Abstract
Background
The American Society of Breast Surgeons recommends genetic testing (GT) for all women with breast cancer (BC), but implementation and uptake of GT has not been well-described.
Methods
A retrospective chart review was performed for newly diagnosed BC patients or patients with a newly identified recurrence of BC seen in a multidisciplinary clinic (MDBC) who were offered genetic counseling (GC) and GT.
Results
The 138 women attending the MDBC had a median age of 54 years and comprised non-Hispanic whites (46%), Asians (28%), Hispanics (17%), blacks (4%), and other (5%). Of the 105 (76%) patients without prior GT, 100 (95%) accepted GC, with 93 (93%) of these 100 patients undergoing GT. The patients meeting the National Comprehensive Cancer Network (NCCN) guidelines for GT were more likely to undergo GT. Testing was performed with a 67- to 84-gene panel, together with an 8- to 9-gene STAT panel if needed. Among 120 patients with reports available, including 33 patients previously tested, 15 (12%) were positive (1 BLM, 1 BRCA1, 3 BRCA2, 1 BRIP1, 1 CFTR, 1 CHEK2, 1 MUTYH, 1 PALB2, 1 PRSS1, 1 RAD50, 1 RET, and 2 TP53), 44 (37%) were negative, and 61 (51%) had an uncertain variant. The median time to STAT results (n = 50) was 8 days. The STAT results were available before surgery for 47 (98%) of the 48 STAT patients undergoing surgery.
Conclusions
New BC patients attending the MDBC demonstrated high rates of acceptance of GC and GT. The combination of GC and GT can offer timely information critical to patient risk assessment and treatment planning.
Multiple professional societies have issued guidelines for genetic testing (GT) for hereditary breast cancer (BC),1–5 and many of these guidelines include a recommendation for testing on a panel of multiple genes via next-generation sequencing technology3–5 and accompanying genetic counseling (GC)1,2,4,5 (Table 1). Current data show that many women who meet standard testing criteria are not being referred for GC or GT,6,7 particularly among underrepresented minorities.7
Table 1.
Guidelines of professional societies for genetic testing
| Professional society, publication year | Next-generation sequencing panels recommended | Genetic counseling recommended | Genetic testing for all women with breast cancer recommended |
|---|---|---|---|
| American Cancer Society/American Society of Clinical Oncology, Survivorship, 2016 | X | ||
| United States Preventive Services Task Force, 2019 | X | X | |
| American Society of Breast Surgery, 2019 | X | X | |
| American College of Obstetricians and Gynecologists, 2019 | X | X | |
| National Comprehensive Cancer Network, 2022.2 | X | X |
In 2019, the American Society of Breast Surgeons (ASBrS) issued guidelines recommending that GT be offered to all women with a current or past diagnosis of BC3 because a significant proportion of women who tested positive did not meet standard testing guidelines.8 The same ASBrS statement supported pre-test counseling by a breast surgeon, genetic counselor, or other knowledgeable medical professional.
For women with a new diagnosis of BC, GT can inform decision-making regarding risk-reducing mastectomy at the time of breast surgery.9 Also, new data suggest that BRCA1- or BRCA2-positive BC patients treated with adjuvant olaparib had an increase in disease-free survival,10 lending further argument to the need to offer GT as part of BC treatment decision-making.
Although breast surgeons express interest in providing GT, many feel they need more knowledge11 or have difficulty interpreting variants of uncertain significance (VUSs).3,11 Most importantly, surgeons must prioritize surgical care and may not have the clinical bandwidth for the standard components of GC such as pedigree extraction, education regarding genetics, testing decision-making discussions, interpretion of test results such as pathogenic variants and VUSs, calculation of future lifetime cancer risk, and discussion of risk for family members.12
Genetics providers including genetic counselors can provide education, order the correct test, and interpret results,12 but patients may not have timely access to a genetic counselor,13 particularly when making surgery and treatment decisions. Given this need, there are opportunities for partnerships between oncology providers and genetics providers to efficiently identify patients needing urgent genetic test results, allowing for the integration of timely and high-quality delivery of genetics services into coordinated oncology care.12
Previous efforts to involve a genetic counselor in a multidisciplinary clinic or to offer rapid GT using strategies that offer alternatives to pre-test GC14–16 or embed a genetic counselor in a surgical clinic have been described.17 However, these programs did not offer GT to all women with BC as recommended by the ASBrS. It is unknown whether all breast patients would be interested in GT at the time of diagnosis or how quickly genetic test results could be available for surgical planning.
