Abstract
Years of research exploring mRNA vaccines for cancer treatment in preclinical and clinical trials have set the stage for the rapid development of mRNA vaccines during the COVID-19 pandemic. Therapeutic cancer vaccines based on mRNA are well tolerated, and the inherent advantage in ease of production, which rivals the best available conventional vaccine manufacture methods, renders mRNA vaccines a promising option for cancer immunotherapy. Technological advances have optimised mRNA-based vaccine stability, structure, and delivery methods, and multiple clinical trials investigating mRNA vaccine therapy are now enrolling patients with various cancer diagnoses. Although therapeutic mRNA-based cancer vaccines have not yet been approved for standard treatment, encouraging results from early clinical trials with mRNA vaccines as monotherapy and in combination with checkpoint inhibitors have been obtained. This Review summarises the latest clinical advances in mRNA-based vaccines for cancer treatment and reflects on future perspectives and challenges for this new and promising treatment approach.
Introduction
The COVID-19 pandemic has directed worldwide focus towards mRNA-based vaccines. Indeed, the foundation for the rapid COVID-19 vaccine development and production was based on years of research exploring mRNA vaccines as a therapeutic strategy against cancer in preclinical and clinical trials.1 mRNA brings several benefits to a vaccine setting (panel ). Firstly, mRNA-based vaccines are well tolerated, easily degraded, and do not integrate into the host genome.2, 3, 4 Secondly, mRNA molecules are non-infectious, and mRNA vaccines have the potential to induce both humoral and cell-mediated immunity (figure ).5, 6 Lastly, the production of mRNA vaccines is fast and inexpensive.7
Panel. Advantages of mRNA vaccines for the treatment of cancer.
-
•
Well tolerated: adverse events are generally manageable and transient
-
•
No genome integration: eliminates the risk of insertional mutagenesis
-
•
Non-infectious: no pathogenic viral agents are used
-
•
Easily degraded: reduces risk of toxicity
-
•
Humoral and cellular immunity: necessary for activating and sustaining anti-tumour responses
-
•
Fast and inexpensive production: laboratory-based and cell-free production
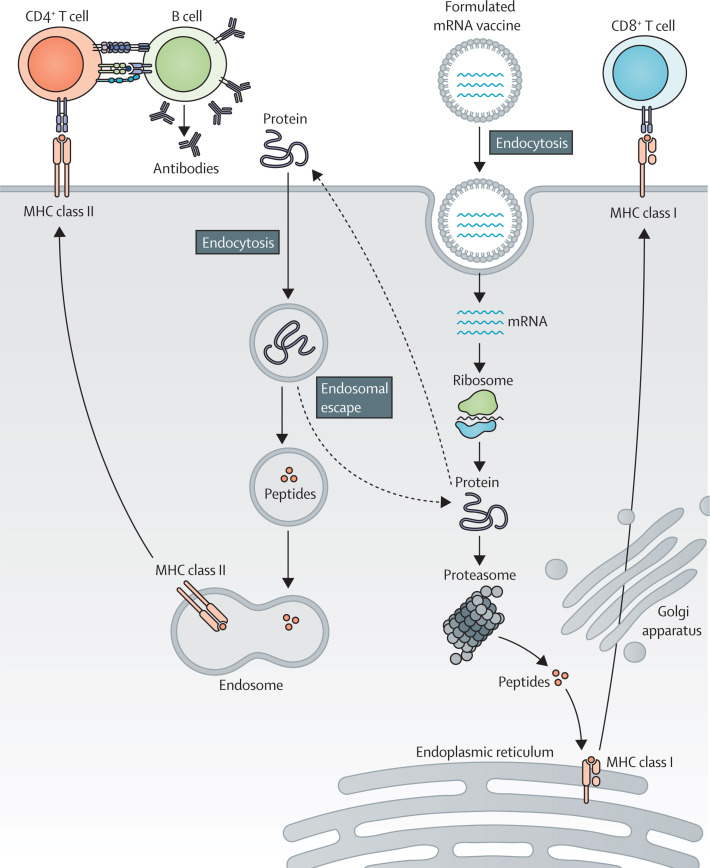
Figure.
mRNA-based vaccine mode of action
mRNA is taken up by antigen-presenting cells and peptides are loaded on MHC class I for antigen-specific CD8+ T-cell activation. Extracellular proteins are cross-presented on MHC class I or loaded on MHC class II for CD4+ T-cell activation. CD4+ T cells can co-activate protein-specific B cells, and B cells can activate CD4+ T cells after B-cell receptor-mediated antigen internalisation.
In 1996, the first mRNA-based cancer vaccine study tested dendritic cells pulsed with RNA in vitro.8 Nowadays, technological advances have led to optimised mRNA structure, stability, and delivery methods, and multiple clinical trials are now enrolling patients with cancer for mRNA-based vaccine treatments (Table 1, Table 2 ).2, 3 mRNA vaccine administration routes include intradermal, subcutaneous, intranasal, intranodal, intramuscular, intratumoural, and intravenous delivery.9 The ex-vivo engineering of autologous dendritic cells with mRNA has been the method of choice for tumour antigen delivery, but most mRNA vaccine approaches focus on direct mRNA administration using lipid nanoparticulate formulation carriers (table 1).
Table 1.
