Abstract
We conducted a systematic review and meta-analysis of the associations between prenatal exposure to persistent organic pollutants (POPs) and childhood obesity. We focused on organochlorines (dichlorodiphenyltrichloroethane [DDT], dichlorodiphenyldichloroethylene [DDE], hexachlorobenzene [HCB] and polychlorinated biphenyls [PCBs]), per- and polyfluoroalkyl substances [PFAS] and polybrominated diphenyl ethers [PBDEs]) that are the POPs more widely studied in environmental birth cohorts so far. We search two databases (PubMed and Embase) through July/09/2021 and identified 33 studies reporting associations with prenatal organochlorine exposure, 21 studies reporting associations with prenatal PFAS and 5 studies reporting associations with prenatal PBDEs. We conducted a qualitative review. Additionally, we performed random-effects meta-analyses of POP exposures, with data estimates from at least three prospective studies, and BMI-z. Prenatal DDE and HCB levels were associated with higher BMI z-score in childhood (beta: 0.12, 95% CI: 0.03, 0.21; I2: 28.1% per study-specific log increase of DDE and beta: 0.31, 95% CI: 0.09, 0.53; I2: 31.9% per study-specific log increase of HCB). No significant associations between PCB-153, PFOA, PFOS, or pentaPBDEs with childhood BMI were found in meta-analyses. In individual studies, there was inconclusive evidence that POP levels were positively associated with other obesity indicators (e.g., waist circumference).
Keywords: pregnancy, persistent organic pollutants, obesity, childhood
Introduction
Childhood obesity is an important public health concern. It is estimated that 10–30% of children in Europe and the US have currently overweight or obesity based on the World Health Organization body mass index (BMI) cutoffs.1 Greater BMI and adiposity in childhood are associated with future risk of type 2 diabetes, cardiovascular morbidity, and mental health problems.2–4 Further, excess weight gained in childhood is difficult to treat and likely to lead to an adulthood with overweight and obesity.5,6 Hence, there is an urgent need to identify early-life risk factors that can be targeted for clinical and public health interventions.
Excess food consumption and a sedentary lifestyle are important risk factors, but other factors may also help explain the current childhood obesity epidemic.7 The environment to which humans are exposed has changed considerably over the last decades due to the exponential growth in the production and use of synthetic chemicals. In the early 2000s, it was proposed that some synthetic chemicals can act as “obesogens” and affect individual susceptibility to obesity and metabolic disease - a hypothesis also known as the “environmental obesogen hypothesis”.8,9
In this context, there has been an increasing interest in the obesogenic effects of persistent organic pollutants (POPs). These include organochlorine compounds (OCs) that were widely used in the past as pesticides [e.g., dichlorodiphenyltrichloroethane (DDT), dichlorodiphenyldichloroethylene (DDE), hexachlorobenzene (HCB)] or in industrial processes [polychlorinated biphenyls (PCBS)], as well as other synthetic chemicals used in various industrial applications and consumer products [e.g., perfluoroalkyl substances (PFAS), polybrominated diphenyl ethers (PBDEs)].10 POPs are ubiquitous, accumulate in living organisms, and have long elimination half-lives.10,11 Human biomonitoring studies have shown widespread exposure to these chemicals.12,13 POPs can act as endocrine disruptors and alter hormonally regulated metabolic processes.14 They have been suggested to predispose to the development of obesity by increasing the number of adipocytes (and fat storage into existing adipocytes), changing the basal metabolic rate, shifting energy balance to favor calorie storage and altering hormonal control of appetite and satiety.14–16
Maternal concentrations of POPs reach the developing fetus through the placenta.17–19 This is of importance as during the intrauterine period vulnerability to pollutant exposures is high due to rapid cellular differentiation and tissue development, as well as incomplete development or function of protective mechanisms, such as xenobiotic metabolism, immune function, and the blood-brain barrier.14,16 Indeed, the “Developmental Origins of Health and Disease” paradigm highlights the importance of pollutant exposures during early development in eliciting metabolic changes and increased disease risk, even after the exposure has occurred.16,20 Growing evidence in animal models suggests that developmental exposure to endocrine-disrupting chemicals can alter normal homeostatic controls over adipogenesis and promote obesity.21–25 Nevertheless, evidence from human prospective studies has been conflicting. Several reviews to date have tried to summarize available human evidence. However, previous reviews were not systematic, provided only a qualitative assessment or did not consider several recent studies.16,26–35
The aim of the present study was to provide an up-to date systematic evaluation and meta-analysis, wherever possible, of available epidemiologic evidence on the prenatal effects of three major POP classes (OCs, PFAS, and PBDEs) on childhood obesity.
Materials and Methods
We followed the Preferred Reporting Items for systematic Reviews and Mata-Analysis (PRISMA) guidelines (Table S1). The review protocol has been registered in PROSPERO (CRD42020173299).
Search Strategy
We systematically searched two databases, PubMed and Embase, from the earliest available online indexing year through July 09, 2021 for studies evaluating the association between prenatal POP exposure and childhood adiposity. Independent searches were carried out for each of the three selected POP classes: 1) OCs, including DDE, DDT, PCBs and HCB; 2) PBDEs, and 3) PFAS. These were combined with detailed search terms related to pregnancy (e.g., intrauterine, fetus, prenatal) and childhood adiposity status (e.g., anthropometry, BMI, waist circumference). Details of the search strategy are presented in Appendix 1. We also manually searched reference lists of recent reviews and extracted articles to identify any additional eligible studies.
Study selection
Studies were eligible for inclusion if they met the following criteria: 1) were original research (i.e., not a review, meta-analysis, abstract, editorial, letter, or commentary); 2) were human studies; 3) assessed one or more POPs of interest in maternal or cord blood samples; and 4) reported data on at least one obesity measure in children and adolescents (<20 years of age). We did not include studies examining birth outcomes. We also restricted publications to the English language. The primary outcome of interest was BMI, as it is widely used both in clinical settings and research studies to assess weight status in childhood and adolescence. Secondary outcomes of interest included waist circumference and/or its ratio with body height, weight-for-height, body fat mass, and skinfold thickness. We also assessed rapid infant growth from birth up to 2 years of age (defined as a z-score change in weight or BMI greater than 0.67), as it is considered an important predictor for future obesity.36,37 Two reviewers (NS, SR) independently performed an initial screening of titles and abstracts and then evaluated potentially relevant studies based on full-text reviews. Any discrepancy was resolved through discussion.
Our literature search and study selection procedure yielded a total of 33 studies assessing prenatal OC exposures (Figure 1A), 21 studies assessing prenatal PFAS exposures (Figure 1B), and five studies assessing prenatal PBDE exposures (Figure 1C).
Figure 1.
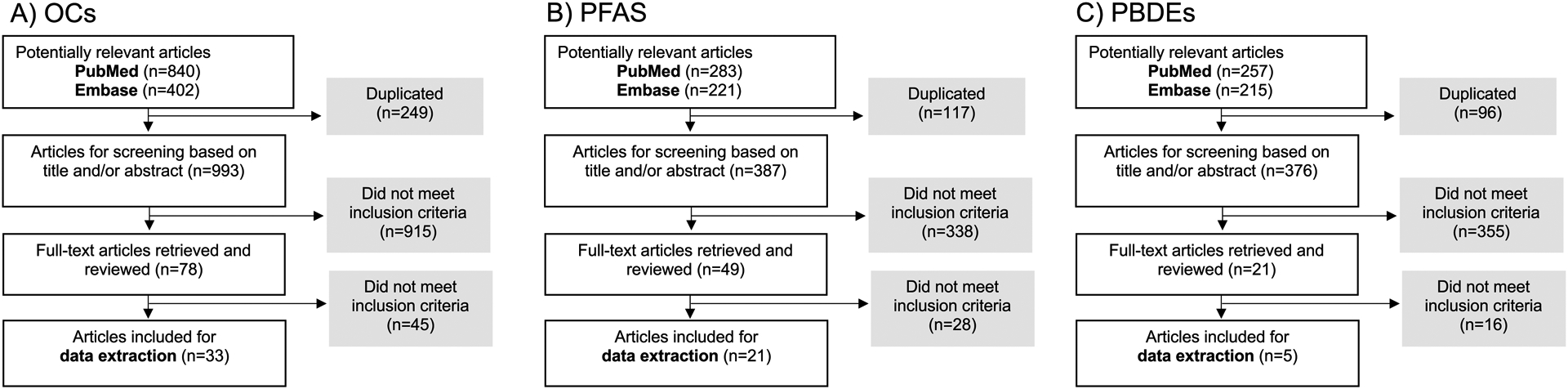
Flowcharts of study selection for a) organochlorine compounds [OCs; polychlorinated biphenyls (PCBs), dichlorodiphenyltrichloroethane and dichlorodiphenyldichloroethylene (DDT and DDE), and hexachlorobenzene (HCB)], b) per- and polyfluoroalkyl substances (PFAS), and c) polybrominated diphenyl ethers (PBDEs).
Data Extraction and Quality Assessment
For each article identified, we extracted information on the following characteristics: first author, publication year, geographic location and name of the cohort, sample size, type of exposure, biological sample used for exposure assessment, gestational age of exposure assessment, results of exposure assessment, primary and secondary outcomes measured, age at time of outcome assessment, adjustment set used in analysis and statistical findings (adjusted β coefficients, ORs and/or RRs with their 95% CIs or SEs). Data were extracted by one reviewer (SR) using standardized forms and independently checked by another (NS) to ensure accuracy. Discrepancies were resolved by discussion.
Two reviewers (SR and NS) independently assessed study quality using the Newcastle-Ottawa Scale (NOS),38 a commonly used tool for assessing quality of observational studies. The NOS evaluates each study by a points system across three domains: selection of participants (scored 0–4 points); comparability (scored 0–2 points); and the ascertainment of outcome (scored 0–3 points). Studies with a NOS score ≥7 were considered as good quality, those with NOS score 4–7 as moderate quality, and those with a NOS score <4 as poor quality. Any discrepancy in scoring between the two reviewers was resolved by discussion or in consultation with a third reviewer (LC). Table S2 shows our quality assessment of each study included in the review.
Data Synthesis and Meta-Analysis
Findings on the associations between prenatal POP exposures and later obesity measures are presented separately for each class of POPs (OCs, PBDEs, and PFAS). To take into account differential growth across the study period of interest, we grouped results into the following age categories of outcome assessment: infancy (0–2 years), childhood (2–9 years), and adolescence (>10 years).39
Initially, we planned to meta-analyze effect estimates between any prenatal POP exposure and obesity measure at each age period that were reported in at least three different prospective studies; i.e., availability of three effect estimates within each age period of outcome assessment was our minimum threshold for conducting a meta-analysis. Due to heterogeneous methodologies used and a limited number of studies assessing certain obesity outcomes of interest, we were able to conduct meta-analysis only for BMI-z, overweight and waist circumference and certain POPs. In meta-analyses, we included studies providing beta coefficients and their measure of uncertainty (95% CI, SE, or other data to calculate variance) that were provided from the most complete adjustment for potential confounders. When studies provided multiple effect estimates by percentile categories of exposure to POPs instead of continuous trends, we considered the worst outcome contrast within the comparison groups (worst-case scenario) by selecting the highest estimate to include in analysis.30,40 Moreover, when studies reported effect estimates for a given pollutant for two time points within each period (infancy, childhood or adolescence), we combined these estimates by means of a fixed-effect meta-analysis.41–44 For multi-center studies, we included site-specific estimates separately in analysis.45–47 We calculated overall estimates by pooling study-specific results using the inverse variance weighted method and a random effects model, which accounts for between-study variation. We evaluated heterogeneity between studies with the Cochran Q test (statistical significance defined as P<0.10) and used I2 to quantify the proportion of the total variation due to that heterogeneity.48,49 Potential publication bias was assessed with visual inspection of funnel plots and use of the Egger test.50 We also performed the Duval and Tweedie nonparametric ‘trim and fill’ method to examine whether such bias, if present, had impacted our overall effect estimates.51 We also examined the influence of individual studies on the overall estimates by omitting one study at a time. Moreover, we re-run meta-analyses, whenever possible, following further inclusion of a study that assessed POP concentrations in maternal 2-week postpartum serum.52 Finally, we performed prespecified analysis stratified by sex, where at least three effect estimates for males and females within each period of outcome assessment were available.
