Abstract
Background
Proton pump inhibitor (PPI) indications are limited to gastrointestinal disorders and ulcer prophylaxis. However, PPIs are among the most frequently prescribed drugs.
Aim
To evaluate the appropriateness of PPI prescriptions and identify predictive factors for inappropriate PPI use.
Design and setting
Observational study using a Dutch primary care database with all new PPI prescriptions between 2016 and 2018.
Method
Individual patient data and details on PPI use were collected. The appropriateness of initiation and continuation of PPI prescriptions was evaluated using the applicable guidelines.
Results
In total, 148 926 patients (aged ≥18 years) from 27 general practices were evaluated. A total of 23 601 (16%) patients started PPI therapy (mean age 57 [SD 17] years, 59% female). Valid PPI indications at initiation were seen in 10 466 PPI users (44%). Predictors for inappropriately initiated PPI use were older age (odds ratio [OR] 1.03, 95% confidence interval [CI] = 1.03 to 1.03), and use of non-selective non-steroidal anti-inflammatory drugs (OR 5.15, 95% CI = 4.70 to 5.65), adenosine diphosphate receptor inhibitors (OR 5.07, 95% CI = 3.46 to 7.41), COX-2 inhibitors (also known as coxibs) (OR 3.93, 95% CI = 2.92 to 5.28), and low-dose aspirin (OR 3.83, 95% CI = 3.07 to 4.77). Despite an initial valid indication, PPI use was inaccurately continued in 32% of patients on short-course therapy for dyspepsia and in 11% of patients on ulcer prophylaxis.
Conclusion
More than half of PPI users in primary care were found to have an inappropriate indication, with unnecessary ulcer prophylaxis related to drug use being one of the leading causes. Future initiatives to reduce PPI use for unnecessary ulcer prophylaxis and timely deprescription if PPI is no longer indicated, are needed.
Keywords: anti-ulcer agents, dyspepsia, inappropriate prescribing, non-steroidal anti-inflammatory drugs, primary health care, proton pump inhibitors
INTRODUCTION
Proton pump inhibitors (PPIs) are among the most prescribed drugs worldwide and are the cornerstone for treating and preventing acid-related disorders.1–4 There use has a major impact as it accounts for at least 37 million euros spent on health care annually in the Netherlands alone.5
PPI therapy is frequently prescribed in the absence of an appropriate indication.6 Examples of inappropriate PPI use are ulcer prophylaxis in patients without risk factors (for example, steroid therapy alone) and overtreatment of functional dyspepsia.6 Inappropriate PPI use may potentially harm patients through adverse drug reactions or drug–drug interactions. Increasing evidence shows that long-term PPI use is associated with severe adverse drug reactions, such as Clostridium difficile colitis , malabsorption of magnesium, osteoporosis, and kidney disease.7–10
Prior studies have identified unnecessary PPI use for ulcer prophylaxis (that is, in patients without risk factors) as an important factor associated with inappropriate PPI therapy.11,12 However, use of certain drugs or clinical conditions that are most predictive for inappropriate PPI use in clinical practice remain largely unknown, which hampers targeted interventions to reduce PPI use. This study aimed to evaluate the appropriateness of PPI therapy in a large primary care setting in the Netherlands and determine predictors for inappropriate PPI use.
METHOD
This study uses real-world, pseudonymised, routine primary care data covering the Leiden/The Hague region in the Netherlands. Continuous updated electronic medical record data from all patients (aged ≥18 years) from GP centres in the Extramural LUMC Academic Network (ELAN) were accessible. A total of 27 general practice centres associated with ELAN (each with 2–6 practising GPs) could be approached for this study, covering 148 926 patients. All practice centres associated with ELAN use an ‘informed opt-out’ procedure, so electronic medical record data of the patients enlisted with these practices can be used for research purposes. No more than 5% of all patients chose informed opt-out. The general practice centres can be characterised as representative for the average Dutch population, randomly spread over rural, suburban, and highly urbanised areas. According to the Dutch healthcare system, all residents primarily contact their GP in the case of a health problem. GPs can deal with routine health issues, including upper gastrointestinal disorders. If indicated, GPs can refer their patient to a specialist.
How this fits in
| Although overuse of proton pump inhibitors (PPIs) is a common issue worldwide, predictors for this remain insufficiently known. This observational study using real-world primary care data identified older age and non-selective non-steroidal anti-inflammatory drug use as most predictive for inappropriate PPI use. The study also showed that unnecessarily continued PPI therapy was common in patients using PPI therapy for dyspepsia or as ulcer prophylaxis. Future initiatives on reducing inappropriate PPI use should target these patient groups. |
Data collection
The database used International Classification of Primary Care (ICPC) codes for medical conditions and Anatomic Therapeutic Chemical (ATC) codes for drug use. Available data included patient characteristics, medical history, drug prescriptions, and GP consultations. Drug prescriptions were linked to the pharmacist’s database in which all pharmacy data from participating GP practices is stored. Therefore, drug prescriptions included all drugs prescribed by GPs and non-prescription medicine in case this was registered by pharmacies. All patients with PPI prescriptions and upper gastrointestinal symptoms or conditions were identified using ATC and ICPC codes (see Supplementary Boxes S1 and S2). The accuracy of ICPC code registration in Dutch general practices is around 90%.13 Data between 2015 and 2018 were available. New PPI usage periods were identified over the years 2016–2018. Data in 2015 were used to confirm that PPI prescriptions were initiated between 2016 and 2018, defined as no PPI use during at least 3 months before the start of the new PPI prescription.
