Abstract
Although cardiovascular disease risk factors relate to COVID-19, the association of estimated atherosclerotic cardiovascular disease (ASCVD) risk with severe COVID-19 is not established. We examined the relation of the pooled-cohort ASCVD risk score to severe COVID-19 among 28,646 subjects from the National COVID Cohort Collaborative database who had positive SARS-CoV-2 test results from April 1, 2020 to April 1, 2021. In addition, 10-year ASCVD risk scores were calculated, and subjects were stratified into low-risk (<5%), borderline-risk (5% to <7.5%), intermediate-risk (7.5% to <20%), and high-risk (>=20%) groups. Severe COVID-19 outcomes (including death, remdesivir treatment, COVID-19 pneumonia, acute respiratory distress syndrome, and mechanical ventilation) occurring during follow-up were examined individually and as a composite in relation to ASCVD risk group across race and gender. Multiple logistic regression, adjusted for age, gender, and race, examined the relation of ASCVD risk group to the odds of severe COVID-19 outcomes. Our subjects had a mean age of 59.4 years; 14% were black and 57% were female. ASCVD risk group was directly related to severe COVID-19 prevalence. The adjusted odds ratio of the severe composite COVID-19 outcome by risk group (vs the low-risk group) was 1.8 (95% confidence interval 1.5 to 2.2) for the borderline-risk, 2.7 (2.3 to 3.2) for the intermediate-risk, and 4.6 (3.7 to 5.6) for the high-risk group. Black men and black women in the high-risk group showed higher severe COVID-19 prevalence compared with nonblack men and nonblack women. Prevalence of severe COVID-19 outcomes was similar in intermediate-risk black men and high-risk nonblack men (approximately 12%). In conclusion, although further research is needed, the 10-year ASCVD risk score in adults ages 40 to 79 years may be used to identify those who are at highest risk for COVID-19 complications and for whom more intensive treatment may be warranted.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus which may progress to coronavirus disease (COVID-19).1 Most patients with COVID-19 are either asymptomatic or exhibit mild symptoms, although some progress to more severe disease. Severe symptoms of COVID-19, as defined by the National Institutes of Health (NIH), include the following: COVID-19–associated pneumonia, blood oxygen saturation <94% on room air at sea level, arterial partial pressure of oxygen to fraction of inspired oxygen ratio <300 mm Hg, respiratory frequency >30 breaths/min, lung infiltrates >50% on imaging, acute respiratory distress syndrome (ARDS), septic shock, multiorgan dysfunction, and/or systemic disease.2 The risk of developing severe COVID-19 is greater in those with a history of cardiovascular disease (CVD).3, 4, 5, 6, 7 This has motivated interest in tools that can help stratify COVID-19 risk. The 10-year Pooled Cohort Equation (PCE) atherosclerotic CVD (ASCVD) risk score was developed from 4 major US prospective studies with wide ethnic diversity and is recommended to guide the use of preventive therapies.8 , 9 Although hypertension, dyslipidemia, diabetes mellitus, and tobacco use have been shown to predict severe COVID-19, the PCE has not been adequately studied in relation to severe COVID-19.3 We examined in a large registry of patients with COVID-19 the association of the PCE score with severe COVID-19, including by gender and race/ethnicity, to help identify those at highest risk of severe COVID-19.
Methods
The National COVID Cohort Collaborative (N3C) from the NIH is a multicenter national data resource that includes deidentified subject data from 23,576 medical centers across the United States (at the time of data analysis).10 The data were collected and transferred under a Johns Hopkins University Reliance Protocol (IRB00249128) or through individual agreements between the NIH and each site. The NIH N3C database includes subjects with any encounter (>95% outpatient) with a healthcare professional after January 1, 2020, including any previous patient visits at the same medical center on or after January 1, 2018; unique deidentified patient identifiers are used to link to available medical history. The database includes 1,475,656 subjects with positive and 6,097,052 with negative SARS-CoV-2 test results, for a total sample cohort of 7,571,708 subjects at the time of analysis. A COVID-19 outcome was defined through diagnosis code U07.1. Our study utilized de-identified data exempt from Institutional Review Board approval at University of California, Irvine.
