Abstract
Coronavirus disease 2019 (COVID-19) has caused a global pandemic and presents a significant danger to public health. Lymphopenia is considered to be the defining characteristic of severe COVID-19, especially in elderly people. Lymphopenia has been suggested as a pivotal factor in disease severity. To minimize mortality in COVID-19 patients, it is essential to have a deeper understanding of the processes behind lymphocytopenia. Recently, myeloid-derived suppressor cells (MDSCs) have been confirmed as a key mediator of lymphopenia. MDSCs are characterized by their powerful capacity to suppress T cells and eventually contribute to the course of illness. Targeting these cells may improve the disease prognosis. In this article, we analyze the available research on MDSCs in lymphopenia and discuss their immunopathologic changes and prospective therapeutic targets in patients with COVID-19 lymphocytopenia.
Keywords: Myeloid-derived suppressor cells, COVID-19, Lymphopenia
Abbreviations: Arg-1, Arginase 1; ATRA, All-trans retinoic acid; BALF, Bronchoalveolar lavage fluid; C/EBPβ, CCAAT/enhancer binding protein beta; cGMP, Cyclic guanosine monophosphate; COX-2, Cyclooxygenase 2; COVID-19, Coronavirus disease 2019; CREB, cAMP-response element binding protein; CS, Cytokine storm; CXCR, CXC-chemokine receptor; DP2, d-type prostanoid receptor 2; FasL, Fas ligand; FATP2, Fatty acid transport protein 2; HLA-DR, Human leukocyte antigen DR, ICL, Innate lymphoid cells; ICU, Intensive care unit; IDO, Indoleamine 2,3-dioxygenase; IFN-γ, Interferon-gamma; IL-6, Interleukin-6; iNOS, Inducible nitric oxide synthase; IRF, Interferon related factor; LDH, Lactate dehydrogenase; IMCs, Immature myeloid cells; LOX-1, Lectin-type oxidized LDL receptor 1; MDSCs, Myeloid-derived suppressor cells; M−MDSCs, Monocytic MDSCs; MMP, Matrix metalloproteinase; MPO, Myeloperoxidase; NE, Neutrophil elastase; NK cells, Natural killer cells; PBMCs, Peripheral blood mononuclear cells; PD-1, Programmed cell death 1; PDE, Phosphodiesterase; PGD2, Prostaglandin D2; PGE2, Prostaglandin E2; PI3K, Phosphoinositide 3-kinase; PMN-/G-MDSCs, Polymorphonuclear/granulocytic MDSCs; ROS, Reactive oxygen species; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; STAT, Signal transducer and activator of transcription; TGF-β1, Transforming growth factor-β1; TNF-α, Tumor necrosis factor alpha; VDR, Vitamin D receptor; 5-FU, 5-Fluorouracil
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a causative agent of coronavirus disease 2019 (COVID-19), which has become a global pandemic that has claimed millions of lives [1]. More than 599 million individuals have been verified to have COVID-19 as of 31 August 2022, causing over 6 million deaths reported to the World Health Organization [2]. When compared to bacterial sepsis patients, COVID-19 patients exhibited less early physiologic derangement but more chronic inflammation and immunosuppression, as well as poorer clinical outcomes [3]. The clinical signs of SARS-CoV-2 infection range from mild to moderate to severe; the majority of infected individuals have mild illness, but severe cases are associated with a greater fatality rate. The common findings of the hematological manifestation of COVID-19 are lymphopenia, anemia, thrombocytopenia, and coagulation abnormalities [4]. The incidence of lymphopenia in COVID-19 patients varies widely, ranging from 25 % to 80 % due to various vaccination rates, countries, detection timing, and the emergence of new variants. Lymphopenia is characterized by drastically reduced numbers of lymphocytes, which are responsible for progressing to the critical stage of the disease [5]. Lymphopenia is generally found in critically ill patients, while mild cases manifest with an upgraded number of lymphocyte counts. Importantly, the decreased number of CD4+ T cells and CD8+ T cells showed a downregulated capability of antiviral cytokine production. Exhaustion markers are upregulated, including PD-1 (programmed cell death 1) and T-cell immunoglobulin and mucin domain-containing-3 [6], [7]. In addition, there was a negative relationship between lymphocyte counts and pulmonary SARS-CoV-2 RNA load [8]. Multiple studies have demonstrated that lymphopenia was recognized as a severity predictor [5], [9]. Given that deep immunosuppression caused by downregulated T cells is likely to exacerbate the conditions, it is rational to restore the function and count of lymphocytes. Potential therapeutic options to reverse lymphopenia and restore its function to orchestrate an efficient immune response against the virus may benefit patients with COVID-19.
Recently, myeloid-derived suppressor cells (MDSCs) have been thought to be a crucial mediator in the pathogenesis of lymphopenia in COVID-19 patients. MDSCs with significant immunosuppressive properties are implicated in pathological immune responses that contribute to the reduction and exhaustion of lymphocytes. Consistent proliferation of these cells may induce significant pathophysiology changes and lead to dismal clinical outcomes. In this review, we analyze the available research on MDSCs in lymphopenia, highlighting immunopathologic alterations and prospective targets for treatment interventions in COVID-19 lymphopenic patients.
2. Pathogenesis of lymphopenia in COVID-19
The underlying pathogenesis of lymphopenia in COVID-19 is complex and makes it hard to explain with a single theory. Several potential mechanisms, such as cytokine storm (CS), may play a leading role in the pathogenesis of lymphopenia. Patients with lymphopenia were found to have higher serum levels of proinflammatory cytokines compared to cases without lymphopenia, possibly because upregulated tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) induce apoptosis in T cells [10]. When the serum levels of cytokines and chemokines were reduced to normal levels, the clinical manifestations improved. Moreover, upgraded proinflammatory cytokines may aggravate lymphopenia in a direct or indirect manner. It is noteworthy that lymphopenia and elevated serum levels of proinflammatory cytokines may form a destructive positive feedback loop [11].
The destruction of secondary lymphoid organs is also responsible for lymphopenia in COVID-19 patients. This disclosure was confirmed by postmortem tissue samples of nonsurvivors of SARS-CoV whose histopathological examination showed degeneration of splenic white pulp, absence of germinal centers and lymph node necrosis accompanied by decreased Tfh cells [12], [13], [14]. Similar results were revealed by Feng et al. [15], who proposed that SARS-CoV-2 decimated lymphatic organs and resulted in activation-induced cell death by inducing secretion of IL-6, followed by a significant decrease in lymphocyte counts.
Infected patients have altered gene expression impacting lymphocyte proliferation, differentiation, and apoptosis. Several genes involved in apoptosis pathways were upregulated [16]. It is likely that lymphopenia is caused by the upregulation of proapoptotic genes. In addition, a report showed that MAP2K7 and SOS1, which are related to T-cell activation signals, were downregulated. Interestingly, after initial treatment, these genes were upregulated compared to those on admission [17].
In addition, some metabolic products are associated with dismal outcomes, such as lactic acid and lactate dehydrogenase (LDH). Exposure to lactic acid may impede the proliferation and function of human cytotoxic T lymphocytes by disturbing lactic acid export [18]. Furthermore, the clinical stage of severe patients may deteriorate when suffering from lactic acid acidosis. A parallel result was found in patients with elevated LDH [19].
Lymphopenia may be attributed to epigenetic alterations, such as the histone methyltransferase G9a [20]. Moreover, lymphopenia closely correlates with upregulated inhibitory immune checkpoint molecules, such as PD-1 and Fas. MDSCs are also implicated in the development of lymphopenia. In fact, there have been numerous attempts to elucidate the pathogenesis of lymphopenia in COVID-19, but no definite etiology has been identified. Further deepening the knowledge of the etiology and pathogenesis of lymphopenia is needed.
