Abstract
The glucan synthase complex of the human pathogenic mold Aspergillus fumigatus has been investigated. The genes encoding the putative catalytic subunit Fks1p and four Rho proteins of A. fumigatus were cloned and sequenced. Sequence analysis showed that AfFks1p was a transmembrane protein very similar to other Fksp proteins in yeasts and in Aspergillus nidulans. Heterologous expression of the conserved internal hydrophilic domain of AfFks1p was achieved in Escherichia coli. Anti-Fks1p antibodies labeled the apex of the germ tube, as did aniline blue fluorochrome, which was specific for β(1–3) glucans, showing that AfFks1p colocalized with the newly synthesized β(1–3) glucans. AfRHO1, the most homologous gene to RHO1 of Saccharomyces cerevisiae, was studied for the first time in a filamentous fungus. AfRho proteins have GTP binding and hydrolysis consensus sequences identical to those of yeast Rho proteins and have a slightly modified geranylation site in AfRho1p and AfRho3p. Purification of the glucan synthase complex by product entrapment led to the enrichment of four proteins: Fks1p, Rho1p, a 100-kDa protein homologous to a membrane H+-ATPase, and a 160-kDa protein which was labeled by an anti-β(1–3) glucan antibody and was homologous to ABC bacterial β(1–2) glucan transporters.
The fungal cell wall, which is specific and essential to fungal life, is mainly constituted of polysaccharides. Among all polysaccharides identified to date in the cell wall, β(1–3) glucans are the most prevalent, and they are present in all yeast and filamentous fungi investigated to date (14). Although β(1–3) glucan biosynthesis has been the subject of intensive research efforts for the last 30 years, the β(1–3) glucan biosynthetic pathway is not fully understood. It has been known since the early studies of Cabib and coworkers (22, 31, 35, 36) that β(1–3) glucans are synthesized from UDP glucose by a membrane protein complex, β(1–3) glucan synthase (EC 2.4.1.34; UDP-glucose/liter 1,3-β-d-glucan–3-β-d-glucosyltransferase). Synthesis occurs on the cytoplasmic side of the plasma membrane, and β(1–3) glucan chains are extruded towards the periplasmic space (15, 35). The glucan synthase complex has been characterized at the molecular level almost exclusively in the yeast Saccharomyces cerevisiae (5, 7, 12, 19, 29) and has been shown to be composed of two proteins: (i) the putative catalytic subunit Fksp, a large-molecular-size (>200 kDa) polypeptide with 16 transmembrane domains (12, 29, 30), and (ii) the regulatory subunit Rho1p, a small-molecular-size GTPase, which stimulates β(1–3) glucan synthase activity in its prenylated form (1, 11, 17, 18, 24, 28, 33).
If the β(1–3) glucan synthase has been extensively analyzed in yeast, then this enzymatic complex has been poorly studied in filamentous fungi. Only one FKS gene had been cloned and sequenced to date in Aspergillus nidulans (23), and neither has a regulatory partner been identified nor has the cellular localization of the glucan synthase complex been investigated.
This study was centered on the characterization of the glucan synthase complex of the filamentous fungus Aspergillus fumigatus, which is a human opportunistic pathogen of increasing importance in human health (26). Here we report (i) the cloning and sequencing of the FKS1 and RHO genes, (ii) the identification of the major proteins which coprecipitate with the Fks1p–Rho1p–β(1–3) glucan complex during product entrapment experiments, and (iii) the localization of the glucan synthase complex at the apices of hyphae.
MATERIALS AND METHODS
Strains and culture media.
Strains CBS 143.89 and 2965B2 were A. fumigatus clinical isolates. The strains were maintained on 2% malt agar slants. Mycelia for DNA extraction were grown for 18 h at 37°C in Sabouraud medium (2% glucose, 1% mycopeptone) (Biokar). Mycelia for glucan synthase assays were produced in the same medium in 2 liters of Biolafitte fermenter at 25°C for 16 h with an agitation of 500 rpm and an aeration of 0.5 liters of air/min (2). Escherichia coli strain DH5α (Biolabs) was used for cloning procedures with pBluescript SK(+) plasmid (Stratagene), and E. coli strain BL21 (Pharmacia) was used for expression with the pGEX4T vector (Pharmacia). Pichia pastoris strain SMD1168 (Invitrogen) was used for expression with the pPIC3 vector (Invitrogen).
Cloning procedures for A. fumigatus FKS1.
Approximately 50,000 recombinant plaques of an A. fumigatus genomic library in λEMBL3 (Stratagene) (a gift of M. Monod, CHUV, Lausanne, Switzerland) were immobilized on nylon membranes (Genescreen; Dupont NEN). These filters were probed with a [α-32P]dCTP-labeled 3.5-kb (KpnI-KpnI) fragment of S. cerevisiae FKS1, provided by A. F. J. Ram (Institute for Molecular Cell Biology, University of Amsterdam, Amsterdam, The Netherlands), under low-stringency hybridization conditions (hybridization and washings at 50°C) (32). Positive plaques were purified, and the DNA was isolated. Agarose gel electrophoresis of restricted recombinant bacteriophage, Southern blotting, and cloning of the positive bands in pBluescript SK(+) plasmid were performed according to standard protocols (34). cDNA of FKS1 was obtained by PCR using a λgt11 (Stratagene) A. fumigatus cDNA library (a kind gift of M. Monod) as template.
Cloning procedure for A. fumigatus RHO.
