Abstract
Summary
The Covid-19 vaccination has been rapidly implemented among patients with cancer. We present two cases of patients with endocrine tumours who developed lymphadenopathy following a Covid-19 vaccination. In the case of a patient with multiple endocrine neoplasia (MEN) 1 syndrome, an 18-fluorodeoxyglucose (18FDG)-PET/CT showed positive axillary lymph nodes. Further work-up with fine needle aspiration showed a reactive pattern following a Covid-19 vaccination in the ipsilateral arm shortly before the 18FDG-PET/CT. A second patient, in follow-up for thyroid cancer, developed clinical supraclavicular lymphadenopathy after a Covid-19 vaccination. Follow-up ultrasound proved the lesion to be transient. These cases demonstrate lymphadenopathy in response to a Covid-19 vaccination in two patients susceptible to endocrine tumours and metastatic disease. With growing evidence about the pattern and occurrence of lymphadenopathy after mRNA Covid-19 vaccination, recommendations for scheduling and interpretation of imaging among cancer patients should be implemented to reduce equivocal findings, overdiagnosis, and overtreatment, while maintaining a good standard of care in oncological follow-up.
Learning points
Reactive lymphadenopathy is very common after an mRNA vaccination against Covid-19 and should be part of the differential diagnosis in patients with endocrine tumours who recently received a Covid-19 mRNA vaccination and present with an ipsilateral lymphadenopathy.
A good vaccine history is essential in assessing the risk for lymphadenopathy and if possible, screening imaging in patients with endocrine tumours should be postponed at least 6 weeks after the previous vaccination.
For now, a multidisciplinary care approach is recommended to determine the necessary steps in the diagnostic evaluation of lymphadenopathy in the proximity of a Covid-19 vaccination.
Patient Demographics: Adult, Female, Male, White, Belgium
Clinical Overview: Thyroid, Tumours and neoplasia
Related Disciplines: Oncology
Publication Details: Unique/unexpected symptoms or presentations of a disease, September, 2022
Background
The Covid-19 pandemic presented one of the biggest healthcare challenges of the 21st century. The worldwide implementation of vaccines has slowed down the pandemic. Cancer patients have been given priority in the vaccination programmes, as their risk for severe Covid-related complications is higher than in the general population (1).
While the implementation of vaccines is proving to be a turning point, they also pose new challenges for healthcare providers. Clinical axillary lymphadenopathy related to vaccination was described in 11% of the patients after the first and in 16% after the second dose of the Moderna vaccine. Likewise, this clinical phenomenon has been described after the Comirnaty-Pfizer/BioNTech vaccine (2). After the broad roll-out of the vaccination campaigns, axillary, cervical, and supraclavicular lymphadenopathies have been widely described in clinical imaging. Observational studies on ultrasound and 18-fluorodeoxyglucose PET/CT (18FDG-PET/CT) scans show these lymphadenopathies in more than half of the subjects (3, 4). Recommendations and considerations are starting to be published for radiologists to interpret these vaccine-related lymphadenopathies. In healthy individuals, watchful waiting and clinical follow-up is the joint advice. In cancer patients, however, new lymph nodes raise the question of disease progression and metastatic disease and consequently, the threshold for further diagnostic procedures is reduced. These considerations have been described for the more common cancers, such as breast cancer, which often metastasizes to the axillary lymph nodes (5, 6).
To the best of our knowledge, limited cases of Covid-19-related lymphadenopathy in patients with endocrine tumours have been published to this date. Hereby, we present two cases of patients with an endocrine tumour in their previous medical history, who presented with lymphadenopathy after a Covid-19 vaccination.
