Abstract
Concentrations of the hormone leptin, which is produced by adipose tissue, increase with increasing BMI, whereas leptin sensitivity often declines with higher BMI. Thus, altered leptin signaling may play a role in reproductive health risks observed with increasing BMI, which include later onset and slow progression of labor. Conflicting evidence from clinical, animal and in vitro studies have suggested that leptin either promotes or inhibits labor. We hypothesized that serum leptin concentrations or serum leptin: body mass index (BMI) ratios in women may be associated with the initiation and progression of labor. Following informed consent, serum samples were collected from 90 women with singleton pregnancies at the time of routine glucose-challenge testing, for measurement of leptin. The potential influence of leptin on gestation length and cervical dilation timing were examined by multiple linear regression. Data were analyzed from 63 participants who met exclusion and inclusion criteria. Leptin concentrations (log-transformed) at 24–28 weeks gestation were not significantly correlated with first trimester BMI . Log serum leptin and leptin: BMI ratio each were significantly associated with shorter total gestation length in uncomplicated, term pregnancies. In contrast, the mid-pregnancy leptin concentrations were not associated with progression of labor, assessed by cervical dilation over time. The association between higher serum leptin and shorter gestation length is consistent with the hypothesis that leptin promotes, or is permissive for, the onset of labor.
Subject terms: Reproductive biology, Reproductive signs and symptoms
Introduction
The hormone leptin is produced by adipose tissue in proportion to overall fat mass and it serves as a signal to the reproductive system of adequate nutritional status, acting as a permissive factor for puberty, cyclicity, and pregnancy1,2. Circulating leptin levels increase with increasing body weight in men, and in both pre- and post- menopausal women3. Thus, some individuals with higher adipose mass are chronically exposed to high leptin levels and become resistant to this hormone4. Even the placenta can become leptin resistant in women with high BMI. For example, leptin receptor expression and leptin-stimulated amino acid transport were significantly reduced in placentas from women with BMI ≥ 30 kg/m2 vs. those from women with BMI 18.5–24.9 kg/m24,5. As a result, pregnant women with high BMI have high circulating leptin levels, but may have reduced leptin signaling. These alterations in leptin concentration and function at high BMI suggest that leptin could play a role in reproductive health risks observed at high BMI.
Over a third of women of childbearing age in the United States have a body mass index (BMI) > 30, and they are at increased risk for slow progression of labor, as well as later onset of labor or longer overall gestation length6–8. Women with a BMI > 30 kg/m2 are at significantly greater risk of having gestation extended past 41–42 weeks6,9,10. The duration of labor is also extended with increasing BMI in several reports11,12. For example, in a study of 612 nulliparous women, the median cervical dilation time from 4 to 10 cm for those with BMI > 29 was significantly longer at 7.9 h versus just 6.2 h in those with a BMI ≤ 26 and 7.5 h for those with a BMI 26.1–2913 and Bishop Scores for cervical ripening were lower upon admission for women with BMI of 30 or greater12. Sensitivity to prostaglandins, which promote cervical ripening, is also reduced in women with BMI > 3014The incidence of intra partum caesarean section for labor dystocia is also higher at higher BMI15–17, Although uterine contractility is reduced18, and even differences in the maternal serum metabolome7 have been observed, the physiological mechanisms that underlie delays in the onset and progression of labor with increasing BMI remain poorly understood. At the same time, the incidence of preterm birth is also higher in women with BMI > 30. The relationship between BMI and preterm birth is U-shaped, with the increasing risk of preterm birth for BMIs above or below the 18–22 range, highlighting the complicated relationship between BMI and initiation of labor19–21.
There is limited, sometimes contradictory, evidence for a role of leptin in the onset and/or progression of labor. Evidence that leptin promotes the onset of labor includes a study in thethe Lepob/ob mouse, which cannot produce leptin, and is infertile. When leptin is given to the Lepob/ob mouse to restore estrous cycles and initiate pregnancy but then withdrawn prior to term, gestation and labor are prolonged22. Similarly, when leptin receptor is knocked out in progesterone receptor-expressing tissues, the average time to deliver each pup is lengthened, a greater number of stillborn pups are observed, and there is increased risk of dystocia23 Consistent with these observations, leptin injections promote cervical softening in the non-pregnant, hormone-primed rat24. Collectively, these studies suggest that leptin ensures that delivery can progress at the appropriate time, and raise the possibility that greater leptin signaling could hasten delivery.
However, evidence from other studies suggests that leptin may delay or slow labor, or have no effect. In mice with just one fully functional copy of the leptin receptor gene, myometrial contractions are not different than those in control mice, although the underlying signaling changes25. In samples from both women and rodent models, incubation of myometrial strips with leptin inhibits contractility, although it should be noted that inhibition occurs at superphysiological concentrations of 1 µM26–29. Cord blood leptin concentrations, reflecting fetal rather than maternal leptin, are positively associated with longer duration of labor30,31. Mid-gestation leptin levels were also significantly higher in women with failed induction of labor in one study, but after adjustment for BMI, were not associated with failed first stage of labor in another32,33. Thus, the question of whether leptin modulates gestation length or progression of labor remains open.
The goal of the present study is to test whether maternal serum leptin concentrations, measured in mid-pregnancy, are significantly associated with the onset of labor, as measured by gestation length, or with the progression of labor, measured by the timing of cervical dilation.
Methods
Patient population
The study was approved by the University of Missouri Health Sciences Institutional Review Board, and all participants provided written informed consent. It was conducted in accordance with all relevant regulations for Human Subjects research, and the Declaration of Helsinki. Women ≥ 18 years of age bearing a singleton pregnancy were recruited from the University of Missouri Women’s and Children’s Hospital, Smiley Lane Clinic, and Missouri OB/GYN Associates in Columbia, Missouri at a regularly scheduled, second trimester obstetric visit prior to oral glucose challenge testing.
Participant data were included in analysis if a body mass index had been recorded in the electronic medical record during the first trimester of pregnancy. Criteria for exclusion from analysis included those conditions that might modify the labor process or shorten gestation length: multiple gestation pregnancy, gestational hypertension, pre-eclampsia, preterm premature rupture of membranes (PPROM), premature rupture of membranes, scheduled or planned cesarean delivery, or fetal malpresentation. Additionally, those who underwent induction of labor by administration of oxytocin (Pitocin), prostaglandin, or manual rupture of membranes for post-term pregnancy as documented in the medical record were excluded from analysis, as these inductions would alter the length of gestation and could affect the rate of cervical dilation34,35. Routine administration of Pitocin to augment uterine contractions was not excluded, but was accounted for in the statistical analysis of cervical dilation timing.
