Abstract
Polycystic ovary syndrome (PCOS) occurs in approximately 10% of all reproductive‐age women, with over 50% of these patients having imaging‐confirmed nonalcoholic fatty liver disease (NAFLD). Whether PCOS increases the risk for more clinically relevant disease, such as nonalcoholic steatohepatitis (NASH), is unclear. Such findings are relevant to prognosticating risk of progressive liver disease in the growing population of young adults with NAFLD. Using weighted discharge data from the United States National Inpatient Sample from 2016 to 2018, we evaluated the association of PCOS with the presence of NASH among reproductive‐age women with NAFLD. The association of PCOS with NASH was assessed by logistic regression, adjusting for demographic and comprehensive metabolic comorbidities. Other causes of hepatic steatosis and chronic liver diseases were excluded. Our analysis included 189,440 reproductive‐age women with NAFLD, 9415 of whom had PCOS. Of those with PCOS, 1390 (15%) had a distinct code for NASH. Women with PCOS were younger (median age, 33 vs. 40 years; p < 0.001) and more likely to have diabetes (37.0% vs. 34.0%), obesity (83.0% vs. 58.0%), dyslipidemia (26.0% vs. 21.0%), and hypertension (38.0% vs. 35.0%) (all p ≤ 0.01). On adjusted analysis accounting for these metabolic comorbidities, PCOS remained independently associated with an increased prevalence of NASH (adjusted odds ratio, 1.22; 95% confidence interval, 1.05–1.42; p = 0.008). Conclusions: Among reproductive‐age women with NAFLD, metabolic risk factors were more common in those with PCOS. Despite adjustment for these metabolic comorbidities, PCOS remained associated with a 22% higher odds of having NASH. These findings support efforts to increase NAFLD screening in young women with PCOS and highlight the potential “head start” in progressive liver disease among young women with PCOS.

INTRODUCTION
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders in reproductive‐age women with a prevalence of 10–15%.[ 1 , 2 ] PCOS is tightly linked with the metabolic syndrome, with most women having insulin resistance as well as a greater risk for dyslipidemia and overweight/obese. Not surprisingly, PCOS is also an established risk factor for nonalcoholic fatty liver disease (NAFLD), with 40%–55% of women with PCOS having imaging‐confirmed hepatic steatosis.[ 3 ] This high risk of NAFLD likely relates to coexisting metabolic disease in PCOS as well as elevated levels of circulating androgens, which also promote hepatic steatosis in women.[ 4 , 5 ] However, whether PCOS is associated with more clinically significant manifestations of NAFLD, such as nonalcoholic steatohepatitis (NASH), remains unclear. Such findings are relevant to the need for routine NAFLD screening in adolescents and young women with PCOS in addition to prognosticating the risk of progressive liver disease among young women with NAFLD.
MATERIAL AND METHODS
This study leveraged the US National Inpatient Sample (NIS), the largest, publicly available, all‐payer inpatient, longitudinal database in the United States. The NIS was developed as part of the Health Cost and Utilization Project, which is sponsored by the Agency for Healthcare Research and Quality. Data are derived from over 7 million hospital admissions annually and include demographic variables and discharge diagnoses categorized by the International Classification of Diseases, tenth revision (ICD‐10) codes.[ 6 ] Weighted, the sample provides a nationally representative estimate of over 35 million hospital stays per year. The NIS does not capture outpatient encounters.
Using NIS data from 2016 to 2018, we evaluated the association of PCOS with the presence of NASH in reproductive‐age women (18–50 years old) and NAFLD defined by ICD‐10 K76.0. The 2016 start date was selected because a dedicated ICD‐10 code for NASH (K75.81) was introduced in the last quarter of 2015. PCOS was defined by ICD code E28.2. We excluded women with any potential non‐NAFLD causes of hepatic steatosis or steatohepatitis, such as alcohol‐related conditions, and women with NAFLD plus other concurrent acute or chronic liver diseases (Table S1).