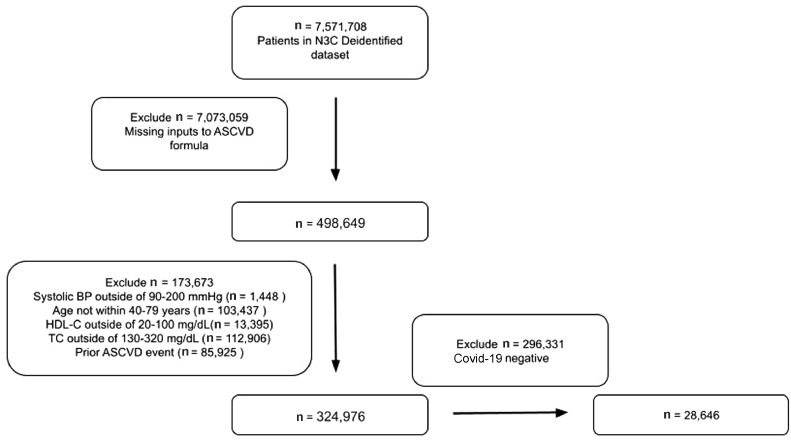
We conducted a retrospective cohort study of adults aged 40 to 79 years across 5,602 N3C sites whose data included all necessary variables to calculate the 10-year ASCVD risk with the PCE using the ASCVD Risk Estimator Plus.11 From the 7,571,708 total subjects whose deidentified data were released for analysis, we obtained an analytical sample of 28,646 subjects without previous CVD who had positive SARS-CoV-2 test results from April 1, 2020 to April 1, 2021, and who had all valid and complete data needed to calculate the 10-year ASCVD risk with the PCE. Figure 1 details the specifics on deriving the analytical sample.
Figure 1.
Analytical N3C COVID-19 patient cohort construction methodology. BP = blood pressure; HDL-C = high-density lipoprotein-C; TC = total cholesterol.
Our primary outcome of interest was the association of severe COVID-19 outcome with 10-year ASCVD risk score. The variables included in the PCE include age, gender, race/ethnicity (black and white/other), total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, treatment for hypertension, history of diabetes, and smoking within the preceding year. When multiple values were present for total cholesterol, high-density lipoprotein cholesterol, or systolic blood pressure, the median of all values was determined and used. After the scores were calculated, subjects were stratified into 4 risk groups: low (<5%), borderline (5% to <7.5%), intermediate (7.5% to <20%) and high (>=20%), per the American College of Cardiology guidelines.11 We defined a severe COVID-19 outcome as observation of any of the following end points, which have been well-documented to correspond to severe COVID-19 illness, during the follow-up period: remdesivir administration, extracorporeal membrane oxygenation, mechanical ventilation, COVID-19 pneumonia, ARDS, and death.12 Palliative and hospice care were also included. Although we did not have access to the specific reasons that physicians prescribed remdesivir, the drug is typically prescribed for severe COVID-19. A composite variable—severe COVID-19—was defined and served as a primary end point.
The data analysis platform provided by the N3C Enclave was used to perform most of the data analysis. In particular, the Code Workbook and Analysis tools were utilized. Python version 3.6 and the Python data analysis libraries PySpark, Pandas, NumPy, Seaborn, and StatsModels were used to clean and organize the data and run logistic regression models.
We first examined the demographic and risk-factor means or prevalences by risk group. We examined the prevalence of severe COVID-19 outcomes by risk group overall and by gender and race/ethnicity. Finally, we conducted unadjusted and adjusted (for age, gender, and race/ethnicity) logistic regression models to estimate the odds ratios of severe COVID-19 infection associated with ASCVD risk score measured continuously (per 10%) and according to low- (reference), borderline-, intermediate-, and high-risk categories. Additional adjusted and unadjusted analyses were conducted by gender and race/ethnicity (black or nonblack).
Results
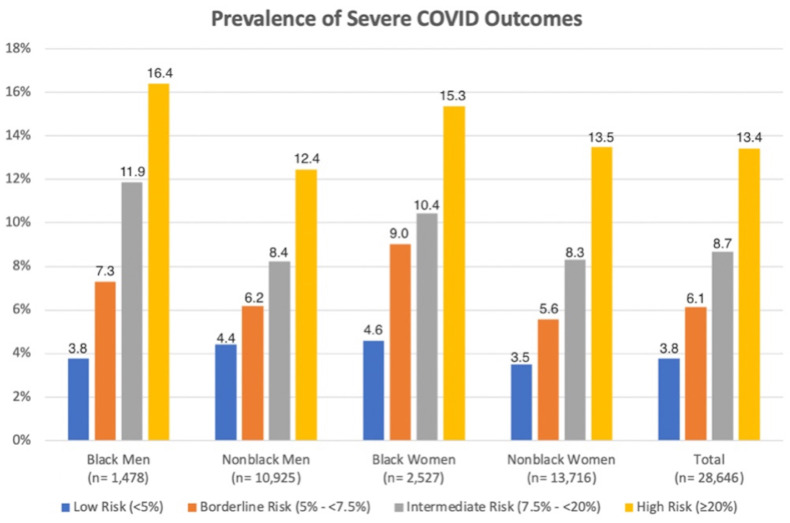
Among our 28,646 included subjects with positive SARS-CoV-2 test results, age averaged 59 years, 14% of participants were black, and 57% were female (Table 1 ). Key demographic and risk factors such as age, race, and gender did not differ significantly between included and excluded subjects. A total of 8,602 participants (30%) were in the low- risk (<5%) group, 3,304 (12%) were in the borderline-risk (5% to <7.5%) group, 9,760 (34%) were in the intermediate-risk (7.5% to <20%) group, and 6,980 (24%) were in the high-risk (>=20%) group. Those in the 10-year ASCVD high-risk (>=20%) group were more likely to be male, be black, have diabetes, have hypertension, and/or have a history of smoking. Subjects with higher 10-year ASCVD risk scores were more likely to experience death, COVID-19 pneumonia, ARDS, remdesivir administration, and mechanical ventilation. In the overall sample, there were 3,492 cases of composite severe COVID-19 outcomes, with 307 all-cause deaths observed. The overall prevalence of composite severe COVID-19 illness for the total cohort was 8.1% (Table 1). Prevalence of composite severe COVID-19 illness in low-, borderline-, intermediate-, and high-risk groups was 4%, 6%, 9%, and 13%, respectively (Figure 2 ).
