Abstract
Purpose
The objective of this study was to explore the diagnostic and prognostic value of interleukin-6 (IL-6) in sepsis patients presenting to the emergency department.
Patients and Methods
A total of 128 patients who visited the emergency department of West Hospital of Beijing Chaoyang Hospital, affiliated to Capital Medical University, from November 2021 to February 2022 were subjected to this study. According to Sepsis-3.0 diagnostic criteria for sepsis, patients were divided into non-sepsis group (65 cases) and sepsis group (63 cases). Demographic data and clinical characteristics of the two patient groups were compared. Serum levels of biomarkers including IL-6, blood urea nitrogen (BUN), and lactic acid (Lac) were compared with Sequential Organ Failure Assessment (SOFA) and Glasgow Coma Scale (GCS) scores. Logistic regression was used to analyze independent risk factors and Receiver Operating Characteristic Curve (ROC) method was used to analyze the Area Under the Curve (AUC) to determine the diagnostic and prognostic value of markers.
Results
Compared with non-sepsis patients, levels of IL-6, PCT, CRP and BUN were significantly higher in sepsis patients (10.84 (4.41–27.01): 92.22 (21.53–201.12), 0.03 (0.01–0.1):0.49 (0.08–3.1), 8.3 (0.5–31.8):39.8(10.3–98.6), 7.01 (4.90–11.74):13.03 (6.93–25.99), all p = 0.001). IL-6, BUN and mean arterial pressure (MAP) were independent risk factors for sepsis diagnosis. AUC values of IL-6, BUN, MAP and IL-6+BUN+MAP were 0.764, 0.696, 0.685, and 0.848, respectively. Lactate, age and SOFA score were independent risk factors for 28-day mortality in sepsis patients. The AUC of Lac, age, SOFA score and Lac+age+SOFA score to predict 28-day death in sepsis patients was 0.679, 0.626, 0.747, and 0.819, respectively.
Conclusion
IL-6 is an independent predictor of sepsis diagnosis, and the combination of blood BUN and MAP has superior diagnostic performance. Lac, age, and SOFA score could effectively predict clinical outcomes in patients with sepsis.
Keywords: interleukin-6, emergency department, Lac, sepsis, SOFA score, mortality
Introduction
Sepsis is a complex disease syndrome characterized by disturbances in the body’s immune, endocrine, and metabolic responses to infection that lead to multiple organ failure, shock, and death. According to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3.0), defined sepsis as a life-threatening organ dysfunction caused by a dysregulated host response to infection. Despite advances in modern medicine, the mortality rate in patients with sepsis is still high, at an estimated 25–30%.2 Therefore, early identification and diagnosis are crucial, as timely intervention and treatment can improve prognosis. Various biomarkers have been studied, including C-reactive protein (CRP) and procalcitonin (PCT), for the diagnosis of sepsis, but their diagnostic potential3 and prognostic value4 are limited. Therefore, new biomarkers or a combination of multiple markers need to be discovered for rapid diagnosis of sepsis and prediction of clinical outcomes. Sepsis is caused by a complex response to systemic inflammation driven by multiple elevated blood biomarkers, including immune cells and cytokines, such as White Blood Cell (WBC) and interleukin 6 (IL-6). This article aimed to determine the diagnostic value of IL-6 in sepsis and its association with 28-day mortality.
Research Objects and Methods
Research Subjects
In total, 128 critically ill patients who were admitted to the Emergency Department of West Hospital of Beijing Chaoyang Hospital Affiliated to Capital Medical University from November 2021 to February 2022 were subjected to this study.
Exclusion Criteria
Pregnancy; patients with malignant tumors, hematological diseases, connective tissue diseases or receiving immunosuppressive therapy.
Ethics
This study complied with the Declaration of Helsinki. The research protocol was approved by Ethics Committee of Beijing Chaoyang Hospital Affiliated to Capital Medical University (approval number: 2021-ke-636). Informed consent was obtained from all patients or their families before receiving treatments and tests.
Grouping
Enrolled patients were grouped according to the Sepsis-3 sepsis criteria. On the basis of infection and Sequential Organ Failure Assessment (SOFA) score ≥2, patients were classified into non-septic symptom group (65 cases) and sepsis group (63 cases).
Data Collection
Patient age, gender, past medical history, vital signs and laboratory and imaging findings. Quick sepsis-related organ failure assessment (qSOFA), SOFA, acute physiology and chronic health evaluation II (APHCHE II) and Glasgow coma were calculated based on clinical data obtained within 24 hours of admission with Glasgow Coma Scale (GCS) as the scoring method (All blood samples were collected from patients upon arrival in the emergency room. IL was determined using Point of Care Testing (POCT) detector (automatic quantum dot fluorescence immunoassay analyzer QD-S1200, Nanjing Novizan Biotechnology Co., Ltd. Nanjing) and corresponding kits. Cardiac troponin I (cTnI), PCT, D-Dimer (D-D) concentrations were measured. Negative results were defined as cTnI <0.5ng/mL, D-Dimer <0.5mg/l, PCT <0.5pg/mL, and IL-6 <7pg/mL. Lac level was detected using automatic blood gas analyzer, with Lac ≥2 mmol/L taken as a positive indicator of sepsis. Other relevant indicators were determined through routine laboratory tests. All cases were followed up by telephone and the 28-day survival of the patients was recorded.
