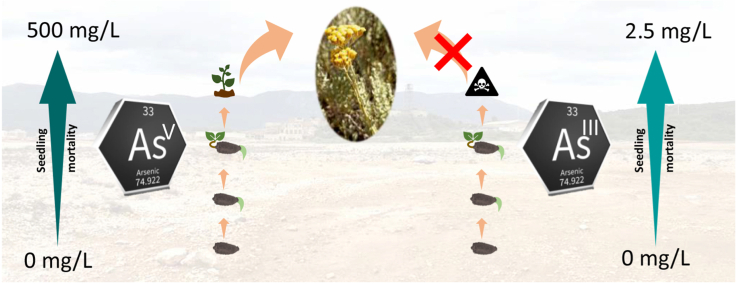
Abstract
Arsenate, As(V), and arsenite, As(III), are the most available arsenicals present in the soil solutions, in particular in mine polluted substrates, and cause several symptoms of toxicity in plants (like inhibition of seed germination and reduction of seedling development). For these reasons, seeds germination studies are essential for the design of phytoremediation activities of mine sites. Seed germination and seedling development of Helichrysum microphyllum subsp. tyrrhenicum, were evaluated at 15 °C using various concentrations of As(V) and As(III) (0–500 mg/L and 0–200 mg/L, respectively). Seeds were harvested (I) into a mine dump contaminated in As, (II) nearby this site, and (III) faraway the As contaminated area and without mine activities. Seed germination, cotyledons emergence, and seedling mortality were evaluated for 90 days. As(V) and As(III) acted differently, showing a much higher toxicity when arsenite was added than arsenate. The taxon was able to germinate, develop cotyledons, and survive under all arsenate concentrations, whereas arsenite acted on these steps already at 2.5 mg/L. Moreover, a linear decrease in cotyledons emergence was assessed with the increase of both arsenicals’ concentrations, as well as a linear decrease of seedling survival under arsenite. The taxon showed great adaptability to As pollution, giving an important contribution in phytoremediation of mining sites.
Keywords: Arsenate, Arsenite, Asteraceae, Mine areas, Mediterranean vascular flora, Phytoremediation
Arsenate; Arsenite; Asteraceae; Mine areas; Mediterranean vascular flora; Phytoremediation.
Graphical abstract
1. Introduction
Arsenic (As) is a word widespread pollutant, and the contamination of waters, soils, and food by As is of health public concern (Chen et al., 2017; Iftikhar et al., 2021; Quaghebeur and Rengel, 2005). It is considered a carcinogenic of “class A″ indeed the international guidelines of “Word Health Organization” (WHO) established restrictive concentration limits in drinking water (10 μg/L; Kumar et al., 2019). The pool of arsenic can be of natural origins (like volcanic eruption), and anthropogenic (i.e. mine activities and related waste materials; Adriano, 2001; Farooq et al., 2016; Li et al., 2007). In the last frame, sulphur (S) ores are the primary source of As (Milton and Johnson, 1999) which often occurs together with other metals and metalloids.
The As chemistry is considered complex by scientific community, mainly because of its several oxidation number and chemical species (inorganic and organic), different levels of toxicity as well as mobility and bioavailability. Generally, inorganic arsenicals are more poisonous than organic ones and pentavalent forms are less poisonous compared with trivalent ones for the living organisms and the whole environment (Adriano, 2001; Quaghebeur and Rengel, 2005): in particular, arsenite, As(III), is sixty times more mobile, poisonous and soluble than arsenate, As(V) (Abbas et al., 2018; Iftikhar et al., 2021). Regardless, different results are available for plants, which shown that the harmful effect of arsenicals depends on the involved taxon and local conditions (Quaghebeur and Rengel, 2005).
When plants species are the final focus of the As intoxication, the most bioavailable arsenical forms must be taken into account: As(V) and As(III) are the most available As species present into soil solutions. In detail, As(V) is common in soils with aerobic conditions, whereas As(III) is more present in anaerobic one (Jiménez et al., 2009). Regardless, both arsenicals cause several symptoms of toxicity such as compromission of seed germination, reduction of plant development, leaf blades’ necrosis and wilting, decrease in leaf surface, and photosynthetic activity (Abbas et al., 2018; Abedin and Meharg, 2002 and reference therein; Shri et al., 2009). Furthermore, in recent years it has been confirmed that during experiments in the presence of a specific arsenic form some plants species are capable to change it to other forms (Zabłudowska et al., 2009; Budzyńska et al., 2021).
The mitigation of As pollution is widely studied when plants are a source of food and this issue is deeply investigated especially in the countries of South-Eastern Asia (i.e. Bangladesh, India and China) where the pollution of irrigation water is the first pool of As contamination for cultivation activities (Abedin and Meharg, 2002; Moulick et al., 2016; Tripathi et al., 2007). Furthermore, the dispersion of As by mine activities and related waste materials in the biogeochemical spheres is another crucial issue, and for this reason, remediation for this phenomenon is of great interest. The soluble arsenic may be removed through conventional techniques such as precipitation (Bolisetty et al., 2019), nanofiltration (Gilhotra et al., 2018) or adsorption (Ma et al., 2013; Sanna Angotzi et al., 2021; 2022), or via direct action for the reclamation of the polluted areas, through phytoremediation. In detail, numerous examples of As tolerance are reported in plants, like Silene vulgaris (Moench) Garcke (Sneller et al., 1999) and even more of hyperaccumulation like Callitriche stagnalis Scop (Robinson et al., 2006). or Pteris vittata L. (Ma et al., 2001). In the Mediterranean Basin, this issue has been studied for several species that grow spontaneously in mine polluted substrates. They were found suitable for phytostabilization, tolerating and accumulating As into roots (Abreu et al., 2008; Carvalho et al., 2020; Márquez-García et al., 2012).
Although the importance of Sardinia for the European mining industry until the XXth century and several As contamination hot spots are nowadays still recognizable among the island (Ardau et al., 2008; Cidu and Biddau, 2007, Cidu et al., 2005; Cidu and Fanfani, 2002; Frau et al., 2005, 2008), no studies concerning As phytoremediation were carried out. Nevertheless, in the last two decades, different native taxa were found tolerant to metals like Zn and Pb (e.g., Bacchetta et al., 2012, 2015, 2017, 2018; Boi et al., 2020a, 2020b, 2021; Cao et al., 2004; Concas et al., 2015; De Giudici et al., 2015, 2017; Jiménez et al., 2005, 2011, 2014, 2021; Medas et al., 2015, 2017). In the last years, more of the efforts were spent investigating H. microphyllum subsp. tyrrhenicum (from now on H. tyrrhenicum) using a multidisciplinary approach that involves botany, chemistry, mineralogy, and environmental engineering. This endemism of Sardinia and Corsica (Fois et al., 2022) is a pioneer taxon of heavy metals polluted substrates (Bacchetta et al., 2003, 2007; Angiolini et al., 2005). Moreover, it is an indifferent edaphic species that grows on a wide altitudinal range. This approach has pointed out its ability to tolerate heavy concentrations of Zn and Pb, storing them in the hypogean organs and behaving as excluder species (Bacchetta et al., 2017, 2018; Boi et al., 2020b, 2021; Cao et al., 2004). Furthermore, ecophysiology of seed germination studies pointed out that seeds can germinate under very high concentrations of Zn and Pb, without the complete inhibition of the process (Boi et al., 2020a). These studies are essential for the design of phytoremediation activities of mine sites; indeed, germination is a critical step where several factors can interfere in this process, for instance metal(loid)s in their growing medium which can hamper germination and/or seedling development (Kranner and Colville, 2011). Moreover, the defense mechanism at this life stage is not fully developed, making plantlets highly sensitive to metal(loid)s (Liu et al., 2005). In this framework, the majority of the literature is focused on edible plant species like rice and wheat, and only a few studies are phytoremediation oriented, even more, if the Mediterranean area and As contamination are considered. Regardless, some studies on Mediterranean species emphasized that ecophysiology of seed germination is an important parameter to consider in remediation action planning (Carvalho et al., 2020; Márquez-García et al., 2013; Lefèvre et al., 2009).