This report describes outcomes related to provision of GT for all BC patients as part of a multidisciplinary clinic. We report the uptake of GC and GT, as well as whether results were available before surgery, and describe the resources allocated to this effort. Results will help guide other programs considering how to provide comprehensive genetics services and align with the ASBrS recommendations for offering GT to all women with BC.
Methods
Patient Population
In 2018, a multidisciplinary breast clinic (MDBC) was established at our institution for patients with newly diagnosed BC or patients with a newly identified recurrence of BC. Patients are scheduled for coordinated care visits on the same day, including breast surgery, medical oncology, radiation oncology, plastic surgery, psychiatry, nutrition, and occupational therapy, with GC. GC, and GT offered to all patients regardless whether the patient meets standard clinical guidelines for GT. Patients who have already undergone GT or have results pending also are given the opportunity to meet with a genetics provider. A nurse navigator collects medical records including pathology reports, summarizes these records, and sends a synopsis to the team in advance of each clinic.
All patients in this review had PPO, HMO, Medicaid, or Medicare insurance. The clinic charged one facility fee, and each physician visit was billed as a separate professional fee. All GC services were included in the facility fee, and GT fees were billed by the outside laboratories with third-party billing. Information about insurance coverage was requested from these laboratories.
GC Procedures
A certified genetic counselor or advanced practice nurse in genetics (AGN-BC) was assigned to cover each clinic, but for clinics with three or more new patients, two providers were assigned. The nurse navigator typically coordinated the GC sessions for the end of the patient’s visit and arranged for the genetics provider to see patients back-to-back.
The GC included elicitation of a three-generation pedigree, discussion of genetics and cancer, consideration of how GT could have an impact on surgery and screening, panel testing, possible types of results, and the impact of testing on family members. Patients typically were invited to participate in two research studies: a cancer genetics registry and a precision oncology study. Each visit typically lasted approximately 30 to 40 min but were not systematically timed.
Most patients were seen in person, but due to the COVID-19 pandemic, patients in the last 5 weeks of the study period received counseling via telemedicine or a telephone call.
The GT typically was performed with a large panel test of 67 to 84 genes offered by one of two major commercial laboratories. If the results could have an impact on surgical decision-making, an 8- or 9-gene BC STAT panel was ordered concurrently from the same lab so that genetic test results could be available more quickly.
When results were reported, the genetics provider placed a telephone call and sent an email to disclose results. A second disclosure of results was made if the STAT panel and full panel were reported separately. Disclosure of results and documentation took approximately 30 min if two phone calls were needed, and approximately 15 min if only one call was needed. A follow-up in-person appointment was recommended for patients identified as having pathogenic or likely pathogenic germline variants, and these appointments typically lasted 45 to 60 min. Genetic test results and family history were captured in Web Progeny (version 10) (Progeny Genetics LLC, Aliso Viejo, CA).
Data Analysis
A retrospective chart review was performed for patients seen in the MDBC between 5 November 2018 and 27 April 2020 to obtain demographic information and to determine whether the patient accepted the offer of GC and GT. The GT results and the result turnaround time, including STAT tests, were extracted from Progeny. For patients undergoing GT, medical records were reviewed to identify the date of BC surgery. Patient characteristics and family history were reviewed to determine whether the National Comprehensive Cancer Network (NCCN) guidelines for GT were met.
To determine wether an association existed between the GT decision and age, the Wilcoxon rank-sum test was applied. The Wilcoxon rank sum-test was chosen so that no assumption would be made about the distribution of the data. To determine whether there as an association between the GT decision and categorical variables (i.e., meeting NCCN guidelines, having a family history, having any children, and having daughters), Pearson’s chi-square test was performed. To handle the small values (< 5) appropriately in some categories for the race/ethnicity and cancer diagnosis variables, Fisher’s exact test was applied to measure the association between the GT decision and these two variables.
Results
All of the 138 patients seen in the MDBC were female with a new diagnosis of BC or ductal carcinoma in situ (DCIS) or a newly diagnosed recurrence of BC. The median age was 54 years (range 32–83 years). Table 2 presents the race and ethnic characteristics of the population, which consisted of 63 (46%) non-Hispanic whites, 39 (28%) Asians, 24 (17%) Hispanic whites, 5 (4%) blacks, 6 (4%) patients of unknown or mixed backgrounds; and 1 (1%) American Indian or Alaska Native.
Table 2.
