Abstract
Purpose
Approximately 25% of cancer patients suffer from chronic cancer-related fatigue (CCRF), which is a complex, multifactorial condition. While there are evidence-based interventions, it remains unclear what treatment works best for the individual patient. This study explored whether baseline characteristics moderated the effect of web-based mindfulness-based cognitive therapy (eMBCT) versus ambulant activity feedback (AAF) and a psycho-education control group (PE) on fatigue in patients suffering from CCRF.
Methods
In a randomized controlled trial, participant suffering from CCRF participated in either eMBCT, AAF, or PE. Complete data of the treatment-adherent sample (≥ 6 sessions) was used to explore whether sociodemographic, clinical, and psychological characteristics at baseline moderated the intervention effect on fatigue severity at 6 months.
Results
A trend showed that baseline fatigue severity and fatigue catastrophizing moderated the intervention effect. That is, at low levels of fatigue severity and catastrophizing, patients benefited more from AAF than from eMBCT and at high levels of fatigue severity and catastrophizing, patients benefited more from eMBCT than from PE.
Conclusions
This study found some preliminary evidence on what treatment works best for the individual suffering from CCRF. These findings emphasize the potential gain in effectiveness of personalizing treatment. An alternative approach that might help us further in answering the question “what treatment works best for whom?” is discussed.
Keywords: Chronic cancer-related fatigue, Fatigue, Cancer survivors, Mindfulness-based cognitive therapy, Activity intervention, Moderation analysis
Introduction
Fatigue is one of the most prevalent and disrupting side effects of cancer and its treatment. This fatigue differs from typical tiredness as it is not alleviated by rest or sleep, nor is it proportional to recent exertion [1]. In approximately 25% of patients, it persists for months to years after completion of cancer treatment [1]. This persisting fatigue is defined as chronic cancer‐related fatigue (CCRF). CCRF interferes with patients’ ability to work, their daily life activities, and social relationships and is often accompanied by distress [1, 2].
Evidence suggests CCRF is a complex multifactorial condition, affected by a range of physiological (e.g., inflammation), clinical (e.g., comorbidities, cancer treatment), and psychosocial factors (e.g., distress) [1]. Following the multifactorial etiology of CCRF, several types of treatments have proven effective in helping patients cope with CCRF. These include both psychosocial interventions and physical activity interventions [3]. So far, it remains unknown what intervention is most suitable for which patient. In order to provide patients with the best and most efficient care, we need to answer the question “What works best for whom?” Therefore, characteristics that influence the direction or magnitude of the effect of such interventions on cancer-related fatigue need to be identified.
Recently, a three-armed randomized controlled trial compared the effects of two online interventions (a psychologist-guided web-based MBCT (eMBCT) and a physiotherapist-guided ambulant activity feedback (AAF)) to an unguided active control group receiving psycho-education (PE) in severely fatigued cancer patients [4]. Results showed that both eMBCT and AAF were superior to PE in reducing fatigue [4]. This three-armed design provides an unprecedented opportunity to conduct moderation analyses and study for which subgroup of patients what type of intervention (i.e., eMBCT, AAF, or PE) is most beneficial. The aim of the current study is to explore the moderator effects of sociodemographic, clinical, and psychological characteristics on the effect of eMBCT, AAF, and PE on fatigue in patients suffering from CCRF.
Methods
This moderation study was embedded in a three-armed randomized controlled trial [5]. The study was approved by the Twente Medical Ethical Committee (P12-26).
Participants and procedure
Cancer survivors were recruited via various channels (e.g., patient organizations, social media, newspapers, health care professionals). Patients were invited to follow a web-based intervention for their fatigue but did not receive the exact content of the interventions in the advertisements. Inclusion criteria were having finished curative-intent cancer treatment at least 3 months previously after any type of cancer diagnosis, suffering from severe fatigue ever since (≥ 35 on the Checklist Individual Strength—Fatigue Severity [CIS-FS] subscale [4]), being ≥ 18 years old at disease onset, no current or former severe psychiatric comorbidity (e.g., suicidal ideation, psychosis), no current substance abuse, no cancer recurrence during study participation, and no dependence on wheelchair for daily activities.
