Abstract
To validate cancer screening programs, experts recommend estimating effects on case fatality rates (CFRs) and cancer‐specific mortality. This study evaluates hepatocellular carcinoma (HCC) screening in patients with cirrhosis for those outcomes using a modeling approach. We designed a Markov model to assess 10‐year HCC‐CFR, HCC‐related, and overall mortality per 100,000 screened patients with compensated cirrhosis. The model evaluates different HCC surveillance intervals (none, annual [12 months], semiannual [6 months], or quarterly [3 months]) and imaging modalities (ultrasound [US] or magnetic resonance imaging [MRI]) in various annual incidences (0.2%, 0.4%, or 1.5%). Compared to no surveillance, 6‐month US reduced the 10‐year HCC‐CFR from 77% to 46%. With annual incidences of 0.2%, 0.4%, and 1.5%, the model predicted 281, 565, and 2059 fewer HCC‐related deaths, respectively, and 187, 374, and 1356 fewer total deaths per 100,000 screened patients, respectively. Combining alpha‐fetoprotein screening to 6‐month US led to 32, 63, and 230 fewer HCC‐related deaths per 100,000 screened patients for annual incidences of 0.2%, 0.4%, and 1.5%, respectively. Compared to 6‐month US, 3‐month US reduced cancer‐related mortality by 14%, predicting 61, 123, and 446 fewer HCC‐related deaths per 100,000 screened patients with annual incidences of 0.2%, 0.4%, and 1.5%, respectively. Compared to 6‐month US, 6‐month MRI (−17%) and 12‐month MRI (−6%) reduced HCC‐related mortality. Compared to 6‐month US, overall mortality reductions ranged from −0.1% to −1.3% when using 3‐month US or MRI. A US surveillance interval of 6 months improves HCC‐related and overall mortality compared to no surveillance. A shorter US interval or using MRI could reduce HCC‐CFR and HCC‐related mortality, with a modest effect on overall mortality.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer‐related death worldwide, with a low 5‐year survival rate of 5% to 10%, which is partly due to delayed diagnoses.[ 1 ] Overall survival increases to 15%–20% in countries with greater access to curative treatments as a result of increased HCC screening rates,[ 2 ] although the latter is only performed in 20%–40% of eligible patients. Despite European Association for the Study of the Liver and American Association for the Study of Liver Diseases (AASLD) guidelines recommending ultrasound (US) imaging at baseline and every 6 months,[ 3 , 4 ] some experts still question the benefits of this protocol.[ 5 ] Overall, the quality/certainty of evidence to support HCC screening is still classified as low to moderate. However, randomized controlled trials are difficult to perform for ethical reasons as well as patient and physician reluctance.[ 6 ] Mathematical modeling is a valuable alternative to obtain further insight into the value of HCC screening.
Defining the optimal primary endpoint when evaluating the benefit of screening in cancer is a complex scientific issue.[ 7 , 8 ] Certain groups support the use of improvement in cancer‐related outcomes as an endpoint, while others support improvement in all‐cause mortality. Breast and colorectal cancer screening programs are the most widely implemented surveillance programs in solid tumors.[ 9 , 10 ] Although they do not reduce overall mortality, they are recommended based on a clear reduction in case fatality and cancer‐specific mortality. Data on the impact of screening on the endpoints used for these surveillance programs are becoming an important issue in HCC.
The incidence of disease is a key factor when evaluating screening programs. The annual incidences of breast and colorectal cancers in the screened population aged 50–75 years are 0.2%–0.4% and 0.15%–0.2%, respectively.[ 11 , 12 ] Surveillance for HCC is recommended in populations with an annual incidence >1.5% based on cost‐effectiveness analysis.[ 13 ] However, this cutoff is now a subject of debate because HCC frequently develops in patients with non‐cirrhotic non‐alcoholic steatohepatitis or hepatitis C virus‐cured patients with advanced fibrosis. To help experts update the incidence thresholds and adapt them to this new epidemiologic paradigm, the absolute reduction in HCC‐related deaths could be compared to those obtained in other established screening programs.
The one‐size‐fits‐all recommendation of semiannual US surveillance is in contrast to the differences in the incidence risk of HCC, tumor biology, and the effectiveness of US, which depends on patients and tumor characteristics. Japanese guidelines on HCC management recommend tailoring the frequency of surveillance to the incidence risk of HCC,[ 14 ] similar to the shortened screening period for breast or colorectal cancers in germline carriers of the breast cancer type 1 (BRCA1) mutation or mismatch‐repair deficiency.[ 15 , 16 ] Based on the negative results of a randomized controlled trial, Western guidelines do not recommend a shortened 3‐month interval.[ 17 ] In addition, the decreased effectiveness of US in patients with obesity suggests that magnetic resonance imaging (MRI) could be used in these cases, although there is little evidence to support this practice.
Using a step‐by‐step, rigorous, modeling approach, we designed a model that updates HCC surveillance outcomes in relation to case fatality rate (CFR) and disease‐specific and all‐cause mortalities for different disease incidences and screening modalities.
MATERIALS AND METHODS
Analytic overview
A monthly multistate Markov model simulated the occurrence, diagnosis, treatment, and follow‐up of HCC in 100,000 56‐year‐old patients (the median age at diagnosis of cirrhosis in previous publications[ 18 , 19 ]) with compensated cirrhosis who complied to HCC surveillance after an initial diagnostic imaging test that excluded HCC. The model assessed the impact of different HCC surveillance strategies on HCC‐CFRs, HCC‐related deaths, and total deaths at 10 years. Because cancer‐related and overall mortality depend on the incidence of cancer, we evaluated the benefit of surveillance according to hypothetical annual HCC incidences: an incidence of 0.2%, which is similar to breast and colorectal cancers; an incidence of 0.4%, which corresponds to the incidence of HCC in patients with cirrhosis who are at low risk[20 ]; and an incidence of 1.5%, the current recommended threshold for HCC screening.[ 21 ]
Model
The Markov model describes the pathway of patients with compensated cirrhosis who undergo imaging surveillance from the diagnosis of cirrhosis to death or 10‐year follow‐up (Figure S1). Patients are at risk of developing decompensated cirrhosis or HCC or to die from non‐liver‐related causes at each monthly cycle of the Markov model. Patients were classified as decompensated in case of clinical events, such as ascites, jaundice, and/or encephalopathy. Consequently, patients without clinical events remained candidates for HCC surveillance in our model as they remain eligible for all therapeutic options and HCC is the main driver of therapeutic decision. In patients with clinical events, the benefit of HCC surveillance was not evaluated because liver transplantation, the only remaining therapeutic option for HCC, is indicated primarily because of liver decompensation in this setting.