In the light of the presence of several As hotspots in Sardinia and considering the results of previous phytoremediation research about H. tyrrhenicum, this work was dedicated to germination and seedling development of this taxon under As stress, both in the As(V) and As(III) forms. Before the evaluation of germination and seedling development, seeds were subjected to a preliminary investigation in terms of As content (and also elementary one), as a solid starting point for the this study. In depth, the purposes of this work were the evaluation of the effect of As speciation in terms of: I) seed germination under different concentrations of As(V) and As(III); II) cotyledons’ emergence under these stress conditions; III) mortality of young seedlings. To the best of our knowledge, our study is the first research that consider As speciation during seed germination and early seedling development of Mediterranean wild species.
2. Materials and methods
2.1. Seed harvesting
Helichrysum tyrrhenicum seeds (achenes) were harvested in different sites of the Sulcis-Iglesiente (SW Sardinia) in the summer of 2020, following its phenological calendar. These sampling areas were already chosen and described in earlier work (Boi et al., 2020b): (I) the mine waste dump of Campo Pisano (CP), (II) nearby this site (OCP), and (III) Campu S'Isca (CSI). CP's plants grow on substrates highly polluted in terms of metal (loid)s (such as As, Cd, Pb, Zn, and mercury, Hg) which are present in the waste materials of the flotation process of galena (PbS) and blende (ZnS) (Boi et al., 2020a). The plants of OCP grow on soils deriving from schists but are, however, naturally subjected to high levels of metal(loids)s and also to the aeolian dispersal of pollutants (Boi et al., 2020a). Plants of CSI grow on an unpolluted substrate and are not subjected to mine impact. For each specimen, 30% of available mature seeds were collected. After seed collection, materials were subjected to a quarantine period of one month (40% relative humidity and ≈ 20 °C) in order to allow a slow post-ripening and evaluate the phytosanitary state of the collected material. Finally, seeds were selected by removing damaged and empty seeds. Before germination tests (see next paragraph), seeds were investigated in terms of As content and elementary composition.
2.2. Compositional analyses of seeds
Before germination tests, seeds were analyzed by SEM-EDX and Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES) to define whether As (and eventually other heavy metals/metalloids) were already present among seed. This step was fundamental in order to select the As concentrations to apply in the following germination tests. SEM-EDX analysis was performed by means of Quanta 200 ESEM (FEI Company, Hillsboro, OR, USA) equipped with a Thermo Scientific EDX probe (Thermo Fisher, Walthman, MA, USA) with an electron beam acceleration of 30 kV and a sample chamber internal residual pressure of 0.75 bar. The images of analyzed areas were acquired in backscattered mode. Seeds were analyzed both on the external surface and on the internal part. To expose the internal area, a cross-section was manually cut with a sharp razor. For each locality, six seeds and twelve sections were selected. On the entire seeds, six square areas (about 100 × 100 mm) were analyzed, and on each section, three areas (about 50 × 50 mm) were selected (see Figure S1 in the supplementary materials). A total of 36 areas on the entire seeds and 36 areas for the sections were analyzed for every sampling site.
The ICP-OES analysis was carried out after seeds mineralization. A portion of the seeds was dried at 40 °C overnight, carefully checked with the help of an optic microscope to remove cuticles, bugs, and anything apart from the seeds themselves, and milled in an Agate mortar. Thirty mg of seeds from each site were digested on hot plate with 1 mL of H2O2 (30 wt.%, Normapur®, from VWR, Leuven, Belgium), 9 mL of HCl (37 wt.%, Aristar® from VWR, Leuven, Belgium), and 3 mL of HNO3 (67 wt.%, Normatom® from VWR, Leuven, Belgium) inside an Erlenmeyer flask equipped with an Allhin condenser. The mixture was heated at 150 °C for 2 h and once cooled, the content was filtered into a 25 mL volumetric flask with a 45 μm acetate cellulose filter and finally analyzed by the ICP–OES Agilent 5110 (Agilent, Santa Clara, CA, USA). The ICP-OES calibration line was performed in the range 0.02–10 mg/L using ICP standard solutions (Merk, Darmstadt, Germany, for As standard Fluka, St. Louise, MO, USA) diluted in HNO3 2%. The analysis was repeated three times in axial mode, at the following wavelengths: aluminum (Al) 396.152 nm, As 188.980 nm, calcium (Ca) 396.847 nm, Cd 214.439 nm, cobalt (Co) 228.615 nm, chromium (Cr) 267.716 nm, copper (Cu) 324.754 nm, iron (Fe) 238.204 nm, potassium (K) 769.897 nm, magnesium (Mg) 279.553 nm, manganese (Mn) 267.610 nm, sodium (Na) 588.995 nm, nickel (Ni) 231.604 nm, Pb 220.353 nm, Zn 213.857 nm.
Table S1 and Figure S2 (see Supplementary materials) show in the details the results of the SEM-EDX and ICP-OES analyses. No As trace were detected using both methods in seeds belonging from the different sites, whereas Pb and Zn can be evidenced in all the samples in low concentrations (<0.1%) only by ICP-OES. Besides the absence of As, it is also worth noting the absence of other hazardous elements that characterize especially the area of Campo Pisano, such as Cd, Sb, and Hg (Boi et al., 2020b).
2.3. Germination tests
2.3.1. Germination substrate
The germination substrate was 1% agar amended with different concentrations of arsenic as As(V) and As(III), prepared in Milli-Q water as follows. First, 1 L of arsenic solutions were prepared in a volumetric flask starting from sodium arsenate dibasic heptahydrate (Na2HAsO4∙7H2O 98%, Sigma St. Louise, MO, USA) as As(V) source (C = 2.5, 25, 50, 100, 200, 500 mg/L) and sodium (meta)arsenite (NaAsO2 90% Aldrich St. Louise, MO, USA) as As(III) source (C = 2.5, 5, 12.5, 25, 50, 100, 200 mg/L). The aqueous solutions were transferred into an Erlenmeyer flask combined with an Allihn condenser and heated at 95 °C under stirring in a hot plate. Then, agar was put into the flask under stirring. After 20 min, the flask was taken out from the hot plate and let to cool down for 5 min. It is important not to let the solution cool down too much to avoid issues in the gel formation. Then the hot solution was poured into 90 mm diameter Petri dishes and let cool down until solidification, avoiding moving them to favor the formation of an uniform gel.
