Abstract
Candida species are a dominant constituent of the human mycobiome and associated with the development of several diseases. Understanding the Candida species metabolism could provide key insights into their ability to cause pathogenesis. Here, we have developed the BioFung database, providing an efficient annotation of protein-encoding genes. Along, with BioFung, using carbohydrate-active enzyme (CAZymes) analysis, we have uncovered core and accessory features across Candida species demonstrating plasticity, adaption to the environment and acquired features. We show a greater importance of amino acid metabolism, as functional analysis revealed that all Candida species can employ amino acid metabolism. However, metabolomics revealed that only a specific cluster of species (AGAu species—C. albicans, C. glabrata and C. auris) utilised amino acid metabolism including arginine, cysteine, and methionine metabolism potentially improving their competitive fitness in pathogenesis. We further identified critical metabolic pathways in the AGAu cluster with biomarkers and anti-fungal target potential in the CAZyme profile, polyamine, choline and fatty acid biosynthesis pathways. This study, combining genomic analysis, and validation with gene expression and metabolomics, highlights the metabolic diversity with AGAu species that underlies their remarkable ability to dominate they mycobiome and cause disease.
Subject terms: Systems analysis, Fungal systems biology
Metabolic differences between Candida species are uncovered using the BioFung database alongside genomic and metabolic analysis.
Introduction
Fungal infections affect around 7.5 million people around the world every year. Within human fungal communities (mycobiome), with the notable exception of the skin, Candida species are the most common group1–3. These species are generally pathobionts, being the most common human fungal pathogens, despite also being commensal organisms4. Candida infections are becoming increasingly concerning, and the World Health Organisation (WHO) has recently emphasised international surveillance for diagnosis and management of fungal infection, particularly Candida albicans infection5–7. Recently, a novel Candida species, Candida auris has been identified with significant mortality and morbidity, as well as a high degree of anti-fungal resistance8,9. There are over 200 Candida species currently identified, but only a handful of these are present in the human microbiota with the ability to cause infection and pathology. Most notable examples of these include C. albicans, C. glabrata, C. dubliniensis, C. tropicalis and C. auris9–15. Candida species, most notably C. albicans and C. glabrata, can give rise to a variety of superficial infections, including oral thrush and vulvovaginal candidiasis, but are also capable of causing a systemic infection with significant mortality16–20. As well as direct infections, fungi such as Candida species have also been associated with oncogenesis through complement activation, demonstrating potential effects of the interaction of fungal species with human host21.
An essential virulence determinant of fungi is their metabolic plasticity22. Fungi are significant in their ability to utilise numerous different anabolic and catabolic sources in their metabolic processes, attributable to switching between carbon and nitrogen sources23. Nutritional availability, environmental factors, competition and pathogenic factors all influence this plasticity24,25. Investigations of Candida species-specific transcriptional regulators of glycolytic genes (e.g. Tye2 and Gal4) and enzymes of the glycolytic pathway (hexose catabolism), indicate these factors play an essential role in central carbon metabolism commonly applied during infection events22,24,26. Glycolytic metabolism can activate virulence factors that initiate hypha formation, activate fermentative pathways, repress gluconeogenesis, and the TCA cycle27–30. Alternatively, C. albicans can switch to gluconeogenesis and the glyoxylate cycle to confer full pathogenesis during systemic candidiasis31–34. Carbohydrate metabolism is coupled with changes of cell wall architecture, host immune response modulation, as well as adherence, biofilm formation, stress response and drug resistance24,35–37. If carbohydrate sources are limited, Candida species can use amino acids and lipids as supplementation for metabolic adaptation22,38–40. Amino acids produced by C. albicans have been shown to drive tissue damage by initiating stress responses and adjusting the surrounding environmental pH, helping induce of host invasion processes35,41–47. Very little is known about the regulation, process and utilisation of amino acid metabolism in Candida39. However, C. albicans is known to use amino acids to replace carbon and other nitrogen sources48. Candida’s ability to convert arginine to urea allows the neutralisation of an acidic environment triggering the development of hyphae and biofilm formation32,49,50. Notably, recent work has shown that C. albicans phagocyted by macrophages induces fatty acid β-oxidation and the glyoxylate pathway to induce hypha formation for escape. In a harsh environment that lacks even a nitrogen source, Candida can recycle and produce its own proteins and polyamines without host nitrate51. Thus, understanding of metabolism and functionality of Candida is instrumental in tackling infection and mortality prevalence52.
Here, we have developed the BioFung tool—a database derived from 128 fungal species using KEGG orthologs (KO) and focused on functional information and interpretation of biological information to address the issue of the lack of resources for functional annotation of fungal genomes. We then go on to apply this database tool to Candida species to identify enriched functionality in specific clusters and further show how it can be integrated with other tools such as CAZyme. In doing so, we demonstrate the power of this tool to make functional analyses of fungal species. Distinct clusters of Candida species were defined based on literature review of contributions to candidemia and mortality (Fig. 1a), cluster of C. albicans, C. glabrata and C. auris referred to as AGAu species. This cluster has a high association with infection and mortality, relative to other Candida species18,53–55. We applied comparative analysis techniques based on gene, protein, and enzyme-substrate levels and identified metabolic pathways in Candida species, such as choline and polyamine pathways. Metabolomics validation along with experimental validation from gene expression confirmed important AGAu cluster difference. This study (1) provides a tool for functional annotation of fungal species, (2) highlights amino acid metabolism importance in AGAu species that remarkably dominate the mycobiome, and (3) identifies potential fungal biomarkers and anti-fungal targets in metabolic pathway.
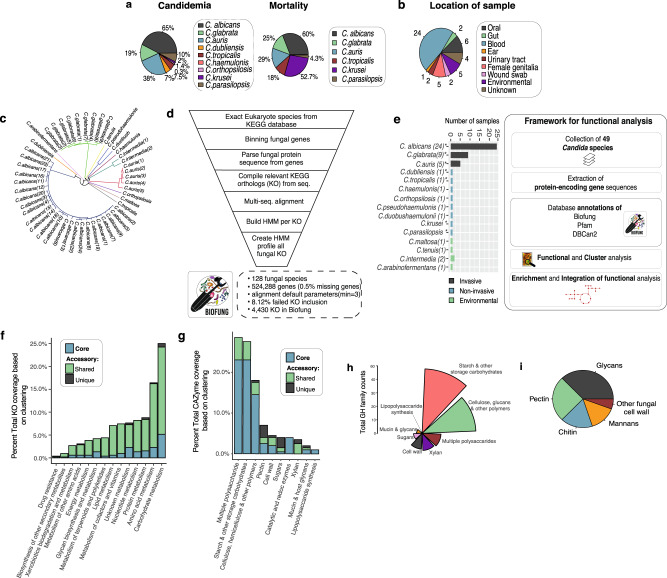
Fig. 1. Exploration of Candida species with BioFung database.
a Contribution of individual Candida species to candidemia and mortality. The impact of each species in AGAu species’ grouping is attributed in this study (literature-based)16–18,20,152. b Candida strain characterisation. Coverage of Candida sample population per species available with the categorisation of species profiled. Numbers around the pie chart signify the number of strain representation in each location. (Supplementary Data 3 for more information about strains and Supplementary Figure 1a for the global representation of Candida strains). c Genome-based phylogenetic tree. The phylogenetic tree was constructed based on average nucleotide identity (ANI) between all strains revealing evolutionary differences across strains (colour coordinated) and indicating distinct metabolic capabilities. See Supplementary Fig. 1b for quality of sequences. d BioFung database creation workflow. Eukaryote annotation from KEGG database parsed to extract all fungal species. They were genes parsed, sequences extracted and reassembled to KO. The multi-sequence alignment was performed on each KO with all corresponding sequence available. HMM, profile built based on each KO and assembled to provide a more accurate annotation of fungal species for KO. e Distribution of Candida species based on sample collection and the framework of protein-encoded genes analysis of Candida strains. Strains isolated from the various location providing relevant clinical association to host mycobiome and environmental species. *indicates clinical strains used for metabolomics. Functional analysis performed on 49 Candida species collected from public repositories. Protein sequences were annotated with Pfam, dbCAN2 and BioFung database for biological information. f Core and accessory overview of the metabolic pathway across Candida strains. Shared genome feature refers to 6–48 species sharing the function and unique genome features is exhibited by less than 5 Candida species denoting accessory functions. g Clustering of carbohydrate-active enzyme profile (CAZyme). Core, shared genome (6–48 strains), and unique genome (<5 strains) illustrates distribution analysis of functions across all Candida species. h Breakdown of GH family substrate-converter activity. Analysis of enzyme function of glycoside hydrolase family across all Candida strains. i Breakdown of cell wall composition of core Candida strains with identification of 49 CAZymes.
