Abstract
Acceleration of global warming has emerged as one of the biggest environmental challenges facing poultry farming. In heat stressed flocks, massive mortality rates and substantial damage to productive performance (eggs and meat) are commonly noticed. Because birds do not have sweat glands, they cannot tolerate high temperatures, especially when combined with high humidity. Under this harsh environmental condition, the birds reduce their feed consumption to decline metabolic energy rate. At an ambient temperature of more than 26°C, the bird increases panting and reduces metabolic rate to get rid of the body's heat increment. There are many scenarios that can be followed to alleviate the adverse effects of heat stress. Management practices, nutritional modification, and/or feed additives are frequently used in poultry farms. However, introducing major genes such as naked neck (Na), frizzle (F), slow feathering (K), and dwarf (dw) could be adopted as a significant solution to improve productive performance in birds raised under high environmental temperatures. The birds carrying these mutations gain popularity owing to their ornamental appearance and highly productive performance at high temperatures. Moreover, utilizing dwarf gene in broiler breeders as a dam line improves adaptability, survivability, and hatchability in flocks kept in hot climates. At hatch, the sex-linked slow feathering mutation has been widely used for wing sexing in some egg-type breeds. The potential use of major genes as a breeding strategy to enhance heat tolerance in chickens has been extensively reviewed.
Key words: major gene, naked neck, frizzle, dwarf, slow feathering
INTRODUCTION
Escalating global temperatures combined with global increases in the number of poultry and the intensification of agriculture has resulted in heat stress becoming an important challenge facing poultry production. Heavy breeds (meat-type) are found to be more sensitive to heat stress than lighter breeds (egg-type). Huge economic losses in egg production and shell quality resulting from heat stress have occurred in laying hens. Under this unusual condition, the bird resorts to increase panting, which leads to excess removal of CO2 from the blood (alkalosis case). According to this physiological mechanism, the availability of blood bicarbonate ions becomes limited for eggshell mineralization. Fortunately, some mutations, such as naked neck, frizzle, slow feathering, and dwarf, confer thermoregulation in hot and humid regions by reducing feather mass or body weight (Gowe and Fairfull, 2008; Desta, 2021). Additionally, reduced feather coverage leaves more dietary protein available to produce more meat yield and egg production. It is strongly recommended to develop commercial strains bearing Na and/or F genes in developing countries suffering from high ambient temperatures (Duah et al., 2020). In a hot environment, the genotypes carrying Na and/or F genes could be called thermo-tolerant chickens (Fathi et al., 2013, 2014). It was observed that use of the naked neck, frizzle, and dwarf genes helps birds to be adapted to heat stress without compromising production performance (Patra et al., 2002; Lin et al., 2006; Islam and Nishibori, 2009; Rajkumar et al., 2010). There are several native chicken breeds with ornamental appearance in Saudi Arabia. Native naked neck chickens are medium-sized with multicolored feathers (mostly black), while frizzle chickens are dark brown with black feathers on the abdomen and tail (Fathi et al., 2017). It is strongly recommended to develop and commercialize naked neck and frizzled birds, especially in developing countries (Duah et al., 2020). Under a harsh tropical environment, incorporating dwarf gene into the broiler industry is strongly recommended (Horst, 1988; Dunnington and Siegel, 1998; Islam, 2005). The dwarf birds exhibited several advantages and pleotropic effects under heat stress, such as better reproductive fitness, lower feed consumption, better feed efficiency, and high disease resistance compared to their normal-sized siblings. A sex-linked dwarf gene has been suggested for use in broiler breeding programs through the heavy dam line to increase fertility and hatchability while saving floor space (Isalm, 2005). In the present review, the influence of major genes on productive performance in chickens has been discussed and reviewed.
FEATHER COVERAGE AND HEAT TOLERANCE
In hot regions, a decline in feather coverage (surface and/or intensity) is of interest to increase productivity in poultry. Heat tolerance and, in turn, growth performance and reproductive traits in poultry can be improved through exploiting some valuable heat tolerant genes such as naked neck (Na), frizzle (F), and slow feathering (K) (Mérat, 1990; Lin et al., 2006; Gowe and Fairfull, 2008; Fathi et al., 2013). These genes have pleotropic effects beyond their impact on feathering rate.
The naked neck gene (Na) is an autosomal incompletely dominant gene located on the third chromosome. It reduces the feather coverage by about 40% in homozygous chickens and 20 to 30% in heterozygous siblings compared to their normal plumage counterparts (Mérat, 1990; Deeb and Cahaner, 1999; Lin et al., 2006; Fathi et al., 2013). The heterozygote state (Nana) shows an isolated feather throat tuft on the neck above the crop (Photo 1), while in the homozygous one (NaNa), the throat tuft is completely absent or reduced to a few units (Photo 2), and this baring extends to the abdomen and ventral side of femurs (Mérat, 1986; Somes, 1990; Fathi et al., 2014). The Na gene could be considered as a marker gene because the feather appearance of different genotypes can be identified by visual examination upon hatching (Photo 3). Galal et al. (2019) found that the naked neck gene improved heat tolerance by increasing HSP70 gene expression rather than by reducing feather cover in Egyptian local breeds raised under heat stress conditions. Additionally, the Na gene increases the size of head appendages (wattle and comb), instigating more body surface to thermoregulation and loss of heat (Chen et al., 2002; El Syaid et al., 2011; Desta, 2021). Reduced feather surface development leaves extra protein for vital physiological functions and produces more eggs and meat. In addition, featherless area reduces the population of lice and mites that are preferentially concentrated under scavenging production systems. (Tabler et al., 2017; Desta, 2021). In cold weather, the Na gene has less advantageous of productive performance, which shows gene by environment interaction (Rajkumar et al., 2011). For extremely appearance, there is an autosomal recessive mutation (scaleless, sc/sc) that causes completely feather loss (featherless birds) and enhances thermotolerance, especially in fast growing broilers (Cahaner et al., 2008; Renaudeau et al., 2012). However, scaleless chicken might be less preferred by some societies (Besbes et al., 2007; Desta et al., 2013; Desta, 2021). In addition to preference, scaleless birds are susceptible to parasites, sunburn, and cold weather, as well as show difficulties in copulating (Bartels, 2003). Consequently, chickens with naked neck are preferred over scaleless ones.
Photo 1.
Heterozygous genotype (Nana) shows an isolated feather tuft on the neck.
Photo 2.
Homozygous genotype (NaNa) exhibits a completely naked neck.
Photo 3.
Feather appearance of naked neck genotypes can be identified by visually examination at hatch.
The frizzle gene (F), an incompletely dominant gene, reduces the intensity of feathers, making the birds dissipate the excess body heat more efficiently. Dong et al. (2018) demonstrated that a deletion allele in KRT75L4 is responsible for the frizzle feather phenotype in Kirin, a Chinese indigenous chicken, and not KRT6A, as in other breeds of frizzle chickens. Frizzling appearance can be identified in newly hatched chicks after 3 to 4 d (Galal and Fathi, 2002). The frizzle gene reduces the feather insulation through curling and reducing the intensity of feathers (Lin et al., 2006; Fathi et al., 2013). In the adult frizzled birds (FF and Ff), the feathers become curled and more delicate compared to the normal case (ff). The contour feathers become curled outward and upward, while rectrices and remiges are less affected but have an irregular appearance. Other structural abnormalities could be noticed in some cases, like thickening of the barbs and barbules, and alteration of the hooklets (Dong et al., 2018). This modification in feather structure reduces the insulating effect of feather coverage. The frizzled appearance is more obvious in a homozygous state (Photo 4) compared to heterozygous siblings (Photo 5). At the older age, the feathers break easily and the bird tends to be completely naked, especially in FF state (Mérat, 1990; Stevens, 1991; Fathi et al., 2013). From the heat tolerance point of view, the frizzle gene behaves as a recessive mutation (Zerjal et al., 2013). No significant difference was detected between heterozygous carriers (Ff) and normally (ff) feathered hens. The combination of Na and F genes gives low feather intensity and more heat dissipation, particularly when the Na exists in a homozygous state (NaNaF-). There is an additive effect in the double heterozygous (Na/Na F/f) broiler (Yunis and Cahaner, 1999). So, the frizzle gene is another potential target for developing heat-tolerant chickens. However, the beneficial effect of the F gene as compared to the Na gene is lower in broilers at high temperatures.
Photo 4.
Frizzled appearance is more obvious in homozygous state (FF).
Photo 5.
Heterozgous genotype (Ff) with curling feathers apperance.
The slow feathering phenomenon is a wonderful sex-linked mutation used for sex identification of day-old chicks in some egg-type strains at hatch, providing an accurate and low-cost effective method. It is considered a multiple allelic trait, which has 4 different alleles (extremely slow, Kn, slow feathering, Ks, delayed feathering, K, and rapid feathering, k+) switched on the same locus (McGibbon, 1977). The direct gene action concerns the growth of feathers’ wing in newly hatched chicks, especially remiges and rectrices. Slow feathering strains inherently have better thermoregulation due to less heat production (Mérat, 1986; Fayeye et al., 2006; Gowe and Fairfull, 2008). An improvement in heat dissipation and economic traits (feed intake, growth rate, fitness) may be expected due to this mutation (Fotsa et al., 2001). Slow feathering locus has pleotropic effects beyond its impact on feathering (Leeson and Walsh, 2004). Its effects on broiler metabolism, immune response, heat tolerance, susceptibility to ascites, fat deposition, and carcass yield are well documented (Crittenden et al., 1987; Singh et al., 2001; Pakdel et al., 2002; Zerehdaran et al., 2004; Khosravinia, 2009). It could be noticed that the slow-feathering chicks are more capable of withstanding post-hatch broiler chicks transported for extended periods without access to feed compared to rapid feathering sibs (Khosravinia and Manafi, 2016). In cold weather, the fast-feathering genotypes are preferred over slow-feathering counterparts in broiler populations to protect the birds against chilling (Fotsa et al., 2001; Khosravinia and Manafi, 2016). Interestingly, some turkey breeds exhibit a similar slow and fast feathering phenomenon. Eventually, gene expression of poor feathering ranges from an almost complete absence of feathers to full feather covering at 4 wk of age (Renema et al., 2008).
