Abstract
Percutaneous thermal ablation (PTA), resection, and liver transplantation are the standard curative options for hepatocellular carcinoma (HCC). Liver transplantation yields the best long‐term outcomes but is limited by graft shortage. Thus, patients with ≤3‐cm HCC are primarily treated by PTA even though recurrence is frequent and may occur outside transplant criteria. Data on non‐transplantable recurrence (NTR) following PTA are lacking, however. We therefore investigated the incidence and predictors of NTR among 213 potentially transplantable patients (cirrhosis, 93%; Child‐Pugh A, 98.6%; alcohol‐related disease, 62%) with ≤3‐cm HCC(s) treated by PTA, to stratify them according to their NTR risk and to improve treatment allocation. During follow‐up (median: 41.2 months), NTR occurred in 18.3% (alpha‐fetoprotein [AFP] model) and 23% (Milan) patients. NTR prediction with competing‐risk analysis and internal validation revealed AFP > 100 ng/ml (subdistribution hazard ratio: 7.28; p < 0.001) and prior HCC (subdistribution hazard ratio: 3.77; p = 0.002) as independent predictors (Harrell's C: 0.76). Based on this model using the AFP score (equally predictive within Milan criteria), patients were stratified into three NTR risk categories: HCC‐naïve with AFP < 100 ng/ml (low risk, n = 108 of 213), non‐HCC naïve with AFP < 100 ng/ml (intermediate risk, n = 92 of 213), AFP ≥ 100 ng/ml (high risk, n = 13 of 213), among whom 9.3% (3.7% [Milan]), 22.8% (25% [Milan]), and 61.5% (38/5% [Milan]) presented NTR (p < 0.001). Median recurrence‐free survival was 4.6, 14.5, and 43.4 months, respectively, in high‐risk, intermediate‐risk, and low‐risk categories (p < 0.001). Median overall survival, which was 19.1 months in high‐risk patients, was not reached otherwise (p < 0.001). Conclusion: Overall, PTA of ≤3‐cm HCC incurs a low NTR risk. Simple and noninvasive predictors (HCC naivety, AFP) accurately stratified patients' risk of NTR, and should help to improve treatment allocation. Patients with AFP ≥ 100 ng/ml have a high risk of NTR, poor recurrence‐free survival, and overall survival. Further studies evaluating preemptive transplantation or adjuvant/neoadjuvant strategies are highly needed in this small patient subset.
Most patients who are eligible for primary PTA remained eligible for salvage LT. Simple and noninvasive covariates (HCC naivety, AFP) obtained before PTA of 3‐cm HCC(s) accurately stratified patients' risk of NTR, ranging from 9.3% to 61.5% (AFP model) and 12% to 53.8% (Milan).

INTRODUCTION
Percutaneous thermal ablation (PTA), resection, and liver transplantation (LT) are the standard curative options for hepatocellular carcinoma (HCC).[ 1 , 2 ] By suppressing both the tumor and the underlying liver disease, LT yields the best long‐term outcomes but is limited by graft shortage and by the risk of progression and eventual dropout from the waiting list.[ 2 , 3 , 4 , 5 ] Consequently, PTA and resection are frequently used upfront in patients with Barcelona Clinic Liver Cancer (BCLC) 0‐A either in a bridge‐to‐transplant strategy or to keep LT as a salvage therapy in case of recurrence.[ 2 ] However, contrary to LT, both PTA and resection suffer from high recurrence rate (60%–80%).[ 2 , 6 ] When occurring, tumor recurrence may be considered non‐transplantable if it exceeds the transplantation criteria such as those defined by the alpha‐fetoprotein (AFP) model[ 7 ] or Milan.[ 8 ] Non‐transplantable recurrence (NTR) is a major cause of precluding salvage LT, which showed comparable overall survival (OS) to primary LT in patients with HCC with compensated cirrhosis.[ 5 ]
NTR is therefore a relevant endpoint for transplantation centers, even though it has been investigated in only a few surgical series,[ 3 , 9 , 10 , 11 ] with predictors mostly determined from pathological analysis of specimen, thereby limiting their impact for upfront treatment choice. Likewise, very limited data on NTR are available in patients treated by PTA, which has become the most frequently used curative treatment of HCC because of its excellent tolerance even in patients with significant portal hypertension or comorbidity, and its improved cost‐effectiveness and comparable oncological outcomes versus resection.[ 12 ] One study[ 13 ] focused on transplantable patients and examined NTR (beyond Milan) after radiofrequency (RF) ablation of small HCC. However, this study only included a subset of patients recommended for PTA by European Association for the Study of the Liver (EASL)/American Association for the Study of Liver Diseases (AASLD)/BCLC,[ 1 , 2 , 14 ] namely HCC‐naïve patients with solitary HCC. Therefore, incidence and predictors of NTR in an unselected population of transplantable patient candidates to PTA remain unknown. This information is crucial to improve upfront treatment choice and to better define the place of salvage LT in early HCC. Additionally, the identification of the patients at risk for NTR would help to design relevant neoadjuvant and/or adjuvant clinical trials.
This study aimed to investigate the incidence and baseline predictors of NTR among potentially transplantable patients with ≤3‐cm HCC(s) treated by PTA and to stratify them according to their risk of NTR to improve treatment allocation.
METHODS
Study population
Consecutive patients who underwent PTA for HCC between January 2015 and December 2020 were included (Figure 1). Data were collected from our prospective database. This retrospective study was approved by our institutional review board (NCT03428321 [www.clinicaltrials.gov]), and written informed consent was obtained from all patients. Inclusion criteria were as follows: HCC diagnosed by histopathology or by EASL imaging criteria, HCC ≤ 30 mm, 1–3 tumor nodules, follow‐up < 3 months, no prior or combined treatment with intra‐arterial therapy, no prior systemic treatment, and potentially transplantable patient (i.e., ≤70 years old, AFP score ≤ 2,[ 7 ] no macroscopic portal vein invasion or extrahepatic metastasis, and no major comorbidity precluding LT).
FIGURE 1.
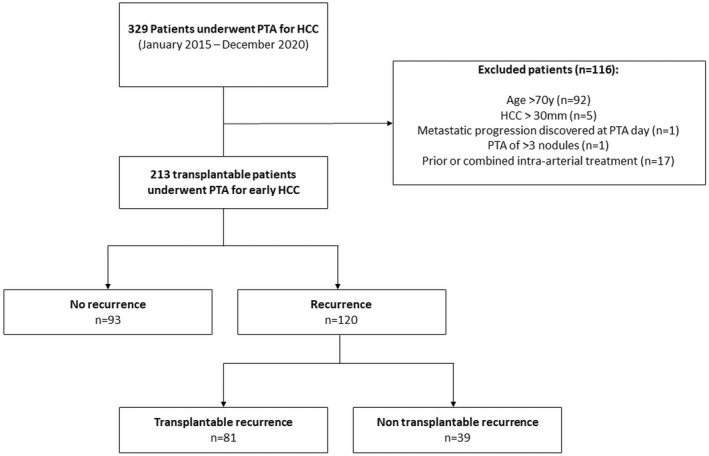
Study flowchart. HCC, hepatocellular carcinoma; PTA, percutaneous thermal ablation.
Patient and liver characteristics were collected, including age, sex, HCC‐naïve status, body mass index, diabetes mellitus, liver steatosis, cirrhosis, cause for hepatopathy, presence of steatotic HCC (defined when signal intensity loss was noted on opposed‐phase compared with in‐phase gradient‐echo images for at least one HCC nodule[ 15 ]), AFP serum level, Child‐Pugh, Model for End‐Stage Liver Disease (MELD) and albumin‐bilirubin (ALBI) scores, and baseline biological data (Table 1).
TABLE 1.