Table 1.
Baseline characteristics of N3C COVID positive patients stratified by 10-year ASCVD risk group*
| Variable | Total cohort(N = 28646) (100%) | Low risk(N = 8602) (30%) | Borderline risk(N = 3304) (12%) | Intermediate risk(N = 9760) (34%) | High risk(N = 6980) (24%) |
|---|---|---|---|---|---|
| Mean age (yrs) | 59.4 (±10.3) | 49.8 (±6.5) | 55.8 (±7.2) | 61.6 (±7.6) | 69.6 (±7.2) |
| Women | 16243 (56.7%) | 6774 (78.8%) | 1921 (58.1%) | 4675 (47.9%) | 2873 (41.2%) |
| Black | 4005 (14.0%) | 824 (9.6%) | 392 (11.9%) | 1432 (14.7%) | 1357 (19.4%) |
| Diabetes Mellitus | 7275 (25.4%) | 718 (8.4%) | 517 (15.7%) | 2267 (23.2%) | 3773 (54.1%) |
| Hypertension Treatment | 11577 (40.4%) | 1582 (18.4%) | 1003 (30.4%) | 4338 (44.5%) | 4654 (66.7%) |
| Smoker | 13425 (46.9%) | 2641 (30.7%) | 1468 (44.4%) | 5098 (52.5%) | 4218 (52.2%) |
| Mean HDL-C (mg/dL) | 52.6 (±15.3) | 57.3 (±15.5) | 52.7 (±14.9) | 50.9 (±14.6) | 49.0 (±14.9) |
| Mean TC (mg/dL) | 190.0 (±36.2) | 193.4 (±33.8) | 196.3 (±36.0) | 190.7 (±37.0) | 182 (±36.7) |
| Mean SBP (mmHg) | 128.8 (±14.1) | 122.7 (±12.5) | 126.5 (±12.3) | 129.9 (±13.1) | 136.2 (±14.2) |
| Severe COVID Outcomes | |||||
| Death | 307 (1.1%) | 25 (0.29%) | 22 (0.67%) | 95 (0.97%) | 165 (2.4%) |
| Remdesivir Administration | 661 (2.3%) | 58 (0.67%) | 57 (1.7%) | 235 (2.4%) | 311 (4.5%) |
| COVID Pneumonia | 1726 (6.0%) | 268 (3.1%) | 160 (4.8%) | 642 (6.6%) | 656 (9.4%) |
| ARDS | 453 (1.6%) | 62 (0.53%) | 46 (1.0%) | 155 (1.3%) | 190 (2.1%) |
| Mechanical Ventilation | 345 (1.2%) | 46 (0.52%) | 33 (6.1%) | 123 (8.7%) | 143 (13.4%) |
| Composite severe COVID | 2312 (8.1%) | 325 (3.8%) | 203 (6.1%) | 847 (8.7%) | 937 (13.4%) |
N (percentage) are reported for categorical variables. N (SD) is reported for continuous variables. Diabetes was defined as having a diagnosis on the electronic health record. Hypertension was defined as taking hypertension medication.
p-values for all variables across risk groups were <.0001.
ARDS = acute respiratory distress syndrome; ASCVD = atherosclerotic cardiovascular disease; HDL-C = high density lipoprotein cholesterol; SBP = systolic blood pressure; TC = total cholesterol.
Figure 2.
Prevalence of composite severe COVID-19 outcomes stratified by ASCVD risk group, race/ethnicity and gender. p<0.001 across risk groups within each stratum.
