Abstract
Ipsilateral transient axillary lymphadenopathy is well-documented following COVID-19 mRNA vaccine administration. Recently, rare mammographic findings of breast tissue changes with co-existing lymphadenopathy have been documented. Current literature on isolated ipsilateral true breast parenchymal changes on diagnostic mammography in symptomatic patients following COVID-19 mRNA vaccine administration is limited. This is one of the first case reports that demonstrates isolated ipsilateral focal asymmetry 5 days after administration of COVID-19 mRNA vaccine followed by complete resolution of symptoms and focal asymmetry confirmed on follow up magnetic resonance imaging. These findings warrant the development of guidelines to reduce unnecessary invasive procedures as part of the workup for possible malignancy.
Keywords: Focal asymmetry, COVID-19, Breast, Mammography
Abbreviations: MRI, magnetic resonance imaging; CC, craniocaudal; MLO, mediolateral oblique; MIP, maximum intensity projection
Introduction
Ipsilateral axillary lymphadenopathy on mammography is well-documented as an adverse effect following administration of COVID-19 mRNA vaccine. Previous incidence of axillary lymphadenopathy following administration of Moderna and Pfizer vaccines was 11.3% and 0.3% respectively with newer data reporting an incidence of 44% [1], [2], [3]. The axillary lymphadenopathy is most likely the result of activation of robust immune defenses after exposure to the vaccine antigen [4]. Prior to the recent COVID-19 pandemic and subsequent increase in vaccine roll out, isolated regional lymphadenopathy has been documented as an adverse effect following administration of other vaccines such as Measles, Human Papilloma Virus and H1N1 influenza vaccines [5], [6], [7]. Recently, rare mammographic findings of true breast parenchymal changes and skin changes with co-existing ipsilateral axillary lymphadenopathy following administration of COVID-19 vaccine have been documented [8]. The documented cases of vaccine associated breast parenchymal changes are thought to arise from a similar inflammatory process described in COVID-19 vaccine associated ipsilateral lymphadenopathy, which supports the self-resolving nature of these findings [8]. To our knowledge, there is little documented in the literature of an isolated new breast focal asymmetry on diagnostic mammography in the absence of ipsilateral axillary lymphadenopathy after administration of the COVID-19 vaccine booster.
Clinical findings
A 52-year-old postmenopausal female with a history of thyroid cancer, diagnosed in 2007, complicated by recurrence and treated with radioactive iodine presented for bilateral diagnostic mammography and bilateral breast ultrasound following the onset of a unilateral palpable firm breast mass with surrounding area of erythema in the upper outer quadrant of the left breast. There was no history of trauma, bruising or recent infection over the area of interest. The patient reported a family history of breast cancer in her paternal aunt. She also had a history of a prior benign left breast biopsy. She had no personal history of dermatologic, rheumatologic, or autoimmune disease. The patient received the Pfizer COVID-19 mRNA booster in the left arm 5 days prior to presenting in clinic and her symptoms began 2 days following vaccine administration.
Diagnostic mammography performed on 12/20/21 demonstrated a new focal asymmetry involving the upper outer quadrant of the left breast. Diagnostic mammogram did not demonstrate any lymphadenopathy. The breast ultrasound performed immediately following the mammogram was unremarkable in the area of interest. A breast MRI was obtained approximately 5 weeks following the initial diagnostic work up and demonstrated no suspicious mass or nonmass enhancement in the left breast upper outer quadrant of the left breast and no evidence of axillary lymphadenopathy. The patient also noted complete resolution of symptoms.
Radiology
Diagnosticmammography: Fig. 1, Fig. 2 and 2.