3. The role of MDSCs in COVID-19
Hematopoietic progenitor cells under normal circumstances transform into common myeloid progenitors, granulocyte–macrophage progenitors, and eventually neutrophils or monocytes. Neutrophils differentiate via progenitor and precursor stages such as myeloblasts, myelocytes, metamyelocytes, and band forms, while monocytes develop from monocyte/macrophage and dendritic cell precursors(MDP) [21]. Under pathologic conditions, MDSCs are differentiated from immature myeloid cells (IMC) via interference with transcription regulators and epigenetic modification. On the other hand, MDSCs maintain their suppressive function and immature state through interactions with cytokines, chemokines, and transcription factors [22]. Interestingly, normal neutrophils or monocytes can be converted into PMN-MDSCs or M−MDSCs through endoplasmic reticulum stress or prostaglandin E2 (PGE2) [23], [24], [25]. Nevertheless, the biological processes responsible for this transformation remain unknown.
MDSCs are heterogeneous immature myeloid cells related to immunoregulation [26]. MDSCs are characterized by suppressing effector T-cell activity and dampening inflammation, contrary to canonical proinflammatory phenotypes. When there is a focal infection, a large number of immune cells are directed to the sites to clear pathogens that may lead to systemic inflammation. Proliferation and stimulation of MDSCs commonly take place to curb dysregulated inflammatory processes. The precise mechanisms causing the growth of MDSCs remain enigmatic; however, they may be related to diverse factors, such as macrophage colony-stimulating factor, granulocyte colony-stimulating factor, granulocyte–macrophage colony stimulating factor, and IL-6 [27].
MDSCs include polymorphonuclear/granulocytic MDSCs (PMN-/G-MDSCs), monocytic MDSCs (M−MDSCs), and early-stage MDSCs which account for less than 5 % of entire MDSCs [28](Table 1 ). And these cells are usually classified according to morphology and functional characteristics. G-MDSCs and M − MDSCs share similar morphology with granulocytes and monocytes, respectively. These cells display dual protective and harmful effects depending on various biological processes. Chronic infections or cancers lead to persistent expansion of MDSCs that may induce T-cell anergy, aggravate tissue damage, and exacerbate the disease [29]. The target of MDSCs is not restricted to dampening T-cell functionality. MDSCs may regulate an extensive array of adaptive and innate immune cells, such as natural killer cells (NK cells), macrophages, and dendritic cells [21]. This is consistent with the observation that COVID-19 patients had NK cells with lower granzyme A levels [30].
Table 1.
Fundamental characteristics of MDSCs, neutrophils, and monocytes.
| PMN-MDSCs | Neutrophils | M−MDSCs | Monocytes | |
|---|---|---|---|---|
| phenotypical markers in mice | CD11b+Ly6G+Ly6Clo | CD11b+LY6G+Ly6Clo | CD11b+Ly6G–Ly6Chi | CD11b+Ly6G−Ly6Chi |
| phenotypical markers in human | CD11b+CD14−CD15+CD66b+HLA-DR– | CD11b+CD14–CD15+CD66b+ | CD11b+CD14+CD15–HLA-DR–/lo | CD11b+CD14+CD15–HLA-DR+ |
| density gradient centrifugation | low | high | low | low |
| novel markers | LOX-1 | NA | CXCR1 | NA |
| function | immunosuppression | activation | immunosuppression | activation |
| molecular characteristics | higher expression of genes involved in the cell cycle, autophagy, G-protein signaling, and CREB pathway | higher expression of NF-κB signaling and lymphotoxin-β receptor signaling, lysosomal and proteosomal protein expression | higher expression of PI3K, IL-6 and TGFβ | higher expression of genes involved in the phagocytosis, immune responses and migration |
| chemokines and receptors | lower expression of chemokine receptors CXCR1 and CXCR2 | higher expression of CXCL4 and CXCL12 and lower expression of CCL3, CCL4, and CXCL2 | higher expression of CXCR2, CXCR4, and CXCR1 | higher expression of CCL2, CXCR1, CD62L and CXCR2 |
| transcription factors | activation of STAT3, C/EBPβ and downregulation of IRF-8 | activation of RUNX1, KLF6, RELB, IRF5, and JUNB | activation of STAT3, C/EBPβ, downregulation of IRF-8 | activation of STAT1/3, C/EBPα-p42, c-Jun/JunB, VDR, IRF1/4/8, and C/EBPβ-LAP |
| biochemical characteristics | Arg-1, ROS, PGE2 | MPO, NE, defensins, cathelicidins, MMP | NO, TGFβ, PGE2, IL-10 | TNF, iNOS, IL-10, IL-6 |
Abbreviations: MDSC: myeloid-derived suppressor cell; PMN-MDSCs: polymorphonuclear MDSCs; M−MDSCs: monocytic MDSCs; LOX1: lectin-type oxidized LDL receptor 1; NO: nitric oxide; NA: not applicable; CXCR: CXC-chemokine receptor; CREB: cAMP-response element binding protein; IL-6: interleukin-6; TGF-β: transforming growth factor-β; PI3K: phosphoinositide 3-kinase; CXCL: CXC-chemokine ligand; CCL: C—C motif chemokine ligand 3; STAT: signal transducer and activator of transcription; C/EBP: CCAAT/enhancer binding protein; IRF: interferon related factor; VDR: vitamin D receptor; Arg-1: arginase 1; ROS: reactive oxygen species; PGE2: prostaglandin E2; MPO: myeloperoxidase; NE: neutrophil elastase; MMP: matrix metalloproteinase; iNOS: inducible nitric oxide synthase; TNF: tumor necrosis factor.
Phenotypic markers of human MDSCs are recognized as CD11b+CD14-CD15+/CD66b+ or CD14+CD15-HLA-DRlo/-. There are no phenotypic markers that can identify these cells from classical neutrophils and monocytes. This also makes it challenging to detect MDSCs based on their phenotypic characteristics. Both PMN-MDSCs and M−MDSCs are low-density granulocytic cells identified by density gradient centrifugation. In recent years, lectin-type oxidized LDL receptor 1 (LOX-1) has been recognized as a specific marker of PMN-MDSCs [31], [32], [33]. This allows for the early identification of subpopulations of MDSCs with immunosuppressive properties by flow cytometry for larger clinical research projects.
A study found dramatic changes in phenotypes and states of MDSCs from proinflammatory to anti-inflammatory, as adoptively transferred MDSCs from mice with early sepsis resulted in higher pro-inflammatory cytokine production and earlier death of naive animals compared to adoptively transplanted MDSCs from the late phase [34]. Epigenetic changes may be involved in the plasticity of these cells, such as DNA methylation and miRNA [35]. Some studies have indicated that several members of the miR-15 family block the PD-1/PD-L1 interaction in cancer, leading to MDSC dysfunction and T-cell activation[36].
The immunosuppressive role of MDSCs has been reported in other conditions, such as cancers and infections. MDSCs are essential for COVID-19 infection. Studies have indicated that the number of MDSCs in the peripheral blood of COVID-19 patients increases while the number of T cells decreases. MDSCs were shown to have increased expression of genes with immunosuppressive effects[37]. At the same time, MDSCs may be engaged in CS, which is one of the major pathophysiological alterations in COVID-19, leading to a poor prognosis and increased mortality. It appears that these cells display various phenotypic characteristics and functional statuses at different stages of the disease.