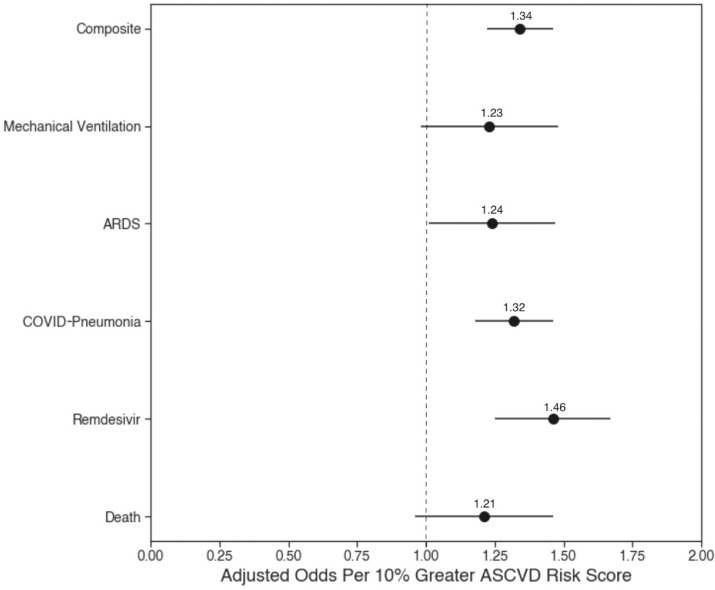
From logistic regression examining the odds of composite severe COVID-19 stratified by 10-year ASCVD risk group (with low-risk [<5%] group as the reference), we showed odds ratios of 1.7 (95% confidence interval [CI] 1.4 to 2.0) for borderline-risk group, 2.4 (95% CI 2.1 to 2.8) for intermediate-risk group, and 3.9 (95% CI 3.5 to 4.5) for high-risk group (p <0.0001 for all results) (Table 2 ). These associations were also significant (p <0.0001) for the individual severe COVID-19 outcomes across 10-year ASCVD higher-risk groups compared with the low-risk group. When adjusted for age, race/ethnicity, and gender, the odds of our composite COVID-19 outcome by risk group (with low-risk [<5%] group as the reference) were 1.8 (95% CI 1.5 to 2.2) for borderline-risk (5% to <7.5%) group, 2.7 (95% CI 2.3 to 3.2) for intermediate-risk (7.5% to <20%) group, and 4.6 (95% CI 3.7 to 5.6) for high-risk (>=20%) group (Table 2). The continuous adjusted (by age, race/ethicity, and gender) logistic regression, displayed in Figure 3 , showed that every 10% increment in 10-year ASCVD risk score was associated with a 34% increase in odds of both composite and individual outcomes which included death, remdesivir administration, COVID-19 pneumonia, ARDS, and mechanical ventilation. The components of the ASCVD risk score that were most strongly associated with the odds of composite severe COVID-19 in the overall study sample were (in order of importance) hypertension, age, diabetes, smoking status, and race (Table 3 ).
Table 2.
Unadjusted and *adjusted categorical logistic regressions for association of severe COVID indicators with 10-year ASCVD risk groups
| Odds ratio (95% CI) of severe COVID outcomes for ASCVD risk tiers compared to low risk (<5%) | Low risk (<5%) | Borderline risk (5-7.5%) | Intermediate risk (7.5% to <20%) | High risk(>=20%) | |
|---|---|---|---|---|---|
| Composite severe COVID-19 |
Unadjusted | 1.0 | 1.7 (1.4-2.0) | 2.4 (2.1-2.8) | 3.9 (3.5-4.5) |
| Adjusted | 1.0 | 1.8 (1.5-2.2) | 2.7 (2.3-3.2) | 4.6(3.7-5.6) | |
| Death | Unadjusted | 1.0 | 2.3 (1.3-4.1) | 3.4 (2.2-5.2) | 8.3 (5.4-12.7) |
| Adjusted | 1.0 | 1.7 (0.90-3.3) | 1.9 (1.0-3.3) | 3.3 (1.73-6.2) | |
| COVID pneumonia | Unadjusted | 1.0 | 1.6 (1.3-1.9) | 2.2 (1.9-2.5) | 3.2 (2.8-5.1) |
| Adjusted | 1.0 | 1.6 (1.4-2.2) | 2.7 (2.2-3.3) | 4.5 (3.52-5.6) | |
| ARDS | Unadjusted | 1.0 | 1.9 (1.3-2.9) | 2.2 (1.7-3.0) | 3.8 (2.9-5.1) |
| Adjusted | 1.0 | 1.8 (1.2-2.7) | 2.1 (1.4-3.0) | 3.7 (2.4-5.7) | |
| Mechanical ventilation | Unadjusted | 1.0 | 1.9 (1.2-29) | 2.4 (1.7-3.3) | 3.9 (2.8-5.4) |
| Adjusted | 1.0 | 1.9 (1.2-2.7) | 2.3 (1.5-3.3) | 3.7 (2.3-6.0) | |
| Remdesivir administration | Unadjusted | 1.0 | 2.6 (1.8-3.7) | 3.6 (2.7-4.9) | 6.9 (5.2-9.1) |
| Adjusted | 1.0 | 2.8 (1.9-4.1) | 4.3 (3.1-6.0) | 9.2 (6.3-13.2) |
Adjusted for age, sex, and race/ethnicity. Composite severe COVID includes any of the negative outcomes. ECMO and hospice not included as indicators due to low sample size.
