Abstract
Background
New-onset atrial fibrillation (AF) during COVID-19 infection is associated with worse cardiovascular outcomes and mortality, with new-onset AF being associated with worse clinical outcomes than recurrent AF. However, it is not known whether a prior history of AF is an independent cardiovascular risk factor predicting worse outcomes in COVID-19 patients. The present investigation sought to determine whether AF should be considered a risk factor for worse outcomes in COVID-19 illness.
Methods
From March 2020-September 2021 patients testing positive for SARS-CoV-2 with a prior AF diagnosis (n = 3623) were propensity matched to non-AF SARS-CoV-2 positive patients (n = 3610). Multivariable Cox hazard regression was used to determine subsequent MACE (all-cause death, myocardial infarction, HF and stroke) risk among patients with and without AF.
Results
COVID-19 patients with a prior history of AF were more likely to be hospitalized, require ICU care, supplemental oxygen, and ventilator support compared COVID-19 patients without a history of AF. There was a 1.40 times higher rate of MACE in the COVID-19 patients with prior AF compared to patients without prior AF (p < 0.0001). The increased rate of MACE in patients with a prior AF was primarily secondary to increases in heart failure hospitalization and death. This finding was confirmed even after controlling for acute AF during COVID-19 illness (HR 1.22, p = 0.0009).
Conclusion
AF history was shown to be an independent risk factor for MACE during a COVID-19 illness. Both recurrent and principally new-onset AF were associated with an increased risk of poor clinical outcomes during COVID-19 illness.
Keywords: Atrial fibrillation, COVID-19, Major adverse cardiovascular events
1. Introduction
Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in a worldwide pandemic with an unprecedented impact on global public health. SARS-CoV-2 infection causes a respiratory infection that can lead to pneumonia, acute respiratory distress syndrome and, in severe cases, can trigger a cytokine storm. A growing body of clinical data suggest that outcomes of COVID-19 are strongly associated with pre-existing comorbidities. In particular, pre-existing cardiovascular disease (CVD) has been associated with poor outcomes in COVID-19 [1], [2], [3], [4]. Specifically, in the presence of pre-existing CVD, COVID-19 infections are known to require more advanced treatment and result in poorer outcomes.
Atrial fibrillation (AF) is a common heart rhythm disorder affecting an estimated 33 million individuals worldwide [5]. Furthermore, AF can lead to serious medical complications such as stroke, heart failure, and mortality. New-onset or worsening AF can be triggered by acute physiological stress and/or illness. Musikantow et al. [6] recently reported acute AF episodes during both influenza and COVID-19 illnesses and showed that the likelihood of arrhythmia is related to inflammation and disease severity, not the specific viral etiology of the illness. Furthermore, studies have shown that new-onset AF during COVID-19 infection is associated with worse cardiovascular outcomes and mortality [7], [8], [9]. Recent data suggests that new-onset AF is associated with worse clinical outcomes than recurrent AF [9]. However, it is not known whether a prior history of AF is a cardiovascular risk factor predicting a worse outcome in COVID-19 patients.
The present investigation sought to determine whether AF should be considered a risk factor for worse outcomes in COVID-19 illness. Specifically, whether a prior history of AF was associated with an increase in major adverse cardiovascular events (MACE) in patients with a COVID-19 illness. Additionally, the effect of acute AF (new-onset or recurrent) during COVID-19 illness on the risk of MACE was evaluated. Finally, we examined whether prior catheter ablation for AF affected MACE risk in patients with a prior history of AF with a COVID-19 illness. We hypothesized that AF is a risk factor for worse outcomes in COVID-19 illness.
2. Methods
2.1. Study population
This study was approved by the Intermountain Healthcare Institutional Review Board with a waiver of consent. Intermountain Healthcare patients aged ≥18 years old that tested positive for SARS-CoV-2 virus from March 1, 2020 to September 30, 2021 were studied. Intermountain Healthcare is a large, nonprofit, electronically integrated healthcare system that includes 24 hospitals and 215 clinics. It provides healthcare for approximately two-thirds of people in the state of Utah and sections of southeastern Idaho and Nevada. Patients were included if they had a positive SARS-CoV-2 test result from either Intermountain Healthcare internal testing or external testing ordered by an Intermountain care provider. Prior history of AF was obtained from the Intermountain Healthcare Enterprise Data Warehouse (EDW). Acute AF during COVID-19 illness was defined as AF on ECG within 30 days of a positive COVID-19 test date and that was captured using the MUSE ECG reporting system.
2.2. Study groups
All patients had a positive SARS-CoV-2 test. This study consisted of four groups; one primary study group and three sub-group populations all with propensity-matched controls. The primary study group compared SARS-CoV-2 positive patients with a history of AF to propensity-matched SARS-CoV-2 positive patients without a history of AF. The subgroup comparisons included: (1) Recurrent AF during COVID-19 illness sub-group – COVID-19 patients with a prior history of AF, comparing those with recurrent AF to those without recurrent AF during their COVID-19 illness. (2) History of AF and prior catheter ablation sub-group – COVID-19 patients with a prior history of AF and a prior AF ablation propensity matched to COVID-19 patients with prior AF without a prior AF ablation. (3) New-onset AF during COVID-19 illness sub-group – COVID-19 patients without a prior AF history with a new onset AF diagnosis (within 30-days of the infection) compared to those without a new-onset.