All tests were 2-sided and statistical significance was defined as P<0.05, unless otherwise stated. Analyses were performed using STATA version 16.1 (StataCorp).
Results
Prenatal OC exposure and childhood obesity
The characteristics of the studies included in our review are shown in Table S3. All studies had a prospective design, with the number of participants in each study ranging from 44 to 2,487. Assessment of study quality by NOS yielded an average score of 8.1 and 25 studies had a score of 7 (good) or above.
DDT and DDE
Studies from Belgium,53–55 Faroes Islands,52,56 France,54 Germany,54,57 Greece,58 Greenland,46 Mexico,59,60 Netherlands,54,61 Norway,45,54 Poland,46 South Africa,62 Spain,63–67 Sweden,45 Ukraine,46 Canada,68 China69 and the US43,44,70–75 reported data on exposure to DDE and/or DDT (Table S3). Given that DDT is rapidly converted to DDE in biological systems76 and there DDE concentrations are substantially higher in human tissues, most studies primarily focused on DDE. The exposure levels of DDE varied largely across studies. Among those studies reporting the exposure levels of p,p’-DDE standardized by lipid content measured in maternal serum, the median concentrations ranged between 82 ng/g lipid44 and 5,700 ng/g lipid.70 For those studies reporting exposures measured in maternal serum by their wet-based values, the median exposure levels of DDE presented narrower estimates ranging from 0.24 ng/L55 to 1.06 ng/L.65
We were able to meta-analyze effect estimates for prenatal exposure to DDE and BMI-z during infancy (0–2 years) and found no significant association (beta: 0.03; 95% CI: −0.01, 0.07; n= 4 estimates) (Figure S1). There was no evidence of between-study heterogeneity (I2: 0.0%; P for heterogeneity= 0.18) or publications bias (Figure S2). Effect estimates remained similar following exclusion of one study at a time or after additional inclusion of one study from the Faroes Islands52 that measured maternal DDE levels shortly after pregnancy (Table S4). Sex-stratified analysis was not feasible. One study reported significantly higher BMI-z with prenatal exposure to DDE in girls but not in boys.62 while another study found a negative association between DDE and BMI-z at 6 months of age in boys only.69 Other studies did not consider sex-specific differences.59,60 Two studies from Mexico assessed prenatal exposure to DDT and BMI-z in infancy, with one study that involved boys only reporting a null association59 and the other showing a positive association in girls but not in boys.62
Many studies also assessed other obesity outcomes during infancy including rapid growth (z-score weight gain > 0.67),58,63 BMI,61,62 and change in weight-for-age,54 though a meaningful meta-analysis was not feasible. Table S5 provides an overview of associations reported between prenatal DDT/DDE and adiposity measures. One study from Spain reported an increase in risk of rapid growth from birth to 6 months with higher exposure to DDE.63 This study also followed children to 14 months and found that prenatal DDE was also associated with increased risk of overweight.63 A European multi-center study also showed that higher prenatal exposure to DDE was associated with greater change in weight-for-age from birth to 24 months.54 A study in Canada found maternal DDE was positively correlated with child weight-gain in the first 2 years of life.57 One study from Greece reported a positive association between prenatal DDE and rapid growth, though the confidence interval (CI) included the null.58 A study from the Netherlands reported that DDE concentration in cord blood or breastmilk was associated with lower BMI trajectory during the first year of life, especially in boys.61
With respect to the childhood period (2–9 years), meta-analysis results showed a positive association of prenatal exposure to DDE with BMI-z (beta: 0.12; 95% CI: 0.03, 0.21; n= 10 estimates) (Figure 2), with no evidence of important between-study heterogeneity (I2: 28.1%; P for heterogeneity= 0.19). There was no evidence of publication bias for DDE with BMI-z, and application of the trim and fill method did not change effect magnitude (Figure S3). When removing one study at a time, the effect estimates did not materially change (Table S6). Sex-stratified meta-analysis analysis was not feasible, as only a limited number of studies provided sex-specific effects.55,58 In individual studies, Delvaux 201455 found a positive association between prenatal exposure to DDE and BMI-z in girls but not in boys, while other studies reported no differences by sex.43,44,46,58,64 Two studies from the US CHAMACOS cohort examined prenatal DDT exposure and BMI-z in children at 743 and 944 years old. While no significant association was found at 7 years of age,43 prenatal p,p’-DDT was associated with higher BMI-z in boys at 9 years of age.44 One study from Spain reported that increased prenatal concentration of DDT was associated with increased BMI-z from 4 until 18 years of age, with no evidence of sex interaction.67
Figure 2.
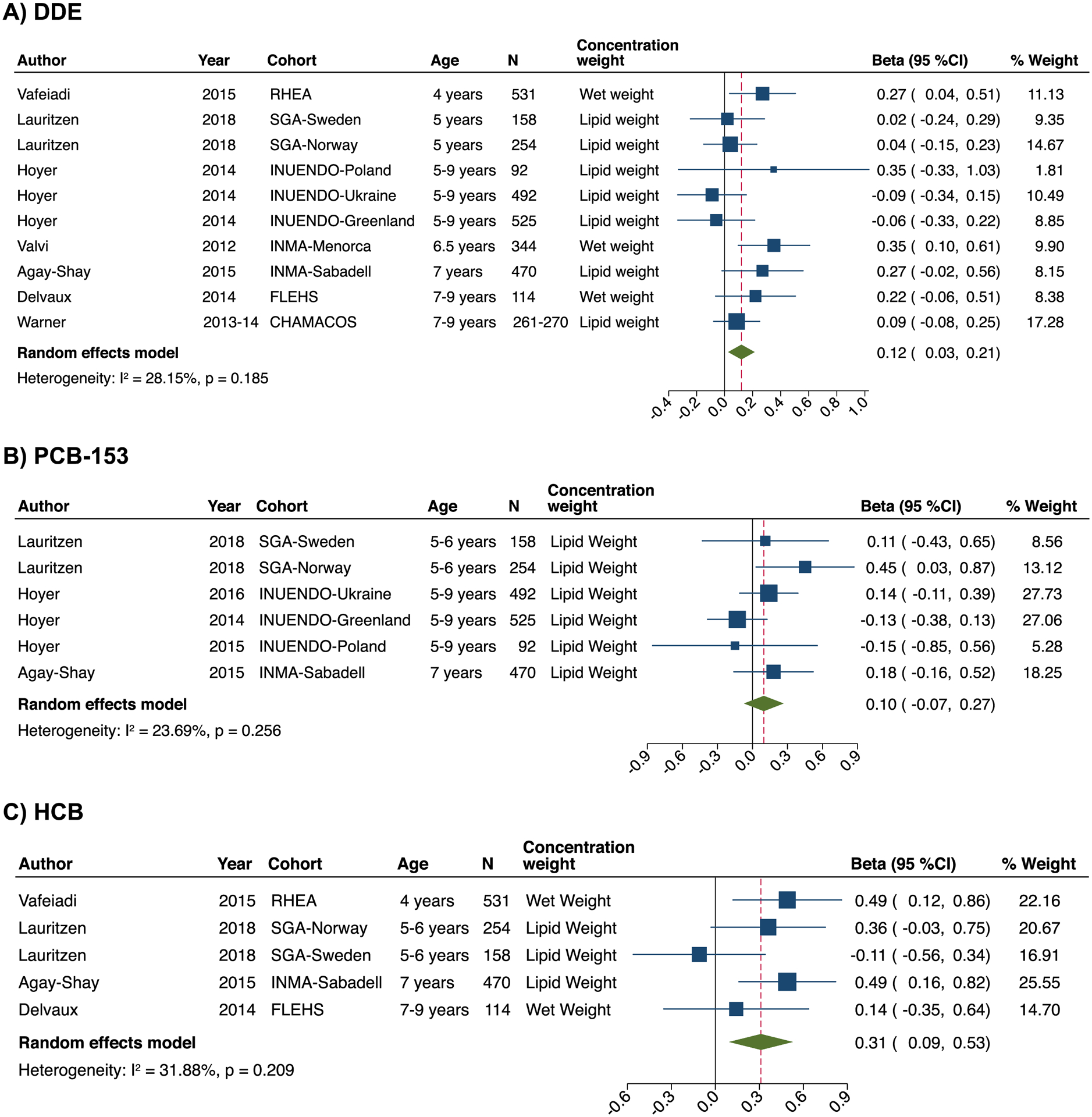
Forest plots for the associations of prenatal exposure to DDE (a), PCB-153 (b), and HCB (c) with BMI-z in childhood (2–11 years). The effect size estimate is the adjusted coefficient regression (beta) with 95% confidence intervals (SD in BMI z-score per unit increase of DDE and PCB-153) for combined sex (boys and female) unless the strata is specifically reported in the cohort label. Horizontal lines denote 95% confidence intervalsand squares denote study-specific point estimates representing continuous organochlorine exposure trends or worst-case scenarios when estimates were provided for different organochlorine exposure percentiles. The size of the squares estimates is proportional to the weight assigned to each study. Diamonds represent pooled estimates from a random effects meta-analysis. The I2 and P-values for heterogeneity are shown. Cohorts: INMA, Infancia y Medio-Ambiente Child and Environment birth cohort, Spain; FLEHS, Flemish Environment and Health Survey, Belgium; INUENDO, Biopersistent organochlorines in diet and human fertility- Epidemiological studies in time to pregnancy and semen quality in Inuit and European populations; SGA, Scandinavian Successive Small-for-Gestational Age births Study, Norway & Sweden; RHEA, The Mother-Child Cohort Study in Greece; CHAMACOS, Center for the Health Assessment of Mothers and Children of Salinas study, the US.
There were heterogeneous methodologies used and/or a limited number of studies assessing other obesity outcomes during childhood (Table S3). A meta-analysis of estimates from three cohorts43–45,77 reporting odds ratios for childhood overweight showed no association with DDE (OR: 1.00; 95% CI: 0.78, 1.27; I2: 28%; P for heterogeneity= 0.24), while two other studies reported a significantly increased relative risk for overweight and/or obesity (RRs: 1.7–3.8) with higher prenatal DDE concentrations.58,65 Results also varied for other obesity measures in childhood (Table S5). For instance, one study from Greece found that higher prenatal DDE exposure was associated with a higher risk for abdominal obesity, defined as waist circumference ≥90th percentile for age, at 4 years of age,58 while another study from the US reported no significant associations of DDE with abdominal obesity risk in children at 743 and 944 years of age.
