Abstract
Objectives
Although adoptive cell therapy with T‐cell receptor‐engineered T cells (TCR‐Ts) has mediated effective antitumor responses in several cancers, senescence of T cells could impair the therapeutic effect of TCR‐Ts. Thus, it is essential to elucidate the characteristics of senescent TCR‐Ts and how to subsequently improve their antitumor effect. Here, we focused on the influence of autophagy on TCR‐Ts, since autophagy is tightly associated with the regulation of T‐cell activation, proliferation and differentiation.
Methods
We first evaluated autophagy level of senescent TCR‐Ts, and then the senescent TCR‐Ts were expanded in vitro for 7 days with and without spermidine treatment, respectively. Furthermore, the proliferative potential, phenotypical characteristics and functionality of the propagated senescent TCR‐Ts were analysed in vitro and in vivo after 7‐day ex vivo expansion.
Results
We found that autophagic flux of senescent TCR‐T cells was significantly impaired. The restoration of autophagic flux via spermidine treatment reduced the expression of inhibitory immunoreceptors (PD‐1, TIM‐3 or LAG‐3), enhanced proliferation and effector functions and subsequently demonstrated the superior in vitro and in vivo antitumor activity of TCR‐Ts.
Conclusion
These data suggest that spermidine treatment presents an opportunity to improve the antitumor effect of TCR‐Ts for the treatment of solid tumors.
Keywords: autophagy, senescence, spermidine, T‐cell receptor, TCR‐T
In our study, we found that autophagic flux of senescent TCR‐T cells was significantly impaired. The restoration of autophagic flux via spermidine treatment reduced the expression of inhibitory immunoreceptors (PD‐1, TIM‐3 or LAG‐3), enhanced proliferation and effector functions and subsequently demonstrated the superior in vitro and in vivo antitumor activity of senescent TCR‐Ts.
Introduction
Adoptive cell therapy with T‐cell receptor‐engineered T cells (TCR‐Ts) has demonstrated effective antitumor activities in several cancers. 1 , 2 Although tumor‐reactive TCRs are critical for the antitumor response of TCR‐Ts, T‐cell function also plays an important role. 3 , 4 As individuals age, the adaptive immune system, in particular T cells, is most susceptible to the deleterious effects of ageing. 4 , 5 Since most cancer patients are aged and suffer from multiple cycles of radiotherapy and chemotherapy, patients' T cells could generally be dysfunctional, and thus, the antitumor activity of corresponding TCR‐Ts could be impaired.
Autophagy is a highly conserved catabolic process that has been demonstrated to be a critical regulator of T‐cell functions, such as intracellular homeostasis, proliferation and survival. 6 , 7 Previous studies reported that autophagy levels of T cells declined in an age‐dependent manner and that a decrease in autophagy levels could cause an inhibitory effect on T‐cell proliferation and cytokine production. 5 , 6 Therefore, the effector function of aged cancer patients' T cells could be impaired because of decreased autophagy levels.
Spermidine, which is a natural polyamine present in all living organisms and is critical for the maintenance of cellular homeostasis, can enhance autophagy via the inhibition of several acetyltransferases; moreover, tissue concentrations of spermidine decrease in an age‐dependent manner in humans. 8 , 9 Thus, it is essential to evaluate whether spermidine treatment could improve the effector function of cancer patients' T cells and further enhance the antitumor activity of corresponding tumor‐reactive TCR‐engineered senescent T cells because of restoration of autophagy damage.
Here, we present data to show that autophagic flux in senescent TCR‐T cells is significantly inhibited. Spermidine‐enhanced autophagic flux can restore the dysfunction of senescent TCR‐Ts and subsequently improve the therapeutic efficacy of tumor‐reactive TCR‐Ts against tumor cells.
Results
Since T‐cell senescence could influence T‐cell autophagic flux, further contributing to impairing the antitumor effect of TCR‐Ts, we primarily evaluated autophagic flux of peripheral T cells (PTCs) of young and old individuals through flow cytometry. When autophagy was inhibited by chloroquine (CQ), which can prohibit LC3‐II degradation by blocking the formation of autolysosomes, LC3‐II levels in young PTCs significantly increased, as expected, but LC3‐II levels in senescent PTCs did not significantly change, indicating inhibition of autophagic flux in senescent PTCs (Figure 1a and b).
Figure 1.