2.3.2. Germination trials
Seeds of the selected taxon, harvested at above mention sites, were sown on the germination substrate and incubate into growth chambers (Sanyo MLR-351, SANYO Electric Co., Ltd), for 90 days with a photoperiod of 12 h of light and 12 of dark at 15 °C (Boi et al., 2020a; Picciau et al., 2019). Each concentration was tested using four replications of 25 seeds.
Concentrations of arsenicals were selected according to the results of compositional analysis on seeds and on the total As concentration of the substrates reported for the Campo Pisano mine dump (Concas, 2014) and confirmed, in this work, to be in the range 60–110 mg/kg by ICP-OES analysis. A seed was considered germinated when the radicle was evident (>1 mm). Seed germination and early seedling development dynamics were analyzed for each concentration of arsenicals by: (I) the Final Germination Percentage (FGP); II) the percentage of germinated seeds which have emerged cotyledon, here defined as “Cotyledons Emergence Percentage” (CEP); III) the mortality of seedlings. The trial was performed at the Sardinian Germplasm Bank (BG-SAR; Porceddu et al., 2017) and at the Department of Chemical and Geological Sciences of the University of Cagliari (Italy).
2.4. Statistical analyses
For the evaluation of the influence of As(V) and As(III), of the collecting sites and their mutual interaction on FGP, CEP and mortality, Generalized Linear Models (GLMs) were used. Statistical differences showed by GLM were then analyzed by a pairwise comparisons (with Bonferroni adjustment). To overcome residual overdispersion, a quasi-poisson error structure and F test on the ANOVA were used (Crawley, 2007). The analyses were performed with the “R v. 3.0.3” software (R Development Core Team, 2014).
3. Results and discussion
3.1. Seed germination
Statistical analysis (Table S2) showed differences (p < 0.05) among sites and concentrations in terms of germination percentage under both arsenate and arsenite. Moreover, no interaction (p > 0.05) between localities and concentration was detected in each case.
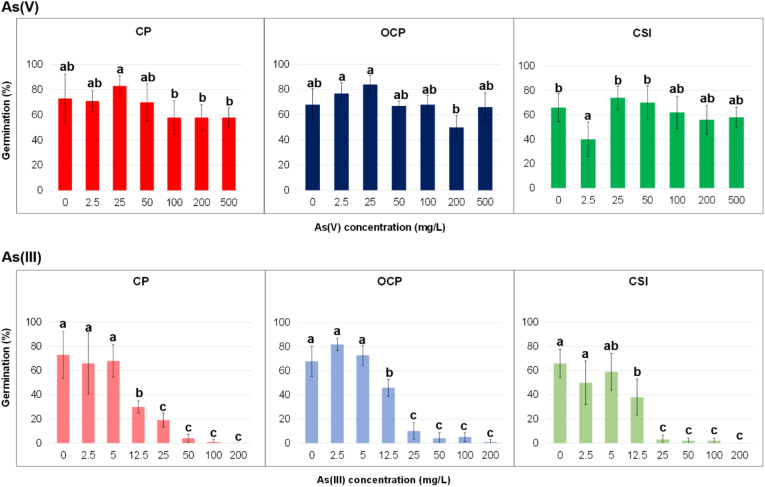
Seed germination of H. tyrrhenicum was not substantially affected by As(V) in any locality tested. Indeed, the final germination percentages (FGP) were in all cases > 60%, (except for 2.5 mg/L in CSI), as shown in Figure 1.
Figure 1.
Final germination percentage (FGP; % mean ± SD) of Helichrysum tyrrhenicum seeds under As(V) and As(III); letters specify significant differences (p < 0.05).
When each locality is considered, differences (p < 0.05) among concentrations were highlighted (Figure 1), even if these differences were sometimes not graphically evident. For instance, in CP, germination was affected in the same way up to 50 mg/L and changed after this concentration, whereas in OCP As(V) acted differently after 25 mg/L.
Regardless, these results suggested that the seeds H. tyrrhenicum were tolerant to the different As(V) concentrations independently on the collecting site, showing an intrinsic specific tolerance towards arsenate. In this case, H. tyrrhenicum showed a dissimilar behaviour than that pointed out for Zn and Pb (Boi et al., 2020a). Moreover, unlike what was observed in this study, several papers (Kranner and Colville, 2011; Li et al., 2005; Márquez-García et al., 2013; Street et al., 2007) showed that the presence of metals is linked to a reduction of germinated seeds for several taxa. As briefly introduced, germination tests under As(V) stress were mainly carried out for crop plants, like wheat and rice, showing a sensitive reduction in seed germination within the increasing of As levels (Hossain et al., 2007; Liu et al., 2005). As far as no crop species, Guterres et al. (2019) showed that some Australian endemic taxa commonly used in local phytoremediation actions, are tolerant towards As(V) during germination. For instance, A. harpophylla has a toxicity threshold > 667.36 μM (≈50 mg/L) of As(V) within the 75% of germinated seeds. Considering the Mediterranean species, seed germination under As stress was recently studied on C. salviifolius (Carvalho et al., 2020), even though the difference in As speciation was not taken into consideration. Since Carvalho et al. (2020) used the pentavalent As, it is possible to make a comparison: germination data of C. salviifolius was <50% at all applied concentrations (from 0 to 5 mg/L), indicating that H. tyrrhenicum is a more tolerant species to As than C. salviifolius.
The correlation between As(V) concentration and FGP was evaluated, but no linear correlation was determined, as shown in Figure S3. Hence, the concentration limit of As(V) ([As(V)]0%), defined as the concentration of As(V) over that germination process is completely inhibited as proposed in the previous study of Boi et al. (2020a) for Zn and Pb stress, was no estimated. The absence of this correlation may suggest that As(V) does not interfere directly with germination in sensu stricto like already observed in H. tyrrhenicum seeds subjected to Pb stress (Boi et al., 2020a).
As far as As(III) is considered, it was able to interfere in the germination process in every locality tested, as shown in Figure 1. From 0 to 5 mg/L, the FGP remained similar, but at 12.5 mg/L it decreased substantially until 30% (CP), and it was almost inhibited (4% for CSI) at 25 mg/L. The process stopped at 100 mg/L for CP and CSI, while at 200 mg/L for OCP. This aspect was also confirmed by statistical analysis carried out on each locality. Indeed, differences (p < 0.05) among concentrations were observed (Figure 1), showing that up to 5 mg/L, As(III) acted similarly and became toxic after 12.5 mg/L (FGP < 50%), both for polluted and unpolluted sites. Li et al. (2007) observed that wheat tolerates As(III) until 5 mg/kg, whilst in the range 5–20 mg/kg it became toxic, lowering the germination percentage, according to our observation. Even if the statistical analysis showed significant difference among localities, the absence of the interaction among concentrations and localities suggests that also for As(III), seeds of unpolluted sites are able to germinate in the same way as those of the polluted ones.