Data on fetal sex, parity, induction of labor, labor augmentation, maternal age, first trimester BMI, gestational weight gain and cervical dilation timing were collected from the electronic medical record.
Serum leptin determination
During routine glucose challenge testing at 24–28 weeks gestation, following overnight fast, an additional blood sample was taken for leptin measurement at 1 h post glucose challenge. The samples were centrifuged for 15 min at 1000 × g for serum separation, and then serum was stored at -80 °C until use. The Human Leptin Quantikine ELISA kit (R&D Systems) was used according to manufacturer’s protocol. Serum samples were diluted 100-fold to fit within the assay range. Briefly, both serum samples and leptin standards were diluted, and added to the coated microplate. After washing, wells were incubated with human leptin conjugate solution for 1 h, and then washed before incubating with substrate solution for an additional 30 min. Results were read on a Biotek plate spectrophotometer (Winooski, VT). To validate the assay, serial dilutions of serum were used to confirm linearity, and known quantities of the leptin standard solution provided with the kit were added to serum samples to assess recovery. The intraassay and interassay coefficients of variation (CV%) were 2.5% and 7.1%, respectively.
Statistical analysis
A sample size calculation was performed for multiple regression analysis by using G*Power Software36. For a power of 0.8, alpha = 0.05, it predicted that 68 subjects would be needed to detect an effect size, F2, of 0.15 on gestation length, the primary outcome, in a regression model with two predictors, where both predictors and dependent variables are continuous. The effect size was calculated by using Cohen’s F2, where F2 = R2/(1-R2)37, with R2 estimated from the impact of BMI on gestation length in multiple regression analyses6,38,39. Additionally, Cohen defines 0.15 as a “medium” effect size37. It was estimated that approximately 25% of patients recruited in mid-pregnancy would be lost to follow-up or develop one of the criteria for exclusion from analysis prior to study completion. Therefore, a goal of recruiting 90 participants was established.
Descriptive statistics were used to summarize patient data using frequency and percentages for categorical variables, and mean ± standard deviation (SD) and the median with interquartile range (IQR) for continuous variables. A linear regression was used to assess the relationship between BMI and serum leptin. As leptin concentrations and leptin:BMI ratios were not normally distributed, these variables were log-transformed to achieve normal distributions. Separate generalized linear regression models were used to assess the effect of either log (leptin) or log (leptin:BMI) on gestation length, controlling for the following confounding variables: 1st trimester BMI, maternal age, gestational weight gain, fetal sex, Pitocin administration and parity. Stepwise backward selection was used to sequentially eliminate variables with the largest p-values to obtain the final regression models.
For analysis of progression of labor, the time at which full effacement or 10 cm cervical dilation was noted in the medical record was considered time 0 for each patient, and each prior recorded observation of cervical dilation was expressed relative to time 040. A generalized estimating equations model (GEE) was used to fit a quadratic model describing the cervical dilation for all subjects, taking repeated measures within subject into account.
All tests were two-sided, with a level of significance 0.05. SAS 9.4® software was used for statistical analysis of data.
Ethics approval and consent to participate
The study was approved by the University of Missouri Health Sciences Institutional Review Board, and all participants provided written informed consent.
Results
Data stratification
Ninety subjects were enrolled and data from nineteen of them were excluded from analysis due to complications considered exclusion criteria, as listed in Materials and Methods (Fig. 1). Leptin data were missing for one subject and BMI values were missing for two additional subjects. Additionally, data from five participants were excluded from analysis due to induction of labor for post-term pregnancy. Data from the remaining 63 subjects were analyzed (Fig. 1). Patient characteristics are displayed in Table 1 for continuous variables, and Table 2 for categorical variables. The mean length of gestation, 275.9 days, is equivalent to 39.4 weeks.
Figure 1.
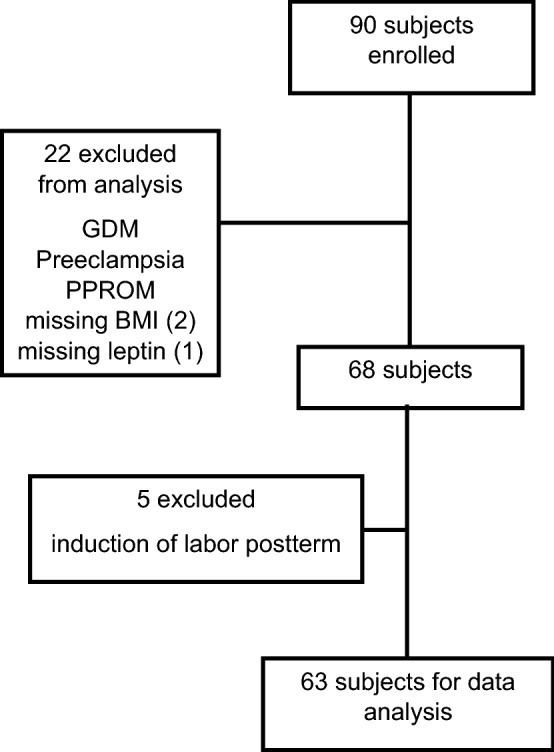
Number of participants enrolled and included in analysis. GDM = gestational diabetes mellitus. PPROM = preterm premature rupture of membranes.
Table 1.
Patient characteristics for continuous variables (N = 63).
| Variable | Mean ± SD | Median | Interquartile range |
|---|---|---|---|
| Maternal age (years) | 27.8 ± 5.0 | 28 | 24–31 |
| 1st trimester body mass index (kg/m2) | 27.0 ± 5.7 | 25 | 23–31 |
| Gestational weight gain (kg) | 11.3 ± 6.0 | 11.5 | 8.4–15.7 |
| Leptin (ng/mL) | 43.9 ± 47.2 | 32.6 | 21.2–45.6 |
| Leptin: BMI ratio | 1.66 ± 1.9 | 1.15 | 0.69–1.7 |
| Length of gestation (days) | 275.9 ± 5.2 | 275 | 273–279 |
Table 2.