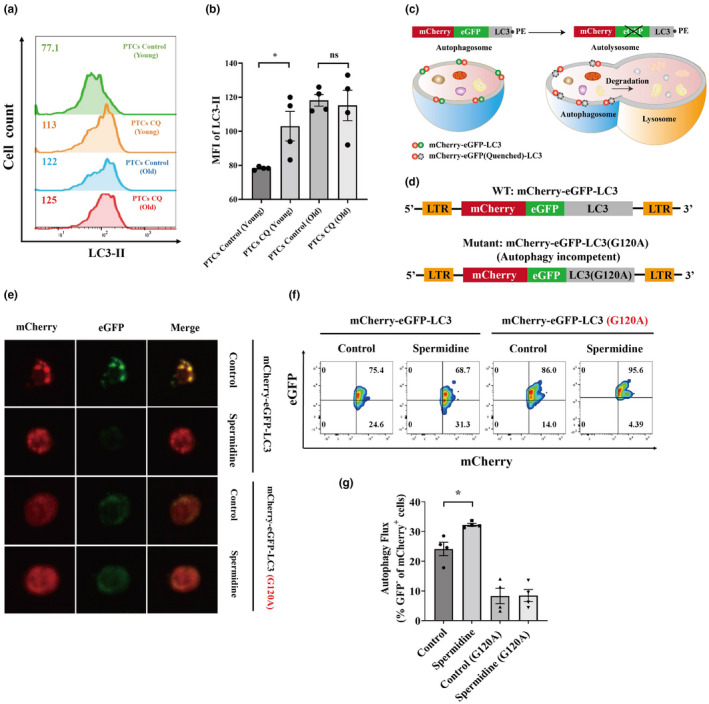
Autophagic flux impairment and restoration of senescent TCR‐Ts. (a, b) Peripheral T cells (PTCs) from young (25–30 years old) and old (more than 60 years old) donors were treated with or without chloroquine (CQ) (50 μm; 12 h), and LC3‐II was detected by flow cytometry. Representative flow cytometric results of LC3‐II in PTCs from young and old donors treated with or without CQ are shown (a). The LC3‐II expression levels in PTCs from young and old donors after CQ treatment are summarised and shown. Data represent mean ± SEM of n = 4 biological replicates (b). (c) Diagram showing the reporting mechanism of the mCherry‐GFP‐LC3 fusion protein. (d) Illustration of the lentiviral constructs encoding the autophagy gene LC3 in conjunction with mCherry and eGFP. Replacement of the LC3b glycine at the amino acid 120 position with alanine was used as an autophagy‐incompetent construct. (e) Representative confocal images defining the GFP and mCherry puncta in PTCs from old donors treated under the indicated conditions. (f, g) Representative flow cytometry plot (f) and quantification (g) of autophagic flux in the indicated conditions by measuring the loss of GFP in mCherry populations. Data represent mean ± SEM of n = 4 biological replicates. *P < 0.05; ns, not significant, the paired t‐test.
Since spermidine can enhance autophagic flux in several cell types, 9 we then evaluated whether spermidine could restore autophagic flux inhibition in senescent PTCs. We used a mCherry‐eGFP‐LC3 fusion reporter system to evaluate autophagic flux in live PTCs. 10 GFP fluorescence is quenched upon fusion of the autophagosome with the acidified lysosome, whereas acid‐insensitive mCherry fluorescence remains until this protein is degraded (Figure 1c). LC3 is hydrolysed to expose Gly120 to create LC3‐I, which is further conjugated with PE to generate LC3‐II, enabling phagophore expansion to obtain autophagosomes. An autophagy‐incompetent construct containing a Gly120 → Ala substitution (G120A) was adopted as a negative control (Figure 1d). Confocal microscopy demonstrated loss of GFP fluorescence in mCherry puncta of spermidine‐treated PTCs, indicating autophagosome‐lysosome fusion (Figure 1e). In addition, flow cytometric analysis confirmed that the proportion of mCherry+GFP− cells was greater in spermidine‐treated PTCs, revealing ongoing autophagic flux (Figure 1f and g). In summary, these data revealed that spermidine could enhance autophagic flux in senescent PTCs.