After providing written informed consent, eligible patients were randomized to eMBCT, AAF, or PE [4]. Participants filled out questionnaires prior randomization (baseline, T0), 1 week after the 9-week intervention period (T1), and at follow-up (6 months, T2).
Interventions
Interventions were similar in duration (9 weeks) and psycho-education content which participants received weekly via a website (eMBCT and AAF) or e-mail (PE). The psycho-education content came from the eMBCT program [7] and included information on fatigue, sleep hygiene, balancing energy during the day, and coping with worrying thoughts. Due to the similarities in duration and psycho-education content, treatment adherence was set at ≥ 6 sessions for all three interventions.
eMBCT
The eMBCT-program is a 9-week web-based psychologist-guided intervention, based on the original MBCT protocol [6] and tailored to patients suffering from CCRF by including cancer- and fatigue-related psycho-education, and adapted movement exercises [7]. The treatment material consisted of nine modules, which the patient could consult by logging on to a password-secured website. Each module involved information about mindfulness, psycho-education on fatigue, and audio-guided mindfulness exercises (body scans, sitting meditations, gentle yoga exercises, and walking meditations). Patients were encouraged to practice and fill out their experiences in a diary on a daily basis. On an agreed-upon day of the week, the therapist replied to these diary entries, thereby guiding the patient through the program.
AAF
AAF is a 9-week web-based physiotherapist-guided protocolled intervention [8] using an activity coaching system that consists of the patient’s smartphone and an accelerometer. Patients set personal activity goals together with the therapist (i.e., (1) activate: becoming more active; (2) temper: taking rest in time; (3) balance: balancing activity and rest throughout the day and especially conserve energy in the morning). The coaching system supports patients in meeting these goals by showing real-time feedback about the accumulated activity relative to a personalized line of reference. Participants received tailored messages on their smartphone in order to increase, decrease, or balance their daily activities in ways that improve their energy levels. The feedback was not coupled to exercise training but to activities that can be easily performed in and around the house or office. For example, depending on the current deviation from the reference line, activating feedback messages included proposed behaviors such as “a nice stroll” or “a brisk walk” while tempering feedback messages included proposed behaviors such as “reading the newspaper.” Via a password-secured website, patients received weekly psycho-education on fatigue. On this website, participants described their experiences in a diary. On an agreed-upon day of the week, the therapist replied to these diary entries, thereby guiding the patient through the program.
PE
PE involved reading weekly non-reply emails for a period of 9 weeks, including the psycho-education on fatigue. In contrast to eMBCT and AAF, adherence was not actively monitored in PE. Only when participants informed the researcher they wanted to drop out before the sixth session, they were considered non-adherent.
Measures
Outcome measure
Fatigue severity was assessed with the 8-item CIS-FS [9]. The CIS has been validated, showed good psychometric properties, and has often been used with cancer survivors [6].
Moderators
Sociodemographic and clinical moderators were assessed via self-report at T0: gender, age, education level, cancer type, and fatigue duration. Baseline fatigue severity was assessed with the CIS-FS [10]. Fatigue catastrophizing was assessed with the Fatigue Catastrophizing Scale (FCS) [6], which showed good psychometric properties and has previously been used in cancer survivors [11]. Sense of control over fatigue (confidence about one’s capacity to change fatigue) was measured with the self-efficacy scale (SE28), specifically adapted for people suffering from chronic fatigue [12]. Perceived activity and concentration were assessed with the 3-item activity subscale and the 5-item concentration subscale of the CIS [12]. Acceptance (non-reactive attitude to inner experiences) and presence (focus on current experiences) were assessed with the 8-item acceptance and 6-item presence subscale of the Freiburg Mindfulness Inventory (FMI) [6], which has been validated in cancer survivors, showing good psychometric properties [13]. The 12-item Multidimensional Scale of Perceived Social Support (MSPSS) assessed social support, which shows good psychometric properties [13, 14].