Patients who develop HCC are diagnosed by either surveillance imaging, depending on the effectiveness of the screening method, or by symptoms. Patients diagnosed with HCC are then treated and are subsequently at risk of death from HCC, non‐HCC liver‐related causes, or non‐liver‐related causes. Survival from HCC depends on the tumor characteristics (tumor size and number of nodules) and treatment options at diagnosis. We applied a 3‐month delay in initiating treatment during which patients (both symptomatic and asymptomatic) had no HCC‐specific mortality[ 22 ] but only mortality similar to that with compensated cirrhosis. Regardless of tumor characteristics, patients with undiagnosed HCC (asymptomatic and unidentified by imaging) are at risk of death from non‐HCC liver‐related causes or non‐liver‐related causes.
Outcomes
We considered the following established outcomes for cancer screening: CFR, defined as the number of HCC‐related deaths over the number of diagnosed HCC (by surveillance or by symptoms); HCC‐related deaths, defined as death due to HCC among 100,000 patients with cirrhosis; and overall mortality as any death that occurred among 100,000 patients with cirrhosis.
To limit lead‐time bias for cancer‐specific outcomes, the follow‐up time was calculated from the diagnosis of cirrhosis for all patients. To analyze cancer‐specific outcomes, we only considered patients who developed HCC within the first 5 years. Outcomes were analyzed 10 years after the start of the surveillance program, allowing at least 5 years of follow‐up after HCC diagnosis, which was considered to be sufficient to assess final HCC‐related outcomes (death or resolution).
The baseline scenario was the comparison between semiannual US surveillance and no surveillance. We then compared other HCC surveillance intervals (quarterly and annually) to semiannual intervals for US imaging and then MRI.
Input data
Decompensation of cirrhosis and mortality
The annual risk of developing decompensated cirrhosis was set at 2.3%,[ 23 , 24 , 25 ] corresponding to a monthly risk of 0.19%. In the same cycle, individuals with decompensated cirrhosis had a risk of death from liver disease of 39% in the first year (4.04% per month), then 11% per year (0.97% per month),[ 26 ] and non‐liver related deaths were derived from French life tables.[ 27 ]
Tumor size
Tumor size varied over time on the basis of constant exponential tumor growth, reflected in a fixed tumor doubling time (TDT) as proposed by Schwartz[ 28 ] as follows:
where D t is tumor size at time t, and D 0 equals the initial diameter of the tumor and was set at 1 cm as the minimum size of a tumor detectable by surveillance that warrants further diagnosis, according to guidelines. The baseline scenario relied on a TDT of 90 days, which was the median observed in the literature.[ 29 , 30 , 31 , 32 , 33 , 34 ]
HCC diagnosis
The working hypothesis was that patients were 100% compliant with HCC surveillance. Thus, HCC screening only depended on the effectiveness of the screening method (US or MRI). The effectiveness of screening (performance in real‐world conditions) is its capacity to detect HCC in relation to the size of the main nodule. The effectiveness of US and MRI for incremental tumor sizes was extracted from the literature to derive a logarithmic continuous model of imaging effectiveness according to tumor size (Supporting Information).
To define the risk of having symptoms leading to a cancer diagnosis in patients between two imaging sessions (interval cancer), we calibrated data from Cucchetti and colleagues[ 35 ] to derive the cumulative risk of symptoms according to tumor size (D t ) as 0.021 × exp(0.5636 × D t ), setting 0.15% as the baseline risk for a size of 1 cm. The monthly incidence of symptoms was recalculated accordingly.
HCC treatment
First, we analyzed previous studies to derive the probability of HCC being uninodular, of having up to three nodules, or more than three nodules according to tumor size (Supporting Information). We then applied tumor stage‐dependent treatment based on tumor characteristics (the presence or absence of symptoms, tumor size, and the number of nodules) 3 months after initial detection to account for the real‐world waiting time between the diagnosis and starting treatment[ 22 , 36 ] (Supporting Information). Waiting time to liver transplantation was set at 12 months for eligible patients, based on French data.[ 37 ]
HCC prognosis
Cancer‐related and overall mortality from HCC after treatment were defined according to published studies (Supporting Information). Death could be related to HCC if it occurred within the first 5 years after treatment. HCC‐related mortality depended on tumor stage, type of treatment, and the presence of symptoms. After an analysis of available studies, we made the conservative assumption that symptomatic HCCs were still eligible for the same therapeutic options as asymptomatic HCCs but with a 15% reduction in 5‐year survival.
The posttreatment risks of non‐HCC liver‐related death and non‐liver‐related death were the same for all patients regardless of treatment (except patients that underwent liver transplantation, which has specific risks) and were ongoing until the end of the 10‐year period.
Patients waiting for treatment or with undiagnosed asymptomatic HCC were not expected to die from HCC‐related mortality[ 22 ] but only from other causes, with a risk identical to that from compensated cirrhosis (0.90% per year [0.08% per month]).
Model validation
Criteria to validate the model included the percentage of patients diagnosed with a tumor <3 cm, the mean size of the diagnosed HCC, and the rate of access to curative treatment in a scenario based on semiannual US surveillance. These outcomes were defined to allow comparison with published outcomes of HCC surveillance. The rate of access to curative treatment was defined as the number of patients that received resection, thermal ablation, or liver transplantation per the number diagnosed with HCC.
Sensitivity analysis
Sensitivity analysis was performed to determine the robustness of our overall conclusions.