Two studies in the US assessed obesity outcomes in adolescence (>10 years).70,72 One study reported positive associations of prenatal exposure to DDE and DDT with BMI-z and waist circumference in boys, but not girls, aged 12 years.72 In contrast, another study that involved boys aged 10–20 years found no significant associations of DDE and DDT with BMI and triceps and subscapular skinfolds.70
PCBs
Studies from Belgium,53,55,78 Canada,79 Faroe Islands52,56 France,78 Germany,78 Greece,58 Greenland,46 Netherlands,61,78,80 Norway,45,78 Poland,46 Slovakia,78 Spain,63–66 Sweden,45 Ukraine,46 the UK,81 and US73,74,82,83 reported data on PCB exposure (Table S3). Median concentrations of PCB-153 (the most commonly reported congener), measured in maternal blood and standardized by lipid context ranged from 11 ng/g lipid46 to 117 ng/g lipid.45 Due to heterogeneity in methodology, including reporting of weight (lipid- or wet-weight) and reporting measures (geometric mean, mean, median), we were not able to establish an exposure range for ∑PCBs.
One study from Belgium examined prenatal exposure to ∑PCBs and BMI-z in infancy and found that increasing concentrations of PCBs were associated with higher BMI-z at ages 1–3 years.53 In contrast, another study found a negative association between PCBs and change in weight from birth to 3 months80 while a multi-center European study reported no association between exposure to PCB-153 and change in weight-for-age from birth to 24 months.54 Other studies assessing rapid growth,58,63 BMI,52,61 overweight status52,63 and weight adjusted for height83 during infancy found no significant associations with PCBs.
Meta-analysis results on prenatal PCB-153 exposure and BMI-z during childhood showed a positive association but the CI included the null (beta: 0.10; 95% CI: −0.07, 0.27; n= 6 estimates). There was no evidence of important between-study heterogeneity (I2: 25.0%; P for heterogeneity= 0.25) (Figure 2). The small number of studies did not allow for a meaningful assessment of publication bias. When removing one study at a time, overall effect estimates for PCB-153 did not significantly change, except with the removal of Hoyer 2014 (INUENDO-Greenland)46 where the effect estimate became slightly larger (beta: 0.18, 95% CI: 0.01, 0.35) (Table S7). Meta-analysis of three studies assessing prenatal exposure to ∑PCB showed a positive association between ∑PCB and childhood BMI-z but the CI included the null (beta: 0.10; 95% CI: −0.02, 0.22; I2: 11.1%; P for heterogeneity= 0.33) (Figure S4). A Spanish study also reported a weak positive association between prenatal ∑PCB and BMI-z from 4 till 18 years of age.67 While sex-stratified meta-analysis was not feasible as most of the studies did not provide sex-specific effects, three studies reported no differences by sex.46,64,67
For other obesity outcomes during childhood, there were heterogeneous methodologies used, thus not allowing meta-analyses. Results varied (Table S8). For instance, two studies from Spain showed higher overweight risk with prenatal exposure to PCB-13864 or ∑PCBs65, while other studies from the US,77 Norway45 and Sweden45 reported no significant association.45,77
One study from Canada examined associations PCB 153 in cord blood and adolescent anthropometry. They found cord PCB 153 was related to higher BMI and fat mass index (kg/m2) in girls but not boys.68
HCB
Studies from Belgium,53,55 Faroes Islands,52 Greece,58 Norway,45 Spain,63,64,66,84 Sweden,45 and the US74 reported data on prenatal HCB exposure (Table S3). We were not able to establish an exposure range due to reporting heterogeneity.
One study of the Spanish INMA cohort reported an increase in risk of rapid growth from birth to 6 months with higher exposure to HCB.63 This study also followed children to 14 months and found that HCB was also associated with increased risk of overweight.63 One study from Greece also examined rapid growth in infancy and reported a positive association with prenatal HCB but with wide CI that included the null.58 A study from the Faroes Islands examining maternal OC concentrations shortly after birth showed a positive association of HCB with BMI-z at 18 months of age.52
Meta-analysis results showed a positive association of prenatal exposure to HCB with BMI-z in childhood (beta: 0.31; 95% CI: 0.09, 0.53; n=5 estimates) (Figure 2). We found no evidence of between-study heterogeneity (I2: 31.9%; P for heterogeneity= 0.21). The small number of studies did not allow for a meaningful assessment of publication bias. Effect estimates remained similar following exclusion of one study at a time or after additional inclusion of one study from the Faroes Islands52 that measured maternal HCB concentrations shortly after pregnancy (Table S9). Sex-stratified meta-analysis was not feasible. Only one study provided effect estimates by sex and showed no difference.58 Agay-Shay 201564 and Delvaux 201455 also reported no significant effect modification by sex. One study from Spain also reported that increased prenatal concentration of HCB was associated with increased BMI-z from 4 until 18 years of age, with no evidence of sex interaction.67
For other obesity outcomes during childhood, there were heterogeneous methodologies used and a limited number of studies assessing each outcome. Results varied (Table S10). For instance, studies from Spain64 and Faroes Islands52 showed a higher risk for overweight with higher maternal HCB concentrations, while two other studies conducted in Norway45 and Sweden45 and the US77 reported no significant association with the odds for overweight. One study from Spain showed that higher cord blood HCB concentrations were associated with increased BF % from 4 till 18 years of age.67
Prenatal PFAS exposure and childhood obesity
The characteristics of the studies included in our review are shown in Table S11. Nineteen studies had a prospective cohort design41,42,45,47,52,61,85–97 and two were case-control studies.75,98 We mainly focused on the legacy PFAS pollutants PFOS and PFOA, as human biomonitoring studies show that these are the most highly detected compounds.99,100 The exposure levels varied widely across studies. Among those assessing maternal plasma or serum, median PFOS concentrations ranged from 2.2 ng/mL93 to 33.8 ng/mL86 and median PFOA concentrations ranged from 1.0 ng/mL47,93 to 5.6 ng/mL.41 Median cord plasma concentrations ranged from 1.6 ng/mL61 to 5.7 ng/mL88 for PFOS and from 0.87 ng/mL61 to 6.74 ng/mL89 for PFOA. Number of participants in each study ranged from 285 to 1915. Assessment of study quality ranged from 6 to 9, with most of the studies having a score of seven (good) or above.
A meta-analysis of 3 studies assessing prenatal PFAS exposure with BMI-z in infancy showed a small inverse association with PFOS and PFOA (beta: −0.007; 95% CI: −0.012, −0.003; n= 3 estimates for PFOS and beta: 0.03; 95% CI: −0.02, 0.08; n= 3 estimates for PFOA) (Figure S5). There was no evidence of between-study heterogeneity for the effect estimates of PFOS (I2: 0.0%; P for heterogeneity= 0.44), but PFOA estimates were heterogeneous across studies (I2: 70.9%; P for heterogeneity= 0.03). There was no evidence of publication bias (Figure S6). Application of the trim and fill method did not change effect magnitude. Effect estimates for both PFOS attenuated following exclusion of the study by Andersen85 (Table S12). When we included a study from the Faroes Islands that assessed maternal PFOS and PFOA levels shortly after birth, we observed that the PFAs effect estimates for BMI-z in infancy were also attenuated. Sex-stratified pooled analysis was not feasible, however, Andersen 2010 reported stronger effect estimates in boys,85 while other studies did not report different associations of BMI-z with PFOS or PFOA between boys and girls.52,92,95 A study from the UK assessed weight adjusted for height in girls only and reported a positive association with PFOS but not PFOA.94 Studies from the Netherlands61 and Taiwan88 reported no significant associations with infant BMI trajectories. Likewise, prenatal PFOS and PFOA exposures were not significantly associated with weight change during infancy.42,92,93 A study from the US measured % fat mass at 5 months of age and reported a positive association with prenatal PFOA in boys,93 while a study from Denmark assessing % fat mass at 3 and 18 months of age showed null associations with prenatal PFOA or PFOS but positive associations with prenatal PFNA and PFDA both in boys and girls aged 3 months.95
With respect to the childhood period (2–9 years), we found no significant associations of prenatal PFOS and PFOA concentrations with BMI-z (beta: 0.00; 95% CI: −0.01, 0.01; n= 10 estimates for PFOS and beta: 0.03; 95% CI: −0.02, 0.08; n= 10 estimates for PFOA) (Figure 3). There was evidence of between-study heterogeneity for the effect estimates of both PFOS (I2: 42.9%; P for heterogeneity= 0.07) and PFOA (I2: 55.5%; P for heterogeneity= 0.02). There was no evidence of publication bias (Figure S7), although the number of included studies was not adequate to allow for clear conclusions. Application of the trim and fill method did not change effect magnitude. Effect estimates for both PFOS and PFOA remained similar following exclusion of one study at a time (Table S13). Exclusion of Lauritzen 2018 (Norway)45 reduced substantially the between-study heterogeneity for the estimate of PFOS, and exclusion of Andersen 201386 or Braun 201687 reduced between-study heterogeneity for the estimate of PFOA. Inclusion of the study from the Faroes Islands52 that assessed PFAS shortly after birth did not materially change the overall effect estimates for PFOS and PFOA. In stratified analyses by sex, no significant associations with BMI-z were observed in either boys or girls (Table S13).
Figure 3.
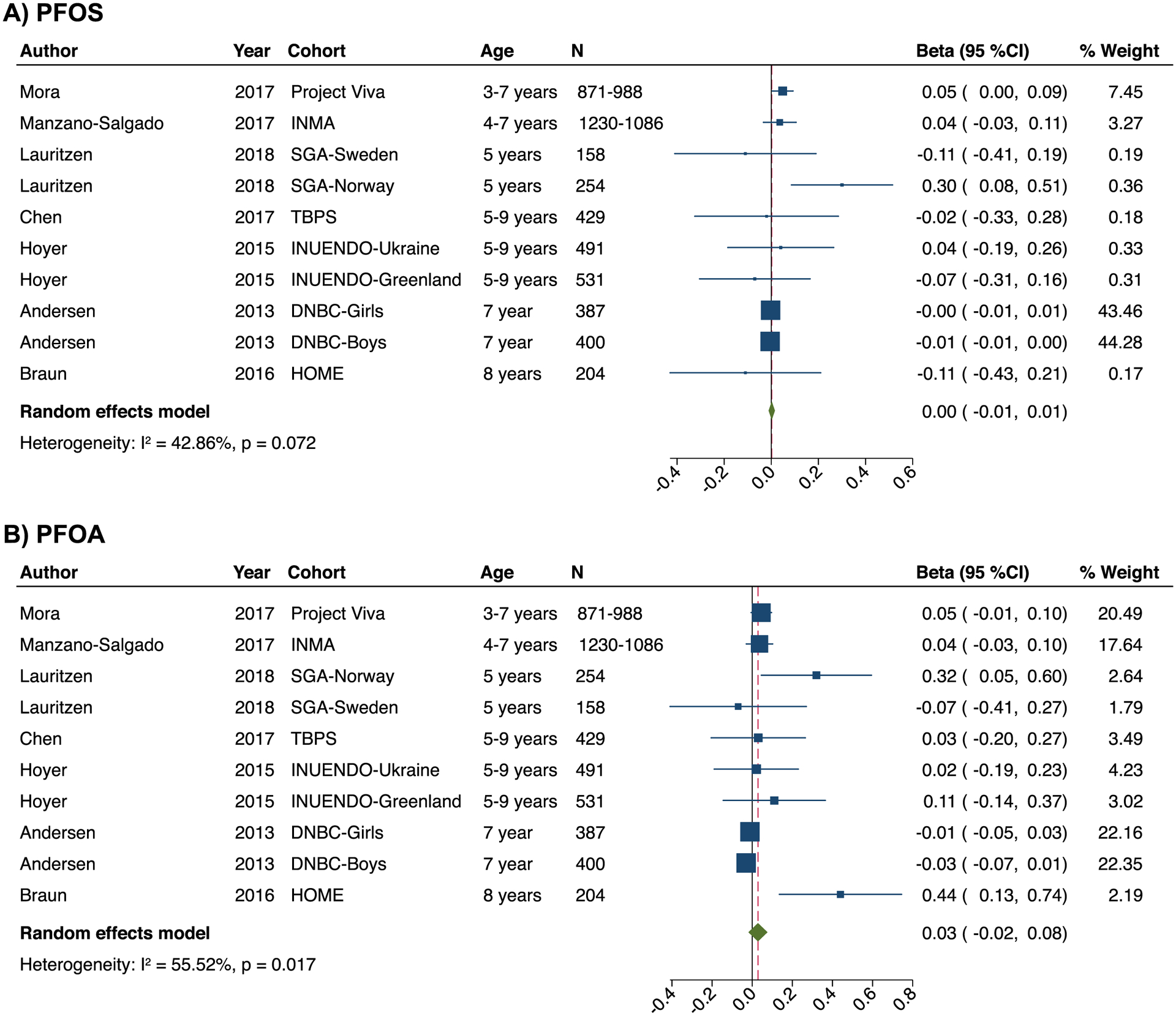
Forest plots for the associations of prenatal exposure to PFOS (a) and PFOA (b) with BMI-z in childhood (2–11 years). The effect size estimate is the adjusted coefficient regression (beta) with 95% confidence intervals (SD in BMI z-score per unit increase of PFOS and PFOA) for combined sex (boys and female) unless the strata is specifically reported in the cohort label. Horizontal lines denote 95% confidence intervalsand squares denote study-specific point estimates representing continuous PFAS exposure trends or worst-case scenarios when estimates were provided for different PFAS exposure percentiles. The size of the squares is proportional to the weight assigned to each study. Diamonds represent pooled estimates from a random effects meta-analysis. The I2 and P-values for heterogeneity are shown. Cohorts: DNBC, Danish National Birth Cohort, Denmark; HOME, Health Outcomes and Measures of the Environment Study, the US; INMA, Infancia y Medio-Ambiente Child and Environment birth cohort, Spain; INUENDO, Biopersistent organochlorines in diet and human fertility- Epidemiological studies in time to pregnancy and semen quality in Inuit and European populations, Greenland & Ukraine; SGA, Scandinavian Successive Small-for-Gestational Age births Study, Norway & Sweden; SPC, Shanghai Prenatal Cohort, China; TBPS, Taiwan Birth Panel Study, Taiwan.