Case presentation
Patient 1
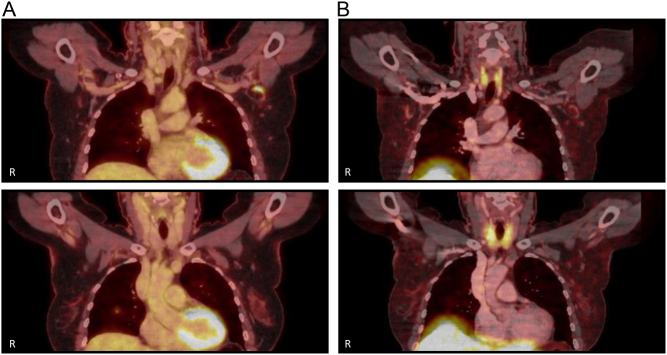
A 60-year-old female patient, in follow-up for a multiple endocrine neoplasia syndrome type 1 (MEN1), came to our clinic in January 2021 for a yearly check-up. MEN1 is a rare, autosomal dominant hereditary syndrome caused by mutations in the MEN1 tumour suppressor gene. The diagnosis is defined clinically by the presence of at least two primary MEN1 tumours (parathyroid, anterior pituitary, and pancreatic islet). This patient already had a resection of a prolactinoma in 1993, a total parathyroidectomy in 2000, and removal of two neuroendocrine tumours (NET) from the pancreas; a lesion positive for glucagon in 1996 and a 9-mm cystic lesion (pT1Nx) in 2011. In 2018, a solitary nodule in the upper lobe of the right lung was discovered. In her current yearly follow-up, the CT scan showed a new lesion in the intermediate lobe of the right lung. For further workup, an 18FDG-PET/CT was performed which showed uptake of FDG in the new lung lesion and also in the left axillary lymph nodes (Fig. 1A). On CT scan, these lymph nodes were described as non-suspicious for neoplastic disease and had not increased in size. However, further investigation was performed due to existing lung lesions and history of pancreas NET tumours.
Figure 1.
Positive 18-fluorodeoxyglucose (18FDG)-PET/CT and negative DOTA-1-NaI3-octreotide (DOTANOC) scan: images of a 60-year-old female patient (patient 1), in follow-up for a multiple endocrine neoplasia syndrome type 1 as further evaluation after the finding of a new lesion in the intermediate lobe of the right lung on CT scan during the yearly check-up. (A) Images of an 18FDG-PET/CT scan with accumulation of FDG in a not enlarged and radiographically non-suspicious lymph node in the left axillary region (top) and accumulation of FDG in a condensation zone in the intermediate lobe of the right lung with a bordering millimetric nodule (bottom). (B) Images of a DOTANOC scan which show no foci with enlarged somatostatin receptor expression, especially in the lesions with increased FDG uptake described in A. R, right side of the patient.
A DOTANOC scan showed no sites with heightened somatostatin receptor expression in the lung or elsewhere (Fig. 1B). Since MEN1 syndrome has been associated with a higher risk of breast cancer and pathological axillary lymph nodes (7), breast ultrasound and mammography were performed, but no abnormalities were seen. Because of the FDG uptake in the lung lesion and the axillary adenopathy, an additional ultrasound-guided fine needle aspiration (FNA) was performed on the axillary lymph node to complete the work-up. The pathological analysis revealed a benign reactive pattern without metastatic disease. Due to the location, depth, and dimension of the lung nodule, FNA was not possible to perform. Further follow-up CT-imaging after 3 and 12 months showed no progression of the lung nodule and the disappearance of the axillary lymphadenopathy.
After all the results were known, the patient mentioned to have received a first Covid-19 vaccination, Comirnaty-Pfizer/BioNTech, in the ipsilateral arm to the axillary lymphadenopathy, 13 days before the initial 18FDG-PET/CT.
Patient 2
A 27-year-old male patient, who had been in follow-up at our clinic after total thyroidectomy in 2019 for a low risk, pT2NxMx, papillary thyroid cancer (PTC), presented with a supraclavicular lymph node on the left side. The initial pathological analysis of his PTC had not shown lymphatic, vascular, or neural invasion. After thyroidectomy, he received adjuvant treatment with radioactive iodine, whereafter single-photon emission computerized tomography (SPECT)/CT of the neck showed no residual lesions. During follow-up with ultrasound after 6 months and with whole-body SPECT/CT after recombinant TSH stimulation after 12 months, no locoregional recurrence or metastases were seen. Furthermore, thyroglobulin levels remained undetectable. His treatment to date consists of levothyroxine.
At the current presentation, he described a swollen and tender nodule in the left supraclavicular region that he noticed for the first time 4 days before the consultation. He also mentioned having received his first Covid-19 vaccination of Comirnaty-Pfizer/BioNTech in the left arm about 2 weeks before the consultation. On clinical examination, a centimetric nodule could be palpated. A blood test was performed which showed a suppressed thyroid-stimulating hormone (0.205 mIU/L) and undetectable thyroglobulin (<0.1 µg/L). An ultrasound was performed to further identify the lymphadenopathy, which showed two supraclavicular lymph nodes on the left side, respectively, 10 × 7 mm and 5 × 5 mm. In the left axillary region, one lymph node was visualized as well. The lymph nodes were hyperaemic with a homogenous thickened cortex.
