Abstract
Purpose Transabdominal ultrasound (US) and magnetic resonance enterography (MRE) are used to assess disease activity and extent in IBD, but their impact on therapeutic decisions is unclear. Therefore, our study has two goals: to compare the usefulness of US and MRE in assessing disease extent and activity in the small and large bowel, and to determine the relevance for clinical decisions in IBD.
Materials and Methods We included 54 IBD patients who had undergone both MRE and US within three months. We used the construct reference standard model to compare MRE and US for detecting inflammation and examined the impact on clinical decisions in IBD patients.
Results In 54 IBD patients (44 patients Crohn’s disease (CD), 5 ulcerative colitis (UC), 5 indeterminate colitis (IC)), 42 patients (77.8%) showed inflammation either in the small or large bowel. Small bowel disease was present in 34 patients (77.3%). Complications were found in 19 patients (35.2%). MRE and US both showed high sensitivity (90.5 and 88.1%) and moderate specificity (50% in MRE and US) for detecting inflammation. MRE revealed higher sensitivity than US for detecting conglomerate tumors without statistical significance (85.7 vs. 71.4%, p=1.0) and equal specificity (97.9 vs 97.7, p=1.0). Therapeutic decisions included steroids in 20 patients (47.6%) and surgery/percutaneous drainage in six patients (14.3%), these decisions were triggered by results of US or MRE in equal distribution.
Conclusion US and MRE have comparable sensitivity and specificity for detecting intestinal inflammation and complications in IBD patients. Therefore, both methods are sufficient for making clinical decisions.
Key words: abdomen, AREAS; STRUCTURES & SYSTEMS; fistula, THEMES; abscess; inflammation; MR imaging
Introduction
The treatment of inflammatory bowel disease (IBD) has evolved from treating symptoms to achieving mucosal healing. The “treat to target approach” demands close monitoring of disease activity involving different methods 1 . For this reason, therapeutic decisions are based on clinical parameters, additional laboratory and fecal markers, enterography results, and endoscopic findings 2 3 . Ileo-colonoscopy is the first-line method to assess disease activity and to monitor the development of malignancies in IBD patients. However, patients are often reluctant to have a colonoscopy because of the invasiveness and pain. Moreover, it is not feasible to repeat the procedure in short time intervals for disease monitoring. Also, it represents an invasive procedure with procedure-related risks such as bowel wall perforation, especially in IBD patients 4 .
Magnetic resonance enterography (MRE) and transabdominal ultrasound (US) are widely available for diagnosing and monitoring disease activity in IBD patients in industrialized countries. Both methods are sometimes combined, e. g., in complicated Crohn´s disease. However, the diagnostic value of each individual method is unclear. In addition, there is suspected uncertainty of inter-observer differences in the outcome of US 5 . On the other hand, US is cheap, easily accessible, and well tolerated compared to MRE 6 7 . However, MRE has a higher accuracy with respect to diagnosing abdominal abscesses and IBD manifestations involving deeper pelvic loops or located proximal to the terminal ileum 8 . Also, MRE shows high accuracy for predicting the need for surgery in patients with Crohn´s disease and strictures 9 . Although several studies point to sufficient diagnostic accuracy of bowel US in comparison to MRE in IBD patients, it is still not clear whether both methods should be combined in order to increase diagnostic sensitivity and to improve disease course by guiding clinicians´ decisions 10 .
We therefore aimed to compare the usefulness of US and MRE for assessing disease extent and activity in the small and large bowel in patients with Crohn´s disease and ulcerative colitis, and to assess the relevance for clinical decisions even if endoscopy is not available.
Materials and Methods
We reviewed the medical records of all 232 patients who underwent MRE between January 2017 and May 2020. In this group, 124 patients were identified with IBD. Sixty-one patients had undergone bowel US and MRE within three months. Six patients were excluded due to a therapy switch between examinations. One patient was excluded due to multiple abscesses on MRE. The remaining 54 patients were included in our analysis. Our collective contained newly diagnosed patients with IBD and patients with established disease in whom relapse was suspected. All US examinations were performed according to the EFSUMB recommendations and clinical guidelines for intestinal ultrasound in inflammatory bowel diseases 11 . Intestinal ultrasound was performed with high-end devices using convex (1–6 MHz) and linear (2–9 and 6–15 MHz) probes (GE Health Care GmbH, Germany (Logiq E7-E9)). The US examinations were performed by experienced (>4 months of practice) physicians and cross-checked by the attending (>10 years of experience). The activity of inflammation was determined by B-scan mode and Doppler mode. Therefore, we used the Limberg score (including hypoechoic wall thickening>4 mm, loss of wall stratification, and increase of vascularity).
