Abstract
Discerning modification to the amino acid sequence of native glucagon can generate specific glucagon receptor (GCGR) antagonists, that include desHis1Pro4Glu9-glucagon and the acylated form desHis1Pro4Glu9(Lys12PAL)-glucagon. In the current study, we have evaluated the metabolic benefits of once-daily injection of these peptide-based GCGR antagonists for 18 days in insulin-resistant high-fat-fed (HFF) mice with streptozotocin (STZ)-induced insulin deficiency, namely HFF-STZ mice. Administration of desHis1Pro4Glu9-glucagon moderately (P < 0.05) decreased STZ-induced elevations of food intake. Body weight was not different between groups of HFF-STZ mice and both treatment interventions delayed (P < 0.05) the onset of hyperglycaemia. The treatments reduced (P < 0.05–P < 0.001) circulating and pancreatic glucagon, whilst desHis1Pro4Glu9(Lys12PAL)-glucagon also substantially increased (P < 0.001) pancreatic insulin stores. Oral glucose tolerance was appreciably improved (P < 0.05) by both antagonists, despite the lack of augmentation of glucose-stimulated insulin release. Interestingly, positive effects on i.p. glucose tolerance were less obvious suggesting important beneficial effects on gut function. Metabolic benefits were accompanied by decreased (P < 0.05–P < 0.01) locomotor activity and increases (P < 0.001) in energy expenditure and respiratory exchange ratio in both treatment groups. In addition, desHis1Pro4Glu9-glucagon increased (P < 0.01–P < 0.001) O2 consumption and CO2 production. Together, these data provide further evidence that peptidic GCGR antagonists are effective treatment options for obesity-driven forms of diabetes, even when accompanied by insulin deficiency.
Keywords: glucagon, glucose homeostasis, insulin sensitivity, high-fat-fed mice, streptozotocin
Introduction
It has been well established that abnormal elevation in circulating glucagon leads to an increase in hepatic glucose production and glycogen metabolism that contribute to hyperglycaemia in diabetes (Unger 1978). For this reason, blockade of glucagon receptor (GCGR) signalling has been widely regarded as a potential therapeutic option to help control blood glucose levels for the treatment of diabetes (Patil et al. 2020, Lafferty et al. 2021). In addition, some recent observations (Wang et al. 2021), coupled with earlier work (Okamoto et al. 2015, 2017), suggest that GCGR blockade can also promote recovery of functional beta-cell mass, with obvious additional benefits for diabetes. Indeed, there are several reports that GCGR knockout (KO) mice are more resistant to beta-cell destruction in response to islet stress (Conarello et al. 2007, Lee et al. 2012).
Various chemical approaches have been taken in an attempt to annul GCGR activity for therapeutic benefit, including small molecules (Mu et al. 2012, Guzman-Perez et al. 2013, Pettus et al. 2020), monoclonal antibodies (Kim et al. 2012, Okamoto et al. 2015, 2017) or antisense oligonucleotides (Liang et al. 2004, Morgan et al. 2019). Although all approaches possess robust glucose-lowering actions, the adverse side effect profile of each has been questioned (Patil et al. 2020, Lafferty et al. 2021). To date, it appears that peptide-based GCGR antagonists offer the best efficacy vs side effect profile (Irwin et al. 2013, O’Harte et al. 2013, Franklin et al. 2014, 2022, McShane et al. 2014). Whether this relates to the composition of the compounds in question, or overall potency and degree of GCGR blockade, remains to be determined. However, a wealth of data suggests that organic peptides, such as desHis1Pro4Glu9-glucagon, represent highly effective GCGR antagonists (O’Harte et al. 2013, Franklin et al. 2022). Indeed, other truncated glucagon-based peptides have recently been shown to yield selective, high potency, GCGR antagonists (Yang et al. 2021), supporting this as an effective approach to decrease GCGR activity. Moreover, an acylated, longer-acting, version of desHis1Pro4Glu9-glucagon has been described, namely desHis1Pro4Glu9-glucagon(Lys12PAL), that also effectively antagonises the GCGR (Franklin et al. 2014). This analogue has a palmitic acid covalently attached to the Lys12 residue of desHis1Pro4Glu9-glucagon via a γ-glutamyl spacer molecule, delivering a significantly extended pharmacodynamic profile (O’Harte et al. 2013). Notably, our previous work fully characterises the in vitro and acute in vivo biological action profile of both desHis1Pro4Glu9-glucagon and desHis1Pro4Glu9-glucagon(Lys12PAL), including effects on cAMP accumulation, insulin secretion, inhibition of glucagon action, glucose disposal and islet hormone secretion (O’Harte et al. 2013, Franklin et al. 2014).
Moreover, we have also previously shown that sustained administration of desHis1Pro4Glu9-glucagon, or its Lys12 acylated counterpart, can reverse aspects of genetically induced and dietary-induced obesity-related diabetes in obese-diabetic (ob/ob) and high-fat-fed (HFF) mice, respectively (O’Harte et al. 2014). However, both these murine models of diabetes are associated with adaptive beta-cell expansion prior to the development of overt diabetes. In this regard, administration of the beta-cell toxin, streptozotocin (STZ), can counter beta-cell compensation and prevent such innate adaptations (Furman 2015). Thus, HFF mice with STZ-induced compromised beta-cells are characterised by obstruction of the classical beta-cell hypertrophy in response to prolonged high-fat feeding (Tanday et al. 2021). Therefore, this HFF-STZ murine model represents an ideal tool to fully explore the positive effects of peptide-based GCGR antagonists in obesity-driven forms of diabetes, where restoration of functional beta-cell mass would be highly advantageous. Notably, the benefits of GCGR blockade are believed to require at least some residual beta-cell function (Damond et al. 2016), which would be the case for HFF-STZ mice (Tanday et al. 2021).
Consequently, in the current study, we have investigated the impact of once-daily treatment with desHis1Pro4Glu9-glucagon or desHis1Pro4Glu9-glucagon(Lys12PAL) in HFF-STZ mice for 18 days. Effects on food and fluid intake as well as body weight and circulating glucose were assessed at regular intervals. The metabolic status of the mice was then examined at the end of the treatment period through glucose and insulin tolerance tests. Finally, aspects of indirect calorimetry and pancreatic hormone content were also investigated. Taken together, we reveal that peptidic GCGR antagonists possess metabolic benefits following STZ-induced beta-cell insult in insulin-resistant HFF mice, which merits further investigation in terms of translation to the clinical setting.