ClinicalTrials.gov-registered mRNA-based cancer vaccine trials by type of formulation
| Trial phase | Target antigen | Cancer type | Combination | Vaccine route of administration | Sponsor | |
|---|---|---|---|---|---|---|
| Lipid nanoparticle formulation | ||||||
| NCT03948763 | 1 | mRNA-5671 (KRAS gene driver mutations) | Non-small-cell lung, pancreatic, and colorectal neoplasms | With pembrolizumab | Intramuscular | Merck Sharp & Dohme |
| NCT03313778 | 1 | mRNA-4157 (personalised cancer vaccine encoding several neoantigens) | Solid tumours (resected) | With pembrolizumab | Intramuscular | Moderna |
| NCT03897881 | 2 | mRNA-4157 (personalised cancer vaccine encoding 20 different mutated neoepitopes) | Melanoma | With pembrolizumab | Intramuscular | Moderna |
| NCT04573140 | 1 | Formulation with pp65 LAMP and tumour mRNA | Glioblastoma | None | Intravenous | University of Florida (Gainesville, FL, USA) |
| Lipoplex formulation | ||||||
| NCT02410733 | 1 | BNT111 (NY-ESO-1 [CTAG1A], tyrosinase, MAGE-A3, and TPTE) | Melanoma | None | Intravenous | BioNTech |
| NCT04526899 | 2 | BNT111 (NY-ESO-1, tyrosinase, MAGE-A3, and TPTE) | Melanoma | With cemiplimab | Intravenous | BioNTech |
| NCT04382898 | 1/2 | BNT112 (PAP, PSA, and three undisclosed antigens) | Prostate | With cemiplimab | Intravenous | BioNTech |
| NCT04534205 | 2 | BNT113 (HPV16 E6 and E7 oncoproteins) | Head and neck squamous cell carcinoma | With pembrolizumab | Intravenous | BioNTech |
| NCT03418480 | 1/2 | BNT113 (HPV16 E6 and E7 oncoproteins) | HPV16-positive solid tumours | With anti-CD40 antibodies | Intravenous | University of Southampton (Southampton, UK) |
| NCT05142189 | 1 | BNT116 (non-small-cell lung cancer tumour-associated antigens) | Non-small-cell lung cancer | With cemiplimab plus docetaxel | Intravenous | BioNTech |
| NCT04486378 | 2 | BNT122 (personalised cancer vaccine encoding individual tumour mutations) | Colorectal | None | Intravenous | BioNTech |
| NCT02316457 | 1 | BNT-114 plus BNT-122 (personalised set of pre-manufactured non-mutated shared tumour-associated antigens plus a personalised cancer vaccine encoding individual tumour mutations) | Triple-negative breast cancer | None | Intravenous | BioNTech |
| NCT04163094 | 1 | BNT115 (ovarian cancer tumour-associated antigens) | Ovarian | With carboplatin plus paclitaxel | Intravenous | University Medical Center Groningen (Groningen, Netherlands) |
| NCT04161755 | 1 | BNT122 (personalised cancer vaccine encoding individual tumour mutations) | Pancreatic | With oxaliplatin, irinotecan, fluorouracil, leucovorin, and atezolizumab | Intravenous | Memorial Sloan Kettering Cancer Center (New York, NY, USA) |
| NCT03815058 | 2 | BNT122 (personalised cancer vaccine encoding individual tumour mutations) | Advanced melanoma | With pembrolizumab | Intravenous | Genentech |
| NCT03289962 | 1 | BNT122 (personalised cancer vaccine encoding individual tumour mutations) | Solid tumours | With atezolizumab | Intravenous | Genentech |
| NCT04503278 | 1/2 | CARVac (CLDN6) | Solid tumours | With chimeric antigen receptor therapy | Intravenous | BioNTech and Gene Therapies |
Trials with recruitment status “not yet recruiting,” “recruiting,” and “active, not recruiting” were found on ClinicalTrials.gov with the search terms “cancer” and “RNA, vaccine” on Feb 7, 2022, and through a PubMed search (see Search strategy and selection criteria panel). HPV=human papillomavirus.
Table 2.
mRNA-based dendritic cell cancer vaccines
| Trial phase | Target antigen | Cancer type | Combination | Vaccine route of administration | Sponsor | |
|---|---|---|---|---|---|---|
| NCT05000801 | Not described | WT1, hTERT, and survivin-loaded dendritic cells | Acute myeloid leukaemia | With follow-up care | Not described | Affiliated Hospital to Academy of Military Medical Sciences (Beijing, China) |
| NCT01686334 | 2 | WT1 | Acute myeloid leukaemia | With follow-up care | Intradermal | Antwerp University Hospital (Antwerp, Belgium) |
| NCT02649829 | 1/2 | WT1 | Pleural mesothelioma | With standard therapy | Intradermal | Antwerp University Hospital |
| NCT04911621 | 1/2 | WT1 | High-grade glioma and diffuse intrinsic pontine glioma | With chemoradiation (with or without standard therapy) | Intradermal | Antwerp University Hospital |
| NCT02649582 | 1/2 | WT1 | Glioblastoma multiforme | With temozolomide | Intradermal | Antwerp University Hospital |
| NCT04157127 | 1 | Pancreatic adenocarcinoma mRNA and lysate | Pancreatic adenocarcinoma | With standard therapy | Intradermal | Baylor College of Medicine (Houston, TX, USA) |
| NCT00639639 | 1 | Cytomegalovirus pp65-LAMP | Glioblastoma multiforme | With autologous lymphocyte transfer and Td | Intradermal | Duke University (Durham, NC, USA) |
| NCT03688178 | 2 (randomised) | Cytomegalovirus pp65-flLAMP | Glioblastoma multiforme | With temozolomide, varlilumab, and Td | Intradermal | Duke University |
| NCT04335890 | 1 | Autologous tumour RNA with gp100, tyrosinase, PRAME, MAGE-A3, IDO, and different driver mutations | Uveal melanoma | With standard therapy | Intravenous | Hasumi International Research Foundation |
| NCT02465268 | 2 (randomised) | HCMV pp65-shLAMP or pp65-flLAMP | Glioblastoma multiforme | With temozolomide, GM-CSF, and Td | Not described | Immunomic Therapeutics |
| NCT01995708 | 1 | CT7, MAGE-A3, and WT1 (Langerhans-type dendritic cells) | Multiple myeloma | With standard treatment | Intradermal | Memorial Sloan Kettering Cancer Center (New York, NY, USA) |
| NCT01456104 | 1 | Trp2 (Langerhans-type dendritic cells) | Melanoma | None | Not described | Memorial Sloan Kettering Cancer Center |
| NCT01197625 | 1/2 | hTERT, survivin, and mRNA from primary prostate cancer tissue | Prostate cancer | None | Not described | Oslo University Hospital (Oslo, Norway) |
| NCT03548571 | 2/3 | hTERT, survivin, and mRNA from autologous tumour stem cells | Glioblastoma multiforme | With temozolomide | Intradermal | Oslo University Hospital |
| NCT01983748 | 3 (randomised) | Autologous tumour RNA | Uveal melanoma | None | Intravenous | University Hospital Erlangen (Erlangen, Germany) |
| NCT03083054 | 1/2 | WT1 | Myelodysplastic syndromes, acute myeloid leukaemia | None | Not described | University of Campinas (Campinas, Brazil) |
| NCT04963413 | 1 | Cytomegalovirus pp65-flLAMP | Glioblastoma multiforme | With temozolomide, GM-CSF, and Td | Not described | University of Florida (Gainesville, FL, USA) |
| NCT03396575 | 1 | TTRNA | Brainstem gliomas | With cyclophosphamide, fludarabine, temozolomide, TTRNA-xALT, autologous haematopoietic stem cells, GM-CSF, and Td | Intradermal | University of Florida |
| NCT01326104 | 1/2 | TTRNA | Medulloblastoma, neuroectodermal tumour | With TTRNA-xALT | Intradermal | University of Florida |
Trials with recruitment status: “not yet recruiting,” “recruiting,” and “active, not recruiting” were found on ClinicalTrial.gov with the search terms: “cancer” and “RNA, vaccine” on Feb 7, 2022, and through a PubMed search (see Search strategy and selection criteria panel). Td=tetanus-diphtheria toxoid vaccine. TTRNA=tumour mRNA-pulsed autologous dendritic cells. TTRNA-xALT=tumour-specific autologous lymphocyte transfer.