Demographics of multidisciplinary breast cancer clinic patients and comparison of acceptors and decliners of genetic testing
| Demographic characteristic | Total (n = 138) n (%) |
Previous GT (n = 33) |
Accepted genetic testing in MDBC (n = 93) n (%) |
Declined genetic testing in MDBC (n = 12) n (%) |
p valueI |
|---|---|---|---|---|---|
| Median age: years (range) | 54 (47–65) | 48 (43–52) | 57 (48–66) | 70.5 (58–79) | 0.006 |
| Breast cancer diagnosis | 0.371 | ||||
| Invasive | 107 (77.5) | 21 (63.6) | 76 (81.7) | 10 (83.3) | |
| DCIS | 21 (15.9) | 5 (15.2) | 15 (16.1) | 1 (8.3) | |
| Local recurrence | 5 (2.2) | 3 (9.1) | 1 (1.1) | 1 (8.3) | |
| Metastatic | 5 (3.6) | 4 (12.1) | 1 (1.1) | 0 (0) | |
| Race/ethnicity | 0.378 | ||||
| Non-Hispanic white | 63 (45.7) | 15 (45.5) | 39 (41.9) | 9 (75.0) | |
| Asian | 39 (28.3) | 8 (24.2) | 28 (30.1) | 3 (25.0) | |
| Hispanic white | 24 (17.4) | 7 (21.2) | 17 (18.3) | 0 (0) | |
| Black or African American | 5 (3.6) | 1 (3.0) | 4 (4.3) | 0 (0) | |
| Other or mixed ancestry | 6 (4.3) | 2 (6.0) | 4 (4.3) | 0 (0) | |
| American Indian or Alaska Native | 1 (0.7) | 0 (0) | 1 (1.1) | 0 (0) | |
| NCCN guidelines met | 0.02 | ||||
| Yes | 95 (68.8) | 28 (84.8) | 63 (67.7) | 4 (33.3) | |
| No | 43 (31.2) | 5 (15.2) | 30 (32.3) | 8 (66.7) | |
| Family historyII | 0.183 | ||||
| Yes | 73 (53.3) | 19 (59.4) | 50 (53.8) | 4 (33.3) | |
| No | 64 (46.7) | 13 (40.6) | 43 (46.2) | 8 (6.77) | |
| Has children | 0.247 | ||||
| Yes | 97 (70.8) | 21 (65.6) | 69 (74.2) | 7 (58.3) | |
| No | 40 (29.2) | 11 (34.4) | 24 (25.8) | 5 (41.7) | |
| Has daughter | 0.806 | ||||
| Yes | 69 (51.9) | 13 (46.4) | 50 (53.8) | 6 (50.0) | |
| No | 64 (48.1) | 15 (53.6) | 43 (46.2) | 6 (50.0) |
MDBC Multidisciplinary Breast Cancer Clinic, DCIS ductal carcinoma in situ, NCCN National Comprehensive Cancer Network
ITwo-group comparison between those who accepted versus those who declined genetic testing in the MDBC
IIHaving a family history is defined as having a first- or second-degree relative with breast or ovarian cancer
All 138 patients were offered GC, and 33 had already completed GT including 12 patients previously seen at our institution, 13 patients tested at outside facilities who did not have GC at the MDBC, and 8 patients who underwent GC at the MDBC visit but who had already had GT pending or completed by an outside facility.
Of the 105 remaining patients, 100 (95%) accepted GC and 5 (5%) declined. Of the 100 patients undergoing GC during the MDBC visit, 93 (93%) underwent GT, and 7 (7%) declined or postponed testing. Among the entire population of 138 patients seen during the study period, 126 (91%) underwent GT, including both the patients who had GT within the MDBC and those tested previously (Fig. 1).
Fig. 1.

Uptake of genetic counseling and testing among multidisciplinary breast cancer clinic patients. GC, genetic counseling; GT, genetic testing. *Includes one woman with incomplete genetic testing who had GC and accepted additional GT. **GC accepted but testing previously ordered at outside facility, with results pending or with results indicating a variant of uncertain significance (VUS)
For the 105 patients offered GT at the MDBC, younger age was a predictor for acceptance of GT. The median age was 57 years for those accepting GT versus 70.5 years for those declining GT (p = 0.006). The patients who met the NCCN guidelines were more likely to accept GT, with 63 (93%) of the 67 patients who met guidelines undergoing GT and 30 (79%) of the 38 patients who did not meet guidelines undergoing GT (p = 0.02). Having a family history of breast or ovarian cancer, having children, or having a daughter was not a significant predictor for acceptance of GT. The proportion of patients undergoing GT was 100% of the Hispanic (17/17) and black (4/4) women, 90% of the Asian women (28/31), and 81% of the non-Hispanic white women (39/48) (p = 0.378).