Our outcome of interest was NASH defined by ICD‐10 K75.81 Covariates included demographics (age, race, rural vs. urban location, insurance status) and metabolic comorbidities, including diabetes (defined as “uncomplicated,” “complicated” for those with kidney, ophthalmic, neurologic, other specified or unspecified complications, and a “composite” value combining both that was used in our adjusted model), obesity, dyslipidemia, and hypertension (see Table S1). Demographic data were collected directly from the NIS database, and metabolic variables were determined by discharge diagnosis codes.
The association of PCOS with NASH was evaluated by multivariate logistic regression, adjusting for relevant demographic and metabolic risk factors by using survey‐specific versions of analysis procedures. Descriptive statistics were compared by PCOS status using Pearson chi‐square or t tests as appropriate. p ≤ 0.05 indicated statistical significance, and analyses were performed with Stata MP 17 (StataCorp, College Station, TX). The NIS data set was purchased by the University of California, San Francisco, and permission was obtained for analysis after completion of a signed data use agreement form. This study was exempt from institutional review board approval, given absence of patient identifiers.
RESULTS
From 2016 to 2018, there were 189,440 reproductive‐age women with NAFLD meeting study inclusion criteria, 9415 of whom had PCOS. Of those with NAFLaD and PCOS, 1390 (15.0%) had a distinct diagnosis of NASH. During the study period, there were no statistically significant differences in temporal trends of NASH prevalence by PCOS status. However, by 2018, the prevalence of NASH among those with PCOS and NAFLD was 16.8% versus 13.3% for women with NAFLD and no PCOS (p = 0.008) (Figure 1).
FIGURE 1.
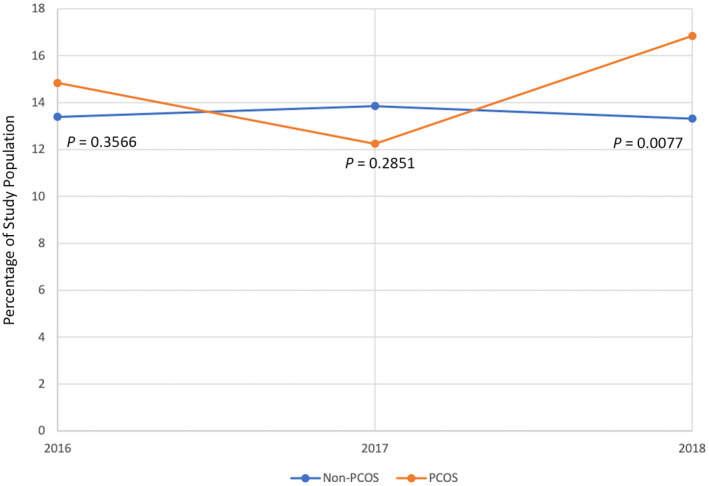
NASH prevalence as percentage of study population by PCOS status from 2016 to 2018. p values are from Fisher's exact test comparing proportion with NASH by PCOS status and year. NASH, nonalcoholic steatohepatitis; PCOS, polycystic ovary syndrome.
Compared to women without PCOS, those with PCOS were younger (median age, 33 vs. 40 years; p < 0.001) with a racial/ethnic distribution of 69.0% White, 17.0% Hispanic, and 7.7% Black, compared to 57.0% White, 24.0% Hispanic, and 13.0% Black among women without PCOS (p < 0.001) (Table 1). Women with PCOS were more likely to have private insurance (59.0% vs. 44.0%) and less likely to have Medicare (7.1% vs. 12.0%), Medicaid (24.0% vs. 33.0%), or other/unknown insurance (9.4% vs. 11.0%) (all p < 0.001). Women with PCOS were more likely to have diabetes (37.0% vs. 34.0%), obesity (83.0% vs. 58.0%), dyslipidemia (26.0% vs. 21.0%), and hypertension (38.0% vs. 35.0%) (all p ≤ 0.01).
TABLE 1.
