Abstract
Background
Progressive supranuclear palsy (PSP) is a parkinsonian-like progressive neurodegenerative syndrome. Key clinical features include ocular motor dysfunction, postural instability, and cognitive dysfunction. Maintaining and improving balance function and gait function are very important for patients with PSP with severe postural dysfunction and repeated falls. In addition, patients with PSP have a poor response to pharmacological treatment; hence, rehabilitation is a key approach in dealing with this syndrome. However, no conclusion on the beneficial effects of rehabilitation for patients with PSP have been established in the literature.
Objectives
The effectiveness of multiple therapeutic exercise program with probable or possible PSP according to the Movement Disorder Society criteria for the clinical diagnosis of PSP was validated.
Methods
Participants underwent multiple therapeutic exercise program customized for each participant, including resistance training, balance training, and walking exercises that were performed for 60–80 minutes a day, 5 days a week for 4 weeks. The outcomes measured were as follows: pull test, Berg Balance Scale (BBS), timed up and go test (TUG), and gait speed test.
Results
A total of 117 patients with PSP were enrolled and the analysis was performed on 20 patients with probable PSP. Four-week rehabilitation significantly improved pull test (p = 0.034) and BBS scores (p = 0.001). There were no significant differences both TUG (p = 0.502) and gait speed (p = 0.813).
Conclusion
The multiple therapeutic exercise program had beneficial effects on balance performance in patients with PSP in 4 weeks and could be an essential element in their rehabilitation. Although this pilot study was conducted without a control group, it provided valuable information for future prospective randomized controlled trials.
Keywords: progressive supranuclear palsy, exercise, rehabilitation, rehabilitation research, balance
Introduction
Progressive supranuclear palsy (PSP) is a parkinsonian-like progressive neurodegenerative syndrome. Key clinical features include ocular motor dysfunction, postural instability, akinesia, and cognitive dysfunction (1, 2). In addition, mobility problems are the most common early feature in PSP, and onset of falls within 1 year has been associated with a worse prognosis (3).
Maintaining and improving balance function and gait function are very important for patients with PSP with severe postural dysfunction and repeated falls. Rehabilitation for idiopathic Parkinson's disease (PD) was proven to be effective for improving the motor and balance function (4–6). Furthermore, rehabilitation combined with pharmacotherapy is known to improve motor symptoms, gait, and quality of life of patients with PD (7, 8). However, patients with PSP respond poorly to pharmacological treatment (2); consequently, rehabilitation could be highly relevant for maintaining the motor function and activities of daily living (ADL) in PSP (9).
Several studies related to rehabilitation interventions for patients with PSP used therapies designed for them. These therapies included balance and eye movement training (10, 11), harness-supported treadmill training (12–14), weighted vests during ambulation (15), and robot-assisted gait training (12, 16), of these, balance exercise (10, 11) and gait training (12–14, 16) indicated potential benefit. However, a recent systematic review concluded that the effects of structured physical activity for patients with advanced PSP remain unknown (17).
Moreover, the impact on patients with PSP of different multiple therapeutic exercise program (including resistance training, balance training, walking exercises) effective for motor and balance function in the elderly and patients with PD has not been investigated in a sufficient manner yet.
Our study aimed to determine whether multiple patient-specific customized therapeutic exercise program are effective in improving balance and motor functions of patients with PSP.
Methods
Participants
We conducted a pre–post study between January 2016 and December 2021 that included probable or possible patients with PSP according to the Movement Disorder Society criteria for the clinical diagnosis of PSP (2) in the National Hospital Organization Higashinagoya National Hospital. The exclusion criteria were: (1) a score of the modified Rankin Scale (mRS) ≥ 5, (2) patients with subtypes except Richardson syndrome (RS), (3) patients who underwent rehabilitation treatment for 4 consecutive weeks in the past, (4) inability to walk without assistance for at least 10 meters, (5) patients who were discharged within 4 weeks, and (6) patients with missing values in the outcome data. Patients were hospitalized for the purpose of rehabilitation. The medication of all participants was not changed to maintain a stable condition during the rehabilitation period.