The clinical efficacy and immunogenicity of mRNA vaccines have been evaluated across cancer diagnoses and administration methods (table 3 ). A few trials have reported durable objective responses in patients with cancer after mRNA-based vaccine treatment, without unmanageable toxic effects.10, 14, 15, 16, 19 mRNA vaccines are promising therapeutic candidates for future cancer treatments, especially in combination with additional immunotherapies.14, 19, 20 However, no phase 3 studies are ongoing, and, at the time of writing, the US Food and Drug Administration (FDA) has not yet approved a therapeutic mRNA-based cancer vaccine.21
Table 3.
A summary of the published results (2017–22) from mRNA cancer vaccine trials by type of formulation
| Trial phase | Target antigen | Cancer type | Patients, n | Combination | Immune response | Clinical response | |
|---|---|---|---|---|---|---|---|
| Non-formulated (naked) | |||||||
| NCT02035956 | 1 | An individualised tumour mutation signature with ten selected neoepitopes for each patient | Melanoma (stages III and IV) | 13 | None | T-cell responses against numerous vaccine neoepitopes | One (8%) patient had complete response and another patient (8%) had partial response10 |
| NCT03394937 | 1 | CD40L, CD70, caTLR4; tumour-associated antigens: tyrosinase, gp100, MAGE-A3, MAGE-C2, and PRAME | Resected melanoma (stages IIc, III, and IV) | 20 | None | Vaccine-induced immune responses in four (40%) of ten patients (low dose) and three (33%) of nine patients (high dose) | Not reported11 |
| Protamine formulation | |||||||
| NCT01817738 | 1/2 | PSA, PSMA, PSCA, STEAP1, PAP, and MUC1 | Metastatic castration-resistant prostate cancer | 197 | None | Not reported | No significant differences in progression-free survival12 |
| NCT00923312 | 1/2 | MAGE-C1, MAGE-C2, NY-ESO-1, survivin, and 5T4 | Non-small-cell lung cancer (stages IIIb and IV) | 46 | None | T-cell responses against at least one tumour-associated antigen in 19 (63%) patients | No objective responses; progression-free survival and overall survival not improved13 |
| NCT01915524 | 1 | MAGE-C1, MAGE-C2, NY-ESO-1, survivin, 5T4, and MUC-1 | Non-small-cell lung cancer (stage IV) | 26 | With local irradiation (with or without pemetrexed and with or without EGFR tyrosine-kinase inhibitor) | Detectable antigen-specific immunity in 21 (84%) patients | One (4%) patient had partial response in combination with chemotherapy treatment, and 12 (46%) patients had stable disease14 |
| Lipoplex formulation | |||||||
| NCT02410733 | 1 | NY-ESO-1, tyrosinase, MAGE-A3, and TPTE | Melanoma | 25 (monotherapy); 17 (combination) | With or without standard PD-1 therapy | Immune responses against a minimum of one tumour-associated antigen in 39 (75%) patients | mRNA vaccine with anti-PD-1 therapy: six (35%) patients had partial response and two (12%) had stable disease; mRNA vaccine monotherapy: three (12%) patients had partial response, and seven (28%) had stable disease15 |
| NCT04503278 | 1/2 | CLDN6 (CARVac) | Solid tumours (CLDN6 CAR T cells with CARVac) | 7 | With CLDN6 CAR T cells | Engraftment of CAR T cells in all patients | Four (57%) patients had partial response and one (14%) patient had stable disease at the 6-week evaluation16, 17 |
| Lipid nanoparticle formulation | |||||||
| NCT03480152 | 1/2 | Neoantigen-specific mRNA | Gastrointestinal cancer | 4 | None | Mutation-specific CD4+ and CD8+ T-cell responses against predicted neoepitopes in three (75%) of four patients | No objective clinical responses18 |
| NCT03313778 | 1 | Personalised cancer vaccine encoding several neoantigens | Solid tumours (resected) | 13 (monotherapy); 19 (combination) | With pembrolizumab | Detectable neoantigen T-cell responses | Vaccine monotherapy: 12 patients were cancer-free on study treatment with a median follow-up of 8 months; combination treatment: one patient had complete response before vaccination, two patients had partial response, five patients had stable disease, five had disease progression, and two had unconfirmed disease progression19 |
CAR=chimeric antigen receptor.
This Review summarises the latest clinical advances in therapeutic mRNA-based cancer vaccines, with a focus on direct mRNA administration methods.
mRNA-based cancer vaccine trials
The aim of mRNA-based vaccination is to induce or boost an effective anti-tumour immune response.22 Synthetic mRNA encoding tumour-associated or tumour-specific antigens is delivered through autologous dendritic cells engineered with mRNA ex vivo or through formulated or non-formulated mRNA injections.23 After vaccination and cellular uptake by antigen-presenting cells, mRNA is transported to the cytoplasm and undergoes antigen processing and enters the MHC presentation cascade. Thus, antigen-presenting cells present tumour-associated antigens on MHC class I and MHC class II to activate CD8+ and CD4+ T cells. In addition, CD4+ T cells can co-activate antigen-specific B cells and induce a humoral immune response. B cells that function as antigen-presenting cells can conversely activate CD4+ T cells after internalisation of extracellular proteins and presentation on B cells’ MHC class II (figure).9, 24
Several clinical trials (eg, NCT04534205, NCT03313778, and NCT04503278) are enrolling patients for various mRNA-based cancer vaccine therapy studies with the aim of inducing an mRNA-based anti-tumour response (Table 1, Table 2).