2.3. Statistical analysis
The propensity matching of the cases and control sets was performed using age, gender, race, ethnicity, prior stroke, prior coronary artery disease (CAD), hypertension and prior heart failure. The propensity score was obtained using logistic regression with a greedy matching method and a caliper of 0.20 for the logit propensity score. Each case was matched to one control. Baseline patient and clinical characteristics were compared using chi-square tests and the Wilcoxon rank sum test. After multiple testing corrections, p ≤ 0.002 were considered significant for these baseline comparisons. The primary outcome was MACE post COVID-19 infection. MACE was defined as a composite outcome of all-cause mortality, myocardial infarction (MI), heart failure hospitalization, and stroke. Secondary outcomes included hospitalization for COVID-19, treatments for COVID-19, and the individual components of MACE – death, MI, heart failure hospitalization and stroke. The MACE outcomes were evaluated using Cox proportional hazard regression, and the hospitalization and treatment outcomes were tested using logistic regression. Significance for outcomes was set at p ≤ 0.003 to allow for multiple testing for each population. SAS 9.4 was used for all analyses.
3. Results
3.1. Baseline characteristics
There were 3623 patients with a positive SARS-CoV-2 test and a prior history of AF, and these patients were propensity matched to 3610 (99.6%) SARS-CoV-2 positive patients without a prior history of AF. The average follow-up time was 294 days from date of positive SARS-CoV-2 test. Patient characteristics, co-morbidities, and use of cardiovascular medications for the entire population are shown in Table 1. The patient groups were fairly well matched on their baseline characteristics including body mass index. The entire study population was predominantly Caucasian (92%), 60% male, with an average age of 69.8 ± 14.0 years. Patients with prior AF were more likely to have chronic obstructive pulmonary disease (19% vs 16%, p = 0.002) and were more likely to be on cardiovascular-related medications than those without a history of AF.
Table 1.
Patient and clinical characteristics for patients with COVID-19 and those with prior AF propensity score matched* to those without prior AF.
| BASELINE CHARACTERISTICS | Prior AF n = 3610 | No Prior AF n = 3610 | p-value |
|---|---|---|---|
| Age, mean ± SD | 70.0 ± 13.9 | 69.7 ± 13.7 | 0.18 |
| Male Sex | 2147 (59.5%) | 2103 (58.3%) | 0.29 |
| Caucasian | 3309 (91.7%) | 3322 (92.0%) | 0.58 |
| Hispanic | 253 (7.0%) | 278 (7.7%) | 0.26 |
| BMI**, mean ± SD | 30.9 ± 7.8 | 30.8 ± 7.3 | 0.67 |
| Comorbidities | |||
| Asthma | 894 (24.8%) | 827 (22.9%) | 0.06 |
| CAD | 1884 (52.2%) | 1848 (51.2%) | 0.40 |
| Cancer (excluding skin) | 700 (19.4%) | 644 (17.8%) | 0.09 |
| COPD | 696 (19.3%) | 575 (15.9%) | 0.002 |
| Depression | 1258 (34.8%) | 1281 (35.5%) | 0.57 |
| Diabetes | 1536 (42.5%) | 1517 (42.0%) | 0.65 |
| Heart Failure | 1591 (44.1%) | 1513 (41.9%) | 0.06 |
| Hyperlipidemia | 2705 (74.9%) | 2671 (74.0%) | 0.36 |
| Hypertension | 3010 (83.4%) | 3024 (83.8%) | 0.66 |
| PAD | 196 (5.4%) | 155 (4.3%) | 0.02 |
| Stroke | 428 (11.9%) | 369 (10.2%) | 0.03 |
| Medications | |||
| ACE/ARBS | 1205 (33.4%) | 996 (27.6%) | <0.0001 |
| Anti-arrhythmic | 664 (18.4%) | 33 (0.9%) | <0.0001 |
| Anti-coagulants | 1786 (49.5%) | 1321 (36.6%) | <0.0001 |
| Beta Blocker | 1326 (36.7%) | 884 (24.5%) | <0.0001 |
| Calcium Channel Blocker | 785 (21.7%) | 444 (12.3%) | <0.0001 |
| Diuretic | 1268 (35.1%) | 926 (25.7%) | <0.0001 |
*Propensity Score Matched (Score based on age, gender, race, ethnicity, prior CAD, prior heart failure, prior stroke and hypertension.).
**BMI was available for a subset of cases (n = 3331) and controls (n = 3073).
NOTE: AF = Atrial fibrillation, SD = standard deviation, CAD = Coronary artery disease, COPD = Chronic obstructive pulmonary disease, PAD = Peripheral artery disease.
p-values based on chi-square tests (categorical variables) and Wilcoxon rank sum tests (continuous variables).