Prenatal PFOS and PFOA concentrations were also not associated with waist circumference in childhood in meta-analyses (beta: −0.06; 95% CI: −0.19, 0.07; n= 4 estimates for PFOS and beta: 0.30; 95% CI: −0.50, 1.09; n= 4 estimates for PFOA), though there was evidence of between-study heterogeneity for the effect estimates of PFOA (I2: 20.5%; P for heterogeneity: 0.287 for PFOS and I2: 85.7%; P for heterogeneity: <0.001 for PFOA) (Figure S8). The small number of studies did not allow for a meaningful assessment of publication bias. Exclusion of one study at a time did not change pooled effect estimates nor reduced between-study heterogeneity for the estimate of PFOA (Table S14). Sex-stratified pooled analysis was not feasible, however, apart from Hartman 201791 which examined associations only in girls, other studies did not report different association between boys and girls.41,87,89
For other obesity outcomes during childhood, there were heterogeneous methodologies used, and results varied (Table S15). For instance, four studies assessed fat mass (in kg, kg/m2, or %), with one reporting a positive association with prenatal exposure to PFOA in US children,87 another reporting an inverse association with both prenatal PFOS and PFOA among British daughters of highly-educated mothers,91 and the others showing no significant associations in US41 or Chinese children.89 A meta-analysis of PFAS effect estimates from three cohorts assessing overweight risk in Greenlandic,47 Ukrainian,47 Faroese52 and US children87 showed no association with PFOS exposure (RR: 0.99; 95% CI: 0.81, 1.20; I2: 0.0%; P for heterogeneity: 0.86) and a trend towards higher risk with increasing PFOA exposure (RR: 1.23; 95% CI: 0.98, 1.55; I2: 24.7%; P for heterogeneity: 0.26) (Figure S9).
Regarding the adolescent period, one study from the US included participants aged 12 age and showed modest increases in central adiposity and risk of overweight/obesity associated with prenatal PFOA and PFHxS exposures.96 Another study from Denmark included participants aged 20 years and reported a positive association of prenatal PFOA exposure with BMI and waist circumference only in females.90
Prenatal PBDE exposure and childhood obesity
The characteristics of studies included in our review are shown in Table S16. Four studies had a prospective cohort design64,101–103 and one was a case-control study.75 We focused mainly on pentaBDEs (BDE-47, BDE-99, BDE-100, BDE-153) given that these were among the most abundant PBDE congeners detected in human tissues.102,104 Geometric mean maternal serum pentaBDE concentrations (in ng/g lipid) ranged from 15.3101 to 20.3102 for BDE-47, from 4.3101 to 4.8102 for BDE-99, from 2.8101 to 4.0102 for BDE-100, and from 2.4101 to 5.3102 for BDE-153. There were heterogeneous methodologies across studies for summing individual congener concentrations, hence, it was not possible to compare concentration ranges for ΣPBDEs. Number of participants in each study ranged from 224 to 470. All studies scored seven or above in our assessment of study quality.
All studies assessed obesity in childhood. In meta-analyses, we observed that pentaPBDE congeners were inversely associated with BMI-z, with the associations for BDE-100 and BDE-153 reaching statistical significance (Figure 4). There was some evidence of between-study heterogeneity for the effect estimates of BDE-153 (I2: 21.8%; P for heterogeneity= 0.28). There was no evidence of publication bias (Figure S10). Exclusion of Guo 2020103 attenuated effect estimates for BDE-100 and BDE-153 (Table S17). Stratified analyses by sex were only feasible for BDE-100 and BDE-153, and showed no significant difference in effect estimates between boys and girls. For the other PBDE congeners, one study reported that prenatal levels were associated with higher BMI-z in boys but lower BMI-z in girls,101 while the other studies from Spain64 and the US102 reported no effect modification by child sex.64,102 Results for other obesity outcomes varied (Table S18). For instance, one study from the US showed that prenatal BDE-153, BDE-100, and ΣPBDE exposures were associated with smaller waist circumference and BDE-153 with lower % body fat in childhood, with no evidence of modification by sex.102 Other studies from the US and Spain reported no significant associations of individual congeners and ΣPBDEs with waist circumference101 and overweight or obesity.64,101
Figure 4.
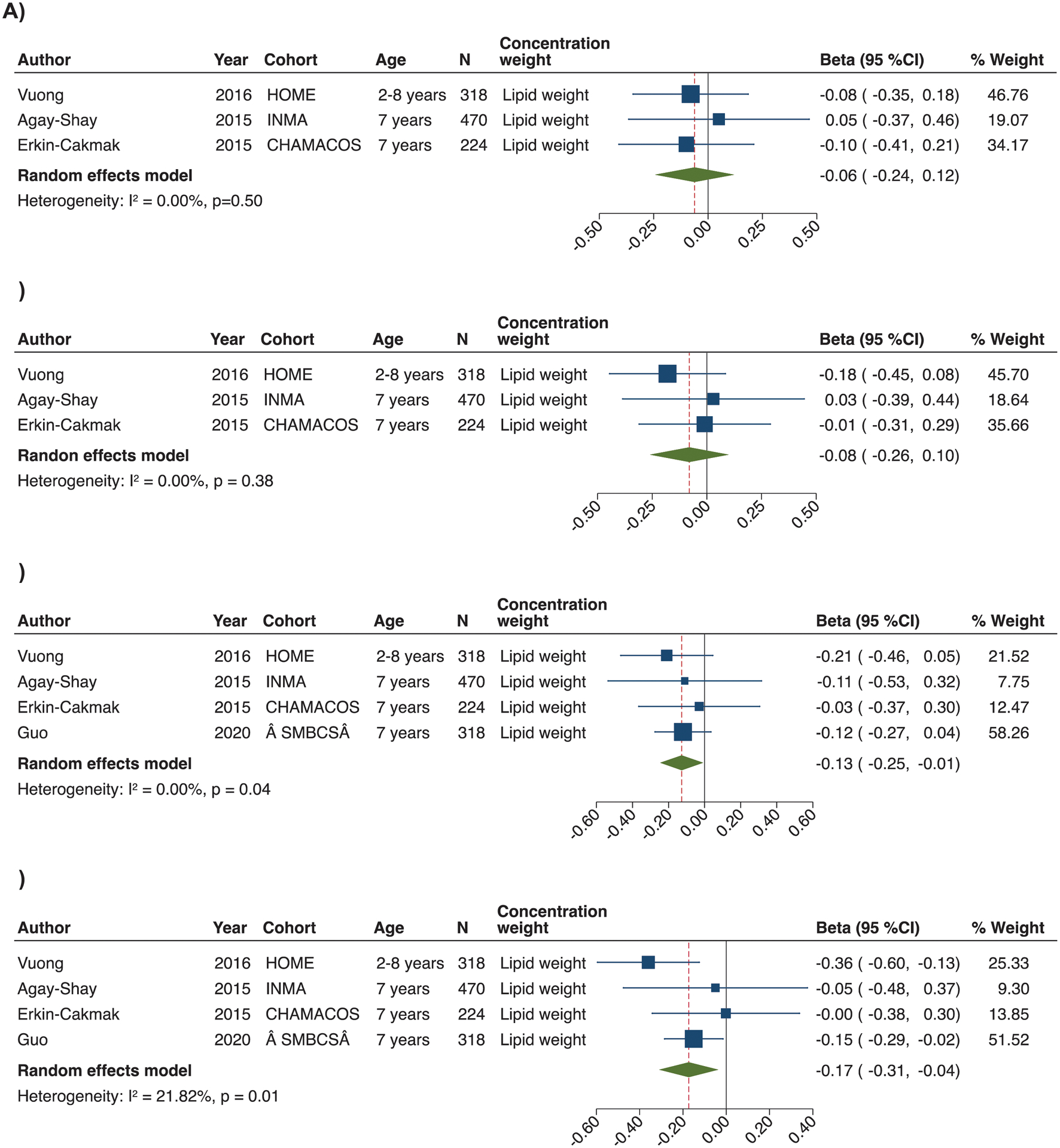
Forest plots for the associations of prenatal exposure to BDE-47 (a), BDE-99 (b), BDE-100 (c) and BDE-153 (d) with BMI-z in childhood (2–11 years). The effect size estimate is the adjusted coefficient regression (beta) with 95% confidence intervals (SD in BMI z-score per unit increase of PBDE congener) for combined sex (boys and female) unless the strata is specifically reported in the cohort label. Horizontal lines denote 95% confidence intervalsand squares denote study-specific point estimates representing continuous PBDE exposure trends or worst-case scenarios when estimates were provided for different PBDE exposure percentiles. The size of the squares is proportional to the weight assigned to each study. Diamonds represent pooled estimates from a random effects meta-analysis. The I2 and P-values for heterogeneity are shown. Cohorts: CHAMACOS, Center for the Health Assessment of Mothers and Children of Salinas study, the US; HOME, Health Outcomes and Measures of the Environment Study, the US; INMA, Infancia y Medio-Ambiente Child and Environment birth cohort, Spain; SMBCS, Sheyang Mini Birth Cohort Study.