To clone RHO genes, degenerated oligonucleotide primers 5′-GG(TC)GA(TC)GG(TC)GC(TC)TG(TC)GG(TC)AA-3′ and 5′-TC(TC)TC(TC)TGGCCGGC(I)GT(GA)TCCCA(I)AG-3′ were designed based on conserved GTP binding and GTP hydrolysis sequences. Primers were used in PCR with the genomic DNA phage library of A. fumigatus as template. An amplified DNA fragment from A. fumigatus genomic DNA was cloned, sequenced, and subsequently used to screen the genomic library. cDNA of RHO genes were obtained by PCR using the λgt11 A. fumigatus cDNA library.
Sequencing and sequence analysis of A. fumigatus FKS1 and RHO genes.
Sequencing of FKS1 and RHO1 from genomic DNA and cDNA was performed at ESGS (Cybergène, Evry, France). DNA sequence data were analyzed using the University of Wisconsin Genetics Computer Group programs (10).
Southern blottings were performed to look for the presence of homologs of AfFKS1 in the A. fumigatus genome. A. fumigatus genomic DNA was digested with BamHI, ClaI, HindIII, and SalI, and the blot was probed with a HindIII fragment of the AfFKS1 gene (bases 1257 to 2354 from the genomic sequence) under low-stringency hybridization conditions (hybridization and washings at 42°C) (32).
Expression of AfFKS1.
The expression of the conserved hydrophilic internal region (IntF) of AfFKS1 was undertaken in E. coli strain BL21 using the expression plasmid pGEX4T1. The IntF fragment (nucleotides [nt] 2943 to 4219) was obtained by PCR using the primers Intgex1,
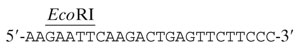 |
(nt 2943 to 2962), and Intgex2,
(antisense, nt 4201 to 4219), and the cDNA of AfFKS1. The PCR fragment was then digested by EcoRI-NotI. The IntF fragment was cloned into the EcoRI/NotI site of pGEX-4T1 in fusion with glutathione S-transferase (GST). After expression following the manufacturer's instructions (Pharmacia), E. coli producing IntF-GST was resuspended in STE buffer (10 mM Tris-HCl, 0.15 M NaCl, 1 mM EDTA) supplemented with 1 mg of lysozyme (Sigma) per ml. After 15 min at 0°C, the extract was sonicated for 1 min in the presence of 1.5% (vol/vol) Sarkosyl to separate the recombinant peptide from the inclusion bodies. After centrifugation at 13,000 × g, 2% (vol/vol) Triton X-100 was added to the supernatant. The solubilized IntF-GST was bound to glutathione-Sepharose 4B beads (Pharmacia) and purified by electroelution after electrophoresis on a 10% separating sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel.
Expression of AfRho1p and purification of recombinant Rho1p.
AfRHO1 cDNA with a CCAAG Kosak consensus sequence located immediately upstream of the ATG translation start and a six-His tag immediately downstream of the ATG start was obtained by PCR and was cloned in the P. pastoris intracellular expression vector pPIC3 at the BamHI/EcoRI site. Recombinant Rho1p was expressed in the P. pastoris SMD1168 strain. Recombinant yeasts were grown until saturation in buffered minimal glycerol medium-yeast extract (BMGY) (Invitrogen), and after 48 h of expression in the presence of methanol (BMMY) (Invitrogen), the P. pastoris yeasts were recovered by centrifugation, washed with water, and disrupted in a Braun MSK homogenizer using glass beads of 0.5-mm diameter for 5 min in a 50 mM sodium phosphate buffer, pH 7.5, containing 1 mM phenylmethylsulfonyl fluoride and 3% glycerol (PPG). Cell walls were removed by centrifugation for 10 min at 4,000 × g. The intracellular extract was centrifuged at 36,500 × g for 1 h. The supernatant (Rho1Sup) was stored at −80°C, and the pellet was resuspended in PPG supplemented with 2% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS). After 30 min of incubation at 4°C, the extract was centrifuged at 36,500 × g for 1 h, and the solubilized proteins (Rho1PSo1) were stored at −80°C. Recombinant Rho1p from Rho1Sup and Rho1Pso1 was purified on Ni2+ columns (Invitrogen) and was eluted with 50 mM histidine.
MMF of A. fumigatus.
A microsomal membrane pellet was recovered after 1 h of centrifugation at 36,500 × g at 4°C of the membrane extract as described previously (2) and was resuspended in EB (50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 0.2 M NaF, 1 M sucrose). This extract was stored under liquid nitrogen and named the microsomal membrane fraction (MMF).
Purification of the glucan synthase complex by product entrapment.
The A. fumigatus glucan synthase complex was purified by product entrapment by following the procedure described by Kelly et al. (23), with the following modifications: (i) solubilization of MMF extract was carried out at room temperature for 30 min with 0.5% polyoxyethylene ether W1 (W1) (Sigma) in EB containing 20 μM GTP-γ-S, and (ii) two incubations with 2 mM UDP glucose at 4°C for 10 min were performed. Solubilization of the proteins from the product entrapment pellet (PE pellet) was done in EB containing 0.1% W1, and centrifugation was done at 60,000 × g for 15 min at 4°C and was repeated once. Combined supernatants were referred to as the product entrapment fraction (PE). Nonspecific binding of W1-solubilized proteins to β(1–3) glucan was assessed by incubating the W1-solubilized MMF extract for 30 min at 25°C with β(1–3) glucan from the PE pellet digested with proteinase K (Sigma) (4 mg for 45 min at 56°C) to remove all proteins associated with the neosynthesized glucan. All fractions were analyzed by SDS-PAGE (25) after having dissolved the samples in reducing denaturing 4× SDS electrophoresis buffer at room temperature without heating.