Materials and methods
Peptides
All peptides were synthesised by Synpeptide (Shanghai, China) at 95% purity, which was confirmed in-house by high-performance liquid chromatography (HPLC) and matrix-assisted laser desorption/ionisation-time of flight (MALDI-TOF) mass spectrometry (MS), as previously described (Lafferty et al. 2020).
Animals
Young male NIH Swiss mice (10-week-old; n = 8) were maintained on high-fat diet (45% fat, 20% protein and 25% carbohydrates; percent of total energy of 26.15 kJ/g; Special Diets Services, Witham, Essex, UK) for 12 weeks, by which stage obesity was clearly manifested. After this period, mice were administered with a single large i.p. dose of STZ (4-h fast, 125 mg/kg bw, dissolved in sodium citrate buffer, pH 4.5). A separate group of HFF mice that did not receive STZ injection were employed as an additional control group. Appropriate numbers of non-diabetic control mice were not available for inclusion in the current study, but the basic phenotypes of HFF mice such as obesity, impaired, glucose tolerance, hyperinsulinaemia and insulin resistance were confirmed.
Chronic in vivo experiments
Groups (n = 8) of HFF-STZ mice received once-daily i.p. injections (10:00 h) of saline vehicle (0.9% (w/v) NaCl), desHis1Pro4Glu9-glucagon or desHis1Pro4Glu9-glucagon(Lys12PAL) (both at 25 nmol/kg bw) for 18 days, starting on the same day that STZ was administered. To acclimatise mice to the injection regimen, all mice received once-daily i.p. injections of saline over a 6-day run in period. Mice were maintained on high-fat diet (45%) throughout the experiment. At regular intervals, cumulative energy and fluid intake, body weight and non-fasting circulating glucose were assessed. At the end of the treatment period, oral and i.p. glucose tolerance (18 mmol/kg bw; i.p. or oral as appropriate; 18-h fasted) as well as insulin sensitivity (5 U/kg bovine insulin; i.p.; non-fasted) tests were conducted. Aspects of indirect calorimetry were measured using an Oxymax Comprehensive Laboratory Animal Monitoring System (CLAMS), with 18 h acclimation prior to recordings (Columbus Instruments, Columbus, OH, USA). Following the acclimatisation period, O2 consumption, CO2 production, respiratory exchange ratio (RER), energy expenditure and locomotor activity were assessed, as described previously (O’Harte et al. 2018). All animal experiments were approved by Ulster University Animal Ethics Review Committee and conducted in accordance with the UK Animals (Scientific Procedures) Act 1986.
Biochemical analyses
Blood samples were obtained from conscious mice via the cut tip on the tail vein, and blood glucose was immediately measured using an Ascencia Contour blood glucose meter (Bayer Healthcare, Newbury, UK). Pancreatic or plasma insulin and glucagon, as appropriate, were measured by in-house RIA (Flatt & Bailey 1981) or commercially available ELISA (EZGLU-30K, Merck Millipore), respectively.
Statistical analyses
Statistical tests were conducted using GraphPad PRISM software (Version 5.0). Values are expressed as mean ± s.e.m. Comparative analyses between groups were performed using a one-way or two-way ANOVA with Bonferroni’s post hoc test, as appropriate. Differences were deemed significant if P < 0.05.
Results
Effects of desHis1Pro4Glu9-glucagon or desHis1Pro4Glu9-glucagon(Lys12PAL) on food and fluid intake, body weight, circulating glucose and glucagon in HFF-STZ mice
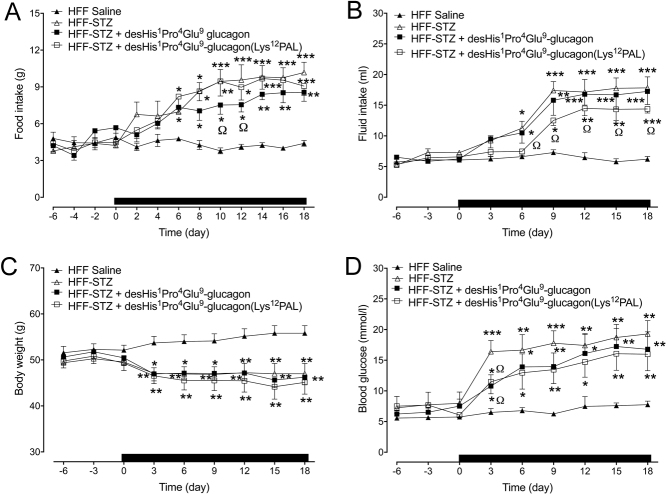
Food intake was significantly (P < 0.05–P < 0.001) increased in all HFF mice that received STZ injection (Fig. 1A). Only treatment with desHis1Pro4Glu9-glucagon led to reductions (P < 0.05) of STZ-induced elevations of food intake that was evident on days 10 and 12 (Fig. 1A). Interestingly, STZ-related increases (P < 0.05–P < 0.001) in fluid intake were partially reversed (P < 0.05) in desHis1Pro4Glu9-glucagon(Lys12PAL)-treated mice, but not by desHis1Pro4Glu9-glucagon (Fig. 1B). Body weight was reduced in all STZ mice, with treatment interventions having no impact on this parameter (Fig. 1C). As expected, STZ administration resulted a significant (P < 0.001) sustained increase in blood glucose levels from day 3 onwards (Fig. 1D). Both desHis1Pro4Glu9-glucagon and desHis1Pro4Glu9-glucagon(Lys12PAL) partially protected (P < 0.05) against STZ-induced elevations of glucose, but these mice still had increased (P < 0.05–P < 0.01) circulating glucose when compared to HFF control mice (Fig. 1D). In terms of circulating glucagon concentrations, STZ treatment increased (P < 0.001) circulating glucagon levels in HFF mice on day 18 when compared to lean controls (51.2 ± 13.6 vs 24.3 ± 8.8 pg/mL, respectively), but this effect was fully reversed by both treatment regimens where circulating glucagon was between 19.8 and 23.4 ± 6.7 pg/mL in these mice on day 18.
Figure 1.
Effects of once-daily administration of desHis1Pro4Glu9-glucagon or desHis1Pro4Glu9-glucagon (Lys12PAL) (each at 25 nmol/kg bw) for 18 days on cumulative food intake (A), cumulative fluid intake (B), body weight (C) and blood glucose (D) in HFF-STZ mice. Measurements were taken 6 days prior to and throughout the treatment period, at regular intervals. The treatment period is highlighted by the horizontal black bar parallel to x-axis. Values are mean ± s.e.m. (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 compared to HFF-STZ saline controls. ΩP < 0.05 compared to HFF saline controls.
