Abstract
Objective:
This study aimed to examine within-race interactions of multiple dimensions of self-reported discrimination with depressive symptoms in relation to carotid intimal-medial thickness (IMT), a subclinical marker of atherosclerosis prospectively implicated in stroke incidence, in middle-aged to older African American and white adults.
Methods:
Participants were a socioeconomically diverse group of 1941 African Americans (56.5%) and whites from the Healthy Aging in Neighborhoods of Diversity across the Life Span study (30–64 years old, 47% men, 45.2% with household income <125% federal poverty threshold) who underwent carotid IMT measurement. Discrimination was assessed across four dimensions (everyday, frequency across various social statuses, racial, and lifetime burden). The Center for Epidemiologic Studies Depression scale was used to assess depressive symptoms.
Results:
In cross-sectional hierarchical regression analyses, two interactions were observed in African Americans: more frequent discrimination across various social statuses (b < 0.001, p = .006) and a higher lifetime discrimination burden (b < 0.001, p = .02) were each related to thicker carotid IMT in those with greater depressive symptoms. No significant findings were observed within whites.
Conclusions:
Among African Americans, those reporting high levels of discrimination and depressive symptoms have increased carotid atherosclerosis and may be at greater risk for clinical end points compared with those reporting one or neither of these risk factors. Findings suggest that assessment of interactive relationships among social and psychological factors may elucidate novel pathways for cardiovascular disease, including stroke, among African Americans.
Keywords: atherosclerosis, health disparities, intimal-medial thickness, subclinical vascular disease, risk factors, psychosocial vulnerabilities
INTRODUCTION
Black-white disparities in stroke risk in the United States exceed 300% between the ages of 45 and 65 years (1). For example, in 2014, it was estimated that 22,384 excess strokes occurred in African Americans relative to whites. Although this racial disparity attenuates at older ages, the ensuing disability, secondary stroke, and mortality risks contribute to a substantial and protracted burden of disease in African Americans. Traditional risk factors and socioeconomic status (SES) explain only ≈50% of the excess risk observed in African Americans (1), prompting researchers to call for the identification of psychosocial factors that may be contributing (1). Ultimately, identifying novel factors associated with early indicators of disease may be a crucial step in reducing the disproportionate stroke burden among African Americans.
The prognostic disease process underlying stroke incidence is atherosclerosis, which can begin early in the life course (2). Carotid intimal-medial thickness (IMT), measured with B-mode carotid ultrasonography, provides an early marker of subclinical atherosclerosis (3). IMT is prospectively linked to stroke incidence in both middle-aged (4) and older adults (5), as well as in minority racial groups, including African Americans (6,7).
Interpersonal discrimination, which is broadly defined as unfair treatment experienced in one on one interactions (8), is an established social stressor and predictor of cardiovascular disease (CVD; (9–14)). African Americans report significantly higher rates of interpersonal discrimination across various race-and non–race-related dimensions than other race groups, especially whites (15–17). Some prior work has demonstrated that specific types of interpersonal discrimination and even expectations of future interpersonal discrimination (18) may be adversely linked to subclinical vascular disease, including IMT, in African Americans (18–20). For example, in the Study of Women’s Health Across the Nation (SWAN), everyday discrimination, which captures minute but chronic insults without specifying the reason for the discrimination, was associated with concurrently greater IMT in African American women (20). However, in a subsequent longitudinal analysis of the same sample, cumulative exposure to everyday discrimination for 10 years was not a significant predictor of IMT in African American women (19). Similarly, everyday discrimination and lifetime discrimination burden were unrelated to IMT in 3029 African Americans in the Jackson Heart Study (21). Studies examining interpersonal discrimination in relation to other subclinical markers of CVD have also been mixed, but largely demonstrate an adverse linkage (22–29).
At least four considerations may shed light on these inconsistent findings. First, interpersonal discrimination is a multidimensional construct that can be captured in several ways. For example, one can focus on whether or not the reason for the discrimination is discerned as being due to specific attributes (e.g., race, age, or sex; (16)) or social statuses occupied by the targeted individual, the frequency of exposure to particular types of discriminatory events (e.g., weekly), the time frame of assessment (e.g., lifetime), and/or the subjective bearing or weight associated with these experiences altogether. Researchers have recently posited that variations in exposure to these different forms of discrimination may yield variations in health end points (8) and accordingly have called for simultaneous examination of various dimensions of discrimination in relation to health disparities (30). For instance, in a recent report from the Jackson Heart Study, major lifetime discrimination and the overall burden of discrimination were associated with greater hypertension risk, whereas everyday discrimination was not. Similarly, a recent report in 26,992 adults in the National Epidemiologic Survey of Alcohol and Related Conditions study demonstrated that reports of interpersonal discrimination from multiple sources (e.g., racial, weight, and sex discrimination) were more strongly predictive of self-reported CVD incidence compared with fewer sources (31). Most prior studies of cardiovascular-related end points, however, have focused on a single dimension of discrimination and therefore may not have fully captured the complexity of discriminatory experiences (9,32). Furthermore, the methodologies used to assess even one type of discrimination may vary between studies and contribute to discrepant results. For example, some CVD risk studies examining non–race-based discrimination (e.g., everyday discrimination; e.g., Refs. (20,23)) assess attributions for these experiences. That is, they use a two-stage approach where the questions initially assess “unfair treatment” without regard for the reason but then pose a follow-up item to ascertain a primary attribution (e.g., race) for these experiences. In contrast, the one-stage approach explicitly focuses on various aspects of the respondent’s experiences with racial discrimination in the primary measure. Emerging evidence demonstrates that these two approaches are distinct and not empirically equivalent (33), yielding significantly different respondent reports (e.g., Refs. (15,34,35)). Some confusion about the consistency of the relation between racial discrimination and CVD end points in the literature may therefore be due to the use of the term racial discrimination to refer to studies examining attributions to race for everyday discrimination, as well as studies explicitly examining racial discrimination.
Second, exploration of discrimination associated with interlocking social statuses may be critical. In this regard, the intersectionality framework posits that discriminatory events linked to the multiple identities that one occupies (e.g., African American race, female sex, and older age) are not independent or unidimensional but instead are interdependent (36,37). It has been suggested that an individual’s experiences with discrimination may be uniquely influenced by these mutually constitutive social statuses (38), and emerging evidence supports this proposition as related to health end points (e.g., Refs. (39,40)). However, to our knowledge, no prior work has examined discrimination related to multiply occupied social statuses (e.g., race, sex, and SES) in association with CVD end points, including subclinical vascular risk markers such as IMT.