Patient characteristics, categorical variables.
| Variable | Frequency (%) | Cumulative frequency (%) |
|---|---|---|
| Parity | ||
| 0 | 32 (50.8%) | 32 (50.8%) |
| 1 | 21 (33.3%) | 53 (84.1%) |
| ≥ 2 | 10 (15.9%) | 63 (100.0%) |
| Fetal sex | ||
| Male | 37 (58.7%) | 37 (58.7%) |
| Female | 26 (41.3%) | 63 (100.0%) |
| Pitocin | ||
| Yes | 34 (54%) | 34 (54%) |
| No | 29 (46%) | 63 (100.0%) |
| BMI category | ||
| < 25 | 28 (44.4%) | 28 (44.4%) |
| 25–29.9 | 16 (25.4%) | 45 (69.8%) |
| ≥ 30 | 19 (30.2%) | 63 (100.0%) |
Relationship between first-trimester BMI and mid-gestation leptin concentrations
The mean leptin concentration at 24–28 weeks gestation for all participants was 43.9 ± 47.2 ng/mL. Serum leptin was not significantly dependent upon first trimester BMI (Fig. 2; β = 0.02, p = 0.18). When BMI was stratified by category, there were no significant differences in leptin concentration amongst those with a BMI less than 25 kg/m2, a BMI between 25 and 29.9 kg/m2, and a BMI of 30 kg/m2and higher (F-value = 1.85, p = 0.16).
Figure 2.
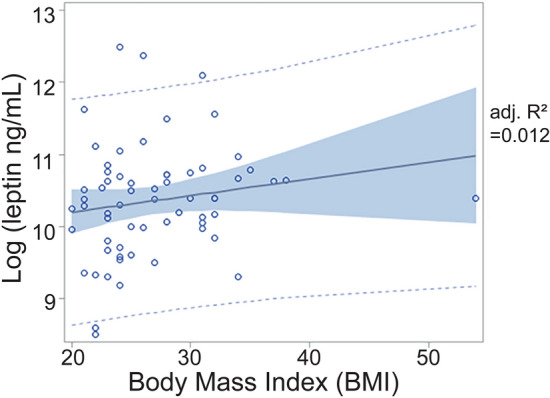
Relationship between body mass index (BMI) in the first trimester and mid-gestation serum leptin concentrations. Leptin concentrations are log-transformed to achieve a normal distribution. There is a weak relationship (β = 0.02, p = 0.18). Each circle represents a single observation, the solid line is the best fit line, and the shaded area indicates the 95% confidence interval.
Relationship between mid-gestation leptin concentrations and length of gestation
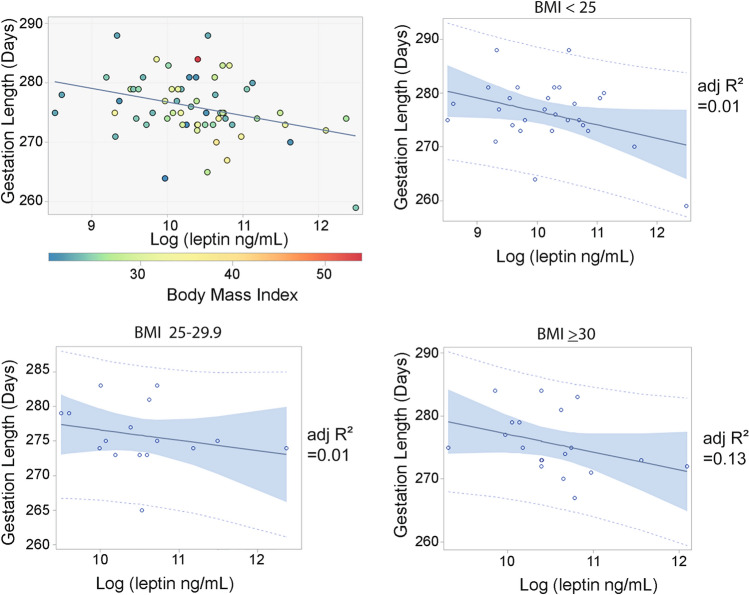
In generalized linear regression analysis, including the variables maternal age, 1st trimester BMI, gestational weight gain, fetal sex, Pitocin administration and parity, only serum leptin concentrations showed a significant, negative correlation with gestation length (p = 0.013, model p = 0.26). Backward selection was performed to remove the non-signficant variables leaving a significant final regression model that included log leptin, gestation weight gain and fetal sex, with log leptin as the most significant predictor (Table 3, Fig. 3a). In a subgroup analysis, the relationship between serum leptin concentration and gestation length was visualized within each BMI category (Fig. 3b–d). Although the sample size of each subcategory was not large enough to draw conclusions, the relationship between leptin and gestation length did not appear to differ between categories.
Table 3.
Final generalized linear model assessing the effect of log leptin and potentially confounding variables on length of gestation after stepwise backward selection.
| Model | N = 63 | R2 = 0.128 | p = 0.043 |
|---|---|---|---|
| Parameter | Estimate | 95% CI | p-value |
| Gestational weight gain (kg) | 0.10 | − 0.12–0.32 | 0.44 |
| Log [leptin (ng/mL)] | − 2.21 | − 4.90 to − 0.53 | 0.0071 |
| Fetal sex (female vs male) | 0.70 | − 1.98–3.38 | 0.60 |
Significant values are in bold.
Figure 3.
(a) Relationship between mid-gestation serum leptin concentrations and total gestation length in days. Leptin concentrations are log-transformed to achieve a normal distribution. For each individual, BMI is indicated by marker color. Gestation length is significantly related to log leptin (p = 0.01) (b) Relationship between serum leptin concentrations and length of gestation for individuals with BMI < 25 (c) Relationship between serum leptin concentrations and length of gestation for individuals with BMI 25–29.9 (d) Relationship between serum leptin concentrations and length of gestation for individuals with BMI > 30. Each circle represents a single observation, the solid line is the linear regression, and the shaded area indicates the 95% confidence interval.