Effects of desHis1Pro4Glu9-glucagon or desHis1Pro4Glu9-glucagon(Lys12PAL) on glucose tolerance and insulin sensitivity in HFF-STZ mice
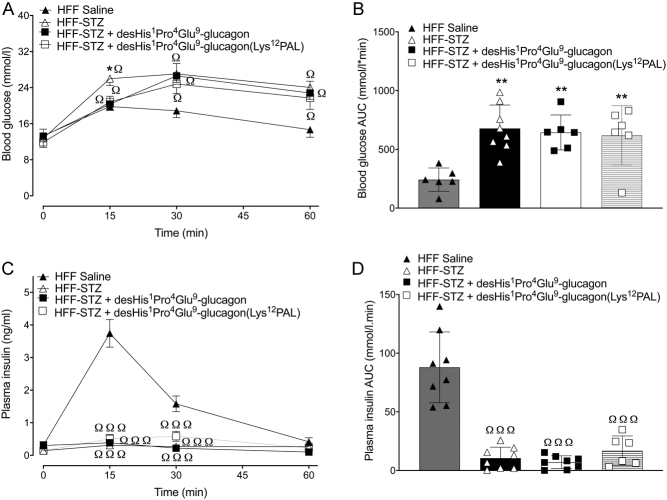
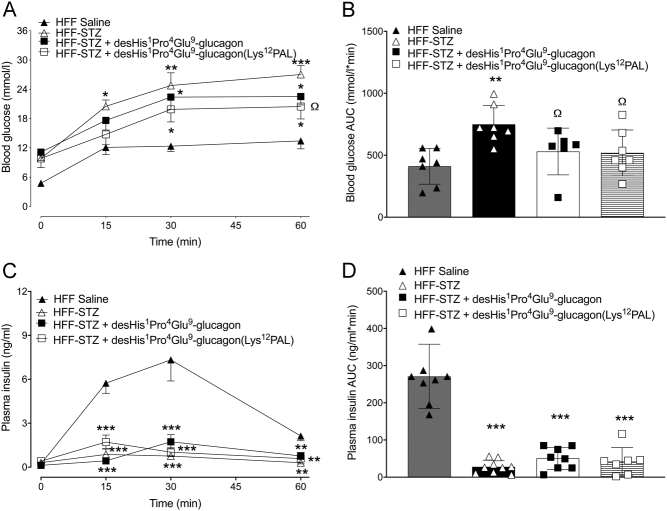
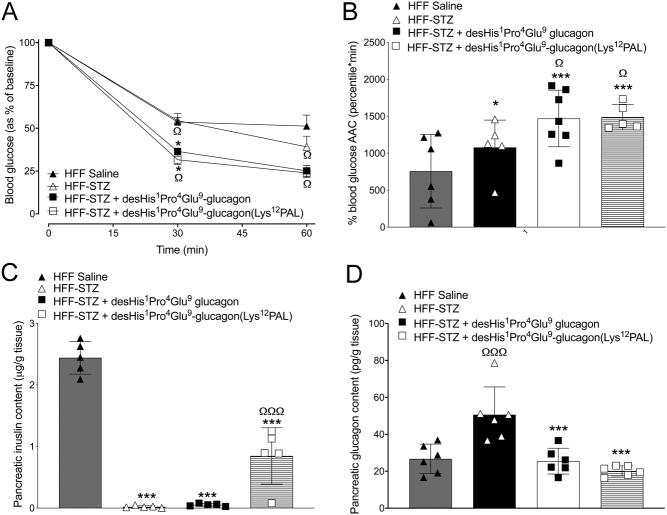
Following an i.p. glucose challenge, glucose levels were significantly lower (P < 0.05) 15 min post injection in both desHis1Pro4Glu9-glucagon and desHis1Pro4Glu9-glucagon(Lys12PAL)-treated HFF-STZ mice when compared to saline controls (Fig. 2A). However, this reduction was not sustained at 30 and 60 min (Fig. 2A), and there was no difference in 0–60 min glucose AUC values between all HFF-STZ groups of mice (Fig. 2B). Glucose-induced insulin secretory responses were almost absent in all HFF-STZ mice, with only control HFF mice displaying any real glucose-induced elevations of insulin concentrations (Fig. 2C and D). The benefits of desHis1Pro4Glu9-glucagon and desHis1Pro4Glu9-glucagon(Lys12PAL) treatment were more prominent following an oral glucose tolerance challenge (Fig. 3A and B). Thus, although individual glucose levels were still elevated in the treatment groups compared to HFF controls (Fig. 3A), 0–60 min AUC values were decreased (P < 0.05) by desHis1Pro4Glu9-glucagon and desHis1Pro4Glu9-glucagon(Lys12PAL) when compared to STZ-diabetic control mice, and not significantly different from HFF controls (Fig. 3B). However, glucose-induced insulin concentrations were not noticeably amplified by either treatment (Fig. 3C and D). In some agreement with this, the hypoglycaemic action of exogenous insulin was significantly (P < 0.05–P < 0.001) augmented by desHis1Pro4Glu9-glucagon and desHis1Pro4Glu9-glucagon(Lys12PAL), when compared to HFF-STZ or HFF control mice (Fig. 4A and B). Interestingly, STZ administration alone also appeared to enhance (P < 0.05) peripheral insulin action in HFF mice (Fig. 4A and B). As anticipated, administration of STZ significantly (P < 0.001) depressed pancreatic insulin content, but 18 days therapy with desHis1Pro4Glu9-glucagon(Lys12PAL) was able to partially reverse (P < 0.001) this detrimental effect (Fig. 4C). STZ also increased (P < 0.001) pancreatic glucagon content of HFF mice, but this effect was fully reversed by both desHis1Pro4Glu9-glucagon and desHis1Pro4Glu9-glucagon(Lys12PAL) treatment (Fig. 4D).
Figure 2.
Effects of once-daily administration of desHis1Pro4Glu9-glucagon or desHis1Pro4Glu9-glucagon(Lys12PAL) (each at 25 nmol/kg bw) for 18 days on i.p. glucose tolerance in HFF-STZ mice (18 mmol/kg bw). Blood glucose (A) and associated plasma insulin responses (C) with respective areas under the curves (B and D) are provided. Values are mean ± s.e.m. (n = 8). *P < 0.05 compared to HFF-STZ saline controls. ΩP < 0.05, ΩΩΩP < 0.001 compared to HFF saline controls.
Figure 3.