3.2. History of AF and COVID-19 illness
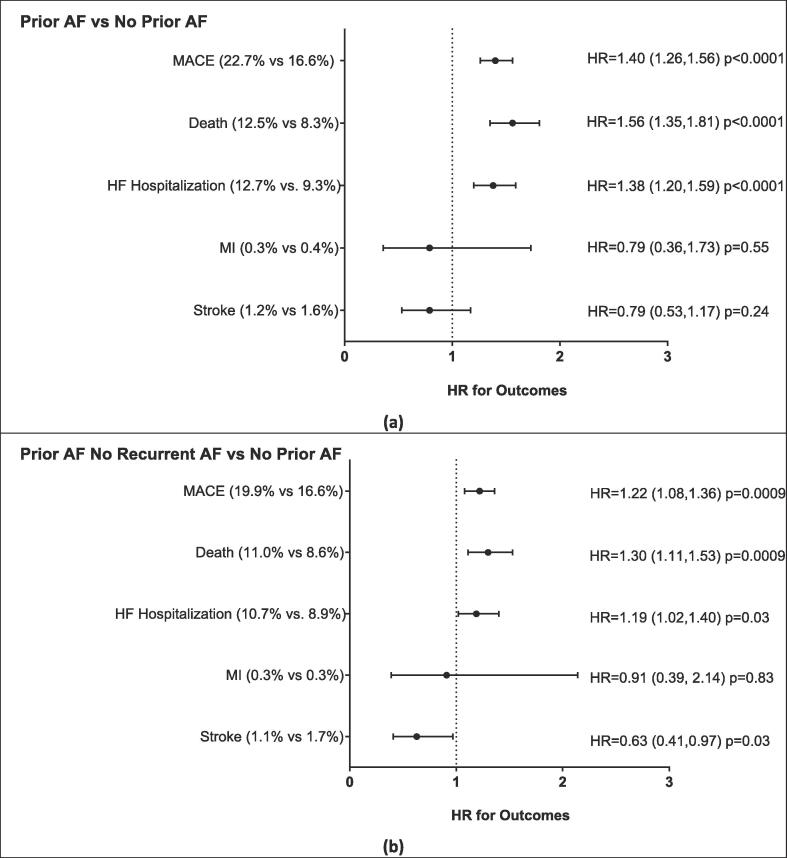
COVID-19 patients with a prior history of AF were more likely to be hospitalized and to require ICU care, supplemental oxygen, and ventilator support compared COVID-19 patients without a history of AF (Table 2, Upper Panel). There were 820 (22.7%) MACE events in COVID-19 patients with prior AF compared to 600 (16.6%) for the patients without prior AF; resulting in a 1.40 times higher rate of MACE in the COVID-19 patients with prior AF compared to COVID-19 patients without prior AF (Fig. 1a). The increased rate of MACE in patients with a prior history of AF was primarily secondary to increases in heart failure hospitalization and death.
Table 2.
COVID-19 clinical severity for patients with prior AF compared to those with no prior AF (top) and prior AF without recurrent AF compared to no prior AF (bottom). Both groups are propensity matched*.
| COVID-19 CLINICAL SEVERITY | Groups |
Odds Ratio (95% CI), p-value | |
|---|---|---|---|
| Prior AF n = 3610 | No Prior AF n = 3610 | ||
| Hospitalization | 1244 (34.5%) | 997 (27.6%) | 1.38 (1.25, 1.52), p < 0.0001 |
| ICU | 460 (12.7%) | 328 (9.1%) | 1.46 (1.26, 1.70), p < 0.0001 |
| Oxygen | 1146 (31.7%) | 903 (25.0%) | 1.39 (1.26, 1.55), p < 0.0001 |
| Ventilator Used | 290 (8.0%) | 153 (4.2%) | 1.97 (1.61, 2.41), p < 0.0001 |
| Prior AF Without Recurrent AF n = 3228 | No Prior AF n = 3228 | ||
| Hospitalization | 946 (29.3%) | 904 (28.0%) | 1.07 (0.96, 1.19), p = 0.25 |
| ICU | 304 (9.4%) | 322 (10.0%) | 1.07 (0.90, 1.26), p = 0.45 |
| Oxygen | 914 (28.3%) | 813 (25.2%) | 1.17 (1.05, 1.31), p = 0.004 |
| Ventilator Used | 213 (6.6%) | 151 (4.7%) | 1.44 (1.16, 1.78), p = 0.0009 |
*Propensity Score Matched (Score based on age, gender, race, ethnicity, prior CAD, prior heart failure, prior stroke and hypertension.)
NOTE: AF = Atrial fibrillation, ICU = Intensive care unit, CI = confidence interval. Odds ratios and p-values are from logistic regression.
Fig. 1.
Major cardiovascular outcomes for patients with COVID-19 with prior AF compared to no prior AF (a) and prior AF without recurrent AF compared to no prior AF (b). NOTE: AF = Atrial fibrillation, MACE = major adverse cardiovascular events (including death, MI, HF hospitalization, and stroke), MI = myocardial infarction, HF = heart failure. p-values based on Cox proportional hazard regression. The average follow-up time was 294 days with the average time to the MACE events being 21 days. Propensity Score Matched. (Score based on age, gender, race, ethnicity, prior CAD, prior heart failure, prior stroke and hypertension.)