Statistical analysis
Analyses were based on complete cases of the treatment-adherent sample (participation in ≥ 6 sessions). Using the SPSS macro PROCESS [15], we evaluated a set of linear regression models to examine whether the effect of treatment (eMBCT vs. AAF vs. PE) on the outcome at T2 (fatigue severity) was moderated by the moderators at T0. Following recommendations by Hayes [16], we used the T0 measure of the outcome as a covariate to correct for individual differences at baseline. The multi-categorical independent variable for treatment condition was dummy-coded, with “0” indicating absence and “1” indicating presence of each treatment condition. eMBCT served as the reference condition. Both dummy variables representing the independent variable of treatment, the moderator, and the two resulting interaction terms between each dummy variable and the moderator were included in the analyses. A significant increase in explained variance (ΔR2) resulting from adding the product terms to the model already containing the dummy variables and the main effect of the moderator was considered evidence for moderation [16].
Simple slope analysis was used to interpret the significant moderation effects [17]. PROCESS for SPSS provides omnibus tests, indicating if there is a difference in estimated outcome between the conditions at three different values of the moderator: 1 SD below the mean (“low”), the mean (“moderate”), and 1 SD above the mean (“high”). When the omnibus test for a specific level of the moderator was significant, pairwise inferences explored if eMBCT differed from AAF or PE at that level of the moderator.
Results
Study sample
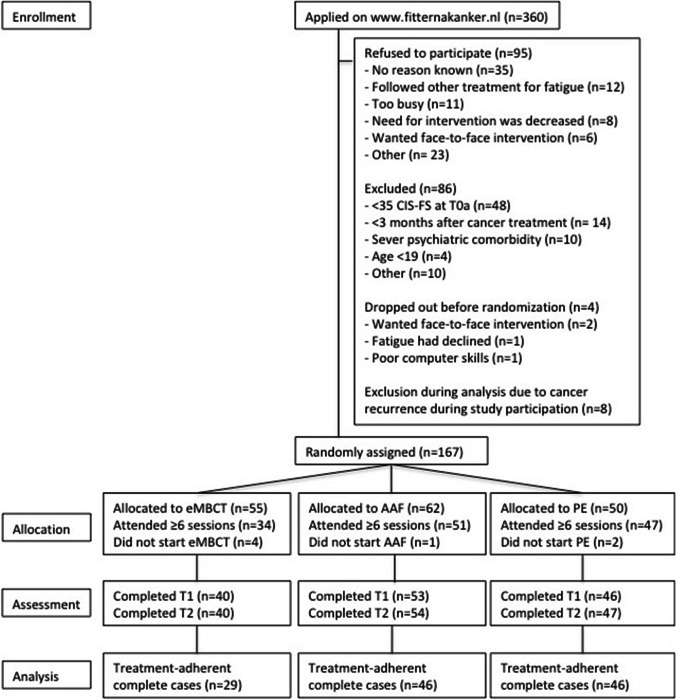
Of the 360 people who applied on the website between March 2013 and June 2015, 95 (26%) refused to participate, 86 (24%) were excluded, 4 (1%) dropped out additionally before randomization, and 8 (2%) were excluded prior analysis due to cancer recurrence during study participation, resulting in 167 participants. See Fig. 1 for participant flowchart. Due to a temporary error in the website’s randomization algorithm, participants were not allocated 1:1:1 for a period of 6 months, resulting in unequal sample sizes for the conditions: eMBCT (n = 55), AAF (n = 62), and PE (n = 50). Of those 167 participants, 132 (79%) were considered treatment-adherent (participated in ≥ 6 sessions). Reasons for dropping out the interventions included lack of confidence the intervention would be effective, no desire for treatment because fatigue had already reduced considerably during the first sessions, and preference for face-to-face contact. Specific reasons for dropping out of eMBCT included the high intensity of the program and difficulties using the eMBCT portal. Reasons for dropping out of AAF were mainly technical problems and poor usability of the accelerometer. Intervention dropout was higher in eMBCT (21/55; 38%) than in AAF (11/62; 18%) and PE (3/50; 6%), which is probably due to the high intensity of the eMBCT program, including daily mindfulness practice. Adherent patients did not differ from non-adherent patients regarding baseline characteristics.