Drug prescription variables
Drug usage periods were calculated by merging repeat (refill) prescriptions. The usage periods of drugs that are known for chronic use or as a treatment during a predefined period of time (corticosteroids, anticoagulants, antidepressants, and spironolactone) were defined as the start date of the first prescription until the end date of the last prescription. For drugs that are potentially used short term (PPIs, H2-blockers, antacids, Heliobacter pylori [ H. pylori ] eradication therapy, and non-steroidal anti-inflammatory drugs [NSAIDs]), a unique usage period was created if the interval between two prescriptions exceeded 3 months. For example, if the interval between the end date of prescription 1 and the start date of prescription 2 was >3 months, the end date of prescription 1 was considered the end date of the first usage period and the start date of prescription 2 as the start date of the second usage period.
End dates of a drug prescription were calculated using the start date, dosage, and usage frequency. To categorise drug prescriptions that did not specify an exact frequency, the lowest possible usage frequency was selected (for example, ‘one to three times daily’ was transformed into ‘one time daily’). Furthermore, on-demand use was converted to one-third of the time used (for example, ‘one time daily, on demand’ was transformed to ‘one time daily, every 3 days’). If the prescribed frequency was not provided, it was replaced by once daily. Finally, if no dosage was available, prescriptions were considered to end after 3 months.
Chronic PPI use was defined as >180 defined daily doses (DDDs)/year, a technical unit measuring drug consumption, as a proxy of >6 months PPI use.14,15 NSAID prescriptions were recorded as high dose if the DDD was exceeded. Lastly, H. pylori eradication therapy was defined as either a fixed-dose combination or the prescription of a PPI with at least two types of antibiotics initiated simultaneously.
Appropriateness of PPI therapy
Appropriateness of PPI therapy was assessed for all patients receiving a new PPI prescription between 2016 and 2018. In case of multiple PPI usage periods in a single patient, appropriateness of PPI use was categorised based on the earliest PPI usage period and scored according to the Dutch College of General Practitioners guideline ‘Upper gastrointestinal symptoms’ (version 2013) and clinical decision rules.16–18 PPI therapy was deemed appropriate if used for
confirmed gastroesophageal reflux disease;
peptic ulcer disease (if registered <3 months before start of PPI);
short-course therapy for dyspepsia (if registered <6 months before start of PPI);
alarm symptoms (for example, haematemesis) if registered <1 month before start of PPI; and
as part of eradication therapy for H. pylori.18
Furthermore, PPI use was determined appropriate for ulcer prophylaxis in high-risk patients when using NSAIDs, low-dose aspirin, or in patients with a history of peptic ulcer disease. To assess if a patient was at high risk of developing gastroduodenal ulcers their age, comorbidities, and concomitant drug use at the time of PPI prescription was evaluated. Chronic use of PPIs is only indicated for severe reflux oesophagitis, Barrett’s oesophagus, Zollinger–Ellison syndrome, and chronic ulcer prophylaxis.19,20 Indications were evaluated based on registered ICPC and ATC codes. ICPC codes of medical conditions such as reflux oesophagitis are only used if confirmed by additional examination such as a gastroscopy. In cases where a medical condition is not confirmed, symptom ICPC codes such as pyrosis were used. Supplementary Box S2 shows all valid PPI indications, including corresponding ICPC and ATC codes.
Appropriate duration of PPI therapy
The accepted duration of PPI use for a temporary indication to treat upper gastrointestinal disorders was limited to 3 months. These include short-course PPI therapy for dyspepsia, treatment of peptic ulcer disease, alarm features such as haematemesis, and H. pylori eradication. If a PPI was started as ulcer prophylaxis, it had to be stopped within 3 months after cessation of the drug that initiated PPI use.
Predictors for inappropriate PPI use
To identify predictive factors for inappropriate PPI use, users of PPIs (PPI use group) were compared with non-users of PPIs (non-PPI use group) who had consulted the GP for upper gastrointestinal conditions as a control group. Factors in the regression model included patient characteristics (for example, age, sex, and body mass index [BMI]), comorbidities (for example, diabetes mellitus and heart failure), anti-reflux medication used before the start of PPIs, and concomitant drug use associated with PPI indications. To allow comparison between inappropriate use in the PPI group and the non-PPI use group, concomitant drug use in the inappropriate PPI use group was not restricted to a specific time interval between 2016 and 2018. This means that all concomitant drug use in the period of 2016–2018 was included regardless of the duration of use or, for the inappropriate PPI use group, interval between PPI use and concomitant drug use.