Measured outcomes
The age, disease duration, sex, subtype, frontal assessment battery (FAB), mini-mental state examination (MMSE), mRS, and the PSP rating scale (PSPRS) scores of all patients were evaluated (18). The balance and basic motor functions were evaluated using the Berg Balance Scale (BBS) (19), timed up and go test (TUG) (20), and gait speed. All patients were evaluated for balance and basic motor functions within 2 days before and 4 weeks after rehabilitation. Participants underwent the following tests:
(1) PSPRS (18): The PSPRS was developed to assess disease severity in patients with PSP. Furthermore, PSPRS assesses characteristic symptoms associated with PSP, including behavioral change, ocular-motor, gait, and balance disfunctions. The maximum total score is 100 points. Higher scores indicate high disease severity. The PSPRS subitem scores and total score were evaluated as baseline, and the scores of V: limb movements and VI: gait and midline were evaluated pre and before interventions.
(2) Pull test (18, 21, 22): The pull test is used for evaluating postural stability (0–4 points) as a component of PSPRS. The examiner stands behind the patient and applies a strong pull on the shoulders with the patient erect with eyes open and feet comfortably apart.
(3) BBS (19): This evaluated the individual's balance abilities during the performance of 14 items (0–4 points per item), such as sitting, standing, and one leg standing, and positional changes. The maximum total score is 56 points. Higher scores indicate good balance ability.
(4) TUG (20): This evaluation consisted of the participant standing up from a sitting position in the chair with a seat height of 40 cm, walking a distance of 3 m, then passing around a cone, returning, and sitting back down in the chair. Comfortable and maximum gait speeds were both measured once each. To assess comfortable speed walking, participants were instructed to walk at their normal comfortable (natural) speed. To assess maximum walking speed, they were asked to walk as fast as possible without running.
(5) Gait speed: Comfortable and maximum gait speed were both measured once each. Participants were required to accelerate and decelerate 2 m before and after the 10 m test distance. To assess comfortable speed walking, participants were instructed to walk at their normal comfortable speed. To assess maximum walking speed, they were asked to walk as fast as possible without running.
This study was not considered about an assessment bias, therefore it included cases that the evaluators are the same professionals in charge of performing the therapies.
Intervention programs
The multiple therapeutic exercise program consisted of balance training, resistance training, range of motion (ROM) exercises, stretching, walking exercises, and ADL training and was customized for each patient by physical and occupational therapists. More details about multiple therapeutic exercise program are provided in Supplementary Table 1. The program was performed for 60–80 mins a day, 5 days a week for 4 weeks.
Statistical analysis
We evaluated the normality of the distribution of all variables using the Shapiro–Wilk test. For comparison before and after the rehabilitation, we used the Wilcoxon signed-rank test or paired t-tests. In addition, we calculated the effect size (r) by the test statistic. Data were reported as mean ± standard deviation for normally distributed data and number for discrete variables. We performed the statistical analysis using the SPSS software, version 26 (IBM Inc., Armonk, NY, USA). A p-value of <0.05 was considered statistically significant.
Results
We enrolled 117 probable or possible patients with PSP. We excluded the following patients who met the exclusion criteria: (1) a score of mRS ≥ 5, n = 53; (2) patients with subtypes except RS, n = 2; (3) patients who underwent rehabilitation treatment for 4 weeks in the past, n = 2; (4) inability to walk without assistance for at least 10 m, n = 12; (5) patients who were discharged within 4 weeks, n = 11; and (6) patients with missing values in the outcome data, n = 17. Consequently, the analysis was performed on 20 patients with probable PSP (Figure 1).
Figure 1.
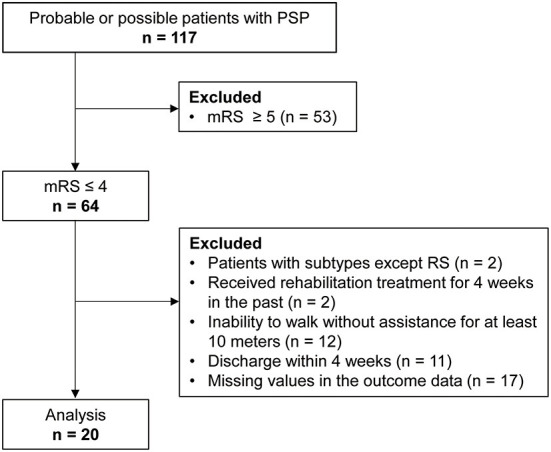
Flow chart.
Table 1 lists the participants' demographic and clinical characteristics.
Table 1.