MRE examinations were performed either at 1.5 T or 3 T. Patients were instructed to drink 1,500 ml of a 2% sorbitol solution during the 60 minutes before undergoing MRE. A 20-mg dose of hyoscine butylbromide (Buscopan, Boehringer Ingelheim, Germany) was intravenously administered directly before the start of the scan 12 . The imaging protocol consisted of coronal and transverse T2-weighted imaging sequences and transverse and coronal T1-weighted fat-suppressed T1-weighted imaging sequences before and after intravenous contrast injection.
MRE examinations were evaluated in consensus by two radiologists (one radiologist with>2 years of experience and one attending with>10 years of experience). Bowel segments with suspicion of inflammation were defined by bowel wall thickness>3 mm and increased vascularity detected by Doppler signal on US or by contrast enhancement of the bowel wall on MRE.
The primary outcome was detection of bowel inflammation by US and MRE in IBD patients validated against a construct reference standard 13 . Each patient’s record was reviewed by one gastroenterologist with>5 years of experience and one attending with>10 years of experience and the suspicion of bowel inflammation was challenged by other established diagnostic markers of disease activity (endoscopy or surgery with inflammation in histology, CRP>5.0 mg/l, white blood cell (WBC) count>11.0×10 9 /L, fecal calprotectin concentration>40 μg/g, clinical symptoms, response to steroid therapy). Then a consensus decision about the presence of bowel inflammation at the time of enterography was made and used as the reference standard ( Supplementary table 1 ).
Table 1 Patient characteristics in our study population of 54 IBD patients at the time of enterography.
| Parameter | All patients n=54 |
|---|---|
| Diagnosis | |
| Crohn’s disease | 44 (81.5%) |
| Ulcerative colitis | 5 (9.3%) |
| Indeterminate colitis | 5 (9.3%) |
| Sex | |
| Female | 38 (70.4%) |
| Male | 16 (29.6%) |
| Age (median; IQR 25–75) | 29.5, 21.0–47.3 years |
| Body mass index (median; IQR 25–75) | 21; 19–24 |
| New diagnosis | 14 (26.0%) |
| Disease duration (median; IQR 25–75) | |
| All patients | 3.7; 0.1–10.4 years |
| Previous surgery | 11 (20.4%) |
| Disease extent in patients with inflammation | N=42/54 |
| Small bowel alone | 18 (33.3%) |
| Colon alone | 12 (22.2%) |
| Small bowel and colon | 12 (22.2%) |
| Time between bowel US and MRE (median; IQR 25–75) | 5; 2.8–28.0 days |
| Calprotectin (median, IQR 25–75) | 952; 482–235 µg/g |
| Mayo score (if endoscopy is available) | N=25 |
| Score 0 | 7 (28%) |
| Score 1 | 4 (16%) |
| Score 2 | 9 (36%) |
| Score 3 | 5 (20%) |
In addition, correct identification of disease extent ((neo-) terminal Ileum vs. colon) and identification of pathological findings such as fistulae, abscesses, stenoses, or conglomerate tumors by US and MRE were compared. These secondary outcomes were also validated against a construct reference standard based on a consensus decision after reviewing each patient’s record.
Descriptive statistics of the baseline data are presented as medians [interquartile range], or as percentages when appropriate. The sensitivity, specificity, accuracy, positive predictive value [PPV], and negative predictive value [NPV] were calculated with a 95% confidence interval using the retrospective final clinical decision as the reference standard. Sensitivities were compared using McNemar’s comparison of paired proportions. Differences in parameters were accepted as statistically significant, when the probability of error for zero hypothesis was less than 5%. All statistical analyses were performed by SPPS version 27 for Mac OS (SPSS Inc., Chicago, IL, USA).
Results
A total of 54 patients with IBD (44 Crohn’s disease (CD), 5 ulcerative colitis (UC) and 5 indeterminate colitis (IC)) were analyzed retrospectively. The median time between US and MRE was 5 days (IQR 2.8–28.0 days). In our cohort the median age was 29.5 years (IQR 21–47 years), and 38 patients (70.4%) were female. Fourteen patients (26.0%) were diagnosed with IBD for the first time. In the remaining 40 patients, the median disease duration was 6.7 years (IQR 2.5–17.2 years).