To expedite surgical decision-making, a STAT test was ordered for 50 patients. The results time for a STAT test was a median of 8 days (range 6–17 days, with one exception of 35 days due to both a billing issue and a lab assay failure). Most of the STAT results (90%, 45/50) were available within 2 weeks. Concurrent panel tests of 67 to 84 genes also were ordered on the day of the clinic visit for 92 patients, and these results were available 12.5 days (range 5–32 days, except for the same case noted earlier) after the date of the visit (Table 3).
Table 3.
Time to genetic test results for testing ordered at a multidisciplinary clinic (n = 93)
| Total patients n (%) |
Median time to result Days (range) |
Proportion of test results available within 14 days (%) | |
|---|---|---|---|
| STAT test ordered (8- or 9-gene panel) | 50 (53.7) | 8 (6–35) | 45/50 (90.0) |
| Large panel test ordered (67- to 84-gene panel) | 92 (98.9) | 12.5 (5–56) | 54/92 (58.7) |
Among the 93 patients with GT ordered at the MDBC, insurance covered the GT for 55 (87%) of the 63 patients who met the NCCN guidelines and for 22 (73%) of the 30 patients who did not meet the NCCN guidelines.
Of the 120 results available for the entire clinic population (Fig. 2), 44 (37%) were negative, 61 (51%) had a VUS, and 15 (12%) were positive (including 1 BLM, 1 BRCA1, 3 BRCA2, 1 BRIP1, 1 CFTR, 1 CHEK2, 1 MUTYH, 1 PALB2, 1 PRSS1, 1 RAD50, 1 RET, and 2 TP53) (Fig. 2). The only pathogenic variants identified in the patients with prior testing were the two TP53 variants. All seven of the patients with pathogenic variants in genes that have an impact on surgical decisions (BRCA1, BRCA2, PALB2, TP53) met the NCCN guidelines for GT for BRCA1 and BRCA2. Six of these patients were surgical candidates, and all six of them received genetic test results before surgery. This included five patients who had GT ordered at the MDBC and one patient who previously knew of her TP53 pathogenic variant. Five of the six surgical candidates ultimately elected bilateral mastectomy, including one who was a candidate for breast conservation, two who were candidates for unilateral mastectomy, and two who already desired bilateral mastectomy at diagnosis due to genetic risk (including the patient with the previously identified TP53 mutation and a patient with a known BRCA2 mutation in a relative). One of the six surgical candidates elected lumpectomy after strongly considering bilateral mastectomy due to her test results. The one CHEK2 carrier identified did not meet the NCCN testing guidelines and elected segmental mastectomy. One of the five metastatic cases in the MDBC had a previously identified TP53 pathogenic variant, but the other metastatic cases had no pathogenic mutation identified.
Fig. 2.
Genetic test results showing the proportion of patients with results that were positive or negative or that indicated a variant of uncertain significance
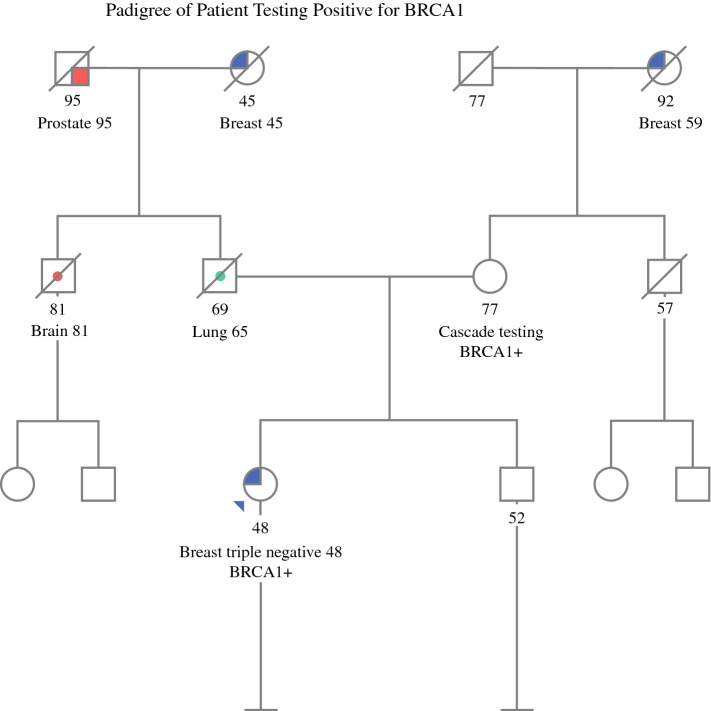
Pathogenic variants were identified in genes with uncertain or no BC risk (BLM, BRIP1, RAD50) and therefore had no change in management for BC screening or surgery. The CFTR, MUTYH, PRSS1, and RET carriers did not meet the standard GT guidelines for conditions related to theose genes, nor did they have an impact on breast screening or surgery. However, the RET pathogenic variant did lead to a preoperative assessment to rule out pheochromocytoma, and the recommendation for the patient was additional screening related to multiple endocrine neoplasia type 2. All the patients carrying pathogenic variants received follow-up GC, including risk calculation of a second primary BC risk. Such counseling included the offer of cascade testing of relatives. An example of a patient testing positive for BRCA1 is shown in Fig. 3. Cascade testing showed that her unaffected 77-year-old mother also carried the BRCA1 mutation.
