Abstract
Nonalcoholic fatty liver disease (NAFLD) is characterized by excessive accumulation of hepatic lipids and metabolic stress-induced liver injury. There are currently no approved effective pharmacological treatments for NAFLD. Traditional Chinese medicine (TCM) has been used for centuries to treat patients with chronic liver diseases without clear disease types and mechanisms. More recently, TCM has been shown to have unique advantages in the treatment of NAFLD. We performed a systematic review of the medical literature published over the last two decades and found that many TCM formulas have been reported to be beneficial for the treatment of metabolic dysfunctions, including Potentilla discolor Bunge (PDB). PDB has a variety of active compounds, including flavonoids, terpenoids, organic acids, steroids and tannins. Many compounds have been shown to exhibit a series of beneficial effects for the treatment of NAFLD, including anti-oxidative and anti-inflammatory functions, improvement of lipid metabolism and reversal of insulin resistance. In this review, we summarize potential therapeutic effects of TCM formulas for the treatment of NAFLD, focusing on the medicinal properties of natural active compounds from PDB and their underlying mechanisms. We point out that PDB can be classified as a novel candidate for the treatment and prevention of NAFLD.
Key words: Nonalcoholic fatty liver disease (NAFLD), Traditional Chinese medicine, Acupuncture, Potentilla discolor Bunge (PDB), Natural active compounds, Anti-oxidative, Anti-inflammatory, Lipid metabolism, Insulin resistance, Endoplasmic reticulum stress, Intestinal microflora
Graphical abstract
This review summarizes the therapeutic effects of traditional Chinese medicine, including herbal formulas and acupuncture, for treating NAFLD, and also emphasizes the medicinal properties of natural active compounds from Potentilla discolor Bunge and their underlying mechanisms.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD), recently also named as metabolic associated fatty liver disease1, is one of the leading causes of chronic liver diseases and one of the most prevalent metabolic disorders worldwide2. NAFLD comprises a series of liver abnormalities, ranging from simple hepatic steatosis to steatohepatitis, liver cirrhosis and hepatocellular carcinoma. Multiple conditions of the metabolic syndrome are regarded as the main risk factors of NAFLD, including obesity, dyslipidemia and type 2 diabetes (T2DM). The “multiple hits” hypothesis3 reveals that several hepatic insults act together in the pathogenesis of NAFLD. The mechanisms contributing to the development of NAFLD include hyperlipidemia, insulin resistance (IR), abnormal adipocyte stimulation, secretion of inflammatory mediators by immune cells and adipose tissue, oxidative stress, endoplasmic reticulum (ER) stress, dysregulation of intestinal microflora, disturbance in genetic and epigenetic functions, dysfunction of mitochondria and environmental and dietary factors4, 5, 6. Due to the complexity of the disease, no effective pharmacological treatments have been currently approved to treat NAFLD.
Nowadays, traditional Chinese medicine (TCM) has been recognized worldwide as a complementary and alternative therapy. Chinese herbs and their extracts have been identified as new sources of potential therapeutic agents for the prevention and treatment of NAFLD7. More specifically, many Chinese medicine formulas containing Potentilla discolor Bunge (PDB) have been found to play a beneficial role in the treatment of metabolic dysfunctions8. PDB was first described in the ‘Meteria Medica for Relief of Famines’, which is the earliest monograph of agronomy and botany of China published in the 14th–15th century9. PDB, growing in temperate zones and mountainous areas, is a dry grass of the Rosaceae species. There are 88 species of PDB in China, which are mainly produced in Shandong, Liaoning and Anhui provinces and are widely used in Hebei, Henan, Inner Mongolia and Hunan provinces10. Extracts of the aerial and underground parts of the plant have been used in formulations for the treatment of several diseases, including inflammations, wounds, cancers, infections induced by bacteria, fungi and viruses, diarrhoea and diabetes mellitus11. In this review, we discuss the medicinal properties of PDB and the underlying mechanisms of its active compounds for the treatment of NAFLD.
2. Therapeutic effects and mechanisms of TCM in treating NAFLD
2.1. Pathogenesis of NAFLD and current therapeutic targets
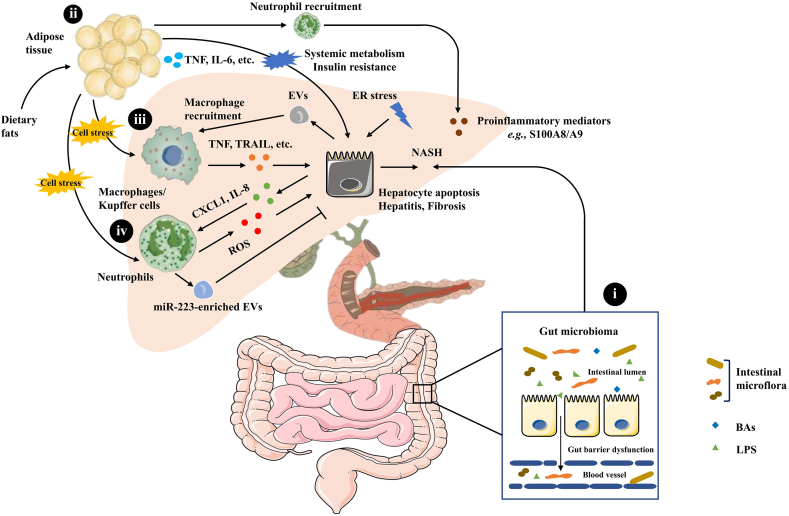
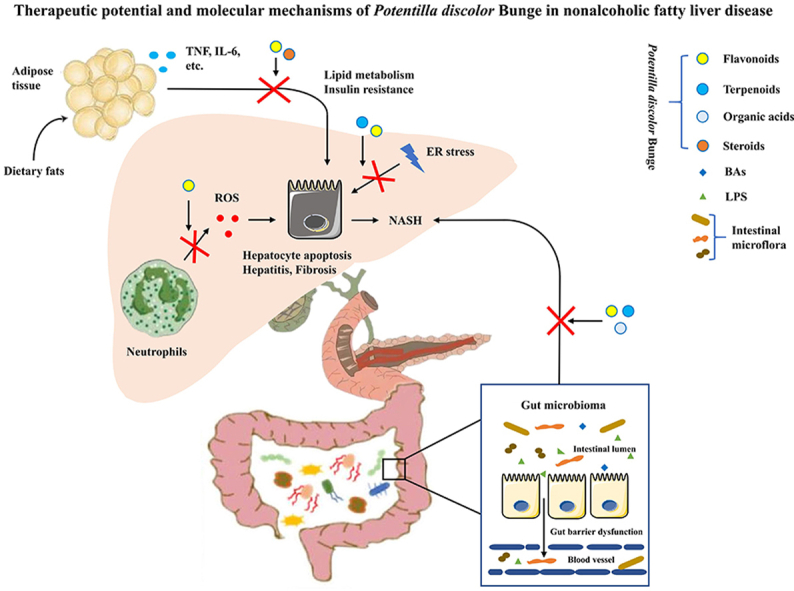
NAFLD is a major cause of liver-related morbidity worldwide, impacting nearly 25% of the global population12,13. The comprehensive inter-tissue crosstalk between the liver, the intestine, adipose tissue, and the nervous system plays a role in the development of NAFLD14,15. Also, the liver immune microenvironment, and particularly macrophages and neutrophils are involved in lipid accumulation and inflammation during NAFLD16 (Fig. 1).
Figure 1.
Pathogenesis of NAFLD: inter-tissue crosstalk between the liver, the intestine, and adipose tissue. (i) Gut barrier dysfunction and disruption of barrier integrity cause translocation of bacteria or bacterial products into the blood circulation, resulting in liver inflammation and the progression towards NASH. (ii) The intricate crosstalk between adipose tissue and the liver contributes to the progression of NAFLD. (iii) KCs produce TNF, TRAIL and FAS ligands through phagocytosis of apoptotic bodies, which subsequently promotes hepatocyte apoptosis, causing hepatitis and fibrosis. EVs released from hepatocytes contribute to hepatic recruitment of monocyte-derived macrophages. (iv) The up-regulation of hepatic chemokines CXCL1 and IL-8 and resulting infiltration of neutrophils are hallmarks of NASH.
2.1.1. Gut microbiome
Gut microbiota play a significant role in the pathogenesis of NAFLD. The gut microbiota is affected by environmental, dietary and host factors, such as gastro-intestinal anatomy and pH17. Gut barrier dysfunction and disruption of barrier integrity cause translocation of bacteria or bacterial products into the blood circulation, which is the essential condition for liver inflammation and the progression of NAFLD towards nonalcoholic steatohepatitis (NASH)18.
2.1.2. Crosstalk between adipose tissue and the liver
The intricate crosstalk between adipose tissue and the liver affects systemic metabolism and IR. Adipose tissue plays an important role in regulating NASH development by secreting adiponectin, leptin, tumor necrosis factor (TNF) and IL-619,20. In addition, some lipid moieties (palmitic acid, ceramide) released by adipocytes also hinder the function of the ER and mitochondria, which causes cell stress and even hepatocyte death21. Hepatocyte death is one of the crucial triggers of liver inflammation in NAFLD progression14. It has been recently found that E-selectin-mediated neutrophil recruitment promotes inflammation and lipolysis in adipose tissue, thereby inducing the release of free fatty acids and proinflammatory adipokines that exacerbate the steatosis-to-NASH progression22.
2.1.3. Macrophages
Liver-resident macrophages, also termed Kupffer cells (KCs), and recruited macrophages play a central role in the progression of NAFLD. KCs are the major source of cytokines and chemokines. KCs produce TNF, TNF-related apoptosis inducing ligand (TRAIL), and fatty acid synthase (FAS) ligands through phagocytosis of apoptotic bodies, which subsequently promotes hepatocyte apoptosis and causes hepatitis and fibrosis23. In addition, extracellular vesicles (EVs) released from hepatocytes contribute to key processes involved in the pathogenesis and progression of NAFLD24, 25, 26. The EVs can promote the expression of proinflammatory cytokines and polarize hepatic macrophages to the proinflammatory (M1) phenotype27, 28, 29. Mixed-lineage kinase 3 induces lipid-treated hepatocytes to release EVs containing C–X–C motif chemokine ligand 10 to recruit macrophages30. Moreover, EVs can contribute to hepatic recruitment of monocyte-derived macrophages31, which results in inflammation32. The identification of the pivotal molecules associated with the dynamic changes of macrophages could be crucial in the quest for novel therapeutic approaches against NAFLD.
2.1.4. Neutrophils
NASH, a more severe type of NAFLD, is accompanied by hepatocellular injury and ballooning with lobular inflammation in addition to lipid accumulation33. The hepatic upregulation of chemokines, including C–X–C motif chemokine ligand 1 (CXCL1) and interleukin (IL)-8, resulting in infiltration of neutrophils in the liver are hallmarks of NASH34, 35, 36. Hepatic overexpression of Cxcl1 is sufficient to drive steatosis-to-NASH progression in high fat diet (HFD)-fed mice through neutrophil-driven reactive oxygen species (ROS) and activation of stress kinases. This can be reversed by IL-22 treatment via the induction of metallothionein37.
In addition, neutrophil-specific microRNA-223 (miR-223) is elevated in hepatocytes and limits NASH progression in obese mice38. This elevation of miR-223 in hepatocytes is due to preferential uptake of miR-223-enriched EVs mainly derived from neutrophils. Once internalized by hepatocytes, the EV-derived miR-223 acts to inhibit hepatic inflammatory and fibrogenic gene expression39.
2.2. Potential therapeutic effects of TCM for treating NAFLD
TCM has been widely used in China and other Asian countries for thousands of years. TCM formulas are developed under the guidance of TCM theory. The therapeutic effects of TCM on NAFLD have been gradually reported in clinical practices, leading to an increased recognition. TCM discriminates between different types of syndromes in different patients with NAFLD, and therefore, diverse prescriptions and treatments are administered to different patients, based on the four properties of Chinese medicinal herbs (cold, hot, warm, cool), five flavors (sour, bitter, sweet, spicy, salty) and efficiency40.
2.2.1. The classical formulas of TCM for the treatment of NAFLD
Based on clinical experience, the pathogeneses of NAFLD can be summarized as the deficiency of spleen, dampness-heat, phlegm and stasis, cold coagulation and qi-stagnation. The syndromes in patients with NAFLD can be classified into the following types41: (i) spleen-deficiency and phlegm-turbid stagnation; (ii) stagnation of liver-qi; (iii) accumulated damp-heat; (iv) stasis blocking channels and (v) deficiency of liver and kidney. According to these TCM syndromes, the treatment principle and the relevant classical formulas to treat NAFLD are as follows: (i) formulas for invigorating spleen, removing dampness and phlegm: shenlingbaizhu powder (Tai Ping Hui Min He Ji Ju Fang)42,43, simiao powder (Cheng Fang Bian Du)44, sanziyangqin decoction (Han Shi Yi Tong)45; (ii) formulas for relieving liver and regulating qi: xiaochaihu decoction (Shang Han Lun)46, chaihushugan powder (Jingyue Complete Library)47, 48, 49; (iii) formulas for clearing heat, promoting dampness and dispersing knot: dachaihu decoction (Treatise on Febrile Disease)50,51, yinchenhao decoction (Shang Han Lun)52; (iv) formulas for promoting blood circulation and dissipating blood stasis: taohongsiwu decoction (The Golden Mirror of Medicine)53; (v) formulas for warming yng and invigorating spleen: chaihulizhong decoction (Shang Han Lun)54, lingguizhugan decoction (Jin Gui Yao Lue)55, 56, 57, fuzilizhong decoction (San Yin Ji–Bing Zheng Fang Lun)58, sini powder (Shang Han Lun)59, 60, 61, ganjianglingzhu decoction (Jin Gui Yao Lue)62. The regulatory effects and relevant mechanisms of the classical formulas of TCM treating NAFLD are shown in Table 142, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62 and Fig. 2.
Table 1.