We evaluated the influence of a reduced effectiveness of US (−25%) to detect HCC. We evaluated the combination of US and alpha‐fetoprotein (AFP) screening, recommended by AASLD, and took into account a recent meta‐analysis[ 38 ] that showed an increase of screening sensitivity by 23%. We considered two alternative scenarios of 50% and 70% adherence to screening.
We also considered an alternative scenario of incidental detection for miscellaneous reasons, such as abdominal symptoms not related to HCC, abnormal liver tests, or urogenital symptoms. For this, we obtained data on the use of medical imaging between 2000 and 2016 in health care systems of the United States.[ 39 ] We considered that 56‐year‐old patients with cirrhosis may have a higher rate of use of incidental abdominal imaging than an individual of the same age from the general population, and consequently we assumed a similar rate to that of individuals from the United States who were older than 65 years old. We calculated that the overall rate of abdominal US was 165 per 1000 person‐years, which led us to consider that 16.5% of the cirrhotic population randomly receive incidental US screening yearly.
Finally, we evaluated the influence of an excess of non‐liver‐related mortality in patients with cirrhosis compared to the general population. We applied a 2.1 relative risk to all‐cause mortality rates as the mean observed in health care settings for patients with compared to without cirrhosis.[ 40 ]
Software
The model was developed using TreeAge Pro 2021 (TreeAge Software, LLC, Williamstown, MA, USA).
RESULTS
Validity of the model
The model predicted that with semiannual US, 69% of HCC would be diagnosed before the largest nodule reached a maximum size of 3 cm. The mean size of HCC at diagnosis would be 2.7 cm, with a rate of access to curative treatment of 59%. These numbers were not affected by variations in the incidence of HCC. These results were similar to previous results of semiannual US, which reported a mean HCC of <3 cm in 63%–70% of patients,[ 17 , 41 ] a median size of 2–2.5 cm,[ 35 , 42 ] and access to curative treatment in 53%–74% of patients[ 17 , 35 , 42 , 43 , 44 ] (Table 1; Table S1 for those with access to liver transplantation).
TABLE 1.
Semiannual US compared to no surveillance for hepatocellular carcinoma
| Model output | No surveillance | 6‐month US |
|---|---|---|
| Interval cancer | N/A | 10% |
| Size of main nodule | ||
| Mean (cm) | 5.5 | 2.7 |
| 1–3 cm | 8% | 69% |
| 3–5 cm | 20% | 24% |
| >5 cm | 71% | 7% |
| Curative treatment | 11% | 59% |
| Case fatality rate | ||
| Overall | 77% | 46% |
| 1–3 cm | 44% | 37% |
| 3–5 cm | 68% | 61% |
| >5 cm | 83% | 83% |
Note: Baseline case scenario relies on a tumor doubling time of 90 days.
Abbreviations: N/A, not applicable; US, ultrasound.
HCC‐CFR at 10 years
Comparing semiannual US surveillance to no surveillance (Table 1), the size of HCC at diagnosis was between 1 and 3 cm in 69% versus 8% of patients, between 3 and 5 cm in 25% versus 20% of patients, and >5 cm in 7% versus 72% of patients, respectively. Interval cancer occurred in 10% of patients in the 6‐month group. CFRs in the semiannual and no‐surveillance groups were 46% and 77%, respectively. Size‐specific CFR in the semiannual and no‐screening groups were 37% and 44% in nodules between 1 and 3 cm, 61% and 68% in nodules between 3 and 5 cm, and 83% and 83% for nodules >5 cm, respectively.
CFRs varied according to the interval and the type of imaging surveillance (Table 2). When US was used, CFR varied from 39% in the 3‐month interval group to 56% in the 12‐month interval group. With MRI, the CFR with a 6‐month interval was 38% while it was 43% with the 12‐month interval.
TABLE 2.
Overall and size‐specific 10‐year case fatality rates, using different surveillance strategies
| Size of main HCC nodule | No surveillance | US | MRI | |||
|---|---|---|---|---|---|---|
| 12 M | 6 M | 3 M | 12 M | 6 M | ||
| Overall | 77% | 56% | 46% | 39% | 43% | 38% |
| 1–3 cm | 44% | 38% | 37% | 37% | 37% | 37% |
| 3–5 cm | 68% | 62% | 61% | 61% | 61% | 61% |
| >5 cm | 83% | 83% | 83% | 82% | 83% | NA a |
Note: Baseline case scenario relies on a tumor doubling time of 90 days.
Abbreviations: M, month; MRI, magnetic resonance imaging; NA, not adequate; US, ultrasound.
NA due to zero hepatocellular carcinoma diagnoses in this subcategory.
Ten‐year mortality according to annual incidence of HCC
Comparing semiannual US surveillance (Table 3) versus no surveillance, 431 versus 712 (annual incidence, 0.2%), 865 versus 1430 (annual incidence, 0.4%), and 3146 versus 5205 (annual incidence, 1.5%) HCC‐related deaths were observed per 100,000 screened patients with cirrhosis. This corresponds to an absolute reduction of 281, 565, and 2059 HCC‐related deaths, respectively. Overall, mortality was reduced by 187, 374, and 1356 deaths per 100,000 screened patients, respectively, with semiannual US compared to no surveillance. Causes of deaths in patients without HCC are provided in Table S2.
TABLE 3.
HCC‐related and overall mortality per 100,000 screened patients according to HCC annual incidence, showing 6‐month US compared to no surveillance
| Annual HCC incidence | HCC‐related deaths | Overall deaths | Death attributable to HCC (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No surveillance (ref.) | 6‐M US | Abs. diff. | Rel. diff. | No surveillance (ref.) | 6‐M US | Abs. Diff. | Rel. Diff. | No surveillance (ref.) | 6‐M US | |
| 0.2% | 712 | 431 | −281 | −39.5% | 19,203 | 19,016 | −187 | −1.0% | 3.7% | 2.3% |
| 0.4% | 1430 | 865 | −565 | −39.5% | 19,905 | 19,531 | −374 | −1.9% | 7.2% | 4.4% |
| 1.5% | 5205 | 3146 | −2059 | −39.6% | 23,595 | 22,239 | −1356 | −5.7% | 22.1% | 14.1% |
Note: Baseline case scenario relies on a tumor doubling time of 90 days.