A generalized regression model was also constructed using leptin:BMI ratio, or BMI-adjusted leptin concentrations, maternal age, BMI, gestational weight gain, fetal sex, oxytocin and parity as predictors of length of gestation (p = 0.27). After backward selection, a final regression model with log leptin:BMI ratio, gestational weight gain and fetal sex as variables showed a significant relationship with gestation length (Table 4, Fig. 4).
Table 4.
Final generalized linear model assessing the effect of log leptin:BMI ratio and potentially confounding variables on length of gestation after stepwise backward selection.
| Model | N = 63 | R2 = 0.139 | p = 0.031 |
|---|---|---|---|
| Parameter | Estimate | 95% CI | p-value |
| Gestational Weight Gain (kg) | 0.12 | − 0.10–0.33 | 0.44 |
| Log [Leptin (ng/mL): BMI (kg/m2)] | − 2.37 | − 4.07 to − 0.66 | 0.0048 |
| Fetal Sex (Female vs Male) | 0.73 | − 1.92–3.39 | 0.58 |
Significant values are in bold.
Figure 4.
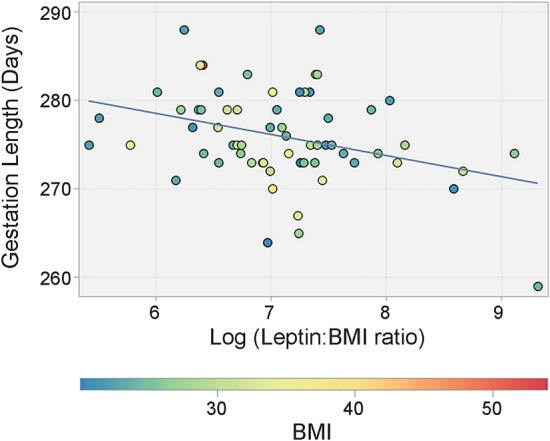
Relationship between mid-gestation serum leptin concentrations, adjusted for BMI, and total gestation length in days. The leptin:BMI ratio is log-transformed to achieve a normal distribution. For each individual, BMI is indicated by marker color.
Relationship between serum leptin concentrations and progression of labor
A generalized estimation model was used to assess the relationship between serum leptin concentration and the progression of labor, controlling for confounding variables (Fig. 5, Table 5). Leptin was not a significant determinant of the labor curve, nor was there any significant relationship with maternal age, gestational weight gain, or oxytocin administration. While the relationships were not statistically significant, there was some indication that BMI, fetal sex and parity influenced cervical dilation.
Figure 5.
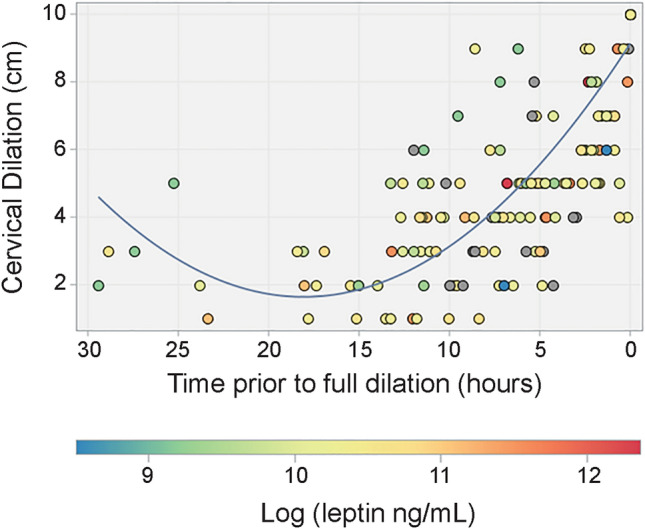
The timing of cervical dilation across all subjects. Time 0 represents the time at which full dilation or 10 cm was achieved. Each marker represents one observation of cervical dilation. The color of each marker indicates log (leptin). Leptin was not a significant factor in timing of cervical dilation (β = −0.06, p = 0.7) when controlling for confounding variables.
Table 5.
Generalized estimating equations model assessing the effect of log of leptin and potential confounding variables on cervical dilation over time.
| Model | N = 63 | ||
|---|---|---|---|
| Parameter | Estimate | 95% CI | p-value |
| Cervical dilation time | −0.823 | −0.939 to −0.708 | < .0001 |
| Cervical dilation time2 | 0.021 | 0.015–0.028 | < .0001 |
| Maternal age | −0.025 | −0.092–0.042 | 0.46 |
| 1st trimester body mass index (kg/m2) | −0.024 | −0.054–0.006 | 0.12 |
| Gestational weight gain (kg) | −0.009 | −0.044–0.026 | 0.61 |
| Log leptin (ng/mL) | −0.058 | −0.401–0.284 | 0.74 |
| Fetal sex (female vs male) | 0.460 | −0.127–1.046 | 0.12 |
| Pitocin (no vs yes) | −0.014 | −0.591–0.562 | 0.96 |
| Parity | |||
| 0 vs ≥ 2 | 0.477 | −0.264–1.218 | 0.21 |
| 1 vs ≥ 2 | −0.380 | −1.01–0.246 | 0.23 |
Discussion
The major finding of this study is that serum leptin concentrations at 24–28 weeks, especially BMI-adjusted leptin concentrations, are negatively associated with gestation length. This suggests that leptin may have a role in promoting the initiation of labor. In contrast, no relationship was observed between serum leptin and cervical dilation time.
Amongst the 63 subjects in this study, there was only a weak relationship between serum leptin concentrations at 24–28 weeks gestation and BMI recorded in the first trimester. It is possible that leptin concentrations were somehow altered by the glucose challenge testing, as insulin regulates leptin production, but only chronic fasting and not acute feeding has been shown to alter serum leptin41. Numerous studies in non-pregnant humans and animal models show a tight correlation between serum leptin concentrations and weight, BMI or adiposity42. However, this relationship is weakened or absent in pregnancy43,44, though in several studies a significant relationship remains45–47. The weakened relationship is not surprising, as the placenta becomes a major source of leptin production during pregnancy, and its production is partially hormone-regulated, whereas adipose tissue is the major leptin source outside of pregnancy43,48–51. Although maternal leptin is inversely associated with gestational weight gain in the first trimester, consistent with its role in suppressing appetite and weight gain, leptin is positively associated with weight gain in the second trimester, suggesting a positive feedback mechanism52. One important implication of the altered leptin-BMI relationship in pregnancy is that leptin:BMI ratio, sometimes used as a measure of leptin resistance, likely does not meaningfully reflect leptin resistance during pregnancy.