Non-formulated (naked) mRNA-based cancer vaccines
Naked or non-formulated mRNA vaccines contain mRNA molecules in a buffer solution.10 The non-formulated vaccines are administered either intradermally or intranodally.10, 25 The administration of non-formulated mRNA intranodally enables the delivery of antigens to antigen-presenting cells at the actual location of T-cell activation, thereby avoiding the requirement for antigen-presenting cell migration.26 Several studies have shown that dendritic cells can take up intranodally injected non-formulated mRNA and induce potent anti-tumour T-cell responses.7, 10
Only a few clinical trials have treated patients with cancer with non-formulated mRNA vaccines in the past 5 years (table 3). In a phase 1 clinical trial, non-formulated mRNA vaccines were administered intranodally in 13 patients with stage III or IV melanoma with stable disease, partial response, or complete response after previous treatment. This neoepitope-targeting vaccine encoded a unique and individualised tumour mutation signature with ten selected neoepitopes for each patient. All patients developed T-cell responses against numerous vaccine-encoded neoepitopes, and vaccine-related clinical responses were observed in two (40%) of the five patients with stage IV melanoma.10 In a recently concluded phase 1 clinical trial (NCT03394937), 20 patients with resected melanoma (stages IIc, III, and IV) received an intranodally injected non-formulated mRNA vaccine (ECI-006). The vaccine included mRNAs encoding three dendritic cell-activating molecules (TriMix) and five tumour-associated antigens (table 3).11 In a second study cohort (NCT03394937), patients with metastatic melanoma with stable disease after 3–12 months of standard treatment received the ECI-006 vaccine in combination with standard anti-PD-1 therapy; results are not yet published. No clinical trials registered at ClinicalTrials.gov are currently recruiting patients for non-formulated mRNA cancer vaccine treatment.
Formulated mRNA-based cancer vaccines
Non-formulated mRNA is easily degraded by extracellular RNases.27 Consequently, several nanocarrier pharmaceutical systems, generally containing polymers such as peptides or lipids, have been developed to optimise mRNA preservation and facilitate mRNA uptake by antigen-presenting cells.3, 28, 29
Protamine-formulated mRNA-based cancer vaccines
Protamines are positively charged polycationic peptides that form complexes with negatively charged mRNA and protect the molecules from degradation.30 Protamine-formulated mRNA vaccines have been evaluated in diverse clinical trials in the form of RNActive vaccines.12, 13, 14 RNActive vaccines incorporate nucleotide modified mRNA molecules complexed with protamine to enhance protein expression and immunogenicity.31 At the time of writing, no clinical trials registered at ClinicalTrials.gov are recruiting patients for protamine-formulated mRNA cancer vaccine studies.
An RNActive vaccine encoding six prostate cancer-specific antigens (CV9104) was investigated in a placebo-controlled phase 1/2 study in patients with metastatic castration-resistant prostate cancer. The vaccine was clinically safe for the patients but did not improve overall survival and progression-free survival compared with the placebo.12
The RNActive immunisation technology was also investigated in a phase 1/2 dose-escalation trial in patients with non-small-cell lung cancer (NSCLC). Patients had reached stable disease after first-line therapy before receiving the protamine-formulated mRNA vaccine (CV9201) encoding five NSCLC tumour-associated antigens. Seven patients with stage IIIb NSCLC and 39 patients with stage IV NSCLC received five intradermal injections of CV9201. The vaccines were well tolerated, and T-cell responses against at least one tumour-associated antigen were detected in 19 (63%) of 30 evaluable patients, but the therapy did not improve overall survival when compared with historical controls.13 A third phase 1b clinical trial evaluated an RNActive vaccine treatment in combination with local irradiation in patients with stage IV NSCLC. In this trial, RNActive encoding six tumour-associated antigens (CV9202; the five tumour-associated antigens used in CV9201 plus MUC-1) was administered to patients intradermally. The patients were divided into three strata according to their NSCLC pathology. Two of the three patient strata continued chemotherapy or tyrosine-kinase inhibitor treatment. The vaccine therapy was well tolerated, and CV9202 antigen-specific immunity was detected in 21 (84%) of 25 evaluable patients. One (3·8%) patient had a partial response and 12 (46·2%) of 26 evaluable patients reached stable disease across the three patient strata.14 The CV9202 vaccine was also evaluated in a recently completed two-arm phase 1/2 study (NCT03164772), in which patients with metastatic NSCLC received CV9202 in combination with either durvalumab (a PD-L1 antibody), or durvalumab plus tremelimumab (anti-CTLA-4 antibody); results are not yet published.
mRNA-based lipoplex vaccines
The mRNA lipoplex vaccine is a hybrid carrier combining a complex of mRNA with a polycationic component, within a lipid shell.32 Positively charged cationic lipids naturally form complexes with negatively charged mRNA and facilitate antigen-presenting cell endocytosis,33 and are therefore often used for the construction of lipoplexes.34 Ongoing mRNA lipoplex vaccine trials are listed in table 1. Clinical trial results are listed in table 3.
A phase 1 dose-escalation trial (NCT02410733) evaluated the safety and efficacy of an mRNA lipoplex vaccine (BNT111) encoding four melanoma tumour-associated antigens in patients with advanced melanoma expressing at least one of the four antigens. BNT111 was the first in the series of fixed combinations of shared cancer antigens (FixVac) mRNA vaccines that included a fixed combination of shared tumour-associated antigens. Immune responses against one or more tumour-associated antigens were detected in over 39 (75%) of 50 patients, and BNT111 induced both CD4+ and CD8+ T-cell responses. 17 patients received BNT111 plus standard anti-PD-1 therapy; six (35%) of these patients had a partial response and two (12%) patients reached stable disease. 25 patients were given single-agent BNT111, with three (12%) patients reaching partial response and seven (28%) patients reaching stable disease.15 FixVac BNT111 is being evaluated in a randomised phase 2 trial (NCT04526899), alone or in combination with the anti-PD-1 antibody cemiplimab, in patients with anti-PD-1-refractory or relapsed unresectable stage III and IV melanoma.