Fig. 3.
Pedigree of a woman with a new diagnosis of breast cancer who tested positive for BRCA1. Cascade testing of family members found that the mutation was inherited from her 77-year-old unaffected mother
Of the 95 patients (68.3%) among the 138 patients in the overall clinic cohort who met the NCCN GT criteria, 91 (96%) underwent testing, and 12 (14%) of the 86 patients with results available were found to be carrying pathogenic variants (1 BRCA1, 3 BRCA2, 1 BLM,1 BRIP1,1 PALB2, 2 TP53, 1 RET, 1 CFTR, 1 PRSS1). Of the 43 (31%) patients who did not meet the criteria, 35 (79.5%) had testing, and 3 (9%) of 34 patients with results available tested positive for a pathogenic variant (1 RAD50, 1 CHEK2, 1 MUTYH heterozygote). Of the 138 patients in the total cohort, 12 patients declined GT, including 5 patients who met the NCCN GT criteria. Two of these five patients had GC but still declined testing.
Of the 120 patients in the MDBC for whom genetic test results were available, 106 completed BC surgery at our institution, 5 did not have surgery due to metastatic BC or other disease, and 9 had surgery at an outside hospital, with no records available. Among the 106 patients for whom surgical records were available, 96 (90.6%) had genetic test results available before surgery. Among 50 patients with STAT GT, results were available before surgery for 47 (98%) of the 48 patients undergoing surgery,.
GC Resources Utilized
In the 55 MDBC clinics that occurred during the study period, the number of GC patients per clinic ranged between 0 and 4 patients. There were 4 clinics with four patients, 8 clinics with three patients, 22 clinics with two patients, 20 clinics with one patient, and 1 clinic with no patients needing GC. The majority of the clinics at our institution (76%) required the efforts of only one genetics provider.
Discussion
When integrated into a multidisciplinary management clinic, the acceptance of GC and GT among all women with BC was very high, with 95% of the women who had no prior genetics evaluation accepting GC, and 93% of the women undergoing GC electing to have GT. Genetic test results ordered on a STAT basis were available in a median of 8 days (> 90% of STAT results reported in less than 2 weeks), and 98% of the women who had STAT testing ordered received their results before surgery.
Among the genetic results available for this clinic population, 15 (12%) of 120 were positive, including 8 (7%) positive results for a gene associated with BC risk (BRCA1, BRCA2, CHEK2, PALB2, or TP53).
Genetic testing can be very helpful for women making decisions about surgery, radiation therapy (as is the case with TP53), or systemic therapy such as olaparib for BRCA1 or BRCA2. If a pathogenic variant is identified in certain cancer-susceptible genes, there are clear recommendations for treatment,5 and the knowledge of such a pathogenic variant can help women make an informed decision at the time of diagnosis. Findings show that timely GT for women with BC has an impact on surgical decision-making, with positive results shown to be strongly associated with the decision to undergo bilateral mastectomy.18 Additionally, and just as importantly, the knowledge that a patient has tested negative for a pathogenic variant may reduce the rate of bilateral mastectomy. A study by Metcalfe et al.19 investigated 788 women with a new diagnosis of BC and reported that 37% of the women were undecided or leaning toward bilateral mastectomy, but that after receiving negative genetic test results, only 15% of the women opted for bilateral mastectomy.
One argument for offering GT to all women with BC is that the NCCN guidelines for referral and testing lead to under-diagnosis of individuals with inherited susceptibility to hereditary BC. In a study of almost 1000 BC patients undergoing an 80-gene panel, 9.4% of those meeting the NCCN guidelines and 7.9% of those not meeting guidelines carried pathogenic variants (p = 0.42). If this panel was reduced to a panel of 11 BC genes, 6.3% of the women meeting the guidelines had positive test results, and 3.5% of the women not meeting guidelines had positive test results.8 Notably, in this study as well as in the current study, pathogenic variants in clinically actionable BC genes were identified more frequently in patients who met the NCCN criteria than in those who do not. This is not surprising because the NCCN guidelines are optimized for the identification of BRCA1 and BRCA2 pathogenic variants.
