Abstract
Human saliva is a complex fluid containing proteins such as salivary cytokines, which can be used for diagnostic purposes, particularly among the pediatric population. This study aimed to assess the concentrations of salivary cytokines in healthy children and adolescents and determine their associations with age, sex, and oral and dental findings. Healthy children and adolescents aged 4–18 years were enrolled in this cross-sectional study. The concentrations of the following salivary cytokines were measured by Luminex technology: IFN-γ, IL-1α, IL-1β, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, IP-10, TNF-α, and VEGF-A. Additionally, oral and dental parameters were recorded using a standardized protocol. A total of 128 participants (mean age, 10.7 years; males, 50.8%) were enrolled. The levels of 1β, IL-6, IL-8, and IL-10 were significantly higher in those with gingivitis. Increased salivary flow rates were negatively correlated with IL-1α, IL-1β, IL-6, IL-8, IL-10, TNF-α, and VEGF-A concentrations. The findings of this study showed that the concentrations of most of the salivary cytokines were positively correlated with age and the presence of oral pathologies (such as gingivitis and caries) and negatively correlated with salivary flow rate.
Subject terms: Cytokines, Dentistry, Paediatrics
Introduction
Cytokine concentrations are measurable in several body fluids, tissues, and cells for diagnostic or prognostic purposes1. Human saliva is a complex fluid that has gained recent interest as a diagnostic sample to detect cytokines. In pediatrics, the use of blood cytokines for diagnostic purposes has most commonly been studied in systemic diseases such as neonatal sepsis, tuberculosis, pneumonia, and neuroinflammatory as well as rheumatological diseases2,3. Salivary cytokines were further assessed as diagnostic biomarkers for oral conditions such as caries, gingivitis, and periodontitis4–6.
Contrary to blood, saliva sampling is a noninvasive procedure; moreover, it does not require trained staff and is cost effective. Thus, saliva collection is particularly useful in instances where the collection of blood is challenging, such as in young children and elderly or patients with anxiety6,7. Only a few studies have analyzed the salivary cytokines in children, and most of the studies were conducted in children with acute lymphatic leukemia to determine the presence of a possible biomarker for graft versus host disease or mucositis8–13. Additionally, a few studies investigated salivary cytokines as markers for caries and gingivitis14–19. Data on the concentrations of salivary cytokines in healthy pediatric populations are negligible. In order to find diagnostic biomarkers in the future that can predict oral complications and support diagnosis of systemic diseases as well as treatment efficacy, we depend on reference values in healthy children. The concentrations of cytokines in blood increase with age until early adolescence20,21; therefore, we hypothesized that the concentrations of salivary cytokines might be associated with age and oral health.
This study aimed to assess the cytokine concentrations in healthy children and adolescents and assess their association with age, sex, salivary flow rate and oral health. The findings might contribute to our understanding and interpretation of salivary cytokine-associated assays in the pediatric population.
Materials and methods
Study population
This prospective observational study comprised healthy children aged 4–18 years who visited the Department of Children and Adolescent Dentistry, University Center for Dental Medicine Basel, University of Basel, Switzerland, for routine dental prophylaxis consultations over a period time of 2 years. The exclusion criteria for this study were as follows: history of acute symptoms of respiratory infections in the preceding 2 weeks (including rhinitis, bronchitis, pharyngitis, cough, and tonsillitis); known allergy to paraffin; hemato-oncological or autoimmune disease; use of systemic antibiotics in the preceding 2 weeks; use of antimicrobial rinsing solutions 12 h before the study; and any vaccination within the preceding 48 h. Written informed consent, parental where required, was obtained for each patient.
Demographic and clinical information were collected from each child in a standardized form using an electronic database (Epidata manager, v.2.0.13.65 and Epidata entry client, v.2.0.10.26 by EpiData Association, Denmark). The following variables were recorded: age; sex; the presence or absence of gingivitis, washable plaque, caries, xerostomia, candidiasis, mucosa blandness, and aphthae and its localization; number of carious, missing, and filled teeth; date of saliva collection; total volume of saliva collected; and the salivary flow rate. All oral findings were assessed and recorded by 7 experienced dentists, who had been previously trained for this study and had obtained detailed information concerning study protocol and procedure. The study was approved by the ethics committee of North–West Switzerland (Number: 2016-00583) and all methods were performed in accordance with the relevant guidelines and regulations.
Saliva collection
The saliva was collected 30–90 min after eating or brushing the teeth in the morning hours between 8 am and 12 noon before the oral cavity was examined or any dental treatment was provided. The sample was obtained by asking the patients to chew on paraffin gum for 2–5 min while tilting their head forward and repeatedly expectorate the saliva into a cup. The saliva volume was measured (1 g equivalent to 1 ml) and a saliva flow rate (ml/min) was calculated. The samples were stored at 4 °C within 20 min. Subsequently, they were centrifuged at 1650 gravity (G) for 15 min before cryopreservation at − 75 °C; only those with a volume of at least 30 μl were retained for further experiments.
