Abstract
Purpose
To evaluate the impact of Covid-19 (Pfizer-BioNTech BNT162b2) third booster dose vaccination during pregnancy on maternal and neonatal outcomes.
Methods
This is a multicenter, retrospective computerized database study. Parturients who delivered in Israel between August and December 2021 with full records of Covid-19 disease and vaccination status were included. Those who received third booster during pregnancy were compared to those who received two doses of vaccine during pregnancy and to unvaccinated parturients. Various adverse maternal and neonatal outcomes were evaluated. Parturients who were previously positive with Covid-19 PCR swabs during pregnancy or before pregnancy were excluded. Univariate analysis was followed by multivariate analysis.
Results
A total of 2583 women were included in the analysis; 626 received the third booster dose of the BNT162b2 Covid-19 vaccine, 1094 received two doses of the vaccine, and 863 unvaccinated women. Maternal and neonatal outcomes were comparable between the study groups. An adjusted multivariable logistic regression analysis demonstrated that receiving the third booster was not associated with an increase in neither composite adverse maternal or neonatal outcome (aOR 0.9; 95% CI [0.65–1.22], p = 0.47; aOR 0.7; 95% CI [0.53–1.035], p= 0.09, respectively) when compared to those who received two doses of the vaccine. However, administration of the third booster dose during pregnancy was associated with a reduced composite adverse neonatal outcome when compared to unvaccinated women (aOR 0.6; 95% CI [0.42–0.86], p = 0.01).
Conclusion
Receiving the third booster dose of the BNT162b2 Covid-19 vaccine during pregnancy is not associated with an increased risk of any adverse maternal outcomes and may be beneficial for the neonates.
Keywords: Health care coverage, Covid-19 vaccine, Booster dose, Pregnancy, Outcome
What does this study add to the clinical work
| In this multicenter retrospective cohort study, we have shown that uptake of the third booster dose of the Covid-19 vaccine during pregnancy is not associated with an increase in composite adverse maternal outcome and may even be associated with reduced adverse neonatal outcomes. Pregnant women should be reassured regarding the safety of the third booster dose the Covid-19 vaccine during pregnancy. |
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in pregnant women is more severe compared to their non-pregnant counterparts especially in the third trimester of pregnancy, with an increased risk of hospital admission, preterm birth, intensive care unit (ICU) stay, mechanical ventilation, and even death [1–4]. Pregnant women with Covid-19 as compared to healthy pregnant women have also higher risk of severe maternal and fetal complications: preeclampsia and thrombosis, preterm delivery, cesarean delivery (CD), mechanical ventilation, ICU admissions, admission to the neonatal intensive care unit (NICU), and maternal and neonatal death [5–8]. All these can be explained as a result of the immunological features of pregnant women who are supposed to be particularly susceptible to intracellular infections as well as immunological disturbances, especially during the late second and third trimesters [9].
Immunization against SARS-CoV-2 with mRNA vaccines remains the most effective way to prevent Covid-19-related morbidity and mortality [10]. However, pregnant women were not included in the initial Covid-19 vaccine trials [10, 11]. Current data demonstrate the efficacy of the two doses—mRNA vaccination for up to 5–6 months, as supported by immunogenicity studies [12, 13].
Although mRNA Covid-19 vaccine safety was not confirmed in randomized trials for pregnant women, several observational studies showed no safety concerns among pregnant women who received mRNA Covid-19 vaccines during pregnancy or for their neonates [14, 15]. In Israel, the Ministry of Health encouraged pregnant women to receive the third booster dose as part of the Israeli national vaccination program. Since the recommendations for a third booster dose continue to evolve, and scant data exist regarding the third booster dose especially in pregnancy [16, 17], we aimed to evaluate the impact of the third Covid-19 booster dose (Pfizer-BioNTech BNT162b2) on maternal and neonatal outcomes.
Methods
Study design
A multicenter retrospective cohort database study was conducted in two university-affiliated medical centers in Jerusalem, Israel. These medical centers serve Jerusalem’s population (> 1,200,000 residents), together the deliveries at these medical centers account for approximately 16% of all deliveries in the state of Israel, with a mean annual volume of 22,000 deliveries.
The National Health Plan covers antepartum and delivery care for all women and neonates; more than 95% of deliveries are managed by the public system. Labor and delivery data were extracted from the medical record database, which is updated in real-time during labor and delivery by attending healthcare professionals and audited periodically by trained technical personnel to confirm the validity of data.
The study population included all women aged 18 years or older, without documented previous positive SARS-CoV-2 polymerase-chain-reaction (PCR) test (in pregnancy or before pregnancy), delivered between August 28 (when the first group of women who received the third booster dose was delivered) and December 31, 2021. Women who received only the first dose of the vaccine and women with current or previous Covid-19 disease were excluded to assess the sole effect of the third booster dose on pregnancy outcomes as compared to women who had two doses of vaccine before and during pregnancy and unvaccinated women with no previous Covid-19 disease.
Upon labor admission, women were questioned regarding their Covid-19 disease and/or vaccine status, as well as potential current Covid-19 symptoms and/or suspicious contacts (travel abroad, contact with active confirmed Covid-19-positive persons or persons in Covid-19 quarantine, etc.). At the time of admission and during hospitalization, all symptomatic women, or those with a primary contact with Covid-19, were tested for SARS-CoV-2, using a real-time reverse-transcription PCR test of nasopharyngeal swabs. We excluded women from the present study if their Covid-19 disease/vaccine status was undocumented or if they were positive for Covid-19 before or during their hospitalization for delivery.