Discussion
We conducted an up-to-date systematic review and meta-analysis of human evidence on the potential childhood obesogenic effects of common POP exposures during the prenatal period. Figure 5 presents a summary of our findings. Overall, we found that prenatal exposures to DDE and HCB were associated with increased BMI in children. For PCBs, PFAS and PBDEs, there was no conclusive evidence that prenatal exposure to these chemicals was associated with obesity development in childhood.
Figure 5.
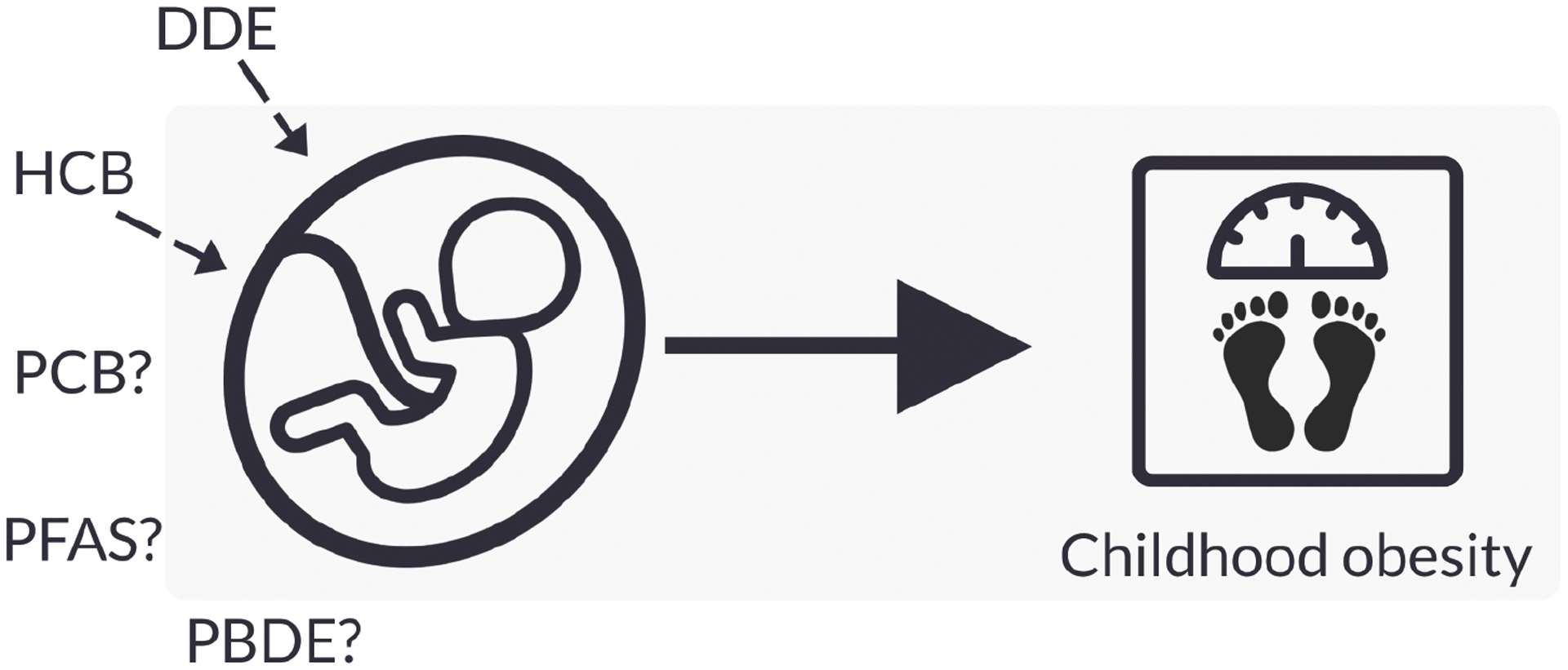
Summary of main findings. Simplified diagram not including confounders or other pathways.
The body of evidence examining potential obesogenic effects of POPs has increased notably over the last years, with a particular focus on exposure during prenatal development. The fetus has long been recognized as especially vulnerable to the effects of environmental pollutants, and fetal exposure is a key feature of the chemical obesogen hypothesis.15 This hypothesis proposes that chemicals can interfere with endocrine and metabolic systems to change growth patterns and induce weight gain and obesity.105
Organochlorine compounds have long been suspected to act as obesogens. Among them, DDE/DDT, PCBs and HCB are the most widely studied in birth cohort research. These compounds exhibit lipophilic properties and bioaccumulate in human tissues.35 DDT is an insecticide widely used for the control of disease (e.g., malaria, typhus) vectors in most countries until the late 20th century. DDE is the main metabolite of DDT, and exposure to DDE occurred as a result of contamination of the environment and food supply.30 PCBs were widely used as lubricants and coolants in electrical appliances, while HCB was used as a pesticide and in the production of fireworks, ammunition, and synthetic rubber through the 1980s. The use of these chemicals has been discontinued in the United States and Europe,106 though their levels in migrants to those areas from areas where OCs are manufactured can be relatively high.107 However, because of their persistence in the environment, the general population is still exposed to these substances at low doses,12,13 and adverse health outcomes related to background population levels of exposure are a major concern.108
Our findings in the meta-analysis that prenatal exposure to DDE was associated with increased BMI in childhood is in line with a 2016 review that classified DDE as “presumed” to be obesogenic for humans based on epidemiological observations and on experimental evidence of increased rodent adiposity, impaired energy expenditure, and adipogenic expansion that were estimated to fall within the range of the human exposures in prospective studies.30 Similarly, the historic Michigan fisheater cohort involving 20–50-year-old women showed that their prenatal DDE exposure was associated with higher BMI,109 while more recently, another study reported that grandmaternal perinatal DDT exposure was associated with a higher granddaughter obesity risk in adulthood.110 Consistent with our results showing an association of prenatal HCB exposure with higher BMI in childhood, an earlier systematic, qualitative review in 2011 that evaluated the potential obesogenic role of POPs in adults and children concluded that HCB exposure was generally associated with an increase in body size.26 In our study, we observed inconclusive evidence to support that prenatal exposures to PCBs exert obesogenic effects in children. The 2011 review suggested that health effects of OCs, and especially of PCBs, could follow non-monotonic dose responses, with positive associations at lower population exposure levels (<1 ng/mg lipid) but inverse or not existing at higher exposures.26 In our meta-analyses involving studies that had varying exposure levels, with geometric mean PCB values being as low as 0.09 ng/mg lipid63, we did not observe significant heterogeneity between study estimates. An analysis of dose–response profiles was not feasible given the variable increments of exposure categories across studies. Pooled analyses with harmonized exposure data across populations with varying background exposure levels are needed to disentangle potential non-monotonic relationships with obesity.
In our review, we also examined the potential obesogenic effects of the legacy PFAS compounds, PFOS and PFOA. These are chemically and thermally stable synthetic compounds that were widely used in various industrial applications and consumer products, including fire-fighting foams, nonstick coatings, water- and stain- repellent textiles.111 PFOS and PFOA have a long elimination half-life in the human body (half-life of serum elimination ~ 3–7 years), and although their production has been phased out in Europe and the United States, human biomonitoring studies still show widespread exposure.99,100,112. Evidence from animal studies indicates that PFAS can alter lipid metabolism, increase body weight, and lead to higher body fat accumulation23,113,114 However, given the species-specific toxicokinetics of PFAS,115 extrapolation from animals to humans is difficult.
Most studies included in this review found that prenatal PFOS and PFOA exposures were not associated with BMI and weight gain during infancy. We also found no conclusive evidence that prenatal PFOS and PFOA exposure is associated with BMI and waist circumference in childhood. Results for other obesity indicators in childhood, such as fat mass, were highly heterogeneous and studied by a very small number of studies, thereby precluding any conclusions. The only study involving older participants, with a mean age of 20 years, reported a positive association of prenatal PFOA with BMI and waist circumference only in girls.90
Background PFAS exposure levels varied widely across studies. An analysis of dose–response profiles across studies was analytically prohibited by variable increments of exposure across studies. Nevertheless, it is unlikely that the variable exposures in different studies explained any inconsistency in the results, as there were neither stronger nor statistically significant effect estimates in populations with higher exposure levels. Associations suggesting potential obesogenic PFAS effects was reported in studies45,47,87 with background exposure levels that were similar to, or lower than, other studies showing null associations (e.g.,42,86,88,98). Previous human studies of PFAS exposure and obesity were largely cross-sectional that could not establish that exposure preceded the outcome.116–118 The prospective European Youth Heart Study recently demonstrated that higher plasma PFOS concentrations during childhood were associated with greater adiposity in adolescence and young adulthood.119 Moreover, a recent trial in adults showed that higher baseline plasma concentrations of PFOS, PFOA, and PFNA were associated with a greater weight regain following diet-induced weight-loss, especially in women, possibly explained by a slower regression of resting metabolic rate levels.120 An important consideration for future human studies is to examine the potential obesogenic effects of short-chain PFAS (≤6 fluorinated carbons) or PFAS ‘alternatives’ (e.g., GenX) which have been introduced as replacements to the legacy compounds and human biomonitoring studies report increasing concentrations.121 Animal studies suggest that these emerging chemicals have the potential to interact with PAR pathways and affect body weight.121
PBDEs are a class of endocrine-disrupting flame retardants used extensively in consumer products such as in furniture, carpet padding, electronics, and textiles. PBDEs are lipophilic, accumulate in living organisms, and have an estimated half-life up to 12 years in humans.122,123 Although production of pentaBDE congeners has been phased out in Europe and the U.S., they continue to be released from older furniture and are commonly found in house dust.124 Experimental models suggest that PBDEs may increase adipogenesis and lead to higher weight gain.25,125–127 In our systematic review, we identified a limited number of human studies examining prenatal PBDE exposure in relation to obesity development in children and found no evidence to support an obesogenic role for these chemicals. Other epidemiologic studies examining postnatal exposures were mostly cross-sectional and did not provide consistent evidence for a relation of PBDEs with increased weight status.128–130
Strong experimental evidence suggests that endocrine disruptors (as the POPs included in this review) exert sex-specific effects, possibly as a result of their ability to interfere with sex hormone pathways.131–134 This is of major relevance when examining the chemical obesogen hypothesis given that there are well-known sex effects on energy metabolism and fat regulation, including the regulation of adipose tissue and leptin signaling, by sex hormones.135–139 In our review, we did not find conclusive evidence that there were differential associations by sex between any of the POPs assessed and obesity. However, sex was largely overlooked, and sex-stratified estimates were not provided by many studies, thus precluding a thorough summary of potential sex differences. Investigation and clear presentation of sex-specific effects in the association of POPs with obesity development constitute an important consideration for future studies in order to unravel sex-specific susceptibility to POPs effects.
Further, studies included in this review have almost exclusively assessed the relationships of single pollutant exposures with obesity, with the exception of a few multipollutants studies.62,64,93 It is clear that pregnant women (and the general population) are not exposed to single chemicals, but to complex real-world mixtures due to common sharing of sources and routes of exposure (e.g., diet). Pollutant exposures (and their measured congeners) may not only be highly correlated within and between the different classes (e.g. PCBs, PFAS, PBDEs)140–142 but also exert interactive (synergistic or antagonistic) effects.143,144 This makes it challenging to identify specific chemicals responsible for certain effects. For instance, we recently followed an exposome-wide approach to systematically assess many suspected environmental obesogens during critical early-life periods and highlighted the contribution of exposure to tobacco smoke and air pollution to the development of childhood obesity.145 This area of research is still in its infancy, but it is rapidly developing with novel statistical techniques that may allow us to identify the complex inter-relationships of chemicals and their health effects.146 Such studies may help both in discovery and in setting priorities for prevention and intervention early in life.