Statistical analysis
Normally distributed data were presented as mean (standard deviation [SD]) and non-normally distributed data as median and interquartile range. χ2-testing was performed to compare categorical variables. Patients were clustered within practices and, therefore, a mixed-model logistic regression was used to determine predictive factors for inappropriate PPI use. A random intercept model was performed with the other factors fixed. Variables with a P-value <0.2 in the univariate analysis were included in the multivariate analysis. A backwards model was used to stepwise eliminate the variables with the highest P-value until all variables in the model had a P-value <0.05. Two-sided testing with a P-value of <0.05 was considered significant. IBM SPSS Statistics (version 25.0) and R (version 4.1.1) packages haven, funnelR, and ggplot2 were used to process and analyse the data.
RESULTS
PPI prescriptions and patient characteristics
In total, 339 816 new PPI prescriptions between 2016 and 2018 in 23 601/148 926 patients (16%) were identified. Merging consecutive (refill) prescriptions resulted in 32 401 PPI usage periods (Figure 1). The prescribed frequency and dosage were not provided in 3190 (1%) and 36 (0.01%) PPI prescriptions, respectively. The number of new PPI usage periods was relatively stable throughout 2016–2018 (11 235 in 2016, 10 955 in 2017, and 10 211 in 2018).
Figure 1.
Selection of PPI usage periods in 2016–2018. PPI = proton pump inhibitor.
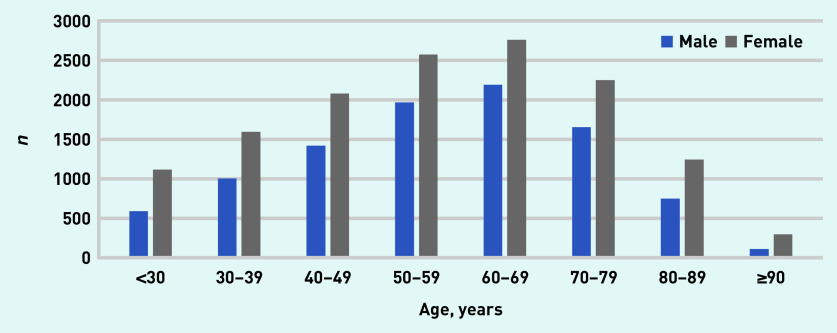
Figure 2 shows the age and sex distribution of all patients with PPI usage periods in 2016–2018. Mean age at initiation of PPI prescription was 57 years (SD 17), of whom 59% were female (Table 1). A total of 2823 (12%) patients were registered as active smokers and 3106 (13%) as active alcohol users. Mean BMI was 28 kg/m2 (SD 6), and diabetes mellitus was registered in 2536 (11%) patients and heart failure in 446 (2%) patients.
Figure 2.
Age and sex of patients with a PPI usage period in 2016–2018. PPI = proton pump inhibitor.
Table 1.
Patient characteristics, based on first PPI usage period per patient
| Characteristic | All PPI users (n= 23 601), n (%)a | Appropriate PPI users (n= 10 466), n (%)a | Inappropriate PPI users (n= 13 135), n (%)a |
|---|---|---|---|
| Sex, female | 13 916 (59) | 6237 (60) | 7679 (58) |
| Age, years, mean (SD) | 57 (17) | 61 (18) | 54 (16) |
| BMI, kg/m2, mean (SD)b | 28 (6) | 28 (5) | 28 (6) |
| Current smoker | 2823 (12) | 1222 (12) | 1601 (12) |
| Use of alcohol | 3106 (13) | 1678 (16) | 1428 (11) |
| Diabetes mellitus | 2536 (11) | 1346 (13) | 1190 (9) |
| Heart failure | 446 (2) | 206 (2) | 240 (2) |
| Rheumatoid arthritis | 125 (1) | 59 (1) | 66 (1) |
| Peptic ulcer disease | 596 (3) | 483 (5) | 113 (1) |
| Reflux oesophagitis | 523 (2) | 523 (5) | 0 (0) |
| Antacid | 884 (4) | 440 (4) | 444 (3) |
| H2RA | 801 (3) | 527 (5) | 274 (2) |
| Non-selective NSAID | 9281 (39) | 4790 (46) | 4491 (34) |
| COX-2 inhibitor | 560 (2) | 165 (2) | 395 (3) |
| Low-dose aspirin | 3048 (13) | 1699 (16) | 1349 (10) |
| Vitamin K antagonist | 926 (4) | 403 (4) | 523 (4) |
| ADP receptor inhibitor | 1030 (4) | 393 (4) | 637 (5) |
| DOAC | 513 (2) | 201 (2) | 312 (2) |
| LMWH | 845 (4) | 436 (4) | 409 (3) |
| Systemic corticosteroid | 1943 (8) | 825 (8) | 1118 (9) |
| SSRI | 1565 (7) | 804 (8) | 761 (6) |
| Trazadone | 43 (0.2) | 23 (0.2) | 20 (0.2) |
| Venlafaxine | 279 (1) | 151 (1) | 128 (1) |
| Duloxetine | 54 (0.2) | 20 (0.2) | 34 (0.3) |
| Spironolactone | 285 (1) | 106 (1) | 179 (1) |
Unless otherwise stated.
n =14 764 missing. ADP = adenosine diphosphate. BMI = body mass index. DOAC = direct-acting oral anticoagulant. H2RA = H2 receptor antagonist. LMWH = low-molecular-weight heparin. NSAID = non- steroidal anti-inflammatory drug. PPI = proton pump inhibitor. SSRI = selective serotonin reuptake inhibitor.