Cytokine measurement
Salivary cytokines were selected based on previous work by Diesch et al.6. In addition, we analyzed other potentially relevant cytokines in order to get a spectrum of cytokines as comprehensive as possible. Batch analyses of the salivary samples were performed using a multiplex cytokine analyzer (Magpix, Luminex Corp. Austin, US) with magnetic bead-based multianalyte panels (Milliplex, Merck Millipore, Schwalbach, Germany). The following cytokines were measured: IFN-γ, interleukin (IL)-1α, IL-1β, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, IFN-γ-inducible protein (IP)-10, tumor necrosis factor (TNF)-α, and vascular endothelial growth factor (VEGF-A). The samples were thawed, centrifuged, and stained according to the manufacturers’ instructions and the protocol for cell culture supernatants. The concentrations of the cytokines were calculated using a log regression standard curve. If the concentrations were below the quantification limit, they were set to half the detection limit (DL), as in the cases of IFN-γ (DL = 1.3), IL-1β (1.6), IL-13 (6.4), TNF-α (6.4), and IP-10 (2.6).
Statistical analyses
The patients were stratified into two age groups: 4–11 (children) and 12–18 (adolescents) years. The descriptive statistics included the frequency (proportion), median (interquartile range [IQR]) or mean ± the standard deviation. The corresponding p-values were derived from significance tests such as the Chi-squared test, Fisher’s exact test, Wilcoxon rank-sum test, or t-test. To predict the association between age and the cytokine level, linear regression models adjusted for sex, gingivitis, caries, and salivary flow rate were used. The models had a nested design to receive separate estimates for the two age groups. The cytokine concentrations had to be log-transformed for the regression analysis as verified by quantile comparison plots and “standardized residuals versus fitted values plots.” Additionally, significance tests were performed for potential nonlinear age dependency and other significant interactions. The back-transformed estimates for the predicted cytokines are presented as the ratio per year of age or the geometric mean ratios for sex and health status. Additionally, the corresponding 95% confidence intervals and p-values are indicated. No adjustments to the significance levels for multiple comparisons were made because of the exploratory nature of the study. All analyses were performed using the statistical program R version 3.5.1 (Austria).
Results
Demographic and clinical profile
Of the 143 children enrolled in the study, 128 were included in the final analysis according to the described exclusion criteria. The mean age was 10.7 ± 4.2 years; 50.8% of the participants were males, 60.2% were children; and 39.8% were adolescents (Table 1). The median amount of saliva collected was 4.2 ml (IQR: 2.6, 6.5), and the mean salivary flow rate was 1.0 ± 0.7 ml/min. Adolescents had higher median saliva quantity and higher mean salivary flow rate than children. Tables 1 and 2 summarize the additional patient characteristics and baseline cytokine concentrations, respectively.
Table 1.
Baseline characteristics of the study participants.
| Child n = 77 | Adolescent n = 51 | Total n = 128 | p-value | |
|---|---|---|---|---|
| Males/femalesa | 41/36 (53.2/46.8) | 24/27 (47.1/52.9) | 65/63 (50.8/49.2) | 0.61 |
| Ageb | 7.8 (2.4) | 15.1 (1.7) | 10.7 (4.2) | < 0.001 |
| Stomatological and dental findings | ||||
| Cariesa | 38 (49.4) | 11 (21.6) | 49 (38.3) | 0.003 |
| Gingivitisa | 14 (18.2) | 21 (41.2) | 35 (27.3) | 0.008 |
| Washable plaquea | 51 (66.2) | 21 (41.2) | 72 (56.2) | 0.009 |
| Xerostomiaa | 0 (0) | 0 (0) | 0 (0) | n.e |
| Candidiasisa | 0 (0) | 1 (2) | 1 (0.8) | 0.40 |
| Decayed teethc | 0.5 (0, 3) | 0 (0, 0) | 0 (0, 1.5) | 0.001 |
| Missing teethc | 0 (0, 1) | 0 (0, 0) | 0 (0, 0.5) | 0.080 |
| Filled teethc | 2 (0, 5) | 1 (0, 3) | 2 (0, 4) | 0.27 |
| Any mucosal changesa | 4 (5.2) | 2 (3.9) | 6 (4.7) | 1 |
| Aphthaea | 2 (2.6) | 0 (0) | 2 (1.6) | 0.52 |
| Saliva total quantity (ml)c | 3.2 (2.1, 4.7) | 6.0 (4.0, 10.7) | 4.2 (2.6, 6.5) | < 0.001 |
| Salivary flow rate (ml/min)b | 0.8 (0.5) | 1.5 (0.8) | 1.0 (0.7) | < 0.001 |
n.e. not estimatable.
aThe values are given as the frequency (and proportion).
bThe values are given as the mean (and the standard deviation).
cThe values are given as the median (and the interquartile range).
Table 2.