Fig. 1.
Flowchart of participants
Eventually, 121 participants (92%) of the treatment-adherent sample completed the T2 assessment: eMBCT (n = 29), AAF (n = 46), or PE (n = 46). Baseline characteristics of the complete cases of the treatment-adherent sample are shown in Table 1. No significant differences were found between interventions, with the exception that eMBCT participants were more severely fatigued at baseline than PE participants (p = 0.021).
Table 1.
Baseline characteristics of treatment-adherent sample with complete data
| eMBCT (n = 29) | AAF (n = 46) | PE (n = 46) | ||
|---|---|---|---|---|
| Demographic characteristics, n (%) | ||||
| Gender | Female | 18 (62.1) | 33 (71.7) | 37 (80.4) |
| Male | 11 (37.9) | 13 (28.3) | 9 (19.6) | |
| Age, M (SD) | 53.24 (11.13) | 58.02 (9.57) | 56.15 (8.66) | |
| Education levelab | Low/middle | 10 (34.5) | 24 (52.2) | 16 (34.8) |
| High | 19 (65.5) | 21 (45.7) | 30 (65.2) | |
| Clinical characteristics, n (%) | ||||
| Type of cancer | Breast | 12 (41.4) | 18 (39.1) | 26 (56.6) |
| Other | 17 (58.6) | 28 (60.9) | 20 (43.5) | |
| Length of fatiguea | < 2 years | 9 (31.0) | 17 (37.0) | 22 (47.8) |
| > 2 years | 20 (69.0) | 28 (60.9) | 24 (52.2) | |
| Psychological characteristics, M (SD) | ||||
| Fatigue severity (CIS-FS)c | 44.56 (6.79) | 42.46 (6.60) | 40.15 (9.35) | |
| Fatigue catastrophizing (FCS) | 21.99 (5.36) | 22.05 (5.97) | 21.40 (5.64) | |
| Sense of control over fatigue (SE28) | 17.52 (2.42) | 18.15 (2.04) | 18.04 (2.80) | |
| Perceived activity (CIS-A) | 10.38 (4.83) | 10.33 (3.77) | 10.98 (5.27) | |
| Concentration (CIS-C) | 16.52 (6.96) | 18.59 (7.21) | 19.24 (7.56) | |
| Acceptance (FMI-A) | 20.59 (3.73) | 21.63 (3.97) | 22.52 (4.81) | |
| Presence (FMI-P) | 16.79 (3.56) | 17.59 (3.11) | 18.30 (3.48) | |
| Social support (MSPPS) | 5.31 (1.18) | 5.75 (0.94) | 5.68 (1.24) | |
aIn AAF, n = 1 is missing; blow/middle = primary and secondary education; high = higher vocational training and university; ceMBCT participants reported a higher level of fatigue severity than PE participants (p = 0.021)
Moderation
Table 2 shows the results of the linear regression models to determine moderation of treatment effect. In all analyses, there was a significant main effect of intervention on fatigue severity at T2, indicating that eMBCT and AAF outperformed PE in reducing fatigue severity. None of the moderation effects resulted in a significant increase in explained variance of T2 fatigue severity. Baseline fatigue severity (p = 0.066) and fatigue catastrophizing (p = 0.084) did show a trend towards an overall moderation effect.
Table 2.