The exposure measure of the study was the third Covid-19 booster dose of Pfizer-BioNTech BNT162b2, administered during the index pregnancy. All women included in the study received the same vaccine preparation for any vaccination.
The group of women who received the third Covid-19 booster dose during pregnancy was compared to:
Women who received two doses of the vaccine during the index pregnancy.
Women who did not receive the vaccine at any time (unvaccinated).
For the study, we defined a composite adverse maternal outcome as our primary outcome: a recorded diagnosis of one or more of the following: chorioamnionitis, postpartum hemorrhage (PPH, estimated blood loss > 1000 mL, and/or hemoglobin drop ≥ 3 g/dL), endometritis, blood products transfusion, CD, ICU admission, maternal hospital length of stay > 5 days for vaginal delivery and > 7 days for CD (the recommended routine postpartum hospital lengths of stay for vaginal deliveries and CD are 48–72 h and 96–120 h, respectively).
The secondary outcome was individual adverse maternal outcomes that comprised the composite adverse maternal outcome as well as individual adverse neonatal outcomes and a composite adverse neonatal outcome measure.
Composite adverse neonatal outcome was defined as one or more of the following recorded in the medical record: intrauterine fetal death (IUFD), 1′ Apgar score ≤ 7, 5′ Apgar score ≤ 7, neonatal intensive care unit (NICU) admission, neonatal asphyxia, intracranial hemorrhage (any severity), meconium aspiration syndrome, jaundice, neonatal seizures, neonatal hypoglycemia, neonatal sepsis (early and late), and mechanical ventilation.
The study was approved by the institutional ethics committee and was performed according to the principles of the Declaration of Helsinki. Data were obtained from medical records and de-identified, with no direct participation of patients; hence, informed consent was waived.
Statistical analysis
Categorical variables were presented as percentages and compared using the Chi-square or Fisher’s exact tests, as appropriate. Continuous variables with a normal distribution were presented as mean and standard deviation, whereas those without a normal distribution were presented as the median and interquartile range (IQR). Comparisons were made using Student’s t-test and Mann–Whitney U test for normally and non-normally distributed data, respectively.
To test the independent association between Covid-19 vaccination and the primary outcome (composite adverse maternal outcome) and one of the secondary outcomes—composite adverse neonatal outcome, two separate multivariable logistic regression models were applied. All variables found to be significantly associated with the risk of each outcome in univariate analysis were included. Accordingly, the multivariate models for risk assessment for the composite adverse maternal outcome were adjusted for: maternal age, parity, previous miscarriage, induction of labor, oxytocin augmentation of labor, and epidural analgesia.
The models were fitted to assess the risk of composite neonatal outcome (secondary outcome) while controlling for: maternal age, previous CD, diabetes (pre-gestational and gestational), multifetal gestation, epidural analgesia, gestational age at delivery, and neonatal birth weight.
All analyses were two-sided, and a p value < 0.05 was considered to indicate statistical significance. Adjusted odds ratios (aOR) and 95% confidence intervals (CI) were computed.
Analyses were carried out using the IBM SPSS version 25 statistical package.
Results
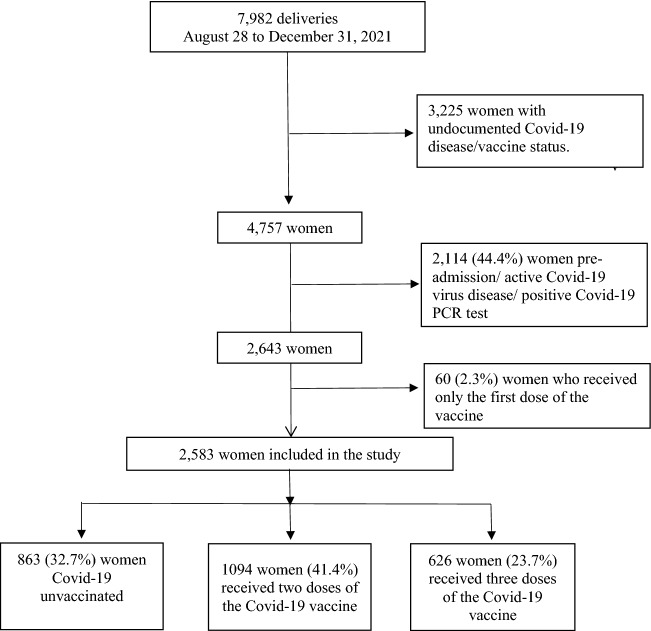
During the study period, 7982 women gave birth at both medical centers participating in the study (SZMC n = 5786 and BHMC n = 2196). Of those, 4757 (59.6%) women had documented Covid-19 disease/vaccine status.
Among women who were not Covid-19 positive on admission or before admission (n = 2643) uptake of the Covid-19 vaccine occurred in 1780 women (67.3%) who received at least one dose of the Covid-19 vaccine, while 863 (32.7%) women did not receive any dose of the Covid-19 vaccine. Of those who received the Covid-19 vaccine, 60 women (2.3%) received 1 dose (not included in the study), 1094 (40.4%) received 2 doses and 626 (23.7%) received 3 doses. Finally, 2583 women were eligible for inclusion in the present study (Fig. 1).