These data suggest that a prior history of AF is a risk factor for a worse outcome in COVID-19 illness. However, it is not clear whether this finding was primarily because of a higher incidence of acute AF during COVID-19 illness in patients with a prior history of AF. As such, a secondary analysis of this cohort was performed excluding patients that had an AF diagnosis within 30 days of SARS-CoV-2 positive test. Specifically, SARS-CoV-2 positive patients with (n = 3228) and without (n = 3228) a prior history of AF and no AF within 30 days of having a positive SARS-CoV-2 test were propensity-matched. COVID-19 patients with a prior history of AF were more likely to require supplemental oxygen and ventilator support than patients without a history of AF (Table 2, Lower Panel). However, there was no difference in rates of hospitalization or ICU care between these groups. As with the primary analysis, COVID-19 patients with a prior history of AF had a 1.22 times increased rate of MACE compared to patients without a history of AF (Fig. 1b). The increased MACE rate was driven primarily by a trend toward increased heart failure hospitalization and an increase in death. Interestingly, COVID-19 patients with a prior history of AF trended to have fewer strokes than patients without a history of AF. These data suggest that independent of acute AF, a prior history of AF is a risk factor of a worse outcome in COVID-19 illness. Yet, acute AF also appears to increase the risk of MACE in COVID-19.
3.3. Recurrent AF during COVID-19 Illness
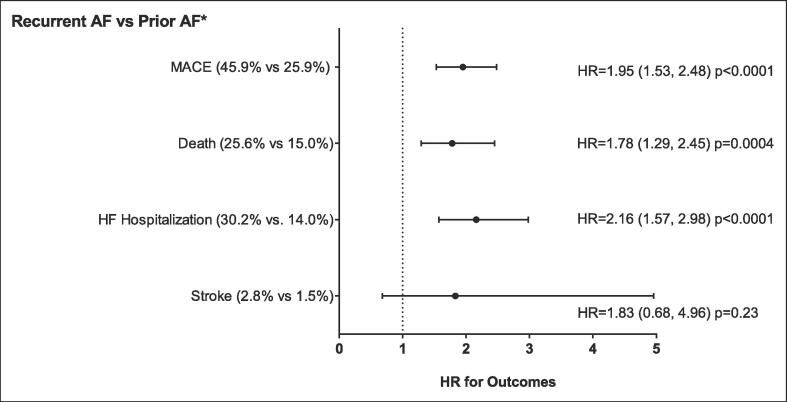
We next sought to better understand the impact of recurrent AF during COVID-19 illness on MACE. COVID-19 patients with prior AF and recurrent AF (n = 394) were propensity-matched to patients with prior AF and no recurrent AF (n = 394). There were no significant differences in the baseline patient characteristics and comorbidities. However, the patients with recurrent AF were less likely to be taking oral anticoagulants and diuretics (Table S1). COVID-19 patients with prior AF and recurrent AF were more likely to be hospitalized, require ICU care, and need supplemental oxygen and a ventilator (Table 3). Additionally, COVID-19 patients with a prior history of AF and recurrent AF during the infection were also more likely to have MACE events (Fig. 2). These data further support an increase in MACE with acute AF during COVID-19.
Table 3.
COVID-19 clinical severity for patients with recurrent AF compared to prior AF without prior AF during infection (propensity score matched*).
| COVID-19 CLINICAL SEVERITY | Recurrent AF n = 394 | Prior AF** n = 394 | Odds Ratio (95% CI), p-value |
|---|---|---|---|
| Hospitalization | 304 (77.2%) | 156 (39.6%) | 5.15 (3.78, 7.03), p < 0.0001 |
| ICU | 142 (36.0%) | 50 (12.7%) | 3.88 (2.70, 5.56), p < 0.0001 |
| Oxygen | 237 (60.2%) | 142 (36.0%) | 2.68 (2.01, 3.57), p < 0.0001 |
| Ventilator Used | 78 (19.8%) | 24 (6.1%) | 3.81 (2.35, 6.16), p < 0.0001 |
*Propensity Score Matched (score based on age, gender, race, ethnicity, prior CAD, prior heart failure, prior stroke and hypertension).
**Prior AF with no recurrent AF within a month of COVID-19 infection.
NOTE: AF = Atrial fibrillation, ICU = Intensive care unit, CI = confidence interval. Odds ratios and p-values are from logistic regression.
Fig. 2.
Major cardiovascular outcomes for patients with COVID-19 with recurrent AF compared to prior AF without recurrent AF during infection. * Prior AF with no recurrent AF within a month of COVID-19 infection. NOTE: AF = Atrial fibrillation, MACE = major adverse cardiovascular events (including death, MI, HF hospitalization, and stroke), HF = heart failure. p-values based on Cox proportional hazard regression. Propensity Score Matched. (Score based on age, gender, race, ethnicity, prior CAD, prior heart failure, prior stroke and hypertension.) Non-fatal MI not included as only one occurred in the recurrent AF group.