Third, in a similar vein, elucidating within-race heterogeneity may yield critical insight into how experiences of discrimination shape CVD disparities between races. Although much of the literature examining interpersonal discrimination and CVD has focused on African Americans, emerging research demonstrates adverse CVD-related outcomes in whites who report these experiences as well (19,41). Presumably, however, the sociohistorical context surrounding race in the United States has shaped divergent bases for the linkages between discrimination and health in whites versus African Americans (39,42). For instance, cognitive and social factors underlie individual perceptions of discrimination (43) and likely lead to qualitatively different experiences in majority versus minority groups (44). As an example, several studies show that whites perceptions’ of being unfairly treated because of their race are driven by growing resentment due to shifts in the racial status hierarchy or the belief that gains by racial minorities reflect a zero-sum game for whites (45–47). In contrast, enduring evidence of race-based inequities in key domains including politics, education, health care, the criminal justice system, and the economy underscores and parallels African Americans’ reports of discrimination in interpersonal-level interactions (48). Previous investigations of interpersonal discrimination and IMT have either focused solely on one racial group (49) or have investigated the moderating role of race (19,20), thereby emphasizing intergroup differences. Although such approaches add to the literature on race, discrimination, and cardiovascular risk, they may obscure potentially vital within-group processes. Therefore, we use a within-group framework to elucidate patterns in the linkages of discrimination with IMT separately within African Americans and within whites.
Fourth, none of the previous studies on discrimination and IMT considered potential psychological risk moderators of this relationship. Specifically, depressive symptoms are an independent predictor of stroke and are more prevalent in those reporting discrimination. Findings from the Health and Retirement Study and the National Health and Nutrition Examination Survey demonstrated that in both African Americans and whites, a) increased depressive symptoms were prospectively associated with a 25% increased stroke risk, with evidence for a dose-response relationship (50); b) a high level of depressive symptoms conferred a 50% to 160% increased stroke risk during a 22-year follow-up compared with a low level of depressive symptoms (51); and c) this latter pattern was most pronounced among African Americans, as greater depression put them at ≈3-fold increased stroke risk (51). Similarly, depressive symptoms predict progression of IMT over time after accounting for traditional cardiovascular risk factors (52,53). In addition, African Americans experience greater chronicity and severity of depressive symptoms (54,55), with symptoms more likely to go untreated (56), compared with whites. Notably, data across five longitudinal studies (57–61) examining the linkage between discrimination and depressive symptoms indicate that a) the two constructs are conceptually distinct and b) generally, various dimensions of interpersonal discrimination precede the onset and worsening of depressive symptoms rather than the reverse.
We posit that the coupling of an interpersonal, socially based susceptibility (discrimination) with an intrapersonal psychological vulnerability (depressive symptoms) may contribute to greater subclinical vascular disease. Broadly, this proposition of a “coupling” effect draws upon an integrative framework including the diathesis-stress model and the biopsychosocial model of racism, the weathering hypothesis, and social-evaluative threat theory (10,62–64). Altogether, this framework suggests that the synergistic effect of a stressful event combined with psychological distress may uniquely increase risk for CVD. Indeed, psychological stressors are thought to influence physiology via activation of specific cognitive and/or affective processes and their related central nervous system underpinnings. Individuals with elevated depressive symptoms may have diminished resources for coping with the stress of chronic interpersonal discrimination and thus show exacerbations in physiological changes that contribute to disease (65). Similarly, events evaluated as threating to an individual’s social identity or standing, such as discriminatory acts, can mobilize emotive reactions, and individuals experiencing higher levels of depressive symptoms may be more vulnerable to the stress-related risk for these events. If such factors are chronically in play, a wear and tear of essential physiological systems would be expected and may emerge on an accelerated trajectory, such as in middle age. Consistent with this conceptualization, an analysis of more than 5000 African Americans in the National Survey of American Life study demonstrated that those who reported high levels of racial discrimination and a history of mood disorder, including depression, were at the greatest risk for CVD (65). However, to our knowledge, no prior studies have assessed whether depressive symptoms exacerbate the relation between interpersonal discrimination and carotid IMT.
The current study examines interactive associations among different dimensions of self-reported discrimination with depressive symptoms in relation to subclinical vascular disease as indicated by carotid IMT in middle-aged to older, community-dwelling African Americans in the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study. We focus primarily on African Americans because of the disproportionate burdens of discrimination and stroke observed in this group. With regard to discrimination, we include a comprehensive assessment of both race-and non–race-based forms of discrimination. Specifically, we focus on measurements that capture everyday discrimination without attribution for the cause, frequency of experiences of discrimination that individuals attribute to various social statuses they concurrently occupy (e.g., age, race, sex, etc.), racial discrimination, and the subjective impact or weight of discrimination throughout the lifetime. We hypothesize that the positive relationships between these four dimenssions of interpersonal-level discrimination and carotid IMT will be more pronounced among African Americans with greater depressive symptoms. We also anticipate that the strength of the relationships may vary across the specific dimensions of discrimination. However, we do not posit a priori hypotheses for each of the four dimensions assessed because of mixed findings across studies examining single dimensions of discrimination (14,19) and few studies examining multiple dimensions (65–68) in relation to CVD end points. Although African Americans are the primary focus, emerging findings document an adverse impact of discrimination in whites, as well. Thus, we also conduct exploratory analyses in whites that are analogous to the primary analyses in African Americans. In addition, we directly examine racial differences in these associations by testing three-way interactions among discrimination indices, depressive symptoms, and race with carotid IMT in the overall sample of African Americans and whites.
We anticipate that associations between discrimination, depressive symptoms, and carotid IMT will be independent of established cardiovascular risk factors (e.g., body mass index [BMI], hypertension, diabetes, CVD, lipid-lowering medication use, cigarette smoking, and alcohol and/or illicit drug use) and key sociodemographic factors (i.e., age, sex, SES). Alternatively, it is plausible that depressive symptoms could serve as an intermediate pathway (69–71) linking discrimination to poorer health. Therefore, we will also explore the potential mediating effect of depressive symptoms on the association between interpersonal-level discrimination and carotid IMT following adjustment for the aforementioned cardiovascular and sociodemographic factors.
MATERIALS AND METHODS
Participants and Procedure
HANDLS is a longitudinal study of health disparities attributable to race and SES in an urban sample. The study’s design and implementation has been described elsewhere (72). Briefly, HANDLS participants are a fixed cohort of African American and white adults recruited via household screenings from an area probability sample of 13 census segments in the city of Baltimore, Maryland, which were selected for their likelihood of yielding representative samples of participants who were African American or white, male or female, and with household incomes of ≥125% or <125% of the 2004 federal poverty level. In addition, participants were deemed eligible for HANDLS if they were between 30 and 64 years of age at baseline. The institutional review board at the National Institute of Environmental Health Sciences approved the HANDLS study protocol.