Statistical Method
SPSS 26.0 software was used for data analysis. Normally distributed data were expressed as mean ± standard deviation (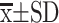 ). Independent samples test was used for comparison of variable between two groups. For comparison of multiple groups, one-way analysis of variance was used. Count data were compared using the χ2 test. Logistic regression was used to analyze the independent risk factors for 28-day mortality. The receiver operator characteristic curve (ROC) was used to evaluate the diagnostic efficacy of biomarkers for sepsis. Pearson correlation coefficient analysis method was used for correlation analysis. The area under the ROC curve was used to evaluate the predictive ability of biomarkers for 28-day mortality, and the Z test was used for comparison. Two-sided test p < 0.05 was considered statistically significant.
). Independent samples test was used for comparison of variable between two groups. For comparison of multiple groups, one-way analysis of variance was used. Count data were compared using the χ2 test. Logistic regression was used to analyze the independent risk factors for 28-day mortality. The receiver operator characteristic curve (ROC) was used to evaluate the diagnostic efficacy of biomarkers for sepsis. Pearson correlation coefficient analysis method was used for correlation analysis. The area under the ROC curve was used to evaluate the predictive ability of biomarkers for 28-day mortality, and the Z test was used for comparison. Two-sided test p < 0.05 was considered statistically significant.
Results
Comparison of Baseline Patient Data Between the Two Groups (Table 1)
Table 1.
Comparison of Baseline Patient Data Between the Non-Sepsis Group and Sepsis Group
| Demographic Characteristics | Non-Septic (n=65) | Sepsis (n=63) | Total (n=128) | P value |
|---|---|---|---|---|
| Age, median (range) | 70(30–90) | 79(34–95) | 72(30–95) | 0.004** |
| Sex, Male, n (%) | 44(67.7) | 40(63.5) | 84(65.6) | 0.511 |
| MAP (mmHg) | 106.47(21.06) | 92.14(23.07) | 97.5(84.75–114) | 0.000*** |
| Comorbidities | ||||
| Acute cerebrovascular disease, n (%) | 19(29.2) | 23(36.5) | 42 (32.8) | 0.686 |
| Diabetes mellitus, n (%) | 22(33.8) | 20(31.7) | 42 (32.8) | 0.220 |
| Cardiovascular disease, n (%) | 28(43.1) | 6(9.5) | 34 (26.6) | 0.001** |
| Chronic lung disease, n (%) | 7(10.8) | 14(22.2) | 21 (16.4) | 0.246 |
| Use of vasopressors, n (%) | 3(4.6) | 19(30.2) | 22 (17.7) | 0.008** |
| Use of mechanical ventilation, n (%) | 8(12.3) | 24(38.1) | 32 (25) | 0.142 |
| APHACHE II | 29(16–39) | 33.56(11.91) | 33(20–41) | 0.054 |
| qSOFA | 0(0–1) | 1(0–1) | 1(0–1) | 0.006** |
| SOFA | 1.75(1.5) | 5(3–6) | 3.3(2.36) | 0.000*** |
| GCS | 15(15–15) | 14(12–15) | 15(13–15) | 0.002** |
| Laboratory value | ||||
| PCT (ng/mL) | 0.03(0.01–0.1) | 0.49(0.08–3.1) | 0.09(0.02–0.57) | 0.000*** |
| CRP (mg/dl) | 8.3(0.5–31.8) | 39.8(10.3–98.6) | 23.1(2.08–69.53) | 0.000*** |
| Lac (mmol/L) | 1.25(0.83–1.88) | 1.5(1–2.8) | 1.4(0.9–2.2) | 0.256 |
| Creatinine (umol/L) | 75.3(63–88.85) | 88.8(55.5–124.7) | 78.7(60.05–101.18) | 0.170 |
| Bilirubin (umol/L) | 16.38 (14.63) | 15.9(9.5–23) | 14.95(9.7–21.73) | 0.147 |
| Platelets (× 1000/μL) | 208.85 (60.59) | 189(124–263) | 206.5(150–255.75) | 0.571 |
| IL-6 (pg/mL) | 10.84(4.41–27.01) | 92.22(21.53–201.12) | 23.09(8.32–115.41) | 0.000*** |
| WBC (×109个/L) | 10.2 (4.25) | 11.1(7–15.75) | 10(6.85) | 0.125 |
| BUN (mmol/L) | 7.01(4.90–11.74) | 13.03(6.93–25.99) | 9.71(5.45–19.19) | 0.001** |
Notes: **Means p < 0.01, ***Means p < 0.001. Normally distributed data are expressed as mean ± standard deviation; skewed distributed data are expressed as median and quartile.