Overall tests of moderation effects (the added value of the moderator x intervention interactions) on fatigue severity at T2. Moderation effects are corrected for the main effect of intervention, the main effect of the moderator, and baseline fatigue severity
| Full model | Overall test of moderation effect | |||||
|---|---|---|---|---|---|---|
| Moderator | F (df) | p | R2 | F (df) | p | R2-change |
| Gender (ref: males) | 6.59 (6, 114) | < 0.001 | 0.258 | 0.41 (2, 114) | 0.665 | 0.005 |
| Age | 6.56 (6, 114) | < 0.001 | 0.257 | 0.31 (2, 114) | 0.734 | 0.004 |
| Education level (ref: low/middle) | 6.86 (6, 113) | < 0.001 | 0.267 | 0.88 (2, 113) | 0.417 | 0.011 |
| Type of cancer (ref: other) | 7.52 (6, 114) | < 0.001 | 0.283 | 2.15 (2, 114) | 0.121 | 0.027 |
| Length of fatigue (ref: < 2 years) | 6.74 (6, 113) | < 0.001 | 0.264 | 0.41 (2, 113) | 0.661 | 0.005 |
| Fatigue severity at T0 | 9.24 (5, 115) | < 0.001 | 0.287 | 2.77 (2, 115) | 0.066 | 0.035 |
| Fatigue catastrophizing | 7.55 (6, 114) | < 0.001 | 0.285 | 2.53 (2, 114) | 0.084 | 0.032 |
| Sense of control over fatigue | 6.63 (6, 114) | < 0.001 | 0.259 | 0.05 (2, 114) | 0.949 | < 0.001 |
| Perceived activity | 6.41 (6, 114) | < 0.001 | 0.252 | 0.01 (2, 114) | 0.988 | < 0.001 |
| Concentration | 7.00 (6, 114) | < 0.001 | 0.269 | 1.17 (2, 114) | 0.316 | 0.015 |
| Acceptance | 6.80 (6, 114) | < 0.001 | 0.264 | 0.59 (2, 114) | 0.555 | 0.008 |
| Presence | 6.74 (6, 114) | < 0.001 | 0.262 | 0.59 (2, 114) | 0.558 | 0.008 |
| Social support | 7.40 (6, 114) | < 0.001 | 0.280 | 2.17 (2, 114) | 0.119 | 0.027 |
Regarding baseline fatigue severity (Fig. 2), the omnibus tests showed that T2 fatigue severity differed between interventions for participants with low (F(2, 115) = 5.87, p = 0.004), moderate (F(2, 115) = 10.53, p < 0.001), and high (F(2, 115) = 6.70, p = 0.002) fatigue levels. Pairwise inferences showed that at low to moderate (CIS-FS = 34.16–42.09) baseline fatigue, eMBCT was outperformed by AAF (B = − 9.44 (3.55), p = 0.009; B = − 4.78 (2.09), p = 0.024, respectively); no differences between eMBCT and PE. At high (CIS-FS = 50.01) baseline fatigue, eMBCT outperformed PE (B = 8.35 (2.73), p = 0.003); no differences between eMBCT and AAF.
Fig. 2.
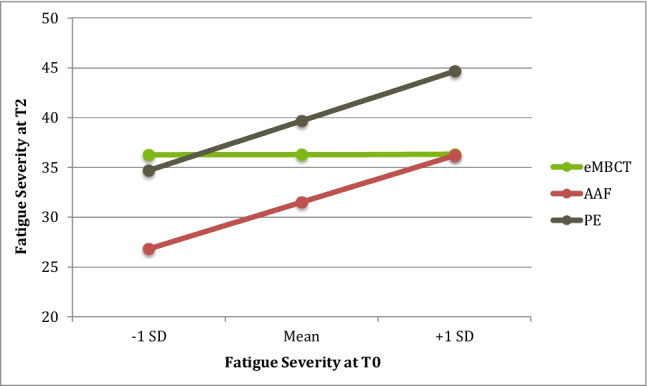
The effect of treatment on T2 fatigue severity at different levels (− 1 SD, Mean, + 1 SD) of the moderator baseline fatigue severity
Regarding fatigue catastrophizing (Fig. 3), the omnibus tests showed that T2 fatigue severity differs between interventions at low (F(2, 114) = 3.22, p = 0.044), moderate (F(2, 114) = 9.88, p < 0.001), and high catastrophizing levels (F(2, 114) = 9.31, p < 0.001). Pairwise inferences showed that at low catastrophizing levels (FCS = 16.12), eMBCT was outperformed by AAF (B = − 6.04 (2.98), p = 0.045); no differences were found between eMBCT and PE. At moderate to high (FCS = 21.79–27.46) catastrophizing levels, eMBCT outperformed PE (B = 4.30 (2.07), p = 0.040; B = 9.05 (2.95), p = 0.003, respectively); no differences were found between eMBCT and AAF.