The present study used data collected during the first wave of HANDLS, which occurred between 2004 and 2009 and consisted of two phases: a) recruitment, written informed consent, and an interview within participants’ homes, and b) medical history, physical examination, and other assessments on mobile medical research vehicles (MRVs) parked within participants’ neighborhoods. After initial screening, participants were excluded from HANDLS if they met any of the following criteria: a) unable to provide informed consent, b) currently pregnant, c) severe developmental disability, d) within 6 months of active cancer treatment (i.e., chemotherapy, radiation, or biological treatments), e) diagnosed as having AIDS, or f) were unable to provide valid government-issued identification or a verifiable address (72). Examinations were performed on those participants whose blood pressure was <160/100 mm Hg. Of the 3720 participants who were recruited, 2801 were deemed eligible for continued participation in the study, of whom 2226 had valid carotid ultrasonography data (56.2% African American, 43.8% white). Participants were deemed ineligible for carotid ultrasonography if they had a) elevated blood pressure at the time of ultrasound (>200/100 mm Hg), b) presence of carotid bruit, c) weight ≥295 lb, or d) inability to lie in a completely supine position for 15 minutes. In addition, participants were excluded if they reported a history of stroke (n = 37), transient ischemic attack (n = 54), heart failure (n = 43), or carotid endartectomy (n = 2). No participants with valid carotid IMT data had a history of aneurysm. Finally, after medical exclusions were applied, participants were excluded if they were missing data for any variables used in the present analyses (n = 165), resulting in a final analysis sample of 1941 participants, of whom 1097 were African American and 844 were white. Of note, missing data for depressive symptoms and total cholesterol were previously imputed in the larger HANDLS sample by regression, with groups stratified by race, poverty status, and sex (<10% of HANDLS participants were missing these variables in the originally).
Measures
Dimensions of Interpersonal-Level Discrimination
The nine-item Everyday Discrimination scale (73) measures the frequency of routine experiences of discrimination without requiring the participant to make an explicit attribution (e.g., race/ethnicity) for the experience. Participants are asked, “In your day-to-day life, how often have any of the following things happened to you?” Examples of items are “How often are you treated with less courtesy than other people?” and “How often do you get worse service at restaurants and stores than other people?” Participants responded to items on a 6-point scale ranging from 1 (almost every day) to 5 (less than once a year), or 6 (never). Responses were reversed scored and summed, such that possible scores ranged from 9 to 54 and higher scores indicating greater everyday discrimination. This scale has been shown to have strong internal consistency (α = .88; (54)) and was similarly strong among African Americans and whites in our sample, α = .85.
Participants completed the 10-item Frequency of Discrimination across Sources measure, which assessed how often they experienced various sources of discrimination. Specifically, these items asked, “Overall how much have you experienced prejudice or discrimination due to…” sex, race, ethnicity, income, age, religion, physical appearance, sexual orientation, health status, and disability. The items do not specify a time frame. Participants responded to items on a 4-point scale ranging from 1 (not at all) to 4 (a lot). Possible scores ranged from 10 to 40, with higher scores indicating greater frequency of discrimination from various sources. This measure was derived from a similar measure used in previous research to assess prevalence of discrimination in health care settings (74). This scale had high internal consistency among African Americans and whites in our sample, α = .85.
Racial discrimination was assessed with a measure previously used in epidemiologic research (75). This six-item inventory ascertains whether participants ever (i.e., across their lifetimes) experienced racial discrimination at school, when getting a job, at work, when getting housing, when getting medical care, and from police or in courts. Participants responded no (0) or yes (1) to each item. Possible scores ranged from 0 to 6, with higher scores indicating greater racial discrimination. These six items were among those included in versions of the Experiences of Discrimination scale, which has strong internal consistency (α = .74) and test-retest reliability (0.70; (15)). In our sample of African Americans and whites, this scale had strong internal consistency, α = .84.
Lifetime discrimination burden was assessed with a two-item measure (16,76,77). Specifically, these items asked, a) “Overall, how much harder has your life been because of discrimination?” and b) “Overall, how much has discrimination interfered with your life?” Participants responded to each item on a 4-point scale ranging from 1 (not at all) to 4 (a lot). Possible scores ranged from 2 to 8, with higher scores indicating greater lifetime discrimination burden. These items have previously been used in epidemiologic studies of African Americans (21,66). In the present sample of African Americans and whites, these two items were strongly correlated, r = 0.79 (p < .001).
Depressive Symptoms
Depressive symptoms were assessed with the 20-item Center for Epidemiologic Studies Depression (CES-D) scale, which has strong psychometric properties (78). Participants responded to items on a 4-point scale ranging from 0 (rarely) to 3 (mostly). Possible scores ranged from 0 to 60, with higher scores indicating greater depressive symptoms. This measure had strong internal consistency among African Americans and whites in our sample, α = .91.
Carotid IMT
High-resolution B-mode ultrasonography of the left common carotid artery was performed with a standard transducer (5.OL45) and equipment (Acuson CV 70; Siemens). The far arterial wall IMT was evaluated as the distance between the intimal-luminal interface and medial-adventitial interface in a region 1.5 cm proximal to the carotid bifurcation. Specific care was taken to measure IMT in areas devoid of plaque. IMT was measured on a frozen-frame image, magnified to achieve higher resolution of detail. The IMT measurement was obtained from five contiguous sites at approximately 1-mm intervals and averaged for analyses. Sonography measurements were performed by a single sonographer and reviewed by a board-certified cardiologist, who adjusted them as necessary.
Adjustment Variables
Similar to our prior work (79), adjustment variables included age, biological sex, SES, BMI, total cholesterol, hypertension, diabetes, CVD, lipid-lowering medication use, cigarette smoking, and alcohol and/or illicit drug use. Participants self-reported their age, and biological sex was coded as 0 (women) and 1 (men). SES was calculated from a composite score that included self-reported annual household income and years of education. Participants were considered higher SES (coded as 0) if they reported a) an annual household income (adjusted for household size) ≥125% of the 2004 federal poverty level and b) ≥12 years of education. Conversely, participants were classified as lower SES (coded as 1) if they reported a) an adjusted annual household income <125% of the 2004 federal poverty level or b) <12 years of education. The Wide Range Achievement Test (WRAT-3) Word Reading subtest was administered at the MRV visit and used as a measure of literacy (80).
During the MRV visit, participants completed a comprehensive physical examination and medical history with a physician or nurse practitioner. BMI was calculated as the ratio of weight to height squared (in kilograms per meter squared), both measured with calibrated equipment. Fasting venous blood specimens for total cholesterol assay were collected on the MRV and analyzed at the NIA Clinical Research Branch Core Laboratory (Baltimore, Maryland) and Quest Diagnostics Inc. (Baltimore, Maryland, and Chantilly, Virginia) using a spectrophotometer (AU5400 Immuno Chemistry Analyzer; Olympus, Center Valley, Pennsylvania). Hypertension and diabetes were represented as dichotomous variables (coded as 0 [absent] and 1 [present]) in the present study. Hypertension was based on self-reported diagnosed hypertension, self-reported use of antihypertensive medications (diuretics, blockers, angiotensin inhibitors, or vasodilators), or resting systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg. Diabetes was based on self-reported diagnosed diabetes, self-reported use of diabetes medications, and/or glucose ≥126 mg/dl (7 mmol/L). Next, a dichotomous CVD cluster variable was created to indicate the absence or presence (coded as 0 and 1, respectively) of self-reported diagnoses of coronary artery disease, myocardial infarction, peripheral artery disease, atrial fibrillation, angioplasty, and/or coronary artery bypass surgery. Other dichotomous adjustment variables were self-reported lipid-lowering medication use (0, never prescribed; 1, ever prescribed), self-reported cigarette smoking status (coded as 0 [never used regularly] and 1 [ever used regularly]), and self-reported alcohol and/or illicit drug use (i.e., alcohol, marijuana, cocaine, and/or opiate use status collapsed into one dichotomous variable; coded as 0 [never used regularly] and 1 [ever used regularly]).