Increases in both serum leptin and leptin:BMI ratio were associated with shorter gestation length, with leptin:BMI ratio being the most significant predictor of gestation length of all the factors examined. The fact that raw leptin concentrations and leptin:BMI ratios changed gestation length in the same direction, and by a similar magnitude, again suggests that leptin:BMI ratio is a BMI–adjusted measure of leptin action, not a measure of leptin resistance.
The median BMI for participants in this study , at 27 ± 5.7 kg/m2, was just above the “normal” BMI range, and slightly lower than the U.S. average for adult women of 29.653. This raised a concern that participants with a high BMI may have been disproportionally excluded from analysis because high BMI is a risk factor for events or conditions, including pre-eclampsia54,55, gestational hypertension8, and planned caesarean section56, for which subjects were excluded. However, when mean BMI, gestational weight gain, maternal age, parity and fetal sex were compared between the 63 subjects included in analysis and the subjects that were excluded, no significant differences were detected (Student’s t-test, Chi-square p > 0.4). Whatever the reason for the lower representation of high BMI subjects in this study, it would be necessary to examine more women of higher BMI to determine with high confidence whether the leptin- gestation length relationship differs with BMI, although we found no evidence for that within this sample.
Of the factors potentially influencing gestation length and cervical dilation considered here, only serum leptin and leptin:BMI ratio had any significant effects. Maternal age and fetal sex were included in the analyses of gestation length and cervical dilation times because of previous evidence that they may play a significant role. Duration of the second stage of labor increases with maternal age, with one study of nearly 32,000 births finding a 97-min increase in average second stage duration in women over 39 compared to those under 2057–60. Similarly, women carrying a male fetus have increased risk of failure to progress61 and a longer first stage of labor, although the delay (0.6 h) is modest62. First pregnancies have been found to be significantly longer than multiparous pregnancies59,63, although others have not found a significant effect of parity60. Like higher BMI, excessive gestational weight gain is associated with prolonged labor complications, cesarean delivery and prolonged gestation lengths64,65, though there are less data available. That none of these factors (maternal age, fetal sex, gestational weight gain, parity) significantly impacted gestation length in this study probably reflects the relatively small sample size, as hundreds, or even thousands of births were examined in the previous studies that did find these factors significant. This is additionally suggested by the partial evidence (p < 0.2) for an impact of BMI, fetal sex and parity on cervical dilation times. That there was no significant relationship between Pitocin administration and gestation length suggests that we were able to sufficiently discriminate between those cases in which it was given to initiate labor, which were excluded from analysis, and cases in which it was given after the natural initiation of labor, which were included.
Given the extensive evidence linking higher BMI to labor dysfunction, the lack of a significant relationship between BMI and gestation length or cervical dilation was unexpected. However, average gestation length may not be as closely related to BMI as the incidence of post-term pregnancy. For example, a study of nearly 150,000 births in Sweden found a significant effect of BMI on average gestation length but the difference between the highest and lowest categories was only 1.5 days, and at least one smaller study found no effect6,60. In contrast, risk of post-term pregnancy is a consistent finding, and meta-analysis showed an odds ratio of 1.75 for gestation length ≥ 42 weeks in women with BMI of at least 409. As those with scheduled induction of labor were excluded from the present analysis, post-term pregnancies were largely not included, and there were too few to analyze as a separate outcome measure. The limited evidence for a relationship between BMI and cervical dilation (p = 0.12) again suggests that there was not a large enough sample size to make a meaningful conclusion.
This study was powered to determine the potential effect of leptin on the initiation of labor, by examining the relationship between leptin concentrations and gestation length, or the time until initiation of labor, as a primary outcome, but also examined the relationship between mid-gestation serum leptin concentrations and cervical dilation, finding no significant effect. Because dilation times were extracted from the electronic medical record, rather than recorded systematically, some patients had only one or a few dilation times recorded. Nonetheless, the lack of an effect of leptin is consistent with a previous study of 766 Swedish women in spontaneous labor, in which there was also no significant association between leptin concentrations during active labor and the duration of labor66.
A potential weakness of this study is that serum leptin concentrations were measured at 24–28 weeks, whereas the outcome measures occur many weeks later. This makes it more difficult to impute a potential causal or mechanistic relationship between serum leptin and the initiation or progression of labor, as leptin concentrations were not actually measured during labor, and may have changed in the intervening time. However, leptin concentrations, after increasing through the second trimester, remain fairly consistent for the rest of pregnancy43,67. Here, blood samples were taken at 24–28 weeks to facilitate patient participation; it was more convenient for participants to provide a second blood sample at the time of glucose challenge testing than to schedule a blood draw specifically for the study, or to undergo sampling while in labor. Additionally, the 24–28 week sampling may also be considered advantageous for its clinical implications, as leptin concentrations as early as 24–28 weeks appear to have some value in predicting gestation length.
Conclusions
The clinical significance of leptin:BMI ratios for the prediction of gestation length is limited, as it explains a small portion of the overall variation in uncomplicated pregnancies. This is true for many other factors that have been shown to significantly relate to gestation length, consistent with the idea that regulation of the initiation of labor in women is complex and robust68,69. Nonetheless, together with data from mouse models, the significant relationship between serum leptin and gestation length found here supports a role for leptin in the initiation of labor, and undercuts the idea that leptin inhibition of myometrial contractions contributes significantly to longer gestation lengths. The physiological mechanisms by which leptin may shorten gestation, and even whether leptin plays a role through central or peripheral signaling have yet to be determined.
Acknowledgements
The authors thank Christina Shearer and the Research Success Core of the Department of Obstetrics and Gynecology for assistance in study design, participant recruitment and sample collection.
Abbreviations
- BMI
Body mass index
- Lep
Leptin
- SD
Standard deviation
- IQR
Interquartile range
- GEE
Generalized estimating equation
Author contributions
K.K.R.B. and L.C.S. conceived the study, interpreted data and wrote the paper. K.K.R.B. and E.S. carried out the study. E.D. and M.G. performed data analysis and edited the paper. E.D. contributed to experimental design.