Fig. 3.
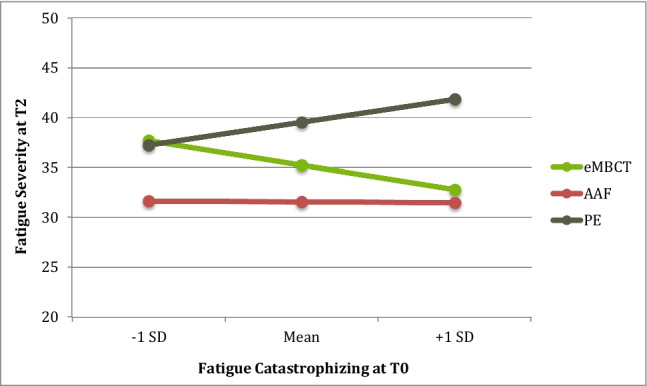
The effect of treatment on T2 fatigue severity at different levels (− 1 SD, Mean, + 1 SD) of the moderator fatigue catastrophizing
Discussion
The present study explored whether baseline sociodemographic, clinical, and psychological characteristics moderated the effect of eMBCT, AAF, and PE on 6-month fatigue severity. Some indication was found (marginally significant effect) that baseline fatigue severity and fatigue catastrophizing moderated the intervention effect on fatigue. None of the other characteristics moderated the intervention effect.
Regarding baseline fatigue severity, additional analyses suggested that in case of high fatigue, patients benefited more from eMBCT than from PE. These findings are in line with a recent individual patient data meta-analysis, showing that cancer patients with clinically relevant fatigue levels benefit more from psychosocial interventions than patients with non-clinical fatigue [17]. In the present study, all patients reported clinically relevant fatigue at baseline, indicating that in cases of “extreme” fatigue, eMBCT outperformed PE. Interestingly, when fatigue was less extreme but still clinically significant, AAF outperformed eMBCT, implying it seems more beneficial for patients to receive support on balancing activities in order to improve their energy levels, rather than train their awareness to help them cope with fatigue. Regarding fatigue catastrophizing, additional analyses suggested that at low catastrophizing levels, patients benefited more from AAF than from eMBCT, and at high catastrophizing levels, patients benefited more from eMBCT than from PE. Previous studies have shown that catastrophizing is an important predictor of fatigue severity and is linked to more daily life interference due to the fatigue [10]. Following these results, our findings suggest that when people catastrophize more, more personal support is warranted and psycho-education does not seem to offer enough support. A more intensive intervention, such as eMBCT or AAF, is required to help patients cope with fatigue. Similar to the moderating trend of baseline fatigue severity, patients benefit more from AAF than from eMBCT when patients catastrophize less. At these lower levels of catastrophzing, potentially less psychological factors might influence the CCRF experience and as such it can be more beneficial for patients to focus on balancing activities (AAF), rather than participate in a psychosocial intervention, such as eMBCT.
Methodological issues
The design of this three-armed RCT in severely fatigued cancer patients provided a unique opportunity to study for which subgroup of patients what intervention is most beneficial. However, when interpreting the findings, the small sample size needs to be taken into account. The study was not powered to conduct moderation analysis and several patients did not adhere to the intervention, resulting in a small sample size. This raises the concern whether the negative findings are due to the study being underpowered rather than the effects being absent. This power issue also needs to be taken into account when interpreting the marginally significant moderation effects of baseline fatigue severity and fatigue catastrophizing. In light of these findings, it should also be noted that, probably due to the high intensity of the program, dropout appeared larger in eMBCT than in the other interventions, leading to a relatively small sample size in the eMBCT arm. Moreover, eMBCT patients reported higher fatigue levels at baseline than patients in AAF and PE. However, by including baseline fatigue in the moderation analysis, we controlled for this baseline difference between conditions. In addition, as with other psycho-oncology research, the majority of participants were middle-aged breast cancer patients. Moreover, the sample was mainly self-selected, resulting in a group of motivated participants. Although this is mostly in line with the characteristics of cancer patients seeking psychosocial support [18], this might limit generalizability to patients with other cancer types who are less motivated to participate in an online fatigue intervention.