Treatment principles and effects of the classical TCM formulas for the treatment of NAFLD.
| Treatment principle | Chinese medicinal formula | Model | Effects of TCM treating NAFLD | Ref. |
|---|---|---|---|---|
| Invigorate spleen, remove dampness and phlegm | Shenlingbaizhu powder (SLBZS) | HFD-induced NAFLD rats CDAA-fed rats |
↓ Hepatic injury ↓ Lipid accumulation ↓ The serum level of endotoxin, TNF-α, IL-1β ↓ TLR4, TRAF6 in the liver tissue ↑ The abundance of intestinal microbiota ↑ The abundance of short-chain fatty acid ↑ Adiponection ↓ SREBP-1c, FAS |
42, 43 |
| Simiao powder (SMS) | HFHS-induced NAFLD mice | ↓ Acly, Fas, Acc, Scd1 ↓ IL-1β, NLRP-3 ↓ The biosynthesis of fatty acids ↑ Insulin secretion pathway ↑ Gut microbiota composition |
44 | |
| Sanziyangqin decoction (SZYQT) | HFD-induced NAFLD mice | ↓ Hepatosteatosis ↓ TNF-α ↓ Inflammatory cell infiltration in liver tissues ↑ Insulin resistance ↑ p-AKT; ↓ apoptosis |
45 | |
| Relieve liver and regulate Qi | Xiaochaihu decoction (XCHT) | Patients with NAFLD | ↑ Lipid metabolism | 46 |
| Chaihushugan powder (CHSGS) | HFD-induced NAFLD/NASH rats High fat and sugar emulsion-induced NAFLD rats |
↓ Enterobacteriaceae, Staphylococcaceae families and Veillonella genus ↑ Anaeroplasma genus ↓ Fat accumulation ↓ Inflammatory factors (TNF-α, IL-1β, IL-18, IL-6) ↓ NLRP3, ASC, CASPASE-1 ↓ TLR4, p-p38 MAPK ↑ Adiponectin; ↓ leptin |
47, 48, 49 | |
| Clear heat, promote dampness and disperse knot |
Dachaihu decoction (DCHT) | Patients with NAFLD High-fat high-fructosediet-induced NAFLD rats |
↓ TNF-α, TGF-β, NF-κB, TLR4 | 50, 51 |
| Yinchenhao decoction (YCHT) | HFD-induced NASH rats | ↓ TNF-α | 52 | |
| Promote blood circulation and dissipate blood stasis | Taohongsiwu decoction (THSWT) | HFHC-induced NAFLD mice | ↓ Hepatic lipid accumulation ↓ C/EBPα, PPARγ, pAMPK; ↑ IRS-1, pAKT ↓ The ratio of BAX to BCL-2 expression |
53 |
| Warm Yang and invigorate spleen | Chaihulizhong decoction (CHLZT) | HFD-induced NAFLD rats A long chain fat emulsion-treated HepG2 cells |
↑ AMPKα, PPAR-γ; ↓ ACC-α, p-ACC-α, SREBP2, HMGR |
54 |
| Lingguizhugan decoction (LGZGT) | HFD-induced NAFLD rats HFD-induced NAFLD mice |
↓ GS, ACC, SREBP-1c, HMGCR ↑ PYGL activity ↓ Hepatosteatosis ↑ Insulin resistance; ↑ Oxygen consumption rate ↓ The expression and protein abundance of lipogenic genes in the liver ↑ Gut microbiota |
55, 56, 57 | |
| Fuzilizhong decoction (FZLZT) | HFD-induced NAFLD rats | ↑ IL-10, IFN-α, IFN-β ↑ p53 signaling; ↓ PPARγ signaling |
58 | |
| Sini powder (SNS) | Stress-induced NAFLD rats HFD-induced NAFLD mice |
↓ Psychological stress ↓ TNF-α ↑ Gut microbiota |
59, 60, 61 | |
|
Ganjianglingzhu decoction (GJLZT) |
HFD-induced NAFLD rats | ↓ CPT1B, rno-miR-138-5p | 62 |
HFD, high-fat diet; CDAA, choline-deficient amino acid-defined diet; HFHS, high fat/high sucrose diet; HFHC, high-fat and high-cholesterol diet; TLR4, toll-like receptor 4; TRAF6, TNF receptor associated factor 6; SREBP-1c, sterol regulatory element binding protein-1c; FAS, fatty acid synthase; Acly, ATP citrate lyase; Acc, acetyl-CoA carboxylase; Scd1, stearoyl-CoA desaturase 1; IL-1β, interleukin-1β; NLRP-3, NLR family containing pyrin domain protein 3; p-AKT, phospho-protein kinase B; TNF-α, tumor necrosis factor-α; CASPASE-1, cysteinyl aspartate specific proteinase; p38 MAPK, mitogen activated protein kinases with molecular weight of 38 kD; TGF-β, transforming growth factor-β; PPAR-γ, peroxisome proliferator-activated receptor-γ; C/EBPα, CCAAT/enhancer binding proteins alpha; IRS-1, insulin receptor substrate 1; BAX, protein of BCL2 associated x; BCL-2, B-cell lymphoma-2; SREBP2, sterol regulatory element binding protein-2; HMGR, 3-hydroxy-3-methyl glutaryl coenzyme A reductase; GS, glycogen synthase; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase; PYGL, glycogen phosphorylase liver type; IL-10, interleukin-10; IFN-β, interferon-β; CPT1B, carnitine palmitoyltransferase.
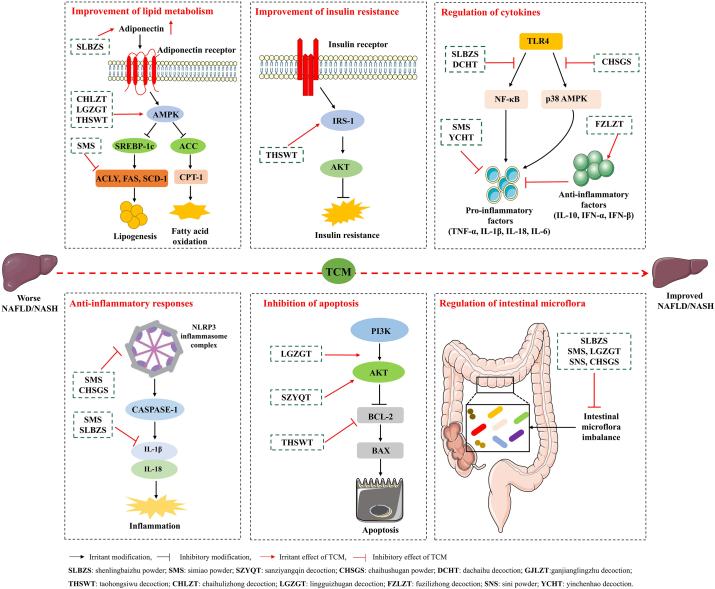
Figure 2.
Mechanisms of the classical TCM formulas for the treatment of NAFLD. The classical formulas of TCM exhibit a series of beneficial effects for the treatment of NAFLD, including improvement of lipid metabolism and IR, regulation of cytokines, anti-inflammatory responses, inhibition of apoptosis, and regulation of intestinal microflora.
2.2.2. Acupuncture for the treatment of NAFLD
Acupuncture, which is a classical TCM method, has been used to treat NAFLD during clinical practice. The safety profile of acupuncture therapy for the treatment of NAFLD is satisfactory. Taichong (LR3), Zusanli (ST36), Fenglong (ST40), and Sanyinjiao (SP6) are the major acupoints63. Electroacupuncture combined with lifestyle control can effectively treat patients with NAFLD by reducing serum leptin levels, increasing the sensitivity of hepatocytes to insulin and improving IR ameliorating blood glycolipid metabolism and reducing hepatic fat, waist circumference and waist-to-hip ratio64, 65, 66. In addition, acupuncture has been shown to ameliorate NAFLD by regulating lipid metabolism67, 68, 69, 70, improving IR71 and ER stress70,72,73, alleviating oxidative stress68,74, inhibiting the expression of inflammatory cytokines68,69,75, and alleviating steatosis, necrosis and inflammatory cell infiltration of liver tissue in NAFLD rat model. Furthermore, acupuncture can alleviate bullous steatosis of liver tissue76, 77, 78, and the expansion and disorder of rough endoplasmic reticulum73 in NAFLD rat model.
3. Beneficial effects of Chinese medicinal formulas containing PDB
According to the Compendium of Materia Medica, PDB has the effects of “clearing heat and cooling blood, detoxification, hemostasis and detumescence”. An increasing number of studies show that many formulas of TCM containing PDB exert beneficial effects for the treatment of metabolic, inflammatory and hematologic diseases. In Table 279, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116 (patient clinical data) and Table 3117, 118, 119, 120, 121, 122, 123, 124 (animal models), we summarize Chinese herbal products containing PDB and their medical application.
Table 2.
Beneficial effects of Chinese medicinal formulas containing PDB in the treatment of patients.
| Disease | Chinese medicinal formula | Composition of herbal mixture | Ref. |
|---|---|---|---|
| T2DM | Fanbaicao mixture | PDB, Corn Silk | 79 |
| Fanbaicao decoction | PDB, Rubus idaeus L, Astragalus mongholicus, Ophiopogon japonicus, Radix Pseudostellariae, Dioscorea opposita Thunb, Polygonatum sibiricum, Salvia miltiorrhiza Bge, Corn Silk, Ilex pubescens Hook, Rehmannia glutinosa, Chinese wolfberry, Dendrobium nobile Lindl, Rehmannia glutinosa, Cornus officinalis | 80 | |
| Jiulongjiangtang decoction | Gentian, Astragalus mongholicus, Poria cocos, Salvia miltiorrhiza Bge, PDB,Atractylodes lancea, Agrimonia pilosa Ledeb, Pueraria lobata, Codonopsis pilosul, Rehmannia glutinosa, Rhizoma Dioscoreae, Schisandra chinensis, Anemarrhena asphodeloides Bunge, Cornus officinalis | 81 | |
| Jiangtangzengmin decoction | Pueraria lobata, Astragalus mongholicus, Codonopsis pilosula, Atractylodes macrocephala, PDB, Lotus leaf, Poria cocos, Salvia miltiorrhiza Bge, Coptis chinensis Franch, Licorice | 82,83 | |
| Zengmin decoction | Euonymus alatus (Thunb.) Sieb, PDB, Trichosanthin, Dioscorea opposita Thunb, Raw Astragalus, Coptis chinensis Franch, Anemarrhena asphodeloides Bunge, Laminaria, Asparagus root, Ophiopogon japonicus, Chinese wolfberry root-bark, Dendrobium nobile Lindl, Polygonatum odoratum | 84 | |
| Yidaozengmin decoction | Radix Bupleuri, Fructus aurantii, Coptis chinensis Franch, Codonopsis pilosula, Atractylodes macrocephala Koidz, Poria cocos, Lotus leaf, Salvia miltiorrhiza Bge, PDB,Pueraria lobata, Licorice | 85 | |
| Xiaokekang No.2 decoction | Atractylodes lancea, Atractylodes macrocephala, Pinellia ternata, Pericarpium Citri Reticulatae, Coptis chinensis, Scutellaria baicalensis, PDB, Radix Scrophulariae, Radix puerariae, Litchi seed | 86 | |
| Qiyupingtang decoction | Astragalus membranaceus, Cornus officinalis, Rehmannia glutinosa, Lilium browmies Thunb, Trichosanthin, Wolfberry, PDB, Cortex rehmanniae, Schisandra chinensis | 87 | |
| Tangniaoning decoction | PDB,Salvia miltiorrhiza, Pangolin, Dalbergia odorifera, Achyranthes bidentata, Astragalus, Atractylodes macrocephala, Pueraria lobata, Sophora flavescens, Coptis chinensis, Bamboo shavings, Trichosanthin | 88 | |
| Antang capsule | Astragalus, Cornus officinalis, Salvia miltiorrhiza, PDB, etc. | 89 | |
| Kuhuang capsule | Bitter melon, Coptis, Pueraria, PDB | 90 | |
| Baihuangjiangtang granule | PDB, Raspberry, Astragalus membranaceus, Ophiopogon japonicus, Pseudostellaria heterophylla, Dioscorea opposita, Polygonatum, Salvia miltiorrhiza, Stigma maydis, Ilex pubescens, Medlar, Dendrobium, Rehmannia glutinosa, Cornus officinalis | 91 | |
| Jiedufuyang decoction | Honeysuckle, PDB, Coptis chinensis, Epimedium, Cynomorium songaricum, Morinda officinalis, Licorice | 92 | |
| Yiqiyangyinhuoxue decoction | Astragalus membranaceus, Epimedium, PDB, Radix paeoniae alba, Radix rehmanniae, Fructus mume, Rhizoma atractylodes, Radix Scrophulariae, Radix puerariae, Radix salviae miltiorrhizae, Radix glycyrrhiza | 93 | |
| Yiqiyangyinqingre decoction | Astragalus, Dioscorea opposita Thunb, Pueraria, Ophiopogon japonicus, Radix rehmanniae, Codonopsis pilosula, Coptis chinensis, PDB, Schisandra chinensis, Cortex rehmanniae, Anemarrhena asphodeloides, Cassia obtusifolia | 94 | |
| Diabetic nephropathy | Yiqijianpihuayu decoction | Raw Astragalus, Salvia miltiorrhiza, PDB, Dioscorea opposita Thunb, Codonopsis pilosula, Leech, Radix rehmanniae, Peach kernel, Atractylodes macrocephalae, Arctium lappa, Angelica sinensis, Rhubarb | 95,96 |
| Yiqihuayu decoction | Raw Astragalus, Leech, Dioscorea opposita Thunb, Codonopsis pilosula, PDB, Radix rehmanniae, Rhizoma atractylodis macrocephalae, Angelica sinensis, Salvia miltiorrhiza, Eupatorium adenophorum, Earthworm and rhubarb | 97 | |
| Yiqihuoxue decoction | Raw Astragalus, Radix Codonopsis, PDB, Cornus officinalis, Chinese yam, Radix rehmanniae, Rhizoma atractylodismacrocephalae, Angelica sinensis, Salvia miltiorrhiza, Eupatorium adenophorum, peach kernel, Safflower and rhubarb | 98 | |
| Tangshenkang mixture | Astragalus membranaceus, Codonopsis pilosula, Angelica sinensis, Radix paeoniae rubra, Rhizoma Chuanxiong, Salvia miltiorrhiza, Peach kernel, Leech, Rehmannia glutinosa, Cornus officinalis, Achyranthes bidentata, Raspberry, Euryale ferox seed, PDB, Honeysuckle, Licorice | 99 | |
| Diabetic peripheral neuropathy | Fanbaicao capsule | PDB, Astragalus membranaceus, Leech, Dioscorea opposita Thunb | 100 |
| Diabetic limb arterial occlusion | Fandihuanwu decoction | PDB, Chinese wolfberry root-bark, Angelica tail, Astragalus, Peach kernel, Dragon, Radix paeoniae rubra, Ligusticum wallichii, Carthamus tinctorious, Achyranthes bidentata | 101, 102 |
| Chronic nephritis with proteinuria | Jiangbai decoction | Lysimachia christinae, Hedyotis diffusa, PDB, Plantain, Tripterygium wilfordii, Cuscuta, Cornus corni, Dried lotus, Cherry, Thicken, Salvia miltiorrhiza, Motherwort, Astragalus, Poria cocos, Atractylodes | 103, 104 |
| Chronic hepatitis B | Medicine of the yao nationality (no compound name) | Acanthopanax, Hypericum japonicum Thunb, Dicliptera chinensis, Ardisia mamillata Hance, Aralia elata, Hugen, Camellia, Sapium sebiferum, Blumea megacephala, Guidianhuo, Selaginella uncinata(Desv.) spring, Melicope pteleifolia, Wild sesame, PDB, Sedum sarmentosum, Abrus cantoniensis, Meizizhen, Acer davidii | 105 |
| Acute mastitis | Fanbaicao decoction | PDB, Taraxacum mongolicum, Purslane, Geranium, Plantago asiatica, Polygonum sibiricum, Dianthus Superbus, Angelica dahurica, Bupleurum chinense, Achyranthes bidentata, Cyperus, Elsholtzia splendens, Isatis indigotica | 106 |
| Bacterial dysentery | Potentilla discolor Bunge | PDB | 107 |
| Yuliyin | Radix pulsatillae, Radix paeoniae rubra, Honeysuckle charcoal, Portulaca oleracea, PDB, Portulaca oleracea, Angelica sinensis, Radix paeoniae rubra, Radix Aucklandiae, Radix glycyrrhiza | 108 | |
| Idiopathic thrombocytopenic purpura | Purpura mixture | Thistle, Thistle, Lotus leaf, Platycladus orientalis, Imperata cylindrica, Palm, Forsythia suspensa, Peony bark, PDB, Bauhinia root (rhubarb), Gardenia jasminoides Ellis, Schizonepeta tenuifolia, Rehmannia glutinosa, Paeonia lactiflora (stir fried with bran) | 109, 110 |
| Zhixuexiaoban decoction | Astragalus mongholicus, Angelica sinensis, Rehmannia glutinosa, Radix rehmanniae, Charred Radix Rubiae, Hairyvein agrimony, Alternanthera philoxeroides, Chinese wolfberry, Fructus Ligustri Lucidi, PDB, Licorice, Rhizoma Cyperi, Jujube | 111 | |
| Acute gouty arthritis | Xiaozhongjiuwei powder (Mongolian medicine, external use) | Euphorbia pekinensis, PDB, Polygonatum odoratum, Turmeric, Acorus calamus, Aconitum kusnezoffii, Asparagus, Rheum subrheum, Rhubarb | 112 |
| Epidemic parotitis | Habuder-9 (Mongolian patent medicine, external use) | PDB, Euphorbia, Rhubarb, Turmeric, Aconitum kusnezoffii, Polygonatum, Polygonatum odoratum, Acorus calamus, Asparagus | 113 |
| Chronic prostatitis | Lebi-balazhuri powder (anal plug) | PDB, Euphorbia, Rhubarb, Rheum subrheum, Polygonatum, Acorus calamus, Turmeric, Asparagus, Aconitum kusnezoffii | 114 |
| Empyrosis | Fanbaicao powder (external application) | PDB | 115 |
| Hemorrhoids | Zhining decoction (fumigation bath) | Caecum, PDB, Verbena officinalis, Galla chinensis, Sanguisorba officinalis, Sophora japonica, Coptis chinensis, Honeysuckle, Artemisia anomala, Angelica sinensis, Angelica dahurica, Schizonepeta tenuifolia, Camphor, etc. | 116 |
PDB, Potentilla discolor Bunge; T2DM, type 2 diabetes.