Results
Development of BioFung database and functional annotation of Candida protein-encoding genes
There is currently a dearth of tools for accurate and complete annotation of fungal genomes. In order to analyse the global functionality of fungal species, we decided to develop a database tool (BioFung) to help solve this problem. The BioFung database takes a list of protein sequences in fasta format (representing a fungal genome) and outputs KEGG orthologues that are then used to annotate the associated genome, giving an overview of the potential functionality. To perform a global functional analysis of Candida species, we collected 49 publicly available genomes of different Candida strains covering 13 different species (Supplementary Table 1). We selected species based on their clinical importance and abundance within the human mycobiome56–60. All 49 Candida strains were isolated from different body sites from people represented in a pie chart demonstrating diverse survival of Candida species and in different geographical locations illustrated on a global map (Fig. 1b and Supplementary Fig. 1a). Comparisons of sequencing platform, scaffold assembly and genome were performed to assess how the quality of published genomes impacts on the annotations (Supplementary Figure 1b, Supplementary Table 1). No distinct impacts were seen based on differences in sequencing processes and genome assembly. Although assessment of similarity of sequence across 49 strains is not a direct assessment of integrity of genome annotation, average nucleotide identity (ANI) using nucleotide sequence reveals that the phylogenetic relationship of all these Candida species are interlinked (Fig. 1c, Method). We observed strain-specific differences in phylogenetic lineages with 11 distinct branches, including branching of C. auris, C. glabrata and C. albicans, implying genetic diversity that could implicitly be interpreted into functional variances. To elucidate functional details for these strains, we built fungi-specific Hidden Markov Models (HMM) using fungal gene clusters, named the BioFung database (Fig. 1d, “Method”)61,62. We analysed 524,288 fungal genes, from 128 fungal species, with a coverage of 4,822 KOs, and 4,430 fungal KO alignments to create BioFung (Supplementary Data 1–3). Comparison of the BioFung database with similar eukaryote-specific HMM sources (Euk90 and Euk100) indicated that BioFung has both higher coverage and specificity of KOs (Supplementary Fig. 2a–c)61,62. BioFung was applied to protein sequences of Aspergillus fumigatus, Aspergillus niger and Aspergillus nidulans for robustness (Supplementary Figure 2d, e). Output of BioFung is compared to representative organisms in the KEGG database (Supplementary Fig. 2f).
The collection of Candida strains used to integrate functional annotations can be categorised into commonly invasive and non-invasive (requiring a secondary factor to cause infection, such as co-morbidity, immunodeficiency) based on the literature (Fig. 1e, Supplementary Table 2, “Method”). These representative samples of Candida were integrated into the functional analysis framework, with a total of 49 Candida species annotated with BioFung, Protein families (Pfam)63 and Carbohydrate-Active enZyme (CAZyme)64 databases. We applied BioFung using the UCLUST algorithm, to establish core genome features (found in all Candida species) and accessory genome features (shared or unique functions)65. In covering KEGG metabolic orthologs, clustering analysis determined a larger number of accessory features of metabolism compared to core characteristics seen in all Candida strains (Fig. 1f, Supplementary Fig. 2g–i, “Method”). Intra-strain analysis of C. albicans across 24 strains sequenced showed largely conserved metabolic pathways and CAZyme profiles (Supplementary Fig. 2j, k).
Identification of global functional annotation profiles in Candida
We next determined the CAZyme profile by mapping the 49 Candida protein sequences to the dbcan2 database64. Doing this allowed us to infer molecular enzyme function64. CAZymes are vital enzymes involved in the metabolism of complex carbohydrates. Approximately 205 unique CAZymes were identified in all Candida strains, with various functions (Fig. 1g, Supplementary Data 4, “Method”). From core Candida genome analysis, annotated enzymes were distributed across 6 active CAZyme families, with an assortment of enzymatic functions. The glycoside hydrolase (GH) family showed the highest degree of core coverage (Supplementary Fig. 3a), with much of the GH family activity in starch and other storage carbohydrates substrate converters (Fig. 1h).
Previous reports have determined the importance of fungal cell wall composition a crucial virulence factor during infection, and assessing CAZyme components of fungal cell wall substrate converters has been extensively researched66. Here, we reveal the presence of pectin lyases, glycan lyases, chitin lyases and mannan lyases (Fig. 1i). Xylan and sugar utilisation appears to be surprisingly present in the accessory genome (Supplementary Data 4). Pectin substrate-conversion enzyme has been identified as the core feature of Candida species’ functional cell wall enzyme, though previously only reported in Candida bodinii67 and frequently seen in the fungal plant pathogen, including Aspergillus Pencillium68. Alongside ß-glucan, mannan and chitin carbohydrate enzyme profiles, Candida cell wall activity includes pectin enzyme activity (Supplementary Table 3).
In addition, we identified 1182 Pfam clans from all Candida strains and re-categorised them into 14 functional clans (Supplementary Data 5). Pfam domain annotation indicating genetic information processing, cell machinery, and metabolism was among the most extensive Pfam domains exhibited (Supplementary Fig. 3b). We assessed the diverse functional association of protein domains by analysing core functional clans, and determined similar patterns of dominance for carbohydrate, amino acid and lipid processing-associated domains (Supplementary Fig. 3c).
The functional and metabolomic activity of clinical AGAu Candida strains
Next, to better explore and understand the link to metabolism and pathogenesis, we clustered Candida species into groups based on the invasive nature of particular species, from literature search of species contribution to candidemia and mortality (Fig. 1a). C. albicans, C. glabrata and the emerging invasive species C. auris were grouped together (AGAu cluster). Alternative Candida species termed non-AGAu group include opportunistic species that require virulence factors or a defective immune system to cause disease pathology as well as environmental Candida species. The AGAu cluster contains those Candida species most commonly associated with clinical pathology, contributing to a higher percentage of mortality and candidemia16–18,53–55,69. This classification of AGAu is analysed and discussed throughout the rest of this paper.
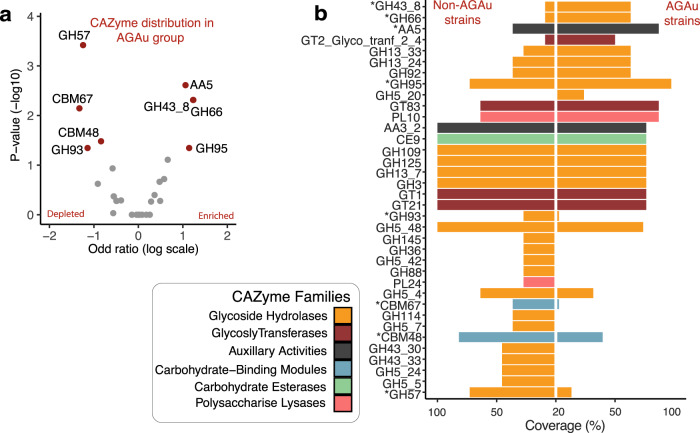
We compared the CAZyme profile coverage of AGAu and non-AGAu groups, with classes highlighted based on colour (Fig. 2a, b, Supplementary Table 4). The CAZyme GH43_8 (substrate conversion of α-L-arabinofuranosidase/β-xylosidase70) was significantly enriched in AGAu possibly involved in the breakdown of complex glucans (Wilcoxon rank-sum test, P < 0.05)71. The identification of significant CAZymes in the AGAu cluster showed carbohydrate conversion of xylan (GH43_8), mucin (GH95), cellulose (GH66) and copper oxidase family (AA5)72–74. The presence of xylan substrate converter is unanticipated as xylan is only commonly found in plant cell walls and more likely present in non-AGAu species. The presence of GH43_8 has not been associated with any other host, bacterial or fungal species. Interestingly, GH66 associated with human oral plaque formation72 and AA5 enzyme has been reportedly linked to fungal defence75. CAZymes seen in non-AGAu species showed carbohydrate-binding module families involved in sugar, polysaccharide and cell wall breakdown, including CBM48.
Fig. 2. CAZyme profile in AGAu Candida strains.
a CAZyme analysis across AGAu and non- AGAu groups. (*Asterisk connotes statistically significant CAZyme, Chi-square tests, p values < 0.05). b CAZyme enrichment and depletion in AGAu strains. Volcano plot showing statistically significant evidence of a relationship between AGAu strains (Chi-square tests, p values < 0.05 and odds ratio on enriched and depleted in AGAu strains, in red).
The AGAu species are morphologically diverse, (C. albicans is dimorphic—capable of growing a both filamentous hyphae and single-celled yeast, whilst C. glabrata and C. auris are yeasts), and they are all potential pathogens. Based on contrasting KO annotations, the genetic potential of pathways between AGAu and non-AGAu Candida strains showed little difference with hypergeometric testing. Nevertheless, there is functional evidence of significance in these pathways present in both clusters, indicating a genetic potential for all Candida strains to undertake these metabolic trajectories (Wilcoxon rank-sum test, P < 0.05; Fig. 3a). All Candida strains notably revealed encoded pathways facilitating carbohydrate catabolism within the system; thus, demonstrating the potential to drive increasing metabolic activity, for example through fructose and mannose metabolism. We did, however, identify significant enrichment of amino acid metabolism with metabolomics highlighting trajectories for arginine, proline, cysteine and methionine metabolism. We also observed significant levels of fatty acid biosynthesis and glutathione metabolism, which have previously been associated with virulence mechanisms76–79.
