Abstract
Background
This review is the third update of a previously published Cochrane Review. The original review, looking at all possible cardioprotective agents, was split and this part now focuses on dexrazoxane only.
Anthracyclines are effective chemotherapeutic agents in the treatment of numerous malignancies. Unfortunately, their use is limited by a dose‐dependent cardiotoxicity. In an effort to prevent or reduce this cardiotoxicity, different cardioprotective agents have been studied, including dexrazoxane.
Objectives
To assess the efficacy of dexrazoxane to prevent or reduce cardiotoxicity and determine possible effects of dexrazoxane on antitumour efficacy, quality of life and toxicities other than cardiac damage in adults and children with cancer receiving anthracyclines when compared to placebo or no additional treatment.
Search methods
We searched CENTRAL, MEDLINE and Embase to May 2021. We also handsearched reference lists, the proceedings of relevant conferences and ongoing trials registers.
Selection criteria
Randomised controlled trials (RCTs) in which dexrazoxane was compared to no additional therapy or placebo in adults and children with cancer receiving anthracyclines.
Data collection and analysis
Two review authors independently performed study selection, data extraction, risk of bias and GRADE assessment of included studies. We analysed results in adults and children separately. We performed analyses according to the Cochrane Handbook for Systematic Reviews of Interventions.
Main results
For this update, we identified 548 unique records. We included three additional RCTs: two paediatric and one adult. Therefore, we included a total of 13 eligible RCTs (five paediatric and eight adult). The studies enrolled 1252 children with leukaemia, lymphoma or a solid tumour and 1269 participants, who were mostly diagnosed with breast cancer.
In adults, moderate‐quality evidence showed that there was less clinical heart failure with the use of dexrazoxane (risk ratio (RR) 0.22, 95% confidence interval (CI) 0.11 to 0.43; 7 studies, 1221 adults). In children, we identified no difference in clinical heart failure risk between treatment groups (RR 0.20, 95% CI 0.01 to 4.19; 3 studies, 885 children; low‐quality evidence). In three paediatric studies assessing cardiomyopathy/heart failure as the primary cause of death, none of the children had this outcome (1008 children, low‐quality evidence). In the adult studies, different definitions for subclinical myocardial dysfunction and clinical heart failure combined were used, but pooled analyses were possible: there was a benefit in favour of the use of dexrazoxane (RR 0.37, 95% CI 0.24 to 0.56; 3 studies, 417 adults and RR 0.46, 95% CI 0.33 to 0.66; 2 studies, 534 adults, respectively, moderate‐quality evidence). In the paediatric studies, definitions of subclinical myocardial dysfunction and clinical heart failure combined were incomparable, making pooling impossible. One paediatric study showed a benefit in favour of dexrazoxane (RR 0.33, 95% CI 0.13 to 0.85; 33 children; low‐quality evidence), whereas another study showed no difference between treatment groups (Fischer exact P = 0.12; 537 children; very low‐quality evidence).
Overall survival (OS) was reported in adults and overall mortality in children. The meta‐analyses of both outcomes showed no difference between treatment groups (hazard ratio (HR) 1.04, 95% 0.88 to 1.23; 4 studies; moderate‐quality evidence; and HR 1.01, 95% CI 0.72 to 1.42; 3 studies, 1008 children; low‐quality evidence, respectively). Progression‐free survival (PFS) was only reported in adults. We subdivided PFS into three analyses based on the comparability of definitions, and identified a longer PFS in favour of dexrazoxane in one study (HR 0.62, 95% CI 0.43 to 0.90; 164 adults; low‐quality evidence). There was no difference between treatment groups in the other two analyses (HR 0.95, 95% CI 0.64 to 1.40; 1 study; low‐quality evidence; and HR 1.18, 95% CI 0.97 to 1.43; 2 studies; moderate‐quality evidence, respectively). In adults, there was no difference in tumour response rate between treatment groups (RR 0.91, 95% CI 0.79 to 1.04; 6 studies, 956 adults; moderate‐quality evidence). We subdivided tumour response rate in children into two analyses based on the comparability of definitions, and identified no difference between treatment groups (RR 1.01, 95% CI 0.95 to 1.07; 1 study, 206 children; very low‐quality evidence; and RR 0.92, 95% CI 0.84 to 1.01; 1 study, 200 children; low‐quality evidence, respectively). The occurrence of secondary malignant neoplasms (SMN) was only assessed in children. The available and worst‐case analyses were identical and showed a difference in favour of the control group (RR 3.08, 95% CI 1.13 to 8.38; 3 studies, 1015 children; low‐quality evidence). In the best‐case analysis, the direction of effect was the same, but there was no difference between treatment groups (RR 2.51, 95% CI 0.96 to 6.53; 4 studies, 1220 children; low‐quality evidence). For other adverse effects, results also varied. None of the studies evaluated quality of life.
If not reported, the number of participants for an analysis was unclear.
Authors' conclusions
Our meta‐analyses showed the efficacy of dexrazoxane in preventing or reducing cardiotoxicity in adults treated with anthracyclines. In children, there was a difference between treatment groups for one cardiac outcome (i.e. for one of the definitions used for clinical heart failure and subclinical myocardial dysfunction combined) in favour of dexrazoxane. In adults, no evidence of a negative effect on tumour response rate, OS and PFS was identified; and in children, no evidence of a negative effect on tumour response rate and overall mortality was identified. The results for adverse effects varied. In children, dexrazoxane may be associated with a higher risk of SMN; in adults this was not addressed. In adults, the quality of the evidence ranged between moderate and low; in children, it ranged between low and very low. Before definitive conclusions on the use of dexrazoxane can be made, especially in children, more high‐quality research is needed.
We conclude that if the risk of cardiac damage is expected to be high, it might be justified to use dexrazoxane in children and adults with cancer who are treated with anthracyclines. However, clinicians and patients should weigh the cardioprotective effect of dexrazoxane against the possible risk of adverse effects, including SMN, for each individual.
For children, the International Late Effects of Childhood Cancer Guideline Harmonization Group has developed a clinical practice guideline.
Keywords: Adult; Child; Humans; Anthracyclines; Anthracyclines/adverse effects; Antibiotics, Antineoplastic; Antibiotics, Antineoplastic/adverse effects; Cardiotonic Agents; Cardiotonic Agents/therapeutic use; Cardiotoxicity; Cardiotoxicity/drug therapy; Cardiotoxicity/etiology; Cardiotoxicity/prevention & control; Dexrazoxane; Dexrazoxane/therapeutic use; Heart Failure; Heart Failure/drug therapy; Leukemia, Myeloid, Acute; Leukemia, Myeloid, Acute/drug therapy; Polyketides; Polyketides/therapeutic use; Systematic Reviews as Topic
Plain language summary
Can the medicine dexrazoxane prevent or reduce heart damage in adults and children with cancer receiving anthracyclines?
Review question We reviewed the evidence regarding the effectiveness of the medicine dexrazoxane to prevent or reduce heart damage in children and adults with cancer treated with anthracycline chemotherapy. We also looked at the possible effects of dexrazoxane on antitumour effectiveness (that is, survival and tumour response rate), quality of life and adverse effects (i.e. unwanted or harmful effects of a treatment) other than cardiac damage.
Background Anthracyclines are effective chemotherapy treatments available for various types of cancer. However, there is a risk of damage to the heart (cardiotoxicity) depending on the cumulative dose (total amount of treatment given over time). Cardiotoxicity may lead to subclinical myocardial dysfunction (when there is evidence from a test that heart function is limited, but the person does not have symptoms), which can progress to clinical heart failure (when the person has symptoms). Dexrazoxane is a medicine with the potential to prevent or reduce this damage.
This review is the third update of a previously published Cochrane Review. The original review, looking at all possible cardioprotective agents (medicines that protect the heart), was split and this review now focuses on dexrazoxane only.
Study characteristics The evidence is current to May 2021.
We found 13 randomised studies (clinical studies where people are randomly put into one of two or more treatment groups) looking at dexrazoxane: 5 studies in children (1252 children with leukaemia, lymphoma or a solid tumour) and 8 studies in adults (1269 adults who were mostly diagnosed with breast cancer).
Key results Our analyses showed that:
‐ in adults, dexrazoxane was able to prevent or reduce heart damage for those treated with anthracyclines; ‐ in children, there was a difference between treatment groups in favour of dexrazoxane for only one of the cardiac (heart‐related) outcomes; namely, clinical heart failure and subclinical myocardial dysfunction combined; ‐ in adults, no evidence of a negative effect on survival or a lower tumour response rate was identified; ‐ in children, no evidence of a lower overall mortality or a lower tumour response rate was identified.
The results for adverse effects varied. Children treated with dexrazoxane might have a higher risk of secondary cancers (i.e. a new cancer). This outcome was not evaluated in adults.
None of the studies evaluated the quality of life of the people who participated.
Before definitive conclusions on the use of dexrazoxane can be made, especially in children, more high‐quality research is needed. We conclude that if the risk of heart damage from anthracyclines is expected to be high, it might be justified to use dexrazoxane in children and adults with cancer who are treated with anthracyclines. However, clinicians and patients should weigh the cardioprotective effect of dexrazoxane against the possible risk of adverse effects, including secondary cancers, for each individual. For children, the International Late Effects of Childhood Cancer Guideline Harmonization Group has developed a clinical practice guideline (www.ighg.org).
Quality of the evidence In children, we assessed the quality of the evidence as low for almost all evaluated outcomes and very low for two outcomes (one definition of clinical heart failure and subclinical myocardial dysfunction combined and one definition of tumour response rate); for the other definitions of these outcomes, we assessed the results as low quality. In adults, we assessed the quality of the evidence as moderate for almost all evaluated outcomes, and as low for two definitions of survival (for the other two definitions of survival as moderate).
The quality of the evidence was limited because of issues with the study design, the small numbers of participants in some studies, or for both reasons.
Summary of findings
Summary of findings 1. Dexrazoxane versus no cardioprotective intervention or placebo for preventing or reducing cardiotoxicity in adults with cancer receiving anthracyclines.
| Dexrazoxane compared with no cardioprotective intervention or placebo for preventing or reducing cardiotoxicity in adults with cancer receiving anthracyclines | ||||||
|
Patient or population: adults with cancer receiving anthracyclines Settings: hospital Intervention: dexrazoxane Comparison: no cardioprotective intervention or placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No cardioprotective intervention or placebo | Dexrazoxane | |||||
|
Clinical heart failure Available case analysis Follow‐up ranged between 1 day and 5.1 years (nm for 5 studies) |
107 per 1000a | 24 per 1000 (12 to 46) | RR 0.22 (0.11 to 0.43) | 1221 (7 studies) | ⊕⊕⊕⊝ Moderateb,c |
In 1 study, none of the participants developed clinical heart failure; the relative effect for that study was not estimable. The available‐case, best‐case and worst‐case analyses showed identical results, including the GRADE assessment. In the worst‐case analysis, there was unexplained heterogeneity (I2 = 52%). |
|
Clinical heart failure and subclinical myocardial dysfunction combined Comparable definitions; see Characteristics of included studies for exact definitions. Available‐case analysis Follow‐up nm |
314 per 1000a | 116 per 1000 (75 to 176) | RR 0.37 (0.24 to 0.56) | 417 (3 studies) | ⊕⊕⊕⊝ Moderatec,d |
The available‐case, best‐case and worst‐case analyses showed identical results, including the GRADE assessment. |
|
Clinical heart failure and subclinical myocardial dysfunction combined Comparable definitions; see Characteristics of included studies for exact definitions. Available‐case analysis Follow‐up ranged between 1 day and 5.1 years |
312 per 1000a | 144 per 1000 (103 to 206) | RR 0.46 (0.33 to 0.66) | 534 (2 studies) | ⊕⊕⊕⊝ Moderatec,e |
The available‐case, best‐case and worst‐case analyses showed identical results, including the GRADE assessment. |
|
Overall survival (Illustrative comparative risks reported as number of alive participants) Follow‐up ranged between 1 day and 5.1 years (nm for 2 studies) |
233 per 1000f | 219 per 1000 (166 to 277) | HR 1.04 (0.88 to 1.23) | Unclear (4 studies) | ⊕⊕⊕⊝ Moderateg,h |
|
|
Progression‐free survival Defined as time from first date of complete response, partial response or stable disease until the date progressive disease was first noticed (Illustrative comparative risks reported as number of participants without progressive disease) Follow‐up nm |
0 per 1000i | 0 per 1000 (0 to 3) | HR 0.62 (0.43 to 0.90) | 164 (1 study) | ⊕⊕⊝⊝ Lowj,k |
All participants in the control group had progression at the end of follow‐up, but as the GRADEpro software was not able to calculate the corresponding risk with an assumed risk of 0%, we used 0.0001% as the assumed risk in the control group instead. |
|
Progression‐free survival Defined as time to progression; starting point nm (Illustrative comparative risks reported as number of participants without progression) Follow‐up nm |
150 per 1000l | 165 per 1000 (70‐297) | HR 0.95 (0.64 to 1.40) | Unclear (1 study) | ⊕⊕⊝⊝ Lowm,n |
|
|
Progression‐free survival Defined as time from randomisation to progression either on or off treatment (Illustrative comparative risks reported as number of participants without progression) Follow‐up ranged between 1 day and 5.1 years |
100 per 1000o | 66 per 1000 (37 to 107) | HR 1.18 (0.97 to 1.43) | Unclear (2 studies) | ⊕⊕⊕⊝ Moderatep,q |
|
|
Tumour response rate Defined as number of complete or partial remissions Available‐case analysis Follow‐up ranged between 1 day and 5.1 years (nm for 4 studies) |
533 per 1000a | 485 per 1000 (421 to 554) | RR 0.91 (0.79 to 1.04) | 956 (6 studies) | ⊕⊕⊕⊝ Moderater,s |
Due to the nature of this outcome (number of participants with a remission), a high event rate is favourable. The available‐case, best‐case and worst‐case analyses showed identical results, including the GRADE assessment. |
| Quality of life | No studies evaluated this outcome | |||||
| Secondary malignant neoplasms | No studies evaluated this outcome | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
CI: confidence interval CTCAEv2: Common Terminology Criteria for Adverse Events, version 2 e.g.: for example HR: hazard ratio LVEF: left ventricular ejection fraction LVFS: left ventricular fractional shortening MUGA: multigated acquisition scan NCI: National Cancer institute nm: not mentioned P: P‐value RR: risk ratio
aThe assumed risk is based on the overall prevalence in the control groups of the included studies. bUnclear risk of selection bias in 5 (71%) studies, high risk of performance bias in 4 (57%) and unclear risk in 1 (14%) of the studies, unclear risk of detection bias in 2 (29%) studies, high risk of selective reporting in 1 (14%) study, unclear risk of other bias in all studies (downgraded 1 level). cWe did not downgrade for imprecision; the total number of events was fewer than 300 (the threshold rule‐of‐thumb value stated in the GRADEpro handbook (GRADEpro handbook), but the effect was large and the 95% CI is small and below no effect. dUnclear risk of selection and other bias in all studies, high risk of performance bias in all studies, unclear risk of detection bias in 1 (33%) study, high risk of attrition bias in 1 (33%) study (downgraded 1 level). eUnclear risk of other bias in all studies (downgraded 1 level). fThe assumed risk is based on the approximate mean percentage of participants alive in the control groups at the final point of the survival curves presented in the included studies. gUnclear risk of selection bias in 2 (50%) studies, high risk of performance bias in 2 (50%) studies, high risk of attrition bias in 1 (25%) study and unclear in 3 studies (75%), unclear risk of other bias in all studies (downgraded 1 level). hWe did not downgrade for imprecision; the number of events and total available participants in the 4 studies was unclear, but based on the maximum number of participants and the assumed baseline risk, we assumed that it was above 300 (the threshold rule‐of‐thumb value stated in the GRADEpro handbook (GRADEpro handbook); the 95% CI includes no effect, but was small. iThe assumed risk is based on the percentage of participants without progression in the control group at the final point of the survival curve presented in the included study (see comments for more information). jUnclear risk of selection bias, detection bias and other bias and a high risk of performance bias in the included study (downgraded 1 level). kAs this was a small study with a total number of events fewer than 300 (the threshold rule‐of‐thumb value stated in the GRADEpro handbook (GRADEpro handbook) without a large effect, we downgraded 1 level, even though the 95% CI was below no effect. lThe assumed risk is based on the approximate percentage of participants without progression in the control group at the final point of the survival curve presented in the included study. mUnclear risk of selection bias, detection bias, attrition bias and other bias and a high risk of performance bias in the included study (downgraded 1 level). nAs this was a small study with a total number of events fewer than 300 (the threshold rule‐of‐thumb value stated in the GRADEpro handbook (GRADEpro handbook), we downgraded 1 level. oThe assumed risk is based on the approximate mean percentage of participants alive in the control groups at the final point of the survival curves presented in the included studies. pUnclear risk of attrition and other bias in both studies (downgraded 1 level). qWe did not downgrade for imprecision; the number of events and available participants in the 2 studies was unclear, but based on the maximum number of participants and the assumed baseline risk we assumed that it was above 300 (the threshold rule‐of‐thumb value stated in the GRADEpro handbook (GRADEpro handbook); the 95% CI includes no effect, but was small. rUnclear risk of selection and detection bias in 4 (67%) studies, high risk of performance bias in 4 (67%) studies, high risk of attrition bias in 3 (50%) studies, unclear risk of other bias in all studies (downgraded 1 level). sWe did not downgrade for imprecision; the total number of events was more than 300 (the threshold rule‐of‐thumb value stated in the GRADEpro handbook (GRADEpro handbook); the 95%CI includes no effect, but was small.
Summary of findings 2. Dexrazoxane versus no cardioprotective intervention for preventing or reducing cardiotoxicity in children with cancer receiving anthracyclines.
| Dexrazoxane compared with no cardioprotective intervention for preventing or reducing cardiotoxicity in children with cancer receiving anthracyclines | ||||||
|
Patient or population: children with cancer receiving anthracyclines Settings: hospital Intervention: dexrazoxane Comparison: no cardioprotective intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No cardioprotective intervention | Dexrazoxane | |||||
|
Clinical heart failure Available‐case analysis Follow‐up ranged between 0.01 and 15 years (nm for 1 study) |
5 per 1000a | 1 per 1000 (0 to 19) | RR 0.20 (0.01 to 4.19) | 885 (3 studies) | ⊕⊕⊝⊝ Lowb,c |
In 2 studies, none of the participants developed clinical heart failure; the relative effect for those studies was not estimable. The available‐case, best‐case and worst‐case analyses showed identical results, including the GRADE assessment. |
|
Cardiomyopathy/heart failure primary cause of death Available‐case analysis Follow‐up ranged between 0 and 15.5 years |
‐ | ‐ | Not estimable (see comments) | 1008 (3 studies) | ⊕⊕⊝⊝ Lowc,d |
In all studies, none of the participants had cardiomyopathy/heart failure as the primary cause of death; the relative effect was not estimable. The available‐case, best‐case and worst‐case analyses were identical, including the GRADE assessment. |
|
Clinical heart failure and subclinical myocardial dysfunction combined Defined as (1) evidence of clinical congestive heart failure, (2) a reduction in LVEF as measured by MUGA to < 45% or (3) a decrease in LVEF as measured by MUGA of > 20 percentage points from baseline. Available‐case analysis Follow‐up nm for randomised participants |
667 per 1000a | 220 per 1000 (87 to 567) | RR 0.33 (0.13 to 0.85) | 33 (1 study) | ⊕⊕⊝⊝ Lowe,f |
The available‐case, best‐case and worst‐case analyses showed identical results, including the GRADE assessment. Study participants were aged between 4 and 24 years, so not all paediatric patients (< 21 years). |
|
Clinical heart failure and subclinical myocardial dysfunction combined Defined as clinical heart failure (no definition provided) or subclinical myocardial dysfunction defined as decreased LVFS; however, it was stated that toxicity was graded according to NCI CTCAEv2 criteria, grade 3 or higher but LVFS is not included in that definition. Best‐case analysis Follow‐up ranged between 0.01 and 15 years |
‐ | ‐ | Not estimable (see comments) | 537 (1 study) | ⊕⊝⊝⊝ Very lowg,h |
For this outcome definition, only one study was available in which one of the treatment groups experienced no events. Thus, we were not able to calculate a RR and we used Fischer's exact test instead (P = 0.12). Only a best‐case analysis could be performed due to an unclear number of participants lost to follow‐up. |
|
Overall mortality (Reported as number of participants who died) Follow‐up ranged between 0 and 15.5 years |
130 per 1000i | 131 per 1000 (95 to 179) | HR 1.01 (0.72 to 1.42) |
1008 (3 studies) | ⊕⊕⊝⊝ Lowc,d |
|
| Progression‐free survival | No studies evaluated this outcome | |||||
|
Tumour response rate Defined as number of complete remissions (no definition of complete remission provided). Best‐case analysis Follow‐up median 2.7 years |
950 per 1000a | 960 per 1000 (903 to 1000) | RR 1.01 (0.95 to 1.07) | 206 (1 study) | ⊕⊝⊝⊝ Very lowh,j |
Due to the nature of this outcome (number of participants with a complete remission), a high event rate is favourable. Only a best‐case analysis could be performed due to an unclear number of participants lost to follow‐up. |
|
Tumour response rate Defined as number of complete responses (i.e. disappearance of active Hodgkin lymphoma (gallium negative, ≥ 70% decrease in the sum of the products of the perpendicular diameters of measurable lesions, and negative bone marrow or bone scan if initially positive)). Available‐case analysis Follow‐up nm (median follow‐up for participants without an event was 5.2 years). |
939 per 1000a | 864 per 1000 (789 to 949) | RR 0.92 (0.84 to 1.01) | 200 (1 study) | ⊕⊕⊝⊝ Lowh,k |
Due to the nature of this outcome (number of participants with a complete response), a high event rate is favourable. The available‐case, best‐case and worst‐case analyses showed identical results, including the GRADE assessment. |
| Quality of life | No studies evaluated this outcome | |||||
| Adverse effects other than cardiac damage | ||||||
|
Secondary malignant neoplasms Available‐case analysis Follow‐up ranged between 0.01 and 15 years (nm for 1 study) |
10 per 1000a | 31 per 1000 (11 to 83) | RR 3.08 (1.13 to 8.38) | 1015 (3 studies) | ⊕⊕⊝⊝ Lowc,l |
The available‐case and worst‐case analyses were identical; the best‐case analysis showed the same direction of effect, but the result was not different between treatment groups (RR 2.51 (0.96 to 6.53). GRADE assessments were comparable for all analyses. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
CI: confidence interval CTCAEv2: Common Terminology Criteria for Adverse Events, version 2 e.g.: for example HR: hazard ratio LVEF: left ventricular ejection fraction LVFS: left ventricular fractional shortening MUGA: multigated acquisition scan NCI: National Cancer institute nm: not mentioned P: P value RR: risk ratio
aThe assumed risk is based on the overall prevalence in the control group(s) of the included study/ies. bUnclear risk of selection and detection bias in 2 (67%) studies, high risk of performance bias in all studies, high risk of attrition bias and selective reporting in 1 (33%) study, unclear risk of other bias in all studies (downgraded 1 level). cAs these were relatively small studies with a total number of events fewer than 300 (the threshold rule‐of‐thumb value stated in the GRADEpro handbook (GRADEpro handbook), we downgraded one level. dUnclear risk of selection and other bias in all studies, high risk of performance bias in all studies (downgraded 1 level). eUnclear risk of selection, detection and other bias, and high risk of performance and attrition bias (downgraded 2 levels). fWe did not downgrade for imprecision; it was a small study but the effect was large, the 95% CI is small and below no effect. gUnclear risk of selection, detection and other bias, high risk of performance and attrition bias (downgraded 2 levels). hAs this was a small study with a total number of events fewer than 300 (the threshold rule‐of‐thumb value stated in the GRADEpro handbook (GRADEpro handbook), we downgraded 1 level. iThe assumed risk is based on the number of participants who died in the control groups of the included studies. jUnclear risk of attrition and other bias, high risk of performance bias and selective reporting (downgraded 2 levels). kUnclear risk of selection, detection and other bias; high risk of performance bias (downgraded 1 level). lUnclear risk of selection, detection and other bias in all studies; high risk of performance bias in all studies (downgraded 1 level).
Background
Description of the condition
Anthracyclines – that is, doxorubicin, epirubicin, idarubicin and daunorubicin – are drugs used in chemotherapy for the treatment of cancer. They are widely used to treat solid tumours and leukaemia in both adults and children. However, their use is limited because treatment with anthracyclines is associated with myocardial damage (Bonadonna 1969; Leerink 2020; Lefrak 1973)
Myocardial damage may lead to subclinical myocardial dysfunction, which is diagnosed by an imaging modality in people without symptoms. This may lead to clinical heart failure, which is a combination of myocardial dysfunction and the presence of related symptoms. Heart failure is one of the most severe long‐term adverse effects in childhood cancer survivors (CCSs) and is associated with increased mortality (Fidler 2017; Mertens 2008). Heart transplantation is the only remaining treatment option for end‐stage heart failure.
There is wide variation in the reported frequency of both subclinical myocardial dysfunction and clinical heart failure. In children, the prevalence of subclinical myocardial dysfunction at a median follow‐up time of up to 23 years after cancer diagnosis or cardiotoxic cancer treatment is more than 56% (Kremer 2002a; Merkx 2021). The cumulative incidence of clinical heart failure can be as high as 16% (0.9 to 40 years after treatment, depending on the specific study) (Feijen 2019b; Kremer 2002b). The risk of subclinical myocardial dysfunction and clinical heart failure depends on the type of anthracycline used and increases with higher cumulative and peak doses (Armstrong 2015; Feijen 2019a; Feijen 2019b; Mulrooney 2020; Van Dalen 2010; Van Dalen 2016). Other important cancer treatment risk factors are radiation therapy involving the heart region, and the use of cyclophosphamide and mitoxantrone (Feijen 2019b). In addition, female sex, existing heart disease, a younger age at diagnosis and presence of traditional cardiovascular risk factors may play a role in the development of heart failure (Chellapandian 2019; Chow 2015; Mulrooney 2020; Van der Pal 2012).
Researchers have investigated whether anthracyclines can be omitted from the treatment regime without reducing survival. A study by Pritchard‐Jones and colleagues, which included a subgroup of children with a Wilms tumour, showed that anthracyclines could safely be excluded from the treatment of this subgroup (Pritchard‐Jones 2015). However, when anthracyclines cannot be avoided (Van Dalen 2014), clinicians may have a clinical dilemma as they balance the efficacy of higher cumulative doses of anthracyclines against the cardiotoxicity associated with these higher doses. In an effort to prevent or reduce this cardiotoxicity, extensive research has been devoted to the identification of methods or drugs capable of ameliorating the toxicity. Several less cardiotoxic anthracycline analogues have been developed, including liposomal anthracyclines (Batist 2001; Fojtu 2017; Hori 2017; Muggia 1991; Muggia 1997; Van Dalen 2010), and the cumulative and peak doses of anthracycline therapy have been reduced (Legha 1982; Lipshultz 1998; Loeffen 2018; Van Dalen 2016; Von Hoff 1979). Despite these efforts, anthracycline‐induced cardiotoxicity (AIC) remains an issue.
Description of the intervention
A different approach to prevent or reduce AIC is the use of cardioprotective agents, of which dexrazoxane (also known as Cardioxane, ICRF‐187; Zinecard, ADR‐529) is the most widely investigated drug. An important question regarding any cardioprotective intervention during anthracycline therapy is whether the cardioprotective drug can reduce any myocardial damage caused by anthracyclines without affecting the antitumour efficacy and without causing other adverse effects, such as alopecia, nausea, vomiting and anaemia.
How the intervention might work
We do not understand exactly the mechanism of how anthracyclines cause myocardial damage. It may be due to lipid peroxidation and the generation of free radicals by anthracycline‐iron complexes. The myocardium is particularly vulnerable to injury from free radicals as it has a lower level of protective enzymes, such as superoxide dismutase, than other tissues (Keizer 1990; Myers 1998). As dexrazoxane chelates iron, it may decrease cardiotoxicity by preventing the formation of free radicals (Gammella 2014). In recent years, interest has grown in another possible contributor to AIC; namely, topoisomerase 2β (TOP2B). This enzyme is highly expressed in cardiomyocytes and causes apoptosis when bound to anthracycline. Animal studies have also suggested that dexrazoxane may prevent cardiotoxicity via inhibition of TOP2B (Deng 2014; Lyu 2007).
Why it is important to do this review
The risk of developing heart failure remains a lifelong threat, especially to children who would otherwise have a long life expectancy after successful treatment for cancer. Therefore, the prevention or reduction of AIC is crucial.
This is the third update of the systematic review on cardioprotective interventions during anthracycline therapy. The review has been split and this update focuses on dexrazoxane alone. Since the last update (Van Dalen 2011), new evidence on dexrazoxane has become available and is included in this update. A second updated review will focus on other cardioprotective interventions.
Objectives
To assess the efficacy of dexrazoxane to prevent or reduce cardiotoxicity and determine possible effects of dexrazoxane on antitumour efficacy, quality of life and toxicities other than cardiac damage in adults and children with cancer receiving anthracyclines when compared to placebo or no additional treatment.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Adults and children with cancer who received anthracycline chemotherapy.
Types of interventions
Intervention: anthracycline therapy together with dexrazoxane.
Control: anthracycline therapy with or without a placebo.
In the design of the study (i.e. according to protocol), it should have been the intention to treat (ITT) both the intervention and control groups with the same cumulative anthracycline dose. The median or mean cumulative anthracycline dose participants actually received should not have differed between the treatment groups by 100 mg/m2 or more of body surface area. Any chemotherapy other than anthracyclines and radiotherapy involving the heart should have been the same in both treatment groups.
Types of outcome measures
Primary outcomes
-
Heart failure:
clinical heart failure (as defined by the authors; including death caused by heart failure)
clinical heart failure (as defined by the authors; including death caused by heart failure) and subclinical myocardial dysfunction (defined as either abnormalities in cardiac function measured by imaging (echocardiography, radionuclide ventriculography or cardiac magnetic resonance imaging) or histological abnormalities scored by the Billingham score (Billingham 1978) on endomyocardial biopsy) combined
Overall survival (OS) or overall mortality
Secondary outcomes
Progression‐free survival (PFS)
Tumour response rate (for adults, defined as the number of complete and partial remissions; for children, defined as the number of complete remissions)
Quality of life (QoL, as defined by the authors)
Toxicities other than cardiac damage (such as secondary malignant neoplasms (SMN), alopecia, nausea, vomiting, stomatitis, diarrhoea, fatigue, anaemia, leukopenia, thrombocytopenia)
Search methods for identification of studies
We imposed no language restrictions.
Electronic searches
We searched the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 1) in the Cochrane Library (searched 7 May 2021);
MEDLINE (PubMed) (from 1966 to 7 May 2021); and
Embase (Ovid) (from 1980 to 7 May 2021).
The search strategies for the different electronic databases (using a combination of controlled vocabulary and text word terms) are detailed in the appendices (Appendix 1, Appendix 2, Appendix 3). These searches included the National Institutes of Health and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP).
Searching other resources
We located information about trials not listed in CENTRAL, MEDLINE or Embase, either published or unpublished, by searching the reference lists of included articles and review articles. In addition, we searched the conference proceedings of the International Society for Paediatric Oncology (SIOP) and the American Society of Clinical Oncology (ASCO) from 1998 to 2020 (see Appendix 4 for search strategies).
Data collection and analysis
Selection of studies
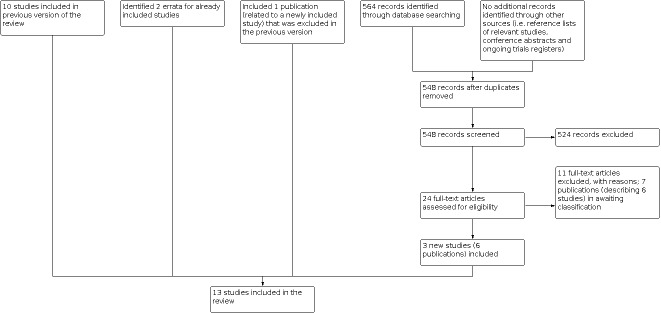
After performing the search strategy described previously, two review authors independently identified studies meeting the inclusion criteria. We obtained the full‐text articles for any study seemingly meeting the inclusion criteria based on the title, abstract, or both, for closer inspection. We resolved any discrepancies by discussion or, when this was not possible, by third‐party arbitration. We clearly stated the details of the reasons for exclusion of any study considered for the review. We included a flow diagram of the selection of studies (Figure 1). When multiple reports of one study were identified, we collated the full‐text results.
1.
Flow diagram of selection of studies
Data extraction and management
Two review authors independently performed the data extraction using standardised data collection forms.
We extracted the characteristics of the participants (for example: age, type of malignancy, stage of disease), intervention (for example: dose, timing), outcome measures, length of follow‐up, details of funding sources and the declaration of interests for each included study. To inform interpretation of the findings, we assessed the similarity of the experimental groups at baseline regarding the most important prognostic indicators (that is, age, prior cardiotoxic therapy, prior cardiac dysfunction and stage of disease). We resolved any discrepancies between review authors by discussion or, when this was not possible, by third‐party arbitration.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias in the included studies (i.e. selection bias, performance bias, detection bias (for each outcome separately), attrition bias (for each outcome separately), reporting bias and other potential sources of bias). We used the risk of bias items as described in the module of Cochrane Childhood Cancer (Module CCG), which are based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved discrepancies between review authors by discussion and needed no third‐party arbitration. We took into account the risk of bias in the included studies in the interpretation of the review's results.
Measures of treatment effect
We analysed dichotomous variables using risk ratios (RR). For the assessment of survival, we used the generic inverse variance function of the Review Manager 5 software (Review Manager 2020) to combine logs of the hazard ratios (HRs). Parmar's method was used to extract the log of the HR and its standard error (SE) from survival curves (Parmar 1998) for the studies of Marty 2006 and Speyer 1992. We digitised the published Kaplan‐Meier survival curves and noted the minimum and maximum duration of follow‐up (Guyot 2012), which are required for Parmar's method. We performed the required calculations in Stata 9 (Stata 2005), using a specially written program, which yielded the reported log(HR) and variance when used on the data presented in table V of Parmar 1998. We presented all results with the corresponding 95% confidence interval (CI).
Unit of analysis issues
Unit of analysis issues were not applicable.
Dealing with missing data
When relevant data regarding study selection, data extraction and risk of bias assessment were missing, we attempted to contact the study authors to retrieve the missing data. If possible, we extracted data by allocated group, irrespective of compliance with the allocated intervention, in order to allow an intention‐to‐treat analysis. If outcome assessments were not available for all participants, we performed an available‐case analysis and, if possible, also a best‐case and worst‐case analysis. The available‐case analysis only includes participants who had an outcome assessment. The best‐case analysis includes all participants and usually assumes that participants without an outcome assessment did not develop the outcome (for example, heart failure). The worst‐case analysis includes all participants and usually assumes that all participants without an outcome assessment developed the outcome. However, for example, for tumour response rate (i.e. number of participants with a remission) this is the opposite: due to the nature of this outcome, 'best case' here means that the participant does have the outcome.
Assessment of heterogeneity
We assessed heterogeneity by both visual inspection of forest plots and by a formal statistical test for heterogeneity; namely, the I2 statistic (we considered I2 > 50% to represent substantial heterogeneity) (Higgins 2011). If we detected substantial heterogeneity, we explored possible reasons for the occurrence of heterogeneity.
Assessment of reporting biases
In addition to the evaluation of reporting bias as described in the Assessment of risk of bias in included studies section, we planned to assess reporting bias by constructing a funnel plot when there was a sufficient number of included studies (i.e. at least 10 studies included in a meta‐analysis); without this number, the power of the test is too low to distinguish chance from real asymmetry (Higgins 2011). Since all meta‐analyses included fewer than 10 studies, this was not applicable.
Data synthesis
We entered data into the Review Manager 5 software provided by Cochrane (Review Manager 2020; RevMan Web 2021). We performed analyses according to the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We performed a meta‐analysis if two or more comparable studies were identified. If this was not the case, we summarised results descriptively. For outcomes where only one study was available and we were unable to calculate a RR as one of the treatment groups experienced no events, we used Fischer's exact test instead (www.graphpad.com/quickcalcs/contingency1.cfm).
Subgroup analysis and investigation of heterogeneity
We planned to analyse data separately for children and adults and different types of tumour (i.e. leukaemia and solid tumours) if there were a sufficient number of trials of adequate size. However, this was not possible for different tumour types, as all adult participants were diagnosed with a solid tumour and data available for children were limited.
Sensitivity analysis
For all outcomes for which pooling was possible, we performed sensitivity analyses for all risk of bias items separately (i.e. excluding studies with a high risk of bias and studies for which the risk of bias was unclear, and comparing the results of studies with a low risk of bias with the results of all available studies; we only performed sensitivity analyses if at least two studies remained in the analysis after exclusion of the studies with a high or unclear risk of bias).