Statistical Analyses
Race-Stratified Moderation Analyses
Statistical analyses were conducted with the Statistical Package for the Social Sciences (SPSS) version 24. Moderation analyses were stratified by race. That is, primary analyses were first run in the African American participants, followed by parallel, exploratory analyses in the white participants. Hierarchical-entry multiple linear regression was used to examine main effects and interactive relations of multiple forms of discrimination (see Dimensions of Interpersonal-Level Discrimination measures) and depressive symptoms with carotid IMT. Three models were run for each discrimination measure. Model 1 included the main effect of discrimination and all covariates. Next, model 2 added the main effect of depressive symptoms. Finally, model 3 added the interaction effect of discrimination by depressive symptoms. If the interaction term was significant, model 3 was retained, and main effects of discrimination and depressive symptoms were not interpreted further (irrespective of their significance across models). Conversely, if the interaction term was nonsignificant, it was removed from the analysis and model 2 was retained as the final model.
The PROCESS macro for SPSS, Version 2.16 (81), was used to probe significant interaction effects using model template 1 (moderation with one moderator) within the package. Within PROCESS, the Johnson-Neyman technique (81) was used to detect regions of significance for the conditional effect of discrimination with carotid IMT across a wide range of CES-D scores. Significant interactions were also plotted at the mean ± 1 SD of depressive symptoms to assist with interpretation.
All adjustment variables were mean centered before analyses. To assist with interpretation of the Johnson-Neyman findings, we present findings from analyses without mean-centering discrimination and depressive symptoms. However, subsequent analyses confirmed that mean centering these variables did not change the results.
Combined-Sample Moderation Analyses
To test racial differences in the discrimination by depressive symptoms interaction, three-way interactions of discrimination measures, depressive symptoms, and race were tested in the overall sample consisting of African Americans and whites.
Mediation Analyses
Alternative mediation analyses were also run to determine whether depressive symptoms mediated associations between discrimination and carotid IMT. Mediation analyses were race stratified, with primary analyses run in the African American participants first, followed by parallel, exploratory analyses in the white participants. Mediation analyses were conducted using a bootstrapping confidence interval approach to examine potential mediating effects of depressive symptoms on the association between multiple forms of discrimination and carotid IMT. Within PROCESS, model template 4 (mediation with one mediator) was used within the sample, and 5000 bootstrap samples and a 95% confidence interval were requested. Mediation analyses adjusted for all covariates described previously.
RESULTS
Sample Descriptives
African Americans were more likely to have lower SES (χ2(1) = 27.09, p < .001), hypertension (χ2(1) = 10.18, p = .001), and a self-reported history of cigarette use (χ2(1) = 5.86, p = .017) than their white counterparts (Table 1). Conversely, white participants were more likely to be women (χ2(1) = 4.25, p = .043) and have a self-reported history of lipid-lowering medication use (χ2(1) = 8.20, p = .005) than African Americans. Whites also had greater total cholesterol (t(1,939) = 4.35, p < .001) and BMI, t(1,939) = 2.76, p = .006), whereas African Americans had greater carotid IMT (t(1,939) = −5.61, p < .001). African Americans endorsed more interpersonal-level discrimination than did whites across all discrimination measures (p values < .001). In the overall sample, 468 (24.1%) of participants self-reported a history of depression, with whites reporting greater rates of depression than African Americans, (χ2(1) = 55.73, p < .001). We also observed that the discrimination measures were moderately to strongly correlated with one another, ranging from r = 0.39 to r = 0.67 (all, p values < .001; Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/PSYMED/A595). Correlations between depressive symptoms and each of the discrimination measures were small, ranging from r = 0.12 to r = 0.26 (all, p values < .001).
TABLE 1.
Participant Characteristics Stratified by Race
| Variable | AA (n = 1097) | Whites (n = 844) | Sig. |
|---|---|---|---|
| Female, n (%) | 571 (52.1) | 479 (56.8) | * |
| Lower SES, n (%)a | 654 (59.6) | 403 (47.7) | *** |
| <High school education/GED, n (%) | 350 (31.9) | 265 (31.4) | |
| Below 125% poverty level, n (%) | 521 (47.5) | 264 (31.3) | *** |
| Age, y | 46.67 ± 9.13 | 46.97 ± 9.25 | |
| Body mass index, kg/m2 | 28.85 ± 7.13 | 29.78 ± 7.54 | ** |
| Total cholesterol, mg/dl | 182.33 ± 38.78 | 190.26 ± 41.10 | *** |
| Hypertension, n (%) | 475 (43.3) | 305 (36.1) | ** |
| Diabetes mellitus, n (%) | 155 (14.1) | 105 (12.4) | |
| ≥1 Cardiovascular disease, n (%)b | 118 (10.8) | 77 (9.1) | |
| Lipid-lowering medications, n (%) | 140 (12.8) | 147 (17.4) | ** |
| Ever used cigarettes, n (%) ever used | 539 (49.1) | 368 (43.6) | * |
| Ever used alcohol or illicit drugs, n (%)c | 635 (57.9) | 539 (60.3) | |
| Depression history, n (%) | 195 (17.8) | 273 (32.3) | *** |
| Everyday discrimination | 21.50 ± 8.83 | 19.90 ± 8.12 | *** |
| Frequency of discrimination across sources | 17.39 ± 5.94 | 14.49 ± 4.82 | *** |
| Racial discrimination | 7.68 ± 1.92 | 6.41 ± 1.04 | *** |
| Lifetime discrimination burden | 3.77 ± 1.77 | 2.85 ± 1.50 | *** |
| Depressive symptoms (CES-D scale) | 14.71 ± 10.83 | 14.86 ± 11.42 | |
| Carotid IMT, mm | 0.70 ± 0.13 | 0.67 ± 0.13 | *** |
AA = African Americans; SES = socioeconomic status; GED = General Educational Development; CES-D = Center for Epidemiologic Studies Depression scale; IMT = intimal-medial thickness.
Values are presented as mean ± SD, unless otherwise indicated. Racial differences were examined with independent-samples t tests and χ2 tests of independence.
p < .05.
p < .01.
p < .001.
Lower SES reflects an annual household income (adjusted for household size) <125% of the 2004 Health and Human Services poverty level and/or educational attainment <high school diploma or GED.
Cardiovascular diseases assessed were coronary artery disease, myocardial infarction, peripheral artery disease, atrial fibrillation, angioplasty, and coronary artery bypass surgery.
Illicit drugs assessed were marijuana, cocaine/crack, and opiates.