Abbreviations: Abs., absolute; diff., difference; HCC, hepatocellular carcinoma; M, month; ref., reference; US, ultrasound.
The absolute reduction in HCC‐related deaths varied according to the screening scenario (Table 4; Figure 1). Compared to semiannual US, quarterly US reduced HCC‐related mortality by 61, 123, and 446 deaths for annual HCC incidences of 0.2%, 0.4%, and 1.5%, respectively. Compared to semiannual US, semiannual MRI reduced cancer‐related mortality by 75, 150, and 544 deaths and annual MRI by 26, 52, and 186 deaths per 100,000 screened patients.
TABLE 4.
HCC‐related and overall mortality per 100,000 screened patients according to HCC annual incidence and surveillance modality and showing annual and quarterly compared to semiannual surveillance
| Annual HCC incidence | 6‐M US (ref.) | US | MRI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 M | 3 M | 12 M | 6 M | ||||||||||
| Deaths | Deaths | Abs. Diff | Rel. Diff. | Deaths | Abs. Diff | Rel. Diff. | Deaths | Abs. Diff | Rel. Diff. | Deaths | Abs. Diff | Rel. Diff. | |
| HCC‐related mortality | |||||||||||||
| 0.2% | 431 | 520 | +89 | +20.6% | 370 | −61 | −14.2% | 405 | −26 | −6.0% | 356 | −75 | −17.4% |
| 0.4% | 865 | 1044 | +179 | +20.7% | 742 | −123 | −14.2% | 813 | −52 | −6.0% | 715 | −150 | −17.3% |
| 1.5% | 3146 | 3799 | +653 | +20.8% | 2700 | −446 | −14.2% | 2960 | −186 | −5.9% | 2602 | −544 | −17.3% |
| Overall mortality | |||||||||||||
| 0.2% | 19,016 | 19,074 | +58 | +0.3% | 18,980 | −36 | −0.2% | 19,003 | −13 | −0.07% | 18,978 | −38 | −0.2% |
| 0.4% | 19,531 | 19,647 | +116 | +0.6% | 19,459 | −72 | −0.4% | 19,504 | −27 | −0.1% | 19,454 | −77 | −0.4% |
| 1.5% | 22,239 | 22,658 | +419 | +1.9% | 21,976 | −293 | −1.3% | 22,141 | −98 | −0.4% | 21,958 | −281 | −1.3% |
Note: Baseline case scenario relies on a tumor doubling time of 90 days.
Abbreviations: Abs., absolute; diff., difference; HCC, hepatocellular carcinoma; M, month; MRI, magnetic resonance imaging; ref., reference; US, ultrasound.
FIGURE 1.
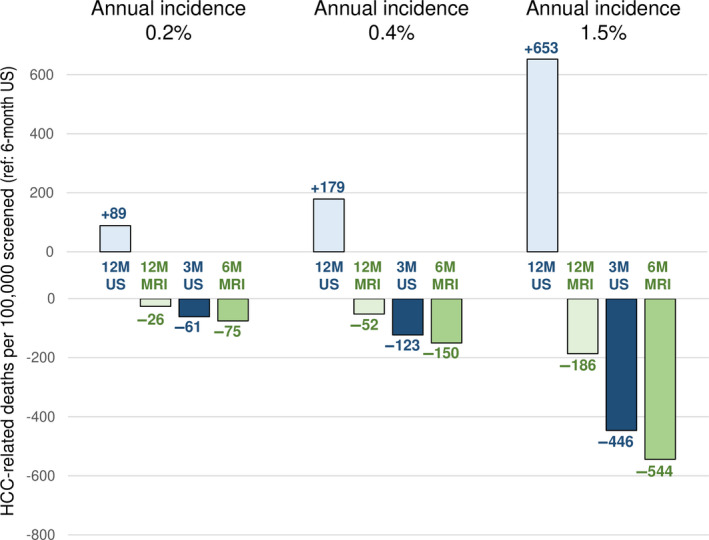
Reduction of HCC‐related deaths in various scenarios of imaging modality and screening interval. Increased (+, upwards) or decreased (−, downwards) absolute numbers of HCC‐related deaths when using annual US (12M US, light blue), annual MRI (12M MRI, light green), quarterly US (3M US, dark blue), or semiannual MRI (6M MRI, dark green), compared to semiannual US (6M US). Left, middle, and right sets of bar graphs are results for annual HCC incidences of 0.2%, 0.4%, and 1.5% respectively. Abbreviations: HCC, hepatocellular carcinoma; M, month; MRI, magnetic resonance imaging; US, ultrasound.
For annual HCC incidences of 0.2%, 0.4%, and 1.5%, quarterly US compared to semiannual US reduced total mortality by 36, 72, and 293 deaths, respectively, corresponding to reductions of total mortality of −0.1%, −0.4%, and −1.3%; semiannual MRI compared to semiannual US reduced total mortality by 38, 77, and 281 deaths, respectively, corresponding to reductions of −0.2%, −0.4%, and −1.3%; annual MRI compared to semiannual US reduced total mortality by 13, 27, and 98 deaths per 100,000 screened patients, corresponding to reductions of <−0.1%, −0.1%, and −0.4%.
Compared to no surveillance, the number of patients needed to screen (NNS) with semiannual US was 356, 177, and 49 to prevent one HCC‐related death and 535, 267, and 74 to prevent one all‐cause death, for annual incidences of 0.2%, 0.4%, and 1.5%, respectively. Compared to semiannual US, the NNS to prevent one HCC‐related death was 1639, 813, and 224 with quarterly US; 3846, 1923, and 538 with annual MRI; and 1333, 667, and 184 with semiannual MRI, for annual incidences of 0.2%, 0.4%, and 1.5%, respectively. Compared to semiannual US, the NNS to prevent one all‐cause death was 2777, 1388, and 341 with quarterly US; 7692, 3703, and 1020 with annual MRI; and 2632, 1299, and 356 with semiannual MRI, for annual incidences of 0.2%, 0.4%, and 1.5%, respectively (Table 5).
TABLE 5.