Abbreviations: SD, Standard Deviation; MAP, Mean Arterial Pressure; qSOFA, Rapid Sequential Organ Failure Score; SOFA, Sequential Organ Failure Score; APHCHE II, Acute Physiology and Chronic Health Assessment II; GCS, Glasgow Coma Scale; PCT, procalcitonin; CRP, C-reactive protein; IL-6, interleukin-6; WBC, white blood cell count.
Comparison of age, gender, comorbidities, scores and biomarkers between the non-sepsis group and sepsis group. In this study, a total of 128 patients were enrolled, including 63 patients with sepsis. Baseline characteristics of these patients (Table 1), including gender, APHACHE II score, and use of mechanical ventilation were not statistically different between the two groups. Patients with sepsis were generally older, had lower mean arterial pressure (MAP), and were more likely to use vasoactive drugs than non-septic patients. Patients with sepsis had significantly higher SOFA scores than non-septic patients. Among biomarkers, IL-6, PCT, CRP, and BUN were significantly elevated in sepsis patients compared with non-sepsis patients (all p = 0.001). However, there was no statistically significant differences in the levels of lactate (p = 0.256), creatinine (p = 0.17), bilirubin (p = 0.147), platelets (p = 0.571) and WBC (p = 0.125) between the two groups.
Logistic Regression Analysis of Biomarkers to Predict Sepsis
SOFA, IL-6, GCS and urea nitrogen had diagnostic efficacy for sepsis (all p < 0.05), indicating that these biomarkers were independent risk factors for sepsis (Table 2).
Table 2.
Logistic Regression Analysis of Blood-Based Biomarkers for Predicting Sepsis
| Independent Predictors | β | SE | Wald | P value | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| SOFA | 1.326 | 0.503 | 6.944 | 0.008** | 3.767 | 1.405 | 10.102 |
| GCS | 0.507 | 0.245 | 4.266 | 0.039* | 1.66 | 1.026 | 2.684 |
| IL-6 | 0.029 | 0.012 | 5.673 | 0.017* | 1.029 | 1.005 | 1.054 |
| qSOFA | 0.807 | 0.928 | 0.757 | 0.384 | 2.241 | 0.364 | 13.811 |
| D-D | −0.253 | 0.19 | 1.772 | 0.183 | 0.776 | 0.535 | 1.127 |
| PCT | −0.03 | 0.43 | 0.005 | 0.944 | 0.97 | 0.418 | 2.254 |
| BUN | 0.079 | 0.039 | 3.999 | 0.046* | 1.082 | 1.002 | 1.169 |
| Constant | −13.237 | 5.054 | 6.859 | 0.009 | 0 | ||
Notes: *Means p < 0.05, **Means p < 0.01.
Abbreviations: CI, confidence interval; OR, odds ratio; IL-6, interleukin-6; PCT, procalcitonin; D-D, D-dimer; qSOFA, Quick Sequential Organ Failure Assessment; blank means none.
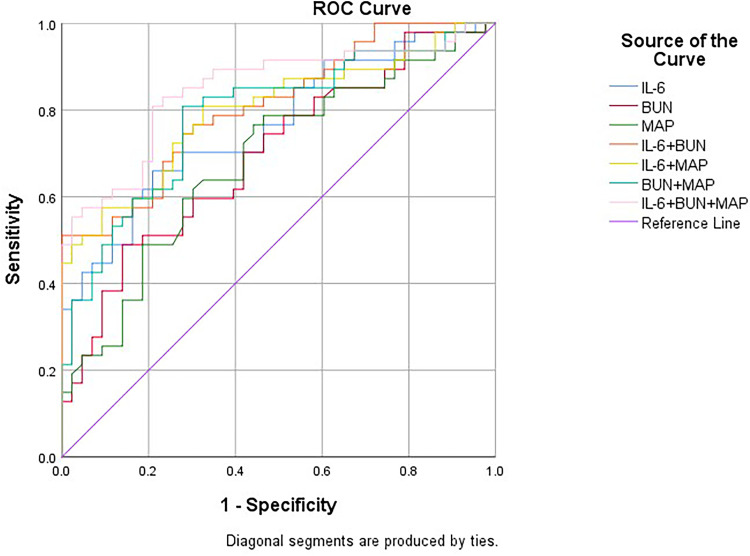
ROC Curve Analysis of Sepsis Diagnostic Biomarkers (Figure 1)
Figure 1.
Receiver operating characteristic (ROC) curves of biomarkers for the diagnosis of sepsis.