Table 3.
Beneficial effects of Chinese medicinal formulas containing PDB in animal models.
| Disease model | Chinese medicinal formula | Composition of herbal mixture | Ref. |
|---|---|---|---|
| T2DM mice | Mixture of fanbaicao and dandelion | PDB, Dandelion | 117 |
| Qibai mixture | Raw Astragalus, PDB | 118 | |
| TCM for clearing heat and replenishing qi | PDB, Raw Astragalus | 119 | |
| T2DM rats | Fanbaicaodanshen mixture | PDB, Salvia miltiorrhiza, Astragalus, Schisandra chinensis, Trichosanthin | 120 |
| Fanbaicao mixture | PDB, Semen Platycladus, Ginseng, Polygala tenuifolia, Schisandra chinensis | 121 | |
| Diabetic nephropathy mice | Tangshenping capsule | Astragalus tablets, Cooked ground yellow, Cornus, White flower snake tongue grass, PDB, Leech | 122 |
| Big-ear white rabbits with hyperlipidemia | Water decoction of Potentilla discolor Bunge | PDB | 123 |
| Hyperlipidemia rats | Water decoction of Potentilla discolor Bunge | PDB | 124 |
PDB, Potentilla discolor Bunge; T2DM, type 2 diabetes.
4. Functions of the main natural active compounds of PDB
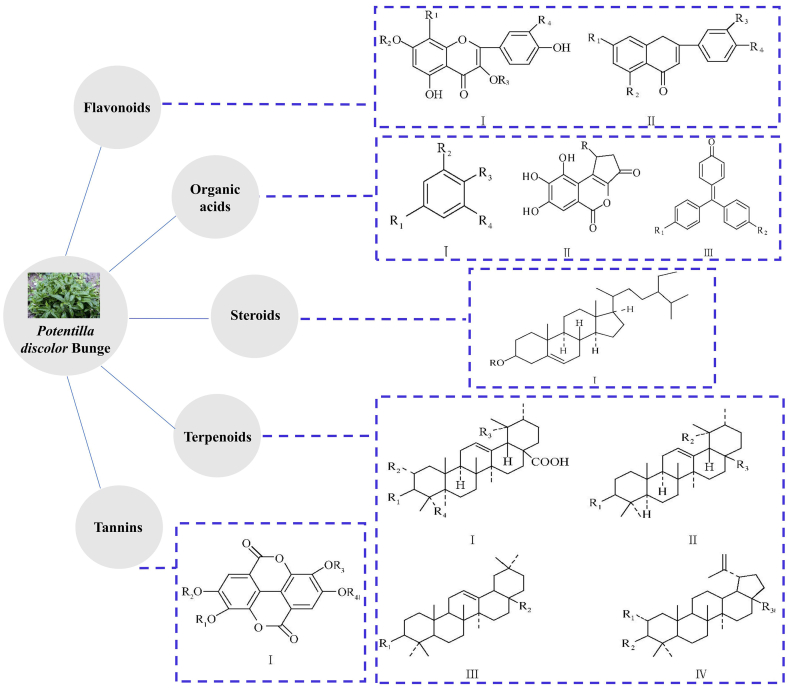
PDB contains a variety of chemiczal components, including flavonoids, terpenoids, organic acids, steroids and tannins125. The structure backbones of the main components of PDB are shown in Fig. 3.
Figure 3.
Structure backbones of the main components of PDB.
Flavonoids, which have different chemical structure subtypes, are one of the main active compounds in PDB, with many pharmacological and physiological activities126. The total flavonoids content in PDB is approximately 20%127. The two main forms of flavonoids are conjugated glycosides and free forms. Terpenoids in PDB are mainly monoterpenes and triterpenes10. The content of monoterpenes is lower than that of the triterpenes, and most of the triterpenes are oleanolic alkane, urethane and their saponins128. The main organic acids in PDB are phenolic acids and fatty acids10. The steroids obtained from PDB are mainly beta-sitosterol and carotene129. Tannins in PDB are mainly ellagic acid and its derivatives130.
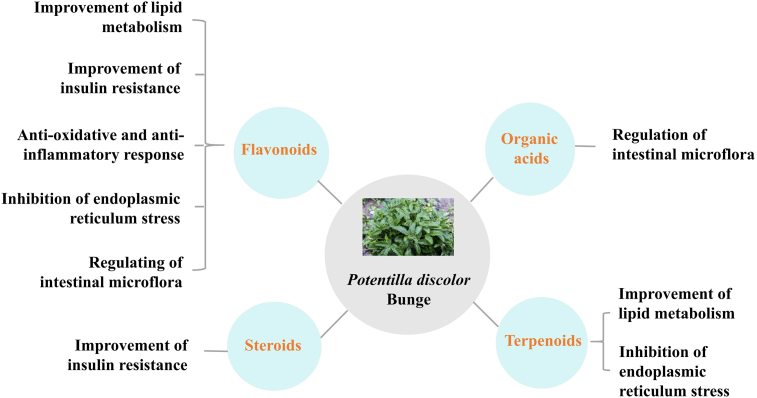
In a large majority of studies in which mouse and rat models were used, the active compounds from PDB have been found to exhibit a series of beneficial effects for the treatment of NAFLD. As such, it was found that flavonoids improve lipid metabolism and IR, reduce oxidative stress, ER stress and inflammation in rodent models131, 132, 133, 134, 135, 136, 137, 138, 139. In addition, flavonoids and organic acids were shown to regulate the intestinal microflora131,137,140. The steroids and terpenoids from PDB also improved IR141 and lipid metabolism142,143, respectively. The latter, also inhibited ER stress143 (Fig. 4).
Figure 4.
Functions of the main natural active compounds of PDB. Flavonoids improve lipid metabolism and IR, reduce oxidative stress and ER stress, and regulate the intestinal microflora. Organic acids regulate the intestinal microflora. The terpenoids improve lipid metabolism and inhibit endoplasmic reticulum stress. The steroids improve IR.
5. Anti-NAFLD mechanisms of the natural active compounds of PDB
Table 4131, 132, 133, 134, 135,140,142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155 summarizes the PDB active compounds that have been shown to improve NAFLD.
Table 4.
Anti-NAFLD mechanisms of natural active compounds of PDB.
| Natural active compound | Chemical structure | Active ingredient content | Model | Mechanism of action | Ref. | PubChem CID | |
|---|---|---|---|---|---|---|---|
| Flavonoids | Quercetin | 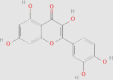 |
0.1086 mg/g144 | HFD-induced NAFLD rats FFA-induced HepG2 db/db mice | ↓ TC, TG ↑ VLDL ↑ Microsomal TG-transfer protein complex ↑ Co-localization of lysosomes with LDs ↓ Accumulation of p62 ↑ IRE1α endonuclease activity ↑ XBP1s ↓ Lipid accumulation ↓ Serum transaminase levels ↓ Serum total bile acids ↑ Lipid distribution, lipid profiles ↓ Histological alterations of liver ↓ IL-1β, IL-6, and TNF-α in liver ↑ FXR1/TGR5 signaling pathway |
131, 145 | 5280343 |
| Quercetin-3-O-β-glucoside | 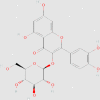 |
Sucrose-fed rats | ↓ Glucose concentration in plasma ↑ AKT phosphorylation |
132 | 5280804 | ||
| Kaempferol | 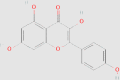 |
0.0611 mg/g144 | HFD-fed mice | ↓ Body weight ↓ FBG, HbA1c ↓ Adipose tissue accumulation ↓ TGs ↑ Lipid metabolism ↓ PPAR-γ and SREBP-1c |
133 | 5280863 | |
| Rutin | 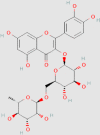 |
0.555 mg/g146 | HFD-induced NAFLD mice HepG2 cells |
↓ TC ↓ The abundance of lipid droplets ↓ Lipid accumulation ↓ Cellular malondialdehyde level ↑ Superoxide dismutase activity ↑ AMPK activity ↑ Anti-oxidative enzymes ↑ PPARα, CPT-1 and CPT-2 ↓ SREBP-1c, DGAT-1, DGAT-2 ↓ HMGCR, GPAT, FAS, ACC |
147, 148 | 5280805 | |
| Apigenin | 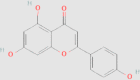 |
0.114 mg/g144 | HFD-induced NAFLD mice Hepa1-6 cells pre-treated with FFA |
↑ Insulin sensitivity ↓ Hepatic steatosis ↓ Macrophages recruitment ↓ IL-1β and IL-18 ↓ Xanthine oxidase(XO) activity ↓ ROS production ↑ NLRP3 inflammasome ↑ Nrf2 ↓ PPAR-γ ↓ TC, TGs, LDL-C, FBG, fasting insulin, HOMA-IR ↑ HDL-C ↑ Glucose tolerance ↓ Hepatic inflammatory necrosis ↑ PPAR-α and PPAR-γ (protein and mRNA expression) |
149, 150, 151 | 5280443 | |
| Luteolin | 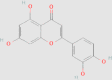 |
0.04 mg/g146 |
db/db mice HepG2 cells Primary hepatocytes from spontaneous type 2 diabetes mellitus model KK-Ay mice |
↓ Novel lipid synthesis ↑ Glycogen storage ↓ LXR-SREBP-1c signaling pathway ↓ FBG, HbA1c, HOMA-IR, TGs in serum and liver ↓ SREBP-1c mRNA expression in liver ↓ FAS activity ↓ Serum TNF-α and TNF-α mRNA expression in liver |
134, 135 | 5280445 | |
| Terpenoids | Ursolic acid | 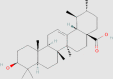 |
0.02436 mg/g152 | T0901317-induced mice HepG2 cells Intestinal cells db/db mice palmitate solution-treated LO2 cells |
↓ Hepatocyte lipid content ↓ LXRα-SREBP-1c signaling pathway ↑ AMPK phosphorylation ↓ Liver weight ↓ ALT and AST ↓ Lipid accumulation ↓ IRE1α activity ↓ JNK phosphorylation ↓ C/EBP homologous protein accumulation ↑ PPARα ↑ Lipid β-oxidation |
142, 143 | 64945 |
| Oleanolic acid | 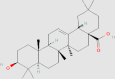 |
High fructose diet-fed rats | ↑ Lipid metabolism ↑ AMPK gene expression ↑ GLUT-4 |
153 | 10494 | ||
| 3-Acetyloleanolic acid | 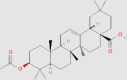 |
HFD-induced NAFLD rats FFA-treated primary rat hepatocytes HepG2 cells |
↓ Body weight, liver weight, TC, TGs and LDC-C ↑ GLUT-2 ↑ Low-density lipoprotein receptor ↑ AMPK phosphorylation |
154 | 151202 | ||
| Steroids | β-Sitosterol | 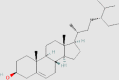 |
Diabetic rats | ↓ Blood glucose, serum insulin, blood lipid, oxidative stress markers, anti-oxidant enzymes ↑ Insulin receptor, GLUT-4 |
155 | 222284 | |
| Organic acids | Gallic acid | 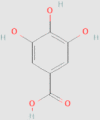 |
0.1086 mg/g144 | HFD-induced NAFLD mice | ↓ Trimethylamine ↓ Trimethylamine -N-oxide ↓ Dimethylamine |
140 | 370 |
AKT, protein kinase B; AMPK, (AMP)-activated protein kinase; db/db mice, leptin receptor deficient diabetic mice; FAS, fatty acid synthase; FBG, fasting blood glucose; FFA, free fatty acid; FXR, farnesoid X receptor; GLUT-4, glucose transporter type 4; HbA1c, glycosylated hemoglobin; HFD, high-fat diet; HOMA-IR, homeostasis model assessment for IR; IRE1α, inositol-requiring transmembrane kinase/endoribonuclease 1α; LDL-C, low-density lipoprotein cholesterol; Nrf2, nuclear factor E2-related factor 2; PPAR-α, peroxisomal proliferator-activated receptor α; ROS, reactive oxygen species; SREBP-1c, sterol regulatory element binding protein-1c; TC, total cholesterol; TG, triglyceride; TGR5, Takeda G protein-coupled receptor 5; VLDL, very low density lipoprotein; XBP1s, X-box binding protein 1.