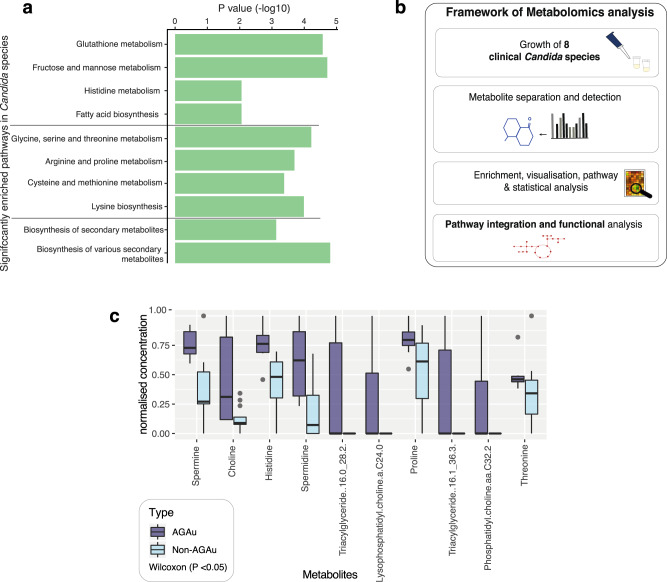
Fig. 3. Comparison of functional and metabolomics in AGAu and non-AGAu strains.
a Pathway enrichment in 49 Candida strains. Significant metabolic pathways highlighted in Candida species indicating genetic pathway potential (Hypergeometric tests on contrasted annotation between AGAu and non-AGAu species, p values < 0.05). b Framework outline of metabolomic analysis of Candida species’ metabolism levels of bioactive metabolites in Candida strains exhausted media. Computer simulations were performed for pathway analysis, and statistical approach was applied for candidate metabolites that have a potential influence on the host. c Enriched metabolites detected in targeted metabolomics between AGAu and non- AGAu groups (Wilcoxon rank-sum tests, P values < 0.05, middle line for median with interquartile range (IQR) and whiskers 1.5× IQR, “Method”).
Metabolomics revealed key metabolic pathways assimilated by AGAu group
To elucidate the metabolic trajectory taking place by each cluster group, we performed metabolomics on a collection of 7 clinical Candida isolates, representing the diverse pathogenic species (Fig. 3b, “Method”). These clinical samples were previously isolated from patients infections53,80–83 and were used to evaluate in vitro the critical metabolic activity predicted by our functional analyses (Supplementary Fig. 4a). These Candida isolates were representative of both AGAu and non-AGAu grouped species. The metabolomics data were used for partial least squares discriminant analysis (PLS-DA), which resulted in distinct cluster separation of significant analyte classes (Supplementary Fig. 4b–d, “Method”). The PLS-DA models identified distinct metabolites feature in AGAu Candida species (Supplementary Fig. 4e).
For example, histidine metabolite production in AGAu species supports this metabolite role in systemic infection and is potential an anti-fungal target84,85. We also identified choline-derived metabolites (choline, phosphatidylcholine and lysophosphatidylcholine) as increased in certain AGAu species (Fig. 3c; Wilcoxon P < 0.05). We identified phosphatidylcholines analyte class as contributing the highest number of features across selected clinical Candida species (Supplementary Fig. 4d), which has been observed previously in the hypervirulent C. albicans (SC5314) strain38–41. Spermine and spermidine were found to be significantly associated with AGAu strains, indicating polyamine metabolism could play a functional role in the increased association with disease pathology of these strains.
Integrative global metabolic map of AGAu Candida species
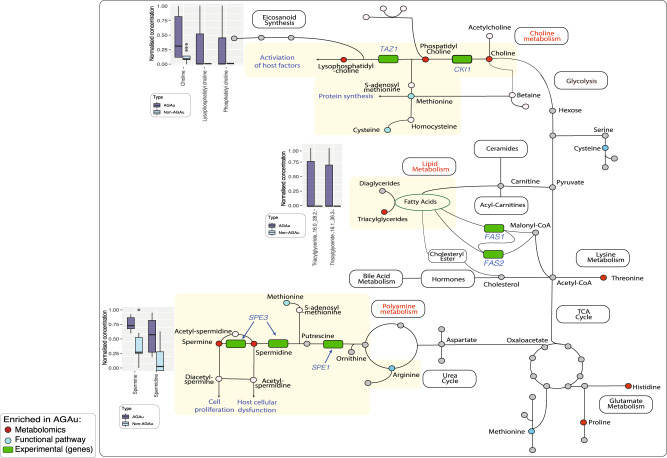
Having identified these pathways in silico, we next determined gene expression levels of essential polyamine (SPE11, SPE3), choline (CKI1, TAZ1) and fatty acid (FAS1, FAS2) pathways in C. albicans (representative of the AGAu cluster) to validate our findings (Supplementary Fig. 5a, Supplementary Table 5 and “Methods”). All 5 genes showed expression, demonstrating the activity of these pathways. Our conclusions of key pathway associations draws importance of nitrogen sources, specifically the metabolism of amino acids, in this process (Fig. 4), although to date, Candida species pathogenesis is better known to be driven by carbon sources22,37,86,87. Increased levels of metabolites were exhibited in AGAu species in the choline pathway, polyamine and fatty acid biosynthesis pathways that are primarily propagated through arginine, cysteine, and methionine pathways (Supplementary Fig. 5b–d). Based on integration of computational and experimental data revealed fundamental metabolic pathways applied AGAu species providing a link to the major advantage shown by AGAu species across the human body with considerable contributions to pathogenesis. These important pathways include polyamine, choline and fatty acid biosynthesis. For instance, the polyamine pathway is thought to be involved in Candida cell proliferation, and in turn, causes host cellular dysfunction by modulating acetylation levels of aminopropyl groups and inducing autophagy, thus increasing cell life span86,88,89. We observed fatty acid biosynthesis production with large numbers of metabolites of triglycerides featuring in PLS-DA and family have previously been reported to promote germination and virulence of AGAu Candida strains (Supplementary Fig. 4d)90,91. Further, fatty acid biosynthesis is vital in fungal cell membrane viability, energy storage, signalling, and cell proliferation—all functions critical in pathogenesis92–95.
Fig. 4. Global map metabolism in AGAu Candida strains.
Enriched pathways observed in functional annotation and further validated by metabolomics with key metabolites markedly significant. The boxplots indicate metabolomics of individual metabolites in corresponding pathway (Wilcoxon rank-sum test, P value < 0.05, median middle line IQR and whiskers 1.5× IQR). *Colour in pathway red identify significant metabolites, blue indicate significant pathway enrichment and green denotes validation with gene expression.
Discussion
In this study, we develop a tool for analysing fungal metabolism—the BioFung database. Here, BioFung was used for building metabolic maps of key Candida strains and the database provides the mycology community with a resource allowing them to dive more deeply into all fungal species’ metabolic capability based on protein-encoding genes. Development of data generation technologies development, tools and database for fungal species is currently in its infancy, despite significant advances in these areas for bacteria and archaea. This database enables detailed mechanistic annotations to optimise our understanding of fungal species. to do this, it uses HMM to provide high specificity for fungal annotation. As such, it is currently the best database available for KEGG-based functional annotation of fungi (Supplementary Fig. 1a–c and Supplementary Data 1–3). Currently, alternative KEGG annotation tools are available including Figfam96, Pathogen-Host Interaction97, GO terms98, Blast2Go99 and InterPro100. However, none of these provides fungal-specific pathway annotation. BioFung is reliant on the KEGG database and current fungal genome annotations, but in doing so is specifically oriented to fungi. Moreover, as updates to KEGG and advances in the functional annotation of fungal genomes become available, they will increase the power of this tool. The robustness and effectiveness of functional annotation of BioFung compared to similar tools have been explored in Supplementary Figure 1d–f, demonstrating the utility of this tool.
Analysis of annotations allowed us to identify the influential AGAu group of Candida strains, highlighting critical metabolic pathways in these strains. In doing so, we developed increased understanding of the metabolism of these strains through integrating multi-omics and experimental data. BioFung can be used extensively to better understand individual fungal species’ metabolic pathways but can be extended to explore metabolic interactions between fungi, other organisms, and within the host mycobiome.
Using BioFung in combination with metabolomics validation indicates that the AGAu species appear to be employing specific pathways in amino acid metabolism to potentially improve their competitive fitness during host pathogenesis. This shows a degree of metabolic plasticity indicative of fungi, where secretion of these metabolites associated with these pathways aiding in better adaptability to growth, virulence factor production, hyphae and biofilm formation39,101, enabling more effective adaptation to a wide variety of environments and habitats. Amino acid metabolism has been proposed as an alternative energy source in stress responses and an alternative to carbon sources for growth. We demonstrate here that all Candida species have an amino acid pathways to employ metabolic remodelling (Fig. 3a)39,42,86. However, integrating metabolomics from strains grown with abundant nutrient source, shows that the AGAu group have significantly more active production of polyamine and choline metabolites, which requires the use of amino acid metabolism for production (Fig. 4). The AGAu group employs arginine, methionine and cysteine metabolism and more extensive exploration and experimental data needed to understand the causal effect of amino acid metabolism. For instance, we have identified a confirmed target pathway for anti-fungal drugs with glutathione metabolism (GSH), attributed to fungal mitochondrial maintenance, preservation of membrane integrity, regulation of transcription factors in stress response and protection against reactive oxygen species. Reducing activity of GSH is under investigation as supplementary aid for anti-fungal drugs (azoles and echinocandin) against resistant strains102–105. This finding verifies that pathway enrichment analysis echoes feasibility in clinical relevance within the host.