Sex-linked slow and fast feathering rates have been widely used in poultry breeding for auto-sexing at hatch. The growth pattern of wing feathers is distinctively different between the rapid- and slow-feathering chicks at hatch with an accuracy of 98% (Sohn et al., 2012). In chicken hybrids produced from both fast- and slow-feathering genotypes, sex identification of newly hatched chicks can be carried out by personnel without training with an average of 96% accuracy (Goger et al., 2017). In fast-feathered day-old female chicks, the primary wing feathers are thicker and longer than the covert feathers, while in slow-feathered day-old male chicks, the primary and covert feathers are similar in length and thickness.
The dwarf gene (dw) is a sex-linked recessive gene associated with reduced body weight by about 40% and 30% in homozygous males and females, respectively compared to the normal status (DwDw, Dwdw, Dw-; Daghir, 2008). There has been a discrepancy regarding the advantage of the dw gene in heat-stressed laying hens. Its effect is more pronounced in a heavy-type breed compared to a lighter one. Under heat stress conditions, introducing dwarf gene becomes more interesting for broiler breeding programs, particularly when a reduction in energy requirements and feed consumption is taken into consideration. A better adaptability of dwarf hens to heat was observed only in one experiment (Sharifi et al., 2010). However, there has been a discrepancy regarding the advantage of the dw gene in heat-stressed laying hens (Wasti et al., 2020). Decuypere et al. (1991) concluded that the inherent heat tolerance of dw genotype in laying hens was uncertain. Under chronic heat stress, it has been found that the dw gene in fast-growing broiler chickens did not improve heat tolerance (Deeb and Cahaner, 1999; Lin et al., 2006).
GROWTH PERFORMANCE AND CARCASS YIELD
At high ambient temperatures, the naked neck genotypes performed significantly better in terms of growth performance, carcass characteristics, and biochemical blood parameters. It was concluded that the ability of the Na chicken to adapt to high temperatures predicts a viable option for the biological alleviation of climate change (Rajkumar et al., 2011). In the F2 generation, the naked neck chickens (NaNa and Nana) had significantly lower feed conversion ratio, body weight, body weight gain, linear body measurements, survivability, and carcass yield compared to their normal plumage (nana) counterparts (Adomako et al., 2014a). Higher meat yield due to the presence of the Na gene is well established (El-Attar and Fathi, 1995; Yalcin et al., 1999; Fathi et al., 2008). Rajkumar et al. (2011) reported that the naked neck chickens showed a significantly greater increase in live weight, dressing yield, thigh and giblet weight than their normal siblings. The increasing thoracic muscles associated with the Na allele might be due to less dietary protein requirement, leaving more protein for muscle development compared with full feather counterparts (Merat, 1990; El-Attar and Fathi, 1995; Reddy et al., 2015). Moreover, naked neck chickens exhibited less cannibalism behavior compared to normally feathered siblings, resulting from a lower protein requirement and a lower mass of feathers (Barua et al., 1998; Deeb and Cahaner, 1999; Gowe and Fairfull, 2008). In Ghana, the indigenous naked neck (Na/na) chickens are preferred over their normal plumage counterparts for meat yield and quality (taste, texture, and water holding capacity) (Duah et al., 2018). Due to a reduced feather cover, naked neck chickens need less time and cost for feather scalding and plucking leaving a carcass smoothly dressed (Moyle et al., 2011; Parker et al., 2014; Tabler et al., 2017). In the developing countries, the naked neck genotypes are preferred in flocks raised under scavenging and free-range systems. The naked neck chickens require less amount of drinking water, which enable them to be adapted for raising in dry lands (Chikumba and Chimonyo, 2014). The naked neck chickens spend more time on foraging compared to normally feathered ones indicating their enhanced adaptation (Mutibvu et al., 2018). Birds having Na or F genes had significantly increased body weight at the onset of laying compared with normally feathered counterparts (Abou-Emera et al., 2017). However, the positive effect of the F gene on the growth rate of broilers is less than the effect of the Na allele under high temperatures (Lin et al., 2006; Pawar et al., 2016). Furthermore, there is an additive effect in the heterozygous gene utilization (Na/na F/f) in broiler chickens (Yunis and Cahaner, 1999; Fisinin and Kavtarashvili, 2015). With respect to the rate of feathering, Khosravinia (2009) reported that a significantly increased live weight and carcass traits were noticed in rapid feathering chickens compared to slow feathering ones of commercial broiler flocks. In turkeys, differences in body weight and carcass characteristics were found between slow feathering males and fast feathering females (Sikur et al., 2004). On the other hand, the presence of sex-linked slow feathering gene in the chicks kept under hot environment (31°C) did not influence growth rate, feed intake, and feed efficiency (Fotsa et al., 2001). In broiler breeders, the sex-linked recessive gene for dwarfism (dw) reduced adult body size by 30 to 40% and lowered metabolic heat production (Lin et al., 2006; Pawar et al., 2016). The dw gene shortens shank length by about 25% in heavy weight breeds compared with 6% in light weight breeds (Photo 6) (Reddy and Siegel, 1977; Hussain et al., 1982). Accordingly, dwarf birds become more suitable for adaptation to hot climate by getting rid of the excess heat through radiation and convection (Horst and Petersen, 1977; Islam, 2005). The advantages of major genes for growth performance and carcass characteristics under hot temperatures are summarized in Table 1.
Table 2.
Effect of major genes on egg production performance and egg quality.
| Trait | Gene function |
Ambient temperature | Reference | |||||
|---|---|---|---|---|---|---|---|---|
| NaNa | Nana | FF | Ff | Na/-F/- | dw | |||
| Body weight at first egg | +7.9 | tropical conditions | Adomako et al., 2014b | |||||
| Age at maturation | −7.3 | −8.1 | −9.1 | −3.5 | hot region | Abou-Emera et al., 2017 | ||
| −12.6 | 32°C | Islam, 2005 | ||||||
| Egg number | −9 | −9.7 | 22°C | Chen et al., 2002 | ||||
| +16.8 | +9.7 | 32°C | ||||||
| +7.5 | +8.6 | 32°C | El-Safty, 2006 | |||||
| +4.97 | +0.49 | +8.47 | 29–34°C | Mahrous and El-Dlebshany, 2011 | ||||
| Egg mass | −24.2 | 32°C | Islam, 2005 | |||||
| +7.48 | +1.65 | +11.62 | 29–34 | Mahrous and El-Dlebshany, 2011 | ||||
| +6.4 | tropical conditions | Adomako et al., 2014b | ||||||
| Egg production | +6.4 | tropical conditions | Adomako et al., 2014b | |||||
| +6.5 | +14.1 | 39.1°C | Fathi et al., 2018 | |||||
| −20.1 | 32°C | Islam, 2005 | ||||||
| Egg weight | +6.5 | +6.4 | 22°C | Chen et al., 2002 | ||||
| +10.8 | +7 | 32°C | ||||||
| −4.6 | 32°C | Islam, 2005 | ||||||
| +1.2 | +0.9 | 32°C | El-Safty, 2006 | |||||
| +1.99 | +0.26 | +2.27 | 29–34°C | Mahrous and El-Dlebshany, 2011 | ||||
| +0.0 | +0.0 | −1.5 | −1.0 | hot region | Abou-Emera et al., 2017 | |||
| +1.4 | +2.0 | 39.1°C | Fathi et al., 2018 | |||||
| Broken eggs | −3.8 | −3.3 | 39.1°C | Fathi et al., 2018 | ||||
| Haugh unit | −0.3 | +0.79 | 32°C | El-Safty, 2006 | ||||
| +3.3 | +2.9 | tropical conditions | Adomako et al., 2014b | |||||
| +2.3 | +3.3 | +2.9 | +2.1 | hot region | Abou-Emera et al., 2017 | |||
| Albumin, % | +3.4 | +1.4 | 32°C | El-Safty, 2006 | ||||
| Yolk, % | −6.2 | −2.9 | 32°C | |||||
| Shell, % | +2.1 | +3.1 | 32°C | |||||
| +2.5 | +0.3 | +4.7 | 29–34°C | Mahrous and El-Dlebshany, 2011 | ||||
| Albumin height | +1.1 | +1.1 | 32°C | El-Safty, 2006 | ||||
| Yolk height | +1.1 | +1.7 | 32°C | |||||
Blank cell indicates data not available, Gene function = (genotype having a mutation –normal case/normal case) × 100.
Photo 6.
Dwarf male (dwdw) shows short shank length (short-legged bird). All photos are taken from the chicken flock belonging to research station, College of Agriculture and Veterinary Medicine, Qassim University, SA.
Table 1.