Person-based approach
The present moderation study provided limited insight into “what works best for whom?” A larger sample size could have helped us to better determine relevant moderators. However, a more person-centered approach might be more appropriate to answer this question. For example, a structured diary technique, such as the experience sampling method, in which participants receive questions multiple times a day for multiple days on end about their symptoms, thoughts, and feelings, allows closely monitoring of fatigue in patients’ daily living environment [19]. This results in an intensive longitudinal dataset, making it possible to examine the interactions between symptoms, cognitions, emotions, and behavior in a detailed, ecologically valid manner at the level of the individual patient [20]. The network approach offers a new way to gain insight into an individual’s symptom dynamics. It theorizes symptoms as elements of a complex dynamical system in which symptoms can trigger one another (e.g., sleep problems lead to fatigue and concentration problems, resulting in loss of enjoyment, which in turn can trigger a depressed mood) [20]. Such symptom networks can provide new insight into how one copes with fatigue, which could be helpful in determining what treatment would be most suitable for the individual patient [21].
Conclusion
In sum, we found some preliminary evidence that baseline fatigue severity and fatigue catastrophizing moderated the effect of eMBCT versus AAF and PE on fatigue severity in CCRF patients. These findings emphasize the potential gain in effectiveness of personalized treatment. This work could help healthcare professionals to find the right treatment for their patients suffering from CCRF. However, more research is needed to substantiate and improve guidance of personalized treatment for CCRF. The network approach [22] might help us further in answering the question “what treatment works best for whom?”.
Author contribution
Fieke Bruggeman-Everts, Marije Wolvers, Miriam Vollenbroek-Hutten, and Marije van der Lee contributed to the study conception and design. Data collection was performed by Fieke Bruggeman-Everts and Marije Wolvers. Melanie Schellekens analyzed the data and wrote the first draft of the manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The work was supported by Alpe d’Huzes/Dutch Cancer society (project number 2011–5264).
Data availability
The data that support the findings of this study are available from the corresponding author, MS, upon reasonable request.
Code availability
N/A.
Declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Approval was granted by the Twente Medical Ethical Committee (P12-26).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
N/A.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thong MSY, van Noorden CJF, Steindorf K, Arndt V. Cancer-related fatigue: causes and current treatment options. Curr Treat Options Oncol. 2020;21:17. doi: 10.1007/s11864-020-0707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bootsma TI, Schellekens MPJ, van Woezik RAM, et al. Experiencing and responding to chronic cancer-related fatigue: a meta-ethnography of qualitative research. Psychooncology. 2020;29:241–250. doi: 10.1002/pon.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961–968. doi: 10.1001/jamaoncol.2016.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruggeman-Everts FZ, Wolvers MDJ, van de Schoot R, et al. Effectiveness of two web-based interventions for chronic cancer-related fatigue compared to an active control condition: results of the “Fitter na kanker” randomized controlled trial. J Med Internet Res. 2017;19:e336. doi: 10.2196/jmir.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolvers MD, Bruggeman-Everts FZ, Van der Lee ML, et al. Effectiveness, mediators, and effect predictors of internet interventions for chronic cancer-related fatigue: the design and an analysis plan of a 3-armed randomized controlled trial. JMIR Res Protoc. 2015;4:e77. doi: 10.2196/resprot.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vercoulen JHMM, Swanink CMA, Fennis JFM, et al. Dimensional assessment of chronic fatigue syndrome. J Psychosom Res. 1994;38:383–392. doi: 10.1016/0022-3999(94)90099-X. [DOI] [PubMed] [Google Scholar]