Cohort characteristics by PCOS status (n = 189,440)
| Characteristic | PCOS (n = 9415) | Non‐PCOS (n = 180,025) | p value |
|---|---|---|---|
| Age, median years (IQR) | 33 (28–39) | 40 (32–46) | <0.001 |
| Race, n (%) | <0.001 | ||
| White | 6320 (69.0%) | 99,360 (57.0%) | |
| Black | 700 (7.7%) | 22,530 (13.0%) | |
| Hispanic | 1525 (17.0%) | 41,165 (24.0%) | |
| Asian or Pacific Islander | 165 (1.8%) | 3565 (2.0%) | |
| Native American | 95 (1.0%) | 1735 (1.0%) | |
| Other | 300 (3.3%) | 6635 (3.8%) | |
| Location (rural vs. urban), n (%) | 0.039 | ||
| Micropolitan counties or smaller | 1340 (14.0%) | 24,235 (13.0%) | |
| Counties of metropolitan areas of 50,000‐999,000 | 3130 (33.0%) | 55,050 (31.0%) | |
| Counties of metropolitan areas of ≥1 million | 4945 (53.0%) | 100,310 (56.0%) | |
| Insurance, n (%) | <0.001 | ||
| Medicare | 665 (7.1%) | 21,120 (12.0%) | |
| Medicaid | 2280 (24.0%) | 60,010 (33.0%) | |
| Private | 5585 (59.0%) | 79,210 (44.0%) | |
| Other/unknown | 885 (9.4%) | 19,685 (11.0%) | |
| Diabetes, n (%) | |||
| Composite | 3465 (37.0%) | 61,125 (34.0%) | 0.012 |
| Uncomplicated | 2190 (23.0%) | 30,130 (17.0%) | <0.001 |
| Complicated a | 1275 (14.0%) | 30,995 (17.0%) | <0.001 |
| Obese, n (%) | 7800 (83.0%) | 104,875 (58.0%) | <0.001 |
| Dyslipidemia, n (%) | 2425 (26.0%) | 38,320 (21.0%) | <0.001 |
| Hypertension, n (%) | 3590 (38.0%) | 63,100 (35.0%) | 0.012 |
Abbreviations: IQR, interquartile range; PCOS, polycystic ovary syndrome.
Complicated defined as diabetes mellitus with kidney, ophthalmic, neurologic, and other specified (e.g., vascular, dermatologic) or unspecified complications.
On adjusted analysis, older age, White race, and most metabolic comorbidities were associated with a higher prevalence of NASH among reproductive‐age women with NAFLD (Table 2). Although not apparent on unadjusted analysis (odds ratio [OR], 1.1; 95% confidence interval [CI], 0.97–1.28; p = 0.17), PCOS was strongly associated with a prevalence of NASH after adjusting for comprehensive demographic and metabolic comorbidities (OR, 1.22; 95% CI, 1.05–1.42; p = 0.008).
TABLE 2.