PE proteins were identified by in-gel digestion after separation on SDS–8% PAGE and Coomassie blue staining followed either by separation of the resulting peptides by liquid chromatography and Edman sequencing as described earlier (4) or by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) analysis and tandem mass spectrometry (MS). For MS, proteins were digested by trypsin in a Progest robot (Abimed). After extraction, peptides were desalted on Ziptip micro columns (Millipore), and eluates either were mixed with MALDI matrix (saturated solution of dihydroxybenzoic acid) on the MALDI-TOF target of the Voyager DE-STR mass spectrometer (Applied Biosystems) or were infused by using the nanoelectrospray source kit (Protana A/S) for the Finnigan TSQ 7000 (Thermoquest). MALDI-TOF (MS) spectra were obtained by using an accelerating voltage of 21 kV in the reflector mode. MS-MS was done on doubly charged protonated molecules using argon (2.5 millitorr) as the collision gas at 20 to 38 eV. MALDI-TOF (MS) and MS-MS data were matched against the National Center for Biotechnology database using the MS-Fit and MS-Edman programs, respectively (assembled into the Protein Prospector package, P. R. Baker and K. R. Clauser, http://prospector.ucsf.edu), over an intranet connection.
ADP-ribosylation assay.
Ten microliters (50 to 100 μg) of MMF, solubilized in 0.3% CHAPS as described by Beauvais et al. (2), was incubated for 60 min at 37°C in the presence of a solution containing 20 μM GTP, 7.5 μM [32P]NAD+ (1.25 μCi), and 15 ng of C3 exoenzyme from Clostridium botulinum (Biomol) in a 60 mM HEPES buffer (pH 8) containing 1.5 mM MgCl2 and 1.5 mM 5′-AMP followed by SDS-PAGE with a 12% separating gel and by autoradiography.
Antibodies.
Purified recombinant Rho1 protein from Rho1Sup (420 μg) produced by P. pastoris and the IntF-GST fusion protein produced by E. coli (300 μg) and purified as described above were used as antigens to raise anti-AfrRho1p and anti-IntF antibodies, respectively, in rabbits (27). The presence of specific antibodies in the animals was verified by Western blotting by using the ECL chemiluminescence detection method of Amersham. Preimmune rabbit serum was collected and used as a control. Murine anti-β(1–3) glucan monoclonal antibody was from Biosupplies Australia (Victoria, Australia).
Localization of AfFks1p and β(1–3) glucan.
Immunolocalization of AfFks1p was attempted on germ tubes. Permeabilization and immunofluorescence studies were performed basically as described by Harris et al. (16), with the following modifications. Coverslips with germ tubes were incubated in phosphate-buffered saline (PBS) containing 5% goat serum (Sigma) (PBS-goat serum) for 1 h at room temperature (RT) before incubation in the anti-IntF or preimmune rabbit antisera diluted 1/20 for 1 h at RT. After being washed, labeling was performed using a goat anti-rabbit fluorescein isothiocyanate conjugate diluted 1/50 (Sigma) for 1 h at RT. Antisera and fluorescein isothiocyanate conjugates were diluted in PBS-goat serum, and all washings were done in PBS-goat serum. After the final PBS washing, germ tubes were observed under a fluorescent microscope with an excitation filter of 450 to 490 nm.
Localization of β(1–3) glucan was done by incubating germ tubes produced in liquid Sabouraud medium for 10 h at 37°C with aniline blue Water Blue (Fluka) at a concentration of 0.1 mg/ml in a phosphate buffer (50 mM, pH 8.5) for 1 h at RT.
Nucleotide sequence accession numbers.
DNA sequences of A. fumigatus FKS1, RHO1, RHO2, RHO3, and RHO4 are available in the GenBank database under the accession numbers U79728, AY007297, AY007298, AY007299, and AY007300, respectively.
RESULTS AND DISCUSSION
Cloning of A. fumigatus FKS1 and production of recombinant Fks1p fragment.
The genomic DNA sequence of the FKS1 optical reading frame (ORF) of A. fumigatus was 5,813 bp long and was interrupted by two introns located at the N terminus (nt 138 to 185) and the C terminus (nt 5466 to 5521). A TATA transcription sequence was found 233 nt upstream of the ATG site. AfFKS1 encoded a predicted protein of 1,903 amino acids with an estimated molecular size of 218 kDa and a pI of 8.17. The amino acid sequence of AfFKS1 was highly similar to other FKS genes present in the databases (37). Interestingly, it was almost identical to the A. nidulans FKS gene, with 90% amino acid identity, identical intron positions, and the presence of the two domains D1 and D2 in the conserved internal hydrophilic fragment homologous (44 and 29% identity, respectively) to the catalytic subunit of bacterial cellulose synthase of Acetobacter xylinum (accession number SP19449) (23). As with the other Fksp proteins, AfFks1p predicts an integral membrane protein with a cytoplasmic N terminus, has 16 transmembrane domains, and contains the putative UDP glucose binding consensus sequence RXTG at the C terminus (AfFks1p, RITG: amino acids [aa] 1565 to 1568) (20, 23, 29, 30, 38).
Southern analysis of genomic DNA isolated from A. fumigatus showed that only a single band hybridized to the HindIII 1.1-kb fragment of AfFKS1 under low stringency (data not shown), suggesting the absence of homologs of AfFKS1. A similar situation was found in Cryptococcus neoformans, where only a single FKS gene was found (38). Disruption of the FKS1 gene was also attempted in A. fumigatus. Transformation of A. fumigatus was performed with a pBluescript SK(+) plasmid containing the AfFKS1 gene interrupted by the pyrG gene or the phleomycin resistance gene. Among the 105 transformants tested, none showed the insertion of the disrupted gene at the AfFKS1 locus (data not shown), whereas disruption of more than 10 different nonessential genes in our laboratory using the same markers and transformation protocols always resulted in gene disruption at the correct locus, with double crossing over for 5 to 10% of the transformants (8, 9, 21; S. Paris, unpublished data; I. Mouyna, unpublished data). The essentiality of the FKS1 gene was recently confirmed (A. Firon, M. C. Grosjean-Cournoyer, A. Beauvais, and C. d'Enfert, Abstr. 21st Fung. Genet. Conf. Asilomar, abstr. 412, 2001) using a strategy previously used with A. nidulans (3) and adapted to A. fumigatus. The essentiality of the FKS gene was also shown in S. cerevisiae when FKS1 and FKS2 were both disrupted (29).