The correlation between As(III) concentration and FGP was calculated and a negative linear correlation was determined as shown in Figure S3. Correlations were calculated from 0 mg/L to the first As value before the experimental zero. The best correlation was recorded in OCP (R2 = 0.9757), followed by CP and CSI (R2 = 0.9733 and R2 = 0.9322). According to the R2 values (Figure S3) and the related linear regression line (Table 1), an As(III) concentration limit of about 30 mg/L for all sites, was estimate.
Table 1.
Concentration limits (mg/L) of As(V) and As(III) on FGP, CEP and mortality (M) of Helichrysum tyrrhenicum and linear regression lines.
| Site | Linear regression line | R2 | Concentration limit (mg/L) | ||
|---|---|---|---|---|---|
| FGP | As(III) | CP | 58.56x – 1.745 | 0.7320 | 34 ± 13 |
| OCP | 86.31x – 3.059 | 0.9652 | 28.2 ± 3.4 | ||
| CSI | 66.02x – 2.515 | 0.9891 | 26.2 ± 2.1 | ||
| CEP | As(V) | CP | 92.68x – 0.314 | 0.9924 | 295 ± 15 |
| OCP | 103.42x – 0.324 | 0.7403 | 319 ± 96 | ||
| CSI | 94.91x – 0.203 | 0.7950 | 467 ± 119 | ||
| As(III) | CP | 93.64x – 7.278 | 0.9672 | 12.9 ± 2.0 | |
| OCP | 106.98x – 8.203 | 0.9248 | 13.0 ± 2.9 | ||
| CSI | 116.85x – 9.115 | 0.9723 | 12.8 ± 1.8 | ||
| M | As(III) | CP | 5.40x + 6.654 | 0.9430 | 14 ± 11 |
| OCP | 21.05x + 7.829 | 0.7843 | 10.1 ± 7.8 | ||
| CSI | 27.41x + 5.807 | 0.9639 | 7.4 ± 2.7 | ||
The toxic effect of As(III) during germination was evident: as far as a comparison between the two arsenicals was concerned, their effect on the germination process was highlighted, showing that the speciation can interfere and differently influence this process. For instance, 25 mg/L of As(V) acted differently than the same concentration of As(III). Abedin and Meharg (2002) showed that rice germination decreased both under As(V) and As(III), and the latter one was found more toxic in the process, as also shown for wheat by Liu et al. (2005). Moreover, Kabata-Pendias (2011 and reference therein) indicate as toxic an As range of 5–20 mg/kg (calculate in mature leaf of different plants), but, in our tests, this range can be considered toxic for germination only in the case of As(III).
3.2. Cotyledons emergence
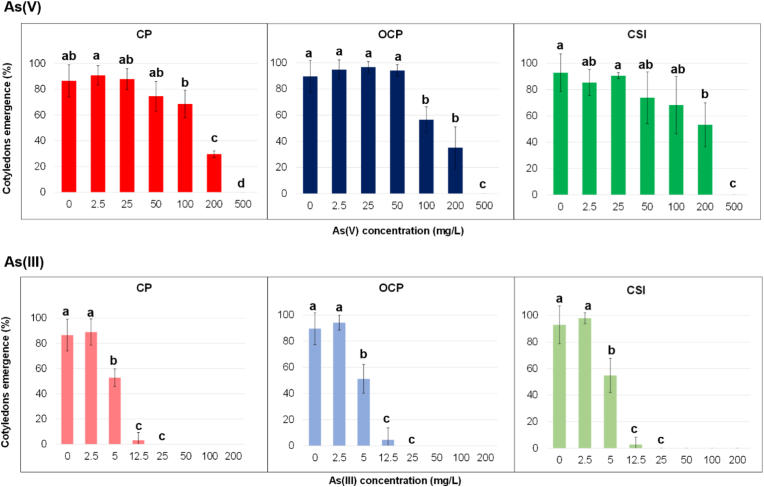
Statistical differences (p < 0.05) were detected for cotyledons emergence only among concentrations, whereas none (p > 0.05) were highlighted among localities as well as in the interaction between localities and concentrations, for both arsenicals. Cotyledons’ emergence (CEP under arsenate stress was however high up to 100 mg/L for CP and CSI (>70%), whilst in OCP at this concentration, CEP decreased substantially, as shown in Figure 2.
Figure 2.
Cotyledons emergence percentage (CEP; % mean ± SD) of Helichrysum tyrrhenicum seeds under As(V) and As(III); letters specify significant differences (p < 0.05).
In detail, CEP decreased substantially between 100 and 200 mg/L for CP, whereas between 50 and 100 mg/L for OCP, showing a lower limit of emergence in the area outside the mine dump. For CSI, the decrease was less sharp than for the other localities. Statistical differences (p < 0.05) among concentrations were highlighted (Figure 2) when each locality is considered, confirming, for example, the sharp decrease of CEP between 100–200 mg/L and 200–500 mg/L for CP and OCP, respectively. The emergence was completely inhibited at 500 mg/L in all the localities tested. In this study, CEP was considered as a growth parameter: as mentioned in the introduction section, As interferes in different ways in the development of plants, causing, for instance, a reduction in terms of roots, shoots, and leaves growth and biomass, also causing damage in the photosynthetic process. However, the emergence of the cotyledons is a fundamental process for plant establishment, so a reduction in their production beyond a certain value of As can be considered a threshold limit for seedling survival and future mature plant establishment, as observed in this study. Since no provenience-dependence was observed for both germination and cotyledons emergence, an intrinsic tolerance of H. tyrrhenicum towards As(V) emerges.
The correlations “CEP–As(V) concentration” were calculated from 0 mg/L to the first As value (200 mg/L) before the experimental zero (500 mg/L), and a negative linear correlation was found, as shown in Figure S4. The best correlation was recorded for CP (R2 = 0.9924), followed by CSI and OCP (R2 = 0.7950 and R2 = 0.7402). According to the R2 values (Figure S4) and the related linear regression equations (Table 1), the concentration limit of As(V) was estimate and defined as the concentration over that the cotyledons were absent. The highest value was obtained for CSI, followed by OCP and CP, ranging from about 300 to 500 mg/L. These results show extremely high resistance in terms of cotyledons emergence both for the seeds from polluted and unpolluted areas, as already detected by statistical analysis.
As observed for germination, Figure 2 shows the different effects of As(III) on CEP (p > 0.05): a concentration of 2.5 mg/L did not affect CEP whilst the other concentrations act differently. Moreover, a negative effect was evident at 5 mg/L, and the emergence was interrupted at 25 mg/L for polluted (CP and OCP) and unpolluted localities (CSI).
The correlations “CEP–As(III) concentrations” were calculated from 0 to 12.5 mg/L. A negative linear correlation was observed, as shown in Figure S4. The best correlation was recorded for CP, followed by CSI and OCP. According to the R2 values (Figure S4) and the related linear regression line (Table 1), we assess the concentration limit of As(III) in cotyledons emergence. The close values around 13 mg/L suggested that both seeds from polluted and unpolluted areas were extremely sensitive to As(III) in terms of CEP, as already detected by statistical analysis (interaction between localities and concentrations). The different effect of the two arsenicals on the emergence of cotyledons is clear, and As(III) appears much more toxic than As(V): the seeds subjected to 25 mg/L of As(V) developed a CEP higher than 80% (CP) while no emergence was detected for As(III). The effect of the two arsenicals is evident, also comparing the concentrations limits of the pentavalent and the trivalent forms (Table 1). Previous studies carried out on wheat and rice showed that As(III) could affect seedling development in a worse manner than As(V) (Liu et al., 2005; Shri et al., 2009).