This is the first systematic review with meta-analysis to examine prenatal exposure to three major POP classes and multiple measures of childhood obesity. We focused on studies which evaluated prenatal exposure in biological samples (maternal or cord blood) using well-established methods. POPs have long half-lives and one biomarker measurement can give a good estimate of past exposure. Although all included studies were prospective, and thus exposures preceded the outcome, we cannot infer causality in the observed associations. Due to the observational nature of included studies, the existence of unmeasured confounders is always possible, and that resulting residual confounding could bias the results. Nevertheless, we included fully adjusted estimates in meta-analysis and reporting of results in an effort to limit risk of confounding, and almost all studies included in this review adjusted for maternal BMI, a major risk factor for childhood obesity that is also associated with blood POPs levels. To strengthen causal inference, future studies should move beyond traditional approaches of simply examining exposure-outcome associations. Examples of methods for improving causal inference in this field include the use of instrumental variables (e.g. examining water contamination level as instrumental variable for PFAS-health associations) and use of negative control exposures (e.g. also examining paternal exposures when assessing effects of maternal exposures); the reader is referred to two excellent articles on this topic.147,148 The studies included in our review varied substantially with respect to exposure metric and outcome evaluated. Thus, we cannot rule out these factors as sources of effect heterogeneity between studies. Although some studies measured POPs in cord and some in maternal blood, concentrations across these sample types have been shown to be highly correlated.149,150 BMI was a main outcome of interest, a measure that incorporates both lean and fat mass; however, a high BMI has been suggested as a sensitive marker for excess adiposity.107 We acknowledge that the magnitude of association between prenatal DDE and BMI observed in our meta-analysis was relatively small; a 0.1-SD increase in BMI-z might not be seem substantial at the individual level. Nevertheless, given the widespread exposure of the general population to this chemical, the aggregated effect at the population level, as measured by a rightward shift in the distribution of obesity, may translate into an important increase in the number of children with overweight. The relatively high degree of unexplained heterogeneity observed in some of our analyses (e.g., prenatal PFOA in relation to BMI-z and waist circumference in childhood) may limit the validity of our conclusions. Further, we assessed the potential for publication bias using well-established methods, however, for most comparisons, the limited number of available studies did not allow a meaningful assessment of such bias. Finally, the POP chemicals studied in this review are just a few chemicals out of the growing list of potentially obesogenic chemicals that are currently under study. The joined effects of potentially obesogenic chemicals that humans are simultaneously exposed to could be significantly larger and require further consideration in future studies.
Conclusion
The current evidence from epidemiological studies suggests that prenatal exposures to DDE and HCB are associated with greater adiposity in childhood. There was insufficient evidence to conclude that prenatal exposure to other POPs, such as PCBs, PFOA, PFOS, and PBDEs has obesogenic effects in children. Follow-up studies taking into account exposure to POP mixtures and potential sex differences are needed to improve our understanding of obesity development in childhood. Such work is critical to inform strategies to tackle the current obesity epidemic.
Supplementary Material
Acknowledgments
This study is an ancillary endeavor of the Science & Technology in childhood Obesity Policy (STOP) project (H2020 SC2; ref. 774548). This study was supported by the National Institute of Environmental Health Science (NIEHS, R01ES030364). Additional funding from NIEHS includes R21ES029681 (Chatzi, McConnell, Conti, Valvi, Stratakis), R01ES029944 (Chatzi, Conti, Valvi, Rock), R01ES030691 (Chatzi, McConnell, Conti, Valvi), R21ES028903 (Chatzi, McConnell, Valvi), P30ES007048 (McConnell, Chatzi, Conti, Stratakis), R21ES029328 (Valvi), and P30ES023515 (Valvi). Additional funding from NIH supported Dr. Conti (P01CA196569, R01CA140561, R01ES016813), and Dr. Stratakis (P30DK048522). Dr La Merill received additional funding from USDA National Institute of Food and Agriculture, Hatch project: 1002182.
Funding information
European Union, Grant/Award Numbers: H2020 SC2, ref. 774548; National Institute of Environmental Health Sciences, Grant/Award Numbers: R01ES030364, R01ES029944, R01ES030691, P30ES007048; National Institutes of Health, Grant/Award Numbers: P30DK048522, R01CA140561, P01CA196569; USDA National Institute of Food and Agriculture, Grant/Award Number: 1002182
References
- 1.Collaboration NCDRF. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park MH, Falconer C, Viner RM, Kinra S. The impact of childhood obesity on morbidity and mortality in adulthood: a systematic review. Obes Rev. 2012;13(11):985–1000. [DOI] [PubMed] [Google Scholar]
- 3.Quek YH, Tam WWS, Zhang MWB, Ho RCM. Exploring the association between childhood and adolescent obesity and depression: a meta-analysis. Obes Rev. 2017;18(7):742–754. [DOI] [PubMed] [Google Scholar]
- 4.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond). 2011;35(7):891–898. [DOI] [PubMed] [Google Scholar]
- 5.Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9(5):474–488. [DOI] [PubMed] [Google Scholar]
- 6.Geserick M, Vogel M, Gausche R, et al. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. N Engl J Med. 2018;379(14):1303–1312. [DOI] [PubMed] [Google Scholar]
- 7.Egusquiza RJ, Blumberg B. Environmental Obesogens and Their Impact on Susceptibility to Obesity: New Mechanisms and Chemicals. Endocrinology. 2020;161(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grun F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147(6 Suppl):S50–55. [DOI] [PubMed] [Google Scholar]
- 9.Baillie-Hamilton PF. Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med. 2002;8(2):185–192. [DOI] [PubMed] [Google Scholar]
- 10.Gore AC, Chappell VA, Fenton SE, et al. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015;36(6):E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gyalpo T, Toms LM, Mueller JF, Harden FA, Scheringer M, Hungerbuhler K. Insights into PBDE Uptake, Body Burden, and Elimination Gained from Australian Age-Concentration Trends Observed Shortly after Peak Exposure. Environ Health Perspect. 2015;123(10):978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porta M, Puigdomenech E, Ballester F, et al. Monitoring concentrations of persistent organic pollutants in the general population: the international experience. Environ Int. 2008;34(4):546–561. [DOI] [PubMed] [Google Scholar]
- 13.U. S. Department of Health and Human Sevices Centers for Disease Control and Prevention. National Report on Human Exposure to Environmental Chemicals. Updated Tables. https://www.cdc.gov/exposurereport/index.html. Published 2019. Accessed May 16, 2020.
- 14.Heindel JJ, Blumberg B, Cave M, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017;68:3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grun F, Blumberg B. Endocrine disrupters as obesogens. Mol Cell Endocrinol. 2009;304(1–2):19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heindel JJ, Newbold R, Schug TT. Endocrine disruptors and obesity. Nat Rev Endocrinol. 2015;11(11):653–661. [DOI] [PubMed] [Google Scholar]
- 17.Needham LL, Grandjean P, Heinzow B, et al. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ Sci Technol. 2011;45(3):1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Covaci A, Jorens P, Jacquemyn Y, Schepens P. Distribution of PCBs and organochlorine pesticides in umbilical cord and maternal serum. Sci Total Environ. 2002;298(1–3):45–53. [DOI] [PubMed] [Google Scholar]
- 19.Sala M, Ribas-Fito N, Cardo E, et al. Levels of hexachlorobenzene and other organochlorine compounds in cord blood: exposure across placenta. Chemosphere. 2001;43(4–7):895–901. [DOI] [PubMed] [Google Scholar]
- 20.Heindel JJ, Vandenberg LN. Developmental origins of health and disease: a paradigm for understanding disease cause and prevention. Curr Opin Pediatr. 2015;27(2):248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Esterik JC, Verharen HW, Hodemaekers HM, et al. Compound- and sex-specific effects on programming of energy and immune homeostasis in adult C57BL/6JxFVB mice after perinatal TCDD and PCB 153. Toxicol Appl Pharmacol. 2015;289(2):262–275. [DOI] [PubMed] [Google Scholar]
- 22.La Merrill M, Karey E, Moshier E, et al. Perinatal exposure of mice to the pesticide DDT impairs energy expenditure and metabolism in adult female offspring. PLoS One. 2014;9(7):e103337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: Low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol. 2009;304(1–2):97–105. [DOI] [PubMed] [Google Scholar]
- 24.Sitarek K, Gralewicz S. Early developmental effects of separate or combined perinatal exposure to methylmercury (MeHg) and 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB 153) in the rat. Int J Occup Med Environ Health. 2009;22(2):89–105. [DOI] [PubMed] [Google Scholar]
- 25.Bondy GS, Lefebvre DE, Aziz S, et al. Toxicologic and immunologic effects of perinatal exposure to the brominated diphenyl ether (BDE) mixture DE-71 in the Sprague-Dawley rat. Environ Toxicol. 2013;28(4):215–228. [DOI] [PubMed] [Google Scholar]
- 26.Tang-Peronard JL, Andersen HR, Jensen TK, Heitmann BL. Endocrine-disrupting chemicals and obesity development in humans: a review. Obes Rev. 2011;12(8):622–636. [DOI] [PubMed] [Google Scholar]
- 27.Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol. 2017;13(3):161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newbold RR, Padilla-Banks E, Snyder RJ, Phillips TM, Jefferson WN. Developmental exposure to endocrine disruptors and the obesity epidemic. Reprod Toxicol. 2007;23(3):290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elobeid MA, Allison DB. Putative environmental-endocrine disruptors and obesity: a review. Curr Opin Endocrinol Diabetes Obes. 2008;15(5):403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cano-Sancho G, Salmon AG, La Merrill MA. Association between Exposure to p,p’-DDT and Its Metabolite p,p’-DDE with Obesity: Integrated Systematic Review and Meta-Analysis. Environ Health Perspect. 2017;125(9):096002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez MC. Prenatal exposure to persistent organic pollutants as a risk factor of offspring metabolic syndrome development during childhood. Rev Environ Health. 2021. [DOI] [PubMed] [Google Scholar]
- 32.Lichtveld K, Thomas K, Tulve NS. Chemical and non-chemical stressors affecting childhood obesity: a systematic scoping review. J Expo Sci Environ Epidemiol. 2018;28(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russ K, Howard S. Developmental Exposure to Environmental Chemicals and Metabolic Changes in Children. Curr Probl Pediatr Adolesc Health Care. 2016;46(8):255–285. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Peterson KE. Maternal Exposure to Synthetic Chemicals and Obesity in the Offspring: Recent Findings. Curr Environ Health Rep. 2015;2(4):339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.La Merrill M, Birnbaum LS. Childhood obesity and environmental chemicals. Mt Sinai J Med. 2011;78(1):22–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life--a systematic review. Obes Rev. 2005;6(2):143–154. [DOI] [PubMed] [Google Scholar]
- 37.Karaolis-Danckert N, Buyken AE, Bolzenius K, Perim de Faria C, Lentze MJ, Kroke A. Rapid growth among term children whose birth weight was appropriate for gestational age has a longer lasting effect on body fat percentage than on body mass index. Am J Clin Nutr. 2006;84(6):1449–1455. [DOI] [PubMed] [Google Scholar]
- 38.Peterson JWV, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa, Ontario, Canada: Ottawa Hospital Research Institute; 2011. [Google Scholar]
- 39.Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC. The age of adolescence. Lancet Child Adolesc Health. 2018;2(3):223–228. [DOI] [PubMed] [Google Scholar]
- 40.Liu P, Yang F, Wang Y, Yuan Z. Perfluorooctanoic Acid (PFOA) Exposure in Early Life Increases Risk of Childhood Adiposity: A Meta-Analysis of Prospective Cohort Studies. Int J Environ Res Public Health. 2018;15(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mora AM, Oken E, Rifas-Shiman SL, et al. Prenatal Exposure to Perfluoroalkyl Substances and Adiposity in Early and Mid-Childhood. Environ Health Perspect. 2017;125(3):467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, et al. Prenatal Exposure to Perfluoroalkyl Substances and Cardiometabolic Risk in Children from the Spanish INMA Birth Cohort Study. Environ Health Perspect. 2017;125(9):097018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warner M, Aguilar Schall R, Harley KG, Bradman A, Barr D, Eskenazi B. In utero DDT and DDE exposure and obesity status of 7-year-old Mexican-American children in the CHAMACOS cohort. Environ Health Perspect. 2013;121(5):631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warner M, Wesselink A, Harley KG, Bradman A, Kogut K, Eskenazi B. Prenatal exposure to dichlorodiphenyltrichloroethane and obesity at 9 years of age in the CHAMACOS study cohort. Am J Epidemiol. 2014;179(11):1312–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lauritzen HB, Larose TL, Oien T, et al. Prenatal exposure to persistent organic pollutants and child overweight/obesity at 5-year follow-up: a prospective cohort study. Environ Health. 2018;17(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoyer BB, Ramlau-Hansen CH, Henriksen TB, et al. Body mass index in young school-age children in relation to organochlorine compounds in early life: a prospective study. Int J Obes (Lond). 2014;38(7):919–925. [DOI] [PubMed] [Google Scholar]