Effects of once-daily administration of desHis1Pro4Glu9-glucagon or desHis1Pro4Glu9-glucagon(Lys12PAL) (each at 25 nmol/kg bw) for 18 days on oral glucose tolerance in HFF-STZ mice (18 mmol/kg bw). Blood glucose (A) and associated plasma insulin responses (C) with respective areas under the curves (B and D) are provided. Values are mean ± s.e.m. (n = 8). *P < 0.05 compared to HFF-STZ saline controls. Ω P < 0.05 compared to HFF saline controls.
Figure 4.
Effects of once-daily administration of desHis1Pro4Glu9-glucagon or desHis1Pro4Glu9-glucagon(Lys12PAL) (each at 25 nmol/kg bw) for 18 days on insulin sensitivity (A) in HFF-STZ mice, with the related area above the curve (B) presented. Insulin was administered by i.p. injection at 5 IU/kg/bw in non-fasted mice. Additionally, effects on pancreatic hormone content were assessed on day 18. Pancreatic glucagon (C) and insulin (D) levels were assessed in excised pancreatic tissue via a commercially available ELISA or in-house RIA, respectively. Values are mean ± s.e.m. (n = 8). *P < 0.05, ***P < 0.001 compared to HFF-STZ saline controls. ΩP < 0.05, ΩΩΩP < 0.001 compared to HFF saline controls.
Effects of desHis1Pro4Glu9-glucagon or desHis1Pro4Glu9-glucagon(Lys12PAL) on indirect calorimetry and locomotor activity in HFF-STZ mice
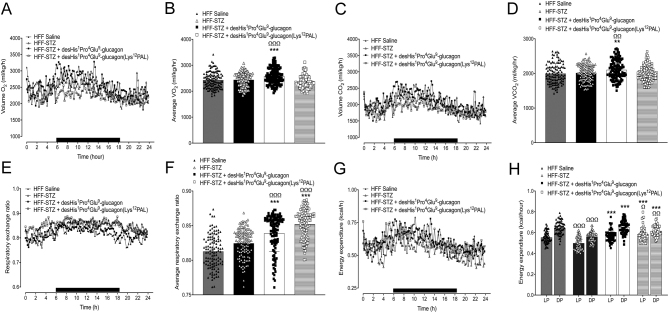
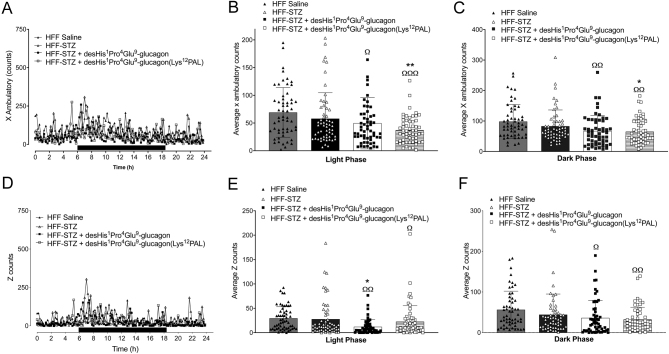
Consumption of O2 was similar in HFF and HFF-STZ mice on day 18, but desHis1Pro4Glu9-glucagon increased (P < 0.001) this parameter (Fig. 5A and B). Consistent with these findings, desHis1Pro4Glu9glucagon also increased CO2 production (P < 0.01) in comparison to both HFF and HFF-STZ control mice (Fig. 5C and D). In addition, desHis1Pro4Glu9-glucagon and desHis1Pro4Glu9-glucagon(Lys12PAL) treatment resulted in a significant (P < 0.001) increase in RER (Fig. 5E and F). Energy expenditure was decreased (P < 0.001) by STZ administration in HFF mice, which was fully reversed by both GCGR antagonists (Fig. 5G and H). Interestingly, both treatment interventions decreased (P < 0.05–P < 0.01) X beam ambulatory breaks vs HFF-STZ controls during both the light and dark phases (Fig. 6A, B and C). A similar effect of desHis1Pro4Glu9-glucagon was also noted in terms of Z-beam breaks during the light phase (P < 0.05), which represent vertical activity levels such as mouse rearing events (Fig. 6D, E and F). Both peptide treatments had significantly (P < 0.05–P < 0.001) reduced X and Z beam breaks when compared to saline-treated HFF controls (Fig. 6A, B, C, D, E and F).
Figure 5.
Effects of once-daily administration of desHis1Pro4Glu9-glucagon or desHis1Pro4Glu9-glucagon(Lys12PAL) (each at 25 nmol/kg bw) for 18 days on O2 consumption (A and B), CO2 production (C and D), respiratory exchange ratio (RER) (E and F) and energy expenditure (G and H) in HFF-STZ mice. Mice were placed in CLAMS metabolic chambers for 18 h to acclimatise, and measurements were obtained over a further 24 h period (12 h dark period as shown by black bar parallel to x-axis) at the end of the treatment period. O2 consumption and CO2 production were measured for 30 s at 25 min intervals (A and C). RER was calculated by dividing VCO2 by VO2 (E and F). Energy expenditure was calculated using RER with the following equation: (3.815 + 1.232 × RER) × VO2 (G). Average energy expenditure is also provided (H), separated into the light (LP) and dark phases (DP). Overall incremental data are presented in panels B, D, F and G, where each data point represents information collected at individual time-points over the 24 h period. Values are mean ± s.e.m. (n = 6). **P < 0.01, ***P < 0.001 compared to HFF-STZ saline controls. ΩP < 0.05, ΩΩP < 0.01, ΩΩΩP < 0.001 compared to HFF saline controls.
Figure 6.
Effects of once-daily administration of desHis1Pro4Glu9-glucagon or desHis1Pro4Glu9-glucagon(Lys12PAL) (each at 25 nmol/kg bw) for 18 days on locomotor activity in HFF-STZ mice. Mice were placed in CLAMS metabolic chambers for 18 h to acclimatise, and measurements were obtained over a further 24 h period (12 h dark period as shown by black bar parallel to x-axis) at the end of the treatment period. Activity counts on x-axis (A, B and C) and z-axis (D, E and F) were recorded at 60-s intervals. Overall incremental data are presented in panels B, C, E and F, where each data point represents information collected at individual time-points over the 24 h period. Values are mean ± s.e.m. (n = 6). *P < 0.05, **P < 0.01 compared to HFF-STZ saline controls. ΩP < 0.05, ΩΩP < 0.01, ΩΩΩP < 0.001 compared to HFF saline controls.