Funding
This work was supported by internal funds from the University of Missouri.
Data availability
The data underlying the manuscript are presented in the manuscript or are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Castracane VD, Henson MC. When did leptin become a reproductive hormone? Semin. Reprod. Med. 2002;20(2):89–92. doi: 10.1055/s-2002-32499. [DOI] [PubMed] [Google Scholar]
- 2.Elias CF, Purohit D. Leptin signaling and circuits in puberty and fertility. Cell. Mol. Life Sci. 2013;79:841–862. doi: 10.1007/s00018-012-1095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jequier E, Tappy L. Regulation of body weight in humans. Physiol. Rev. 1999;79(2):451–480. doi: 10.1152/physrev.1999.79.2.451. [DOI] [PubMed] [Google Scholar]
- 4.Chou SH, Mantzoros C. 20 years of leptin: Role of leptin in human reproductive disorders. J. Endocrinol. 2014;223(1):T49–62. doi: 10.1530/JOE-14-0245. [DOI] [PubMed] [Google Scholar]
- 5.Farley DM, Choi J, Dudley DJ, Li C, Jenkins SL, Myatt L, et al. Placental amino acid transport and placental leptin resistance in pregnancies complicated by maternal obesity. Placenta. 2010;31(8):718–724. doi: 10.1016/j.placenta.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Denison FC, Price J, Graham C, Wild S, Liston WA. Maternal obesity, length of gestation, risk of postdates pregnancy and spontaneous onset of labour at term. BJOG. 2008;115(6):720–725. doi: 10.1111/j.1471-0528.2008.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson NS, Frediani JK, Corwin EJ, Dunlop A, Jones D. Metabolomic pathways predicting labor dystocia by maternal body mass index. AJP Rep. 2020;10(1):e68–e77. doi: 10.1055/s-0040-1702928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson NS, Hernandez TL, Hurt KJ. Parturition dysfunction in obesity: Time to target the pathobiology. Reprod. Biol. Endocrinol. 2015;13:135. doi: 10.1186/s12958-015-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heslehurst N, Vieira R, Hayes L, Crowe L, Jones D, Robalino S, et al. Maternal body mass index and post-term birth: A systematic review and meta-analysis. Obes. Rev. 2017;18(3):293–308. doi: 10.1111/obr.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharya S, Campbell DM, Liston WA, Bhattacharya S. Effect of body mass index on pregnancy outcomes in nulliparous women delivering singleton babies. BMC Public Health. 2007;7:168. doi: 10.1186/1471-2458-7-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pevzner L, Powers BL, Rayburn WF, Rumney P, Wing DA. Effects of maternal obesity on duration and outcomes of prostaglandin cervical ripening and labor induction. Obstet Gynecol. 2009;114(6):1315–1321. doi: 10.1097/AOG.0b013e3181bfb39f. [DOI] [PubMed] [Google Scholar]
- 12.Norman SM, Tuuli MG, Odibo AO, Caughey AB, Roehl KA, Cahill AG. The effects of obesity on the first stage of labor. Obstet. Gynecol. 2012;120(1):130–135. doi: 10.1097/AOG.0b013e318259589c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vahratian A, Zhang J, Troendle JF, Savitz DA, Siega-Riz AM. Maternal prepregnancy overweight and obesity and the pattern of labor progression in term nulliparous women. Obstet. Gynecol. 2004;104(5 Pt 1):943–951. doi: 10.1097/01.AOG.0000142713.53197.91. [DOI] [PubMed] [Google Scholar]
- 14.Gauthier T, Mazeau S, Dalmay F, Eyraud JL, Catalan C, Marin B, et al. Obesity and cervical ripening failure risk. J. Matern. Fetal. Neonatal. Med. 2012;25(3):304–307. doi: 10.3109/14767058.2011.575485. [DOI] [PubMed] [Google Scholar]
- 15.Walsh J, Foley M, O'Herlihy C. Dystocia correlates with body mass index in both spontaneous and induced nulliparous labors. J. Matern. Fetal. Neonatal. Med. 2011;24(6):817–821. doi: 10.3109/14767058.2010.531313. [DOI] [PubMed] [Google Scholar]
- 16.Cedergren MI. Non-elective caesarean delivery due to ineffective uterine contractility or due to obstructed labour in relation to maternal body mass index. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009;145(2):163–166. doi: 10.1016/j.ejogrb.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Roman H, Goffinet F, Hulsey TF, Newman R, Robillard PY, Hulsey TC. Maternal body mass index at delivery and risk of caesarean due to dystocia in low risk pregnancies. Acta Obstet. Gynecol. Scand. 2008;87(2):163–170. doi: 10.1080/00016340701762975. [DOI] [PubMed] [Google Scholar]
- 18.Luca AM, Carvalho JCA, Ramachandran N, Balki M. The effect of morbid obesity or advanced maternal age on oxytocin-induced myometrial contractions: An in vitro study. Can. J. Anaesth. 2020;67(7):836–846. doi: 10.1007/s12630-020-01615-6. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Chen Y, Lin Z, Lin W, Liu Y, Ou W, et al. Association of pre-pregnancy body mass index with adverse pregnancy outcome among first-time mothers. PeerJ. 2020;8:e10123. doi: 10.7717/peerj.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slack E, Best KE, Rankin J, Heslehurst N. Maternal obesity classes, preterm and post-term birth: A retrospective analysis of 479,864 births in England. BMC Pregnancy Childbirth. 2019;19(1):434. doi: 10.1186/s12884-019-2585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cnattingius S, Villamor E, Johansson S, Edstedt Bonamy AK, Persson M, Wikstrom AK, et al. Maternal obesity and risk of preterm delivery. JAMA. 2013;309(22):2362–2370. doi: 10.1001/jama.2013.6295. [DOI] [PubMed] [Google Scholar]
- 22.Mounzih K, Qiu J, Ewart-Toland A, Chehab FF. Leptin is not necessary for gestation and parturition but regulates maternal nutrition via a leptin resistance state. Endocrinology. 1998;139(12):5259–5262. doi: 10.1210/endo.139.12.6523. [DOI] [PubMed] [Google Scholar]
- 23.Pennington, K.A., Oestreich, A.K., Cataldo, K.H., Fogliatti, C.M., Lightner, C., Lydon, J.P. et al. Conditional knockout of leptin receptor in the female reproductive tract reduces fertility due to parturition defects in mice. Biol. Reprod. (2022). [DOI] [PubMed]
- 24.Baumgartner, W.W. Effects of Leptin on the Rat Cervix: University of Illinois at Urbana-Champaign, (2003).