Since autophagy plays a central role in T‐cell activation, differentiation and survival, it is necessary to assess whether restoration of autophagic flux could improve the effector function of senescent TCR‐Ts. To evaluate the influence on TCR‐Ts, we adopted a tumor‐reactive TCR that was previously isolated from tumor‐infiltrating lymphocytes in a patient with oesophageal cancer and was reactive to corresponding tumor cells from this patient. 11 PTCs were treated with spermidine for 2 days and TCR was introduced into PTCs to obtain TCR‐Ts, and then TCR‐T were treated with spermidine for another 5 days and the transduction efficiency, phenotype and antitumor activities of TCR‐T were evaluated. The schematic workflow was shown in Supplementary figure 1a. We primarily found that spermidine treatment significantly enhanced the transduction efficiency of TCR‐Ts (Figure 2a and b). Furthermore, flow cytometric analysis demonstrated that spermidine‐treated TCR‐Ts exhibited decreased the expression of PD‐1, TIM‐3 and LAG‐3, and moreover, the frequencies of double inhibitory immunoreceptors coexpressing TCR‐Ts with spermidine treatment significantly decreased (Figure 2c–e). In addition, we found that spermidine‐treated TCR‐Ts exhibited enhanced proliferative capacity based on Ki67 expression (Figure 2f and g), and spermidine‐treated TCR‐Ts stimulated by PMA/ionomycin or OKT3 produced more IFN‐γ than TCR‐Ts without spermidine treatment (Figure 2h–k). In addition, we found that spermidine had the similar effects on CD4+ and CD8+ TCR‐T cells (Supplementary figure 1b–e). These data collectively indicated that spermidine‐treated senescent TCR‐Ts are less prone to exhaustion, with lower expression levels of PD‐1, TIM‐3 and LAG‐3 and stronger proliferation and cytokine production abilities.
Figure 2.
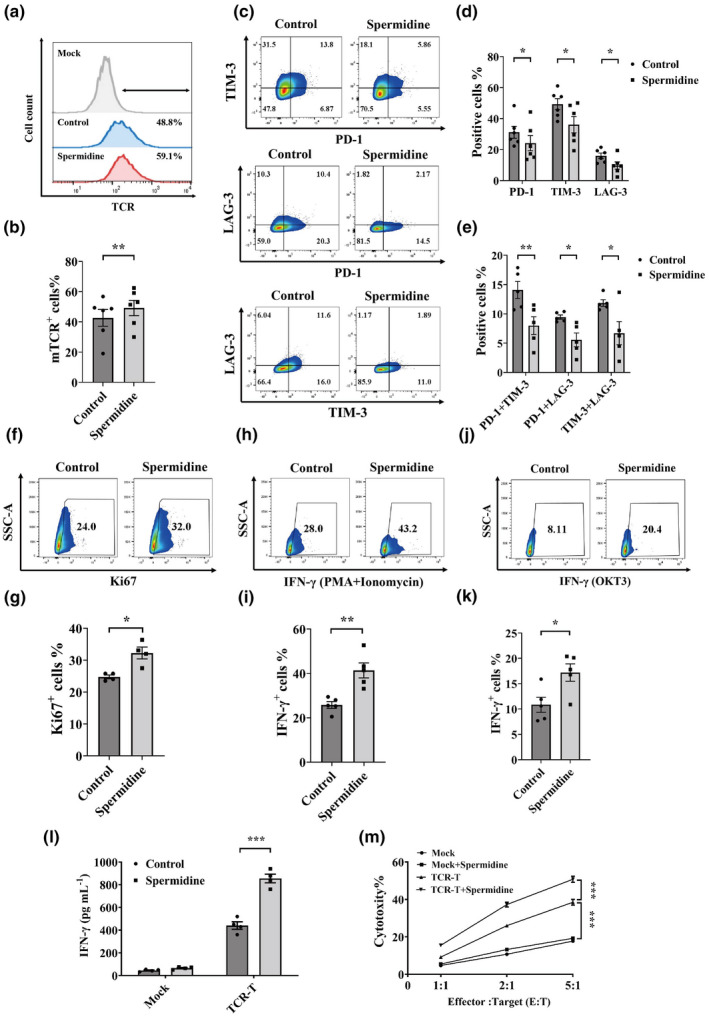
Restoring autophagic flux improves the in vitro antitumor activity of senescent TCR‐Ts. (a, b) Representative flow cytometric results of transduction efficiency measured by staining PTCs from old donors with an antimurine TCR‐β chain constant region antibody (a). The transduction efficiency results are summarised. Data represent mean ± SEM of n = 6 biological replicates (b). (c–e) Representative flow cytometric plots of TCR‐Ts expressing inhibitory immunoreceptors (PD‐1, TIM‐3 and LAG‐3) with or without spermidine treatment are shown (c). A statistical summary of the proportions of PD‐1+, TIM‐3+ or LAG‐3+ TCR‐Ts with or without spermidine treatment is shown. Data represent mean ± SEM of n = 6 biological replicates (d). Statistical summary of double‐positive TCR‐Ts (PD‐1 + TIM‐3; PD‐1 + LAG‐3; TIM‐3 + LAG‐3) with or without spermidine treatment is shown. Data represent mean ± SEM of n = 5 biological replicates (e). (f, g) Representative flow cytometric plots showing the proportions of Ki67+ TCR‐Ts with or without spermidine treatment (f). A statistical summary of the proportions of Ki67+ TCR‐Ts with or without spermidine treatment is shown. Data represent mean ± SEM of