Salivary cytokine concentrations in the study population.
| Child n = 77 | Adolescent n = 51 | Total n = 128 | p-value | |
|---|---|---|---|---|
| IFN-γ | 1.1 (0.6, 2.7) | 1.1 (0.6, 2.5) | 1.1 (0.6, 2.7) | 0.86 |
| IL-1α | 733 (402, 1433) | 806 (404, 1534) | 756 (398, 1471) | 0.60 |
| IL-1β | 16.1 (8.1, 34.2) | 17.1 (6.9, 41.2) | 16.1 (7.7, 36.6) | 0.75 |
| IL-4 | 1.4 (0.9, 2) | 1.6 (0.9, 2.1) | 1.4 (0.9, 2) | 0.40 |
| IL-5 | 0.9 (0.6, 1.2) | 1.1 (0.7, 1.5) | 0.9 (0.6, 1.3) | 0.032 |
| IL-6 | 3.3 (1.4, 8.5) | 2.6 (1.3, 5.1) | 2.9 (1.4, 8.1) | 0.39 |
| IL-8 | 297 (152, 590) | 222 (137, 714) | 282 (147, 609) | 0.66 |
| IL-10 | 7.6 (4.1, 11.8) | 7.6 (4.7, 11.5) | 7.6 (4.3, 11.8) | 0.92 |
| IL-13 | 9.0 (3.2, 15.4) | 8.6 (3.3, 21.3) | 9.0 (3.2, 18.9) | 0.40 |
| IP-10 | 39.6 (16.7, 103) | 63.7 (22.7, 112) | 52.6 (17.7, 105) | 0.35 |
| TNF-α | 7.1 (4.5, 12.4) | 7.8 (4.4, 13.1) | 7.7 (4.4, 13.1) | 0.72 |
| VEGF-A | 164 (110, 249) | 154 (70, 263) | 163 (88, 255) | 0.39 |
The values are given as the median (and the interquartile range).
Salivary cytokines
The median concentrations of the 12 cytokines had a range from 0.9 to 756 pg/ml (Table 2). Two cytokines (IFN-γ and IL-13) were excluded from further analysis because more than a third of the concentrations were below the lower limit of quantification (43.4% and 34.1%, respectively). A quantifiable concentration was detected in more than 90% of the samples for the remaining cytokines. In total, 7 out of 10 cytokines, including IL-1α, IL-1β, IL-4, IL-5, IP-10, TNF-α, and VEGF-A, showed higher median concentrations in adolescents than in children. IL-5 was the only cytokine that was significantly higher in adolescents. The median concentrations of IL-6 and IL-8 were higher in children, whereas that of IL-10 was similar in both groups.
Dental examinations
Dental examinations revealed the presence of washable plaque in 56.2%, caries in 38.3%, and gingivitis in 27.3% of the participants (Table 1). Overall, plaque, caries, or gingivitis was not detected in 30.5% of the participants. Gingivitis was more frequent in adolescents (41.2%) than in children (18.2%). By contrast, caries and washable plaque were observed more frequently in children than adolescents (49.4% vs. 21.6% and 66.2% vs. 41.2%, respectively). Supplementary Table 1 presents the characteristics and findings of the oral examinations in participants with and without gingivitis. Participants with gingivitis were significantly older than those without gingivitis (12.8 vs. 9.9 years; p < 0.001); moreover, they presented with increased saliva quantity and flow rate.
Supplementary Table 2 presents the characteristics and clinical profiles of participants with and without caries. The group with caries had a mean age of 8.8 ± 3.9 years; gingivitis was observed in 24.5% and washable plaque in 77.6% of the participants. Both saliva quantity and flow rate were decreased in those with caries.
Influence of age, sex, dental health, and salivary flow rate on cytokine concentration
Age