5.1. Improvement of lipid metabolism
The abnormal lipid metabolism during NAFLD involves synthesis, uptake and oxidation of FA, triglycerides (TG) synthesis, and very low density lipoprotein (VLDL) secretion156. When carbohydrates are in excess, they are converted into FA by acetyl-CoA carboxylase, FAS and stearoyl-CoA desaturase and subsequently esterified to TG. The liver X receptors (LXRs) are multifunctional nuclear receptors that control lipid homeostasis. LXRs can be activated by glucose at physiological concentrations in the liver157. Therefore, LXRs provide a transcriptional switch that integrates hepatic glucose metabolism and FA synthesis158. Inhibition of LXRs transactivation may be beneficial for NAFLD. In addition, the mRNAs encoding enzymes in the biosynthetic pathway of FA can be regulated by sterol regulatory element binding protein-1c (SREBP-1c) that is a critical molecule involved in lipid synthesis. Adenosine 5′-monophosphate-activated protein kinase (AMPK) is known to regulate glucose and lipid metabolism, which plays vital roles in FAS and gluconeogenesis. Once AMPK is activated, the uptake of FA β-oxidation in the mitochondria is increased, with a concomitant increase of glucose uptake through the translocation of Glucose transporter type 4 (GLUT-4). In addition, peroxisomal proliferator-activated receptor α (PPAR-α) plays a central role in FA β-oxidation. The gene carnitine palmitoyl transferase1/2 involved in FA β-oxidation is regulated by PPAR-α. Accumulating evidence suggests that several natural active ingredients from PDB play an important role in improving lipid metabolism, as discussed below.
5.1.1. Luteolin
Luteolin, a natural flavonoid, has been shown to have strong anti-oxidant and anti-inflammatory activities159. Luteolin can improve hepatic steatosis by repressing hepatic TG accumulation and novel lipid synthesis in leptin receptor deficient diabetic (db/db) mice, which is correlated with the inhibition of LXR-SREBP-1c signaling pathway. In HepG2 cells and primary hepatocytes134, luteolin inhibits LXR activation and thus reduces the accumulation of TGs. In addition, luteolin indirectly activates Sirt1/AMPK pathway by up-regulating nicotinamide phosphoribosyl transferase expression levels, subsequently increasing β-oxidation and inhibiting lipogenesis136.
5.1.2. Ursolic acid (UA)
UA is the natural pentacyclic triterpenoid carboxylic acid, which has many medicinal properties, such as anti-tumorigenic, anti-obesity, anti-oxidative, anti-inflammatory, anti-fibrotic and anti-atherosclerotic properties141,160. UA significantly inhibits the activity of LXRα response element by competitively binding to LXRα ligand binding region, which demonstrates that UA is a natural LXRα antagonist. In addition, UA reduces hepatic lipid contents through increasing AMPK phosphorylation142. Another recent study showed that UA meaningfully reduces the degree of hepatic steatosis by down-regulating the expression levels of PPAR-α and carnitine palmitoyltransferase 1 A (CPT1A), which plays an essential role in the transport of FA into mitochondria for β-oxidation143.
5.1.3. Oleanolic acid (OA)
OA is a natural triterpenoid compound, which widely exists in many plants. It has been demonstrated that OA plays a wide range of biological effects, including anti-oxidation, renal protection, liver protection and anti-cancer effects161, 162, 163. One study in HFD-induced NAFLD model shows153 that the administration of OA significantly increases AMPK and CPT-1 levels, which decreases lipid accumulation and promotes the uptake of FA by mitochondria for β-oxidation. Another study shows that OA can sensitize cells to insulin and suppress the hormone-sensitive lipase, which inhibits lipolysis in adipose tissue and consequently decreases serum TGs and VLDL-C particles164. OA also ameliorates hepatic oxidative stress and lowers the SREBP and intrahepatic TGs levels164.
5.1.4. 3-Acetyloleanolic acid (3Ac-OA)
3Ac-OA is a derivative of oleanolic acid, which can significantly reduce body weight, liver weight and serum total cholesterol (TC), TG, low-density lipoprotein cholesterol (LDL-C) levels in HFD-fed rats by ameliorating hepatic lipid accumulation154. In vitro, 3Ac-OA decreases intracellular levels of TC and TG and the number of lipid droplets in free fatty acids (FFA)-treated primary rat hepatocytes. Moreover, 3Ac-OA significantly increases the expression levels of GLUT-2 and LDL receptor, phosphorylated AMPK and protein kinase B (AKT) and glycogen synthase kinase 3β in the liver tissues of HFD-fed rats154.
5.2. Improvement of IR
IR is one of the important pathogeneses of NAFLD. IR can lead to the increase of liver lipid synthesis and the inhibition of FA β-oxidation and lipolysis, which leads to hepatic steatosis. At present, homeostasis model assessment for IR (HOMA-IR) is the gold standard for measuring IR. HOMA-IR increases with the severity of NAFLD. In addition, studies show that Tumor necrosis factor-α (TNF-α) directly disrupts the role of intracellular calcium in beta cells and then induces IR165,166. Accumulating evidence suggests that several natural active ingredients from PDB play an important role in IR in the development of NAFLD, as discussed below.
5.2.1. Quercetin
Quercetin, one of the most abundant flavonoids, is found naturally as glycosides, such as quercetin-3-O-β-rutinoside or quercetin-3-O-β-glucoside. Quercetin treatment decreases IR and NAFLD activity score by modulating lipid metabolism gene expression, cytochrome P450 2E1 dependent lipoperoxidation and related lipotoxicity, which reduces the intrahepatic lipid accumulation130. Quercetin-3-O-β-glucoside can promote AKT phosphorylation in gastrocnemius muscles that are the most important tissue to determine whole-body insulin sensitivity132. The activation of insulin signaling pathway induced by AKT may contribute to the reduction of plasma glucose concentration and IR132.
5.2.2. Luteolin
Luteolin increases hepatic FA oxidation and decreases hepatic lipogenesis, which improves the hepatic insulin sensitivity and increases the insulin receptor substrate expression167. Luteolin-7-O-glucoside (LUG) is one of the O-glycosides of luteolin. Luteolin and LUG can decrease serum fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c), insulin levels and HOMA-IR index in the spontaneous T2DM mouse model135. Luteolin and LUG significantly decrease SREBP-1c levels in the liver135. The activity of FAS, which is positively correlated with TG levels, is notably lower in the luteolin and LUG groups than in the control group135. Besides, luteolin and LUG notably reduce TNF-α levels in serum and liver135.
5.2.3. Kaempferol
Kaempferol, one of the flavonols, which is a subclass of flavonoids, has many medicinal properties such as anti-oxidative, anti-carcinogenic, anti-diabetic, antimicrobial and cardio-protective properties168. Oral administration of kaempferol significantly improves FBG and decreases glucose tolerance in HFD-induced obese mice, which is associated with reduction of hepatic glucose production and improvement of whole-body insulin sensitivity138. Kaempferol is an inhibitor of hepatic pyruvate carboxylase activity133. It inhibits gluconeogenesis through suppressing pyruvate carboxylase and glucose-6 phosphatase activity133. In addition, kaempferol also improves hepatic glucose metabolism by activating AKT and glucokinase. It has also been shown that kaempferol glycoside (KG) fractions reduce body weight, adipose tissue and TG levels in HFD-fed mice133. KG treatment also decreases the levels of FBG and HbA1c and improves IR. In addition, KG decreases peroxisome proliferator-activated receptor-γ (PPAR-γ) and SREBP-1c expression levels, which is correlated with the decrease of adipose tissue accumulation and the improvement of lipid metabolism and IR133.
5.2.4. Apigenin
Apigenin is a member of the flavone subclass of flavonoids present in fruits and vegetables. Previous research showed that apigenin can decrease serum TC, TG, LDL-C, FBG and fasting insulin levels, and increase high-density lipoprotein cholesterol levels in the HFD-induced NASH rats151. In addition, apigenin can notably decrease HOMA-IR and increase PPAR-α and PPAR-γ levels in the liver. These results show that apigenin alleviates hepatic steatosis and inflammatory necrosis through improving IR, glucose tolerance and lipid metabolism151.
5.2.5. β-Sitosterol
β-Sitosterol is a plant sterol, and its chemical structure is similar to cholesterol. β-Sitosterol has anti-diabetes, anti-cancer, anti-arthritis, hypolipidemic and hepatoprotective properties155. It normalizes serum levels of glucose, insulin, lipids, oxidative stress markers and anti-oxidant enzymes in diabetic rats through the regulation of insulin receptor and GLUT-4141.
5.3. Anti-oxidative and anti-inflammatory responses
Oxidative stress in the liver is one of the hits in the pathogenesis of NAFLD. The chronic inflammatory state of the liver is closely associated with IR, inflammatory cytokines and hepatic steatosis. Neutrophils can produce ROS, subsequently activate stress kinases (e.g., ASK1 and p38 MAPK), and induce liver injury169. CXCL1 or IL-8 can induce hepatic neutrophil infiltration and promote the progression of fatty liver to NASH in HFD-fed mice, which is mediated via the p47Phox-dependent production of ROS by neutrophils. By inducing hepatic metallothionein IL-22Fc is able to attenuate hepatic ROS production, stress kinase activation and the inflammatory functions of hepatocyte-derived EVs, and thereby ameliorates CXCL1-driven NASH37. As described below, several PDB active ingredients also have anti-oxidative and anti-inflammatory properties.
5.3.1. Luteolin
Luteolin inactivates nuclear factor-κB and decreases the inflammatory levels of IL-6, Interleukin-1β (IL-1β) and TNF-α136. Furthermore, hepatic ROS production is significantly attenuated by luteolin administration, which indicates that oral intake of luteolin exerts the anti-oxidant effects in the liver139.
5.3.2. Rutin
Rutin is a natural flavonoid and has many biological functions, including anti-oxidative, anti-inflammatory, anti-cancer, neuroprotective and hepatoprotective functions170, 171, 172, 173. Rutin has also hypolipidemic and hepatoprotective effects in NAFLD147,148. Rutin reduces the cellular malondialdehyde levels and increases the expression levels of anti-oxidant enzymes147. It restores the superoxide dismutase activity, which inhibits the accumulation of lipids in liver cells and reduces oxidative damage simultaneously148.
5.3.3. Apigenin
Apigenin has a variety of biological activities, such as anti-oxidative, anti-inflammatory, anti-apoptotic, anti-mutagenic and anti-tumorigenic properties172,174, 175, 176, 177. Apigenin can alleviate HFD-induced liver injury in mice by increasing insulin sensitivity, reducing liver lipid accumulation, improving hepatic steatosis and reducing macrophages recruitment149. These protective effects may be correlated with the activation of NLRP3 inflammasome, the decreased expression of IL-1β and IL-18, the inhibition of xanthine oxidase activity and the reduction of ROS production149. In addition, apigenin has been shown to ameliorate lipid metabolism and oxidative stress through regulating nuclear factor E2-related factor 2 (Nrf2) (a master regulator of lipid metabolism homeostasis and oxidative stress) and PPAR-γ150. It has been confirmed that apigenin promotes the entry of Nrf2 into the nucleus, and thereby considerably activates Nrf2 to inhibit the expression of PPAR-γ150.
5.4. Inhibition of endoplasmic reticulum (ER) stress
ER stress is a major contributor in the development of hepatic steatosis. ER is crucial for the formation of lipid droplets and is pivotal for VLDL assembly and the progression of hepatic steatosis. ER homeostasis is maintained through an adaptive mechanism termed the unfolded protein response. This adaptive mechanism is mediated by inositol-requiring transmembrane kinase/endoribonuclease 1α (IRE1α), which is responsible for producing spliced X-box binding protein 1 (XBP1s) and protein kinase R-like ER kinase, and activating transcription factor 6α178. In addition, C/EBP homologous protein is a critical molecule involved in ER stress and ER stress-induced apoptosis178. There is increasing evidence to suggest that several natural active compounds from PDB play a central role in endoplasmic reticulum stress in the development of NAFLD.
5.4.1. Quercetin
Quercetin can activate IRE1α and ameliorate hepatic steatosis and ER stress induced by high cholesterol179,180. A study reports that quercetin reduces the levels of hepatic TG and TC and increases the levels of hepatic VLDL, and up-regulates XBP1s expression in the HFD-fed rats145. Additionally, microsomal TG-transfer protein complex expression is also increased by quercetin. Moreover, quercetin increases co-localization of lysosomes and lipid droplets, accompanied by the decreasing accumulation of autophagy related protein p62145. Collectively, these findings demonstrate that quercetin plays anti-NAFLD effects by inducing the hepatic VLDL assembly and lipophagy through the IRE1α/XBP1s pathway145.