Demographic and clinical characteristics of participants.
| N = 20 | |
|---|---|
| Age, years | 72.3 ± 6.2 (61–83) |
| Disease duration, months | 29.3 ± 18.2 (10–82) |
| Sex, men/women | 13/7 |
| FAB, score | 12.2 ± 3.2 (6–17) |
| MMSE, score | 26.0 ± 2.6 (22–30) |
| mRS, score | 3.3 ± 0.6 (2–4) |
| PSPRS, score | |
| I | 7.3 ± 2.4 (3–11) |
| II | 3.2 ± 2.4 (0–8) |
| III | 2.7 ± 1.2 (1–5) |
| IV | 6.7 ± 3.9 (0–12) |
| V | 3.9 ± 1.3 (2–7) |
| VI | 8.2 ± 2.1 (6–12) |
| Total | 31.8 ± 10.0 (16–50) |
Data were reported as mean ± standard deviation (minimum—maximum) and numbers.
Table 2 shows the results of PSPRS V: Limb motor and VI: Gait and midline pre (0W) and post (4W) rehabilitation. Four-week rehabilitation significantly improved V: Gait and midline total scores (p = 0.004, r = 0.645). Similarly, significant improvements were observed in the VI subitems of arising from chair (p = 0.007, r = 0.607), and postural stability (p = 0.034, r = 0.474).
Table 2.
Each subitem (V: Limb motor and VI: Gait and midline) score of PSPRS pre (0W) and post (4W) rehabilitation.
| Pre | Post | p-value | Effect size | ||
|---|---|---|---|---|---|
| V. Limb motor | Limb rigidity (full score = 4) |
1.4 ± 0.9 (0–3) |
1.3 ± 0.9 (0–3) |
0.655 | 0.100 |
| Limb dystonia (full score = 4) |
0.3 ± 0.6 (0–2) |
0.3 ± 0.5 (0–1) |
1.000 | 0.000 | |
| Finger tapping (full score = 2) |
0.9 ± 0.5 (0–2) |
0.8 ± 0.5 (0–2) |
0.564 | 0.129 | |
| Toe tapping (full score = 2) |
1.0 ± 0.4 (0–2) |
0.9 ± 0.4 (0–2) |
0.317 | 0.224 | |
| Apraxia of hand movement (full score = 2) |
0.1 ± 0.3 (0–1) |
0.1 ± 0.2 (0–1) |
0.317 | 0.224 | |
| Tremor in any part (full score = 2) |
0.3 ± 0.4 (0–1) |
0.3 ± 0.5 (0–2) |
1.000 | 0.000 | |
| Total (full score = 16) |
3.9 ± 1.6 (2–7) |
3.6 ± 1.9 (0–7) |
0.406 | 0.186 | |
| VI.Gait and midline | Neck rigidity or dystonia (full score = 16) |
1.4 ± 0.9 (0–3) |
1.3± 0.9 (0–3) |
0.083 | 0.387 |
| Arising from chair (full score = 4) |
1.7 ± 0.7 (1–3) |
1.2 ± 0.8 (1–3) |
0.007* | 0.607 | |
| Gait (full score = 4) |
1.7 ± 0.7 (1–3) |
1.5 ± 0.7 (1–3) |
0.102 | 0.365 | |
| Postural stability (full score = 4) |
2.0 ± 0.8 (1–3) |
1.7 ± 0.8 (1–3) |
0.034* | 0.474 | |
| Sitting down (full score = 4) |
1.5 ± 0.5 (1–2) |
1.3 ± 0.6 (1–2) |
0.102 | 0.365 | |
| Total (full score = 20) |
8.2 ± 2.1 (5–12) |
6.9 ± 2.6 (5–11) |
0.004* | 0.645 |
Data were reported as mean ± standard deviation (minimum—maximum). *Indicates p < 0.05.
Table 3 shows the results of the motor function pre (0W) and post (4W) rehabilitation, and the plot data are shown in Supplementary Figure 1 in the Supplemental material. Four-week rehabilitation significantly improved pull test results (p = 0.034, r = 0.474, Supplementary Figure 1A) and BBS total scores (p = 0.001, r = 0.679, Supplementary Figure 1B). There were no significant differences between pre and post in TUG (comfortable: p = 0.502, r = 0.150; maximum: p = 0.956, r = 0.013, Supplementary Figure 1C) and gait speed (comfortable: p = 0.813, r = 0.055; maximum: p = 0.566, r = 0.133, Supplementary Figure 1D). These results suggest that 4-week rehabilitation improved postural control and balance function in PSP.