It is important for clinicians to consider when implementing a universal testing program that a broad panel will identify pathogenic variants in unexpected and “off-target” genes such as RET. In addition, pathogenic variants will be identified in genes that have no impact on BC management but increase risk for other cancers such BRIP1 as well as genes unlikely to be associated with cancer risk but that still require discussion, such as BLM.
The range of possible genetic test results highlights an opportunity for collaboration between surgeons and genetics providers to improve patient understanding and management of both BC-related and non-BC-related findings. Also, although some other genetic findings may not have a direct impact on BC treatment, they can have an impact on future screening or prevention, such as a BRIP1 carrier undergoing oophorectomy. Given the high rate for survival of BC, optimizing future cancer prevention is extremely important.
Multiple studies have shown that despite increased awareness and availability, together with decreased costs, GT remains underutilized among BC patients. A population-based study estimated that 36% of BC patients met the eligibility criteria for testing but that only 15% underwent testing.20 A recent study of Surveillance, Epidemiology, and End Results (SEER) data in California and Georgia showed that only about 25% of women with BC had GT between 2012 and 2019.21 In a recent study of 397 patients presenting at an academic institution with a new diagnosis of BC and no prior GT, 212 (53%) met the NCCN criteria for GT, and among these 212 patients, 45 (21%) were missed and not tested, mostly because a family history of breast or ovarian cancer was not recognized.22
A universal approach to testing has the potential to more reliably reach individuals with the highest probability of pathogenic variants, those who meet NCCN guidelines, those with pathogenic variants less easily recognized by standard guidelines, and those who desire testing for personal and family risk assessment. Previous reports have found racial disparities among BC patients, with non-Hispanic whites more likely to be referred for GC and GT,7,23–25 but no significant differences in uptake of GT.25
Offering GT to all BC patients has the potential to increase access and mitigate referral biases that can exacerbate racial and ethnic disparities.7 Our study did note a somewhat higher uptake of GT among Hispanic and Asian women than among non-Hispanic whites, although the number of decliners was too small for a valid statistical analysis. Further research is needed to determine whether the uptake differs among various racial and ethnic groups and whether disparities can be resolved by offering universal GT for BC.
Although universal GT of BC patients is recommended by the ASBrS and has been encouraged by some providers,26 access to GT still may be limited. The opportunity for a patient to meet with a genetic counselor for pre-test GC is not always available and should not be a barrier to GT. Therefore, “mainstreaming” germline GT for patients with BC has been proposed, with providers offering point-of-care GT.13,26 Such models have been developed for BC patients who have a high mutation probability, with GT facilitated by oncology providers and patients receiving follow-up GC if they test positive or have other risks.27,28
For patients with other types of cancer, universal GT has been offered using alternative service delivery models without pre-test GC. For example, in ovarian cancer, the NCCN guidelines have recommended GT for all patients since 2007, but it is estimated that only 55% of patients with ovarian cancer diagnosed between 2015 and 2021 have undergone testing.29 Targeted interventions such as telegenetics, GCs embedded in clinics, GT facilitated by non-genetics providers, and reflex tumor somatic GT have substantially increased GT uptake.29 Pancreatic cancer is another example for which universal GT is recommended and strategies to expand access at the point of care have been successful.30
Expanding universal testing to BC patients will require an integrated, multidisciplinary approach. Although it may be ideal to use genetic counselors for pre-test counseling or to discuss complex results when needed such as pathogenic variants and VUS results,31,32 this may not be possible in many settings. Nonetheless, partnerships between oncology providers and genetics providers can ensure safe and high-quality genetics care by developing streamlined processes for test ordering, results disclosure, and timely access to GC when needed. Some key points for successful implementation of integrating universal GT in a BC surgical clinic are shown in Table 4.
Table 4.