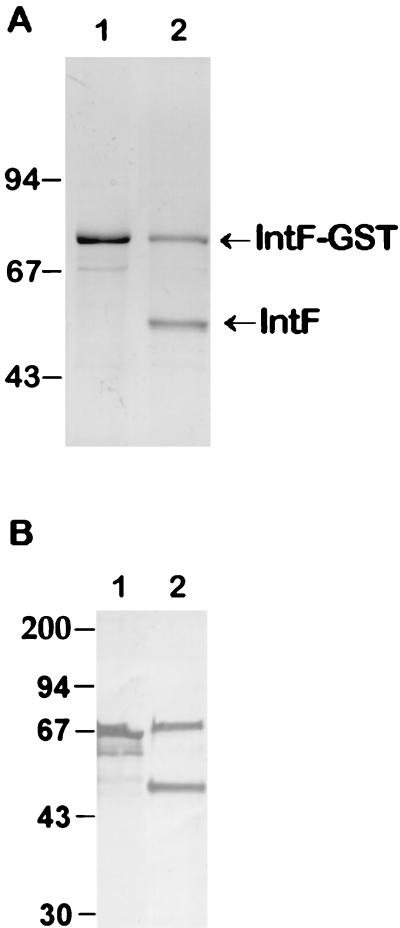
Attempts to produce the entire recombinant protein AfFks1p using the baculovirus expression system failed because transcription in the insect cell resulted in the production of a truncated mRNA (data not shown). All attemps to obtained heterologous expression of Fksp have, indeed, failed to date (M. Kurtz, personal communication). It was then decided to express part of the protein in E. coli. The conserved hydrophilic internal fragment (IntF) (aa 841 to 1265) of the A. fumigatus amino acid sequence was selected to be expressed in E. coli. A GST fusion protein with a molecular size of 74 kDa was produced which released a polypeptide of 48 kDa after human thrombin (Sigma) digestion (0.25 U of thrombin in PBS containing 2.5 mM CaCl2 for 1 h at RT), corresponding to the expected molecular size of IntF (Fig. 1). This protein was used to raise anti-AfFks1p antibodies in rabbit serum (anti-IntF antiserum). The N-terminal fragment (aa 1 to 387) could also be expressed in E. coli, but in contrast, expression of the C terminus was unsuccessful (aa 1441 to 1904) (data not shown).
FIG. 1.
Expression of IntF in E. coli (A) Coomassie blue staining of proteins after SDS-PAGE with a 10% separating gel. Lane 1, IntF-GST fusion protein; lane 2, IntF polypeptide after thrombin digestion (note that the digestion was incomplete with two polypeptides corresponding to the undigested IntF-GST and IngF). (B) Immunolabeling of IntF-GST and IntF with anti-IntF antibodies (1/1,000 dilution). Lanes 1 and 2 correspond to lanes 1 and 2 of panel A. No labeling was seen when lanes 1 and 2 of panel A were incubated with preimmune sera (not shown). The numbers at the left of each panel are molecular sizes in kDa.
Cloning of A. fumigatus RHO genes and production of recombinant AfRho1p.
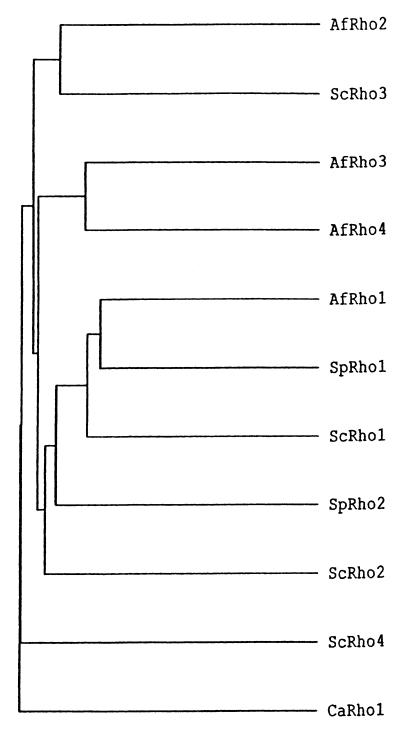
In A. fumigatus, four RHO genes (RHO1 through RHO4) were cloned. However, only RHO1 was studied, because it showed the highest amino acid homology to RHO1 of the yeast S. cerevisiae (Fig. 2).
FIG. 2.
Phylogenetic dendrogram of Rho proteins of A. fumigatus (AfRho1, AfRho2, AfRho3, AfRho4), S. cerevisiae (ScRho1, ScRho2, ScRho3, ScRho4), C. albicans (CaRho1), and S. pombe (SpRho1, SpRho2).