3.4. History of AF and prior catheter ablation and COVID-19 Illness:
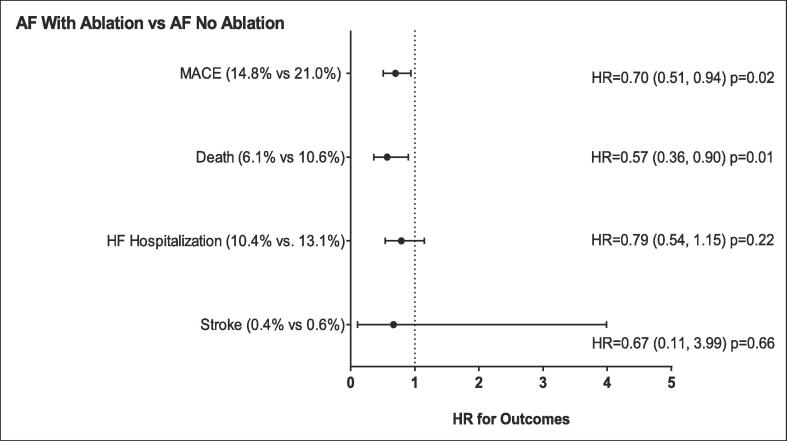
We next sought to understand the role of prior catheter ablation for AF in COVID-19 patients with a prior history of AF. Specifically, COVID-19 patients with a history of AF and prior catheter ablation (n = 472) were propensity-matched to COVID-19 patients with prior AF without prior catheter ablation (n = 472). While the patient characteristics and comorbidities were similar, the rates of cardiovascular medication use were higher in the patients with a prior ablation (Table S2). Importantly, the incidence of recurrent AF during COVID-19 illness in patient with prior catheter ablation was 5.1% vs 10.2% in patients without a prior catheter ablation (p = 0.003). The COVID-19 patients with a history of AF and prior catheter ablation were less likely to be hospitalized, require ICU care, and need oxygen (Table 4). Also, there was a trend toward fewer MACE events in patients with a history of AF and prior catheter ablation (Fig. 3).
Table 4.
COVID-19 clinical severity for patients with prior AF with ablation compared to those without ablation (propensity score matched*).
| COVID-19 CLINICAL SEVERITY | AF With Ablation n = 472 | AF Without Ablations n = 472 | Odds Ratio (95% CI), p-value |
|---|---|---|---|
| Hospitalization | 106 (22.5%) | 161 (34.1%) | 0.56 (0.42, 0.75), p < 0.0001 |
| ICU | 30 (6.4%) | 74 (15.7%) | 0.37 (0.23, 0.57), p < 0.0001 |
| Oxygen | 96 (20.3%) | 141 (29.9%) | 0.60 (0.45, 0.81), p = 0.0008 |
| Ventilator Used | 25 (5.3%) | 44 (9.3%) | 0.54 (0.33, 0.91), p = 0.02 |
*Propensity Score Matched (Score based on age, gender, race, ethnicity, prior CAD, prior heart failure, prior stroke and hypertension).
NOTE: AF = Atrial fibrillation, ICU = Intensive care unit, CI = confidence interval. Odds ratios and p-values are from logistic regression.
Fig. 3.
Major cardiovascular outcomes for patients with COVID-19 with prior AF with ablation compared to those without ablation. NOTE: AF = Atrial fibrillation, MACE = major adverse cardiovascular events (including death, MI, HF hospitalization, and stroke), HF = heart failure. p-values based on Cox proportional hazard regression. Propensity Score Matched. (Score based on age, gender, race, ethnicity, prior CAD, prior heart failure, prior stroke and hypertension.) Non-fatal MI not included as only one occurred in the AF ablation group.
3.5. New-onset AF during COVID-19 illness
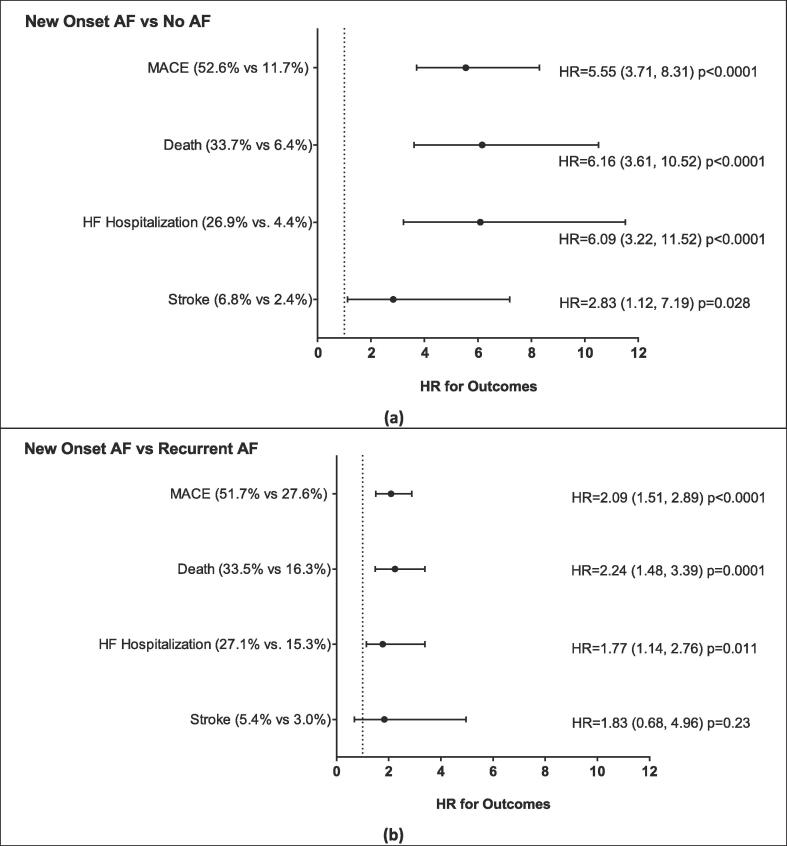
Next, we evaluated the impact of new-onset AF on MACE during COVID-19 illness. COVID-19 patients without a history of AF and new-onset of AF (n = 249) were propensity-matched to COVID-19 patients without new-onset AF (n = 249). There were no baseline differences in these groups (Table S3). Patients with new-onset AF were significantly more likely to need hospitalization and ICU care (Table 5, Top Panel). Importantly, COVID-19 patients with new-onset AF had a marked increase in MACE events compared to patients without new-onset AF (Fig. 4a). This increase in MACE was driven by an increase in heart failure hospitalization and death. Additionally, there was a trend toward increased stroke rates in COVID-19 patient with new-onset AF.