43 out of 54 patients (76.8%) had not undergone previous surgery. Seven patients had undergone prior ileocecal resection or right hemicolectomy resulting in a neo-terminal ileum, and two patients had undergone left hemicolectomy. One patient had had a colectomy and another patient a proctocolectomy in the past. Endoscopic findings with histology were available in 25 patients. Calprotectin values at the time of enterography were available in 26 patients. Laboratory results including CRP and blood count as well as information on the clinical course (fever, abdominal pain, and diarrhea) were available in all 54 patients ( Table 1 ).
According to the construct reference standard, 42 patients (78.0%) were considered to have luminal bowel inflammation at the time of imaging. Eighteen patients had isolated inflammation in the small bowel, compared to twelve patients with isolated colonic inflammation. In the remaining 12 patients, the colon and small bowel were affected simultaneously. Complications defined as abscesses, conglomerate tumors, fistulae, or stenoses were found in 19 patients (35.2%). 9 patients (16.7%) had more than one complication.
Clinical decisions in these 42 patients led to initiation of steroid treatment in 20 patients, antibiotic treatment in 5 patients, initiation, change or adaptation of antibody treatment in 8 patients, and surgery or placement of percutaneous drainage of an abscess in 6 patients. Three patients received other treatments (5-aminosalicylic acid and azathioprine) ( Table 2 ).
Table 2 Summary of all clinical decisions following US and MRE results.
| Clinical decision | Number of patients | Percentage detected in US | Percentage detected in MRE |
|---|---|---|---|
| Steroids | 20 | 19 (95.0%) | 18 (90.0%) |
| Antibiotic treatment | 5 | 4 (80.0%) | 4 (80.0%) |
| Surgery/drainage | 6 | 6 (100%) | 6 (100%) |
| Change of antibody | 8 | 6 (75.0%) | 8 (100%) |
| Other (e. g., 5-ASA*) | 3 | 3 (100%) | 3 (100%) |
*5-ASA: 5-aminosalicylic acid.
Disease activity
37 of 42 patients (68.5%) with luminal bowel inflammation were correctly detected by US. Five patients escaped detection with US, and in six cases, US led to false-positive results. MRE identified 38 patients (67.9%) with bowel inflammation correctly and missed four. There were also six false-positive cases with MRE. Detailed information regarding ultrasound and MRE findings is given in Tables 3 and 4 .
Table 3 Comparison of results of US and MRE in our study population of 54 IBD patients, validated by the reference standard.
| Ultrasound | MRE | Total validated | |||||||
|---|---|---|---|---|---|---|---|---|---|
| tp* | tn | fp | fn | tp | tn | fp | fn | ||
| Small bowel and/or colon inflammation | 37 | 6 | 6 | 5 | 38 | 6 | 6 | 4 | 42/54 |
| (Neo) terminal Ileum inflammation | 22 | 24 | 4 | 3 | 24 | 27 | 1 | 1 | 25/54 |
| Colon inflammation | 20 | 25 | 3 | 5 | 18 | 23 | 5 | 7 | 25/54 |
| Stenoses | 4 | 43 | 4 | 3 | 4 | 44 | 3 | 3 | 7/54 |
| Fistulae | 4 | 47 | 0 | 3 | 6 | 47 | 0 | 1 | 7/54 |
| Conglomerate tumors | 5 | 46 | 1 | 2 | 6 | 46 | 1 | 1 | 7/54 |
| Abscesses | 6 | 42 | 2 | 4 | 8 | 44 | 0 | 2 | 10/54 |
* tp: true positive; tn: true negative; fp: false positive; fn: false negative.
Table 4 Statistical comparison of results of US and MRE for detecting inflammation and complications in our IBD patient cohort.