3.3. Mortality
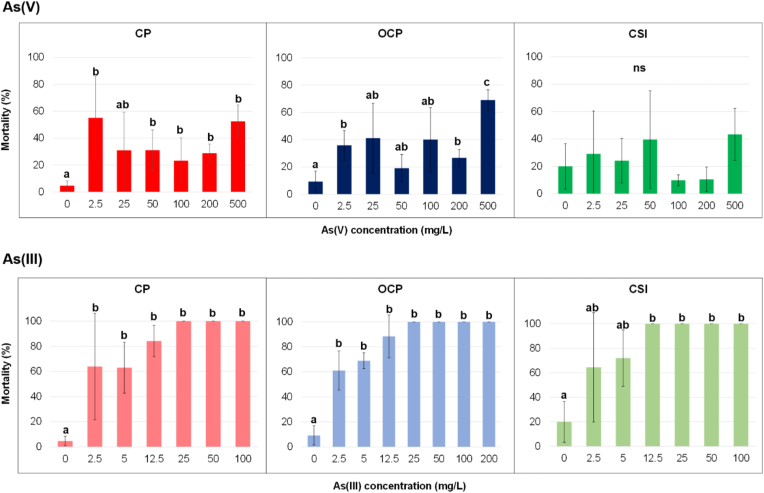
For As(V), statistical analysis (Table S2) highlighted differences (p < 0.05) occurring only among concentrations in terms of mortality, whereas none were detected among localities as well as in the interaction between localities and concentration (p > 0.05). On the contrary, for As(III), no differences (p > 0.05) among concentrations of As(III), localities, and interaction between them were detected. When each locality is separately taken into account (Figure 3), and considering the standard deviations, the mortality remains substantially similar at all As(V) concentrations with the exception of 500 mg/L (even if the statistical analysis showed significant differences among concentrations for CP and OCP).
Figure 3.
Mortality (% mean ± SD) of Helichrysum tyrrhenicum seedlings under As(V) and As(III); letters specify significant differences (p < 0.05); ns = no significant differences (p > 0.05).
In particular, the mortality was < 40% in all the localities from 2.5 to 200 mg/L of As(V), excluding the 2.5 mg/L for CP, whereas it increased to 500 mg/L for all localities and reached 70% for OCP. Moreover, for the CP and OCP sites, the mortality at 500 mg/L is five and seven times higher than 0 mg/L, respectively, while in CSI, no significant difference is evident, probably due to the high standard deviation associated with the mortality at 0 mg/L of As(V).
Beyond the calculated values of R2 that might suggest the presence of a linear correlation at least for CP and OCP between the mortality (%) and the As(V) concentrations (Figure S5), the high standard deviations prevent any further estimation of the concentration limit.
As shown in Figure 3, the presence of As(III) influenced the mortality already at 2.5 mg/L (> 60%), remaining similar at 5 mg/L, whereas the mortality increased up to 80% at 12.5 mg/L for CP and OCP, becoming lethal between 12.5 (CSI) and 25 mg/L (CP, OCP). If each locality is considered, differences (p < 0.05) in mortality are highlighted among concentrations, confirming that even an As(III) concentration of 2.5 mg/L can be toxic for plant survival.
As far as a correlation between As(III) concentrations and mortality (%) is considered, a positive linear correlation was observed, as suggested by the calculated R2 (Figure S5). Hence, in this case, it was possible to calculate the As(III) concentration limit where the mortality was total (Table 1). Seeds of CSI showed the lowest As(III) concentration limit (about 7 mg/L), followed by OCP and CP (about 10 and 14 mg/L).
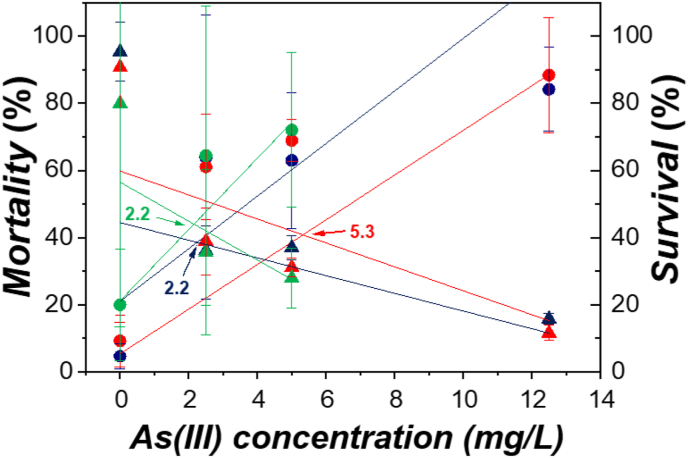
If the lines of mortality and survival were plotted together, the point where they intersect would correspond to the 50% of mortality or survival, hence a limit where the As(III) becomes toxic (Figure 4). In detail, this limit was reached first by CSI and OCP seeds (2.2 mg/L) and then by CP (5.3 mg/L), showing a higher threshold limit of the polluted site (CP) than unpolluted one (CSI).
Figure 4.
Comparison between mortality and survival (% mean ± SD) of Helichrysum tyrrhenicum seedlings under different As(III) concentrations (mg/L) and linear correlation; R2 = R square. The color arrows indicate the 50% of mortality (survival).
Finally, As(III) does not hamper the seeds' germination and cotyledon's emergence (even if at very low concentration), but the seedlings do not resist in the following growth steps and finally die.
4. Conclusion
Helichrysum tyrrhenicum proved to be a tolerant plant towards As(V) in the germination process and early seedling development. However, it is worthy to note that speciation is an important parameter when planning phytoremediation projects or ecotoxicological assessment studies. Indeed, in this study, the effect of the different arsenical species was demonstrated already in the early development stages, confirming the much higher phytotoxicity of As(III). Therefore, not only the final germination percentage (FGP) but also the cotyledons emergence percentage (CEP) can be considered an important growth indicator for ecotoxicological study. The germination tests under different speciation of the same metalloid here reported, provide useful information about the relative local abundance of the As species in the studied localities. Indeed, no data about As speciation at the mine site are still available, but we can suppose that As(III) is not present, not phytoavailable, or below the survival concentration limit (i.e., 2.2 mg/L) in the mine dump of Campo Pisano, since mature plants can be found there. Although the arsenic contents might be high in the contaminated substrates, most As could be associated with the minerals fraction. Only the soluble arsenic in the substrates can impact the seed germination and plant development, and this As portion would likely be limited to seeds applied to substrates. Considering that As(V) is much more persistent in the environment than As(III), an As(V) tolerant plant canopy that can prevent its dispersion in the surrounding area is of fundamental importance. Hence, taking into consideration the high tolerance to As(V) during germination and seedling development of H. tyrrhenicum, we argue that this taxon is proper for phytoremediation of As(V) contaminated areas.