An increased proportion of MDSCs was associated with a longer hospital stay, high rates of nosocomial infections, and clinical worsening [38]. The frequency of MDSCs has been suggested as a prognostic indicator in cancer patients [39]. It is noted that these cells may also predict COVID-19 severity. Patients with severe COVID-19 were shown to have a greater frequency of MDSCs than healthy controls[40]. Patients admitted to the intensive care unit (ICU) had a higher population of these cells than non-ICU patients[41], [42]. A multivariate regression study conducted in Italy suggested a correlation between PMN-MDSCs and poor clinical outcomes[43]. In the convalescent phase, M−MDSCs are reduced to levels comparable to those in healthy controls[44]. A study conducted in Japan indicated that transient expansion of MDSCs was observed in survivors, while it was not found in non-survivors [45]. In contrast, an Italian study showed an expansion of PMN-MDSCs in non-survivors but not in survivors [43]. This result may be explained by the versatile roles of MDSCs in the different stages of disease. Early expansion of MDSCs may impair tissue repair, while later-stage expansion may facilitate disease recovery. It has been proposed that the increased MDSC frequency independently correlates with fatal outcomes after adjusting for other confounders.
4. MDSCs in COVID-19 patients with lymphopenia
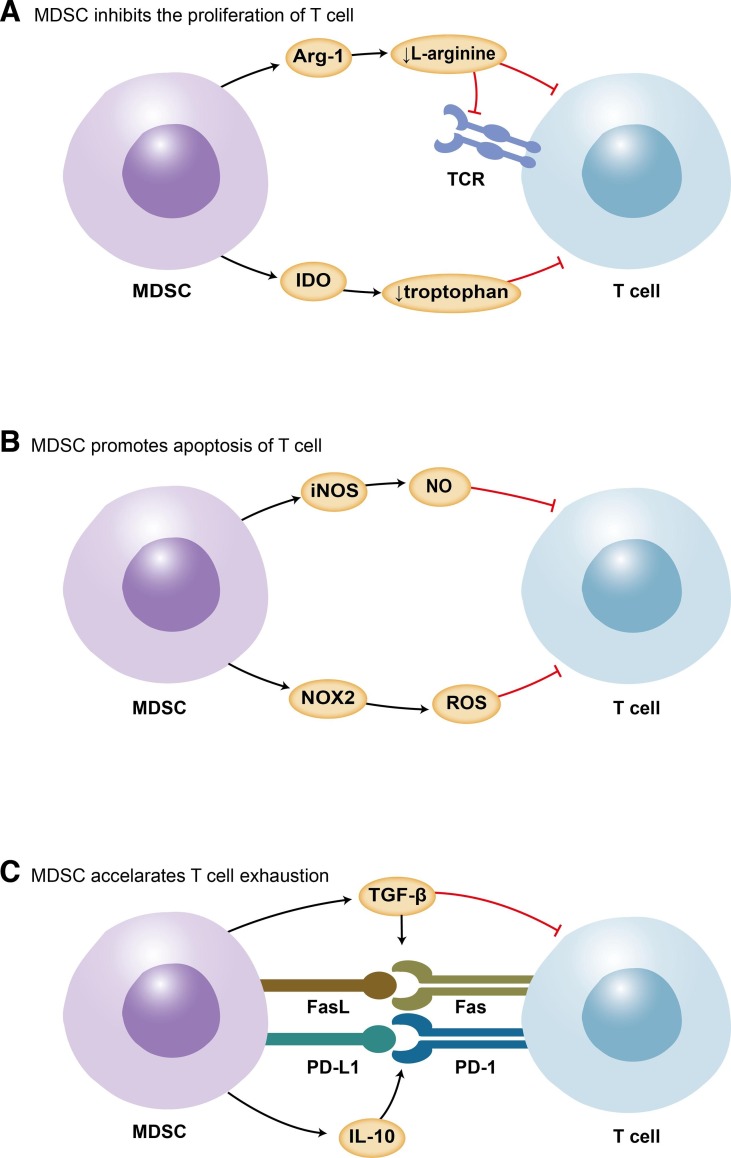
MDSCs are essential in tissue repair, immunotolerance, and preventing unrestrained inflammation in late-stage illness, indicating their favorable features[46]. In some cases, aberrant activation of MDSCs is driven by persistent inflammatory signals, such as tissue trauma, chronic infections, inflammatory mediators, and epigenetic mechanisms[21], [47]. IMCs, which usually mature into classical neutrophils and monocytes, develop into MDSCs with immunosuppressive functions[48], [49]. MDSCs will sustain their immature condition and not be able to undergo maturation. It has been demonstrated that MDSCs release a number of chemicals and alter transcription factors that contribute to T-cell anergy, apoptosis, and, ultimately, lymphopenia (Fig. 1 )[50]. The finding that in vitro MDSC reduction enhanced T-cell activities validated their putative in vivo suppressive role. Using single-cell RNA sequencing in COVID-19 patients and healthy donors, peripheral blood mononuclear cells (PBMCs), as well as paired bronchoalveolar lavage fluid (BALF), indicated that the expansion of MDSC-like monocytes was related to lymphopenia[51].
Fig. 1.
The involvement of MDSCs in COVID-19-associated lymphopenia. MDSCs play a crucial role in lymphopenia. Mechanisms include suppression of the proliferation of T cells by Arg-1 and IDO (A); promotion of apoptosis of T cells via iNOS and ROS (B); induction of PD-1/PD-L1 and Fas/FasL interaction through IL-10 and TGF-β, leading to T-cell exhaustion(C). MDSCs: myeloid-derived suppressor cells; Arg-1: arginase 1; PD-L1: programmed cell death ligand 1; PD-1: programmed cell death protein 1; FasL: Fas ligand; ROS: reactive oxygen species; NOX2: NADPH oxidase 2; iNOS: inducible nitric oxide synthase; IDO: indoleamine 2,3-dioxygenase; TGF-β: transforming growth factor-β; IL-10: interleukin-10.
4.1. Inhibiting proliferation of lymphocytes
Cytokines and mediators secreted by MDSCs, such as indoleamine 2,3-dioxygenase (IDO) and arginase 1 (Arg-1)[52], can undermine the proliferation of T cells while promoting the generation of regulatory T cells[40]. l-arginine is involved not only in TCR synthesis and antigen recognition but also in T-cell proliferation[53], [54]. There is a correlation between the degradation of l-arginine by MDSC-secreted Arg-1 and the impairment of T-cell receptor signaling, endothelial function, and coagulation[55], [56]. The formation of nitric oxide, which modulates endothelial function, was decreased by arginase with high avidity.[56] A recent study reported a dramatically decreased T-cell proliferative capability in vitro, but it can be restored with arginine supplementation[57]. However, l-arginine supplementation was reported to have little effect on improving immune functioning in humans. Administration timing, specific patients, and nutritional regimens may play a role. Citrulline administration was reported to be more efficacious than arginine in restoring T-cell proliferation and mitochondrial function in a septic animal model by raising arginine availability[58], [59]. This discrepancy may be explained by the absence of first-pass metabolism of citrulline. And regulatory T cells with potency of suppressing effector T cells were shown to be downregulated after citrulline administration. IDO produced by MDSCs was found to activate the stress-response kinase GCN2 and contribute to impairing the proliferation of T cells[60], [61]. A recent study showed that the proliferation of GCN2-ablated T cells was not suppressed in a mouse model. Tryptophan, which flavors the activation of T cells, is catabolized by IDO[62].
4.2. Promoting apoptosis of lymphocytes
MDSC-derived reactive oxygen species (ROS) and its downstream product peroxynitrate nitrate at the T-cell receptor[63]. Thus, T cells lose their capacity to attach to the phosphorylated major histocompatibility complex and are not able to react to particular antigens, leading to antigen-specific T-cell tolerance[64]. ROS may be involved in activation-induced cell death[65]. Enhanced expression of NADPH oxidase mRNA was observed in COVID-19 patients in conjunction with a substantial proliferation of MDSCs, which allows for the possibility that PMN-MDSCs induce ROS-mediated tissue damage[66]. In addition, NO produced by inducible nitric oxide synthase (iNOS) is associated with interference with the JAK-STAT signaling pathway and initiates apoptosis via nitration of certain cytokines[67]. INOS, in collaboration with arginase-1, can nitrosylate TCR to generate reactive nitrogen oxide species that suppress T cells or trigger apoptosis[68].