Association of PCOS with NASH, adjusted for baseline demographic and metabolic covariates
| Characteristic | Univariate analysis | Multivariate analysis a | ||
|---|---|---|---|---|
| OR (95% CI) | p value | AOR (95% CI) | p value | |
| PCOS | 1.11 (0.97–1.28) | 0.170 | 1.22 (1.05–1.42) | 0.008 |
| Age | 1.03 (1.03–1.04) | <0.001 | 1.03 (1.02–1.03) | <0.001 |
| Race (ref. group, White) | ||||
| Black | 0.56 (0.50–0.63) | <0.001 | 0.59 (0.52–0.66) | <0.001 |
| Hispanic | 0.66 (0.59–0.74) | <0.001 | 0.78 (0.70–0.87) | <0.001 |
| Asian or Pacific Islander | 0.41 (0.31–0.55) | <0.001 | 0.50 (0.37‐ | <0.001 |
| Native American | 1.29 (0.95–1.75) | 0.097 | 0.66) | 0.265 |
| Other | 0.64 (0.45–0.90) | 0.011 | 1.19 (0.88–1.60) 0.76 (0.53–1.08) | 0.125 |
| Location (ref. group, urban) | ||||
| Metropolitan 50,000‐999,000 | 1.34 (1.21–1.48) | <0.001 | 1.17 (1.06–1.29) | 0.002 |
| Micropolitan | 1.85 (1.66–2.06) | <0.001 | 1.46 (1.31–1.63) | <0.001 |
| Insurance (ref. group, Medicare) | ||||
| Medicaid | 0.46 (0.41–0.51) | <0.001 | 0.60 (0.54–0.67) | <0.001 |
| Private | 0.43 (0.39–0.48) | <0.001 | 0.54 (0.49–0.60) | <0.001 |
| Other/unknown | 0.42 (0.36–0.48) | <0.001 | 0.53 (0.45–0.61) | <0.001 |
| Diabetes | 1.99 (1.85–2.15) | <0.001 | 1.83 (1.68–2.00) | <0.001 |
| Obese | 1.08 (0.99–1.17) | 0.079 | 1.07 (0.98–1.17) | 0.123 |
| Dyslipidemia | 1.27 (1.17–1.37) | <0.001 | 0.91 (0.84–0.99) | 0.048 |
| Hypertension | 1.07 (1.01–1.15) | 0.034 | 0.83 (0.77–0.89) | <0.001 |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; NASH, nonalcoholic steatohepatitis; OR, odds ratio; PCOS, polycystic ovary syndrome; ref., reference.
Composite diabetes (uncomplicated plus complicated as defined in Table 1) used for adjustment in multivariate analysis.
DISCUSSION
In this large, nationally representative, population‐based study, we identified a more than 20% higher odds of prevalent NASH among reproductive‐age women with NAFLD and PCOS, despite these women being on average 7 years younger than women without PCOS. Although PCOS is an established risk group for NAFLD,[ 7 ] the current study supports the influence of PCOS on clinically significant liver disease. These findings have implications for NAFLD risk stratification in women with PCOS and support the need for routine NAFLD/NASH screening in women with PCOS.
PCOS occurs in an estimated 10–15% of reproductive‐age women, and its prevalence continues to rise.[ 1 , 2 , 8 ] Approximately half of reproductive‐age women with PCOS have imaging‐confirmed hepatic steatosis compared to 25% in the general population.[ 9 ] In our prior study of reproductive‐age women with biopsy‐confirmed NAFLD, we did not find PCOS to be associated with the presence of NASH, although we did identify PCOS as a risk factor for more severe histologic features, including advanced fibrosis.[ 10 ] Advanced fibrosis was also evident at a younger age than in women without PCOS, underscoring their risk for potentially early onset and accelerated disease. Such findings are supported by the current large epidemiologic database, which facilitated greater power to also detect differences in prevalent NASH by PCOS status, a finding that was independent of comprehensive metabolic risk factors.
The high prevalence of NAFLD among women with PCOS is particularly relevant as NASH is now the leading indication for liver transplantation in women[ 3 ] and the most common cause of chronic liver disease in young adults.[ 11 ] A recent population‐based observational study found that the prevalence of NAFLD among young adults aged 20–39 years old had increased from 9.22% in 1990 to 12.17% by 2017.[ 12 ] Practically speaking, this translates to an increasing number of reproductive‐age women presenting to a liver clinic for NAFLD evaluation and care. Given the influence of PCOS on clinically significant liver disease, hepatology providers should routinely inquire about PCOS and its related symptoms (hirsutism and irregular menses). Furthermore, the identification of PCOS in the liver clinic can allow women with suspected PCOS to be referred for specialist PCOS care.