Baseline characteristics of patients
| Characteristic | N (%) or median (IQR) |
|---|---|
| Patients | n = 213 |
| Age, years (median [IQR]) | 62 (56–65) |
| Sex (n, %) | |
| Male | 179 (84) |
| Female | 34 (16) |
| ASA score (n, %) | |
| 1–2 | 112 (52.6) |
| 3–4 | 101 (47.4) |
| Diabetes (n, %) | |
| No | 141 (66.2) |
| Yes | 72 (33.8) |
| Metformin treatment (n, %) | 39 (18.3) |
| Statin treatment (n, %) | 32 (15) |
| BMI (kg/m2) | 27 (24–30) |
| Prior curative treatment (n, %) | |
| Naive patient | 98 (46) |
| Yes | 115 (54) |
| Liver disease | |
| Cirrhosis (n, %) | |
| No | 15 (7) |
| Yes | 198 (93) |
| Causes of liver disease (n, %) | |
| Alcohol | 132 (62%) |
| Viral hepatitis B or C | 46 (21.6%) |
| Other liver disease | 35 (16.4) |
| Steatosis (n, %) | |
| Absent | 140 (65.7) |
| Present | 73 (34.3) |
| MRI quantification, % (median [IQR]) | 3 (2–6) |
| Child‐Pugh class (n, %) | |
| A | 208 (97.7%) |
| B | 5 (2.3) |
| MELD score (median [IQR]) | 8 (7–10) |
| Laboratory data (median [IQR]) | |
| AFP (ng/ml) | 5.7 (3.4–11.45) |
| AFP > 100 ng/ml (n, %) | |
| Total bilirubin (μmol/L) | 11.7 (8–18) |
| Albumin (g/L) | 40.8 (38–43.7) |
| Prothrombin activity (%) | 83 (72–97) |
| AST (UI/ml) | 34.5 (24–48) |
| ALT (UI/ml) | 27 (19–38) |
| GGT (UI/ml) | 100.5 (51–222) |
| Platelet count (×10/mm3) | 122 (81–174) |
| Neutrophils (×10/mm3) | 3.35 (2.35–4.07) |
| Lymphocytes (×10/mm3) | 1.42 (1.05–2.03) |
| Monocytes (×10/mm3) | 0.52 (0.28–0.67) |
| Creatinine (μmol/L) | 72 (63–85) |
| ALBI score | |
| 1 | 144 (72) |
| 2 | 56 (28) |
| HCC | |
| Tumor size (median [IQR]) | 16 (13–20) |
| <20 mm | 173 (81.2%) |
| >20 mm | 40 (18.8%) |
| No. of nodules per patient (n, %) | |
| 1 | 157 (73.7%) |
| 2 | 42 (19.7%) |
| 3 | 14 (6.6%) |
| At least one biopsy‐proven nodule (n, %) | 47 (22) |
| Steatotic HCC (n, %) | 40 (18.8) |
| Subcapsular location (n, %) | 80 (37.6) |
| PTA | |
| PTA modality (n, %) | |
| Radiofrequency | 103 (48.4) |
| Microwave | 110 (51.6) |
| Imaging guidance (n, %) | |
| US guidance | 112 (52.6) |
| CT guidance | 101 (47.4) |
Note: Unless otherwise indicated, results are expressed as numbers (percentages).
Abbreviations: ALBI, albumin‐bilirubin; ALT, alanine aminotransferase; ASA, American Society of Anesthesiologists; AST, aspartate aminotransferase; BMI, body mass index; CT, computed tomography; GGT, gamma‐glutamyltransferase; IQR, interquartile range; MELD, Model for End‐Stage Liver Disease; MRI, magnetic resonance imaging; SIR, Society of Interventional Radiology; US, ultrasonography.
PTA
Treatment by PTA was decided during our biweekly multidisciplinary meeting on liver tumors. Our local policy favors PTA as a first‐line option in patients with early HCC (BCLC 0‐A). Other treatment options are considered in the case of Child‐Pugh score > B7; World Health Organization performance status >1; prothrombin time ratio < 50%, platelet count < 50 G/L, perihilar tumor, or history of biliary‐digestive anastomosis or endoscopic sphincterotomy. In compliance with the French allocation policy, PTA in BCLC 0‐A is used as a bridge to LT in enlisted patients with well‐preserved liver function. In the case of complete treatment by PTA, there is a temporary inactivation on the waiting list without the allocation points awarded based on the waiting time being lost.[ 16 ] Rules implemented in 2016 lead us to grant exception points to patients with transplantable recurrence occurring >6 months after PTA.[ 17 ]
All procedures were performed under general anesthesia in a multimodality interventional suite as previously reported,[ 18 ] using RF or microwave (MW) device, depending on the operator's choice. It was intended to have a 5–10‐mm ablation margin around the tumor. Ultrasonography (US) was the first‐line guidance modality. No PTA had been excluded for technical reasons (tumor location, visibility, or accessibility) due to multimodal imaging guidance (US was the preferred guidance modality, but ethiodized oil tagging and computed tomography [CT] guidance were used whenever necessary). Contrast‐enhanced CT (portal phase) was performed immediately after the procedure both to evaluate the ablation zone (i.e., the area of low attenuation) and to detect potential complications.
Follow‐up and outcomes
Clinical and biological evaluations (including AFP level and liver function test), together with imaging follow‐up by contrast‐enhanced magnetic resonance imaging [MRI], were performed 6 weeks after PTA and every 3 months thereafter. In addition, a chest CT scan was conducted every 6 months. Complete ablation observed on the first follow‐up MRI was considered the primary treatment success. Secondary treatment success was treatment success observed only after a second PTA performed within 8 weeks after the first one.
During follow‐up, the first three occurrences of both local and distant recurrences (according to usual definitions[ 15 , 19 ]) were recorded. In the case of tumor relapse, the following characteristics of tumor recurrence were collected on follow‐up imaging: number of HCC nodules according to EASL criteria, size of the largest nodule, presence of portal vein invasion (PVI) as detected by MRI (from subsegmental to main PVI), and presence of extrahepatic metastasis. In addition, AFP serum level at recurrence was also noted. NTR was defined as any of the following: hepatic recurrence with AFP score > 2, PVI, or ≥1 extrahepatic metastasis.
In case of recurrence, all patients were rereviewed by the multidisciplinary tumor board. Due to graft shortage, our policy was to re‐ablate intrahepatic recurrence whenever possible and to consider liver resection or salvage LT otherwise. Intra‐arterial therapies were used if patients were not candidates to curative options or as a bridging or downstaging therapy to LT, in keeping with the concept of stage migration.[ 2 ]
Statistical analysis
Continuous variables were described using means ± SD or medians and interquartile range (IQR), depending on the normality of their distribution. Median (and 95% confidence interval [CI]) follow‐up was calculated using the reverse Kaplan–Meier method. Categorical variables were compared with the Fischer's exact test, and continuous variables with the two‐sided t test or Kruskal‐Wallis test, as appropriate.
Occurrence of NTR after PTA was analyzed in a competing‐risks framework, with transplantation and death as competing events. Covariates associated with NTR were analyzed using Fine‐Gray proportional subdistribution hazards models. Significant covariates at univariate analysis were included in the multivariate model, and subdistribution hazard ratios (SHRs) were calculated. We also computed Harrell's C‐statistic for discrimination (where no predictive discrimination would have a Harrell's C index of 0.5 and perfect separation of patients, a Harrell's C index of 1.0). The final multivariate model was internally validated using bootstrapping (200 replications). A stratification of patients according to their NTR risk was obtained based on SHR of covariates. The results regarding NTR according to Milan criteria were considered exploratory, as our allocation policy for LT followed the AFP model.