Table 3.
Motor function pre (0W) and post (4W) rehabilitation.
| Pre | Post | p-value | Effect size | ||
|---|---|---|---|---|---|
| Pull test, score | 2.0 ± 0.8 (1–3) | 1.7 ± 0.8 (0–3) | 0.034* | 0.474 | |
| BBS, score | 40.6 ± 8.2 (20–52) | 44.6± 8.5 (20–56) | 0.001* | 0.679 | |
| TUG, sec | Comfortable | 15.3 ± 3.7 (10.6–26.4) | 14.9 ± 3.5 (10.4–22.0) | 0.502 | 0.150 |
| Maximum | 12.5 ± 3.1 (8.0–20.2) | 12.5 ± 2.9 (8.4–20.1) | 0.956 | 0.013 | |
| Gait speed, m/min | Comfortable | 56.6 ± 12.9 (28.3–83.3) | 57.4 ± 13.4 (26.4–82.8) | 0.813 | 0.055 |
| Maximum | 74.8 ± 13.8 (48.9–113.0) | 73.3 ± 16.6 (43.8–111.7) | 0.566 | 0.133 | |
Data were reported as mean ± standard deviation (minimum—maximum).
*Indicates p < 0.05.
Table 4 shows the results of each subitem of BBS pre (0W) and post (4W) rehabilitation, and the plot data are shown in Supplementary Figure 2 in the Supplemental material. There were significant improvements in the items of reaching forward with outstretched arm (p = 0.011, r = 0.566), turning to look behind (p = 0.039, r = 0.461), turning 360 degrees (p = 0.046, r = 0.447), standing with one foot in front (p = 0.047, r = 0.445), and standing on one foot (p = 0.009, r = 0.588).
Table 4.
Each subitem score of Berg Balance Scale pre (0W) and post (4W) rehabilitation.
| Pre | Post | p-value | Effect size | |
|---|---|---|---|---|
| Sitting to standing | 3.4 ± 0.8 (2–4) | 3.6 ± 0.8 (1–4) | 0.453 | 0.168 |
| Standing unsupported | 3.5 ± 0.5 (3–4) | 3.7 ± 0.5 (3–4) | 0.102 | 0.365 |
| Sitting unsupported | 4.0 ± 0.0 (4–4) | 4.0 ± 0.0 (4–4) | 1.000 | 0.224 |
| Standing to sitting | 3.5 ± 0.7 (2–4) | 3.5 ± 1.0 (0–4) | 0.705 | 0.085 |
| Transfers | 3.0 ± 0.8 (1–4) | 3.2 ± 0.8 (1–4) | 0.157 | 0.316 |
| Standing with eyes closed | 3.6 ± 0.5 (3–4) | 3.5 ± 0.5 (3–4) | 0.564 | 0.129 |
| Standing with feet together | 3.3 ± 0.7 (1–4) | 3.5 ± 0.6 (2–4) | 0.257 | 0.254 |
| Reaching forward with outstretched arm | 3.1 ± 1.1 (0–4) | 3.6 ± 0.9 (0–4) | 0.011* | 0.566 |
| Retrieving object from floor | 3.0 ± 1.1 (0–4) | 3.1 ± 1.1 (0–4) | 0.083 | 0.387 |
| Turning to look behind | 3.4 ± 1.1 (0–4) | 3.9 ± 0.5 (2–4) | 0.039* | 0.461 |
| Turning 360 degrees | 1.8 ± 1.0 (0–4) | 2.3 ± 1.0 (0–4) | 0.046* | 0.447 |
| Placing alternate foot on stool | 2.0 ± 1.4 (0–4) | 2.3 ± 1.3 (0–4) | 0.165 | 0.310 |
| Standing with one foot in front | 2.0 ± 1.4 (0–4) | 2.5 ± 1.2 (0–4) | 0.047* | 0.445 |
| Standing on one foot | 1.2 ± 1.0 (0–3) | 2.0 ± 1.4 (0–4) | 0.009* | 0.588 |
Data were reported as mean ± standard deviation (minimum—maximum).
*Indicates p < 0.05.