A follow-up ultrasound was performed 10 weeks later, which showed a decrease in the size of the lymph nodes with again benign imaging aspects. Six weeks before that second ultrasound, the patient had also received his second dose of the Covid-19 vaccination with Comirnaty-Pfizer/BioNTech.
Differential diagnosis
In both patients, the most important differential diagnosis was the metastatic disease of their respective previous neoplastic disease. Both patients had no prior history of a haematological illness and there were no arguments for another systemic illness to account for the lymphadenopathy. In the first patient, pathological analysis showed no evidence of malignancy. Reactive lymphadenopathy secondary to the Covid-19 vaccination was the most plausible diagnosis in both patients, based on their recent Covid-19 vaccination in the ipsilateral arm to the lymphadenopathy, the radiographic benign imaging aspects, and the reactive histological pattern in patient 1.
Discussion
To date and to the best of our knowledge, there are a limited number of cases of Covid-19 vaccination-associated lymphadenopathy in patients with endocrine tumours described in the literature.
Lymphadenopathy related to Covid-19 vaccination has been described, since its implementation in December 2020 (2). Reactive lymphadenopathy has already been reported after other vaccines, such as the vaccines for influenza and human papillomavirus. Data suggest that mRNA vaccines have a more potent immune stimulatory potential and thus are inherently more immunogenic as compared to other traditional vaccines. This might account for the more frequent and longer-lasting lymphadenopathies found on imaging after the current mRNA vaccines (4). Therefore, if possible, it is recommended to schedule routine imaging, such as screening, either before vaccination or at least 6 weeks after the final vaccination dose (8). This might eliminate some false-positive results for cancer and consequently, the necessity for further examinations and diagnostic tests and the accompanying distress for patients and healthcare providers.
This strategy, however, is not always feasible in clinical practice. Furthermore, avid axillary lymph node uptake on 18FDG-PET/CT can be detected beyond 6 weeks after the second dose of the mRNA-based Covid-19 vaccination (9). Thus, in management strategies, it is essential to consider the higher prevalence of lymphadenopathy visible on imaging after a Covid-19 vaccination in patients with a history of cancer because of the regular follow-up and imaging studies in this group (3, 4, 10).
In a general recommendation for radiologists, axillary lymphadenopathy found in a patient with a vaccination history of less than 6 weeks at the time of their screening needs no additional follow-up. Whenever the patient has a history of cancer, current recommendations state that a multidisciplinary consensus should be reached to decide whether to perform a biopsy or to do follow-up imaging (8). The European Society of Breast Imaging, for example, recommends managing ipsilateral axillary lymphadenopathy to the vaccination side according to the metastatic risk (6).
Currently, an overall management plan for patients with a cancer history is lacking. The abovedescribed cases show two different ways of follow-up. In the patient with MEN1 syndrome, there was a significant fear of a new malignancy because of the existing lung lesions and history of pancreas NET tumours. At the time of presentation, the first case reports of vaccination-related lymphadenopathy were only being published and this was initially not considered a possible differential diagnosis. Therefore, after multidisciplinary evaluation, a DOTANOC scan and FNA were performed. In the patient with a history of thyroid cancer, consecutive ultrasound was opted for, because of a low risk of recurrent disease, based on previous risk stratification, the undetectable thyroglobulin levels, the benign aspects of the lymphadenopathy on imaging and the proximity to a Covid-19 mRNA vaccine in the ipsilateral arm. In hindsight, the second approach might have been equally suitable for the patient with MEN1 syndrome.
The pitfall of a multidisciplinary evaluation, as often advised, is that it may lead to a broad and diverse spectrum of management strategies without standardization. In the current absence of standard management plans, some general recommendations are to inject the Covid-19 vaccine in the arm or the antero-lateral thigh contralateral to the side of the neoplasia (6, 10). Patient instruction about the possibility of the appearance of lymphadenopathy both clinically and on imaging is needed to aid shared decision-making afterwards (8, 10). Thirdly, recording and sharing the details about recent vaccination, such as time, lateralization, type of vaccine, etc., is another vital point (6, 8). Finally, it is important to note that while it is advised to schedule routine imaging, either before or at least 6 weeks after the final vaccination, delaying vaccination is not recommended because of its global consequences and because cancer patients count as a high-risk group (1, 8, 10).