Recurrence‐free survival (RFS) was defined as the time from PTA until the first recurrence, death, or last follow‐up. Patients who underwent LT were censored at transplantation date. Overall survival (OS) was defined as the interval between PTA and death (any cause) or last follow‐up. Survival curves were estimated using the Kaplan–Meier method and compared with the log‐rank test. Cox proportional‐hazards models were built to compute the hazard ratios (HRs) of covariates with their 95% CI. A robust variance estimator was used systematically. Log linearity was checked using fractional polynomials. To avoid overfitting, we applied for each multivariate model the rule of 1 independent variable per 10 events included.
All analyses were performed with the Stata software, version 16.1 (Stata Corp.). A p‐value < 0.05 was considered significant.
RESULTS
Study population and baseline characteristics
Between January 2015 and December 2020, 329 consecutive patients underwent PTA for HCC at our institution. A total of 116 patients were excluded due to (a) age > 70 years (n = 92), (b) HCC > 30 mm (n = 5), (c) metastatic progression discovered at PTA day (n = 1), (d) PTA of > 3 nodules (n = 1), and (e) combined or prior intra‐arterial treatment (n = 17). Therefore, 213 patients (median age: 62 years [IQR: 56–65 years]; 84% men [179 of 213]) who underwent PTA of 283 small HCC nodules (one, two, and three HCC nodules in 157, 42, and 14 patients, respectively) were included in this study. Cirrhosis was noted in 93% (198 of 213) patients, primarily alcohol‐related (62%) with preserved liver function in most cases (Child‐Pugh A in 98.6%, median MELD score of 8 [IQR: 7–10] and ALBI score 1–2 in 94.4%).
Non‐HCC‐naïve patients (n = 115; 54%) underwent prior curative treatment (resection in 25, PTA in 90) without any intra‐arterial or systemic therapy. Initial HCC was solitary in 87 patients (median diameter: 23 mm [range: 10–38]) and multifocal in 28 patients (median number of nodules: 2 [range: 2–3]; median diameter: 19 mm [range: 10–34]).
Among our 213 patients, 97 (45.5%) were listed for LT during the study period. The main indication for enlistment was HCC (94.8%; 92 of 97). Among the 38 patients (out of 97 on the waiting list) who were listed before recurrence, only 14 (36.8%; 14 of 38) received LT after a median delay of 17.5 months (range: 3–76); 14 (36.8%; 14 of 38) dropped out due to NTR or death; 2 refused to receive LT; and the others (n = 8) were placed on temporary contraindication after curative treatment. Among the 26 patients (out of 97 on the waiting list) who were listed after HCC recurrence (84.6% after 2018, as MELD exception points were proposed by then in case of HCC recurrence remaining within transplant criteria), 13 received LT after a median delay of 16 months (range: 1–28); 9 dropped out due to NTR or death; and the others (n = 4) refused LT.
Tumor biopsy was performed in 22% patients (47 of 213). HCC median size was 16 mm (IQR: 13–20), with subcapsular location in 37.6% (80 of 213) patients.
PTA was performed with RF in 103 patients (48.4%) and MW in 110 patients (51.6%). Guidance imaging modality was US in 51.6% of cases.
Follow‐up and outcome
Primary and secondary treatment success was obtained in 98.9% and 100% of patients, respectively. No PTA‐related death was recorded. After a median follow‐up of 41.2 months (95% CI: 36.4–45.2), 16% (34 of 213) patients were transplanted and 23% (49 of 213) died.
At least one recurrence was observed in 56.3% (120 of 213) patients during follow‐up. Local recurrence was 12.4% at 1 year (19.7% during follow‐up). No tumor track seeding was noted. Median RFS was 20.8 months (95% CI: 15.6–26.8) (Figure 2). Median OS was not reached. OS rates at 1, 2, 3, 4, and 5 years were 96.5% (95% CI: 92.9–98.3), 84.3% (95% CI: 78–88.9), 78.6% (95% CI: 71.4–84.1), 74.1% (95% CI: 65.5–80.9), and 68.2% (95% CI: 58.3–76.3), respectively (Figure 2).
FIGURE 2.
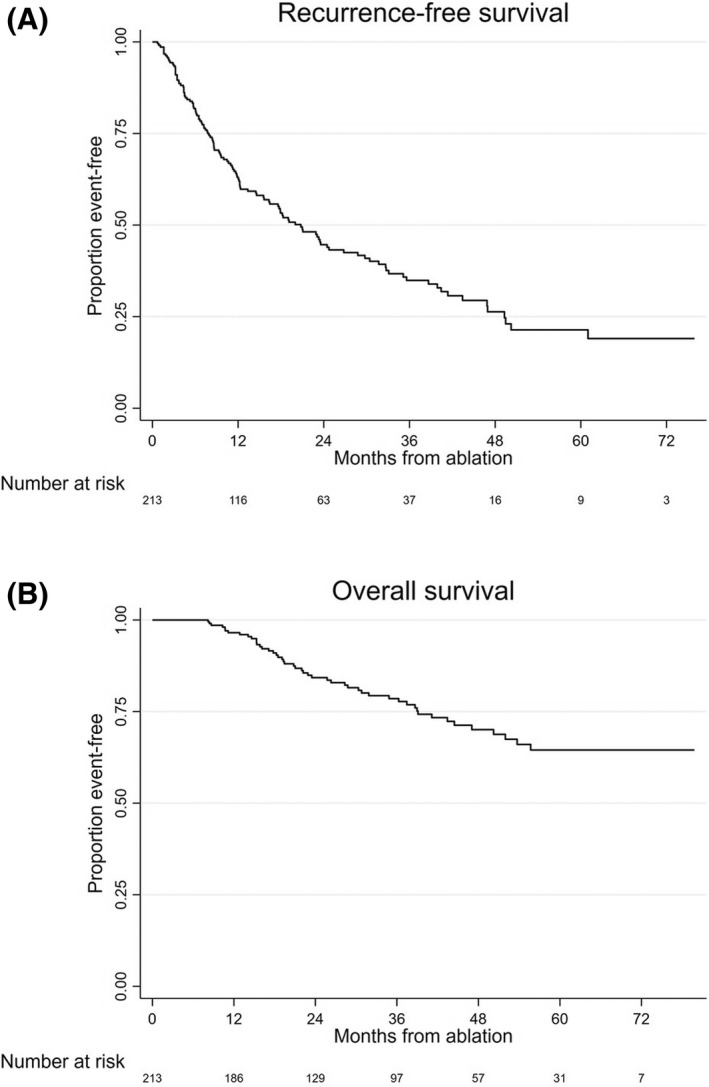
Recurrence‐free (A) and overall (B) survival of study population.
First distant recurrence was detected in 53% (113 of 213) patients after a median time‐to‐recurrence of 9.6 months (IQR: 4.4–18.3) from PTA. This first distant recurrence presented with >3 nodules in 8% (9 of 113), PVI in 6.2% (7 of 113), or ≥1 extrahepatic metastasis in 11.5% (13 of 113). At first distant recurrence, median tumor diameter was 14 mm (IQR: 11–18), whereas median AFP level was 5.7 ng/ml (IQR: 3.4–5.7).
Overall, NTR occurred in 18.3% (39 of 213) patients during follow‐up, determining a 1‐year, 2‐year, and 3‐year NTR cumulative incidence of 8.5%, 15.6% and 17.6%, respectively. Median time‐to‐NTR from PTA was 12.8 months (IQR: 7.7–22.8) in these patients. NTR occurred as the first recurrence in 56.4% (22 of 39) of cases. In patients presenting NTR, 5.1% (2 of 39) had >3 HCC nodules, 17.9% (7 of 39) had PVI, and 15.4% (6 of 39) showed ≥1 extrahepatic metastasis. At NTR, the largest tumor nodule had a median diameter of 26 mm (IQR: 18–50), whereas median AFP level was 92 ng/ml (IQR: 5–1633). NTR occurred in the same segment as PTA in only 12.8% (5 of 39) of the cases.