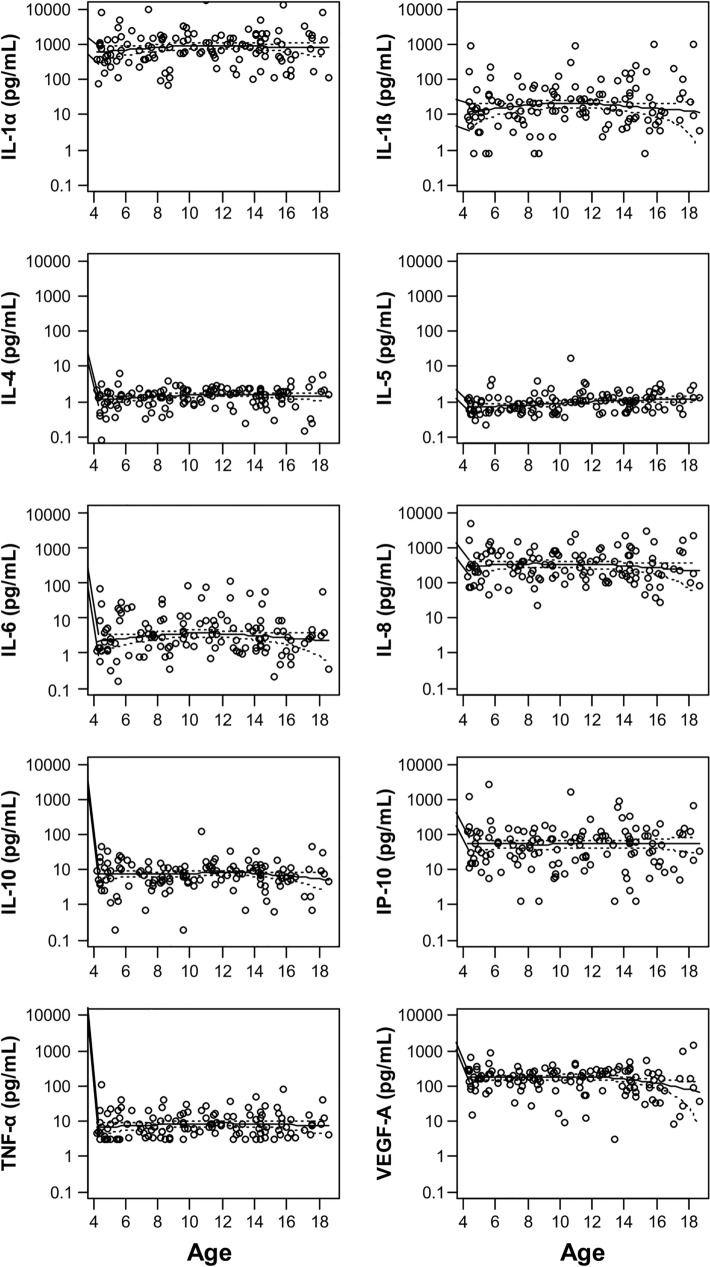
The concentrations of all the cytokines were stable over the years, as indicated by the local polynomial regression lines (Fig. 1). Linear regression models with a nested design were calculated to quantify the changes by age in children and adolescents separately (Table 3). The regression models were adjusted for sex, gingivitis, and salivary flow rate. In children, significant annual increases of 11.6%, 15.4%, 8.7%, and 9.3% were observed in IL-1α, IL-1β, IL-4, and IL-5, respectively. Although IL-1β was also substantially increased in adolescents by 22.7% per year, this was only a trend. Similarly, IL-5, and IL-6 demonstrated increasing trends in adolescents and children, respectively, per year.
Figure 1.
Graphs showing the concentrations of the cytokines across the years (age). A fixed log scale ranging from 0.1 pg/ml to 10 μg/ml was used. The local polynomial regression lines (loess function, degree of smoothing, 2/3) with 95% confidence limits are shown.
Table 3.
Relative annual changes in the cytokine concentration (ratio per year of age).
| Cytokine | Ratios (95% CI) per year of age in children; p-value | Ratios (95% CI) per year of age in adolescents; p-value |
|---|---|---|
| IL-1α | 1.116 (1.006, 1.238); p = 0.039 | 1.104 (0.933, 1.305); p = 0.25 |
| IL-1β | 1.154 (1.002, 1.329); p = 0.048 | 1.227 (0.972, 1.549); p = 0.085 |
| IL-4 | 1.087 (1.012, 1.169); p = 0.023 | 1.025 (0.910, 1.155); p = 0.68 |
| IL-5 | 1.093 (1.028, 1.162); p = 0.005 | 1.092 (0.987, 1.208); p = 0.086 |
| IL-6 | 1.112 (0.982, 1.260); p = 0.093 | 0.980 (0.798, 1.203); p = 0.84 |
| IL-8 | 1.023 (0.922, 1.134); p = 0.67 | 1.036 (0.873, 1.228); p = 0.69 |
| IL-10 | 1.057 (0.958, 1.167); p = 0.27 | 1.002 (0.851, 1.179); p = 0.99 |
| IP-10 | 0.962 (0.833, 1.111); p = 0.59 | 0.944 (0.748, 1.191); p = 0.62 |
| TNF-α | 1.039 (0.961, 1.123); p = 0.33 | 1.059 (0.932, 1.204); p = 0.38 |
| VEGF-A | 0.956 (0.867, 1.055); p = 0.37 | 0.929 (0.790, 1.093); p = 0.37 |
A separate linear regression model was performed for each cytokine. The models had a nested design to receive separate estimates for children and adolescents and were further adjusted for sex, gingivitis, caries, and salivary flow rate.
Sex
Generally, no significant differences in cytokine concentrations between males and females were observed by linear regression analysis (see Supplementary Table 3). Considering the effect sizes, the concentrations of 8 out of 10 cytokines (VEGF-A, IL-1β, IL-4, IL-5, IL-6, IL-10, IP-10, and TNF-α) were slightly higher in males, with the highest difference seen in IL-5 (39%); alternatively, the concentrations of IL-1α and IL-8 were marginally higher in females.
Gingivitis
The concentrations of IL-1β, IL-6, IL-8 and IL-10 were elevated in the presence of gingivitis (Supplementary Table 1). These findings were confirmed by the linear regression models (Supplementary Table 3), where a 2.0, 2.5, 1.6, and 1.6-fold higher concentration was observed for IL-1β, IL-6, IL-8, and IL-10, respectively.