5.4.2. UA
UA significantly reduces the liver weight, serum ALT/AST levels and hepatic steatosis in leptin receptor deficient diabetic mice (db/db mice). Moreover, it also decreases lipid accumulation in LO2 cells exposed to palmitic acid143. In addition, UA treatment inhibits the hyperlipidemia-induced IRE1α activation, JNK phosphorylation and C/EBP homologous protein accumulation in the livers of db/db mice and cultured hepatocytes143. Furthermore, UA treatment normalizes the down-regulated protein levels of PPAR-α, which plays a central role in FA β-oxidation. These results suggest that UA improves NAFLD by increasing lipid β-oxidation and inhibiting ER stress143.
5.5. Regulation of intestinal microflora
The intestinal microflora and their metabolites, including bile acids (BAs), branched-chain amino acids and tryptophan catabolites, regulate the intestinal homeostasis and may contribute to the pathogenesis of NAFLD. The metabolites exhibit multiple effects on the development of NAFLD through saccharolytic and proteolytic fermentation. For example, short chain fatty acids maintain the gut barrier and reduce pro-inflammatory cytokine secretion in the liver181. The mechanisms by which BAs contribute to the development of NAFLD involve two major receptor molecules: the nuclear farnesoid X receptor (FXR) (mainly activated by primary BAs) and the Takeda G protein-coupled receptor 5 (TGR5) (mainly activated by secondary BAs)182,183. Activation of FXR reduces hepatic inflammation and maintains the intestinal barrier by inhibiting LPS-stimulated nuclear factor-κB activation184. Moreover, choline acquired through the diet can be further metabolized by the microbiome from trimethylamine into trimethylamine-N-oxide185. Trimethylamine-N-oxide has been suggested to induce the development of NAFLD by multiple mechanisms, such as aggravating hepatic IR, increasing adipose tissue inflammation and reducing the levels of BAs produced by enzymes186. In recent years, studies have shown that several natural active ingredients from PDB play an important role in regulating intestinal flora in the course of NAFLD progression. Those active ingredients are discussed below.
5.5.1. Quercetin
Quercetin can revert the gut microbiota imbalance and the linked endotoxemia-mediated TLR-4 pathway activation, which results in the inhibition of inflammasome response and reticulum stress pathway activation and the deregulation of lipid metabolism gene expression137. Quercetin significantly reduces serum transaminase levels and T2DM-induced liver histological characteristics. In addition, quercetin restores the levels of superoxide dismutase, catalase and glutathione, and reduces total BAs levels and lipid accumulation in the liver of db/db mice131. In vitro, quercetin eliminates lipid droplets and restores the up-regulated TC and TG levels. Mechanistic studies have shown that quercetin activates the FXR1/TGR5 signaling pathway that is involved in the regulation of T2DM-induced lipid metabolism during NAFLD131.
5.5.2. Gallic acid (GA)
GA, an endogenous plant phenol, has potent free radical scavenging properties and anti-oxidative activities187, 188, 189. Lower levels of methylamine-associated metabolites including trimethylamine, trimethylamine-N-oxide and dimethylamine are found in GA treatment HFD-fed mice compared with the control group140. GA is able to reduce the elevation of choline metabolism in the gut microflora present in HFD-fed mice and as such improve hepatic steatosis140.
6. Challenges and suggestions
The application of TCM for the treatment of NAFLD has been reported in many Asian countries including China, India and Japan. However, the clinical effects of TCM for the treatment of NAFLD have not been yet recognized by regulatory agencies such as the U.S. Food and Drug Administration. Clinical trials for the evaluation of the safety and efficacy of PDB as a potential anti-NAFLD therapeutic are still necessary for regulatory acceptance. In this paper we investigated the mechanisms by which the natural active compounds of PDB may improve NAFLD using experimental models. Yet, clinical data, in which the mode-of-action of the therapeutical effects of natural active compounds of PDB are described, are still missing. Moreover, pharmacokinetic data of the PDB compounds, such as drug dose variance and absorbance rates cannot be extrapolated from animal models and need also to be determined in patients during clinical trials.
7. Summary
The prevalence of NAFLD is reaching pandemic proportions, and since the pathogenesis of this disease is very complex, there are currently no approved effective drugs for its treatment. Therefore, it is urgent to develop novel efficient therapeutic and preventative strategies for NAFLD. More and more studies are paying attention to TCM. PDB has been known since ancient times for its curative properties. In this paper, we provide an overview of the current knowledge of the pathogenesis of NAFLD, and summarize the anti-NAFLD properties of PDB, providing the underlying mechanisms of its natural active compounds. Luteolin, UA, OA, 3Ac-OA, quercetin, kaempferol, apigenin, β-sitosterol, rutin and GA were found to ameliorate NAFLD characteristics. Interesting, these compounds exert their anti-NAFLD effects through different mechanisms, including improving lipid metabolism and IR, reducing oxidative stress and inflammation, inhibiting ER stress, and regulating intestinal microflora. These beneficial effects of the natural active compounds of PDB support the notion that PDB can be considered as a potential novel candidate for the treatment and prevention of NAFLD. As such, the PDB natural active compounds may represent new sources for the development of new drugs or dietary supplements against NAFLD.
However, some questions remain to be addressed. On one hand, a systematic meta-analysis of the available publications about traditional Chinese medicines containing PBD still needs to be conducted. On the other hand, the hepatotoxicity and nephrotoxicity induced by PDB also needs investigation. The increase of well-designed preclinical and clinical studies to investigate the therapeutical effects of TCM, will hopefully validate the benefits of PDB as a therapeutical agent for the treatment of NAFLD in the future.
Acknowledgments
This work was partly supported by National Natural Science Foundation of China (Nos. 82074155, 81874436, 81973773, China); “Shuguang Program” supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission (No. 18SG39, China); Program of Shanghai Academic/Technology Research Leader (No. 20XD1423500, China); Clinical Research Plan of SHDC (No. SHDC2020CR3089B, China); Shanghai Key Clinical Specialty Construction Project (No. shslczdzk01201, China); Shanghai Frontier Research Base of Disease and Syndrome Biology of inflammatory cancer transformation (No. 2021KJ03-12, China); Shanghai Sailing Program (No. 20YF1450200, China); Shanghai Collaborative Innovation Center of Industrial Transformation of Hospital TCM Preparation (China).
Author contributions
Man Li, Yueqiu Gao and Robim M. Rodrigues: proposition proposal, design and final revision; Longshan Ji and Qian Li: organizational framework and construction, paper drafting; Yong He: revision and analysis; Xin Zhang and Zhenhua Zhou: collected data and provided materials; Yating Gao, Miao Fang, and Zhuo Yu: revision.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Robim M. Rodrigues, Email: robim.marcelino.rodrigues@vub.be.
Yueqiu Gao, Email: gaoyueqiu@shutcm.edu.cn.
Man Li, Email: liman121000@shutcm.edu.cn.
References
- 1.Eslam M., Sanyal A.J., George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated aatty liver disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z., Tacke F., Arrese M., Chander Sharma B., Mostafa I., Bugianesi E., et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69:2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- 3.Noureddin M., Sanyal A.J. Pathogenesis of NASH: the impact of multiple pathways. Curr Hepat Rep. 2018;17:350–360. doi: 10.1007/s11901-018-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dufour J.F., Caussy C., Loomba R. Combination therapy for non-alcoholic steatohepatitis: rationale, opportunities and challenges. Gut. 2020;69:1877–1884. doi: 10.1136/gutjnl-2019-319104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kefala G., Tziomalos K. Apoptosis signal-regulating kinase-1 as a therapeutic target in nonalcoholic fatty liver disease. Expet Rev Gastroenterol Hepatol. 2019;13:189–191. doi: 10.1080/17474124.2019.1570136. [DOI] [PubMed] [Google Scholar]
- 6.Thanapirom K., Tsochatzis E.A. Non-alcoholic fatty liver disease (NAFLD) and the quest for effective treatments. Hepatobiliary Surg Nutr. 2019;8:77–79. doi: 10.21037/hbsn.2018.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan T., Yan N., Wang P., Xia Y., Hao H., Wang G., et al. Herbal drug discovery for the treatment of nonalcoholic fatty liver disease. Acta Pharm Sin B. 2020;10:3–18. doi: 10.1016/j.apsb.2019.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L., Yang J., Chen X.Q., Zan K., Wen X.D., Chen H., et al. Antidiabetic and antioxidant effects of extracts from Potentilla discolor Bunge on diabetic rats induced by high fat diet and streptozotocin. J Ethnopharmacol. 2010;132:518–524. doi: 10.1016/j.jep.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 9.Lu Y.T., Gao C.B., Fu B. Textual research on the herb of Potentilla discolor Bunge. Hunan J Tradit Chin Med. 2019;35:126–128. [Google Scholar]
- 10.Huang F.B., Wu J., Sheng W.B., Peng C.G., Wang W., Zhang Z.Q., et al. Research progress on chemical constituents and pharmacological activities of Potentilla freyniana Bornm. J Hunan Univ Chin Med. 2020;40:1039–1044. [Google Scholar]
- 11.Wang S.S., Wang D.M., Pu W.J., Li D.W. Phytochemical profiles, antioxidant and antimicrobial activities of three Potentilla species. BMC Compl Alternative Med. 2013;13:321. doi: 10.1186/1472-6882-13-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F., Zhou J., Wang W., Zhang X.J., Ji Y.X., Zhang P., et al. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: a systematic review and meta-analysis. Hepatology. 2019;70:1119–1133. doi: 10.1002/hep.30702. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J., Zhou F., Wang W., Zhang X.J., Ji Y.X., Zhang P., et al. Epidemiological features of NAFLD from 1999 to 2018 in China. Hepatology. 2020;71:1851–1864. doi: 10.1002/hep.31150. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim S.H., Hirsova P., Gores G.J. Non-alcoholic steatohepatitis pathogenesis: sublethal hepatocyte injury as a driver of liver inflammation. Gut. 2018;67:963–972. doi: 10.1136/gutjnl-2017-315691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parthasarathy G., Revelo X., Malhi H. Pathogenesis of nonalcoholic steatohepatitis: an overview. Hepatol Commun. 2020;4:478–492. doi: 10.1002/hep4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H., Mehal W., Nagy L.E., Rotman Y. Immunological mechanisms and therapeutic targets of fatty liver diseases. Cell Mol Immunol. 2021;18:73–91. doi: 10.1038/s41423-020-00579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothschild D., Weissbrod O., Barkan E., Kurilshikov A., Korem T., Zeevi D., et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 18.Jiang W., Wu N., Wang X., Chi Y., Zhang Y., Qiu X., et al. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 2015;5:8096. doi: 10.1038/srep08096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adolph T.E., Grander C., Grabherr F., Tilg H. Adipokines and non-alcoholic fatty liver disease: multiple interactions. Int J Mol Sci. 2017;18:1649. doi: 10.3390/ijms18081649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polyzos S.A., Kountouras J., Mantzoros C.S. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 2019;92:82–97. doi: 10.1016/j.metabol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Marra F., Svegliati-Baroni G. Lipotoxicity and the gut–liver axis in NASH pathogenesis. J Hepatol. 2018;68:280–295. doi: 10.1016/j.jhep.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues R.M., He Y., Hwang S., Bertola A., Mackowiak B., Ahmed Y.A., et al. E-selectin-dependent inflammation and lipolysis in adipose tissue exacerbate steatosis-to-NASH progression via S100A8/9. Cell Mol Gastroenterol Hepatol. 2022;13:151–171. doi: 10.1016/j.jcmgh.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canbay A., Feldstein A.E., Higuchi H., Werneburg N., Grambihler A., Bronk S.F., et al. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- 24.Szabo G., Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2017;14:455–466. doi: 10.1038/nrgastro.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu D., Zhu H., Wang H. Extracellular vesicles in non-alcoholic fatty liver disease and alcoholic liver disease. Front Physiol. 2021;12:707429. doi: 10.3389/fphys.2021.707429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malhi H. Emerging role of extracellular vesicles in liver diseases. Am J Physiol Gastrointest Liver Physiol. 2019;317:G739–G749. doi: 10.1152/ajpgi.00183.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsova P., Ibrahim S.H., Krishnan A., Verma V.K., Bronk S.F., Werneburg N.W., et al. Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology. 2016;150:956–967. doi: 10.1053/j.gastro.2015.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X.L., Pan Q., Cao H.X., Xin F.Z., Zhao Z.H., Yang R.X., et al. Lipotoxic hepatocyte-derived exosomal microRNA 192-5p activates macrophages through rictor/akt/forkhead box transcription factor o1 signaling in nonalcoholic fatty liver disease. Hepatology. 2020;72:454–469. doi: 10.1002/hep.31050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Z., Zhong L., Li P., He K., Qiu C., Zhao L., et al. Cholesterol impairs hepatocyte lysosomal function causing M1 polarization of macrophages via exosomal miR-122-5p. Exp Cell Res. 2020;387:111738. doi: 10.1016/j.yexcr.2019.111738. [DOI] [PubMed] [Google Scholar]
