Abstract
Nonalcoholic fatty liver disease (NAFLD) is defined by excessive accumulation of lipid droplets within hepatocytes. The STE20‐type kinases comprising the germinal center kinase III (GCKIII) subfamily – MST3, MST4, and STK25 – decorate intrahepatocellular lipid droplets and have recently emerged as critical regulators of the initiation and progression of NAFLD. While significant advancement has been made toward deciphering the role of GCKIII kinases in hepatic fat accumulation (i.e., steatosis) as well as the aggravation of NAFLD into its severe form nonalcoholic steatohepatitis (NASH), much remains to be resolved. This review provides a brief overview of the recent studies in patient cohorts, cultured human cells, and mouse models, which have characterized the function of MST3, MST4, and STK25 in the regulation of hepatic lipid accretion, meta‐inflammation, and associated cell damage in the context of NAFLD/NASH. We also highlight the conflicting data and emphasize future research directions that are needed to advance our understanding of GCKIII kinases as potential targets in the therapy of NAFLD and its comorbidities. Conclusions: Several lines of evidence suggest that GCKIII proteins govern the susceptibility to hepatic lipotoxicity and that pharmacological inhibition of these kinases could mitigate NAFLD development and aggravation. Comprehensive characterization of the molecular mode‐of‐action of MST3, MST4, and STK25 in hepatocytes as well as extrahepatic tissues is important, especially in relation to their impact on carcinogenesis, to fully understand the efficacy as well as safety of GCKIII antagonism.
Nonalcoholic fatty liver disease (NAFLD) is defined by excessive accumulation of lipid droplets within hepatocytes. The STE20‐type kinases comprising the GCKIII subfamily ‐ MST3, MST4, and STK25 ‐ decorate intrahepatocellular lipid droplets and have recently emerged as critical regulators of the initiation and progression of NAFLD. This review provides a brief overview of the recent studies in patient cohorts, cultured human cells, and mouse models, which have characterized the function of MST3, MST4, and STK25 in the regulation of hepatic lipid accretion, meta‐inflammation, and associated cell damage in the context of NAFLD/NASH.

INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD), defined as fatty infiltration in > 5% of hepatocytes (steatosis) in the absence of excessive alcohol consumption, is emerging as a leading cause of chronic liver disease worldwide.[ 1 , 2 ] In a subgroup of patients with NAFLD, the disease aggravates to nonalcoholic steatohepatitis (NASH), which in addition to liver lipid accumulation is characterized by local inflammation and hepatocellular damage in the form of ballooning and apoptosis, with different degrees of hepatic fibrosis.[ 3 ] NAFLD contributes to the pathogenesis of type 2 diabetes mellitus and cardiovascular disease, and patients with NASH are also at risk of progressing into cirrhosis, liver failure, and hepatocellular carcinoma (HCC).[ 1 , 4 , 5 , 6 ] Despite extensive research being invested in this area of high unmet medical need, the molecular pathogenesis of NAFLD and NASH remains elusive, and no approved drugs are available for their treatment.[ 7 ]
Among genetic variants that confer susceptibility to NAFLD/NASH, the best characterized are a single‐nucleotide polymorphism (rs738409) in the PNPLA3 (patatin‐like phospholipase domain containing 3) gene and a splice variant (rs72613567:TA) in the HSD17B13 gene,[ 8 , 9 ] both of which encode proteins anchored to the intrahepatocellular lipid droplets. Importantly, recent evidence has revealed that the STE20‐type kinases comprising the GCKIII subfamily (MST3, MST4, and STK25) also regulate the initiation and aggravation of NAFLD via association with hepatocellular lipid droplets. Together, these data highlight the critical function of liver lipid droplet proteome in NAFLD etiology.
In the following sections, we discuss the newly described roles of GCKIII kinases in the molecular pathogenesis of NAFLD and NASH and explore the underlying mechanisms.
DOMAIN STRUCTURE OF THE HUMAN GCKIII SUBFAMILY OF STE20 KINASES
The human kinome features a large branch of STE20 kinases, named after the founding member yeast Sterile20 kinase involved in the mating pathway.[ 10 ] Mammalian STE20‐type kinases (~30 described to date) are distinguished by a high degree of homology within the catalytic domain, and they fall into two subfamilies: the p21‐activated kinases (PAKs) with a C‐terminal kinase domain and an N‐terminal p21 GTPase‐binding domain and the germinal center kinases (GCKs), which have an N‐terminal kinase domain and lack GTPase‐binding domains.[ 11 ] Functionally, STE20 kinases have been reported to regulate a broad range of biological processes including cell differentiation, proliferation, apoptosis, motility, polarity, metastasis, angiogenesis, and stress responses.[ 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 ]
MST3 (also known as STK24), MST4 (also known as STK26 or MASK), and STK25 (also known as YSK1 or SOK1) comprise the GCKIII subgroup of STE20 proteins. The members of the GCKIII family are ubiquitously expressed and have been implicated in the regulation of Golgi integrity, cytoskeletal organization, cell polarity, proliferation and migration, apoptosis, as well as neuronal and immune functions.[ 21 , 22 , 23 , 24 , 25 , 26 , 27 ] In humans, MST3 is located on chromosome 13q32.2, whereas MST4 and STK25 are located on Xq26.2 and 2q37.3, respectively.
The kinases in the GCKIII subgroup are the shortest within the STE20 family, with the human proteins ranging from 416 to 431 amino acids in length. At the N‐terminal end of GCKIIIs, a short variable sequence is followed by a highly conserved kinase domain of 251 residues (about 90% similarity in amino acid sequence).[ 19 , 20 , 25 , 28 , 29 ] This catalytic domain encompasses the adenosine triphosphate–binding (ATP) site (GXGX[F]GX16K) and a protein substrate binding site, which also contains the STE20 signature peptide (GTPFWMAPE) (Figure 1).[ 12 , 30 ] Molecular modeling and crystallographic studies have revealed that the GCKIII catalytic domains display a typical structure of protein kinase domains, with a smaller N‐lobe and a larger C‐lobe, and the substrate‐binding cleft placed between them.[ 25 , 31 , 32 , 33 , 34 , 35 , 36 ] Notably, the lysine residue (Lys49 in STK25 and Lys53 in MST3/MST4) and the threonine residue (Thr174 in STK25 and Thr178 in MST3/MST4) within the kinase domain have been identified as instrumental for the catalytic activity of GCKIII kinases.[ 29 , 33 , 37 , 38 , 39 ] Rather than requiring activation by upstream kinase(s), GCKIIIs appear to be activated by constitutive autophosphorylation of Thr174/178, suggesting that the primary regulation of MST3, MST4, and STK25 activity lies at the level of inhibition of the Thr174/178 dephosphorylation by protein phosphatase(s).[ 25 , 33 , 40 , 41 , 42 , 43 ]
FIGURE 1.