Among these metabolites identified are the polyamines, including spermine and spermidine. Polyamines play critical roles in normal cell physiology. Spermine is essential for Candida hyphal formation, playing a pivotal role in Candida invasion106. Spermidine, meanwhile, drives genetic modification in fungi by regulating cell cycle and translating the modification of eukaryotic initiation factor (eIF)107–109. Excessive polyamines prolong yeast survival via delayed DNA degradation, increasing the likelihood of mutations that could contribute to the development of anti-fungal resistance110,111. These mutations are an important consideration, given that C. glabrata and C. auris are heavily associated with rising anti-fungal resistance112–114. Polyamines have also been shown to be anti-inflammatory, depending on the microenvironment, potentially explaining the additional benefits of secondary metabolites to Candida species by modulating host immune responses115. The use of polyamines is not limited to fungi. Bacteria use polyamines to create and maintain biofilms in order to withstand host defences as well as promoting cancers116–118. Viruses use polyamines to promote cell proliferation, thereby promoting their propagation and spread. Intervention in polyamine synthesis has a high degree of potential as a target for antimicrobials. DNA viruses upregulate polyamine synthesis in host during infection and blocking polyamine synthesis is a strategy used in broad-spectrum anti-viral119–121. These examples along with our findings here indicate that manipulating polyamine secretion from Candida species is a realistic target for therapeutic intervention of associated diseases.
Choline metabolism is a critical function for both microbial and host physiology, as demonstrated by the increase seen in AGAu Candida species’ related metabolites. Disruption of phospholipid biosynthesis in fungi can occur through inhibition of phosphatidylcholine synthesis, showing preventing virulence within the systemic mice model122–125. Further, acetylcholine is essential in the formation of the chitin wall characteristic of fungi126,127. Along with the bacteriome, Candida species contribute towards host acquisition of choline. As understanding of choline metabolism is in its infancy, further investigation of host–mycobiome interactions is needed, potentially providing insights for repurposing potential therapeutic intervention. For instance, lack of choline in humans drives liver dysfunction due to the accumulation of lipids within hepatocytes, which can lead to fatty liver diseases and even hepatic liver cancer128–132. We acknowledge the limitation of using in vitro metabolomics experiments as the carbon source of the microbial media used (SAB) is not representative of the profile of sources available during human host infection. However, use of these metabolomics datasets does give an indication of potential differences in the metabolic potential of different Candida species, indicating that this approach would have real value in exploring clinical metabolomics datasets from different fungal infections.
Functional analysis indicates that both the AGAu and non-AGAu groups show a high degree of metabolic plasticity (Fig. 3a)56,59,60,133. CAZymes also demonstrated functional differences seen between AGAu and non-AGAu groups. The unexpected findings of xylan in AGAu cluster, that is normally present in plant cell walls. We contemplate that AGAu clusters are pathobionts in humans, they are also common environmental fungi, and thus the enrichment of functions related to breakdown of wood biomass potentially reflect the range of environments and nutrient sources that can be utilised by the AGAu cluster. It is possible that this is a further reflection of their virulence, demonstrating that one of their main virulence attributes is an ability to thrive in a variety of environments.
The AGAu group show GH66, which has previously only been associated with the human oral microbiome and as a potential marker for plaque formation72. Given that C. albicans is a constituent of oral plaque, this is consistent with clinical data (Fig. 2a, b)134. The function of GH43_8 found in the AGAu group is inconclusive but was recently detected in bacteria as β-galactofuranosidase70. Although the modes of action for both GH43_8 and AA5 are currently unknown their enrichment in the AGAu group may provide a function-targeted biomarker for Candida infections135,136. We also highlight fatty acid biosynthesis pathway in AGAu species with significant levels of triglycerides production detected (Fig. 3c). Fatty acid synthesis has been identified in Candida species previously, with focus on OLE1, FAS1 & FAS2 genes as key indicators to pathogenesis and virulence76–79. This validates the notion of targeting fatty acid biosynthesis pathway within Candida species to disrupt Candida overgrowth in the host.
Our study has addressed the need for functional data and tools for fungal species by developing the BioFung resource using the KEGG database. This enables detailed mechanistic pathway analysis of fungi. Our integrative analysis of the AGAu group (associated with the disease pathogenesis) highlighted key pathways that potentially increase virulence and have associated effects in the host. We hypothesise that these markers can aid in identifying routes for intervention in invasive infection and suggest polyamine, choline and fatty acid biosynthesis metabolism as potential targets for further investigation. The presence of these metabolites from AGAu Candida species potentially directly affects host homoeostasis with the mycobiome and adversely affects the host during infection. As such, the AGAu Candida species’ metabolic reprogramming may present a method of controlling interaction and infection with these fungi. Finally, we focus on fungal metabolism exploration and distinctively towards amino acid metabolism, playing a more significant role in virulence and pathogenicity.
Methods
BioFung database construction
Kyoto Encyclopedia of Genes and Genomes (KEGG) database were downloaded for the investigation of all 128 fungal species (3GB file size) from eukaryote database (5GB file size) (downloaded on August 2019)137. Around 1,210,746 genes, which are annotated with 4717 KEGG orthology (KO), were selected among 128 fungal species genes. There were 6071 fungal genes missing sequence to place into KO, and 105 KO failed in multi-sequence alignment due to default settings (minimum of 3 genes sequence required). Those genes per each KO were performed multiple-sequence alignment by ClustalW and generated Hidden Markov Model (HMM) profiles using the hmm-build function of HMMER software (Fig. 1A for workflow and supplementary 2a for coverage)138,139. BioFung database is a fungal-specific HMM model made up of 4,722 KOs was freely shared via Github repository (https://github.com/sysbiomelab/BioFung). Missing KO from fungal species was not added due to missing gene sequences from KEGG, or the low number of sequences per KO (<3), thereby failed to perform ClustalW alignment (See details in Supplementary Data 3).
BioFung quality assessment
Quality check was performed by comparing BioFung HMMs to pre-trained HMMS for eukaryotes (euk90 and euk100 version 91.0) from Raven Toolbox57,58, and we observed that BioFung coverage was much higher than both eukaryote profiles (Supplementary Fig. 1c). Application of BioFung to Aspergillus species present in KEGG database were compared to assess robustness (Supplementary Fig. 2f).
Application of BioFung and other functional annotations
Fungal KO annotation of each species was performed by HMM scanning of BioFung HMM models by HMMER software. An in-depth exploratory analysis was performed by manually checking KO annotations of individual species. Pathway abundance for AGAu and non-AGAu species was performed using KEGG pathway annotations. Hypergeometric testing uses hypergeometric distribution for pathways and computing p value with Wilcoxon rank-sum test (<0.05). CAZyme annotations were performed by mapping Candida protein sequences using HMMs of dbCAN2 database64. Substrate conversion of CAZyme families was checked based on literature review68,140–146. Candida protein sequences to map against Pfam-A families using HMMs, that are fully annotated and curated above a threshold63. Pfam clans’ annotations were sub-set into a broader annotation based on a reported standard function of protein domains (please see Supplementary Data 5).
Genome sequence collection
Genome sequences of 49 Candida species were collected from NCBI database with version release 45 of Ensemble Fungi (date accessed: April 2019)147,148. Applied assembly strategy and sequencing platform is associated to predicting gene function, thus supplementary information of sample strain, genome ID, ENA ID, Biosample ID, sequence platform, year of collection, sample location of collection, sample tuple and available biological annotation has been provided in Supplementary Table 1. The quality of the sequences was checked to look at GC content, scaffold and genome size (see Supplementary Fig. 1b). Nucleotide sequences were used with Average Nucleotide Identity (ANI) to determine the phylogenetic relationship and determine differences between strains using Pyani package149.
Contrasted functional annotation of Candida species grouping
Presence and absence of microbial annotations, i.e., prevalence, was tested for significance based on condition using Chi-squared tests and odd ratio. Percentage coverage of each was also tested between AGAu and non-AGAu Candida species (Supplementary Data 6). Contrasted functional annotations were checked on individual strains and placed into presence/absence to perform chi-squared for significance (<0.05), and the odds ratio was performed to identify enriched and depleted in AGAu samples. Additional significant functional annotations are seen in AGAu cluster (Supplementary Table 4).
Clustering of protein sequences
Core, shared, and unique proteins were identified based on sequence similarity by a clustering approach called UCLUST algorithm65. In short, UCLUST algorithm was applied to identify similarity in protein-encoding gene sequences by clustering and unique protein sequences were identified if included in singleton protein clusters. Core proteins were identified if corresponding proteins from all 49 species were included in the same cluster. Shared proteins were selected if they did not belong to unique and core proteins. Protein sequence clusters were selected based on a threshold 0.5 for representative seed sequence, a default threshold in UCLUST software65 (Supplementary Fig. 2g). Based on definitions of the core, shared, and unique proteins, we were able to determine the core, shared and unique annotations for KO and CAZymes, accordingly.
Strain growth
8 strains of Candida species (C. albicans (SC5314), C. dubliniensis (CD36), C. tropicalis (CBS94), C. glabrata (CBS 138), C. auris (47477), C. parapsilosis (73/037), and C. krusei (CBS573)). Strains were grown in 5ml liquid sabouraud dextrose broth (Thermo Scientific-Oxoid microbiology, UK)150. All strains were grown in a shaking incubator 95rpm at the temperature of 25 °C. Timepoint measurement of growth was taken to measure the exponential and stationary phase of the optical density of 1 at 600 nm absorbance (iEMS Ascent absorbance 96-well plate reader).