Future studies are planned, and they will include seed germination tests under combined As(V)–As(III) stress, under multi-metals and metalloids (Cd, Pb, Zn, As), under As(V) and As(III) in the presence of PGP bacteria, also for other plants growing spontaneously in these sites.
Declarations
Author contribution statement
Maria Enrica Boi, Marco Sanna Angotzi & Elodia Musu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Marco Porceddu: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Valentina Mameli: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Gianluigi Bacchetta: Conceived and designed the experiments; Wrote the paper.
Carla Cannas: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Regione Autonoma della Sardegna (CESA Project _Piano Sulcis,) for the funding of the grants of M. E. Boi, M. Sanna Angotzi and E. Musu, and Ministero dell’Istruzione, dell’Università e della Ricerca PON AIM (PON Ricerca e Innovazione 2014–2020–Azione I.2–DD n. 407 del 27febbraio 2018 “Attraction and International Mobility”, Cult-GeoChim project AIM1890410-3) for financing the fixed-term fellowship of V. Mameli.
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We acknowledge the CeSAR (Centro Servizi d’Ateneo per la Ricerca) of the University of 514 Cagliari, Italy, for ESEM-EDX measurements performed with of FEI Company Quanta 200 ESEM equipped with a Thermo Scientific EDX probe.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Abbas G., Murtaza B., Bibi I., Shahid M., Niazi N.K., Khan M., Amjad M., Hussain M. Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Publ. Health. 2018;15:59. doi: 10.3390/ijerph15010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedin M.J., Meharg A.A. Relative toxicity of arsenite and arsenate on germination and early seedling growth of rice (Oryza sativa L.) Plant Soil. 2002;243:57–66. [Google Scholar]
- Abreu M.M., Tavares M.T., Batista M.J. Potential use of Erica andevalensis and Erica australis in phytoremediation of sulphide mine environments: São Domingos, Portugal. J. Geochem. Explor. 2008;96:210–222. [Google Scholar]
- Adriano D.C. Springer; New York: 2001. Trace Elements in the Terrestrial Environment. [Google Scholar]
- Angiolini C., Bacchetta G., Brullo S., Casti M., Giusso del Galdo G., Guarino R. The vegetation of mining dumps in SW-Sardinia. Feddes Repert. 2005;116:243–276. [Google Scholar]
- Ardau C., Cidu R., Da Pelo S., Dadea C., Fanfani L., Frau F., Musu E., Lattanzi P. Proceedings 10th IMWA congress “Mine Water and the Environment” 2 – 5 June 2008. 2008. Arsenic in mining environments: evidences from Sardinia (Italy) Czech Republic. [Google Scholar]
- Bacchetta G., Brullo S., Mossa L. Note sul genere Helichrysum miller (Asteraceae) in Sardegna. Ital. Bot. 2003;35:217–225. [Google Scholar]
- Bacchetta G., Casti M., Zavattero L. Analisi della vegetazione del distretto minerario di Montevecchio (Sardegna sud-occidentale) Fitosociologia. 2007;44:83–108. [Google Scholar]
- Bacchetta G., Cao A., Cappai G., Carucci A., Casti M., Fercia M.L., Lonis R., Mola F. A field experiment on the use of Pistacia lentiscus L. and Scrophularia canina L. subsp. bicolor (Sibth. et Sm.) Greuter for the phytoremediation of abandoned mining areas. Plant Biosyst. 2012;146:1054–1063. [Google Scholar]
- Bacchetta G., Cappai G., Carucci A., Tamburini E. Use of native plants for the remediation of abandoned mine sites in Mediterranean semiarid environments. Bull. Environ. Contam. Toxicol. 2015;94:326–333. doi: 10.1007/s00128-015-1467-y. [DOI] [PubMed] [Google Scholar]
- Bacchetta G., Boi M.E., Cappai G., De Giudici G., Piredda M. Proceedings of the 15th International Conference on Environmental Science and Technology. 2017. Phytoremediation of Sardinian abandoned mine site: a preliminary study on the use of Helichrysum microphyllum Cambess. subsp. tyrrhenicum Bacch., Brullo & Giusso.https://cest2017.gnest.org/sites/default/fles/presentation_fle_list/cest2017_01066_oral_paper .pdf Rhodes, Greece. [Google Scholar]
- Bacchetta G., Boi M.E., Cappai G., De Giudici G., Piredda M., Porceddu M. Metal tolerance capability of Helichrysum microphyllum Cambess. subsp. tyrrhenicum Bacch., Brullo & Giusso: a candidate for phytostabilization in abandoned mine sites. Bull. Environ. Contam. Toxicol. 2018;101:758–765. doi: 10.1007/s00128-018-2463-9. [DOI] [PubMed] [Google Scholar]
- Boi M.E., Porceddu M., Cappai G., De Giudici G., Bacchetta G. Effects of zinc and lead on seed germination of Helichrysum microphyllum subsp. tyrrhenicum, a metal-tolerant plant. Int. J. Environ. Sci. Technol. 2020;17:1917–1928. [Google Scholar]
- Boi M.E., Medas D., Aquilanti G., Bacchetta G., Birarda G., Cappai G., Carlomagno I., Casu M.A., Gianoncelli A., Meneghini C., Piredda M., Podda F., Porceddu M., Rimondi V., Vaccari L., De Giudici G. Mineralogy and Zn chemical speciation in a soil-plant system from a metal-extreme environment: a study on Helichrysum microphyllum subsp. tyrrhenicum (Campo Pisano Mine, SW Sardinia, Italy) Minerals. 2020;10:259. [Google Scholar]
- Boi M.E., Cappai G., De Giudici G., Medas D., Piredda M., Porceddu M., Bacchetta G. Ex situ phytoremediation trial of Sardinian mine waste using a pioneer plant species. Environ. Sci. Pollut. Res. 2021;28:55736–55753. doi: 10.1007/s11356-021-14710-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolisetty S., Peydayesh M., Mezzenga R. Sustainable technologies for water purification from heavy metals: review and analysis. Chem. Soc. Rev. 2019;48:463–487. doi: 10.1039/c8cs00493e. [DOI] [PubMed] [Google Scholar]
- Budzyńska S., Niedzielski P., Mleczek M. Time-dependent changes of arsenic and its selected forms in a hydroponic experiment with Quercus robur L. J. Hazard Mater. 2021;405 doi: 10.1016/j.jhazmat.2020.124244. [DOI] [PubMed] [Google Scholar]
- Cao A., Cappai G., Carucci A., Muntoni A. Selection of plants for zinc and lead phytoremediation. J. Environ. Sci. Health. 2004;39:1011–1024. doi: 10.1081/ese-120028410. [DOI] [PubMed] [Google Scholar]
- Carvalho L.C., Vieira C., Abreu M.M., Magalhães M.C.F. Physiological response of Cistus salviifolius L. to high arsenic concentrations. Environ. Geochem. Health. 2020;42:2305–2319. doi: 10.1007/s10653-019-00389-1. [DOI] [PubMed] [Google Scholar]