The number of T lymphocytes may also be influenced by MDSCs via the process of cell-to-cell contact. MDSCs extensively express PD ligand 1 (PD-L1), Fas ligand (FasL), and galectin-9, which are responsible for triggering T-cell anergy[69]. It has been shown that CD8+ T cells cultured with galectin-9 have a diminished ability to produce perforin and granzyme, but their activity can be restored when this route is inhibited.
4.3. Regulating lymphocyte exhaustion
T cells undergo significant alterations during persistent infection, including T-cell exhaustion. Exhausted T cells significantly produce inhibitory checkpoint molecules such as PD-1, and their function gradually diminishes. The increased expression of PD-1 correlated with the depletion of T cells during septic conditions[70]. Several studies have demonstrated that IL-10 seems to stimulate the production of PD-L1 on MDSCs. Membrane-bound transforming growth factor-β1 (TGF-β1) produced by MDSCs may promote the development of T-cell exhaustion. A recent study showed that TGF-β1 in tumor mice impeded interferon-gamma (IFN-γ) production and NK-cell functions[71]. When the patients were in the recovery period, MDSCs were reduced along with the depletion of TGF-β[29]. The expansion of MDSCs correlated with decreased IFN-γ production, which can be reversed by inhibiting TGF-β or iNOS but not Arg-1[43]. A study showed that TGF antagonists revived T cells’ cytokine-producing ability[72]. Furthermore, exposure to PGE2 may promote the formation of monocytic MDSCs from dendritic cells[24], [73]. PGE2 and cyclooxygenase 2 (COX-2) are involved in a positive feedback loop, and this loop is partially responsible for immunosuppressive effects as well as cellular accumulation.
The key molecular properties of M−MDSCs and PMN-MDSCs are comparable, which allow them to inhibit immunological responses, such as the production of Arg-1 and S100A8/A9. However, the subsets of MDSCs exhibit suppressive potential in different ways. PMN-MDSCs predominantly use ROS, Arg-1, and PGE2 to suppress the immunological system, while M−MDSCs function via NO, IL-10, and TGF[74], [75]. The upcoming outcomes of clinical studies assessing arginine, anti-PD1 antibodies, and COX-2 inhibitors for the treatment of lymphopenia will guide the design of investigations intended to decipher the molecular pathways behind MDSC-induced lymphopenia.
5. MDSCs as therapeutic targets
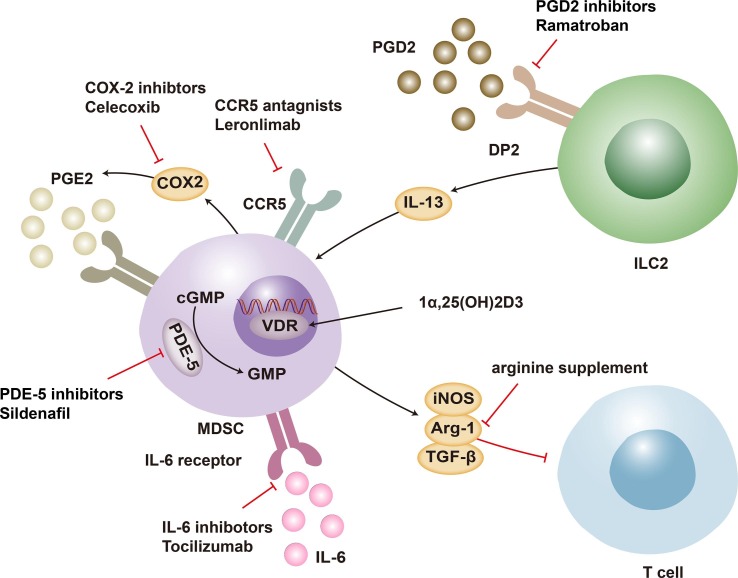
To lessen the number of deaths caused by COVID-19, a wide variety of antiviral drugs have been tested in clinical studies and shown to be effective in treating infected people. In addition to antiviral drugs and vaccines aimed at viral entry, therapeutics aimed at boosting an effective host immune response have become a promising and potent focus. The significant importance of T cells has been underlined for eliminating pathogens and orchestrating an effective immune response[76]. MDSC accumulation mediates the suppression of T cells and has unfavorable effects on individuals who have severe COVID-19. Diverse therapeutic approaches have been suggested to suppress the detrimental role and maintain the protective role of MDSCs in patients. Targeting MDSCs by delaying myeloid bone marrow cell differentiation, blocking recruitment to affected organs, or changing the microenvironment appears to be potential ways to raise lymphocyte counts. Several molecular processes responsible for the immunosuppressive effect of MDSCs in COVID-19 can be rationally treated utilizing knowledge gained from cancer research. Given the detrimental effects of MDSCs, a variety of medications have been proposed to improve clinical outcomes (Fig. 2 ).
Fig. 2.
Strategies for targeting MDSCs. The therapeutic approaches that target the proliferation, differentiation, and reprogramming of MDSCs may assist in the recovery from lymphopenia in COVID-19 patients. MDSC-induced lymphopenia is mediated by PGE2 generated from arachidonic acid through COX-2 catalysis. MDSCs express COX-2, which contributes to their own immunosuppressive function. Inhibitors of COX-2 interrupt this positive feedback loop. 1,25(OH)2D3 penetrates cells and binds to VDR, hence inhibiting MDSC activation. The PDEs catalyze the hydrolysis of cGMP. PDE-5 drugs that enhance cGMP limit the production of iNOS and Arg-1, leading to reducing MDSC-mediated T-cell suppression. CCR5 inhibitors reduce the recruitment of MDSCs, hence impeding their interaction with lymphocytes. As an immunotherapy for lymphopenia, a PGD2 receptor antagonist of PGD2/DP2 signaling is suggested, given that PGD2 is essential for MDSC overexpression through DP2 receptor signaling. It has been hypothesized that l-arginine inhibits the immunosuppressive activity of MDSCs. Moreover, IL-6 receptor inhibitors may improve HLA-DR expression and decrease M−MDSC synthesis in plasma. MDSC: myeloid-derived suppressor cell; COVID-19: Coronavirus disease 2019; PGE2: prostaglandin E2; COX-2, cyclooxygenase 2; VDR: vitamin D receptor; cGMP: cyclic guanosine monophosphate; PDE: phosphodiesterase; iNOS: inducible nitric oxide synthase; Arg-1: arginase 1; PGD2: prostaglandin D2; DP2: d-type prostanoid receptor 2; IL-6: interleukin-6; ILC2: innate lymphoid cells.
5.1. Eliminating MDSCs
The proliferation of MDSCs is usually accompanied by decreased lymphocyte count, suggesting rational therapeutic options for patients. l-arginine has been proposed to dampen the immunosuppressive function of MDSCs that help maintain endothelial function, platelet equilibrium and cardiovascular homeostasis[77], [78]. CD4+ and CD8+ T lymphocytes showed an increased rate of proliferation through arginine supplementation[57]. It has been proposed that IL-6 may induce emergency myelopoiesis leading to the generation of M−MDSCs and act as a prognostic indicator of poor disease outcomes[79]. IL-6 receptor inhibitors may upgrade HLA-DR expression, reduce the production of M−MDSCs in plasma, and bring clinical benefit to patients[80]. The authors implied that the raised proportion of MDSCs was coupled with the elevated serum level of IL-6, which is the hallmark of inflammation and provides advice for clinicians to recognize severe COVID-19 from moderate cases. It was not seen that administration of an IL-6 inhibitor brought about a reduction in MDSCs matched for age, sex, and disease severity[81]. Whether IL-6 antagonists directly affect MDSC frequency still needs to be fully investigated. 1α, 25-dihydroxyvitamin D3 has the potential to serve as an effective therapeutic strategy that inhibits the proliferation of MDSCs via the IL-6 signaling pathway[82]. The active form of vitamin D, 1α, 25-dihydroxyvitamin D3, lowers MDSC suppressive activity by 70 %, especially in the early phases of development[83]. Vitamin D suppresses miR-155 expression associated with MDSC activation[84]. Low dosages of zebularine, a DNA methylation blocker, downregulated the expression of IDO in colon cancer mice, suggesting that it might offer a possible therapeutic approach to COVID-19 patients[85]. A phosphodiesterase-5 antagonist has been demonstrated to inhibit the activity of various mediators, such as Arg-1 and iNOS, resulting in suppressing MDSC function[86], [87].