- 25.Harrod JS, Rada CC, Pierce SL, England SK, Lamping KG. Altered contribution of RhoA/Rho kinase signaling in contractile activity of myometrium in leptin receptor-deficient mice. Am. J. Physiol. Endocrinol. Metab. 2011;301(2):E362–E369. doi: 10.1152/ajpendo.00696.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wendremaire M, Bardou M, Peyronel C, Hadi T, Sagot P, Morrison JJ, et al. Effects of leptin on lipopolysaccharide-induced myometrial apoptosis in an in vitro human model of chorioamnionitis. Am. J. Obstet. Gynecol. 2011;205(4):363e1–9. doi: 10.1016/j.ajog.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 27.Moynihan AT, Hehir MP, Glavey SV, Smith TJ, Morrison JJ. Inhibitory effect of leptin on human uterine contractility in vitro. Am. J. Obstet. Gynecol. 2006;195(2):504–509. doi: 10.1016/j.ajog.2006.01.106. [DOI] [PubMed] [Google Scholar]
- 28.Mumtaz S, AlSaif S, Wray S, Noble K. Inhibitory effect of visfatin and leptin on human and rat myometrial contractility. Life Sci. 2015;125:57–62. doi: 10.1016/j.lfs.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Srinivasan G, Parida S, Pavithra S, Panigrahi M, Sahoo M, Singh TU, et al. Leptin receptor stimulation in late pregnant mouse uterine tissue inhibits spontaneous contractions by increasing NO and cGMP. Cytokine. 2021;137:155341. doi: 10.1016/j.cyto.2020.155341. [DOI] [PubMed] [Google Scholar]
- 30.Logan CA, Thiel L, Bornemann R, Koenig W, Reister F, Brenner H, et al. Delivery mode, duration of labor, and cord blood adiponectin, leptin, and C-reactive protein: Results of the population-based ulm birth cohort studies. PLoS ONE. 2016;11(2):e0149918. doi: 10.1371/journal.pone.0149918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahmood NA, Mathew J, Kang B, DeBari VA, Khan MA. Broadening of the red blood cell distribution width is associated with increased severity of illness in patients with sepsis. Int. J. Crit. Illn. Inj. Sci. 2014;4(4):278–282. doi: 10.4103/2229-5151.147518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zera CA, McElrath TF, Rifas-Shiman SL, Parker M, Rich-Edwards JW, Gillman MW. A prospective study of second trimester leptin level and failed first stage of labor. Reprod. Sci. 2010;17(3):211A. [Google Scholar]
- 33.Santillan, D.A., Cowman, W.L., Scroggins, S.M., Santillan, M.K. Abstract P321: Differential leptin levels are associated with hypertensive disorders of pregnancy and adverse pregnancy outcomes. Hypertension. 68(suppl_1): AP321-AP (2016).
- 34.ACOG Practice Bulletin No ACOG practice bulletin no 107: Induction of labor. Obstet. Gynecol. 2009;114(2 Pt 1):386–397. doi: 10.1097/AOG.0b013e3181b48ef5. [DOI] [PubMed] [Google Scholar]
- 35.Osilla, E.V., Sharma, S.O. StatPearls. (Treasure Island (FL), 2021).
- 36.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 37.Cohen, J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, N.J.: L. Erlbaum Associates; xxi, 567 (1998).
- 38.Mittendorf R, Chorzempa LM, Quinlan MP, Herschel M, Williams MA. Length of pregnancy in African Americans: Validation of a new predictive rule. J. Natl. Med. Assoc. 1999;91(9):523–527. [PMC free article] [PubMed] [Google Scholar]
- 39.Barisic T, Mandic V, Barac I. Associations of body mass index and gestational weight gain with term pregnancy outcomes. Mater. Sociomed. 2017;29(1):52–57. doi: 10.5455/msm.2017.29.52-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vahratian A, Troendle JF, Siega-Riz AM, Zhang J. Methodological challenges in studying labour progression in contemporary practice. Paediatr. Perinat. Epidemiol. 2006;20(1):72–78. doi: 10.1111/j.1365-3016.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- 41.Sinha MK, Caro JF. Clinical aspects of leptin. Vitam. Horm. 1998;54:1–30. doi: 10.1016/S0083-6729(08)60919-X. [DOI] [PubMed] [Google Scholar]
- 42.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996;334(5):292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 43.Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, et al. Nonadipose tissue production of leptin: Leptin as a novel placenta-derived hormone in humans. Nat. Med. 1997;3(9):1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- 44.Butte NF, Hopkinson JM, Nicolson MA. Leptin in human reproduction: Serum leptin levels in pregnant and lactating women. J. Clin. Endocrinol. Metab. 1997;82(2):585–589. doi: 10.1210/jcem.82.2.3731. [DOI] [PubMed] [Google Scholar]
- 45.Stefaniak M, Dmoch-Gajzlerska E, Mazurkiewicz B, Gajzlerska-Majewska W. Maternal serum and cord blood leptin concentrations at delivery. PLoS ONE. 2019;14(11):e0224863. doi: 10.1371/journal.pone.0224863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Highman TJ, Friedman JE, Huston LP, Wong WW, Catalano PM. Longitudinal changes in maternal serum leptin concentrations, body composition, and resting metabolic rate in pregnancy. Am. J. Obstet. Gynecol. 1998;178(5):1010–1015. doi: 10.1016/S0002-9378(98)70540-X. [DOI] [PubMed] [Google Scholar]
- 47.Verhaeghe J, Pintiaux A, Van Herck E, Hennen G, Foidart JM, Igout A. Placental GH, IGF-I, IGF-binding protein-1, and leptin during a glucose challenge test in pregnant women: Relation with maternal body weight, glucose tolerance, and birth weight. J. Clin. Endocrinol. Metab. 2002;87(6):2875–2882. doi: 10.1210/jcem.87.6.8569. [DOI] [PubMed] [Google Scholar]