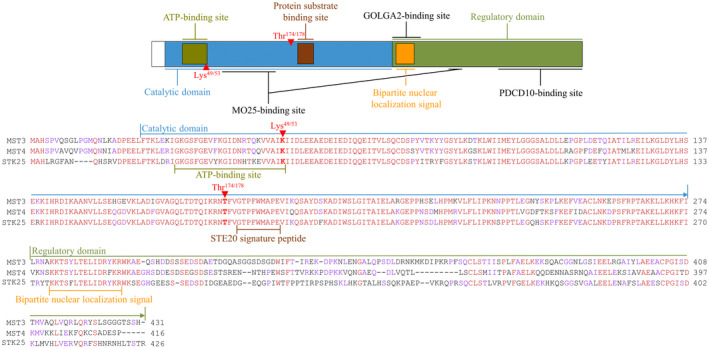
Domain structure and alignment of human GCKIII kinases. Sequences of MST3 (GenPept accession number NP_001027467), MST4 (GenPept accession number NP_057626), and STK25 (GenPept accession number NP_001258906) were aligned using Clustal Omega (1.2.2; Conway Institute UCD, Dublin, Ireland). Fully, partially, or not aligned sequences are shown in red, violet, or black, respectively. ATP, adenosine triphosphate.
The regulatory domain of MST3, MST4, and STK25 lies at the C‐terminal to the catalytic domain and carries a putative bipartite nuclear localization signal at its N‐terminal end, which is relatively well conserved (Figure 1).[ 44 , 45 ] This sequence is followed by a variable region of 119–134 residues (about 20% similarity in amino acid sequence), which is predicted to be highly disordered and is suggested to interact with various signaling molecules and regulatory proteins.[ 25 , 30 ] As an example, the regulatory domain of all three GCKIIIs has been shown to heterodimerize with PDCD10 (programmed cell death 10, also known as CCM3).[ 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 ] The regulatory region of GCKIIIs also interacts with GOLGA2 (Golgin subfamily A member 2, also known as GM130) in the control of Golgi assembly.[ 25 ] Furthermore, through both regulatory and catalytic domains, GCKIIIs associate with MO25 protein in the LKB1/STRAD/MO25 complex.[ 34 , 35 , 59 , 60 ] The regulatory regions appear to be involved in homodimerization of GCKIII kinases, although the exact residues engaged in these interactions are not clearly determined.[ 29 , 61 ] Notably, several caspase cleavage sites are predicted in silico within the regulatory domain of MST3, MST4, and STK25, some of which have been experimentally verified (Figure 1).[ 25 , 37 , 38 , 44 , 62 , 63 , 64 , 65 , 66 ]
GCKIII KINASES DECORATE INTRAHEPATOCELLULAR LIPID DROPLETS
Intracellular lipid droplets are composed of a neutral lipid core of primarily triacylglycerol (TAG) and cholesterol esters surrounded by a phospholipid monolayer that harbors specific proteins.[ 67 , 68 , 69 ] By using immunofluorescence microscopy, we found that MST3, MST4, and STK25 are exclusively localized around lipid droplets in human hepatocytes (exemplified by STK25 in Figure 2).[ 70 , 71 , 72 ] Consistently, GCKIII kinases are associated with hepatic lipid droplets in mice, as demonstrated by global proteomic analysis of the lipid droplet fraction isolated from steatotic livers of obese mice as well as using immunofluorescence microscopy of mouse liver sections (Figure 2).[ 71 , 72 , 73 , 74 ] In contrast, MST4 and STK25 have been shown to localize to the Golgi apparatus in the human cervical carcinoma cell line (HeLa) and human embryonic kidney cell line (HEK293), where they function in a signaling cascade required for cell migration and polarization.[ 29 , 49 , 50 ] MST3 has been reported to localize predominantly to the cytosol in HeLa and HEK293 cells; however, following caspase cleavage, the protein translocates to the nucleus inducing apoptosis.[ 37 , 44 ] Similarly, a cleaved form of STK25 can translocate from the Golgi to the nucleus in HEK293 cells after chemical anoxia, promoting cell death.[ 38 ] Together, these observations suggest that GCKIII kinases display a different subcellular localization pattern in the liver compared with extrahepatic tissues. Furthermore, it is important to investigate whether the localization of GCKIII kinases may change in hepatocytes in response to challenges such as fasting, when very few lipid droplets are present.
FIGURE 2.
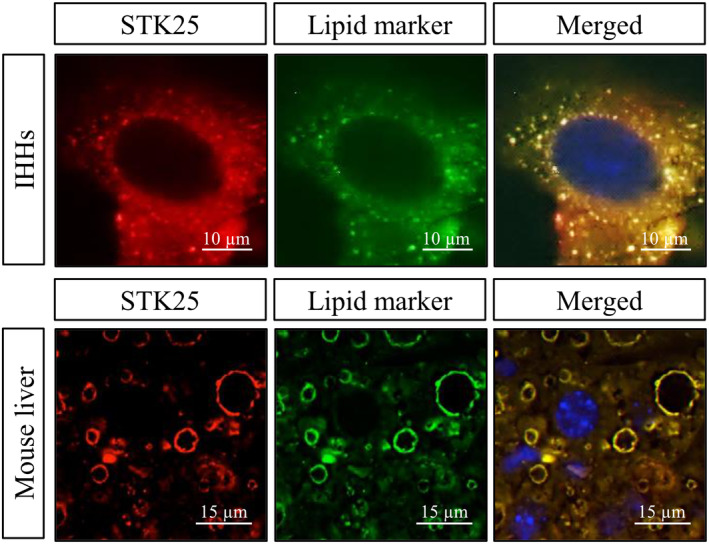
STK25 associates with intrahepatocellular lipid droplets. Representative immunofluorescence images of oleate‐loaded immortalized human hepatocytes (IHHs) and liver sections from high fat–fed mice, where the merged images show colocalization of STK25 and lipid droplets. (Figure adapted from Refs. 70 and 74).
HEPATIC EXPRESSION OF GCKIII KINASES CORRELATES WITH THE SEVERITY OF HUMAN NAFLD
We have reported a significant positive correlation between MST3, MST4, and STK25 transcript abundance in human liver biopsies and all three individual lesions of the NAFLD Activity Score (NAS) used for clinical diagnosis (i.e., histological scores of hepatic steatosis, lobular inflammation, and hepatocellular ballooning) as well as total NAS (n = 62; Figure 3A–D).[ 71 , 72 , 75 ] Moreover, messenger RNA (mRNA) levels of MST3, MST4, and STK25 were about 2–3‐fold higher in subjects with NAS ≥ 5 (defines definite NASH; n = 24) compared to participants with NAS ≤ 4 (defines simple steatosis or borderline NASH; n = 38) (Figure 3E).[ 71 , 72 , 75 ] We also found a positive correlation between the hepatic expression of GCKIII kinases and the liver fat content assessed by magnetic resonance spectroscopy (Figure 3F).[ 70 , 71 , 72 , 75 ]
FIGURE 3.