Fig. 1.
Study population schematic flowchart
Comparisons between women who received three doses of the vaccine (n = 626) to those who received two doses of the vaccine (n = 1094) and to those who received none (n = 863) with regards to demographics and obstetric characteristics are presented in Table 1. Women who received the third booster dose of vaccine were older and had higher rates of previous CD and obesity as compared to the other study groups. Markedly, they were of higher gravidity and parity as compared to those who received two doses of vaccine. In comparison to the unvaccinated women, they had higher rates of diabetes mellitus (pre-gestational and gestational), and anemia (Hb < 11gr%) on admission to labor, as well as immigrant status (i.e., having been born outside of Israel), as compared to unvaccinated women.
Table 1.
Demographic and obstetric characteristics of the study groups
| Covid-19 two-dose vaccinated n = 1094 |
Covid-19 booster vaccinated n = 626 |
p value | Covid-19 unvaccinated n = 863 |
p value | |
|---|---|---|---|---|---|
| Maternal age, years | 28.9 ± 5.7 | 30.5 ± 6 | < 0.01 | 28.4 ± 6.2 | < 0.01 |
| Gravidity | 3 [2–5] | 4 [2–6] | < 0.01 | 4 [2–6] | 0.29 |
| Parity | 3 [2–5] | 3 [2–5] | < 0.01 | 3 [2–5] | 0.06 |
| Previous miscarriages | 325 (29.7%) | 212 (33.9%) | 0.07 | 271 (31.4%) | 0.32 |
| Previous cesarean delivery, any | 131 (12%) | 117 (18.7%) | < 0.01 | 93 (10.8%) | < 0.01 |
| Primiparous | 254 (23.2%) | 110 (17.6%) | 0.01 | 185 (21.4%) | 0.06 |
| Fertility treatments (any) | 16 (1.5%) | 10 (1.6%) | 0.83 | 24 (2.8%) | 0.13 |
| Hypertensive disorders of pregnancy | 13 (1.2%) | 12 (1.9%) | 0.22 | 10 (1.2%) | 0.23 |
| Diabetes (pre-gestational + gestational) | 59 (5.4%) | 45 (7.2%) | 0.13 | 36 (4.2%) | 0.01 |
| Obesity (BMI ≥ 30) | 54 (4.9%) | 50 (8%) | 0.01 | 34 (3.9%) | < 0.01 |
| Multifetal gestation | 17 (1.6%) | 5 (0.8%) | 0.18 | 6 (0.7%) | 0.82 |
| Positive records of smoking habit | 26 (2.5%) | 13 (2.1%) | 0.66 | 16 (1.9%) | 0.75 |
| Immigranta | 144 (15.3%) | 83 (14.6%) | 0.69 | 48 (7.3%) | < 0.01 |
| Anemia (Hb < 11gr%) upon admission for labor and delivery | 139 (12.7%) | 64 (10.2%) | 0.12 | 122 (14.1%) | 0.02 |
Data are mean ± standard deviation; median [IQR], number (%)
BMI Body Mass Index
aBorn outside of Israel
Primary outcome
The rate of composite adverse maternal outcome was comparable among all study groups. An adjusted multivariable logistic regression analysis for significant covariates and confounders demonstrated that vaccination of the third booster dose of Covid-19 vaccine was not associated with the maternal composite adverse outcome when compared to women who received two doses (aOR 0.9; 95% CI [0.65–1.22], p = 0.47) or unvaccinated women (aOR 0.73; 95% CI [0.52–1.08], p = 0.08) (Table 2).
Table 2.
Multivariate logistic regression analysis for the association between vaccination with the third Covid-19 booster dose and composite maternal outcomes (adjusted odds ratio)
| p value | aOR | 95% CI | ||
|---|---|---|---|---|
| Comparison with women who received two doses of the vaccine | ||||
| Parity | < 0.01 | 0.76 | 0.68 | 0.85 |
| Oxytocin augmentation of labor | 0.13 | 1.38 | 0.91 | 2.08 |
| Induction of labor | 0.25 | 1.39 | 0.79 | 2.43 |
| Epidural analgesia | 0.34 | 1.21 | 0.82 | 1.80 |
| Third Covid-19 booster dose vs. two doses | 0.47 | 0.89 | 0.65 | 1.22 |
| Previous miscarriages | 0.69 | 0.96 | 0.78 | 1.17 |
| Maternal age, years | 0.93 | 1.00 | 0.96 | 1.03 |
| Comparison with unvaccinated women | ||||
| Parity | < 0.01 | 0.73 | 0.68 | 0.81 |
| Oxytocin augmentation of labor | 0.03 | 1.58 | 1.04 | 2.40 |
| Third Covid-19 booster dose vs. none | 0.08 | 0.73 | 0.52 | 1.03 |
| Induction of labor | 0.15 | 1.60 | 0.85 | 3.04 |
| Maternal age, years | 0.31 | 1.02 | 0.98 | 1.06 |
| Previous miscarriages | 0.36 | 1.09 | 0.90 | 1.32 |
| Epidural analgesia | 0.79 | 1.05 | 0.71 | 1.57 |
CI confidence interval, aOR adjusted odds ratio
Secondary outcomes
Individual adverse maternal outcomes are presented in Table 3. Women who received the third booster dose of the vaccine during pregnancy had higher rates of CDs, mostly planned CDs, higher rates of blood products transfusion as compared to those who received two doses of vaccine, and longer hospitalization as compared to the unvaccinated group. However, they had lower rates of meconium-stained amniotic fluid as compared to both groups.