- Chen G., Feng T., Li Z., Chen Z., Chen Y., Wang H., Xiang Y. Influence of sulfur on the arsenic phytoremediation using Vallisneria natans (Lour.) Hara. Bull. Environ. Contam. Toxicol. 2017;99:411–414. doi: 10.1007/s00128-017-2135-1. [DOI] [PubMed] [Google Scholar]
- Cidu R., Fanfani L. Overview of the environmental geochemistry of mining districts in southwestern Sardinia, Italy. Geochem. Explor. Environ. Anal. 2002;2:243–251. [Google Scholar]
- Cidu R., Biddau R., Secci G. Proceedings of the 9th International Mine Water Congress (IMWC) 2005. Legacy at Abandoned Mines: Impact of Mine Wastes on Surface Waters; pp. 247–252. Oviedo, Spain. [Google Scholar]
- Cidu R., Biddau R. Transport of trace elements under different seasonal conditions: effects on the quality of river water in a Mediterranean area. Appl. Geochem. 2007;22:2777–2794. [Google Scholar]
- Concas S. Ph.D. thesis, Università di Cagliari. 2014. http://veprints.unica.it/943/
- Concas S., Lattanzi P., Bacchetta G., Barbafieri M., Vacca A. Zn, Pb and Hg contents of Pistacia lentiscus L. grown on heavy metal rich soils: implications for phytostabilization. Water Air Soil Pollut. 2015;226:340–355. [Google Scholar]
- Crawley M.J. The R Book. Wiley; Chichester: 2007. [Google Scholar]
- De Giudici G., Medas D., Meneghini C., Casu M.A., Giannoncelli A., Iadecola A., Podda S., Lattanzi P. Microscopic bio mineralization processes and Zn bioavailability: a synchrotron- based investigation of Pistacia lentiscus L. root. Environ. Sci. Pollut. Res. Int. 2015;22:19352–19361. doi: 10.1007/s11356-015-4808-9. [DOI] [PubMed] [Google Scholar]
- De Giudici G., Pusceddu C., Medas D., Meneghini C., Gianoncelli A., Rimondi V., Podda F., Cidu R., Lattanzi P., Wanty R.B., Kimball B.A. The role of natural biogeochemical barriers in limiting metal loading to a stream affected by mine drainage. Appl. Geochem. 2017;6:124–135. [Google Scholar]
- Farooq M.A., Islama F., Alia B., Najeebc U., Maod B., Gilla R.A., Guijun Yane G., Siddiquef K.H.M., Zhoua W. Arsenic toxicity in plants: cellular and molecular mechanisms of its transport and metabolism. Environ. Exp. Bot. 2016;132:42–52. [Google Scholar]
- Fois M., Farris E., Calvia G., Campus G., Fenu G., Porceddu M., Bacchetta G. The endemic vascular flora of Sardinia: a dynamic checklist with an overview of biogeography and conservation status. Plants. 2022;11:601. doi: 10.3390/plants11050601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frau F., Rossi A., Ardau C., Biddau R., Da Pelo S., Atzei D., Licheri C., Cannas C., Capitan G.C. Determination of arsenic speciation in complex environmental samples by the combined use of TEM and XPS. Microchim. Acta. 2005;151:189–201. [Google Scholar]
- Frau F., Ardau C., Fanfani L. Environmental geochemistry and mineralogy of lead at the old mine area of Baccu Locci (south-east Sardinia, Italy) J. Geochem. Explor. 2008;100:105–115. [Google Scholar]
- Gilhotra V., Das L., Sharma A., Kang T.S., Singh P., Dhuria R.S., Bhatti M.S. Electrocoagulation technology for high strength arsenic wastewater: process optimization and mechanistic study. J. Clean. Prod. 2018;198:693–703. [Google Scholar]
- Guterres J., Rossato L., Doley D., Pudmenzky A., Bee C., Cobena V. Assessing germination characteristics of Australian native plant species in metal/metalloid solution. J. Hazard Mater. 2019;364:173–181. doi: 10.1016/j.jhazmat.2018.10.019. [DOI] [PubMed] [Google Scholar]
- Hossain M.A., Uddin M.N., Sarwar A.K.M.G. Toxicity of arsenic on germination and seedling growth of rice. J. Bangladesh Soc. Agric. Sci. Technol. 2007;4:153–156. [Google Scholar]
- Iftikhar S., Turan V., Tauqeer H.M., Rasool B., Zubair M., Rahman M., Khan M.A., Shamim Akhtar S., Khan S.A., Basharat Z., Zulfiqar I., Iqbal J., Iqbal M., Adnan Ramzani P.M. In: Handbook of Bioremediation. Hasanuzzaman M., Prasad M.N.V., editors. Academic Press; 2021. Phytomanagement of As-contaminated matrix: physiological and molecular basis; pp. 61–79. [Google Scholar]
- Jiménez E.M., Penalosa J.M., Esteban E., Bernalb M.P. Feasibility of arsenic phytostabilisation using Mediterranean shrubs: impact of root mineralisation on as availability in soils. J. Environ. Monit. 2009;11:1375–1380. doi: 10.1039/b822335a. [DOI] [PubMed] [Google Scholar]
- Jiménez M.N., Fernandez E., Navarro E.B., Contini E., Casti M., Bacchetta G. Livelli di metalli pesanti in Dittrichia viscosa (L.) Greuter, Cistus salviifolius L. e Euphorbia cupanii Bertol. ex Moris su suoli contaminati e non contaminati dalle attività estrattive nell’Iglesiente (Sardegna sudoccidentale) Inf. Bot. Ital. 2005;37:794–795. [Google Scholar]
- Jiménez M.N., Bacchetta G., Casti M., Navarro F.B., Lallena A.M., Fernández-Ondoño E. Potential use in phytoremediation of three plant species growing on contaminated mine-tailing soils in Sardinia. Ecol. Eng. 2011;37:392–398. [Google Scholar]
- Jiménez M.N., Bacchetta G., Casti M., Navarro F.B., Lallena A.M., Fernández-Ondoño E. Study of Zn, Cu and Pb content in plants and contaminated soils in Sardinia. Plant Biosyst. 2014;148:419–428. [Google Scholar]
- Jiménez M.N., Bacchetta G., Navarro F.B., Casti M., Fernández-Ondoño E. Native plant capacity for gentle remediation in heavily polluted mines. Appl. Sci. 2021;11:1769. [Google Scholar]
- Kabata-Pendias A. CRC Press; Boca Raton: 2011. Trace Elements in Soils and Plants. [Google Scholar]
- Kranner I., Colville L. Metals and seeds: biochemical and molecular implications and their significance for seed germination. Environ. Exp. Bot. 2011;72:93–105. [Google Scholar]
- Kumar K., Gupta D., Mosa K.A., Ramamoorthy K., Sharma P. In: Plant-Metal Interactions. Srivastava S., Srivastava A., Suprasanna P., editors. Springer; 2019. Arsenic transport, metabolism and possible mitigation strategies in plants; pp. 141–158. [Google Scholar]
- Lefèvre I., Marchal G., Corréal E., Zanuzzi A., Lutts S. Variation in response to heavy metals during vegetative growth in Dorycnium pentaphyllum Scop. Plant Growth Regul. 2009;59:1–11. [Google Scholar]