Salivary flow rate
The analysis of the cofactors in the linear regression models revealed that salivary flow rate had the most significant effect on the cytokine concentrations (see Supplementary Table 3). Except for IL-4, IL-5, and IP-10, the concentrations of all other measured cytokines were significantly reduced with the increase in the salivary flow rate. The greatest reduction in cytokine concentration per flow rate increase of 1 ml/min was seen for IL-1β (− 59%), IL-6 (− 50%), and IL-1α (− 47%).
Caries
Caries appeared to have a minor impact on cytokine concentration, as indicated in Supplementary Tables 2 and 3.
Discussion
This is the first study to present a comprehensive cytokine profile of children and adolescents, particularly in relation to age, sex, and oral health status. In comparison, former studies investigating cytokines in healthy patients included a smaller number of patients22–26, measured fewer cytokines1,27–29 or examined patients belonging to a lower age range1,22,25,28,30–32.
As quantified using linear regression models, the concentration of the salivary cytokines demonstrated a slight increase with the increase in age in both children and adolescents. This finding is in line with the increase in cytokine concentrations in blood, as reported by Decker et al.1. In their study comprising 271 healthy children aged 0–12 years, age-dependent increases in the concentrations of IL-10, IL-6, IL-4, and IL-2 (in unstimulated samples); IL-4, IFN-γ, and TNF-α (in stimulated samples); and IL-2, IL-6, and IP-10 (in C. albicans-stimulated samples) were observed. Similarly, age-dependent increases in the concentrations of TNF-α, IFN-γ, IP-10, and IL-12p70 were reported in stimulated blood samples from children (0–12 months; n = 30) and healthy adults (n = 30)33. Stowe et al.34, who measured the plasma cytokine profiles in 1411 people aged 25–91 years, observed an increase in the level of IL-6 with aging. However, the findings of the present study were not in accordance with those reported by Riis et al.35, who measured the concentrations of cytokines in the saliva and serum of 114 healthy adolescent girls; the concentrations of IFN-γ, IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12p70, and TNF-α were found to be lower in older girls. This discrepancy in results may be attributed to the differences in the study samples and demographics and the potential influence of a smoking habit in the study by Riis et al.
Gingivitis is a common oral finding associated with local inflammation36. In the current study, gingivitis was associated with an increase in the concentration of all the cytokines. Santos et al. reported similar findings in unstimulated saliva from 71 children with cerebral palsy, wherein gingival inflammation was related to the increased concentrations of the cytokines (IL-1β, IL-6, IL-8, and TNF-α)19. By contrast, Belstrøm et al. reported negative correlations with the salivary cytokine concentrations of IL-1β, VEGF, IL-8, IL-1ra, and MCP-1 in patients with early gingivitis18. There is a dearth of studies on the association between cytokine concentration and gingivitis; however, studies on oral inflammatory conditions, such as mucositis, periodontitis, and peri-implantitis, have been published. For instance, different proinflammatory cytokines are thought to play essential roles in the pathogenesis of oral mucositis10, and the severity of the disease has been shown to correlate with the intensity of the production of these cytokines37. Furthermore, the concentrations of IL-6, TNF-α10, and buccal TNF-α (oncological patients)11 are reported to be increased in mucositis. Ertugrul et al. demonstrated that the severity of periodontitis correlated with the expression level of IL-838. Liskmann et al. reported elevated IL-6 and IL-10 levels in patients with peri-implantitis39. The elevated concentration of IL-6 in children with gingivitis in the current study likely mirrors the underlying pathophysiology of local inflammation. The more frequently observed gingivitis in adolescents could be explained by the fact that there is an age-related tendency for the development of gingivitis, with children showing a lower severity of gingivitis than adults. Certain oral findings are rare or even absent in our study population. This was to be expected, as these findings are not likely to be seen in healthy young individuals.
To the best of our knowledge, the correlation between oral inflammation and salivary flow rate has not been described thus far. Saliva is important for the maintenance of oral health40. The concentrations of all cytokines examined in this study decreased with the increase in the salivary flow rate. This observation could be explained by the dilution of the cytokines due to the high salivary flow rate; alternatively, the antiinflammatory and antibiotic components in the saliva might exert a protective effect against oral inflammation, which has been reported recently41,42. Furthermore, the salivary flow rate is reported to be reduced in chronic periodontitis, a subcategory of oral inflammation43.
Because of the limited number of participants in this study, it was not possible to perform a complete logistic regression analysis with all the cytokines integrated as cofactors without the problem of overfitting. Therefore, separate linear regression models were used to predict each cytokine. Longitudinal study designs using larger samples and more diverse populations are needed to further characterize the cytokine expression and its association with the altered conditions in the oral cavity.
Conclusion
In summary, the concentrations of the cytokines were correlated with age, sex, and gingivitis in this study. The proinflammatory cytokines IL-6 and IL-1β were prominently associated with gingivitis, suggesting that the levels of these cytokines could predict or confirm oral inflammation. Furthermore, the salivary flow rate influenced the cytokine concentrations; however, the interpretation of this finding remains unclear. The findings of this study confirm the feasibility of measuring salivary cytokines. Nonetheless, additional studies are required to ascertain the prognostic significance of cytokines in oral health in the pediatric population.