Table 3.
Obstetric and maternal outcomes among the study groups
| Covid-19 two-dose vaccinated n = 1094 |
Covid-19 booster vaccinated n = 626 |
p value | Covid-19 unvaccinated n = 863 |
p value | |
|---|---|---|---|---|---|
| Gestational age at delivery | 39.4 ± 1.5 | 39.1 ± 1.5 | < 0.01 | 39.4 ± 1.5 | < 0.01 |
| Gestational age at delivery < 34 weeks | 12 (1.1%) | 6 (1%) | 0.79 | 5 (0.6%) | 0.40 |
| Gestational age at delivery < 37 weeks | 36 (3.3%) | 25 (4%) | 0.45 | 37 (4.3%) | 0.78 |
| Induction of labor | 61 (5.6%) | 28 (4.5%) | 0.32 | 35 (4.1%) | 0.69 |
| Oxytocin augmentation of labor | 686 (62.7%) | 362 (57.8%) | 0.05 | 507 (58.7%) | 0.72 |
| Epidural analgesia | 634 (58%) | 353 (56.4%) | 0.53 | 382 (44.3%) | < 0.01 |
| Meconium-stained amniotic fluid | 186 (17%) | 83 (13.3%) | 0.04 | 194 (22.5%) | < 0.01 |
| Chorioamnionitis | 23 (2.1%) | 13 (2.1%) | 0.97 | 20 (2.3%) | 0.76 |
| Cesarean delivery | 144 (13.2%) | 105 (16.8%) | 0.04 | 91 (10.5%) | < 0.01 |
| Planned cesarean delivery | 68 (6.2%) | 63 (10.1%) | < 0.01 | 35 (4.1%) | < 0.01 |
| In labor cesarean | 63 (5.8%) | 32 (5.1%) | 0.57 | 40 (4.6%) | 0.67 |
| Home/car delivery | 11 (1%) | 10 (1.6%) | 0.28 | 14 (1.6%) | 0.97 |
| Vacuum-assisted delivery | 80 (7.3%) | 43 (6.9%) | 0.73 | 47 (5.4%) | 0.26 |
| Hospitalization length, days | 1.9 ± 1 | 1.9 ± 1.1 | 0.42 | 1.7 ± 1 | 0.01 |
| Prolonged hospital stay | 6 (0.5%) | 7 (1.1%) | 0.19 | 2 (0.2%) | 0.03 |
| Episiotomy | 97 (8.9%) | 62 (9.9%) | 0.48 | 107 (12.4%) | 0.13 |
| Maternal ICU admissions | 0 (0%) | 1 (0.2%) | 0.19 | 2 (0.2%) | 0.76 |
| Postpartum hemorrhage | 96 (8.8%) | 41 (6.5%) | 0.10 | 77 (8.9%) | 0.09 |
| Placental abruption | 10 (0.9%) | 9 (1.4%) | 0.32 | 23 (2.7%) | 0.11 |
| Hemoglobin drop, g/dL | 1.3 ± 1 | 1.3 ± 1 | 0.81 | 1.2 ± 1 | 0.31 |
| Hemoglobin drop > 4 g/dL | 26 (2.4%) | 12 (1.9%) | 0.53 | 21 (2.4%) | 0.50 |
| Puerperal fever | 54 (4.9%) | 24 (3.8%) | 0.29 | 35 (4.1%) | 0.83 |
| Blood products transfusion | 8 (0.7%) | 13 (2.1%) | 0.01 | 8 (0.9%) | 0.06 |
| Composite adverse maternal outcome | 144 (13.2%) | 68 (10.9%) | 0.16 | 113 (13.1%) | 0.19 |
Data are mean ± standard deviation; number (%)
ICU intensive care unit
aComposite adverse maternal outcome was defined as one of the following: cesarean delivery, prolonged hospitalization, postpartum hemorrhage, blood products transfusion, maternal ICU admissions, chorioamnionitis, and endometritis
Individual adverse neonatal outcomes are presented in Table 4. We did not identify any neonatal outcome that differed among the study groups.
Table 4.