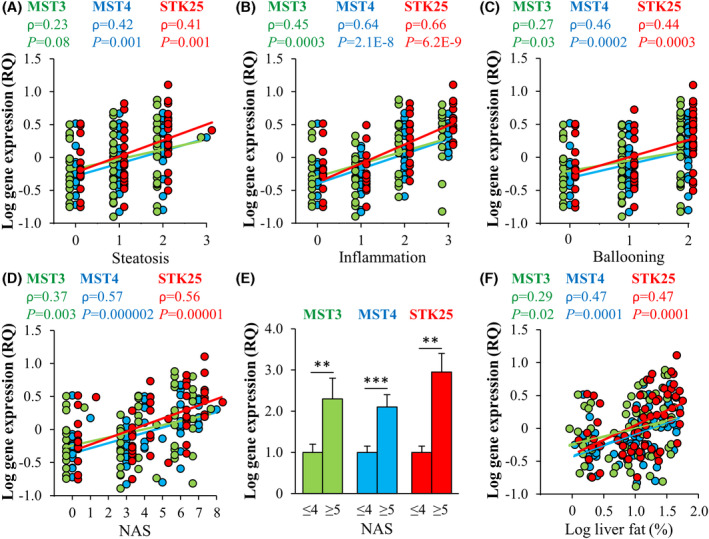
Hepatic expression of GCKIII kinases is positively correlated with nonalcoholic fatty liver disease (NAFLD) severity. Correlation between messenger RNA (mRNA) levels in human liver biopsies and the individual histological lesions of NAFLD Activity Score (NAS; A–C), the total NAS (D), and subjects with low versus high NAS (E). (F) Correlation between mRNA levels and hepatic fat content measured by magnetic resonance spectroscopy. RQ, relative quantification. (Figure combines results from several publications (70, 71, 72 and 75).
GCKIII KINASES CELL‐AUTONAMOUSLY CONTROL LIPID STORAGE AND METABOLIC STRESS IN HUMAN HEPATOCYTES
Our recent studies reveal that small interfering RNA silencing of MST3, MST4, or STK25 in human hepatocytes markedly reduces intracellular lipid accumulation, which is mediated by increased β‐oxidation and very low–density lipoprotein (VLDL)–TAG secretion (i.e., output), combined with decreased TAG synthesis (i.e., input) (Figure 4).[ 70 , 71 , 72 ] Importantly, MST3‐, MST4‐, or STK25‐deficient hepatocytes are also substantially protected against oxidative and endoplasmic reticulum (ER) stress (Figure 4),[ 71 , 72 , 73 , 75 ] which are considered the key triggers of disease progression from simple liver steatosis to NASH.[ 76 , 77 , 78 ] Reciprocally, a substantial increase in lipid deposition as well as oxidative/ER stress was detected in human hepatocytes overexpressing GCKIII kinases.[ 70 , 71 , 72 , 75 ] With these data describing the functional implications of modified MST3, MST4, and STK25 abundance on lipid partitioning and metabolic stress being highly consistent, the underlying mechanisms of GCKIII signaling in the regulation of intrahepatocellular lipid homeostasis still remain elusive. Further unbiased investigations combining global phosphoproteomics, interactomics, and substrate mapping would be valuable to identify the interaction partners and direct targets of GCKIII kinases to decipher their molecular mode‐of‐action in hepatic lipid metabolism. It would also be interesting to use advanced imaging (e.g., superresolution microscopy or correlative light‐electron microscopy) to examine whether GCKIII kinases affect the interactions of liver lipid droplets with other cellular organelles including ER, mitochondria, peroxisomes, and lysosomes.
FIGURE 4.
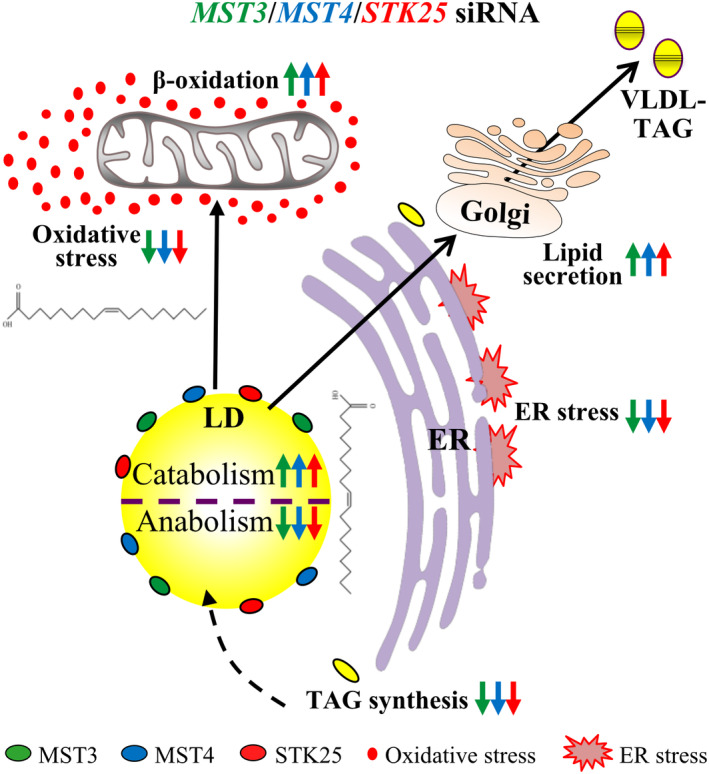
Proposed mechanisms through which GCKIII kinases control liver lipotoxicity. Silencing of MST3, MST4, or STK25 in human hepatocytes lowers fat storage by inhibiting lipid droplet anabolism through reduced triacylglycerol (TAG) synthesis, and by stimulating lipid droplet catabolism through enhanced β‐oxidation and very low–density lipoprotein (VLDL)–TAG secretion, resulting in protection against oxidative and endoplasmic reticulum (ER) stress. LD, lipid droplet. (Figure combines results from several publications (70, 71, 72, 73 and 75).
The possible role of MST3, MST4, and STK25 in glucose metabolism has not been studied in detail in hepatocytes. However, in vivo data support the impact of GCKIII kinases on the control of glucose and insulin homeostasis (see subsequent section). Of note, STK25 was shown to negatively regulate the aerobic glycolysis in colorectal cancer cells via mammalian target of rapamycin (mTOR) signaling.[ 79 ]
SILENCING OF STK25 OR MST3 HINDERS THE DEVELOPMENT OF NASH IN MICE
The main pathophysiological characteristics of human NALFD can be replicated in rodent models by different dietary challenges. We found that, when challenged with a NASH‐inducing diet, the livers from Stk25 knockout mice and mice treated with Stk25‐targeting antisense oligonucleotides (ASOs) display substantial suppression of integral NASH features compared with wild‐type livers: Along with less steatosis, we observed attenuation of oxidative stress, inflammation, and fibrosis, combined with reduced hepatocellular damage (Figure 5).[ 80 , 81 , 82 ] This improvement of the liver phenotype correlates with a decrease in the hepatic levels of acetyl‐CoA carboxylase (ACC) protein, a key regulator of both lipid oxidation and synthesis.[ 80 , 81 , 82 ] Reciprocally, we show that diet‐induced NASH is markedly aggravated in Stk25‐overexpressing transgenic versus wild‐type mice.[ 74 , 75 ] Of note, high‐fat–fed Stk25 −/− mice also accumulate less ectopic fat in extrahepatic tissues such as skeletal muscle, kidney, and vascular wall, and have healthier adipose tissue,[ 82 , 83 , 84 , 85 ] which is accompanied by improved whole‐body glucose tolerance and insulin sensitivity[ 82 ] compared with wild‐type littermates.