At the time of PPI prescription, 9281 (39%) patients used non-selective NSAIDs and 3048 (13%) patients used low-dose aspirin. Antacids were prescribed before the start of PPI in 884 (4%) and H2-blockers in 801 (3%) patients.
In 16 328 (69%) patients, PPIs were prescribed for <3 months. A total of 6794 (29%) patients in the PPI use group fulfilled the criteria for chronic PPI use.
Appropriateness at start of PPI therapy
A total of 10 466 (44%) patients had an appropriate indication for PPI use at the start of PPI therapy. The indications for PPI use was equally distributed between treatment for upper gastrointestinal conditions (n = 4749, 20%) and ulcer prophylaxis (n = 5382, 23%). Table 2 and Figure 3 show the indications for appropriately prescribed PPIs. Dyspepsia was the leading upper gastrointestinal symptom in patients with a PPI (n = 3260, accounting for 69% of PPIs started as treatment of upper gastrointestinal disorders). Use of non-selective NSAIDs and low-dose aspirin use were responsible for 78% (n = 4191) and 17% (n = 935) of ulcer prophylaxis indications, respectively.
Table 2.
PPI indications
| Variable | PPI users (n= 23 601), n (%) |
|---|---|
| Treatment of upper gastrointestinal disorders | 4749 (20) |
| Temporary indication | |
| Dyspepsia | 3260 (14) |
| Peptic ulcer disease | 40 (0.2) |
| Alarm features (for example, haematemesis) | 106 (0.4) |
| Eradication of Heliobacter pylori | 73 (0.3) |
| Chronic indication | |
| Oesophageal disease (for example, Barrett’s oesophagus) | 458 (2) |
| Reflux oesophagitis | 347 (1) |
| Multiple PPI indications as treatment of upper gastrointestinal disorders | 465 (2) |
|
| |
| Ulcer prophylaxis | 5382 (23) |
| Non-selective NSAID | 4191 (18) |
| Low-dose aspirin | 935 (4) |
| COX-2 inhibitor | 82 (0.3) |
| Peptic ulcer disease in medical historya | 71 (0.3) |
| and usage of coumarins | 10 (0.04) |
| and usage of DOAC | 6 (0.03) |
| and usage of LMWH | 2 (0.008) |
| and usage of ADP receptor inhibitor | 13 (0.06) |
| and usage of thrombolytics | 0 (0) |
| and usage of systemic corticosteroid | 15 (0.06) |
| and usage of SSRI | 8 (0.03) |
| and usage of venlafaxine | 0 (0) |
| and usage of duloxetine | 0 (0) |
| and usage of trazodone | 0 (0) |
| and usage of spironolactone | 3 (0.01) |
| overlay in comedication use | 14 (0.06) |
| Multiple PPI indications as ulcer prophylaxis | 103 (0.4) |
|
| |
| Both treatment of upper gastrointestinal disorders and ulcer prophylaxis | 335 (1) |
|
| |
| No accepted indication | 13 135 (56) |
If not already in combination with NSAID or low-dose aspirin usage. ADP = adenosine diphosphate. DOAC = direct- acting oral anticoagulant. LMWH = low-molecular-weight heparin. NSAID = non-steroidal anti- inflammatory drug. PPI = proton pump inhibitor. SSRI = selective serotonin reuptake inhibitor.
Figure 3.