While a large number of oncological patients have already received at least two and even three doses of a Covid-19 vaccine, these recommendations remain valid as invitations for a fourth dose are currently being sent out to this high-risk group. Furthermore, there is a significant variation in the vaccination rate worldwide.
In conclusion, vaccination has proven the turning point in the Covid-19 pandemic; however, a new healthcare challenge arises. Distinguishing reactive lymphadenopathy from metastatic disease in patients with a cancer history is necessary and challenging. Overdiagnosis, overtreatment, and increasing psychological stress for the patient and the provider should be avoided in order to maintain a good standard of care in the follow-up of an oncological patient, where the final goal must be to increase survival (5, 8). General recommendations for the interpretation of imaging among cancer patients after a Covid-19 vaccination are necessary. In the meantime, screening should be planned around the vaccination and transparent communication with the patient and between healthcare providers remains essential.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written informed consent for publication of their clinical details and/or clinical images was obtained from both patients.
Author contribution statement
Iris Dirven is an internist-in-training and was the main author. All authors were involved in the clinical care of the patient. All authors contributed to the reviewing and editing process and approved the final version of the manuscript.
References
- 1.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang Het al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet: Oncology 202021335–337. ( 10.1016/S1470-2045(2030096-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention. U.S. COVID-19 Vaccine Product Information, 2021. [Google Scholar]
- 3.Igual-Rouilleault AC, Soriano I, Quan PL, Fernández-Montero A, Elizalde A, Pina L. Unilateral axillary adenopathy induced by COVID-19 vaccine: US follow-up evaluation. European Radiology 2021323199–3206. ( 10.1007/s00330-021-08309-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skawran S, Gennari AG, Dittli M, Treyer V, Muehlematter UJ, Maurer A, Burger IA, Mader C, Messerli O, Grünig Het al. [18F]FDG uptake of axillary lymph nodes after COVID-19 vaccination in oncological PET/CT: frequency, intensity, and potential clinical impact. European Radiology 202232508–516. ( 10.1007/s00330-021-08122-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagen C, Nowack M, Messerli M, Saro F, Mangold F, Bode PK. Fine needle aspiration in COVID-19 vaccine-associated lymphadenopathy. Swiss Medical Weekly 2021151 w20557. ( 10.4414/smw.2021.20557) [DOI] [PubMed] [Google Scholar]
- 6.Schiaffino S, Pinker K, Magni V, Cozzi A, Athanasiou A, Baltzer PAT, Camps Herrero J, Clauser P, Fallenberg EM, Forrai Get al. Axillary lymphadenopathy at the time of COVID-19 vaccination: ten recommendations from the European Society of Breast Imaging (EUSOBI). Insights into Imaging 202112119. ( 10.1186/s13244-021-01062-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Leeuwaarde RS, Dreijerink KM, Ausems MG, Beijers HJ, Dekkers OM, De Herder WW, Van Der Horst-Schrivers AN, Drent ML, Bisschop PH, Havekes Bet al. MEN1-dependent breast cancer: indication for early screening? Results from the Dutch MEN1 Study Group. Journal of Clinical Endocrinology and Metabolism 20171022083–2090. ( 10.1210/jc.2016-3690) [DOI] [PubMed] [Google Scholar]
- 8.Lehman CD, D’Alessandro HA, Mendoza DP, Succi MD, Kambadakone A, Lamb LR. Unilateral lymphadenopathy after COVID-19 vaccination: a practical management plan for radiologists across specialties. Journal of the American College of Radiology 202118843–852. ( 10.1016/j.jacr.2021.03.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eshet Y, Tau N, Alhoubani Y, Kanana N, Domachevsky L, Eifer M. Prevalence of increased FDG PET/CT axillary lymph node uptake beyond 6 weeks after mRNA COVID-19 vaccination. Radiology 2021300E345–E347. ( 10.1148/radiol.2021210886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McIntosh LJ, Bankier AA, Vijayaraghavan GR, Licho R, Rosen MP. COVID-19 vaccination-related uptake on FDG PET/CT: an emerging dilemma and suggestions for management. American Journal of Roentgenology 2021217975–983. ( 10.2214/AJR.21.25728) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a