5.2. Targeting MDSC recruitment
A substantial number of MDSCs have been discovered in the lungs of nonsurvivors of COVID-19, suggesting that when infection develops, MDSCs are attracted to the site of infection to restrict infection. Diverse cytokines or chemokines, including IL-6, IL-10, TNF-α, and CCL2/5, stimulate the migration of MDSCs to sites of chronic inflammation[88]. MDSCs may interfere with T-cell expansion at infection sites in this phase. The efficacy of Leronlimab on the outcomes of COVID-19 is now the subject of several ongoing clinical investigations. Leronlimab, a CCR5 inhibitor, has been demonstrated to restore the CD4/CD8 ratio and resolve SARS-CoV-2 viremia[89]. Furthermore, elevated IL-8 levels were related to a high frequency of MDSCs. It is likely that IL-8 acts as a chemokine signal that contributes to the recruitment and mobilization of MDSCs and is a treatment alternative[45], [90]. Furthermore, as the PGE-COX-2 axis plays a crucial role in MDSC production and function, COX-2 inhibitors might reduce not only recruitment of MDSCs to afflicted areas but also rates of tumorigenesis[91], [92]. Ramatroban, a receptor antagonist of PGD2/DP2 signaling, is proposed as an immunotherapy for lymphopenia since PGD2 is required for MDSC upregulation via DP2 receptor signaling[93]. 5-Fluorouracil (5-FU) has been administered to boost the Th1-cell response in cancer and thus may provide clinical benefits for patients. An investigation demonstrated that 5-FU was able to lessen the number of MDSCs, which in turn led to a reduction in the production of IL-8, IL-10, IL-17, and IL-22 but an increase in the production of IFN-γ[81].
S100A8/A9 upregulates the expression of arginase and consequently raises immunosuppressive function, which may provide another rational therapeutic choice[39]. A S100A8/9 inhibitor, such as Paquinimod, reduced lung injury and viral load in mouse models[94]. In addition, a group of neutrophils was increased with an unrestrained immune response in the early stage of COVID-19, and administration of paquinimod diminished these neutrophils and restored antiviral responses. Paquinimod and leronlimab are offered as potential therapeutic approaches that require more research to validate their efficacy.
5.3. Reprogramming MDSCs
Prospective agents may be created to reprogram myeloid cells to treat hyperinflammation and the immune dysregulation that ensues. All-trans retinoic acid (ATRA) is commonly used to treat myeloid leukemia. It provides a glimmer of hope to COVID-19 patients, given its role in the induction of MDSC differentiation and ROS-neutralizing agent production[95]. ATRA has been demonstrated to prompt viral clearance in vitro[96].
Therapeutic strategies aimed at reprogramming MDSCs can be considered for the treatment of COVID-19. Metabolic factors are involved in MDSC-inducing T-cell anergy, such as fatty acid transport protein 2 (FATP2), suggesting a viable therapy option for COVID-19 patients[97]. The subsequently synthesized PGE2 has been recognized as a modulator of PMN-MDSCs. A few studies also implied a correlation between STAT3 signaling and MDSC function.
The function of MDSCs depends on the stage of disease, viral load, and illness severity. These cells may be protective in later stages by dampening unrestrained inflammation. However, the early expansion of MDSCs may compromise host immunity and cause lymphopenia. Concerning possible targets for MDSCs in COVID-19, the illness stage and severity must be specified. For this therapy strategy to be useful rather than harmful to the patients, identifying the disease’s stage and severity remains vital. It is difficult to determine whether the function of MDSCs affects disease outcomes.
6. Conclusions and perspectives
Due to the lack of specific antiviral agents, severe COVID-19 patients with lymphopenia and comorbidities still suffer from a high rate of mortality. It is necessary to determine rational approaches to elevate absolute lymphocyte counts and diminish the dysregulated immune response. MDSCs are related to the pathogenesis of lymphopenia and are responsible for the suppression of immunological defenses in the later phase of disease. High frequencies of PMN-MDSCs and M−MDSCs in the blood and their immunosuppressive function have been confirmed in severe COVID-19 patients. The data collected on MDSCs provides evidence for the potential application of treatment techniques aimed at lowering MDSC number/function.
The inhibiting impact of MDSCs on T lymphocytes was evaluated, but their potential impact on B lymphocytes was not investigated. Besides, there are no phenotypic markers that can distinguish these cells from classical neutrophils and monocytes. Further investigation is required to identify these cells with novel markers, which may facilitate better standardization and ultimately the identification of more targeted therapy targets. Several therapeutic approaches have been identified as rational methods underlying the pathological alteration of MDSCs. Certain cytokines such as IL-6 and CCL2 were shown to be elevated in line with the rise in the number and activity of MDSCs, suggesting that these cytokines were involved in the proliferation and activation of these cells. In fact, some randomized controlled trials with cytokine antagonists failed to demonstrate an increase in survival, underlining the requirement for their logical design and administration at the optimum time and dose. Personalized immunotherapy may bring a ray of hope for resolving the deficiency in host immunity. It is yet unclear whether short-term expansion of MDSCs is harmful to patients. Further studies are necessary to better our understanding of the mechanisms by which MDSCs contribute to lymphopenia during COVID-19.
Authorship.
T.L., wrote the original draft of the manuscript. F.Z. and F.C., revised the manuscript. All authors contributed to the critical revision of the manuscript and approved the final version.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81974426).
Data availability
No data was used for the research described in the article.
References
- 1.Wu C., et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Internal Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.W. H. O., WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int,2022(1 September, 2022).