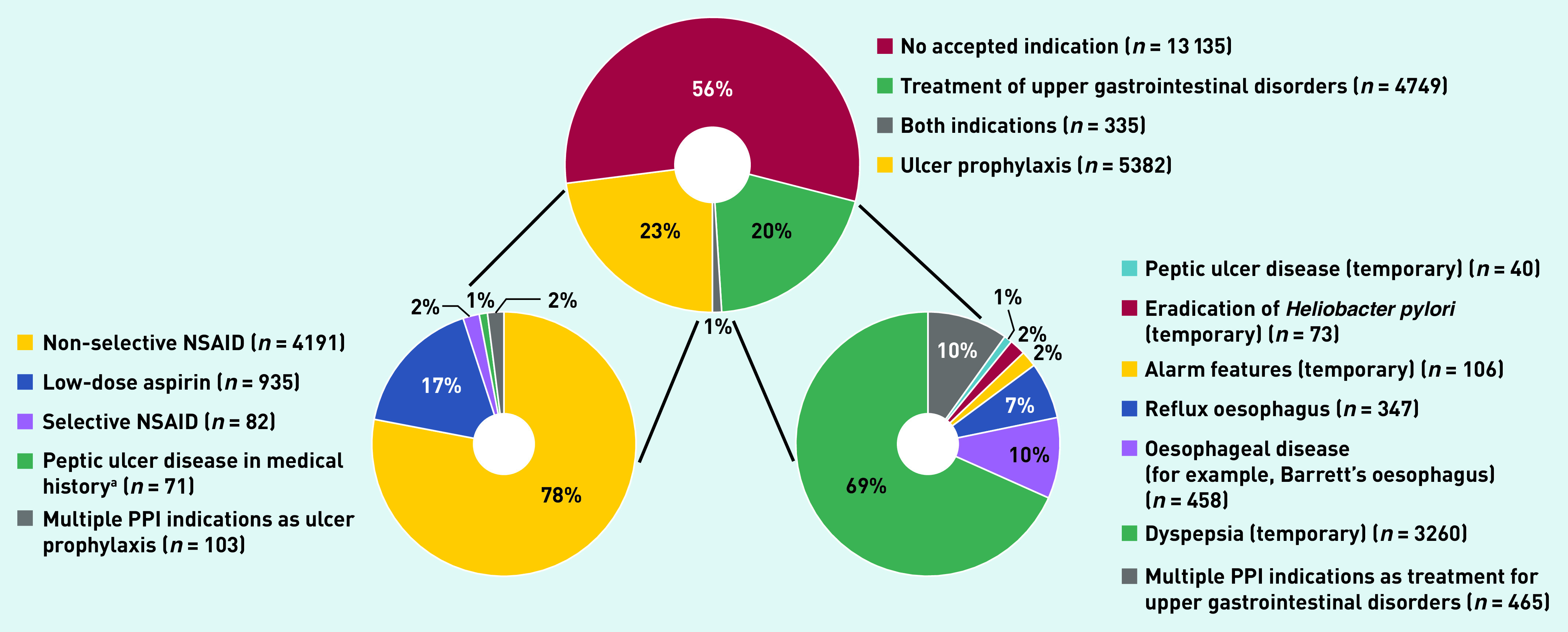
PPI indications. aIf not already in combination with NSAID or low-dose aspirin usage. NSAID = non-steroidal anti-inflammatory drug. PPI = proton pump inhibitor.
An inappropriate PPI indication was identified in 13 135 (56%) patients. In this group, 8493 patients (65%) used drugs associated with an indication as ulcer prophylaxis at the time of PPI prescription. These drugs primarily included non-selective NSAIDs (34%), low-dose aspirin (10%), and systemic corticosteroids (9%) (Table 1).
Appropriateness of PPI therapy ranged from 47% to 67% between general practices (P<0.001, Supplementary Table S1 and Supplementary Figure S1).
Appropriate duration of PPI therapy
Of patients receiving a short course of PPI for dyspepsia, 1042/3260 (32%) did not stop PPI treatment within 3 months. In 3944/5717 (69%) PPI users with an appropriate indication for PPI as ulcer prophylaxis, the drug that initiated PPI treatment was stopped during follow-up. Despite stopping, 446 (11%) patients continued PPI use for >3 months. This included 311 (70%) patients that used a PPI as ulcer prophylaxis for non-selective NSAIDs use, 118 (26%) for low-dose aspirin use, 5 (1%) for COX-2 inhibitor (also known as coxib) use, and 12 (3%) patients with a history of peptic ulcer disease combined with comedication use that is associated with a higher bleeding risk (data not shown).
Predictors for inappropriate PPI therapy
A total of 13 135 patients in the inappropriate PPI use group were compared with 3155 patients in the non-PPI use group (see Supplementary Table S2). Variables with substantial missing data (BMI), not fully registered data (smoking, alcohol use, and antacid and H2-blocker use), small numbers (rheumatoid arthritis, peptic ulcer disease, and spironolactone), or a direct association with an appropriate PPI indication (reflux oesophagitis) were excluded.
Predictors for inappropriate PPI use were age (odds ratio [OR] 1.03 increment per year, 95% confidence interval [CI] = 1.03 to 1.03) and drug use associated with PPI indications (Table 3). Non-selective NSAID use (OR 5.15, 95% CI = 4.70 to 5.65) and adenosine diphosphate receptor inhibitor use (OR 5.07, 95% CI = 3.46 to 7.41) had the strongest association with inappropriate PPI use, followed by COX-2 inhibitor use (OR 3.93, 95% CI = 2.92 to 5.28) and low-dose aspirin use (OR 3.83, 95% CI = 3.07 to 4.77).
Table 3.