Moderation Results
Race-Stratified Moderation Results
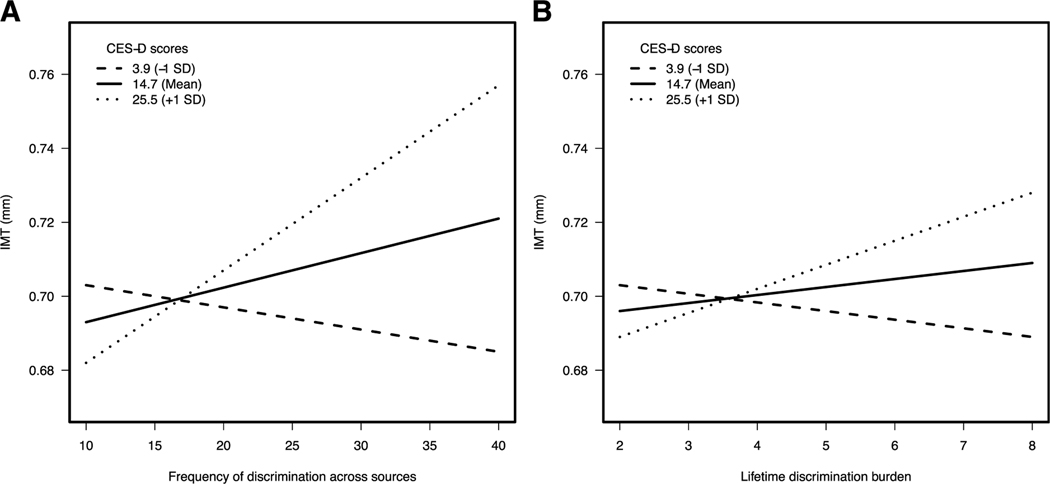
Among African Americans, findings revealed a significant main effect of frequency of discrimination across sources before adjustment for depressive symptoms (b = 0.001, β = 0.06, p = .045), such that greater scores on the measure were associated with greater carotid IMT (see model 1 in Table 2). This main effect attenuated to nonsignificance after adjustment for depressive symptoms (b = 0.001, β = 0.06, p = .052; see model 2 in Table 2). In addition, this main effect was not interpreted further after subsequent analyses revealed it to be subsumed within a significant two-way interaction of frequency of discrimination across sources by depressive symptoms (b < 0.01, β = 0.26, p = .006; see model 3 in Table 2). Subsequent analyses using the Johnson-Neyman technique revealed that greater frequency of discrimination across sources was associated with greater carotid IMT at all CES-D scores >16.7 (62nd percentile; all, p values < .05; Supplementary Table 2, Supplemental Digital Content 1, http://links.lww.com/PSYMED/A595).
TABLE 2.
Independent and Interactive Relations of Frequency of Discrimination Across Sources and Depressive Symptoms With Carotid Intima-Medial Thickness Among African Americans (n = 1097)
| Variable | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Age | 0.004*** | 0.004*** | 0.004 *** |
| Male sex | 0.025** | 0.026** | 0.027 ** |
| Lower SES | −0.007 | −0.007 | −0.007 |
| Body mass index | 0.003*** | 0.003*** | 0.003 *** |
| Total cholesterol | <0.001 | <0.001 | <0.001 |
| Hypertension | 0.019* | 0.018* | 0.019 * |
| Diabetes mellitus | 0.012 | 0.012 | 0.013 |
| Cardiovascular disease | 0.006 | 0.006 | 0.006 |
| Lipid-lowering medication use | 0.002 | 0.002 | 0.002 |
| Ever used cigarettes | 0.006 | 0.005 | 0.006 |
| Ever used alcohol and/or illicit drugs | 0.014 | 0.014 | 0.013 |
| Frequency of discrimination across sources | 0.001* | 0.001 | −0.001 |
| Depressive symptoms | <0.001 | −0.002 * | |
| Frequency of discrimination across sources by depressive symptoms | <0.001 ** |
SES = socioeconomic status.
Model 3 (shown in bold) was retained as the final regression model.
p < .05.
p < .01.
p < .001.
We observed no main effect of lifetime discrimination burden on carotid IMT among African Americans before adjustment for depressive symptoms (b < 0.01, β = 0.01 p = .807) or after adjustment for depressive symptoms (b < 0.01, β < 0.01, p = .955; see models 1 and 2 in Table 3). However, findings revealed a significant two-way interaction of lifetime discrimination burden by depressive symptoms (b < 0.01, β = 0.19, p = .021; see model 3 in Table 3) in this group. The Johnson-Neyman technique demonstrated that greater lifetime discrimination burden was associated with greater carotid IMT at CES-D scores >20.82 (75th percentile; all, p values < .05; Supplementary Table 3, Supplemental Digital Content 1, http://links.lww.com/PSYMED/A595). As shown in Figure 1, among African American participants with high depressive symptoms (i.e., CES-D score of 25.53, +1 SD above mean), greater carotid IMT was associated with greater a) frequency of discrimination from various sources (b = 0.003, p = .001) and b) lifetime discrimination burden (b = 0.01, p = .018).
TABLE 3.
Independent and Interactive Relations of Lifetime Discrimination Burden and Depressive Symptoms With Carotid Intima-Medial Thickness Among African Americans (n = 1097)
| Variable | Model 1 | Model 2 | Model 3 | Sig. |
|---|---|---|---|---|
| Age | 0.004 | 0.004 | 0.004 | *** |
| Male sex | 0.025 | 0.026 | 0.027 | ** |
| Lower SES | −0.007 | −0.008 | −0.008 | |
| Body mass index | 0.003 | 0.003 | 0.003 | *** |
| Total cholesterol | <0.001 | <0.001 | <0.001 | |
| Hypertension | 0.01 | 0.019 | 0.018 | * |
| Diabetes mellitus | 0.014 | 0.014 | 0.014 | |
| Cardiovascular disease | 0.007 | 0.007 | 0.008 | |
| Lipid-lowering medication use | 0.002 | 0.001 | 0.001 | |
| Ever used cigarettes | 0.006 | 0.006 | 0.006 | |
| Ever used alcohol and/or illicit drugs | 0.014 | 0.014 | 0.013 | |
| Lifetime discrimination burden | 0.003 | 0.003 | −0.004 | |
| Depressive symptoms | <0.001 | <0.001 | ||
| Lifetime discrimination burden by depressive symptoms | <0.001 | * |
SES = socioeconomic status.
Model 3 (shown in bold) was retained as the final regression model.
p < .05.
p < .01.
p < .001.
FIGURE 1.
Significant moderating effect of depressive symptoms on the association between carotid IMT and frequency of discrimination across sources (A) and lifetime discrimination burden among African American participants (B). * p < .05. IMT = intimal-medial thickness; CES-D = Center for Epidemiologic Studies Depression scale.