Tips for successful integration of genetic testing for all new breast cancer patients
| Optimization of patient intake to inquire about family history & previous genetic testing |
| Pre-test counseling or education offered to any interested breast cancer patient |
| Implementation of a STAT-testing process for patients having upfront surgery |
| Consideration of the availability of genetic counseling resources in determining the number of genes to include on a genetic testing panel |
| Excellent communication between genetics and surgical teams regarding results, particularly positive and VUS results |
| Development of a location for genetic test results storage in the electronic medical record |
| Opportunity for a patient testing positive to meet with a genetic counselor to discuss cancer risks and cascade testing |
VUS variant of uncertain significance
In summary, tremendous opportunities exist for surgeons and the BC care team to partner with genetics professionals to develop efficient integration of genetics evaluation at the time of a BC diagnosis. A multidisciplinary clinic can successfully integrate GT for all breast cases with a high acceptance rate, successfully incorporating genetic test results into treatment planning.
Acknowledgments
We acknowledge the following sources of research support: National Institutes of Health grants R35-CA197461 and P30-CA14089. We thank our interns, Sydney Fraser, Rebecca Schapiro, and Amber Sher, who performed a chart review and pedigree analysis of study participants.
Disclosure
Irene Kang has accepted consulting fees and speaker bureau fees for Puma Biotechnology and consulting fees for Bristol Myers Squibb. Ketan Patel received textbook royalties from Elsevier. The remaining authors have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol. 2016;34:611–635. doi: 10.1200/JCO.2015.64.3809. [DOI] [PubMed] [Google Scholar]
- 2.Owens DK, Davidson KW, Krist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:652–665. doi: 10.1001/jama.2019.10987. [DOI] [PubMed] [Google Scholar]
- 3.Manahan ER, Kuerer HM, Sebastian M, et al. consensus guidelines on genetic testing for hereditary breast cancer from the American Society of Breast Surgeons. Ann Surg Oncol. 2019;26:3025–3031. doi: 10.1245/s10434-019-07549-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists Hereditary cancer syndromes and risk assessment: ACOG committee opinion, no. 793. Obstet Gynecol. 2019;134:e143-9. doi: 10.1097/AOG.0000000000003562. [DOI] [PubMed] [Google Scholar]
- 5.Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw. 2021;19:77–102. doi: 10.6004/jnccn.2021.0001. [DOI] [PubMed] [Google Scholar]
- 6.Stuckey A, Febbraro T, Laprise J, Wilbur JS, Lopes V, Robison K. Adherence patterns to National Comprehensive Cancer Network guidelines for referral of women with breast cancer to genetics professionals. Am J Clin Oncol. 2016;39:363–367. doi: 10.1097/COC.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 7.Kurian AW, Ward KC, Howlader N, et al. Genetic testing and results in a population-based cohort of breast cancer patients and ovarian cancer patients. J Clin Oncol. 2019;37:1305–1315. doi: 10.1200/JCO.18.01854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beitsch PD, Whitworth PW, Hughes K, et al. Underdiagnosis of hereditary breast cancer: are genetic testing guidelines a tool or an obstacle? J Clin Oncol. 2019;37:453–460. doi: 10.1200/JCO.18.01631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiba A, Hoskin TL, Hallberg EJ, et al. Impact that timing of genetic mutation diagnosis has on surgical decision-making and outcome for BRCA1/BRCA2 mutation carriers with breast cancer. Ann Surg Oncol. 2016;23:3232–3238. doi: 10.1245/s10434-016-5328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384:2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35:2232–2239. doi: 10.1200/JCO.2016.71.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cragun D, Vadaparampil S, Scherr C, Pal T. Comment on “Can breast surgeons provide breast cancer genetic testing? An American Society of Breast Surgeons Survey”. Ann Surg Oncol. 2017;24:588–589. doi: 10.1245/s10434-017-6190-y. [DOI] [PubMed] [Google Scholar]
- 13.Milliron KJ, Griggs JJ. Advances in genetic testing in patients with breast cancer, high-quality decision making, and responsible resource allocation. J Clin Oncol. 2019;37:445–447. doi: 10.1200/JCO.18.01952. [DOI] [PubMed] [Google Scholar]
- 14.Wevers MR, Aaronson NK, Bleiker EMA, et al. Rapid genetic counseling and testing in newly diagnosed breast cancer: patients’ and health professionals’ attitudes, experiences, and evaluation of effects on treatment decision making. J Surg Oncol. 2017;116:1029–1039. doi: 10.1002/jso.24763. [DOI] [PubMed] [Google Scholar]