- 3.Loftus T.J., Ungaro R., Dirain M., Efron P.A., Mazer M.B., Remy K.E., Hotchkiss R.S., Zhong L., Bacher R., Starostik P., Moldawer L.L., Brakenridge S.C. Overlapping but Disparate Inflammatory and Immunosuppressive Responses to SARS-CoV-2 and Bacterial Sepsis: An Immunological Time Course Analysis. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.792448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M., Psaltopoulou T., Gerotziafas G., Dimopoulos M.A. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., Lee M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou P., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azkur A.K., Akdis M., Azkur D., Sokolowska M., van de Veen W., Brüggen M.-C., O’Mahony L., Gao Y., Nadeau K., Akdis C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y., Liao W., Wan L., Xiang T., Zhang W. Correlation Between Relative Nasopharyngeal Virus RNA Load and Lymphocyte Count Disease Severity in Patients with COVID-19. Viral Immunol. 2021;34(5):330–335. doi: 10.1089/vim.2020.0062. [DOI] [PubMed] [Google Scholar]
- 9.Huang G., Kovalic A.J., Graber C.J. Prognostic Value of Leukocytosis and Lymphopenia for Coronavirus Disease Severity. Emerg. Infect. Dis. 2020;26(8):1839–1841. doi: 10.3201/eid2608.201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front. Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fathi N., Rezaei N. Lymphopenia in COVID-19: Therapeutic opportunities. Cell Biol. Int. 2020;44(9):1792–1797. doi: 10.1002/cbin.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang Z.-W., Zhang L.-J., Zhang S.-J., Meng X., Li J.-Q., Song C.-Z., Sun L., Zhou Y.-S., Dwyer D.E. A clinicopathological study of three cases of severe acute respiratory syndrome (SARS) Pathology. 2003;35(6):526–531. doi: 10.1080/00313020310001619118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicholls J.M., Poon L.L.M., Lee K.C., Ng W.F., Lai S.T., Leung C.Y., Chu C.M., Hui P.K., Mak K.L., Lim W., Yan K.W., Chan K.H., Tsang N.C., Guan Y., Yuen K.Y., Peiris J.S.M. Lung pathology of fatal severe acute respiratory syndrome. Lancet (London, England) 2003;361(9371):1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhan J., Deng R., Tang J., Zhang B., Tang Y., Wang J.K., Li F., Anderson V.M., McNutt M.A., Gu J. The spleen as a target in severe acute respiratory syndrome, FASEB journal : official publication of the Federation of American Societies for. Experimental Biology. 2006;20(13):2321–2328. doi: 10.1096/fj.06-6324com. [DOI] [PubMed] [Google Scholar]
- 15.Feng Z., Diao B., Wang R., Wang G., Wang C., Tan Y., Liu L., Wang C., Liu Y., Liu Y. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. MedRxiv. 2020 [Google Scholar]
- 16.Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z., Wu H., Lin Y., Zhang M., Zhang Q., Shi M., Liu Y., Zhou Y., Lan K., Chen Y. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouyang Y., Yin J., Wang W., Shi H., Shi Y., Xu B., Qiao L., Feng Y., Pang L., Wei F., Guo X., Jin R., Chen D. Downregulated Gene Expression Spectrum and Immune Responses Changed During the Disease Progression in Patients With COVID-19. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020;71(16):2052–2060. doi: 10.1093/cid/ciaa462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer K., Hoffmann P., Voelkl S., Meidenbauer N., Ammer J., Edinger M., Gottfried E., Schwarz S., Rothe G., Hoves S., Renner K., Timischl B., Mackensen A., Kunz-Schughart L., Andreesen R., Krause S.W., Kreutz M. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109(9):3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 19.Henry B.M., Aggarwal G., Wong J., Benoit S., Vikse J., Plebani M., Lippi G. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. The American journal of emergency medicine. 2020;38(9):1722–1726. doi: 10.1016/j.ajem.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.L. Wang, et al., Novel gene-specific translation mechanism of dysregulated, chronic inflammation reveals promising, multifaceted COVID-19 therapeutics, bioRxiv (2020).
- 21.Veglia F., Perego M., Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018;19(2):108–119. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Condamine T., Gabrilovich D.I. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32(1):19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar V., Cheng P.Y., Condamine T., Mony S., Languino L.R., McCaffrey J.C., Hockstein N., Guarino M., Masters G., Penman E., Denstman F., Xu X.W., Altieri D.C., Du H., Yan C., Gabrilovich D.I. CD45 Phosphatase Inhibits STAT3 Transcription Factor Activity in Myeloid Cells and Promotes Tumor-Associated Macrophage Differentiation. Immunity. 2016;44(2):303–315. doi: 10.1016/j.immuni.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obermajer N., Muthuswamy R., Lesnock J., Edwards R.P., Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118(20):5498–5505. doi: 10.1182/blood-2011-07-365825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiu B., Lin Y., Grote D.M., Ziesmer S.C., Gustafson M.P., Maas M.L., Zhang Z., Dietz A.B., Porrata L.F., Novak A.J., Liang A.B., Yang Z.Z., Ansell S.M. IL-10 induces the development of immunosuppressive CD14(+) HLA-DRlow/- monocytes in B-cell non-Hodgkin lymphoma. Blood Cancer J. 2015;5 doi: 10.1038/bcj.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson E.A., et al. Metabolic programs define dysfunctional immune responses in severe COVID-19 patients. Cell Rep. 2021;34(11) doi: 10.1016/j.celrep.2021.108863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schrijver I.T., Théroude C., Roger T. Myeloid-Derived Suppressor Cells in Sepsis. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bronte V., Brandau S., Chen S.-H., Colombo M.P., Frey A.B., Greten T.F., Mandruzzato S., Murray P.J., Ochoa A., Ostrand-Rosenberg S., Rodriguez P.C., Sica A., Umansky V., Vonderheide R.H., Gabrilovich D.I. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agrati C., et al. Expansion of myeloid-derived suppressor cells in patients with severe coronavirus disease (COVID-19) Cell Death Differ. 2020;27(11):3196–3207. doi: 10.1038/s41418-020-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazzoni A., et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. Journal of Clinical Investigation. 2020;130(9):4694–4703. doi: 10.1172/JCI138554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Condamine T., et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol. 2016;1(2) doi: 10.1126/sciimmunol.aaf8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H.R., Park S.M., Seo S.U., Jung I., Yoon H.I., Gabrilovich D.I., Cho B.C., Seong S.Y., Ha S.J., Youn J.I. The Ratio of Peripheral Regulatory T Cells to Lox-1(+) Polymorphonuclear Myeloid-derived Suppressor Cells Predicts the Early Response to Anti-PD-1 Therapy in Patients with Non-Small Cell Lung Cancer. Am J Respir Crit Care Med. 2019;199(2):243–246. doi: 10.1164/rccm.201808-1502LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coudereau R., Waeckel L., Cour M., Rimmele T., Pescarmona R., Fabri A., Jallades L., Yonis H., Gossez M., Lukaszewicz A.-C., Argaud L., Venet F., Monneret G., Grp R.S. Emergence of immunosuppressive LOX-1+PMN-MDSC in septic shock and severe COVID-19 patients with acute respiratory distress syndrome. J. Leukoc. Biol. 2022;111(2):489–496. doi: 10.1002/JLB.4COVBCR0321-129R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brudecki L., Ferguson D.A., McCall C.E., Gazzar M.E. Myeloid-derived suppressor cells evolve during sepsis and can enhance or attenuate the systemic inflammatory response. Infect. Immun. 2012;80(6):2026–2034. doi: 10.1128/IAI.00239-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su Y., Qiu Y., Qiu Z., Qu P. MicroRNA networks regulate the differentiation, expansion and suppression function of myeloid-derived suppressor cells in tumor microenvironment. J Cancer. 2019;10(18):4350–4356. doi: 10.7150/jca.35205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang M.M., Liu Q.F., Mi S.P., Liang X., Zhang Z.Q., Su X.M., Liu J.Y., Chen Y.Y., Wang M.M., Zhang Y.A., Guo F.H., Zhang Z.J., Yang R.C. Both miR-17-5p and miR-20a Alleviate Suppressive Potential of Myeloid-Derived Suppressor Cells by Modulating STAT3 Expression. J. Immunol. 2011;186(8):4716–4724. doi: 10.4049/jimmunol.1002989. [DOI] [PubMed] [Google Scholar]