How PCOS influences NASH risk may relate, in part, to more severe metabolic disease as 30%–60% of women with PCOS are overweight/obese[ 13 ] and 44%–85% have insulin resistance or frank diabetes.[ 14 ] However, the current study showed that NASH risk remained independent of coexisting metabolic comorbidities, including diabetes, obesity, and dyslipidemia. The current study could not evaluate the contribution of androgens to study outcomes; this is relevant as androgens are associated with severity of hepatic steatosis in women with PCOS.[ 4 ] In a prior study of predominantly patients without PCOS, higher testosterone levels did confer a greater severity of NASH, although findings were only evident in the youngest subgroup of reproductive‐age women,[ 15 ] which may relate to the natural decline in testosterone levels with premenopausal aging.[ 16 ] Whether androgens promote liver injury in women with PCOS, a population that maintains elevated androgens throughout premenopausal years, remains to be elucidated. Mechanisms by which androgens may influence NASH include androgenic effects on visceral adiposity as well as production of lipotoxic lipid species that have been shown to promote NASH in the general population.[ 17 , 18 ] More granular data on the role of androgens on liver injury and progression in women with PCOS, including potential mechanistic pathways, are needed.
The current findings also support the need to consider routine NAFLD/NASH screening in women with PCOS. The American Society for Reproductive Medicine and European Society of Human Reproduction and Embryology's current International guidelines, the most widely used in PCOS management, recommend screening for metabolic risk factors among women with PCOS, including hypertension, dyslipidemia, and insulin resistance/diabetes,[ 19 ] but do not include liver tests to screen for NAFLD/NASH. Given the high NAFLD prevalence in approximately half of patients with PCOS and current findings supporting greater risk for NASH, the inclusion of a liver panel as part of routine metabolic screening in this young population of high‐risk women should be considered.
The current study has several important strengths, including the use of a large racially diverse cohort with NASH‐specific coding. However, our data set relied on ICD‐10 coding; thus, we were unable to evaluate histologic data or laboratory parameters related to NASH or severity of NASH. The NIS also does not capture coding from the outpatient setting, where the majority of NAFLD cases are managed. Inpatients may have abnormal liver tests for other reasons, with potential miscoding as NAFLD/NASH, although such miscoding is unlikely to differ by PCOS status. Depending on the indication for hospital admission, particularly for liver‐related events, PCOS may not be deemed relevant for inclusion as a discharge diagnosis. Thus, the prevalence of NASH among reproductive‐age women with PCOS is likely underestimated. Despite these concerns, notable differences in the risk of NASH by PCOS status were observed.
In summary, we identified an increased risk of prevalent NASH among women with PCOS that was independent of metabolic risk factors, and was apparent despite the younger age of PCOS patients. Our findings underscore the need for a routine inquiry of PCOS by hepatology providers caring for young women with NAFLD. The findings also further support the need to consider routine NAFLD screening by the larger community of providers caring for PCOS. Such efforts may help to mitigate the risk of liver disease progression in this high‐risk population with early onset disease.
AUTHOR CONTRIBUTIONS
Monika Sarkar developed the study concept; contributed to the study design, data acquisition and interpretation; and provided key revisions to the draft manuscript. Stephanie Maldonado contributed to study design, performed statistical analyses and data interpretation, and drafted the initial manuscript. Joshua Grab contributed to data acquisition, interpretation and analyses, and manuscript editing. Connie Wang contributed to data analyses and manuscript editing. Heather Huddleston and Marcelle Cedars contributed to manuscript editing.
FUNDING INFORMATION
National Institutes of Health, K23 award; Grant Number: DK111944; University of California San Francisco, Liver Center core facilities; Grant Number: P30 DK026743.
CONFLICTS OF INTEREST
Monika Sarkar and Heather Huddleston have received grant support from Zydus Pharmaceuticals.