FIGURE 5.
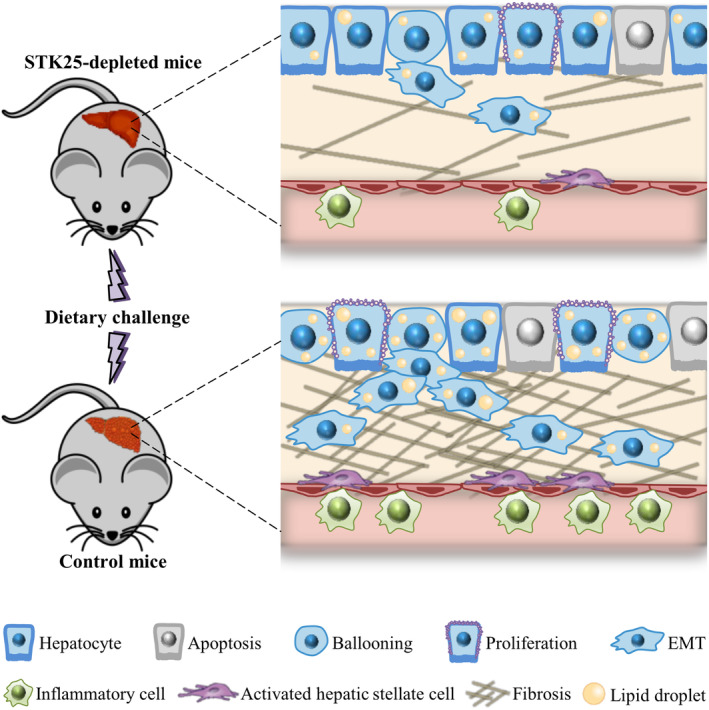
A schematic model: STK25 antagonism protects mice against diet‐induced nonalcoholic steatohepatitis (NASH) and hepatocellular carcinoma (HCC) by suppressing lipotoxicity‐induced inflammatory and profibrotic responses, reducing hepatocellular ballooning and apoptosis, decreasing compensatory proliferation and epithelial–mesenchymal transition (EMT). (Figure combines results from several publications (75, 80, 81, 82 and 96).
Similarly to the silencing of STK25, we found that mice treated with Mst3‐targeting ASOs are significantly protected against diet‐induced NASH, which is also paralleled by a lower abundance of liver ACC.[ 86 ] In light of the recent evidence of an association and causal link between NASH and insulin resistance,[ 5 , 87 ] our results are in line with a study by Iglesias et al. reporting that Mst3 genetrap mice (which have a significant but not complete reduction of Mst3 expression) fed with a high‐fat diet display improved hepatic insulin sensitivity compared with wild‐type controls.[ 88 ] In the livers from high fat–fed mice, the loss of MST3 was also shown to increase the signaling of the insulin receptor pathway at the level of AKT phosphorylation, accompanied by high activity of mTOR complex 2.[ 88 ] In contrast, Qin et al. found that elimination of MST3 in mice promoted high‐fat diet–induced systemic glucose intolerance and insulin resistance, and attenuated phosphorylation of AKT in adipose tissue and liver.[ 89 ] It is possible that the differences in the genetic background of mouse stains studied, and composition of the high‐fat diets used, have contributed to this variability in results.
To date, there are no studies in nonclinical in vivo models that implicate MST4 in the development of NAFLD/NASH. However, there is some indirect evidence suggesting that MST4 may regulate liver fibrosis,[ 90 ] and MST4 has also been described as a critical mediator limiting inflammatory responses in extrahepatic tissues.[ 23 , 91 ]
ROLE OF GCKIII KINASES IN HCC
Recently, NASH has been reported in several studies as a rapidly increasing underlying etiology of HCC,[ 92 , 93 , 94 ] which is one of the most fatal and fastest‐growing cancers.[ 95 ] Interestingly, we found that the genetic ablation of STK25 protects mice against NASH‐driven HCC via reduced hepatocellular apoptosis and lowered compensatory proliferation, by a mechanism that involves the suppression of hepatic lipotoxicity and inactivation of liver signal transducer and activator of transcription 3 (STAT3), extracellular signal‐regulated kinase 1/2 (ERK1/2), and p38 signaling—the key pathways implicated in human HCC (Figure 5).[ 96 ] In line with these observations, short hairpin RNA knockdown of STK25 has been demonstrated to inhibit tumor growth in human HCC xenografts in nude mice.[ 97 ] The silencing of STK25 also suppresses proliferation, migration, and invasion in human hepatocarcinoma cells in vitro, which is accompanied by a lower expression of the markers of epithelial–mesenchymal transition (EMT) and augmented autophagic flux.[ 96 , 97 ] Furthermore, high STK25 expression was found to be correlated with adverse clinicopathological characteristics and poor survival in patients with HCC.[ 97 ]
A report by Lim et al. has described STK25 as an activator of the Hippo pathway in the liver by phosphorylation of LATS.[ 98 ] The Hippo signaling is known to maintain tissue homeostasis and organ size by negatively regulating the oncogenic transcriptional co‐activators yes‐associated protein 1 (YAP) and transcriptional co‐activator with PDZ‐binding motif (TAZ).[ 99 ] Consistently, STK25 knockdown was reported to result in a moderate increase in liver weight in aged mice; however, hepatic carcinomas were not detected.[ 98 ] In contrary, a recent study by Bae et al. has identified STK25 as a suppressor of the Hippo pathway in human embryonic kidney cells by inhibition of STE20‐type kinase MST2.[ 100 ] In all, STK25 may display both positive and negative impact on Hippo signaling depending on the downstream targets involved.