Neonatal outcomes among the study groups
| Covid-19 two-dose vaccinated n = 1094 |
Covid-19 booster vaccinated n = 626 |
p value | Covid-19 unvaccinated n = 863 |
p value | |
|---|---|---|---|---|---|
| Birthweight, g | 3311.4 ± 449.2 | 3275.7 ± 448.1 | 0.11 | 3298.3 ± 459.4 | 0.35 |
| LGA | 103 (9.4%) | 55 (8.8%) | 0.66 | 81 (9.4%) | 0.69 |
| SGA | 59 (5.4%) | 41 (6.5%) | 0.33 | 55 (6.4%) | 0.89 |
| Male gender | 570 (52.1%) | 310 (49.5%) | 0.30 | 445 (51.6%) | 0.44 |
| 1-minute Apgar score ≤ 7 | 39 (3.6%) | 27 (4.3%) | 0.44 | 42 (4.9%) | 0.62 |
| 5-minute Apgar score ≤ 7 | 20 (1.8%) | 16 (2.6%) | 0.31 | 22 (2.5%) | 0.99 |
| Intrauterine fetal death | 58 (5.3%) | 24 (3.8%) | 0.17 | 41 (4.8%) | 0.39 |
| NICU admission | 0 (0%) | 0 (0%) | N/A | 0 (0%) | N/A |
| Meconium aspiration syndrome | 23 (2.1%) | 12 (1.9%) | 0.79 | 34 (3.9%) | 0.03 |
| Jaundice | 4 (0.4%) | 1 (0.2%) | 0.45 | 2 (0.2%) | 0.76 |
| TTN | 4 (0.4%) | 1 (0.2%) | 0.45 | 2 (0.2%) | 0.76 |
| Mechanical ventilation (any) | 4 (0.4%) | 1 (0.2%) | 0.45 | 4 (0.5%) | 0.32 |
| Neonatal seizures | 2 (0.2%) | 2 (0.3%) | 0.57 | 1 (0.1%) | 0.39 |
| Hypoglycemia | 10 (0.9%) | 8 (1.3%) | 0.48 | 13 (1.5%) | 0.71 |
| Sepsis (any) | 0 (0%) | 0 (0%) | N/A | 1 (0.1%) | 0.39 |
| Encephalopathy | 0 (0%) | 0 (0%) | N/A | 0 (0%) | N/A |
| Intracranial hemorrhage (all grades) | 0 (0%) | 0 (0%) | N/A | 0 (0%) | N/A |
| Birth asphyxia | 1 (0.1%) | 0 (0%) | 0.45 | 2 (0.2%) | 0.23 |
| Composite adverse neonatal outcome | 123 (11.2%) | 65 (10.4%) | 0.58 | 114 (13.2%) | 0.10 |
Data are mean ± standard deviation; number (%)
LGA Large for gestational age, SGA small-for-gestational-age, NICU neonatal intensive care unit, TTN transient tachypnea of the newborn
aComposite adverse neonatal outcome was defined as one of the following: Intrauterine fetal death, 1′ Apgar score < 7, 5′ Apgar score < 7, NICU admission, neonatal asphyxia, jaundice, neonatal hypoglycemia, meconium aspiration, TTN, mechanical ventilation, sepsis, seizures, intracranial hemorrhage, and encephalopathy
In univariate analysis, the composite adverse neonatal outcome also did not differ between the groups, however, in multivariate analysis, the third booster dose of the Covid-19 vaccine during pregnancy was not associated with an increased neonatal risk as compared to women who received two doses (aOR 0.7; 95% CI [0.53–1.035], p= 0.09). Yet, when compared to unvaccinated women, the risk for neonatal composite adverse outcomes was significantly reduced (aOR 0.6; 95% CI [0.42–0.86], p = 0.01) (Table 5).
Table 5.
Multivariate logistic regression analysis for the association between vaccination with the third Covid-19 booster dose and composite neonatal outcomes (adjusted odds ratio)
| p value | aOR | 95% CI | ||
|---|---|---|---|---|
| Comparison with women who received two doses of the vaccine | ||||
| Diabetes (pre-gestational + gestational) | < 0.01 | 2.88 | 1.75 | 4.76 |
| Gestational age at delivery, weeks | < 0.01 | 0.82 | 0.73 | 0.93 |
| Neonatal birthweight, g | 0.01 | 1.00 | 1.00 | 1.00 |
| Previous cesarean delivery, any | 0.03 | 1.57 | 1.04 | 2.38 |
| Third Covid-19 booster dose vs. two doses | 0.09 | 0.74 | 0.53 | 1.05 |
| Epidural analgesia | 0.62 | 0.92 | 0.66 | 1.27 |
| Maternal age, years | 0.72 | 1.01 | 0.98 | 1.03 |
| Multifetal gestation | 0.73 | 0.83 | 0.29 | 2.40 |
| Comparison with unvaccinated women | ||||
| Diabetes (pre-gestational + gestational) | < 0.01 | 3.69 | 2.15 | 6.31 |
| Gestational age at delivery, weeks | < 0.01 | 0.74 | 0.65 | 0.84 |
| Third Covid-19 booster dose vs. none | 0.01 | 0.60 | 0.42 | 0.86 |
| Neonatal birthweight, g | 0.07 | 1.00 | 1.00 | 1.00 |
| Maternal age, years | 0.34 | 1.01 | 0.99 | 1.04 |
| Epidural analgesia | 0.80 | 0.96 | 0.68 | 1.34 |
| Previous cesarean delivery, any | 0.84 | 0.95 | 0.59 | 1.53 |
| Multifetal gestation | 0.90 | 0.91 | 0.22 | 3.80 |
CI Confidence interval, aOR adjusted odds ratio
Discussion
Main findings
In this multicenter retrospective cohort study, we have shown that uptake of the third booster dose of the Covid-19 vaccine in women who were not Covid-19 positive during pregnancy or admission for delivery was 67.3% (1780/2643). Even though 32.7% of those included in the study did not receive any dose of the Covid-19 vaccine before and during pregnancy, 40.4% received two doses and 23.7% received three doses. Some demographic and obstetric differences exist between those who received the third booster dose and those vaccinated twice or not at all. Despite the higher obstetric risk of women who received the third booster dose (older women of higher gravidity and parity, with higher rates of previous CD and obesity), their composite adverse obstetric outcomes did not differ from those who received two doses or none at all. The multivariable analysis confirmed this finding. Furthermore, neonatal outcomes were comparable between those who received two doses and those who received the third booster dose, however women who received the third booster dose had a reduced rate of composite adverse neonatal outcomes as compared to unvaccinated women (aOR 0.6; 95% CI [0.42–0.86], p= 0.01).