Collection and targeted metabolomics on fungal extracellular matrix
Mid-exponential phase indicates bioactive metabolites and time points for the extraction of extracellular metabolites (see Supplementary Figure 4a). Five hundred microlitres of extracellular medium, proximity to the pellet was removed from growing fungal cells. Samples were placed through a 20 µm Whatman filter and snap-frozen in liquid nitrogen. Targeted metabolomics performed using the MxP Quant500 kit (Biocrates, Austria). Partial Least Square—Discriminant Analysis (PLS-DA) was performed on targeted metabolomics of fungal extracellular matrices and media as control, using ropls package151. First, PLS-DA was performed to distinguish between Candida samples and control (media). Further, PLS-DA was performed to distinguish between AGAu species and non-AGAu Candida samples. PLS-DA indicated a significant difference between AGAu clusters (Supplementary Data 7). Further analysis of metabolite concentrations of targeted metabolomics was normalised, and the Wilcoxon rank-sum test was performed to identify critical metabolites (<0.05). Boxplot mid-line notes median with interquartile range (IQR) with whiskers 1.5x IQR.
Validation experiment
RNA was extracted from 3 biological repeats C. albicans (SC5139) using RNA Qiagen Powersoil kit adapted with bead beating with interval placement on dry ice and additional 100 µl of isopropanol. DNAse clean-up performed using RNA clean-up and concentration kit (NORGEN, Biotek corporation). Primers designed for specific amplification of genes SPE1 targeting Ornithine Carboxylase, SPE3 gene for spermidine synthase, CKI1 specific for bifunctional choline kinase/ethanolamine kinase, TAZ1 gene focused on lysophosphatidylcholine acyltransferase and FAS1/FAS2 gene target for fatty acid synthase (Supplementary Table 5 for primer information). These primers are specific for C. albicans. Other Candida species only predicted gene ontology-based on C. albicans and Saccharomyces annotation (http://www.candidagenome.org/cgi-bin/GO/goAnnotation.pl?dbid=CAL0000224407&seq_source=C.%20auris%20B8441). Conventional RT-qPCR was performed to identify the expression of these critical pathways for samples, two standard curve analysis with RDN25 which encodes the 25s rRNA subunit and error bars are representative of mean ± SD.
Statistics and reproducibility
All statistical analyses were performed using R software v3.6.3. In the analysis for functional pathway annotation with BioFung hypergeometric testing uses hypergeometric distribution and p value computed with Wilcoxon rank-sum test (<0.05) using built into R version 3.6.2 package. We further conducted comparison of species with CAZyme annotation by applying chi-squared (<0.05) to determine significant CAZyme and odd ratio analysis indicated presence and absence of these CAZyme in AGAu species. To test significant metabolites between AGAu and non-AGAu species, Wilcoxon rank-sum test (≤0.05) was performed on normalised concentration of significant critical metabolites.
Strains of Candida species analysis was based on availability of protein sequence at the time of data collection at NCBI database. Metabolomics was performed for triplicate biological experiments and placed through MaxQuant500 kit that was analysed with PLS-DA. qPCR validation with C. albicans was performed for each primer in triplicates
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This study was supported by Engineering and Physical Sciences Research Council (EPSRC), EP/S001301/1, Biotechnology Biological Sciences Research Council (BBSRC) BB/S016899/1, Science for Life Laboratory, Swedish National Infrastructure for Computing at SNIC through Uppsala Multidisciplinary Centre for Advanced Computational Science (UPPMAX) under projects SNIC 2020-5-222, SNIC 2019/3-226, SNIC 2020/6-153, SNIC 2021/5-248, SNIC 2022/5-334 and King’s College London computational infrastructure facility, Rosalind (https://rosalind.kcl.ac.uk) for high-performance computing. We thank Professor Bernhard Hube for kindly sending C. parapsilosis strain.
Author contributions
N.B, D.M. and S.S. conceived the project. N.B. performed all sample preparation, metagenomics, metabolomics, gene expression data preparation and extraction protocols for the paper. N.B. developed the pipeline, analysis, and made all the figures. N.B. wrote and drafted the figures and manuscript. S.L. advised on design, statistical and functional analysis of the data. T.J.P advised on streamlining BioFung pipeline. A.P. and S.S.N. processed prepared samples on qPCR platform. J.N. and M.U. provided critical feedback on the data and manuscript. All authors read, edited, and reviewed the manuscript.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Karli Montague-Cardoso. Peer reviewer reports are available.
Data availability
BioFung is public open access database that can be downloaded from GitHub repository: https://github.com/sysbiomelab/BioFung.
Code availability
The instruction and the pipeline scripts for BioFung can be found at our GitHub repository https://github.com/sysbiomelab/BioFung. BioFung.hmm file was uploaded via GitHub large file storage (lfs) on the GitHub page. Additionally, BioFung is on an automated pipeline with Nextflow v21.04.1 and either singularity v3.8.3 or docker 20.10.7. All software executed by the pipeline is containerised meaning that no additional installation is required for both local or high-performance computing. ReadME file contains code for usage and example fasta.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
David L. Moyes, Email: david.moyes@kcl.ac.uk
Saeed Shoaie, Email: saeed.shoaie@kcl.ac.uk.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-022-03955-z.
References
- 1.Kam AP, Xu J. Diversity of commensal yeasts within and among healthy hosts. Diagn. Microbiol. Infect. Dis. 2002;43:19–28. doi: 10.1016/S0732-8893(02)00364-4. [DOI] [PubMed] [Google Scholar]
- 2.Bougnoux M-E, et al. Multilocus sequence typing reveals intrafamilial transmission and microevolutions of Candida albicans isolates from the human digestive tract. J. Clin. Microbiol. 2006;44:1810–1820. doi: 10.1128/JCM.44.5.1810-1820.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angebault C, et al. Candida albicans is not always the preferential yeast colonizing humans: a study in Wayampi Amerindians. J. Infect. Dis. 2013;208:1705–1716. doi: 10.1093/infdis/jit389. [DOI] [PubMed] [Google Scholar]
- 4.Moyes DL, Richardson JP, Naglik JR. Candida albicans-epithelial interactions and pathogenicity mechanisms: scratching the surface. Virulence. 2015;6:338–346. doi: 10.1080/21505594.2015.1012981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meeting on global surveillance of antimicrobial resistance invasive Candida infections. https://www.who.int/news-room/events/detail/2018/04/24/default-calendar/meeting-on-global-surveillance-of-antimicrobial-resistance-invasive-candida-infections.
- 6.Invasive fungal infections: a creeping public health threat. ASM.orghttps://www.asm.org/Articles/2018/September/Invasive-Fungal-Infections-A-Creeping-Public-Healt.
- 7.Fungal Disease Frequency | Gaffi - global action fund for fungal infections. https://www.gaffi.org/why/fungal-disease-frequency/.
- 8.Nett JE. Candida auris: an emerging pathogen “incognito”? PLoS Pathog. 2019;15:e1007638. doi: 10.1371/journal.ppat.1007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lockhart SR, et al. Thinking beyond the common Candida species: need for species-level identification of Candida due to the emergence of multidrug-resistant Candida auris. J. Clin. Microbiol. 2017;55:3324–3327. doi: 10.1128/JCM.01355-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin. Microbiol. Infect. 2014;20:5–10. doi: 10.1111/1469-0691.12539. [DOI] [PubMed] [Google Scholar]
- 11.Yapar N. Epidemiology and risk factors for invasive candidiasis. Ther. Clin. Risk Manag. 2014;10:95–105. doi: 10.2147/TCRM.S40160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spampinato C, Leonardi D. Candida infections, causes, targets, and resistance mechanisms: traditional and alternative antifungal agents. BioMed. Res. Int. 2013;2013:e204237. doi: 10.1155/2013/204237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkinson, H. F. & Douglas, L. J. Interactions Between Candida Species And Bacteria In Mixed Infections. In: Polymicrobial Diseases (ASM Press, 2002).