Multiple active clinical trials are assessing FixVac mRNA lipoplex vaccines. A phase 1/2, four-arm expansion trial (NCT04382898) is evaluating the cancer vaccine BNT112, encoding five tumour-associated antigens, alone or in combination with cemiplimab in patients with metastatic castration-resistant prostate cancer. The FixVac BNT115 encodes three ovarian-specific tumour-associated antigens and is being evaluated in a phase 1 study (NCT04163094), administered both before and in combination with adjuvant and neoadjuvant chemotherapies in patients with ovarian cancer. A randomised phase 2 clinical trial (NCT04534205) is evaluating the anti-human papillomavirus (HPV) -16-derived oncoprotein-encoding mRNA BNT113 in combination with the PD-1 inhibitor pembrolizumab in patients with HPV16-positive and PD-L1-positive head and neck squamous cell carcinoma. BNT113 is also being evaluated in a two-arm, phase 1/2 vaccine dose-escalation study (NCT03418480) in patients with previously treated HPV16-positive head and neck squamous cell carcinoma or advanced HPV16-positive head and neck squamous cell carcinoma. A fifth clinical trial (NCT05142189) is evaluating the FixVac vaccine BNT116 in combination with cemiplimab or docetaxel in a phase 1 study in patients with advanced or metastatic NSCLC.
In addition to FixVac vaccines, several studies are exploring the mRNA lipoplex vaccine platform called individualised neoantigen-specific immunotherapy (iNeST) or BNT122. iNeST includes mRNA lipoplex vaccines that encode individual tumour mutations, and the treatment is being evaluated in clinical trials across multiple solid tumour diagnoses (NCT03289962, NCT03815058, NCT04486378, and NCT04161755). iNeST is also being assessed in combination with another lipoplex-formulated mRNA encoding tumour-associated antigens (BNT114) and RNA encoding p53 in patients with triple-negative breast cancer (NCT02316457). Finally, an ongoing phase 1/2 clinical study (NCT04503278) is investigating a CLDN6-encoding mRNA lipoplex vaccine, CARvac, in patients with relapsed or refractory CLDN6-positive advanced solid tumours. CARVac is administered intravenously in combination with an autologous CLDN6 targeting CAR T-cell therapy, BNT211, and aims to improve CAR T-cell therapy. Early data showed that four (57%) of seven evaluable patients treated with CLDN6 CAR T-cell therapy and CARVac together had partial response and one (14%) patient had stable disease at the 6-week evaluation; ongoing responses were reported at the 12-week evaluation. Efficacy data showed no dose-limiting, drug-related serious adverse events in the evaluated patients. Manageable, low-grade cytokine release syndrome was observed in eight patients in total.16, 17
mRNA-based lipid nanoparticle vaccines
Lipid nanoparticle vaccines consist of ionisable lipids, cholesterol, phospholipids, and lipid-linked polyethylene glycol derivates.3 Cholesterol and phospholipids increase stability and support the bilayer structure of the lipid nanoparticle vaccines.35 Polyethylene glycol prevents mRNA-plasma protein binding, thereby increasing the nanoparticle's circulation period. Lipid nanoparticle vaccine properties are affected by changes in pH levels, which facilitate mRNA encapsulation and host-cell endocytosis.34 The mRNA nanoparticle vaccine carrier system recently gained attention following the approval of the first two lipid nanoparticle SARS-CoV-2 vaccines.36, 37 Ongoing mRNA nanoparticle vaccine trials are listed in table 1 and clinical trial results are listed in table 3.
Two lipid nanoparticle mRNA cancer vaccines encoding several neoantigens (mRNA-4157) are being assessed in combination with pembrolizumab as adjuvant therapy in patients with high-risk cutaneous melanoma following complete resection (NCT03313778 and NCT03897881). The same vaccine template (mRNA-4157) was examined as a monotherapy in patients with completely resected solid tumours (NCT03313778), and in combination with pembrolizumab in patients with unresectable solid tumours. The treatment induced neoantigen-specific T cells and did not lead to serious adverse events (grade 3 or worse). 13 patients received mRNA-4157 monotherapy, and all patients except one remained free of cancer on study treatment, with a median follow-up of 8 months. Of the 19 evaluable patients receiving combination treatment, one (5%) patient had a complete response before vaccination, two (11%) patients had a partial response, five (36%) patients had stable disease, five (36%) had confirmed disease progression, and two (11%) had unconfirmed disease progression.19 A phase 1/2 trial evaluated a neoantigen-specific mRNA nanoparticle vaccine in four patients with gastrointestinal cancer (table 3).18 A phase 1 clinical trial (NCT03948763) is now evaluating the lipid nanoparticle-based mRNA cancer vaccine mRNA-5671, which targets four KRAS mutations. The mRNA vaccine is either administered as monotherapy or in combination with pembrolizumab in patients with KRAS-mutated NSCLC, colorectal cancer, or pancreatic cancer (table 1).
mRNA-based dendritic cell cancer vaccines
Dendritic cells have been of particular interest in immune therapy approaches because of their unique ability not only to initiate immunity, but also to control and regulate the type of immune response, making them attractive candidates as vehicles for mRNA delivery.7, 38 Over the past 30 years, research has focused on generating an ex-vivo population of antigen-loaded dendritic cells that are able to stimulate robust and long-lasting CD8+ and CD4+ T-cell responses in patients with cancer.39 Of note, though, is the current inability to fully recapitulate the development of immunopotent dendritic cells ex vivo for effective anti-tumour immune responses.40 Obtaining the source of dendritic cells and the ex-vivo manipulation of them in addition to antigen preparation and loading are laborious and time-consuming compared with the manufacture of formulated and non-formulated mRNA vaccines.41 Ongoing mRNA dendritic cell vaccine clinical trials are listed in table 2.
In general, mRNA-loaded dendritic cell vaccines induce modest T-cell responses and have low clinical efficacy.42 However, some studies suggest that the mRNA-based dendritic vaccines can prevent or delay disease relapse and potentially prolong overall survival.20, 43 In the past 5 years of published trials, dendritic cell vaccines have been investigated in patients with various cancer diagnoses either as monotherapy or in combination with chemotherapy or immunotherapy. Patients with metastatic renal cell carcinoma were given dendritic cells loaded with amplified tumour RNA and mRNA encoding CD40L in combination with the tyrosine-kinase inhibitor sunitinib in a phase 3 trial;44 however, the vaccine did not significantly improve patient survival. In a phase 2 trial,45 patients with metastatic castration-resistant prostate cancer were given dendritic cells loaded with mRNA encoding tumour-associated antigens, but the vaccine did not significantly improve patient survival either. In a phase 2 trial, patients with advanced melanoma were given TriMix dendritic cells with tumour-associated antigen-encoding mRNA in combination with the anti-CTLA-4 antibody ipilimumab. Of the 39 treated patients, 15 (38%) reached either a partial response or a complete response with the combination therapy, but no direct comparison was made between responses to the two treatments.20 Patients with acute myeloid leukaemia in remission were vaccinated with dendritic cells loaded with a tumour-associated antigen-encoding mRNA. 5-year overall survival of the vaccinated patients compared favourably with historical controls, and prevention or delay of relapse was observed in 43% of patients.43 Finally, patients with glioblastoma multiforme were given dendritic cells loaded with mRNA encoding a cytomegalovirus antigen in different phase 1 clinical studies (eg, NCT00639639, NCT00626483, and NCT02529072). The choice of antigen was based on the high expression of cytomegalovirus proteins in glioblastomas. Trial results indicate long-lasting overall survival compared with non-transfected dendritic cell vaccines and historical controls.46, 47 Six active clinical trials are evaluating mRNA-based dendritic cell vaccines in different treatment combinations in patients with glioblastoma multiforme (NCT03688178, NCT02465268, NCT00639639, NCT04963413, NCT02649582, and NCT03548571; table 2).