Evidence supporting the involvement of MST4 in HCC is contradictory. A high level of liver MST4 expression, associated with large tumor size, microvascular invasion, and presence of intrahepatic metastasis, has been reported as an adverse prognostic factor for overall survival and time to recurrence after hepatectomy in patients with HCC.[ 101 ] In the same study, the knockdown of MST4 in human HCC cell lines (MHCC‐97H and SMMC7721 cells) was shown to inhibit proliferation, colony formation, and invasion, whereas up‐regulation of MST4 appeared to aggravate these processes by promoting EMT via the activation of ERK signaling.[ 101 ] Conversely, a low MST4 expression has also been reported to correlate with HCC progression and poor prognosis, and MST4 inactivation was found to induce EMT in human HCC cell lines (Bel‐7402, Bel‐7404, and Huh7 cells), promoting their migratory and invasive potential in vitro, and facilitating intrahepatic metastasis in vivo, with the opposite phenotype observed when MST4 is overexpressed.[ 102 , 103 ] Similarly to STK25, MST4 is suggested to act in the Hippo signaling pathway: MST4 is described as a bona fide kinase of YAP, phosphorylating YAP at Thr83 and causing an impaired nuclear importing of YAP.[ 104 , 105 ]
MST3 has also been implicated in the Hippo pathway: Under certain circumstances, MST3 directly phosphorylates the nuclear Dbf2‐related kinase, which in turn phosphorylates and inhibits YAP.[ 106 , 107 ] The possible role of MST3 in HCC initiation and progression is yet to be explored; however, MST3 has been reported to act both as a positive or negative regulator of tumorigenesis in extrahepatic tissues.[ 40 , 108 , 109 , 110 ]
CONCLUSIONS
Recent translational studies in patient cohorts, cultured human cells, and mouse models provide several lines of experimental evidence suggesting that GCKIII kinases MST3, MST4, and STK25 are important components of the hepatocellular lipotoxic milieu. Antagonizing hepatic GCKIII signaling appears to enable targeting of the initiating nexus for lipid‐triggered liver injury in NAFLD, warranting further investigations in basic biology as well as clinical implications of these kinases.
Funding information
Supported by a grant from the Swedish Research Council.
CONFLICT OF INTEREST
Nothing to report.
Mahlapuu M, Caputo M, Xia Y, Cansby E. GCKIII kinases in lipotoxicity: Roles in NAFLD and beyond. Hepatol Commun. 2022;6:2613–2622. 10.1002/hep4.2013
REFERENCES
- 1. Powell EE, Wong VW, Rinella M. Non‐alcoholic fatty liver disease. Lancet. 2021;397:2212–24. [DOI] [PubMed] [Google Scholar]
- 2. Geier A, Tiniakos D, Denk H, Trauner M. From the origin of NASH to the future of metabolic fatty liver disease. Gut. 2021;70:1570–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323:1175–83. [DOI] [PubMed] [Google Scholar]
- 4. Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16:411–28. [DOI] [PubMed] [Google Scholar]
- 5. Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus—mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18:599–612. [DOI] [PubMed] [Google Scholar]
- 6. Anstee QM, Mantovani A, Tilg H, Targher G. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2018;15:425–39. [DOI] [PubMed] [Google Scholar]
- 7. Vuppalanchi R, Noureddin M, Alkhouri N, Sanyal AJ. Therapeutic pipeline in nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2021;18:373–92. [DOI] [PubMed] [Google Scholar]
- 8. Abul‐Husn NS, Cheng X, Li AH, Xin Y, Schurmann C, Stevis P, et al. A protein‐truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med. 2018;378:1096–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–34. [DOI] [PubMed] [Google Scholar]
- 11. Strange K, Denton J, Nehrke K. Ste20‐type kinases: evolutionarily conserved regulators of ion transport and cell volume. Physiology (Bethesda). 2006;21:61–8. [DOI] [PubMed] [Google Scholar]
- 12. Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 2001;11:220–30. [DOI] [PubMed] [Google Scholar]
- 13. Zhao ZS, Manser E. PAK family kinases: physiological roles and regulation. Cell Logist. 2012;2:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu A, Jiang X. p21‐Activated kinases as promising therapeutic targets in hematological malignancies. Leukemia. 2021;36:315–26. [DOI] [PubMed] [Google Scholar]
- 15. Kumar R, Sanawar R, Li X, Li F. Structure, biochemistry, and biology of PAK kinases. Gene. 2017;605:20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Radu M, Semenova G, Kosoff R, Chernoff J. PAK signalling during the development and progression of cancer. Nat Rev Cancer. 2014;14:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ye DZ, Field J. PAK signaling in cancer. Cell Logist. 2012;2:105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whale A, Hashim FN, Fram S, Jones GE, Wells CM. Signalling to cancer cell invasion through PAK family kinases. Front Biosci (Landmark Ed). 2011;16:849–64. [DOI] [PubMed] [Google Scholar]
- 19. Delpire E. The mammalian family of sterile 20p‐like protein kinases. Pflugers Arch. 2009;458:953–67. [DOI] [PubMed] [Google Scholar]
- 20. Ling P, Lu TJ, Yuan CJ, Lai MD. Biosignaling of mammalian Ste20‐related kinases. Cell Signal. 2008;20:1237–47. [DOI] [PubMed] [Google Scholar]
- 21. Ultanir SK, Yadav S, Hertz NT, Oses‐Prieto JA, Claxton S, Burlingame AL, et al. MST3 kinase phosphorylates TAO1/2 to enable Myosin Va function in promoting spine synapse development. Neuron. 2014;84:968–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matsuki T, Matthews RT, Cooper JA, van der Brug MP, Cookson MR, Hardy JA, et al. Reelin and stk25 have opposing roles in neuronal polarization and dendritic Golgi deployment. Cell. 2010;143:826–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jiao S, Zhang Z, Li C, Huang M, Shi Z, Wang Y, et al. The kinase MST4 limits inflammatory responses through direct phosphorylation of the adaptor TRAF6. Nat Immunol. 2015;16:246–57. [DOI] [PubMed] [Google Scholar]
- 24. Tang J, Ip JP, Ye T, Ng YP, Yung WH, Wu Z, et al. Cdk5‐dependent Mst3 phosphorylation and activity regulate neuronal migration through RhoA inhibition. J Neurosci. 2014;34:7425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sugden PH, McGuffin LJ, Clerk A. SOcK, MiSTs, MASK and STicKs: the GCKIII (germinal centre kinase III) kinases and their heterologous protein‐protein interactions. Biochem J. 2013;454:13–30. [DOI] [PubMed] [Google Scholar]