- 14.Pannanusorn S, Fernandez V, Römling U. Prevalence of biofilm formation in clinical isolates of Candida species causing bloodstream infection. Mycoses. 2013;56:264–272. doi: 10.1111/myc.12014. [DOI] [PubMed] [Google Scholar]
- 15.Colombo AL, et al. Global distribution and outcomes for Candida species causing invasive candidiasis: results from an international randomized double-blind study of caspofungin versus amphotericin B for the treatment of invasive candidiasis. Eur. J. Clin. Microbiol. Infect. Dis. 2003;22:470–474. doi: 10.1007/s10096-003-0973-8. [DOI] [PubMed] [Google Scholar]
- 16.Pfaller MA, et al. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of candida in 2,496 Patients: Data from the Prospective Antifungal Therapy (PATH) registry 2004–2008. PLoS ONE. 2014;9:e101510. doi: 10.1371/journal.pone.0101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindberg E, Hammarström H, Ataollahy N, Kondori N. Species distribution and antifungal drug susceptibilities of yeasts isolated from the blood samples of patients with candidemia. Sci. Rep. 2019;9:3838. doi: 10.1038/s41598-019-40280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adam R, et al. 378. Candida auris fungemia: risk factors and outcome. Open Forum Infect. Dis. 2018;5:S147–S147. doi: 10.1093/ofid/ofy210.389. [DOI] [Google Scholar]
- 19.de Almeida JN, et al. Candida haemulonii complex species, Brazil, January 2010-March 2015. Emerg. Infect. Dis. 2016;22:561–563. doi: 10.3201/eid2203.151610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez-Lopez A, et al. Prevalence and susceptibility profile of Candida metapsilosis and Candida orthopsilosis: results from population-based surveillance of candidemia in Spain. Antimicrob. Agents Chemother. 2008;52:1506–1509. doi: 10.1128/AAC.01595-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aykut B, et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature. 2019;574:1–4. doi: 10.1038/s41586-019-1608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown AJP, Brown GD, Netea MG, Gow NAR. Metabolism impacts upon candida immunogenicity and pathogenicity at multiple levels. Trends Microbiol. 2014;22:614–622. doi: 10.1016/j.tim.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miramón P, Lorenz MC. A feast for Candida: metabolic plasticity confers an edge for virulence. PLoS Pathog. 2017;13:e1006144. doi: 10.1371/journal.ppat.1006144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandai D, et al. The evolutionary rewiring of ubiquitination targets has reprogrammed the regulation of carbon assimilation in the pathogenic yeast Candida albicans. mBio. 2012;3:e00495–12. doi: 10.1128/mBio.00495-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chew SY, et al. Physiologically relevant alternative carbon sources modulate biofilm formation, cell wall architecture, and the stress and antifungal resistance of Candida glabrata. Int. J. Mol. Sci. 2019;20:3172. doi: 10.3390/ijms20133172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandai, D., Tabana, Y. & Sandai, R. Carbon sources attribute to pathogenicity in Candida albicans. In Candida Albicans (InTechOpen, 2018). 10.5772/intechopen.73454.
- 27.Tucey TM, et al. Glucose homeostasis is important for immune cell viability during Candida challenge and host survival of systemic fungal infection. Cell Metab. 2018;27:988–1006.e7. doi: 10.1016/j.cmet.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moyes DL, et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532:64–68. doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naglik JR, Gaffen SL, Hube B. Candidalysin: discovery and function in Candida albicans infections. Curr. Opin. Microbiol. 2019;52:100–109. doi: 10.1016/j.mib.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodaki A, et al. Glucose promotes stress resistance in the fungal pathogen Candida albicans. Mol. Biol. Cell. 2009;20:4845–4855. doi: 10.1091/mbc.e09-01-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deacon, J. W. In Fungal Biology (John Wiley & Sons, 2013).
- 32.Yin Z, et al. Glucose triggers different global responses in yeast, depending on the strength of the signal, and transiently stabilizes ribosomal protein mRNAs. Mol. Microbiol. 2003;48:713–724. doi: 10.1046/j.1365-2958.2003.03478.x. [DOI] [PubMed] [Google Scholar]
- 33.Childers DS, et al. The rewiring of ubiquitination targets in a pathogenic yeast promotes metabolic flexibility, host colonization and virulence. PLoS Pathog. 2016;12:e1005566. doi: 10.1371/journal.ppat.1005566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorenz MC, Bender JA, Fink GR. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot. Cell. 2004;3:1076–1087. doi: 10.1128/EC.3.5.1076-1087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ene IV, Brown AJP. Integration of metabolism with virulence in Candida albicans. Fungal Genom. 2014;13:349–370. doi: 10.1007/978-3-642-45218-5_14. [DOI] [Google Scholar]
- 36.Ene IV, et al. Carbon source-induced reprogramming of the cell wall proteome and secretome modulates the adherence and drug resistance of the fungal pathogen Candida albicans. Proteomics. 2012;12:3164–3179. doi: 10.1002/pmic.201200228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ene IV, et al. Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell. Microbiol. 2012;14:1319–1335. doi: 10.1111/j.1462-5822.2012.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tudzynski B, et al. Nitrogen regulation of fungal secondary metabolism in fungi. Front. Microbiol. 2014;5:656. doi: 10.3389/fmicb.2014.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garbe E, Vylkova S. Role of amino acid metabolism in the virulence of human pathogenic fungi. Curr. Clin. Microbiol. Rep. 2019;6:108–119. doi: 10.1007/s40588-019-00124-5. [DOI] [Google Scholar]
- 40.Ramachandra S, et al. Regulatory networks controlling nitrogen sensing and uptake in Candida albicans. PLoS ONE. 2014;9:e92734. doi: 10.1371/journal.pone.0092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maidan MM, Thevelein JM, Van Dijck P. Carbon source induced yeast-to-hypha transition in Candida albicans is dependent on the presence of amino acids and on the G-protein-coupled receptor Gpr1. Biochem. Soc. Trans. 2005;33:291–293. doi: 10.1042/BST0330291. [DOI] [PubMed] [Google Scholar]
- 42.Vylkova S, et al. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. mBio. 2011;2:e00055–11. doi: 10.1128/mBio.00055-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hudson DA, et al. Identification of the dialysable serum inducer of germ-tube formation in Candida albicans. Microbiology. 2004;150:3041–3049. doi: 10.1099/mic.0.27121-0. [DOI] [PubMed] [Google Scholar]
- 44.Kaur R, Ma B, Cormack BP. A family of glycosylphosphatidylinositol-linked aspartyl proteases is required for virulence of Candida glabrata. Proc. Natl Acad. Sci. USA. 2007;104:7628–7633. doi: 10.1073/pnas.0611195104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng S, et al. Profiling of Candida albicans gene expression during intra-abdominal candidiasis identifies biologic processes involved in pathogenesis. J. Infect. Dis. 2013;208:1529–1537. doi: 10.1093/infdis/jit335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schrevens S, et al. Methionine is required for cAMP-PKA-mediated morphogenesis and virulence of Candida albicans. Mol. Microbiol. 2018;108:258–275. doi: 10.1111/mmi.13933. [DOI] [PubMed] [Google Scholar]
- 47.Silao FGS, et al. Mitochondrial proline catabolism activates Ras1/cAMP/PKA-induced filamentation in Candida albicans. PLoS Genet. 2019;15:e1007976. doi: 10.1371/journal.pgen.1007976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han T-L, Cannon RD, Gallo SM, Villas-Bôas SG. A metabolomic study of the effect of Candida albicans glutamate dehydrogenase deletion on growth and morphogenesis. Npj Biofilms Microbiomes. 2019;5:1–14. doi: 10.1038/s41522-019-0086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 2003;67:400–428. doi: 10.1128/MMBR.67.3.400-428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richardson JP, Moyes DL. Adaptive immune responses to Candida albicans infection. Virulence. 2015;6:327–337. doi: 10.1080/21505594.2015.1004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mayer FL, et al. The novel Candida albicans transporter Dur31 Is a multi-stage pathogenicity factor. PLoS Pathog. 2012;8:e1002592. doi: 10.1371/journal.ppat.1002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Global and temporal state of the human gut microbiome in health and disease. https://www.researchsquare.com (2021).
- 53.Moran C, Grussemeyer CA, Spalding JR, Benjamin DKJ, Reed SD. Candida albicans AND non-albicans bloodstream infections in adult and pediatric patients: comparison of mortality and costs. Pediatr. Infect. Dis. J. 2009;28:433–435. doi: 10.1097/INF.0b013e3181920ffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hirano R, Sakamoto Y, Kudo K, Ohnishi M. Retrospective analysis of mortality and Candida isolates of 75 patients with candidemia: a single hospital experience. Infect. Drug Resistance. 2015;8:199–205. doi: 10.2147/IDR.S80677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horn DL, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin. Infect. Dis. 2009;48:1695–1703. doi: 10.1086/599039. [DOI] [PubMed] [Google Scholar]
- 56.Nash AK, et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome. 2017;5:153–153. doi: 10.1186/s40168-017-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sam Q, Chang M, Chai L. The fungal mycobiome and its interaction with gut bacteria in the host. Int. J. Mol. Sci. 2017;18:330–330. doi: 10.3390/ijms18020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seed PC. The human mycobiome. Cold Spring Harb. Perspect. Med. 2015;5:a019810–a019810. doi: 10.1101/cshperspect.a019810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galloway-Peña JR, Kontoyiannis DP. The gut mycobiome: the overlooked constituent of clinical outcomes and treatment complications in patients with cancer and other immunosuppressive conditions. PLoS Pathog. 2020;16:e1008353. doi: 10.1371/journal.ppat.1008353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huseyin CE, O’Toole PW, Cotter PD, Scanlan PD. Forgotten fungi-the gut mycobiome in human health and disease. FEMS Microbiol. Rev. 2017;41:479–511. doi: 10.1093/femsre/fuw047. [DOI] [PubMed] [Google Scholar]
- 61.Agren R, et al. The RAVEN toolbox and its use for generating a genome-scale metabolic model for Penicillium chrysogenum. PLoS Comput. Biol. 2013;9:e1002980. doi: 10.1371/journal.pcbi.1002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.SysBioChalmers/RAVEN. GitHubhttps://github.com/SysBioChalmers/RAVEN.