n = 4 biological replicates (g). (h, i) Representative flow cytometric plots showing the proportions of IFN‐γ+ TCR‐Ts with or without spermidine treatment under PMA + ionomycin stimulation (h). The proportions of IFN‐γ+ TCR‐Ts with or without spermidine treatment under PMA + ionomycin stimulation are summarised. Data represent mean ± SEM of n = 5 biological replicates (i). (j, k) Representative flow cytometric plots demonstrating the proportions of IFN‐γ+ TCR‐Ts with or without spermidine treatment under OKT3 stimulation (j). The proportions of IFN‐γ+ TCR‐Ts with or without spermidine treatment under OKT3 stimulation are summarised. Data represent mean ± SEM of n = 5 biological replicates (k). (l) IFN‐γ secretion from TCR‐Ts and spermidine‐treated TCR‐Ts with or without coculture with autologous tumor cells is shown. Data represent mean ± SEM of n = 4 biological replicates. (m) A statistical summary of the CFSE‐based cytotoxicity assay of TCR‐Ts with and without spermidine treatment at different E:T ratios is shown. Data represent mean ± SEM of n = 4 biological replicates. All analyses were performed by the paired t‐test, except for m where one‐way ANOVA (Tukey's post‐test) was used. *P < 0.05, **P < 0.01, ***P < 0.001.
Since enhanced autophagic flux could restore dysfunction and exhaustion of senescent TCR‐Ts, it is necessary to further evaluate whether spermidine‐treated senescent TCR‐Ts could enhance the therapeutic effect against autologous tumor cells in comparison with TCR‐Ts without spermidine treatment. We primarily found higher IFN‐γ secretion levels in spermidine‐treated TCR‐Ts upon stimulation of autologous tumor cells compared with TCR‐Ts without spermidine treatment (Figure 2l). Next, we found that spermidine‐treated TCR‐Ts had significantly higher cytotoxic activity against autologous tumor cells than TCR‐Ts without spermidine treatment, as measured with a flow cytometry‐based cytotoxicity assay (Figure 2m). To test the in vivo antitumor activity of TCR‐Ts, we delivered a (5 × 10 6 ) dose of TCR‐Ts intravenously into mice following subcutaneous inoculation of autologous tumor cells (Figure 3a). Although spermidine‐untreated TCR‐Ts induced significant tumor regression, mice treated with an equal number of spermidine‐treated TCR‐Ts exhibited significantly higher antitumor activity than untreated TCR‐Ts (Figure 3b). Moreover, flow cytometry analysis showed that significantly higher numbers of transferred TCR‐T cells were detected in tumor tissue of mice infused with spermidine‐treated TCR‐T cells than in mice infused with untreated TCR‐T cells on Day 12 after tumor implantation (Figure 3c and d). In aggregate, these data demonstrated that restoration of autophagic flux could significantly improve the therapeutic effect of senescent TCR‐Ts against autologous tumor cells. Meanwhile, we found that spermidine did not influence autophagic flux, transduction efficiency, phenotype and function of TCR‐Ts from young individuals (25–30 years old) (Supplementary figure 2a–h).
Figure 3.