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | ||
|---|---|---|---|---|---|---|
| Inflammation, overall | US | 88.1 | 50.0 | 86.1 | 54.6 | 79.6 |
| MRE | 90.5 | 50.0 | 86.4 | 60.0 | 86.4 | |
| US+MRE | 97.6 | 33.3 | 83.7 | 80.0 | 83.3 | |
| Inflammation, (neo) terminal Ileum | US | 88.0 | 85.7 | 84.6 | 88.9 | 86.8 |
| MRE | 96.0 | 96.4 | 96.0 | 96.4 | 96.2 | |
| Inflammation, colon | US | 80.0 | 89.3 | 87 | 83.3 | 84.9 |
| MRE | 72.0 | 82.1 | 78.3 | 76.7 | 77.3 | |
| Stenoses | US | 57.1 | 91.5 | 50.0 | 93.5 | 87 |
| MRE | 57.1 | 93.6 | 57.1 | 93.6 | 88.9 | |
| Fistulae | US | 57.1 | 100 | 100 | 95.0 | 94.4 |
| MRE | 85.7 | 100 | 100 | 97.9 | 98.2 | |
| Conglomerate | US | 71.4 | 97.9 | 83.3 | 95.8 | 94.4 |
| MRE | 85.7 | 97.9 | 85.7 | 97.9 | 96.3 | |
| Abscesses | US | 60.0 | 95.5 | 75.0 | 91.3 | 88.9 |
| MRE | 80.0 | 100 | 100 | 95.7 | 96.3 |
The findings correspond to a sensitivity of 88.1% (95% CI: 74.4–96.0%) and a specificity of 50.0% (95% CI: 21.1–78.9%) for US. MRE had a sensitivity of 90.5% (95% CI: 77.4–97.3%) and specificity of 50.0% (95% CI: 21.1% to 78.9%). The accuracy was 79.6% (95% CI: 66.5% to 89.4%) for US and 81.5% (95% CI: 68.6–90.8%) for MRE. There was no statistically significant difference in sensitivities between US and MRE (p=1.0). When including the discordant findings and thereby combining the results of US and MRE, 41 of 42 patients with inflammation were detected and sensitivity increased to 97.62% (95% CI: 87.43–99.94%). Specificity, however, decreased to 33.3% (95% CI: 9.9–65.1%).
In the subgroup of patients who received colonoscopy, inflammation of the colon or (neo-) terminal ileum was discovered in 18 of 25 patients. Sonography detected 15 cases and MRE discovered 14. Using only histological inflammation as a reference, the sensitivity for detecting inflammation of the colon or (neo-) terminal was 83.3% (95% CI: 58.6 - 96.4%) and the specificity was 28.6% (95% CI: 3.67–71.0%) for US. MRE had a sensitivity of 77.8% (95% CI: 52.4–93.6%) and a specificity of 37.5% (95% CI: 8.5 to 75.5%). The accuracy was 68.0% (95% CI: 46.5 to 85.0%) for US and 65.4% (95% CI: 44.3–83.8%) for MRE. There was also no statistically significant difference in sensitivities between US and MRE (p=1.0).
In our cohort, only 9 patients showed a BMI≥25 kg/m 2 . In this group, the sensitivity was lower with 75.0% (95% CI: 34.9–96.8%) for US and 87.5% (95% CI: 47.4–99.7%) for MRE.
Disease extent
25 patients had confirmed disease involvement of the terminal or (neo-) terminal ileum. US correctly detected 22 cases (88%) and missed 3 cases. In four instances, US returned false-positive findings. MRE identified 24 cases (96%) correctly. Only one patient with disease involvement of the terminal ileum escaped detection with MRE. These findings correspond with a sensitivity for detecting inflammation of the terminal or neo-terminal ileum of 88.0% (95% CI: 68.8–97.5%) and a specificity of 85.7% (95% CI: 67.3–96.0%) for US. The sensitivity for MRE is 96.0% (95% CI: 79.7–99.9%) and the specificity is 96.4% (95% CI: 81.7–99.9%). The accuracy is 86.8% (95% CI: 74.7–94.5%) for US and 96.2% (95% CI: 87.0 - 99.5%) for MRE. There was no statistically significant difference in sensitivities between US and MRE (p=0.63).
25 patients had confirmed disease involvement of the colon. US detected 20 (80%) and missed 5 cases while 5 false-positive findings occurred. MRE correctly identified 18 patients (72%) with colonic inflammation. Seven patients were overlooked by MRE, and five findings were false positives. The sensitivity for detecting disease involvement of the colon was 80.0% (95% CI: 59.3–93.2%) for US and 72.0% (95% CI: 50.6–87.9%) for MRE. The specificities were 89.3% (95% CI: 71.8–97.7%) and 82.1% (95% CI: 63.1–93.9%), respectively. The accuracy was 84.9% (95% CI: 72.4–93.2%) for US and 77.3% (95% CI: 63.8–87.7%) for MRE. There was no statistically significant difference in sensitivities between US and MRE (p=0.73).