ROC curve analysis showed that IL-6 had a significantly higher predictive efficiency than urea nitrogen and MAP. A composite method including all three indicators of sepsis can have a better diagnostic efficiency. The ROC curve analysis results are shown in Table 3 and Figure 1. The value of these biomarkers in the diagnosis of sepsis is reflected by their high sensitivity and specificity. Specifically, IL-6, urea nitrogen and MAP had a sensitivity of 68%, 49% and 75%, respectively, and specificity of 83%, 86% and 55%, respectively. When combined, the combination of IL-6, urea nitrogen and MAP had a higher diagnostic efficiency (Z = 2.03, p = 0.02) than individual biomarkers, with sensitivity and specificity of 81% and 79%, respectively (Table 3). Pearson correlation analysis showed that IL-6 was positively correlated with SOFA score (p < 0.01) (Table 4).
Table 3.
Diagnostic Value of Serum IL-6, BUN and MAP for Sepsis
| Independent Predictors | AUC | 95% CI | P value | Cutoff | Sensitivity | Specificity | Youden Index | Positive Likelihood Ratio | Negative Likelihood Ratio | |
|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 | 0.764 | 0.668 | 0.861 | 0*** | 49.01 | 0.683 | 0.828 | 0.511 | 3.971 | 0.383 |
| BUN | 0.696 | 0.589 | 0.804 | 0.001** | 14.015 | 0.489 | 0.864 | 0.353 | 3.596 | 0.591 |
| MAP | 0.685 | 0.575 | 0.795 | 0.003** | 102.833 | 0.746 | 0.554 | 0.3 | 1.673 | 0.458 |
| IL-6+ BUN | 0.809 | 0.722 | 0.895 | 0*** | 0.74 | 0.511 | 1 | 0.511 | 0.489 | |
| IL-6+MAP | 0.798 | 0.706 | 0.89 | 0*** | 0.358 | 0.825 | 0.734 | 0.559 | 3.102 | 0.238 |
| BUN +MAP | 0.786 | 0.692 | 0.881 | 0*** | 0.425 | 0.809 | 0.727 | 0.536 | 2.963 | 0.263 |
| IL-6+ BUN +MAP | 0.848 | 0.766 | 0.929 | 0*** | 0.467 | 0.809 | 0.791 | 0.6 | 3.871 | 0.241 |
Notes: **Means p < 0.01, ***Means p < 0.001.
Abbreviations: IL-6, interleukin-6; CI, confidence interval; OR, odds ratio; blank means no item.
Table 4.
Pearson Correlation Analysis of IL-6 and Other Biomarkers
| Variable | IL-6 | |
|---|---|---|
| r | P | |
| SOFA | 0.341 | <0.01 |
| qSOFA | 0.174 | >0.05 |
| PCT | −0.018 | >0.05 |
| CRP | 0.221 | <0.05 |
| WBC | 0.061 | >0.05 |
Abbreviations: SOFA, Sequential Organ Failure Assessment; qSOFA, Quick Sequential Organ Failure Assessment; PCT, procalcitonin; CRP, C-reactive protein; IL-6, interleukin-6; WBC, white blood cell count; r, Pearson correlation coefficient.
Biomarkers Predict 28-Day Mortality in Sepsis Patients
In this study, there were 63 patients with sepsis. Univariate regression analysis results showed (Table 5) that age, GCS, WBC, BUN, SOFA score, and qSOFA score were statistically significant. Logistic regression analysis showed that lactate, age and SOFA score were independent risk factors for 28-day death in sepsis patients (p < 0.05) (Table 6).
Table 5.