Discussion
The interplay between pancreatic alpha- and beta-cell signalling is intriguing, with secretions from beta-cells directly inhibiting alpha-cell function, whilst alpha-cells release factors that are stimulatory for beta-cells (English & Irwin 2019, Moede et al. 2020). Coupled with recent awareness that mature beta-cells de-differentiate into alpha-cells (Weir et al. 2013) and that alpha-cells can act as progenitors for functional beta-cells (Habener & Stanojevic 2012), modulation of alpha-cell activity could hold promise for the treatment of diabetes. Indeed, alpha-to-beta-cell lineage conversion is enhanced in GCGR KO mice (Damond et al. 2016), and more recently, human alpha-cells were shown to be capable of reprogramming into glucose-sensitive insulin-secreting cells to help ameliorate diabetes in mice (Furuyama et al. 2019). In this regard, inhibition of GCGR signalling has long been considered as a potential means of effectively controlling blood glucose levels (Unger 1978).
In the current study, we employed a high single dose of STZ as an established method to induce beta-cell cytotoxicity and perturb insulin secretory function in HFF mice (Deeds et al. 2011, Millar et al. 2017). Thus, HFF mice are classically characterised by the manifestation of insulin resistance leading to subsequent compensatory beta-cell expansion and hyperinsulinaemia (Ahrén et al. 2010). Notably, saline-treated control HFF mice did not present with overt hyperglycaemia, but as expected, glucose intolerance was evident following a glucose challenge likely as a result of dietary-induced insulin resistance (Ahrén et al. 2010). However, hyperglycaemia was clearly apparent in all GCGR antagonist-treated HFF-STZ mice by day 18, treatment intervention appeared to delay onset. Indeed, the acylated GCGR analogue partially restored pancreatic insulin concentrations, which may be linked to the more protracted bioactive profile of desHis1Pro4Glu9-glucagon(Lys12PAL) over desHis1Pro4Glu9-glucagon (Franklin et al. 2014). In agreement, GCGR blockade has recently been demonstrated to promote recovery of functional beta-cell mass in diabetic mice (Wang et al. 2021). Regrettably, a technical issue during tissue processing thwarted our efforts to investigate aspects of pancreatic islet morphology, including beta-cell mass and turnover, that would help to validate our observations. Interestingly, the augmented pancreatic insulin content, which was particularly apparent in desHis1Pro4Glu9-glucagon(Lys12PAL)-treated HFF-STZ mice, was not matched by prominent improvements in insulin secretory responses or glucose levels. Thus, further investigation of beta-cell secretory function and responsiveness would be required to uncover the relationship between increased insulin stores and translation to more obvious improvements of metabolism in these mice, although improvements in insulin action might also be important in this regard. Circulating and pancreatic glucagon levels were reduced in all GCGR antagonist-treated HFF-STZ, which contrasts with observations using small molecule GCGR inhibitors (Mu et al. 2011, 2012), but complements previous work with peptidic GCGR antagonists (Franklin et al. 2014, McShane et al. 2014, O’Harte et al. 2014). This also highlights the improved adverse effect profile of peptidic GCGR antagonists over other methods employed to inhibit GCGR signalling. As such, rebound hyperglycaemia has been observed on treatment termination with some small molecule GCGR antagonists (Sloop et al. 2004), likely because of their actions to elevate circulating glucagon.
Benefits on glucose tolerance were more apparent following an oral as opposed to i.p. glucose challenge in desHis1Pro4Glu9-glucagon and desHis1Pro4Glu9-glucagon(Lys12PAL)-treated HFF-STZ mice. In accordance with this, GCGR antagonism-mediated improvements in glycaemic control have been suggested to be dependent on functional GLP-1 receptors (Gu et al. 2010). Indeed, more recent studies have demonstrated that GCGR blockade can promote intestinal L-cell proliferation (Lang et al. 2020a) and inhibit L-cell apoptosis (Lang et al. 2020b), leading to elevated GLP-1 synthesis and secretion. In agreement, inhibition of the incretin hormone degrading enzyme, dipeptidyl peptidase-4, improves the effectiveness of GCGR inhibition in diabetic mice (Mu et al. 2011). It has also been suggested that combined GLP-1 receptor activation and GCGR inhibition possesses beneficial actions (Claus et al. 2007). Unfortunately, we were unable to measure circulating GLP-1 concentrations in the current study due to the limited volume of blood that can be withdrawn from mice. However, we have recently shown that combined administration of a peptidic GCGR antagonist, with the well-characterised GLP-1 receptor mimetic exendin-4, exerts limited additive metabolic benefits (Franklin et al. 2022). Thus, activation of receptors for the sister incretin hormone of GLP-1, namely glucose-dependent insulinotropic polypeptide, may offer a more attractive paradigm in terms of combination therapy with GCGR antagonism (McShane et al. 2016). However, intestinal L-cell number has also been demonstrated to be reduced by STZ administration (Vasu et al. 2015), which could represent another confounding factor in our current observations. Thus, both pancreatic beta-cells and enteroendocrine L-cells appear to have limited antioxidant defence mechanisms (Lenzen 2008, Vasu et al. 2015). Although, in this respect, it should be noted that by their very nature, intestinal mucosal cell turnover is rapid, with efficient cellular replacement by differentiating stem cells that arise from intestinal crypts (Roth & Gordon 1990, Schonhoff et al. 2004).
Of note is the improvement of glucose handling in the absence of any real augmentation of insulin concentrations, this being despite elevated pancreatic insulin content in desHis1Pro4Glu9-glucagon(Lys12PAL)-treated HFF-STZ mice. It follows that insulin action must be enhanced in these mice, which was indeed apparent following exogenous insulin injection. Similar observations have been made previously following STZ treatment in GCGR KO mice (Lee et al. 2011). In the absence of GCGR signalling, hepatic glucose output and the positive effects of GCGR signalling on basal metabolic rate are also likely to be much reduced (Breton et al. 1983). In good accord with this, in the current study, the peptidic GCGR antagonists both decreased physical activity in HFF-STZ mice. However, the ability of desHis1Pro4Glu9-glucagon(Lys12PAL), and particularly desHis1Pro4Glu9-glucagon, to increase energy expenditure does contrast with this notion, but this may simply highlight the plasticity of signalling pathways involved in energy homeostasis (Smith et al. 2018). The slight difference in efficacy between desHis1Pro4Glu9-glucagon and desHis1Pro4Glu9-glucagon(Lys12PAL) in terms of indirect calorimetry data could be related to free vs albumin-bound drug, where it is often considered that albumin binding reduces bioactivity of peptides (Miyakawa et al. 2013). However, more detailed pharmacokinetic studies, that are outside the scope of the current investigation, would be required to confirm this. In a similar fashion, there were also slight differences between the effect of both peptides on food and fluid intake. Thus, desHis1Pro4Glu9-glucagon had a mild and transient impact on moderating STZ-induced elevations of feeding, whereas desHis1Pro4Glu9-glucagon(Lys12PAL) exerted more enduring effects to counter increased fluid intake in HFF-STZ mice. Although peptide pharmacodynamic profiles may also be important in this observation, we are unable to discount alterations in the passage of either peptide through the blood–brain barrier and the subsequent impact on hypothalamic circuits that regulate energy intake and thirst (Woods 2013).