- 47.Hoyer BB, Ramlau-Hansen CH, Vrijheid M, et al. Anthropometry in 5- to 9-Year-Old Greenlandic and Ukrainian Children in Relation to Prenatal Exposure to Perfluorinated Alkyl Substances. Environ Health Perspect. 2015;123(8):841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 49.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. [DOI] [PubMed] [Google Scholar]
- 52.Karlsen M, Grandjean P, Weihe P, Steuerwald U, Oulhote Y, Valvi D. Early-life exposures to persistent organic pollutants in relation to overweight in preschool children. Reprod Toxicol. 2017;68:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verhulst SL, Nelen V, Hond ED, et al. Intrauterine exposure to environmental pollutants and body mass index during the first 3 years of life. Environ Health Perspect. 2009;117(1):122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iszatt N, Stigum H, Verner MA, et al. Prenatal and Postnatal Exposure to Persistent Organic Pollutants and Infant Growth: A Pooled Analysis of Seven European Birth Cohorts. Environ Health Perspect. 2015;123(7):730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delvaux I, Van Cauwenberghe J, Den Hond E, et al. Prenatal exposure to environmental contaminants and body composition at age 7–9 years. Environ Res. 2014;132:24–32. [DOI] [PubMed] [Google Scholar]
- 56.Tang-Peronard JL, Heitmann BL, Andersen HR, et al. Association between prenatal polychlorinated biphenyl exposure and obesity development at ages 5 and 7 y: a prospective cohort study of 656 children from the Faroe Islands. Am J Clin Nutr. 2014;99(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kronke AA, Jurkutat A, Schlingmann M, et al. Persistent organic pollutants in pregnant women potentially affect child development and thyroid hormone status. Pediatr Res. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vafeiadi M, Georgiou V, Chalkiadaki G, et al. Association of Prenatal Exposure to Persistent Organic Pollutants with Obesity and Cardiometabolic Traits in Early Childhood: The Rhea Mother-Child Cohort (Crete, Greece). Environ Health Perspect. 2015;123(10):1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cupul-Uicab LA, Hernandez-Avila M, Terrazas-Medina EA, Pennell ML, Longnecker MP. Prenatal exposure to the major DDT metabolite 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) and growth in boys from Mexico. Environ Res. 2010;110(6):595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garced S, Torres-Sanchez L, Cebrian ME, Claudio L, Lopez-Carrillo L. Prenatal dichlorodiphenyldichloroethylene (DDE) exposure and child growth during the first year of life. Environ Res. 2012;113:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Cock M, de Boer MR, Lamoree M, Legler J, van de Bor M. First year growth in relation to prenatal exposure to endocrine disruptors - a Dutch prospective cohort study. Int J Environ Res Public Health. 2014;11(7):7001–7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coker E, Chevrier J, Rauch S, et al. Association between prenatal exposure to multiple insecticides and child body weight and body composition in the VHEMBE South African birth cohort. Environ Int. 2018;113:122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valvi D, Mendez MA, Garcia-Esteban R, et al. Prenatal exposure to persistent organic pollutants and rapid weight gain and overweight in infancy. Obesity (Silver Spring). 2014;22(2):488–496. [DOI] [PubMed] [Google Scholar]
- 64.Agay-Shay K, Martinez D, Valvi D, et al. Exposure to Endocrine-Disrupting Chemicals during Pregnancy and Weight at 7 Years of Age: A Multi-pollutant Approach. Environ Health Perspect. 2015;123(10):1030–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valvi D, Mendez MA, Martinez D, et al. Prenatal concentrations of polychlorinated biphenyls, DDE, and DDT and overweight in children: a prospective birth cohort study. Environ Health Perspect. 2012;120(3):451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mendez MA, Garcia-Esteban R, Guxens M, et al. Prenatal organochlorine compound exposure, rapid weight gain, and overweight in infancy. Environ Health Perspect. 2011;119(2):272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guil-Oumrait N, Valvi D, Garcia-Esteban R, et al. Prenatal exposure to persistent organic pollutants and markers of obesity and cardiometabolic risk in Spanish adolescents. Environ Int. 2021;151:106469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tahir E, Cordier S, Courtemanche Y, et al. Effects of polychlorinated biphenyls exposure on physical growth from birth to childhood and adolescence: A prospective cohort study. Environ Res. 2020;189:109924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang C, Fang J, Sun X, et al. Prenatal exposure to organochlorine pesticides and infant growth: A longitudinal study. Environ Int. 2021;148:106374. [DOI] [PubMed] [Google Scholar]
- 70.Gladen BC, Klebanoff MA, Hediger ML, et al. Prenatal DDT exposure in relation to anthropometric and pubertal measures in adolescent males. Environ Health Perspect. 2004;112(17):1761–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heggeseth B, Harley K, Warner M, Jewell N, Eskenazi B. Detecting Associations between Early-Life DDT Exposures and Childhood Growth Patterns: A Novel Statistical Approach. PLoS One. 2015;10(6):e0131443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Warner M, Ye M, Harley K, Kogut K, Bradman A, Eskenazi B. Prenatal DDT exposure and child adiposity at age 12: The CHAMACOS study. Environ Res. 2017;159:606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gladen BC, Ragan NB, Rogan WJ. Pubertal growth and development and prenatal and lactational exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene. J Pediatr. 2000;136(4):490–496. [DOI] [PubMed] [Google Scholar]
- 74.Cupul-Uicab LA, Klebanoff MA, Brock JW, Longnecker MP. Prenatal exposure to persistent organochlorines and childhood obesity in the US collaborative perinatal project. Environ Health Perspect. 2013;121(9):1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gross RS, Ghassabian A, Vandyousefi S, et al. Persistent organic pollutants exposure in newborn dried blood spots and infant weight status: A case-control study of low-income Hispanic mother-infant pairs. Environ Pollut. 2020;267:115427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.(ATSDR) AfTSaDR. Toxicological Profile for DDT, DDE, and DDD. 2002. Sep. [PubMed]
- 77.Cupul-Uicab L, Klebanoff M, Brock J, Longnecker M. Prenatal Exposure to Persistent Organochlorines and Childhood Obesity in the US Collaborative Perinatal Project. Environ Health Perspect. 2013;121(9):1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iszatt N, Stigum H, Verner MA, et al. Prenatal and Postnatal Exposure to Persistent Organic Pollutants and Infant Growth: A Pooled Analysis of Seven European Birth Cohorts. Environ Health Perspect. 2015;123(7):730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dallaire R, Dewailly E, Ayotte P, et al. Growth in Inuit children exposed to polychlorinated biphenyls and lead during fetal development and childhood. Environ Res. 2014;134:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patandin S, Koopman-Esseboom C, de Ridder MA, Weisglas-Kuperus N, Sauer PJ. Effects of environmental exposure to polychlorinated biphenyls and dioxins on birth size and growth in Dutch children. Pediatr Res. 1998;44(4):538–545. [DOI] [PubMed] [Google Scholar]
- 81.Wang A, Jeddy Z, Sjodin A, Taylor EV, Marks KJ, Hartman TJ. Prenatal exposure to Polychlorinated Biphenyls and body fatness in girls. Chemosphere. 2019;236:124315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blanck HM, Marcus M, Rubin C, et al. Growth in girls exposed in utero and postnatally to polybrominated biphenyls and polychlorinated biphenyls. Epidemiology. 2002;13(2):205–210. [DOI] [PubMed] [Google Scholar]
- 83.Jackson LW, Lynch CD, Kostyniak PJ, McGuinness BM, Louis GM. Prenatal and postnatal exposure to polychlorinated biphenyls and child size at 24 months of age. Reprod Toxicol. 2010;29(1):25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smink A, Ribas-Fito N, Garcia R, et al. Exposure to hexachlorobenzene during pregnancy increases the risk of overweight in children aged 6 years. Acta Paediatr. 2008;97(10):1465–1469. [DOI] [PubMed] [Google Scholar]
- 85.Andersen CS, Fei C, Gamborg M, Nohr EA, Sorensen TI, Olsen J. Prenatal exposures to perfluorinated chemicals and anthropometric measures in infancy. Am J Epidemiol. 2010;172(11):1230–1237. [DOI] [PubMed] [Google Scholar]
- 86.Andersen CS, Fei C, Gamborg M, Nohr EA, Sorensen TI, Olsen J. Prenatal exposures to perfluorinated chemicals and anthropometry at 7 years of age. Am J Epidemiol. 2013;178(6):921–927. [DOI] [PubMed] [Google Scholar]
- 87.Braun JM, Chen A, Romano ME, et al. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study. Obesity (Silver Spring). 2016;24(1):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen MH, Ng S, Hsieh CJ, Lin CC, Hsieh WS, Chen PC. The impact of prenatal perfluoroalkyl substances exposure on neonatal and child growth. Sci Total Environ. 2017;607–608:669–675. [DOI] [PubMed] [Google Scholar]
- 89.Chen Q, Zhang X, Zhao Y, et al. Prenatal exposure to perfluorobutanesulfonic acid and childhood adiposity: A prospective birth cohort study in Shanghai, China. Chemosphere. 2019;226:17–23. [DOI] [PubMed] [Google Scholar]
- 90.Halldorsson TI, Rytter D, Haug LS, et al. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environ Health Perspect. 2012;120(5):668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hartman TJ, Calafat AM, Holmes AK, et al. Prenatal Exposure to Perfluoroalkyl Substances and Body Fatness in Girls. Child Obes. 2017;13(3):222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shoaff J, Papandonatos GD, Calafat AM, et al. Prenatal Exposure to Perfluoroalkyl Substances: Infant Birth Weight and Early Life Growth. Environ Epidemiol. 2018;2(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Starling AP, Adgate JL, Hamman RF, Kechris K, Calafat AM, Dabelea D. Prenatal exposure to per- and polyfluoroalkyl substances and infant growth and adiposity: the Healthy Start Study. Environ Int. 2019;131:104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maisonet M, Terrell ML, McGeehin MA, et al. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ Health Perspect. 2012;120(10):1432–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jensen RC, Andersen MS, Larsen PV, et al. Prenatal Exposures to Perfluoroalkyl Acids and Associations with Markers of Adiposity and Plasma Lipids in Infancy: An Odense Child Cohort Study. Environ Health Perspect. 2020;128(7):77001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu Y, Li N, Papandonatos GD, et al. Exposure to Per- and Polyfluoroalkyl Substances and Adiposity at Age 12 Years: Evaluating Periods of Susceptibility. Environ Sci Technol. 2020;54(24):16039–16049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li N, Liu Y, Papandonatos GD, et al. Gestational and childhood exposure to per- and polyfluoroalkyl substances and cardiometabolic risk at age 12 years. Environ Int. 2021;147:106344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martinsson M, Nielsen C, Bjork J, et al. Intrauterine exposure to perfluorinated compounds and overweight at age 4: A case-control study. PLoS One. 2020;15(3):e0230137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kannan K, Corsolini S, Falandysz J, et al. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol. 2004;38(17):4489–4495. [DOI] [PubMed] [Google Scholar]
- 100.CDC. Fourth National Report on Human Exposure to Environmental Chemicals, updated tables, January 2019. [PubMed]
- 101.Erkin-Cakmak A, Harley KG, Chevrier J, et al. In utero and childhood polybrominated diphenyl ether exposures and body mass at age 7 years: the CHAMACOS study. Environ Health Perspect. 2015;123(6):636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vuong AM, Braun JM, Sjodin A, et al. Prenatal Polybrominated Diphenyl Ether Exposure and Body Mass Index in Children Up To 8 Years of Age. Environ Health Perspect. 2016;124(12):1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guo J, Miao W, Wu C, et al. Umbilical cord serum PBDE concentrations and child adiposity measures at 7 years. Ecotoxicol Environ Saf. 2020;203:111009. [DOI] [PubMed] [Google Scholar]
- 104.Chen A, Chung E, DeFranco EA, Pinney SM, Dietrich KN. Serum PBDEs and age at menarche in adolescent girls: analysis of the National Health and Nutrition Examination Survey 2003–2004. Environ Res. 2011;111(6):831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect. 2012;120(6):779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stockholm Convention on Persistent Organic Pollutants. http://chm.pops.int/TheConvention/POPsReviewCommittee/ReportsandDecisions/tabid/3309/Default.aspx. Published 2009. Accessed May 18, 2020.
