gov/ct2/show/NCT02995733 (first received 16 December 2016).
Nuhoglu 2001 {published data only}
- Nuhoglu Y, Bahceciler NN, Barlan IB, Mujdat Basaran M. The effectiveness of high-dose inhaled budesonide therapy in the treatment of acute asthma exacerbations in children. Annals of Allergy, Asthma & Immunology 2001;86(3):318-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
O'Connor 2010 {published data only}
- O'Connor BJ, Kilfeather S, Cheung D, Kafé H, Blagden MD, Schlösser N, et al. Efficacy and safety of ciclesonide in patients with severe asthma: a 12-week, double-blind, randomized, parallel-group study with long-term (1-year) follow-up. Expert Opinion on Pharmacotherapy 2010;11(17):2791-803. [DOI: 10.1517/14656566.2010.526603] [DOI] [PubMed] [Google Scholar]
Papi 2022 {published data only}
- NCT03769090. A study to assess the efficacy and safety of budesonide/albuterol metered-dose inhaler (BDA MDI/PT027) in adults and children 4 years of age or older with asthma (MANDALA). clinicaltrials.gov/ct2/show/NCT03769090 (first received 7 December 2018).
- Papi A, Chipps BE, Beasley R, Panettieri RA, Israel E, Cooper M, et al. Albuterol-budesonide fixed-dose combination rescue inhaler for asthma. New England Journal of Medicine 2022;386(22):2071-83. [DOI] [PubMed] [Google Scholar]
Pedersen 2009 {published data only}
- Pedersen S, Engelstätter R, Weber HJ, Hirsch S, Barkai L, Emeryk A, et al. Efficacy and safety of ciclesonide once daily and fluticasone propionate twice daily in children with asthma. Pulmonary Pharmacology and Therapeutics 2009;22(3):214-20. [DOI] [PubMed] [Google Scholar]
Prentice 2017 {published data only}
- Prentice B, Homaira N, Jaffe A. Increased doses of inhaled corticosteroids during home management of asthma flare-ups do not reduce the need for systemic steroids. Journal of Paediatrics and Child Health 2017;53(9):915-7. [DOI] [PubMed] [Google Scholar]
Razi 2008 {published data only}
- Razi CH, Turktas I, Bakirtas A. Comparison of single 2000-microg dose treatment vs. sequential repeated-dose 500-microg treatments with nebulized budesonide in acute asthma exacerbations. Annals of Allergy, Asthma & Immunology 2008;100(4):370-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rodrigo 1998 {published data only}
- Rodrigo G, Rodrigo C. Inhaled flunisolide for acute severe asthma. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Pt 1):698-703. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rodrigo 2005 {published data only}
- Rodrigo GJ. Comparison of inhaled fluticasone with intravenous hydrocortisone in the treatment of adult acute asthma. American Journal of Respiratory and Critical Care Medicine 2005;171(11):1231-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schuh 2000 {published data only}
- Schuh S, Reisman J, Alshehri M, Dupuis A, Corey M, Arseneault R, et al. A comparison of inhaled fluticasone and oral prednisone for children with severe acute asthma. New England Journal of Medicine 2000;343(10):689-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schuh 2006 {published data only}
- Schuh S, Dick PT, Stephens D, Hartley M, Khaikin S, Rodrigues L, et al. High-dose inhaled fluticasone does not replace oral prednisolone in children with mild to moderate acute asthma. Pediatrics 2006;118(2):644-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sekerel 2005 {published data only}
- Sekerel BE, Sackesen C, Tuncer A, Adalioglu G. The effect of nebulized budesonide treatment in children with mild to moderate exacerbations of asthma. Acta Paediatrica 2005;94(10):1372-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Singhi 1999 {published data only}
- Singhi S, Banerjee S, Nanjundaswamy H. Inhaled budesonide in acute asthma. Journal of Paediatrics and Child Health 1999;35(5):483-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Svedmyr 1995 {published data only}
- Svedmyr J, Nyberg E, Asbrink Nilsson E, Hedlin G. Intermittent treatment with inhaled steroids for deterioration of asthma due to upper respiratory tract infections. Acta Paediatrica 1995;84(8):884-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Volovitz 1998 {published data only}
- Volovitz B, Bentur L, Finkelstein Y, Mansour Y, Shalitin S, Nussinovitch M, et al. Effectiveness and safety of inhaled corticosteroids in controlling acute asthma attacks in children who were treated in the emergency department: a controlled comparative study with oral prednisolone. Journal of Allergy and Clinical Immunology 1998;102(4 Pt 1):605-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Weinberger 2016 {published data only}