Summary of findings and assessment of the certainty of the evidence
We prepared summary of findings tables based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021), and using GRADEpro software (GRADEpro GDT). We presented the following outcomes: heart failure, OS, PFS, tumour response rate, QoL and secondary malignant neoplasms (SMN). Two review authors independently assessed the quality of the evidence (i.e. very low, low, moderate or high quality) for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which takes into account study limitations (risk of bias), inconsistency, indirectness, imprecision and publication bias.
Results
Description of studies
Results of the search
At the start of the third update, we split the original review to address dexrazoxane separately. Consequently, the search results below only discuss studies on dexrazoxane.
Up to and including the second update, we included 10 studies that addressed dexrazoxane: DFCI 95‐01 (study ID was Lipshultz 2004 in the 2011 review update); Galetta 2005; Lopez 1998; Marty 2006; P9425 (study ID was Schwartz 2009 in the 2011 review update); Speyer 1992; Swain 1997a(088001); Swain 1997a(088006); Venturini 1996; Wexler 1996. An overview of the full search results and study flow for the second review update can be found in Van Dalen 2011 and in Appendix 5.
For the third update, our searches in CENTRAL, MEDLINE and Embase yielded 564 records. After removing duplicates, we screened the titles or abstracts (or both) of 548 records. We excluded 524 records as they clearly did not meet the inclusion criteria. We obtained the remaining 24 full‐text articles and assessed these for inclusion. We identified three new studies (six publications) eligible for inclusion: P9404, P9426, and Sun 2016. Of the remaining 18 publications, five described five new studies that did not meet the eligibility criteria for inclusion in the review. We added seven publications (six studies) to the studies awaiting classification, either because they were conference abstracts, ongoing trial registry entries of studies for which some preliminary results are already available in conference abstracts (but no full‐text publications are available yet) or they are awaiting translation. The final six publications were associated with included studies; we collated these with their respective studies.
We identified no additional eligible studies after scanning the reference lists of relevant articles and conference proceedings. We identified errata for two already included studies (P9425; Speyer 1992). Furthermore, we checked (26 May 2021) if new information was available on the studies listed in the Characteristics of ongoing studies and the Characteristics of studies awaiting classification tables in the second update of this review. For two of the three ongoing studies previously listed, results were now available and identified in the electronic database searches of this update. Therefore, only one ongoing study remains (Characteristics of ongoing studies). For the studies awaiting classification, no new information was available. Finally, cardiac data became available for the P9426 study, so we could include long‐term follow‐up data on other outcomes for the third update (Tebbi 2007; previously excluded).
In order to comply with Cochrane policy, 12 publications labelled as 'excluded studies' in the previous versions of this review, which were associated with various included studies, are now collated with their respective included studies.
In summary, we included a total of 13 studies in the third update of this systematic review. See Figure 1 for a flow diagram of the selection of studies.
Included studies
Of the 13 included RCTs, seven RCTs addressed dexrazoxane solely in adults (Galetta 2005; Marty 2006; Speyer 1992; Sun 2016; Swain 1997a(088001); Swain 1997a(088006); Venturini 1996), four RCTs investigated the effects of dexrazoxane solely in children (DFCI 95‐01; P9404; P9425; P9426), and two RCTs included both children and adults (Lopez 1998; Wexler 1996). We categorised the study of Wexler 1996 as paediatric since the age at diagnosis was maximum 24 years (range 4 to 24). We included the study of Lopez 1998 in the adult category as the median age at diagnosis was 50+ years (range 14 to 75). The same study group conducted three of the studies: P9404 investigated leukaemia and non‐Hodgkin lymphoma; P9425 investigated intermediate‐ and high‐risk Hodgkin lymphoma; and P9426 investigated low‐risk Hodgkin lymphoma. The Swain studies both investigated dexrazoxane for women with breast cancer but investigated different stages of disease and applied different treatments.
The baseline characteristics of the participants in these studies are summarised below; more detailed information can be found in the Characteristics of included studies table.
Adults
The total number of participants in the eight adult studies was 1269 (622 in the dexrazoxane groups and 647 in the control groups). In five studies, the control groups did not receive a cardioprotective intervention (N = 327) and in three studies, the control group received a placebo (N = 340) (Sun 2016; Swain 1997a(088001); Swain 1997a(088006)). All participants were diagnosed with a solid tumour of which the majority had advanced breast cancer. Participants were treated with doxorubicin in three studies (Speyer 1992; Swain 1997a(088001); Swain 1997a(088006)), with epirubicin in four studies (Galetta 2005; Lopez 1998; Sun 2016; Venturini 1996), and with either epirubicin or doxorubicin in one study (Marty 2006). The ratio of dexrazoxane to anthracycline dose varied between studies and was ether 6.25:1, 10:1 or 20:1. In four studies, adults in the dexrazoxane groups and control groups received comparable cumulative anthracycline doses (Lopez 1998; Marty 2006; Sun 2016; Venturini 1996); in one study, the mean cumulative anthracycline was 150 mg/m2 higher in thedexrazoxane group compared to the control group (Speyer 1992); and in three studies, it was unclear whether cumulative anthracycline doses were comparable (Galetta 2005; Swain 1997a(088001); Swain 1997a(088006)).
Children
The total number of participants in the five paediatric studies was 1252 (632 in the dexrazoxane groups and 620 in the control groups). None of the children in the control groups received a cardioprotective intervention or placebo. One study included children with a solid tumour, including a Ewing sarcoma family tumour (Wexler 1996). Two studies included children with Hodgkin lymphoma (P9425; P9426). One study included children with leukaemia (DFCI 95‐01), and another study included children with leukaemia or non‐Hodgkin lymphoma (P9404). All studies used doxorubicin for cancer treatment. The ratio of dexrazoxane to anthracycline dose varied between studies and was either 10:1 (DFCI 95‐01; P9404; P9425; P9426), or 20:1 (Wexler 1996). In two studies, it was unclear if children in the intervention and control groups received similar cumulative anthracycline doses (DFCI 95‐01; P9425). In two studies, the cumulative anthracycline dose was not mentioned, but it was either stated that all children received the same cumulative dose (P9404), or that the received dose was in high compliance with the prescribed dose (P9426). In one study, the median cumulative anthracycline dose was 100 mg/m2 higher in thedexrazoxane group as compared to the control group (Wexler 1996).
Excluded studies
In this review update, there are eight excluded studies (Getz 2019; Li 2013; Massida 1997; Neto 2006; Paiva 2005; Rabinovich 2012; Tap 2019; Wang 2020). The primary reasons for exclusion were: ineligible study design (three studies); ineligible intervention or control (three studies); and ineligible outcome measurement (e.g. no cardiac outcomes or cardiac function not measured by echocardiography or radionuclide ventriculography).
Risk of bias in included studies
See the risk of bias section of the Characteristics of included studies table and Figure 2 for detailed judgements of risk of bias for each included study and the support for the judgements made.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study (+ = low risk of bias, ‐ = high risk of bias, ? = unclear risk of bias)
Allocation
For evaluating selection bias, we assessed random sequence generation and allocation concealment.
Adults
Two studies applied both random sequence generation and concealed treatment allocation, and thus we assessed the risk of selection bias as low (Swain 1997a(088001); Swain 1997a(088006)). For the six remaining studies in adults, the risk of selection bias was unclear: in three studies, both random sequence generation and allocation concealment were unclear (Galetta 2005; Lopez 1998; Speyer 1992); in one study, random sequence generation was applied, but allocation concealment was unclear (Sun 2016); and in two studies, treatment allocation was concealed, but random sequence generation was unclear (Marty 2006; Venturini 1996).
Children
One study applied both random sequence generation and concealed treatment allocation, and thus we assessed the risk of selection bias as low (DFCI 95‐01). For the four remaining studies in children, the risk of selection bias was unclear: in three studies, both random sequence generation and allocation concealment were unclear (P9404; P9425; P9426); and in one study, random sequence generation was applied, but allocation concealment was unclear (Wexler 1996).
Blinding
For evaluating performance bias, we assessed blinding of participants and personnel. For evaluating detection bias, we scored blinding of outcome assessors separately for all outcomes with the exception of overall survival/overall mortality and adverse effects other than cardiac damage and diagnosed by laboratory tests. Since blinding is not relevant for these outcomes, we judged the risk of bias as low. Not all studies assessed all outcomes.
Adults
The risk of performance bias was low in two studies (Swain 1997a(088001); Swain 1997a(088006)), high in five studies (Galetta 2005; Lopez 1998; Marty 2006; Speyer 1992; Venturini 1996), and unclear in one study (Sun 2016). For clinical heart failure, the risk of detection bias was low in five studies (Marty 2006; Speyer 1992; Swain 1997a(088001); Swain 1997a(088006); Venturini 1996), and unclear in two studies (Lopez 1998; Sun 2016). For clinical heart failure and subclinical myocardial dysfunction combined, the risk of detection bias was low in five studies (Marty 2006; Speyer 1992; Swain 1997a(088001); Swain 1997a(088006); Venturini 1996), and unclear in one study (Lopez 1998). For tumour response rate, the risk of detection bias was low in two studies (Swain 1997a(088001); Swain 1997a(088006)), and unclear in four studies (Lopez 1998; Marty 2006; Speyer 1992; Venturini 1996). For progression‐free survival (PFS), the risk of detection bias was low in two studies (Swain 1997a(088001); Swain 1997a(088006)), and unclear in two studies (Marty 2006; Speyer 1992). For adverse effects other than cardiac damage and those not diagnosed by a laboratory test, the risk of detection bias was low in two studies (Swain 1997a(088001); Swain 1997a(088006)), and unclear in five studies (Lopez 1998; Marty 2006; Speyer 1992; Sun 2016; Venturini 1996).
Children
The risk of performance bias was high in all five studies. For clinical heart failure, the risk of detection bias was low in one study (DFCI 95‐01), and unclear in two studies (P9404; P9425). For cardiomyopathy/heart failure as primary cause of death, the risk of detection bias was low in all studies assessing this outcome (P9404; P9425; P9426). For tumour response rate, the risk of detection bias was low in one study (DFCI 95‐01), and unclear in the other study (P9425). For clinical heart failure and subclinical myocardial dysfunction combined (P9404; Wexler 1996), and adverse effects other than cardiac damage and those not diagnosed by laboratory tests (DFCI 95‐01; P9404; P9425; P9426), the risk of detection bias was unclear in all studies assessing these outcomes.
Incomplete outcome data
For evaluating attrition bias, we assessed incomplete outcome data for all outcomes separately. A maximum of 10% of participants with missing data in each treatment arm was acceptable. Not all outcomes were assessed by all studies.
Adults
We assessed the risk of attrition bias as low for clinical heart failure in all studies addressing the outcome (Lopez 1998; Marty 2006; Speyer 1992; Sun 2016; Swain 1997a(088001); Swain 1997a(088006); Venturini 1996). For clinical heart failure and subclinical myocardial dysfunction combined, the risk of attrition bias was low in four studies (Lopez 1998; Marty 2006; Swain 1997a(088001); Swain 1997a(088006)), high in one study (Venturini 1996), and unclear in one study (Speyer 1992). For overall survival (OS), the risk of attrition bias was high in one study (Marty 2006), and unclear in three studies (Speyer 1992; Swain 1997a(088001); Swain 1997a(088006)). For tumour response rate, the risk of attrition bias was low for three studies (Lopez 1998; Speyer 1992; Venturini 1996), and high for three studies (Marty 2006; Swain 1997a(088001); Swain 1997a(088006)). For PFS, the risk of attrition bias was low in one study (Marty 2006), and unclear in three studies (Speyer 1992; Swain 1997a(088001); Swain 1997a(088006)). For toxicities other than cardiac damage, the risk of attrition bias was low in five studies (Lopez 1998; Marty 2006; Speyer 1992; Sun 2016; Venturini 1996), and unclear in two studies (Swain 1997a(088001); Swain 1997a(088006)).
Children
For clinical heart failure, we assessed the risk of attrition bias as low in two studies (P9404; P9425), and high in one study (DFCI 95‐01). The risk of attrition bias was high for clinical heart failure and subclinical myocardial dysfunction combined in both studies addressing this outcome (P9404; Wexler 1996). The risk of attrition bias was low for cardiomyopathy/heart failure as primary cause of death (P9404; P9425; P9426), overall mortality (P9404; P9425; P9426), and secondary malignant neoplasms (SMN) (DFCI 95‐01; P9404; P9425; P9426). For tumour response rate, the risk of attrition bias was low in one study (P9425), and unclear in the other study (DFCI 95‐01). For toxicities other than cardiac damage with the exception of SMN, the risk of attrition bias was low in two studies (P9404; P9425), and high in one study (P9426).
Selective reporting
For evaluating reporting bias, we assessed selective reporting. The predefined expected outcomes were cardiotoxicity (clinical, asymptomatic or both) and overall survival.
Adults
We assessed the risk of reporting bias as low in six studies (Lopez 1998; Marty 2006; Speyer 1992; Swain 1997a(088001); Swain 1997a(088006); Venturini 1996), and high in two studies (Galetta 2005; Sun 2016). For Galetta 2005, it should be noted that the primary objective of this study was to assess QT‐dispersion on electrocardiogram (ECG), not to assess heart failure.
Children
We assessed the risk of reporting bias as low in four studies (P9404; P9425; P9426; Wexler 1996), and high in one study (DFCI 95‐01).
Other potential sources of bias
For evaluating other potential sources of bias, we assessed the following items: block randomisation in unblinded trials, baseline imbalance between treatment groups related to outcome (prior cardiotoxic treatment (anthracyclines and cardiac irradiation), age, gender, stage of disease and prior cardiac dysfunction) and different lengths of follow‐up between treatment arms.
Adults
The risk of other potential sources of bias was unclear for all included studies. For a detailed description of the different items, see the risk of bias section of the Characteristics of included studies table.
Children
The risk of other potential sources of bias was unclear for all included studies. For a detailed description of the different items, see the risk of bias section of the Characteristics of included studies table.
Effects of interventions
Not all articles allowed data extraction for all endpoints (see the Characteristics of included studies table for detailed descriptions of the extractable endpoints in each study).
Clinical heart failure
Adults
We could extract data on clinical heart failure from seven studies with a total of 1249 participants (Lopez 1998; Marty 2006; Speyer 1992; Sun 2016; Swain 1997a(088001); Swain 1997a(088006); Venturini 1996). The available‐case analysis (1221 participants) showed a benefit in favour of dexrazoxane treatment (RR 0.22, 95% CI 0.11 to 0.43; P < 0.001; moderate‐quality evidence; Analysis 1.1; Table 1; Figure 3); there were 11 cases among the 596 available participants in the dexrazoxane group and 67 cases among the 625 control participants. The relative effect of Sun 2016 was not estimable for the meta‐analysis since none of the participants developed clinical heart failure. Intention‐to‐treat (ITT) analyses (1249 participants) showed a comparable difference between the treatment groups: the RR for the best‐case scenario (i.e. 11 cases among 612 participants in the dexrazoxane group and 79 cases among 637 participants in the control group) was 0.22 (95% CI 0.11 to 0.43; P < 0.001; moderate‐quality evidence; Analysis 1.2). The RR for the worst‐case scenario (i.e. 27 cases among 612 participants in the dexrazoxane group and 79 cases among 637 participants in the control group) was 0.42 (95% CI 0.21 to 0.84; P = 0.01; moderate‐quality evidence; Analysis 1.3). Unexplained significant heterogeneity (I2 = 52%) appeared in this analysis.
1.1. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 1: Clinical heart failure available‐case
3.

Forest plot of comparison: 1 Dexrazoxane versus no dexrazoxane or placebo, outcome: 1.1 Clinical heart failure available‐case.
1.2. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 2: Clinical heart failure best‐case
1.3. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 3: Clinical heart failure worst‐case
Children
We could extract data on clinical heart failure from three studies with a total of 885 participants (DFCI 95‐01; P9404; P9425). The available‐case analysis of clinical heart failure showed no difference between the treatment groups (RR 0.20, 95% CI 0.01 to 4.19; P = 0.30; low‐quality evidence; Analysis 1.1; Table 2; Figure 3). There were zero cases among the 447 available participants in the dexrazoxane group and two cases among the 438 available control participants. The relative effects of DFCI 95‐01 and P9404 were not estimable for the meta‐analysis since none of the participants developed clinical heart failure. ITT analyses (959 participants) also showed no difference between the treatment groups: the RR for the best‐case scenario (no cases among 485 participants in the dexrazoxane group and 2 cases among 474 control participants) was 0.20 (95% CI 0.01 to 4.19; P = 0.30; low‐quality evidence; Analysis 1.2). The relative effects of DFCI 95‐01 and P9404 were not estimable, again as a result of zero events in both treatment groups. The RR for the worst‐case scenario (i.e. 38 cases among 485 participants in the dexrazoxane group and 38 cases among 474 participants in the control group) was 0.99 (95% CI 0.68 to 1.43; P = 0.95; low‐quality evidence; Analysis 1.3). The relative effect of P9404 was not estimable as a result of zero events in both treatment groups.
We excluded the study of Wexler 1996 from this analysis since, in this study, it was not possible to separate cases of clinical heart failure and subclinical myocardial dysfunction.
Cardiomyopathy/heart failure as primary cause of death
Adults
The outcome cardiomyopathy/heart failure as primary cause of death was not assessed in any of the studies with adults.
Children
We could extract data on cardiomyopathy/heart failure as primary cause of death from three studies with a total of 1008 participants (P9404; P9425; P9426). Since all studies reported zero events in both the dexrazoxane group (507 participants) and control group (501 participants), the relative effect was not estimable in the available‐case analysis (low‐quality evidence; Analysis 1.4; Table 2). ITT analyses (best‐case and worst‐case) showed identical results.
1.4. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 4: Cardiomyopathy/heart failure primary cause of death available‐case (best‐case and worst‐case identical results)
In these three studies, two participants (both from the control group; as results were provided only for the three studies combined (P9404; P9425; P9426), it is not known from which individual study these children came) died as a result of cardiomyopathy/heart failure listed as a secondary cause of death. No difference was identified (data not shown): RR 0.20 (95% CI 0.01 to 4.11; P = 0.29).
Heart failure (that is, clinical heart failure and subclinical myocardial dysfunction combined)
We split the analysis of heart failure (that is, clinical heart failure and subclinical myocardial dysfunction combined) into separate analyses with comparable definitions because the definitions used in the included studies were too different to pool them all together. See Characteristics of included studies for exact definitions.
Adults
Data on heart failure could be extracted from four studies using comparable definitions (Lopez 1998; Marty 2006; Speyer 1992; Venturini 1996). The available‐case analysis was based on the results of Lopez 1998, Marty 2006 and Venturini 1996 with a total of 417 participants and showed a benefit for dexrazoxane treatment (RR 0.37, 95% CI 0.24 to 0.56; P < 0.001; moderate‐quality evidence; Analysis 1.5; Table 1; Figure 4); there were 24 cases among the 207 available participants in the dexrazoxane group and 66 cases among the 210 control participants. ITT analyses demonstrated the same benefit of dexrazoxane. The RR for the worst‐case scenario (i.e. 49 cases among 232 participants in the dexrazoxane group and 79 among 223 control participants; a total of 455 participants) was 0.60 (95% CI 0.42 to 0.86; P = 0.006; moderate‐quality evidence; Analysis 1.7). For the best‐case scenario the study of Speyer 1992 was added which resulted in a total of 605 participants. The RR of the best‐case scenario (i.e. 30 cases among 308 participants in the dexrazoxane group and 103 among 297 control participants) was 0.29 (95% CI 0.19 to 0.44; P < 0.001; moderate‐quality evidence; Analysis 1.6).
1.5. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 5: Heart failure (i.e. clinical heart failure and subclinical myocardial dysfunction combined) available‐case
4.

Forest plot of comparison: 1 Dexrazoxane versus no dexrazoxane or placebo, outcome: 1.5 Heart failure (i.e. clinical and subclinical heart failure combined) available‐case.
1.7. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 7: Heart failure (i.e. clinical heart failure and subclinical myocardial dysfunction combined) worst‐case
1.6. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 6: Heart failure (i.e. clinical heart failure and subclinical myocardial dysfunction combined) best‐case
Data on heart failure could be extracted from two other studies with a total of 534 participants using another comparable definition (Swain 1997a(088001); Swain 1997a(088006)). The available‐case analysis showed a benefit for dexrazoxane treatment (RR 0.46, 95% CI 0.33 to 0.66; P < 0.001; moderate‐quality evidence; Analysis 1.5; Table 1; Figure 4); there were 36 cases among the 249 available participants in the dexrazoxane group and 89 cases among the 285 control participants. ITT analyses demonstrated the same benefit of dexrazoxane: both the RR for the worst‐case scenario and for the best‐case scenario were identical to the available‐case analysis.
We excluded the study of Galetta 2005 because it did not evaluate clinical heart failure and therefore the results included only cases of subclinical myocardial dysfunction. We excluded the study of Sun 2016 from this analysis because it addressed only clinical heart failure.
It should be noted that participants from the studies of Lopez 1998, Marty 2006, Speyer 1992, Swain 1997a(088001), Swain 1997a(088006) and Venturini 1996 who suffered from clinical heart failure were also included in the meta‐analysis of clinical heart failure as mentioned above.
Children
Data on heart failure defined as (1) evidence of clinical congestive heart failure, (2) a reduction in left ventricular ejection fraction (LVEF) as measured by multigated acquisition scan (MUGA) to less than 45%, or (3) a decrease in LVEF as measured by MUGA of greater than 20 percentage points from baseline could be extracted from one study with a total of 33 participants (Wexler 1996). The available‐case analysis showed a benefit for dexrazoxane treatment (RR 0.33, 95% CI 0.13 to 0.85; P = 0.02; low‐quality evidence; Analysis 1.5; Table 2; Figure 4); there were 4 cases among the 18 available participants in the dexrazoxane group and 10 cases among the 15 control participants. ITT analyses showed similar results: the RR for the worst‐case scenario (i.e. 6 cases among 20 participants in the dexrazoxane group and 13 among 18 participants in the control group; total of 38 participants) was 0.42 (95% CI 0.20 to 0.86; P = 0.02; low‐quality evidence; Analysis 1.7), and the RR for the best‐case scenario (i.e. 4 cases among 20 participants in the dexrazoxane group and 10 cases among 18 control participants; total of 38 participants) was 0.36 (95% CI 0.14 to 0.95; P = 0.04; low‐quality evidence; Analysis 1.6).
Data on heart failure defined as clinical heart failure (no definition provided) or subclinical myocardial dysfunction defined as decreased left ventricular fractional shortening (LVFS) could be extracted from one study with a total of 537 participants (P9404). We were not able to calculate a RR since there was only study available in which one of the treatment groups experienced no events (zero cases among 273 participants in the dexrazoxane group and three cases among 264 participants in the control group). Therefore, we used Fischer's exact test instead (P = 0.12; very low‐quality evidence). Only a best‐case analysis could be performed because it was unclear how many participants were lost to follow‐up.
It should be noted that participants from the study of P9404 who suffered from clinical heart failure were also included in the meta‐analysis of clinical heart failure as mentioned above.
We excluded the study of P9425 since their results only include cases of clinical heart failure. In the study of DFCI 95‐01, the necessary information on the occurrence of subclinical myocardial dysfunction was not provided.
Overall survival (OS)
Adults
Data on OS could be extracted from four studies (Marty 2006; Speyer 1992; Swain 1997a(088001); Swain 1997a(088006)). Two studies (Swain 1997a(088001); Swain 1997a(088006)) presented HRs with 95% CIs, and the remaining two studies provided survival curves (Marty 2006; Speyer 1992).
The meta‐analysis showed no difference between the treatment groups (HR 1.04, 95% CI 0.88 to 1.23, P = 0.65; moderate‐quality evidence; Analysis 1.8; Table 1; Figure 5; number of participants included in the analysis unclear).
1.8. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 8: Overall survival
5.

Forest plot of comparison: 1 Dexrazoxane versus no dexrazoxane or placebo, outcome: 1.8 Overall survival.
We excluded the study of Venturini 1996 from this analysis since it did not include the two participants who did not receive any chemotherapy in the evaluation of survival. We excluded the study of Lopez 1998 from this analysis since we were not able to reliably extract data needed to use Parmar's method for the assessment of survival for this study. None of the excluded studies showed differences between the treatment groups.
Median overall survival durations of the individual studies are shown in Table 3. No differences between the treatment arms were found.
1. Survival (adults): dexrazoxane versus control treatment.
| Study | Median progression‐free survival* | Median overall survival |
| Marty 2006 | 7.8 months versus 7 months | 13.5 months versus 16 months |
| Speyer 1992 | 10.1 months versus 9.4 months | 18.3 months versus 16.7 months |
| Swain 1997a(088001) | 254 days versus 260 days | 598 days versus 551 days |
| Swain 1997a(088006) | 233 days versus 249 days | 458 days versus 553 days |
* Different definitions for progression‐free survival are used; see Characteristics of included studies for exact definition per study
Children
Data on OS could not be extracted from any of the studies in children.
We excluded the study of Wexler 1996 from this analysis since it was impossible to separate the three non‐randomised participants from the randomised participants in the dexrazoxane group. However, in this study, there was no significant difference in overall survival between the treatment groups. We excluded P9404 from this analysis since we were not able to reliably extract data needed to use Parmar's method for the assessment of overall survival. In addition, more long‐term follow‐up data on overall mortality were available.
Overall mortality
Adults
Overall mortality was not assessed in the studies in adults.
Children
Data on overall mortality could be extracted from three studies with 1008 participants in total (P9404; P9425; P9426). The included studies presented hazard rations (HRs) with 95% CIs. The meta‐analysis demonstrated no difference between the treatment groups (HR 1.01, 95% CI 0.72 to 1.42, P = 0.96; low‐quality evidence; Analysis 1.9; Table 2; Figure 6). Median overall survival durations for each individual study were not provided.
1.9. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 9: Overall mortality
6.

Forest plot of comparison: 1 Dexrazoxane versus no dexrazoxane or placebo, outcome: 1.9 Overall mortality.
Progression‐free survival
Adults
Data on PFS could be extracted from four studies (Marty 2006; Speyer 1992; Swain 1997a(088001); Swain 1997a(088006)). The Swain 1997a(088001) and Swain 1997a(088006) studies presented HRs with 95% CIs and the other two studies provided survival curves (Marty 2006; Speyer 1992).
As not all studies used comparable definitions of progression‐free survival, we split this analysis into three separate analyses. See Characteristics of included studies for exact definitions.
The study of Marty 2006 assessed PFS in 164 participants and defined it as time from first date of complete response, partial response or stable disease until the date progressive disease was first noticed. The analysis showed a difference in favour of dexrazoxane treatment (HR 0.62, 95% CI 0.43 to 0.90; P = 0.01; low‐quality evidence; Analysis 1.10; Table 1; Figure 7).
1.10. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 10: Progression‐free survival
7.

Forest plot of comparison: 1 Dexrazoxane versus no dexrazoxane or placebo, outcome: 1.10 Progression‐free survival.
The study of Speyer 1992 defined PFS as time to progression; however, they did not mention the starting point nor the number of participants assessed. In this analysis, there was no difference between the treatment groups (HR 0.95, 95% CI 0.64 to 1.40; P = 0.80; low‐quality evidence; Analysis 1.10; Table 1; Figure 7).
The Swain 1997a(088001) and Swain 1997a(088006) studies defined PFS as time from randomisation to progression either on or off treatment. It was unclear how many participants were assessed for PFS in these studies. The analysis demonstrated no difference between the treatment groups (HR 1.18, 95% CI 0.97 to 1.43; P = 0.10; moderate‐quality evidence; Analysis 1.10; Table 1; Figure 7).
We excluded the study of Venturini 1996 from this analysis since it did not include the two participants who did not receive any chemotherapy in the evaluation of survival. We excluded the study of Lopez 1998 from this analysis since we were not able to reliably extract the data needed to use Parmar's method for the assessment of survival for this study. However, none of the excluded studies showed differences between the treatment arms.
Median progression‐free survival durations of the individual studies are shown in Table 3. No differences between the treatment arms were found.
Children
Data on PFS could not be extracted from any of the studies in children.
Tumour response rate
Tumour response rate was defined as the number of participants in complete and partial remission for adult studies and the number of participants in complete remission for paediatric studies. Please note that due to the nature of this measurement, a high event rate is favourable. Therefore, in the figure of this analysis 'favours control' is on the left and 'favours dexrazoxane' is on the right, as opposed to the figures for the other analyses.
Adults
We could extract data on tumour response rate from six studies with a total of 956 participants (Lopez 1998; Marty 2006; Speyer 1992; Swain 1997a(088001); Swain 1997a(088006); Venturini 1996). These studies used comparable criteria to assess tumour response rate. The studies Swain 1997a(088001) and Swain 1997a(088006)) included only participants with evaluable disease. The available‐case analysis demonstrated no difference between the treatment groups (RR 0.91, 95% CI 0.79 to 1.04; P = 0.16; moderate‐quality evidence; Analysis 1.11; Table 1; Figure 8); there were 223 complete and partial responses among 468 participants randomised to dexrazoxane and 260 among 488 randomised to the control group. ITT analyses (1021 participants) also showed no difference between the treatment groups: the RR for the worst‐case scenario (i.e. 223 cases among 503 participants in the dexrazoxane group and 260 cases among 518 participants in the control group) was 0.89 (95% CI 0.78 to 1.01; P = 0.07; moderate‐quality evidence; Analysis 1.13), and the RR for the best‐case scenario (i.e. 258 cases among 503 participants in the dexrazoxane group and 290 cases among 518 control participants) was 0.94 (95% CI 0.82 to 1.08; P = 0.37; moderate‐quality evidence; Analysis 1.12).
1.11. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 11: Response rate available‐case
8.

Forest plot of comparison: 1 Dexrazoxane versus no dexrazoxane or placebo, outcome: 1.11 Response rate available‐case.
1.13. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 13: Response rate worst‐case
1.12. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 12: Response rate best‐case
Children
We could extract data on tumour response rate from two studies. As no comparable definitions were used, we split this analysis into two separate analyses.
The DFCI 95‐01 study did not provide a definition of complete remission and only a best‐case analysis could be performed because it was unclear how many participants were lost to follow‐up. It demonstrated no difference between the treatment groups (RR 1.01, 95% CI 0.95 to 1.07; P = 0.69; very low‐quality evidence; Analysis 1.12; Table 2); there were 101 complete remissions among 105 participants randomised to dexrazoxane and 96 among 101 randomised to the control group.
The P9425 study defined complete response as disappearance of active Hodgkin lymphoma (gallium negative, ≥ 70% decrease in the sum of the products of the perpendicular diameters of measurable lesions, and negative bone marrow or bone scan if initially positive). The available‐case analysis demonstrated no difference between the treatment groups (RR 0.92, 95% CI 0.84 to 1.01; P = 0.07; low‐quality evidence; Analysis 1.11; Table 2; Figure 8); there were 87 complete responses among 101 participants randomised to dexrazoxane and 93 among 99 randomised to the control group. ITT analyses also showed no difference between the treatment groups: the RR for the worst‐case scenario (i.e. 87 cases among 107 participants in the dexrazoxane group and 93 cases among 109 participants in the control group) was 0.95 (95% CI 0.85 to 1.07 to 1.01, P = 0.43; low‐quality evidence; Analysis 1.13), and the RR for the best‐case scenario (i.e. 93 cases among 107 participants in the dexrazoxane group and 103 cases among 109 control participants) was 0.92 (95% CI 0.84 to 1.00; P = 0.06; low‐quality evidence; Analysis 1.12).
We excluded the study of Wexler 1996 from this analysis since it was impossible to separate the three non‐randomised participants from the randomised participants in the dexrazoxane group.
Quality of life (QoL)
None of the studies evaluated QoL.
Adverse effects
Since all participants receiving chemotherapy will suffer from side effects, we decided to analyse only the severe and life‐threatening effects. For studies using the Eastern Cooperative Oncology Group (ECOG) (Oken 1982), World Health Organization (WHO) (Miller 1981), or National Cancer Institute (NCI) Common Toxicity Criteria (CTC), currently known as Common Terminology Criteria for Adverse Events (CTCAE) (for different versions, see: ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm), we defined this as grade 3 (severe) or grade 4 (life‐threatening); for the study of Speyer 1992 we excluded the two lowest grades reported. For studies that did not provide definitions we used severe cases (Sun 2016), or all cases (P9426). Secondary malignant neoplasm (SMN) was considered as a severe side effect irrespective of the availability of an exact definition. We classified the adverse effects based on the (organ) system involved. It was possible to perform meta‐analyses for adverse effects for which more than one RCT was available. For adverse effects for which only one RCT was available, we provide descriptive results (all RRs, 95% CIs and P values mentioned below are calculated in Review Manager 5 with the random‐effects model, unless stated otherwise). The timing and frequency of the evaluation of the side effects in the different studies was not clear. Not all studies addressed all adverse effects. For results not included as a figure, see Analysis 1.14, Analysis 1.15, Analysis 1.16, Analysis 1.17, Analysis 1.18, Analysis 1.19, Analysis 1.20, Analysis 1.21, Analysis 1.22, Analysis 1.23 and Analysis 1.24 for more detailed information.
1.14. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 14: Adverse effects: Secondary malignant neoplasms (Children)
1.15. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 15: Adverse effects: Haematological effects (Adults)
1.16. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 16: Adverse effects: Haematological effects (Children)
1.17. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 17: Adverse effects: Immune system/infectious effects (Adults)
1.18. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 18: Adverse effects: Immune system/infectious effects (Children)
1.19. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 19: Adverse effects: Gastrointestinal effects (Adults)
1.20. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 20: Adverse effects: Gastrointestinal effects (Children)
1.21. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 21: Adverse effects: Neurological effects (Adults)
1.22. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 22: Adverse effects: Neurological (Children)
1.23. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 23: Adverse effects: Other (Adults)
1.24. Analysis.

Comparison 1: Dexrazoxane versus no dexrazoxane or placebo, Outcome 24: Adverse effects: Other (Children)
Adults
Data on adverse effects could be extracted from seven studies: Lopez 1998 and Venturini 1996 used the WHO criteria; Swain 1997a(088001) and Swain 1997a(088006) used the ECOG criteria, and Marty 2006 used the CTC (version 2). The study of Speyer 1992 provided definitions of the different adverse effects used in the study without a reference. Sun 2016 did not provide definitions.
Children
Data on adverse effects could be extracted from four RCTs: P9404 and P9425 used the CTCAEv2.0. For the studies of DFCI 95‐01 and P9426, no definitions were provided. We excluded the study of Wexler 1996 from this analysis since this study did not report the number of participants having suffered an adverse effect.
Secondary malignant neoplasm (SMN)
Adults
SMN was not assessed in the studies with adults.
Children
Data could be extracted from four studies (DFCI 95‐01; P9404; P9425; P9426). The available‐case analysis was based on the results of P9404, P9425 and P9426 with a total of 1015 participants and showed a difference in favour of the control group (RR 3.08, 95% CI 1.13 to 8.38; P = 0.03; low‐quality evidence; Analysis 1.14; Table 2; Figure 9). There were 16 cases of SMN among the 512 available participants in the dexrazoxane group and 5 cases among the 503 control participants. ITT analyses demonstrated the following results: the results for the worst‐case scenario were identical to the available‐case analysis. For the best‐case scenario, the study of DFCI 95‐01 could be added which resulted in a total of 1220 participants. The results of the best‐case scenario (i.e. 16 cases among 617 participants in the dexrazoxane group and 6 cases among the 607 participants in the control group) showed the same direction of effect, but now the result was not different between the treatment groups (RR 2.51, 95% CI 0.96 to 6.53; P = 0.06; low‐quality evidence).
9.

Forest plot of comparison: 1 Dexrazoxane versus no dexrazoxane or placebo, outcome: 1.14 Adverse effects: Secondary malignant neoplasms (Children).
In the dexrazoxane group, there were seven cases with acute myeloid leukaemia (AML), five cases with brain tumours, two cases with papillary carcinoma, one case with osteosarcoma and one case with myelodysplastic syndrome. In the control group, there were three cases with AML, one case with myeloid sarcoma, one case with lymphoma and one case with melanoma (see Table 4 for more information).