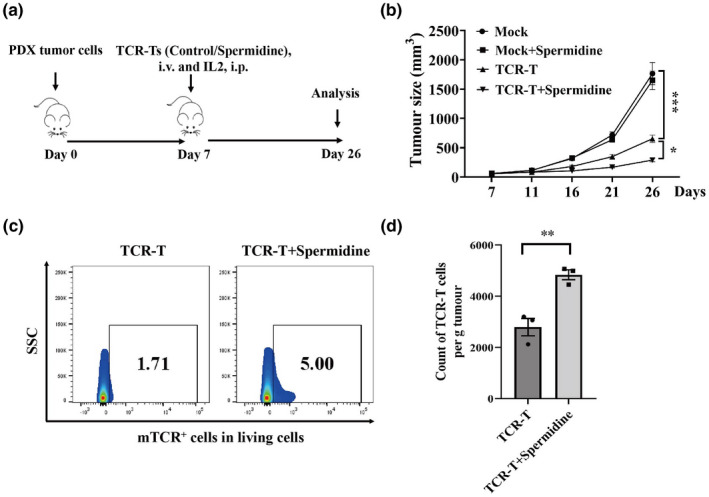
Restoring autophagic flux improves the in vivo antitumor activity of senescent TCR‐Ts. (a) The flow diagram of the in vivo adoptive experiment using TCR‐Ts with and without spermidine treatment is shown. (b) The growth curves of tumors in PDX mice infused with TCR‐Ts with and without spermidine treatment are shown. Data represent mean ± SEM of n = 5 mice per group, one‐way ANOVA (Tukey's posttest). The results are representative of two independent experiments. (c, d) Representative flow cytometric plots indicating the infiltration of transferred untreated TCR‐Ts or spermidine‐treated TCR‐Ts in tumors from PDX mice is shown on day 12 after tumor implantation (c). A statistical summary of counts of transferred TCR‐T cells per g tumor is shown (d). Data represent mean ± SEM of n = 3 biological replicates, the t‐test. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Adoptive cell transfer of TCR‐Ts is a promising treatment for cancer. 12 , 13 Since the number of TCR‐Ts for clinical application could be more than 109, it is essential for in vitro culture and expansion of TCR‐Ts for 7–14 days to obtain enough TCR‐Ts. In our study, we found that although autophagic flux was impaired in senescent TCR‐Ts, autophagic flux could be restored through 7 days of spermidine treatment, which could contribute to the restoration of dysfunction and exhaustion as well as the enhanced therapeutic effect of TCR‐Ts.
Accumulating evidence indicates that senescent T cells demonstrate autophagic flux impairment; moreover, decreased autophagy levels have an important impact on aged T‐cell function. 4 , 14 , 15 Since most cancer patients are aged and suffer from multiple cycles of radiotherapy and chemotherapy, autophagic flux and the effector function of cancer patients' T cells could generally be impaired. Previous studies demonstrated that spermidine could enhance autophagic flux in multiple cell types, including T cells, 9 , 16 and we found that spermidine treatment not only restored the autophagic flux of senescent TCR‐Ts but also reversed the dysfunction and exhaustion of TCR‐Ts and improved the therapeutic effect of TCR‐Ts against tumor cells.
Although the identification of tumor‐specific TCRs is essential for the clinical application of TCR‐T therapy, and moreover, we have presented multiple approaches to obtain tumor‐reactive TCRs, 11 , 17 T‐cell function also plays an important role in the antitumor activity of TCR‐Ts, especially for PTCs of cancer patients who are generally aged and suffer from multiple cycles of radiotherapy and chemotherapy. Therefore, spermidine treatment could be applied to enhance antitumor activity of TCR‐Ts for future clinical application.
Autophagy could be associated with not only T‐cell function but also the function of tumor cells. One recent study demonstrated that autophagy inhibition of tumor cells could enhance antitumor ability by restoring immunoproteasome activity and MHC class I–mediated antigen presentation of tumor cells. 18 However, our study found that senescent TCR‐T cells with restoration of autophagic flux demonstrated reversal of dysfunction and exhaustion and subsequently improved antitumor activity. Thus, since autophagy could have different effects on TCR‐Ts and tumor cells, in our study, spermidine was only added to the in vitro culture of TCR‐Ts, which did not affect tumor cells and could achieve better treatment outcomes.
In conclusion, senescent T cells demonstrated more serious autophagic flux impairment. Restoration of autophagic flux in senescent TCR‐Ts through spermidine treatment could contribute to the restoration of dysfunction and exhaustion as well as the enhanced therapeutic effect of TCR‐Ts, which offers a very attractive therapeutic approach to improve the antitumor ability of TCR‐Ts for the treatment of solid tumors.
Methods
Cell line, primary tumor cells and xenograft model
This study was approved by the Institutional Review Board of the Peking University School of Oncology, China. Tumor samples from a 63‐year‐old woman with oesophageal squamous cell carcinoma (ESCC) from Peking University Cancer Hospital were used to obtain primary tumor cells and a patient‐derived xenograft (PDX) model, as previously described. 11 HEK 293‐FT cells, a packaging cell line used to produce high‐titre lentivirus supernatants, were purchased from ATCC.
Isolation and culture of peripheral T cells
Peripheral blood mononuclear cells (PBMCs) from old cancer patients (more than 60 years old) and young donors (25–30 years old) at Beijing Cancer Hospital (Beijing, China) were obtained by Ficoll‐Paque (GE Healthcare, UK) gradient centrifugation, and then peripheral T cells (PTCs) were isolated using a Dynabeads® Untouched™ Human T Cells Kit (Thermo Fisher Scientific, USA) for the following experiments. After finishing the test of viability, PBMCs were cultured in T‐cell culture medium, which consisted of X‐VIVO 15 serum‐free medium (Lonza, USA) appended with GlutaMAX (Thermo Fisher, USA), IL2 (50 U mL−1; Peprotech, USA), IL‐7 (10 ng mL−1; Peprotech, USA), IL‐15 (10 ng mL−1, Peprotech, USA), OKT3 antibody (50 ng mL−1; ACRO, USA) and anti‐CD28 antibody (1 μg mL−1; T&L Biotechnology, China) in 37 °C incubator with 5% CO2.