Table 5.
COVID-19 clinical severity for patients with new AF onset compared to those with no AF (top) and those with new AF onset compared to those with recurrent AF (bottom). Both groups are propensity matched*.
| COVID-19 CLINICAL SEVERITY | Groups |
Odds Ratio (95% CI), p-value | |
|---|---|---|---|
| New AF Onset n = 249 | No AF n = 249 | ||
| Hospitalization | 202 (81.1%) | 55 (22.1%) | 15.16 (9.80, 23.5), p < 0.0001 |
| ICU | 125 (50.2%) | 12 (4.85%) | 19.90 (10.59, 37.40), p < 0.0001 |
| Oxygen | 84 (33.7%) | 46 (18.5%) | 2.25 (1.49, 3.40), p = 0.0001 |
| Ventilator Used | 24 (9.6%) | 5 (2.0%) | 5.21 (1.95, 13.88), p = 0.001 |
| New AF n = 203 | Recurrent AF n = 203 | ||
| Hospitalization | 168 (82.8%) | 142 (70.0%) | 2.06 (1.29, 3.31), p = 0.0026 |
| ICU | 98 (48.3%) | 66 (32.5%) | 1.94 (1.30, 2.90), p = 0.0013 |
| Oxygen | 72 (35.5%) | 110 (54.2%) | 0.47 (0.31, 0.69), p = 0.0002 |
| Ventilator Used | 22 (10.8%) | 35 (17.2%) | 0.58 (0.33, 1.04), p = 0.07 |
Fig. 4.
Major cardiovascular outcomes for patients with COVID-19 with new onset AF compared to those with no AF (a) and those with new onset AF compared to those with recurrent prior AF (b). NOTE: AF = Atrial fibrillation, MACE = major adverse cardiovascular events (including death, MI, HF hospitalization, and stroke), HF = heart failure. p-values based on Cox proportional hazard regression. Propensity Score Matched. (Score based on age, gender, race, ethnicity, prior CAD, prior heart failure, prior stroke and hypertension.) Non-fatal MI not included as only one occurred in the new AF, no prior AF, and recurrent AF groups.
Finally, we sought to determine whether MACE rates were different between patients with new-onset AF compared to patients with recurrent AF during COVID-19 illness. COVID-19 patients with new-onset of AF (n = 249) were propensity-matched to COVID-19 patients with recurrent AF (n = 249). Patients with new-onset AF were more likely to be hospitalized and require ICU care but less likely to require oxygen (Table 5, Lower Panel). Interestingly, COVID-19 patients with new-onset AF had a higher rate of MACE compared to patients with recurrent AF (Fig. 4b). Specifically, patients with new-onset AF trended to have more heart failure hospitalizations and an increased risk of death compared to patients with recurrent AF.
3.6. COVID-19 illness severity
This study has shown that a prior history of AF and acute AF during COVID-19 illness is associated with increase MACE events. However, these worse clinical outcomes are also in the setting of increase COVID-19 illness severity. As such, secondary analyses stratifying for COVID-19 illness severity in this patient cohort were performed. COVID-19 illness severity was stratified as follows: (1) outpatient, (2) non-ICU inpatient, and (3) ICU inpatient. As with the primary analysis, when stratifying for COVID-19 illness severity, COVID-19 patients with a prior history of AF had 1.22 times increased rate of MACE events compared to patients without a history of AF (Supplemental Table 4). Similarly, there was 1.2 times increase rate of MACE events in COVID patients with a prior history of AF without recurrent AF when compared to patients without a prior of AF. Finally, consistent with the primary subgroup analyses acute AF during COVID (whether new onset or recurrent) was associated with increased MACE rates even after stratifying for COVID-19 illness severity (Supplemental Table 4). In contrast, when stratifying for COVID-19 illness severity, there was no difference in MACE events between COVID-19 patients with a history of AF and prior catheter ablation compared to patients with a history of AF and no prior catheter ablation (Supplemental Table 4).
4. Discussion
The key findings of this study of AF and COVID-19 illness are: (1) a prior history of AF increases the risk of MACE during COVID-19 illness that is independent of acute AF; (2) recurrent AF during COVID-19 illness is a marker of even greater risk of MACE; and (3) new-onset AF markedly increases the risk of MACE during COVID-19 illness and is a stronger marker of risk than recurrent AF. Overall, these data support the premise that both a prior history of AF and acute AF are risk factors for worse clinical outcomes in COVID-19 illness.