The RHO1 ORF was 947 bases long. The ORF was interrupted by four introns spanning nt 69 to 164, 193 to 314, 513 to 605, and 741 to 776. The cDNA (600 nt) coded for a protein of 21.5 kDa. The amino acid sequence of AfRho1p was highly homologous to that of Rho1p of Schizosaccharomyces pombe, S. cerevisiae, and Candida albicans, showing 85, 79, and 60% identity, respectively (Fig. 3). Amino acid sequences of Rho1p of A. fumigatus, S. cerevisiae, C. albicans, and S. pombe presented similar motifs for the GTP binding and GTP hydrolysis sites (Fig. 3). The recognition sequence for prenylation, CTIL, was also present at the C-terminal end of the protein but was different from those of yeast (Fig. 3) (18). In AfRho1p, the first aliphatic amino acid (a1) of the yeast consensus sequence Ca1a2L was replaced by the polar amino acid threonine. The same modification was found in AfRho3p, whereas in AfRho2p the sequence was identical to the one found in yeast. AfRHO4 was not fully sequenced.
FIG. 3.
Alignment of Rho1 proteins of A. fumigatus (AfRho1), S. pombe (SpRho1), S. cerevisiae (ScRho1), and C. albicans (CaRho1). GTP binding and GTP hydrolysis domains are indicated by stars and dots, respectively. Geranyl-geranylation domains of each Rho1p are underlined.
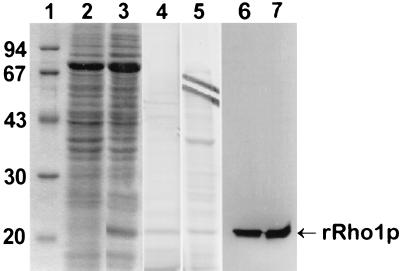
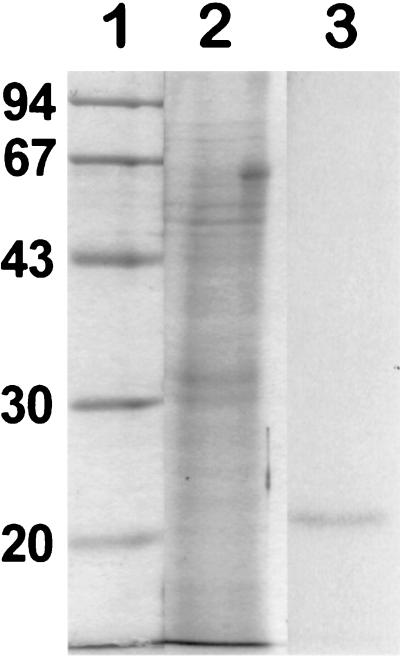
Recombinant AfRho1p was expressed in P. pastoris. A polypeptide of 21 kDa was found in the cell lysates (Fig. 4). This 21-kDa polypeptide was recovered in the cellular extract both in the Rho1Sup and in the Rho1PSo1 fractions. Recombinant Rho1p from Rho1Sup purified from a Ni2+ column (Fig. 4) was used to raise anti-AfrRho1p antibodies in rabbits. The antiserum specifically reacted with the recombinant AfRho1p (Fig. 4), whereas control membranes of P. pastoris were completely negative, indicating that the antiserum did not recognize Rho1p of P. pastoris (data not shown).
FIG. 4.
Recombinant Rho1p (rRho2p). P. pastoris proteins were separated by SDS-PAGE with a 12% separating gel followed by Coomassie blue staining of molecular size standards in kDa (lane 1), cell lysate of control P. pastoris without rRho1p (lane 2), cell lysate of P. pastoris expressing rRho1p after methanol induction (lane 3), and purified rRho1p from Rho1Sup (lane 4) and Rho1PSol (lane 5) fractions of recombinant P. pastoris. Lanes 6 and 7, immunolabeling of rRho1p from lanes 3 and 4, respectively, with the antiserum against the recombinant AfRho1p (1/15,000 dilution). No labeling was seen when the lysate shown in lane 2 was incubated with anti-AfrRho1p antibodies (not shown).
Analysis of the protein complex associated with newly synthesized β(1–3) glucans.
Incubation of the W1-solubilized membrane proteins of A. fumigatus with UDP glucose resulted in the isolation of a protein mixture which coprecipitated with the β(1–3) glucan synthesized and which still displayed a glucan synthase activity. In A. fumigatus, W1 detergent was an activator (1.5- to 3-fold increase in glucan synthase activity in the presence of 0.5% W1), whereas it inhibited the glucan synthase activity in A. nidulans (23). The product entrapment method achieved a 240-fold purification in one cycle of product entrapment (Table 1). Another cycle of entrapment did not result in further purification (data not shown). The proteins associated with β(1–3) glucan were investigated by immunoblotting using anti-IntF and anti-AfrRho1p antisera and by mass spectrometry.
TABLE 1.
Purification of β(1-3) glucan synthase from microsomal membranes of A. fumigatus by product entrapment
| Fraction | Total protein (mg) | Total activity (nmol/min) | Specific activity (nmol/min/mg) | Purification (fold) |
|---|---|---|---|---|
| Microsomal membranes | 74.7 | 194 | 2.6 | 1 |
| Solubilized membranesa | 39.2 | 325 | 8.3 | 3.2 |
| Product entrapment | 0.1 | 62 | 622 | 240 |
The extraction buffer contained Tris (50 mM), EDTA (1 mM), sucrose (1 M), NaF (0.2 M), and W1 (0.5%).