Growth performance and carcass characteristics as affected by major genes.
| Trait | Gene function |
Ambient temperature | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NaNa | Nana | FF | Ff | Na/-F/- | dw | ||||
| Live body weight | 4 wks | −13.1 | −4.2 | 24°C | Fathi et al., 2005 | ||||
| 6 wks | +8.0 | <45°C | Rajkumar et al., 2011 | ||||||
| +3.8 | +5.7 | tropical | Adomako et al., 2014a | ||||||
| 8 wks | +9.5 | +7.4 | 13.8 | 26°C | Mahrous et al., 2008 | ||||
| 12 wks | +6.9 | +9.3 | 27°C | Fathi et al., 2008 | |||||
| 16 wks | −2.0 | 21°C | El-Safty and Fathi, 2004 | ||||||
| +7.9 | ˂45°C | Rajkumar et al. 2011 | |||||||
| 20 wks | −1.28 | −0.4 | 32°C | El-Safty, 2006 | |||||
| +17.1 | +6.4 | Singh et al., 2001 | |||||||
| 40 wks Adult |
−29.3 | 32°C | Islam, 2005 | ||||||
| −30/−40* | hot | Daghir, 2008 | |||||||
| +12.5 | +13.7 | −7.6 | +2.7 | hot | Abou-Emera et al., 2017 | ||||
| FCR | −5.8 | −1.9 | −7.0 | 26°C | Mahrous et al., 2008 | ||||
| −0.01 | >12 | Rajkumar et al., 2011 | |||||||
| +0.01 | <45 |
Rajkumar et al., 2011 Mahrous and El-Dlebshany, 2011 |
|||||||
| −5.5 | −3.5 | −9.1 | 29-34 | ||||||
| −3.2 | −5.3 | tropical | Adomako et al., 2014a | ||||||
| +8.5 | 32°C | Islam, 2005 | |||||||
| Mortality rate | −42.8 | −40.7 |
+8.3 |
tropical |
Adomako et al., 2014a Adomako et al., 2014b |
||||
| Dressed carcass | +0.2 | 21 | El-Safty and Fathi, 2004 | ||||||
| +5.6 | >12 | Rajkumar et al., 2011 | |||||||
| +4.4 | <45 | ||||||||
| +7.5 | +5.5 | tropical | Adomako et al., 2014a | ||||||
| +3.3 | +1.9 | Reddy et al., 2015 | |||||||
| Feather | −43.2 | −18.5 | 27°C | Fathi et al., 2008 | |||||
| Breast muscles | +6.4 | +6.4 | 24–32°C | Deeb and Cahaner, 1999 | |||||
| -12.8 | 21 | El-Safty and Fathi, 2004 | |||||||
| +6.9 | 27°C | Fathi et al., 2008 | |||||||
| +9.5 | +0.7 | >12 | Rajkumar et al., 2011 | ||||||
| +3.3 | <45 | ||||||||
| +33.1 | +29.8 | tropical | Adomako et al., 2014a | ||||||
| Drumstick | +1.2 | 21 | El-Safty and Fathi, 2004 | ||||||
| +17.1 | +17.1 | 27°C | Fathi et al., 2008 | ||||||
| Thigh | +13.6 | 21 | El-Safty and Fathi, 2004 | ||||||
| +19.8 | +5.6 | 27°C | Fathi et al., 2008 | ||||||
| +5.0 | ˂45°C | Rajkumar et al. 2011 | |||||||
| +8.2 | +4.9 | tropical | Reddy et al., 2015 | ||||||
| Giblets | −1.7 | 21 | El-Safty and Fathi, 2004 | ||||||
| +16.5 | +13.0 | 27°C | Fathi et al., 2008 | ||||||
| +14.2 | >12°C | Rajkumar et al. 2011 | |||||||
| +5.6 | ˂45°C | ||||||||
| Abdominal fat | −79.3 | 21 | El-Safty and Fathi, 2004 | ||||||
| −59.6 | −49.5 | 27°C | Fathi et al., 2008 | ||||||
| −6.3 | >12°C | Rajkumar et al. 2011 | |||||||
| −20.4 | ˂45°C | ||||||||
Blank cell indicates data not available, Gene function = (genotype having a mutation –normal case/normal case) × 100, FCR = feed conversion ratio,
Reduction% in females and males, respectively.
EGG PRODUCTION AND EGG QUALITY
It is well known that egg production and quality deteriorate under hot weather conditions. In general, many reports stated that the presence of Na or F gene significantly increased laying performance and eggshell quality, especially breaking strength in laying hens kept at high environmental temperatures (Mérat, 1990; Galal, 2000; Singh et al., 2001; Mahrous et al., 2008; Abou-Emera et al., 2017). Because of their enhanced heat dissipation, both frizzle genotypes (homozygous and heterozygous) have relatively higher meat and egg production than wild type individuals under tropical conditions (Mathur, 2003; Adomako et al., 2014b). The superiority of the homozygous state (FF) was evident for all egg quantity and quality traits. Besides, the presence of Na and F genes in laying hens raised under low ambient temperature significantly increased shell thickness and, in turn, improved shell strength (Fathi et al., 2019). The heterozygous naked neck (Nana) genotype significantly improved the Haugh unit and shell thickness compared to the normally feathered one. Additionally, the naked neck and frizzled genotypes attained sexual maturity earlier than normal plumage hens (Abou-Emera et al., 2017).
Islam (2005) found that the dwarf hens attained sexual maturity earlier than normal size sisters at hot environment (30°C). Besides, the existence of dw gene reduced vitellogenesis, resulting in a greater reduction of defective eggs, such as double yolked, shell less, soft shelled eggs and, in turn, higher normal hatching eggs (Anonym, 2003; Islam, 2005). Nevertheless, slow-feathered chickens show a reduction in egg production and need a higher amount of dietary energy (Wilson et al., 2007). It was found that the slow feathering gene (K) adversely affected fertility, embryonic mortality, hatchability, and egg production traits, except for egg weight at 22°C rearing temperature (Goger et al., 2017). However, Mincheva et al. (2012) reported that the egg production traits of hybrids were not negatively affected by the slow feathering (K) gene.
In terms of ultrastructural properties of eggshell, Mahrous (2012) reported that Nanaff and NanaFf genotypes exhibited a good confluence of mammillary caps and early fusion among calcite columns compared to the nanaff genotype. In an intensive eggshell ultrastructural study, Radwan (2014) pointed to a positive effect on the increasing length of the palisade layer of eggs laid by hens carrying Na and/or F genes. Additionally, the presence of the Na gene in a single state or combined with the F gene significantly increased the total mammillary thickness compared to eggs from the nanaff genotype (Mahrous and Galal, 2011). It is well known that the palisade layer provides the stiffness of the eggshell, thus imparting strength to the egg (Bain, 1992). A weak attachment between caps and the inner shall membrane resulting from increased type B bodies was clearly observed in eggs produced from the normally feathered genotype compared with those produced from naked neck and frizzle siblings (Mahrous, 2012). Tables 2 and 3 show the effects of major genes on productive performance and eggshell quality, respectively.
Table 3.
Shell quality properties of eggs produced from different genotypes.
| Trait | Gene function |
Ambient temperature | Reference | |||||
|---|---|---|---|---|---|---|---|---|
| NaNa | Nana | FF | Ff | Na/-F/- | dw | |||
| Breaking strength | +10 | +9 | hot region | Galal and Fathi, 2002 | ||||
| −5.7 | 32°C | Islam, 2005 | ||||||
| +19.6 | +15.2 | 32°C | El-Safty, 2006 | |||||
| +3.7 | +4.4 | 5.9 | 29–34°C | Mahrous and El-Dlebshany, 2011 | ||||
| +6.1 | +14.6 | +1.9 | +5.6 | hot region | Abou-Emera et al., 2017 | |||
| Mammillary thickness | +16.8 | +15.3 | Mahrous, 2012 | |||||
| Shell thickness | +5.4 | +4.2 | 22°C | Chen et al., 2002 | ||||
| +6.8 | +1.6 | 32°C | ||||||
| +5.9 | +2.9 | 32°C | El-Safty, 2006 | |||||
| +2.6 | +1.8 | +3.5 | 29–34°C | Mahrous and El-Dlebshany, 2011 | ||||
| +9.4 | +6.3 | tropical conditions | Adomako et al., 2014b | |||||
| +2.7 | +4.3 | +1.0 | +1.1 | hot region | Abou-Emera et al., 2017 | |||
Blank cell indicates data not available, Gene function = (genotype having a mutation –normal case/normal case) × 100.