Pre‐ablation model for NTR
The following covariates were associated with NTR in univariate analysis: AFP > 100 ng/ml (SHR: 6.35; p < 0.001), steatotic HCC (SHR: 0.3; p = 0.044), multifocality (SHR: 2.07; p = 0.027), and nonnaïve HCC (SHR: 3; p = 0.001) (Table 2). Pre‐ablation multivariate model for NTR revealed AFP > 100 ng/ml (SHR: 7.28 [95% CI: 2.63–20.15]; p < 0.001) and nonnaïve HCC (SHR: 3.77 [95% CI: 1.65–8.65]; p = 0.002) as independent predictors. Both predictors were internally validated using bootstrapping. Harrell's C index was 0.76, indicating a good discrimination ability of the model.
TABLE 2.
Pre‐ablation model for NTR (competing‐risk regression)
| Variables | Univariate analysis | Multivariate analysis | Bootstrapping (200 replications) | |||
|---|---|---|---|---|---|---|
| SHR (95% CI) | p | SHR (95% CI) | p | SHR (95% CI) | p | |
| Patients | ||||||
| Age (>65 vs. ≤65 years) | 0.48 (0.21–1.11) | 0.08 | ||||
| Sex (female vs. male) | 0.85 (0.37–1.97) | 0.70 | ||||
| BMI (≥30 vs. <30) | 1.4 (0.66–2.97) | 0.38 | ||||
| ASA (>2 vs. ≤2) | 0.84 (0.45–1.56) | 0.45 | ||||
| Diabetes | 1.19 (0.62–2.29) | 0.59 | ||||
| Metformin treatment | 0.70 (0.27–1.79) | 0.46 | ||||
| Statin treatment | 0.85 (0.33–2.17) | 0.74 | ||||
| Non‐HCC naive | 3 (1.54–5.85) | 0.001 | 3.77 (1.64–8.64) | 0.002 | 3.77 (1.38–10.31) | 0.010 |
| Cirrhosis | 1.32 (0.33–5.16) | 0.68 | ||||
| Steatosis | 0.79 (0.40–1.56) | 0.51 | ||||
| Child‐Pugh (B vs. A) | 0.97 (0.10–8.91) | 0.98 | ||||
| Cause of liver disease (vs. alcohol) | ||||||
| Viral hepatitis | 1.09 (0.54–2.23) | 0.801 | ||||
| Other | 0.14 (0.2–1.02) | 0.059 | ||||
| Laboratory data | ||||||
| AFP (≥100 vs. <100 ng/ml) | 6.35 (2.67–15.11) | <0.001 | 7.28 (2.63–20.14) | <0.001 | 7.28 (1.78–29.79) | 0.006 |
| Prothrombin time | 0.99 (0.97–1.02) | 0.77 | ||||
| Platelet count (≥100 vs. <100 ×10/mm3) | 1.08 (0.52–2.26) | 0.83 | ||||
| Albumin | 0.98 (0.92–1.04) | 0.53 | ||||
| Bilirubin | 1.01 (0.96–1.06) | 0.64 | ||||
| Neutrophils | 0.91 (0.72–1.15) | 0.46 | ||||
| Lymphocytes | 0.81 (0.6–1.11) | 0.2 | ||||
| Monocytes | 0.73 (0.24–2.18) | 0.58 | ||||
| MELD (>9 vs. ≤9) | 1.03 (0.52–2.03) | 0.92 | ||||
| ALBI score (2 vs. 1) | 0.92 (0.47–1.80) | 0.81 | ||||
| HCC | ||||||
| Multifocal (vs. unifocal) | 2.07 (1.08–3.94) | 0.027 | 1.67 (0.75–3.71) | 0.20 | 1.67 (0.64–4.38) | 0.64 |
| Tumor size (<2 vs. ≥2 cm) | 1.26 (0.57–2.78) | 0.56 | ||||
| Steatotic HCC | 0.3 (0.09–0.96) | 0.044 | 0.54 (0.157–1.88) | 0.335 | 0.54 (0–196.11) | 0.84 |
| Subcapsular location | 1.21 (0.64–2.29) | 0.54 | ||||
| PTA | ||||||
| Modality (MW vs. RF) | 1.9 (0.97–3.67) | 0.07 | ||||
| Guidance (US vs. CT) | 1.46 (0.78–2.75) | 0.24 | ||||
| Harrell's C statistic: 0.76 | ||||||
Abbreviations: SHR, subdistribution hazard ratio; CI, confidence interval.
Data in bold are statistically significant (i.e., p < 0.05).
Stratification of NTR risk
Given the approximately doubled SHR of AFP > 100 ng/ml compared with that of the nonnaïve patient, a 1‐point score was attributed to nonnaïve patients and 2‐point score was awarded in patients with AFP > 100 ng/ml. Therefore, the score varied from 0 to 3. Stratification of patients determining low risk, intermediate risk, and high risk of NTR was then established as follows: The low‐risk group included patients both HCC‐naïve and with AFP < 100 ng/ml (score = 0); the intermediate‐risk group consisted of patients both nonnaïve and with AFP < 100 ng/ml (score = 1); and the high‐risk group included patients with AFP ≥ 100 ng/ml, regardless of HCC naivety (score ≥ 2).
NTR (AFP model) according to NTR risk stratification
Overall, 50.7% (108 of 213), 43.2% (92 of 213), and 6.1% (13 of 213) patients were classified in the low‐risk, intermediate‐risk, and high‐risk categories for NTR, respectively, among whom 9.3% (10 of 108), 22.8% (21 of 92), and 61.5% (8 of 13) presented NTR (p < 0.001) (Table 3). NTR occurred as the first recurrence in 2.8% (3 of 108), 15.2% (14 of 92), and 38.5% (5 of 13), respectively, in the low‐risk, intermediate‐risk, and high‐risk categories (p < 0.001). Compared with low‐risk patients, SHR for intermediate‐risk patients was 2.7 (95% CI: 1.29–5.6; p = 0.008), whereas it was 10.4 (95% CI: 3.83–28.1; p = 0.008) for high‐risk patients (Harrell's C index: 0.71). NTR cumulative incidence in the low‐risk, intermediate‐risk, and high‐risk categories was 4.1%, 10.7% and 35.3%, respectively, at 1 year; 7.9%, 19.9%, and 57.5% at 2 years; 9.1%, 22.5%, and 62.6% at 3 years; 10.7%, 26.2%, and 68.9% at 4 years (Figure 3). In patients presenting with NTR, median time‐to‐NTR was 16.8 months, 12.8 months, and 5.4 months, respectively, in low‐risk, intermediate‐risk, and high‐risk categories (p < 0.001).
TABLE 3.
NTR cumulative incidence and survival according to pre‐ablative NTR model
| Low risk (n = 08; 50.7%) HCC‐naïve and AFP < 100 ng/ml | Intermediate risk (n = 92; 43.2%) Prior HCC and AFP < 100 ng/ml | High risk (n = 13; 6.1%) AFP ≥ 100 ng/ml | |
|---|---|---|---|
| AFP model | |||
| NTR (%) | |||
| Overall b | 9.3% | 22.8% | 61.5% |
| At first recurrence b | 2.8% | 15.2% | 38.5% |
| at 1 year | 4.1% | 10.7% | 35.3% |
| at 2 years | 7.9% | 19.9% | 57.5% |
| at 3 years | 9.1% | 22.5% | 62.6% |
| at 4 years | 10.7% | 26.2% | 68.9% |
| Time to NTR a , b (median [IQR]) | 16.8 months (6.7–40.4) | 12.8 months (10.3–21.1) | 5.4 months (4–13.6) |
| Milan criteria | |||
| NTR (%) | |||
| Overall b | 12% | 31.5% | 53.8% |
| At first recurrence b | 3.7% | 25% | 38.5% |
| at 1 year | 6.1% | 17.4% | 32.6% |
| at 2 years | 10.4% | 28.2% | 49.7% |
| at 3 years | 12% | 32% | 55.1% |
| at 4 years | 13.7% | 36% | 60.4% |
| Time to NTR a , b (median [IQR]) | 15.2 months (10.7–28.1) | 10.5 months (8.4–15.8) | 5.6 months (3.5–14.7) |
| Survival | |||
| RFS (median [95% CI]) | 43.4 months (23.6–50.2) | 14.5 months (8.6–18.3) | 4.6 months (2.2–23.5) |
| OS (median [95% CI]) | NR | NR | 19.1 months (10.3–NR) |
Abbreviations: NR, not reached; OS, overall survival; RFS, recurrence‐free survival.