- 15.Kishan AU, Gomez CL, Dawson NA, et al. Increasing appropriate BRCA1/2 mutation testing: the role of family history documentation and genetic counseling in a multidisciplinary clinic. Ann Surg Oncol. 2016;23:634–641. doi: 10.1245/s10434-016-5545-0. [DOI] [PubMed] [Google Scholar]
- 16.Quinn VF, Meiser B, Kirk J, et al. Streamlined genetic education is effective in preparing women newly diagnosed with breast cancer for decision making about treatment-focused genetic testing: a randomized controlled noninferiority trial. Genet Med. 2017;19:448–456. doi: 10.1038/gim.2016.130. [DOI] [PubMed] [Google Scholar]
- 17.Pederson HJ, Hussain N, Noss R, et al. Impact of an embedded genetic counselor on breast cancer treatment. Breast Cancer Res Treat. 2018;169:43–46. doi: 10.1007/s10549-017-4643-4. [DOI] [PubMed] [Google Scholar]
- 18.Dettwyler SA, Thull DL, McAuliffe PF, et al. Timely cancer genetic counseling and testing for young women with breast cancer: impact on surgical decision-making for contralateral risk-reducing mastectomy. Breast Cancer Res Treat. 2022;194:393–401. doi: 10.1007/s10549-022-06619-y. [DOI] [PubMed] [Google Scholar]
- 19.Metcalfe KA, Eisen A, Poll A, et al. Frequency of contralateral prophylactic mastectomy in breast cancer patients with a negative BRCA1 and BRCA2 rapid genetic test result. Ann Surg Oncol. 2021;28:4967–4973. doi: 10.1245/s10434-021-09855-6. [DOI] [PubMed] [Google Scholar]
- 20.Childers CP, Childers KK, Maggard-Gibbons M, Macinko J. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800–3806. doi: 10.1200/JCO.2017.73.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurian AW, Ward KC, Abrahamse P, et al. Time trends in receipt of germline genetic testing and results for women diagnosed with breast cancer or ovarian cancer, 2012–2019. J Clin Oncol. 2021;39(15):1631. doi: 10.1200/JCO.20.02785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alberty-Oller JJ, Weltz S, Santos A, et al. Adherence to NCCN guidelines for genetic testing in breast cancer patients: who are we missing? Ann Surg Oncol. 2021;28:281–286. doi: 10.1245/s10434-020-09123-z. [DOI] [PubMed] [Google Scholar]
- 23.Cragun D, Weidner A, Lewis C, et al. Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer. 2017;123:2497–2505. doi: 10.1002/cncr.30621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chapman-Davis E, Zhou ZN, Fields JC, et al. Racial and ethnic disparities in genetic testing at a hereditary breast and ovarian cancer center. J Gen Intern Med. 2021;36:35–42. doi: 10.1007/s11606-020-06064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson JM, Pepin A, Thomas R, et al. Racial disparities in breast cancer hereditary risk assessment referrals. J Genet Couns. 2020;29:587–593. doi: 10.1002/jgc4.1250. [DOI] [PubMed] [Google Scholar]
- 26.Rajagopal PS, Catenacci DVT, Olopade OI. The time for mainstreaming germline testing for patients with breast cancer is now. J Clin Oncol. 2019;37:2177–2178. doi: 10.1200/JCO.19.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beard C, Monohan K, Cicciarelli L, James PA. Mainstream genetic testing for breast cancer patients: early experiences from the Parkville Familial Cancer Centre. Eur J Hum Genet. 2021;29:872–880. doi: 10.1038/s41431-021-00848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kemp Z, Turnbull A, Yost S, et al. Evaluation of cancer-based criteria for use in mainstream BRCA1 and BRCA2 genetic testing in patients with breast cancer. JAMA Netw Open. 2019;2:e194428. doi: 10.1001/jamanetworkopen.2019.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin J, Sharaf RN, Saganty R, et al. Achieving universal genetic assessment for women with ovarian cancer: are we there yet? A systematic review and meta-analysis. Gynecol Oncol. 2021;162(2):506–516. doi: 10.1016/j.ygyno.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker EJ, Goldberg D, Gordon KM, et al. Implementation of an embedded in-clinic genetic testing station to optimize germline testing for patients with pancreatic adenocarcinoma. Oncologist. 2021;26:e1982–e1991. doi: 10.1002/onco.13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wain KE, Azzariti DR, Goldstein JL, et al. Variant interpretation is a component of clinical practice among genetic counselors in multiple specialties. Genet Med. 2020;22:785–792. doi: 10.1038/s41436-019-0705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berliner JL, Cummings SA, Boldt Burnett B, Ricker CN. Risk assessment and genetic counseling for hereditary breast and ovarian cancer syndromes: practice resource of the National Society of Genetic Counselors. J Genet Couns. 2021;30:342–360. doi: 10.1002/jgc4.1374. [DOI] [PubMed] [Google Scholar]