Interpretation
Previous studies have shown that the rate of vaccination uptake ranged from 20.8% to 21.8% to 23% to 28.5% to 40.2% and 41% [14–16, 18–21]. In one of these studies, which examined women’s attitudes and fears regarding the Covid‑19 vaccination, pregnant women show very low vaccination willingness (13.8%), mostly because of lack of scientific evidence on the safety of the vaccination during pregnancy and the fear to harm the fetus or infant [21]. However, while later studies have shown that women who received the Covid-19 vaccine did not have an increased rate of obstetric complications as compared to women who did not receive the vaccine, including rates of preterm birth, small-for-gestational-age (SGA), postpartum hemorrhage (PPH), chorioamnionitis and CD, the rate of vaccination uptake among pregnant women remain still low as compere to the general population [14–16].
The safety of the vaccine as mentioned above in terms of obstetrical complications along with the safety of the vaccine in the general population [10] and later as evident in the obstetric population [22] and the reduced risk of SARS-CoV-2 infection in pregnant women [23] all may have a led to the increase in the uptake of the Covid-19 vaccine as noted in our study as opposed to previous studies.
Only recently the safety and efficacy of a third booster dose of BNT162b2 Covid-19 vaccine were reported from a placebo-controlled, randomized, phase 3 trial, in the general population [24]. It was noted that local and systemic reactogenicity events from the third booster dose were generally of low grade and there were no new safety signals identified, and no cases of myocarditis or pericarditis were reported, while the relative vaccine efficacy was 95.3%.
Hence, the current recommendation of the American College of Obstetricians and Gynecologists to administer the third booster dose of the Covid-19 vaccine [25] may be supported by the safety and efficacy studies in the general population along with the current study, which demonstrates its safety in terms of maternal and neonatal outcomes.
Scarce data exist regarding the third booster dose in pregnancy. In one study [26] that described the association of gestational age at Covid-19 vaccination, history of SARS-CoV-2 infection, and a vaccine booster dose, with maternal and umbilical cord antibody levels at delivery, only 20 women out of 1359 vaccinated women received the third booster dose, and it was associated with the highest maternal and umbilical cord antibody levels. Other than this study we have failed to find a report on the third booster dose in pregnancy. Hence, even though our study results echo the results on the safety of the Covid-19 vaccine in general in the obstetric population, i.e. we showed no adverse maternal or obstetrical complications, additional studies are needed to state this with certainty.
While the third booster dose of the Covid-19 vaccine during pregnancy was not associated with an increased neonatal risk as compared to women who received two doses, when compared to unvaccinated women, the third booster dose of the Covid-19 vaccine during significantly reduced the risk for neonatal composite adverse outcomes. Notably, in univariate analysis, only meconium aspiration syndrome rates were found significantly lower among women who received the third booster dose compared to unvaccinated women. In a recent systematic review and meta-analysis who evaluated the neonate’s outcome, two doses of the vaccine were observed to be protective against NICU admissions [27]. In the study of Dick et al. who examined some neonate’s outcomes of women who received the third booster dose, the rates of Umbilical artery pH<7.1 was significantly lower among the third doses group compared to unvaccinated women [28]. This interesting finding, obviously this is not due to the protection towards Covid-19 since all groups did not develop Covid-19 infection. A possible explanation, may be attributed to the healthier medical behavior of women who choose to receive the vaccine including better hygiene, wearing masks adequate perinatal follow-up and nutritional and supplementation vitamins. Good compliance to pandemic restrictions may protect from other and several other obstetrics risks as some studies showing better obstetric outcomes in pregnancy during the pandemic as compared to before the pandemic [29], however, since the biological mechanisms underlying each outcome are distinct, this finding should be further evaluated in future studies.
Our study did not address the reduction in the risk of SARS-CoV-2 in pregnant women who received the third booster dose, we also have not studied the transfer of protective antibodies to the neonate. However, studies have previously shown that pregnant women who receive the Covid-19 vaccine have lower rates of SARS-CoV-2 infection and severe infection [23] and women who received the vaccine during pregnancy transfer protective antibodies to their neonates [30]. Hence, having the added value of the third booster dose along with the lack of harm in terms of obstetrical complications, in addition to the possible added benefit in terms of neonatal outcomes, and antibody transfer, the net benefit of the third booster dose administered to our pregnant patients appears clear.
While the evidences regarding Covid-19 vaccination’s safety during pregnancy are increasing, some medical practitioners still suggest caution with Covid-19 vaccine in the first trimester and many women prefer to avoid from vaccination during the embryogenesis [21]. Although the current data did not support any concerns regarding first trimester vaccination including miscarriages and congenital malformations rates [19, 31, 32], this important information should be further studied to increase compliance rates and decrease Covid-19 morbidity.