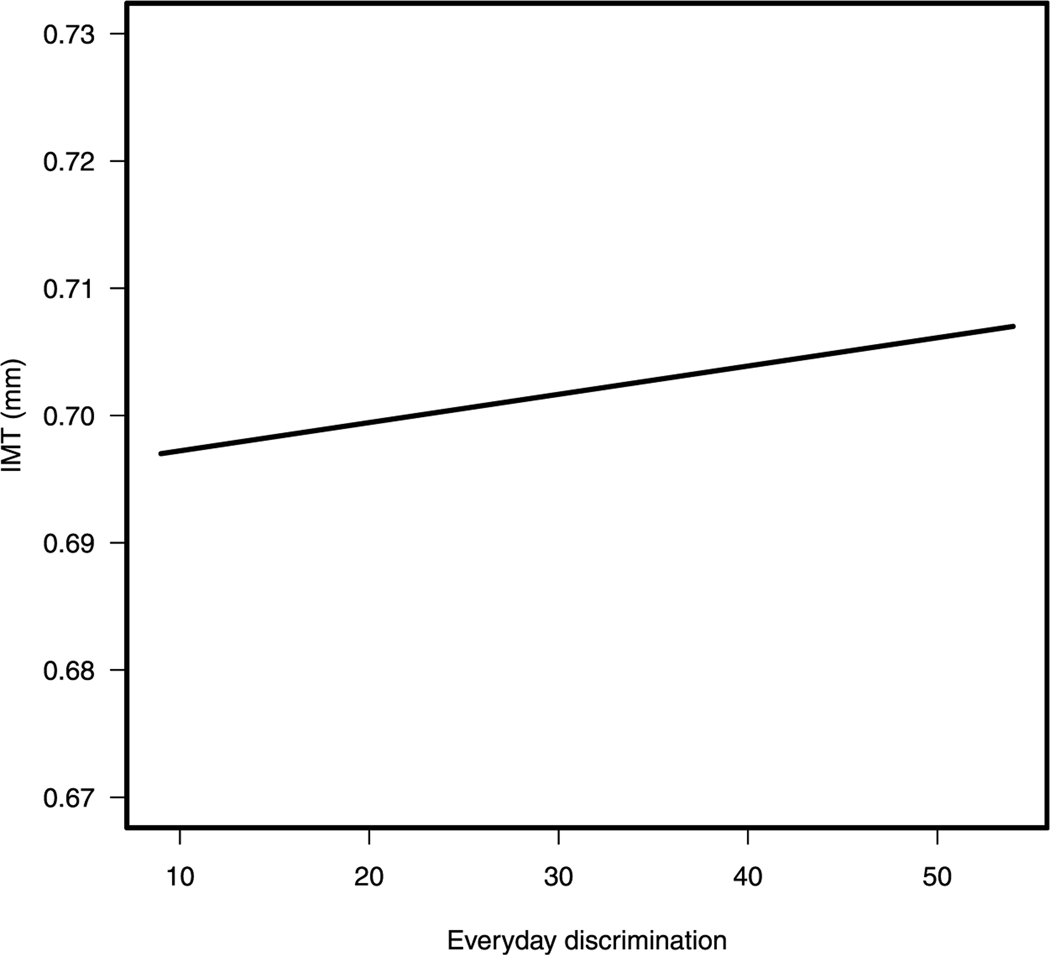
Further analyses revealed a significant main effect of everyday discrimination with carotid IMT among African Americans before adjustment for depressive symptoms (b = 0.001, β = 0.07, p = .017) and after adjustment for depressive symptoms (b = 0.001, β = 0.07, p = .021; see models 1 and 2 in Table 4, respectively). As shown in Figure 2, greater everyday discrimination was associated with greater carotid IMT in this group. The interaction of everyday discrimination by depressive symptoms was nonsignificant (b < 0.01, β = 0.08, p = .221; see model 3 in Table 4).
TABLE 4.
Independent and Interactive Relations of Everyday Discrimination and Depressive Symptoms With Carotid Intima-Medial Thickness Among African Americans (n = 1097)
| Variable | Model 1 | Model 2 | Model 3 | Sig. |
|---|---|---|---|---|
| Age | 0.004 | 0.004 | 0.004 | *** |
| Male sex | 0.026 | 0.026 | 0.026 | ** |
| Lower SES | −0.007 | −0.007 | −0.007 | |
| Body mass index | 0.003 | 0.003 | 0.003 | *** |
| Total cholesterol | <0.001 | <0.001 | <0.001 | |
| Hypertension | 0.019 | 0.019 | 0.019 | * |
| Diabetes mellitus | 0.013 | 0.013 | 0.013 | |
| Cardiovascular disease | 0.007 | 0.007 | 0.007 | |
| Lipid-lowering medication use | 0.003 | 0.002 | 0.002 | |
| Ever used cigarettes | 0.006 | 0.006 | 0.006 | |
| Ever used alcohol and/or illicit drugs | 0.013 | 0.013 | 0.013 | |
| Everyday discrimination | 0.001 | 0.001 | 0.001 | * |
| Depressive symptoms | <0.001 | <0.001 | ||
| Everyday discrimination by depressive symptoms | <0.001 |
SES = socioeconomic status.
Model 2 (shown in bold) was retained as the final regression model.
p < .05.
p < .01.
p < .001.
FIGURE 2.
Significant main effect of everyday discrimination with carotid IMT among African Americans. IMT = intimal-medial thickness.
Findings also revealed a) nonsignificant main effects of racial discrimination before adjustment for depressive symptoms (b < .01, β = −0.01, p = .825) and after adjustment for depressive symptoms (b < 0.01, β = −0.01, p = .757; see models 1 and 2 in Supplementary Table 4, Supplemental Digital Content 1, http://links.lww.com/PSYMED/A595), and b) a nonsignificant interaction of racial discrimination by depressive symptoms (b < 0.01, β = 0.22, p = .095; see model 3 in Supplementary Table 4, Supplemental Digital Content 1, http://links.lww.com/PSYMED/A595), in association with carotid IMT among African Americans. Finally, across analyses, there were no significant main effects of depressive symptoms in the African American participants before adjustment for interaction terms (all, p values ≥ .514; see model 2 in Tables 2–4 and Supplementary Table 4, Supplemental Digital Content 1, http://links.lww.com/PSYMED/A595).
Next, among whites, there were no significant main effects of a) everyday discrimination (b < 0.01, β < 0.01, p = .900), b) frequency of discrimination across sources (b < 0.01, β = −0.01, p = .870), (c) racial discrimination (b < 0.01, β < 0.01, p = .972), or d) lifetime discrimination burden (b < .01, β = 0.01, p = .807) with carotid IMT; these effects remained nonsignificant after adjustment for depressive symptoms (all, p values ≥ .744). Main effects of depressive symptoms with carotid IMT among whites were also nonsignificant across analyses (all, p values ≥ .414). Likewise, among whites, there were no significant interactions of a) everyday discrimination by depressive symptoms (b < 0.01, β = 0.01, p = .824), b) frequency of discrimination across sources by depressive symptoms (b < 0.01, β = 0.02, p = .535), c) racial discrimination by depressive symptoms (b < 0.01, β = 0.02, p = .557), or d) lifetime discrimination burden by depressive symptoms (b < 0.01, β = 0.03, p = .400) with carotid IMT. Results of these exploratory analyses are shown in Supplementary Tables 5–8, Supplemental Digital Content 1, http://links.lww.com/PSYMED/A595.