Among patients presenting NTR.
Indicates significant differences among the three risk categories (p < 0.001).
FIGURE 3.
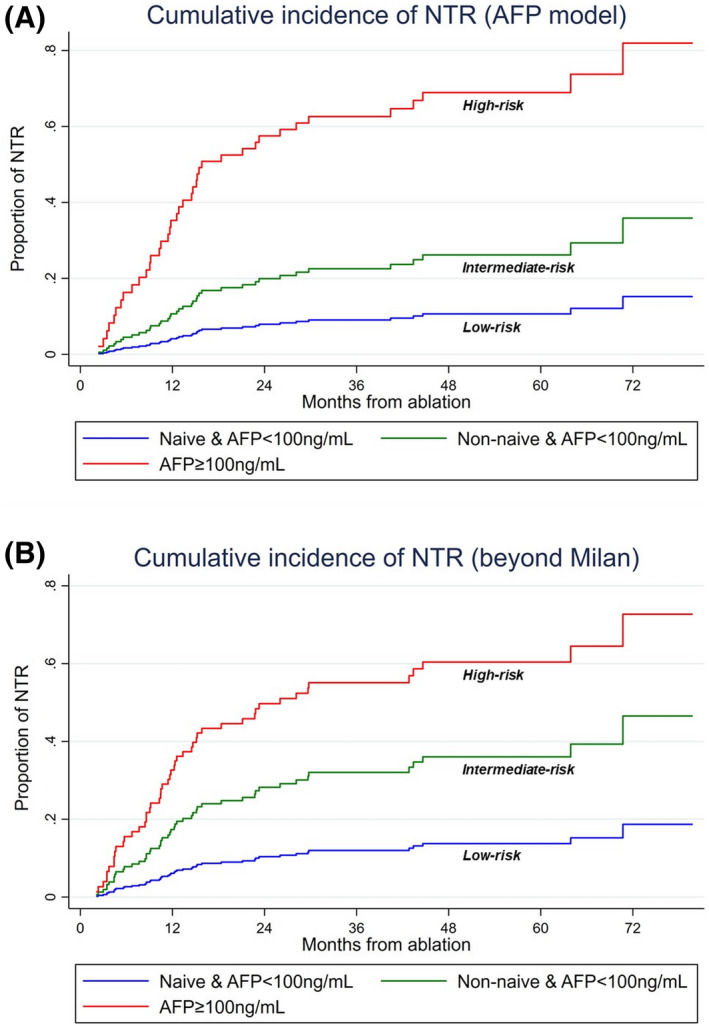
Cumulative incidence of non‐transplantable recurrence (NTR) according to the alpha‐fetoprotein (AFP) model (A) and Milan criteria (B) by NTR risk categories.
NTR (beyond Milan) according to NTR risk stratification
NTR risk stratification system yielded similar results for NTR beyond Milan criteria (Table 3). Overall, NTR beyond Milan occurred in 23% (49 of 213) patients during follow‐up, determining a 1‐year, 2‐year, 3‐year, and 4‐year NTR cumulative incidence of 11.7%, 20.2%, 22.9%, and 25.3%, respectively. Median time‐to‐NTR from PTA was 11.7 months (IQR: 6.7–21.1) in these patients. In the low‐risk, intermediate‐risk, and high‐risk categories for NTR, 12% (13 of 108), 31.5% (29 of 92), and 53.9% (7 of 13) presented NTR (p < 0.001). NTR occurred at the first recurrence in 3.7% (4 of 108), 25% (23 of 92), and 38.5% (5 of 13), respectively, in the low‐risk, intermediate‐risk, and high‐risk categories (p < 0.001).
Compared with low‐risk patients, SHR for intermediate‐risk patients was 3 (95% CI: 1.6–5.7; p = 0.001), whereas it was 6.3 (95% CI: 2.4–16.3; p < 0.001) for high‐risk patients (Harrell's C index: 0.71). NTR cumulative incidence in low‐risk, intermediate‐risk, and high‐risk categories was 6.1%, 17.4%, and 32.6% at 1 year; 10.4%, 28.2%, and 49.75% at 2 years; and 12%, 32%, and 55.1% at 3 years; 13.7%, 36%, and 60.4% at 4 years; respectively (Figure 3 and Table 3). In patients presenting NTR (beyond Milan), median time‐to‐NTR was 15.2 months (IQR: 10.7–28.1), 10.5 months (IQR: 8.4–15.8), and 5.6 months (IQR: 3.5–14.7), respectively, in low‐risk, intermediate‐risk, and high‐risk categories (p < 0.001).
RFS and OS according to NTR risk stratification
NTR risk category was also significantly (p < 0.001) associated with RFS (intermediate risk vs. low risk: HR = 2.51; p < 0.001; high risk vs. low risk: HR = 5.57; p < 0.001) and OS (intermediate risk vs. low risk: HR = 2.59; p = 0.009; high risk vs. low risk: HR = 15.47; p < 0.001) (Figure 4). Median RFS was 4.6 months (95% CI: 2.2–23.5), 14.5 months (95% CI: 8.6–18.3), and 43.4 months (23.6–50.2) in patients, respectively, in high‐risk, intermediate‐risk, and low‐risk categories. Median OS was not reached in intermediate‐risk and low‐risk patients, whereas it was of 19.1 months (10.3–NR) in high‐risk patients.
FIGURE 4.
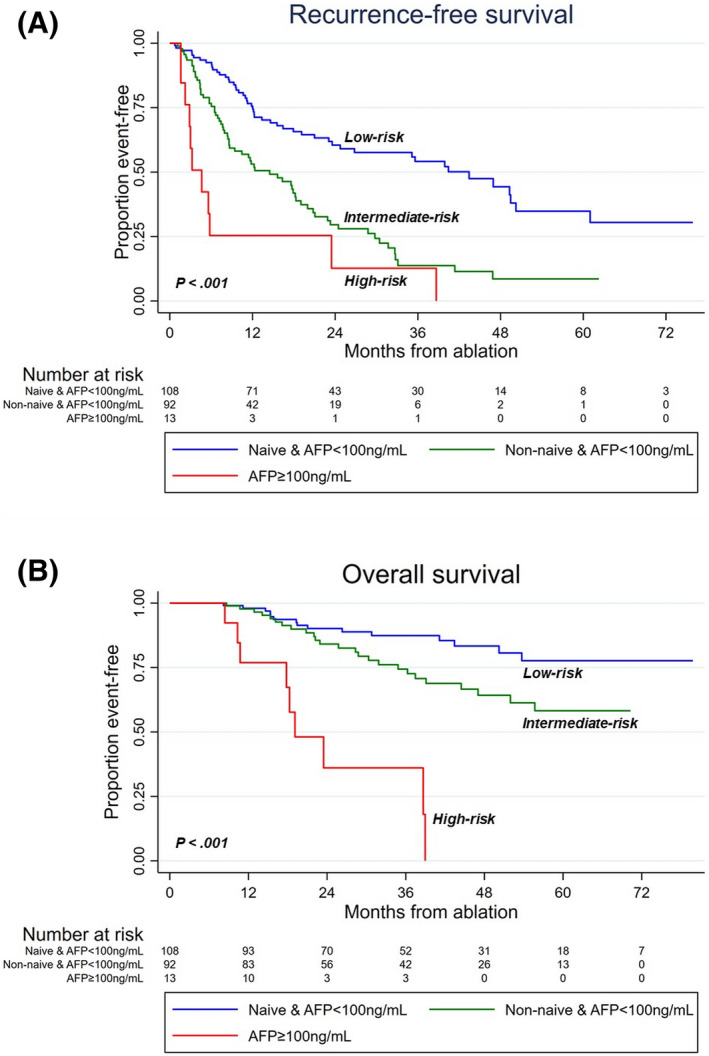
Recurrence‐free (A) and overall (B) survival according to the NTR risk categories.