- 7.Bruggeman Everts FZ, van der Lee ML, de Jager ME. Web-based individual mindfulness-based cognitive therapy for cancer-related fatigue - a pilot study. Internet Interv. 2015;2:200–213. doi: 10.1016/j.invent.2015.03.004. [DOI] [Google Scholar]
- 8.Wolvers MD, Vollenbroek-Hutten MM (2015) An mHealth intervention strategy for physical activity coaching in cancer survivors. In: Proceedings of the 1st International Workshop on Personalisation and Adaptation in Technology for Health (PATH 2015). 23rd conference on User Modeling, Adaptation and Personalization. CEUR-WS, Dublin
- 9.Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: a new approach to preventing relapse. New York: Guilford Press; 2002. [Google Scholar]
- 10.Abrahams HJG, Gielissen MFM, Schmits IC, et al. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: a meta-analysis involving 12 327 breast cancer survivors. Ann Oncol. 2016;27:965–974. doi: 10.1093/annonc/mdw099. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen PB, Andrykowski MA, Thors CL. Relationship of catastrophizing to fatigue among women receiving treatment for breast cancer. J Consult Clin Psychol. 2004;72:355–361. doi: 10.1037/0022-006X.72.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goedendorp MM, Gielissen MFM, Verhagen CAHHVM, Bleijenberg G. Development of fatigue in cancer survivors: a prospective follow-up study from diagnosis into the year after treatment. J Pain Symptom Manage. 2013;45:213–222. doi: 10.1016/j.jpainsymman.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Walach H, Buchheld N, Buttenmüller V, et al. Measuring mindfulness—the Freiburg mindfulness inventory (FMI) Pers Individ Dif. 2006;40:1543–1555. doi: 10.1016/j.paid.2005.11.025. [DOI] [Google Scholar]
- 14.Bruggeman-Everts FZ, Van der Lee ML, Van ‘tHooft EFM, Nyklíček I. Validation of the Dutch Freiburg mindfulness inventory in patients with medical illness. SAGE Open. 2017;7:2158244017705936. doi: 10.1177/2158244017705936. [DOI] [Google Scholar]
- 15.Zimet GGD, Powell SS, Farley GK, et al. Psychometric characteristics of the multidimensional scale of perceived social support. J Pers Assess. 1990;55:610–617. doi: 10.1207/s15327752jpa5503&4_17. [DOI] [PubMed] [Google Scholar]
- 16.Hayes AF (2017) Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. Guilford publications
- 17.Hayes AF, Montoya AK. A tutorial on testing, visualizing, and probing an interaction involving a multicategorical variable in linear regression analysis. Commun Methods Meas. 2017;11:1–30. doi: 10.1080/19312458.2016.1271116. [DOI] [Google Scholar]
- 18.Lukkahatai N, Saligan LN. Association of catastrophizing and fatigue: a systematic review. J Psychosom Res. 2013;74:100–109. doi: 10.1016/j.jpsychores.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garssen B, Van der Lee M, Van der Poll A, et al. Characteristics of patients in routine psycho-oncological care, and changes in outcome variables during and after their treatment. Psychol Health. 2016;31:1237–1254. doi: 10.1080/08870446.2016.1204447. [DOI] [PubMed] [Google Scholar]
- 20.Csikszentmihalyi M, Larson R. Validity and reliability of the experience-sampling method. J Nerv Ment Dis. 1987;175:526–536. doi: 10.1097/00005053-198709000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Borsboom D, Cramer AOJ. Network analysis: an integrative approach to the structure of psychopathology. Annu Rev Clin Psychol. 2013;9:91–121. doi: 10.1146/annurev-clinpsy-050212-185608. [DOI] [PubMed] [Google Scholar]
- 22.van der Lee ML, Schellekens MPJ. Capturing the complexity of mental disorders in the medically ill: the network approach on behavioral medicine. Transl Behav Med. 2020;10:812–816. doi: 10.1093/tbm/ibz019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, MS, upon reasonable request.
N/A.