REPRODUCTION PERFORMANCE
It is well recognized that fertility and hatchability represent significant factors affecting reproductive performance in poultry breeding programs. Major genes affecting fertility and hatchability of chickens kept under high environmental temperatures have been reported (Mérat, 1990; Renema et al., 2008; Ajayi and Agaviezor, 2016). In general, the hatchability percentage in naked neck chickens was low due to the high incidence of late embryonic mortality (Mérat, 1986; Rajkumar et al., 2012). Embryonic mortality in the late developing stage is significantly increased, and the homozygous genotype (NaNa) is more affected than the heterozygous embryos (Nana). The possible reason for the Na gene-induced embryonic mortality is still not clear (Sharifi et al., 2010). However, Peters (2005) concluded that the Na gene is likely to be lethal when present in a dominant form. Nevertheless, better fertility and hatchability estimates were recorded in chickens carrying the Na gene at high and humid temperatures (Ladjali et al., 1995). Furthermore, Sharifi et al. (2010) reported that the normally feathered genotype (nana) showed an inferior performance in reproductive traits, viz. fertility, hatchability, and number of live chicks in comparison with the homozygous genotype (NaNa) under thermal stress. Among Nigerian local chickens, the frizzle gene is more desirable in improving fertility and hatchability traits, while the naked neck gene appears to have a lethal effect on embryonic development (Adeleke et al., 2012). Similarly, Peters et al. (2008) found that frizzled cockerels produced higher semen quality, including volume, motility, and concentration compared with their naked neck counterparts. In terms of slow feathering mutation, it was observed that fast feathering genotypes had higher hatching traits than those of slow feathering ones (Goger et al., 2017). Slow-feathered turkeys show inferior reproductive efficiency compared with their fast-feathered counterparts (Renema et al., 2008). Moreover, slow-feathered chickens show late sexual maturity (Wilson et al., 2007). Better reproductive fitness (fertility and hatchability) and more hatching eggs were observed in dwarf breeders compared to normal-sized siblings raised in the tropics (Islam, 2005).
IMMUNITY AND HEALTH
There is no doubt that some major genes are believed to increase the bird's adaptability to defeat heat stress and improve disease resistance as well. Numerous reports concluded that Na and F genes could be used in selection programs of chicken breeds raised in hot climates to improve immunocompetence traits and decrease mortality rate. Lower mortality rate and higher disease resistance were reported for naked neck birds over normally feathered siblings (Mahrous et al., 2008; Islam and Nishibori, 2009; Fathi et al., 2019). In the tropics, higher disease resistance and lower mortality rate were associated with birds carrying Na, F, and dw genes compared to those having normal plumage (Njenga et al., 2005). The naked neck gene significantly increased cell-mediated responses. Homozygous naked neck (NaNa) chickens had a higher response to phytohemagglutinin-P (PHA-P) challenges compared with both heterozygous (Nana) and normally feathered (nana) genotypes at high ambient temperatures (Nazmi, 2006; Galal, 2008). Additionally, El-Safty, 2006 noticed that the heterozygous genotype of the naked neck gene had a significantly greater dermal swelling response to PHA-P compared to the normally feathered one in laying hens kept under winter conditions. At low ambient temperatures, the presence of Na and F genes greatly enhanced cellular immunity, particularly in heterozygous naked neck status (Fathi et al., 2019). In the laying hen population, Fathi et al. (2005) and Abou-Emera et al. (2017) demonstrated that the presence of Na and F genes significantly increased cell mediated response at all interval times as compared to normally feathered genotype. Similarly, Patra et al. (2004) confirmed that both the NaNa and Nana genotypes have better cellular mediated immunity when compared to the nana sibs. On the other hand, Martin et al. (1989) and Haunshi (1999) did not find a positive effect of Na and F genes on cell-mediated response to concanavalin A.
In terms of humoral immunity, the Na gene significantly increased antibody titers against to SRBC (Fathi et al., 2005; Galal and Mahrous, 2011; Rajkumar et al., 2011). Nevertheless, Haunshi et al. (2002) reported that there were no significant differences in antibody response to SRBC in heterozygous naked neck and frizzled birds when compared with their normally feathered counterparts. Several studies have revealed that the Na gene has more resistance against bacterial, viral, and parasitic infections. Naked neck birds have a higher carbon clearance index, that is, higher phagocytosis compared to normal birds (Fathi et al., 2005; Nazmi, 2006; Mahrous, 2008; El Syaid et al., 2011). Conversely, Haunshi et al. (2002) reported that there was no difference between naked neck and normally feathered genotypes for the ability of phagocytosis. Naked neck chickens did not show signs of sudden death or ascites syndrome (Gonzales et al., 1998, 1999).
Dwarf laying hens have better resistance to Marek's disease, showing lower mortality in birds injected with Marek's disease under heat stress (Dunnington and Siegel, 1998). In addition, Islam (2005) stated that sex-linked dwarf (dw) birds may have more competent T-cell subpopulations and weaker B-cell reactivity compared to normal ones. Dwarf chickens had consistently higher or equivalent cell-mediated immunity responses compared to their normal counterparts (Klingensmith et al., 1983). On the other hand, no adverse effect was found due to introducing dwarf and slow feathering genes on the immunocompetence of chickens (Klingensmith et al., 1983; Bacon et al., 1986). Records of immune response and lymphoid organs according to several authors are summarized in Table 4.
Table 4.
Summarized effects of major genes on lymphoid organs and immune response parameters.
| Trait | Gene function |
Ambient temperature | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NaNa | Nana | FF | Ff | Na/-F/- | dw | ||||
| Bursa% | +82.6 | −4.3 | +52.2 | 26°C | Mahrous et al., 2008 | ||||
| −11.79 | +19.29 | 24°C | Fathi et al., 2005 | ||||||
| Spleen% | +9.1 | +59.1 | +63.6 | 26°C | Mahrous et al., 2008 | ||||
| +23.73 | +12.29 | 24°C | Fathi et al., 2005 | ||||||
| Thymus% | +55.6 | +5.6 | +44.4 | 26°C | Mahrous et al., 2008 | ||||
| −19.84 | +23.89 | 24°C | Fathi et al., 2005 | ||||||
| Antibody against SRBC | +26.9 | +14.7 | +14.9 | 26°C | Mahrous et al., 2008 | ||||
| +51.5 | +48.9 | 34–42°C | Rajkumar et al., 2010 | ||||||
| +34.6 | >12°C | Rajkumar et al. 2011 | |||||||
| +0.9 | ˂45°C | ||||||||
| CMI | +183.33 | +133.33 | +83.33 | 26°C | Mahrous et al., 2008 | ||||
| +17.1 | >12°C | Rajkumar et al. 2011 | |||||||
| +19.7 | ˂45°C | ||||||||
| 72 h | +91.7 | +50.0 | +108.3 | +50.0 | hot region | Abou-Emera et al., 2017 | |||
| +75.0 | +162.5 | 39.1°C | Fathi et al., 2018 | ||||||
| 24 h | +34.5 | +24.1 | 32°C | Galal, 2008 | |||||
| NDV titre | +21 | +19.7 | 34–42°C | Rajkumar et al., 2010 | |||||
| +8.1 | >12°C | Rajkumar et al. 2011 | |||||||
| +4.0 | ˂45°C | ||||||||
| +1.2 | +1.8 | 39.1°C | Fathi et al., 2018 | ||||||
| Phagocytic index |
+24.4 | +11.8 | +30.9 | 26°C | Mahrous et al., 2008 | ||||
Blank cell indicates data not available.
Gene function = (genotype having a mutation –normal case/normal case) × 100; CMI, cell mediated immunity resulting from phytohemagglutinin response; NDV, Newcastle disease virus.
BLOOD HEMATOLOGY AND BIOCHEMISTRY
In naked neck chickens raised under high ambient temperatures, the increased growth rate may be associated with a high concentration of triiodothyronine hormone (Decuypere et al., 1993; Lin et al., 2006). It also reduces fat deposition in skin and breast muscles, which increases heat dissipation via the neck and bare surface (Raju et al., 2004). The existence of the Na gene reduces triglycerides and cholesterol content (very low and low-density lipoprotein), while it increases high-density lipoprotein (Patra et al., 2002; Rajkumar et al., 2010; Mahrous and El-Dlebshany, 2011). Accordingly, blood cholesterol and the accumulation of fat could be reduced (Rajkumar et al., 2010). In addition, Duah et al. (2020) demonstrated that the nanaff genotype birds had significantly higher cholesterol levels than in both the Nanaff and NanaFf genotype states. In terms of health, the indigenous naked neck genotype appears to be superior due to its low fat and cholesterol content. On the other hand, there were insignificant differences in the concentrations of cholesterol, triglycerides, and thyroid hormones among naked, frizzle and normally feathered genotypes (Peters et al., 2011; Fathi et al., 2019). At low ambient temperatures, the Na and F genes increased hemoglobin concentration, white blood cells, and oxygen demand compared to the normal plumage genotype (Monnet et al., 1979 ; Yahav et al., 1998; Peters et al., 2011). A higher haematocrit value in birds carrying the Na gene was observed (Yahav et al., 1998; Galal, 2008; El-Safty, 2006). The Heterophil (H)/Lymphocyte (L) ratio has been considered as the indicator of stress condition in chickens. Rajkumar et al. (2011) demonstrated that the naked neck chickens had a significantly lower H/L ratio than their normal siblings, indicating less heat stress under high temperatures. An increased H/L ratio was found in the normal plumage genotype compared to the naked neck one under low ambient temperature (El-Safty, 2006). According to the findings of Mahrous et al. (2008), introducing the Na or F gene, either alone or in combination, significantly increased plasma total protein and globulin levels compared to the normal plumage state (nanaff). When compared to frizzled and naked neck genotypes, the normally feathered birds had higher serum glucose, urea, and creatinine levels (Peters et al., 2011). Under summer conditions, the lipid peroxidation concentration was higher in normally feathered birds compared to naked neck sibs, resulting in higher concentration of malondialdehyde (MDA) in the blood (Rajkumar et al., 2011).
In dwarf laying hens, the naked neck gene (single or double segregation) had a significantly lower cholesterol level as compared with normal sized siblings (Galal et al., 2007). In terms of liver function, the inclusion of dw, Na, and their combination greatly enhanced liver function by reducing liver enzymes (GOT and GPT) as compared to Dw-nana counterparts. Moreover, the inclusion of dw gene in a single manner or in combination with the Na gene significantly reduced plasma T3 compared to normal (Dw-nana) birds (Galal et al., 2007). Blood parameters as affected by major genes are listed in Table 5.