Univariate Regression Analysis of Biomarkers for Predicting 28-Day Mortality in Patients with Sepsis
| Independent Predictors | F | Significance | t | df | P value | Mean Difference | Sandard Error | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|
| Age | 1.81 | 0.183 | 2.368 | 61 | 0.021* | −8.694 | 3.671 | −16.035 | −1.354 |
| Sex | 0.485 | 0.489 | 0.257 | 61 | 0.798 | −0.038 | 0.149 | −0.337 | 0.26 |
| MAP | 0.289 | 0.593 | 0.138 | 61 | 0.89 | 0.913 | 6.601 | −12.287 | 14.114 |
| APHACHE II | 0.056 | 0.814 | 0.705 | 43 | 0.485 | −2.719 | 3.856 | −10.496 | 5.058 |
| qSOFA | 23.236 | 0 | 4.554 | 41.207 | 0.000*** | −0.604 | 0.133 | −0.872 | −0.336 |
| SOFA | 1.185 | 0.281 | 2.839 | 61 | 0.006** | −1.522 | 0.536 | −2.593 | −0.45 |
| PCT | 1.925 | 0.17 | 1.124 | 61 | 0.266 | −1.215 | 1.081 | −3.377 | 0.947 |
| CRP | 0.192 | 0.663 | 0.709 | 45 | 0.482 | 16.433 | 23.187 | −63.133 | 30.268 |
| Lac | 2.392 | 0.128 | 1.879 | 51 | 0.066 | −1.1 | 0.585 | −2.274 | 0.075 |
| Creatinine | 1.013 | 0.318 | 1.044 | 61 | 0.3 | 27.855 | 26.672 | −81.189 | 25.48 |
| Bilirubin | 0.296 | 0.589 | 0.28 | 61 | 0.78 | 1.496 | 5.334 | −9.171 | 12.162 |
| GCS | 1.183 | 0.281 | 3.151 | 61 | 0.003** | 2.398 | 0.761 | 0.876 | 3.919 |
| IL-6 | 0.111 | 0.74 | 0.394 | 61 | 0.695 | 36.68 | 93.029 | 222.704 | 149.343 |
| WBC | 2.44 | 0.126 | 3.003 | 43 | 0.004** | −5.927 | 1.974 | −9.908 | −1.947 |
| BUN | 3.085 | 0.086 | 2.785 | 45 | 0.008** | 15.551 | 5.584 | −26.797 | −4.304 |
Notes: *Means p < 0.05, **Means p < 0.01, ***Means p < 0.001.
Abbreviations: MAP, mean arterial pressure; qSOFA, Quick Sequential Organ Failure Assessment; SOFA, Sequential Organ Failure Score; APHCHE II, Acute Physiology and Chronic Health Assessment II; GCS, Glasgow Coma Scale; PCT, Procalcitonin; CRP, C-reactive protein; IL-6, interleukin-6; WBC, white blood cell count; Lac, lactate; CI, confidence interval.
Table 6.
Logistic Regression Analysis of Biomarkers for Predicting 28-Day Mortality in Patients with Sepsis
| Independent Predictors | β | SE | Wald | df | P value | OR | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| SOFA | 0.694 | 0.305 | 5.156 | 1 | 0.023* | 2.001 | 1.1 | 3.641 |
| IL-6 | −0.001 | 0.001 | 0.57 | 1 | 0.45 | 0.999 | 0.996 | 1.002 |
| Lac | 0.519 | 0.218 | 5.64 | 1 | 0.018* | 1.68 | 1.095 | 2.577 |
| BUN | 0.033 | 0.026 | 1.632 | 1 | 0.201 | 1.034 | 0.982 | 1.088 |
| Agw | 0.131 | 0.059 | 4.934 | 1 | 0.026* | 1.14 | 1.016 | 1.28 |
| Constant | −16.881 | 6.089 | 7.685 | 1 | 0.006 | 0 | ||
Note: *Means p < 0.05.
Abbreviations: SOFA, Sequential Organ Failure Assessment; IL-6, interleukin-6; Lac, lactate; CI, confidence interval; OR, odds ratio.
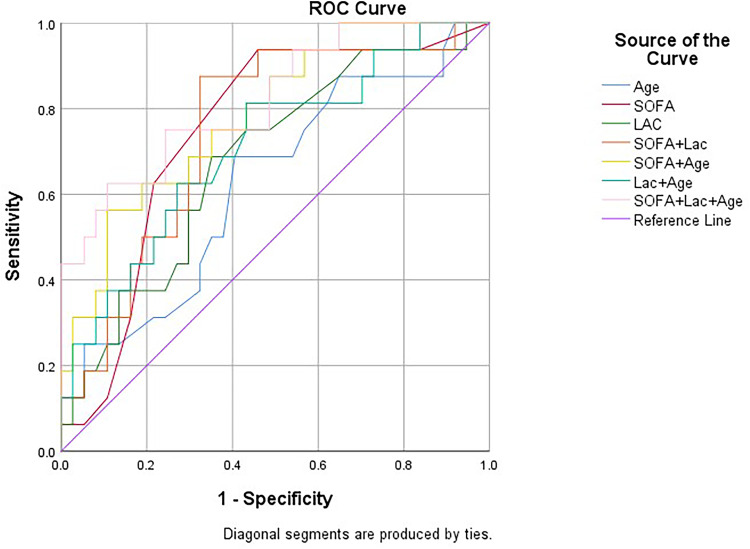
ROC curve analysis results (Table 7, Figure 2) indicated the value of these biomarkers in predicting 28-day mortality in sepsis patients. Specifically, the sensitivity and specificity were respectively 70.6% and 67.4% for age, 94.1% and 13% for SOFA and 68.8% and 64.9% for Lac. Compared with the SOFA score alone, the combination of age, Lac and SOFA score had higher predictive efficiency in predicting 28-day mortality in sepsis patients, with a sensitivity and specificity of 62.5% and 89.2%, respectively, but no statistical significance (Z = 1.06, p = 0.14).