2. Overview of secondary malignant neoplasm (SMN) cases and their primary tumours in the dexrazoxane and control groups.
| Dexrazoxane group | Control group | ||||
| Primary tumour | SMN | Primary tumour | SMN | ||
| 1 | HL | MDS | 1 | HL | AML |
| 2 | HL | Papillary carcinoma | 2 | HL | AML |
| 3 | HL | AML | 3 | ALL | Melanoma |
| 4 | HL | AML | 4 | L‐NHL | Myeloid sarcoma |
| 5 | HL | AML | 5 | T‐ALL + CNS | AML |
| 6 | HL | AML | 6 | T‐ALL | DLBL |
| 7 | HL | AML | |||
| 8 | HL | Osteosarcoma | |||
| 9 | T‐ALL | AML | |||
| 10 | T‐ALL | AML | |||
| 11 | L‐NHL | Astrocytoma | |||
| 12 | L‐NHL | Astrocytoma | |||
| 13 | T‐ALL | Glioblastoma multiforme | |||
| 14 | T‐ALL + CNS | Glioblastoma multiforme | |||
| 15 | T‐ALL | Medulloblastoma | |||
| 16 | L‐NHL | Papillary carcinoma ‐ thyroid | |||
P9426: numbers 1 to 5 of the dexrazoxane group and number 1 of the control group P9425: numbers 6 to 8 of the dexrazoxane group and number 2 of the control group DFCI 95‐01: number 3 of the control group P9404: numbers 9 to 16 of the dexrazoxane group and 4 to 6 of the control group
ALL: acute lymphoblastic leukaemia AML: acute myeloid leukaemia CNS: central nervous system DLBL: diffuse large B‐cell lymphoma DOX: doxorubicin ETOP: etoposide HDM: high‐dose methotrexate HL= Hodgkin lymphoma L‐NHL: lymphoblastic non‐Hodgkin lymphoma MDS: myelodysplastic syndrome RT: radiotherapy SMN: secondary malignant neoplasm T‐ALL: T‐cell acute lymphoblastic leukaemia
Haematological effects
Adults
Thrombocytopenia
Data on thrombocytopenia (defined as grade 3 or 4 according to WHO or the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events, version 2 (CTCAEv2) criteria, which were comparable) could be extracted from three studies with a total of 452 participants (Lopez 1998; Marty 2006; Venturini 1996). The available‐case analysis showed no difference between the treatment groups (RR 1.03, 95% CI 0.48 to 2.20; P = 0.94). There were 11 cases among the 229 available participants in the dexrazoxane group and 11 cases among the 223 participants in the control group. The relative effects of Venturini 1996 were not estimable for the meta‐analysis since none of the participants developed thrombocytopenia. ITT analyses demonstrated comparable results (455 participants). For more details, see Analysis 1.15.
Neutropenia
Data on neutropenia (defined as grade 3 or 4 according to WHO or CTCAEv2 criteria, which were comparable) could be extracted from two studies with a total of 292 participants (Lopez 1998; Marty 2006). The available‐case analysis showed no difference between the treatment groups (RR 1.05, 95% CI 0.96 to 1.15; P = 0.32). There were 91 cases among the 147 available participants in the dexrazoxane group and 88 cases among the 145 participants in the control group. ITT analyses demonstrated comparable results (293 participants). For more details, see Analysis 1.15.
Abnormal granulocyte count at nadir
Data on abnormal granulocyte count at nadir (defined as grade 3 or 4 according to ECOG criteria) could be extracted from two studies (Swain 1997a(088001); Swain 1997a(088006)). Only the best‐case scenario (i.e. 221 cases among the 249 participants in the dexrazoxane group and 244 cases among the 285 in the control group; total of 534 participants) was analysed since the number of missing data was unclear. The results showed no difference between the treatment groups (RR 1.04, 95% CI 0.96 to 1.13; P = 0.29). For more details, see Analysis 1.15.
Abnormal granulocyte count at recovery
Data on abnormal granulocyte count at recovery (defined as grade 3 or 4 according to ECOG criteria) could be extracted from two studies (Swain 1997a(088001); Swain 1997a(088006)). Only the best‐case scenario (i.e. 42 cases among the 249 participants in the dexrazoxane group and 57 cases among the 285 in the control group; total of 534 participants) was analysed since the number of missing data was unclear. The results showed no difference between the treatment groups (RR 0.85, 95% CI 0.59 to 1.21; P = 0.36). For more details, see Analysis 1.15.
Abnormal white blood cell count at nadir
Data on abnormal white blood cell count at nadir (defined as grade 3 or 4 according to ECOG criteria) could be extracted from two studies with a total of 534 participants (Swain 1997a(088001); Swain 1997a(088006)). Only the best‐case scenario (195 cases among the 249 participants in the dexrazoxane group and 193 cases among the 285 in the control group; total of 534 participants) was analysed since the number of missing data was unclear. The results showed a difference in favour of the control treatment (RR 1.16, 95% CI 1.05 to 1.29; P = 0.004). For more details, see Analysis 1.15.
Abnormal white blood cell count at recovery
Data on abnormal white blood cell count at recovery (defined as grade 3 or 4 according to ECOG criteria) could be extracted from two studies (Swain 1997a(088001); Swain 1997a(088006)). Only the best‐case scenario (i.e. 14 cases among the 249 participants in the dexrazoxane group and 23 cases among the 285 in the control group; total of 534 participants) was analysed since the number of missing data was unclear. The results showed no difference between the treatment groups (RR 0.69, 95% CI 0.36 to 1.31; P = 0.26). For more details, see Analysis 1.15.
Abnormal platelet count at nadir
Data on abnormal platelet count at nadir (defined as grade 3 or 4 according to ECOG criteria) could be extracted from two studies (Swain 1997a(088001); Swain 1997a(088006)). Only the best‐case scenario (i.e. 21 cases among the 249 participants in the dexrazoxane group and 26 cases among the 285 in the control group; total of 534 participants) was analysed since the number of missing data was unclear. The results showed no difference between the treatment groups (RR 0.88, 95% CI 0.42 to 1.84; P = 0.73). For more details, see Analysis 1.15.
Abnormal platelet count at recovery
Data on abnormal platelet count at recovery (defined as grade 3 or 4 according to ECOG criteria) could be extracted from two studies (Swain 1997a(088001); Swain 1997a(088006)). Only the best‐case scenario (i.e. two cases among the 249 participants in the dexrazoxane group and 3 cases among the 285 in the control group; total of 534 participants) was analysed since the number of missing data was unclear. The results showed no difference between the treatment groups (RR 0.84, 95% CI 0.16 to 4.42; P = 0.83). For more details, see data and Analysis 1.15.
Anaemia
Data on anaemia (defined as grade 3 or 4 according to WHO or CTCAEv2 criteria, which were comparable) could be extracted from three studies with a total of 452 participants (Lopez 1998; Marty 2006; Venturini 1996). The available‐case analysis showed no difference between the treatment groups (RR 1.37, 95% CI 0.79 to 2.39; P = 0.26). There were 27 cases among the 229 available participants in the dexrazoxane group and 19 cases among the 223 participants in the control group. ITT analyses demonstrated comparable results (455 participants). For more details, see Analysis 1.15.
Myelosuppression
Data on severe myelosuppression (definition not provided) could be extracted from one study with a total of 108 participants (Sun 2016). The available‐case analysis showed no difference between the treatment groups (RR 2.00, 95% CI 0.19 to 21.41; P = 0.57). There were two cases among the 54 available participants in the dexrazoxane group and one among the 54 participants in the control group. ITT analyses demonstrated comparable results (110 participants). For more details, see Analysis 1.15.
Leukopenia
Data on leukopenia (defined as grade 3 or 4 according to WHO or CTCAEv2 criteria, which were comparable) could be extracted from two studies with a total of 324 participants (Marty 2006; Venturini 1996). The available‐case analysis showed no difference between the treatment groups (RR 1.10, 95% CI 0.66 to 1.83; P = 0.71). There were 27 cases among the 167 available participants in the dexrazoxane group and 23 cases among the 157 participants in the control group. ITT analyses demonstrated comparable results (326 participants). For more details, see Analysis 1.15.
Children
Lymphocytes
Data on lymphocytes (no definition provided) could be extracted from one study with a total of 222 participants (P9426). The available‐case analysis showed no difference between the treatment groups (RR 1.04, 95% CI 0.07 to 16.37; P = 0.98). There was one case among the 109 available participants in the dexrazoxane group and one case among the 113 participants in the control group. ITT analyses demonstrated comparable results (225 participants). For more details, see Analysis 1.16.
Haemoglobin
Data on haemoglobin could be extracted from two studies (P9425; P9426); however, we analysed the studies separately because P9426 did not provide a definition for haemoglobin. P9425 used grade 3 or 4 according to the CTCAEv2 criteria. In both studies, the available‐case analysis demonstrated a difference in favour of the control group: for P9426, the RR was 2.96 (95% CI 1.31 to 6.72; P = 0.009), there were 20 cases among the 109 available participants in the dexrazoxane group and 7 cases among the 113 participants in the control group (222 participants in total); for P9425, the RR was 1.48 (95% CI 1.13 to 1.95; P = 0.005), there were 64 cases among the 106 available participants in the dexrazoxane group and 44 cases among the 108 participants in the control group (214 participants in total). ITT analyses demonstrated comparable results for both P9426 (255 participants) and P9425 (216 participants). For more details, see Analysis 1.16.
White blood cell count
Data on white blood cell count (no definition provided) could be extracted from one study with a total of 222 participants (P9426). The available‐case analysis showed a difference in favour of the control group (RR 1.87, 95% CI 1.30 to 2.68; P < 0.001). There were 54 cases among the 109 available participants in the dexrazoxane group and 30 cases among the 113 participants in the control group. ITT analyses demonstrated comparable results (255 participants). For more details, see Analysis 1.16.
Thrombosis
Data on thrombosis (defined as grade 3 or 4 according to NCI CTCAEv2 criteria) could be extracted from one study with a total of 214 participants (P9425). The available‐case analysis demonstrated no difference between the treatment groups (RR 4.08, 95% CI 0.46 to 35.87; P = 0.21). There were four cases among the 106 available participants in the dexrazoxane group and one case among the 108 participants in the control group. ITT analyses demonstrated comparable results (216 participants). For more details, see Analysis 1.16.
Platelets
Data on platelets could be extracted from two studies (P9425; P9426); however, we analysed the studies separately because P9426 did not provide a definition for platelets. P9425 used grade 3 or 4 according to the NCI CTCAEv2 criteria. In the study of P9426, the available‐case analysis demonstrated no difference between the treatment groups (RR 1.87, 95% CI 0.90 to 3.86; P = 0.09). There were 18 cases among the 109 available participants in the dexrazoxane group and 10 cases among the 113 participants in the control group (222 participants in total). ITT analyses demonstrated comparable results (255 participants). For more details, see Analysis 1.16.
In the study of P9425, the available‐case analysis demonstrated a difference in favour of the control group (RR 2.45, 95% CI 1.79 to 3.35; P < 0.001). There were 77 cases among the 106 available participants in the dexrazoxane group and 33 cases among the 108 participants in the control group (214 participants in total). ITT analyses demonstrated comparable results (216 participants). For more details, see Analysis 1.16.
Absolute neutrophil count
Data on absolute neutrophil count could be extracted from two studies (P9425; P9426); however, we analysed the studies separately because P9426 did not provide a definition for absolute neutrophil count grade 3 or 4. P9425 used grade 3 or 4 according to CTCAEv2 criteria. In the study of P9426, the available‐case analysis demonstrated a difference in favour of the control group (RR 1.27, 95% CI 1.03 to 1.58; P = 0.02). There were 75 cases among the 109 available participants in the dexrazoxane group and 61 cases among the 113 participants in the control group (222 participants in total). ITT analyses (255 participants) demonstrated comparable results regarding the worst‐case scenario with a RR of 1.23 (95% CI 1.03 to 1.47; P = 0.02), but for the best‐case scenario there was no difference between the treatment groups with a RR of 1.24 (95% CI 0.98 to 1.56; P = 0.07).
In the study of P9425, the available‐case analysis demonstrated a difference in favour of the control group (RR 1.10, 95% CI 1.00 to 1.20; P = 0.04). There were 100 cases among the 106 available participants in the dexrazoxane group and 93 cases among the 108 participants in the control group (214 participants in total). ITT analyses demonstrated comparable results (216 participants). For more details, see Analysis 1.16.
Haematological effects
Data on haematological effects (defined as grade 3 or 4 according to CTCAEv2 criteria) could be extracted from one study with a total of 537 participants (P9404). The available‐case analysis showed no difference between the treatment groups (RR 0.99, 95% CI 0.94 to 1.05; P = 0.77). There were 243 cases among the 273 available participants in the dexrazoxane group and 237 cases among the 264 participants in the control group. ITT analyses demonstrated identical results since there were no missing data in this study.
Immune system/infectious effects
Adults
Fever
Data on fever could be extracted from three studies (Swain 1997a(088001); Swain 1997a(088006); Venturini 1996); however, we performed two separate analyses as the definitions used were not comparable. Data on fever (grade 3 or 4 according to ECOG criteria) could be extracted from two trials (Swain 1997a(088001); Swain 1997a(088006)). Only the best‐case scenario (i.e. 25 cases among the 249 participants in the dexrazoxane group and 20 cases among the 285 participants in the control group; total of 534 participants) was analysed since the number of missing data was unclear. The results showed no difference between the treatment groups (RR 1.43, 95% CI 0.81 to 2.54; P = 0.22).
Data on fever (defined as grade 3 or 4 according to the WHO criteria) could be extracted from one study (Venturini 1996). There was one case among the 82 available participants in the dexrazoxane group and zero cases among the 78 participants in the control group. We were not able to calculate a RR since there was only one study available and one of its treatment groups experienced no events. Therefore, we used Fischer's exact test instead (P = 1.00). Best‐case and worst‐case scenarios showed identical results (162 participants). For more details and data, see Table 5.
3. Dexrazoxane versus no dexrazoxane or placebo (Fischer's exact tests).
| Outcome | Study | Definition | n/N dexrazoxane | n/N controls | Analysis | Fischer's exact P value |
| Children | ||||||
| Heart failure (i.e. clinical and subclinical heart failure combined) | P9404 | No definition of clinical heart failure provided; subclinical myocardial dysfunction defined as decreased LVFS; however it was stated that toxicity was graded according to NCI CTCAEv2 criteria, grade 3 or higher but LVFS is not included in that definition | 0/273 | 3/264 | Best‐case | 0.12 |
| Adults | ||||||
| Bone pain grade 3 or 4 | Marty 2006 | CTCAEv2 criteria | 0/85 | 4/79 | Best‐case, worst‐case and available‐case (identical results) | 0.0517 |
| Pyrexia grade 3 or 4 | Marty 2006 | CTCAEv2 criteria | 2/85 | 0/79 | Best‐case, worst‐case and available‐case (identical results) | 0.50 |
| Constipation grade 3 or 4 | Marty 2006 | CTCAEv2 criteria | 1/85 | 0/79 | Best‐case, worst‐case and available‐case (identical results) | 1.00 |
| Mucosal inflammation grade 3 or 4 | Marty 2006 | CTCAEv2 criteria | 0/85 | 1/79 | Best‐case, worst‐case and available‐case (identical results) | 0.48 |
| Fever grade 3 or 4 | Venturini 1996 | WHO criteria | 1/82 | 0/78 | Available‐case (best‐case and worst‐case identical results) | 1.00 |
| Diarrhoea grade 3 or 4 | Venturini 1996 | WHO criteria | 0/82 | 0/78 | Available‐case (best‐case and worst‐case identical results) | 1.00 |
| Phlebitis grade 3 or 4 | Venturini 1996 | WHO criteria | 0/82 | 2/78 | Available‐case (best‐case and worst‐case identical results) | 0.24 |
| Fatigue grade 3 or 4 | Venturini 1996 | WHO criteria | 4/82 | 0/78 | Available‐case (best‐case identical result; worst‐case significant difference in favour of the control group P = 0.03) | 0.12 |
| Hand foot syndrome grade 3 or 4 | Venturini 1996 | WHO criteria | 1/82 | 0/78 | Available‐case (best‐case and worst‐case identical results) | 1.00 |
CTCAEv2: Common Terminology Criteria for Adverse Events, version 2 LVFS: left ventricular fractional shortening n: number nm: not mentioned P: P value WHO: World Health Organization
Febrile bone marrow aplasia
Data on febrile bone marrow aplasia (defined as grade 3 or 4 according to the CTCAEv2 criteria) could be extracted from one study with a total of 164 participants (Marty 2006). The available‐case analysis showed no difference between the treatment groups (RR 3.72, 95% CI 0.42 to 32.55; P = 0.24). There were four cases among the 85 available participants in the dexrazoxane group and one case among the 79 participants in the control group. ITT analyses demonstrated identical results since there were no missing data.
Febrile neutropenia
Data on febrile neutropenia (defined as grade 3 or 4 according to the CTCAEv2 criteria) could be extracted from one study with a total of 164 participants (Marty 2006). The available‐case analysis showed no difference between the treatment groups (RR 1.27, 95% CI 0.62 to 2.59; P = 0.52). There were 15 cases among the 85 available participants in the dexrazoxane group and 11 cases among 79 participants in the control group. ITT analyses demonstrated identical results since there were no missing data.
Fever with positive blood cultures
Data on fever with positive blood cultures (no reference provided) could be extracted from one study with a total of 150 participants (Speyer 1992). The available‐case analysis showed no difference between the treatment groups (RR 0.65, 95% CI 0.11 to 3.77; P = 0.63). There were two cases among the 76 available participants in the dexrazoxane group and three cases among 74 participants in the control group. ITT analyses demonstrated identical results since there were no missing data.
Fever with other positive cultures
Data on fever with other positive cultures (no reference provided) could be extracted from one study with a total of 150 participants (Speyer 1992). The available‐case analysis showed no difference between the treatment groups (RR 1.95, 95% CI 0.37 to 10.31; P = 0.43). There were four cases among the 76 available participants in the dexrazoxane group and two cases among the 74 participants in the control group. ITT analyses demonstrated identical results since there were no missing data.
Pyrexia
Data on pyrexia (defined as grade 3 or 4 according to the CTCAEv2 criteria) could be extracted from one study with a total of 164 participants (Marty 2006). There were two cases among the 85 available participants in the dexrazoxane group and zero cases among the 79 participants in the control group. We were not able to calculate a RR since there was only study available in which one of the treatment groups experienced no events. Therefore, we used Fischer's exact test instead (P = 0.50). Best‐case and worst‐case scenarios showed identical results. For more details and data, see Table 5.
Children
Sepsis
Data on sepsis could be extracted from two studies (P9425; P9426); however, we analysed the studies separately as P9426 reported only that the sepsis was caused by bacteria and provided no further information. P9425 used grade 3 or 4 according to the CTCAEv2 criteria. In both studies, the available‐case analysis demonstrated no difference between the treatment groups: for P9426, the RR was 1.04 (95% CI 0.07 to 16.37; P = 0.98), there was one case among the 109 available participants in the dexrazoxane group and one case among the 113 participants in the control group (222 participants in total); for P9425, the RR was 2.04 (95% CI 0.96 to 4.33; P = 0.06), there were 18 cases among the 106 available participants in the dexrazoxane group and 9 cases among the 108 participants in the control group (214 participants in total). ITT analyses demonstrated comparable results for both P9426 (255 participants) and P9425 (216 participants). For more details, see Analysis 1.18.
Infection
Data on infection could be extracted from three studies (P9404; P9425; P9426); however, we analysed the results of P9426 separately because it did not provide the definition it used. P9404 and P9425 used grade 3 or 4 according to the CTCAEv2 criteria; for P9425, in addition to stating that the criteria were used, for this outcome the authors also explicitly stated "not otherwise specified/unknown". In both analyses, the available‐case analysis demonstrated no difference between the treatment groups; for P9426, the RR was 0.35 (95% CI 0.04 to 3.27; P = 0.35), there was one case among the 109 available participants in the dexrazoxane group and 13 cases among the 113 participants in the control group (222 participants in total); for the meta‐analysis of P9404 and P9425, the RR was 1.24 (95% CI 0.78 to 1.97; P = 0.35), there were 248 cases among the 379 available participants in the dexrazoxane group and 216 cases among the 372 participants in the control group (751 participants in total). Unexplained substantial heterogeneity was detected (I² = 91%). ITT analyses demonstrated comparable results for both P9426 (255 participants) and the meta‐analysis of P9404 and P9425 (753 participants). For more details, see Analysis 1.18.
Allergic reaction
Data on allergic reaction could be extracted from two studies (P9425; P9426); however, we analysed the studies separately because P9426 did not provide a definition for allergic reaction. P9425 used grade 3 or 4 according to the CTCAEv2 criteria. In both studies, the available‐case analysis demonstrated no difference between the treatment groups: for P9426, the RR was 0.26 (95% CI 0.03 to 2.28; P = 0.22), there was one case among the 109 available participants in the dexrazoxane group and four cases among the 113 participants in the control group (222 participants in total); for P9425, the RR was 3.57 (95% CI 0.76 to 16.78; P = 0.11), there were seven cases among the 106 available participants in the dexrazoxane group and two cases among the 108 participants in the control group (214 participants in total). ITT analyses demonstrated comparable results for both P9426 (255 participants) and P9425 (216 participants). For more details, see Analysis 1.18.
Gastrointestinal effects
Adults
Nausea
Data on nausea could be extracted from four studies (Marty 2006; Swain 1997a(088001); Swain 1997a(088006); Venturini 1996); however, we performed two separate analyses as the definitions used were not comparable. Data on nausea (defined as grade 3 or 4 according to the CTCAEv2 or ECOG criteria, which were comparable) could be extracted from three studies (Marty 2006; Swain 1997a(088001); Swain 1997a(088006)). The available‐case analysis was based on the results of Marty 2006 with a total of 164 participants and showed no difference between the treatment groups (RR 0.19, 95% CI 0.02 to 1.56; P = 0.12). There was one case among the 85 available participants in the dexrazoxane group and five cases among the 79 participants in the control group. ITT analyses demonstrated the following results: the RR for the worst‐case scenario was identical since there were no missing data in the study of Marty 2006. For the best‐case scenario, the studies Swain 1997a(088001) and Swain 1997a(088006) were added, which resulted in a total of 698 participants. The best‐case scenario (i.e. 46 cases among 334 participants in the dexrazoxane group and 77 cases among 364 participants in the control group) demonstrated a benefit for dexrazoxane treatment (0.70, 95% CI 0.50 to 0.97; P = 0.03). The studies Swain 1997a(088001) and Swain 1997a(088006) could only be added to the best‐case scenario as the number of missing participants was unclear.
Data on nausea (defined as grade 3 or 4 according to the WHO criteria) could be extracted from one study (Venturini 1996). The available‐case analysis showed no differences between treatment groups (RR 0.95, 95% CI 0.25 to 3.67; P = 0.94; 160 participants). There were four cases among the 82 available participants in the dexrazoxane group and four cases among the 78 participants in the control group. Best‐case and worst‐case scenarios showed comparable results (162 participants).
Vomiting
Data on vomiting could be extracted from four studies (Marty 2006; Swain 1997a(088001); Swain 1997a(088006); Venturini 1996); however, we performed two separate analyses as the definitions used were not comparable. Data on vomiting (defined as grade 3 or 4 according to the CTCAEv2 or ECOG criteria, which were comparable) could be extracted from three studies (Marty 2006; Swain 1997a(088001); Swain 1997a(088006)). The available‐case analysis was based on the results of Marty 2006 with a total of 164 participants and showed no difference between the treatment groups (RR 0.15, 95% CI 0.02 to 1.26; P = 0.08). There was one case among the 85 available participants in the dexrazoxane group and six cases among the 79 participants in the control group. ITT analyses also demonstrated no difference between the treatment groups. The RR for the worst‐case scenario was identical since there were no missing data in the study of Marty 2006. For the best‐case scenario, the studies Swain 1997a(088001) and Swain 1997a(088006) were added which resulted in a total of 698 participants. The RR for the best‐case scenario (i.e. 42 cases among 334 participants in the dexrazoxane group and 60 cases among the 364 participants in the control group) was 0.71 (95% CI 0.37 to 1.39; P = 0.32). The studies Swain 1997a(088001) and Swain 1997a(088006) could only be added to the best‐case scenario as the number of missing participants was unclear.
Data on vomiting (defined as grade 3 or 4 according to the WHO criteria) could be extracted from one study (Venturini 1996). The available‐case analysis showed no differences between treatment groups (RR 1.11, 95% CI 0.39 to 3.16; P = 0.85; 160 participants). There were seven cases among the 82 available participants in the dexrazoxane group and six cases among the 78 participants in the control group. Best‐case and worst‐case scenarios showed comparable results (162 participants).
Nausea and vomiting
Data on nausea and vomiting could be extracted from two studies (Lopez 1998; Speyer 1992); however, we analysed the studies separately since the definitions differed. In the study of Lopez 1998, the available‐case analysis demonstrated no difference between the treatment groups in nausea and vomiting grade 3 or 4 according to WHO criteria (RR 0.32, 95% CI 0.09 to 1.11; P = 0.07). There were 3 cases among the 62 available participants in the dexrazoxane group and 10 cases among the 66 participants in the control group (128 participants in total). ITT analyses demonstrated comparable results (129 participants). For more details, see Analysis 1.19.
The study of Speyer 1992 divided the results on nausea and vomiting into "controllable" and "intractable". The available‐case analysis on controllable nausea and vomiting demonstrated no difference between the treatment groups (RR 1.07, 95% CI 0.81 to 1.40; P = 0.46). There were 46 cases among 76 available participants in the dexrazoxane group and 42 among 74 in the control group (150 participants in total). The available‐case analysis on intractable nausea and vomiting also demonstrated no difference between the treatment groups (RR 0.39, 95% CI 0.08 to 1.95; P = 0.25). There were two cases among the 76 available participants in the dexrazoxane group and five among 74 in the control group (150 participants in total). ITT analyses demonstrated identical results for both definitions since there were no missing data.
Stomatitis
Data on stomatitis could be extracted from six studies (Lopez 1998; Marty 2006; Speyer 1992; Swain 1997a(088001); Swain 1997a(088006); Venturini 1996); however, we subdivided the analysis into four groups since the studies used different definitions (see Characteristics of included studies).
First, the studies of Lopez 1998 and Venturini 1996 used the same definition. The available‐case analysis demonstrated no difference between the treatment groups (RR 0.96, 95% CI 0.38 to 2.44; P = 0.94). There were 13 cases among the 144 available participants in the dexrazoxane group and 14 cases among the 144 participants in the control group (288 participants in total). ITT analyses demonstrated comparable results (291 participants).
The study of Speyer 1992 was also analysed separately. This study divided the results on stomatitis into "ulcers can eat" and "ulcers cannot eat". The available‐case analysis on ulcers can eat demonstrated no difference between the treatment groups (RR 0.89, 95% CI 0.40 to 1.96; P = 0.76). There were 10 cases among the 76 available participants in the dexrazoxane group and 11 cases among the 74 participants in the control group (150 participants in total). The available‐case analysis on ulcers cannot eat also demonstrated no difference between the treatment groups (RR 0.42, 95% CI 0.11 to 1.55; P = 0.25). There were three cases among the 76 available participants in the dexrazoxane group and seven cases among the 74 participants in the control group (150 participants in total). ITT analyses demonstrated identical results for both definitions since there were no missing data.
Lastly, the studies of Marty 2006, Swain 1997a(088001) and Swain 1997a(088006) used comparable definitions. The available‐case analysis was based on the results of Marty 2006 with a total of 164 participants and showed no difference between the treatment groups (RR 0.19, 95% CI 0.02 to 1.56; P = 0.12). There was one case among the 85 available participants in the dexrazoxane group and five cases among the 79 participants in the control group. ITT analyses demonstrated the following results: the RR for the worst‐case scenario was identical since there were no missing data in the study of Marty 2006. For the best‐case scenario, the studies Swain 1997a(088001) and Swain 1997a(088006) were added which resulted in a total of 698 participants. The best‐case scenario (i.e. 15 cases among 334 participants in the dexrazoxane group and 25 cases among the 364 participants in the control group) demonstrated no difference between the treatment groups (0.70, 95% CI 0.38 to 1.30; P = 0.26). The studies Swain 1997a(088001) and Swain 1997a(088006) could only be added to the best‐case scenario as the number of missing participants was unclear.
In summary, all the analyses on stomatitis demonstrated no difference between the treatment groups. For more details, see Analysis 1.19.
Diarrhoea
Data on diarrhoea could be extracted from four studies (Marty 2006; Swain 1997a(088001); Swain 1997a(088006); Venturini 1996); however, we subdivided the analysis into three groups since the studies used different definitions.
First, the study of Marty 2006 was analysed separately. Diarrhoea was defined as grade 3 or 4 according to the CTCAEv2 criteria. The available‐case analysis showed no difference between the treatment groups (RR 0.93, 95% CI 0.06 to 14.61; P = 0.96). There was one case among the 85 available participants in the dexrazoxane group and one among 79 in the control group (164 participants in total). ITT analyses demonstrated identical results since there were no missing data.
Second, the studies of Swain 1997a(088001) and Swain 1997a(088006) used the same definition. Diarrhoea was defined as grade 3 or 4 according to the ECOG criteria. Only the best‐case scenario (i.e. 10 cases among the 249 participants in the dexrazoxane group and 10 cases among the 285 participants in the control group; total of 534 participants) was analysed since the number of missing data was unclear. The results showed no difference between the treatment groups (RR 1.15, 95% CI 0.40 to 3.30; P = 0.79).
For more details, see Analysis 1.19.
Third, the study of Venturini 1996 was analysed separately. Diarrhoea was defined as grade 3 or 4 according to the WHO criteria. There were no cases in both treatment groups (82 available participants in the dexrazoxane group and 78 available participants in the control group; 160 participants in total). We were not able to calculate a RR since there was only one study available and both treatment groups experienced no events. Therefore, we used Fischer's exact test instead (P = 1.00). Best‐case and worst‐case scenarios showed identical results (162 participants). For more details and data, see Table 5.
Constipation
Data on constipation (defined as grade 3 or 4 according to the CTCAEv2 criteria) could be extracted from one study with a total of 164 participants (Marty 2006). There was one case among the 85 available participants in the dexrazoxane group and zero cases among the 79 participants in the control group. We were not able to calculate a RR since there was only one study available in which one of the treatment groups experienced no events. Therefore, we used Fischer's exact test instead (P = 1.0). The best‐cases and worst‐case scenarios showed identical results. For more data and details, see Table 5.
Mucosal inflammation
Data on mucosal inflammation (defined as grade 3 or 4 according to the CTCAEv2 criteria) could be extracted from one study with a total of 164 participants (Marty 2006). There were zero cases among the 85 available participants in the dexrazoxane group and one case among the 79 participants in the control group. We were not able to calculate a RR since there was only one study available in which one of the treatment groups experienced no events. Therefore, we used Fischer's exact test instead (P = 0.48). The best‐cases and worst‐case scenarios showed identical results. For more data and details, see Table 5.
Children
Nausea
Data on nausea (no definition provided) could be extracted from one study with a total of 222 participants (P9426). The available‐case analysis showed no difference between the treatment groups (RR 1.04, 95% CI 0.15 to 7.23; P = 0.97). There were two cases among the 109 available participants in the dexrazoxane group and two cases among the 113 participants in the control group. ITT analyses demonstrated comparable results (255 participants). For more details, see Analysis 1.20.
Vomiting
Data on vomiting (no definition provided) could be extracted from one study with a total of 222 participants (P9426). The available‐case analysis showed no difference between the treatment groups (RR 0.62, 95% CI 0.15 to 2.54; P = 0.51). There were three cases among the 109 available participants in the dexrazoxane group and five cases among the 113 participants in the control group. ITT analyses demonstrated comparable results (255 participants). For more details, see Analysis 1.20.
Nausea or vomiting
Data on nausea or vomiting (defined as grade 3 or 4 according to the CTCAEv2 criteria) could be extracted from one study with a total of 214 participants (P9425). The available‐case analysis demonstrated no difference between the treatment groups (RR 1.02, 95% CI 0.44 to 2.35; P = 0.96). There were 10 cases among the 106 available participants in the dexrazoxane group and 10 cases among the 108 participants in the control group. ITT analyses demonstrated comparable results (216 participants). For more details, see Analysis 1.20.
Stomatitis
Data on stomatitis (defined as grade 3 or 4 according to the CTCAEv2 criteria) could be extracted from one study with a total of 214 participants (P9425). The available‐case analysis demonstrated no difference between the treatment groups (RR 0.99, 95% CI 0.64 to 1.51; P = 0.95). There were 30 cases among the 106 available participants in the dexrazoxane group and 31 cases among the 108 participants in the control group. ITT analyses demonstrated comparable results (216 participants). For more details, see Analysis 1.20.
Mucositis
Data on mucositis (defined as grade 3 or 4 according to the CTCAEv2 criteria) could be extracted from one study with a total of 537 participants (P9404). The available‐case analysis showed a benefit for dexrazoxane treatment (RR 0.61, 95% CI 0.41 to 0.92; P = 0.02). There were 33 cases among the 273 available participants in the dexrazoxane group and 52 cases among the 264 participants in the control group. ITT analyses demonstrated identical results since there were no missing data in this study.
Typhlitis
Data on typhlitis (defined as grade 3 or 4 according to the CTCAEv2 criteria) could be extracted from one study with a total of 214 participants (P9425). The available‐case analysis demonstrated no difference between the treatment groups (RR 3.06, 95% CI 0.85 to 10.98; P = 0.09). There were nine cases among the 106 available participants in the dexrazoxane group and three cases among the 108 participants in the control group. ITT analyses demonstrated comparable results (216 participants). For more details, see Analysis 1.20.
Neurological effects
Adults
Neurotoxicity
Data on neurotoxicity (grade 3 or 4 according to the ECOG criteria) could be extracted from two trials (Swain 1997a(088001); Swain 1997a(088006)). Only the best‐case scenario (i.e. two cases among the 249 participants in the dexrazoxane group and five cases among the 285 participants in the control group; total of 534 participants) was analysed since the number of missing data was unclear. The results showed no difference between the treatment groups (RR 0.62, 95% CI 0.03 to 13.45; P = 0.76). However, unexplained heterogeneity was detected (I² = 63%). For more details, see Analysis 1.21.
Children
Central nervous system
Data on central nervous system grade 3 or 4 toxicity (according to the CTCAEv2 criteria) could be extracted from two studies with a total of 751 participants (P9404; P9425). P9425 explicitly stated that central nervous system included mood, cortical and cerebellar. The available‐case analysis demonstrated no difference between the treatment groups (RR 1.21, 95% CI 0.72 to 2.03; P = 0.48). There were 29 cases among the 379 available participants in the dexrazoxane group and 23 cases among the 372 participants in the control group. ITT analyses demonstrated comparable results (753 participants). For more details, see Analysis 1.22.
Peripheral nervous system
Data on peripheral nervous system grade 3 or 4 toxicity (according to the CTCAEv2 criteria) could be extracted from one study with a total of 214 participants (P9425). The available‐case analysis demonstrated no difference between the treatment groups (RR 0.68, 95% CI 0.12 to 3.98; P = 0.67). There were two cases among the 106 available participants in the dexrazoxane group and three cases among the 108 participants in the control group. ITT analyses demonstrated comparable results (216 participants). For more details, see Analysis 1.22.
Other effects
Adults
Liver damage
Data on severe liver damage (no definition provided) could be extracted from one study with a total of 108 participants (Sun 2016). The available‐case analysis showed no difference between the treatment groups (RR 1.00, 95% CI 0.06 to 18.58; P = 1.0). There was one case among the 54 available participants in the dexrazoxane group and one case among 54 participants in the control group. ITT analyses demonstrated comparable results (110 participants). For more details, see Analysis 1.23.
Pain on injection
Data on pain on injection (grade 3 or 4 according to the ECOG criteria) could be extracted from two trials (Swain 1997a(088001); Swain 1997a(088006)). Only the best‐case scenario (i.e. four cases among the 249 participants in the dexrazoxane group and three cases among the 285 participants in the control group; total of 534 participants) was analysed since the number of missing data was unclear. The results showed no difference between the treatment groups (RR 1.51, 95% CI 0.34 to 6.73; P = 0.59).
Phlebitis
Data on phlebitis could be extracted from three trials (Swain 1997a(088001); Swain 1997a(088006); Venturini 1996); however, we subdivided the analysis into two groups since the studies used different definitions. Swain 1997a(088001) and Swain 1997a(088006) defined phlebitis as grade 3 or 4 according to the ECOG criteria. Only the best‐case scenario (i.e. four cases among the 249 participants in the dexrazoxane group and three cases among the 285 participants in the control group; total of 534 participants) was analysed since the number of missing data was unclear. The results showed no difference between the treatment groups (RR 1.53, 95% CI 0.34 to 6.90; P = 0.58).
Venturini 1996 defined phlebitis as grade 3 or 4 according to the WHO criteria. There was no case among the 82 available participants in the dexrazoxane group and two cases among the 78 participants in the control group. We were not able to calculate a RR since there was only one study available in which one of the treatment groups experienced no events. Therefore, we used Fischer's exact test instead (P = 0.24). Best‐case and worst‐case scenarios showed comparable results (162 participants). For more details and data, see Table 5.