Table 5.
Blood parameters as affected by major genes.
| Trait | Gene function |
Ambient temperature | Reference | |||||
|---|---|---|---|---|---|---|---|---|
| NaNa | Nana | FF | Ff | Na/-F/- | dw | |||
| Total protein | +22.8 | +30.2 | 20 | Fathi et al., 2005 | ||||
| +6.1 | +4.1 | 32°C | Galal, 2008 | |||||
| +17.6 | 15.4 | +13.4 | 26 | Mahrous et al., 2008 | ||||
| +6.4 | >12 | Rajkumar et al., 2011 | ||||||
| −1.8 | <45 | |||||||
| Albumen | +31.6 | +26.3 | 20 | Fathi et al., 2005 | ||||
| 32°C | Galal, 2008 | |||||||
| Globulin | +10.4 | +35.6 | 20 | Fathi et al., 2005 | ||||
| +19.8 | +11.0 | 32°C | Galal, 2008 | |||||
| +104.5 | +38.5 | +98.9 | 26 | Mahrous et al., 2008 | ||||
| −15.3 | −9.5 | Rajkumar et al., 2010 | ||||||
| Total cholesterol | −3.8 | −7.0 | −5.7 | 29–34°C | Mahrous and El-Dlebshany, 2011 | |||
| −11.5 | >12°C | Rajkumar et al. 2011 | ||||||
| −11.5 | ˂45°C | |||||||
| LDL | −32.7 | −13.4 | Rajkumar et al., 2010 | |||||
| HDL | +19.5 | +16.5 | ||||||
| Hematocrit | +2.5 | +1.0 | 32°C | Galal, 2008 | ||||
| H/L ratio | +14.21 | +7.45 | 24°C | Fathi et al., 2005 | ||||
| +15.4 | >12°C | Rajkumar et al. 2011 | ||||||
| −12.7 | ˂45°C | |||||||
Blank cell indicates data not available.
Gene function = (genotype having a mutation –normal case/normal case) × 100; LDL; low-density lipoprotein; HDL, high-density lipoprotein; H/L, Heterophils/ Lymphocytes ratio.
THE INTERACTION AMONG MAJOR GENES
It seems that the heat tolerance was improved in the double heterozygous chickens (NaNaFf) compared with the single state genotypes (Yunis and Cahaner, 1999; Mahrous, 2008). In terms of productive performance, several studies have reported that the combination of Na and F genes appeared to have a positive interaction on body weight, feed conversion, egg production, disease resistance, and immune response, particularly at high ambient temperatures (Mahrous and El-Dlebshany, 2011; Fathi et al., 2013, 2014). When compared to the normal genotype, introducing the Na gene alone or in combination with an F gene significantly improved egg quality and/or shell quality (thickness and breaking strength; Zulkifli et al., 1992; Mahrous and El-Dlebshany, 2011). Moreover, a favorable effect on ultrastructural properties was noticed in eggs laid by laying hens carrying a combination of Na and F genes (Mahrous and Galal, 2011; Radwan, 2014). In addition, Missohou et al. (2003) found that the interaction between the dwarf and frizzle genes was only positive for growth traits and egg number in Senegal's tropical climate. On the other hand, there was no gene-by-gene interaction when frizzle-dwarfed birds (FFdw-) were kept at a moderate ambient temperature (24°C). The genotype combining the 2 major genes (FFdw‑) proved to be inferior to the normally feathered dwarf genotype (ffdw‑) for laying traits but superior in fertility (Sharifi et al., 2010).
CONCLUSIONS
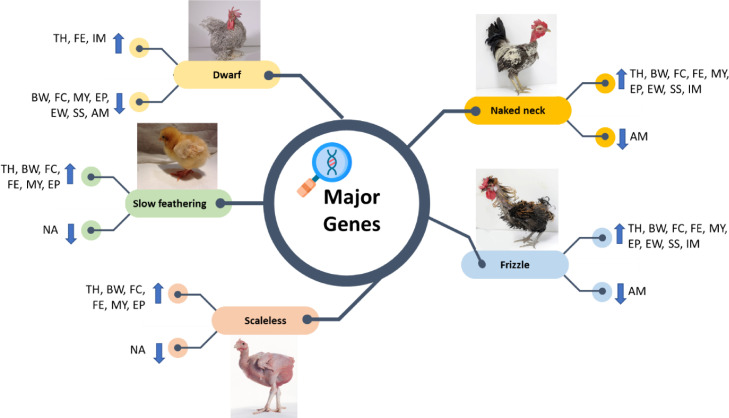
Heat stress is considered the biggest concern affecting productive performance in the poultry industry. It has been established that the Na and F genes (alone or in combined status) are highly tolerant to high environmental conditions and can improve both growth performance and egg production. Implanting a sex-linked dwarf gene in broiler breeder maternal lines improves the profitability of the project under hot climate. Lower requirements for housing and feed, better survivability and fertility, improved hatchability, increased feed efficiency, and resilient resistance to disease could be achieved. Using sex-linked slow feathering mutation for auto-sexing in chicks of some egg-type strains at hatch provides an accurate and low-cost effective method. Table 6 summarizes and describes the morphological appearance, direct action, and pleotropic associated effects of the major genes under hot environmental conditions. Additionally, a schematic diagram illustrating the improvement or deterioration that occurred in the productive traits of genotypes carrying major genes is given (Figure 1).
Table 6.
Description of morphological appearance and pleotropic effects of the major genes under hot environmental conditions.
| Mutation | Symbol | Dominance | Direct gene action/description | Pleotropic effects | Photo |
|---|---|---|---|---|---|
| Naked neck (Na) | Na ˃ na | Incompletely dominant autosomal gene | Reduces the feather coverage by about 40% in homozygous and 20-30% in heterozygous chickens. The neck becomes completely bare in homozygous with feather tuft in heterozygous state. | • Heat shock proteins is rapidly synthesized. • Enhances immunocompetence. • Improves eggshell quality. • Increases breast meat. • Increases body weight and egg production. |
 |
| Frizzle (F) | F ˃ f | Incompletely dominant autosomal gene | Feathers become curling outward and more delicate. Reduces feather intensity. | • Enhanced heat dissipation. • Increases meat yield and egg production. • Enhances immunocompetence. |
 |
| Dwarfism (dw) | Dw ˃ dw | Recessive sex-linked gene | Reduces body weight by about 30-40% in females and homozygous males. Shortens shank length by about 25% in heavy weight breeds. |
• Improves feed converstion rate. • Reduces metabolic heat output • Decreases defective eggs and increases settable eggs. • Better resistanse to diseases. • Enhances immunocompetence. |
 |
| Slow feathering (K)1 | Kn˃ Ks ˃ K˃ k | Recessive sex-linked multiple allelic gene | Plumage development is greatly delayed during the first weeks of juvenile life. The feathers become curling outward and upward. The primary remiges evidently longer than the coverlets in newly hatched female chicks. | • Better thermoregulation due to increased heat loss during early growth. • Day-old sex determination (autosexing) by flight feathers. |
 |
| Scaleless (sc)2 | Sc ˃ sc | Recessive autosomal gene | Causes entire loss of feathers (featherless birds). | • Enhances thermotolerance, particulary in heavy breeds. • Improves feed efficiency. • Tolerates heat stress (over 40C) for a long period. • Increases meat yield, especially breast muscles. • Decreases dietary protein requirements. • Decreases scalding and defeathering costs. |
 |
The photos belong to research station, College of Agriculture and Veterinary Medicine, Qassim University, SA.
Figure 1.
The schematic diagram shows the effect of major genes on productive traits in chickens raised at hot ambient temperature. The arrow pointing up indicates improvement and the arrow pointing down indicats deterioration. Abbreviations: AM, age at maturation; BW, body weight; EP, egg production; EW, egg weight; FC, feed consumption; FE, feed efficiency; MY, meat yield; SS, shell strength; IM, immunocompetence; NA, not applicable; TH, thermotolerance.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Abou-Emera O.K., Ali U., Galal A., El-Safty S., Abdel-Hameid E., Fathi M.M. Evaluation of genetic diversity of naked neck and frizzle genotypes based on microsatellite markers. Int. J. Poult. Sci. 2017;16:118–124. [Google Scholar]
- Adeleke M.A., Peters S.O., Ozoje M.O., Ikeobi C.O.N., Bamgbose A.M., Adebambo O.A. Effect of crossbreeding on fertility, hatchability and embryonic mortality of Nigerian local chickens. Trop. Anim. Health. Prod. 2012;44:505–510. doi: 10.1007/s11250-011-9926-x. [DOI] [PubMed] [Google Scholar]
- Adomako K., Olympio O.S., Hagan J.K., Hamidu J.A. Growth performance of crossbred naked neck and normal feathered laying hens kept in tropical villages. Br. Poult. Sci. 2014;55:701–708. doi: 10.1080/00071668.2014.960805. [DOI] [PubMed] [Google Scholar]
- Adomako K., Olympio O.S., Hagan J.K., Hamidu J.A. Effect of the frizzle gene (F) on egg production and egg quality of laying hens kept in tropical villages. Br. Poult. Sci. 2014;55:709–714. doi: 10.1080/00071668.2014.963026. [DOI] [PubMed] [Google Scholar]
- Ajayi F.O., Agaviezor B.O. Fertility and hatchability performance of pure and crossbred indigenous chicken strains in the high rainforest zone of Nigeria. Int. J. Livest. Prod. 2016;7:141–144. [Google Scholar]
- Anonym. 2003. A report on the growth hatching egg production performance of i-757 (sex-linked dwarf gene), Arbor Acres and MPK broilers strain in Bangladesh. Bhagalpur, Bajitpur, Kishoregonj, Bangladesh.