Supplementary Information
Acknowledgements
The authors thank all the children and their parents who participated in the study. We also thank Aurora Frei, Sybille Chettata, Edith Corneo, Diana Opitz, and Imbach Monika for their substantial contributions to this research. Moreover, we are grateful to the foundation “Stiftung für krebskranke Kinder Regio Basiliensis” for their support, which allowed us to conduct this study.
Author contributions
T.D.-F., C.F., and N.R. conceived and designed the study. N.F. did the laboratory analysis. C.H.R., T.D.-F., C.F., N.R., and U.S. were responsible for the analysis, interpretation of data, and writing of the manuscript. N.F. and A.F. revised the manuscript critically for intellectual content. The final version for publication was approved by all authors.
Funding
This project was funded by the “Stiftung für krebskranke Kinder Regio Basiliensis,” Basel, Switzerland.
Data availability
The data set from the study are held securely in coded form at the Ambulantes Studienzentrum (ASZ) of the University Children’s hospital of Basel. The data underlying this article will be shared on reasonable request to the corresponding authors after granting prespecified criteria for confidential access.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-20475-2.
References
- 1.Decker ML, Gotta V, Wellmann S, Ritz N. Cytokine profiling in healthy children shows association of age with cytokine concentrations. Sci. Rep. 2017;7(1):17842. doi: 10.1038/s41598-017-17865-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kothur K, Wienholt L, Brilot F, Dale RC. CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: A systematic review. Cytokine. 2016;77:227–237. doi: 10.1016/j.cyto.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Monastero RN, Pentyala S. Cytokines as biomarkers and their respective clinical cutoff levels. Int. J. Inflamm. 2017;2017:4309485. doi: 10.1155/2017/4309485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dikova VR, Principe S, Bagan JV. Salivary inflammatory proteins in patients with oral potentially malignant disorders. J. Clin. Exp. Dent. 2019;11(7):e659–e664. doi: 10.4317/jced.55917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahibzada HA, Khurshid Z, Khan RS, Naseem M, Siddique KM, Mali M, Zafar MS. Salivary IL-8, IL-6 and TNF-α as potential diagnostic biomarkers for oral cancer. Diagnostics. 2017;7:2. doi: 10.3390/diagnostics7020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diesch T, Filippi C, Fritschi N, Filippi A, Ritz N. Cytokines in saliva as biomarkers of oral and systemic oncological or infectious diseases: A systematic review. Cytokine. 2021;143:155506. doi: 10.1016/j.cyto.2021.155506. [DOI] [PubMed] [Google Scholar]
- 7.Szabo YZ, Slavish DC. Measuring salivary markers of inflammation in health research: A review of methodological considerations and best practices. Psychoneuroendocrinology. 2021;124:105069. doi: 10.1016/j.psyneuen.2020.105069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krzaczek PM, Mitura-Lesiuk M, Zawitkowska J, Petkowicz B, Wilczyńska B, Drabko K. Salivary and serum concentrations of selected pro- and antiinflammatory cytokines in relation to oral lesions among children undergoing maintenance therapy of acute lymphoblastic leukemia. Contemp. Oncol. 2019;23(2):81–86. doi: 10.5114/wo.2019.85878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pels E. Comparison of saliva interleukin-2 concentration to the condition of gums in children with acute lymphoblastic leukaemia during anti-tumour treatment. Cancer Chemother. Pharmacol. 2015;76(1):205–210. doi: 10.1007/s00280-015-2750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morales-Rojas T, Viera N, Morón-Medina A, Alvarez CJ, Alvarez A. Proinflammatory cytokines during the initial phase of oral mucositis in patients with acute lymphoblastic leukaemia. Int. J. Paediatr. Dent. 2012;22(3):191–196. doi: 10.1111/j.1365-263X.2011.01175.x. [DOI] [PubMed] [Google Scholar]
- 11.Fall-Dickson JM, Ramsay ES, Castro K, Woltz P, Sportés C. Oral mucositis-related oropharyngeal pain and correlative tumor necrosis factor-alpha expression in adult oncology patients undergoing hematopoietic stem cell transplantation. Clin. Ther. 2007;29:2547–2561. doi: 10.1016/j.clinthera.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Resende RG, Correia-Silva FJ, Arão TC, Silva TA, Abreu MH, Bittencourt H, Gomez RS. Investigation of functional IL-10 gene polymorphism and IL-10 levels in acute graft-versus-host disease. J. Clin. Immunol. 2010;30(3):465–473. doi: 10.1007/s10875-010-9377-6. [DOI] [PubMed] [Google Scholar]