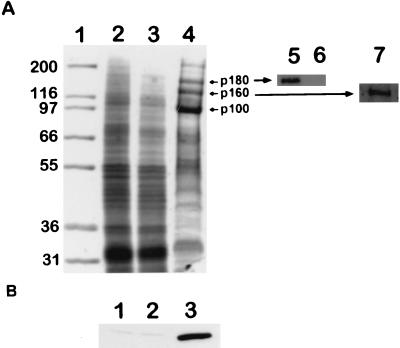
Antiserum against IntF strongly reacted with a protein in the product entrapment extract, migrating at 180 kDa (p180, Fig. 5A, lanes 4 and 6). MALDI-TOF (MS) and nanoelectrospray MS-MS confirmed that this band corresponded to AfFks1p. Immunolabeling of a membrane preparation prior to product entrapment with the same anti-IntF antiserum was negative even at high concentrations (1/40 dilution) (data not shown) (Fig. 5A, lanes 3 and 6). Similar enrichment of Fks1p was obtained in S. cerevisiae (33). Antiserum directed against anti-AfrRho1p reacted with a 21-kDa protein with the expected molecular size of AfRho1p in a membrane extract, and this protein was highly enriched in product entrapment (Fig. 5B). The identity and functionality of the 21-kDa protein were confirmed by ADP-ribosylation, which was specific for the Rho1 protein (5), using the C3 exoenzyme and [32P]NAD+ on membrane-solubilized proteins from A. fumigatus (Fig. 6).
FIG. 5.
Analysis of the β(1-3) glucan synthase complex after product entrapment. (A) Coomassie blue staining of proteins (15 μg of protein per lane) separated by SDS-PAGE with an 8% separating gel of molecular size standards in kDa (lane 1), MMF (lane 2), W1-solubilized MMF (lane 3), and PE (lane 4). Inserts show immunoblotting of PE (lane 5) and MMF (lane 6) with anti-IntF (1/1,000 dilution) and PE (lane 7) with an anti-β(1-3) glucan antibody (1/1,000 dilution). MMF remained negative after incubation with anti-IntF diluted 1/40 (not shown). (B) Immunoblotting with anti-AfrRho1p antibodies (1/15,000 dilution) of MMF (lane 1), solubilized MMF (lane 2), and PE (lane 3). A total of 2.6 μg of protein per lane after separation of the proteins by SDS-PAGE with a 12% separating gel was used.
FIG. 6.
ADP-ribosylation of Rho1p in A. fumigatus. Lane 1, molecular size standards in kDa; lane 2, Coomassie blue staining of CHAPS-solubilized MMF proteins separated by SDS-PAGE with a 12% separating gel; lane 3, autoradiography of CHAPS-solubilized MMF incubated with 7.5 μM [32P]NAD+ and 15 ng of C3 exoenzyme for 1 h at 37°C followed by SDS-PAGE (12% separating gel).
Two other proteins of large molecular size with apparent sizes of 100 and 160 kDa were enriched in the PE (p100 and p160, Fig. 5A, lane 4). These two proteins were only enriched when the glucan synthase was functional. Indeed, when a solubilized membrane preparation was incubated with β(1–3) glucan purified from the PE pellet treated with protease, the 100- and 160-kDa (as well as AfFks1p and AfRho1p) proteins were not found in the β(1– 3) glucan pellet recovered by centrifugation, indicating that the enrichment of these proteins did not result from their nonspecific binding to neosynthesized β(1–3) glucan. A similar electrophoretic gel pattern was obtained during PE purification of A. nidulans glucan synthase (23). Two peptide sequences, TVYFGEIGEK and KXGEMLVVLGRP, were obtained for the 160-kDa protein. They matched with the ABC transporter pmr1 of Penicillium digitatum (accession number AB010442), which showed approximately 40% similarity with two ABC bacterial transporters, chrA in Agrobacterium tumefaciens (13) and ndrA in Rhizobium meliloti (37), which are involved in the export of β(1–2) glucans in these organisms. This 160-kDa protein reacted with an anti-β(1–3) glucan antibody, suggesting that this protein was bound to glucan (Fig. 5A, lane 7). Peptides DNLGRKTRSKA, DSERLIHG, DEYTALNRYL, AREILSYN, KADEFARV, KYTPFDG, and KYQVVEMLQQ were obtained for the 100-kDa protein and were matched with a plasma membrane H+-ATPase of A. nidulans (accession number AF043332), migrating at 110 kDa in the PE extract SDS-PAGE gel from A. nidulans (23).
Cellular localization of glucan synthase.
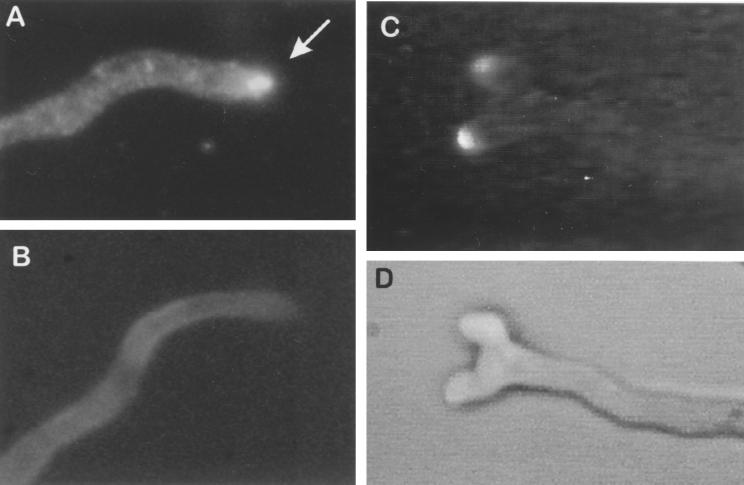
When aniline blue, a fluorochrome specific for β(1–3) glucans (6), was added to growing germ tubes, the apex of the germ tube was the point most intensively labeled by the aniline blue, indicating that the newly synthesized β(1–3) glucan was produced at the apex of the germ tube (Fig. 7). The apex also was positively labeled with the anti-IntF antiserum. This result showed that AfFks1p was localized at the apical growing region of the mycelium (Fig. 7). Similar results were found in yeasts where Fksp was localized in the bud (7).