- 37.Arunachalam P.S., et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science. 2020;369(6508):1210–1220. doi: 10.1126/science.abc6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schrijver I.T., Théroude C., Roger T. Myeloid derived suppressor cells sepsis. Front. Immunol. 2019;10(FEB) doi: 10.3389/fimmu.2019.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L., Chang E.W.Y., Wong S.C., Ong S.M., Chong D.Q.Y., Ling K.L. Increased Myeloid-Derived Suppressor Cells in Gastric Cancer Correlate with Cancer Stage and Plasma S100A8/A9 Proinflammatory Proteins. J. Immunol. 2013;190(2):794–804. doi: 10.4049/jimmunol.1202088. [DOI] [PubMed] [Google Scholar]
- 40.Jimenez-Cortegana C., Liro J., Palazon-Carrion N., Salamanca E., Sojo-Dorado J., de la Cruz-Merino L., Pascual A., Rodriguez-Bano J., Sanchez-Margalet V. Increased Blood Monocytic Myeloid Derived Suppressor Cells but Low Regulatory T Lymphocytes in Patients with Mild COVID-19. Viral Immunol. 2021;34(9):639–645. doi: 10.1089/vim.2021.0044. [DOI] [PubMed] [Google Scholar]
- 41.Silvin A., et al. Elevated Calprotectin and Abnormal Myeloid Cell Subsets Discriminate Severe from Mild COVID-19. Cell. 2020;182(6) doi: 10.1016/j.cell.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silvin A., et al. Elevated Calprotectin and Abnormal Myeloid Cell Subsets Discriminate Severe from Mild COVID-19. Cell. 2020;182(6):1401-+. doi: 10.1016/j.cell.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sacchi A., et al. Early expansion of myeloid-derived suppressor cells inhibits SARS-CoV-2 specific T-cell response and may predict fatal COVID-19 outcome. Cell Death Dis. 2020;11(10) doi: 10.1038/s41419-020-03125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Falck-Jones S., et al. Functional monocytic myeloid-derived suppressor cells increase in blood but not airways and predict COVID-19 severity. Journal of Clinical Investigation. 2021;131(6) doi: 10.1172/JCI144734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takano T., et al. Myeloid cell dynamics correlating with clinical outcomes of severe COVID-19 in Japan. Int. Immunol. 2021;33(4):241–247. doi: 10.1093/intimm/dxab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ou L., Shi Y., Dong W., Liu C., Schmidt T.J., Nagarkatti P., Nagarkatti M., Fan D., Ai W. Kruppel-Like Factor KLF4 Facilitates Cutaneous Wound Healing by Promoting Fibrocyte Generation from Myeloid-Derived Suppressor Cells. J, Invest. Dermatol. 2015;135(5):1425–1434. doi: 10.1038/jid.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostrand-Rosenberg S., Sinha P., Figley C., Long R., Park D., Carter D., Clements V.K. Frontline Science: Myeloid-derived suppressor cells (MDSCs) facilitate maternal–fetal tolerance in mice. J. Leukoc. Biol. 2017;101(5):1091–1101. doi: 10.1189/jlb.1HI1016-306RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Condamine T., Mastio J., Gabrilovich D.I. Transcriptional regulation of myeloid-derived suppressor cells. J. Leukoc. Biol. 2015;98(6):913–922. doi: 10.1189/jlb.4RI0515-204R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ostrand-Rosenberg S., Beury D.W., Parker K.H., Horn L.A. Survival of the fittest: how myeloid-derived suppressor cells survive in the inhospitable tumor microenvironment. Cancer Immunol. Immunother. 2020;69(2):215–221. doi: 10.1007/s00262-019-02388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thibaudin M., Fumet J.D., Bon M., Hampe L., Limagne E., Ghiringhelli F. Immunological features of coronavirus disease 2019 in patients with cancer, European journal of cancer (Oxford. England. 1990;139(2020):70–80. doi: 10.1016/j.ejca.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu G., Qi F.R., Li H.J., Yang Q.T., Wang H.Y., Wang X., Liu X.J., Zhao J.J., Liao X.J., Liu Y., Liu L., Zhang S.Y., Zhang Z. The differential immune responses to COVID-19 in peripheral and lung revealed by single-cell RNA sequencing. Cell Discovery. 2020;6(1) doi: 10.1038/s41421-020-00225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kusmartsev S., Gabrilovich D.I. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J. Immunol. 2005;174(8):4880–4891. doi: 10.4049/jimmunol.174.8.4880. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez P.C., Quiceno D.G., Ochoa A.C. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109(4):1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.P.C. Rodriguez, A.H. Zea, J. DeSalvo, K.S. Culotta, J. Zabaleta, D.G. Quiceno, J.B. Ochoa, A.C. Ochoa, L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes, Journal of immunology (Baltimore, Md : 1950) 171(3) (2003) 1232-1239. [DOI] [PubMed]
- 55.Cassetta L., Baekkevold E.S., Brandau S., Bujko A., Cassatella M.A., Dorhoi A., Krieg C., Lin A., Loré K., Marini O., Pollard J.W., Roussel M., Scapini P., Umansky V., Adema G.J. Deciphering myeloid-derived suppressor cells: isolation and markers in humans, mice and non-human primates. Cancer Immunol. Immunother. 2019;68(4):687–697. doi: 10.1007/s00262-019-02302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dean M.J., et al. Severe COVID-19 Is Characterized by an Impaired Type I Interferon Response and Elevated Levels of Arginase Producing Granulocytic Myeloid Derived Suppressor Cells. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.695972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reizine F., et al. SARS-CoV-2-Induced ARDS Associates with MDSC Expansion, Lymphocyte Dysfunction, and Arginine Shortage. J. Clin. Immunol. 2021;41(3):515–525. doi: 10.1007/s10875-020-00920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reizine F., et al. Beneficial effects of citrulline enteral administration on sepsis-induced T cell mitochondrial dysfunction. PNAS. 2022;119(8) doi: 10.1073/pnas.2115139119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agarwal U., Didelija I.C., Yuan Y., Wang X.Y., Marini J.C. Supplemental Citrulline Is More Efficient Than Arginine in Increasing Systemic Arginine Availability in Mice. J. Nutr. 2017;147(4):596–602. doi: 10.3945/jn.116.240382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munn D.H., Sharma M.D., Baban B., Harding H.P., Zhang Y.H., Ron D., Mellor A.L. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22(5):633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 61.Harden J.L., Egilmez N.K. Indoleamine 2,3-Dioxygenase and Dendritic Cell Tolerogenicity. Immunol Invest. 2012;41(6–7):738–764. doi: 10.3109/08820139.2012.676122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith C., Chang M.Y., Parker K.H., Beury D.W., DuHadaway J.B., Flick H.E., Boulden J., Sutanto-Ward E., Soler A.P., Laury-Kleintop L.D., Mandik-Nayak L., Metz R., Ostrand-Rosenberg S., Prendergast G.C., Muller A.J. IDO Is a Nodal Pathogenic Driver of Lung Cancer and Metastasis Development. Cancer Discovery. 2012;2(8):722–735. doi: 10.1158/2159-8290.CD-12-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mukund K., Nayak P., Ashokkumar C., Rao S., Almeda J., Betancourt-Garcia M.M., Sindhi R., Subramaniam S. Immune Response in Severe and Non-Severe Coronavirus Disease 2019 (COVID-19) Infection: A Mechanistic Landscape. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.738073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohl K., Tenbrock K. Reactive Oxygen Species as Regulators of MDSC-Mediated Immune Suppression. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.02499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hildeman D.A., Mitchell T., Kappler J., Marrack P. T cell apoptosis and reactive oxygen species. J Clin Invest. 2003;111(5):575–581. doi: 10.1172/JCI18007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Delgado-Roche L., Mesta F. Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch. Med. Res. 2020;51(5):384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.R.M. Bingisser, P.A. Tilbrook, P.G. Holt, U.R. Kees, Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway, Journal of immunology (Baltimore, Md : 1950) 160(12) (1998) 5729-5734. [PubMed]