It is established that glucagon plays an important role in lipid oxidation and metabolism (Galsgaard et al. 2019), and our observed increases in RER evoked by sustained GCGR antagonism likely partly reflects this. Thus, carbohydrate oxidation drives RER to a value closer to 1.0, with fatty acid oxidation reducing this to 0.7 (Rosenkilde et al. 2010, Purdom et al. 2018). Hyperaminoacidaemia has also been reported following inhibition of GCGR signalling and assessment of plasma amino acid levels would have been interesting in this regard (Richter et al. 2022). The impact of the high-fat background diet (45%), enduring insulin deficiency and small GCGR antagonist-induced changes in food intake and body weight need to be considered in terms of overall effects on carbohydrate metabolism. In that respect, GCGR KO mice are reported to be resistant to high-fat feeding-induced obesity (Conarello et al. 2007), but the possibility for life-long adaptations in these animals should not be overlooked. However, differences in the magnitude of GCGR signalling annulment between genetic and chemical receptor blockade could also be a factor. Thus, similar to the current setting, prolonged treatment with a small molecule GCGR antagonist did not affect body weight in HFF mice (Mu et al. 2011), this being despite increased energy expenditure with desHis1Pro4Glu9-glucagon and desHis1Pro4Glu9-glucagon(Lys12PAL) therapy. The current treatment regimen entailed once-daily peptide treatment for 18 days and whether extended dosing periods would lead to more discernible benefits on metabolism in HFF-STZ mice still needs to be established.
In summary, the current study establishes that peptide-based GCGR antagonism exerts notable benefits in obesity-driven forms of diabetes, even in the presence of insulin deficiency. As well as delaying the onset of hyperglycaemia, desHis1Pro4Glu9-glucagon, and particularly desHis1Pro4Glu9-glucagon(Lys12PAL), improved glucose handling and insulin action in addition to augmenting pancreatic insulin stores. Our observations further support the promise of peptidic GCGR antagonists as a new class of drugs for the management of various forms of diabetes.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by an Invest Northern Ireland Proof-of-Concept grant (PoC106), a Department for the Economy, Northern Ireland PhD studentship and Ulster University Selective Research Funding
Data availability statement
The authors declare that the data supporting the findings of this study are available within the article. Any additional raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contribution statement
N I and F O H conceived/designed and supervised the study. N I, R A L and P R F drafted the manuscript. L M McS and Z J F and participated in the conduct/data collection and analysis and interpretation of data. All authors revised the manuscript critically for intellectual content and approved the final version of the manuscript.
References
- Ahrén J, Ahrén B, Wierup N.2010Increased β-cell volume in mice fed a high-fat diet: a dynamic study over 12 months. Islets 2353–356. ( 10.4161/isl.2.6.13619) [DOI] [PubMed] [Google Scholar]
- Breton L, Clot JP, Baudry M.1983Effects of glucagon on basal metabolic rate and oxidative phosphorylation of rat liver mitochondria. Hormone and Metabolic Research 15429–432. ( 10.1055/s-2007-1018747) [DOI] [PubMed] [Google Scholar]
- Claus TH, Pan CQ, Buxton JM, Yang L, Reynolds JC, Barucci N, Burns M, Ortiz AA, Roczniak S, Livingston JNet al. 2007Dual-acting peptide with prolonged glucagon-like peptide-1 receptor agonist and glucagon receptor antagonist activity for the treatment of type 2 diabetes. Journal of Endocrinology 192371–380. ( 10.1677/JOE-06-0018) [DOI] [PubMed] [Google Scholar]
- Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, Ronan J, Liu F, Roy RS, Zhu Let al. 2007Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia 50142–150. ( 10.1007/s00125-006-0481-3) [DOI] [PubMed] [Google Scholar]
- Damond N, Thorel F, Moyers JS, Charron MJ, Vuguin PM, Powers AC, Herrera PL.2016Blockade of glucagon signaling prevents or reverses diabetes onset only if residual β-cells persist. eLife 5 e13828. ( 10.7554/eLife.13828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeds MC, Anderson JM, Armstrong AS, Gastineau DA, Hiddinga HJ, Jahangir A, Eberhardt NL, Kudva YC.2011Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Laboratory Animals 45131–140. ( 10.1258/la.2010.010090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- English A, Irwin N.2019Nonclassical islet peptides: pancreatic and extrapancreatic actions. Clinical Medicine Insights: Endocrinology and Diabetes 121179551419888871. ( 10.1177/1179551419888871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt PR, Bailey CJ.1981Abnormal plasma glucose and insulin responses in heterozygous lean (ob/+) mice. Diabetologia 20573–577. ( 10.1007/BF00252768) [DOI] [PubMed] [Google Scholar]
- Franklin ZJ, O’Harte FPM, Irwin N.2014Effects of short-term chemical ablation of glucagon signalling by peptide-based glucagon receptor antagonists on insulin secretion and glucose homeostasis in mice. Biological Chemistry 395433–442. ( 10.1515/hsz-2013-0224) [DOI] [PubMed] [Google Scholar]
- Franklin ZJ, Lafferty RA, Flatt PR, McShane LM, O’Harte FPM, Irwin N.2022Metabolic effects of combined glucagon receptor agonism and glucagon-like peptide-1 receptor agonism in high fat fed mice. Biochimie 19960–67. ( 10.1016/j.biochi.2022.04.005) [DOI] [PubMed] [Google Scholar]
- Furman BL.2015Streptozotocin-induced diabetic models in mice and rats. Current Protocols in Pharmacology 705.47.1–5.47.20. ( 10.1002/0471141755.ph0547s70) [DOI] [PubMed] [Google Scholar]