Discussion
The present study shows that multiple therapeutic exercise program can improve the balance function in patients with PSP. As no standard pharmacological treatment them has been established yet (recall that PSP has poor response to Levodopa) (9, 23), therapeutic exercise is a key non-pharmacological approach for maintaining their motor function. However, the eventual positive effects of therapeutic exercise in patients with PSP have been insufficiently documented yet (17); therefore, our investigation is among the first to reveal the effectiveness of therapeutic exercise for these patients. This study excluded a control group. Therefore, this limitation hinders the applicability of the results. However, this pilot study provided valuable information for future prospective randomized controlled trials.
PSPRS
In PSP with similar severity, rehabilitation interventions improve PSPRS items V: Limb motor and VI: Gait and midline (12, 13). In this study, multiple therapeutic exercises improved items VI and VI subitems; arising from chair and postural stability post-intervention. Therefore, rehabilitation was expected to enhance the stability of gait and basic movements such as standing, sitting, etc., in patients with PSP.
Balance functions
The pull tests and BBS provided useful measures for changes that are related to balance function. The pull test easily evaluates postural instability and predicts falls in PD (21, 24). The BBS is a wellaccepted, comprehensive evaluation of balance that has excellent reliability and validity with older adults (25). It can also continuously monitor balance function and predict falls in patients with PD and neurodegenerative diseases (26, 27).
In the pre-intervention evaluation of balance function, the pull test showed that the participants had moderate postural instability. The total BBS score of participants who had moderate balance dysfunction was 40.6. In PD, the fall risk cutoff score on BBS reported by Dibble was 54 of 56 (28), that reported by Landers was 44 (29); the participants of this study had a higher fall risk. The results of the BBS subitems (Table 4) showed particularly low values for turning 360 degrees, placing alternate foot on a stool, standing with one foot in front, and standing on one foot. These results suggest that participants have particularly impaired anticipatory postural adjustments (APA), reactive postural adjustments (RPA) and the more challenging balance items. The main lesions of PSP are the substantia nigra, subthalamic nucleus, brain stem, globus pallidus, tegmental portion pons, subthalamic nucleus, cerebellar dentate nucleus, and frontal lobe (1, 30). Postural instability is the primary symptom of PSP, which impairs the ability to maintain balance while standing, rising, seated, changing direction, and walking, making the patient susceptible to falls. The balance dysfunction observed in patients with PSP is primarily a disruption of reactive and anticipatory postural coordination and is associated with dysfunction related to the basal ganglia and brainstem. In addition, cerebellar disturbances may potentiate postural instability.
A few recent reports have assessed the effect of several rehabilitation programs (12–14, 31, 32) for balance function in PSP. In the post-intervention assessment of balance function, our multiple therapeutic exercise program were found to improve balance function in patients with PSP, similar to earlier studies.
Several subsystems contribute to postural stability, such as the functional level of motor systems, anticipatory postural control, dynamic stability, static stability, sensory integration, functional stability limits, reactive postural control, cognitive influences, and verticality (33). Balance training was conducted, including exercise to cope with postural changes when moving the body voluntarily as an intervention for APA disorders and exercise to control posture against external disturbances as an intervention for RPA disorders. In addition, ROM exercises adjusted the participants' optimal postural alignment by improving ROM of limited joints, and strength training focusing on antigravity muscles was performed to maintain posture. These multiple approaches may have improved the balance function such as RPA, APA, static stability, functional stability limits in patients with PSP.
Motor functions
The TUG and gait speed provided useful measures for changes that are related to motor function and the risk of fall. A previous study reported fall risk cutoff score for TUG comfortable time to be 11.5 s in PD (34), whereas another reported it to be 13.5 s in elderly persons (35); the score in this study was 15.3 s. The fall risk cutoff score reported for comfortable 10-m gait speed was 1.1–1.2 m/sec in PD (36) and 1.0 m/sec in elderly persons (37), whereas the score in the present study was 56.6 m/min (converted value 0.94 m/sec). In the pre-intervention evaluation of motor function, participants' motor function was found to be lower than both patients with PD and elderly persons, who were at a higher risk for falls.
In patients with PD, therapeutic exercise, including ROM, stretching, balance training, resistance training, and treadmill walking, was effective for improving motor function, muscle strength, balance function, and gait speed (4, 8). The effect of the same multiple therapeutic exercise program was found to be beneficial for balance function in patients with PSP as well; however, their gait function did not improve as expected.