Combined-Sample Moderation Results
In the overall sample, there were no significant three-way interactions among a) everyday discrimination by depressive symptoms by race (b < .01, β = .05, p = .645), b) frequency of discrimination across sources by depressive symptoms by race (b < .01, β = .18, p = .196), c) racial discrimination by depressive symptoms by race (b < .01, β = .10, p = .672), and (d) lifetime discrimination burden by depressive symptoms by race (b < .01, β = .11, p = .334) with carotid IMT. Results of these analyses are shown in Supplementary Tables 9–12, Supplemental Digital Content 1, http://links.lww.com/PSYMED/A595.
Mediation Results
Finally, across all analyses, depressive symptoms did not significantly mediate associations between any of the discrimination indices and carotid IMT among African Americans or whites (all 95% confidence intervals overlapped with 0; data not shown).
DISCUSSION
In an urban sample of middle-aged and older adults, we identified interaction effects between dimensions of interpersonal-level discrimination and depressive symptoms in relation to early stage carotid atherosclerosis in African Americans. Specifically, African Americans reporting either a) greater exposure to discrimination across multiple sources or b) greater lifetime discrimination burden, in conjunction with increased levels of depressive symptoms, had greater average carotid IMT than did those reporting lower levels of discrimination or depressive symptoms. In secondary analyses, race did not moderate the relations among depressive symptoms, discrimination, and IMT across whites and African Americans, and depressive symptoms did not mediate any of the relationships between discrimination and IMT.
Discrimination, depressive symptoms, and their interaction were not associated with IMT among white participants. There are several potential reasons for the lack of analogous findings in whites. For one, reports of all types of discrimination, along with IMT levels, were higher among African Americans than among whites, which may suggest the presence of threshold effects. African Americans, in facing more severe, frequent, or long-standing interpersonal discrimination, may begin to develop atherosclerosis on an earlier or more accelerated trajectory compared with whites, especially when considered alongside other risk factors (i.e., elevated rates of hypertension in African Americans). In addition, or alternatively, the processes underlying perceptions and reports of discrimination could be qualitatively different between these two racial/ethnic groups (45,46,82), such that these experiences have more of a deleterious cardiovascular impact in African Americans. Continuing to examine the associations between psychosocial factors and cardiovascular risk factors from a within-race perspective may contribute to our understanding of racial disparities in cardiovascular outcomes. This approach may be particularly useful when seeking to explicate factors uniquely linked to the lived experience of particular racial groups, as discrimination is for African Americans.
Relatedly, race was not a statistically significant moderator of the associations among discrimination indices, depressive symptoms, and carotid IMT in the overall sample. This may suggest that there are truly no racial differences between African Americans and whites in the linkages of interpersonal discrimination and depressive symptoms to IMT. Alternatively, the underlying process linking interpersonal discrimination to IMT may be dissimilar in African Americans and whites. The literature is quite clear that African Americans experience more discrimination (15–17) and also have greater carotid IMT compared with whites, with racial differences in IMT potentially stronger in younger adult samples (83–86), such as HANDLS participants. In addition, the overarching hypothesis in health disparities research is that African Americans are more severely affected by CVD end points and that the related subclinical disease processes begin at younger ages. Thus, it may not be appropriate to directly contrast these two racial groups when examining relationships among these variables. We also know that assessment of race alone is not an adequate proxy for the lived and dynamic experience of race, especially among historically marginalized racial groups such as African Americans. Thus, factors more closely aligned with the experience of a minority racial group may be best assessed for within-group variability, as critical distinctions may be lost if emphasis is only placed on interpreting differences across or between a majority and minority racial group. Here, owing to the racial disparities in discrimination and IMT, and limitations in the contribution of traditional risk factors, beyond the question of whether there are race differences, we sought to elucidate whether and how discrimination, a central aspect of life for African Americans, impacts IMT (86,87). Learning more about such within-group variability may lead to the development of more effective policies specific to minorities.
To our knowledge, no previous studies have examined depression as a moderator of the relationship between interpersonal discrimination and IMT. However, our results are consistent with a prior study of African Americans, in which the combination of both racial discrimination and a history of a mood disorder (depression, dysthymia, or bipolar disorder) was associated with self-reported CVD, as defined by hypertension, atherosclerosis, myocardial infarction, or stroke (57). Several biopsychosocial mechanisms could account for this effect. With regard to psychosocial factors, theoretical models of discrimination and health among African Americans have emphasized the potential moderating role of psychological factors, which may influence how individuals recognize, appraise, and react to stressors (10,88). For instance, prior work has suggested that relationships between discrimination and health outcomes may be buffered by an active coping style or increased social support (89,90), both of which may be limited in those with elevated depressive symptoms. Frequent exposure to discrimination may also promote psychological weathering and/or heightened perceptions of social threat (61,64). Individuals who are simultaneously experiencing depressive symptoms along with exposure to discrimination may be more attuned to instances of discrimination and/or experience them as more psychologically taxing than those with fewer symptoms. Over time, this may lead to physiological changes that harm the cardiovascular system. Specifically, depressive symptoms are associated with a host of neuroendocrine, immune, and autonomic perturbations that heighten cardiovascular risk (91,92). Thus, in those with symptoms of depression, physiological responses to the stress of discrimination may be amplified and, when experienced repeatedly, could contribute to an accelerated progression of atherosclerosis (93–95). Finally, depressive symptoms are linked to a variety of unhealthy behaviors such as smoking (61,89), difficulty with medication adherence (96), and poor sleep (97,98), any of which could exacerbate cardiovascular risk among those experiencing discrimination. Additional work exploring the behavioral and physiological sequelae of the combination of higher discrimination and greater depressive symptoms could shed light on these potential pathways.
The current study demonstrates the importance of examining discrimination as a multidimensional construct. Here, we observed that discriminatory experiences linked to multiple social categories one concurrently occupies, including race, ethnicity, sex, age, and SES, as well as the overall weight or burden of discriminatory experiences across one’s lifetime were implicated in early atherosclerosis. This work echoes recent findings that suggest that reports of interpersonal discrimination from multiple sources (e.g., racial, weight, and sex discrimination) were related to self-reported CVD incidence (23). It is plausible that the synergistic nature of holding various social statuses for which one may be targeted for discrimination in varying or consistent degrees is especially potent. This particular finding may exemplify the need for further examination of discrimination and health within an intersectionality framework. This framework may be apt for such work as it posits that multiple social categories intersect at the microlevel of the individual experience to reproduce various macrolevels and mesolevels, interconnecting systems of disadvantage (36,37), in turn, promoting and maintaining heath disparities. Next steps should seek to explicate whether and how specific social-status based discriminatory experiences, such as racial discrimination, may be especially deleterious in the context of exposure to other social status–based discrimination (e.g., sex) and if combinations of particular social statuses may further compromise or protect against adverse effects. In this regard, examination of the intersectionality paradox—where individuals occupy low (e.g., racial minority and physically challenged) and high (e.g., higher SES) status social identities—may further reveal subgroups for whom greater or lesser health disparities have previously been unknown.