ARDS = acute respiratory distress syndrome; ASCVD = atherosclerotic cardiovascular disease; CI = confidence interval; OR = odds ratio.
Figure 3.
Age, race/ethnicity, and gender-adjusted odds of severe COVID-19 outcomes per 10% greater 10-year ASCVD risk score.
Table 3.
Odds ratios for the association of risk factors with severe COVID infection overall and within demographic groups
| Risk factor | Overall N = 28,646 | Black women N = 2,527 | Nonblack women N = 13,716 | BlackMen N = 1,478 | Nonblack men N = 10,925 | Wald Chi- square (overall) |
|---|---|---|---|---|---|---|
| Age OR (95% CI) |
1.3 (1.3-1.5) |
1.5 (1.3-1.7) |
1.5 (1.4-1.6) |
1.3 (1.1-1.5) |
1.3 (1.2-1.4) | 158.8 |
| Sex | 1.3 (1.1-1.4) |
- | - | - | - | 1.6 |
| Race/ethnicity | 1.6 (1.4-1.8) |
- | - | - | - | 33.6 |
| Systolic BP | 1.0 (0.97-1.1) |
0.97 (0.50-.91) |
1.1 (1.0-1.2) |
0.90 (0.78-1.0) |
0.89 (0.83-0.96) |
49.0 |
| Hypertension treatment | 3.2 (2.8-3.5) |
2.6 (1.9-3.6) |
3.9 (3.4-4.6) |
2.4 (1.7-3.5) |
2.5 (2.2-2.9) |
231.04 |
| TC | 0.79 (0.75-0.85) |
0.81 (0.70-0.93) |
0.79 (0.73-0.85) |
0.80 (0.67-0.96) |
0.90 (0.77-0.90) |
0.1 |
| HDL-C | 0.70 (0.66-0.73) |
0.70 (0.61-0.82) |
0.70 (0.64-0.75) |
0.73 (0.60-0.89) |
0.69 (0.63-0.76) |
146.4 |
| Diabetes | 2.0 (1.9-1.3) |
1.8 (1.4-2.4) |
2.3 (1.9-2.7) |
1.7 (1.2-2.3) |
1.8 (1.5-2.1) |
68.0 |
| History of smoking | 1.6 (1.5-1.8) |
1.4 (1.1-1.9) |
1.7 (1.4-1.9) |
1.15 (0.84 -1.6) |
1.6 (1.4-1.8) |
47.6 |
Composite severe COVID includes any of the negative outcomes. ECMO and hospice not included as indicators due to low sample size. For all numeric factors except for age (TC, HDL-C, and SBP) the unit of change in the logistic regression model was a single standard deviation from the mean. For age, the unit of change was a 10 year timespan. For categorical factors (diabetes, smoking status, and treatment for hypertension), the reference group was the lowest risk category.
ARDS = acute respiratory distress syndrome; ASCVD = atherosclerotic cardiovascular disease; BP = blood pressure; CI = confidence interval; HDL-C = high density lipoprotein cholesterol; OR = odds ratio; TC = total cholesterol.
Prevalence of composite severe COVID-19 was higher in black women than in equivalent-risk nonblack women across all ASCVD risk groups. A similar trend was seen when comparing black men with nonblack men within the same ASCVD risk group; however, the prevalence of composite severe COVID-19 was lower in low-risk black men than in low-risk nonblack men (Figure 2). The prevalence of severe COVID-19 in intermediate-risk black was similar to that of high-risk nonblack men.
Moreover, in adjusted analyses, every 10% increment in 10-year ASCVD risk score was associated with significantly higher odds of composite severe COVID-19 illness among men and women and among black and nonblack persons; women and black persons demonstrated greater odds (40% and 30%, respectively) (Table 4 ). There was also an increased adjusted odds of composite severe COVID-19 with increasing ASCVD risk group among women and men and in black and nonblack persons. Within the same risk group, women demonstrated greater odds of composite severe COVID-19 than did men, and black persons demonstrated greater odds than did nonblack persons.
Table 4.
Adjusted continuous and categorical logistic regressions for association of composite severe COVID-19 with 10-year ASCVD risk groups examined by gender and race/ethnicity*
| Women N = 16,243 | Men N = 12,403 |
Black people N = 4,005 | Non-black people N = 24,641 | |
|---|---|---|---|---|
| ASCVD risk score per 10% |
1.4 (1.4-1.5) |
1.3 (1.3-1.4) |
1.3 (1.1-1.4) |
1.4 (1.3-1.4) |
| Low risk (<5%) (reference group) |
1.0 |
1.0 |
1.0 |
1.0 |
| Borderline risk (5-<7.5%) |
1.7 (1.4-2.2) |
1.6 (1.2-2.4) |
2.3 (1.3-3.7) |
1.6 (1.3-2.0) |
| Intermediate risk (7.5-<20%) |
2.6 (2.2-3.1) |
2.4 (1.7-3.1) |
2.9 (1.9-4.3) |
2.5 (2.1-2.9) |
| High risk (≥20%) |
4.6 (4.3-5.4) |
3.7 (2.8-4.9) |
4.5 (3.0-6.6) |
4.0 (3.5-4.7) |
P values all <0.0001. Shown are odds ratios (95% confidence intervals).