Anorexia
Data on anorexia (grade 3 or 4 according to the ECOG criteria) could be extracted from two trials (Swain 1997a(088001); Swain 1997a(088006)). Only the best‐case scenario (i.e. 23 cases among the 249 participants in the dexrazoxane group and 27 cases among the 285 participants in the control group; total of 534 participants) was analysed since the number of missing data was unclear. The results showed no difference between the treatment groups (RR 0.97, 95% CI 0.57 to 1.65; P = 0.91).
Alopecia
Data on alopecia could be extracted from four studies (Marty 2006; Speyer 1992; Swain 1997a(088001); Swain 1997a(088006)); however, we subdivided the analysis into two groups since the studies used different definitions (see Characteristics of included studies).
First, the study of Speyer 1992 was analysed separately and the available‐case analysis demonstrated no difference between the treatment groups (RR 1.02, 95% CI 0.91 to 1.13; P = 0.74). There were 69 cases among the 76 available participants in the dexrazoxane group and 66 cases among the 74 participants in the control group (150 participants in total). ITT analyses demonstrated identical results since there were no missing data.
The studies of Marty 2006, Swain 1997a(088001) and Swain 1997a(088006) used comparable criteria. The available‐case analysis was based on the results of Marty 2006 with a total of 164 participants and showed no difference between the treatment groups (RR 1.19, 95% CI 0.64 to 2.24; P = 0.58). There were 18 cases among the 85 available participants in the dexrazoxane group and 14 cases among the 79 participants in the control group. ITT analyses demonstrated comparable results. The RR for the worst‐case scenario was identical since there were no missing data in the study of Marty 2006. For the best‐case scenario, the studies Swain 1997a(088001) and Swain 1997a(088006) were added which resulted in a total of 698 participants. The RR of the best‐case scenario (i.e. 227 cases among 334 participants in the dexrazoxane group and 251 cases among 364 participants in the control group) was 1.01 (95% CI 0.94 to 1.09; P = 0.75). The studies Swain 1997a(088001) and Swain 1997a(088006) could only be added to the best‐case scenario as the number of missing participants was unclear.
For more details, see Analysis 1.23.
Asthenia
Data on asthenia (grade 3 or 4 according to the CTCAEv2 criteria) could be extracted from one study with a total of 164 participants (Marty 2006). The available‐case analysis showed no difference between the treatment groups (RR 0.93, 95% CI 0.13 to 6.44; P = 0.94). There were two cases among the 85 available participants in the dexrazoxane group and two cases among the 79 participants in the control group. ITT analyses demonstrated identical results since there were no missing data.
Fatigue
Data on fatigue could be extracted from two studies (Marty 2006; Venturini 1996); however, as definitions were not comparable, we performed separate analyses.
Data on fatigue (grade 3 or 4 according to the CTCAEv2 criteria) could be extracted from one study with a total of 164 participants (Marty 2006). The available‐case analysis showed no difference between the treatment groups (RR 2.79, 95% CI 0.30 to 26.25; P = 0.37). There were three cases among the 85 available participants in the dexrazoxane group and one case among the 79 participants in the control group. ITT analyses demonstrated identical results since there were no missing data.
Data on fatigue (grade 3 or 4 according to the WHO criteria) could be extracted from one study with a total of 160 available participants (Venturini 1996). There were four cases among the 82 available participants in the dexrazoxane group and zero cases among the 78 participants in the control group. We were not able to calculate a RR since there was only one study available in which one of the treatment groups experienced no events. Therefore, we used Fischer's exact test instead (P = 0.12). Best‐case analysis showed an identical result, while the worst‐case analysis showed a significant difference (P = 0.03) in favour of the control group (162 participants). For more details and data, see Table 5.
Bone pain
Data on bone pain (grade 3 or 4 according to the CTCAEv2 criteria) could be extracted from one study with a total of 164 participants (Marty 2006). There were zero cases among the 85 available participants in the dexrazoxane group and four cases among the 79 participants in the control group. We were not able to calculate a RR since there was only one study available in which one of the treatment groups experienced no events. Therefore, we used Fischer's exact test instead (P = 0.052).The best‐case and worst‐case scenarios showed identical results (see Table 5).
Hand‐foot syndrome
Data on hand‐foot syndrome (grade 3 or 4 according to the WHO criteria) could be extracted from one study with a total of 160 available participants (Venturini 1996). There was one case among the 82 available participants in the dexrazoxane group and no cases among the 78 participants in the control group. We were not able to calculate a RR since there was only one study available in which one of the treatment groups experienced no events. Therefore, we used Fischer's exact test instead (P = 1.00).The best‐case and worst‐case scenarios showed comparable results (see Table 5).
Children
Pulmonary
Data on pulmonary grade 3 or 4 toxicity (according to the CTCAEv2 criteria) could be extracted from one study with a total of 214 participants (P9425). The available‐case analysis demonstrated a difference in favour of the control group (RR 4.42, 95% CI 1.30 to 15.05; P = 0.02). There were 13 cases among the 106 available participants in the dexrazoxane group and 3 cases among the 108 participants in the control group. ITT analyses demonstrated comparable results (216 participants). For more details, see Analysis 1.24.
Sensitivity analyses for the risk of bias criteria
The results of the sensitivity analyses were consistent among the trials and did not differ from the overall analyses for all meta‐analyses.
Discussion
Myocardial damage due to anthracycline chemotherapy is a considerable, serious problem. It reduces QoL and can even cause premature death. Also, when myocardial damage occurs during therapy, the maximum cumulative dose of anthracyclines needs to be limited, and as a result, the efficacy of anthracycline chemotherapy will be reduced. There is thus a need for cardioprotective strategies, such as the use of dexrazoxane. This is the third update of this Cochrane Review evaluating the existing evidence on dexrazoxane.
Summary of main results
We identified 13 RCTs that were eligible for inclusion in the review: eight in adults and five in children. With this update, we added one new RCT in adults and two new RCTs in children. To ascertain the efficacy of a cardioprotective intervention, the best study design – provided that the design and execution are correct – is a randomised controlled trial in which the only difference between intervention and control groups is the use of the cardioprotective intervention. Although non‐randomised studies have been published, due to the high risk of bias associated with these study designs, we did not include them in this systematic review.
In contrast to previous versions of this review, we now present results separately for adults and children (i.e. participants less than 22 years of age). Because of differences in, for example, background risks of cardiac disease in these populations (Armstrong 2013; Feijen 2019b; Groenewegen 2020; Van Dalen 2006), developmental changes and differences in the body composition of children, results might not be (easily) interchangeable (Kearns 2003).
We summarise the results in adults and children by outcome below (see also Table 1 and Table 2).
For clinical heart failure, our meta‐analysis in adults showed a benefit in favour of the use of dexrazoxane (RR 0.22, 95% CI 0.11 to 0.43; 7 studies). In children, we identified no difference in clinical heart failure between treatment groups (RR 0.20, 95% CI 0.01 to 4.19; 3 studies). Three paediatric studies also assessed cardiomyopathy/heart failure as the primary cause of death. None of the participants had this outcome, but two control group participants died as a result of cardiomyopathy/heart failure listed as a secondary cause of death. No difference between treatment groups was identified (RR 0.20, 95% CI 0.01 to 4.11).
For subclinical myocardial dysfunction and clinical heart failure combined, we performed two separate pooled analyses for the adult studies based on the definitions used: there was a benefit in favour of the use of dexrazoxane for both available‐case meta‐analyses (RR 0.37, 95% CI 0.24 to 0.56; 3 studies; and RR 0.46, 95% CI 0.33 to 0.66; 2 studies, respectively). The paediatric studies also used different definitions, precluding a pooled analysis. One study showed a benefit in favour of the use of dexrazoxane (RR 0.33, 95% CI 0.13 to 0.85), whereas another study showed no difference between treatment groups (RR not estimable; best‐case analysis only).
However, an important question regarding any cardioprotective intervention during anthracycline therapy is whether the cardioprotective drug could decrease the cardiotoxicity by anthracyclines without reducing the antitumour efficacy and without negative effects on toxicities other than cardiac damage. The antitumour efficacy is reflected by survival and tumour response rate. Overall survival and progression‐free survival were only reported in adult RCTs (no new data in the update) and overall mortality was only reported in paediatric RCTs (all newly included in the update). The meta‐analyses of both overall survival in adults and overall mortality in children showed no difference between the treatment groups (HR 1.04, 95% 0.88 to 1.23; 4 studies, and HR 1.01, 95% CI 0.72 to 1.42; 3 studies, respectively). We pooled the results on progression‐free survival into one meta‐analysis in the previous update, which demonstrated no difference between the treatment groups. However, after re‐evaluating the definitions used in the different studies, in this update, we deemed them to be too heterogeneous to pool. We subdivided progression‐free survival into three analyses based on the comparability of the definitions. We found a longer progression‐free survival in favour of the use of dexrazoxane in one study (HR 0.62, 95% CI 0.43 to 0.90) and we found no difference between the treatment groups for the other two analyses (HR 0.95, 95% CI 0.64 to 1.40; 1 study, and HR 1.18, 95% CI 0.97 to 1.43; 2 studies, respectively). In adults, there was no difference in tumour response rate between treatment groups (RR 0.91, 95% CI 0.79 to 1.04; 6 studies, available‐case analysis; no new data in the update). We subdivided tumour response rate in children into two analyses based on the comparability of definitions and identified no difference between treatment groups (RR 1.01, 95% CI 0.95 to 1.07; 1 study, only best‐case analysis; and RR 0.92, 95% CI 0.84 to 1.01; 1 study, available‐case analysis, respectively).
One of the most important adverse effects to investigate is the occurrence of secondary malignant neoplasms (SMN). Thus far, only paediatric studies have assessed this outcome. Since the previous update of this review, two studies could be added to the pooled analysis. The direction of effect remained the same, but the difference between the treatment groups changed in some analyses. The available‐ and worst‐case analyses were identical and showed a difference in favour of the control group (RR 3.08, 95% CI 1.13 to 8.38; 3 RCTs). In the best‐case analysis (the only analysis performed in the previous update) a fourth study could be added. It showed the same direction of effect but the result was not different between treatment groups (RR 2.51, 95% CI 0.96 to 6.53, 4 RCTs).
Regarding the other adverse effects (grade 3 or higher), it was possible to pool data for some adverse effects (available‐case, best‐case and/or worst‐case analyses), but for others, only descriptive results are available. Compared to the second update of this review (Van Dalen 2011), we have added data on the adverse effects hand‐foot syndrome, myelosuppression and liver damage for adults. We have added data on the following adverse effects for children: abnormal lymphocytes, haemoglobin, white blood cell count, platelets, absolute neutrophil count, haematological effects, sepsis, infection, allergic reaction, nausea, vomiting, mucositis and central nervous system effects.
In adults, there was a higher risk of abnormal white blood cell count at nadir in the dexrazoxane group. The haematologic effects that showed no difference between treatment groups were thrombocytopenia, neutropenia, abnormal granulocyte count at nadir and at recovery, abnormal white blood cell count at recovery, abnormal platelet count at nadir and at recovery, anaemia, myelosuppression (one study) and leukopenia. All analyses included two pooled studies unless otherwise stated. In children, there was a higher risk of abnormal haemoglobin (two individual studies) and abnormal white blood cell count (one study) in the dexrazoxane group. For both platelets (either a difference in favour of the control group (one study) or no difference between treatment groups (one study)) and absolute neutrophil count (a difference in favour of the control group in most analyses, but no difference in one analysis; two individual studies), inconsistent results were identified. The following haematologic effects showed no difference between treatment groups: lymphocytes (one study), thrombosis (one study), and haematological effects (one study).
None of the immune system/infectious effects showed a difference between the treatment groups. In adults, fever (two pooled studies; one individual study), febrile bone marrow aplasia (one study), febrile neutropenia (one study), fever with either positive blood or other cultures (both one study) and pyrexia (one study) were evaluated. In children, sepsis (two individual studies), infection (two pooled studies (unexplained heterogeneity was identified) and one individual study), and allergic reaction (two individual studies) were evaluated.
In adults, for nausea the best‐case analysis demonstrated a lower risk of nausea in the dexrazoxane group (three pooled studies), but the available‐ and worst‐case analysis demonstrated no difference between treatment groups (both one study); one individual study showed no difference between treatment groups irrespective of type of analysis. The gastrointestinal effects that showed no difference between treatment groups were vomiting (three pooled studies best‐case analyses, other analyses one study; one individual study), nausea and vomiting (two individual studies), stomatitis (one individual study; two pooled studies; three pooled studies best‐case analyses, other analyses one study), diarrhoea (two individual studies and two pooled studies), constipation (one study), and mucosal inflammation (one study). In children, there was a lower risk of mucositis in the dexrazoxane group (one study). The following effects showed no difference between treatment groups: nausea, vomiting, nausea or vomiting, stomatitis and typhlitis (all in one study).
None of the neurological effects showed a difference between the treatment groups. These outcomes were neurotoxicity in adults (two pooled studies; unexplained heterogeneity was identified) and central and peripheral nervous system in children (two pooled studies and one individual study, respectively).
For other effects, in adults, none of the other effects showed a difference between the treatment groups. These were liver damage (one study), pain on injection (two pooled studies), phlebitis (two pooled studies; one individual study), anorexia (two pooled studies), alopecia (one individual study and three pooled studies), asthenia (one study), and bone pain (one study). For fatigue (two individual studies), only in a worst‐case analyses was a difference in favour of the control group identified. In children, there was a higher risk of pulmonary effects in the dexrazoxane group (one study).
In summary, for adverse effects other than cardiac and SMN, results varied. For some haematological effects (adults and children), pulmonary effects (children) and other effects (adults), there was a difference in favour of the control group, although not always consistent in all analyses. For some gastrointestinal effects (adults and children), there was a difference in favour of the dexrazoxane group, but again not always consistent in all analyses. For most adverse effects, no difference between treatment groups was identified.
It should be noted that data were not available for all outcomes of interest. None of the included studies evaluated quality of life.
Overall completeness and applicability of evidence
The evidence from adults demonstrated a cardioprotective effect of dexrazoxane. The evidence in children is less clear; only for one cardiac outcome was a difference reached. However, 'no evidence of effect' is not the same as 'evidence of no effect'. The reason that no difference between treatment groups was identified could be, as with all other outcomes, due to the number of participants included in these studies being too small to detect a difference (i.e. low power). Also, anthracycline‐induced cardiotoxicity is dose‐dependent (Feijen 2019b), and in some of the studies participants received a relatively low cumulative anthracycline dose. Furthermore, heart failure can develop not only during anthracycline therapy, but also years after the end of treatment (Armstrong 2013; Feijen 2019b), so the length of follow‐up could have been too short to detect a difference between the treatment groups.
At the moment, dexrazoxane is not routinely used in children and adults who receive anthracyclines as part of their cancer treatment. This caution might be driven by the suspicion of interference with antitumour efficacy (that is, tumour response rate and survival) and by the occurrence of SMN.
Our (meta‐)analyses of antitumour efficacy either showed results in favour of the dexrazoxane group or no difference between participants who were treated with or without dexrazoxane (in children, PFS was not evaluated). Also, the value of tumour response rate for predicting survival is not clear (Cooper 2020; Odaimi 1987; Pierga 2001). In our (meta‐)analyses of both OS and PFS, either a difference in favour of the dexrazoxane group (which included the individual study which identified a difference in tumour response rate (Swain 1997a(088001)) was found or no difference between the dexrazoxane and control group. It should be noted that the study that identified a difference in PFS in favour of the dexrazoxane group used a rather unconventional definition (i.e. time from first date of complete response, partial response or stable disease until the date progressive disease was first noticed). We cannot be sure how that affected the results.
Only paediatric RCTs evaluated SMN and the results were slightly different depending on the analysis method used (i.e. available‐case, best‐case, worst‐case), but the direction of effect, in favour of the control group, was the same in all analyses. Although we cannot rule out that dexrazoxane might increase the risk of SMN, when interpreting these results it should be kept in mind that, although the only difference between treatment groups in these RCTs should have been the presence or absence of dexrazoxane, it is possible that other factors influenced the occurrence of SMN.
For example, etoposide is associated with an increased risk of SMN (Le Deley 2003; Seif 2015; Travis 2013). In some of the included studies, participants did receive etoposide (P9425; P9426), possibly with different cumulative doses in the dexrazoxane and control groups. Etoposide, anthracyclines and dexrazoxane all interfere with topoisomerase II and, hypothetically, this combination may have a synergistic effect on cell proliferation as suggested by an in vitro study on cardiotoxicity (Nemade 2018). Topoisomerase inhibitors are associated with secondary haematologic malignancies, such as acute myeloid leukaemia (AML) and myelodysplastic syndrome (MDS), which mainly occur within three years after therapy. The latency time of secondary solid tumours caused by chemotherapy is more than 10 years (Hawkins 2020). The median follow‐up time of the studies evaluating SMN ranged from 4.6 to 9.4 years; for some included participants, follow‐up was only 0.01 year.
Radiation therapy is also an important risk factor of SMN (Hawkins 2020). Again, in some of the included studies, participants did receive radiation therapy, possibly with differences between the dexrazoxane and control groups. And some of the identified SMN are located within the radiation field. So we cannot exclude the possibility that radiation therapy plays a role in the occurrence of SMN in our included studies.
The same is true for other potential risk factors for SMN, such as other chemotherapeutic agents and genetic susceptibility (Turcotte 2018).
Unfortunately, there are too few included studies to reliably perform subgroup analyses in order to further investigate reasons for the possible increased risk of SMN in the dexrazoxane group (Higgins 2011); the risk of possible confounding should also not be forgotten. However, when analysing only studies that included etoposide in their treatment regime (P9425; P9426), or studies that included cranial irradiation (DFCI 95‐01; P9404), the direction of effect remained the same (results not shown).
It should be noted that, although there might be a higher risk of SMN in children treated with dexrazoxane, mortality due to a second cancer did not differ between treatment groups according to a publication addressing three of the four paediatric studies with SMN data included in this review (P9404; P9425; P9426; Chow 2015 reference): HR 1.24 (95% CI 0.49 to 3.15). This result was based on 10 SMN deaths in the dexrazoxane group and 8 in the control group after a median follow‐up of 12.4 years. A more recent study by Chow and colleagues showed similar results when including data from all four paediatric RCTs included in this review: HR 1.17 (95% CI 0.51 to 2.70; 12 SMN deaths in the dexrazoxane group and 10 in the control group), but only approximately 28% of participants from the DFCI 95‐01 study could be included (Chow 2021). The median follow‐up duration for this outcome is not completely clear, but might be 18.6 years as reported for the study overall. Unfortunately, at the moment, no data on the total number of SMN cases (so not only deaths) with increased follow‐up are available to update the current analysis.
In one of the five paediatric studies and in three of the eight adults studies, participants in the intervention and control groups received comparable cumulative anthracycline doses. Although according to the review's protocol, participants in both treatment groups should have received the same anthracycline dose, the actual received cumulative dose was not reported in three paediatric studies. However, in these three paediatric studies, the following information was reported: all participants received the same cumulative dose (P9404); the received dose was in high compliance with prescribed dose (P9426); and there were virtually no dose reductions (P9425). In one paediatric study (Wexler 1996), and in one adult study (Speyer 1992), participants in the dexrazoxane group received a higher cumulative anthracycline dose (100 mg/m2 or more) than participants in the control group. So despite a higher cumulative anthracycline dose received in the dexrazoxane group, there was still a lower rate of cardiotoxicity. In four adult studies, it was unclear if participants in the intervention and control groups received similar cumulative anthracycline doses. If participants in the control group received a higher cumulative anthracycline dose than participants treated with dexrazoxane, this could have led to an overestimation of the cardioprotective effect of dexrazoxane (and vice versa). This uncertainty should also be kept in mind when interpreting the results of the secondary outcomes (tumour response rate, survival and adverse effects).
In the included studies, different ratios of dexrazoxane to anthracyclines were used. We did not analyse the effect of these different ratios on the outcomes.
The applicability of our results to current clinical practice might be limited since the majority of the included studies were executed at the end of last century. Supportive care and anticancer treatments have since improved considerably.
Finally, data were not available for all outcomes of interest. As a result, we cannot draw conclusions regarding those outcomes, but they are of course important for clinical practice.
We are awaiting (additional) results of the currently ongoing study (N = 1) and the studies which await classification (N = 12).
Quality of the evidence
In adults, we graded the quality of the evidence as moderate for almost all evaluated outcomes (downgraded one level for study limitations). We graded two of the three PFS outcomes (using different definitions) as low (downgraded an additional level for imprecision); we graded the third PFS outcome/definition as moderate.
In children, we graded the quality of the evidence as low for almost all evaluated outcomes (downgraded either two levels for study limitations or one level for study limitations and one level for imprecision). We graded two outcomes as very low quality (one definition of clinical heart failure and subclinical myocardial dysfunction combined and one definition of tumour response rate) (downgraded two levels for study limitations and one level for imprecision).
In many studies, bias could not be ruled out due to lack of reporting. However, this is the best evidence available now from RCTs evaluating dexrazoxane as a cardioprotective intervention in children and adults with cancer treated with anthracyclines.
Potential biases in the review process
This systematic review used a very broad search strategy for identifying eligible studies. Thus, although it is unlikely that we missed eligible studies, it is never possible to completely rule out reporting bias.
Since the search strategy included search terms for cardiotoxicity, it is possible that for outcomes other than cardiotoxicity, more evidence is available than identified in this review. Also, in this systematic review, cardiotoxicity was evaluated as a binary outcome; that is, the number of participants below and above the cut‐off value for an abnormal result. Some studies have evaluated cardiotoxicity as a continuous outcome, but in doing so, it is possible that participants with good and bad values balance each other out, resulting in an adequate mean value. This can give the impression that there is no problem, while for some participants this might not be true. Therefore, we did not include these data.
Authors' conclusions
Implications for practice.
Our meta‐analyses showed the efficacy of dexrazoxane in preventing or reducing cardiotoxicity in adults treated with anthracyclines. In children, there was only a difference between treatment groups for one of the cardiac outcomes (in favour of dexrazoxane). In adults, no evidence of a negative effect on tumour response rate, overall survival (OS) and progression‐free survival (PFS) was identified. In children, no evidence of a negative effect on tumour response rate and overall mortality was identified. The results for adverse effects varied, but there might be a higher risk of some haematological effects (adults and children) and pulmonary effects (children) and a lower risk of some gastrointestinal effects (adults and children) for those treated with dexrazoxane compared to control. Children treated with dexrazoxane might have a higher risk of secondary malignant neoplasms (SMN); in adults, this outcome was not addressed. In adults, the quality of the evidence ranged between moderate and low; in children, between low and very low.
We conclude that if the risk of cardiac damage is expected to be high, it might be justified to use dexrazoxane in children and adults with cancer treated with anthracyclines. However, clinicians and patients should weigh the cardioprotective effect of dexrazoxane against the possible risk of adverse effects, including SMN, for each individual. For children, the International Late Effects of Childhood Cancer Guideline Harmonization Group has developed a clinical practice guideline (De Baat 2022).
Implications for research.
Before definitive conclusions on the use of dexrazoxane can be made, especially in children, more high‐quality research is needed. Future trials should preferably be randomised controlled trials (RCTs). They should be performed in homogeneous study populations (for example, with regard to tumour diagnosis and stage of disease) to help us reach firmer conclusions about antitumour efficacy and other outcomes. They should have a long‐term follow‐up using valid outcome definitions (including for cardiotoxicity, antitumour efficacy, survival and adverse effects). The number of included participants should be sufficient to obtain the power needed for the results to be reliable. We are awaiting the results of the studies currently being performed in children. The performance of an individual participant data analysis is another possibility to assess the effect of dexrazoxane.
What's new
| Date | Event | Description |
|---|---|---|
| 7 May 2021 | New citation required and conclusions have changed | Summary of most important changes when compared to second update: The original review was split and this part now focuses on dexrazoxane only. We analysed adult and paediatric data separately. New randomised controlled trials and additional data on some already included studies were added. We updated the risk of bias criteria. In previous versions of the review, best‐case analyses were performed; starting with this update we present results from available‐case, best‐case and worst‐case analyses where possible. |
| 7 May 2021 | New search has been performed | The search for eligible studies was updated to 7 May 2021. |
History
Protocol first published: Issue 7, 2002
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | Contact details updated. |
| 24 February 2015 | Amended | Contact details updated. |
| 11 February 2015 | Amended | Contact details updated. |
| 27 March 2014 | Amended | Contact details updated. |
| 26 February 2014 | Amended | Contact details updated. |
| 9 May 2011 | New search has been performed | The search for eligible studies was updated to November 2010. |
| 9 May 2011 | New citation required and conclusions have changed | Summary of most important changes in results of this second update when compared to the first update of this review: We identified a new randomised controlled trial (RCT) on the use of amifostine (no eligible data on amifostine were available before). Also, we identified a new RCT on the use of dexrazoxane and long‐term follow‐up data of an already included RCT on dexrazoxane. Finally, we identified a new ongoing trial (on enalapril maleate) and two new trials awaiting assessment (on telmirsartan and the combination of hydroprednisone and gluthatione). Again, only for dexrazoxane pooling of results was possible and for the occurrence of cardiotoxicity, response rate and survival the conclusions did not change. More information on adverse effects became available including secondary malignant neoplasms. |
| 19 August 2008 | Amended | Converted to new review format. |
| 18 February 2008 | New citation required and conclusions have changed | Substantive amendment |
| 10 July 2007 | Amended | New studies found and included or excluded: 01/04/07 Conclusions changed: 10/07/07 Summary of most important changes in results of the update when compared to the original review: as opposed to the original review, there was no evidence for a lesser tumour response rate with the use of dexrazoxane. For adverse effects now pooling of results was possible: only for one adverse effect (abnormal white blood cell count at nadir) a difference in favour of the control group was identified. The search for eligible studies was updated to April 2007 using an updated search strategy and including eight new possible cardioprotective agents. And as opposed to the original review, for the update we searched in ongoing trials databases. Instead of pooling results when three or more randomised controlled trials (RCTs) were available, we now pooled results of two or more RCTs. Instead of focusing only on the primary outcome (heart failure) when assessing the quality of included studies, we now assessed the quality criteria blinding of the outcome assessor and completeness of follow‐up for all outcomes separately. Prior cardiac dysfunction was added as a baseline characteristic. Sex, age per treatment group, anthracycline peak dose, anthracycline infusion duration, cumulative anthracycline doses in the intervention and control groups, and a description of other chemotherapy and / or radiotherapy in the study protocol were added to the table of included studies. Five new RCTs were included: one addressing L‐carnitine, one addressing carvedilol and three additional ones addressing dexrazoxane. We also identified six ongoing studies and seven studies awaiting assessment evaluating different cardioprotective agents; characteristics of these trials are provided. Again, only for dexrazoxane pooling of results was possible and for the occurrence of cardiotoxicity and survival the conclusions did not change. As opposed to the original review, now there was no evidence for a lesser tumour response rate with the use of dexrazoxane. For adverse effects now pooling of results was possible: only for one adverse effect (abnormal white blood cell count at nadir) a difference in favour of the control group was identified. We conclude that if the risk of cardiac damage is expected to be high, it might be justified to use dexrazoxane in patients with cancer treated with anthracyclines. However, for each individual patient clinicians should weigh the cardioprotective effect of dexrazoxane against the possible risk of adverse effects. |
Acknowledgements
We would like to thank Rob Scholten, Marianne van de Wetering, Jan Tijssen, Piet Bakker, Heleen van der Pal and the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Editorial office for their advice and support; and Joanne Platt, Information Specialist at Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers, for checking the search strategies for the third update and running them. Also, we would like to thank Alexey Nabatov for translating Russian articles. In addition, we are grateful to Huib Caron and Heather Dickinson, authors of the previous versions of this review and protocol, who have not been involved in this update. Finally, we would like to thank our funders for the financial support which made it possible to perform earlier versions of this systematic review: Foundation of Pediatric Cancer Research (SKK) Amsterdam, the Netherlands; Jacques H. de Jong Foundation, the Netherlands; Knowledge and Research Center for Alternative Medicine (ViFAB) / Danish Cancer Society, Denmark; Stichting Kinderen Kankervrij (KiKa), the Netherlands; the Dutch Heart Foundation. The Editorial Base of Cochrane Childhood Cancer has been funded by KiKa and is located in the Prinsess Máxima Center for Pediatric Oncology, Utrecht, the Netherlands.
For the Speyer 1992 study and both Swain 1997a(088001) and Swain 1997a(088006) studies, the hazard ratio and associated statistics were initially calculated using an Excel spreadsheet provided by Dr MG Hart of the Department of Clinical Neurosciences, Western General Hospital, Edinburgh, United Kingdom. For the second update of this review, the necessary data for the survival analyses were (re)calculated using a spreadsheet developed by Heather Dickinson.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Group. The views and opinions expressed here are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, the National Health Service (NHS), or the Department of Health.
The authors and Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Team are grateful to the following peer reviewers for their time and comments: Daniel Mulrooney, Paul Nathan and Roderick Skinner.
Appendices
Appendix 1. Search strategy for Cochrane Central Register of Controlled Trials (CENTRAL)
(1) For the dexrazoxane we used the following subject headings and text words:
MeSH descriptor: [Razoxane] explode all trees or dexrazoxan* or cardioxan* or zinecar* or razoxan* or piperazin* or totect* or savene* or "ADR‐529" or "ADR 529" or "ADR529" or "ICRF‐187" or "ICRF 187" or "ICRF187" or "ADR‐5" or "ICRF" or "NSC‐169780" or "NSC 169780" or "NSC169780"
(2) For anthracyclines we used the following subject headings and text words:
MeSH descriptor: [Anthracyclines] explode all trees or anthracyclin* or "4‐demethoxydaunorubicin" or "4 demethoxydaunorubicin" or "4‐desmethoxydaunorubicin" or "4 desmethoxydaunorubicin" or "IMI 30" or "IMI30" or "IMI‐30" or "NSC 256439" or "NSC‐256439" or "NSC256439" or idarubic* or "4'‐epiadriamycin" or "4' epiadriamycin" or "4'‐epidoxorubicin" or "4' epidoxorubicin" or "4'‐epi‐doxorubicin" or "4' epi doxorubicin or 4'‐epi‐adriamycin" or "4' epi adriamycin" or "4'‐epi‐DXR" or "4' epi DXR" or farmorubicin or "IMI‐28" or "IMI 28" or "IMI28" or "NSC 256942" or "NSC‐256942" or "NSC256942" or epirubic* or adriablastin* or adriblastin or "DOX‐SL" or "DOX SL" or doxorubic* or adriamyc* or "dauno‐rubidomycine" or dauno rubidomycin or rubidomycin or rubomycin or daunomycin or cerubidine or daunoblastin or daunoblastine or daunorubic* or rubidomyc* or "NSC‐82151" or "NSC 82151" or "NSC82151" or daunoxome or daunosom* or doxil or caelyx or myocet
Searches were combined as (1) AND (2).
For the previous versions a slightly different search strategy was used as presented in Van Dalen 2011.
Appendix 2. Search strategy for MEDLINE (PubMed)
(1) For the dexrazoxane we used the following subject headings and text words:
exp Dexrazoxane/ or (dexrazoxan* or cardioxan* or zinecar* or razoxan* or piperazin* or totect* or savene*).mp. or ("ADR‐529" or "ADR 529" or "ADR529" or "ICRF‐187" or "ICRF 187" or "ICRF187" or "ADR‐5" or "ICRF" or "NSC‐169780" or "NSC 169780" or "NSC169780").mp.
(2) For anthracyclines we used the following subject headings and text words:
exp Anthracyclines/ or (anthracyclin* or "4‐demethoxydaunorubicin" or "4 demethoxydaunorubicin" or "4‐desmethoxydaunorubicin" or "4 desmethoxydaunorubicin" or "IMI 30" or "IMI30" or "IMI‐30" or "NSC 256439" or "NSC‐256439" or "NSC256439" or idarubic* or "4'‐epiadriamycin" or "4' epiadriamycin" or "4'‐epidoxorubicin" or "4' epidoxorubicin" or "4'‐epi‐doxorubicin" or "4' epi doxorubicin or 4'‐epi‐adriamycin" or "4' epi adriamycin" or "4'‐epi‐DXR" or "4' epi DXR" or farmorubicin or "IMI‐28" or "IMI 28" or "IMI28" or "NSC 256942" or "NSC‐256942" or "NSC256942" or epirubic* or adriablastin* or adriblastin or adriamycin or "DOX‐SL" or "DOX SL" or doxorubic* or adriamyc* or "dauno‐rubidomycine" or dauno rubidomycin or rubidomycin or rubomycin or daunomycin or cerubidine or daunoblastin or daunoblastine or daunorubic* or rubidomyc* or "NSC‐82151" or "NSC 82151" or "NSC82151" or daunoxome or daunosom* or doxil or caelyx or myocet).mp.
(3) For randomised controlled trials we used the highly sensitive search strategy for identifying reports of randomised controlled trials:
randomized controlled trial.pt. or controlled clinical trial.pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ti. or groups.ab.
Searches were combined as (1) AND (2) AND (3).
For the previous versions slightly different search strategies were used as presented in Van Dalen 2011. For the original search (August 2002) and for the first update (April 2007) we used the highly sensitive search strategy for identifying reports of randomised controlled trials (all phases) as described in the Cochrane Handbook (Higgins 2006). For the second update (2011) the filter described in Higgins 2008 was used.
Appendix 3. Search strategy for EMBASE (Ovid)
(1) For the dexrazoxane we used the following subject headings and text words:
exp razoxane/ or (dexrazoxan* or cardioxan* or zinecar* or razoxan* or piperazin* or totect* or savene*).mp.or ("ADR‐529" or "ADR 529" or "ADR529" or "ICRF‐187" or "ICRF 187" or "ICRF187" or "ADR‐5" or "ICRF" or "NSC‐169780" or "NSC 169780" or "NSC169780").mp.
(2) For anthracyclines we used the following subject headings and text words:
exp Anthracyclines/ or (anthracyclin* or "4‐demethoxydaunorubicin" or "4 demethoxydaunorubicin" or "4‐desmethoxydaunorubicin" or "4 desmethoxydaunorubicin" or "IMI 30" or "IMI30" or "IMI‐30" or "NSC 256439" or "NSC‐256439" or "NSC256439" or idarubic* or "4'‐epiadriamycin" or "4' epiadriamycin" or "4'‐epidoxorubicin" or "4' epidoxorubicin" or "4'‐epi‐doxorubicin" or "4' epi doxorubicin or 4'‐epi‐adriamycin" or "4' epi adriamycin" or "4'‐epi‐DXR" or "4' epi DXR" or farmorubicin or "IMI‐28" or "IMI 28" or "IMI28" or "NSC 256942" or "NSC‐256942" or "NSC256942" or epirubic* or adriablastin* or adriblastin or "DOX‐SL" or "DOX SL" or doxorubic* or adriamyc* or "dauno‐rubidomycine" or dauno rubidomycin or rubidomycin or rubomycin or daunomycin or cerubidine or daunoblastin or daunoblastine or daunorubic* or rubidomyc* or "NSC‐82151" or "NSC 82151" or "NSC82151" or daunoxome or daunosom* or doxil or caelyx or myocet).mp.
(3) For randomised controlled trials we used the following subject headings and text words:
crossover procedure/ or double‐blind procedure/ or randomized controlled trial/ or single‐blind procedure/ or random*.mp. or factorial*.mp. or (crossover* or cross over* or cross‐over*).mp. or placebo*.mp. or (double* adj blind*).mp. or (singl* adj blind*).mp. or assign*.mp. or allocat*.mp. or volunteer*.mp.
Searches were combined as (1) AND (2) AND (3).
For the previous versions a slightly different search strategy was used as presented in Van Dalen 2011.
[mp = title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]; [ti,ab=title, abstract]; [sh=subject heading]
Appendix 4. Search strategy for conference proceedings
For the 2011 to 2020 editions of the SIOP conferences the pdf files were assessed using these terms:
dexrazoxan, cardioxan, zinecar, razoxan, piperazin, ICRF, totect, savene.
For the 2011 to 2020 editions of the ASCO conferences we did an advanced search on https://meetinglibrary.asco.org/:
Keywords: dexrazoxane OR cardioxane OR zinecard OR razoxane OR piperazine OR ICRF OR totect OR savene
Meeting: ASCO annual meeting
Dates: 2020, 2019, 2018, 2017, 2016, archive
Media: abstracts
Appendix 5. Overview of the full search results and study flow for the second review update
Up to and including the second update, we included a total of 11 articles that fulfilled all the criteria for including studies in this review (three new in the first update; three new in the second update). The total number of identified RCTs was 10 since one of the articles provided the results of two RCTs (Swain 1997a(088001); Swain 1997a(088006)), and two articles provided long‐term follow‐up data of an already included RCT (see DFCI 95‐01; Barry 2008 and Lipshultz 2010 references). We excluded fifteen articles. We included six studies as awaiting classification, either because they did not provide enough information to assess eligibility for this review and we did not succeed in contacting the authors or they were conference abstracts. We identified three new ongoing trials.