Number needed to prevent one death 10 years after the start of screening, according to HCC annual incidence and surveillance modality
| Annual HCC incidence | 6‐M US vs. no surveillance | 3‐M US vs. 6‐M US | 12‐M MRI vs. 6‐M US | 6‐M MRI vs. 6‐M US |
|---|---|---|---|---|
| HCC‐related mortality | ||||
| 0.2% | 356 | 1639 | 3846 | 1333 |
| 0.4% | 177 | 813 | 1923 | 667 |
| 1.5% | 49 | 224 | 538 | 184 |
| Overall mortality | ||||
| 0.2% | 535 | 2777 | 7692 | 2632 |
| 0.4% | 267 | 1388 | 3703 | 1299 |
| 1.5% | 74 | 341 | 1020 | 356 |
Note: Baseline case scenario relies on a tumor doubling time of 90 days.
Abbreviations: HCC, hepatocellular carcinoma; M, month; MRI, magnetic resonance imaging; US, ultrasound.
Sensitivity analysis
Sensitivity analysis was performed by decreasing the effectiveness of US screening to detect the presence of HCC. For semiannual US, a 25% reduction in effectiveness resulted in an 11% increase in the CFR and HCC‐related mortality (CFR, 51% vs. 46%; HCC‐related mortality: 477 vs. 431, 957 vs. 865, and 3483 vs. 3146 for annual incidences of 0.2%, 0.4%, and 1.5%, respectively). In this scenario, quarterly rather than semiannual US resulted in a reduction of 79, 159, and 579 HCC‐related deaths and 50, 100, and 361 total deaths per 100,000 screened patients for annual incidences of 0.2%, 0.4%, and 1.5%, respectively. Semiannual MRI decreased HCC‐related mortality by 121, 242, and 881 deaths and total mortality by 68, 137, and 497 deaths compared to semiannual US. Finally, annual MRI decreased HCC‐related mortality by 72, 144, and 523 deaths and total mortality by 43, 87, and 314 deaths compared to semiannual US for the same incidences.
Compared to US alone, combining AFP to semiannual US imaging led to an increase of screening sensitivity and a decrease of CFR and HCC‐related mortality (CFR, 43% vs. 46%; HCC‐related mortality: 399 vs. 431, 802 vs. 865, and 2916 vs. 3146 for annual incidences of 0.2%, 0.4%, and 1.5%, respectively).
When screening adherence to semiannual US was 70%, CFR and HCC‐related mortality increased by 15% compared to 100% adherence (CFR, 52% vs. 46%; HCC‐related mortality: 487 vs. 431, 979 vs. 865, and 3562 vs. 3146 for annual incidences of 0.2%, 0.4% and 1.5%, respectively). When screening adherence to semiannual US was 50%, CFR and HCC‐related mortality increased by 25% compared to 100% adherence (CFR, 58% vs. 46%; HCC‐related mortality: 537 vs. 431, 1079 vs. 865, and 3926 vs. 3146 for annual incidences of 0.2%, 0.4%, and 1.5%, respectively).
When compared to no screening, the scenario of incidental detection (16.5% of the cirrhotic population randomly receiving abdominal imaging) resulted in a reduction of CFR and HCC‐related mortality (CFR, 67% vs. 77%; HCC‐related mortality: 627 vs. 712, 1259 vs. 1430, and 4580 vs. 5205 for annual incidences of 0.2%, 0.4%, and 1.5%, respectively).
Increasing non‐liver‐related mortality led to small decreases in HCC‐related mortality in all surveillance scenarios but did not affect the differences between scenarios. When comparing 6‐month US to no surveillance, overall mortality was reduced by 0.7%, 1.4%, and 4.5% vs. 1%, 1.9%, and 5.7% in the baseline analysis for annual incidences of 0.2%, 0.4%, and 1.5%, respectively. All results of sensitivity analysis are provided in Tables S3–S5.
DISCUSSION
The evaluation of CFR and the absolute numbers of cancer‐related and overall deaths provides additional insight into the value of HCC surveillance. Compared to no surveillance, semiannual US screening decreases the CFR and HCC‐related and all‐cause mortality. Even in the scenario with the lowest annual incidence of 0.2%, the extent of the decrease in cancer‐related mortality is close to that observed with other established cancer screening protocols. This questions the optimal incidence threshold for HCC screening. Moreover, reducing the interval of US to 3 months improves CFR and cancer‐related mortality, although it does not significantly change overall mortality. The use of MRI instead of US also improves cancer‐related outcomes. When US is unsuitable, such as in patients with obesity, annual MRI could be an acceptable option.
The optimal endpoint to evaluate screening programs is a subject of debate. While all‐cause mortality is a clear endpoint that is free of bias, it is not suitable to identify the specific decrease in death from the target disease. When all‐cause mortality is used as an endpoint, identifying the effect of screening requires huge sample sizes and extended follow‐up. A modeling study for breast cancer screening has suggested that 300,000 participants per arm followed up for 16 to 26 years were needed to show a reduction in all‐cause mortality of between 0.4% and 1.8%.[ 45 ] Consequently, health authorities recommend screening for breast, colorectal, and lung cancers only due to their effect on cancer‐related mortality. This was achieved for colorectal cancer with biennial fecal immunological testing (an estimated 236 fewer cancer deaths)[ 46 ] and breast cancer with an annual mammogram (eight fewer cancer deaths).[ 47 ] Thus, the evaluation of HCC screening should evaluate both total and cancer‐specific mortalities but as with other cancers should focus on the latter to help experts design new strategies.