Adjusted for age, sex, and race/ethnicity. Composite severe COVID includes any of the negative severe COVID outcomes.
ASCVD = atherosclerotic cardiovascular disease; CI = confidence interval; OR = odds ratio.
Discussion
We show that higher estimated 10-year ASCVD risk, measured continuously and by risk group, is significantly associated with a greater likelihood of severe COVID-19 (death, remdesivir administration, COVID-19 pneumonia, ARDS, and mechanical ventilation), both individually and as a composite. Black persons in the highest ASCVD risk category had higher prevalences of severe COVID-19 than did their nonblack counterparts.
Our study is the first major investigation to examine ASCVD estimated risk using the well-validated and recommended PCE in relation to composite severe COVID-19 outcomes. Other investigators have shown that individual measures of smoking history, diabetes, and hypertension are negatively associated with severe COVID-19 outcomes.3 Studies have also examined the Framingham Risk Score in relation to severe COVID-19 outcomes and infection in less-generalizable samples.8, 9, 10 The PCE is recommended for ASCVD risk assessment, and our study illustrates its potential to stratify risk of severe COVID-19.
Hypertension, age, diabetes, race, and smoking status are the largest contributors to our model's predictive ability. This may be related to the fact that inflammation, which is common in most of these comorbidities, may predispose a subject to an exaggerated, dysregulated, and potentially deleterious immune response.13 , 14 Thus, these indicators and their underlying conditions could be targets for treatment to reduce the risk of severe COVID-19 outcomes. It is not surprising that age is one of the strongest predictors, as age is the predominant variable in ASCVD risk calculation and is among the strongest predictors of death from severe COVID-19 disease.15
In adddition, our analysis highlights specific race and gender groups most at risk— most notably, black men and black women. Others have highlighted the increased prevalence of CVD in black communities, indicating contributing factors such as obesity, unstable housing status, lack of health insurance, poor diet and exercise, and even residential zip code, which can predispose to adverse health outcomes.16, 17, 18, 19, 20 These disparities in social determinants of health are strongly associated with greater CVD prevalence within the black population, which explains the persistence of black persons in the intermediate- and high-risk groups (15% and 19%, respectively) (Table 1). Although the PCE includes black or white/other race/ethnicity, it does not account for social determinants of health. This helps to explain why, across all risk groups, black women experienced an increased prevalence of severe COVID-19 compared with nonblack women in the same ASCVD risk group. Black men experienced a higher prevalence of severe COVID-19 compared with nonblack men in the same ASCVD risk group across all risk stratifications except low-risk. Overall prevalence of severe COVID-19 in intermediate-risk black men was similar to that in high-risk nonblack men. This study demonstrates the increased importance of prevention, education, and awareness in these and other groups with disparities in severe COVID-19.
Other machine-learning prediction models have been effectively able to predict risk of intensive care unit admission and death because of COVID-19, but had smaller sample sizes and highly specific patient populations; some were limited to a single health system and a only few months of data collection.21, 22, 23, 24 Other models calculating risk of death or intensive care unit admission did not explore variables such as rescue treatment (remdesivir), mechanical ventilation, ARDS, and pneumonia.25 In comparison, our study utilized a large range of more than 5,000 clinical sites across the country and included patients with up to 2 years of relevant medical history. This enabled us to examine the PCE in relation to a large study population representative of US adults seeking healthcare for COVID-19 and to explore a variety of potential negative outcomes seen in severe cases of COVID-19, thus expanding its potential applicability.
Beyond efficacy, the most important characteristics of a prediction model are accessibility and applicability. Previous studies have developed prediction models using various laboratory markers such as lactate dehydrogenase—a cellular respiration enzyme that is present in nearly all body tissues and that when elevated may indicate tissue damage or disease—to predict mortality risk from COVID-19.20 , 26 , 27 Although these models are accurate, they are not readily applicable for screening and prevention in the general population. They are more applicable to hospitalized patients for the purpose of prioritizing resource utilization to minimize morbidity and mortality. There has also been an increase in the number of prognostic models that use computed tomography (CT).21 , 28 These models have their own limitations, as CT imaging is not acquired on every patient with positive SARS-CoV-2 reverse-transcription polymerase chain reaction test results.29 CT scans are indicated only when there is suspected lung pathology and imaging would significantly alter the clinical decision making. Furthermore, unnecessary radiation exposure and fiscal cost would result from using CT scans much more frequently for purposes of risk stratification.30 The 10-year ASCVD risk score requires no laboratory or imaging workup, can be used in most patients with COVID-19 and without previous CVD, and can be calculated readily by both clinicians and patients themselves in outpatient settings. This can help clinicians advise and educate patients on their potential risks for severe COVID-19 outcomes in a more personalized and specific manner. Although its application may be limited in more acute settings, the PCE can be a valuable tool in the outpatient and primary care setting for educating patients not only about their risks of ASCVD but about how these may relate to their risks of severe COVID-19.