Mixed-model multivariate logistic regression analysis for inappropriate PPI use
| Category | Univariate logistic regression | Multivariate logistic regression | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Sex, male | 1.13 | 1.04 to 1.23 | — | — |
| Age, increment per year | 1.03 | 1.03 to 1.03 | 1.03 | 1.03 to 1.03 |
| Diabetes mellitus | 1.58 | 1.35 to 1.84 | — | — |
| Heart failure | 1.37 | 1.08 to 1.75 | — | — |
| Non-selective NSAID | 3.44 | 3.16 to 3.74 | 5.15 | 4.70 to 5.65 |
| COX-2 inhibitor | 4.92 | 3.70 to 6.54 | 3.93 | 2.92 to 5.28 |
| Low-dose aspirin | 5.31 | 4.32 to 6.52 | 3.83 | 3.07 to 4.77 |
| Vitamin K antagonist | 1.97 | 1.57 to 2.49 | — | — |
| ADP receptor inhibitor | 8.57 | 5.94 to 12.35 | 5.07 | 3.46 to 7.41 |
| DOAC | 3.55 | 2.56 to 4.92 | 2.54 | 1.80 to 3.57 |
| LMWH | 4.58 | 3.50 to 5.99 | 2.91 | 2.20 to 3.85 |
| Systemic corticosteroids | 3.08 | 2.69 to 3.53 | 2.37 | 2.05 to 2.74 |
| SSRI | 1.66 | 1.41 to 1.96 | 1.77 | 1.49 to 2.11 |
| SNRI | 2.37 | 1.66 to 3.38 | 2.18 | 1.49 to 3.19 |
ADP = adenosine diphosphate. DOAC = direct-acting oral anticoagulant. LMWH = low-molecular-weight heparin. NSAID = non-steroidal anti-inflammatory drug. OR = odds ratio. PPI = proton pump inhibitor. SNRI = serotonin and norepinephrine reuptake inhibitor. SSRI = selective serotonin reuptake inhibitor.
DISCUSSION
Summary
This study found that, at the time of analysis, more than half of PPIs prescribed in primary care were not adequately indicated. The most important predictors for inappropriate PPI therapy were age and the use of drugs, such as non-selective NSAIDs, for which ulcer prophylaxis is only indicated in high-risk patients. One-third of PPIs started as short-course therapy for dyspepsia and one-tenth of PPIs started as ulcer prophylaxis were continued after the indication was no longer valid.
Strengths and limitations
The data used were obtained directly from electronic medical records without a pre-known research purpose. Using real-world data allows accurate investigation of current clinical practice. Moreover, a strength of this study is the large size, which allowed a detailed assessment of PPI appropriateness. Also, as most PPIs are prescribed in a primary care setting in the Netherlands, this population is representative for assessing the appropriateness of PPI use.18
This study is, however, limited by its retrospective design. First, patient characteristics, registered comorbidity, and comedication prescriptions were used as a proxy to determine appropriateness of PPI use. Some assumptions were needed for comorbidity stages and duration of comedication use; however, appropriateness of PPI therapy was always given the benefit of the doubt. Second, not all anti-reflux medication use was known as non-prescription drug registration was incomplete; however, apart from including drugs prescribed by GPs, non-prescription drug use registered by pharmacists was also included. Moreover, as a proxy for general non-PPI users, non-PPI users who consulted the GP for upper gastrointestinal conditions were used as a control group for the logistic regression analysis. Nonetheless, two groups without valid PPI indication were compared to determine possible predictors for inappropriate PPI use. Furthermore, some variables, such as BMI and alcohol use, could not be included in the logistic regression analysis because of missing data. Finally, total duration of PPI use and number of patients with chronic use may have been underestimated as there was only access to data up to 2018.
Comparison with existing literature
The high percentage of inappropriately initiated PPI prescriptions (56%) corresponds with an earlier Dutch study.21 In contrast to the current study, those authors had no access to electronic primary care patient records, which may potentially overestimate the number of inappropriate users of PPI. Another study from Denmark had similar access to primary care source data and showed that 25% of patients had an invalid PPI indication.22 However, appropriateness could have been overestimated in that study as all patients using NSAIDs or aspirin were considered as appropriate users of PPI. Moreover, bias could have been introduced as the prescribing physicians collected the data themselves, which was not the case in the current study as real-world data was extracted by authors with no role in prescribing this PPI therapy.
Patients using non-selective NSAIDs were identified as having the highest odds for inappropriate PPI use. This finding is in line with previous studies.11,12,21 A questionnaire study showed that inappropriate PPI therapy as ulcer prophylaxis was recommended by 35% of GPs and internists when starting NSAIDs in low-risk patients.23 As non-selective NSAIDs are frequently prescribed, its use in low-risk patients is likely one of the leading causes of inappropriate PPI use.24
Moreover, patient age was found to be predictive for inappropriate PPI therapy. This could be related to the increasing number of drugs patients use when age increases.25 A previous study showed that the number of drugs used was a predictor of inappropriate PPI therapy in older people.26 A possible explanation could be that physicians tend to prescribe ulcer prophylaxis more often in frail older people regardless of a valid indication.27
Another important risk factor for inappropriate PPI use is unjustified continuation of temporary indicated PPIs in patients with dyspepsia or as ulcer prophylaxis, as already suggested by prior studies.28,29 Not explicitly informing patients that PPI treatment is of limited duration and lack of physician follow-up may lead to unjustified continuation of PPI therapy. Furthermore, rebound symptoms may complicate discontinuation of PPI use and, in the case of dyspepsia, suggesting an alternative therapy such as lifestyle measures to patients can be challenging.30,31
Implications for practice
By identifying predictors for PPI overuse, the current study provides possible targets for future interventions to reduce inappropriate PPI use. Previous studies have shown different interventions that successfully reduce inappropriate PPI use, such as prescriber and patient education, PPI use evaluation, and self-management plans.21,32,33 However, sustainable and time-efficient strategies are lacking. One potential strategy is a close collaboration between GPs and pharmacists to double-check PPI indications and to stress discontinuing inappropriate PPI therapy. Furthermore, GPs could also play a role when they notice that PPIs are inappropriately prescribed by medical specialists in secondary care.