Cell staining and flow cytometry
PTCs were pelleted in tubes and washed with phosphate‐buffered saline (PBS). First, fixable viability stain 780 (FVS780) was used to stain the cells protected from light for 15 min at room temperature, indicating the viability of the cells. Then, antibodies against membrane molecules, including CD3 (Clone: HIT3α), CD4 (Clone: L200), CD8 (Clone: RPA‐T8), CD45 (Clone: HI30), PD‐1 (Clone: EH12.1), TIM‐3 (Clone: 7D3) and LAG‐3 (Clone: T47‐530), were used to stain cells for 15 min at room temperature protected from light. All of the above antibodies were from BD Biosciences, except for the anti‐mouse TCR‐β constant region (Clone: H5‐597, eBioscience, USA) for detecting the transduction efficiency of TCR‐Ts.
For the intracellular molecules, cells were fixed and permeabilized using fixation and permeabilization buffer (BD Biosciences) for 15 min at room temperature first. After two washes with perm/wash buffer (BD Biosciences), the cells were stained with intracellular antibodies in wash buffer including Ki67 (Clone: B56) and IFN‐γ (Clone: B27) (Source: BD Biosciences, USA) for 15 min without light exposure. After two washes with PBS, the cells were loaded on a flow cytometry machine for detection. Specifically, intracellular IFN‐γ detection was required prior to overnight operation by GolgiPlug in 1: 1000 work concentration, a protein transporter inhibitor (BD Biosciences, USA).
For LC3 staining, 1‐h fixation and permeabilization against exposure to light at room temperature was required after FVS780 staining. Then, the cells were washed twice with perm/wash buffer, and Alexa Fluor 488‐labelled LC3 antibody (Cell Signalling Technology, USA) in perm/wash buffer was mixed with the fixed cells at a dilution of 1:50 for 1 h at room temperature. After discarding of the LC3 antibody by centrifugation and washing twice with PBS, the surface markers mentioned above were used to stain the cells as described above. Cells were washed twice with PBS prior to flow cytometric analysis. Notably, for Figure 1b–h, PBMCs were treated with chloroquine (CQ) (Sigma Aldrich, USA) at a concentration of 50 μm for 12 h prior to LC3 detection.
Cells were acquired on BD FACSAria and BD FACSCelesta™ SORP. Data collected were analysed further by FlowJo version 10.6.2 software (BD Biosciences, USA).
Lentivirus construction and transduction of PTCs
To evaluate the antitumor activity of TCR‐T cells, we adopted a tumor‐reactive TCR that was previously identified and isolated from tumor‐infiltrating lymphocytes (TILs) in a patient with ESCC. 11 In brief, TILs from ESCC were cocultured with autologous tumor cells and activated T cells (CD137+) were sorted into 96‐well PCR plates by single‐cell sorting of flow cytometry. And then, the TCR of each T cell was obtained through single‐cell TCR sequencing, and the most dominant TCR was approved to be tumor‐specific TCR which was used in our study. TCRα/β chains were synthesised (GenScript) and cloned into our lentivirus vector. TCRs were constructed in a β‐α chain order, and their constant regions were replaced by mouse counterparts modified with hydrophobic substitution and added disulfide bonds as previously described, 11 which not only was convenient for the detection of TCR‐T cells but also improved TCR pairing and TCR/CD3 stability. The sequences of wild‐type and mutant mCherry‐eGFP‐LC3 were obtained from the Addgene database, and the plasmids were synthesised by GenScript Bioscience Company (Nanjing, China). The target fragment in the plasmid was cloned into the lentiviral vector pCDH‐EF1‐MCS‐T2A‐Puro with two restriction sites, EcoR I and BamH I. Then, the above‐mentioned lentivectors were generated by cotransduction of 293F T cells with a lentivector and packaging plasmids using PEI MAX 40000 (Polysciences Inc. USA). The lentiviral supernatants were harvested at 48 and 72 h after transfection and concentrated using ultracentrifugation at 20 000 g for 90 min at 4°C. Then, activated T cells were transduced by concentrated lentivirus with 8 μg mL−1 polybrene (Sigma–Aldrich, USA). 19 , 20 Transduction of PTCs was conducted as previously delineated. 17 In brief, the PTCs were stimulated in T‐cell media for 2 days before transduction, and activated T cells were transduced by concentrated lentivirus at the presence of 8 μg mL−1 polybrene (Sigma–Aldrich, USA). For spermidine‐treatment group, PTCs were treated in T‐cell media with spermidine for 2 days, and activated T cells were transduced by concentrated lentivirus at the presence of 8 μg mL−1 polybrene (Sigma–Aldrich, USA) to obtain TCR‐T. And then, TCR‐T were treated with spermidine for another 5 days, and the transduction efficiency, phenotype and antitumor activities of TCR‐T were evaluated. This workflow was shown in Supplementary figure 1a. For mCherry‐eGFP‐LC3 transduction, spermidine was administered to the transduced cells after 2 days. Flow cytometry was conducted after 7 days focussing on mCherry and eGFP.