DISCUSSION
In the present study, we assessed the value of upfront PTA in all potentially transplantable patients with NTR as an indicator of failure. The AFP score, which combines AFP level, number of tumor nodules and tumor size, has replaced the Milan criteria in France since January 2013.[ 3 , 7 ] Only a few studies have investigated the risk of NTR following PTA of early HCC,[ 13 , 20 , 21 ] and they either exceeded or restricted their inclusion criteria with regard to validated indications of PTA for HCC. Tsuchiya et al. included larger tumors (solitary HCC up to 5 cm),[ 20 ] whereas Cho et al. focused solely on solitary HCC.[ 21 ] Additionally, neither of them selected potentially transplantable patients as in our study. Doyle et al. specifically investigated this population, but they included only HCC‐naïve patients with solitary HCC.[ 13 ] All of these studies were conducted more than 15 years ago[ 20 , 21 ] or over a long study period (2000–2015),[ 13 ] whereas the state of the art in PTA has considerably changed over the past 10 years with the emergence of new ablation techniques (such as MW) or multimodal imaging guidance.[ 18 ] Non‐HCC‐naïve patients are often excluded from HCC series or clinical trials, whereas both re‐ablation and ablation of intrahepatic recurrence following resection are valid options because of the excellent tolerance and oncological outcome of PTA, provided that the tumor burden is limited (one to three nodules, ≤3 cm) and liver function and health status are preserved.[ 2 , 11 , 22 , 23 , 24 ] Thus, we aimed to include over a short and recent period of time all potentially transplantable patients with one to three ≤3‐cm nodule(s), whether naïve or not naïve, in order to (a) stick to EASL/AASLD/BCLC recommendations[ 1 , 2 , 14 ] (especially regarding multifocal tumors), (b) better reflect daily practice (non‐HCC‐naïve patients are frequently referred to PTA for small HCC,[ 23 ] as reflected in our series), and (c) incorporate the current state of the art in PTA.
With a 68.2% OS rate at 5 years, PTA meets the expectations as a curative option (i.e., median OS > 5 years).[ 2 ] Recurrence after PTA of small HCC occurs in 60%–80% of patients at 5 years, in keeping with our results (56.3%).[ 6 , 13 , 20 , 25 ] Interestingly, we showed that a large majority (67.5%) of patients who recurred remained within transplantation criteria and were therefore potentially eligible to salvage LT. By comparison, this rate was 62% for resection in a French study that was also based on the AFP model and that provided a comparable follow‐up.[ 3 ] The overall NTR incidence was only 18.3% (AFP model) and 23% (Milan) in our 213 patients, confirming PTA as a valid curative option with limited risk of NTR. Doyle et al. reported a comparable 27.6% NTR occurrence (beyond Milan) after radiofrequency ablation (RFA), although only solitary HCC in HCC‐naïve patients were selected.[ 13 ] Our NTR rate also compares favorably with the 15%–67% rate reported in surgical series,[ 3 , 4 , 11 ] although patients slightly differ (larger tumors, less advanced liver disease).
It is not possible to primarily transplant all patients with early HCC with preserved liver function, given the shortage of deceased donors in Western countries. Specifically, access to LT for T1 HCC is based solely on the MELD score in our nationwide graft allocation system. In this context, improving the selection of patients for first‐line PTA is highly desirable, especially if further chance for salvage LT is preserved. This would also avoid complications and immunosuppression resulting from primary LT. On resection specimen, histological features such as microvascular invasion, satellite nodules, or tumor differentiation have been identified as predictors of early or advanced recurrence,[ 26 ] or recurrence beyond Milan.[ 9 , 11 ] However, they are by definition obtained after resection, whereas preoperative predictors are needed to improve treatment allocation. Considerable advances have been made in HCC prognostication based on pathological features.[ 27 , 28 ] In the context of PTA, the biopsy of small HCC is challenging due both to frequent poor visibility or accessibility under US[ 6 , 18 , 29 , 30 , 31 ] and to the risk to further compromise PTA due to bleeding or post‐biopsy imaging modifications. Thus, only 22% of our patients underwent tumor biopsy. Consequently, we performed a competing‐risk analysis to stratify PTA patients into three categories based on covariates easy to obtain noninvasively before treatment. Interestingly, the model established using the AFP score was equally predictive within Milan criteria in our cohort.
The low‐risk group (naïve patients with AFP < 100 ng/ml) yielded a very low risk (9.3% AFP model, 12% beyond Milan) of developing NTR and showed long median RFS (43.4 months) and excellent OS (89.1% at 3 years). In addition, salvageability by LT at recurrence was theoretically preserved, as NTR had occurred at first recurrence in only 2.8% of low‐risk patients. This group of very good prognosis after upfront PTA represented half (50.7%) of the potentially transplantable patients. In other words, naïve patients with AFP < 100 ng/ml are very good candidates to upfront PTA with preserved chances of being further transplanted.
The intermediate‐risk category consists of the patients with a past history of HCC treatment but AFP < 100 ng/ml. Although salvage LT is theoretically recommended for recurrent HCC,[ 2 ] PTA or resection may be proposed either instead of or as a bridge‐to‐transplant strategy, due to graft shortage, insufficient recognition of salvage LT indication,[ 3 ] and to potential risks of dropout from the waiting list (especially from NTR). From a pragmatic standpoint, given the high proportion of patients presenting HCC recurrence (56.3% in our series), the systematic use of salvage LT is not realistic without seriously compromising equity in organ allocation. All LT policies are facing the same issue—the complex balance between utility and equity in a context of graft shortage—which is even more relevant in the post–COVID‐19 period. Although, by definition, all patients on the transplant list require transplantation, the severity of the disease and therefore the urgency of the transplant can differ greatly from one patient to another. The exclusive application of the principle of utility therefore results in impeding access to candidates whose disease is not life‐threatening in the short term. In our study, the median MELD score was 8 (IQR: 7–10), which meant no chance of getting a transplant in an allocation policy based on the MELD score only. The principle of utility therefore needed to be counterbalanced by the principle of equity, which guarantees for each candidate a similar probability of access to graft, regardless of the nature of the disease. This led to the development of the HCC MELD exception in many countries, including France. However, the proportion of patients receiving a priority listing with an HCC MELD exception has increased in the 2000s. Consequently, patients with HCC were given an unfair advantage in organ allocation with respect to non‐HCC patients listed for LT according to their MELD scores.[ 32 ]
In France, a strategy for delaying access to LT in patients with HCC receiving a waiting treatment by resection or PTA was implemented in two phases: from 2015, encouraging the placement of patients on temporary contraindication after curative treatment; and from 2018, awarding of MELD exception points for allowing accelerated access to LT in case of recurrence after curative treatment. Interestingly, Mazzaferro et al. proposed a quite similar allocation system, in which priority for enlistment was given based on treatment response (after bridge or downstaging) instead of initial tumor burden.[ 33 ]
At recurrence and despite HCC MELD exception points, waiting time remained long given the scarcity of donors, as observed in our series (waiting period for transplanted patients was 16 months). Provided that fewer than three HCC nodules were less than 3 cm, re‐ablation was therefore favored as upfront treatment. Before 2018, re‐ablated patients were placed under temporary contraindication. By 2018, MELD exception points were officially endorsed in case of tumor recurrence (within AFP score < 2), and the patients who were candidates for LT were enlisted. Of note, only 50% (13 of 26) were eventually transplanted in our series.