Complications
Complications defined as abscess, fistulae, stenoses, or conglomerate tumors were present in 19 patients (35.2%). 9 patients (16.7%) had more than one complication.
Seven patients had stenoses. US and MRE both correctly detected four of these case. In addition, US returned four false-positive findings and MRE three. The sensitivities for detecting bowel stenoses were 57.1 (95% CI: 18.4–90.1%) for both US and MRE with specificities of 91.5% (95% CI: 79.6–97.6%) for US and 93.6% (95% CI: 82.5–98.7%) for MRE. There was no statistically significant difference in sensitivities between US and MRE for the detection of stenoses (p=1.0).
Seven patients had one or more fistulae. MRE correctly detected these in six cases and US in four. There were no false-positive findings in US and MRE. The sensitivity was 57.1% (95% CI: 18.4–90.1%) for US and 85.7% (95% CI: 42.1–99.6%) for MRE. The specificity was 100% (95% CI: 92.4 to 100%) for both modalities. There was no statistically significant difference in sensitivities between US and MRE for the detection of fistulae (p=0.63).
There were seven patients with conglomerate tumors. US identified five of these cases and MRE six. US and MRE both led to one false-positive finding. The sensitivity was 71.4% (95% CI: 29.0–96.3%) for US and 85.7% (95% CI: 42.1–99.6%) for MRE. The specificity was 97.9% (95% CI: 88.7–100%) for US and MRE. There was no statistically significant difference in sensitivities between US and MRE for the detection of conglomerate tumors (p=1.0)
Abscesses were found in ten patients, six identified via US and seven by MRE. In two cases, US returned false-positive findings. The sensitivity was 60.0% (95% CI: 26.2–87.8%) for US and 80.0% (95% CI: 44.4–97.5%) for MRE. The specificity was 95.5% (95% CI: 84.5–99.4%) for US and 100% (95% CI: 92.0–100%) for MRE. There was no statistically significant difference in sensitivities between US and MRE for the detection of abscesses (p=1.0).
Using multivariate logistic regression, known markers for complications such as elevated CRP, WBC, or fever did not predict the presence of abscesses or fistulae in patients with suspected inflammation on sonography. Examples of matching US and MRE findings in four patients are shown in Figs. 1 2 3 for terminal ileitis ( Fig. 1a, b ), left-sided colitis ( Fig. 2a–d ) and terminal ileitis, enteroenteric fistulae, and abscess ( Fig. 3a–c ).
Fig. 1.
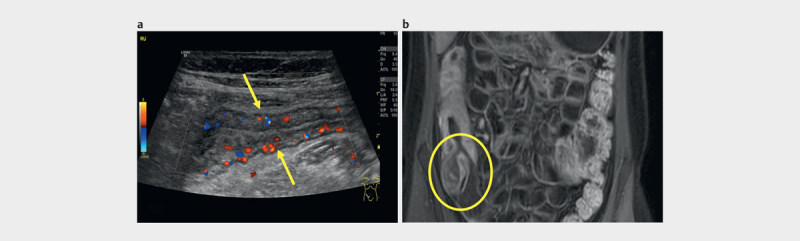
4.9 year old patient with terminal ileitis: a ) longitudinal US image of terminal ileum with thickened wall, narrowed lumen and hyperperfusionhypervascularity; b ) thickened bowel wall with hyperperfusion of the terminal ileum (circle) and ascending colon on postcontrast T1-weighted MRE images in coronal plane.
Fig. 2.

5.9 year old patient with left-sided colitis: a ) longitudinal US image of descending colon with wall thickening (0.58 cm) and total loss auf wall stratification (arrows); b ) long-segment wall thickening and narrowed lumen of the left colon (circle) on postcontrast T1-weighted MRE images in transverse plane; c ) 56 year old patient with Crohn’s disease with left-sided colitis: increase of vascularity; d ) 56 year old patient with Crohn’s disease with left-sided colitis: hypoechoic wall thickening > 4 mm.
Fig. 3.