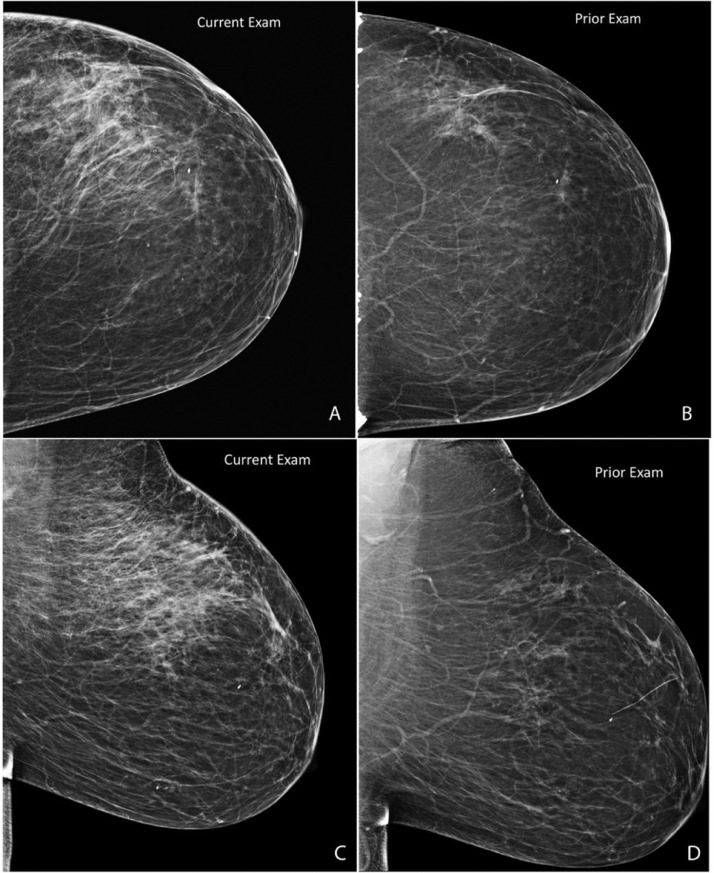
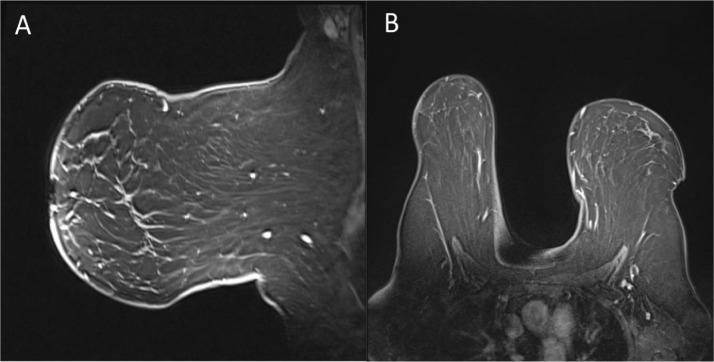
Fig. 1.
Routine current CC (A) and MLO (C) views with tomosynthesis were obtained and compared to prior CC (B) and MLO (D) 2- dimensional views. Current images demonstrate a new focal asymmetry involving the majority of the left breast upper outer quadrant, middle third to posterior third depth.
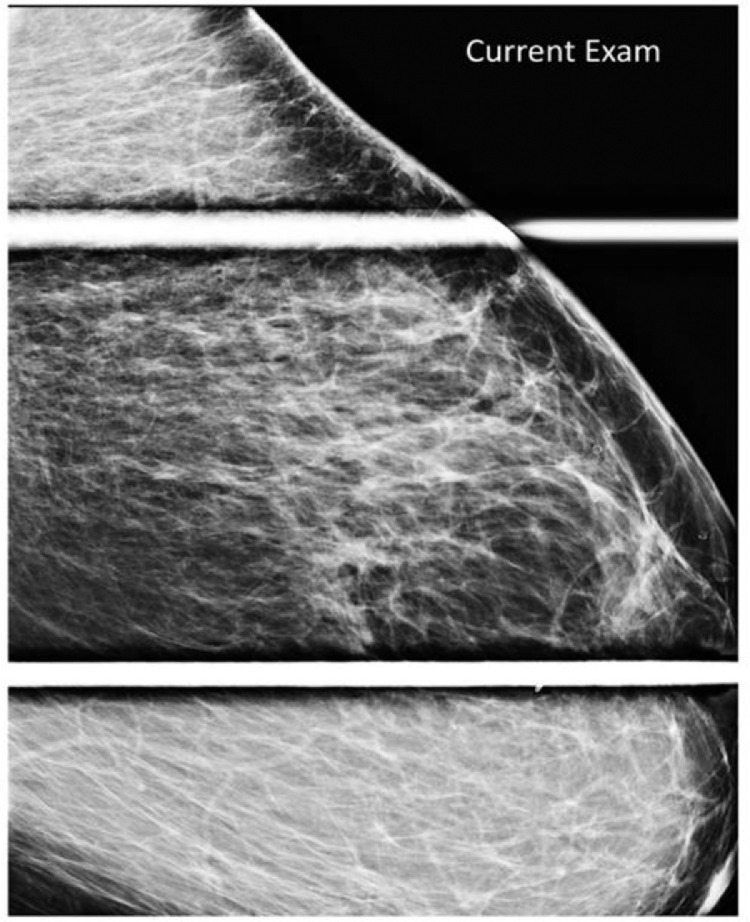
Fig. 2.