- Furuyama K, Chera S, van Gurp L, Oropeza D, Ghila L, Damond N, Vethe H, Paulo JA, Joosten AM, Berney Tet al. 2019Diabetes relief in mice by glucose-sensing insulin-secreting human α-cells. Nature 56743–48. ( 10.1038/s41586-019-0942-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galsgaard KD, Pedersen J, Knop FK, Holst JJ, Wewer Albrechtsen NJ.2019Glucagon receptor signalling and lipid metabolism. Frontiers in Physiology 10 413. ( 10.3389/fphys.2019.00413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Winters KA, Motani AS, Komorowski R, Zhang Y, Liu Q, Wu X, Rulifson IC, Sivits Jr G, Graham Met al. 2010Glucagon receptor antagonist-mediated improvements in glycemic control are dependent on functional pancreatic GLP-1 receptor. American Journal of Physiology: Endocrinology and Metabolism 299E624–E632. ( 10.1152/ajpendo.00102.2010) [DOI] [PubMed] [Google Scholar]
- Guzman-Perez A, Pfefferkorn JA, Lee EC, Stevens BD, Aspnes GE, Bian J, Didiuk MT, Filipski KJ, Moore D, Perreault Cet al. 2013The design and synthesis of a potent glucagon receptor antagonist with favorable physicochemical and pharmacokinetic properties as a candidate for the treatment of type 2 diabetes mellitus. Bioorganic and Medicinal Chemistry Letters 233051–3058. ( 10.1016/j.bmcl.2013.03.014) [DOI] [PubMed] [Google Scholar]
- Habener JF, Stanojevic V.2012α-Cell role in β-cell generation and regeneration. Islets 4188–198. ( 10.4161/isl.20500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin N, Franklin ZJ, O’Harte FPM.2013DesHis1Glu9-glucagon-(mPEG) and desHis 1Glu9(Lys30PAL)-glucagon: long-acting peptide-based pegylated and acylated glucagon receptor antagonists with potential antidiabetic activity. European Journal of Pharmacology 70943–51. ( 10.1016/j.ejphar.2013.03.041) [DOI] [PubMed] [Google Scholar]
- Kim WD, Lee YH, Kim MH, Jung SY, Son WC, Yoon SJ, Lee BW.2012Human monoclonal antibodies against glucagon receptor improve glucose homeostasis by suppression of hepatic glucose output in diet-induced obese mice. PLoS ONE 7 e50954. ( 10.1371/journal.pone.0050954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty RA, Tanday N, Flatt PR, Irwin N.2020Generation and characterisation of C-terminally stabilised PYY molecules with potential in vivo NPYR2 activity. Metabolism: Clinical and Experimental 111 154339. ( 10.1016/j.metabol.2020.154339) [DOI] [PubMed] [Google Scholar]
- Lafferty RA, O’Harte FPM, Irwin N, Gault VA, Flatt PR.2021Proglucagon-derived peptides as therapeutics. Frontiers in Endocrinology 12689678. ( 10.3389/fendo.2021.689678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S, Yang J, Yang K, Gu L, Cui X, Wei T, Liu J, Le Y, Wang H, Wei Ret al. 2020aGlucagon receptor antagonist upregulates circulating GLP-1 level by promoting intestinal L-cell proliferation and GLP-1 production in type 2 diabetes. BMJ Open Diabetes Research and Care 8 e001025. ( 10.1136/bmjdrc-2019-001025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S, Wei R, Wei T, Gu L, Feng J, Yan H, Yang J, Hong T.2020bGlucagon receptor antagonism promotes the production of gut proglucagon-derived peptides in diabetic mice. Peptides 131 170349. ( 10.1016/j.peptides.2020.170349) [DOI] [PubMed] [Google Scholar]
- Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH.2011Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes 60391–397. ( 10.2337/db10-0426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Berglund ED, Wang MY, Fu X, Yu X, Charron MJ, Burgess SC, Unger RH.2012Metabolic manifestations of insulin deficiency do not occur without glucagon action. PNAS 10914972–14976. ( 10.1073/pnas.1205983109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzen S.2008The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 51216–226. ( 10.1007/s00125-007-0886-7) [DOI] [PubMed] [Google Scholar]
- Liang Y, Osborne MC, Monia BP, Bhanot S, Gaarde WA, Reed C, She P, Jetton TL, Demarest KT.2004Reduction in glucagon receptor expression by an antisense oligonucleotide ameliorates diabetic syndrome in db/db mice. Diabetes 53410–417. ( 10.2337/diabetes.53.2.410) [DOI] [PubMed] [Google Scholar]
- McShane LM, Franklin ZJ, O’Harte FPM, Irwin N.2014Ablation of glucagon receptor signalling by peptide-based glucagon antagonists improves glucose tolerance in high fat fed mice. Peptides 6095–101. ( 10.1016/j.peptides.2014.08.002) [DOI] [PubMed] [Google Scholar]
- McShane LM, Irwin N, O’Flynn D, Franklin ZJ, Hewage CM, O’Harte FPM.2016Glucagon receptor antagonist and GIP agonist combination for diet-induced obese mice. Journal of Endocrinology 229319–330. ( 10.1530/JOE-15-0463) [DOI] [PubMed] [Google Scholar]
- Millar P, Pathak N, Parthsarathy V, Bjourson AJ, O’Kane M, Pathak V, Moffett RC, Flatt PR, Gault VA.2017Metabolic and neuroprotective effects of dapagliflozin and liraglutide in diabetic mice. Journal of Endocrinology 234255–267. ( 10.1530/JOE-17-0263) [DOI] [PubMed] [Google Scholar]
- Miyakawa N, Nishikawa M, Takahashi Y, Ando M, Misaka M, Watanabe Y, Takakura Y.2013Gene delivery of albumin binding peptide-interferon-gamma fusion protein with improved pharmacokinetic properties and sustained biological activity. Journal of Pharmaceutical Sciences 1023110–3118. ( 10.1002/jps.23493) [DOI] [PubMed] [Google Scholar]
- Moede T, Leibiger IB, Berggren PO.2020Alpha cell regulation of beta cell function. Diabetologia 632064–2075. ( 10.1007/s00125-020-05196-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan ES, Tai LJ, Pham NC, Overman JK, Watts LM, Smith A, Jung SW, Gajdošík M, Krššák M, Krebs Met al. 2019Antisense inhibition of glucagon receptor by IONIS-GCGRRx improves type 2 diabetes without increase in hepatic glycogen content in patients with type 2 diabetes on stable metformin therapy. Diabetes Care 42585–593. ( 10.2337/dc18-1343) [DOI] [PubMed] [Google Scholar]
- Mu J, Jiang G, Brady E, Dallas-Yang Q, Liu F, Woods J, Zycband E, Wright M, Li Z, Lu Ket al. 2011Chronic treatment with a glucagon receptor antagonist lowers glucose and moderately raises circulating glucagon and glucagon-like peptide 1 without severe alpha cell hypertrophy in diet-induced obese mice. Diabetologia 542381–2391. ( 10.1007/s00125-011-2217-2) [DOI] [PubMed] [Google Scholar]