- 26. Zalvide J, Fidalgo M, Fraile M, Guerrero A, Iglesias C, Floridia E, et al. The CCM3‐GCKIII partnership. Histol Histopathol. 2013;28:1265–72. [DOI] [PubMed] [Google Scholar]
- 27. Pombo CM, Iglesias C, Sartages M, Zalvide JB. MST Kinases and Metabolism. Endocrinology. 2019;160:1111–8. [DOI] [PubMed] [Google Scholar]
- 28. Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995;9:576–96. [PubMed] [Google Scholar]
- 29. Preisinger C, Short B, De Corte V, Bruyneel E, Haas A, Kopajtich R, et al. YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14‐3‐3zeta. J Cell Biol. 2004;164:1009–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pombo CM, Force T, Kyriakis J, Nogueira E, Fidalgo M, Zalvide J. The GCK II and III subfamilies of the STE20 group kinases. Front Biosci. 2007;12:850–9. [DOI] [PubMed] [Google Scholar]
- 31. Ko TP, Jeng WY, Liu CI, Lai MD, Wu CL, Chang WJ, et al. Structures of human MST3 kinase in complex with adenine, ADP and Mn2+. Acta Crystallogr D Biol Crystallogr. 2010;66:145–54. [DOI] [PubMed] [Google Scholar]
- 32. Record CJ, Chaikuad A, Rellos P, Das S, Pike AC, Fedorov O, et al. Structural comparison of human mammalian ste20‐like kinases. PLoS One. 2010;5:e11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fuller SJ, McGuffin LJ, Marshall AK, Giraldo A, Pikkarainen S, Clerk A, et al. A novel non‐canonical mechanism of regulation of MST3 (mammalian Sterile20‐related kinase 3). Biochem J. 2012;442:595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shi Z, Jiao S, Zhang Z, Ma M, Zhang Z, Chen C, et al. Structure of the MST4 in complex with MO25 provides insights into its activation mechanism. Structure. 2013;21:449–61. [DOI] [PubMed] [Google Scholar]
- 35. Hao Q, Feng M, Shi ZB, Li CC, Chen M, Wang WJ, et al. Structural insights into regulatory mechanisms of MO25‐mediated kinase activation. J Struct Biol. 2014;186:224–33. [DOI] [PubMed] [Google Scholar]
- 36. Mehellou Y, Alessi DR, Macartney TJ, Szklarz M, Knapp S, Elkins JM. Structural insights into the activation of MST3 by MO25. Biochem Biophys Res Commun. 2013;431:604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang CY, Wu YM, Hsu CY, Lee WS, Lai MD, Lu TJ, et al. Caspase activation of mammalian sterile 20‐like kinase 3 (Mst3). Nuclear translocation and induction of apoptosis. J Biol Chem. 2002;277:34367–74. [DOI] [PubMed] [Google Scholar]
- 38. Nogueira E, Fidalgo M, Molnar A, Kyriakis J, Force T, Zalvide J, et al. SOK1 translocates from the Golgi to the nucleus upon chemical anoxia and induces apoptotic cell death. J Biol Chem. 2008;283:16248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jiang H, Wang WW, Zhang Y, Yao WW, Jiang JY, Qin B, et al. Cell polarity kinase MST4 cooperates with cAMP‐dependent kinase to orchestrate histamine‐stimulated acid secretion in gastric parietal cells. J Bio Chem. 2015;290:28272–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lu TJ, Lai WY, Huang CY, Hsieh WJ, Yu JS, Hsieh YJ, et al. Inhibition of cell migration by autophosphorylated mammalian sterile 20‐like kinase 3 (MST3) involves paxillin and protein‐tyrosine phosphatase‐PEST. J Biol Chem. 2006;281:38405–17. [DOI] [PubMed] [Google Scholar]
- 41. Chen CB, Ng JKW, Choo PH, Wu W, Porter AG. Mammalian sterile 20‐like kinase 3 (MST3) mediates oxidative‐stress‐induced cell death by modulating JNK activation. Biosci Rep. 2009;29:405–15. [DOI] [PubMed] [Google Scholar]
- 42. Schinkmann K, Blenis J. Cloning and characterization of a human STE20‐like protein kinase with unusual cofactor requirements. J Biol Chem. 1997;272:28695–703. [DOI] [PubMed] [Google Scholar]
- 43. Osada S, Izawa M, Saito R, Mizuno K, Suzuki A, Hirai S, et al. YSK1, a novel mammalian protein kinase structurally related to Ste20 and SPS1, but is not involved in the known MAPK pathways. Oncogene. 1997;14:2047–57. [DOI] [PubMed] [Google Scholar]
- 44. Lee WS, Hsu CY, Wang PL, Huang CY, Chang CH, Yuan CJ. Identification and characterization of the nuclear import and export signals of the mammalian Ste20‐like protein kinase 3. FEBS Lett. 2004;572:41–5. [DOI] [PubMed] [Google Scholar]
- 45. Thiriet M. Intracellular Signaling Mediators in the Circulatory and Ventilatory Systems. Berlin, Germany: Springer Science & Business Media; 2012. [Google Scholar]
- 46. Ceccarelli DF, Laister RC, Mulligan VK, Kean MJ, Goudreault M, Scott IC, et al. CCM3/PDCD10 heterodimerizes with germinal center kinase III (GCKIII) proteins using a mechanism analogous to CCM3 homodimerization. J Biol Chem. 2011;286:25056–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rual JF, Venkatesan K, Hao T, Hirozane‐Kishikawa T, Dricot A, Li N, et al. Towards a proteome‐scale map of the human protein‐protein interaction network. Nature. 2005;437:1173–8. [DOI] [PubMed] [Google Scholar]
- 48. Ma X, Zhao H, Shan J, Long F, Chen Y, Chen Y, et al. PDCD10 interacts with Ste20‐related kinase MST4 to promote cell growth and transformation via modulation of the ERK pathway. Mol Biol Cell. 2007;18:1965–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Voss K, Stahl S, Schleider E, Ullrich S, Nickel J, Mueller TD, et al. CCM3 interacts with CCM2 indicating common pathogenesis for cerebral cavernous malformations. Neurogenetics. 2007;8:249–56. [DOI] [PubMed] [Google Scholar]
- 50. Fidalgo M, Fraile M, Pires A, Force T, Pombo C, Zalvide J. CCM3/PDCD10 stabilizes GCKIII proteins to promote Golgi assembly and cell orientation. J Cell Sci. 2010;123:1274–84. [DOI] [PubMed] [Google Scholar]
- 51. Zhang M, Dong L, Shi ZB, Jiao S, Zhang Z, Zhang WQ, et al. Structural mechanism of CCM3 heterodimerization with GCKIII kinases. Structure. 2013;21:680–8. [DOI] [PubMed] [Google Scholar]
- 52. Xu XY, Wang XY, Zhang Y, Wang DC, Ding JJ. Structural basis for the unique heterodimeric assembly between cerebral cavernous malformation 3 and germinal center kinase III. Structure. 2013;21:1059–66. [DOI] [PubMed] [Google Scholar]