Our present study does have a few limitations. Most importantly, the N3C database includes only subjects who had visits to a medical center. Moreover, we could not verify that indicators of severe illness were specific to severe COVID-19 disease. For example, it is possible that patients died for reasons unrelated to severe COVID-19 disease, but we did not have the data to determine whether this was the case. Importantly, with our limited follow-up time, some severe outcomes may have occurred after follow-up; however, this would have likely resulted in even greater statistical power to detect associations than we had in our study. Additionally, the dataset included limited information on insurance status, socioeconomic status, maximum education levels attained, and other potentially important social determinants of health that would have been of interest to include in our study. Finally, we did not have sufficiently large numbers of subjects from race/ethnic groups other than black and white to further stratify our analyses.
In conclusion, we show that ASCVD higher risk groups display higher prevalences and odds of severe COVID-19 outcomes, with greater disparities in outcomes noted among black persons at the highest estimated ASCVD risk. Although further research is needed, the 10-year ASCVD risk score in adults 40 to 79 years old may be used to identify those at highest risk for COVID-19 complications and for whom more intensive treatment may be warranted. Future studies may also analyze how robust the association between 10-year ASCVD risk score and COVID-19 prognosis remains with new variants.
Disclosures
The authors have no conflicts of interest to declare.
Footnotes
Drs. Arif and Stefanko contributed equally to this work.
Funding: None.
See page 22 for disclosure information.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2022.08.011.
Appendix. Supplementary materials
References
- 1.Hu Y, Sun J, Dai Z, Deng H, Li X, Huang Q, Wu Y, Sun L, Xu Y. Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/. Accessed on February 2, 2022. [PubMed]
- 3.Izcovich A, Ragusa MA, Tortosa F, Lavena Marzio MA, Agnoletti C, Bengolea A, Ceirano A, Espinosa F, Saavedra E, Sanguine V, Tassara A, Cid C, Catalano HN, Agarwal A, Foroutan F, Rada G. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin C, Zhou L, Hu Z, Yang S, Zhang S, Chen M, Yu H, Tian DS, Wang W. Clinical characteristics and outcomes of COVID-19 patients with a history of stroke in Wuhan, China. Stroke. 2020;51:2219–2223. doi: 10.1161/STROKEAHA.120.030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng YD, Meng K, Guan HQ, Leng L, Zhu RR, Wang BY, He MA, Cheng LX, Huang K, Zeng QT. [Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV] Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:450–455. doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 7.He XW, Lai JS, Cheng J, Wang MW, Liu YJ, Xiao ZC, Xu C, Li SS, Zeng HS. [Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients] Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:456–460. doi: 10.3760/cma.j.cn112148-20200228-00137. [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi WT, Michos ED, Flueckiger P, Blaha M, Sandfort V, Herrington DM, Burke G, Yeboah J. Impact of replacing the Pooled Cohort Equation with other cardiovascular disease risk scores on atherosclerotic cardiovascular disease risk assessment (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2016;118:691–696. doi: 10.1016/j.amjcard.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Haendel MA, Chute CG, Bennett TD, Eichmann DA, Guinney J, Kibbe WA, Payne PRO, Pfaff ER, Robinson PN, Saltz JH, Spratt H, Suver C, Wilbanks J, Wilcox AB, Williams AE, Wu C, Blacketer C, Bradford RL, Cimino JJ, Clark M, Colmenares EW, Francis PA, Gabriel D, Graves A, Hemadri R, Hong SS, Hripscak G, Jiao D, Klann JG, Kostka K, Lee AM, Lehmann HP, Lingrey L, Miller RT, Morris M, Murphy SN, Natarajan K, Palchuk MB, Sheikh U, Solbrig H, Visweswaran S, Walden A, Walters KM, Weber GM, Zhang XT, Zhu RL, Amor B, Girvin AT, Manna A, Qureshi N, Kurilla MG, Michael SG, Portilla LM, Rutter JL, Austin CP, Gersing KR, N3C Consortium. The National COVID Cohort Collaborative (N3C): rationale, design, infrastructure, and deployment. J Am Med Inform Assoc. 2021;28:427–443. doi: 10.1093/jamia/ocaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ASCVD Risk Estimator +. Available at: https://tools.acc.org/ascvd-risk-estimatorplus/#!/calculate/estimate. Accessed on July 26, 2022.