Challenges and future perspectives for mRNA-based cancer vaccines
The number of clinical trials with therapeutic mRNA cancer vaccines is rapidly expanding, taking advantage of recent research advances that have optimised mRNA delivery, simplified administration methods, and improved translational efficiency.2, 3 Despite substantial progress, several challenges to mRNA vaccine immunogenicity and efficacy remain. Thus, one of the most important advances in therapeutic clinical cancer vaccines is the ability to identify individual cancer neoantigens. However, identifying tumour-specific mutations or non-conforming sequences and predicting corresponding neoepitopes for individual HLA alleles remains difficulut.48, 49 Furthermore, the technological and regulatory hurdles that will arise from the need for rapid and large-scale good manufacturing practice production of individualised mRNA vaccines are future obstacles that will need to be addressed.
Another challenge is to validate the most feasible vaccine administration methods. The administration route determines mRNA distribution and influences vaccine efficacy. mRNA that is injected intradermally and subcutaneously is easily processed by regional antigen-presenting cells, but the administrations often induce considerable local injection-site reactions.50 Intranasal administered mRNA reaches antigen-presenting cells in the peripheral lymph nodes, and intranodal injections reach lymphatic antigen-presenting cells directly, but the delivery methods are cumbersome and only allow for small injection volumes.7, 9 The same limitations apply to intratumoural injections, and this administration route primarily aims to induce local inflammation with mRNA encoding co-activating molecules.51 Muscle tissue is highly vascularised, contains diverse immune cells for mRNA processing, and intramuscular injection induces fewer injection-site reactions in general. Intramuscular administration is, therefore, a common and feasible vaccination route, and the current approved mRNA SARS-CoV-2 vaccines are administered intramuscularly.52, 53 Intravenous injections allow mRNA to reach numerous lymphoid organs, and this administration method has been shown to induce a robust CD8+ T-cell response compared with local injections.40, 54, 55 CD8+ T cells have a central role in anti-tumour responses, and intravenous injection is the most common direct administration route in active therapeutic mRNA cancer vaccine trials (table 1).
Most mRNA-based cancer vaccines are therapeutic rather than prophylactic, and require multiple administrations and substantial vaccine potency to induce a tumour response when given as monotherapy. Monotherapy mRNA-based vaccines could be an effective treatment for patients diagnosed with early-stage cancer or in an adjuvant setting, but it appears unlikely that the vaccines will succeed as a monotherapy treatment for advanced cancers because of challenges regarding the highly immunosuppressive tumour microenvironment of this setting.
Therapeutic mRNA cancer vaccines are more likely to succeed in combination with other immunotherapeutic treatment methods such as immune checkpoint inhibitors, oncolytic viruses, and adoptive cell therapy. Indeed, patients receiving these combinations show encouraging clinical treatment responses across cancer diagnoses.14, 17, 19, 20 There is a need for new treatment combinations that increase response rates and progression-free survival without inducing severe side-effects, and an mRNA cancer vaccine with low toxicity is an obvious combination partner. Several trials are already combining mRNA vaccines with checkpoint inhibitors (Table 1, Table 2, Table 3). Moderna (MA, USA) has recently expanded its vaccine development programme with a new checkpoint-targeting cancer vaccine, mRNA-4359. The mRNA vaccine encodes indoleamine 2,3-dioxygenase and PD-L1 antigens, and will be administered to patients with NSCLC and advanced or metastatic cutaneous melanoma.56 In addition, BioNTech (Mainz, Germany)combines the mRNA-based FixVac platform with cemiplimab for patients with various cancer diagnoses (ie, NCT04526899, NCT04382898, and NCT05142189). Together with Regeneron (NY, USA), BioNTech is now planning a phase 1/2 trial for a first-line treatment for late-stage NSCLC.57
The future for therapeutic mRNA-based cancer vaccines is promising. Many clinical trials of mRNA vaccines are still early phase studies, but the field is moving fast. For example, in November, 2021, BioNTech received an FDA Fast Track Designation for BNT111 in patients with stage III or IV melanoma on the basis of the phase 1 trial results listed in table 3 (NCT02410733).15, 58
Conclusion
The rapid development and worldwide approval of mRNA vaccines against SARS-CoV-2 have showcased the vast potential of mRNA technology.36, 37 The response to the COVID-19 pandemic has leveraged data from years of research to improve the design of therapeutic mRNA cancer vaccines.1 Results from early clinical trials have shown only modest indications of clinical efficacy. However, with the optimisation of mRNA vaccine structure, stability, and delivery methods, and with the associated advantages of personalised preparations, low manufacturing costs, and the fast and scalable production required for a patient group that often experiences rapid disease progression (panel), mRNA vaccines are reaching their potential as a future crucial strategy for cancer treatment.
Search strategy and selection criteria
References were found through searches on PubMed with the Medical Subject Headings terms “RNA” or “RNA, Messenger” and “cancer vaccines” for articles published between Jan 1, 2017, and Jan 1, 2022. For tables 1 and 2, trials with recruitment status “not yet recruiting,” “recruiting,” and “active, not recruiting” were found on ClinicalTrials.gov. with the search terms “cancer” and “RNA, vaccine” on Feb 7, 2022, and through a PubMed search. A 5-year period was chosen to focus solely on the most recent clinical studies and patient data. All human clinical trials published in English with a full journal text available were reviewed. The additional references were relevant references from the selected search results and applicable abstracts based on clinical mRNA cancer vaccine trials.