- 53. Wang R, Wu ST, Yang X, Qian YD, Choi JP, Gao R, et al. Pdcd10‐Stk24/25 complex controls kidney water reabsorption by regulating Aqp2 membrane targeting. JCI Insight. 2021;6:e142838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zheng X, Xu C, Di Lorenzo A, Kleaveland B, Zou Z, Seiler C, et al. CCM3 signaling through sterile 20‐like kinases plays an essential role during zebrafish cardiovascular development and cerebral cavernous malformations. J Clin Invest. 2010;120:2795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goudreault M, D'Ambrosio LM, Kean MJ, Mullin MJ, Larsen BG, Sanchez A, et al. A PP2A phosphatase high density interaction network identifies a novel striatin‐interacting phosphatase and kinase complex linked to the cerebral cavernous malformation 3 (CCM3) protein. Mol Cell Proteomics. 2009;8:157–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang Y, Tang WW, Zhang HF, Niu XF, Xu YK, Zhang JS, et al. A network of interactions enables CCM3 and STK24 to coordinate UNC13D‐driven vesicle exocytosis in neutrophils. Dev Cell. 2013;27:215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yin H, Shi Z, Jiao S, Chen C, Wang W, Greene MI, et al. Germinal center kinases in immune regulation. Cell Mol Immunol. 2012;9:439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Voss K, Stahl S, Hogan BM, Reinders J, Schleider E, Schulte‐Merker S, et al. Functional analyses of human and zebrafish 18‐amino acid in‐frame deletion pave the way for domain mapping of the cerebral cavernous malformation 3 protein. Hum Mutat. 2009;30:1003–11. [DOI] [PubMed] [Google Scholar]
- 59. Filippi BM, de los Heros P, Mehellou Y, Navratilova I, Gourlay R, Deak M, et al. MO25 is a master regulator of SPAK/OSR1 and MST3/MST4/YSK1 protein kinases. EMBO J. 2011;30:1730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. ten Klooster JP, Jansen M, Yuan J, Oorschot V, Begthel H, Di Giacomo V, et al. Mst4 and ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Dev Cell. 2009;16:551–62. [DOI] [PubMed] [Google Scholar]
- 61. Chen XD, Cho CY. Downregulation of SOK1 promotes the migration of MCF‐7 cells. Biochem Biophys Res Commun. 2011;407:389–92. [DOI] [PubMed] [Google Scholar]
- 62. Zhou J, Shao Z, Kerkela R, Ichijo H, Muslin AJ, Pombo C, et al. Serine 58 of 14‐3‐3zeta is a molecular switch regulating ASK1 and oxidant stress‐induced cell death. Mol Cell Biol. 2009;29:4167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Turowec JP, Zukowski SA, Knight JDR, Smalley DM, Graves LM, Johnson GL, et al. An unbiased proteomic screen reveals caspase cleavage is positively and negatively regulated by substrate phosphorylation. Mol Cell Proteomics. 2014;13:1184–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lin CY, Wu HY, Wang PL, Yuan CJ. Mammalian Ste20‐like protein kinase 3 induces a caspase‐independent apoptotic pathway. Int J Biochem Cell B. 2010;42:98–105. [DOI] [PubMed] [Google Scholar]
- 65. Martin DDO, Beauchamp E, Berthiaume LG. Post‐translational myristoylation: fat matters in cellular life and death. Biochimie. 2011;93:18–31. [DOI] [PubMed] [Google Scholar]
- 66. Dan I, Ong SE, Watanabe NM, Blagoev B, Nielsen MM, Kajikawa E, et al. Cloning of MASK, a novel member of the mammalian germinal center kinase III subfamily, with apoptosis‐inducing properties. J Biol Chem. 2002;277:5929–39. [DOI] [PubMed] [Google Scholar]
- 67. Gluchowski NL, Becuwe M, Walther TC, Farese RV Jr. Lipid droplets and liver disease: from basic biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2017;14:343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mashek DG. Hepatic lipid droplets: a balancing act between energy storage and metabolic dysfunction in NAFLD. Mol Metab. 2021;50:101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Bio. 2019;20:137–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Amrutkar M, Kern M, Nunez‐Duran E, Stahlman M, Cansby E, Chursa U, et al. Protein kinase STK25 controls lipid partitioning in hepatocytes and correlates with liver fat content in humans. Diabetologia. 2016;59:341–53. [DOI] [PubMed] [Google Scholar]
- 71. Cansby E, Kulkarni NM, Magnusson E, Kurhe Y, Amrutkar M, Nerstedt A, et al. Protein kinase MST3 modulates lipid homeostasis in hepatocytes and correlates with nonalcoholic steatohepatitis in humans. FASEB J. 2019;33:9974–89. [DOI] [PubMed] [Google Scholar]
- 72. Caputo M, Cansby E, Kumari S, Kurhe Y, Nair S, Stahlman M, et al. STE20‐type protein kinase MST4 controls NAFLD progression by regulating lipid droplet dynamics and metabolic stress in hepatocytes. Hepatol Commun. 2021;5:1183–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nerstedt A, Kurhe Y, Cansby E, Caputo M, Gao L, Vorontsov E, et al. Lipid droplet‐associated kinase STK25 regulates peroxisomal activity and metabolic stress response in steatotic liver. J Lipid Res. 2020;61:178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Amrutkar M, Cansby E, Nunez‐Duran E, Pirazzi C, Stahlman M, Stenfeldt E, et al. Protein kinase STK25 regulates hepatic lipid partitioning and progression of liver steatosis and NASH. FASEB J. 2015;29:1564–76. [DOI] [PubMed] [Google Scholar]
- 75. Amrutkar M, Chursa U, Kern M, Nunez‐Duran E, Stahlman M, Sutt S, et al. STK25 is a critical determinant in nonalcoholic steatohepatitis. FASEB J. 2016;30:3628–43. [DOI] [PubMed] [Google Scholar]
- 76. Schuster S, Cabrera D, Arrese M, Feldstein AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15:349–64. [DOI] [PubMed] [Google Scholar]
- 77. Li X, Ramadori P, Pfister D, Seehawer M, Zender L, Heikenwalder M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat Rev Cancer. 2021;21:541–57. [DOI] [PubMed] [Google Scholar]
- 78. Kucukoglu O, Sowa JP, Mazzolini GD, Syn WK, Canbay A. Hepatokines and adipokines in NASH‐related hepatocellular carcinoma. J Hepatol. 2021;74:442–57. [DOI] [PubMed] [Google Scholar]
- 79. Wu F, Gao P, Wu W, Wang Z, Yang J, Di J, et al. STK25‐induced inhibition of aerobic glycolysis via GOLPH3‐mTOR pathway suppresses cell proliferation in colorectal cancer. J Exp Clin Cancer Res. 2018;37:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cansby E, Nunez‐Duran E, Magnusson E, Amrutkar M, Booten SL, Kulkarni NM, et al. Targeted delivery of Stk25 antisense oligonucleotides to hepatocytes protects mice against nonalcoholic fatty liver disease. Cell Mol Gastroenterol Hepatol. 2019;7:597–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nunez‐Duran E, Aghajan M, Amrutkar M, Sutt S, Cansby E, Booten SL, et al. Serine/threonine protein kinase 25 antisense oligonucleotide treatment reverses glucose intolerance, insulin resistance, and nonalcoholic fatty liver disease in mice. Hepatol Commun. 2018;2:69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Amrutkar M, Cansby E, Chursa U, Nunez‐Duran E, Chanclon B, Stahlman M, et al. Genetic disruption of protein kinase STK25 ameliorates metabolic defects in a diet‐induced type 2 diabetes model. Diabetes. 2015;64:2791–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cansby E, Caputo M, Gao L, Kulkarni NM, Nerstedt A, Stahlman M, et al. Depletion of protein kinase STK25 ameliorates renal lipotoxicity and protects against diabetic kidney disease. JCI Insight. 2020;5:e140483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sutt S, Cansby E, Paul A, Amrutkar M, Nunez‐Duran E, Kulkarni NM, et al. STK25 regulates oxidative capacity and metabolic efficiency in adipose tissue. J Endocrinol. 2018;238:187–202. [DOI] [PubMed] [Google Scholar]