- 30.Ibrahim S.H., Hirsova P., Tomita K., Bronk S.F., Werneburg N.W., Harrison S.A., et al. Mixed lineage kinase 3 mediates release of C–X–C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology. 2016;63:731–744. doi: 10.1002/hep.28252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Q., Furuta K., Lucien F., Gutierrez Sanchez L.H., Hirsova P., Krishnan A., et al. Integrin β1-enriched extracellular vesicles mediate monocyte adhesion and promote liver inflammation in murine NASH. J Hepatol. 2019;71:1193–1205. doi: 10.1016/j.jhep.2019.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dasgupta D., Nakao Y., Mauer A.S., Thompson J.M., Sehrawat T.S., Liao C.Y., et al. IRE1A stimulates hepatocyte-derived extracellular vesicles that promote inflammation in mice with steatohepatitis. Gastroenterology. 2020;159:1487–1503.e17. doi: 10.1053/j.gastro.2020.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rinella M.E., Tacke F., Sanyal A.J., Anstee Q.M. Report on the AASLD/EASL joint workshop on clinical trial endpoints in NAFLD. Hepatology. 2019;70:1424–1436. doi: 10.1002/hep.30782. [DOI] [PubMed] [Google Scholar]
- 34.Gao B., Tsukamoto H. Inflammation in alcoholic and nonalcoholic fatty liver disease: friend or foe? Gastroenterology. 2016;150:1704–1709. doi: 10.1053/j.gastro.2016.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertola A., Bonnafous S., Anty R., Patouraux S., Saint-Paul M.C., Iannelli A., et al. Hepatic expression patterns of inflammatory and immune response genes associated with obesity and NASH in morbidly obese patients. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 37.Hwang S., He Y., Xiang X., Seo W., Kim S.J., Ma J., et al. Interleukin-22 ameliorates neutrophil-driven nonalcoholic steatohepatitis through multiple targets. Hepatology. 2020;72:412–429. doi: 10.1002/hep.31031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He Y., Hwang S., Cai Y., Kim S.J., Xu M., Yang D., et al. MicroRNA-223 ameliorates nonalcoholic steatohepatitis and cancer by targeting multiple inflammatory and oncogenic genes in hepatocytes. Hepatology. 2019;70:1150–1167. doi: 10.1002/hep.30645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He Y., Rodrigues R.M., Wang X., Seo W., Ma J., Hwang S., et al. Neutrophil-to-hepatocyte communication via LDLR-dependent miR-223-enriched extracellular vesicle transfer ameliorates nonalcoholic steatohepatitis. J Clin Invest. 2021;131 doi: 10.1172/JCI141513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi T., Wu L., Ma W., Ju L., Bai M., Chen X., et al. Nonalcoholic fatty liver disease: pathogenesis and treatment in traditional Chinese medicine and western medicine. Evid Based Complement Alternat Med. 2020;2020:8749564. doi: 10.1155/2020/8749564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji Y.M., Mao Q.G. Study on TCM constitution and correlative syndromes of nonalcoholic fatty liver patients. Medical Information. 2018;31:46–52. [Google Scholar]
- 42.Zhang Y., Tang K., Deng Y., Chen R., Liang S., Xie H., et al. Effects of shenling baizhu powder herbal formula on intestinal microbiota in high-fat diet-induced NAFLD rats. Biomed Pharmacother. 2018;102:1025–1036. doi: 10.1016/j.biopha.2018.03.158. [DOI] [PubMed] [Google Scholar]
- 43.Tang K.R., Deng Y.J., Zheng C.Y., Nie H., Pan M.X., Chen R.S., et al. Prevention of nonalcoholic hepatic steatosis by shenling baizhu powder: involvement of adiponectin-induced inhibition of hepatic SREBP-1c. Oxid Med Cell Longev. 2020;2020:9701285. doi: 10.1155/2020/9701285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han R.T., Qiu H.H., Zhong J., Zhen N.N., Li B.B., Hong Y., et al. Si miao formula attenuates non-alcoholic fatty liver disease by modulating hepatic lipid metabolism and gut microbiota. Phytomedicine. 2021;85:153544. doi: 10.1016/j.phymed.2021.153544. [DOI] [PubMed] [Google Scholar]
- 45.Li Y., Liu Y., Yang M., Wang Q., Zheng Y., Xu J., et al. A study on the therapeutic efficacy of San Zi Yang Qin decoction for non-alcoholic fatty liver disease and the underlying mechanism based on network pharmacology. Evid Based Complement Alternat Med. 2021;2021:8819245. doi: 10.1155/2021/8819245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu W.Y., Luo H., Xiong M., Shen T. Xiaochaihu decoction for nonalcoholic fatty liver disease: a protocol for a systematic review and meta-analysis. Medicine (Baltim) 2021;100 doi: 10.1097/MD.0000000000025267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang Y.J., Zhang Y.P., Deng Y.J., Liang S., He Y.F., Chen Y.N., et al. Chaihu-Shugan-San decoction modulates intestinal microbe dysbiosis and alleviates chronic metabolic inflammation in NAFLD rats via the NLRP3 inflammasome pathway. Evid Based Complement Alternat Med. 2018;2018:9390786. doi: 10.1155/2018/9390786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Q.H., Xu Y.J., Feng G.F., Hu C.F., Zhang Y.P., Cheng S.B., et al. p38 MAPK signal pathway involved in anti-inflammatory effect of Chaihu-Shugan-San and Shen-Ling-Bai-Zhu-San on hepatocyte in non-alcoholic steatohepatitis rats. Afr J Tradit Complementary Altern Med. 2013;11:213–221. doi: 10.4314/ajtcam.v11i1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang W.N., Li D., Jiang T., Guo J., Chen Y.F., Wang J., et al. Protective effects of Chaihu Shugan San, on nonalcoholic fatty liver disease in rats with insulin resistance. Chin J Integr Med. 2018;24:125–132. doi: 10.1007/s11655-016-2252-4. [DOI] [PubMed] [Google Scholar]
- 50.Fan Y.H., Miao B.Q. Treatment of 63 cases of nonalcoholic fatty liver disease with Dachaihu decoction. Mongol J Traditional Chinese Medicine. 2014;33:9. [Google Scholar]
- 51.Yang J.M., Sun Y., Wang M., Zhang X.L., Zhang S.J., Gao Y.S., et al. Regulatory effect of a Chinese herbal medicine formula on non-alcoholic fatty liver disease. World J Gastroenterol. 2019;25:5105–5119. doi: 10.3748/wjg.v25.i34.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen S.D., Fan Y., Xu W.J. Effects of YinChenHao decoction (see text) for non-alcoholic steatohepatitis in rats and study of the mechanism. J Tradit Chin Med. 2011;31:220–223. doi: 10.1016/s0254-6272(11)60045-9. [DOI] [PubMed] [Google Scholar]
- 53.Park S.H., Lee J.E., Lee S.M., Lee J., Seo C.S., Hwang G.S., et al. An unbiased lipidomics approach identifies key lipid molecules as potential therapeutic targets of Dohongsamul-tang against non-alcoholic fatty liver diseases in a mouse model of obesity. J Ethnopharmacol. 2020;260:112999. doi: 10.1016/j.jep.2020.112999. [DOI] [PubMed] [Google Scholar]
- 54.Zhang M., Yuan Y., Wang Q., Li X., Men J., Lin M. The Chinese medicine chai hu li zhong tang protects against non-alcoholic fatty liver disease by activating AMPKα. Biosci Rep. 2018;38 doi: 10.1042/BSR20180644. BSR20180644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dang Y.Q., Hao S.J., Zhou W.J., Zhang L., Ji G. The traditional Chinese formulae Ling-Gui-Zhu-Gan decoction alleviated non-alcoholic fatty liver disease via inhibiting PPP1R3C mediated molecules. BMC Compl Alternative Med. 2019;19:8. doi: 10.1186/s12906-018-2424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu M.Z., Hao S.J., Liu T., Yang L.L., Zheng P.Y., Zhang L., et al. Lingguizhugan decoction improves non-alcoholic fatty liver disease by altering insulin resistance and lipid metabolism related genes: a whole trancriptome study by RNA-Seq. Oncotarget. 2017;8:82621–82631. doi: 10.18632/oncotarget.19734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu M.T., Huang Y.J., Zhang T.Y., Tan L.B., Lu X.F., Qin J. Lingguizhugan decoction attenuates diet-induced obesity and hepatosteatosis viagut microbiota. World J Gastroenterol. 2019;25:3590–3606. doi: 10.3748/wjg.v25.i27.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang J.Y., Ma W., Mei Q.C., Song J.F., Shu L., Zhang S., et al. Protective effect of FuZi LiZhong decoction against non-alcoholic fatty liver disease via anti-inflammatory response through regulating p53 and PPARγ signaling. Biol Pharm Bull. 2020;43:1626–1633. doi: 10.1248/bpb.b20-00053. [DOI] [PubMed] [Google Scholar]
- 59.Wei S.S., Yang H.J., Huang J.W., Lu X.P., Peng L.F., Wang Q.G. Traditional herbal formula sini powder extract produces antidepressant-like effects through stress-related mechanisms in rats. Chin J Nat Med. 2016;14:590–598. doi: 10.1016/S1875-5364(16)30069-3. [DOI] [PubMed] [Google Scholar]
- 60.Cheng F.F., Ma C.Y., Wang X.Q., Zhai C.M., Wang G.L., Xu X.L., et al. Effect of traditional Chinese medicine formula Sinisan on chronic restraint stress-induced nonalcoholic fatty liver disease: a rat study. BMC Compl Alternative Med. 2017;17:203. doi: 10.1186/s12906-017-1707-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu F., Li Y.M., Feng T.T., Wu Y., Zhang H.X., Jin G.Y., et al. Freeze-dried Si-Ni-San powder can ameliorate high fat diet-induced non-alcoholic fatty liver disease. World J Gastroenterol. 2019;25:3056–3068. doi: 10.3748/wjg.v25.i24.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dang Y.Q., Xu J.J., Zhu M.Z., Zhou W., Zhang L., Ji G. Gan-Jiang-Ling-Zhu decoction alleviates hepatic steatosis in rats by the miR-138-5p/CPT1B axis. Biomed Pharmacother. 2020;127:110127. doi: 10.1016/j.biopha.2020.110127. [DOI] [PubMed] [Google Scholar]
- 63.Chen P., Zhong X., Dai Y., Tan M., Zhang G., Ke X., et al. The efficacy and safety of acupuncture in nonalcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. Zhongguo Zhen Jiu. 2021;100 doi: 10.1097/MD.0000000000027050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dong C., Zhang C.R., Xue B.Y., Miu W.F., Fang N.Y., Li K., et al. Electroacupuncture combined with lifestyle control on obese nonalcoholic fatty liver disease: a randomized controlled trial. Zhongguo Zhen Jiu. 2020;40:129–134. doi: 10.13703/j.0255-2930.20190201-k00034. [DOI] [PubMed] [Google Scholar]
- 65.Xie J.P., Liu G.L., Li W., Gu Q., Qiao J.L., Zhang H., et al. Study on optimization parameters of electroacupuncture at Fenglong (ST 40) for adjusting blood lipids. Zhongguo Zhen Jiu. 2007;27:39–43. [PubMed] [Google Scholar]
- 66.Chen W., Xie J., Wang J.M. Clinical research and related indicators of change of treating NAFLD by electro-acupunctue. Chin J Clin Res. 2012;4:28. [Google Scholar]
- 67.Han J., Guo X., Meng X.J., Zhang J., Yamaguchi R., Motoo Y., et al. Acupuncture improved lipid metabolism by regulating intestinal absorption in mice. World J Gastroenterol. 2020;26:5118–5129. doi: 10.3748/wjg.v26.i34.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meng X.J., Guo X., Zhang J., Moriya J., Kobayashi J., Yamaguchi R., et al. Acupuncture on ST36, CV4 and KI1 suppresses the progression of methionine- and choline-deficient diet-induced nonalcoholic fatty liver disease in mice. Metabolites. 2019;9:299. doi: 10.3390/metabo9120299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen X.L., Tang C.L., Xie H., Tang N.Z., Gao J., Liu R.J. Effect of electro-acupuncture on hepatic Toll-like receptor 4 and nuclear factor κB expressions in rats with non-alcoholic fatty liver disease. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34:1584–1588. [PubMed] [Google Scholar]
- 70.Yu M., Li G., Tang C.L., Gao R.Q., Feng Q.T., Cao J. Effect of electroacupunctrue stimulation at "Fenglong” (ST 40) on expression of SREBP-1 c in non-alcoholic fatty liver disease rats. Zhen Ci Yan Jiu. 2017;42:308–314. [PubMed] [Google Scholar]
- 71.Feng W.Q., Zeng Z.H., Zhuo L.S. Influence of electroacupuncture on insulin-resistance in nonalcoholic fatty liver rats. Zhen Ci Zhen Jiu. 2008;33:111–115. [PubMed] [Google Scholar]
- 72.Zhang Y., Tang C.L., Tian Y., Yuan H.Z., Yang H., Tang N.Z., et al. Effect of electroacupunctrue on ERp57 in NAFLD rats. Si Chuan Da Xue Xue Bao Yi Xue Ban. 2016;47:208–213. [PubMed] [Google Scholar]
- 73.Zhang Y., Tang C.L., Tian Y., Yuan H.Z., Gao R.Q., Cao J. Effects of electroacupuncture combined with dietary control on liver endoplasmic reticulum stress in rats with non-alcoholic fatty liver disease. Zhongguo Zhen Jiu. 2016;36:951–956. doi: 10.13703/j.0255-2930.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 74.Wang H.Y., Liang C.M., Cui J.W., Pan L., Hu H., Fang H.J. Acupuncture improves hepatic lipid metabolism by suppressing oxidative stress in obese nonalcoholic fatty liver disease rats. Zhen Ci Yan Jiu. 2019;44:189–194. doi: 10.13702/j.1000-0607.180650. [DOI] [PubMed] [Google Scholar]