The fall risk cutoff score for both TUG and gait speed suggests that greater speed is associated with a lower risk of falls in patients with PD and the elderly. In contrast, as postural instability appears in the early stages in patients with PSP, it leads to difficulties in balance control and thereby to an increased risk of falls. As the disease progresses, gait instability due to cerebellar damage is observed in addition to symptoms of parkinsonism, such as rush symptoms and frozen gait (38). Therefore, we argue that primary rehabilitation for patients with PSP should focus on improving their gait stability instead of their gait speed. This study did not assess gait stability; therefore, evidence on the effects of gait stability on fall reduction needs to be validated in the future.
Limitations
This study has several limitations. First is the fact that it was a retrospective study in a single facility involving a small sample size, the lack of a control group, and absence of follow-ups. Further prospective multicenter studies with a larger sample size, randomized controlled trial setting, and follow-up of long-term rehabilitation can help validate and support our findings. Second, in some cases, the intervention and evaluation were conducted by the same therapists, and there might be concerns about the assessment bias. Therefore, pre-determination and arrangement are needed in which intervention and evaluation are not performed by the same therapists in further prospective studies. Lastly, we lacked the assessment of the quality of life (QoL). This study mainly focused on motor and balance functions of PSP, but the impact of the therapeutical intervention on patients' QoL should be known. In the future, the effect of rehabilitation on QoL of patients with PSP by PSP-QoL (39) or EQ-5D (40) should be investigated.
Conclusion
In this pre–post study, multiple 4-week long therapeutic exercise program known to improve several functions of patients suffering from PD were shown to induce beneficial effects on balance function in patients with PSP.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the National Hospital Organization Higashinagoya Hospital (approval number 30-11). Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. All participants were verbally informed of the study and their consent was obtained.
Author contributions
NM and YT prepared and repeatedly revised the manuscript. IA is responsible for the ethics application and contributed to the development, procedure, and funding for research. YT and IA revised the manuscript. All authors reviewed the manuscript and provided final approval for the manuscript.
Funding
This work was supported by Grants-in Aid from the Research Committee of CNS Degenerative Diseases, Research on Policy Planning and Evaluation for Rare and Intractable Diseases, Health, Labor, and Welfare Sciences Research Grants, the Ministry of Health, and Labor and Welfare, Japan (20FC1049 to IA).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the patients and their families for their contributions. We would also like to thank the doctors (Department of Neurology) and physical therapists (Department of Rehabilitation) at the National Hospital Organization Higashinagoya National Hospital for their support.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.955893/full#supplementary-material
References
- 1.Steele JC, Richardson JC, Olszewski J. Progressive supranuclear palsy. a heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol. (1964) 10:333–59. 10.1001/archneur.1964.00460160003001 [DOI] [PubMed] [Google Scholar]
- 2.Hoglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord. (2017) 32:853–64. 10.1002/mds.26987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nath U, Ben-Shlomo Y, Thomson RG, Lees AJ, Burn DJ. Clinical features and natural history of progressive supranuclear palsy: a clinical cohort study. Neurology. (2003) 60:910–6. 10.1212/01.WNL.0000052991.70149.68 [DOI] [PubMed] [Google Scholar]
- 4.Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord. (2008) 23:631–40. 10.1002/mds.21922 [DOI] [PubMed] [Google Scholar]