- 68.S. Kusmartsev, D.I. Gabrilovich, STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion, Journal of immunology (Baltimore, Md : 1950) 174(8) (2005) 4880-4891. [DOI] [PubMed]
- 69.Ruan W.-S., Feng M.-X., Xu J., Xu Y.-G., Song C.-Y., Lin L.-Y., Li L., Lu Y.-Q. Early Activation of Myeloid-Derived Suppressor Cells Participate in Sepsis-Induced Immune Suppression via PD-L1/PD-1 Axis. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Venet F., Demaret J., Gossez M., Monneret G. Myeloid cells in sepsis-acquired immunodeficiency. Ann. N. Y. Acad. Sci. 2021;1499(1):3–17. doi: 10.1111/nyas.14333. [DOI] [PubMed] [Google Scholar]
- 71.Li H.Q., Han Y.M., Guo Q.L., Zhang M.G., Cao X.T. Cancer-Expanded Myeloid-Derived Suppressor Cells Induce Anergy of NK Cells through Membrane-Bound TGF-beta 1. J. Immunol. 2009;182(1):240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 72.Sacchi A., et al. Early expansion of myeloid-derived suppressor cells inhibits SARS-CoV-2 specific T-cell response and may predict fatal COVID-19 outcome. Cell Death Dis. 2020;11(10):921. doi: 10.1038/s41419-020-03125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Obermajer N., Kalinski P. Generation of myeloid-derived suppressor cells using prostaglandin E2. Transplantation Research. 2012;1(1):15. doi: 10.1186/2047-1440-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Veglia F., Sanseviero E., Gabrilovich D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021;21(8):485–498. doi: 10.1038/s41577-020-00490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Atanackovic D., et al. Deep dissection of the antiviral immune profile of patients with COVID-19. Communications Biology. 2021;4(1) doi: 10.1038/s42003-021-02852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao J.C., Zhao J.X., Perlman S. T Cell Responses Are Required for Protection from Clinical Disease and for Virus Clearance in Severe Acute Respiratory Syndrome Coronavirus-Infected Mice. J Virol. 2010;84(18):9318–9325. doi: 10.1128/JVI.01049-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sacchi A., Grassi G., Notari S., Gili S., Bordoni V., Tartaglia E., Casetti R., Cimini E., Mariotti D., Garotto G., Beccacece A., Marchioni L., Bibas M., Nicastri E., Ippolito G., Agrati C. Expansion of Myeloid Derived Suppressor Cells Contributes to Platelet Activation by L-Arginine Deprivation during SARS-CoV-2 Infection. Cells. 2021;10(8) doi: 10.3390/cells10082111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gambardella J., Khondkar W., Morelli M.B., Wang X., Santulli G., Trimarco V. Arginine and Endothelial Function. Biomedicines. 2020;8(8) doi: 10.3390/biomedicines8080277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang J., Jiang M.M., Chen X., Montaner L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020;108(1):17–41. doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giamarellos-Bourboulis E.J., et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020;27(6):992-+. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tomić S., Đokić J., Stevanović D., Ilić N., Gruden-Movsesijan A., Dinić M., Radojević D., Bekić M., Mitrović N., Tomašević R., Mikić D., Stojanović D., Čolić M. Reduced Expression of Autophagy Markers and Expansion of Myeloid-Derived Suppressor Cells Correlate With Poor T Cell Response in Severe COVID-19 Patients. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.614599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kloc M., Ghobrial R.M., Lipińska-Opałka A., Wawrzyniak A., Zdanowski R., Kalicki B., Kubiak J.Z. Effects of vitamin D on macrophages and myeloid-derived suppressor cells (MDSCs) hyperinflammatory response in the lungs of COVID-19 patients. Cell. Immunol. 2021;360 doi: 10.1016/j.cellimm.2020.104259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fleet J.C., Burcham G.N., Calvert R.D., Elzey B.D., Ratliff T.L. 1α, 25 Dihydroxyvitamin D (1,25(OH)D) inhibits the T cell suppressive function of myeloid derived suppressor cells (MDSC) J Steroid Biochem Mol Biol. 2020;198 doi: 10.1016/j.jsbmb.2019.105557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bruns H., Bottcher M., Qorraj M., Fabri M., Jitschin S., Dindorf J., Busch L., Jitschin R., Mackensen A., Mougiakakos D. CLL-cell-mediated MDSC induction by exosomal miR-155 transfer is disrupted by vitamin D. Leukemia. 2017;31(4):985–988. doi: 10.1038/leu.2016.378. [DOI] [PubMed] [Google Scholar]
- 85.Liu H., Xue Z.T., Sjogren H.O., Salford L.G., Widegren B. Low dose Zebularine treatment enhances immunogenicity of tumor cells. Cancer Lett. 2007;257(1):107–115. doi: 10.1016/j.canlet.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 86.Lin S.Y., Wang J., Wang L.H., Wen J., Guo Y.D., Qiao W.G., Zhou J.Q., Xu G.L., Zhi F.C. Phosphodiesterase-5 inhibition suppresses colonic inflammation-induced tumorigenesis via blocking the recruitment of MDSC. American Journal of Cancer Research. 2017;7(1):41–52. [PMC free article] [PubMed] [Google Scholar]
- 87.Serafini P., Meckel K., Kelso M., Noonan K., Califano J., Koch W., Dolcetti L., Bronte V., Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J. Exp. Med. 2006;203(12):2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu G., Qi F., Li H., Yang Q., Wang H., Wang X., Liu X., Zhao J., Liao X., Liu Y., Liu L., Zhang S., Zhang Z. The differential immune responses to COVID-19 in peripheral and lung revealed by single-cell RNA sequencing. Cell Discovery. 2020;6(1):73. doi: 10.1038/s41421-020-00225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patterson B.K., et al. CCR5 inhibition in critical COVID-19 patients decreases in fl ammatory cytokines, increases CD8 T-cells, and decreases SARS-CoV2 RNA in plasma by day 14. International Journal of Infectious Diseases. 2021;103:25–32. doi: 10.1016/j.ijid.2020.10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tobin R.P., Jordan K.R., Kapoor P., Spongberg E., Davis D., Vorwald V.M., Couts K.L., Gao D.X., Smith D.E., Borgers J.S.W., Robinson S., Amato C., Gonzalez R., Lewis K.D., Robinson W.A., Borges V.F., McCarter M.D. IL-6 and IL-8 Are Linked With Myeloid-Derived Suppressor Cell Accumulation and Correlate With Poor Clinical Outcomes in Melanoma Patients. Front. Oncol. 2019;9 doi: 10.3389/fonc.2019.01223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fujita M., Kohanbash G., Fellows-Mayle W., Hamilton R.L., Komohara Y., Decker S.A., Ohlfest J.R., Okada H. COX-2 Blockade Suppresses Gliomagenesis by Inhibiting Myeloid-Derived Suppressor Cells. Cancer Res. 2011;71(7):2664–2674. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sandler R.S., Halabi S., Baron J.A., Budinger S., Paskett E., Keresztes R., Petrelli N., Pipas J.M., Karp D.D., Loprinzi C.L., Steinbach G., Schilsky R. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N. Engl. J. Med. 2003;348(10):883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 93.Gupta A., Chander Chiang K. Prostaglandin D2 as a mediator of lymphopenia and a therapeutic target in COVID-19 disease. Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guo Q., et al. Induction of alarmin S100A8/A9 mediates activation of aberrant neutrophils in the pathogenesis of COVID-19. Cell Host Microbe. 2021;29(2):222–235.e224. doi: 10.1016/j.chom.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nefedova Y., Fishman M., Sherman S., Wang X., Beg A.A., Gabrilovich D.I. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 2007;67(22):11021–11028. doi: 10.1158/0008-5472.CAN-07-2593. [DOI] [PubMed] [Google Scholar]
- 96.Morita T., Miyakawa K., Jeremiah S.S., Yamaoka Y., Sada M., Kuniyoshi T., Yang J.W., Kimura H., Ryo A. All-Trans Retinoic Acid Exhibits Antiviral Effect against SARS-CoV-2 by Inhibiting 3CLpro Activity. Viruses-Basel. 2021;13(8) doi: 10.3390/v13081669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Veglia F., et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature. 2019;569(7754):73-+. doi: 10.1038/s41586-019-1118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.