FIG. 7.
(A and B) Immunofluorescence of a germ tube of A. fumigatus labeled with anti-IntF antibodies and goat anti-IgG fluorescein. (A) Anti-IntF antiserum, arrow indicates the apex of the germ tube; (B) preimmune serum. (C and D) Labeling of a germ tube of A. fumigatus with aniline blue fluorochrome. (C) Epifluorescence; (D) transmitted light.
ACKNOWLEDGMENTS
We thank H. Chaabihi (Quantum Biogene) and his collaborators for the construction of the expression AfFKS1 vector for the baculovirus expression system, J.-P. Le Caer at the Ecole Supérieure de Physique et de Chimie Industrielle, Paris, France, for conducting mass spectrometry experiments, and C. Fudali from Aventis Hoechst Marion Roussel and J. Dalayer from the Pasteur Institute for Edman sequencing experiments.
REFERENCES
- 1.Arellano M, Duran A, Pérez P. Rho1 GTPase activates the (1–3)β-d-glucan synthase and is involved in Schizosaccharomyces pombe morphogenesis. EMBO J. 1996;15:4584–4591. [PMC free article] [PubMed] [Google Scholar]
- 2.Beauvais A, Drake R, Ng K, Diaquin M, Latgé J P. Characterization of the 1,3-β-glucan synthase of Aspergillus fumigatus. J Gen Microbiol. 1993;139:3071–3078. doi: 10.1099/00221287-139-12-3071. [DOI] [PubMed] [Google Scholar]
- 3.Brookman J L, Denning D W. Molecular genetics in Aspergillus fumigatus. Curr Opin Microbiol. 2000;3:468–474. doi: 10.1016/s1369-5274(00)00124-7. [DOI] [PubMed] [Google Scholar]
- 4.Bruneau J M, Yea C M, Spinella-Jaegle S, Fudali C, Woodward K, Robson P A, Sautes C, Westwood R, Kuo E A, Williamson R A, Ruuth E. Purification of human dihydro-orotate dehydrogenase and its inhibition by A77 1726, the active metabolite of leflunomide. Biochem J. 1998;336:299–303. doi: 10.1042/bj3360299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabib E, Drgonova J, Drgon T. Role of small G proteins in yeast cell polarization and wall biosynthesis. Annu Rev Biochem. 1998;67:307–333. doi: 10.1146/annurev.biochem.67.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Currier H B, Stugger S. Aniline blue and fluorescence microscopy of callose in bulb scales of Allium cepa L. Protoplasma. 1956;45:552–559. [Google Scholar]
- 7.Delley P A, Hall M N. Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J Cell Biol. 1999;147:163–174. doi: 10.1083/jcb.147.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.d'Enfert C. Selection of multiple disruption events in Aspergillus fumigatus using the orotidine-5′-decarboxylase gene, pyrG, as a unique transformation marker. Curr Genet. 1996;30:76–82. doi: 10.1007/s002940050103. [DOI] [PubMed] [Google Scholar]
- 9.d'Enfert C, Fontaine T. Molecular characterization of the Aspergillus nidulans treA gene encoding an acid trehalase required for growth on trehalose. Mol Microbiol. 1997;24:203–216. doi: 10.1046/j.1365-2958.1997.3131693.x. [DOI] [PubMed] [Google Scholar]
- 10.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz M, Sanchez Y, Bennett T, Sun C R, Godoy C, Tamanoi F, Duran A, Perez P. The Schizosaccharomyces pombe cwg2+ gene codes for the β subunit of a geranylgeranyltransferase type I required for β-glucan synthesis. EMBO J. 1993;12:5245–5254. doi: 10.1002/j.1460-2075.1993.tb06220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douglas C M, Foor F, Marrinan J A, Morin N, Nielsen J B, Dahl A M, Mazur P, Baginsky W, Li W, El-Sherbeini M, Clemas J A, Mandala S M, Frommer B R, Kurtz M B. The Saccharomyces cerevisiae FKS1 (ETG1) gene encodes an integral membrane protein which is a subunit of 1,3-β-d-glucan synthase. Proc Natl Acad Sci USA. 1994;91:12907–12911. doi: 10.1073/pnas.91.26.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fath M J, Kolter R. ABC transporters: bacterial exporters. Microbiol Rev. 1993;57:995–1017. doi: 10.1128/mr.57.4.995-1017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontaine T, Mouyna I, Hartland R P, Paris S, Latgé J P. From the surface to the inner layer of the fungal cell wall. Biochem Soc Trans. 1996;25:194–199. doi: 10.1042/bst0250194. [DOI] [PubMed] [Google Scholar]
- 15.Girard V, Fèvre M. β-1-4- and β-1-3-glucan synthases are associated with the plasma membrane of the fungus Saprolegnia. Planta. 1984;160:400–406. doi: 10.1007/BF00429755. [DOI] [PubMed] [Google Scholar]
- 16.Harris S D, Morrele J L, Hamer J E. Identification and characterization of Aspergillus nidulans mutants defective in cytokinesis. Genetics. 1994;136:517–532. doi: 10.1093/genetics/136.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue S B, Qadota H, Arisawa M, Anraku Y, Watanabe T, Ohya Y. Signaling toward yeast 1,3-β-glucan synthesis. Cell Struct Funct. 1996;21:395–402. doi: 10.1247/csf.21.395. [DOI] [PubMed] [Google Scholar]
- 18.Inoue S B, Qadota H, Arisawa M, Watanabe T, Ohya Y. Prenylation of Rho1p is required for activation of yeast 1,3-β-glucan synthase. J Biol Chem. 1999;274:38113–38124. doi: 10.1074/jbc.274.53.38119. [DOI] [PubMed] [Google Scholar]