- Li W.Q., Khan M.A., Yamaguchi S., Kamiya Y. Effects of heavy metals on seed germination and early seedling growth of Arabidopsis thaliana. Plant Growth Regul. 2005;46:45–50. [Google Scholar]
- Li C., Feng S., Shao Y., Jiang L., Lu X., Hou X. Effects of arsenic on seed germination and physiological activities of wheat seedlings. J. Environ. Sci. 2007;19:725–732. doi: 10.1016/s1001-0742(07)60121-1. [DOI] [PubMed] [Google Scholar]
- Liu X., Zhang S., Shan X., Zhu Y.G. Toxicity of arsenate and arsenite on germination, seedling growth and amylolytic activity of wheat. Chemosphere. 2005;61:293–301. doi: 10.1016/j.chemosphere.2005.01.088. [DOI] [PubMed] [Google Scholar]
- Ma J., Zhu Z., Chen B., Yang M., Zhou H., Li C., Yu F., Chen J. One-pot, large-scale synthesis of magnetic activated carbon nanotubes and their applications for arsenic removal. J. Mater. Chem. A. 2013;1:4662–4666. [Google Scholar]
- Ma L.Q., Komar K.M., Tu C., Zhang W.H., Cai Y., Kennelley E.D. A fern that hyperaccumulates arsenic. Nature. 2001;409:579. doi: 10.1038/35054664. [DOI] [PubMed] [Google Scholar]
- Márquez-García B., Pérez-López R., Ruíz-Chancho M.J., López-Sánchez J.F., Rubio R., Abreu M.M., Nieto J.M., Córdoba F. Arsenic speciation in soils and Erica andevalensis Cabezudo & Rivera and Erica australis L. From São Domingos mine area. Portugal. J. Geochem. Explor. 2012;119–120:51–59. [Google Scholar]
- Márquez-García B., Márquez C., Sanjosé I., Nieva F.J.J., Rodríguez-Rubio P., Muñoz-Rodríguez A.F. The effects of heavy metals on germination and seedling characteristics in two halophyte species in Mediterranean marshes. Mar. Pollut. Bull. 2013;70:119–124. doi: 10.1016/j.marpolbul.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Medas D., De Giudici G., Casu M.A., Musu E., Giannoncelli A., Iadecola A., Meneghini C., Tamburini E., Sprocati A.R., Turnau K., Lattanzi P. Microscopic processes ruling the bioavailability of Zn to roots of Euphorbia pithyusa L. pioneer plant. Environ. Sci. Technol. 2015;49:1400–1408. doi: 10.1021/es503842w. [DOI] [PubMed] [Google Scholar]
- Medas D., De Giudici G., Pusceddu C., Casu M.A., Birarda G., Vaccari L., Giannoncelli A., Meneghini C. Impact of Zn excess on biomineralization processes in Juncus acutus grown in mine polluted sites. J. Hazard Mater. 2017;370:98–107. doi: 10.1016/j.jhazmat.2017.08.031. [DOI] [PubMed] [Google Scholar]
- Milton A., Johnson M. Arsenic in the food chains of a revegetated metalliferous mine tailings pond. Chemosphere. 1999;39:765–779. doi: 10.1016/s0045-6535(99)00012-0. [DOI] [PubMed] [Google Scholar]
- Moulick D., Ghosh D., Santra S.C. Evaluation of effectiveness of seed priming with selenium in rice during germination under arsenic stress. Plant Physiol. Biochem. 2016;109:571–578. doi: 10.1016/j.plaphy.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Picciau R., Serra S., Porceddu M., Bacchetta G. Seed traits and germination behaviour of four Sardinian populations of Helichrysum microphyllum ssp. tyrrhenicum (Asteraceae) along an altitudinal gradient. Plant Biol. 2019;21:498–506. doi: 10.1111/plb.12903. [DOI] [PubMed] [Google Scholar]
- Porceddu M., Santo A., Orrù M., Meloni F., Ucchesu M., Picciau R., Sarigu M., Cuena Lombraña A., Podda L., Sau S., Fogu M.G., Bacchetta G. Seed conservation actions for the preservation of plant diversity: the case of the Sardinian Germplasm Bank (BG-SAR) Plant Sociol. 2017;54:111–117. [Google Scholar]
- Quaghebeur M., Rengel Z. Arsenic speciation governs arsenic uptake and transport in terrestrial plants. Microchim. Acta. 2005;151:141–152. [Google Scholar]
- R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2014. R: a Language and Environment for Statistical Computing. [Google Scholar]
- Robinson B., Kim N., Marchetti M., Moni C., Schroeter L., van den Dijssel C., Georgie Milne C., Clothier B. Arsenic hyperaccumulation by aquatic macrophytes in the Taupo volcanic Zone, New Zealand. Environ. Exp. Bot. 2006;58:206–215. [Google Scholar]
- Sanna Angotzi M., Mameli V., Cara C., Borchert K.B.L., Steinbach C., Boldt R., Schwarz D., Cannas C. Meso- and macroporous silica-based arsenic adsorbents: effect of pore size, nature of the active phase, and silicon release. Nanoscale Adv. 2021;3:6100–6113. doi: 10.1039/d1na00487e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna Angotzi M., Mameli V., Fantasia A., Cara C., Secci F., Enzo S., Gerina M., Cannas C. As(III, V) uptake from nanostructured Iron Oxides and Oxyhydroxides: the complex interplay between sorbent surface chemistry and Arsenic equilibria. Nanomaterials. 2022;12:136. doi: 10.3390/nano12030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shri M., Kumar S., Chakrabarty D., Trivedi P.K., Mallick S., Misra P., Shukla D., Mishra S., Srivastava S., Tripathi R., Tuli R. Effect of arsenic on growth, oxidative stress, and anti oxidant system in rice seedlings. Ecotoxicol. Environ. Saf. 2009;72:1102–1110. doi: 10.1016/j.ecoenv.2008.09.022. [DOI] [PubMed] [Google Scholar]
- Sneller F.E.C., Van Heerwaarden L.M., Kraaijeveld-Smit F.J.L., Ten Bookum W.M., Koevoets P.L.M., Schat H., Verkleij J.A.C. Toxicity of arsenate in Silene vulgaris, accumulation and degradation of arsenate-induced phytochelatins. New Phytol. 1999;144:223–232. [Google Scholar]
- Street R.A., Kulkarni M.G., Stirk W.A., Southway C., Van Staden J. Toxicity of metal elements on germination and seedling growth of widely used medicinal plants belonging to Hyacinthaceae. Bull. Environ. Contam. Toxicol. 2007;79:371–376. doi: 10.1007/s00128-007-9237-0. [DOI] [PubMed] [Google Scholar]
- Tripathi R.D., Srivastava S., Mishra S., Singh N., Tuli R., Dharmendra K., Gupta D.K., Maathuis F.J.M. Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol. 2007;25:158–165. doi: 10.1016/j.tibtech.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Zabłudowska E., Kowalska J., Jedynak Ł., Wojas S., Skłodowska A., Antosiewicz D.M. Search for a plant for phytoremediation – what can we learn from field and hydroponic studies? Chemosphere. 2009;77:301–307. doi: 10.1016/j.chemosphere.2009.07.064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.