- Weinberger M. COUNTERPOINT: is escalation of the inhaled corticosteroid dose appropriate for acute loss of asthma control in an attempt to reduce need for oral corticosteroids in children? No. Chest 2016;150(3):490-2. [DOI] [PubMed] [Google Scholar]
Wilson 1990 {published data only}
- Wilson NM, Silverman M. Treatment of acute, episodic asthma in preschool children using intermittent high dose inhaled steroids at home. Archives of Disease in Childhood 1990;65(4):407-10. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yousef 2012 {published data only}
- Yousef E, Hossain J, Mannan S, Skorpinski E, McGeady S. Early intervention with high-dose inhaled corticosteroids for control of acute asthma exacerbations at home and improved outcomes: a randomized controlled trial. Allergy and Asthma Proceedings 2012;33(6):508-13. [DOI] [PubMed] [Google Scholar]
- Yousef E, Hossain J, Mannnan S. Ineffectiveness of high-dose inhaled corticosteroids for control of pre-exacerbation asthma symptoms [abstract]. Journal of Allergy and Clinical Immunology 2011;127(2 Suppl 1):AB85. [Google Scholar]
Additional references
Barnes 1993
- Barnes NC, Marone G, Di Maria GU, Visser S, Utama I, Payne SL. A comparison of fluticasone propionate, 1 mg daily, with beclomethasone dipropionate, 2 mg daily, in the treatment of severe asthma. European Respiratory Journal 1993;6:877-85. [PMID: ] [PubMed] [Google Scholar]
Cardet 2020
- Cardet JC, Busse PJ, Carroll JK, Casale TB, Coyne-Beasley T, Dixon-Williams S, et al. Adherence to adding inhaled corticosteroids to rescue therapy in a pragmatic trial with adults with asthma: a pilot study. Annals of Allergy, Asthma & Immunology 2020;124(5):487-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 5 September 2022. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI] [PubMed] [Google Scholar]
Fields 2001
- Fields AP. Meta-analysis of correlation coefficients: a Monte Carlo comparison of fixed- and random-effects methods. Psychological Methods 2001;6(2):161-80. [DOI] [PubMed] [Google Scholar]
Fuhlbrigge 2012
- Fuhlbrigge A, Peden D, Apter AJ, Mauger D, Teague WG, Blaisdell C, et al. Asthma outcomes: exacerbations. Journal of Allergy and Clinical Immunology 2012;129(3):S34-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibson 2004
- Gibson PG, Powell H. Written action plans for asthma: an evidence-based review of the key components. Thorax 2004;59(2):94-9. [DOI: 10.1136/thorax.2003.011858] [DOI] [PMC free article] [PubMed] [Google Scholar]
GINA 2015
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention 2015. ginasthma.org/wp-content/uploads/2016/01/GINA_Report_2015_Aug11-1.pdf (accessed 17 February 2015).
GINA 2021
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf (accessed 8 August 2021).
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 23 September 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Greenland 1985
- Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow-up data. Biometrics 1985;41:55-68. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2016
- Higgins JPT, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I, et al. A revised tool for assessing risk of bias in randomized trials. Cochrane Database of Systematic Reviews 2016;10 Suppl 1:29-30. [DOI: 10.1002/14651858.CD201601] [DOI] [Google Scholar]
Higgins 2021
- Higgins JPT, Li T, Sterne J on behalf of the RoB 2 working group on crossover trials. Revised Cochrane risk of bias tool for randomized trials (RoB 2). Additional considerations for crossover trials (preliminary tool version, 18 March 2021). www.riskofbias.info/welcome/rob-2-0-tool/rob-2-for-crossover-trials (accessed prior to 4 September 2022).
Holt 2001
- Holt S, Suder A, Weatherall M, Cheng S, Shirtcliffe P, Beasley R. Dose-response relation of inhaled fluticasone propionate in adolescents and adults with asthma: meta-analysis. BMJ 2001;323(7307):253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kupczyk 2014
- Kupczyk M, ten Brinke A, Sterk PJ, Bel EH, Papi A, Chanez P, et al. Frequent exacerbators - a distinct phenotype of severe asthma. Clinical and Experimental Allergy 2014;44(2):212-21. [DOI: 10.1111/cea.12179] [DOI] [PubMed] [Google Scholar]
Lemiere 2003
- Lemiere C, Bai T, Balter M, Bayliff C, Becker A, Boulet LP, et al. Adult Asthma Consensus Guidelines Update 2003. Canadian Respiratory Journal 2004;11(Suppl A):9A-33A. [DOI] [PubMed] [Google Scholar]
Marshall 2018
- Marshall IJ, Noel-Storr AH, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner’s guide. Research Synthesis Methods 2018;9(4):602-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2017
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. In: Global Evidence Summit; 2017 Sep 13-16; Cape Town. 2017.