Data and analyses
Comparison 1. Dexrazoxane versus no dexrazoxane or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Clinical heart failure available‐case | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 Adults | 7 | 1221 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.11, 0.43] |
| 1.1.2 Children | 3 | 885 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.19] |
| 1.2 Clinical heart failure best‐case | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 Adults | 7 | 1249 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.11, 0.43] |
| 1.2.2 Children | 3 | 959 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.19] |
| 1.3 Clinical heart failure worst‐case | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.3.1 Adults | 7 | 1249 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.21, 0.84] |
| 1.3.2 Children | 3 | 959 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.68, 1.43] |
| 1.4 Cardiomyopathy/heart failure primary cause of death available‐case (best‐case and worst‐case identical results) | 3 | 1008 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.4.1 Children | 3 | 1008 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.5 Heart failure (i.e. clinical heart failure and subclinical myocardial dysfunction combined) available‐case | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.5.1 Adults (comparable definition 1) | 3 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.24, 0.56] |
| 1.5.2 Adults (comparable definition 2) | 2 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.33, 0.66] |
| 1.5.3 Children (comparable definition 1) | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.13, 0.85] |
| 1.6 Heart failure (i.e. clinical heart failure and subclinical myocardial dysfunction combined) best‐case | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.6.1 Adults (comparable definition 1) | 4 | 605 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.19, 0.44] |
| 1.6.2 Adults (comparable definition 2) | 2 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.33, 0.66] |
| 1.6.3 Children (comparable definition 1) | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.14, 0.95] |
| 1.7 Heart failure (i.e. clinical heart failure and subclinical myocardial dysfunction combined) worst‐case | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.7.1 Adults (comparable definition 1) | 3 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.42, 0.86] |
| 1.7.2 Adults (comparable definition 2) | 2 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.33, 0.66] |
| 1.7.3 Children (comparable definition 1) | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.20, 0.86] |
| 1.8 Overall survival | 4 | Hazard Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.8.1 Adults | 4 | Hazard Ratio (IV, Random, 95% CI) | 1.04 [0.88, 1.23] | |
| 1.9 Overall mortality | 3 | Hazard Ratio (IV, Random, 95% CI) | 1.01 [0.72, 1.42] | |
| 1.9.1 Children | 3 | Hazard Ratio (IV, Random, 95% CI) | 1.01 [0.72, 1.42] | |
| 1.10 Progression‐free survival | 4 | Hazard Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.10.1 Adults (PFS defined as time from first date of complete response, partial response or stable disease until the date progressive disease was first noticed) | 1 | Hazard Ratio (IV, Random, 95% CI) | 0.62 [0.43, 0.90] | |
| 1.10.2 Adults (PFS defined as time to progression; starting point nm) | 1 | Hazard Ratio (IV, Random, 95% CI) | 0.95 [0.64, 1.40] | |
| 1.10.3 Adults (PFS defined as time from randomisation to progression either on or off treatment) | 2 | Hazard Ratio (IV, Random, 95% CI) | 1.18 [0.97, 1.43] | |
| 1.11 Response rate available‐case | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.11.1 Adults | 6 | 956 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.79, 1.04] |
| 1.11.2 Children (complete response defined as disappearance of active Hodgkin lymphoma (gallium negative, ≥ 70% decrease in the sum of the products of the perpendicular diameters of measurable lesions, and negative bone marrow or bone scan if initially positive)) | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.84, 1.01] |
| 1.12 Response rate best‐case | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.12.1 Adults | 6 | 1021 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.08] |
| 1.12.2 Children (complete response defined as disappearance of active Hodgkin lymphoma (gallium negative, ≥ 70% decrease in the sum of the products of the perpendicular diameters of measurable lesions, and negative bone marrow or bone scan if initially positive)) | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.84, 1.00] |
| 1.12.3 Children (no definition of complete remission provided) | 1 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.95, 1.07] |
| 1.13 Response rate worst‐case | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.13.1 Adults | 6 | 1021 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.01] |
| 1.13.2 Children (complete response defined as disappearance of active Hodgkin lymphoma (gallium negative, ≥ 70% decrease in the sum of the products of the perpendicular diameters of measurable lesions, and negative bone marrow or bone scan if initially positive)) | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.07] |
| 1.14 Adverse effects: Secondary malignant neoplasms (Children) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.14.1 Secondary malignant neoplasms available‐case | 3 | 1015 | Risk Ratio (M‐H, Random, 95% CI) | 3.08 [1.13, 8.38] |
| 1.14.2 Secondary malignant neoplasms best‐case | 4 | 1220 | Risk Ratio (M‐H, Random, 95% CI) | 2.51 [0.96, 6.53] |
| 1.14.3 Secondary malignant neoplasms worst‐case | 3 | 1015 | Risk Ratio (M‐H, Random, 95% CI) | 3.08 [1.13, 8.38] |
| 1.15 Adverse effects: Haematological effects (Adults) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.15.1 Thrombocytopenia grade 3 or 4 (WHO/CTCAEv2 criteria)available‐case | 3 | 452 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.48, 2.20] |
| 1.15.2 Thrombocytopenia grade 3 or 4 (WHO/CTCAEv2 criteria) best‐case | 3 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.47, 2.17] |
| 1.15.3 Thrombocytopenia grade 3 or 4 (WHO/CTCAEv2 criteria) worst‐case | 3 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.58, 2.46] |
| 1.15.4 Neutropenia grade 3 or 4 (WHO/CTCAEv2 criteria) available‐case | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.96, 1.15] |
| 1.15.5 Neutropenia grade 3 or 4 (WHO/CTCAEv2 criteria) best‐case | 2 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.94, 1.14] |
| 1.15.6 Neutropenia grade 3 or 4 (WHO/CTCAEv2 criteria) worst‐case | 2 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.96, 1.15] |
| 1.15.7 Abnormal granulocyte count at nadir grade 3 or 4 (ECOG criteria) best‐case | 2 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.96, 1.13] |
| 1.15.8 Abnormal granulocyte count at recovery grade 3 or 4 (ECOG criteria) best‐case | 2 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.59, 1.21] |
| 1.15.9 Abnormal white blood cell count at nadir grade 3 or 4 (ECOG criteria) best‐case | 2 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.05, 1.29] |
| 1.15.10 Abnormal white blood cell count at recovery grade 3 or 4 (ECOG criteria) best‐case | 2 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.36, 1.31] |
| 1.15.11 Abnormal platelet count at nadir grade 3 or 4 (ECOG criteria) best‐case | 2 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.42, 1.84] |
| 1.15.12 Abnormal platelet count at recovery grade 3 or 4 (ECOG criteria) best‐case | 2 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.16, 4.42] |
| 1.15.13 Anaemia grade 3 or 4 (WHO/CTCAEv2 criteria) available‐case | 3 | 452 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.79, 2.39] |
| 1.15.14 Anaemia grade 3 or 4 (WHO/CTCAEv2 criteria) best‐case | 3 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.78, 2.35] |
| 1.15.15 Anaemia grade 3 or 4 (WHO/CTCAEv2 criteria) worst‐case | 3 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.82, 2.73] |
| 1.15.16 Severe myelosuppresion (no definition provided) available‐case | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.19, 21.41] |
| 1.15.17 Severe myelosuppresion (no definition provided) best‐case | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.19, 21.42] |
| 1.15.18 Severe myelosuppresion (no definition provided) worst‐case | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.26, 8.63] |
| 1.15.19 Leukopenia grade 3 or 4 (WHO/CTCAEv2 criteria) available‐case | 2 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.66, 1.83] |
| 1.15.20 Leukopenia grade 3 or 4 (WHO/CTCAEv2 criteria) best‐case | 2 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.66, 1.82] |
| 1.15.21 Leukopenia grade 3 or 4 (WHO/CTCAEv2 criteria) worst‐case | 2 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.71, 1.93] |
| 1.16 Adverse effects: Haematological effects (Children) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.16.1 Lymphocytes (no definition provided) available‐case | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.07, 16.37] |
| 1.16.2 Lymphocytes (no definition provided) best‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.06, 15.94] |
| 1.16.3 Lymphocytes (no definition provided) worst‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.65, 2.22] |
| 1.16.4 Haemoglobin grade 3 or 4 (NCI CTCAEv2 criteria) available‐case | 1 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.13, 1.95] |
| 1.16.5 Haemoglobin grade (no definition provided) available‐case | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 2.96 [1.31, 6.72] |
| 1.16.6 Haemoglobin grade 3 or 4 (NCI CTCAEv2 criteria) best‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.12, 1.95] |
| 1.16.7 Haemoglobin grade (no definition provided) best‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 2.88 [1.26, 6.57] |
| 1.16.8 Haemoglobin grade 3 or 4 (NCI CTCAEv2 criteria) worst‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.12, 1.93] |
| 1.16.9 Haemoglobin grade (no definition provided) worst‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [1.09, 2.77] |
| 1.16.10 White blood cell count (no definition provided) available‐case | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [1.30, 2.68] |
| 1.16.11 White blood cell count (no definition provided) best‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [1.25, 2.63] |
| 1.16.12 White blood cell count (no definition provided) worst‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [1.22, 2.13] |
| 1.16.13 Thrombosis grade 3 or 4 (NCI CTCAEv2 criteria) available‐case | 1 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 4.08 [0.46, 35.87] |
| 1.16.14 Thrombosis grade 3 or 4 (NCI CTCAEv2 criteria) best‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 4.07 [0.46, 35.87] |
| 1.16.15 Thrombosis grade 3 or 4 (NCI CTCAEv2 criteria) worst‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 2.55 [0.51, 12.84] |
| 1.16.16 Platelets grade 3 or 4 (NCI CTCAEv2 criteria) available‐case | 1 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 2.45 [1.79, 3.35] |
| 1.16.17 Platelets grade (no definition provided) available‐case | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.90, 3.86] |
| 1.16.18 Platelets grade 3 or 4 (NCI CTCAEv2 criteria) best‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 2.45 [1.79, 3.36] |
| 1.16.19 Platelets (no definition provided) best‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.87, 3.78] |
| 1.16.20 Platelets grade 3 or 4 (NCI CTCAEv2 criteria) worst‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [1.77, 3.27] |
| 1.16.21 Platelets (no definition provided) worst‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.93, 2.27] |
| 1.16.22 Absolute neutrophil count grade 3 or 4 (NCI CTCAEv2 criteria) available‐case | 1 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.00, 1.20] |
| 1.16.23 Absolute neutrophil count grade (no definition provided) available‐case | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.03, 1.58] |
| 1.16.24 Absolute neutrophil count grade 3 or 4 (NCI CTCAEv2 criteria) best‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.00, 1.20] |
| 1.16.25 Absolute neutrophil count (no definition provided) best‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.98, 1.56] |
| 1.16.26 Absolute neutrophil count grade 3 or 4 (NCI CTCAEv2 criteria) worst‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.00, 1.20] |
| 1.16.27 Absolute neutrophil count (no definition provided) worst‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.03, 1.47] |
| 1.16.28 Hematological effects grade 3 or 4 (NCI CTCAEv2 criteria) available‐case (best‐case and worst‐case identical results) | 1 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.94, 1.05] |
| 1.17 Adverse effects: Immune system/infectious effects (Adults) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.17.1 Fever grade 3 or 4 ( ECOG criteria) best‐case | 2 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.81, 2.54] |
| 1.17.2 Febrile bone marrow aplasia grade 3 or 4 (CTCAEv2 criteria) available‐case (best‐case and worst‐case identical results) | 1 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 3.72 [0.42, 32.55] |
| 1.17.3 Febrile neutropenia grade 3 or 4 (CTCAEv2 criteria) available‐case (best‐case and worst‐case identical results) | 1 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.62, 2.59] |
| 1.17.4 Fever with positive blood cultures (no reference provided) available‐case (best‐case and worst‐case identical results) | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.11, 3.77] |
| 1.17.5 Fever with other positive cultures (no reference provided) available‐case (best‐case and worst‐case identical results) | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.37, 10.31] |
| 1.18 Adverse effects: Immune system/infectious effects (Children) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.18.1 Sepsis grade 3 or 4 (NCI CTCAEv2 criteria) available‐case | 1 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [0.96, 4.33] |
| 1.18.2 Sepsis (bacteria; further definition not provided) available‐case | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.07, 16.37] |
| 1.18.3 Sepsis grade 3 or 4 (NCI CTCAEv2 criteria) best‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [0.96, 4.33] |
| 1.18.4 Sepsis (bacteria; further definition not provided) best‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.06, 15.94] |
| 1.18.5 Sepsis grade 3 or 4 (NCI CTCAEv2 criteria) worst‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [0.94, 3.97] |
| 1.18.6 Sepsis (bacteria; further definition not provided) worst‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.65, 2.22] |
| 1.18.7 Infection grade 3 or 4 (NCI CTCAEv2 criteria; for Schwartz 2009 explicitly stated not otherwise specified/unknown) available‐case | 2 | 751 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.78, 1.97] |
| 1.18.8 Infection (definition not provided) available‐case | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.27] |
| 1.18.9 Infection grade 3 or 4 (NCI CTCAEv2 criteria; for Schwartz 2009 explicitly stated not otherwise specified/unknown) best‐case | 2 | 753 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.78, 1.97] |
| 1.18.10 Infection (definition not provided) best‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.19] |
| 1.18.11 Infection grade 3 or 4 (NCI CTCAEv2 criteria; for Schwartz 2009 explicitly stated not otherwise specified/unknown) worst‐case | 2 | 753 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.79, 1.95] |
| 1.18.12 Infection (definition not provided) worst‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.59, 1.93] |
| 1.18.13 Allergic reaction grade 3 or 4 (NCI CTCAEv2 criteria) available‐case | 1 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 3.57 [0.76, 16.78] |
| 1.18.14 Allergic reaction (definition not provided) available‐case | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.03, 2.28] |
| 1.18.15 Allergic reaction grade 3 or 4 (NCI CTCAEv2 criteria) best‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 3.57 [0.76, 16.78] |
| 1.18.16 Allergic reaction (definition not provided) best‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.22] |
| 1.18.17 Allergic reaction grade 3 or 4 (NCI CTCAEv2 criteria) worst‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 2.72 [0.74, 9.97] |
| 1.18.18 Allergic reaction (definition not provided) worst‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.56, 1.81] |
| 1.19 Adverse effects: Gastrointestinal effects (Adults) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.19.1 Nausea grade 3 or 4 (CTCAEv2 criteria) available‐case | 1 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.02, 1.56] |
| 1.19.2 Nausea grade 3 or 4 (CTCAEv2/ECOG criteria) best‐case | 3 | 698 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.50, 0.97] |
| 1.19.3 Nausea grade 3 or 4 (CTCAEv2 criteria) worst‐case | 1 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.02, 1.56] |
| 1.19.4 Vomiting grade 3 or 4 (CTCAEv2 criteria) available‐case | 1 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.02, 1.26] |
| 1.19.5 Vomiting grade 3 or 4 (CTCAEv2/ECOG criteria) best‐case | 3 | 698 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.37, 1.39] |
| 1.19.6 Vomiting grade 3 or 4 (CTCAEv2 criteria) worst‐case | 1 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.02, 1.26] |
| 1.19.7 Nausea and vomiting ‐ controllable (reference not provided) available‐case (best‐case and worst‐case identical results) | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.81, 1.40] |
| 1.19.8 Nausea and vomiting ‐ vomiting intractable (reference not provided) available‐case (best‐case and worst‐case identical results) | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.08, 1.95] |
| 1.19.9 Nausea and vomiting grade 3 or 4 (WHO criteria) available‐case | 1 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.09, 1.11] |
| 1.19.10 Nausea and vomiting grade 3 or 4 (WHO criteria) best‐case | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.09, 1.09] |
| 1.19.11 Nausea and vomiting grade 3 or 4 (WHO criteria) worst‐case | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.14, 1.27] |
| 1.19.12 Stomatitis grade 3 or 4 (WHO criteria) available‐case | 2 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.38, 2.44] |
| 1.19.13 Stomatitis grade 3 or 4 (WHO criteria) best‐case | 2 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.38, 2.37] |
| 1.19.14 Stomatitis grade 3 or 4 (WHO criteria) worst‐case | 2 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.42, 3.24] |
| 1.19.15 Stomatitis grade 3 or 4 (CTCAEv2 criteria) available‐case | 1 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.82] |
| 1.19.16 Stomatitis grade 3 or 4 (CTCAEv2/ECOG criteria) best‐case | 3 | 698 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.38, 1.30] |
| 1.19.17 Stomatitis grade 3 or 4 (CTCAEv2 criteria) worst‐case | 1 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.82] |
| 1.19.18 Stomatitis (ulcers can eat) (no reference provided) available‐case (best‐case and worst‐case identical results) | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.40, 1.96] |
| 1.19.19 Stomatitis (ulcers cannot eat) (no reference provided) available‐case (best‐case and worst‐case identical results) | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.11, 1.55] |
| 1.19.20 Diarrhoea grade 3 or 4 (CTCAEv2 criteria) available‐case (best‐case and worst‐case identical results) | 1 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.06, 14.61] |
| 1.19.21 Diarrhoea grade 3 or 4 (ECOG criteria) best‐case | 2 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.40, 3.30] |
| 1.19.22 Nausea grade 3 or 4 (WHO criteria) available‐case | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.25, 3.67] |
| 1.19.23 Nausea grade 3 or 4 (WHO criteria) best‐case | 1 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.24, 3.59] |
| 1.19.24 Nausea grade 3 or 4 (WHO criteria) worst‐case | 1 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.41, 4.75] |
| 1.19.25 Vomiting grade 3 or 4 (WHO criteria) available‐case | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.39, 3.16] |
| 1.19.26 Vomiting grade 3 or 4 (WHO criteria) best‐case | 1 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.38, 3.08] |
| 1.19.27 Vomiting grade 3 or 4 (WHO criteria) worst‐case | 1 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.52, 3.73] |
| 1.20 Adverse effects: Gastrointestinal effects (Children) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.20.1 Nausea (no definition provided) available‐case | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.15, 7.23] |
| 1.20.2 Nausea (no definition provided) best‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.14, 7.05] |
| 1.20.3 Nausea (no definition provided) worst‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.65, 2.16] |
| 1.20.4 Vomiting (no definition provided) available‐case | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.15, 2.54] |
| 1.20.5 Vomiting (no definition provided) best‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.15, 2.48] |
| 1.20.6 Vomiting (no definition provided) worst‐case | 1 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.60, 1.85] |
| 1.20.7 Nausea or vomiting grade 3 or 4 (NCI CTCAEv2 criteria) available‐case | 1 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.44, 2.35] |
| 1.20.8 Nausea or vomiting grade 3 or 4 (NCI CTCAEv2 criteria) best‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.44, 2.35] |
| 1.20.9 Nausea or vomiting grade 3 or 4 (NCI CTCAEv2 criteria) worst‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.46, 2.25] |
| 1.20.10 Stomatitis grade 3 or 4 (NCI CTCAEv2 criteria) available‐case | 1 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.64, 1.51] |
| 1.20.11 Stomatitis grade 3 or 4 (NCI CTCAEv2 criteria) best‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.64, 1.51] |
| 1.20.12 Stomatitis grade 3 or 4 (NCI CTCAEv2 criteria) worst‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.65, 1.50] |
| 1.20.13 Mucositis grade 3 or 4 (NCI CTCAEv2 criteria) available‐case (best‐case and worst‐case identical results) | 1 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.41, 0.92] |
| 1.20.14 Typhlitis grade 3 or 4 (NCI CTCAEv2 criteria) available‐case | 1 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 3.06 [0.85, 10.98] |
| 1.20.15 Typhlitis grade 3 or 4 (NCI CTCAEv2 criteria) best‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 3.06 [0.85, 10.98] |
| 1.20.16 Typhlitis grade 3 or 4 (NCI CTCAEv2 criteria) worst‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 2.55 [0.82, 7.87] |
| 1.21 Adverse effects: Neurological effects (Adults) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.21.1 Neurotoxicity (ECOG criteria) grade 3 or 4 best‐case | 2 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.03, 13.45] |
| 1.22 Adverse effects: Neurological (Children) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.22.1 Central nervous system grade 3 or 4 (NCI CTCAEv2 criteria; for Schwartz 2009 explicitly stated that it includes mood, cortical and cerebellar) available‐case | 2 | 751 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.72, 2.03] |
| 1.22.2 Central nervous system grade 3 or 4 (NCI CTCAEv2 criteria; for Schwartz 2009 explicitly stated that it includes mood, cortical and cerebellar) best‐case | 2 | 753 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.72, 2.03] |
| 1.22.3 Central nervous system grade 3 or 4 (NCI CTCAEv2 criteria; for Schwartz 2009 explicitly stated that it includes mood, cortical and cerebellar) worst‐case | 2 | 753 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.72, 2.02] |
| 1.22.4 Peripheral nervous system grade 3 or 4 (NCI CTCAEv2 criteria) available‐case | 1 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.12, 3.98] |
| 1.22.5 Peripheral nervous system grade 3 or 4 (NCI CTCAEv2 criteria) best‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.12, 3.98] |
| 1.22.6 Peripheral nervous system grade 3 or 4 (NCI CTCAEv2 criteria) worst‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.18, 3.33] |
| 1.23 Adverse effects: Other (Adults) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.23.1 Severe liver damage (no definition provided) available‐case | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.06, 15.58] |
| 1.23.2 Severe liver damage (no definition provided) best‐case | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.06, 15.59] |
| 1.23.3 Severe liver damage (no definition provided) worst‐case | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.15, 6.85] |
| 1.23.4 Pain on injection grade 3 or 4 (ECOG criteria) best‐case | 2 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.34, 6.73] |
| 1.23.5 Phlebitis grade 3 or 4 (ECOG criteria) best‐case | 2 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.34, 6.90] |
| 1.23.6 Anorexia grade 3 or 4 (ECOG criteria) best‐case | 2 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.57, 1.65] |
| 1.23.7 Alopecia grade 3 or 4 (CTCAEv2 criteria) available‐case | 1 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.64, 2.24] |
| 1.23.8 Alopecia grade 3 or 4 (CTCAEv2/ECOG criteria) best‐case | 3 | 698 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.94, 1.09] |
| 1.23.9 Alopecia grade 3 or 4 (CTCAEv2 criteria) worst‐case | 1 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.64, 2.24] |
| 1.23.10 Alopecia severe (reference not provided) available‐case (best‐case and worst‐case identical results) | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.91, 1.13] |
| 1.23.11 Asthenia grade 3 or 4 (CTCAEv2 criteria) available‐case (best‐case and worst‐case identical results) | 1 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.13, 6.44] |
| 1.23.12 Fatigue grade 3 or 4 (CTCAEv2 criteria) available‐case (best‐case and worst‐case identical results) | 1 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 2.79 [0.30, 26.25] |
| 1.24 Adverse effects: Other (Children) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.24.1 Pulmonary grade 3 or 4 (NCI CTCAEv2 criteria; explicitly stated that it includes diffusion capacity for carbon momoxide, vital capacity, pulmonary/functional and oxygen saturation) available‐case | 1 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 4.42 [1.30, 15.05] |
| 1.24.2 Pulmonary grade 3 or 4 (NCI CTCAEv2 criteria; explicitly stated that it includes diffusion capacity for carbon momoxide, vital capacity, pulmonary/functional and oxygen saturation) best‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 4.41 [1.29, 15.05] |
| 1.24.3 Pulmonary grade 3 or 4 (NCI CTCAEv2 criteria; explicitly stated that it includes diffusion capacity for carbon momoxide, vital capacity, pulmonary/functional and oxygen saturation) worst‐case | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 3.57 [1.21, 10.49] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
DFCI 95‐01.
| Study characteristics | ||
| Methods | Computer‐generated randomisation was performed centrally at the Quality Assurance Office for Clinical Trials (permuted block design with institutional balancing to ensure that a treatment imbalance within an institution was no greater than 3 participants). | |
| Participants | 206 children (age for all randomised participants nm: see notes; 120 boys and 86 girls) with high‐risk acute lymphoblastic leukaemia (ALL), treated with multiagent chemotherapy (including doxorubicin: see notes) and CNS irradiation. No prior anthracycline therapy. No prior cardiac radiotherapy. Some of the participants were diagnosed with prior cardiac dysfunction (by either echocardiography or the cardiac marker troponin T; definition used nm), but the exact number of participants was nm. | |
| Interventions | Dexrazoxane (10:1 ratio of dexrazoxane to doxorubicin; IV bolus up to 15 minutes immediately before doxorubicin) (N = 105) versus no cardioprotective intervention (N = 101). | |
| Outcomes | Heart failure (clinical heart failure defined as congestive heart failure or other symptomatic cardiac disease) Tumour response rate (no definition of complete remission provided) Adverse effects (no definition provided) |
|
| Notes | Median length of follow‐up: 2.7 years Median age: 7.5 years in intervention group and 7.3 years in control group According to protocol, children in both treatment groups should have received a cumulative doxorubicin dose of 300 mg/m2 (peak dose (i.e. maximal dose received in 1 week) 30 mg/m2; infusion duration nm). Long‐term follow‐up data of this study have been published (DFCI 95‐01; Barry 2008 and Lipshultz 2010 references). Both articles included 205 randomised participants (105 in the dexrazoxane group and 100 in the control group) as opposed to the original publication, which included 206 randomised participants. Lipshultz 2010 provided long‐term follow‐up data (median follow up in the dexrazoxane group 6.2 years; range 3 to 7.7 years and in the control group 5.7 years; range 2.8 to 7.6 years) on clinical heart failure for 134 of the 205 randomised participants, i.e. 68 of the 105 participants in the dexrazoxane group and 66 of the 100 participants in the control group. These were participants for which data were available after treatment completion. It was stated that children leaving the study did not differ in any clinical characteristic from those who stayed in the study. The median cumulative anthracycline dose in the dexrazoxane group was 300 mg/m2 (range 300 mg/m2 to 300 mg/m2) and in the control group it was also 300 mg/m2 (range 288 mg/m2 to 300 mg/m2) with an infusion duration up to 15 minutes (push or bolus). Barry 2008 provided long‐term follow‐up data (median follow up 6.2 years) on secondary malignant neoplasms. Gender: 64 males (61%) males and 41 (39%) females in dexrazoxane group and 56 (55%) males and 45 females (45%) in the control group (DFCI 95‐01 primary reference); 27 (40%) males and 41 (60%) females in the dexrazoxane group and 30 (45%) males and 36 (55%) females in the control group (Lipshultz 2010); in Barry 2008 nm. Stage of disease per treatment group: in both treatment groups all high‐risk ALL. Funding sources: grants from the National Institutes of Health (CA 68484, CA 79060, CA 55576, CA 06516, HL 59837, HR96041, HL 53392, and HL 72705), Pfizer, and Roche Diagnostics (DFCI 95‐01 primary reference); grants from the US National Institutes of Health (HL072705, HL078522, HL053392, CA127642, CA068484, HD052104, AI50274, CA068484, HD052102, HL087708, HL079233, HL004537, HL087000, HL007188, HL094100, HL095127, and HD80002), Children’s Cardiomyopathy Foundation, University of Miami Women’s Cancer Association, Lance Armstrong Foundation, Roche Diagnostics, Pfizer, and Novartis (Lipshultz 2010); grant from the National Institutes of Health (CA 68484) (Barry 2008). Declaration of interests: one of the authors has received investigator‐initiated research grant support from Pfizer, the manufacturer of dexrazoxane (Zinecard), and Roche Diagnostics, the manufacturer of the troponin T assay used in this study. Neither company had any active involvement in the study. This author also reports having received an honorarium as a consultant for Chiron, which manufactures a product related to dexrazoxane (Cardioxane) (DFCI 95‐01 primary reference); one of the authors received investigator‐initiated grants from Pfizer, Novartis and Roche Diagnostics to help support this study. Other authors have received payment for consultancy work (+/‐ stock or stock options) from Enzon Pharmaceuticals, ELISA Pharmaceuticals and/or Genzyme Corporation. The funding sources had no role in the study design, data collection, data analysis, data interpretation, or writing of the report (Lipshultz 2010); some authors received compensation for consultant or advisory roles from Chiron and Enzon Pharmaceuticals, honoraria from Enzon Pharmaceuticals, research funding from Pfizer, Novartis, Chiron and Enzon Pharmaceuticals (Barry 2008). The Vrooman 2011 publication provided long‐term follow‐up data of this study, but provided only information of dexrazoxane participants and results could thus not be used for this review. The Lipshultz 2012 publication also provided long‐term follow‐up data of this study, but a shorter follow‐up than in the Lipshultz 2010 publication and no new information was provided; thus we did not use information from this publication. The Moghrabi 2007 publication again provided long‐term follow‐up data, but no new information was provided and thus results were not included in the review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation was performed centrally |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation was performed centrally. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to the received intervention (either dexrazoxane or no cardioprotective intervention). |
| Blinding of outcome assessors (detection bias) ‐ clinical heart failure | Low risk | The outcome assessors of clinical heart failure (at long‐term follow‐up) were blinded to treatment. |
| Blinding of outcome assessors (detection bias) ‐ tumour response rate | Low risk | Central investigators providing summary study results remained blinded throughout the study. |
| Blinding of outcome assessors (detection bias) ‐ toxicities other than cardiac damage (not diagnosed by laboratory tests) | Unclear risk | No information on blinding of outcome assessors provided for toxicities other than cardiac damage |
| Incomplete outcome data (attrition bias) ‐ clinical heart failure | High risk | Clinical heart failure at long‐term follow‐up evaluated in 65% of participants in the dexrazoxane group and 66% of the control group (68/105 of the dexrazoxane group and 66/100 of the control group) |
| Incomplete outcome data (attrition bias) ‐ tumour response rate | Unclear risk | Unclear in how many participants tumour response rate was evaluated |
| Incomplete outcome data (attrition bias) ‐ SMN | Low risk | SMN evaluated in 95% or 96% of participants in the dexrazoxane group and 95% or 94% of the control group (for 1 participant it was not clear from which treatment group he/she was missing). |
| Selective reporting (reporting bias) | High risk | Not all expected outcomes were reported (e.g. overall survival was missing) |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: unclear (information on blinding of outcome assessors for some of the outcomes not provided; block randomisation was used). Baseline imbalance between treatment groups related to outcome (prior cardiotoxic treatment (anthracyclines and cardiac irradiation), age, gender, stage of disease and prior cardiac dysfunction): unclear (no prior cardiotoxic treatment, but unclear how many in each treatment group had prior cardiac dysfunction; all other factors comparable) Difference in length of follow‐up between treatment groups: unclear (length of follow‐up not mentioned for DFCI 95‐01 primary reference and Barry 2008 reference; for the Lipshultz 2010 reference, there was a difference in length of follow‐up between treatment groups, but relevance unclear) |
Galetta 2005.
| Study characteristics | ||
| Methods | Method of randomisation not clear. | |
| Participants | 20 participants (median age 54 years (all < 60 years); 11 males and 9 females) with non‐Hodgkin lymphoma (stage 2, 3 or 4) treated with epirubicin (cumulative dose nm; peak dose (i.e. maximal dose received in 1 week) 40 mg/m2; bolus infusion), cyclophosphamide, etoposide, prednisolone, vincristine, methotrexate, aracytin and bleomycin. No prior anthracyclines. No prior cardiac radiotherapy. No prior cardiac dysfunction (defined as uncontrolled congestive heart failure; baseline resting LVEF < 50%). | |
| Interventions | Dexrazoxane (10:1 ratio of dexrazoxane to epirubicin; IV infusion over 15 minutes immediately after epirubicin) (n = 10) versus no cardioprotective intervention (n = 10) | |
| Outcomes | The primary objective of this study was to assess QT‐dispersion on ECG, not to assess heart failure. Data on subclinical myocardial dysfunction were available, but not on clinical heart failure; results were thus not eligible for the review. | |
| Notes | Length of follow‐up nm. Age in intervention and control group nm. Cumulative anthracycline dose per treatment group nm. Gender in intervention and control group nm. Stage of disease per treatment group nm. Funding sources nm. Declaration of interests nm. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the method of randomisation was provided. |
| Allocation concealment (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the method of randomisation was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to the received intervention (either dexrazoxane or no cardioprotective intervention). |
| Selective reporting (reporting bias) | High risk | Not all expected outcomes were reported (e.g. overall survival was missing; subclinical myocardial dysfunction was reported but was not eligible for inclusion in the review) |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: unclear (information on both method of randomisation and blinding of outcome assessors not provided). Baseline imbalance between treatment groups related to outcome (prior cardiotoxic treatment (anthracyclines and cardiac irradiation), age, gender, stage of disease and prior cardiac dysfunction): unclear (no prior cardiotoxic treatment and no prior cardiac dysfunction, but age, gender, stage of disease not reported) Difference in length of follow‐up between treatment groups: unclear (length of follow‐up nm) |
Lopez 1998.
| Study characteristics | ||
| Methods | Method of randomisation not clear. | |
| Participants | 129 participants (aged 14 to 75 years; sex: 20 males and 109 females) with metastatic breast cancer (N = 95) or advanced soft tissue sarcoma (N = 34) treated with epirubicin (cumulative dose for all randomised participants nm: see notes; peak dose (i.e. maximal dose received in 1 week) 160 mg/m2; bolus infusion). No prior anthracycline therapy. Prior cardiac radiotherapy possible in 18 participants in the dexrazoxane group and 13 participants in the control group (< 20 Gy on the heart). No prior cardiac dysfunction (defined as congestive heart failure; resting LVEF < 45%). | |
| Interventions | Dexrazoxane (1000 mg/m2 dexrazoxane versus 160 mg/m2 epirubicin; IV infusion over 15 minutes 30 minutes before epirubicin) (N = 63) versus no cardioprotective intervention (N = 66). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as NYHA class 2,3 or 4; subclinical myocardial dysfunction defined as a decrease in left ventricular ejection fraction as measured by MUGA to less than 45% or a decrease from baseline of >= 20% and no development of clinical heart failure later on). Tumour response rate (according to standard WHO criteria: a 50% decrease (or 30% decrease in one diameter) was required for assessable disease). Adverse effects (according to WHO criteria). |
|
| Notes | Length of follow‐up nm. Median age in intervention group for breast cancer: 55 years and for soft tissue sarcoma: 55 years; median age in control group for breast cancer: 58 years and for soft tissue sarcoma: 51 years. Cumulative anthracycline dose in intervention group: median 960 mg/m2; cumulative anthracycline dose in control group: median 880 mg/m2. Gender: 57 (86%) females and 9 (14%) males in the dexrazoxane group and 52 (83%) females and 11 (17%) males in control group. Stage of disease per treatment group comparable. Funding sources nm. Declaration of interests nm. The Vici 1998 publication was a duplicate publication of this study; we did not use information from this publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the method of randomisation was provided. |
| Allocation concealment (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the method of randomisation was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to the received intervention (either dexrazoxane or no cardioprotective intervention). |
| Blinding of outcome assessors (detection bias) ‐ clinical heart failure | Unclear risk | No information on blinding of outcome assessors provided for clinical heart failure |
| Blinding of outcome assessors (detection bias) ‐ clinical heart failure and subclinical myocardial dysfunction combined | Unclear risk | No information on blinding of outcome assessors provided for both clinical heart failure and subclinical myocardial dysfunction |
| Blinding of outcome assessors (detection bias) ‐ tumour response rate | Unclear risk | No information on blinding of outcome assessors provided for tumour response |
| Blinding of outcome assessors (detection bias) ‐ toxicities other than cardiac damage (diagnosed by laboratory tests) | Low risk | No information on blinding of outcome assessors provided, but as blinding is not relevant for outcomes diagnosed by laboratory test, we judged this outcome at low risk of detection bias for the following adverse effects: thrombocytopenia, neutropenia, anaemia. |
| Blinding of outcome assessors (detection bias) ‐ toxicities other than cardiac damage (not diagnosed by laboratory tests) | Unclear risk | No information on blinding of outcome assessors provided for toxicities other than cardiac damage not diagnosed by a laboratory test (i.e. all toxicities not mentioned above). |
| Incomplete outcome data (attrition bias) ‐ clinical heart failure | Low risk | Clinical heart failure evaluated in 94% of participants in both treatment groups (59/63 of the dexrazoxane group and 62/66 of the control group). |
| Incomplete outcome data (attrition bias) ‐ clinical heart failure and subclinical myocardial dysfunction combined | Low risk | Clinical heart failure and subclinical myocardial dysfunction both evaluated in 94% of participants in both treatment groups (59/63 of the dexrazoxane group and 62/66 of the control group). |
| Incomplete outcome data (attrition bias) ‐ tumour response rate | Low risk | Tumour response evaluated in 97% of participants in the dexrazoxane group and 98% of the control group (61/63 of the dexrazoxane group and 65/66 of the control group). |
| Incomplete outcome data (attrition bias) ‐ toxicities other than cardiac damage with the exception of SMN | Low risk | Toxicities other than cardiac damage evaluated in 98% of participants in the dexrazoxane group and 100% of the control group (62/63 of the dexrazoxane group and 66/66 of the control group). |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported (although overall survival was not eligible for inclusion in the review). |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: unclear (information on both method of randomisation and blinding of outcome assessors not provided). Baseline imbalance between treatment groups related to outcome (prior cardiotoxic treatment (anthracyclines and cardiac irradiation), age, gender, stage of disease and prior cardiac dysfunction): unclear (no prior cardiac dysfunction; age, sex and stage of disease well balanced; prior cardiotoxic treatment unclear) Difference in length of follow‐up between treatment groups: unclear (length of follow‐up nm) |
Marty 2006.