- 63.El-Gebali S, et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019;47:D427–D432. doi: 10.1093/nar/gky995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang H, et al. dbCAN2: a meta server for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2018;46:W95–W101. doi: 10.1093/nar/gky418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 66.Zhao Z, Liu H, Wang C, Xu J-R. Erratum to: Comparative analysis of fungal genomes reveals different plant cell wall degrading capacity in fungi. BMC Genomics. 2014;15:6. doi: 10.1186/1471-2164-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakagawa T, et al. A methylotrophic pathway participates in pectin utilization by Candida boidinii. Appl. Environ. Microbiol. 2000;66:4253–4257. doi: 10.1128/AEM.66.10.4253-4257.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barrett K, Jensen K, Meyer AS, Frisvad JC, Lange L. Fungal secretome profile categorization of CAZymes by function and family corresponds to fungal phylogeny and taxonomy: example Aspergillus and Penicillium. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-61907-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sfeir, et al. Breakthrough bloodstream infections caused by echinocandin-resistant Candida tropicalis: an emerging threat to immunocompromised patients with hematological malignancies. J. Fungi. 2020;6:20–20. doi: 10.3390/jof6010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mewis K, Lenfant N, Lombard V, Henrissat B. Dividing the large glycoside hydrolase family 43 into subfamilies: a motivation for detailed enzyme characterization. Appl. Environ. Microbiol. 2016;82:1686–1692. doi: 10.1128/AEM.03453-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mewis, K. Functional Metagenomic Screening for Glycoside Hydrolases (University of British Columbia, 2016).
- 72.Cantarel, B. L., Lombard, V. & Henrissat, B. Complex carbohydrate utilization by the healthy human microbiome. PLoS ONE7, e28742 (2012). [DOI] [PMC free article] [PubMed]
- 73.Katayama T, et al. Molecular cloning and characterization of Bifidobacterium bifidum 1,2-α-l-fucosidase (AfcA), a novel inverting glycosidase (Glycoside Hydrolase Family 95) J. Bacteriol. 2004;186:4885–4893. doi: 10.1128/JB.186.15.4885-4893.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manzo N, et al. Carbohydrate-active enzymes from pigmented Bacilli: a genomic approach to assess carbohydrate utilization and degradation. BMC Microbiol. 2011;11:198. doi: 10.1186/1471-2180-11-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramzi AB, Me MLC, Ruslan US, Baharum SN, Muhammad NAN. Insight into plant cell wall degradation and pathogenesis of Ganoderma boninense via comparative genome analysis. PeerJ. 2019;7:e8065. doi: 10.7717/peerj.8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nguyen LN, Gacser A, Nosanchuk JD. The stearoyl-coenzyme A desaturase 1 is essential for virulence and membrane stress in Candida parapsilosis through unsaturated fatty acid production. Infect. Immun. 2011;79:136–145. doi: 10.1128/IAI.00753-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rodrigues, M. M. In Fatty Acid Synthase 1 in Candida albicans Virulence and the In Vitro Effects of Fluconazole, Tetracycline in Combinatorial Therapy. Masters theses (University of Tennessee, KnoxvilleUnivers, 2012). https://trace.tennessee.edu/cgi/viewcontent.cgi?referer=https://www.google.com/&httpsredir=1&article=2437&context=utk_gradthes
- 78.Southard SB, Cihlar RL. Analysis and expression of the Candida albicans FAS2 gene. Gene. 1995;156:133–138. doi: 10.1016/0378-1119(95)00033-3. [DOI] [PubMed] [Google Scholar]
- 79.Zhao X, Cihlar RL. Isolation and sequence of the Candida albicans FAS1 gene. Gene. 1994;147:119–124. doi: 10.1016/0378-1119(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 80.Odds FC, Brown AJ, Gow NA. Candida albicans genome sequence: a platform for genomics in the absence of genetics. Genome Biol. 2004;5:230. doi: 10.1186/gb-2004-5-7-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bader O, et al. Gross karyotypic and phenotypic alterations among different progenies of the Candida glabrata CBS138/ATCC2001 reference strain. PLoS ONE. 2012;7:e52218. doi: 10.1371/journal.pone.0052218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Douglass AP, et al. Population genomics shows no distinction between pathogenic Candida krusei and environmental Pichia kudriavzevii: one species, four names. PLoS Pathog. 2018;14:e1007138. doi: 10.1371/journal.ppat.1007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barchiesi F, et al. Experimental induction of fluconazole resistance in Candida tropicalis ATCC 750. Antimicrob. Agents Chemother. 2000;44:1578–1584. doi: 10.1128/AAC.44.6.1578-1584.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alcazar-Fuoli L. Amino acid biosynthetic pathways as antifungal targets for fungal infections. Virulence. 2016;7:376–378. doi: 10.1080/21505594.2016.1169360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jastrzębowska K, Gabriel I. Inhibitors of amino acids biosynthesis as antifungal agents. Amino Acids. 2015;47:227–249. doi: 10.1007/s00726-014-1873-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013;4:119–128. doi: 10.4161/viru.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lorenz MC, Fink GR. The glyoxylate cycle is required for fungal virulence. Nature. 2001;412:83–86. doi: 10.1038/35083594. [DOI] [PubMed] [Google Scholar]
- 88.Minois N, Carmona-Gutierrez D, Madeo F. Polyamines in aging and disease. Aging. 2011;3:716–732. doi: 10.18632/aging.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Valdés-Santiago L, Ruiz-Herrera J. Stress and polyamine metabolism in fungi. Front. Chem. 2014;1:42. doi: 10.3389/fchem.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noverr MC, Huffnagle GB. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect. Immun. 2004;72:6206–6210. doi: 10.1128/IAI.72.11.6206-6210.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nguyen LN, Trofa D, Nosanchuk JD. Fatty acid synthase impacts the pathobiology of Candida parapsilosis in vitro and during mammalian infection. PLoS ONE. 2009;4:e8421. doi: 10.1371/journal.pone.0008421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pan, J., Hu, C. & Yu, J.-H. Lipid biosynthesis as an antifungal target. J. Fungi4, 50 (2018). [DOI] [PMC free article] [PubMed]
- 93.Gajewski J, Pavlovic R, Fischer M, Boles E, Grininger M. Engineering fungal de novo fatty acid synthesis for short chain fatty acid production. Nat. Commun. 2017;8:14650. doi: 10.1038/ncomms14650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tehlivets O, Scheuringer K, Kohlwein SD. Fatty acid synthesis and elongation in yeast. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids. 2007;1771:255–270. doi: 10.1016/j.bbalip.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 95.Rattray JB, Schibeci A, Kidby DK. Lipids of yeasts. Bacteriol. Rev. 1975;39:197–231. doi: 10.1128/br.39.3.197-231.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meyer F, Overbeek R, Rodriguez A. FIGfams: yet another set of protein families. Nucleic Acids Res. 2009;37:6643–6654. doi: 10.1093/nar/gkp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Urban M, et al. PHI-base: the pathogen–host interactions database. Nucleic Acids Res. 2019;48:gkz904. doi: 10.1093/nar/gkz904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harris MA, et al. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 2004;32:D258–D261. doi: 10.1093/nar/gkh036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Conesa A, et al. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 100.Blum M, et al. The InterPro protein families and domains database: 20 years on. Nucleic Acids Res. 2021;49:D344–D354. doi: 10.1093/nar/gkaa977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Desai JV, et al. Candida albicans hyphae: from growth initiation to invasion. J. Fungi. 2018;4:10. doi: 10.3390/jof4010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miramón P, et al. A family of glutathione peroxidases contributes to oxidative stress resistance in Candida albicans. Med. Mycol. 2014;52:223–239. doi: 10.1093/mmy/myt021. [DOI] [PubMed] [Google Scholar]
- 103.Liu Y, et al. Glutathione reductase promotes fungal clearance and suppresses inflammation during systemic Candida albicans Infection. J. Immunol. 2018;200:50.1–50.1. doi: 10.4049/jimmunol.1701686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.I P, Ra P, Mj P. Glutathione, altruistic metabolite in fungi. Adv. Microb. Physiol. 2004;49:1–76. doi: 10.1016/S0065-2911(04)49001-8. [DOI] [PubMed] [Google Scholar]
- 105.Maras B, et al. Glutathione metabolism in Candida albicans resistant strains to fluconazole and micafungin. PLoS ONE. 2014;9:e98387. doi: 10.1371/journal.pone.0098387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dorighetto Cogo AJ, et al. Spermine modulates fungal morphogenesis and activates plasma membrane H + -ATPase during yeast to hyphae transition. Biol. Open. 2018;7:bio029660. doi: 10.1242/bio.029660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hoyt, M. A. & Davis, R. H. Polyamines in Fungi. In Biochemistry and Molecular Biology (eds. Brambl, R. & Marzluf, G. A.) 335–347 (Springer, 2004).