Table 7.
Predictive Value of Lac, Age and SOFA Score in Predicting 28-Day Mortality in Patients with Sepsis
| Independent Predictors | AUC | 95% CI | P value | Cutoff | Sensitivity | Specificity | Youden Index | Positive Likelihood Ratio | Negative Likelihood Ratio | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.626 | 0.461 | 0.791 | 0.149 | 80.5 | 0.706 | 0.674 | 0.38 | 2.166 | 0.436 |
| SOFA | 0.747 | 0.606 | 0.889 | 0.005** | 2.5 | 0.941 | 0.13 | 0.071 | 1.082 | 0.454 |
| Lac | 0.679 | 0.524 | 0.834 | 0.040* | 1.7 | 0.688 | 0.649 | 0.337 | 1.96 | 0.481 |
| SOFA+Lac | 0.748 | 0.607 | 0.889 | 0.004** | 0.247 | 0.875 | 0.676 | 0.551 | 2.701 | 0.185 |
| SOFA+ Age | 0.785 | 0.656 | 0.915 | 0.001** | 0.466 | 0.588 | 0.891 | 0.479 | 5.394 | 0.462 |
| Lac+ Age | 0.711 | 0.558 | 0.864 | 0.015* | 0.281 | 0.813 | 0.568 | 0.381 | 1.882 | 0.329 |
| SOFA+Lac+ Age | 0.819 | 0.694 | 0.945 | 0.000*** | 0.44 | 0.625 | 0.892 | 0.517 | 5.787 | 0.42 |
Notes: *Means p < 0.05, **Means p < 0.01, ***Means p < 0.001.
Abbreviations: SOFA, Sequential Organ Failure Assessment; Lac, lactate; CI, confidence interval.
Figure 2.
Receiver operating characteristic (ROC) curves of biomarkers for predicting 28-day mortality in sepsis patients.
Discussion
In recent years, despite significant progress in clinical diagnosis and treatment of sepsis, its morbidity and mortality remain high. According to the 2016 Society for Critical Care Medicine (SCCM) and European Society for Critical Care Medicine (ESICM) jointly updated definitions, sepsis should be defined as life-threatening organ dysfunction caused by a dysregulated host response to infection.5 Considering the limitations of diagnostic criteria and the lack of clinically relevant data for many patients, finding biomarkers for early diagnosis of sepsis is critical.
IL-6 is an important pro-inflammatory factor in the initial stage of inflammation,6 which induces multiple cells to synthesize and secrete acute-phase proteins. During infection, it promotes the production and activation of neutrophils, proliferation and differentiation of B cells and T cells, and production of immunoglobulins. The level of IL-6 in healthy people is extremely low, generally not exceeding 7 pg/mL, but it increases rapidly in the serum of patients with sepsis within 2 hours of infection onset.7 Higher levels of IL-6 are associated with sepsis diagnosis,8 severity of organ dysfunction,3 and mortality.9 Song and others10 showed that IL-6 has a better diagnostic and prognostic value in patients with sepsis and septic shock than PCT. The results of a research by Thao et al11 also showed that ≥86% reduction in IL-6 level within 24 hours after admission to the ICU was a predictor of survival in patients with sepsis and septic shock. However, Ricarte-Bratti et al12 showed that the change of IL-6 within 72 hours after hospitalization rather than the level of IL-6 at the time of diagnosis in patients can predict the clinical prognosis of patients. Contrastingly, the results of logistic regression analysis in the present study showed that serum IL-6, BUN and MAP were independent risk factors for the diagnosis of sepsis. Based on AUC analysis, the AUC of IL-6 in the diagnosis of sepsis was significantly higher than that of BUN and MAP, suggesting good diagnostic performance (Z = 2.03, p = 0.02). However, in the logistic regression model, IL-6 was not significantly associated with 28-day mortality in patients with sepsis, suggesting that IL-6 cannot predict mortality in patients with sepsis. Differences in conclusions may be due to differences in hospital departments, including emergency departments and intensive care units, or in the severity of illness in the study population.
BUN is a waste product of protein metabolism in the liver that is carried through the blood to the kidneys, where it is filtered out of the blood, which increases BUN levels. Sepsis can significantly reduce renal blood flow and renal function. Researchers such as Xiao juan13 have shown that higher BUN levels are associated with the presence and severity of neonatal sepsis. Considering its relationship with renal organ dysfunction at an early stage of sepsis, BUN shows better diagnostic performance. Sepsis can cause capillary leakage, which decreases the effective circulating blood volume, resulting in a decrease in mean arterial pressure. As the disease progresses further, septic shock, cell damage and organ dysfunction may occur. In this study, there was a statistically significant difference in MAP between sepsis and non-septic patients (p < 0.05). Logistic regression analysis showed that MAP could be used as an independent risk factor for the diagnosis of sepsis, indicating that MAP contributes to diagnosis of sepsis.