Representative left MLO spot compression view redemonstrating the large focal asymmetry of the left breast upper outer quadrant.
Ultrasound: Fig. 3
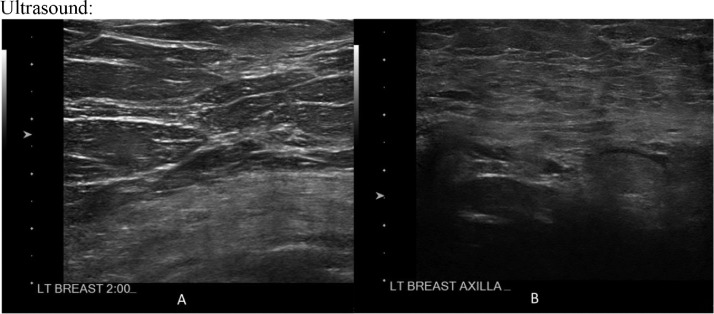
Fig. 3.
Static representative ultrasound image of the left breast upper outer quadrant, labeled as 2:00 (A), demonstrating no suspicious cystic or solid abnormality. Static representative ultrasound image of the left axilla (B) demonstrating normal morphology left axillary lymph nodes with preservation of normal architecture.
MRI: The patient presented for MRI 5 weeks following the initial diagnostic work up (Fig. 4, Fig. 5, Fig. 6).
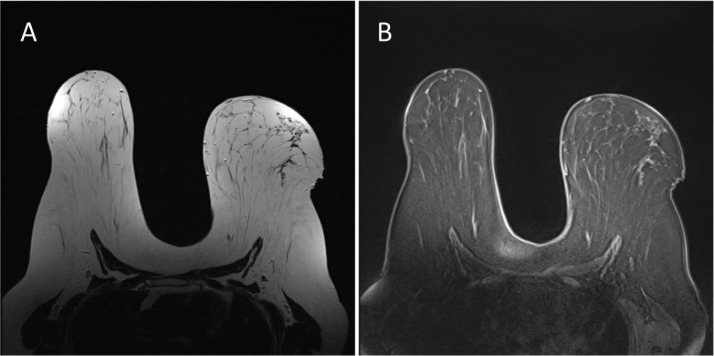
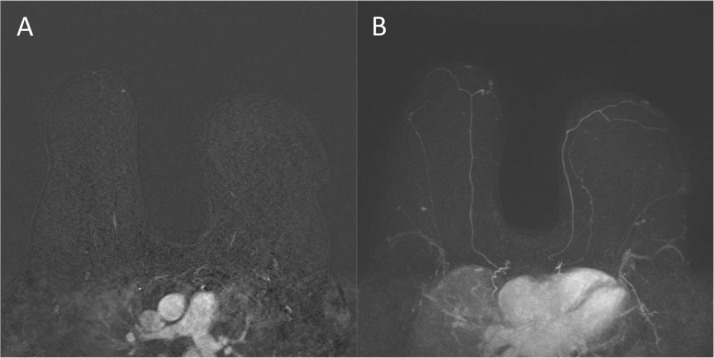
Fig. 4.