1.9 year old patient with terminal ileitis and enteroenteric fistulae and abscess: a ) longitudinal US image of terminal ileum with thickened wall and enteroenteric fistulae (arrow) between the terminal ileum and another small bowel loop; b ) terminal ileum with wall thickening and hyperperfusion (circle) as well as enteroenteric fistula (arrow) and large abscess (small arrow) on postcontrast T1-weighted MRE images in transverse plane c ) transversal US image of large abscess with surrounding hyperechogenic mesenterium and a small hypoechogenic area at 5 o’clock (arrow) as a sign for developing fistula.
Discussion
This retrospective study compared the usefulness and accuracy of US and MRE for detecting inflammation in IBD patients in a real-life setting.
In our study, bowel US and MRE revealed comparable sensitivity and specificity for detecting intestinal inflammation in IBD patients with a disease flare. We observed a trend towards higher sensitivity of MRE for detecting complications such as stenoses and abscesses, although this finding did not prove to be statistically significant. Most importantly, bowel US and MRE were shown to be equally important and sufficient for making clinical decisions. Interestingly, when both methods are combined, the sensitivity further increases to 97.6% while the specificity decreases. Still, in certain indications, a combination of both methods might be useful, e. g., when several disease locations are suspected.
When considering the most suitable method for each patient, clinicians have to factor in several aspects and practical issues of different diagnostic tools. US is easy to use and can be repeated without limitations. Also, direct interaction with the patient enhances the outcome of this method 14 . Our study supports the fact that bowel US can achieve sufficient accuracy to diagnose disease activity in IBD patients. On the contrary, bowel US has been shown to have decreased sensitivity in adipose patients. This finding was confirmed in our study. Additionally, follow-up data are needed to further evaluate the diagnostic accuracy of US and MRE in detecting response to therapy. Only a few prospective studies to assess therapy response via bowel US have been performed so far. They have shown that despite reduced bowel wall thickness, a clear correlation between mucosal healing defined via the gold standard and US findings could not be established 15 16 .
Allocca et al. showed comparable accuracy for both methods when compared to the standard reference method (colonoscopy) in guiding clinical decisions in patients with Crohn´s disease 10 . However, another recent study by Taylor et al. found higher sensitivity and specificity of MRE for detecting small bowel disease in Crohn´s disease patients 17 . Most studies are focusing on Crohn´s disease patients, but US is also recommended and used for detecting disease activity and complications in patients with ulcerative colitis 18 .
MRE is considered the current diagnostic standard for small bowel assessment and for detecting complications in IBD patients 19 . MRE is also suitable for therapy follow-up of IBD patients 8 . On the other hand, it entails higher invasiveness compared to bowel US due to the need for the administration of intravenous contrast agent, and it is more time-consuming. Our study indicates the superiority of MRE for finding complications in IBD patients, although the difference was not statistically significant. This suggests that MRE might be the preferred method in patients with suspicion of complications.
The first and main limitation of our study is its retrospective nature. Second, we only included a small number of patients with complications weakening the statistical outcome in this patient group. Third, due to the retrospective analysis, endoscopic data were only available for a limited number of patients. Due to a lack of endoscopic findings, our reference standard is not as strong compared to other prospective studies. However, we were able to rely on other clinical data and markers to support the diagnosis of IBD flare.