This online publication has been corrected. The corrected version first appeared at thelancet.com/oncology on October 7, 2022
Declaration of interests
IMS reports having lectured for or having had advisory board relationships with Bristol Myers Squibb, MSD, Sanofi Aventis, Pierre Fabre, IO Biotech, Novartis, TILT Biotherapeutics, and Novo Nordisk; research grants from Bristol Myers Squibb, Adaptimmune, Lytix Biopharma, IO Biotech, and TILT Biotherapeutics; and is a co-founder and shareholder for IO Biotech, a company that is developing peptide vaccines targeting immune regulation. IO Biotech is a spin-out of Copenhagen University Hospital and has no approved products at the time of writing; an IDO and PD-L1 targeting peptide vaccine for melanoma has been granted Breakthrough Therapy designation by the FDA and is being tested in a phase 3 trial (NCT05155254). The same vaccine is also in phase 2 trials (NCT05077709 and NCT05280314) in other cancer types (including bladder cancer and head and neck cancer). An additional peptide vaccine targeting arginase is being tested in solid cancers in a phase 1 trial. IO Biotech has no interests in RNA vaccines. JBH reports research grants from Amgen, Asher Bio, BioNTech, Bristol Myers Squibb, MSD, and Novartis; had advisory board relationships with Achilles Therapeutics, BioNTech, Bristol Myers Squibb, Ipsen, Iovance Bio, Instil Bio, MSD, Merck Serono, Neogene Therapeutics, Novartis, Pfizer, PokeAcel, Roche, Sanofi, and T-Knife; and holds stock options in Neogene Therapeutics, a company that is developing T-cell receptor gene modified T cells targeting neoantigens. The first neoantigen-specific T-cell receptor gene therapy for the treatment of solid cancers is planned for testing in a phase 1 clinical trial. Neogene Therapeutics has no interests in RNA vaccines. CLL and ÖM declare no competing interests.
Contributors
CLL did the literature and clinical trial searches, created the tables and figure, and wrote the original draft. JBH, ÖM, and IMS contributed to the writing of the original draft and revisions.
References
- 1.Dolgin E. The tangled history of mRNA vaccines. Nature. 2021;597:318–324. doi: 10.1038/d41586-021-02483-w. [DOI] [PubMed] [Google Scholar]
- 2.Karikó K, Muramatsu H, Welsh FA, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16:1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan S, Rosenecker J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017;24:133–143. doi: 10.1038/gt.2017.5. [DOI] [PubMed] [Google Scholar]
- 4.Thess A, Grund S, Mui BL, et al. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol Ther. 2015;23:1456–1464. doi: 10.1038/mt.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowalzik F, Schreiner D, Jensen C, Teschner D, Gehring S, Zepp F. mRNA-based vaccines. Vaccines. 2021;9:390. doi: 10.3390/vaccines9040390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faghfuri E, Pourfarzi F, Faghfouri AH, Abdoli Shadbad M, Hajiasgharzadeh K, Baradaran B. Recent developments of RNA-based vaccines in cancer immunotherapy. Expert Opin Biol Ther. 2021;21:201–218. doi: 10.1080/14712598.2020.1815704. [DOI] [PubMed] [Google Scholar]
- 7.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines— a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahin U, Derhovanessian E, Miller M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 11.Arance Fernandez AM, Baurain J-F, Vulsteke C, et al. A phase I study (E011-MEL) of a TriMix-based mRNA immunotherapy (ECI-006) in resected melanoma patients: analysis of safety and immunogenicity. J Clin Oncol. 2019;37 [Google Scholar]
- 12.Stenzl A, Feyerabend S, Syndikus I, et al. Results of the randomized, placebo-controlled phase I/IIB trial of CV9104, an mRNA based cancer immunotherapy, in patients with metastatic castration-resistant prostate cancer (mCRPC) Ann Oncol. 2017;28:v408–v409. [Google Scholar]
- 13.Sebastian M, Schröder A, Scheel B, et al. A phase I/IIa study of the mRNA-based cancer immunotherapy CV9201 in patients with stage IIIB/IV non-small cell lung cancer. Cancer Immunol Immunother. 2019;68:799–812. doi: 10.1007/s00262-019-02315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papachristofilou A, Hipp MM, Klinkhardt U, et al. Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. J Immunother Cancer. 2019;7:38. doi: 10.1186/s40425-019-0520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahin U, Oehm P, Derhovanessian E, et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585:107–112. doi: 10.1038/s41586-020-2537-9. [DOI] [PubMed] [Google Scholar]
- 16.Mackensen A, Koenecke C, Haanen J, et al. BNT211: a phase I/II trial to evaluate safety and efficacy of CLDN6 CAR-T cells and vaccine-mediated in vivo expansion in patients with CLDN6-positive advanced solid tumors. J Immunother Cancer. 2021;9 [Google Scholar]
- 17.Haanen J, Gunther J, Orleans NEW, et al. BNT211: a phase I trial to evaluate safety and efficacy of CLDN6 CAR-T cells and CARVac-mediated in vivo expansion in patients with CLDN6-positive advanced solid tumors. 2022. https://www.abstractsonline.com/pp8/#!/10517/presentation/20144
- 18.Cafri G, Gartner JJ, Zaks T, et al. mRNA vaccine–induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J Clin Invest. 2020;130:5976–5988. doi: 10.1172/JCI134915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burris HA, Patel MR, Cho DC, et al. A phase I multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in patients with resected solid tumors and in combination with pembrolizumab in patients with unresectable solid tumors. J Clin Oncol. 2019;37 [Google Scholar]
- 20.De Keersmaecker B, Claerhout S, Carrasco J, et al. TriMix and tumor antigen mRNA electroporated dendritic cell vaccination plus ipilimumab: link between T-cell activation and clinical responses in advanced melanoma. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20:41. doi: 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beck JD, Reidenbach D, Salomon N, et al. mRNA therapeutics in cancer immunotherapy. Mol Cancer. 2021;20:69. doi: 10.1186/s12943-021-01348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019;4:7. doi: 10.1038/s41541-019-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong S, Zhang Z, Liu H, et al. B cells are the dominant antigen-presenting cells that activate naive CD4+ T cells upon immunization with a virus-derived nanoparticle antigen. Immunity. 2018;49:695–708.e4. doi: 10.1016/j.immuni.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Rittig SM, Haentschel M, Weimer KJ, et al. Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients. Mol Ther. 2011;19:990–999. doi: 10.1038/mt.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diken M, Kreiter S, Selmi A, et al. Selective uptake of naked vaccine RNA by dendritic cells is driven by macropinocytosis and abrogated upon DC maturation. Gene Ther. 2011;18:702–708. doi: 10.1038/gt.2011.17. [DOI] [PubMed] [Google Scholar]