- 19.Inoue S B, Takewaki N, Takasuka T, Mio T, Adachi M, Fujii Y, Miyamoto C, Arisawa M, Furuichi Y, Watanabe T. Characterization and gene cloning of 1,3-β-d-glucan synthase from Saccharomyces cerevisiae. Eur J Biochem. 1995;231:845–854. doi: 10.1111/j.1432-1033.1995.tb20770.x. [DOI] [PubMed] [Google Scholar]
- 20.Ishiguro J, Saitou A, Duran A, Ribas J C. cps1+, a Schizosaccharomyces pombe gene homolog of Saccharomyces cerevisiae FKS genes whose mutation confers hypersensitivity to cyclosporin A and papulacandin B. J Bacteriol. 1997;179:7653–7662. doi: 10.1128/jb.179.24.7653-7662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaton-Ogay K, Paris S, Huerre M, Quadroni M, Falchetto R, Togni G, Latgé J P, Monod M. Cloning and disruption of the gene encoding an extracellular metalloprotease of Aspergillus fumigatus. Mol Microbiol. 1994;14:917–928. doi: 10.1111/j.1365-2958.1994.tb01327.x. [DOI] [PubMed] [Google Scholar]
- 22.Kang M S, Cabib E. Regulation of fungal cell wall growth: a guanine nucleotide-binding, proteinaceous component required for activity of (1-3)-β-d-glucan synthase. Proc Natl Acad Sci USA. 1986;83:5808–5812. doi: 10.1073/pnas.83.16.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly R, Register E, Hsu M J, Kurtz M B, Nielsen J B. Isolation of a gene involved in 1,3-β-glucan synthesis in Aspergillus nidulans and purification of the corresponding protein. J Bacteriol. 1996;178:4381–4391. doi: 10.1128/jb.178.15.4381-4391.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondoh O, Tachibana Y, Ohya Y, Arisawa M, Watanabe T. Cloning of the RHO1 gene from Candida albicans and its regulation of β-1,3-glucan synthesis. J Bacteriol. 1997;179:7734–7741. doi: 10.1128/jb.179.24.7734-7741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Latgé J P. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latgé J P, Moutaouakil M, Debeaupuis J P, Bouchara J P, Haynes K, Prévost M C. The 18-kilodalton antigen secreted by Aspergillus fumigatus. Infect Immun. 1991;59:2586–2594. doi: 10.1128/iai.59.8.2586-2594.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazur P, Baginsky W. In vitro activity of 1,3-β-d-glucan synthase requires the GTP-binding protein Rho1. J Biol Chem. 1996;271:14604–14609. doi: 10.1074/jbc.271.24.14604. [DOI] [PubMed] [Google Scholar]
- 29.Mazur P, Morin N, Baginsky W, El-Sherbeini M, Clemas J A, Nielsen J B, Foor F. Differential expression and function of two homologous subunits of yeast 1,3-β-d-glucan synthase. Mol Cell Biol. 1995;15:5671–5681. doi: 10.1128/mcb.15.10.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mio T, Adachi-Shimizu M, Tachibana Y, Tabuchi H, Inoue S B, Yabe T, Yamada-Okabe T, Arisawa M, Watanabe T, Yamada-Okabe H. Cloning of the Candida albicans homolog of Saccharomyces cerevisiae GSC1/FKS1 and its involvement in β1,3-glucan synthesis. J Bacteriol. 1997;179:4096–4105. doi: 10.1128/jb.179.13.4096-4105.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mol P C, Park H M, Mullins J T, Cabib E. A. GTP-binding protein regulates the activity of (1-3)-β-glucan synthase, an enzyme directly involved in yeast cell wall morphogenesis. J Biol Chem. 1994;269:31267–31274. [PubMed] [Google Scholar]
- 32.Monod M, Togni G, Hube B, Sanglard D. Multiplicity of genes encoding secreted aspartic proteinases in Candida species. Mol Microbiol. 1994;13:357–368. doi: 10.1111/j.1365-2958.1994.tb00429.x. [DOI] [PubMed] [Google Scholar]
- 33.Qadota H, Python C P, Inoue S B, Arisawa M, Anraku Y, Zheng Y, Watanabe T, Levin D E, Ohya Y. Identification of yeast Rho1p GTPase as regulatory subunit of 1,3-β-glucan synthase. Science. 1996;272:279–281. doi: 10.1126/science.272.5259.279. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Shematek E M, Braatz J A, Cabib E. Biosynthesis of the yeast cell wall. I. Preparation and properties of β(1-3)glucan synthetase. J Biol Chem. 1980;255:888–894. [PubMed] [Google Scholar]
- 36.Shematek E M, Cabib E. Biosynthesis of the yeast cell wall. II. Regulation of β(1-3)glucan synthetase by AP and GTP. J Biol Chem. 1980;255:895–902. [PubMed] [Google Scholar]
- 37.Stanfield S W, Ielpi L, O'Brochta D, Helinski D R, Ditta G S. The ndvA gene product of Rhizobium meliloti is required for β1(1→2)glucan production and has homology to the ATP-binding export protein HlyB. J Bacteriol. 1988;170:3523–3530. doi: 10.1128/jb.170.8.3523-3530.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson J R, Douglas C M, Li W, Jue C K, Pramanik B, Yuan X, Rude T H, Toffaletti D L, Perfect J R, Kurtz M B. A glucan synthase FKS1 homolog in Cryptococcus neoformans is single copy and encodes an essential function. J Bacteriol. 1999;181:444–453. doi: 10.1128/jb.181.2.444-453.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]