The SOFA score14 is a scoring system used to determine sepsis-related organ dysfunction based on assessed levels of respiratory, cardiovascular, coagulation, renal, and nervous system dysfunction in patients. Shi et al15 reported that the SOFA score of the non-survivor group was significantly higher than that of the survivor group (8.9 ± 2.1 vs 5.4 ± 2.2). In another recent study by Kim et al,4 a mean SOFA score of 5 was found in the non-survivor group was 5, which was significantly higher than that in the survivor group. Similarly, in the present study, SOFA score was shown to effectively predict mortality in patients with sepsis, with a sensitivity of 94.1% and a specificity of 13%.
Lac levels are used as markers of peripheral perfusion, oxygenation adequacy, and impaired microvascular function, which are sensitive but nonspecific to metabolic stress in the body.16,17 Kanashvili et al16 reported that Lac increased with the severity of sepsis and had a good predictive value for the prognosis of patients with sepsis. In this study, lactate level was shown to be an independent risk factor for 28-day mortality in patients with sepsis, but showed no superiority as a predictor of sepsis prognosis when used alone.
A study by Si-Huei Lee et al18 showed that the incidence of sepsis was disproportionately increased in the elderly and elderly patients. Among the older populations, those aged ≥85 years had a sepsis rate of 9414 cases per 100,000 population, 31 times the incidence in adults (18–64 years) and 3 times the rate in those aged 65–84 years. The current study also shows that age is one of the risk factors for mortality in patients with sepsis, considering that elderly patients have more underlying diseases and are prone to organ failure, which affect clinical prognosis. The combination of Lac, age and SOFA score can effectively predict 28-day mortality in sepsis patients.
Both the delivery and analysis of a specimen in the laboratory take time, which limits rapid diagnosis and treatment. The POCT19 method used in this study has the advantages of rapidity, simplicity, high efficiency, and short inspection period. It can yield the inspection indicators required by doctors in a short time after the patient is admitted to the hospital and support targeted medication according to the inspection indicators. So that patients can obtain timely intervention and treatment.
In the present study, logistic regression analysis showed that IL-6, MAP and BUN were independent risk factors for the diagnosis of sepsis. ROC curve analysis showed that the predictive efficiency of IL-6 was significantly higher than that of BUN and MAP. Takahashi et al20 showed that the value of IL-6 in the diagnosis of sepsis is higher than that of PCT and CRP, which is consistent with the results of our study. Additionally, our study showed that IL-6 and mortality in patients with sepsis were not significantly associated, suggesting that IL-6 could be a potential biomarker for diagnosing sepsis, but not predicting 28-day mortality in sepsis patients. Combining MAP and BUN can improve their diagnostic efficiency for sepsis. Logistic regression analysis indicated that lactate, age and SOFA score were independent risk factors for 28-day death in sepsis patients. Among the three indicators, SOFA score showed a better predictive value, which was consistent with the results of a previous study,1 indicating that SOFA score significantly correlated with clinical outcomes in patients with sepsis. Lac and age can also predict clinical outcomes in patients with sepsis.
The present study has limitations. First, because it was a single-center study, the results may not apply to other centers. Second, the small sample size used inevitably leads to statistical bias. Therefore, further multicenter studies with larger sample sizes are needed. In addition, some enrolled patients received anti-infectives, fluids, or vasopressors before being transferred to our hospital. These treatments could have affected our biomarker levels.
Conclusion
IL-6 is an independent predictor of sepsis diagnosis and can be a potential biomarker for sepsis diagnosis. In combination with BUN and MAP, it has a higher diagnostic efficiency. Lac, age and SOFA score can effectively predict the clinical outcome of sepsis in patients.
Acknowledgments
The authors would like to thank Maolin Chen, Ye Zhang, and Yudan Cao for their help in collecting data.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Abbreviations
BUN, blood urea nitrogen; Lac, lactic acid; ROC, Receiver Operating Characteristic Curve; AUC, Area Under the Curve; POCT, Point of Care Testing; cTnI, Cardiac troponin I; D-D, D-Dimer; SD, Standard Deviation; MAP, Mean Arterial Pressure; qSOFA, Rapid Sequential Organ Failure Score; SOFA, Sequential Organ Failure Score; APHCHE II, Acute Physiology and Chronic Health Assessment II; GCS, Glasgow Coma Scale; PCT, procalcitonin; CRP, C-reactive protein; IL-6, interleukin-6; WBC, white blood cell count; CI, confidence interval; OR, odds ratio.
Data Sharing Statement
All data generated or analysed during this study are included in this published article.
Ethics Approval and Consent to Participate
This study complied with medical ethics standards. The research protocol was approved by the Ethics Committee of Beijing Chaoyang Hospital Affiliated to Capital Medical University (approval number: 2021-ke-636). Informed consent was obtained from all patients or their families before receiving treatments and tests.