The French program was one of the first worldwide to implement a national strategy of delayed access to transplantation for patients with HCC receiving curative treatment by resection or PTA. This provided a unique opportunity to investigate the risk of NTR in non‐HCC‐naïve patients and to explore whether re‐ablation could be an acceptable alternative to salvage LT in this frequent but commonly overlooked population.
Compared with low‐risk patients, the NTR incidence was higher (SHR = 2.7) but remained acceptable, as 22.8% eventually presented NTR. In comparison, a recent surgical series examining the risk of NTR according to the AFP model in HCC‐naïve patients reported a 20.9% overall NTR rate, although only 65.5% of patients had cirrhosis.[ 3 ] A risk score incorporating pathological examination of liver explant from resection (n = 1023 patients) validated a risk of NTR (beyond Milan) of 18%, even in the most favorable category.[ 4 ] The limited risk of NTR in the intermediate‐risk category challenges the recommendation to refer all of these patients to salvage LT, even though when occurring, NTR was the first recurrence in most (66%–80%, depending on the criteria).
NTR occurred outside liver segments treated by PTA in most cases (87.2%) and during the first 2 years after treatment as in surgical series,[ 3 , 4 ] in keeping with the greater risk of cancer dissemination from the original tumor during this period.[ 34 ] Thus, NTR mostly results from overlooked microscopic multifocality, suggesting that anatomical resection would have probably not performed better. In intermediate‐risk versus low‐risk patients, recurrence was more frequent, and RFS and OS were significantly reduced (HR = 2.5 and 5.6, respectively). All of this calls for adjuvant/neoadjuvant treatments to PTA. Although none is validated to date, four phase 3 randomized controlled trials (RCTs) are currently ongoing with nivolumab (NCT03383458), pembrolizumab (NCT03867084), durvalumab ± bevacizumab (NCT03847428), or atezolizumab + bevacizumab (NCT04102098) as adjuvant treatment versus placebo for resected/ablated patients at high risk of recurrence. However, these trials have four limitations: First, they all mix resected and ablated patients with different inclusion criteria for each treatment modality; second, they consider at high risk of recurrence tumors exceeding international recommendations (>3 cm and/or >3 nodules) at various degrees depending on the trial; third, by their adjuvant‐only design, they do not take the opportunity of potential synergies between PTA and immunotherapies; fourth and surprisingly, prior HCC treatment is a noninclusion criterion in these trials, even though the risk of recurrence is basically increased. We are conducting a multicenter phase 2 RCT (NCT04727307) in France that is dedicated to ablation only, comparing RFA alone with neoadjuvant atezolizumab followed by RFA and adjuvant atezolizumab/bevacizumab. In this trial, non‐HCC‐naïve patients can be included, and HCC naivety is part of the stratification factors. The optimal treatment strategy of recurrent HCC within BCLC 0‐A is definitely worth exploring.
AFP ≥ 100 ng/ml, which defines high‐risk patients, had a particularly negative impact, as upfront PTA resulted in high NTR risk (61.5%) and poor RFS and OS. AFP reflects aggressive tumor biology and has been included in different transplantation selection criteria.[ 7 , 35 , 36 ] Close surveillance following PTA is advisable to detect recurrence before progression beyond transplantation criteria. Enhancement of post‐PTA follow‐up is unrealistic, as our policy was actually to perform MRI every 3 months for life, whereas imaging follow‐up is usually lightened after 2 years following PTA.[ 13 , 20 ] Salvage LT remains theoretically applicable in 61.5% of high‐risk patients, as 38.5% present with NTR at first recurrence. However, we can anticipate a high rate of dropout, given the 35.3% risk of NTR at 1 year and the median time‐to‐NTR of 4.5 months after PTA. From a pragmatic perspective, offering preemptive LT in these high‐risk patients representing only 6.1% of PTA candidates would have limited impact on organ supply and is a realistic option. Based on the Metroticket 2.0 and AFP models, approximately 50% of 5‐year OS can be expected,[ 7 , 36 ] which is far greater than the OS observed in these patients with upfront PTA (median: 19.1 months). However, the high risk of rapid NTR suggests that such preemptive LT should be prioritized, raising the difficult question of graft allocation priority in a context in which prioritizing HCC with aggressive tumor biology over advanced liver disease without HCC may be ethically questionable. In this high‐risk category, adjuvant/neoadjuvant trials are therefore also needed.
Several limitations to our study must be acknowledged. First, this is a retrospective, single‐center study, although data came from a prospectively maintained database. An RCT comparing upfront PTA to LT in small HCCs, although ideal, is very unlikely to be conducted, which emphasizes the role of robust retrospective data that could help design further studies or clinical trials focusing on subpopulation at intermediate or high risk of NTR. Second, our model requires external validation, even though it has been internally validated using bootstrapping. Third, we investigated the NTR risk after PTA of small HCC using the AFP model that was actually used in our country during the whole study period. As mentioned previously, the results regarding Milan criteria are exploratory. The NTR risk in the context of other policies such as University of California San Francisco, up to seven, or Metroticket 2.0 remains to be investigated. The strengths of this study include the selection of all potentially transplantable patients in line with current recommendations, the stringent follow‐up (every 3 months for life; none lost to follow‐up before recurrence or death), and the competing‐risk analysis of a relevant outcome for transplantation centers.
CONCLUSIONS
Most patients who are eligible for primary PTA remain eligible for salvage LT. Simple and noninvasive covariates (HCC naivety and AFP) obtained before PTA of ≤3‐cm HCC(s) accurately stratified patients' risk of NTR, ranging from 9.3% to 61.5% (AFP model) and 12% to 53.8% (Milan). Patients without history of HCC and with low AFP are excellent candidates to upfront PTA, with excellent outcome and preserved chances for salvage transplantation. Patients with recurrent HCC have an increased risk of NTR, which remains acceptable. Adjuvant/neoadjuvant trials should certainly focus on this frequently overlooked subpopulation. Finally, patient candidates for PTA with AFP ≥ 100 ng/ml have a high risk of NTR and poor RFS and OS. Clinical trials evaluating preemptive transplantation and/or adjuvant/neoadjuvant strategies are highly needed in this small subset of patients.
CONFLICT OF INTEREST
Nothing to report.
ACKNOWLEDGMENT
The authors thank Sandrine Guinodeau for help with the English language.
Gozzo C, Hermida M, Herrero A, Panaro F, Cassinotto C, Mohamad AM et al. Non‐transplantable recurrence after percutaneous thermal ablation of ≤3‐cm HCC: Predictors and implications for treatment allocation. Hepatol Commun. 2022;6:2975–2987. 10.1002/hep4.2063
Cecilia Gozzo and Margaux Hermida contributed equally to this work.