- 13.Resende RG, Abreu MH, de Souza LN, Silva ME, Gomez RS, Correia-Silva FJ. Association between IL1B (+3954) polymorphisms and IL-1β levels in blood and saliva, together with acute graft-versus-host disease. J. Interferon. Cytokine Res. 2013;33(7):392–397. doi: 10.1089/jir.2012.0111. [DOI] [PubMed] [Google Scholar]
- 14.Sharma V, Gupta N, Srivastava N, Rana V, Chandna P, Yadav S, Sharma A. Diagnostic potential of inflammatory biomarkers in early childhood caries: A case control study. Clin. Chim. Acta. 2017;471:158–163. doi: 10.1016/j.cca.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 15.Cogulu D, Onay H, Ozdemir Y, Ozkinay F, Kutukculer N, Eronat C. Associations of interleukin (IL)-1β, IL-1 receptor antagonist, and IL-10 with dental caries. J. Oral Sci. 2015;57(1):31–36. doi: 10.2334/josnusd.57.31. [DOI] [PubMed] [Google Scholar]
- 16.Menon MM, Balagopal RV, Sajitha K, Parvathy K, Sangeetha GB, Arun XM, Sureshkumar J. Evaluation of salivary interleukin-6 in children with early childhood caries after treatment. Contemp. Clin. Dent. 2016;7(2):198–202. doi: 10.4103/0976-237X.183059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gornowicz A, Bielawska A, Bielawski K, Grabowska SZ, Wójcicka A, Zalewska M, Maciorkowska E. Pro-inflammatory cytokines in saliva of adolescents with dental caries disease. Ann. Agric. Environ. Med. 2012;19(4):711–716. [PubMed] [Google Scholar]
- 18.Belstrøm D, Damgaard C, Könönen E, Gürsoy M, Holmstrup P, Gürsoy UK. Salivary cytokine levels in early gingival inflammation. J. Oral Microbiol. 2017;9(1):1364101. doi: 10.1080/20002297.2017.1364101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos M, Diniz MB, Guaré RO, Ferreira MCD, Gutierrez GM, Gorjão R. Inflammatory markers in saliva as indicators of gingival inflammation in cerebral palsy children with and without cervical motor control. Int. J. Paediatr. Dent. 2017;27(5):364–371. doi: 10.1111/ipd.12270. [DOI] [PubMed] [Google Scholar]
- 20.Wiegering V, Eyrich M, Wunder C, Günther H, Schlegel PG, Winkler B. Age-related changes in intracellular cytokine expression in healthy children. Eur. Cytokine Netw. 2009;20(2):75–80. doi: 10.1684/ecn.2009.0149. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann F, Albert MH, Arenz S, Bidlingmaier C, Berkowicz N, Sedlaczek S, Till H, Pawlita I, Renner ED, Weiss M, Belohradsky BH. Intracellular T-cell cytokine levels are age-dependent in healthy children and adults. Eur. Cytokine Netw. 2005;16(4):283–288. [PubMed] [Google Scholar]
- 22.Smart JM, Kemp AS. Ontogeny of T-helper 1 and T-helper 2 cytokine production in childhood. Pediatr. Allergy Immunol. 2001;12(4):181–187. doi: 10.1034/j.1399-3038.2001.012004181.x. [DOI] [PubMed] [Google Scholar]
- 23.Gasparoni A, Ciardelli L, Avanzini A, Castellazzi AM, Carini R, Rondini G, Chirico G. Age-related changes in intracellular TH1/TH2 cytokine production, immunoproliferative T lymphocyte response and natural killer cell activity in newborns, children and adults. Biol. Neonate. 2003;84(4):297–303. doi: 10.1159/000073638. [DOI] [PubMed] [Google Scholar]
- 24.Kleiner G, Marcuzzi A, Zanin V, Monasta L, Zauli G. Cytokine levels in the serum of healthy subjects. Mediators Inflamm. 2013;2013:434010. doi: 10.1155/2013/434010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaisman N, Zaruk Y, Shirazi I, Kaysar N, Barak V. The effect of fish oil supplementation on cytokine production in children. Eur. Cytokine Netw. 2005;16(3):194–198. [PubMed] [Google Scholar]
- 26.Chipeta J, Komada Y, Zhang XL, Deguchi T, Sugiyama K, Azuma E, Sakurai M. CD4+ and CD8+ cell cytokine profiles in neonates, older children, and adults: increasing T helper type 1 and T cytotoxic type 1 cell populations with age. Cell Immunol. 1998;183(2):149–156. doi: 10.1006/cimm.1998.1244. [DOI] [PubMed] [Google Scholar]
- 27.Upham JW, Lee PT, Holt BJ, Heaton T, Prescott SL, Sharp MJ, Sly PD, Holt PG. Development of interleukin-12-producing capacity throughout childhood. Infect. Immun. 2002;70(12):6583–6588. doi: 10.1128/IAI.70.12.6583-6588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berdat PA, Wehrle TJ, Küng A, Achermann F, Sutter M, Carrel TP, Nydegger UE. Age-specific analysis of normal cytokine levels in healthy infants. Clin. Chem. Lab. Med. 2003;41(10):1335–1339. doi: 10.1515/CCLM.2003.204. [DOI] [PubMed] [Google Scholar]