- 107.Rosa-Silva HTD, Panzenhagen AC, Schmidtt V, et al. Hepatic and neurobiological effects of foetal and breastfeeding and adulthood exposure to methylmercury in Wistar rats. Chemosphere. 2020;244:125400. [DOI] [PubMed] [Google Scholar]
- 108.World Health Organization. Persistent Organic Pollutants: Impact on Child Health. WHO Document Production Services. https://www.who.int/ceh/publications/persistent_organic_pollutant/en/. Published 2010. Accessed May 16, 2020.
- 109.Karmaus W, Osuch JR, Eneli I, et al. Maternal levels of dichlorodiphenyl-dichloroethylene (DDE) may increase weight and body mass index in adult female offspring. Occup Environ Med. 2009;66(3):143–149. [DOI] [PubMed] [Google Scholar]
- 110.Cirillo PM, La Merrill MA, Krigbaum NY, Cohn BA. Grandmaternal Perinatal Serum DDT in Relation to Granddaughter Early Menarche and Adult Obesity: Three Generations in the Child Health and Development Studies Cohort. Cancer Epidemiol Biomarkers Prev. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lindstrom AB, Strynar MJ, Libelo EL. Polyfluorinated compounds: past, present, and future. Environ Sci Technol. 2011;45(19):7954–7961. [DOI] [PubMed] [Google Scholar]
- 112.Hu XC, Andrews DQ, Lindstrom AB, et al. Detection of Poly- and Perfluoroalkyl Substances (PFAS) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ Sci Technol Lett. 2016;3(10):344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lv Z, Li G, Li Y, et al. Glucose and lipid homeostasis in adult rat is impaired by early-life exposure to perfluorooctane sulfonate. Environ Toxicol. 2013;28(9):532–542. [DOI] [PubMed] [Google Scholar]
- 114.Yu N, Wei S, Li M, et al. Effects of Perfluorooctanoic Acid on Metabolic Profiles in Brain and Liver of Mouse Revealed by a High-throughput Targeted Metabolomics Approach. Sci Rep. 2016;6:23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99(2):366–394. [DOI] [PubMed] [Google Scholar]
- 116.Lin CY, Chen PC, Lin YC, Lin LY. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes Care. 2009;32(4):702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Timmermann CA, Rossing LI, Grontved A, et al. Adiposity and glycemic control in children exposed to perfluorinated compounds. J Clin Endocrinol Metab. 2014;99(4):E608–614. [DOI] [PubMed] [Google Scholar]
- 118.Nelson JW, Hatch EE, Webster TF. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect. 2010;118(2):197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Domazet SL, Grontved A, Timmermann AG, Nielsen F, Jensen TK. Longitudinal Associations of Exposure to Perfluoroalkylated Substances in Childhood and Adolescence and Indicators of Adiposity and Glucose Metabolism 6 and 12 Years Later: The European Youth Heart Study. Diabetes Care. 2016;39(10):1745–1751. [DOI] [PubMed] [Google Scholar]
- 120.Liu G, Dhana K, Furtado JD, et al. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: A prospective study. PLoS Med. 2018;15(2):e1002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brase RA, Mullin EJ, Spink DC. Legacy and Emerging Per- and Polyfluoroalkyl Substances: Analytical Techniques, Environmental Fate, and Health Effects. Int J Mol Sci. 2021;22(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Geyer HJ, Schramm KW, O. DP, Aune M, Feicht A, Fried KW. Terminal elimination halflives of the brominated flame retardants TBBPA, HBCD, and lower brominates PBDEs in humans. Organohalogen Compounds. 2004;66:3867–3872. [Google Scholar]
- 123.Trudel D, Scheringer M, von Goetz N, Hungerbuhler K. Total consumer exposure to polybrominated diphenyl ethers in North America and Europe. Environ Sci Technol. 2011;45(6):2391–2397. [DOI] [PubMed] [Google Scholar]
- 124.Jones-Otazo HA, Clarke JP, Diamond ML, et al. Is house dust the missing exposure pathway for PBDEs? An analysis of the urban fate and human exposure to PBDEs. Environ Sci Technol. 2005;39(14):5121–5130. [DOI] [PubMed] [Google Scholar]
- 125.Suvorov A, Battista MC, Takser L. Perinatal exposure to low-dose 2,2’,4,4’-tetrabromodiphenyl ether affects growth in rat offspring: what is the role of IGF-1? Toxicology. 2009;260(1–3):126–131. [DOI] [PubMed] [Google Scholar]
- 126.Hoppe AA, Carey GB. Polybrominated diphenyl ethers as endocrine disruptors of adipocyte metabolism. Obesity (Silver Spring). 2007;15(12):2942–2950. [DOI] [PubMed] [Google Scholar]
- 127.Kamstra JH, Hruba E, Blumberg B, et al. Transcriptional and epigenetic mechanisms underlying enhanced in vitro adipocyte differentiation by the brominated flame retardant BDE-47. Environ Sci Technol. 2014;48(7):4110–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lim JS, Lee DH, Jacobs DR Jr. Association of brominated flame retardants with diabetes and metabolic syndrome in the U.S. population, 2003–2004. Diabetes Care. 2008;31(9):1802–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Windham GC, Pinney SM, Sjodin A, et al. Body burdens of brominated flame retardants and other persistent organo-halogenated compounds and their descriptors in US girls. Environ Res. 2010;110(3):251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Turyk ME, Anderson HA, Steenport D, Buelow C, Imm P, Knobeloch L. Longitudinal biomonitoring for polybrominated diphenyl ethers (PBDEs) in residents of the Great Lakes basin. Chemosphere. 2010;81(4):517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shi Z, Zhang H, Ding L, Feng Y, Xu M, Dai J. The effect of perfluorododecanonic acid on endocrine status, sex hormones and expression of steroidogenic genes in pubertal female rats. Reprod Toxicol. 2009;27(3–4):352–359. [DOI] [PubMed] [Google Scholar]
- 132.Benninghoff AD, Bisson WH, Koch DC, Ehresman DJ, Kolluri SK, Williams DE. Estrogen-like activity of perfluoroalkyl acids in vivo and interaction with human and rainbow trout estrogen receptors in vitro. Toxicol Sci. 2011;120(1):42–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kjeldsen LS, Bonefeld-Jorgensen EC. Perfluorinated compounds affect the function of sex hormone receptors. Environ Sci Pollut Res Int. 2013;20(11):8031–8044. [DOI] [PubMed] [Google Scholar]
- 134.Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol. 2011;73:135–162. [DOI] [PubMed] [Google Scholar]
- 135.Palmisano BT, Zhu L, Eckel RH, Stafford JM. Sex differences in lipid and lipoprotein metabolism. Mol Metab. 2018;15:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shi H, Clegg DJ. Sex differences in the regulation of body weight. Physiol Behav. 2009;97(2):199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chang E, Varghese M, Singer K. Gender and Sex Differences in Adipose Tissue. Curr Diab Rep. 2018;18(9):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee MJ, Fried SK. Sex-dependent Depot Differences in Adipose Tissue Development and Function; Role of Sex Steroids. J Obes Metab Syndr. 2017;26(3):172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol. 2009;30(3):396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kalloo G, Wellenius GA, McCandless L, et al. Exposures to chemical mixtures during pregnancy and neonatal outcomes: The HOME study. Environ Int. 2020;134:105219. [DOI] [PubMed] [Google Scholar]
- 141.Robinson O, Basagana X, Agier L, et al. The Pregnancy Exposome: Multiple Environmental Exposures in the INMA-Sabadell Birth Cohort. Environ Sci Technol. 2015;49(17):10632–10641. [DOI] [PubMed] [Google Scholar]
- 142.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119(6):878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Warner M, Rauch S, Coker ES, et al. Obesity in relation to serum persistent organic pollutant concentrations in CHAMACOS women. Environ Epidemiol. 2018;2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Braun JM, Gennings C, Hauser R, Webster TF. What Can Epidemiological Studies Tell Us about the Impact of Chemical Mixtures on Human Health? Environ Health Perspect. 2016;124(1):A6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vrijheid M, Fossati S, Maitre L, et al. Early-Life Environmental Exposures and Childhood Obesity: An Exposome-Wide Approach. Environ Health Perspect. 2020;128(6):67009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gibson EA, Nunez Y, Abuawad A, et al. An overview of methods to address distinct research questions on environmental mixtures: an application to persistent organic pollutants and leukocyte telomere length. Environ Health. 2019;18(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bind MA. Causal Modeling in Environmental Health. Annu Rev Public Health. 2019;40:23–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pearce N, Vandenbroucke JP, Lawlor DA. Causal Inference in Environmental Epidemiology: Old and New Approaches. Epidemiology. 2019;30(3):311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jarrell J, Chan S, Hauser R, Hu H. Longitudinal assessment of PCBs and chlorinated pesticides in pregnant women from Western Canada. Environ Health. 2005;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vizcaino E, Grimalt JO, Fernandez-Somoano A, Tardon A. Transport of persistent organic pollutants across the human placenta. Environ Int. 2014;65:107–115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