- 5.Keus SH, Munneke M, Nijkrake MJ, Kwakkel G, Bloem BR. Physical therapy in Parkinson's disease: evolution and future challenges. Mov Disord. (2009) 24:1–14. 10.1002/mds.22141 [DOI] [PubMed] [Google Scholar]
- 6.Yitayeh A, Teshome A. The effectiveness of physiotherapy treatment on balance dysfunction and postural instability in persons with Parkinson's disease: a systematic review and meta-analysis. BMC Sports Sci Med Rehabil. (2016) 8:17. 10.1186/s13102-016-0042-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frazzitta G, Maestri R, Bertotti G, Riboldazzi G, Boveri N, Perini M, et al. Intensive rehabilitation treatment in early Parkinson's disease: a randomized pilot study with a 2-year follow-up. Neurorehabil Neural Repair. (2015) 29:123–31. 10.1177/1545968314542981 [DOI] [PubMed] [Google Scholar]
- 8.Radder DLM, Ligia Silva de Lima A, Domingos J, Keus SHJ, van Nimwegen M, Bloem BR, et al. Physiotherapy in Parkinson's disease: a meta-analysis of present treatment modalities. Neurorehabil Neural Repair. (2020) 34:871–80. 10.1177/1545968320952799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litvan I. Parkinsonian features: when are they Parkinson disease? JAMA. (1998) 280:1654–5. 10.1001/jama.280.19.1654 [DOI] [PubMed] [Google Scholar]
- 10.Zampieri C, Di Fabio RP. Balance and eye movement training to improve gait in people with progressive supranuclear palsy: quasi-randomized clinical trial. Phys Ther. (2008) 88:1460–73. 10.2522/ptj.20070302 [DOI] [PubMed] [Google Scholar]
- 11.Zampieri C, Di Fabio RP. Improvement of gaze control after balance and eye movement training in patients with progressive supranuclear palsy: a quasi-randomized controlled trial. Arch Phys Med Rehabil. (2009) 90:263–70. 10.1016/j.apmr.2008.07.024 [DOI] [PubMed] [Google Scholar]
- 12.Clerici I, Ferrazzoli D, Maestri R, Bossio F, Zivi I, Canesi M, et al. Rehabilitation in progressive supranuclear palsy: effectiveness of two multidisciplinary treatments. PLoS One. (2017) 12:e0170927. 10.1371/journal.pone.0170927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Pancrazio L, Bellomo RG, Franciotti R, Iodice P, Galati V, D'Andreagiovanni A, et al. Combined rehabilitation program for postural instability in progressive supranuclear palsy. NeuroRehabilitation. (2013) 32:855–60. 10.3233/NRE-130909 [DOI] [PubMed] [Google Scholar]
- 14.Suteerawattananon M, MacNeill B, Protas EJ. Supported treadmill training for gait and balance in a patient with progressive supranuclear palsy. Phys Ther. (2002) 82:485–95. 10.1093/ptj/82.5.485 [DOI] [PubMed] [Google Scholar]
- 15.Wallace R, Abbott C, Gibson-Horn C, Skubic M. In-home measurement of the effect of strategically weighted vests on ambulation. Annu Int Conf IEEE Eng Med Biol Soc. (2013) 2013:949–52. 10.1109/EMBC.2013.6609659 [DOI] [PubMed] [Google Scholar]
- 16.Sale P, Stocchi F, Galafate D, De Pandis MF, Le Pera D, Sova I, et al. Effects of robot assisted gait training in progressive supranuclear palsy (PSP): a preliminary report. Front Hum Neurosci. (2014) 8:207. 10.3389/fnhum.2014.00207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slade SC, Finkelstein DI, McGinley JL, Morris ME. Exercise and physical activity for people with progressive supranuclear palsy: a systematic review. Clin Rehabil. (2020) 34:23–33. 10.1177/0269215519877235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golbe LI, Ohman-Strickland PA. A clinical rating scale for progressive supranuclear palsy. Brain. (2007) 130:1552–65. 10.1093/brain/awm032 [DOI] [PubMed] [Google Scholar]
- 19.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. (1992) 83 Suppl 2:S7-11. [PubMed] [Google Scholar]
- 20.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. (1991) 39:142–8. 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 21.Hunt AL, Sethi KD. The pull test: a history. Mov Disord. (2006) 21:894–9. 10.1002/mds.20925 [DOI] [PubMed] [Google Scholar]
- 22.Munhoz RP, Li JY, Kurtinecz M, Piboolnurak P, Constantino A, Fahn S, et al. Evaluation of the pull test technique in assessing postural instability in Parkinson's disease. Neurology. (2004) 62:125–7. 10.1212/WNL.62.1.125 [DOI] [PubMed] [Google Scholar]