| Study characteristics | ||
| Methods | Randomisation was performed centrally using a permuted block design, which was stratified by centre and thus by type of anthracycline used and dose of dexrazoxane (open‐label study). | |
| Participants | 164 participants (median age 52 years (range 30 to 76); all females) with advanced or metastatic breast cancer treated with either epirubicin or doxorubicin (cumulative dose: see notes; peak dose (i.e. maximal dose received in 1 week) see notes; infusion duration nm). Prior anthracycline therapy in both treatment groups (median cumulative dose similar in both: dexrazoxane group: a median cumulative doxorubicin dose of 290 mg/m2 (range 30 to 650) in 46 participants and a median cumulative epirubicin dose of 421 mg/m2 (range 231 to 599) in 42 participants; some participants were treated with both doxorubicin and epirubicin; control group: a median cumulative doxorubicin dose of 243 mg/m2 (range 60 to 480) in 44 participants and a median cumulative epirubicin dose of 360 mg/m2 (range 94 to 599) in 38 participants; some participants were treated with both doxorubicin and epirubicin). Prior cardiac radiotherapy was possible for 74 participants randomised to dexrazoxane and 62 participants in the control group (dose nm). No prior cardiac dysfunction (defined as congestive heart failure; a normal LVEF according to the lower limit of the normal range in use in the centre). | |
| Interventions | Dexrazoxane (20:1 ratio of dexrazoxane to doxorubicin and 10:1 ratio to epirubicin; IV infusion over 15 minutes 30 minutes prior to anthracycline infusion) (N = 85) versus no cardioprotective intervention (N = 79). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as clinical signs of cardiac insufficiency (graded according to NYHA criteria); subclinical myocardial dysfunction defined as 1) a reduction in LVEF by 10% absolute percentage points or more as measured by MUGA scan or 15% or more as measured by echocardiography, 2) a reduction in absolute LVEF as measured by echocardiography or MUGA scan to a value below 45%). Tumour response rate (according to WHO criteria). Overall survival (defined as time from first date of study treatment to death or date of last contact for living participants). Progression‐free survival (defined as time from first date of complete response, partial response or stable disease until the date progressive disease was first noticed). Adverse effects (according to CTCv2 criteria). |
|
| Notes | Length of follow‐up nm. Median age in intervention group 50 years; median age in control group 52 years. The cumulative anthracycline dose was calculated as all anthracyclines received during this study and prior to it using 50 mg/m2 epirubicin = 90 mg/m2 doxorubicin. The median cumulative anthracycline dose in the dexrazoxane group was 669 mg/m2 (range 247 to 936); the median anthracycline peak dose (i.e. maximal dose received in 1 week) was 80 mg/m2 (range 37 to 116). The median cumulative anthracycline dose in the control group was 608 mg/m2 (range 244 to 900); the median anthracycline peak dose was 80 mg/m2 (40 to 120). Gender: all females in both intervention and control group. Stage of disease per treatment group: 11 (13%) stage I, 48 (56%) stage II, 19 (22%) stage III, 5 (6%) stage IV, for 2 participants nm in the dexrazoxane group and 11 (14%) stage I, 42 (53%) stage II, 13 (16%) stage III, 11 (14%) stage IV, for 2 participants nm in the control group. Funding sources: Chiron Biopharmaceuticals Declaration of interests nm. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed centrally |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to the received intervention (either dexrazoxane or no cardioprotective intervention) |
| Blinding of outcome assessors (detection bias) ‐ clinical heart failure | Low risk | The outcome assessors of clinical heart failure were blinded to treatment. |
| Blinding of outcome assessors (detection bias) ‐ clinical heart failure and subclinical myocardial dysfunction combined | Low risk | The outcome assessors of both clinical heart failure and subclinical cardiac damage were blinded to treatment. |
| Blinding of outcome assessors (detection bias) ‐ overall survival/overall mortality | Low risk | No information on blinding of outcome assessors provided, but as blinding is not relevant for the outcome of overall survival, we judged this outcome at low risk of detection bias. |
| Blinding of outcome assessors (detection bias) ‐ tumour response rate | Unclear risk | No information on blinding of outcome assessors provided for tumour response |
| Blinding of outcome assessors (detection bias) ‐ progression‐free survival | Unclear risk | No information on blinding of outcome assessors provided for PFS |
| Blinding of outcome assessors (detection bias) ‐ toxicities other than cardiac damage (diagnosed by laboratory tests) | Low risk | No information on blinding of outcome assessors provided, but as blinding is not relevant for outcomes diagnosed by laboratory test, we judged this outcome at low risk of detection bias for the following adverse effects: thrombocytopenia, neutropenia, anaemia, leukopenia. |
| Blinding of outcome assessors (detection bias) ‐ toxicities other than cardiac damage (not diagnosed by laboratory tests) | Unclear risk | No information on blinding of outcome assessors provided for toxicities other than cardiac damage not diagnosed by a laboratory test (i.e. all toxicities not mentioned above). |
| Incomplete outcome data (attrition bias) ‐ clinical heart failure | Low risk | Clinical heart failure evaluated in 93% of participants in the dexrazoxane group and 94% of the control group (79/85 of the dexrazoxane group and 74/79 of the control group). |
| Incomplete outcome data (attrition bias) ‐ clinical heart failure and subclinical myocardial dysfunction combined | Low risk | Clinical heart failure evaluated in 93% of participants in the dexrazoxane group and 94% of the control group (79/85 of the dexrazoxane group and 74/79 of the control group); subclinical myocardial dysfunction evaluated in 92% of participants in the dexrazoxane group and 94% of the control group (78/85 of the dexrazoxane group and 74/79 of the control group). |
| Incomplete outcome data (attrition bias) ‐ overall survival/overall mortality | High risk | Overall survival evaluated in 80% of participants in both treatment groups (68/85 of the dexrazoxane group and 63/79 of the control group). |
| Incomplete outcome data (attrition bias) ‐ tumour response rate | High risk | Tumour response evaluated in 89% of participants in the dexrazoxane group and 94% of the control group (76/85 of the dexrazoxane group and 74/79 of the control group). |
| Incomplete outcome data (attrition bias) ‐ progression‐free survival | Low risk | PFS evaluated in 100% of participants in both treatment groups. |
| Incomplete outcome data (attrition bias) ‐ toxicities other than cardiac damage with the exception of SMN | Low risk | Toxicities other than cardiac damage evaluated in 100% of participants in both treatment groups. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: unclear (information on blinding of outcome assessors for some of the outcomes not provided; block randomisation was used). Baseline imbalance between treatment groups related to outcome (prior cardiotoxic treatment (anthracyclines and cardiac irradiation), age, gender, stage of disease and prior cardiac dysfunction): unclear (prior cardiac irradiation unclear; all other factors including prior anthracyclines comparable between treatment groups) Difference in length of follow‐up between treatment groups: unclear (length of follow‐up nm) |
P9404.
| Study characteristics | ||
| Methods | Method of randomisation not clear (stratified by disease (T‐ALL versus NHL) and presence of CNS disease at diagnosis). | |
| Participants | 537 children (mean age 9.8 years; 407 males and 130 females) with T‐ALL (N = 362; stage unclear) or L‐NHL (N = 174; stage III or IV) (for 1 participant the diagnosis was nm) treated with doxorubicin (cumulative dose nm, but according to protocol all participants should have received 360 mg/m2 and it was reported that all participants received the same cumulative dose; peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm), vincristine, prednisone, methotrexate (some participants), mercaptopurine, Escherichia coli L‐asparaginase, intrathecal chemotherapy and cranial radiation). Prior anthracyclines no. Prior cardiac radiotherapy possible (emergency mediastinal radiotherapy for severe respiratory distress was allowed), but numbers nm. Prior cardiac dysfunction nm. | |
| Interventions | Dexrazoxane (300 mg/m2 in 10:1 ratio of dexrazoxane to doxorubicin; IV bolus infusion immediately before each doxorubicin dose; exact infusion duration nm) (n = 273) versus no cardioprotective intervention (n = 264). | |
| Outcomes | Heart failure (i.e. clinical heart failure (no definition provided); primary cause of death cardiomyopathy/heart failure; subclinical myocardial dysfunction defined as decreased LVFS; however it was stated that toxicity was graded according to NCI CTCAEv2 criteria, grade 3 or higher but LVFS is not included in that definition) Overall mortality (time from cancer diagnosis to death) Adverse effects (according to NCI CTCAEv2) |
|
| Notes | Length of follow‐up: median 9.2 years (range 0.01 to 15.0) (in intervention group 9.4 years (0.01 to 15) and in control group 8.9 years (0.02 to 14.7)) Mean age in intervention group 9.9 years and in control group 9.7 years Cumulative anthracycline dose per treatment group nm, but it was reported that all participants received the same cumulative dose. Gender: 69 (25.3%) females and 204 (74.7%) males in the dexrazoxane group and 61 (23.1%) females and 203 (76.9%) males in control group. Stage of disease per treatment group: for T‐ALL nm. For L‐NHL in intervention group: stage III N = 52 (19%) and stage IV N = 33 (12.1%) and in the control group stage III N = 67 (25.4%) and stage IV N = 22 (8.3%). Long‐term follow‐up data for overall mortality and primary cause of death cardiomyopathy/heart failure have been published, these outcomes were not included in the original publication by P9404 (P9426); all 537 randomised participants were included; median follow‐up was 12.4 years (range 0 to 15.5). Funding sources: supported in part by the Clinical Trials Evaluation Program of the National Cancer Institute, National Institutes of Health grants No. U10 CA098543, U10 CA098413, U10 CA180886, and U10 CA180899, and the Michael Garil Fund. A complete listing of grant support for research conducted by the Pediatric Oncology Group and Children’s Cancer Study Group before initiation of the Children’s Oncology Group grant in 2003 is available online at http://www.childrensoncologygroup.org/admin/grantinfo.htm (P9404) and grants No. U10 CA09543 and K07 CA151775 from the US National Institutes of Health, by St Baldrick’s Foundation, and by the Leukemia and Lymphoma Society for the Children’s Oncology Group study (Effects of Dexrazoxane Hydrochloride on Biomarkers Associated With Cardiomyopathy and Heart Failure After Cancer Treatment [HEART (ALTE11C2)]) (P9426). Declaration of interests: some authors reported research funding by Amgen, MedImmune, Bristol‐Myers Squibb, Becton Dickinson, Pfizer, Roche and consulting or advisory roles and speakers' bureau by Clinigen Group, Jazz Pharmaceuticals, Sigma Tau Pharmaceuticals (P9404) and some authors reported research funding by Merck, Roche Diagnostics, Pfizer and consulting or advisory roles and speakers' bureau by Clinigen Group, Jazz Pharmaceuticals, Sigma Tau Pharmaceuticals and travel, accommodation and expenses by Clinigen Group (P9426). The Asselin 2012 publication was a conference proceeding of this study; we did not use information from this publication. The Fernandez 2017 publication was a duplicate publication of this study, but as no data on dexrazoxane versus control participants was provided we did not use any information from this publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the method of randomisation was provided. |
| Allocation concealment (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the method of randomisation was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to the received intervention (either dexrazoxane or no cardioprotective intervention). |
| Blinding of outcome assessors (detection bias) ‐ clinical heart failure | Unclear risk | No information on blinding of outcome assessors provided for clinical heart failure |
| Blinding of outcome assessors (detection bias) ‐ cardiomyopathy/heart failure as primary cause of death | Low risk | The outcome assessors of cardiomyopathy/heart failure as primary cause of death were blinded to treatment. |
| Blinding of outcome assessors (detection bias) ‐ clinical heart failure and subclinical myocardial dysfunction combined | Unclear risk | The outcome assessors for subclinical myocardial dysfunction were blinded to the treatment assignment of the participants, but no information on blinding of outcome assessors provided for clinical heart failure |
| Blinding of outcome assessors (detection bias) ‐ overall survival/overall mortality | Low risk | No information on blinding of outcome assessors provided, but as blinding is not relevant for the outcome overall mortality, we judged this outcome at low risk of detection bias. |
| Blinding of outcome assessors (detection bias) ‐ toxicities other than cardiac damage (not diagnosed by laboratory tests) | Unclear risk | No information on blinding of outcome assessors provided for toxicities other than cardiac damage (haematologic effects might include toxicities diagnosed by laboratory tests only and thus at a low risk of bias, but as this was not further specified, we judged this outcome to be at unclear risk). |
| Incomplete outcome data (attrition bias) ‐ clinical heart failure | Low risk | Clinical heart failure evaluated in 100% of participants in both treatment groups. |
| Incomplete outcome data (attrition bias) ‐ cardiomyopathy/heart failure as primary cause of death | Low risk | Cardiomyopathy/heart failure as primary cause of death evaluated in 100% of participants in both treatment groups. |
| Incomplete outcome data (attrition bias) ‐ clinical heart failure and subclinical myocardial dysfunction combined | High risk | Clinical heart failure evaluated in 100% of all participants in both treatment groups; for subclinical myocardial dysfunction, an overall number for the actual time point included for each participant in the analyses is missing, but subclinical myocardial dysfunction was evaluated in between 30% (82/273) and 11% (31/273) of participants in the intervention group and between 32% (84/264) and 8% (21/264) in the control group at different time points, so we judged this to be a high risk of bias. |
| Incomplete outcome data (attrition bias) ‐ overall survival/overall mortality | Low risk | Overall mortality evaluated in 100% of participants in both treatment groups. |
| Incomplete outcome data (attrition bias) ‐ toxicities other than cardiac damage with the exception of SMN | Low risk | Toxicities other than cardiac damage were evaluated in 100% of participants in both treatment groups. |
| Incomplete outcome data (attrition bias) ‐ SMN | Low risk | SMN evaluated in 100% of participants in both treatment groups. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: unclear (information on both method of randomisation and blinding of some of the outcome assessors not provided). Baseline imbalance between treatment groups related to outcome (prior cardiotoxic treatment (anthracyclines and cardiac irradiation), age, gender, stage of disease and prior cardiac dysfunction): unclear for prior cardiac radiotherapy, stage of disease and prior cardiac dysfunction; other items no imbalance Difference in length of follow‐up between treatment groups: comparable between treatment groups |
P9425.
| Study characteristics | ||
| Methods | Method of randomisation not clear. | |
| Participants | 216 children (mean age 14 years (range 4 to 21); 140 males and 76 females) with intermediate or high risk Hodgkin lymphoma (stage IB N = 1, stage II N = 81, stage III N = 52, stage IV N = 70, stage unknown N = 12) treated with multiagent chemotherapy including doxorubicin (cumulative dose nm (according to protocol 180 mg/m2 for participants with rapid early response and 300 mg/m2 for participants with slow early response; it was stated that there were virtually no dose reductions); peak dose (i.e. maximal dose received in 1 week) 60 mg/m2; infusion duration nm). Participants received 21 Gy of radiotherapy to mantle if it involved Hodgkin lymphoma; pericardial infusions, lung disease or pericardial involvement were treated with 10.5 Gy (no further information provided). Prior anthracycline therapy nm. Prior cardiac radiotherapy nm. Prior cardiac dysfunction nm (definition nm). | |
| Interventions | Dexrazoxane (300 mg/m2 in 10:1 ratio of dexrazoxane to doxorubicin; IV bolus infusion immediately before each doxorubicin dose; exact infusion duration nm) (N = 107) versus no cardioprotective intervention (N = 109). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined according to NCI‐CTCv2.0 criteria; primary cause of death cardiomyopathy/heart failure) Overall mortality (time from cancer diagnosis to death) Tumour response rate (complete response defined as disappearance of active Hodgkin lymphoma (gallium negative, ≥ 70% decrease in the sum of the products of the perpendicular diameters of measurable lesions, and negative bone marrow or bone scan if initially positive). Adverse effects (according to NCI‐CTCv2.0 criteria). |
|
| Notes | Length of follow‐up nm (median follow‐up for participants without an event was 5.2 years). Median age in dexrazoxane group 14.8 years (range 3.7 to 20) and in control group 14.9 years (range 5.6 to 20.8). Cumulative anthracycline dose per treatment group nm. No significant difference in number of participants with rapid and slow early response between treatment groups identified (P = 0.07). It was stated that dexrazoxane was also given on day 7 (besides on day 0 and 1 together with doxorubicin) (in P9426 this was reported to be day 8 in the same 10:1 ratio). Gender: 37 (34.6%) females and 70 (65.4%) males in the dexrazoxane group and 39 (35.8%) females and 70 (64.2%) males in control group. Stage of disease per treatment group: nm. Long‐term follow‐up data for overall mortality and primary cause of death cardiomyopathy/heart failure have been published, these outcomes were not included in the original publication by P9425 (P9426); all 216 randomised participants were included; median follow‐up was 13 years (range 0 to 14.7). Funding sources: the Chair’s Grant U10 CA98543 and the Statistics and Data Center (Grant U10 CA98413) of the Children’s Oncology Group (COG) from the National Cancer Institute, National Institutes of Health, Bethesda, MD. A complete listing of grant support for research conducted by CCG and POG before initiation of the COG grant in 2003 is available online at http://www.childrensoncologygroup.org/admin/grantinfo.htm (P9425) and grants No. U10 CA09543 and K07 CA151775 from the US National Institutes of Health, by St Baldrick’s Foundation, and by the Leukemia and Lymphoma Society for the Children’s Oncology Group study (Effects of Dexrazoxane Hydrochloride on Biomarkers Associated With Cardiomyopathy and Heart Failure After Cancer Treatment [HEART (ALTE11C2)]) (P9426). Declaration of interests: the authors declare no competing financial interests (P9425) and some authors reported research funding by Merck, Roche Diagnostics, Pfizer and consulting or advisory roles and speakers' bureau by Clinigen Group, Jazz Pharmaceuticals, Sigma Tau Pharmaceuticals and travel, accommodation and expenses by Clinigen Group (P9426). Results on secondary malignant neoplasms were also described in the Tebbi 2007 publication, but as more extended data were available in the other publications we did not use data from the Tebbi 2007 paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the method of randomisation was provided. |
| Allocation concealment (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the method of randomisation was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to the received intervention (either dexrazoxane or no cardioprotective intervention). |
| Blinding of outcome assessors (detection bias) ‐ clinical heart failure | Unclear risk | No information on blinding of outcome assessors provided for clinical heart failure. |
| Blinding of outcome assessors (detection bias) ‐ cardiomyopathy/heart failure as primary cause of death | Low risk | The outcome assessors of cardiomyopathy/heart failure as primary cause of death were blinded to treatment. |
| Blinding of outcome assessors (detection bias) ‐ overall survival/overall mortality | Low risk | No information on blinding of outcome assessors provided, but as blinding is not relevant for the outcome overall mortality, we judged this outcome at low risk of detection bias. |
| Blinding of outcome assessors (detection bias) ‐ tumour response rate | Unclear risk | No information on blinding of outcome assessors provided for tumour response. |
| Blinding of outcome assessors (detection bias) ‐ toxicities other than cardiac damage (diagnosed by laboratory tests) | Low risk | No information on blinding of outcome assessors provided, but as blinding is not relevant for outcomes diagnosed by laboratory test, we judged this outcome at low risk of detection bias for the following adverse effects: anaemia, absolute neutrophil count, platelets. |
| Blinding of outcome assessors (detection bias) ‐ toxicities other than cardiac damage (not diagnosed by laboratory tests) | Unclear risk | No information on blinding of outcome assessors provided for toxicities other than cardiac damage not diagnosed by a laboratory test (i.e. all toxicities not mentioned above). |
| Incomplete outcome data (attrition bias) ‐ clinical heart failure | Low risk | Clinical heart failure evaluated in 99% of participants in both treatment groups (106/107 of the dexrazoxane group and 108/109 of the control group). |
| Incomplete outcome data (attrition bias) ‐ cardiomyopathy/heart failure as primary cause of death | Low risk | Cardiomyopathy/heart failure as primary cause of death evaluated in 100% of participants in both treatment groups. |
| Incomplete outcome data (attrition bias) ‐ overall survival/overall mortality | Low risk | Overall mortality evaluated in 100% of participants in both treatment groups. |
| Incomplete outcome data (attrition bias) ‐ tumour response rate | Low risk | Tumour response evaluated in 94% (101/107) of the dexrazoxane group and 91% (99/109) of the control group. |
| Incomplete outcome data (attrition bias) ‐ toxicities other than cardiac damage with the exception of SMN | Low risk | Toxicities other than cardiac damage evaluated in 99% of participants in both treatment groups (106/107 of the dexrazoxane group and 108/109 of the control group). |
| Incomplete outcome data (attrition bias) ‐ SMN | Low risk | SMN evaluated in 100% of participants in both treatment groups. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: unclear (information on both method of randomisation and blinding of outcome assessors not provided) Baseline imbalance between treatment groups related to outcome (prior cardiotoxic treatment (anthracyclines and cardiac irradiation), age, gender, stage of disease and prior cardiac dysfunction): unclear (similar for age and gender, all other factors unclear) Difference in length of follow‐up between treatment groups: unclear (length of follow‐up nm) |
P9426.
| Study characteristics | ||
| Methods | Method of randomisation not clear. | |
| Participants | 255 children (median age 14 years (range 2.1 to 20.9); 150 males and 105 females) with low risk HL (N = 61 stage IA, N = 179 IIA, N = 14 IIIA1 and N = 1 nm)treated with doxorubicin (cumulative dose nm, but according to study protocol this ranged from 100 to 200 mg/m2 and it was stated that received dose was in high compliance with prescribed dose); peak dose (i.e. maximal dose received in 1 week) 25mg/m2; infusion duration: push), vincristine, bleomycin, and etoposide followed, at least for some children, by involved region consolidative radiotherapy. Prior anthracyclines no. Prior cardiac radiotherapy no. Prior cardiac dysfunction nm. | |
| Interventions | Dexrazoxane (250 mg/m2 in 10:1 ratio of dexrazoxane to doxorubicin; IV bolus/push infusion maximal 30 minutes before each doxorubicin dose (see notes)) (N = 127) versus no cardioprotective intervention (N = 128). | |
| Outcomes | Heart failure (i.e. primary cause of death cardiomyopathy/heart failure) Overall mortality (time from cancer diagnosis to death) Adverse effects (definition nm). |
|
| Notes | Length of follow‐up: median 12.4 years (range 1.4 to 15) (in intervention group 12.4 years (1.4 to 15) and in control group 12.4 years (1.8 to 14.8)). Median age at treatment in intervention group 14.2 years (range 2.1 to 20.9) and in control group 13.9 years (3.7 to 19.7). Cumulative anthracycline dose per treatment group nm. Gender: 53 (41.7%) females and 74 (58.3%) males in the dexrazoxane group and 52 (40.6%) females and 76 (59.4%) males in control group. Stage of disease per treatment group: all low risk. Data for secondary malignancies have been published (Tebbi 2007); 262 randomised participants were included (seven more than in P9426 primary reference); median follow‐up was 55.5 months from date of enrolment. Gender 153 males and 109 females; median age at diagnosis 12.5 years. It was stated that dexrazoxane was also given prior to bleomycin. Data for adverse effects other than secondary malignancies have been published (Tebbi 2012); all 255 randomised participants were included; median follow‐up time 7.5 years in participants alive without an event. Age was reported as median 13 years (range 2 to 20); unclear if this is age at diagnosis or age at treatment and therefore unclear if different from P9426 primary reference. It was stated that dexrazoxane was also given prior to bleomycin. Funding sources: supported by Grants No. U10 CA09543 and K07 CA151775 from the US National Institutes of Health, by St Baldrick’s Foundation, and by the Leukemia and Lymphoma Society for the Children’s Oncology Group study (Effects of Dexrazoxane Hydrochloride on Biomarkers Associated With Cardiomyopathy and Heart Failure After Cancer Treatment [HEART (ALTE11C2)]) (P9426 primary reference); not reported (Tebbi 2007); QARC GRANT NIH/NCI CA29511, the Chair’s Grant U10 CA98543‐08 and Statistics and Data Center Grant U10 CA98413‐08 of the Children’s Oncology Group from the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA. A complete listing of grant support for research conducted by CCG and POG before initiation of the COG grant in 2003 is available online at: http://www.childrensoncologygroup.org/admin/grantinfo.htm (Tebbi 2012). Declaration of interests: some authors reported research funding by Merck, Roche Diagnostics, Pfizer and consulting or advisory roles and speakers' bureau by Clinigen Group, Jazz Pharmaceuticals, Sima Tau Pharmaceuticals and travel, accommodation and expenses by Clinigen Group (P9426 primary reference); nothing to declare (Tebbi 2007; Tebbi 2012). The Chow 2014 publication is a conference proceeding of this study; we did not use information from this publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the method of randomisation was provided. |
| Allocation concealment (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the method of randomisation was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to the received intervention (either dexrazoxane or no cardioprotective intervention). |
| Blinding of outcome assessors (detection bias) ‐ cardiomyopathy/heart failure as primary cause of death | Low risk | The outcome assessors of cardiomyopathy/heart failure as primary cause of death were blinded to treatment. |
| Blinding of outcome assessors (detection bias) ‐ overall survival/overall mortality | Low risk | The outcome assessors of overall mortality were blinded to treatment. |
| Blinding of outcome assessors (detection bias) ‐ toxicities other than cardiac damage (diagnosed by laboratory tests) | Low risk | No information on blinding of outcome assessors provided, but as blinding is not relevant for outcomes diagnosed by laboratory test, we judged this outcome at low risk of detection bias for the following adverse effects: white blood cell count, absolute neutrophil count, lymphs, platelets and haemoglobin. |
| Blinding of outcome assessors (detection bias) ‐ toxicities other than cardiac damage (not diagnosed by laboratory tests) | Unclear risk | No information on blinding of outcome assessors provided for toxicities other than cardiac damage not diagnosed by a laboratory test (i.e. all toxicities not mentioned above). |
| Incomplete outcome data (attrition bias) ‐ cardiomyopathy/heart failure as primary cause of death | Low risk | Cardiomyopathy/heart failure as primary cause of death evaluated in 100% of participants in both treatment groups. |
| Incomplete outcome data (attrition bias) ‐ overall survival/overall mortality | Low risk | Overall mortality evaluated in 100% of participants in both treatment groups. |
| Incomplete outcome data (attrition bias) ‐ toxicities other than cardiac damage with the exception of SMN | High risk | Toxicities other than cardiac damage evaluated in 86% of participants in the dexrazoxane group and 88% in the control group (109/127 of the dexrazoxane group and 113/128 of the control group). |
| Incomplete outcome data (attrition bias) ‐ SMN | Low risk | SMN evaluated in 100% of participants in both treatment groups. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: unclear (information on both method of randomisation and blinding of some of the outcome assessors not provided). Baseline imbalance between treatment groups related to outcome (prior cardiotoxic treatment (anthracyclines and cardiac irradiation), age, gender, stage of disease and prior cardiac dysfunction): unclear for stage of disease and prior cardiac dysfunction; other items no imbalance Difference in length of follow‐up between treatment groups: comparable between treatment groups for P9426 primary reference; for Tebbi 2007 and Tebbi 2012 nm. |
Speyer 1992.
| Study characteristics | ||
| Methods | Method of randomisation not clear (participants were stratified by prior adjuvant cyclophosphamide, methotrexate and 5FU and cardiac risk factors; blocks of 10 participants within each stratum). | |
| Participants | 150 participants (aged 27 to 76 years; all females) with breast cancer (stage nm) treated with doxorubicin (cumulative dose range 25 to 2150 mg/m2; peak dose (i.e. maximal dose received in 1 week) 50 mg/m2; infusion duration 5 to 10 minutes), 5FU and cyclophosphamide. No prior anthracycline therapy. Prior cardiac radiotherapy possible in 28 participants (14 in each treatment group). No prior cardiac dysfunction (defined as congestive heart failure; LVEF on resting MUGA scan < 0.50). | |
| Interventions | Dexrazoxane (20:1 ratio of dexrazoxane to doxorubicin; IV infusion over 15 minutes 30 minutes before doxorubicin) (N = 76) versus no cardioprotective intervention (N = 74). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as NYHA class 2,3 or 4 i.e. any signs and symptoms of clinical congestive heart failure; subclinical myocardial dysfunction defined as NYHA class 1 i.e. a decrease in LVEF as measured by MUGA of ≥ 20% from baseline or a decrease in LVEF as measured by MUGA to < 45% or an endomyocardial biopsy score ≥ 2 according to the Billingham scale (Billingham 1978)). Tumour response rate (according to ECOG criteria) Overall survival (defined as time to survival; starting point nm) Progression‐free survival (defined as time to progression; starting point nm) Adverse effects (definitions provided, but not according to specific criteria) |
|
| Notes | An endomyocardial biopsy was not performed in all participants. Length of follow‐up nm. Age in intervention group: mean 55.5 years and median 58 years; age in control group: mean 56.2 years and median 58 years. Cumulative anthracycline dose in intervention group: mean 558 mg/m2 (range 50 to 2150); cumulative anthracycline dose in control group: mean 407.4 mg/m2 (range 25 to 950). Gender: all females in both treatment groups. Stage of disease per treatment group nm. Funding sources: grant no. 36524 from the United States Public Health Service, by grants no. CA‐16087 and CRC‐RR‐99 from the National Institutes of Health, by the Lila Motley Foundation, and by the Chemotherapy Foundation. Dexrazoxane was supplied by the NCI. Declaration of interests nm. The Speyer 1988 and Speyer 1990 publications are duplicate publications of this study; we did not use information from these publications. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the method of randomisation was provided. |
| Allocation concealment (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the method of randomisation was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to the received intervention (either dexrazoxane or no cardioprotective intervention). |
| Blinding of outcome assessors (detection bias) ‐ clinical heart failure | Low risk | The outcome assessors of clinical heart failure were blinded to treatment. |
| Blinding of outcome assessors (detection bias) ‐ clinical heart failure and subclinical myocardial dysfunction combined | Low risk | The outcome assessors of both clinical heart failure and subclinical cardiac damage were blinded to treatment. |
| Blinding of outcome assessors (detection bias) ‐ overall survival/overall mortality | Low risk | No information on blinding of outcome assessors provided, but as blinding is not relevant for the outcome overall survival, we judged this outcome at low risk of detection bias. |
| Blinding of outcome assessors (detection bias) ‐ tumour response rate | Unclear risk | No information on blinding of outcome assessors provided for tumour response |
| Blinding of outcome assessors (detection bias) ‐ progression‐free survival | Unclear risk | No information on blinding of outcome assessors provided for PFS |
| Blinding of outcome assessors (detection bias) ‐ toxicities other than cardiac damage (not diagnosed by laboratory tests) | Unclear risk | No information on blinding of outcome assessors provided for toxicities other than cardiac damage |
| Incomplete outcome data (attrition bias) ‐ clinical heart failure | Low risk | Clinical heart failure evaluated in 100% of all participants in both treatment groups. |
| Incomplete outcome data (attrition bias) ‐ clinical heart failure and subclinical myocardial dysfunction combined | Unclear risk | Clinical heart failure evaluated in 100% of all participants in both treatment groups; unclear in how many participants subclinical myocardial dysfunction was evaluated. |
| Incomplete outcome data (attrition bias) ‐ overall survival/overall mortality | Unclear risk | Unclear in how many participants overall survival was evaluated |
| Incomplete outcome data (attrition bias) ‐ tumour response rate | Low risk | Tumour response evaluated in 100% of all participants in both treatment groups |
| Incomplete outcome data (attrition bias) ‐ progression‐free survival | Unclear risk | Unclear in how many participants PFS was evaluated |
| Incomplete outcome data (attrition bias) ‐ toxicities other than cardiac damage with the exception of SMN | Low risk | Toxicities other than cardiac damage evaluated in 100% of all participants in both treatment groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: unclear (information on blinding of outcome assessors for some of the outcomes not provided; block randomisation was used). Baseline imbalance between treatment groups related to outcome (prior cardiotoxic treatment (anthracyclines and cardiac irradiation), age, gender, stage of disease and prior cardiac dysfunction): unclear (age and gender comparable, no prior anthracyclines, no prior cardiac dysfunction, stage of disease and prior cardiac irradiation unclear) Difference in length of follow‐up between treatment groups: unclear (length of follow‐up nm) |
Sun 2016.
| Study characteristics | ||
| Methods | Randomisation was performed at a 1:1 ratio using a randomisation number table. | |
| Participants | 110 participants (age for all randomised participants nm: see notes; gender nm) with diabetes type 2 and early stage breast cancer (either stage I or II, no numbers provided for all randomised participants) treated with epirubicin (cumulative dose nm, but according to protocol all participants should have received 480 mg/m2; peak dose nm; infusion duration nm) and cyclophosphamide. No prior anthracycline therapy. No prior cardiac radiotherapy. No prior cardiac dysfunction (defined as congestive heart failure, existing cardiovascular symptoms, LVEF < 50%, cardiomyopathy). | |
| Interventions | Dexrazoxane (800 mg/m2 in 10:1 ratio of dexrazoxane to epirubicin; IV 30 minutes prior to epirubicin; infusion duration nm) (n = 55) versus placebo (0.9% NaCl solution; IV 30 minutes prior to epirubicin; infusion duration nm) (n = 55). | |
| Outcomes | Heart failure (clinical heart failure defined as symptoms of heart failure) Adverse effects (no definition provided) |
|
| Notes | Length of follow‐up nm (but no significant difference between treatment groups); treatment duration was 126 days and postchemotherapy measurements were reported. Age in treatment groups nm, but for the participants with a cardiac outcome assessment, it was mean 53.82 years (N = 51) in the dexrazoxane group and 55.25 years in the control group (N = 52). Gender per treatment group: nm for the different treatment groups. Stage of disease per treatment group: nm, but for the participants with a cardiac outcome assessment: in dexrazoxane N = 9/51 (17.6%) stage I and N = 42/51 (82.4%) stage II, and in the control group: N = 10/52 stage I (19.2%) and N = 42/52 (80.8%) stage II. Funding sources: Health and Family Planning Commission of Hebei Province, 2016, key projects of medical science research in Hebei Province (project no.20160646). It is unclear if this was actual funding. Declaration of interests: the authors had no conflicts of interest to disclose. The ChiCTR‐IPR‐16007759 publication is the ongoing trial registration of this study; we did not use information from this publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation number table was used. |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment was provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were blinded to the received intervention (either dexrazoxane or placebo), but no information about blinding of personnel was provided. |
| Blinding of outcome assessors (detection bias) ‐ clinical heart failure | Unclear risk | No information on blinding of outcome assessors provided for clinical heart failure |
| Blinding of outcome assessors (detection bias) ‐ toxicities other than cardiac damage (diagnosed by laboratory tests) | Low risk | No information on blinding of outcome assessors provided, but as blinding is not relevant for outcomes diagnosed by laboratory test we judged this outcome at low risk of detection bias for the following adverse effect: myelosuppression |
| Blinding of outcome assessors (detection bias) ‐ toxicities other than cardiac damage (not diagnosed by laboratory tests) | Unclear risk | No information on blinding of outcome assessors provided for toxicities other than cardiac damage not diagnosed by a laboratory test (no definition of liver damage was provided, but we assume that that diagnosis involved more than only a laboratory test). |
| Incomplete outcome data (attrition bias) ‐ clinical heart failure | Low risk | Clinical heart failure evaluated in 92.7% of participants in the dexrazoxane group and 94.5% in the control group (51/55 of the dexrazoxane group and 52/55 of the control group). |
| Incomplete outcome data (attrition bias) ‐ toxicities other than cardiac damage with the exception of SMN | Low risk | Toxicities other than cardiac damage evaluated in 98.2% in both treatment groups (54/55 participants). |
| Selective reporting (reporting bias) | High risk | Not all expected outcomes were reported (e.g. overall survival was missing). |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: not applicable Baseline imbalance between treatment groups related to outcome (prior cardiotoxic treatment (anthracyclines and cardiac irradiation), age, gender unclear, stage of disease and prior cardiac dysfunction): unclear (gender), no prior anthracyclines, no prior cardiac dysfunction, no prior cardiac irradiation, stage of disease and age comparable. Difference in length of follow‐up between treatment groups: no, observation period not significantly different. |
Swain 1997a(088001).