REFERENCES
- 1. EASL Clinical Practice Guidelines . Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 2. Reig M, Forner A, Rimola J, Ferrer‐Fábrega J, Burrel M, Garcia‐Criado A, et al. BCLC strategy for prognosis prediction and treatment recommendation Barcelona Clinic Liver Cancer (BCLC) staging system. The 2022 update. J Hepatol. 2022;76:681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gelli M, Sebagh M, Porcher R, Romanelli E, Vibert E, Sa Cunha A, et al. Liver resection for early hepatocellular carcinoma: preoperative predictors of non transplantable recurrence and implications for treatment allocation. Ann Surg. 2020;272:820–6. [DOI] [PubMed] [Google Scholar]
- 4. Zheng J, Chou JF, Gönen M, Vachharajani N, Chapman WC, Majella Doyle MB, et al. Prediction of hepatocellular carcinoma recurrence beyond milan criteria after resection: validation of a clinical risk score in an international cohort. Ann Surg. 2017;266:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murali AR, Patil S, Phillips KT, Voigt MD. Locoregional therapy with curative intent versus primary liver transplant for hepatocellular carcinoma: systematic review and meta‐analysis. Transplantation. 2017;101:e249–57. [DOI] [PubMed] [Google Scholar]
- 6. Nault JC, Sutter O, Nahon P, Ganne‐Carrie N, Seror O. Percutaneous treatment of hepatocellular carcinoma: state of the art and innovations. J Hepatol. 2018;68:783–97. [DOI] [PubMed] [Google Scholar]
- 7. Duvoux C, Roudot‐Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, et al.; Liver Transplantation French Study Group . Liver transplantation for hepatocellular carcinoma: a model including α‐fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986–94.e3; quiz e14‐5. [DOI] [PubMed] [Google Scholar]
- 8. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9. [DOI] [PubMed] [Google Scholar]
- 9. Fuks D, Dokmak S, Paradis V, Diouf M, Durand F, Belghiti J. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention‐to‐treat analysis. Hepatology. 2012;55:132–40. [DOI] [PubMed] [Google Scholar]
- 10. Scatton O, Zalinski S, Terris B, Lefevre JH, Casali A, Massault PP, et al. Hepatocellular carcinoma developed on compensated cirrhosis: resection as a selection tool for liver transplantation. Liver Transplant. 2008;14:779–88. [DOI] [PubMed] [Google Scholar]
- 11. Kim M, Kim T, Lee HY, Hong SY, Wang HJ, Kim BW. Risk factors for beyond Milan recurrence after hepatic resection for single hepatocellular carcinoma no larger than 5 centimeters. Liver Transplant. 2021;27:1116–29. [DOI] [PubMed] [Google Scholar]
- 12. Cassinotto C, Nogue E, Morell M, Panaro F, Molinari N, Guiu B. Changing trends in hepatocellular carcinoma management: results from a nationwide database in the last decade. Eur J Cancer. 2021;146:48–55. [DOI] [PubMed] [Google Scholar]
- 13. Doyle A, Gorgen A, Muaddi H, Aravinthan AD, Issachar A, Mironov O, et al. Outcomes of radiofrequency ablation as first‐line therapy for hepatocellular carcinoma less than 3cm in potentially transplantable patients. J Hepatol. 2019;70:866–73. [DOI] [PubMed] [Google Scholar]
- 14. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–80. [DOI] [PubMed] [Google Scholar]
- 15. Hermida M, Preel A, Assenat E, Piron L, Cassinotto C, Ursic‐Bedoya J, et al. Small steatotic HCC: a radiological variant associated with improved outcome after ablation. Hepatol Commun. 2021;5:689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brusset B, Dumortier J, Cherqui D, Pageaux GP, Boleslawski E, Chapron L, et al. Liver transplantation for hepatocellular carcinoma: a real‐life comparison of milan criteria and AFP model. Cancers (Basel). 2021;13:2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Durand F, Antoine C, Soubrane O. Liver transplantation in France. Liver Transplant. 2019;25:763–70. [DOI] [PubMed] [Google Scholar]
- 18. Hermida M, Cassinotto C, Piron L, Aho‐Glele S, Guillot C, Schembri V, et al. Multimodal percutaneous thermal ablation of small hepatocellular carcinoma: predictive factors of recurrence and survival in Western Patients. Cancers (Basel). 2020;12:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Preel A, Hermida M, Allimant C, Assenat E, Guillot C, Gozzo C, et al. Uni‐, bi‐ or trifocal hepatocellular carcinoma in Western patients: recurrence and survival after percutaneous thermal ablation. Cancers (Basel). 2021;13:2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsuchiya K, Asahina Y, Tamaki N, Yasui Y, Hosokawa T, Ueda K, et al. Risk factors for exceeding the Milan criteria after successful radiofrequency ablation in patients with early‐stage hepatocellular carcinoma. Liver Transplant. 2014;20:291–7. [DOI] [PubMed] [Google Scholar]
- 21. Cho JY, Choi MS, Lee GS, Sohn W, Ahn J, Sinn DH, et al. Clinical significance and predictive factors of early massive recurrence after radiofrequency ablation in patients with a single small hepatocellular carcinoma. Clin Mol Hepatol. 2016;22:477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chan AC, Chan SC, Chok KS, Cheung TT, Chiu DW, Poon RT, et al. Treatment strategy for recurrent hepatocellular carcinoma: salvage transplantation, repeated resection, or radiofrequency ablation? Liver Transplant. 2013;19:411–9. [DOI] [PubMed] [Google Scholar]
- 23. Okuwaki Y, Nakazawa T, Kokubu S, Hidaka H, Tanaka Y, Takada J, et al. Repeat radiofrequency ablation provides survival benefit in patients with intrahepatic distant recurrence of hepatocellular carcinoma. Am J Gastroenterol. 2009;104:2747–53. [DOI] [PubMed] [Google Scholar]
- 24. Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947–55. [DOI] [PubMed] [Google Scholar]
- 25. N'Kontchou G, Mahamoudi A, Aout M, Ganne‐Carrié N, Grando V, Coderc E, et al. Radiofrequency ablation of hepatocellular carcinoma: long‐term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009;50:1475–83. [DOI] [PubMed] [Google Scholar]
- 26. Sala M, Fuster J, Llovet JM, Navasa M, Solé M, Varela M, et al. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: an indication for salvage liver transplantation. Liver Transplant. 2004;10:1294–300. [DOI] [PubMed] [Google Scholar]
- 27. Calderaro J, Ziol M, Paradis V, Zucman‐Rossi J. Molecular and histological correlations in liver cancer. J Hepatol. 2019;71:616–30. [DOI] [PubMed] [Google Scholar]
- 28. Nault JC, Martin Y, Caruso S, Hirsch TZ, Bayard Q, Calderaro J, et al. Clinical impact of genomic diversity from early to advanced hepatocellular carcinoma. Hepatology. 2020;71:164–82. [DOI] [PubMed] [Google Scholar]
- 29. Mauri G, Cova L, De Beni S, Ierace T, Tondolo T, Cerri A, et al. Real‐time US‐CT/MRI image fusion for guidance of thermal ablation of liver tumors undetectable with US: results in 295 cases. Cardiovasc Intervent Radiol. 2015;38:143–51. [DOI] [PubMed] [Google Scholar]
- 30. Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, et al. Ten‐year outcomes of percutaneous radiofrequency ablation as first‐line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89–97. [DOI] [PubMed] [Google Scholar]
- 31. Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, et al. Radiofrequency ablation for hepatocellular carcinoma in so‐called high‐risk locations. Hepatology. 2006;43:1101–8. [DOI] [PubMed] [Google Scholar]
- 32. Mehta N, Dodge JL, Goel A, Roberts JP, Hirose R, Yao FY. Identification of liver transplant candidates with hepatocellular carcinoma and a very low dropout risk: implications for the current organ allocation policy. Liver Transplant. 2013;19:1343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mazzaferro V. Squaring the circle of selection and allocation in liver transplantation for HCC: an adaptive approach. Hepatology. 2016;63:1707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–7. [DOI] [PubMed] [Google Scholar]
- 35. Fan J, Yang GS, Fu ZR, Peng ZH, Xia Q, Peng CH, et al. Liver transplantation outcomes in 1,078 hepatocellular carcinoma patients: a multi‐center experience in Shanghai. China J Cancer Res Clin Oncol. 2009;135:1403–12. [DOI] [PubMed] [Google Scholar]
- 36. Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology. 2018;154:128–39. [DOI] [PubMed] [Google Scholar]