Our results show that HCC screening improves cancer‐related mortality. Importantly, even in a scenario with 0.2% annual incidence, equivalent to breast and colorectal cancer incidence in the general population between 50 and 74 years old, semiannual US prevents 281 HCC‐related deaths per 100,000 patients screened compared to no surveillance. It is important to emphasize that the effectiveness of cancer screening depends on the CFRs, which vary according to tumor sites. CFR for breast cancer with a tumor size <3 cm is around 10% while it is 43% for tumors >5 cm.[ 47 ]In HCC, the CFR with semiannual US was 37% and 83% for tumors <3 cm and >5 cm, respectively. The probability of early detection outside of screening also depends on the tumor site. In unscreened patients, colorectal cancer and HCC are often detected at advanced stages due to symptoms while breast cancer can still be diagnosed at a relatively early stage due to its anatomical localization. All these elements show the complexity of evaluating screening programs in different solid tumors. However, the marked reduction in HCC‐related deaths even in the scenario of lowest incidence emphasizes the need for studies to reconsider the optimal cutoff for HCC screening. The current threshold of 1.5% annual incidence for HCC screening was defined in 1996 in a cost‐effectiveness analysis that should be reassessed with updated costs and outcomes.[ 13 ]
In a scenario of 1.5% annual incidence, all‐cause mortality was reduced by 5.7%, from 23,595 deaths to 22,239 deaths, with 10 years of semi‐annual HCC surveillance of 100,000 patients. All‐cause mortality has not been shown to improve with other cancer screening programs. It is important to note that HCC screening is applied to patients with underlying disease while other screening programs apply to the general population.
Although scientific societies widely recommend semiannual US surveillance, certain experts suggest tailoring the screening interval. Our model predicts that quarterly US would reduce HCC‐related mortality compared to semiannual US. Most clinical studies addressing such questions have been underpowered, and none have used cancer‐related mortality as an endpoint. The randomized controlled trial comparing a US interval of 3 and 6 months in 1340 patients did not assess cancer‐related or overall survival.[ 17 ] However, this sample is smaller than those used in other solid tumor screening programs. As an example, the pivotal study for colorectal cancer screening included 46,551 patients to detect a significant difference in cancer‐related mortality between the biennial‐screening group and the control group with 2.2% and 2.7%, respectively.[ 46 ] Only a sample of this size could provide sufficient evidence for scientific societies to recommend colorectal cancer screening.
In the present study, either semiannual or annual MRI reduced HCC‐related mortality compared to semiannual US. Machine availability and costs are major limits to consider this method in a screening program. However, an abbreviated MRI could help alleviate those limitations as it has shown high sensitivity and specificity for early stage HCC. Additional studies are needed to standardize acquisition sequences for HCC screening. Our model shows that combining AFP to US imaging at an interval of 3 months may be an interesting option for patients suitable for US screening. For patients unsuitable for US screening due to reduced sensitivity, such as for patients with obesity, the reduction of HCC‐related mortality observed with MRI in our study calls for the additional evaluation of this strategy.
This study confirms the value of mathematical modeling to evaluate HCC surveillance.[ 48 , 49 , 50 ] Nevertheless, this approach has certain limitations. First, the data used in the model were obtained from multiple sources and assumptions. We therefore focused on the most robust data available. Second, we assumed that patients were 100% compliant to HCC surveillance and that diagnosed patients were 100% fit for HCC treatment. We chose this hypothesis because expert guidelines recommend only applying screening to patients who are fit for HCC treatment. We agree that this is not a conservative assumption, but this makes it possible to evaluate the impact of the different strategies, all other things being equal. However, we also evaluated two alternative scenarios of 50% and 70% adherence to US screening. Third, our baseline scenario of “no surveillance” may not be realistic as incidental detection may occur. Nevertheless, sensitivity analysis considered the use of medical imaging for reasons unrelated to HCC surveillance. Even in this scenario, the benefit of HCC surveillance was still observed even when compared to this “real‐life” scenario. Last, we considered a conservative assumption of similar non‐liver‐related mortality in patients with cirrhosis compared to the general population. Some studies suggest that patients with cirrhosis may have an excess risk of non‐liver‐related mortality.[ 40 ] However, they included patients with cirrhosis identified in health care settings, with the highest probability of comorbidities that are risk factors of liver disease progression but also of non‐liver‐related events. Non‐liver‐related mortality also depends on the etiology of liver disease, and risk factors associated with each etiology vary according to geographic areas. Nevertheless, we performed a sensitivity analysis to consider a 2.1 higher risk of non‐liver‐related mortality compared to the general population. Even such increase had small impact on the benefit of surveillance and did not change our conclusions.
In conclusion, the present study supports the inclusion of cancer‐related outcomes, such as CFRs, in the evaluation of screening for HCC. Semiannual US improves cancer‐related and overall mortality compared to no surveillance. Shortening the interval of US to 3 months or using MRI as an option in patients for whom US is unsuitable was found to be effective for cancer‐related outcomes.
AUTHOR CONTRIBUTIONS
Philippe Mathurin and Sylvie Deuffic‐Burban conceived the study. Massih Ningarhari, Abbas Mourad, Philippe Mathurin, and Sylvie Deuffic‐Burban contributed to the conception and design of the analysis. Massih Ningarhari, Abbas Mourad, and Sylvie Deuffic‐Burban performed the analysis. All authors contributed to the interpretation. Massih Ningarhari, Philippe Mathurin, and Sylvie Deuffic‐Burban drafted the work. All authors critically revised the study for intellectual content and approved the final version for publication. All authors agree to be accountable for all aspects of the work by ensuring that questions related to the accuracy or integrity of any part of this study are appropriately investigated and resolved.
CONFLICT OF INTEREST
Massih Ningarhari received speaker fees from Ipsen. Guillaume Lassailly received speaker fees from Gilead and Novartis. Philippe Mathurin received speaker fees from and is an advisor for AbbVie, Gilead Sciences, Ipsen, Eisai, MSD, and Bayer Healthcare; he consults for MSD, Ipsen, Eisal, AbbVie, Sanofi, Gilead Sciences, Servier, and Bayer Healthcare. Sylvie Deuffic‐Burban has received consultancy honoraria from Intercept. The other authors have nothing to report.
Supporting information
Appendix S1 Supporting information.
Ningarhari M, Mourad A, Delacôte C, Ntandja Wandji L‐C, Lassailly G & Louvet A et al. Benefits of tailored hepatocellular carcinoma screening in patients with cirrhosis on cancer‐specific and overall mortality: A modeling approach. Hepatol Commun. 2022;6:2964–2974. 10.1002/hep4.2059
Massih Ningarhari and Abbas Mourad contributed equally to this work as first authors.