| Study characteristics | ||
| Methods | Block randomisation according to a prospectively prepared randomisation list (a separate list was prepared for each investigational site and within each site, the assignments were stratified relative to the presence or absence of cardiac risk factors and on the basis of measurable versus nonmeasurable disease). | |
| Participants | 349 participants (aged 25 to 84 years; all females) with breast cancer (stage III or IV) treated with doxorubicin (cumulative dose < 100 to 2700 mg/m2; peak dose (i.e. maximal dose received in 1 week) 50 mg/m2; infusion duration nm), fluorouracil and cyclophosphamide. No prior anthracycline therapy. Prior cardiac radiotherapy in 20 participants in the dexrazoxane group and 14 participants in the control group (dose nm). No prior cardiac dysfunction (defined as a LVEF < lower limit of normal percentage for the participating institution, obtained within 4 weeks before study entry; documented history of congestive heart failure or cardiomyopathy within 6 months before entry). | |
| Interventions | Dexrazoxane (10:1 ratio of dexrazoxane to doxorubicin; slow IV push or rapid‐drip IV infusion between 15 and 30 minutes before doxorubicin) (N = 168) versus placebo (N = 181). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as 2 or more of the following: cardiomegaly established by radiography, basilar rales, S3 gallop or paroxysmal nocturnal dyspnoea, orthopnoea, or significant dyspnoea on exertion; subclinical myocardial dysfunction defined as 1) decline in LVEF as measured by MUGA from baseline of ≥ 10% below the institution's LLN, 2) a decline in LVEF as measured by MUGA of at least 20% from baseline or 3) decline in LVEF as measured by MUGA to at least 5% below the institution's LLN). Tumour response rate (according to ECOG criteria). Overall survival (defined as time from randomisation to death). Progression‐free survival (defined as time from randomisation to progression either on or off treatment). Adverse effects (according to ECOG criteria). |
|
| Notes | Length of follow‐up: in the intervention group median 532 days (range 1 to 1863); in the control group median 511 days (range 1 to 1652). Median age in intervention group: 58 years; median age in control group: 56 years. Cumulative anthracycline dose in intervention and control group nm. Gender: all females in both treatment groups. Stage of disease per treatment group nm. Funding sources: Pharmacia & Upjohn Inc (Pharmacia Inc; Adria laboratories), Kalamazoo MI. Declaration of interests: nm, but some authors affiliated with funding source. The Weisberg 1992, Tonkin 1998, Rosenfeld 1992 and Bates 1997 publications are duplicate publications of this study; we did not use information from these publications. The same was true for the Swain 1997 publication which was not the primary publication of this study; in addition this publication also reported on a non‐randomised controlled trial which was not eligible for our review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A prospectively prepared randomisation list was used. |
| Allocation concealment (selection bias) | Low risk | All assignments to treatment were made under double‐blind conditions. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All assignments to treatment were made under double‐blind conditions. |
| Blinding of outcome assessors (detection bias) ‐ clinical heart failure | Low risk | All subsequent clinical assessments were made under double‐blind conditions. |
| Blinding of outcome assessors (detection bias) ‐ clinical heart failure and subclinical myocardial dysfunction combined | Low risk | All subsequent clinical assessments were made under double‐blind conditions. |
| Blinding of outcome assessors (detection bias) ‐ overall survival/overall mortality | Low risk | All subsequent clinical assessments were made under double‐blind conditions. |
| Blinding of outcome assessors (detection bias) ‐ tumour response rate | Low risk | All subsequent clinical assessments were made under double‐blind conditions. |
| Blinding of outcome assessors (detection bias) ‐ progression‐free survival | Low risk | All subsequent clinical assessments were made under double‐blind conditions. |
| Blinding of outcome assessors (detection bias) ‐ toxicities other than cardiac damage (diagnosed by laboratory tests) | Low risk | All subsequent clinical assessments were made under double‐blind conditions. |
| Blinding of outcome assessors (detection bias) ‐ toxicities other than cardiac damage (not diagnosed by laboratory tests) | Low risk | All subsequent clinical assessments were made under double‐blind conditions. |
| Incomplete outcome data (attrition bias) ‐ clinical heart failure | Low risk | Clinical heart failure evaluated in 100% of all participants in both treatment groups. |
| Incomplete outcome data (attrition bias) ‐ clinical heart failure and subclinical myocardial dysfunction combined | Low risk | Clinical heart failure and subclinical cardiac damage both evaluated in 100% of all participants in both treatment groups. |
| Incomplete outcome data (attrition bias) ‐ overall survival/overall mortality | Unclear risk | Unclear in how many participants overall survival was evaluated |
| Incomplete outcome data (attrition bias) ‐ tumour response rate | High risk | Tumour response evaluated in 90% of participants with measurable disease in the dexrazoxane group and 89% of the control group (127/141 in the dexrazoxane group and 136/152 in the control group). |
| Incomplete outcome data (attrition bias) ‐ progression‐free survival | Unclear risk | Unclear in how many participants PFS was evaluated |
| Incomplete outcome data (attrition bias) ‐ toxicities other than cardiac damage with the exception of SMN | Unclear risk | Unclear in how many participants toxicities other than cardiac damage were evaluated |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: not applicable Baseline imbalance between treatment groups related to outcome (prior cardiotoxic treatment (anthracyclines and cardiac irradiation), age, gender, stage of disease and prior cardiac dysfunction): unclear risk (no prior cardiac dysfunction and no prior anthracyclines, stage of disease unclear; all other factors comparable between treatment groups) Difference in length of follow‐up between treatment groups: low risk (comparable follow‐up in both treatment groups) |
Swain 1997a(088006).
| Study characteristics | ||
| Methods | Block randomisation according to a prospectively prepared randomisation list (a separate list was prepared for each investigational site and within each site, the assignments were stratified relative to the presence or absence of cardiac risk factors and on the basis of measurable versus nonmeasurable disease). | |
| Participants | 185 participants (aged 23 to 79 years; all females) with breast cancer (stage IIIB or IV) treated with doxorubicin (cumulative dose < 100 to 1750 mg/m2; peak dose (i.e. maximal dose received in 1 week) 50 mg/m2; infusion duration nm), fluorouracil and cyclophosphamide. No prior anthracycline therapy. Prior cardiac radiotherapy in 3 participants in the dexrazoxane group and 9 participants in the control group (dose nm). No prior cardiac dysfunction (defined as an LVEF < lower limit of normal percentage for the participating institution, obtained within 4 weeks before study entry; documented history of congestive heart failure or cardiomyopathy within 6 months before entry). | |
| Interventions | Dexrazoxane (10:1 ratio of dexrazoxane to doxorubicin; slow IV push or rapid‐drip IV infusion between 15 and 30 minutes before doxorubicin) (N = 81) versus placebo (N = 104). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as 2 or more of the following: cardiomegaly established by radiography, basilar rales, S3 gallop or paroxysmal nocturnal dyspnoea, orthopnoea, or significant dyspnoea on exertion; subclinical myocardial dysfunction defined as 1) decline in LVEF as measured by MUGA from baseline of ≥ 10% below the institution's LLN, 2) a decline in LVEF as measured by MUGA of at least 20% from baseline or 3) decline in LVEF as measured by MUGA to at least 5% below the institution's LLN). Tumour response rate (according to ECOG criteria). Overall survival (defined as time from randomisation to death). Progression‐free survival (defined as time from randomisation to progression either on or off treatment). Adverse effects (according to ECOG criteria). |
|
| Notes | Length of follow‐up: in the intervention group median 397 days (6 to 1393); in the control group median 517 days (range 29 to 1429). Median age in intervention group: 56 years; median age in control group: 59.5 years. Cumulative anthracycline dose in intervention and control group nm. Gender: all females in both treatment groups. Stage of disease per treatment group nm. Funding sources: Pharmacia & Upjohn Inc (Pharmacia Inc; Adria laboratories), Kalamazoo MI. Declaration of interests: nm, but some authors affiliated with funding source. The Weisberg 1992, Bates 1997, Rosenfeld 1992 and Tonkin 1998 publications are duplicate publications of this study; we did not use information from these publications. The same was true for the Swain 1997 publication which was not the primary publication of this study; in addition this publication also reported on a non‐randomised controlled trial which was not eligible for our review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A prospectively prepared randomisation list was used |
| Allocation concealment (selection bias) | Low risk | All assignments to treatment were made under double‐blind conditions. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All assignments to treatment were made under double‐blind conditions. |
| Blinding of outcome assessors (detection bias) ‐ clinical heart failure | Low risk | All subsequent clinical assessments were made under double‐blind conditions. |
| Blinding of outcome assessors (detection bias) ‐ clinical heart failure and subclinical myocardial dysfunction combined | Low risk | All subsequent clinical assessments were made under double‐blind conditions. |
| Blinding of outcome assessors (detection bias) ‐ overall survival/overall mortality | Low risk | All subsequent clinical assessments were made under double‐blind conditions. |
| Blinding of outcome assessors (detection bias) ‐ tumour response rate | Low risk | All subsequent clinical assessments were made under double‐blind conditions. |
| Blinding of outcome assessors (detection bias) ‐ progression‐free survival | Low risk | All subsequent clinical assessments were made under double‐blind conditions. |
| Blinding of outcome assessors (detection bias) ‐ toxicities other than cardiac damage (diagnosed by laboratory tests) | Low risk | All subsequent clinical assessments were made under double‐blind conditions. |
| Blinding of outcome assessors (detection bias) ‐ toxicities other than cardiac damage (not diagnosed by laboratory tests) | Low risk | All subsequent clinical assessments were made under double‐blind conditions. |
| Incomplete outcome data (attrition bias) ‐ clinical heart failure | Low risk | Clinical heart failure evaluated in 100% of all participants in both treatment groups. |
| Incomplete outcome data (attrition bias) ‐ clinical heart failure and subclinical myocardial dysfunction combined | Low risk | Clinical heart failure and subclinical cardiac damage both evaluated in 100% of all participants in both treatment groups. |
| Incomplete outcome data (attrition bias) ‐ overall survival/overall mortality | Unclear risk | Unclear in how many participants overall survival was evaluated |
| Incomplete outcome data (attrition bias) ‐ tumour response rate | High risk | Tumour response evaluated in 90% of participants with measurable disease in the dexrazoxane group and 89% of the control group (46/54 in the dexrazoxane and 61/69 in the control group). |
| Incomplete outcome data (attrition bias) ‐ progression‐free survival | Unclear risk | Unclear in how many participants PFS was evaluated |
| Incomplete outcome data (attrition bias) ‐ toxicities other than cardiac damage with the exception of SMN | Unclear risk | Unclear in how many participants toxicities other than cardiac damage were evaluated |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: not applicable Baseline imbalance between treatment groups related to outcome (prior cardiotoxic treatment (anthracyclines and cardiac irradiation), age, gender, stage of disease and prior cardiac dysfunction): unclear risk (no prior cardiac dysfunction and no prior anthracyclines, stage of disease unclear; all other factors comparable between treatment groups) Difference in length of follow‐up between treatment groups: difference in length of follow‐up between treatment groups, but relevance unclear |
Venturini 1996.
| Study characteristics | ||
| Methods | TiuRandomisation was performed by a phone call to the study coordination centre (participants were stratified before randomisation by institution and according to previously received adjuvant chemotherapy with anthracyclines). | |
| Participants | 162 participants (median age 57 years (range 32 to 74); all females) with breast cancer (metastatic, locally advanced (IIIB) or inflammatory: comparable between treatment groups) treated with therapy including epirubicin (cumulative dose for all randomised participants range 0 to 1440 mg/m2; peak dose (i.e. maximal dose received in 1 week) 60 or 120 mg/m2; infusion duration nm). Prior anthracycline therapy: yes (see notes). Prior cardiac radiotherapy: yes (see notes). No prior cardiac dysfunction (defined as history of congestive heart failure, unless full recovery was documented; resting LVEF < 50%). | |
| Interventions | Dexrazoxane (10:1 ratio of dexrazoxane to epirubicin; IV infusion over 15 minutes, beginning 30 minutes before epirubicin) (N = 84) versus no cardioprotective intervention (N = 78). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as NYHA class 2, 3 or 4; subclinical myocardial dysfunction defined as LVEF as measured by MUGA ≤ 45% or ≥ 20 EF units as compared to baseline). Tumour response rate (according to WHO criteria). Adverse effects (according to WHO criteria). |
|
| Notes | Length of follow‐up nm. Median age in intervention and control group: 57 years. Cumulative anthracycline dose in intervention group: mean 702 mg/m2 (range 0 to 1440); cumulative anthracycline dose in control group: mean 713 mg/m2 (range 120 to 1200). This included prior anthracycline therapy (doxorubicin versus epirubicin = 1:2). Prior cumulative anthracycline dose in intervention group (N = 14): median 410 mg/m2 (range 180 to 800); prior cumulative anthracycline dose in control group (N = 11): median 360 mg/m2 (range 240 to 600). Prior cardiac radiotherapy in 11 participants treated with dexrazoxane and in 13 control participants (dose nm). Gender: all females in both treatment groups. Stage of disease per treatment group: 92% metastatic and 8% locally advanced in dexrazoxane group and 96% metastatic and 4% locally advanced in control group. Funding sources: Associazione Italiana per la Ricerca sul Cancro, Milano, Italy, 1996. Declaration of interests: nm, but at least one author from Chiron (manufacturer of dexrazoxane). The Michelotti 2000 publication is a duplicate publication of this study; we did not use information from this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on the sequence generation process provided |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by a phone call to the study coordination centre |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to the received intervention (either dexrazoxane or no cardioprotective intervention) |
| Blinding of outcome assessors (detection bias) ‐ clinical heart failure | Low risk | The nuclear physicians and cardiologists were blinded to the treatment assignment of the participants. |
| Blinding of outcome assessors (detection bias) ‐ clinical heart failure and subclinical myocardial dysfunction combined | Low risk | The nuclear physicians and cardiologists were blinded to the treatment assignment of the participants. |
| Blinding of outcome assessors (detection bias) ‐ tumour response rate | Unclear risk | No information on blinding of outcome assessors provided for tumour response |
| Blinding of outcome assessors (detection bias) ‐ toxicities other than cardiac damage (diagnosed by laboratory tests) | Low risk | No information on blinding of outcome assessors provided, but as blinding is not relevant for outcomes diagnosed by laboratory test, we judged this outcome at low risk of detection bias for the following adverse effects: thrombocytopenia, anaemia, leukopenia. |
| Blinding of outcome assessors (detection bias) ‐ toxicities other than cardiac damage (not diagnosed by laboratory tests) | Unclear risk | No information on blinding of outcome assessors provided for toxicities other than cardiac damage (not diagnosed by laboratory tests). |
| Incomplete outcome data (attrition bias) ‐ clinical heart failure | Low risk | Clinical heart failure evaluated in 98% of participants in the dexrazoxane group and 100% of the control group (82/84 of the dexrazoxane group and 78/78 of the control group). |
| Incomplete outcome data (attrition bias) ‐ clinical heart failure and subclinical myocardial dysfunction combined | High risk | Clinical heart failure evaluated in 98% of participants in the dexrazoxane group and 100% of the control group (82/84 of the dexrazoxane group and 78/78 of the control group); subclinical myocardial dysfunction evaluated in 83% of participants in the dexrazoxane group and 95% of the control group (70/84 of the dexrazoxane group and 74/78 of the control group). |
| Incomplete outcome data (attrition bias) ‐ tumour response rate | Low risk | Tumour response evaluated in 98% of participants in the dexrazoxane group and 100% of the control group (82/84 of the dexrazoxane group and 78/78 of the control group). |
| Incomplete outcome data (attrition bias) ‐ toxicities other than cardiac damage with the exception of SMN | Low risk | Toxicities other than cardiac damage evaluated in 98% of participants in the dexrazoxane group and 100% of the control group (82/84 of the dexrazoxane group and 78/78 of the control group). |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported (although overall survival was not eligible for inclusion in the review). |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: unclear (information on both method of randomisation and blinding of outcome assessors for some of the outcomes not provided). Baseline imbalance between treatment groups related to outcome (prior cardiotoxic treatment (anthracyclines and cardiac irradiation), age, gender, stage of disease and prior cardiac dysfunction): low risk (no prior cardiac dysfunction, all other factors comparable between groups). Difference in length of follow‐up between treatment groups: unclear (length of follow‐up nm) |
Wexler 1996.
| Study characteristics | ||
| Methods | Participants underwent a computer‐generated 1:1 factorial randomisation (open‐label trial). | |
| Participants | 41 participants (aged 4 to 24 years; 26 males and 15 females) with one of the Ewing sarcoma family of tumours (stage comparable between treatment groups) treated with doxorubicin (cumulative dose for all randomised participants range 70 to 410 mg/m2; peak dose (i.e. maximal dose received in 1 week) 50 or 70 mg/m2; infusion duration 15 minutes); 38 participants were randomised, whereas 3 participants received dexrazoxane without randomisation), vincristine, etoposide, cyclophosphamide and ifosfamide and if necessary, radiotherapy for local control. No prior anthracycline therapy. No prior cardiac radiotherapy. No prior cardiac dysfunction (defined as LVEF < 45%). | |
| Interventions | Dexrazoxane (20:1 ratio of dexrazoxane to doxorubicin; IV infusion over 15 minutes immediately before doxorubicin) (N = 20) versus no cardioprotective intervention (N = 18). | |
| Outcomes | Heart failure (defined as (1) evidence of clinical congestive heart failure, (2) a reduction in LVEF as measured by MUGA to < 45% or (3) a decrease in LVEF as measured by MUGA of > 20 percentage points from baseline). | |
| Notes | Length of follow‐up: median potential 39 months (37 months for the intervention group; 40 months for the control group) (including 3 non‐randomised participants). Median age in intervention group: 18.5 years; median age in control group: 15.5 years (including 3 non‐randomised participants). Cumulative anthracycline dose in the intervention group: median 410 mg/m2 (range 140 to 410); cumulative anthracycline dose in the control group: median 310 mg/m2 (range 70 to 410). Gender per treatment group nm (but reported including 3 non‐randomised participants and based on that information comparable between groups). Stage of disease per treatment group nm (but reported including 3 non‐randomised participants and based on that information comparable between groups). Funding sources nm. Declaration of interests nm. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to the received intervention (either dexrazoxane or no cardioprotective intervention). |
| Blinding of outcome assessors (detection bias) ‐ clinical heart failure and subclinical myocardial dysfunction combined | Unclear risk | The nuclear medicine physicians were blinded to the treatment assignment of the participants, but no information on blinding of outcome assessors provided for clinical heart failure |
| Incomplete outcome data (attrition bias) ‐ clinical heart failure and subclinical myocardial dysfunction combined | High risk | Clinical heart failure and subclinical myocardial dysfunction both evaluated in 90% of participants in the dexrazoxane group and 83% of the control group (18/20 of the dexrazoxane group and 15/18 of the control group). |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported (although overall survival was not eligible for inclusion in the review). |
| Other bias | Unclear risk |
Block randomisation in unblinded trials: unclear (information on both method of randomisation and blinding of outcome assessors for some of the outcomes not provided (in sufficient detail)). Baseline imbalance between treatment groups related to outcome (prior cardiotoxic treatment (anthracyclines and cardiac irradiation), age, gender, stage of disease and prior cardiac dysfunction): low risk (no prior cardiotoxic treatment and cardiac dysfunction; all other factors comparable between treatment groups) Difference in length of follow‐up between treatment groups: low risk (comparable length of follow‐up between treatment groups) |
ALL: acute lymphoblastic leukaemia CCG: Children's Cancer Group CNS: central nervous system CTCv2: Common Toxicity Criteria, version 2 CTCAEv2: Common Terminology Criteria for Adverse Events, version 2 ECG: electrocardiogram ECOG: Eastern Cooperative Oncology Group EF: ejection fraction 5FU: 5‐fluorouracil Gy: Gray HL: Hodgkin lymphoma i.e.: that is/namely IV: intravenous LLN: lower limit of normal L‐NHL: lymphoblastic non‐Hodgkin lymphoma LVEF: left ventricular ejection fraction LVFS: left ventricular fractional shortening MUGA: multiple gated acquisition scan mg/m2: milligram per square metre n: number NCI: National Cancer Institute nm: not mentioned NHL: non‐Hodgkin lymphoma NYHA: New York Heart Association POG: Pediatric Oncology Group T‐ALL: T‐cell acute lymphoblastic leukaemia WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Getz 2019 | Not a randomised controlled trial |
| Li 2013 | No randomisation of dexrazoxane versus control |
| Massida 1997 | Cardiac function not measured by echocardiography or radionuclide ventriculography |
| Neto 2006 | Not a randomised controlled trial |
| Paiva 2005 | Not a randomised controlled trial |
| Rabinovich 2012 | Likely not a randomised controlled trial; no cardiac outcomes; and chemotherapy other than anthracyclines and radiotherapy involving the heart region not the same in both treatment groups |
| Tap 2019 | No randomisation of dexrazoxane versus control |
| Wang 2020 | No adequate information on heart failure provided |
Characteristics of studies awaiting classification [ordered by study ID]
Aggarwal 2018.
| Methods | Method of randomisation not clear. |
| Participants | 105 survivors of paediatric ALL or Hodgkin lymphoma (mean age at follow‐up 27.7 years; N = 47 females and N = 58 males; stage of disease at diagnosis nm) treated with doxorubicin (cumulative dose 100 to 360 mg/m2; peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm). Prior anthracycline therapy nm. Prior cardiac radiotherapy nm. Prior cardiac dysfunction nm. |
| Interventions | Dexrazoxane (ratio to doxorubicin 10:1; timing in relation to anthracycline nm) (N = 57) versus no cardioprotective intervention (N = 48). |
| Outcomes | Heart failure (i.e. different abnormalities on speckle tracking echocardiography); in the abstract no dichotomous results were provided. |
| Notes | Unclear if this is an ongoing or completed study. Length of follow‐up 16.5 years since cancer diagnosis. Mean cumulative anthracycline dose in the dexrazoxane group was 282 mg/m2 and in the control group 275 mg/m2. Mean age at echocardiogram of the dexrazoxane group was 27.3 years and of the control group 28.1 years. |
Cardenas 2003.
| Methods | Method of randomisation not clear. |
| Participants | Children (number of children: see notes) (aged 1 to 18 years; sex nm) with cancer (type and stage nm) treated with anthracyclines (analogue nm; cumulative dose nm; peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm). No prior anthracycline therapy. No prior cardiac radiotherapy. Prior cardiac dysfunction nm. |
| Interventions | Dexrazoxane (ratio to anthracycline nm; timing in relation to anthracycline nm) versus no cardioprotective intervention (number of participants in each group nm). |
| Outcomes | Heart failure (subclinical heart failure defined as "a drop of the 20% as a basic value for FEV or that it was progressive" as measured by echocardiography): no significant difference between both groups. Number of participants that remained free of illness at 24 months: no difference between both groups. Adverse effects: no differences between both groups. |
| Notes | Unclear if this is an ongoing or completed study. Length of follow‐up nm. Cumulative anthracycline dose per treatment group nm. Age per treatment group nm. Number of included children is unclear (in methods N = 50; in results N = 52). |
Chow 2016.
| Methods | Method of randomisation not clear. |
| Participants | 94 survivors of paediatric T‐cell ALL (stage nm) or low, intermediate or high risk Hodgkin lymphoma (mean age at time of study 28 years; N = 40 females and N = 54 males) treated with doxorubicin (cumulative dose average 279 mg/m2; peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm). No prior anthracycline therapy. No prior cardiac radiotherapy. Prior cardiac dysfunction before the long‐term follow‐up: N = 2 in dexrazoxane group clinical cardiomyopathy. |
| Interventions | Dexrazoxane (ratio to doxorubicin 10:1; intravenous bolus before each doxorubicin dose) (N = 51) versus no cardioprotective intervention (N = 43). |
| Outcomes | Heart failure (i.e. clinical cardiomyopathy and different abnormalities on echocardiography): fractional shortening < 28% in 2 dexrazoxane and 1 control participant; no results of other parameters presented. |
| Notes | These are preliminary analyses. Median 16 years since diagnosis. Cumulative anthracycline dose per treatment group nm. Age per treatment group nm. Only a subset of participants (N = 43) had ejection fraction evaluable. |
De Berranger 2006.
| Methods | Method of randomisation not clear. |
| Participants | 16 children (median age 8.5 years; 9 boys and 7 girls) with acute leukaemia (11 AML; 5 ALL; stage nm) treated with doxorubicin containing therapy (cumulative dose 450 mg/m2 for AML and 310 mg/m2 for ALL; peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm). Prior anthracycline therapy nm. Prior cardiac radiotherapy nm. Prior cardiac dysfunction nm. |
| Interventions | Dexrazoxane (1 g for 50 mg of doxorubicin equivalent dose; timing in relation to anthracycline nm) (N = 8) versus no cardioprotective intervention (N = 8). |
| Outcomes | Heart failure (i.e. mean LVSF and mean wall stress before chemotherapy and 1 year after diagnosis): all values were comparable. Survival: no difference in disease‐free and overall survival. Adverse effects: 2 participants in the dexrazoxane group had severe hepatic toxicity (WHO criteria grade 3 or more); no other toxicity WHO criteria more than grade 1 observed. |
| Notes | Unclear if this is an ongoing or completed study. Median follow‐up 28.5 months. Cumulative anthracycline dose per treatment group nm. Age per treatment group nm. |
Feldmann 1992.
| Methods | Method of randomisation not clear. |
| Participants | 155 participants (median age 66 years; sex: see notes) with advanced small cell lung cancer treated with doxorubicin containing chemotherapy (cumulative dose nm; peak dose (i.e. maximal dose received in 1 week) 50 mg/m2; infusion duration nm). Prior anthracycline therapy nm. Prior cardiac radiotherapy nm. Prior cardiac dysfunction nm. |
| Interventions | Dexrazoxane (10:1 ratio of study drug to doxorubicin; within 30 minutes prior to doxorubicin by IV bolus) versus placebo (number of participants in each group nm). |
| Outcomes | Heart failure (defined as cardiac events): difference in favour of the dexrazoxane group. Other toxicities and tumour response rate: level of significance not mentioned. |
| Notes | Unclear if this is an ongoing or completed study. Length of follow‐up nm. Cumulative anthracycline dose per treatment group nm. Median age in both treatment groups 66 years. 70% of dexrazoxane participants were male and 62% of the control participants. 105 participants were evaluable: 43 in the intervention group and 62 in the control group. |
Jackowska 2003.
| Methods | Unclear if this is a randomised controlled trial. |
| Participants | 107 children (age nm; sex nm) with acute lymphoblastic leukaemia (stage nm) treated with either doxorubicin or daunorubicin containing therapy (cumulative dose 120 mg/m2; peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm). Prior anthracycline therapy nm. Prior cardiac radiotherapy nm. Prior cardiac dysfunction nm. |
| Interventions | Dexrazoxane (ratio to anthracycline nm; timing in relation to anthracycline nm) (N = 43) versus no cardioprotective intervention (N = 64). |
| Outcomes | Toxicities other than cardiotoxicity. |
| Notes | Unclear if this is an ongoing or completed study. Unclear if cardiotoxicity is evaluated in this study. Length of follow‐up nm. Cumulative anthracycline dose per treatment group nm. Age per treatment group nm. |
Liu 2013.
| Methods | Method of randomisation not clear. |
| Participants | People (age nm, sex nm) with acute nonlymphocytic leukaemia (stage nm) treated with daunorubicin (cumulative dose nm; peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm). Prior anthracycline therapy nm. Prior cardiac radiotherapy nm. Prior cardiac dysfunction nm. |
| Interventions | Dexrazoxane (ratio to anthracycline nm; timing in relation to anthracycline nm) (N = 20) versus no cardioprotective intervention (N = 22). |
| Outcomes | Heart failure (i.e. different abnormalities on echocardiography); in the abstract no clear dichotomous results were provided. |
| Notes | Unclear if this is an ongoing or completed study. Length of follow‐up nm. Cumulative anthracycline dose per treatment group nm. Age per treatment group nm. |
Saad El‐Din 2003.
| Methods | Method of randomisation not clear. |
| Participants | 46 children (age nm; sex nm) with standard risk acute lymphoblastic leukaemia treated with doxorubicin containing chemotherapy (cumulative dose nm; peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm). Prior anthracycline therapy nm. Prior cardiac radiotherapy nm. Prior cardiac dysfunction nm. |
| Interventions | Dexrazoxane (15:1 ratio of study drug to doxorubicin; prior to doxorubicin) versus no cardioprotective intervention (number of participants in each group nm). |
| Outcomes | Heart failure (subclinical heart failure on, among others, echocardiography and MUGA scan; definitions nm): difference in favour of dexrazoxane. Tumour response rate (defined as the number of participants in complete remission): no difference between groups. Adverse effects (definition nm): similar in both groups. |
| Notes | Unclear if this is an ongoing or completed study. 41 out of 46 randomised participants were evaluated. Length of follow‐up nm. Cumulative anthracycline dose per treatment group nm. Age per treatment group nm. |
Ten Bokkel‐Huinink 1992.
| Methods | Method of randomisation not clear. |
| Participants | 112 participants (age: see notes; sex nm) with breast cancer (stage nm) treated with doxorubicin containing chemotherapy (cumulative dose nm; peak dose (i.e. maximal dose received in 1 week) 50 mg/m2; infusion duration nm). Prior anthracycline therapy nm. Prior cardiac radiotherapy and prior cardiac dysfunction well balanced between treatment groups (number of participants nm). |
| Interventions | Dexrazoxane (20:1 ratio of study drug to doxorubicin; within 30 minutes prior to doxorubicin) (N = 57) versus no cardioprotective intervention (N = 55). |
| Outcomes | Heart failure (clinical cardiomyopathy or subclinical heart failure as measured by gated pool ejection fraction; definitions nm): no difference between treatment groups. Tumour response rate (complete and partial remission; definitions nm): not influenced by dexrazoxane. Adverse effects (definitions nm): slightly increased myelosuppression with dexrazoxane; no increase in other toxicities. |
| Notes | Unclear if this is an ongoing or completed study. Length of follow‐up nm. Cumulative anthracycline dose per treatment group nm. Mean age in intervention group 46 years; mean age in control group 45 years. The Ten Bokkel‐Huinink 1990 publication is a duplicate publication of this study; we did not use information from this publication. |
Vejpongsa 2014.
| Methods | Unclear if this is a randomised controlled trial. |
| Participants | 23 participants (age nm; sex nm) with newly diagnosed sarcoma (stage nm) treated with doxorubicin (cumulative dose nm, but ≥ 150 mg/m2; peak dose (i.e. maximal dose received in 1 week) nm; infusion duration in the control group 72 hours, infusion duration in the dexrazoxane group nm). No prior anthracycline therapy. Prior cardiac radiotherapy nm. Prior cardiac dysfunction yes (some participants had pretreatment elevation of high sensitivity troponin T, exact number nm). |
| Interventions | Dexrazoxane (ratio of study drug to doxorubicin nm; timing of administration nm) (N = 10) versus no cardioprotective intervention (N = 13) |
| Outcomes | Heart failure (different abnormalities on speckle tracking echocardiography); in the abstract no clear dichotomous results were provided. |
| Notes | Unclear if this study is eligible for inclusion. Unclear if this is an ongoing or completed study. Length of follow‐up nm. Cumulative anthracycline dose per treatment group nm. Age per treatment group nm. |
Wang 2013.
| Methods | Method of randomisation not clear. |
| Participants | 122 breast cancer participants (stage nm; mean age at time of study nm; sex nm) treated with epirubicin (cumulative dose average nm (four cycles of adjuvant chemotherapy); peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm). Prior anthracycline therapy nm. Prior cardiac radiotherapy nm. Prior cardiac dysfunction: nm. |
| Interventions | Dexrazoxane (ratio to doxorubicin 10:1; route and timing of dexrazoxane delivery nm) (N = 61) versus no cardioprotective intervention (N = 61). |
| Outcomes | Heart failure (subclinical heart failure based on LVEF; diagnostic test nm); in the abstract no dichotomous results were provided. Adverse effects (no definition provided) Bone marrow suppression: in the intervention group; grade III N = 15 (24.6%), grade IV N = 3 (4.9%) and in the control group; grade III N = 6 (9.8%), grade IV N = 3 (5.5%). Neutrophil count: in the abstract no dichotomous results were provided. |
| Notes | The full text is available in Chinese, results presented here are based on the English abstract. As of yet, unclear if this study is eligible for inclusion. Median time since diagnosis nm. Cumulative anthracycline dose per treatment group nm. Age per treatment group nm. |
Zhuang 2012.
| Methods | Method of randomisation not clear. |
| Participants | 120 participants with haematological malignancies (exact diagnosis nm; stage nm; mean age at time of study nm; sex nm) treated with adriamycin (cumulative dose nm (all groups received two complete cycles of chemotherapy); peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm). Prior anthracycline therapy nm. Prior cardiac radiotherapy nm. Prior cardiac dysfunction nm. |
| Interventions | Shen Mai injection without dexrazoxane (N = 30) versus Shen Mai injection + dexrazoxane (ratio to doxorubicin 10:1; fast intravenous drip 30 minutes prior to anthracyclines) (N = 30) versus dexrazoxane only (ratio to doxorubicin nm, route and timing nm) (N = 30) versus no cardioprotective intervention (N = 30). |
| Outcomes | Heart failure (subclinical heart failure based on echocardiographic LVEF); in the abstract no dichotomous results were provided. |
| Notes | The full text is available in Chinese, results presented here are based on the English abstract. As of yet, unclear if this study is eligible for inclusion. Median time since diagnosis nm. Cumulative anthracycline dose per treatment group nm. Age per treatment group nm. |
ALL: acute lymphoblastic leukaemia AML: acute myeloid leukaemia FEV: ejection fraction LVEF: left ventricular ejection fraction LVSF: left ventricular shortening fraction mg/m2: milligram per square meter MUGA: multiple gated acquisition scan n: number nm: not mentioned WHO: World Health Organization
Characteristics of ongoing studies [ordered by study ID]
NCT00016276.
| Study name | Phase III randomised study of doxorubicin and cyclophosphamide with or without dexrazoxane followed by paclitaxel with or without trastuzumab (Herceptin) followed by surgery and radiotherapy with or without trastuzumab in women with HER‐2+ stage IIIA or IIIB or regional stage IV breast cancer |
| Methods | Randomised controlled trial |
| Participants | Women with HER‐2+ stage IIIA or IIIB or regional stage IV breast cancer (minimal age 18 years) |
| Interventions | Dexrazoxane versus no cardioprotective intervention |
| Outcomes | Cardiac toxicity, tumour response rate, survival, other toxicities |
| Starting date | May 2001 |
| Contact information | Study chair Mark L Graham |
| Notes | ‐ |
HER‐2: human epidermal growth factor receptor 2
Differences between protocol and review
Differences between protocol and review should be read as differences between second and third review updates.
General
The original review (Van Dalen 2011) was split and this part now focuses on dexrazoxane only.
The author team was adjusted.
The term subclinical heart failure was changed into subclinical myocardial dysfunction to be more in line with current practice.
We made some small changes and clarifications to be in line with the latest version of the Cochrane Methodological Expectations of Cochrane Intervention Reviews (MECIR) standards (such as including headers and information on reporting bias/funnel plots, declarations of interest and conflict of interest in included studies, not mentioning the significance level of differences between treatment groups).
Background
The information in the background was updated where necessary.
Methods
To be in line with current diagnostic options, we added cardiac magnetic resonance imaging as an imaging method eligible for the detection of heart failure.
Based on a comment by one of the peer reviewers for children, tumour response rate was defined as the number of complete remissions (instead of the number of complete and partial remissions).
Instead of searching the ISRCTN register, we searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) to be in line with MECIR standards.
The information specialist checked if changes in the search strategies were necessary; if so, these changes are explained in the Appendices.
Search strategies for the conference proceedings were added to the Appendices.
We adapted the risk of bias criteria (RoB1; Higgins 2011). All publications (including those already included in earlier versions of the review) were scored using the new risk of bias criteria.
We used a random‐effects model throughout the review.
In previous versions of the review, best‐case analyses were performed. Starting with the third update, we have presented results from available‐case, best‐case and worst‐case analyses where possible.
We included summary of findings tables and performed GRADE assessments.
Results
We included a flow diagram showing the selection of studies.
Publications labelled as 'excluded studies' in the previous versions of this review, which were associated with various included studies, are now collated with their respective included study, to be in line with Cochrane methodological standards.
Heart failure and subclinical myocardial dysfunction combined: in previous versions, we erroneously did not pool the results of the two studies by Swain and colleagues. This was corrected in the third update.
Progression‐free survival (PFS): after re‐evaluation of the definitions of PFS used by included trials, we decided that they were not similar enough to pool all studies. We adjusted the analyses.
We analysed adult and paediatric data separately.
Characteristics of included studies table: we added information on gender and stage of disease per treatment group and definition of prior cardiac dysfunction.