MRE and bowel US are valid and useful diagnostic tools for diagnosing small and large bowel disease in IBD patients. Each method is sufficient for making clinical decisions, and both methods should not necessarily be combined since this leads to less specificity. Bowel US is the appropriate tool for monitoring disease activity in the small intestine and colon, whereas MRE should be employed in patients with suspected complications.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Supplementary Material
References
- 1.Peyrin-Biroulet L, Sandborn W, Sands B E et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. The American journal of gastroenterology. 2015;110:1324–1338. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi T, Siegmund B, Le Berre C et al. Ulcerative colitis. Nature reviews Disease primers. 2020;6:74. doi: 10.1038/s41572-020-0205-x. [DOI] [PubMed] [Google Scholar]
- 3.Colombel J F, D’Haens G, Lee W J et al. Outcomes and Strategies to Support a Treat-to-target Approach in Inflammatory Bowel Disease: A Systematic Review. Journal of Crohn’s & colitis. 2020;14:254–266. doi: 10.1093/ecco-jcc/jjz131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navaneethan U, Parasa S, Venkatesh P G et al. Prevalence and risk factors for colonic perforation during colonoscopy in hospitalized inflammatory bowel disease patients. Journal of Crohn’s & colitis. 2011;5:189–195. doi: 10.1016/j.crohns.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Calabrese E, Kucharzik T, Maaser C et al. Real-time Interobserver Agreement in Bowel Ultrasonography for Diagnostic Assessment in Patients With Crohn’s Disease: An International Multicenter Study. Inflammatory bowel diseases. 2018;24:2001–2006. doi: 10.1093/ibd/izy091. [DOI] [PubMed] [Google Scholar]
- 6.Allocca M, Fiorino G, Bonovas S et al. Accuracy of Humanitas Ultrasound Criteria in Assessing Disease Activity and Severity in Ulcerative Colitis: A Prospective Study. Journal of Crohn’s & colitis. 2018;12:1385–1391. doi: 10.1093/ecco-jcc/jjy107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryant R V, Friedman A B, Wright E K et al. Gastrointestinal ultrasound in inflammatory bowel disease: an underused resource with potential paradigm-changing application. Gut. 2018;67:973–985. doi: 10.1136/gutjnl-2017-315655. [DOI] [PubMed] [Google Scholar]
- 8.Panes J, Bouhnik Y, Reinisch W et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. Journal of Crohn’s & colitis. 2013;7:556–585. doi: 10.1016/j.crohns.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Schulberg J D, Wright E K, Holt B A et al. Magnetic resonance enterography for predicting the clinical course of Crohn’s disease strictures. Journal of gastroenterology and hepatology. 2020;35:980–987. doi: 10.1111/jgh.14908. [DOI] [PubMed] [Google Scholar]
- 10.Allocca M, Fiorino G, Bonifacio C et al. Comparative Accuracy of Bowel Ultrasound Versus Magnetic Resonance Enterography in Combination With Colonoscopy in Assessing Crohn’s Disease and Guiding Clinical Decision-making. Journal of Crohn’s & colitis. 2018;12:1280–1287. doi: 10.1093/ecco-jcc/jjy093. [DOI] [PubMed] [Google Scholar]
- 11.Nylund K, Maconi G, Hollerweger A et al. EFSUMB Recommendations and Guidelines for Gastrointestinal Ultrasound. Ultraschall in Med. 2017;38:e1–e15. doi: 10.1055/s-0042-115853. [DOI] [PubMed] [Google Scholar]
- 12.Derlin T, Laqmani A, Veldhoen S et al. Magnetic resonance enterography for assessment of intestinal graft-versus-host disease after allogeneic stem cell transplantation. Eur Radiol. 2015;25:1229–1237. doi: 10.1007/s00330-014-3503-4. [DOI] [PubMed] [Google Scholar]
- 13.Rutjes A W, Reitsma J B, Coomarasamy A.Evaluation of diagnostic tests when there is no gold standard. A review of methods Health Technol Assess 200711iiiix-51 [DOI] [PubMed] [Google Scholar]
- 14.Valette P J, Rioux M, Pilleul F et al. Ultrasonography of chronic inflammatory bowel diseases. European radiology. 2001;11:1859–1866. doi: 10.1007/s003300101065. [DOI] [PubMed] [Google Scholar]
- 15.Laterza L, Ainora M E, Garcovich M et al. Bowel contrast-enhanced ultrasound perfusion imaging in the evaluation of Crohn’s disease patients undergoing anti-TNFalpha therapy. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2020 doi: 10.1016/j.dld.2020.08.005:. [DOI] [PubMed] [Google Scholar]
- 16.Quaia E, Migaleddu V, Baratella E et al. The diagnostic value of small bowel wall vascularity after sulfur hexafluoride-filled microbubble injection in patients with Crohn’s disease. Correlation with the therapeutic effectiveness of specific anti-inflammatory treatment. European journal of radiology. 2009;69:438–444. doi: 10.1016/j.ejrad.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Taylor S A, Mallett S, Bhatnagar G et al. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn’s disease (METRIC): a multicentre trial. The lancet Gastroenterology & hepatology. 2018;3:548–558. doi: 10.1016/S2468-1253(18)30161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maaser C, Petersen F, Helwig U et al. Intestinal ultrasound for monitoring therapeutic response in patients with ulcerative colitis: results from the TRUST&UC study. Gut. 2020;69:1629–1636. doi: 10.1136/gutjnl-2019-319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maaser C, Sturm A, Vavricka S R et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. Journal of Crohn’s & colitis. 2019;13:144–164. doi: 10.1093/ecco-jcc/jjy113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