Philippe Mathurin and Sylvie Deuffic‐Burban contributed equally to this work as senior authors.
Contributor Information
Philippe Mathurin, Email: philippe.mathurin@chu-lille.fr.
Sylvie Deuffic‐Burban, Email: sylvie.burban@inserm.fr.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 2. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55. [DOI] [PubMed] [Google Scholar]
- 3. Heimbach J, Kulik LM, Finn R, Sirlin CB, Abecassis M, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–80. [DOI] [PubMed] [Google Scholar]
- 4. European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer . EASL‐EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. Erratum in: J Hepatol. 2012;56:1430. [DOI] [PubMed] [Google Scholar]
- 5. Singal AG. CON: hepatocellular carcinoma surveillance: in need of higher‐quality data. Am J Gastroenterol. 2017;112:1634–5. [DOI] [PubMed] [Google Scholar]
- 6. Yopp A, Waljee A, Singal AG. Does surveillance for hepatocellular carcinoma benefit patients with compensated cirrhosis? Gastroenterology. 2014;147:935–6. [DOI] [PubMed] [Google Scholar]
- 7. Penston J. Should we use total mortality rather than cancer specific mortality to judge cancer screening programmes? Yes. BMJ. 2011;343:d6395. [DOI] [PubMed] [Google Scholar]
- 8. Prasad V, Lenzer J, Newman DH. Why cancer screening has never been shown to “save lives”–and what we can do about it. BMJ. 2016;352:h6080. [DOI] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention . Breast cancer screening guidelines for women. https://www.cdc.gov/cancer/breast/pdf/breast‐cancer‐screening‐guidelines‐508.pdf (2020). Accessed 12 Jul 2022. [Google Scholar]
- 10. American Medical Association . Colorectal cancer: screening. https://www.uspreventiveservicestaskforce.org/home/getfilebytoken/2Wc3FRHpVDPX2jT_WzjEXX (2021). Accessed 12 Jul 2022. [Google Scholar]
- 11. SEER Cancer Statistics Review 1975–2016 . Cancer of the female breast (invasive). https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_04_breast.pdf (2021). Accessed 12 Jul 2022. [Google Scholar]
- 12. Kehm RD, Lima SM, Swett K, Mueller L, Yang W, Gonsalves L, et al. Age‐specific trends in colorectal cancer incidence for women and men, 1935‐2017. Gastroenterology. 2021;161:1060–62.e3. [DOI] [PubMed] [Google Scholar]
- 13. Sarasin FP, Giostra E, Hadengue A. Cost‐effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child‐Pugh class A cirrhosis. Am J Med. 1996;101:422–34. [DOI] [PubMed] [Google Scholar]
- 14. Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, et al. Evidence‐based clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2013 update (3rd JSH‐HCC guidelines). Hepatol Res. 2015;45:123–7. [DOI] [PubMed] [Google Scholar]
- 15. Paluch‐Shimon S, Cardoso F, Sessa C, Balmana J, Cardoso MJ, Gilbert F, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO clinical practice guidelines for cancer prevention and screening. Ann Oncol. 2016;27(Suppl 5):v103–10. Erratum in: Ann Oncol. 2017;28(Suppl 4):iv167‐8. [DOI] [PubMed] [Google Scholar]
- 16. Stoffel EM, Mangu PB, Gruber SB, Hamilton SR, Kalady MF, Lau MW, et al. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology clinical practice guideline endorsement of the familial risk‐colorectal cancer: European Society for Medical Oncology clinical practice guidelines. J Clin Oncol. 2015;33:209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trinchet JC, Chaffaut C, Bourcier V, Degos F, Henrion J, Fontaine H, et al. Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire (GRETCH). Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3‐ and 6‐month periodicities. Hepatology. 2011;54:1987–97. [DOI] [PubMed] [Google Scholar]
- 18. Trinchet JC, Bourcier V, Chaffaut C, Ait Ahmed M, Allam S, Marcellin P, et al. Complications and competing risks of death in compensated viral cirrhosis (ANRS CO12 CirVir prospective cohort). Hepatology. 2015;62:737–50. [DOI] [PubMed] [Google Scholar]
- 19. Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ganne‐Carrie N, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, et al. Nomogram for individualized prediction of hepatocellular carcinoma occurrence in hepatitis C virus cirrhosis (ANRS CO12 CirVir). Hepatology. 2016;64:1136–47. [DOI] [PubMed] [Google Scholar]
- 21. European Association for the Study of the Liver . EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. Erratum in: J Hepatol. 2019;70:817. [DOI] [PubMed] [Google Scholar]
- 22. Lim C, Bhangui P, Salloum C, Gomez‐Gavara C, Lahat E, Luciani A, et al. Impact of time to surgery in the outcome of patients with liver resection for BCLC 0‐A stage hepatocellular carcinoma. J Hepatol. 2017;68:100–8. [DOI] [PubMed] [Google Scholar]
- 23. Vilar‐Gomez E, Calzadilla‐Bertot L, Wai‐Sun Wong V, Castellanos M, Aller‐de la Fuente R, Metwally M, et al. Fibrosis severity as a determinant of cause‐specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi‐national cohort study. Gastroenterology. 2018;155:443–57.e17. [DOI] [PubMed] [Google Scholar]
- 24. Nahon P, Lescat M, Layese R, Bourcier V, Talmat N, Allam S, et al. Bacterial infection in compensated viral cirrhosis impairs 5‐year survival (ANRS CO12 CirVir prospective cohort). Gut. 2017;66:330–41. [DOI] [PubMed] [Google Scholar]
- 25. Ganne‐Carrie N, Chaffaut C, Bourcier V, Archambeaud I, Perarnau JM, Oberti F, et al. Estimate of hepatocellular carcinoma incidence in patients with alcoholic cirrhosis. J Hepatol. 2018;69:1274–83. [DOI] [PubMed] [Google Scholar]
- 26. D'Amico G, Garcia‐Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–31. [DOI] [PubMed] [Google Scholar]