4.1. History of AF is a risk factor of worse clinical outcomes in COVID-19 illness
In the current study, we demonstrate that a prior history of AF is an independent risk factor for increased MACE during COVID-19. This finding supports the earlier findings of Paris et al. [10] that a history of AF was associated with worse clinical outcomes in COVID-19 illness. This study included 697 patients with COVID-19 illness of which 15% had a prior history of AF. After controlling for confounding factors, a prior history of AF in this study was associated with a 1.73 times increased risk of death. However, this study did not control for the presence of acute AF during COVID-19 illness. In the present study of more than 7000 patients with COVID-19 illness, without controlling for acute AF, we found a 1.4 times increased risk of MACE and a 1.6 times increased risk of death in patients with a prior history of AF compared to patients without a prior history of AF.
In the current study, the incidence of recurrent AF was 11% compared to 7% for new-onset AF. This is within the range of previously reported AF incidences (recurrent and new-onset) during COVID-19 illness. [6], [9], [11] To exclude the potential confounding effect of acute AF on our primary analysis we performed a separate sensitivity analysis excluding patients with acute AF during COVID-19 illness. Specifically, we found a 1.2 times increased risk of MACE and a 1.3 times increased risk of death in patients with a prior history of AF. Furthermore, this increased risk of MACE persisted after stratifying for COVID-19 illness severity. Overall, these data support a history of AF as an independent risk factor of worse clinical outcomes in COVID-19 illness. Yet, these data also suggest that acute AF during COVID-19 illness also portends risk for worse clinical outcomes.
4.2. Acute AF during COVID-19 illness predicts worse clinical outcomes
Acute AF has been identified as a marker of worse outcomes in patients with acute illnesses such as sepsis and acute heart failure [12], [13], [14]. More recently, Mountatonakis et al. [9] studied patients hospitalized with a positive COVID-19 test and acute onset AF propensity matched to patients without acute onset AF. In this study, acute onset AF was associated with a 1.46 times higher in-hospital mortality. In contrast, patients with new-onset AF had a 1.18 times increased risk of in-hospital mortality compared to patients with recurrent AF. The findings of the current study support those of Mountatonakis et al. [9] that acute AF is associated with worse clinical outcomes. Specifically, we found that recurrent AF was associated with a 1.95 times increased risk of MACE and a 1.78 times increased risk of death compared to patients with a prior history of AF but without recurrent AF.
Given these findings it could be postulated that treatments that reduce the risk of acute AF during COVID-19 illness may improve clinical outcomes. Catheter ablation for the treatment of AF significantly reduces risk of AF recurrence and is superior to antiarrhythmic drug therapy. Interestingly, we found a trend toward a 30% decreased risk of MACE and a 43% decreased risk of death in COVID-19 patients with a prior history of AF that had previously been treated with catheter ablation. These data suggest that prior catheter ablation for AF may decrease the risk of a poor clinical outcome in COVID-19. Also, in this study, patients with prior catheter ablation were also more likely to have previously been treated with an anti-arrhythmic drug. As would be predicted, patients with prior catheter ablation for AF had a much lower incidence of recurrent AF during COVID-19 illness than patients without a prior catheter ablation (5.1% vs 10.2%). Also, when stratifying for COVID-19 illness severity, there was no change in MACE events in patient with a prior catheter ablation compared to patients with prior history of AF but no prior catheter ablation. As such, we postulate that treatments that reduce the risk of AF recurrence could improve clinical outcomes in patients with a prior history of AF that develop COVID-19 illness in part by reducing the severity of clinical illness. Overall, these data also suggest that acute AF is not just a marker of a more severe clinical illness but may be a driver of more severe clinical illness. Further research is needed to better identify the clinical benefit of treatments that reduce recurrent AF during acute illness.
Finally, we found that new-onset AF during COVID-19 illness was associated with a 5.55 times higher risk of MACE and a 6.16 times increased risk of death compared to COVID-19 patients without new-onset AF. New-onset AF demonstrated a much higher risk of a poor clinical outcome in the current study compared to that reported by Mountatonakis et al. [9] Our study included both hospitalized and non-hospitalized patients compared to only hospitalized patients in the Mountatonakis et al. [9] study. As such, it is likely that the non-AF group in our study had a lower severity of illness. It is also likely that new-onset AF is a marker of a more severe clinical illness that would portend a worse outcome. Consistent with this postulate, both our study and Mountatonakis et al. [9] demonstrated an increased risk of poor clinical outcomes in COVID-19 patients with new-onset AF compared to recurrent AF.
In conclusion, the mechanisms underlying the increased risk of poor clinical outcomes during COVID-19 illness in patients with a prior history of AF and/or acute AF remain unclear. Furthermore, it is not clear whether these risks are specific to COVID-19 illness or apply across a spectrum of acute illness. Recent research suggests that acute AF during both influenza and COVID-19 illnesses is secondary to acute inflammation and disease severity. Also, there did not appear to be a difference in the incidence of acute AF between acute influenza and COVID-19 illness. Future research is needed to better define the underlying mechanisms driving worse clinical outcomes associated with both a prior history of AF and acute AF during acute illnesses, like COVID-19.