Mcivor 1998
- Mcivor RA, Pizzichini E, Turner MO, Hussack P, Hargreave FE, Sears MR. Potential masking effects of salmeterol on airway inflammation in asthma. American Journal of Respiratory and Critical Care Medicine 1998;158(3):924-30. [DOI: 10.1164/ajrccm.158.3.9802069] [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2018
- Noel-Storr AH, Project Transform team. Cochrane Crowd: new ways of working together to produce health evidence. In: Evidence Live 2018; 2018 Jun 18-20; Oxford, UK. 2018.
Nwaru 2020
- Nwaru BI, Ekström M, Hasvold P, Wiklund F, Telg G, Janson C. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. European Respiratory Journal 2020;55(4):1901872. [DOI: 10.1183/13993003.01872-2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramsahai 2019
- Ramsahai JM, Hansbro PM, Wark PAB. Mechanisms and management of asthma exacerbations. American Journal of Respiratory and Critical Care Medicine 2019;199(4):423-32. [DOI: 10.1164/rccm.201810-1931CI] [DOI] [PubMed] [Google Scholar]
Reddel 2009
- Reddel HK, Taylor DR, Bateman ED, Boulet L-P, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. American Journal of Respiratory and Critical Care Medicine 2009;180(1):59-99. [DOI: 10.1164/rccm.200801-060ST] [DOI] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.14.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Risk of bias 2 resources webpage
- Current version of RoB 2. riskofbias.info (accessed prior to 22 September 2022).
Schünemann 2021
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Sears 2008
- Sears MR. Epidemiology of asthma exacerbations. Journal of Allergy and Clinical Immunology 2008;122(4):662-8. [DOI: 10.1016/j.jaci.2008.08.003] [DOI] [PubMed] [Google Scholar]
Soriano 2015
- Soriano JB, Abajobir AA, Abate KH, Abera SF, Agrawal A, Ahmed MB, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respiratory Medicine 2017;5(9):691-706. [DOI: 10.1016/S2213-2600(17)30293-X] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soriano 2017
- Soriano JB, Kendrick PJ, Paulson KR, Gupta V, Vos T, GBD Chronic Respiratory Disease Collaborators, et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respiratory Medicine 2020;8(6):585-96. [DOI: 10.1016/S2213-2600(20)30105-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
Stovold 2014
- Stovold E, Beecher D, Foxlee R, Noel-Storr A. Study flow diagrams in Cochrane systematic review updates: an adapted PRISMA flow diagram. Systematic Reviews 2014;3:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Supplementary file 1
- Flemyng E, Kew K. DOSE-AST supplementary file data. Figshare URL https://doi.org/10.6084/m9.figshare.15797838.v1 2021. [DOI: 10.6084/m9.figshare.15797838.v1] [DOI]
Supplementary file 2
- Flemyng E, Kew K. DOSE-AST RoB2 concensus data file. Figshare URL https://doi.org/10.6084/m9.figshare.15752415 2021. [DOI: 10.6084/m9.figshare.15752415] [DOI]
Thomas 2017
- Thomas J, Noel-Storr AH, Marshall I, Wallace B, McDonald S, Mavergames C, et al. Living Systematic Review Network. Living Systematic Reviews: 2. Combining Human and Machine Effort. Journal of Clinical Epidemiology 2017;S0895-4356(17):30604-2. [DOI] [PubMed] [Google Scholar]
Volmer 2018
- Volmer T, Effenberger T, Trautner C, Buhl R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. European Respiratory Journal 2018;52(4):1800703. [DOI: 10.1183/13993003.00703-2018] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kew 2016
- Kew KM, Quinn M, Quon BS, Ducharme FM. Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2016, Issue CD007524. Art. No: CD007524. [DOI: 10.1002/14651858.CD007524.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quon 2009
- Quon BJ, FitzGerald M, Ducharme FM, Lasserson TJ, Lemière C. Increased versus stable doses of inhaled steroids for exacerbations of chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD007524. [DOI: 10.1002/14651858.CD007524] [DOI] [Google Scholar]
Quon 2010
- Quon BS, FitzGerald JM, Lemière C, Shahidi N, Ducharme FM. Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2010, Issue 12. Art. No: CD007524. [DOI: 10.1002/14651858.CD007524.pub3] [DOI] [PubMed] [Google Scholar]