- 75.Ma B.Q., Li P., An H.Y., Song Z.M. Electroacupuncture attenuates liver inflammation in nonalcoholic fatty liver disease rats. Inflammation. 2020;43:2372–2378. doi: 10.1007/s10753-020-01306-w. [DOI] [PubMed] [Google Scholar]
- 76.Zeng Z.H., Zeng M.H., Huang X.K., Chen R., Yu H. Effect of electroacupuncture stimulation of back-shu points on expression of TNF-alpha and lipid peroxidation reaction in the liver tissue in non-alcoholic fatty liver disease rats. Zhen Ci Yan Jiu. 2014;39:288–292. [PubMed] [Google Scholar]
- 77.Zeng Z.H., Feng W.Q., Zhuo L.S. Influence of electroacupuncture on cytochrome P450 2E1 expression, oxidation, anti-oxidation in non-alcoholic fatty liver tissue. J Fourth Mil Med Univ. 2008;11:994–997. [Google Scholar]
- 78.Bai C.Y., Zhuo L.S., Zhu Y., Fu Y. Effect of electroacupuncture on the expression of leptin and leptin receptor in hypothalamus of rats with nonalcoholic fatty liver. Zhen Ci Yan Jiu. 2010;35:277–280. [PubMed] [Google Scholar]
- 79.Zhang L., Wang H.X., Chen Y., Ouyang S.S. Treatment of 36 cases of type II diabetes compound fubai grass mixture. Chin J Mod Drug Appl. 2007;1:50–51. [Google Scholar]
- 80.Luo X.L., Ma Z.J., Liu H.L., Li M. Clinical observation of compound white grass decoction on 60 patients with type 2 diabetes. Guid J Tradit Chin Med Pharm. 2015;21:62–63. [Google Scholar]
- 81.Jin Y.S., Zhang Z.Q., Mei X.H., Zhang S., Xie L.C., Liu B.G. Effect of Jiulong Jiangtang decoction combined with acupuncture on blood lipid in patients with type 2 diabetes mellitus. Hubei J Tradit Chin Med. 2004;6:36. [Google Scholar]
- 82.Jing L.Y. 33 cases of insulin resistance in elderly patients with type 2 diabetes mellitus by Jiang Tang Zeng Min Tang. Forum Tradit Chin Med. 2017;32:44–45. [Google Scholar]
- 83.Liu R.X., Zeng J.P., Cui D.Z., Yang Q.F. Clinical study on Jiangtang Zengmin decoction in treating insulin resistance in elderly patients with type 2 diabetes. J Shandong Univ Tradit Chin Med. 2016;40:439–441. [Google Scholar]
- 84.Wang Y., Wang N., Mao L.H., Hu L. Clinical study of Chinese medicine Zeng Min decoction on insulin resistance in type 2 diabetes mellitus. Practical Clin J Integrated Tradit Chin West Med. 2008;3:27–28. [Google Scholar]
- 85.Jiang J., Liu R.X., Zhang T., Qian Y.D. Efficacy of the Shugan Yunpi and Huazhuo Jiedu herapy on adiponectin, leptin and insulin resistance in the patients with type 2 diabetes mellitus. Clin J Chin Med. 2017;9:17–19. [Google Scholar]
- 86.Tang X.Z., Luo Y. Treatment of 69 cases of latent latent type 2 diabetes with the method of invigorating spleen and removing dampness and clearing away heat. Henan Tradit Chin Med. 2006;8:44–45. [Google Scholar]
- 87.Yuan M.H., Ma X.B., Zhang X.K., Su L.Y. Study on treatment of type 2 diabetes by glucophage with Qi Yu Ping Tang(Qi Yin deficiency syndrome) J Shanxi Univ Chin Med. 2016;39:63–65. [Google Scholar]
- 88.Wang Z.Q. Clinical study of activating blood and supplementing qi in treating diabetes mellitus. Henan Tradit Chin Med. 2001;21:33–34. [Google Scholar]
- 89.Lin X.Q. Clinical observation on treating diabetes mellitus type 2 with the Antang capsule. Clin J Chin Med. 2018;10:107–109. [Google Scholar]
- 90.Li M.Y., Wang Q.Z., Cai Q.M., Wu W.F. Treatment of insulin resistance in type 2 diabetes mellitus by pioglitazone combined with self made bitter Huang capsule: a clinical observation. Chin Med Mod Distance Ed China. 2011;9:176. [Google Scholar]
- 91.Li M., Ma Z.J., Luo X.L., Liu H.L. Treatment of type 2 diabetes by bai huang jiangtang granule: a clinical observation of 60 cases. Nei Mongol J Tradit Chin Med. 2016;35:51. [Google Scholar]
- 92.Wu J.J., Huang J.R., Huang W., Pan F.M., Huang X.W. Clinical observation of jiedu fuyang prescription in treating type 2 diabetes mellitus. Mod J Integr Tradit Chin West Med. 2014;23:713–714. [Google Scholar]
- 93.Fan H. Clinical observation on treatment of type 2 diabetes with Yiqi Yangyin Huoxue decoction. Hubei J Tradit Chin Med. 2006;28:19–21. [Google Scholar]
- 94.Liu C., Ge H. Effect of boosting qi and nourishing yin on type 2 diabetes patients with serum CysC and HbA1C. Chin J Biochem Pharm. 2016;36:53–55. [Google Scholar]
- 95.Wang W.J. The clinical curative effect of early diabetic nephropathy with Yiqi Jianpi Huayu decoction. Chin J Ethnomed Ethnopharmacy. 2015;24:93–94. [Google Scholar]
- 96.Peng J., Qi Y.H., Huang K., Yan S.F. Observation of curative effect on replenishing spleen Qi and removing blood stasis decoction in preventing early diabetic nephropathy. Chin Mod Med. 2012;19:101–102. [Google Scholar]
- 97.Wu X.Q., Peng J. Treatment of early stage of diabetic nephropathy by Yiqi Huayu decoction: a clinical observation of 26 cases. Chin Med Herald. 2008;5:81–82. [Google Scholar]
- 98.Wang X.F., Zhao C.D., Zhang H.L. Treatment of early stage of diabetic nephropathy by Yiqi Huoxue tang: a clinical observation of 40 cases. J Chin Med. 2006;38:46–47. [Google Scholar]
- 99.Zhu Y.H., Wei T., Liiu J.X. Treatment of 30 cases of early diabetic nephropathy with Yiqi Huoxue, kidney preserving and detoxicating methods. China Naturopathy. 2006;14:6–7. [Google Scholar]
- 100.Dong C.H. Clinical observation onpotentilla discolor bunge capsule for diabetic peripheral neuropathy. Chin J Control Endemic Dis. 2016;31:566. [Google Scholar]
- 101.Song C.L., Wang X.L., Wang T. Plowing huan wu tang combined with western treatment of diabetic peripheral arterial occlusive clinical observation of 35 cases. J Chin Prac Tradit Chin Med. 2015;29:133–135. [Google Scholar]
- 102.Xu C.K., Qu Z.Q. Clinical observation on treating diabetic limb arterial occlusive disease by Fandihuanwu decoction. Lining J Tradit Chin Med. 2006;33:835–836. [Google Scholar]
- 103.Liu C.Y., Liu H. Observation on therapeutic effect of Jiangbai decoction on 25 cases of chronic nephritis proteinuria. Chin Med J Metallurgical Ind. 2008;25:709–710. [Google Scholar]
- 104.Zhao C.D., Bao L.Y., Wu C.S. 30 cases of chronic nephritis proteinuria treated with Jiangbai decoction. J Tradit Chin Med. 2002;43:202–203. [Google Scholar]
- 105.Lu J.M., Liu G.B. Clinical study on Yao medicine in the treatment of chronic viral hepatitis B. Hebei J Tradit Chin Med. 2006;28:253–254. [Google Scholar]
- 106.Xu P., Zhou C.F., Chen S.W. Treatment of 36 patients of mastitlis by discolor cinquefoil herb and yellow cock-tree bark. China Naturopathy. 2003;11:39. [Google Scholar]
- 107.Yuncheng district health and epidemic prevention station Yuanqu County Health and epidemic prevention station. Treatment of 68 cases of bacillary dysentery with Fanbaicao injection. Shanxi Med J. 1977:16–17. [Google Scholar]
- 108.Yan Y.Q., Jin Q.X., Liu X.Z. Clinical study of integrated Chinese and western medicine therapy on bacillary dysentery. Hebei J Tradit Chin Med. 2011;33:1495–1497. [Google Scholar]
- 109.Zhu X.Q., Chen P., Qiu Z.C., Zhao L., Huang Z.D., Hu X.Y., et al. Effects of purpura mixture on cytokines in patients of primary immune thrombocytopenia with syndrome of blood-heat. Shanghai J Tradit Chin Med. 2017;51:90–92. [Google Scholar]
- 110.Ying P.P., Qiu Z.C., Chen P., Wang Y.J. Clinical observation of purpura mixture in the treatment of chronic ITP. Liaoning J Trad Chin Med. 2002;29:667–668. [Google Scholar]
- 111.Zhan T.S., Xiao R.F. Treatment of thrombocytopenic purpura with self-made Zhixue Xiaoban decoction. Lishizhen Med Mater Med Res. 2005;16:340–341. [Google Scholar]
- 112.Wang Y.S. Effect of Tongfeng decoction combined with Mongolian medicine Xiaozhong Jiuwei powder on acute gouty arthritis. J Clin Med Literature. 2015;2:1540. [Google Scholar]
- 113.Qing G.L., A D.Y. Clinical observation on 37 cases of mumps treated with Mongolian western medicine. J Med Pharm Chinese Minorities. 1995;1:36. [Google Scholar]
- 114.Bu R.B.Y.E., Tong D.G.L., Zha G. Treatment of 20 cases of chronic prostatitis by anal embolization with Lebi Balajuri. J Med Pharm Chin Minorities. 1997;3:16–17. [Google Scholar]
- 115.Yan C.F., Lv Y. Analysis of clinical application value of fanbaicao powder. Shenzhen J Integr Tradit Chin West Med. 2014;24:113–114. [Google Scholar]
- 116.Zou B. 124 cases of hemorrhoids treated with Zhining decoction fumigation bath. J Ext Ther Tradit Chin Med. 2007;16:35. [Google Scholar]
- 117.Cheng S.B., Wang H.X., Yan P., Zou T.X. Therapeutic effect of Potentilla discolor and dandelion mixture on diabetic mice. Seek Med Ask Med. 2013;11:446–447. [Google Scholar]
- 118.Zhang D.M., Lou L.X., Wu A.M., Lv X.Y., Hu Z.J., Zhang Y.H., et al. Effects of astragalus membranaceus and Potentilla discolor mixture on insulin resistance and its related mRNA expressions in KKAy mice with type 2 diatetes. J Chin Intergr Med. 2012;10:821–826. doi: 10.3736/jcim20120714. [DOI] [PubMed] [Google Scholar]
- 119.Hu Z.J., Liu H.F., Zhang Y.H., Nie B., Zhang D.M., Lou L.X., et al. Influence of medicinals with actions of clearing heat and tonifying qi on insulin sensitivity in KKay mice with early type 2 diabetes. J Beijing Univ Tradit Chin Med. 2012;35:607–610. [Google Scholar]
- 120.Sheng Q.W., Wu Y.H., Jin Y., Jin Y.H., Jin X.S., Zhu Y.Y., et al. Study on molecular mechanism of the mixture of Potentilla discolor bunge and Salvia miltiorrhiza bunge on the expression of PPAR-γ gene in type 2 diabetic rat model. J Med Sci Yanbian Univ. 2015;38:178–181. [Google Scholar]
- 121.Guo X.M., Yu S.T. Effect of Potentilla discolor mixture on the expression of nerve growth factor and nerve fiber protein in hippocampus of type 2 diabetic rats. Chin J Clin Rehabil. 2005;9:82–83. [Google Scholar]
- 122.Cai J.W., Zhao Z.J., Zhang X.X., Miao Y.H., Wang T., Ge D.Y., et al. Renal protective effect of tangshiping capsule on diabetic nephropathy KK-Ay mice and its effect on Wnt/β-catenin signal transduction pathway. Chin J Exp Tradit Med Form. 2019;25:96–102. [Google Scholar]
- 123.Meng L.Y., Yan X.H., Zhang D.D., Jia Bin. Effect of Potentilla discolor on hyperlipidemia model animals. Acta Chin Med Pharmacol. 2009;37:41–42. [Google Scholar]
- 124.Li Y.M., Xu L., Zheng H.H. Hypolipidemic and antioxidant effects of Potentilla discolor on hyperlipidemia model in rats. Chin J Anim Hus Vet Med. 2012;7:23–24. [Google Scholar]
- 125.Sun Y., Deng Y.R., Wang Y., Lian L.L. Research progress on chemical constituents and pharmacological activities of Potentilla discolor. Chin Tradit Patent Med. 2016;38:639–645. [Google Scholar]
- 126.Qin H.W., Sun H., Wang X.D., Sun J.Y., Zhang J., Yang Q.M., et al. Chemical constituents of Potentilla discolor. Zhong Yao Cai. 2020;43:339–343. [Google Scholar]
- 127.Fu L.M. Extraction technology and chemical composition analysis of total flavonoids from Potentilla discolor. J Clin Med Literature. 2017;4:4713–4714. [Google Scholar]
- 128.Shao F.F. Henan University of Science and Technology; Luoyang: 2010. Experimental study on hypoglycemic and lipid-lowering effect and mechanism of Potentilla discolor [dissertation] pp. 12–14. [Google Scholar]
- 129.Li Y.Y., Xiao C.M., Yao M., Zeng X.Y., Xiao X.H., Zhu C.Q. Study on chemical constituents of triterpenoids from Potentilla discolor. Zhong Yao Cai. 2013;36:1099–1101. [PubMed] [Google Scholar]
- 130.Sun C. Chemical constituents of Potentilla discolor Bunge. Sci Tech Inf. 2015;13:243. [Google Scholar]
- 131.Yang H., Yang T., Heng C., Zhou Y., Jiang Z., Qian X., et al. Quercetin improves nonalcoholic fatty liver by ameliorating inflammation, oxidative stress, and lipid metabolism in db/db mice. Phytother Res. 2019;33:3140–3152. doi: 10.1002/ptr.6486. [DOI] [PubMed] [Google Scholar]
- 132.Phuwamongkolwiwat P., Suzuki T., Hira T., Hara H. Fructooligosaccharide augments benefits of quercetin-3-O-β-glucoside on insulin sensitivity and plasma total cholesterol with promotion of flavonoid absorption in sucrose-fed rats. Eur J Nutr. 2014;53:457–468. doi: 10.1007/s00394-013-0546-2. [DOI] [PubMed] [Google Scholar]
- 133.Zang Y., Zhang L., Igarashi K., Yu C. The anti-obesity and anti-diabetic effects of kaempferol glycosides from unripe soybean leaves in high-fat-diet mice. Food Funct. 2015;6:834–841. doi: 10.1039/c4fo00844h. [DOI] [PubMed] [Google Scholar]
- 134.Yin Y., Gao L., Lin H., Wu Y., Han X., Zhu Y., et al. Luteolin improves non-alcoholic fatty liver disease in db/db mice by inhibition of liver X receptor activation to down-regulate expression of sterol regulatory element binding protein 1c. Biochem Biophys Res Commun. 2017;482:720–726. doi: 10.1016/j.bbrc.2016.11.101. [DOI] [PubMed] [Google Scholar]
- 135.Zang Y., Igarashi K., Li Y. Anti-diabetic effects of luteolin and luteolin-7-O-glucoside on KK-Ay mice. Biosci Biotechnol Biochem. 2016;80:1580–1586. doi: 10.1080/09168451.2015.1116928. [DOI] [PubMed] [Google Scholar]
- 136.Zhu Y., Liu R., Shen Z., Cai G. Combination of luteolin and lycopene effectively protect against the "two-hit” in NAFLD through Sirt1/AMPK signal pathway. Life Sci. 2020;256:117990. doi: 10.1016/j.lfs.2020.117990. [DOI] [PubMed] [Google Scholar]