- 12.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 13.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Farsalinos K, Barbouni A, Poulas K, Polosa R, Caponnetto P, Niaura R. Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: a systematic review and meta-analysis. Ther Adv Chronic Dis. 2020;11 doi: 10.1177/2040622320935765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blagosklonny MV. From causes of aging to death from COVID-19. Aging (Albany NY) 2020;12:10004–10021. doi: 10.18632/aging.103493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson TA, Morton F, Jacobson KL, Sharma S, Garcia DC. An assessment of obesity among African-American women in an inner city primary care clinic. J Natl Med Assoc. 2002;94:1049–1057. [PMC free article] [PubMed] [Google Scholar]
- 17.Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA, Jr, Willis M, Yancy CW. American Heart Association Council on Epidemiology and Prevention, Council on Cardiovascular Disease in the Young, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Functional Genomics and Translational Biology, Stroke Council. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136:e393–e423. doi: 10.1161/CIR.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 18.Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, Davey-Smith G, Dennison-Himmelfarb CR, Lauer MS, Lockwood DW, Rosal M, Yancy CW. American Heart Association Council on Quality of Care and Outcomes Research, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, Council on Lifestyle and Cardiometabolic Health, Stroke Council. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132:873–898. doi: 10.1161/CIR.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 19.Diez Roux AV. Neighborhoods and health: what do we know? what should we do? Am J Public Health. 2016;106:430–431. doi: 10.2105/AJPH.2016.303064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barber S, Hickson DA, Wang X, Sims M, Nelson C, Diez-Roux AV. Neighborhood disadvantage, poor social conditions, and cardiovascular disease incidence among African American adults in the Jackson Heart Study. Am J Public Health. 2016;106:2219–2226. doi: 10.2105/AJPH.2016.303471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie J, Hungerford D, Chen H, Abrams ST, Li S, Wang G, Wang Y, Kang H, Bonnett L, Zheng R, Li X, Tong Z, Du B, Qiu H, Toh CH. Development and external validation of a prognostic multivariable model on admission for hospitalized patients with COVID-19. medRxiv. Preprint posted online April 7, 2020. 10.1101/2020.03.28.20045997. [DOI]
- 22.Yue H, Yu Q, Liu C, Huang Y, Jiang Z, Shao C, Zhang H, Ma B, Wang Y, Xie G, Zhang H, Li X, Kang N, Meng X, Huang S, Xu D, Lei J, Huang H, Yang J, Ji J, Pan H, Zou S, Ju S, Qi X. Machine learning-based CT radiomics method for predicting hospital stay in patients with pneumonia associated with SARS-CoV-2 infection: a multicenter study. Ann Transl Med. 2020;8:859. doi: 10.21037/atm-20-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan M, Yin W, Tao Z, Tan W, Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS One. 2020;15 doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Z, Chen A, Hou W, Graham JM, Li H, Richman PS, Thode HC, Singer AJ, Duong TQ. Prediction model and risk scores of ICU admission and mortality in COVID-19. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yadaw AS, Li YC, Bose S, Iyengar R, Bunyavanich S, Pandey G. Clinical features of COVID-19 mortality: development and validation of a clinical prediction model. Lancet Digit Health. 2020;2:e516–e525. doi: 10.1016/S2589-7500(20)30217-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan L, Zhang HT, Goncalves J, Xiao Y, Wang M, Guo Y, Sun C, Tang X, Jing L, Zhang M, Huang X, Xiao Y, Cao H, Chen Y, Ren T, Wang F, Xiao Y, Huang S, Tan X, Huang N, Jiao B, Cheng C, Zhang Y, Luo A, Mombaerts L, Jin J, Cao Z, Li S, Xu H, Yuan Y. An interpretable mortality prediction model for COVID-19 patients. Nat Mach Intell. 2020;2:283–288. [Google Scholar]
- 27.Gong J, Ou J, Qiu X, Jie Y, Chen Y, Yuan L, Cao J, Tan M, Xu W, Zheng F, Shi Y, Hu B. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020;71:833–840. doi: 10.1093/cid/ciaa443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, Zha Y, Li W, Wu Q, Li X, Niu M, Wang M, Qiu X, Li H, Yu H, Gong W, Bai Y, Li L, Zhu Y, Wang L, Tian J. A fully automatic deep learning system for COVID-19 diagnostic and prognostic analysis. Eur Respir J. 2020;56 doi: 10.1183/13993003.00775-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garg M, Prabhakar N, Bhalla AS, Irodi A, Sehgal I, Debi U, Suri V, Agarwal R, Yaddanapudi LN, Puri GD, Sandhu MS. Computed tomography chest in COVID-19: when & why? Indian J Med Res. 2021;153:86–92. doi: 10.4103/ijmr.IJMR_3669_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Royal HD. Effects of low level radiation-what's new? Semin Nucl Med. 2008;38:392–402. doi: 10.1053/j.semnuclmed.2008.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.