- 27. Institut National D'Etudes Demographiques . Taux de mortalité par sexe et âge. https://www.ined.fr/fr/tout‐savoir‐population/chiffres/france/mortalite‐cause‐deces/taux‐mortalite‐sexe‐age/ (2022). Accessed 12 Jul 2022. [Google Scholar]
- 28. Schwartz M. A biomathematical approach to clinical tumor growth. Cancer. 1961;14:1272–94. [DOI] [PubMed] [Google Scholar]
- 29. Sheu JC, Sung JL, Chen DS, Yang PM, Lai MY, Lee CS, et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology. 1985;89:259–66. [DOI] [PubMed] [Google Scholar]
- 30. Okazaki N, Yoshino M, Yoshida T, Suzuki M, Moriyama N, Takayasu K, et al. Evaluation of the prognosis for small hepatocellular carcinoma based on tumor volume doubling time. A preliminary report. Cancer. 1989;63:2207–10. [DOI] [PubMed] [Google Scholar]
- 31. Woo HY, Jang JW, Choi JY, Bae SH, You CR, Rha SE, et al. Tumor doubling time after initial response to transarterial chemoembolization in patients with hepatocellular carcinoma. Scand J Gastroenterol. 2010;45:332–9. [DOI] [PubMed] [Google Scholar]
- 32. Villa E, Critelli R, Lei B, Marzocchi G, Camma C, Giannelli G, et al. Neoangiogenesis‐related genes are hallmarks of fast‐growing hepatocellular carcinomas and worst survival. Results from a prospective study. Gut. 2016;65:861–9. [DOI] [PubMed] [Google Scholar]
- 33. Kim JK, Kim HD, Jun MJ, Yun SC, Shim JH, Lee HC, et al. Tumor volume doubling time as a dynamic prognostic marker for patients with hepatocellular carcinoma. Dig Dis Sci. 2017;62:2923–31. Erratum in: Dig Dis Sci. 2017;62:3259. [DOI] [PubMed] [Google Scholar]
- 34. Rich NE, John BV, Parikh ND, Rowe I, Mehta N, Khatri G, et al. Hepatocellular carcinoma demonstrates heterogeneous growth patterns in a multicenter cohort of patients with cirrhosis. Hepatology. 2020;72:1654–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cucchetti A, Trevisani F, Pecorelli A, Erroi V, Farinati F, Ciccarese F, et al. Estimation of lead‐time bias and its impact on the outcome of surveillance for the early diagnosis of hepatocellular carcinoma. J Hepatol. 2014;61:333–41. [DOI] [PubMed] [Google Scholar]
- 36. Tsilimigras DI, Hyer JM, Diaz A, Moris D, Bagante F, Ratti F, et al. Impact of time‐to‐surgery on outcomes of patients undergoing curative‐intent liver resection for BCLC‐0, A and B hepatocellular carcinoma. J Surg Oncol. 2021;123:381–8. [DOI] [PubMed] [Google Scholar]
- 37. Agence de la Biomédecine . Organes: greffe hépatique. https://rams.agence‐biomedecine.fr/sites/default/files/pdf/2021‐09/ABM_PG_Organes_Foie2020.pdf (2020). Accessed 12 Jul 2022. [Google Scholar]
- 38. Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta‐analysis. Gastroenterology. 2018;154:1706–18.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith‐Bindman R, Kwan ML, Marlow EC, Theis MK, Bolch W, Cheng SY, et al. Trends in use of medical imaging in US health care systems and in Ontario, Canada, 2000–2016. JAMA. 2019;322:843–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ratib S, Fleming KM, Crooks CJ, Walker AJ, West J. Causes of death in people with liver cirrhosis in England compared with the general population: a population‐based cohort study. Am J Gastroenterol. 2015;110:1149–58. [DOI] [PubMed] [Google Scholar]
- 41. Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta‐analysis. PLoS Med. 2014;11:e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Santi V, Trevisani F, Gramenzi A, Grignaschi A, Mirici‐Cappa F, Del Poggio P, et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol. 2010;53:291–7. [DOI] [PubMed] [Google Scholar]
- 43. Costentin CE, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, et al. Compliance with hepatocellular carcinoma surveillance guidelines associated with increased lead‐time adjusted survival of patients with compensated viral cirrhosis: a multi‐center cohort study. Gastroenterology. 2018;155:431–42.e10. [DOI] [PubMed] [Google Scholar]
- 44. van Meer S, de Man RA, Coenraad MJ, Sprengers D, van Nieuwkerk KM, Klumpen HJ, et al. Surveillance for hepatocellular carcinoma is associated with increased survival: results from a large cohort in the Netherlands. J Hepatol. 2015;63:1156–63. [DOI] [PubMed] [Google Scholar]
- 45. Heijnsdijk EAM, Csanadi M, Gini A, Ten Haaf K, Bendes R, Anttila A, et al. All‐cause mortality versus cancer‐specific mortality as outcome in cancer screening trials: a review and modeling study. Cancer Med. 2019;8:6127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, et al. Long‐term mortality after screening for colorectal cancer. N Engl J Med. 2013;369:1106–14. [DOI] [PubMed] [Google Scholar]
- 47. Welch HG, Prorok PC, O'Malley AJ, Kramer BS. Breast‐cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375:1438–47. [DOI] [PubMed] [Google Scholar]
- 48. Mourad A, Deuffic‐Burban S, Ganne‐Carrie N, Renaut‐Vantroys T, Rosa I, Bouvier AM, et al. Hepatocellular carcinoma screening in patients with compensated hepatitis C virus (HCV)‐related cirrhosis aware of their HCV status improves survival: a modeling approach. Hepatology. 2014;59:1471–81. [DOI] [PubMed] [Google Scholar]
- 49. Yang JD, Mannalithara A, Piscitello AJ, Kisiel JB, Gores GJ, Roberts LR, et al. Impact of surveillance for hepatocellular carcinoma on survival in patients with compensated cirrhosis. Hepatology. 2018;68:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Parikh ND, Singal AG, Hutton DW, Tapper EB. Cost‐effectiveness of hepatocellular carcinoma surveillance: an assessment of benefits and harms. Am J Gastroenterol. 2020;115:1642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information.