Strengths and limitations
Strengths of this study include its multicenter design, its meticulous data collection, and all records were derived from a real-time updated electronic database, minimizing the possibility of bias. Nonetheless, the present study has several caveats. (1) The retrospective design raises the possibility of biases inherent to such investigations. (2) We cannot exclude possible unknown factors (such as socioeconomic status) which may impact the findings; however, all health and vaccine costs of the population are covered by the National Health Care system. Vaccination was free and easily accessible to all. Thus, the socioeconomic status would likely have little or no influence on the rate of vaccination. (3) During the duration of the study, SZMC and BHMC did not screen all women who gave birth, and vaccination was universally available, independent of previous Covid-19 infection status. This may have led to the inclusion of women with previous asymptomatic disease. If this was an unintended bias then we might expect it to be similar in both groups, while if it was intended (women did not disclose their previous Covid-19 symptoms or did not present for nasopharyngeal swabs), we expect this to affect the non-vaccinated rather the vaccinated groups. Since we found no difference in adverse outcomes, and Covid-19 supposedly adversely affects the outcomes investigated in the present study, we may assume that this was not the case. (4) We have no information on the interval between the second or third booster dose of vaccination and the delivery; in some, the interval might have been too short to reveal adverse outcomes. (5) The gestational week at the time of vaccination is also a missed important variable that may affect maternal and neonatal outcome especially in the first trimester; this should be further evaluated in future studies. (6) The most significant limitation is that the current investigation may have lacked sufficient statistical power to detect small but clinically relevant differences in infrequent outcomes. Furthermore, the generalizability of our findings to other populations with different characteristics may be difficult and remains to be determined. (7) Documented Covid-19 disease/vaccine status was available for only 60% of women delivered at the SZMC and BHMC during the study period, however, they had overall similar demographics and obstetric characteristics.
Conclusion
In this retrospective multicenter study, we have shown that the third booster dose is not associated with an increase in composite adverse maternal outcome and may even be associated with reduced adverse neonatal outcomes; hence, pregnant women should be offered the third booster dose regardless of the pregnancy.
Acknowledgements
None.
Author contributions
MR: protocol development, data collection and management, data analysis, and manuscript writing/editing. RR: protocol development, data collection and management, data analysis, and manuscript writing/editing. YWW: data collection and management, and manuscript writing/editing. SGG: protocol development, data collection and management, data analysis, and manuscript writing/editing. HYS: protocol development, data collection and management, data analysis, and manuscript writing/editing.
Funding
None.
Declarations
Availability of materials
Not available.
Conflict of interest
The authors report no conflict of interest.
Ethical approval
The study was approved by the institutional ethics committee (011-22-SZMC), which is responsible for both the SZMC and BHMC. The study was performed following the principles of the Declaration of Helsinki. Data were obtained from medical records and de-identified, with no direct participation of patients; hence, informed consent was waived.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Misgav Rottenstreich and Reut Rotem have contributed equally to the manuscript.
Sorina Grisaru-Granovsky and Hen Y. Sela have contributed equally to the manuscript.
References
- 1.Khedmat L, Mohaghegh P, Veysizadeh M, Hosseinkhani A, Fayazi S, Mirzadeh M. Pregnant women and infants against the infection risk of COVID-19: a review of prenatal and postnatal symptoms, clinical diagnosis, adverse maternal and neonatal outcomes, and available treatments. Arch Gynecol Obstet. 2022;306(2):323–335. doi: 10.1007/s00404-021-06325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delahoy MJ, Whitaker M, O’Halloran A, Chai SJ, Kirley PD, Alden N, et al. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19—COVID-NET, 13 states, March 1–August 22, 2020. Morb Mortal Wkly Rep. 2020;69(38):1347. doi: 10.15585/mmwr.mm6938e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. Morb Mortal Wkly Rep. 2020;69(44):1641. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodworth KR, Olsen EO, Neelam V, Lewis EL, Galang RR, Oduyebo T, et al. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy—SET-NET, 16 jurisdictions, March 29–October 14, 2020. Morb Mortal Wkly Rep. 2020;69(44):1635. doi: 10.15585/mmwr.mm6944e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirbeyk M, Saghazadeh A, Rezaei N. A systematic review of pregnant women with COVID-19 and their neonates. Arch Gynecol Obstet. 2021;304(1):5–38. doi: 10.1007/s00404-021-06049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antoun L, El Taweel N, Ahmed I, Patni S, Honest H. Maternal COVID-19 infection, clinical characteristics, pregnancy, and neonatal outcome: a prospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2020;252:559–562. doi: 10.1016/j.ejogrb.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahlberg M, Neovius M, Saltvedt S, Söderling J, Pettersson K, Brandkvist C, et al. Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA. 2020;324(17):1782–1785. doi: 10.1001/jama.2020.19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020 doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrer-Oliveras R, Mendoza M, Capote S, Pratcorona L, Esteve-Valverde E, Cabero-Roura L, et al. Immunological and physiopathological approach of COVID-19 in pregnancy. Arch Gynecol Obstet. 2021;304(1):39–57. doi: 10.1007/s00404-021-06061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pregnant women: scientific and ethical considerations for inclusion in clinical trials guidance for industry. [cited 2021 Apr 25]. https://www.fda.gov/files/drugs/published/Pregnant-Women--Scientific-and-Ethical-Considerations-for-Inclusion-in-Clinical-Trials.pdf. Accessed 25 April 2021