Acknowledgments
The authors would like to thank the Dutch National Health Care Institute initiative of reducing proton pump inhibitors (reference number: 2019027556) (https://english.zorginstituutnederland.nl) and are grateful to RP Akkermans for assisting in the statistical analysis of this study.
Funding
This study was funded in full by the Dutch National Health Care Institute (grant reference: 2019027556).
Ethical approval
Formal medical ethical review was waived for this study (Medical Ethical Committee region Arnhem-Nijmegen, reference: 2020–6394).
Data
Data are available from the corresponding author on reasonable request.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
Jacqueline Sarah Frankema and Edwin Johannes Heeregrave are employees of the Dutch National Health Care Institute and were involved in the process of allocating funding for this study. Joost Paulus Hubertus Drenth has received research funding from Gilead and AbbVie, is a participant on the COIN-B data safety monitoring board, and is Chair of the Dutch Society of Hepatology. Mattijs Everard Numans has received research funding from the Dutch Stomach, Liver and Bowel Foundation and is a member of the Dutch College of General Practitioners guideline committees ‘Upper gastrointestinal tract’ and ‘Liver’. Peter Derk Siersema has received research funding from Pentax, The E-Nose Company, Lucid Diagnostics, Micro-Tech, Motus GI, Magentiq Eye, and has served as an advisor for Motus GI. The remaining authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Lanza FL, Chan FK, Quigley EM, et al. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104(3):728–738. doi: 10.1038/ajg.2009.115. [DOI] [PubMed] [Google Scholar]
- 2.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108(3):308–328. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]
- 3.Kavitt RT, Lipowska AM, Anyane-Yeboa A, Gralnek IM. Diagnosis and treatment of peptic ulcer disease. Am J Med. 2019;132(4):447–456. doi: 10.1016/j.amjmed.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314(17):1818–1831. doi: 10.1001/jama.2015.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Healthcare Institute Netherlands [Reimbursement 2017–2021 for ATC subgroup A02B: drugs for peptic ulcers and gastroesophageal reflux]. [Article in Dutch] https://www.gipdatabank.nl/databank?infotype=g&label=00-totaal&tabel_g_00-totaal=B_01-basis&tabel_h_00-totaal=B_01-basis&geg=vg&spec=&item=A02B (accessed 29 Jun 2022).
- 6.Savarino V, Marabotto E, Zentilin P, et al. Proton pump inhibitors: use and misuse in the clinical setting. Expert Rev Clin Pharmacol. 2018;11(11):1123–1134. doi: 10.1080/17512433.2018.1531703. [DOI] [PubMed] [Google Scholar]
- 7.Furuya-Kanamori L, Stone JC, Clark J, et al. Comorbidities, exposure to medications, and the risk of community-acquired clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36(2):132–141. doi: 10.1017/ice.2014.39. [DOI] [PubMed] [Google Scholar]
- 8.Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435–2442. doi: 10.1001/jama.2013.280490. [DOI] [PubMed] [Google Scholar]
- 9.Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706–715. doi: 10.1053/j.gastro.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Islam MM, Poly TN, Walther BA, et al. Adverse outcomes of long-term use of proton pump inhibitors: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(12):1395–1405. doi: 10.1097/MEG.0000000000001198. [DOI] [PubMed] [Google Scholar]
- 11.Parente F, Cucino C, Gallus S, et al. Hospital use of acid-suppressive medications and its fall-out on prescribing in general practice: a 1-month survey. Aliment Pharmacol Ther. 2003;17(12):1503–1506. doi: 10.1046/j.1365-2036.2003.01600.x. [DOI] [PubMed] [Google Scholar]
- 12.Sattayalertyanyong O, Thitilertdecha P, Auesomwang C. The inappropriate use of proton pump inhibitors during admission and after discharge: a prospective cross-sectional study. Int J Clin Pharm. 2020;42(1):174–183. doi: 10.1007/s11096-019-00955-8. [DOI] [PubMed] [Google Scholar]
- 13.Jabaaij L, Njoo K, Visscher S, et al. [Improve your reporting, use the EPD-scan-h]. [Article in Dutch]. Huisarts en Wetenschap. 2009;52:240–246. [Google Scholar]
- 14.World Health Organization Collaborating Centre for Drug Statistics Methodology. ATC/DDD index 2022. 2021. https://www.whocc.no/atc_ddd_index (accessed 29 Jun 2022).