Confocal assay
As mentioned above, PTCs were transduced with mCherry‐eGFP‐LC3, indicating autophagic flux. Then, PTCs were dropped onto confocal dishes which were precoated with polylysine for 24 h (Sigma–Aldrich, USA), and PTCs were incubated with the precoated dishes at 37°C for 30 min to 1 h for adhesion to the confocal dish. After incubation and confirmation of the adhesion of the transduced cells, the culture medium was removed, and the adhered cells were washed by PBS twice before the confocal observation. The whole process above was conducted under light‐free conditions. Finally, after washing twice with PBS, the cells were covered with 90% glycerol for the following observation. Immunofluorescence observation was accomplished by confocal laser scanning microscopy (LSM780) (ZEISS, Germany).
IFN‐γ ELISA
PTCs were cultured in medium with or without spermidine for 2 days and then transduced with tumor‐reactive TCRs. Transduction efficiency was tested after 4 days, and TCR‐Ts with or without spermidine treatment were cocultured with corresponding tumor cells in 96‐well plates at an effector:target ratio (E:T ratio) of 5:1. 11 IFN‐γ secretion was detected after 24 h of coculture from the cell supernatant. PTCs without transduction of TCR with and without spermidine treatment were regarded as mock groups.
In vitro cell toxicity assay
A CFSE‐based cell cytotoxicity assay was used to measure the efficacy of TCR‐T cell killing. The autologous tumor cells were stained with 2 μm CFSE for 30 min followed by PBS washing, and staining was stopped with FBS‐containing medium for 10 min. The CFSE‐stained tumor cells were cocultured with T cells at E:T ratios of 1:1, 2:1, and 5:1 for 6 h in a 37°C incubator. After coculture, 1 μg mL−1 propidium iodide (PI) (BD Biosciences, USA) was added to stain the target cells for 15 min at room temperature, and then the samples were analysed with an Accuri C6 FlowCytometer (BD Biosciences, USA).
In vivo adoptive TCR‐T experiment
First, each NOD‐SCID mouse was implanted subcutaneously with autologous tumor cells with Matrigel (Biocoat, USA) as previously described. 11 Next, 3 × 106 TCR‐T cells were intravenously infused into tumor‐bearing NOD‐SCID mice simultaneously with intraperitoneal administration of IL‐2 (3000 IU per mouse) on Day 7. Tumor volume was determined by calliper measurement of perpendicular diameters of each tumor and was calculated using the following formula: tumor volume (mm3) = [(length) × (width) × (width)]/2.
Statistical analysis
Statistical analyses were performed with Stata 11.0 for Windows (StataCorp LP) using the two‐tailed Student's t‐test and one‐way ANOVA. In all cases, statistical significance was considered when P < 0.05. Error bars show the mean ± standard error of the mean (SEM), and P‐values are represented as follows: *P < 0.05, **P < 0.01, ***P < 0.001.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
Chaoting Zhang: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; software; supervision; validation; visualization; writing – original draft; writing – review and editing. Yizhe Sun: Conceptualization; data curation; formal analysis; methodology; resources; software; validation; visualization; writing – original draft; writing – review and editing. Shance Li: Methodology; visualization. Luyan Shen: Methodology; supervision. Xia Teng: Data curation; methodology. Yefei Xiao: Data curation; visualization. Ping Zhou: Conceptualization; formal analysis; project administration; writing – review and editing. Zheming Lu: Conceptualization; formal analysis; funding acquisition; project administration; writing ‐ review and editing.