Consent for Publication
Written informed consent for publication was obtained from all participants.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Li W, Wang M, Zhu B, Zhu Y, Xi X. Prediction of median survival time in sepsis patients by the SOFA score combined with different predictors. Burns Trauma. 2020;8:z6. doi: 10.1093/burnst/tkz006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392(10141):75–87. doi: 10.1016/S0140-6736(18)30696-2 [DOI] [PubMed] [Google Scholar]
- 3.Jekarl DW, Kim JY, Lee S, et al. Diagnosis and evaluation of severity of sepsis via the use of biomarkers and profiles of 13 cytokines: a multiplex analysis. Clin Chem Lab Med. 2015;53(4):575–581. [DOI] [PubMed] [Google Scholar]
- 4.Kim H, Hur M, Moon HW, Yun YM, Di Somma S. Multi-marker approach using procalcitonin, presepsin, galectin-3, and soluble suppression of tumorigenicity 2 for the prediction of mortality in sepsis. Ann Intensive Care. 2017;7(1):27. doi: 10.1186/s13613-017-0252-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a16295. doi: 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson DK, Huffman KM, Kraus WE, Kraus VB. Critical appraisal of four IL-6 immunoassays. PLoS One. 2012;7(2):e30659. doi: 10.1371/journal.pone.0030659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cong S, Ma T, Di X, Tian C, Zhao M, Wang K. Diagnostic value of neutrophil CD64, procalcitonin, and interleukin-6 in sepsis: a meta-analysis. Bmc Infect Dis. 2021;21(1):384. doi: 10.1186/s12879-021-06064-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barre M, Behnes M, Hamed S, et al. Revisiting the prognostic value of monocyte chemotactic protein 1 and interleukin-6 in the sepsis-3 era. J Crit Care. 2018;43:21–28. doi: 10.1016/j.jcrc.2017.08.024 [DOI] [PubMed] [Google Scholar]
- 10.Song J, Park DW, Moon S, et al. Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: a prospective controlled study according to the Sepsis-3 definitions. Bmc Infect Dis. 2019;19(1):968. doi: 10.1186/s12879-019-4618-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thao P, Tra TT, Son NT, Wada K. Reduction in the IL-6 level at 24 h after admission to the intensive care unit is a survival predictor for Vietnamese patients with sepsis and septic shock: a prospective study. BMC Emerg Med. 2018;18(1):39. doi: 10.1186/s12873-018-0191-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricarte-Bratti JP, Jaime-Albarrán NY, Montrull HL, Brizuela NY. IL-6, MMP 3 and prognosis in previously healthy sepsis patients. Rev Fac Cien Med Univ Nac Cordoba. 2017;74(2):99–106. doi: 10.31053/1853.0605.v74.n2.14608 [DOI] [PubMed] [Google Scholar]
- 13.Li X, Li T, Wang J, et al. Higher blood urea nitrogen level is independently linked with the presence and severity of neonatal sepsis. Ann Med. 2021;53(1):2192–2198. doi: 10.1080/07853890.2021.2004317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on ”sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016 [DOI] [PubMed] [Google Scholar]
- 15.Shi Y, Peng JM, Hu XY, Wang Y. The utility of initial procalcitonin and procalcitonin clearance for prediction of bacterial infection and outcome in critically ill patients with autoimmune diseases: a prospective observational study. Bmc Anesthesiol. 2015;15:137. doi: 10.1186/s12871-015-0122-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanashvili B, Saganelidze K, Ratiani L. The role of procalcitonin and blood lactic acid values in prognosis of sepsis and septic shock in polytrauma patients. Georgian Med News. 2018;279:102–107. [PubMed] [Google Scholar]
- 17.Sanaei DA, Alizadeh S, Karimi A, Khalifeh M, Shoja SA. Diagnostic value of lactate, procalcitonin, ferritin, serum-C-reactive protein, and other biomarkers in bacterial and viral meningitis: a cross-sectional study. Medicine. 2017;96(35):e7637. doi: 10.1097/MD.0000000000007637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SH, Hsu TC, Lee MG, et al. Nationwide trend of sepsis: a comparison among octogenarians, elderly, and young adults. Crit Care Med. 2018;46(6):926–934. doi: 10.1097/CCM.0000000000003085 [DOI] [PubMed] [Google Scholar]
- 19.Dalton J. Communications with lab and POCT users. Pract Lab Med. 2021;25:e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi W, Nakada TA, Yazaki M, Oda S. Interleukin-6 levels act as a diagnostic marker for infection and a prognostic marker in patients with organ dysfunction in intensive care units. Shock. 2016;46(3):254–260. doi: 10.1515/cclm-2014-0607 [DOI] [PubMed] [Google Scholar]