- 29.Yerkovich ST, Wikström ME, Suriyaarachchi D, Prescott SL, Upham JW, Holt PG. Postnatal development of monocyte cytokine responses to bacterial lipopolysaccharide. Pediatr. Res. 2007;62(5):547–552. doi: 10.1203/PDR.0b013e3181568105. [DOI] [PubMed] [Google Scholar]
- 30.Reikie BA, Adams RC, Ruck CE, Ho K, Leligdowicz A, Pillay S, Naidoo S, Fortuno ES, 3rd, de Beer C, Preiser W, Cotton MF, Speert DP, Esser M, Kollmann TR. Ontogeny of Toll-like receptor mediated cytokine responses of South African infants throughout the first year of life. PLoS ONE. 2012;7(9):e44763. doi: 10.1371/journal.pone.0044763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raes M, Scholtens PA, Alliet P, Hensen K, Jongen H, Boehm G, Vandenplas Y, Rummens JL. Exploration of basal immune parameters in healthy infants receiving an infant milk formula supplemented with prebiotics. Pediatr. Allergy Immunol. 2010;21(2 Pt 2):e377–e385. doi: 10.1111/j.1399-3038.2009.00957.x. [DOI] [PubMed] [Google Scholar]
- 32.Eriksson M, Sartono E, Martins CL, Balé C, Garly ML, Whittle H, Aaby P, Pedersen BK, Yazdanbakhsh M, Erikstrup C, Benn CS. A comparison of ex vivo cytokine production in venous and capillary blood. Clin. Exp. Immunol. 2007;150(3):469–476. doi: 10.1111/j.1365-2249.2007.03515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen M, Leuridan E, Zhang T, De Wit D, Willems F, Van Damme P, Goldman M, Goriely S. Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLoS ONE. 2010;5(4):e10407. doi: 10.1371/journal.pone.0010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stowe RP, Peek MK, Cutchin MP, Goodwin JS. Plasma cytokine levels in a population-based study: relation to age and ethnicity. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65(4):429–433. doi: 10.1093/gerona/glp198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riis JL, Out D, Dorn LD, Beal SJ, Denson LA, Pabst S, Jaedicke K, Granger DA. Salivary cytokines in healthy adolescent girls: Intercorrelations, stability, and associations with serum cytokines, age, and pubertal stage. Dev. Psychobiol. 2014;56(4):797–811. doi: 10.1002/dev.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James P, Worthington HV, Parnell C, Harding M, Lamont T, Cheung A, Whelton H, Riley P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst. Rev. 2017;3(3):008676. doi: 10.1002/14651858.CD008676.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonis ST. Pathobiology of oral mucositis: novel insights and opportunities. J. Support Oncol. 2007;5(9 Suppl 4):3–11. [PubMed] [Google Scholar]
- 38.Ertugrul AS, Sahin H, Dikilitas A, Alpaslan N, Bozoglan A. Comparison of CCL28, interleukin-8, interleukin-1β and tumor necrosis factor-alpha in subjects with gingivitis, chronic periodontitis and generalized aggressive periodontitis. J. Periodontal. Res. 2013;48(1):44–51. doi: 10.1111/j.1600-0765.2012.01500.x. [DOI] [PubMed] [Google Scholar]
- 39.Liskmann S, Vihalemm T, Salum O, Zilmer K, Fischer K, Zilmer M. Correlations between clinical parameters and interleukin-6 and interleukin-10 levels in saliva from totally edentulous patients with peri-implant disease. Int. J. Oral Maxillofac. Implants. 2006;21(4):543–550. [PubMed] [Google Scholar]
- 40.Amerongen AV, Veerman EC. Saliva–the defender of the oral cavity. Oral Dis. 2002;8(1):12–22. doi: 10.1034/j.1601-0825.2002.1o816.x. [DOI] [PubMed] [Google Scholar]
- 41.Oz HS, Chen T, Ebersole JL. A model for chronic mucosal inflammation in IBD and periodontitis. Dig. Dis. Sci. 2010;55(8):2194–2202. doi: 10.1007/s10620-009-1031-x. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi K, Furuta M, Takeshita T, Shibata Y, Shimazaki Y, Akifusa S, Ninomiya T, Kiyohara Y, Yamashita Y. Risk factors for reduced salivary flow rate in a Japanese population: The Hisayama Study. Biomed. Res. Int. 2015;2015:381821. doi: 10.1155/2015/381821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vallabhan CG, Sivarajan S, Shivkumar AD, Narayanan V, Vijayakumar S, Indhuja RS. Assessment of salivary flow rate in patients with chronic periodontitis. J. Pharm. Bioallied. Sci. 2020;12(Suppl 1):S308–s312. doi: 10.4103/jpbs.JPBS_92_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data set from the study are held securely in coded form at the Ambulantes Studienzentrum (ASZ) of the University Children’s hospital of Basel. The data underlying this article will be shared on reasonable request to the corresponding authors after granting prespecified criteria for confidential access.