- 27.Tsui NBY, Ng EKO, Lo YMD. Stability of endogenous and added RNA in blood specimens, serum, and plasma. Clin Chem. 2002;48:1647–1653. [PubMed] [Google Scholar]
- 28.Midoux P, Pichon C. Lipid-based mRNA vaccine delivery systems. Expert Rev Vaccines. 2015;14:221–234. doi: 10.1586/14760584.2015.986104. [DOI] [PubMed] [Google Scholar]
- 29.Reichmuth AM, Oberli MA, Jaklenec A, Langer R, Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther Deliv. 2016;7:319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng C, Zhang C, Walker PG, Dong Y. Formulation and delivery technologies for mRNA vaccines. Curr Top Microbiol Immunol. 2020;2:1–40. doi: 10.1007/82_2020_217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kallen K-J, Heidenreich R, Schnee M, et al. A novel, disruptive vaccination technology: self-adjuvanted RNActive(®) vaccines. Hum Vaccin Immunother. 2013;9:2263–2276. doi: 10.4161/hv.25181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Persano S, Guevara ML, Li Z, et al. Lipopolyplex potentiates anti-tumor immunity of mRNA-based vaccination. Biomaterials. 2017;125:81–89. doi: 10.1016/j.biomaterials.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malone RW, Felgner PL, Verma IM. Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci USA. 1989;86:6077–6081. doi: 10.1073/pnas.86.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wasungu L, Hoekstra D. Cationic lipids, lipoplexes and intracellular delivery of genes. J Control Release. 2006;116:255–264. doi: 10.1016/j.jconrel.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 35.Kowalski PS, Rudra A, Miao L, Anderson DG. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol Ther. 2019;27:710–728. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol. 1998;161:1313–1319. [PubMed] [Google Scholar]
- 39.Benteyn D, Heirman C, Bonehill A, Thielemans K, Breckpot K. mRNA-based dendritic cell vaccines. Expert Rev Vaccines. 2015;14:161–176. doi: 10.1586/14760584.2014.957684. [DOI] [PubMed] [Google Scholar]
- 40.Kranz LM, Diken M, Haas H, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 41.Perez CR, De Palma M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat Commun. 2019;10 doi: 10.1038/s41467-019-13368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smits ELJM, Anguille S, Cools N, Berneman ZN, Van Tendeloo VFI. Dendritic cell-based cancer gene therapy. Hum Gene Ther. 2009;20:1106–1118. doi: 10.1089/hum.2009.145. [DOI] [PubMed] [Google Scholar]
- 43.Anguille S, Van de Velde AL, Smits EL, et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood. 2017;130:1713–1721. doi: 10.1182/blood-2017-04-780155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Figlin RA, Tannir NM, Uzzo RG, et al. Results of the ADAPT phase 3 study of rocapuldencel-T in combination with sunitinib as first-line therapy in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2020;26:2327–2336. doi: 10.1158/1078-0432.CCR-19-2427. [DOI] [PubMed] [Google Scholar]
- 45.Kongsted P, Borch TH, Ellebaek E, et al. Dendritic cell vaccination in combination with docetaxel for patients with metastatic castration-resistant prostate cancer: a randomized phase II study. Cytotherapy. 2017;19:500–513. doi: 10.1016/j.jcyt.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Batich KA, Mitchell DA, Healy P, Herndon JE, Sampson JH. Once, twice, three times a finding: reproducibility of dendritic cell vaccine trials targeting cytomegalovirus in glioblastoma. Clin Cancer Res. 2020;26:5297–5303. doi: 10.1158/1078-0432.CCR-20-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peters KB, Archer GE, Norberg P, et al. Safety of nivolumab in combination with dendritic cell vaccines in recurrent high-grade glioma. J Clin Oncol. 2019;37 [Google Scholar]
- 48.Bidram M, Zhao Y, Shebardina NG, et al. mRNA-based cancer vaccines: a therapeutic strategy for the treatment of melanoma patients. Vaccines. 2021;9 doi: 10.3390/vaccines9101060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gubin MM, Artyomov MN, Mardis ER, Schreiber RD. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J Clin Invest. 2015;125:3413–3421. doi: 10.1172/JCI80008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He Q, Gao H, Tan D, Zhang H, Wang J. mRNA cancer vaccines: advances, trends and challenges. Acta Pharm Sin B. 2022 doi: 10.1016/j.apsb.2022.03.011. https://www.sciencedirect.com/science/article/pii/S2211383522001198?via%3Dihub published online March 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haabeth OAW, Blake TR, McKinlay CJ, et al. Local delivery of OX40L, CD80, and CD86 mRNA kindles global anticancer immunity. Cancer Res. 2019;79:1624–1634. doi: 10.1158/0008-5472.CAN-18-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moyer TJ, Zmolek AC, Irvine DJ. Beyond antigens and adjuvants: formulating future vaccines. J Clin Invest. 2016;126:799–808. doi: 10.1172/JCI81083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas KS. Intramuscular injections for COVID-19 vaccinations. J Nucl Med Technol. 2021;49:11–12. doi: 10.2967/jnmt.121.262049. [DOI] [PubMed] [Google Scholar]
- 54.Broos K, Van der Jeught K, Puttemans J, et al. Particle-mediated intravenous delivery of antigen mRNA results in strong antigen-specific T-cell responses despite the induction of type I interferon. Mol Ther Nucleic Acids. 2016;5:e326. doi: 10.1038/mtna.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sayour EJ, De Leon G, Pham C, et al. Systemic activation of antigen-presenting cells via RNA-loaded nanoparticles. Oncoimmunology. 2017;6 doi: 10.1080/2162402X.2016.1256527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moderna Moderna research product pipeline. https://www.modernatx.com/research/product-pipeline?
- 57.BioNTech and Regeneron expand strategic collaboration to advance clinical development of FixVac and Libtayo® (cemiplimab) combination in NSCLC. https://investors.biontech.de/news-releases/news-release-details/biontech-and-regeneron-expand-strategic-collaboration-advance/ No authors listed.
- 58.BioNTech receives FDA fast track designation for its FixVac candidate BNT111 in advanced melanoma. https://investors.biontech.de/news-releases/news-release-details/biontech-receives-fda-fast-track-designation-its-fixvac No authors listed.