- 48.Ashworth CJ, Hoggard N, Thomas L, Mercer JG, Wallace JM, Lea RG. Placental leptin. Rev. Reprod. 2000;5(1):18–24. doi: 10.1530/ror.0.0050018. [DOI] [PubMed] [Google Scholar]
- 49.Bi S, Gavrilova O, Gong DW, Mason MM, Reitman M. Identification of a placental enhancer for the human leptin gene. J. Biol. Chem. 1997;272(48):30583–30588. doi: 10.1074/jbc.272.48.30583. [DOI] [PubMed] [Google Scholar]
- 50.Chardonnens D, Cameo P, Aubert ML, Pralong FP, Islami D, Campana A, et al. Modulation of human cytotrophoblastic leptin secretion by interleukin-1alpha and 17beta-oestradiol and its effect on HCG secretion. Mol. Hum. Reprod. 1999;5(11):1077–1082. doi: 10.1093/molehr/5.11.1077. [DOI] [PubMed] [Google Scholar]
- 51.Coya R, Martul P, Algorta J, Aniel-Quiroga MA, Busturia MA, Senaris R. Progesterone and human placental lactogen inhibit leptin secretion on cultured trophoblast cells from human placentas at term. Gynecol. Endocrinol. 2005;21(1):27–32. doi: 10.1080/09513590500099305. [DOI] [PubMed] [Google Scholar]
- 52.Lacroix M, Battista MC, Doyon M, Moreau J, Patenaude J, Guillemette L, et al. Higher maternal leptin levels at second trimester are associated with subsequent greater gestational weight gain in late pregnancy. BMC Pregnancy Childbirth. 2016;16:62. doi: 10.1186/s12884-016-0842-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheryl, D., Fryar, D.K.-M., Qiuping, G. and Cynthia, O. Mean body weight, weight, waist circumference, and body mass index among adults: United States, 1999–2000 through 2015–2016. In: National Center for Health S, editor. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2018.
- 54.Poorolajal J, Jenabi E. The association between body mass index and preeclampsia: A meta-analysis. J. Matern. Fetal Neonatal. Med. 2016;29(22):3670–3676. doi: 10.3109/14767058.2016.1140738. [DOI] [PubMed] [Google Scholar]
- 55.He XJ, Dai RX, Hu CL. Maternal prepregnancy overweight and obesity and the risk of preeclampsia: A meta-analysis of cohort studies. Obes. Res. Clin. Pract. 2020;14(1):27–33. doi: 10.1016/j.orcp.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Poobalan AS, Aucott LS, Gurung T, Smith WC, Bhattacharya S. Obesity as an independent risk factor for elective and emergency caesarean delivery in nulliparous women–systematic review and meta-analysis of cohort studies. Obes. Rev. 2009;10(1):28–35. doi: 10.1111/j.1467-789X.2008.00537.x. [DOI] [PubMed] [Google Scholar]
- 57.Greenberg MB, Cheng YW, Sullivan M, Norton ME, Hopkins LM, Caughey AB. Does length of labor vary by maternal age? Am. J. Obstet. Gynecol. 2007;197(4):428e1–7. doi: 10.1016/j.ajog.2007.06.058. [DOI] [PubMed] [Google Scholar]
- 58.Rasmussen S, Bungum L, Hoie K. Maternal age and duration of labor. Acta Obstet. Gynecol. Scand. 1994;73(3):231–234. doi: 10.3109/00016349409023445. [DOI] [PubMed] [Google Scholar]
- 59.Bergsjo P, Denman DW, 3rd, Hoffman HJ, Meirik O. Duration of human singleton pregnancy. A population-based study. Acta Obstet. Gynecol. Scand. 1990;69(3):197–207. doi: 10.3109/00016349009028681. [DOI] [PubMed] [Google Scholar]
- 60.Jukic AM, Baird DD, Weinberg CR, McConnaughey DR, Wilcox AJ. Length of human pregnancy and contributors to its natural variation. Hum Reprod. 2013;28(10):2848–2855. doi: 10.1093/humrep/det297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheiner E, Levy A, Katz M, Hershkovitz R, Leron E, Mazor M. Gender does matter in perinatal medicine. Fetal Diagn Ther. 2004;19(4):366–369. doi: 10.1159/000077967. [DOI] [PubMed] [Google Scholar]
- 62.Cahill AG, Roehl KA, Odibo AO, Zhao Q, Macones GA. Impact of fetal gender on the labor curve. Am. J. Obstet. Gynecol. 2012;206(4):335e1–5. doi: 10.1016/j.ajog.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 63.Mittendorf R, Williams MA, Berkey CS, Cotter PF. The length of uncomplicated human gestation. Obstet. Gynecol. 1990;75(6):929–932. [PubMed] [Google Scholar]
- 64.Johnson JW, Longmate JA, Frentzen B. Excessive maternal weight and pregnancy outcome. Am. J. Obstet. Gynecol. 1992;167(2):353–370. doi: 10.1016/S0002-9378(11)91414-8. [DOI] [PubMed] [Google Scholar]
- 65.Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes: A systematic review and Meta-analysis. JAMA. 2017;317(21):2207–2225. doi: 10.1001/jama.2017.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carlhall S, Kallen K, Thorsell A, Blomberg M. Maternal plasma leptin levels in relation to the duration of the active phase of labor. Acta Obstet. Gynecol. Scand. 2018;97(10):1248–1256. doi: 10.1111/aogs.13380. [DOI] [PubMed] [Google Scholar]
- 67.Domali E, Messinis IE. Leptin in pregnancy. J. Matern. Fetal. Neonatal. Med. 2002;12(4):222–230. doi: 10.1080/jmf.12.4.222.230. [DOI] [PubMed] [Google Scholar]
- 68.Mendelson CR, Gao L, Montalbano AP. Multifactorial regulation of myometrial contractility during pregnancy and parturition. Front. Endocrinol. (Lausanne) 2019;10:714. doi: 10.3389/fendo.2019.00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Norwitz, E.R., Bonney, E.A., Snegovskikh, V.V., Williams, M.A., Phillippe, M., Park, J.S. et al. Molecular regulation of parturition: The role of the decidual clock. Cold Spring Harb Perspect Med. 5(11) (2015). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying the manuscript are presented in the manuscript or are available from the corresponding author on reasonable request.