- 12.Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas SJ, Moreira ED, Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rottenstreich M, Sela HY, Rotem R, Kadish E, Wiener-Well Y, Grisaru-Granovsky S. Covid-19 vaccination during the third trimester of pregnancy: rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study. BJOG. 2022;129(2):248–255. doi: 10.1111/1471-0528.16941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dick A, Rosenbloom JI, Gutman-Ido E, Lessans N, Cahen-Peretz A, Chill HH. Safety of SARS-CoV-2 vaccination during pregnancy—obstetric outcomes from a large cohort study. BMC Pregnancy Childbirth. 2022;22(1):166. doi: 10.1186/s12884-022-04505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blakeway H, Prasad S, Kalafat E, Heath PT, Ladhani SN, Le Doare K, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226(2):236.e1–236.e14. doi: 10.1016/j.ajog.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalafat E, Magee LA, von Dadelszen P, Heath P, Khalil A. COVID-19 booster doses in pregnancy and global vaccine equity. Lancet. 2022 doi: 10.1016/S0140-6736(22)00166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wainstock T, Yoles I, Sergienko R, Sheiner E. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine. 2021;39(41):6037–6040. doi: 10.1016/j.vaccine.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipkind HS, Vazquez-Benitez G, DeSilva M, Vesco KK, Ackerman-Banks C, Zhu J, et al. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth—eight integrated health care organizations, United States, December 15, 2020–July 22, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(1):26–30. doi: 10.15585/mmwr.mm7101e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fell DB, Dhinsa T, Alton GD, Török E, Dimanlig-Cruz S, Regan AK, et al. Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. JAMA. 2022 doi: 10.1001/jama.2022.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaal NK, Zöllkau J, Hepp P, Fehm T, Hagenbeck C. Pregnant and breastfeeding women’s attitudes and fears regarding the COVID-19 vaccination. Arch Gynecol Obstet. 2022;306(2):365–372. doi: 10.1007/s00404-021-06297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bookstein Peretz S, Regev N, Novick L, Nachshol M, Goffer E, Ben-David A, et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol. 2021;58(3):450–456. doi: 10.1002/uog.23729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldshtein I, Nevo D, Steinberg DM, Rotem RS, Gorfine M, Chodick G, et al. Association Between BNT162b2 Vaccination and Incidence of SARS-CoV-2 Infection in Pregnant Women. JAMA. 2021;326(8):728–735. doi: 10.1001/jama.2021.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreira EDJ, Kitchin N, Xu X, Dychter SS, Lockhart S, Gurtman A, et al. Safety and efficacy of a third dose of BNT162b2 Covid-19 vaccine. N Engl J Med. 2022 doi: 10.1056/NEJMoa2200674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.COVID A. Vaccination Considerations for Obstetric–Gynecologic Care. Online (Stand: 18.11. 2021): https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-forobstetric-gynecologic-care
- 26.Yang YJ, Murphy EA, Singh S, Sukhu AC, Wolfe I, Adurty S, et al. Association of gestational age at coronavirus disease 2019 (COVID-19) vaccination, history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and a vaccine booster dose with maternal and umbilical cord antibody levels at delivery. Obstet Gynecol. 2022;139(3):373–380. doi: 10.1097/AOG.0000000000004693. [DOI] [PubMed] [Google Scholar]
- 27.Hameed I, Khan MO, Nusrat K, Mahmood S, Nashit M, Malik S, et al. Is it safe and effective to administer COVID-19 vaccines during pregnancy a systematic review and meta-analysis. Am J Infect Control. 2022 doi: 10.1016/j.ajic.2022.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dick A, Rosenbloom JI, Karavani G, Gutman-Ido E, Lessans N, Chill HH. Safety of third SARS-CoV-2 vaccine (booster dose) during pregnancy. Am J Obstet Gynecol MFM. 2022;4(4):100637. doi: 10.1016/j.ajogmf.2022.100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemon L, Edwards RP, Simhan HN. What is driving the decreased incidence of preterm birth during the coronavirus disease 2019 pandemic? Am J Obstet Gynecol MFM. 2021;3(3):100330. doi: 10.1016/j.ajogmf.2021.100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rottenstreich A, Zarbiv G, Oiknine-Djian E, Vorontsov O, Zigron R, Kleinstern G, et al. Timing of SARS-CoV-2 vaccination during the third trimester of pregnancy and transplacental antibody transfer: a prospective cohort study. Clin Microbiol Infect. 2022;28(3):419–425. doi: 10.1016/j.cmi.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magnus MC, Gjessing HK, Eide HN, Wilcox AJ, Fell DB, Håberg SE. Covid-19 vaccination during pregnancy and first-trimester miscarriage. N Engl J Med. 2021;385(21):2008–2010. doi: 10.1056/NEJMc2114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldshtein I, Steinberg DM, Kuint J, Chodick G, Segal Y, Shapiro Ben David S, et al. Association of BNT162b2 COVID-19 vaccination during pregnancy with neonatal and early infant outcomes. JAMA Pediatr. 2022 doi: 10.1001/jamapediatrics.2022.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]