Contributions of authors
Esmée de Baat searched the "other search resources" and identified the studies meeting the inclusion criteria; performed data extraction and risk of bias assessment of the included studies; analysed data and interpreted the results; provided a clinical perspective; wrote and revised the manuscript. Renée Mulder provided a methodological perspective; performed the GRADE assessments; critically reviewed the manuscript. Saro Armenian provided a clinical perspective; critically reviewed the manuscript. Elizabeth Feijen provided a clinical perspective; critically reviewed the manuscript. Heynric Grotenhuis provided a clinical perspective; critically reviewed the manuscript. Melissa Hudson: provided a clinical perspective; critically reviewed the manuscript. Annelies Mavinkurve‐Groothuis: provided a clinical perspective; critically reviewed the manuscript. Leontien Kremer provided a methodological and clinical perspective; acted as a third‐party arbitrator; critically reviewed the manuscript. Elvira van Dalen searched the "other search resources" and identified the studies meeting the inclusion criteria; performed data extraction and risk of bias assessment of the included studies; performed the GRADE assessments; analysed data and interpreted the results; provided a methodological and clinical perspective; wrote and revised the manuscript.
All authors approved the final version.
Sources of support
Internal sources
-
None, Other
none
External sources
-
The Dutch Heart Foundation [Grant CVON2015‐21], Netherlands
Grant number CVON2015‐21
Declarations of interest
ECdB: none known RLM: none known SA: contributed to the P9404 study included in this review; however, he was not involved in data extraction, risk of bias or GRADE assessment EAMF: none known HBG: none known MMH: none known AMCMG: none known LCMK: none known ECvD: none known
New
References
References to studies included in this review
DFCI 95‐01 {published data only}
- Barry EV, Vrooman LM, Dahlberg SE, Neuberg DS, Asselin BL, Athale UH, et al. Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukaemia treated with dexrazoxane. Journal of Clinical Oncology 2008;26:1106-11. [DOI] [PubMed] [Google Scholar]
- Lipshultz SE, Miller TL, Scully RE, Lipsitz SR, Rifai N, Silverman LB, et al. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: associations with long-term echocardiographic outcomes. Journal of Clinical Oncology 2012;30(10):1042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. New England Journal of Medicine 2004;351:145-53. [DOI] [PubMed] [Google Scholar]
- Lipshultz SE, Scully RE, Lipsitz SR, Sallan SE, Silverman LB, Miller TL, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncology 2010;11:950-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghrabi A, Levy DE, Asselin B, Barr R, Clavell L, Hurwitz C, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood 2007;103:896-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrooman LM, Neuberg DS, Stevenson KE, Asselin BL, Athale UH, Clavell L, et al. The low incidence of secondary acute myelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: a report from the Dana-Farber Cancer Institute ALL Consortium. European Journal of Cancer 2011;47(9):1373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Galetta 2005 {published data only}
- Galetta F, Franzoni F, Cervetti G, Cecconi N, Carpi A, Petrini M, et al. Effect of epirubicin-based chemotherapy and dexrazoxane supplementation on QT dispersion in non-Hodgkin lymphoma patients. Biomedicine & Pharmacotherapy 2005;59:541-4. [DOI] [PubMed] [Google Scholar]
Lopez 1998 {published data only}
- Lopez M, Vici P, Di Lauro L, Conti F, Paoletti G, Ferraironi A, et al. Randomized prospective clinical trial of high-dose epirubicin and dexrazoxane in patients with advanced breast cancer and soft tissue sarcomas. Journal of Clinical Oncology 1998;16:86-92. [DOI] [PubMed] [Google Scholar]
- Vici P, Ferraironi A, Di Lauro L, Carpano S, Conti F, Belli F, et al. Dexrazoxane cardioprotection in advanced breast cancer patients undergoing high-dose epirubicin treatment. La Clinica Terapeutica 1998;149:15-20. [PubMed] [Google Scholar]
Marty 2006 {published data only}
- Marty M, Espie M, Llombart A, Monnier A, Rapoport BL, Stahalova V. Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane) in advanced/metastatic breast cancer patients treated with anthracycline-based chemotherapy. Annals of Oncology 2006;17:614-22. [DOI] [PubMed] [Google Scholar]
P9404 {published data only}
- Asselin B, Devidas M, Zhou T, Camitta BM, Lipsultz SE. Cardioprotection and safety of dexrazoxane (DRZ) in children treated for newly diagnosed T-cell acute lymphoblastic leukemia (T-ALL) or advanced stage lymphoblastic leukemia (T-LL). Journal of Clinical Oncology 2012;30(15 Suppl):9504. [https://ascopubs.org/doi/abs/10.1200/jco.2012.30.15_suppl.9504] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin BL, Devidas M, Chen L, Franco VI, Pullen J, Borowitz MJ, et al. Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T-cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-Hodgkin lymphoma: a report of the Children's Oncology Group Randomized Trial Pediatric Oncology Group 9404. Journal of Clinical Oncology 2016;34(8):854-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin BL, Devidas M, Chen L, Franco VI, Pullen J, Borowitz MJ, et al. Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T-cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-Hodgkin lymphoma: a report of the Children's Oncology Group Randomized Trial Pediatric Oncology Group 9404. Journal of Clinical Oncology 2016;34(8):Erratum for Journal of Clinical Oncology 2017; 35(18): 2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow EJ, Asselin BL, Schwartz CL, Doody DR, Leisenring WM, Aggarwal S, et al. Late mortality after dexrazoxane treatment: a report from the Children's Oncology Group. Journal of Clinical Oncology 2015;33(24):2639-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández KS, Schwartz CL, Chen L, Constine LS, Chauvenet A, Alarcón PA. Outcome of adolescents and young adults compared to children with Hodgkin lymphoma treated with response-based chemotherapy on pediatric protocols: a Children's Oncology Group report. Pediatric Blood and Cancer 2017;64(12):e26681. [DOI] [PMC free article] [PubMed] [Google Scholar]
P9425 {published data only}
- Chow EJ, Asselin BL, Schwartz CL, Doody DR, Leisenring WM, Aggarwal S, et al. Late mortality after dexrazoxane treatment: a report from the Children's Oncology Group. Journal of Clinical Oncology 2015;33(24):2639-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CL, Constine LS, Villaluna D, London WB, Hutchison RE, Sposto R, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425. Blood 2009;114:2051-9: Erratum for: Blood 2016;128(4):605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CL, Constine LS, Villaluna D, London WB, Hutchison RE, Sposto R, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425. Blood 2009;114(10):2051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebbi CK, London WB, Friedman F, Villaluna D, De Alarcon PA, Constine LS, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. Journal of Clinical Oncology 2007;25:493-500. [DOI] [PubMed] [Google Scholar]
P9426 {published data only}
- Chow EJ, Asselin B, Schwartz CL, Doody D, Leisenring WM, Aggarwal S, et al. Late mortality and relapse after dexrazoxane (DRZ) treatment: an update from the Children's Oncology Group (COG). Journal of Clinical Oncology 2014;32(15 Suppl):10024. [https://ascopubs.org/doi/abs/10.1200/jco.2014.32.15_suppl.10024] [Google Scholar]
- Chow EJ, Asselin BL, Schwartz CL, Doody DR, Leisenring WM, Aggarwal S, et al. Late mortality after dexrazoxane treatment: a report from the Children's Oncology Group. Journal of Clinical Oncology 2015;33(24):2639-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebbi CK, London WB, Friedman D, Villaluna D, De Alarcon PA, Constine LS, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. Journal of Clinical Oncology 2007;25(5):493-500. [DOI] [PubMed] [Google Scholar]
- Tebbi CK, Mendenhall NP, London WB, Williams JL, Hutchison RE, Fitzgerald TJ, et al. Response-dependent and reduced treatment in lower risk Hodgkin lymphoma in children and adolescents, results of P9426: a report from the Children's Oncology Group. Pediatric Blood and Cancer 2012;59(7):1259-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Speyer 1992 {published data only (unpublished sought but not used)}
- Speyer JL, Green MD, Kramer E, Rey M, Sanger J, Ward C, et al. Protective effect of the bispiperazinedione ICRF-187 against doxorubicin-induced cardiac toxicity in women with advanced breast cancer. New England Journal of Medicine 1988;319:745-52. [DOI] [PubMed] [Google Scholar]
- Speyer JL, Green MD, Sanger J, Zeleniuch-Jacquotte A, Kramer E, Rey M, et al. A prospective randomized trial of ICRF-187 for prevention of cumulative doxorubicin-induced cardiac toxicity in women with breast cancer. Cancer Treatment Reviews 1990;17:161-3. [DOI] [PubMed] [Google Scholar]
- Speyer JL, Green MD, Zeleniuch-Jacquotte A, Wernz JC, Rey M, Sanger J, et al. ICRF-187 permits longer treatment with doxorubicin in women with breast cancer. Journal of Clinical Oncology 1992;10(1):117-27: Erratum for: Journal of Clinical Oncology 1992;10(5):867. [DOI] [PubMed] [Google Scholar]
- Speyer JL, Green MD, Zeleniuch-Jacquotte A, Wernz JC, Rey M, Sanger J, et al. ICRF-187 permits longer treatment with doxorubicin in women with breast cancer. Journal of Clinical Oncology 1992;10(1):117-27. [DOI] [PubMed] [Google Scholar]
Sun 2016 {published data only}
- ChiCTR-IPR-16007759. Cardiotoxicity induced by epirubicin-based chemotherapy for early stage breast cancer patients with diabetes and the protection of dexrazoxane. Chinese Clinical Trial Registry: www.chictr.org.cn 2016.
- Sun F, Qi X, Geng C, Li X. Dexrazoxane protects breast cancer patients with diabetes from chemotherapy-induced cardiotoxicity. American Journal of the Medical Sciences 2015;349(5):406-12. [DOI] [PubMed] [Google Scholar]
- Sun F, Shi J, Geng C. Dexrazoxane improves cardiac autonomic function in epirubicin-treated breast cancer patients with type 2 diabetes. Medicine (Baltimore) 2016;95(44):e5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Swain 1997a(088001) {published data only}
- Bates M, Lieu D, Zagari M, Spiers A, Williamson T. A pharmacoeconomic evaluation of the use of dexrazoxane in preventing anthracycline-induced cardiotoxicity in patients with stage IIIB or IV metastatic breast cancer. Clinical Therapeutics 1997;19:167-84. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CS, Weisberg SR, York RM, Jones SE, Spicer DV, Khojasteh A, et al. Prevention of adriamycin cardiomyopathy with dexrazoxane (ADR-529, ICRF-187). In: Proceedings of ASCO. Vol. 11. 1992:62.
- Swain SM, Whaley FS, Gerber MC, Ewer MS, Bianchine JR, Gams RA. Delayed administration of dexrazoxane provides cardioprotection for patients with advanced breast cancer treated with doxorubicin-containing therapy. Journal of Clinical Oncology 1997;15:1333-40. [DOI] [PubMed] [Google Scholar]
- Swain SM, Whaley FS, Gerber MC, Weisberg S, York M, Spicer D, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. Journal of Clinical Oncology 1997;15:1318-32. [DOI] [PubMed] [Google Scholar]
- Tonkin K, Bates M, Lieu D, Arundell E, Williamson TY, Zagari M. Dexrazoxane cardioprotection for patients receiving FAC chemotherapy: a pharmacoeconomic evaluation. Annals of Cancer Control Research 1996;6:458-73. [PubMed] [Google Scholar]
- Weisberg SR, Rosenfeld CS, York RM, Jones SE, Spicer DV, Khojasteh A, et al. Dexrazoxane (ADR-529, ICRF-187, Zinecard) protects against doxorubicin induced chronic cardiotoxicity. In: Proceedings of ASCO . Vol. 11. 1992:91.
Swain 1997a(088006) {published data only}
- Bates M, Lieu D, Zagari M, Spiers A, Williamson T. A pharmacoeconomic evaluation of the use of dexrazoxane in preventing anthracycline-induced cardiotoxicity in patients with stage IIIB or IV metastatic breast cancer. Clinical Therapeutics 1997;19:167-84. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CS, Weisberg SR, York RM, Jones SE, Spicer DV, Khojasteh A, et al. Prevention of adriamycin cardiomyopathy with dexrazoxane (ADR-529, ICRF-187). In: Proceedings of ASCO. Vol. 11. 1992:62.
- Swain SM, Whaley FS, Gerber MC, Ewer MS, Bianchine JR, Gams RA. Delayed administration of dexrazoxane provides cardioprotection for patients with advanced breast cancer treated with doxorubicin-containing therapy. Journal of Clinical Oncology 1997;15:1333-40. [DOI] [PubMed] [Google Scholar]
- Swain SM, Whaley FS, Gerber MC, Weisberg S, York M, Spicer D, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. Journal of Clinical Oncology 1997;15:1318-32. [DOI] [PubMed] [Google Scholar]
- Tonkin K, Bates M, Lieu D, Arundell E, Williamson TY, Zagari M. Dexrazoxane cardioprotection for patients receiving FAC chemotherapy: a pharmacoeconomic evaluation. Annals of Cancer Control Research 1996;6:458-73. [PubMed] [Google Scholar]
- Weisberg SR, Rosenfeld CS, York RM, Jones SE, Spicer DV, Khojasteh A, et al. Dexrazoxane (ADR-529, ICRF-187, Zinecard) protects against doxorubicin induced chronic cardiotoxicity. In: Proceedings of ASCO. Vol. 11. 1992:91.
Venturini 1996 {published data only}
- Michelotti A, Venturini M, Tibaldi C, Bengala C, Gallo L, Carnino F, et al. Single agent epirubicin as first line chemotherapy for metastatic breast cancer patients. Breast Cancer Research and Treatment 2000;59:133-9. [DOI] [PubMed] [Google Scholar]
- Venturini M, Michelotti A, Del Mastro L, Gallo L, Carnino F, Garrone O, et al. Multicenter randomized controlled clinical trial to evaluate cardioprotection of dexrazoxane versus no cardioprotection in women receiving epirubicin chemotherapy for advanced breast cancer. Journal of Clinical Oncology 1996;14:3112-20. [DOI] [PubMed] [Google Scholar]
Wexler 1996 {published data only}
- Wexler LH, Andrich MP, Venzon D, Berg SL, Weaver-McClure L, Chen CC, et al. Randomized trial of the cardioprotective agent ICRF-187 in pediatric sarcoma patients treated with doxorubicin. Journal of Clinical Oncology 1996;14:362-72. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Getz 2019 {published data only}
- Getz KD, Sung L, Gerbing RB, Alonzo TA, Li Y, Huang YV, et al. Occurrence and resolution of anthracycline cardiotoxicity and impact on treatment outcomes among children treated on the AAML1031 clinical trial: a report from the children's oncology group (Abstract ASH Annual Meeting). Blood 2019;134(Suppl 1):331. [https://ashpublications.org/blood/article/134/Supplement_1/331/426197/Occurrence-and-Resolution-of-Anthracycline] [Google Scholar]
Li 2013 {published data only}
- Li C, Zhou X, Song X. Protective effects of traditional Chinese medicine injections against anthracycline-induced cardiotoxicity. Anti-tumor Pharmacy 2013;3(5):[No pagination]. [Google Scholar]
Massida 1997 {published data only}
- Massidda B, Fenu MA, Ionta MT, Tronci M, Foddi MR, Montaldo C, et al. Early detection of the anthracycline-induced cardiotoxicity. A non-invasive haemodynamic study. Anticancer Research 1997;17:663-8. [PubMed] [Google Scholar]
Neto 2006 {published data only}
- Neto RP, Petrilli AS, Silva CM, Campos Filho O, Oporto VM, Gomes LD, et al. Left ventricular systolic function assessed by echocardiography in children and adolescents with osteosarcoma treated with doxorubicin alone or in combination with dexrazoxane. Arquivos Brasileiros de Cardiologia 2006;87:699-706. [DOI] [PubMed] [Google Scholar]
Paiva 2005 {published data only}
- Paiva MG, Petrilli AS, Moises VA, Macedo CR, Tanaka C, Campos O. Cardioprotective effect of dexrazoxane during treatment with doxorubicin: a study using low-dose dobutamine stress echocardiography. Pediatric Blood and Cancer 2005;45:902-8. [DOI] [PubMed] [Google Scholar]
Rabinovich 2012 {published data only}
- Rabinovich A, Weiss D, Weissman M, Novack V, Levi I. Dexrazoxane does not affect treatment outcome in non-Hodgkin's lymphoma patients. American Journal of Hematology 2012;87(8):830-2. [DOI] [PubMed] [Google Scholar]
Tap 2019 {published data only}
- Tap WD, Wagner AJ, Papai Z, Ganjoo KN, Yen C, Schoffski P, et al. ANNOUNCE: a randomized, placebo (PBO)-controlled, double-blind, phase (Ph) III trial of doxorubicin (dox) + olaratumab versus dox + PBO in patients (pts) with advanced soft tissue sarcomas (STS). Journal of Clinical Oncology 2019;37(18 Suppl):LBA3. [https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.18_suppl.LBA3] [Google Scholar]
Wang 2020 {published data only}
- Wang Y, Lu C, Li H, Liu K, Yu M, Zhang P. 3D-STI evaluation of the effect of dexrazoxane on the mechanical properties of right ventricular myocardium in breast cancer patients treated with pirarubicin. Annals of Palliative Medicine 2020;9(3):1187-97. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Aggarwal 2018 {published data only}
- Aggarwal S, Chow EJ, Sasaki N, Doody DR, Armenian SH, Asselin BL, et al. Long-term cardioprotective effects of dexrazoxane infusion during anthracycline chemotherapy: a children's oncology group speckle echocardiography study. Journal of the American Society of Echocardiography (Abstract ASE Annual Meeting) 2018;31(6):B14-5. [Google Scholar]
Cardenas 2003 {published data only}
- Cardenas R, Mercado CG, Rivela LR, Calderon EC. Effectiveness and safety of dexrazoxane as a cardioprotector in Mexican children with cancer. Medical and Pediatric Oncology 2003;41:PL001. [Google Scholar]
Chow 2016 {published data only}
- Chow EJ, Doody DR, Armenian SH, Aggarwal S, Baker KS, Bhatia S, et al. Effect of dexrazoxane on heart function among long-term survivors of childhood leukemia and lymphoma: a report from the children's oncology Group (COG) (Abstract ASH Annual Meeting). Blood 2016;128(22):696. [Google Scholar]
- NCT01790152. Effects of dexrazoxane hydrochloride on biomarkers associated with cardiomyopathy and heart failure after cancer treatment. clinicaltrials.gov/ct2/show/NCT01790152 (first received 13 February 2013).
De Berranger 2006 {published data only}
- De Berranger E, Vaksmann G, Thebaud-Leculee E, Wyckaert I, Mazingue F, Francart C et al. Results of randomized study on dexrazoxane administration in children with de novo acute leukemia. Journal of Clinical Oncolgy 2006;24(18):9037. [Google Scholar]
Feldmann 1992 {published data only}
- Feldmann JE, Jones SE, Weisberg SR, Gandars DR, Lyman GH, York RM, et al. Advanced small cell lung cancer treated with CAV (cyclophosphamide + adriamycin + vincristine) chemotherapy and the cardioprotective agent dexrazoxane (ADR-529, ICRF-187, Zinecard). In: ASCO Annual Meeting. Vol. 11. 1992:296.
Jackowska 2003 {published data only}
- Jackowska T, Rokicka-Milewska R, Matysiak M, Balwierz W, Kowalczyk J, Wachowiak J, et al. Assessment of dexrazoxane tolerance in leukemic children receiving anthracyclines. Medical and Pediatric Oncology 2003;41:PD003. [Google Scholar]
Liu 2013 {published data only}
- Liu H, Yanqiu L, Fengjuan Y, Hong L, Cuilling L, Rui F, et al. Cardiac diastolic dysfunction may hold promise as markers of cardiotoxicity during daunorubicin treatment in acute nonlymphocytic leukemia patients (Abstract Asia Pacific Heart Congress). Heart 2013;99(3):A273. [Google Scholar]
Saad El‐Din 2003 {published data only}
- Saad El-Din I, El Saban K, Abdel Rahman K, Gabaly M. A randomized clinical trial to evaluate cardioprotective effect of ICRF-187 in children with acute lymphoblastic leukemia. Medical and Pediatric Oncology 2003;41:O109. [Google Scholar]
Ten Bokkel‐Huinink 1992 {published data only}
- Ten Bokkel-Huinink WW, Rodenhuis S, Schreuder JE, Dubbelman R, Bierhorst F, Van Tinteren H, et al. ICRF 187 protects against doxorubicin induced cardiomyopathy. Annals of Oncology 1990;1:106. [Google Scholar]
- Ten Bokkel-Huinink WW, Schreuder JE, Dubbelman R, Bierhorst F, Van Tinteren H, Dalesio O, et al. ICRF 187 protects against doxorubicin induced cardiomyopathy. Annals of Oncology 1992;3(Suppl 1):114 (Abstract number 221). [Google Scholar]
Vejpongsa 2014 {published data only}
- Vejpongsa P, Chen M, Araujo DM, Massey MR, Banchs J, Acholonu SA, et al. Comparing the efficacy of primary prevention strategies in doxorubicin-induced cardiotoxicity using echocardiography and high-sensitivity troponin T (Abstract AHA Scientific Sessions and Resuscitation Science Symposium). Circulation 2014;130:A17234. [Google Scholar]
Wang 2013 {published data only}
- Wang P, Zhang S, Zhang XB, Li WJ, Hao XM, Zhang J. Protective effect of dexrazoxane on cardiotoxicity in breast cancer patients who received anthracycline-containing chemotherapy. Chinese Journal of Oncology 2013;35(2):135-9. [DOI] [PubMed] [Google Scholar]
Zhuang 2012 {published data only}
- Zhuang H, Zhang Y, Cai J, Shen J. Clinical study on anthracycline cardiotoxicity reduction by dexrazoxane combined with Shen Mai injection. Chinese Journal of Clinical Oncology 2012;39(6):348-51. [Google Scholar]
References to ongoing studies
NCT00016276 {published data only}
- NCT00016276. Phase III randomised study of doxorubicin and cyclophosphamide with or without dexrazoxane followed by paclitaxel with or without trastuzumab (herceptin) followed by surgery and radiotherapy with or without trastuzumab in women with HER-2+ stage IIIA or IIIB or regional stage IV breast cancer. www.controlled-trials.com (first posted 4 September 2003).
Additional references
Armstrong 2013
- Armstrong GT, Oeffinger KC, Chen Y, Kawashima T, Yasui Y, Leisenring W, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. Journal of Clinical Oncology 2013;31:3673-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Armstrong 2015
- Armstrong GT, Joshi VM, Ness KK, Marwick TH, Zhang N, Srivastava D, et al. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: results from the St. Jude Lifetime Cohort Study. Journal of the American College of Cardiology 2015;65:2511-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Batist 2001
- Batist G, Ramakriskan G, Sekhar Rao C, Chandrasekharan A, Gutheil J, Guthrie T, et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized multicenter trial of metastatic breast cancer. Journal of Clinical Oncology 2001;19:1444-54. [DOI] [PubMed] [Google Scholar]
Billingham 1978
- Billingham ME, Mason JW, Bristow MR, Daniels JR. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treatment Reports 1978;62(6):865-72. [PubMed] [Google Scholar]
Bonadonna 1969
- Bonadonna G, Morfandini S. Cardiac toxicity of daunorubicin. Lancet 1969;1:837. [PubMed] [Google Scholar]
Chellapandian 2019
- Chellapandian D, Pole JD, Nathan PC, Sung L. Congestive heart failure among children with acute leukemia: a population-based matched cohort study. Leukemia & Lymphoma 2019;20:385-94. [DOI] [PubMed] [Google Scholar]
Chow 2015
- Chow EJ, Chen Y, Kremer LC, Breslow NE, Hudson MM, Armstrong GT, et al. Individual prediction of heart failure among childhood cancer survivors. Journal of Clinical Oncology 2015;33:394-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chow 2021
- Chow EJ, Aplenc R, Vrooman LM, Doody DR, Huang YV, Aggarwal S, et al. Late health outcomes after dexrazoxane treatment: a report from the Children’s Oncology Group. Cancer 2021;0:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2020
- Cooper K, Tappenden P, Cantrell A, Ennis K. A systematic review of meta-analyses assessing the validity of tumour response endpoints as surrogates for progression-free or overall survival in cancer. British Journal of Cancer 2020;123(11):1686-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Baat 2022
- De Baat EC, Dalen EC, Mulder RL, Hudson MM, Ehrhardt MJ, Engels FK, et al. Primary cardioprotection with dexrazoxane in childhood cancer patients expected to receive anthracyclines: recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Child & Adolescent Health 2022;published online September 26. [DOI: 10.1016/S2352-4642(22)00239-5] [DOI] [PubMed] [Google Scholar]
Deng 2014
- Deng S, Yan T, Jendrny C, Nemecek A, Vincetic M, Gödtel-Armbrust U, et al. Dexrazoxane may prevent doxorubicin-induced DNA damage via depleting both topoisomerase II isoforms. BMC Cancer 2014;14:842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feijen 2019a
- Feijen EA, Leisenring WM, Stratton KL, Ness KK, Van der Pal HJ, Van Dalen EC, et al. Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late-onset cardiotoxicity. JAMA Oncolocy 2019;5(6):864-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feijen 2019b
- Feijen EA, Font-Gonzalez A, Van der Pal HJ, Kok WE, Geskus RB, Ronckers CM, et al. Risk and temporal changes of heart failure among 5-year childhood cancer survivors: a DCOG-LATER study. Journal of the American Heart Association 2019;8(1):e009122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fidler 2017
- Fidler MM, Reulen RC, Henson K, Kelly J, Cutter D, Levitt GA, et al. Population-based long-term cardiac-specific mortality among 34 489 five-year survivors of childhood cancer in Great Britain. Circulation 2017;135:951-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fojtu 2017
- Fojtu M, Gumulec J, Stracina T, Raudenska M, Skotakova A, Vaculovicova M, et al. Reduction of doxorubicin-induced cardiotoxicity using nanocarriers: a review. Current Drug Metabolism 2017;18(3):237-63. [DOI] [PubMed] [Google Scholar]
Gammella 2014
- Gammella E, Maccarinelli F, Buratti P, Recalcati S, Cairo G. The role of iron in anthracycline cardiotoxicity. Frontiers in Pharmacology 2014;5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed prior to 25 May 2022. Hamilton (ON): McMaster University (developed by Evidence Prime), 2022. Available at gradepro.org.
Groenewegen 2020
- Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. European Journal of Heart Failure 2020;22(88):1342-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyot 2012
- Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Medical Research Methodology 2012;12(9):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawkins 2020
- Hawkins M, Bhatia S, Henderson TO, Nathan PC, Yan A, Teepen JC, et al. Subsequent primary neoplasms: risks, risk factors, surveillance, and future research. Pediatric Clinics of North America 2020;67(6):1135-54. [DOI] [PubMed] [Google Scholar]
Higgins 2006
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated September 2006]. John Wiley & Sons, Ltd, In: The Cochrane Library, Issue 4, 2006. Chichester, UK.
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane-handbook.org, 2008.
Higgins 2011
- Higgins JP, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Hori 2017
- Hori H, Kudoh T, Nishimura S, Oda M, Yoshida M, Hara J, et al. Acute and late toxicities of pirarubicin in the treatment of childhood acute lymphoblastic leukemia: results from a clinical trial by the Japan Association of Childhood Leukemia Study. International Journal of Oncology 2017;22(2):387-96. [DOI] [PubMed] [Google Scholar]
Kearns 2003
- Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology-drug disposition, action, and therapy in infants and children. New England Journal of Medicine 2003;349(12):1157-67. [DOI] [PubMed] [Google Scholar]
Keizer 1990
- Keizer HG, Pinedo HM, Schuurhuis GJ, Joenje H. Doxorubicin (adriamycin): a critical review of free radical-dependent mechanisms of cytotoxicity. Pharmacology and Therapeutics 1990;47:219-31. [DOI] [PubMed] [Google Scholar]
Kremer 2002a
- Kremer LC, Van Dalen EC, Offringa M, Voute PA. Frequency and risk factors of anthracycline-induced heart failure in children: a systematic review. Annals of Oncology 2002;13:819-29. [DOI] [PubMed] [Google Scholar]
Kremer 2002b
- Kremer LC, Van Dalen EC, Offringa M, Voute PA. Frequency and risk factors of anthracycline-induced heart failure in children: a systematic review. Annals of Oncology 2002;13:503-12. [DOI] [PubMed] [Google Scholar]
Le Deley 2003
- Le Deley M, Leblanc T, Shamsaldin A, Raquin M, Lacour B, Sommelet D, et al. Risk of secondary leukemia after a solid tumor in childhood according to the dose of epipodophyllotoxins and anthracyclines: a case-control study by the Société Française d'Oncologie Pédiatrique. Journal of Clinical Oncology 2003;21(6):1074-81. [DOI] [PubMed] [Google Scholar]
Leerink 2020
- Leerink JM, Baat EC, Feijen EA, Bellersen L, Van Dalen EC, Grotenhuis HB, et al. Cardiac disease in childhood cancer survivors: risk prediction, prevention, and surveillance: JACC CardioOncology State-of-the-Art Review. Journal of the American College of Cardiology: CardioOncology 2020;2:363-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefrak 1973
- Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973;32:302-14. [DOI] [PubMed] [Google Scholar]
Legha 1982
- Legha SS, Benjamin RS, Mackay B, Ewer M, Wallace S, Valdivieso M, et al. Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Annals of Internal Medicine 1982;96:133-9. [DOI] [PubMed] [Google Scholar]
Lipshultz 1998
- Lipshultz SE, Sallan SE, Giantris AL, Lipsitz SR, Dalton V, Colan SD. 48 hour continuous doxorubicin infusion is not cardioprotective in children assessed 18 months later: the DFCI 91001 ALL protocol. Proceedings of the American Society of Clinical Oncology 1998;17:528a. [Google Scholar]
Loeffen 2018
- Loeffen EA, Van Dalen EC, Mulder RL, Van de Wetering MD, Kremer LC, Tissing WJ. The duration of anthracycline infusion should be at least one hour in children with cancer: a clinical practice guideline. Pediatric Blood & Cancer 2018;65:Epub. [DOI] [PubMed] [Google Scholar]
Lyu 2007
- Lyu YL, Kerrigan JE, Lin CP, Azarova AM, Tsai YC, Ban Y, et al. Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Research 2007;67(18):8839-46. [DOI] [PubMed] [Google Scholar]
Merkx 2021
- Merkx R, Leerink JM, De Baat EC, Feijen EA, Kok WE, Mavinkurve-Groothuis AM, et al. A symptomatic systolic dysfunction on contemporary echocardiography in anthracycline-treated long-term childhood cancer survivors: a systematic review. Journal of Cancer Survivorship 2021;Epub:Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mertens 2008
- Mertens AC, Liu Q, Neglia JP, Potter JD, Nesbit ME Jr, Ruccione K, et al. Cause specific late mortality among 5-year survivors of childhood cancer: the childhood cancer survivor study. Journal of the National Cancer Institute 2008;100:1368-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 1981
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207-14. [DOI] [PubMed] [Google Scholar]
Muggia 1991
- Muggia FM, Green MD. New anthracycline antitumor antibiotics. Critical Reviews in Oncology/hematology 1991;11:43-64. [DOI] [PubMed] [Google Scholar]
Muggia 1997
- Muggia FM, Hainsworth JD, Jeffers S, Miller P, Groshen S, Tan M, et al. Phase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by liposomal encapsulation. Journal of Clinical Oncology 1997;15:987-93. [DOI] [PubMed] [Google Scholar]
Mulrooney 2020
- Mulrooney DA, Hyun G, Ness KK, Ehrhardt MJ, Yasui Y, Duprez D, et al. Major cardiac events for adult survivors of childhood cancer diagnosed between 1970 and 1999: report from the Childhood Cancer Survivor Study cohort. BMJ 2020;368:l6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Myers 1998
- Myers C. The role of iron in doxorubicin-induced cardiomyopathy. Seminars in Oncology 1998;25 Suppl 10:10-14. [PubMed] [Google Scholar]
Nemade 2018
- Nemade H, Chaudhari U, Acharya A, Hescheler J, Hengstler JG, Papadopoulos S, et al. Cell death mechanisms of the anti-cancer drug etoposide on human cardiomyocytes isolated from pluripotent stem cells. Archives of Toxicology 2018;92:1507–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Odaimi 1987
- Odaimi M, Ajani J. High-dose chemotherapy. American Journal of Clinical Oncology 1987;10:123-32. [DOI] [PubMed] [Google Scholar]
Oken 1982
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology 1982;5:649-55. [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815-34. [DOI] [PubMed] [Google Scholar]
Pierga 2001
- Pierga JY, Robain M, Jouve M, Asselain B, Dieras V, Beuzeboc P, et al. Response to chemotherapy is a major parameter-influencing long-term survival of metastatic breast cancer patients. Annals of Oncology 2001;12:231-7. [DOI] [PubMed] [Google Scholar]
Pritchard‐Jones 2015
- Pritchard-Jones K, Bergeron C, Camargo B, Van den Heuvel-Eibrink MM, Acha T, Godzinski J, et al. Omission of doxorubicin from the treatment of stage II-III, intermediate-risk Wilms' tumour (SIOP WT 2001): an open-label, non-inferiority, randomised controlled trial. Lancet 2015;386(9999):1156-64. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
RevMan Web 2021 [Computer program]
- Review Manager Web (RevMan Web). Version 3.1.2. The Cochrane Collaboration, 2021. Available at revman.cochrane.org.
Seif 2015
- Seif AE, Walker DM, Li Y, Huang YV, Kavcic M, Torp K, et al. Dexrazoxane exposure and risk of secondary acute myeloid leukemia in pediatric oncology patients. Pediatric Blood & Cancer 2015;62(4):704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stata 2005 [Computer program]
- Stata Statistical Software. Version Release 9. StataCorp LP, College Station, TX: StataCorp, 2005.
Travis 2013
- Travis LB, Demark Wahnefried W, Allan JM, Wood ME, Ng AK. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nature Reviews. Clinical Oncology 2013;10(5):289-301. [DOI] [PubMed] [Google Scholar]
Turcotte 2018
- Turcotte LM, Neglia JP, Reulen RC, Ronckers CM, Van Leeuwen FE, Morton LM, et al. Risk, risk factors, and surveillance of subsequent malignant neoplasms in survivors of childhood cancer: a review. Journal of Clinical Oncology 2018;36(21):2145-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Dalen 2006
- Van Dalen EC, Van der Pal HJ, Kok WE, Caron HN, Kremer LC. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. European Journal of Cancer 2006;42(18):3191-8. [DOI] [PubMed] [Google Scholar]
Van Dalen 2010
- Van Dalen EC, Michiels EM, Caron HN, Kremer LC. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database of Systematic Reviews 2010, Issue 5. Art. No: CD005006. [DOI: 10.1002/14651858.CD005006.pub3] [DOI] [PubMed] [Google Scholar]
Van Dalen 2014
- Van Dalen EC, Raphaël MF, Caron HN, Kremer LC. Treatment including anthracyclines versus treatment not including anthracyclines for childhood cancer. Cochrane Database of Systematic Reviews 2014, Issue 9. Art. No: CD006647. [DOI: 10.1002/14651858.CD006647.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Dalen 2016
- Van Dalen EC, Van der Pal HJ, Kremer LC. Different dosage schedules for reducing cardiotoxicity in people with cancer receiving anthracycline chemotherapy. Cochrane Database of Systematic Reviews 2016, Issue 3. Art. No: CD005008. [DOI: 10.1002/14651858.CD005008.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Pal 2012
- Van der Pal HJ, Van Dalen EC, Van Delden E, Van Dijk IW, Kok WE, Geskus RB, et al. High risk of symptomatic cardiac events in childhood cancer survivors. Journal of Clinical Oncology 2012;30(13):1429-37. [DOI] [PubMed] [Google Scholar]
Von Hoff 1979
- Von Hoff DD, Layard MW, Basa P, Davis HL Jr, Von Hoff AL, Rozencweig M, et al. Risk factors for doxorubicin-induced congestive heart failure. Annals of Internal Medicine 1979;91:710-7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Van Dalen 2002
- Van Dalen EC, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database of Systematic Reviews 2002, Issue 3. Art. No: CD003917. [DOI: 10.1002/14651858.CD003917] [DOI] [PubMed] [Google Scholar]
Van Dalen 2005
- Van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database of Systematic Reviews 2005, Issue 2. Art. No: CD003917. [DOI: 10.1002/14651858.CD003917.pub2] [DOI] [PubMed] [Google Scholar]
Van Dalen 2008
- Van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No: CD003917. [DOI: 10.1002/14651858.CD003917.pub3] [DOI] [PubMed] [Google Scholar]
Van Dalen 2011
- Van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database of Systematic Reviews 2011, Issue 6. Art. No: CD003917. [DOI: 10.1002/14651858.CD003917.pub4] [DOI] [Google Scholar]