- 15.Haastrup PF, Jarbøl DE, Thompson W, et al. When does proton pump inhibitor treatment become long term? A scoping review. BMJ Open Gastroenterol. 2021;8(1):e000563. doi: 10.1136/bmjgast-2020-000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Base TRDPAaH [Medical Pharmaceutical Decision Rule: ulcer prophylaxis for NSAID use]. [Article in Dutch] 2013. https://docplayer.nl/74611440-Maagbescherming-bij-nsaid-gebruik-107.html (accessed 22 Aug 2022).
- 17.Base TRDPAaH [Medical Pharmaceutical Decision Rule: Ulcer prophylaxis for salicylate/coxib use]. [Article in Dutch] 2014. https://docplayer.nl/57588870-Maagbescherming-bij-salicylaat-coxibgebruik-108.html (accessed 22 Aug 2022).
- 18.Numans ME, de Wit NJ, Dirven JAM, et al. [Dutch College of General Practitioners guideline ‘Upper gastrointestinal symptoms’]. [Article in Dutch] Huisarts en Wetetenschap. 2013;56:26–35. [Google Scholar]
- 19.Shaheen NJ, Falk GW, Iyer PG, et al. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol. 2016;111(1):30–50. doi: 10.1038/ajg.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito T, Igarashi H, Uehara H, Jensen RT. Pharmacotherapy of Zollinger-Ellison syndrome. Expert Opin Pharmacother. 2013;14(3):307–321. doi: 10.1517/14656566.2013.767332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Vliet EP, Steyerberg EW, Otten HJ, et al. The effects of guideline implementation for proton pump inhibitor prescription on two pulmonary medicine wards. Aliment Pharmacol Ther. 2009;29(2):213–221. doi: 10.1111/j.1365-2036.2008.03875.x. [DOI] [PubMed] [Google Scholar]
- 22.Jarbol DE, Lykkegaard J, Hansen JM, et al. Prescribing of proton-pump inhibitors: auditing the management and reasons for prescribing in Danish general practice. Fam Pract. 2019;36(6):758–764. doi: 10.1093/fampra/cmz025. [DOI] [PubMed] [Google Scholar]
- 23.Murthy SK, Kauldher S, Targownik LE. Physicians’ approaches to the use of gastroprotective strategies in low-risk non-steroidal anti-inflammatory drug users. Aliment Pharmacol Ther. 2006;23(9):1365–1372. doi: 10.1111/j.1365-2036.2006.02873.x. [DOI] [PubMed] [Google Scholar]
- 24.Jones R. Nonsteroidal anti-inflammatory drug prescribing: past, present, and future. Am J Med. 2001;110(1A):4S–7S. doi: 10.1016/s0002-9343(00)00627-6. [DOI] [PubMed] [Google Scholar]
- 25.Qato DM, Alexander GC, Conti RM, et al. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300(24):2867–2878. doi: 10.1001/jama.2008.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voukelatou P, Vrettos I, Emmanouilidou G, et al. Predictors of inappropriate proton pump inhibitors use in elderly patients. Curr Gerontol Geriatr Res. 2019;2019:7591045. doi: 10.1155/2019/7591045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laine L, Bombardier C, Hawkey CJ, et al. Stratifying the risk of NSAID-related upper gastrointestinal clinical events: results of a double-blind outcomes study in patients with rheumatoid arthritis. Gastroenterology. 2002;123(4):1006–1012. doi: 10.1053/gast.2002.36013. [DOI] [PubMed] [Google Scholar]
- 28.Van Soest EM, Siersema PD, Dieleman JP, et al. Persistence and adherence to proton pump inhibitors in daily clinical practice. Aliment Pharmacol Ther. 2006;24(2):377–385. doi: 10.1111/j.1365-2036.2006.02982.x. [DOI] [PubMed] [Google Scholar]
- 29.Chau SH, Sluiter RL, Hugtenburg JG, et al. Cost-utility and budget impact analysis for stopping the inappropriate use of proton pump inhibitors after cessation of NSAID or low-dose acetylsalicylic acid treatment. Drugs Aging. 2020;37(1):67–74. doi: 10.1007/s40266-019-00713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reimer C, Sondergaard B, Hilsted L, et al. Proton-pump inhibitor therapy induces acid-related symptoms in healthy volunteers after withdrawal of therapy. Gastroenterology. 2009;137(1):80–87. doi: 10.1053/j.gastro.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 31.Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med. 2006;166(9):965–971. doi: 10.1001/archinte.166.9.965. [DOI] [PubMed] [Google Scholar]
- 32.Lin D, Eke C, Cai C, et al. Decreasing overall and inappropriate proton pump inhibitor use: perspective from a large safety-net healthcare system. Clin Gastroenterol Hepatol. 2020;18(4):763–766.e2. doi: 10.1016/j.cgh.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Murie J, Allen J, Simmonds R, de Wet C. Glad you brought it up: a patient-centred programme to reduce proton-pump inhibitor prescribing in general medical practice. Qual Prim Care. 2012;20(2):141–148. [PubMed] [Google Scholar]