Supporting information
Supplementary figures 1 and 2
Acknowledgments
This work was supported by Natural Science Foundation of China [Grant No. 81972880 to ZL, Grant No. 82003246 to CZ,Grant No. 82260474]; Capital's Funds for Health Improvement and Research (Grant No. 2022‐1‐1022 to ZL, 2020‐4‐1028 to CZ); Hainan Province Clinical Medical Center; Open Project funded by Key Laboratory of Carcinogenesis and Translational Research, Ministry of Education/Beijing (2022 Open Project‐1); Cooperation Fund of Beijing Cancer Hospital and Beijing Institute for Cancer Research; Clinical Medicine Plus X ‐ Young Scholars Project (PKU2022LCXQ036), Peking University, the Fundamental Research Funds for the Central Universities.
Contributor Information
Ping Zhou, Email: ping.zhou@hainmc.edu.cn.
Zheming Lu, Email: luzheming@bjmu.edu.cn.
References
- 1. Ecsedi M, McAfee MS, Chapuis AG. The anticancer potential of T cell receptor‐engineered T cells. Trends Cancer 2021; 7: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ellis GI, Sheppard NC, Riley JL. Genetic engineering of T cells for immunotherapy. Nat Rev Genet 2021; 22: 427–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao Y, Shao Q, Peng G. Exhaustion and senescence: two crucial dysfunctional states of T cells in the tumor microenvironment. Cell Mol Immunol 2020; 17: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mittelbrunn M, Kroemer G. Hallmarks of T cell aging. Nat Immun 2021; 22: 687–698. [DOI] [PubMed] [Google Scholar]
- 5. Nikolich‐Zugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immun 2018; 19: 10–19. [DOI] [PubMed] [Google Scholar]
- 6. Xia H, Green DR, Zou W. Autophagy in tumor immunity and therapy. Nat Rev Cancer 2021; 21: 281–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang L, Das JK, Kumar A et al. Autophagy in T‐cell differentiation, survival and memory. Immunol Cell Biol 2021; 99: 351–360. [DOI] [PubMed] [Google Scholar]
- 8. Madeo F, Bauer MA, Carmona‐Gutierrez D, Kroemer G. Spermidine: a physiological autophagy inducer acting as an anti‐aging vitamin in humans? Autophagy 2019; 15: 165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eisenberg T, Knauer H, Schauer A et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol 2009; 11: 1305–1314. [DOI] [PubMed] [Google Scholar]
- 10. Vodnala SK, Eil R, Kishton RJ et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science 2019; 363: eaau0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tan Q, Zhang C, Yang W et al. Isolation of T cell receptor specifically reactive with autologous tumor cells from tumor‐infiltrating lymphocytes and construction of T cell receptor engineered T cells for esophageal squamous cell carcinoma. J Immunother Cancer 2019; 7: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nagarsheth NB, Norberg SM, Sinkoe AL et al. TCR‐engineered T cells targeting E7 for patients with metastatic HPV‐associated epithelial cancers. Nat Med 2021; 27: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chapuis AG, Egan DN, Bar M et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post‐transplant. Nat Med 2019; 25: 1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Phadwal K, Alegre‐Abarrategui J, Watson AS et al. A novel method for autophagy detection in primary cells: impaired levels of macroautophagy in immunosenescent T cells. Autophagy 2012; 8: 677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Puleston DJ, Zhang H, Powell TJ et al. Autophagy is a critical regulator of memory CD8+ T cell formation. Elife 2014; 3: e03706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carriche GM, Almeida L, Stüve P et al. Regulating T‐cell differentiation through the polyamine spermidine. J Allergy Clin Immunol 2021; 147: e311. [DOI] [PubMed] [Google Scholar]
- 17. Zhang C, Tan Q, Li S et al. Induction of EBV latent membrane protein‐2A (LMP2A)‐specific T cells and construction of individualized TCR‐engineered T cells for EBV‐associated malignancies. J Immunother Cancer 2021; 9: e002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deng J, Thennavan A, Dolgalev I et al. ULK1 inhibition overcomes compromised antigen presentation and restores antitumor immunity in LKB1‐mutant lung cancer. Nat Cancer 2021; 2: 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang C, Palashati H, Rong Z et al. Pre‐depletion of TRBC1+ T cells promotes the therapeutic efficacy of anti‐TRBC1 CAR‐T for T‐cell malignancies. Mol Cancer 2020; 19: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu H, Lei W, Zhang C et al. CD19‐specific CAR T cells that express a PD‐1/CD28 chimeric switch‐receptor are effective in patients with PD‐L1‐positive B‐cell lymphoma. Clin Cancer Res 2021; 27: 473–484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures 1 and 2