Supporting information
Appendix S1 Supporting Information
Maldonado SS, Grab J, Wang CW, Huddleston H, Cedars M, Sarkar M. Polycystic ovary syndrome is associated with nonalcoholic steatohepatitis in women of reproductive age . Hepatol Commun. 2022;6:2634–2639. 10.1002/hep4.2039
REFERENCES
- 1. Ndefo UA, Eaton A, Green MR. Polycystic ovary syndrome: a review of treatment options with a focus on pharmacological approaches. P T. 2013;38(6):336–55. [PMC free article] [PubMed] [Google Scholar]
- 2. Azziz R. Polycystic ovary syndrome. Obstet Gynecol. 2018;132(2):321–36. [DOI] [PubMed] [Google Scholar]
- 3. Yuan L, Kardashian A, Sarkar M. NAFLD in women: unique pathways, biomarkers and therapeutic opportunities. Curr Hepatol Rep. 2019;18(4):425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jones H, Sprung VS, Pugh CJ, Daousi C, Irwin A, Aziz N, et al. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97(10):3709–16. [DOI] [PubMed] [Google Scholar]
- 5. Sarkar MA, Suzuki A, Abdelmalek MF, Yates KP, Wilson LA, Bass NM, et al. Testosterone is associated with nonalcoholic steatohepatitis and fibrosis in premenopausal women with NAFLD. Clin Gastroenterol Hepatol. 2021;19(6):1267–74.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agency for Healthcare Research and Quality . HCUP: healthcare cost and utilization project. http://www.hcup‐us.ahrq.gov/nisoverview.jsp (2021). Accessed Jan 2, 2022. [PubMed]
- 7. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57. [DOI] [PubMed] [Google Scholar]
- 8. Hoeger KM, Dokras A, Piltonen T. Update on PCOS: consequences, challenges, and guiding treatment. J Clin Endocrinol Metabol. 2021;106(3):e1071–83. [DOI] [PubMed] [Google Scholar]
- 9. Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69(6):2672–82. [DOI] [PubMed] [Google Scholar]
- 10. Sarkar M, Terrault N, Chan W, Cedars MI, Huddleston HG, Duwaerts CC, et al. Polycystic ovary syndrome (PCOS) is associated with NASH severity and advanced fibrosis. Liver Int. 2020;40(2):355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doycheva I, Watt KD, Alkhouri N. Nonalcoholic fatty liver disease in adolescents and young adults: the next frontier in the epidemic. Hepatology. 2017;65(6):2100–9. [DOI] [PubMed] [Google Scholar]
- 12. Zhang X, Wu M, Liu Z, Yuan H, Wu X, Shi T, et al. Increasing prevalence of NAFLD/NASH among children, adolescents and young adults from 1990 to 2017: a population‐based observational study. BMJ Open. 2021;11(5):e042843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Gynecology . ACOG Practice Bulletin No. 194: polycystic ovary syndrome. Obstet Gynecol. 2018;131(6):e157–e171. Erratum in: Obstet Gynecol. 2020;136(3):638.29794677 [Google Scholar]
- 14. Jeanes YM, Reeves S. Metabolic consequences of obesity and insulin resistance in polycystic ovary syndrome: diagnostic and methodological challenges. Nutr Res Rev. 2017;30(1):97–105. [DOI] [PubMed] [Google Scholar]
- 15. Rajagopal S, Whetstone S, Sarkar M. Relevance of reproductive health to comprehensive hepatology care. Lancet Gastroenterol Hepatol. 2021;6(4):260–2. [DOI] [PubMed] [Google Scholar]
- 16. Zumoff B, Strain GW, Miller LK, Rosner W. Twenty‐four‐hour mean plasma testosterone concentration declines with age in normal premenopausal women. J Clin Endocrinol Metab. 1995;80(4):1429–30. [DOI] [PubMed] [Google Scholar]
- 17. Anjani K, Lhomme M, Sokolovska N, Poitou C, Aron‐Wisnewsky J, Bouillot JL, et al. Circulating phospholipid profiling identifies portal contribution to NASH signature in obesity. J Hepatol. 2015;62(4):905–12. [DOI] [PubMed] [Google Scholar]
- 18. O'Reilly MW, Kempegowda P, Walsh M, Taylor AE, Manolopoulos KN, Allwood JW, et al. AKR1C3‐mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(9):3327–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. International PCOS Network. Recommendations from the international evidence‐based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110(3):364–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information