- Bacon L.D., Salter D.W., Motta J.V., Crittendon L.B., Ogasawara F.X. Cryopreservation of chicken semen of inbred or specialized strains. Poult. Sci. 1986;65:1965–1971. doi: 10.3382/ps.0651965. [DOI] [PubMed] [Google Scholar]
- Bain M.M. Eggshell strength: a relationship between the mechanism of failure and the ultrastructural organization of the mammillary layer. Br. Poult. Sci. 1992;33:303–319. [Google Scholar]
- Bartels T. Variations in the morphology, distribution, and arrangement of feathers in domesticated birds. J. Exp. Zool. 2003;298B:91–108. doi: 10.1002/jez.b.28. [DOI] [PubMed] [Google Scholar]
- Barua A., Howlider M.A.R., Yoshimura M. Indigenous naked neck fowl of Bangladesh. Worlds Poult. Sci. J. 1998;54:279–286. [Google Scholar]
- Besbes B., Tixier-Boichard M., Hoffmann I., Jain G.L. Future trends for poultry genetic resources. Proceedings of the International Conference of Poultry in the 21st Century. Avian Influenza and Beyond; Bangkok, 5-7 November 2007; 2007. [Google Scholar]
- Cahaner A., Ajuh J.A., Siegmund-Schultze M., Azoulay Y., Druyan S., Zárate A.V. Effects of the genetically reduced feather coverage in naked neck and featherless broilers on their performance under hot conditions. Poult. Sci. 2008;87:2517–2527. doi: 10.3382/ps.2008-00284. [DOI] [PubMed] [Google Scholar]
- Chen C.F., Bordas A., Tixier-Boichard M. Montpellier; France: 2002. Effect of high ambient temperature and naked neck genotype on egg production in purebred and crossbred dwarf brown-egg layers selected for improved clutch length, Proceedings of the 7th World Congress on Genetics Applied to Livestock Production, August 19-23. [Google Scholar]
- Chikumba N., Chimonyo M. Effects of water restriction on the growth performance, carcass characteristics and organ weights of Naked Neck and Ovambo chickens of Southern Africa. Asian-Australas. J. Anim. Sci. 2014;27:974–980. doi: 10.5713/ajas.2013.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden L.B., McMahon S., Halpern M.S., Faldly A.M. Embryonic infection with the endogenous avian leucosis virus Rous-associated virus-O alters response to exogenous avian leukosis infection. J. Virol. 1987;61:722–725. doi: 10.1128/jvi.61.3.722-725.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daghir N.J. 2nd ed. CABI; Oxfordshire, UK: 2008. Poultry Production in Hot Climates. [Google Scholar]
- Decuypere E., Buyse J., Mérat P., Zoons J., Vloeberghs I. Growth, abdominal fat content, heat production and plasma hormone levels of naked-neck and control broiler chickens. Anim. Prod. 1993;57:483–490. [Google Scholar]
- Decuypere E., Huybrechts L.M., Kuhn E.R., Tixier-Boichard M., Merat P. Physiological alterations associated with the chicken sex-linked dwarfing gene. Crit. Rev. Poult. Biol. 1991;3:191–221. [Google Scholar]
- Deeb N., Cahaner A. The effect of naked neck genotypes, ambient temperature and feeding status and their interaction on body temperature and performance of broilers. Poult. Sci. 1999;78:1341–1346. doi: 10.1093/ps/78.10.1341. [DOI] [PubMed] [Google Scholar]
- Desta T.T. The genetic basis and robustness of naked neck mutation in chicken. Trop. Anim. Health Prod. 2021;53:95. doi: 10.1007/s11250-020-02505-1. [DOI] [PubMed] [Google Scholar]
- Desta T.T., Dessie T., Bettridge J., Lynch S.E., Melese K., Collins M., Christley R.M., Wigley P., Kaiser P., Terfa Z., Mwacharo J.M., Hanotte O. Signature of artificial selection and ecological landscape on morphological structures of Ethiopian village chickens. Anim. Gene. Res. 2013;52:1–13. [Google Scholar]
- Dong J., He C., Wang Z., Li Y., Li S., Tao L., Chen J., Li D., Yang F., Li N., Zhang Q., Zhang L., Wang G., Akinyemi F., Meng H., Du B. A novel deletion in KRT75L4 mediates the frizzle trait in a Chinese indigenous chicken. Genet. Sel. Evol. 2018;50:68. doi: 10.1186/s12711-018-0441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duah K.K., Essuman E.K., Olympio O.S., Akwetey W., Gyimah V., Yeboah J.O. Consumers’ acceptability of indigenous cockerel. Poult. Sci. 2018;97:1768–1773. doi: 10.3382/ps/pex451. [DOI] [PubMed] [Google Scholar]
- Duah K.K., Essuman E.K., Boadu V.G., Olympio O.S., Akwetey W. Comparative study of indigenous chickens on the basis of their health and performance. Poult. Sci. 2020;99:2286–2292. doi: 10.1016/j.psj.2019.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnington E.A., Sigel P.B. Comparison of sex-linked dwarf genes in chickens from two sources when introgressed into unrelated genetic backgrounds. Br. Poult. Sci. 1998;39:216–220. doi: 10.1080/00071669889141. [DOI] [PubMed] [Google Scholar]
- El Syaid A.G., Kamel I., Mahrous M.Y. Productive performance and immunocompetence parameters of naked neck and normally feathered chicken genotypes got from different maternal lines. Egypt. Poult. Sci. 2011;31:379–392. [Google Scholar]
- EL-Attar A.H., Fathi M.M. Incorporating naked neck gene, Na, to increase breast meat yield of dressed chickens. Egypt. Poult. Sci. 1995;15:285–297. [Google Scholar]
- El-Safty S.A. Influence of naked neck, frizzle, crest genes and their triple segregation on productivity of layer chickens under hot environmental conditions. Egypt. Poult. Sci. 2006;26:1253–1267. [Google Scholar]
- El-Safty S.A., Fathi M.M. Proceedings of 9th Conf. Agric. Dev. Res., Faculty of Agric. Ain Shams Univ; Cairo, Egypt: 2004. Impact of frizzle, crest genes and their interaction on some phenotypic and genetic parameters of male chickens under winter conditions of Egypt. [Google Scholar]
- Fathi M.M., Galal A., El-Safty S.A., Abdel-Fattah S.A. Impact of naked neck and frizzle genes on cell mediated immunity of chickens. Egypt. Poult. Sci. 2005;25:1055–1067. [Google Scholar]
- Fathi M.M., Galal A., El-Safty S., Mahrous M. Naked neck and frizzle genes for improving chickens raised under high ambient temperature: I. Growth performance and egg production. Worlds Poult. Sci. J. 2013;69:813–832. [Google Scholar]
- Fathi M.M., Galal A., El-Safty S., Mahrous M. Naked neck and frizzle genes for improving chickens raised under high ambient temperature: II. Blood parameters and immunity. Worlds Poult. Sci. J. 2014;70:165–172. [Google Scholar]
- Fathi M.M., Ebeid T.A., Rayan G.N., El-Safty S.A., Abou-Emera O.K. Genetic differences in eggshell ultrastructural properties of Saudi native chicken breeds kept at high ambient temperatures. Czech J. Anim. Sci. 2018;63:363–370. [Google Scholar]
- Fathi M.M., El-Attar A.H., Ali U.M., Nazmi A. Effect of naked neck gene on carcase composition and immunocompetence in chicken. Br. Poult. Sci. 2008;49:103–110. doi: 10.1080/00071660802005137. [DOI] [PubMed] [Google Scholar]
- Fathi M.M., AL-Homidan I., Abou-Emera O.K., AL-Moshawah A. Characterisation of Saudi native chicken breeds: a case study of morphological and productive traits. Worlds Poult. Sci. J. 2017;73:916–927. [Google Scholar]
- Fathi M.M., Al-Homidan I.H., Rayan G.N., El-Safty S.A., Ebeid T.A., Abou-Emera O.K. Laying performance, immune response and antioxidant properties of hens segregating for naked neck and frizzle genes under low ambient temperature. Czech J. Anim. Sci. 2019;64:216–225. [Google Scholar]
- Fayeye T.R., Ayorinde K.L., Ojo V., Adesina O.M. Frequency and influence of some major genes on body weight and body size parameters of Nigerian local chickens. Livest. Res. Rural Dev. 2006;18:37–41. [Google Scholar]
- Fisinin V.I., Sh. Kavtarashvili A. Heat stress in poultry. II. Methods and techniques for prevention and alleviation (review) Agric. Biol. 2015;50:431–443. [Google Scholar]
- Fotsa J.C., Mérat P., Bordas A. Effect of the slow (K) or rapid (k+) feathering gene on body and feather growth and fatness according to ambient temperature in a Leghorn × brown egg type cross. Genet. Sel. Evol. 2001;33:659–670. doi: 10.1186/1297-9686-33-6-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galal A. Pleiotropic effects of naked neck, frizzled and double segregation genes on some phenotypic and genetic parameters of chickens under hot environmental conditions. Egypt. Poult. Sci. 2000;20:945–960. [Google Scholar]
- Galal A. Immunocompetence and some hematological parameters of naked neck and normally feathered chicken. J. Poult. Sci. 2008;45:89–95. [Google Scholar]
- Galal A.A., Ahmed M.H., Ali U.M., Younis H.H. Influence of Naked Neck gene on laying performance and some haematological parameters of dwarfing hens. Int. J. Poult. Sci. 2007;6:807–813. [Google Scholar]
- Galal A., Fathi M.M. Introducing crest gene to enhance productive performance of naked neck chickens under moderate ambient temperatures. Egypt. Poult. Sci. 2002;22:611–628. [Google Scholar]
- Galal A., Mahrous M. Productive performance and immunocompetence parameters of naked necks and normally feathered chicken genotypes issued from different maternal lines. Poult. Sci. 2011;90(Suppl. 1):8. Proceedings of 100th Poultry Science Annual Meeting, America's Center, St. Louis, Missouri, USA. [Google Scholar]
- Galal A., Radwan L.M., Rezik H.H., Ayoub H. Expression levels of HSP70 and CPT-1 in three local breeds of chickens reared under normal or heat stress conditions after the introduction of the naked neck gene. J. Therm. Biol. 2019;80:113–118. doi: 10.1016/j.jtherbio.2018.12.018. [DOI] [PubMed] [Google Scholar]
- Goger H., Demirtas S.E., Yurtogullari S. Determination effects of slow (K) and fast (k+) feathering gene on egg production and hatching traits in laying hens. Asian J. Anim. Vet. Adv. 2017;12:247–253. [Google Scholar]
- Gonzales E., Mogyca N.S.S., Varoli Júnior J.C., Takita T.S., Loddi M.M. O tempo de jejum do neonato afeta o desempenho do frango de corte na idade de abate. Rev. Bras. Ciênc. Avíc. Suppl. 1999;1:12. [Google Scholar]
- Gonzales E., Buyse J., Loddi M.M., Takita T.S., Buys N., Decuypere E. Performance, incidence of metabolic disturbances and endocrine variables of food-restricted male broiler chickens. Br. Poult. Sci. 1998;39:671–678. doi: 10.1080/00071669888557. [DOI] [PubMed] [Google Scholar]
- Gowe R.S., Fairfull R.W. In: Pages 13–29 in Poultry Production in Hot Climates. Daghir N.J., editor. CABI International, Cromwell Press; Trowbridge: 2008. Breeding for resistance to heat stress. [Google Scholar]
- Haunshi, S. 1999. Studies on general immune competence in specialized chicken populations. M.V.Sc. Thesis submitted in Poultry Science, IVRI, Izatnagar, UP, India.