- 85. Cansby E, Kumari S, Caputo M, Xia Y, Porosk R, Robinson J, et al. Silencing of STE20‐type kinase STK25 in human aortic endothelial and smooth musclecells is atheroprotective. Commun Biol. 2022;5:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Caputo M, Kurhe Y, Kumari S, Cansby E, Amrutkar M, Scandalis E, et al. Silencing of STE20‐type kinase MST3 in mice with antisense oligonucleotide treatment ameliorates diet‐induced nonalcoholic fatty liver disease. FASEB J. 2021;35:e21567. [DOI] [PubMed] [Google Scholar]
- 87. Chao HW, Chao SW, Lin H, Ku HC, Cheng CF. Homeostasis of glucose and lipid in non‐alcoholic fatty liver disease. Int J Mol Sci. 2019;20:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Iglesias C, Floridia E, Sartages M, Porteiro B, Fraile M, Guerrero A, et al. The MST3/STK24 kinase mediates impaired fasting blood glucose after a high‐fat diet. Diabetologia. 2017;60:2453–62. [DOI] [PubMed] [Google Scholar]
- 89. Qin Q, Shou J, Li M, Gu M, Meng Z, Xu P, et al. Stk24 protects against obesity‐associated metabolic disorders by disrupting the NLRP3 inflammasome. Cell Rep. 2021;35:109161. [DOI] [PubMed] [Google Scholar]
- 90. Luo Y, Huo Y, Song P, Zhang X, Liao M. Validation and functional analysis of the critical proteins in combination with taurine, epigallocatechin gallate and genistein against liver fibrosis in rats. Biomed Pharmacother. 2019;115:108975. [DOI] [PubMed] [Google Scholar]
- 91. Luan D, Zhang Y, Yuan L, Chu Z, Ma L, Xu Y, et al. MST4 modulates the neuro‐inflammatory response by regulating IkappaBalpha signaling pathway and affects the early outcome of experimental ischemic stroke in mice. Brain Res Bull. 2020;154:43–50. [DOI] [PubMed] [Google Scholar]
- 92. Dyson J, Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60:110–7. [DOI] [PubMed] [Google Scholar]
- 93. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–44. [DOI] [PubMed] [Google Scholar]
- 94. Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population‐based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26:2183–91. [DOI] [PubMed] [Google Scholar]
- 95. Llovet JM, Zucman‐Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [DOI] [PubMed] [Google Scholar]
- 96. Kurhe Y, Caputo M, Cansby E, Xia Y, Kumari S, Anand SK, et al. Antagonizing STK25 signaling suppresses the development of hepatocellular carcinoma through targeting metabolic, inflammatory, and pro‐oncogenic pathways. Cell Mol Gastroenterol Hepatol. 2022;13:405–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhang Y, Xu J, Qiu Z, Guan Y, Zhang X, Zhang X, et al. STK25 enhances hepatocellular carcinoma progression through the STRN/AMPK/ACC1 pathway. Cancer Cell Int. 2022;22:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lim S, Hermance N, Mudianto T, Mustaly HM, Mauricio IPM, Vittoria MA, et al. Identification of the kinase STK25 as an upstream activator of LATS signaling. Nat Commun. 2019;10:1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Russell JO, Camargo FD. Hippo signalling in the liver: role in development, regeneration and disease. Nat Rev Gastroenterol Hepatol. 2022;19:297–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bae SJ, Ni L, Luo X. STK25 suppresses Hippo signaling by regulating SAV1‐STRIPAK antagonism. Elife. 2020;9:e54863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lin ZH, Wang L, Zhang JB, Liu Y, Li XQ, Guo L, et al. MST4 promotes hepatocellular carcinoma epithelial‐mesenchymal transition and metastasis via activation of the p‐ERK pathway. Int J Oncol. 2014;45:629–40. [DOI] [PubMed] [Google Scholar]
- 102. Dian MJ, Li J, Zhang XL, Li ZJ, Zhou Y, Zhou W, et al. MST4 negatively regulates the EMT, invasion and metastasis of HCC cells by inactivating PI3K/AKT/Snail1 axis. J Cancer. 2021;12:4463–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hao WC, Zhong QL, Pang WQ, Dian MJ, Li J, Han LX, et al. MST4 inhibits human hepatocellular carcinoma cell proliferation and induces cell cycle arrest via suppression of PI3K/AKT pathway. J Cancer. 2020;11:5106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. An L, Nie P, Chen M, Tang Y, Zhang H, Guan J, et al. MST4 kinase suppresses gastric tumorigenesis by limiting YAP activation via a non‐canonical pathway. J Exp Med. 2020;217:e20191817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chen M, Zhang H, Shi Z, Li Y, Zhang X, Gao Z, et al. The MST4‐MOB4 complex disrupts the MST1‐MOB1 complex in the Hippo‐YAP pathway and plays a pro‐oncogenic role in pancreatic cancer. J Biol Chem. 2018;293:14455–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Stegert MR, Hergovich A, Tamaskovic R, Bichsel SJ, Hemmings BA. Regulation of NDR protein kinase by hydrophobic motif phosphorylation mediated by the mammalian Ste20‐like kinase MST3. Mol Cell Biol. 2005;25:11019–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rausch V, Hansen CG. The hippo pathway, YAP/TAZ, and the plasma membrane. Trends Cell Biol. 2020;30:32–48. [DOI] [PubMed] [Google Scholar]
- 108. Chen YL, Wang CY, Fang JH, Hsu HP. Serine/threonine‐protein kinase 24 is an inhibitor of gastric cancer metastasis through suppressing CDH1 gene and enhancing stemness. Am J Cancer Res. 2021;11:4277–93. [PMC free article] [PubMed] [Google Scholar]
- 109. Lee KT, Chang CL, Li CY, Song H, Shan YS, Lai MD. The oncogenic role of MST3 in human gastric cancer. Am J Cancer Res. 2018;8:2130–9. [PMC free article] [PubMed] [Google Scholar]
- 110. Madsen CD, Hooper S, Tozluoglu M, Bruckbauer A, Fletcher G, Erler JT, et al. STRIPAK components determine mode of cancer cell migration and metastasis. Nat Cell Biol. 2015;17:68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]