Axial T2 (A) image and Axial T1 fat-saturated (B) image (pre-contrast) demonstrating no abnormalities in the left breast upper outer quadrant.
Fig. 5.
Axial fat-saturated T1 (A) subtracted postcontrast image demonstrating no suspicious mass or nonmass enhancement in the left breast upper outer quadrant. Representative MIP (B) image demonstrating no suspicious enhancement in the left breast upper outer quadrant.
Fig. 6.
Representative sagittal (A) and axial (B) fat-saturated postcontrast images demonstrating no evidence of axillary lymphadenopathy.
Discussion
To our knowledge, this is one of the first case reports related to true breast parenchymal changes following the COVID-19 booster in the absence of reactive axillary lymphadenopathy. Locklin et al. reported breast parenchymal changes following administration of COVID-19 vaccine with co-existing lymphadenopathy with subsequent resolution of clinical symptoms and imaging findings following a 4–6-week interval [8]. In this case report, as well as the other cases described by Locklin et al., the timeline of breast parenchymal changes following vaccination administration and subsequent complete resolution on imaging aligns with the well-researched timeline and resolution of isolated COVID-19 vaccine related ipsilateral axillary lymphadenopathy [8]. The presence of isolated mammographic breast parenchymal changes such as a developing asymmetry or a focal asymmetry, can result in unnecessary testing and invasive procedures as part of the recommended workup for malignancy. This novel finding of breast parenchymal changes, specifically a focal asymmetry in the absence of unilateral axillary lymphadenopathy following COVID-19 vaccination warrants the development of guidelines to reduce unnecessary imaging and invasive biopsies. Similarly, vaccine history and timeframe for administration remain important when evaluating for the likely etiology of isolated new focal asymmetry in both asymptomatic and symptomatic patients particularly in the setting of recent ipsilateral extremity vaccine administration. Further research focusing on the pathophysiology of true breast parenchymal changes following COVID-19 vaccination should be explored to broaden our understanding of the different individual responses observed among the recently vaccinated.
Patient consent
The patient provided written informed consent for the use of their pertinent medical history, detailed case presentation, and relevant radiographic imaging for publication of this case report with the understanding that all patient identifiers would be removed prior to submission.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Wolfson S, Kim E, Plaunova A, Bukhman R, Sarmiento RD, Samreen N, et al. Axillary adenopathy after COVID-19 vaccine: no reason to delay screening mammogram. Radiology. 2022 doi: 10.1148/radiol.213227. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moderna COVID-19 vaccine: vaccines and related biological products advisory committee meeting, 2020. Available at: https://www.fda.gov/media/144434/download Accessed April 2022. [DOI] [PubMed]
- 3.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim J, Lee SA, Khil EK, Byeon SJ, Kang HJ, Choi JA. COVID-19 vaccine-related axillary lymphadenopathy in breast cancer patients: case series with a review of literature. Semin Oncol. 2021;48(4-6):283–291. doi: 10.1053/j.seminoncol.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coates EE, Costner PJ, Nason MC, Herrrin DM, Conant S, Herscovitch P, et al. Lymph node activation by PET/CT following vaccination with licensed vaccines for human papillomaviruses. Clin Nucl Med. 2017;42(5):329–334. doi: 10.1097/RLU.0000000000001603. [DOI] [PubMed] [Google Scholar]
- 6.Shirone N, Shinkai T, Yamane T, Uto F, Yoshimura H, Tamai H, et al. Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann Nucl Med. 2012;26(3):248–252. doi: 10.1007/s12149-011-0568-x. [DOI] [PubMed] [Google Scholar]
- 7.Dorfman R.F., Herweg J.C. Live, attenuated measles virus vaccine. Inguinal lymphadenopathy complicating administration. JAMA. 1966;198(3):320–321. [PubMed] [Google Scholar]
- 8.Locklin J.N., Woodard G.A. Mammographic and sonographic findings in the breast and axillary tail following a COVID-19 vaccine. Clin Imaging. 2021;80:202–204. doi: 10.1016/j.clinimag.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]