- Haunshi S., Sharma D., Nayal L.M.S., Singh D.P., Singh R.V. Effect of naked neck (Na) and frizzle gene (F) on immune competence in chickens. Br. Poult. Sci. 2002;43:28–32. doi: 10.1080/00071660120109863. [DOI] [PubMed] [Google Scholar]
- Horst P. Native fowl as reservoir for genomes and major genes with direct and indirect effects on production adaptability. Page 105 in Proceedings of the 18th World Poultry Congress; Nagoya, Japan; 1988. [Google Scholar]
- Horst P., Petersen J. Influence of dwarf gene (dw) on laying hen breeding. Arch. Geflügelk. 1977;41:246–252. [Google Scholar]
- Hussain S.A., Horst P., Mukherjee T.K., Tawfik E.S. Genotype-environment interaction in layers involving dwarf gene (dw) and its normal (Dw) allele. Anim. Prod. Health Trop. 1982:195–198. [Google Scholar]
- Islam M.A. Sex-linked Dwarf gene for broiler production in hot-humid climates. Asian-Australas. J. Anim. Sci. 2005;18:1662–1668. [Google Scholar]
- Islam M.A., Nishibori M. Indigenous naked neck chicken: a valuable genetic resource for Bangladesh. Worlds Poult. Sci. J. 2009;65:125–138. [Google Scholar]
- Khosravinia H. Effect of the slow (K) or rapid (k+) feathering gene on carcass related traits of broiler chickens selected for breast and thighs weight. Genetika. 2009;45:112–118. [PubMed] [Google Scholar]
- Khosravinia H., Manafi M. Broiler chicks with slow-feathering (K) or rapid-feathering (k+) genes: effects of environmental stressors on physiological adaptive indicators up to 56 h posthatch. Poult. Sci. 2016;95:1719–1725. doi: 10.3382/ps/pew107. [DOI] [PubMed] [Google Scholar]
- Klingensmith P.M., Donahoe J.P., Stephans J.F. The effect of sex-linked dwarfing genes dw on the immune response of broiler breeder chickens. Poult. Sci. 1983;62:733–740. doi: 10.3382/ps.0620733. [DOI] [PubMed] [Google Scholar]
- Ladjali K., Tixier-Boichard M., Bordas A., Merat P. Cytogenetic study of early chicken embryo: effect of naked neck gene and high ambient temperature. Poult. Sci. 1995;74:903–909. doi: 10.3382/ps.0740903. [DOI] [PubMed] [Google Scholar]
- Leeson S., Walsh T. Feathering in commercial poultry: I. Feather growth and composition. Worlds Poult. Sci. J. 2004;60:42–51. [Google Scholar]
- Lin H., Jiao C., Buyse J., Decuypere E. Strategies for preventing heat stress in poultry. Worlds Poult. Sci. J. 2006;62:71–86. [Google Scholar]
- Mahrous, M. Y. 2008. Effect of interaction between some major and marker genes on immune response and productive performance of chickens. Ph.D. Thesis, Ain Shams University, Egypt.
- Mahrous M.Y. Eggshell ultrastructure of naked neck, frizzle and normally feathered genotype chickens. Egypt. J. Anim. Prod. 2012;48:69–78. [Google Scholar]
- Mahrous M.Y., El-Dlebshany A.E.M. Reduction of the cholesterol content of eggs by introducing naked neck (Na) and frizzle (F) genes in laying hens. Egypt. Poult. Sci. 2011;31:817–824. [Google Scholar]
- Mahrous M.Y., Galal A. Eggshell ultrastructure of naked neck, frizzle and normally feathered genotype chickens. Poult. Sci. 2011;90(Suppl. 1):8. Proceedings of 100th Poultry Science Annual Meeting, America’s Center, St. Louis, Missouri, USA. [Google Scholar]
- Mahrous M., Galal A., Fathi M.M., El-Dein A.Z. Impact of naked neck (Na) and frizzle (F) genes on growth performance and immunocompetence in chickens. Int. J. Poult. Sci. 2008;7:45–54. [Google Scholar]
- Martin A., Gross W.B., Siegel P.B. IgG and IgM responses in high and low antibody selected lines of chickens. J. Heredity. 1989;80:249–252. doi: 10.1093/oxfordjournals.jhered.a110844. [DOI] [PubMed] [Google Scholar]
- Mathur P.K. In: Page 546 in poultry Genetics, Breeding and Biotechnology. Muir W.M., Aggrey S.E., editors. CABI Pub; Wallingford: 2003. Genotype-environment interactions: problems associated with selection for increased production. [Google Scholar]
- Mcgibbon H. A Sex-linked mutation affecting rate of feathering in chickens. Poult. Sci. 1977;56:872–875. [Google Scholar]
- Mérat P. Potential usefulness of the Na (naked neck) gene in poultry production. Worlds Poult. Sci. J. 1986;42:124–142. [Google Scholar]
- Mérat P. In: Pages 429–467 in Poultry Breeding and Genetics. Crawford R.D., editor. Elsevier; Amsterdam, The Netherlands: 1990. Pleiotropic and associated effects of major genes. [Google Scholar]
- Mincheva N., Lalev M., Oblakova M., Hristakieva P., Ivanova I. Effect of feathering alleles (K/k+) on laying performance, hatchability parameters and some body measurements in two lines of white Plymouth Rock hens. Biotechnol. Anim. Husb. 2012;28:405–414. [Google Scholar]
- Missohou A., Dieng A., Horst P., Zarate V.A., Nesseim T., Tchedre K. Effect of dwarf (dw) and frizzle (F) genes on the performance of layers under Senegalese conditions. Trop. Anim. Health Prod. 2003;35:373–380. doi: 10.1023/a:1025149523151. [DOI] [PubMed] [Google Scholar]
- Monnet L.E., Bordas A., Merat P. Gene cou nu et performances de croissance selon la temperature cher le poulet. Ann. Genet. Sel. Anim. 1979;11:397–412. doi: 10.1186/1297-9686-11-4-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle J.R., Wideman R.F., Whipple S.M., Yoho D.E., Bramwell R.K. Urolithiasis in male boiler breeders. Int. J. Poult. Sci. 2011;10:839–841. [Google Scholar]
- Mutibvu T., Chimonyo M., Halimani T.E. Effects of strain and sex on the behaviour of free-range slow-growing chickens raised in a hot environment. J. Appl. Anim. Res. 2018;46:224–231. [Google Scholar]
- Nazmi, A. 2006. Study on immunogenetic differences in naked neck and normally feathered chickens. M.Sc. Thesis, Ain Shams University, Egypt.
- Njenga, S.K., P. Sorensen., and P.N. Nyaga. 2005. Pages 1–93 in The productive performance of the different phenotypes of indigenous poultry in coastal Kenya. Master of Science Thesis, Danish Institute of Agriculture Sciences, Tjee, Denmark.