4.3. Limitations
This study has important limitations that are inherent to all retrospective studies. Acute AF was identified using ECG data and a new diagnosis of AF. As such, it is possible we may have missed acute AF cases, particularly recurrent AF, that was identified only on in-patient telemetry monitoring and did not result in a new AF diagnosis code. Similarly, misclassification of baseline characteristics and missed outcomes are possible. However, missing some of these factors is not anticipated to be biased with regard to the AF status. Given the retrospective nature of this study, it is possible that confounding risk factors for worsening clinical outcomes during COVID-19 illness were considered and compliance with AF related therapies (acute and chronic) were not measured and may have affected the findings of this study. Finally, because our data are observational and are limited to assessing associations, so conclusions regarding causation between AF (prior history or acute AF) and worse clinical outcomes in COVID-19 illness, are not possible.
5. Conclusions
Based on the findings of the present study, a prior history of AF is an independent risk factor for major adverse cardiovascular events during COVID-19 illness. Also, both recurrent and, principally, new-onset AF are associated with an increased risk of poor clinical outcomes during COVID-19 illness. Further research is needed to better determine whether acute AF is causally associated with poor clinical outcomes are merely a marker of more severe illness.
6. Sources of funding
This study was internally funded.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Dr Cutler is on the Heartline Study Steering Committee for Janssen Scientific Affairs/Johnson & Johnson. Dr. May has received grants from the NIH (RECOVER) and PCORnet (RECOVER EHR and for the Characterization of Patients with Vascular Disease and Atrial Fibrillation and Patients with Heart Failure in PCORnet Data). Dr. May is also on a Data Safety Monitoring Board for CAF (funded by Boehringer Ingelheim). Dr. Miller owns stock from Abbvie, Corvus Pharmaceuticals, and Bolt Biotherapeutics. All other authors declare that there are no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2022.101127.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y.i., Zhang L.i., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L.i., Xie J., Wang G., Jiang R., Gao Z., Jin Q.i., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y.i., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B.o., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., Lee M. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in washington state. JAMA. 2020;323(16):1612. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chugh S.S., Havmoeller R., Narayanan K., Singh D., Rienstra M., Benjamin E.J., Gillum R.F., Kim Y.-H., McAnulty J.H., Zheng Z.-J., Forouzanfar M.H., Naghavi M., Mensah G.A., Ezzati M., Murray C.J.L. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musikantow D.R., Turagam M.K., Sartori S., Chu E., Kawamura I., Shivamurthy P., Bokhari M., Oates C., Zhang C., Pumill C., Malick W., Hashemi H., Ruiz-Maya T., Hadley M.B., Gandhi J., Sperling D., Whang W., Koruth J.S., Langan M.-N., Sofi A., Gomes A., Harcum S., Cammack S., Ellsworth B., Dukkipati S.R., Bassily-Marcus A., Kohli-Seth R., Goldman M.E., Halperin J.L., Fuster V., Reddy V.Y. Atrial fibrillation in patients hospitalized with COVID-19: incidence, predictors, outcomes, and comparison to influenza. JACC Clin. Electrophysiol. 2021;7(9):1120–1130. doi: 10.1016/j.jacep.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inciardi R.M., Adamo M., Lupi L., Cani D.S., Di Pasquale M., Tomasoni D., et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur. Heart J. 2020;41:1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inciardi R.M., Adamo M., Lupi L., Metra M. Atrial fibrillation in the COVID-19 era: simple bystander or marker of increased risk? Eur. Heart J. 2020;41:3094. doi: 10.1093/eurheartj/ehaa576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mountantonakis S.E., Saleh M., Fishbein J., Gandomi A., Lesser M., Chelico J., Gabriels J., Qiu M., Epstein L.M. Atrial fibrillation is an independent predictor for in-hospital mortality in patients admitted with SARS-CoV-2 infection. Heart Rhythm. 2021;18(4):501–507. doi: 10.1016/j.hrthm.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paris S., Inciardi R.M., Lombardi C.M., Tomasoni D., Ameri P., Carubelli V., et al. Implications of atrial fibrillation on the clinical course and outcomes of hospitalized COVID-19 patients: results of the Cardio-COVID-Italy multicentre study. Europace. 2021;23:1603–1611. doi: 10.1093/europace/euab146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatla A., Mayer M.M., Adusumalli S., Hyman M.C., Oh E., Tierney A., Moss J., Chahal A.A., Anesi G., Denduluri S., Domenico C.M., Arkles J., Abella B.S., Bullinga J.R., Callans D.J., Dixit S., Epstein A.E., Frankel D.S., Garcia F.C., Kumareswaram R., Nazarian S., Riley M.P., Santangeli P., Schaller R.D., Supple G.E., Lin D., Marchlinski F., Deo R. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17(9):1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells G.L., Morris P.E. Incidence and prognosis of atrial fibrillation in patients with sepsis. Cardiol. Res. 2011;2:293–297. doi: 10.4021/cr108w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mountantonakis S.E., Grau-Sepulveda M.V., Bhatt D.L., Hernandez A.F., Peterson E.D., Fonarow G.C. Presence of atrial fibrillation is independently associated with adverse outcomes in patients hospitalized with heart failure: an analysis of get with the guidelines-heart failure. Circ. Heart Fail. 2012;5(2):191–201. doi: 10.1161/CIRCHEARTFAILURE.111.965681. [DOI] [PubMed] [Google Scholar]
- 14.Qian J., Kuang L., Chen F., Liu X., Che L. Prognosis and management of new-onset atrial fibrillation in critically ill patients. BMC Cardiovasc. Disord. 2021;21:231. doi: 10.1186/s12872-021-02039-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.