- 108.Tome ME, Gerner EW. Cellular eukaryotic initiation factor 5A content as a mediator of polyamine effects on growth and apoptosis. Biol. Signals. 1997;6:150–156. doi: 10.1159/000109121. [DOI] [PubMed] [Google Scholar]
- 109.Belda-Palazón, B. et al. Biochemical quantitation of the eIF5A hypusination in Arabidopsis thaliana uncovers ABA-dependent regulation. Front. Plant Sci. 5, 202 (2014). [DOI] [PMC free article] [PubMed]
- 110.Yu Y, et al. Position of eukaryotic translation initiation factor eIF1A on the 40S ribosomal subunit mapped by directed hydroxyl radical probing. Nucleic Acids Res. 2009;37:5167–5182. doi: 10.1093/nar/gkp519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chattopadhyay MK, et al. Microarray studies on the genes responsive to the addition of spermidine or spermine to a Saccharomyces cerevisiae spermidine synthase mutant. Yeast. 2009;26:531–544. doi: 10.1002/yea.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bhattacharya S, Sae-Tia S, Fries BC. Candidiasis and mechanisms of antifungal resistance. Antibiotics. 2020;9:312. doi: 10.3390/antibiotics9060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hendrickson JA, Hu C, Aitken SL, Beyda N. Antifungal resistance: a concerning trend for the present and future. Curr. Infect. Dis. Rep. 2019;21:47. doi: 10.1007/s11908-019-0702-9. [DOI] [PubMed] [Google Scholar]
- 114.Silva S, et al. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012;36:288–305. doi: 10.1111/j.1574-6976.2011.00278.x. [DOI] [PubMed] [Google Scholar]
- 115.Choi YH, Park HY. Anti-inflammatory effects of spermidine in lipopolysaccharide-stimulated BV2 microglial cells. J. Biomed. Sci. 2012;19:31. doi: 10.1186/1423-0127-19-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li B, et al. Polyamine-independent growth and biofilm formation, and functional spermidine/spermine N-acetyltransferases in Staphylococcus aureus and Enterococcus faecalis. Mol. Microbiol. 2019;111:159–175. doi: 10.1111/mmi.14145. [DOI] [PubMed] [Google Scholar]
- 117.Patel CN, et al. Polyamines are essential for the formation of plague biofilm. J. Bacteriol. 2006;188:2355–2363. doi: 10.1128/JB.188.7.2355-2363.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Villanueva MT. Bacterial biofilms may feed colon cancer. Nat. Rev. Cancer. 2015;15:320–320. [Google Scholar]
- 119.Li MMH, MacDonald MR. Polyamines: small molecules with a big role in promoting virus infection. Cell Host Microbe. 2016;20:123–124. doi: 10.1016/j.chom.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 120.Firpo MR, Mounce BC. Diverse functions of polyamines in virus infection. Biomolecules. 2020;10:628. doi: 10.3390/biom10040628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mounce, B. C., Olsen, M. E., Vignuzzi, M. & Connor, J. H. Polyamines and their role in virus infection. Microbiol. Mol. Biol. Rev. 81, e00029-17 (2017). [DOI] [PMC free article] [PubMed]
- 122.Tams RN, et al. Overproduction of phospholipids by the kennedy pathway leads to hypervirulence in Candida albicans. Front. Microbiol. 2019;10:86. doi: 10.3389/fmicb.2019.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Klig LS, Friedli L, Schmid E. Phospholipid biosynthesis in Candida albicans: regulation by the precursors inositol and choline. J. Bacteriol. 1990;172:4407–4414. doi: 10.1128/jb.172.8.4407-4414.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang J, et al. Phospholipid homeostasis plays an important role in fungal development, fungicide resistance and virulence in Fusarium graminearum. Phytopathol. Res. 2019;1:16. doi: 10.1186/s42483-019-0023-9. [DOI] [Google Scholar]
- 125.Chen Y-L, et al. Phosphatidylserine synthase and phosphatidylserine decarboxylase are essential for cell wall integrity and virulence in Candida albicans. Mol. Microbiol. 2010;75:1112–1132. doi: 10.1111/j.1365-2958.2009.07018.x. [DOI] [PubMed] [Google Scholar]
- 126.A, A. & Sm, K. Acetylcholine induces yeast to hyphal form transition in Candida albicans. Fungal Genomics Biol. 8, p154 (2018).
- 127.Markham P, Robson GD, Bainbridge BW, Trinci APJ. Choline: Its role in the growth of filamentous fungi and the regulation of mycelial morphology. FEMS Microbiol. Rev. 1993;10:287–300. doi: 10.1111/j.1574-6968.1993.tb05872.x. [DOI] [PubMed] [Google Scholar]
- 128.Mehedint MG, Zeisel SH. Choline’s role in maintaining liver function: new evidence for epigenetic mechanisms. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16:339–345. doi: 10.1097/MCO.0b013e3283600d46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sherriff JL, O’Sullivan TA, Properzi C, Oddo J-L, Adams LA. Choline, its potential role in nonalcoholic fatty liver disease, and the case for human and bacterial genes. Adv. Nutr. 2016;7:5–13. doi: 10.3945/an.114.007955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brown AL, et al. Dietary choline supplementation attenuates high-fat-diet–induced hepatocellular carcinoma in mice. J. Nutr. 2020;150:775–783. doi: 10.1093/jn/nxz315. [DOI] [PubMed] [Google Scholar]
- 131.Sun S, et al. Choline and betaine consumption lowers cancer risk: a meta-analysis of epidemiologic studies. Sci. Rep. 2016;6:35547. doi: 10.1038/srep35547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou R, et al. Higher dietary intakes of choline and betaine are associated with a lower risk of primary liver cancer: a case-control study. Sci. Rep. 2017;7:679. doi: 10.1038/s41598-017-00773-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Forbes JD, Bernstein CN, Tremlett H, Van Domselaar G, Knox NC. A fungal world: could the gut mycobiome be involved in neurological disease? Front. Microbiol. 2019;10:3249–3249. doi: 10.3389/fmicb.2018.03249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zijnge V, et al. Oral biofilm architecture on natural teeth. PLoS ONE. 2010;5:e9321. doi: 10.1371/journal.pone.0009321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Arfi Y, et al. Characterization of salt-adapted secreted lignocellulolytic enzymes from the mangrove fungus Pestalotiopsis sp. Nat. Commun. 2013;4:1810. doi: 10.1038/ncomms2850. [DOI] [PubMed] [Google Scholar]
- 136.Kumar V, et al. Poplar carbohydrate-active enzymes: whole-genome annotation and functional analyses based on RNA expression data. Plant J. 2019;99:589–609. doi: 10.1111/tpj.14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49:D545–D551. doi: 10.1093/nar/gkaa970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 139.Eddy SR. What is a hidden Markov model? Nat. Biotechnol. 2004;22:1315–1316. doi: 10.1038/nbt1004-1315. [DOI] [PubMed] [Google Scholar]
- 140.Kovatcheva-Datchary P, et al. Simplified intestinal microbiota to study microbe-diet-host interactions in a mouse model. Cell Rep. 2019;26:3772–3783. doi: 10.1016/j.celrep.2019.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhao Z, Liu H, Wang C, Xu J-R. Comparative analysis of fungal genomes reveals different plant cell wall degrading capacity in fungi. BMC Genomics. 2013;14:274. doi: 10.1186/1471-2164-14-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Borin GP, et al. Comparative secretome analysis of Trichoderma reesei and Aspergillus niger during growth on sugarcane biomass. PLoS ONE. 2015;10:e0129275. doi: 10.1371/journal.pone.0129275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Baroncelli R, et al. Gene family expansions and contractions are associated with host range in plant pathogens of the genus Colletotrichum. BMC Genomics. 2016;17:555. doi: 10.1186/s12864-016-2917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Looi HK, et al. Genomic insight into pathogenicity of dematiaceous fungus Corynespora cassiicola. PeerJ. 2017;5:e2841. doi: 10.7717/peerj.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wegmann U, et al. Complete genome of a new Firmicutes species belonging to the dominant human colonic microbiota (‘Ruminococcus bicirculans’) reveals two chromosomes and a selective capacity to utilize plant glucans. Environ. Microbiol. 2014;16:2879–2890. doi: 10.1111/1462-2920.12217. [DOI] [PubMed] [Google Scholar]
- 146.Geisler-Lee J, et al. Poplar carbohydrate-active enzymes. Gene identification and expression analyses. Plant Physiol. 2006;140:946–962. doi: 10.1104/pp.105.072652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sayers, E. W. et al. Database resources of the national centre for biotechnology information. Nucleic Acids Res.. 47, D23 (2019). [DOI] [PMC free article] [PubMed]
- 148.Cunningham F, et al. Ensembl 2022. Nucleic Acids Res. 2022;50:D988–D995. doi: 10.1093/nar/gkab1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pritchard, L., Glover, R. H., Humphris, S., Elphinstone, J. G. & Toth, I. K. Genomics and taxonomy in diagnostics for food security: soft-rotting enterobacterial plant pathogens. Analytical Methods8, 12–24 (2016).
- 150.Oxoid - Product detail. http://www.oxoid.com/UK/blue/prod_detail/prod_detail.asp?pr=CM0041.
- 151.Thévenot EA, Roux A, Xu Y, Ezan E, Junot C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses. J. Proteome Res. 2015;14:3322–3335. doi: 10.1021/acs.jproteome.5b00354. [DOI] [PubMed] [Google Scholar]
- 152.Almeida, J. N. de et al. Candida haemulonii complex species, Brazil, January 2010–March 2015. Emerg. Infect. Dis. J.22. 10.3201/eid2203.151610 (2016). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
BioFung is public open access database that can be downloaded from GitHub repository: https://github.com/sysbiomelab/BioFung.
The instruction and the pipeline scripts for BioFung can be found at our GitHub repository https://github.com/sysbiomelab/BioFung. BioFung.hmm file was uploaded via GitHub large file storage (lfs) on the GitHub page. Additionally, BioFung is on an automated pipeline with Nextflow v21.04.1 and either singularity v3.8.3 or docker 20.10.7. All software executed by the pipeline is containerised meaning that no additional installation is required for both local or high-performance computing. ReadME file contains code for usage and example fasta.