- Pakdel A., Van Arendonk J.A.M., Vereijken A.L.J., Boven-huis H. Direct and maternal genetic effects for ascites-related traits in broilers. Poult. Sci. 2002;81:1273–1279. doi: 10.1093/ps/81.9.1273. [DOI] [PubMed] [Google Scholar]
- Parker H.M., Kiess A.S., Santa Rosa P., McDaniel C.D. Selection for the parthenogenetic trait in Chinese Painted Quail (Coturnix chinensis) affects hatchability parameters. Poult. Sci. 2014;93:664–672. doi: 10.3382/ps.2013-03527. [DOI] [PubMed] [Google Scholar]
- Patra B.N., Bais R.K.S., Sharma D., Singh B.P., Prasad R.B., Bhushan B. Immunocompetence status of white plumage naked neck versus normally feathered broilers in tropical climates. Asian-Australas. J. Anim. Sci. 2004;14:560–563. [Google Scholar]
- Patra B.N., Bais R.K.S., Prasad R.B., Singh B.P. Performance of naked neck versus normally feathered coloured broilers for growth, carcass traits and blood biochemical parameters in tropical climate. Asian-Australas. J. Anim. Sci. 2002;15:1776–1783. [Google Scholar]
- Pawar S.S., Sajjanar B., Lonkar V.D., Nitin K.P., Kadam A.S., Nirmale A.V., Brahmane M.P., Bal S.K. Assessing and mitigating the impact of heat stress in poultry. Adv. Anim. Vet. Sci. 2016;4:332–341. [Google Scholar]
- Peters, S. O. 2005. Page 213 in Variation in semen quality, reproductive performance and growth of artificially inseminated strains of pure and crossbred chickens. Ph.D. Thesis, University of Agriculture, Nigeria.
- Peters S.O., Shoyebo O.D., Iiori B.M., Ozoje M.O., Ikeobi C.O.N., Adebambo O.A. Semen quality traits of seven strains of chickens raised in the humid tropics. Int. J. Poult. Sci. 2008;7:949–953. [Google Scholar]
- Peters S.O., Gunn H.H., Imumorin I.G., Agaviezor B.O., Ikeobi C.O.N. Haematological studies on frizzled and naked neck genotypes of Nigerian native chickens. Trop. Anim. Health Prod. 2011;3:631–638. doi: 10.1007/s11250-010-9743-7. [DOI] [PubMed] [Google Scholar]
- Radwan L.M. The effect of eggshell ultrastructure on hatchability percentage of eggs laid from naked neck, frizzled and normally feathered hens. Egypt. Poult. Sci. 2014;34:397–413. [Google Scholar]
- Rajkumar U., Reddy B.L., Rajaravindra K.S., Niranjan M., Bhattacharya T.K., Chatterjee R.N., Panda A.K., Reddy M.R., Sharma R.P. Effect of naked neck gene on immune competence, serum biochemical and carcass traits in chickens under a tropical climate. Asian-Australas. J. Anim. Sci. 2010;23:867–872. [Google Scholar]
- Rajkumar U., Reddy M.R., Rama Rao S.V., Radhika K., Shanmugam M. Evaluation of growth, carcass, immune response and stress parameters in Naked neck chicken and their normal siblings under tropical winter and summer temperatures. Asian-Australas. J. Anim. Sci. 2011;24:509–516. [Google Scholar]
- Rajkumar U., Rajaravindra K.S., Haunshi S., Niranjan M., Bhattacharya T.K., Chatterjee R.N. Genetic architecture of growth and production parameters in a laying cycle of 72weeks in naked neck chickens. Indian J. Anim. Sci. 2012;82:615–619. [Google Scholar]
- Raju M.V.L.N., Shyam Sunder G., Chawak M.M., Rama Rao S.V., Sadagopan V.R. Response of naked neck (Nana) and normal (nana) broiler chickens to dietary energy level in a subtropical climate. Br. Poult. Sci. 2004;45:186–193. doi: 10.1080/00071660410001715786. [DOI] [PubMed] [Google Scholar]
- Reddy M.V., Preetam V.C., Reddy A.R., Rajkumar U., Reddy V.R., Gautham K., Hareesh D., Vishnu P.G. Phenotypic characterization of indian naked neck chicken under tropical climatic conditions. Asian J. Anim. Vet. Adv. 2015;10:527–536. [Google Scholar]
- Reddy P.R.K., Siegel P.B. Selection for body weight at eight weeks of age. 14. Effects of the sex-linked dwarf gene. Poult. Sci. 1977;56:1004–1010. [Google Scholar]
- Renaudeau D., Collin A., Yahav S., De Basilio V., Gourdine J.L., Collier R.J. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal. 2012;6:707–728. doi: 10.1017/S1751731111002448. [DOI] [PubMed] [Google Scholar]
- Renema R.A., Sikur V.R., Robinson F.E., Korver D.R., Zuidhof M.J. Effects of nutrient density and age at photostimulation on carcass traits and reproductive efficiency in fast and slowfeathering turkey hens. Poult. Sci. 2008;87:1897–1908. doi: 10.3382/ps.2007-00431. [DOI] [PubMed] [Google Scholar]
- Sharifi A.R., Horst P., Simianer H. The effect of naked neck gene and ambient temperature and their interaction on reproductive traits of heavy broiler dams. Poult. Sci. 2010;89:1360–1371. doi: 10.3382/ps.2009-00593. [DOI] [PubMed] [Google Scholar]
- Sikur V.R., Robinson F.E., Korver D.R., Renema R.A., Zuidhof M.J. Effects of nutrient density on growth and carcass traits in fast and slowfeathering female turkeys. Poult. Sci. 2004;83:1507–1517. doi: 10.1093/ps/83.9.1507. [DOI] [PubMed] [Google Scholar]
- Singh C.V., Kumar D., Singh Y.P. Potential usefulness of the plumage reducing naked neck (Na) gene in poultry production at normal and high ambient temperatures. Worlds Poult. Sci. J. 2001;57:139–156. [Google Scholar]
- Sohn S.H., Park D.B., Song H.R., Cho E.J., Kang B., Suh O. Genotype frequencies of the sex-linked feathering and their phenotypes in domestic chicken breeds for the establishment of auto-sexing strains. J. Anim. Sci. Technol. 2012;54:267–274. [Google Scholar]
- Somes R.G., Jr. In: Pages 169–208 in Poultry Breeding and Genetics. Crawford R.D., editor. Elsevier; Amsterdam, Netherlands: 1990. Mutations and major variants of plumage and skin in chickens. editor. [Google Scholar]
- Stevens L. CABI Publishing; Cambridge, UK: 1991. Genetics and Evolution of the Domestic Fowl. [Google Scholar]
- Tabler J.M., Rigney M.M., Berman G.J., Gopalakrishnan S., Heude E., Al-Lami H.A., Yannakoudakis B.Z., Fitch R.D., Carter C., Vokes S., Liu K.J., Tajbakhsh S., Egnor S.R., Wallingford J.B. Cilia-mediated hedgehog signaling controls form and function in the mammalian larynx. eLife. 2017;6:e19153. doi: 10.7554/eLife.19153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasti S., Sah N., Mishra B. Impact of heat stress on poultry health and performances, and potential mitigation strategies. Animals. 2020;10:1266. doi: 10.3390/ani10081266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson H.R., Jacop J.P. Method of Sexing Day-Old Chicks. University of Florida, Cooperative Extension Service, Fact Sheet PS-21; Gainesville, FL: 2007. [Google Scholar]
- Yahav S., Luger D., Cahaner A., Dotan M., Rusal M., Hurwitz S. Thermoregulation in naked neck chickens subjected to different ambient temperatures. Br. Poult. Sci. 1998;39:133–138. doi: 10.1080/00071669889510. [DOI] [PubMed] [Google Scholar]
- Yalcin S., Ozkan S., Acikgoz Z., Ozkan K. Effect of dietary methionine on performance, carcass characteristics and breast meat composition of heterozygous naked neck (Na/na+) birds under spring and summer conditions. Br. Poult. Sci. 1999;40:688–694. doi: 10.1080/00071669987098. [DOI] [PubMed] [Google Scholar]
- Yunis R., Cahaner A. The effects of the naked neck (Na) and frizzle (F) genes on growth and meat yield of broilers and their interactions with ambient temperatures and perennial growth rate. Poult. Sci. 1999;78:1347–1352. doi: 10.1093/ps/78.10.1347. [DOI] [PubMed] [Google Scholar]
- Zerehdaran S., Vereijken A.L.J., Van Arendonk J.A.M., Waaij E.H. Estimation of genetic parameters for fat deposition and carcass traits in broilers. Poult. Sci. 2004;83:305–313. doi: 10.1093/ps/83.4.521. [DOI] [PubMed] [Google Scholar]
- Zerjal T., Gourichon D., Rivet B., Bordas A. Performance comparison of laying hens segregating for the frizzle gene under thermoneutral and high ambient temperatures. Poult. Sci. 2013;92:1474–1485. doi: 10.3382/ps.2012-02840. [DOI] [PubMed] [Google Scholar]
- Zulkifli I., Yamada Y., Khadijah W., Vidyadaran M.K., Dahlan I. The effect of sex-linked dwarf, naked neck and frizzle genes on the egg quality traits of laying hens under tropical conditions. J. Vet. Malaysia. 1992;4:33–39. [Google Scholar]