- Mu J, Qureshi SA, Brady EJ, Muise ES, Candelore MR, Jiang G, Li Z, Wu MS, Yang X, Dallas-Yang Qet al. 2012Anti-diabetic efficacy and impact on amino acid metabolism of GRA1, a novel small-molecule glucagon receptor antagonist. PLoS ONE 7 e49572. ( 10.1371/journal.pone.0049572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Harte FPM, Franklin ZJ, Rafferty EP, Irwin N.2013Characterisation of structurally modified analogues of glucagon as potential glucagon receptor antagonists. Molecular and Cellular Endocrinology 38126–34. ( 10.1016/j.mce.2013.07.014) [DOI] [PubMed] [Google Scholar]
- O’Harte FPM, Franklin ZJ, Irwin N.2014Two novel glucagon receptor antagonists prove effective therapeutic agents in high-fat-fed and obese diabetic mice. Diabetes, Obesity and Metabolism 161214–1222. ( 10.1111/dom.12360) [DOI] [PubMed] [Google Scholar]
- O’Harte FPM, Parthsarathy V, Hogg C, Flatt PR.2018Long-term treatment with acylated analogues of apelin-13 amide ameliorates diabetes and improves lipid profile of high-fat fed mice. PLoS ONE 13 e0202350. ( 10.1371/journal.pone.0202350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Kim J, Aglione J, Lee J, Cavino K, Na E, Rafique A, Kim JH, Harp J, Valenzuela DMet al. 2015Glucagon receptor blockade with a human antibody normalizes blood glucose in diabetic mice and monkeys. Endocrinology 1562781–2794. ( 10.1210/en.2015-1011) [DOI] [PubMed] [Google Scholar]
- Okamoto H, Cavino K, Na E, Krumm E, Kim SY, Cheng X, Murphy AJ, Yancopoulos GD, Gromada J.2017Glucagon receptor inhibition normalizes blood glucose in severe insulin-resistant mice. PNAS 1142753–2758. ( 10.1073/pnas.1621069114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil M, Deshmukh NJ, Patel M, Sangle GV.2020Glucagon-based therapy: past, present and future. Peptides 127170296. ( 10.1016/j.peptides.2020.170296) [DOI] [PubMed] [Google Scholar]
- Pettus JH, D’Alessio D, Frias JP, Vajda EG, Pipkin JD, Rosenstock J, Williamson G, Zangmeister MA, Zhi L, Marschke KB.2020Efficacy and safety of the glucagon receptor antagonist RVT-1502 in type 2 diabetes uncontrolled on metformin monotherapy: a 12-week dose-ranging study. Diabetes Care 43161–168. ( 10.2337/dc19-1328) [DOI] [PubMed] [Google Scholar]
- Purdom T, Kravitz L, Dokladny K, Mermier C.2018Understanding the factors that effect maximal fat oxidation. Journal of the International Society of Sports Nutrition 15 3. ( 10.1186/s12970-018-0207-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter MM, Galsgaard KD, Elmelund E, Knop FK, Suppli MP, Holst JJ, Winther-Sørensen M, Kjeldsen SAS, Albrechtsen NJW.2022The liver-alpha cell axis in health and in disease. Diabetes 711852–1861. ( 10.2337/dbi22-0004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkilde M, Nordby P, Nielsen LB, Stallknecht BM, Helge JW.2010Fat oxidation at rest predicts peak fat oxidation during exercise and metabolic phenotype in overweight men. International Journal of Obesity 34871–877. ( 10.1038/ijo.2010.11) [DOI] [PubMed] [Google Scholar]
- Roth KA, Gordon JI.1990Spatial differentiation of the intestinal epithelium: analysis of enteroendocrine cells containing immunoreactive serotonin, secretin, and substance P in normal and transgenic mice. PNAS 876408–6412. ( 10.1073/pnas.87.16.6408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonhoff SE, Giel-Moloney M, Leiter AB.2004Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Developmental Biology 270443–454. ( 10.1016/j.ydbio.2004.03.013) [DOI] [PubMed] [Google Scholar]
- Sloop KW, Cao JX, Siesky AM, Zhang HY, Bodenmiller DM, Cox AL, Jacobs SJ, Moyers JS, Owens RA, Showalter ADet al. 2004Hepatic and glucagon-like peptide-1-mediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors. Journal of Clinical Investigation 1131571–1581. ( 10.1172/JCI20911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RL, Soeters MR, Wüst RCI, Houtkooper RH.2018Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocrine Reviews 39489–517. ( 10.1210/er.2017-00211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanday N, English A, Lafferty RA, Flatt PR, Irwin N.2021Benefits of sustained upregulated unimolecular GLP-1 and CCK receptor signalling in obesity-diabetes. Frontiers in Endocrinology 12 674704. ( 10.3389/fendo.2021.674704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH.1978Role of glucagon in the pathogenesis of diabetes: the status of the controversy. Metabolism: Clinical and Experimental 271691–1709. ( 10.1016/0026-0495(7890291-3) [DOI] [PubMed] [Google Scholar]
- Vasu S, Moffett RC, McClenaghan NH, Flatt PR.2015Responses of GLP1-secreting L-cells to cytotoxicity resemble pancreatic β-cells but not α-cells. Journal of Molecular Endocrinology 5491–104. ( 10.1530/JME-14-0214) [DOI] [PubMed] [Google Scholar]
- Wang MY, Dean ED, Quittner-Strom E, Zhu Y, Chowdhury KH, Zhang Z, Zhao S, Li N, Ye R, Lee Yet al. 2021Glucagon blockade restores functional β-cell mass in type 1 diabetic mice and enhances function of human islets. PNAS 118 e2022142118. ( 10.1073/pnas.2022142118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir GC, Aguayo-Mazzucato C, Bonner-Weir S.2013β-Cell dedifferentiation in diabetes is important, but what is it? Islets 5233–237. ( 10.4161/isl.27494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC.2013Metabolic signals and food intake. Forty years of progress. Appetite 71440–444. ( 10.1016/j.appet.2012.08.016) [DOI] [PubMed] [Google Scholar]
- Yang B, Gelfanov VM, Perez-Tilve D, DuBois B, Rohlfs R, Levy J, Douros JD, Finan B, Mayer JP, DiMarchi RD.2021Optimization of truncated glucagon peptides to achieve selective, high potency, full antagonists. Journal of Medicinal Chemistry 644697–4708. ( 10.1021/acs.jmedchem.0c02069) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article. Any additional raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.



 This work is licensed under a
This work is licensed under a