- 23.Lamb R, Rohrer JD, Lees AJ, Morris HR. Progressive supranuclear palsy and corticobasal degeneration: pathophysiology and treatment options. Curr Treat Options Neurol. (2016) 18:42. 10.1007/s11940-016-0422-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munhoz RP, Teive HA. Pull test performance and correlation with falls risk in Parkinson's disease. Arq Neuropsiquiatr. (2014) 72:587–91. 10.1590/0004-282X20140082 [DOI] [PubMed] [Google Scholar]
- 25.Berg KO, Maki BE, Williams JI, Holliday PJ, Wood-Dauphinee SL. Clinical and laboratory measures of postural balance in an elderly population. Arch Phys Med Rehabil. (1992) 73:1073–80. [PubMed] [Google Scholar]
- 26.Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys Ther. (2008) 88:733–46. 10.2522/ptj.20070214 [DOI] [PubMed] [Google Scholar]
- 27.Qutubuddin AA, Pegg PO, Cifu DX, Brown R, McNamee S, Carne W. Validating the berg balance scale for patients with Parkinson's disease: a key to rehabilitation evaluation. Arch Phys Med Rehabil. (2005) 86:789–92. 10.1016/j.apmr.2004.11.005 [DOI] [PubMed] [Google Scholar]
- 28.Dibble LE, Christensen J, Ballard DJ, Foreman KB. Diagnosis of fall risk in Parkinson disease: an analysis of individual and collective clinical balance test interpretation. Phys Ther. (2008) 88:323–32. 10.2522/ptj.20070082 [DOI] [PubMed] [Google Scholar]
- 29.Landers MR, Backlund A, Davenport J, Fortune J, Schuerman S, Altenburger P. Postural instability in idiopathic Parkinson's disease: discriminating fallers from nonfallers based on standardized clinical measures. J Neurol Phys Ther. (2008) 32:56–61. 10.1097/NPT.0b013e3181761330 [DOI] [PubMed] [Google Scholar]
- 30.Kovacs GG, Lukic MJ, Irwin DJ, Arzberger T, Respondek G, Lee EB, et al. Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol. (2020) 140:99–119. 10.1007/s00401-020-02158-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolai S, Mirelman A, Herman T, Zijlstra A, Mancini M, Becker C, et al. Improvement of balance after audio-biofeedback. a 6-week intervention study in patients with progressive supranuclear palsy. Z Gerontol Geriatr. (2010) 43:224–8. 10.1007/s00391-010-0125-6 [DOI] [PubMed] [Google Scholar]
- 32.Croarkin E, Robinson K, Stanley CJ, Zampieri C. Training high level balance and stepping responses in atypical progressive supranuclear palsy: a case report. Physiother Theory Pract. (2022) 1-12. 10.1080/09593985.2022.2032509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sibley KM, Beauchamp MK, Van Ooteghem K, Straus SE, Jaglal SB. Using the systems framework for postural control to analyze the components of balance evaluated in standardized balance measures: a scoping review. Arch Phys Med Rehabil. (2015) 96:122–32 e29. 10.1016/j.apmr.2014.06.021 [DOI] [PubMed] [Google Scholar]
- 34.Nocera JR, Stegemoller EL, Malaty IA, Okun MS, Marsiske M, Hass CJ, et al. Using the Timed Up & Go test in a clinical setting to predict falling in Parkinson's disease. Arch Phys Med Rehabil. (2013) 94:1300–5. 10.1016/j.apmr.2013.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. (2000) 80:896–903. 10.1093/ptj/80.9.896 [DOI] [PubMed] [Google Scholar]
- 36.Lindholm B, Nilsson MH, Hansson O, Hagell P. The clinical significance of 10-m walk test standardizations in Parkinson's disease. J Neurol. (2018) 265:1829–35. 10.1007/s00415-018-8921-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imms FJ, Edholm OG. Studies of gait and mobility in the elderly. Age Ageing. (1981) 10:147–56. 10.1093/ageing/10.3.147 [DOI] [PubMed] [Google Scholar]
- 38.Takamatsu Y, Matsuda N, Aiba I. The combination of short-step and wide-based gait is a gait characteristic in progressive supranuclear palsy: a retrospective, cross-sectional study. Eur Geriatr Med. (2019) 10:809–15. 10.1007/s41999-019-00211-2 [DOI] [PubMed] [Google Scholar]
- 39.Schrag A, Selai C, Quinn N, Lees A, Litvan I, Lang A, et al. Measuring quality of life in PSP: the PSP-QoL. Neurology. (2006) 67:39–44. 10.1212/01.wnl.0000223826.84080.97 [DOI] [PubMed] [Google Scholar]
- 40.Group TE. EuroQol-a new facility for the measurement of health-related quality of life. Health policy. (1990) 16:199–208 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.