- 137.Porras D., Nistal E., Martínez-Flórez S., Pisonero-Vaquero S., Olcoz J.L., Jover R., et al. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut–liver axis activation. Free Radic Biol Med. 2017;102:188–202. doi: 10.1016/j.freeradbiomed.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 138.Alkhalidy H., Moore W., Wang A., Luo J., McMillan R.P., Wang Y., et al. Kaempferol ameliorates hyperglycemia through suppressing hepatic gluconeogenesis and enhancing hepatic insulin sensitivity in diet-induced obese mice. J Nutr Biochem. 2018;58:90–101. doi: 10.1016/j.jnutbio.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sagawa H., Naiki-Ito A., Kato H., Naiki T., Yamashita Y., Suzuki S., et al. Connexin 32 and luteolin play protective roles in non-alcoholic steatohepatitis development and its related hepatocarcinogenesis in rats. Carcinogenesis. 2015;36:1539–1549. doi: 10.1093/carcin/bgv143. [DOI] [PubMed] [Google Scholar]
- 140.Chao J., Huo T.I., Cheng H.Y., Tsai J.C., Liao J.W., Lee M.S., et al. Gallic acid ameliorated impaired glucose and lipid homeostasis in high fat diet-induced NAFLD mice. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Silva F.S., Oliveira P.J., Duarte M.F. Oleanolic, ursolic, and betulinic acids as food supplements or pharmaceutical agents for type 2 diabetes: promise or illusion? J Agric Food Chem. 2016;64:2991–3008. doi: 10.1021/acs.jafc.5b06021. [DOI] [PubMed] [Google Scholar]
- 142.Lin Y.N., Wang C.C.N., Chang H.Y., Chu F.Y., Hsu Y.A., Cheng W.K., et al. Ursolic acid, a novel liver X receptor α (LXRα) antagonist inhibiting ligand-induced nonalcoholic fatty liver and drug-induced lipogenesis. J Agric Food Chem. 2018;66:11647–11662. doi: 10.1021/acs.jafc.8b04116. [DOI] [PubMed] [Google Scholar]
- 143.Li J.S., Wang W.J., Sun Y., Zhang Y.H., Zheng L. Ursolic acid inhibits the development of nonalcoholic fatty liver disease by attenuating endoplasmic reticulum stress. Food Funct. 2015;6:1643–1651. doi: 10.1039/c5fo00083a. [DOI] [PubMed] [Google Scholar]
- 144.Wang J., Jiao Q., Wang H.B., Song H.M. Research progress on chemical constituents, quality evaluation and pharmacological activities of Potentilla discolor. Chin Tradit Patent Med. 2016;38:1590–1593. [Google Scholar]
- 145.Zhu X., Xiong T., Liu P., Guo X., Xiao L., Zhou F., et al. Quercetin ameliorates HFD-induced NAFLD by promoting hepatic VLDL assembly and lipophagy via the IRE1a/XBP1s pathway. Food Chem Toxicol. 2018;114:52–60. doi: 10.1016/j.fct.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 146.Sun Y., Wang Y., Tan B.L., Deng Y.R. Comparison of six flavonoid components of closely-related plants agrimonia pilosa, Potentilla chinensis and Potentilla discolor. Zhong Yao Cai. 2016;39:991–995. [PubMed] [Google Scholar]
- 147.Liu Q., Pan R., Ding L., Zhang F., Hu L., Ding B., et al. Rutin exhibits hepatoprotective effects in a mouse model of non-alcoholic fatty liver disease by reducing hepatic lipid levels and mitigating lipid-induced oxidative injuries. Int Immunopharm. 2017;49:132–141. doi: 10.1016/j.intimp.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 148.Wu C.H., Lin M.C., Wang H.C., Yang M.Y., Jou M.J., Wang C.J. Rutin inhibits oleic acid induced lipid accumulation via reducing lipogenesis and oxidative stress in hepatocarcinoma cells. J Food Sci. 2011;76:T65–T72. doi: 10.1111/j.1750-3841.2010.02033.x. [DOI] [PubMed] [Google Scholar]
- 149.Lv Y., Gao X., Luo Y., Fan W., Shen T., Ding C., et al. Apigenin ameliorates HFD-induced NAFLD through regulation of the XO/NLRP3 pathways. J Nutr Biochem. 2019;71:110–121. doi: 10.1016/j.jnutbio.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 150.Feng X., Yu W., Li X., Zhou F., Zhang W., Shen Q., et al. Apigenin, a modulator of PPARγ, attenuates HFD-induced NAFLD by regulating hepatocyte lipid metabolism and oxidative stress via Nrf2 activation. Biochem Pharmacol. 2017;136:136–149. doi: 10.1016/j.bcp.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 151.Shi T., Zhuang R., Zhou H., Wang F., Shao Y., Cai Z. Effect of apigenin on protein expressions of PPARs in liver tissues of rats with nonalcoholic steatohepatitis. Zhonghua Gan Zang Bing Za Zhi. 2015;23:124–129. doi: 10.3760/cma.j.issn.1007-3418.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 152.Zhang Y., Zhang L.M., Qi Y.X., Hao J.F., Li F.R., Gao Y.S. Determination of ursolic acid in Potentilla discolor Bge. by HPLC. Chin Pharm. 2006;16:20–21. [Google Scholar]
- 153.Matumba M.G., Ayeleso A.O., Nyakudya T., Erlwanger K., Chegou N.N., Mukwevho E. Long-term impact of neonatal intake of oleanolic acid on the expression of AMP-activated protein kinase, adiponectin and inflammatory cytokines in rats fed with a high fructose diet. Nutrients. 2019;11:226. doi: 10.3390/nu11020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ou-Yang Q., Xuan C.X., Wang X., Luo H.Q., Liu J.E., Wang L.L., et al. 3-Acetyl-oleanolic acid ameliorates non-alcoholic fatty liver disease in high fat diet-treated rats by activating AMPK-related pathways. Acta Pharmacol Sin. 2018;39:1284–1293. doi: 10.1038/aps.2017.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ponnulakshmi R., Shyamaladevi B., Vijayalakshmi P., Selvaraj J. In silico and in vivo analysis to identify the antidiabetic activity of beta sitosterol in adipose tissue of high fat diet and sucrose induced type-2 diabetic experimental rats. Toxicol Mech Methods. 2019;29:276–290. doi: 10.1080/15376516.2018.1545815. [DOI] [PubMed] [Google Scholar]
- 156.Kawano Y., Cohen D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol. 2013;48:434–441. doi: 10.1007/s00535-013-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mitro N., Mak P.A., Vargas L., Godio C., Hampton E., Molteni V., et al. The nuclear receptor LXR is a glucose sensor. Nature. 2007;445:219–223. doi: 10.1038/nature05449. [DOI] [PubMed] [Google Scholar]
- 158.Maczewsky J., Sikimic J., Bauer C., Krippeit-Drews P., Wolke C., Lendeckel U., et al. The LXR ligand T0901317 acutely inhibits insulin secretion by affecting mitochondrial metabolism. Endocrinology. 2017;158:2145–2154. doi: 10.1210/en.2016-1941. [DOI] [PubMed] [Google Scholar]
- 159.Seelinger G., Merfort I., Schempp C.M. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008;74:1667–1677. doi: 10.1055/s-0028-1088314. [DOI] [PubMed] [Google Scholar]
- 160.Wang X., Ikejima K., Kon K., Arai K., Aoyama T., Okumura K., et al. Ursolic acid ameliorates hepatic fibrosis in the rat by specific induction of apoptosis in hepatic stellate cells. J Hepatol. 2011;55:379–387. doi: 10.1016/j.jhep.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 161.Madlala H.P., Van Heerden F.R., Mubagwa K., Musabayane C.T. Changes in renal function and oxidative status associated with the hypotensive effects of oleanolic acid and related synthetic derivatives in experimental animals. PLoS One. 2015;10 doi: 10.1371/journal.pone.0128192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yu Z., Sun W., Peng W., Yu R., Li G., Jiang T. Pharmacokinetics in vitro and in vivo of two novel prodrugs of oleanolic acid in rats and its hepatoprotective effects against liver injury induced by CCl4. Mol Pharm. 2016;13:1699–1710. doi: 10.1021/acs.molpharmaceut.6b00129. [DOI] [PubMed] [Google Scholar]
- 163.Liese J., Abhari B.A., Fulda S. Smac mimetic and oleanolic acid synergize to induce cell death in human hepatocellular carcinoma cells. Cancer Lett. 2015;365:47–56. doi: 10.1016/j.canlet.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 164.Gamede M., Mabuza L., Ngubane P., Khathi A. Plant-derived oleanolic acid ameliorates markers associated with non-alcoholic fatty liver disease in a diet-induced pre-diabetes rat model. Diabetes Metab Syndr Obes. 2019;12:1953–1962. doi: 10.2147/DMSO.S218626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Agrawal N.K., Kant S. Targeting inflammation in diabetes: newer therapeutic options. World J Diabetes. 2014;5:697–710. doi: 10.4239/wjd.v5.i5.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 167.Kwon E.Y., Jung U.J., Park T., Yun J.W., Choi M.S. Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in mice with diet-induced obesity. Diabetes. 2015;64:1658–1669. doi: 10.2337/db14-0631. [DOI] [PubMed] [Google Scholar]
- 168.Duan L., Ding W., Liu X., Cheng X., Cai J., Hua E., et al. Biosynthesis and engineering of kaempferol in saccharomyces cerevisiae. Microb Cell Factories. 2017;16:165. doi: 10.1186/s12934-017-0774-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ma J., Cao H., Rodrigues R.M., Xu M., Ren T., He Y., et al. Chronic-plus-binge alcohol intake induces production of proinflammatory mtDNA-enriched extracellular vesicles and steatohepatitis via ASK1/p38MAPKα-dependent mechanisms. JCI Insight. 2020;5 doi: 10.1172/jci.insight.136496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Selloum L., Bouriche H., Tigrine C., Boudoukha C. Anti-inflammatory effect of rutin on rat paw oedema, and on neutrophils chemotaxis and degranulation. Exp Toxicol Pathol. 2003;54:313–318. doi: 10.1078/0940-2993-00260. [DOI] [PubMed] [Google Scholar]
- 171.Alinejad B., Ghorbani A., Sadeghnia H.R. Effects of combinations of curcumin, linalool, rutin, safranal, and thymoquinone on glucose/serum deprivation-induced cell death. Avicenna J Phytomed. 2013;3:321–328. [PMC free article] [PubMed] [Google Scholar]
- 172.Sadeghnia H.R., Yousefsani B.S., Rashidfar M., Boroushaki M.T., Asadpour E., Ghorbani A. Protective effect of rutin on hexachlorobutadiene-induced nephrotoxicity. Ren Fail. 2013;35:1151–1155. doi: 10.3109/0886022X.2013.815546. [DOI] [PubMed] [Google Scholar]
- 173.Janbaz K.H., Saeed S.A., Gilani A.H. Protective effect of rutin on paracetamol-and CCl4-induced hepatotoxicity in rodents. Fitoterapia. 2002;73:557–563. doi: 10.1016/s0367-326x(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 174.Vinayagam R., Xu B. Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutr Metab (Lond) 2015;12:60. doi: 10.1186/s12986-015-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Muthenna P., Akileshwari C., Saraswat M., Bhanuprakash Reddy G. Inhibition of advanced glycation end-product formation on eye lens protein by rutin. Br J Nutr. 2012;107:941–949. doi: 10.1017/S0007114511004077. [DOI] [PubMed] [Google Scholar]
- 176.Boden G., Chen X., Stein T.P. Gluconeogenesis in moderately and severely hyperglycemic patients with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2001;280:E23–E30. doi: 10.1152/ajpendo.2001.280.1.E23. [DOI] [PubMed] [Google Scholar]
- 177.Vetter S.W. Glycated serum albumin and AGE receptors. Adv Clin Chem. 2015;72:205–275. doi: 10.1016/bs.acc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 178.Walter P., Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 179.Wiseman R.L., Zhang Y., Lee K.P., Harding H.P., Haynes C.M., Price J., et al. Flavonol activation defines an unanticipated ligand-binding site in the kinase-RNase domain of IRE1. Mol Cell. 2010;38:291–304. doi: 10.1016/j.molcel.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Woo M., Kim M., Noh J.S., Song Y.O. Kimchi methanol extracts attenuate hepatic steatosis induced by high cholesterol diet in low-density lipoprotein receptor knockout mice through inhibition of endoplasmic reticulum stress. J Funct Foods. 2017;32:218–225. [Google Scholar]
- 181.Hoyles L., Fernández-Real J.M., Federici M., Serino M., Abbott J., Charpentier J., et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat Med. 2018;24:1070–1080. doi: 10.1038/s41591-018-0061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chávez-Talavera O., Tailleux A., Lefebvre P., Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152:1679–1694.e3. doi: 10.1053/j.gastro.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 183.Mouzaki M., Loomba R. Insights into the evolving role of the gut microbiome in nonalcoholic fatty liver disease: rationale and prospects for therapeutic intervention. Therap Adv Gastroenterol. 2019;12 doi: 10.1177/1756284819858470. 1756284819858470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Carr R.M., Reid A.E. FXR agonists as therapeutic agents for non-alcoholic fatty liver disease. Curr Atherosclerosis Rep. 2015;17:500. doi: 10.1007/s11883-015-0500-2. [DOI] [PubMed] [Google Scholar]
- 185.Meroni M., Longo M., Dongiovanni P. The role of probiotics in nonalcoholic fatty liver disease: a new insight into therapeutic strategies. Nutrients. 2019;11:2642. doi: 10.3390/nu11112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chu H., Duan Y., Yang L., Schnabl B. Small metabolites, possible big changes: a microbiota-centered view of non-alcoholic fatty liver disease. Gut. 2019;68:359–370. doi: 10.1136/gutjnl-2018-316307. [DOI] [PubMed] [Google Scholar]
- 187.Hsu C.L., Yen G.C. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br J Nutr. 2007;98:727–735. doi: 10.1017/S000711450774686X. [DOI] [PubMed] [Google Scholar]
- 188.Jang A., Srinivasan P., Lee N.Y., Song H.P., Lee J.W., Lee M., et al. Comparison of hypolipidemic activity of synthetic gallic acid-linoleic acid ester with mixture of gallic acid and linoleic acid, gallic acid, and linoleic acid on high-fat diet induced obesity in C57BL/6 Cr Slc mice. Chem Biol Interact. 2008;174:109–117. doi: 10.1016/j.cbi.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 189.Peng C.H., Liu L.K., Chuang C.M., Chyau C.C., Huang C.N., Wang C.J. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J Agric Food Chem. 2011;59:2663–2671. doi: 10.1021/jf1043508. [DOI] [PubMed] [Google Scholar]