We also observed that the burden of discrimination across one’s lifetime may be particularly meaningful in understanding the impact of discrimination on subclinical vascular disease. This finding is consistent with prior reports demonstrating that the weight of discrimination is adversely linked with CVD end points (66–68). For instance, prior studies in African Americans observed that greater lifetime burden was associated with greater white matter lesion volume (subclinical marker of cerebrovascular disease; [68]), hypertension prevalence (66), and poorer health behaviors (i.e., smoking and sleep; (67)). This finding may reflect the importance of explicitly capturing the individuals’ perception of how their experiences with discrimination have influenced them, in addition to assessing the frequency and types of discrimination they have faced. Specifically, understanding one’s sense of how discriminatory experiences have blocked opportunities and created difficulties for him/her may shed light on the lived consequences or fallout of discriminatory experiences not fully captured in measures that take more of a quantitative focus.
Notably, everyday discrimination and racial discrimination, which assess the frequency of experiences with relatively minor discriminatory events and whether one has experienced racial discrimination across specific settings, respectively, were not related to carotid IMT in this sample. With regard to everyday discrimination, mixed findings have emerged across prior studies (19,66,67,99). Although there is some evidence that attributions to race for experiences of everyday discrimination are linked with greater IMT in African Americans (20), studies explicitly examining racial discrimination in relation to IMT are lacking. Drawing on the discrimination findings overall, it is possible that exposure to discrimination associated with multiple social statuses, including racial discrimination, is particularly noxious for African Americans in ways that everyday discrimination and racial discrimination alone may not fully capture.
The varied findings for different dimensions of discrimination warrant further examination. For instance, a multimethod approach that uses daily diaries via ecological momentary assessment and retrospective self-report measured longitudinally overall multiple time points to assess various dimensions of discrimination and their related burden would provide rich insight into whether and how specific discriminatory experiences compound one another, especially when related to other social statuses. Future work aimed at disentangling discrimination in relation to various social identities may hold promise in forwarding understanding of differential linkages to CVD risk.
Our findings have several clinical and public health implications. Given the propensity for CVD in African Americans, these findings suggest that implementing assessments to capture a broadened definition of self-reported discrimination alongside depressive symptoms at routine health visits would be beneficial, with the hope that optimizing management of these stressors would indirectly improve cardiovascular health vis à vis psychological, behavioral, and physiological pathways. Periodic IMT testing to assess the progression of arterial narrowing and the presence of plaques in African Americans and other populations with an established greater burden of CVD risk and social stressors may also be a consideration.
Although individual-level interventions may provide some support, the challenges highlighted by the current findings and the related mounting body of literature (9,12,14,69,90,100–103) demonstrate that a multilevel response is required. Such a response must target the various systemic and long-standing sources of social inequity, which sustain health disparities. For instance, local state and city policies to ameliorate residential segregation and related neighborhood economic disenfranchisement, race-based police stops, and inequity in economic and resource distribution across school districts would be realistic and key targets for change. In sum, the accumulating evidence documenting individual-and structural-level discrimination and racism as critical bases for the protracted social, economic, and health inequities African Americans endure should serve as a catalyst for recognizing discrimination and racism as social determinants of health and in turn greater investment in their reduction to promote equity.
We did not observe independent associations of discrimination or depressive symptoms with IMT, which is inconsistent with some existing data (4,51). However, this may not be completely surprising given the biopsychosocial model (10) and other conceptualizations of mechanistic pathways linking psychosocial factors to biological processes and subsequent disease (104), which emphasize the complex interplay among multiple variables. Thus, a single level of analysis focused on any one specific psychological or social factor may constrain observation of existing linkages.
This study had some limitations. The use of a dichotomous SES variable may not have captured the full range of socioeconomic variability among the sample. However, HANDLS investigators based their initial area probability recruitment on a division of household income at 125% of the 2004 federal poverty level, with the goal of recruiting sufficient numbers of participants from both low-and moderate-income levels. In addition, HANDLS investigators could not add additional measures of SES, given that many HANDLS participants were unable to accurately estimate their annual incomes or overall wealth, and were used only sporadically. As a result, our most comprehensive estimate of individual-level SES depends on the investigators’ initial ascertainment of poverty status and self-reported high school or greater educational attainment. Of note, methods of SES measurement vary widely in epidemiologic research owing to several study-specific factors, such as social groups and outcomes of interest and feasibility of measurement (105).
In addition, because the observed associations were cross-sectional, temporal associations cannot be established. It is important to determine whether the interactive association between discrimination and depressive symptoms predicts increases in IMT over time. Replication in other samples of African Americans in various regions across the United States is recommended, as geographic variations in experiences with discrimination (106) and CVD, including stroke (107), are well documented among African Americans. Examination of these linkages in other racial minority groups is also recommended before clinical application of these findings. Strong evidence demonstrates that reports of depressive symptoms are fairly stable over time (108). However, assessment of discrimination and depressive symptoms across similar time frames may be helpful as well. Also, similar to prior reports our findings suggest that depressive symptoms and discrimination are two distinct constructs (58–61). However, it is plausible that depressive symptoms may influence an individual’s recall and interpretation of interpersonal interactions. Cigarette smoking was assessed using a dichotomous variable, which is unlikely to fully capture variability in lifetime cigarette smoking. However, asking about ever versus never smoking is a standard epidemiologic item used in nationally representative studies (e.g., Ref. (109)). Finally, use of a single sonographer is a limitation, as we could not estimate reliability with interrater agreement. In addition, the sonographer was not blind to participants’ race or sex, which may have introduced bias. However, use of a single sonographer increases confidence that consistent procedures were followed within and between participants, and therefore may also be viewed as a strength. Sonography was also reviewed by a board-certified cardiologist to ensure validity.
The primary strength of this investigation involves its assessment of the interactive relation between self-reported interpersonal discrimination, a chronic social stressor for racial minorities, and depressive symptoms, an established CVD risk factor, in relation to carotid IMT. Concurrent assessment of multiple dimensions of discrimination is especially important, as it allows for greater precision in identifying the aspects of this chronic stressor that may be particularly pernicious with regard to atherosclerosis. Finally, we focused on these linkages in a sample of middle-aged and older African Americans, a group at increased risk for thicker IMT and subsequent cardiovascular events.
SUMMARY
In this urban, community-dwelling, socioeconomically diverse cohort of adults, African Americans who reported both higher levels of discrimination across several dimensions and greater depressive symptoms demonstrated the greatest carotid IMT. Discrimination and depressive symptoms were not associated with carotid IMT in whites. These findings suggest that singular consideration of social and psychological factors may obscure optimal conceptualization of subclinical vascular risk among racial minorities, as well as clinical end points, such as stroke. In the clinical setting, interventions focused on alleviating depressive symptoms among individuals who are experiencing discrimination may benefit from addressing the inequity of their social experiences. Ultimately, consideration of psychosocial risk factors central to the experience of African Americans may be warranted to intervene on their greater risk for atherosclerosis and stroke.
Supplementary Material
Acknowledgments
We wish to thank the HANDLS participants for their continued commitment to the study as well the HANDLS research team. The National Institute on Aging Intramural Research Program of the National Institutes of Health performed this research.
Source of Funding and Conflict of Interest: We would like to acknowledge our funding sources: K01AG043581 (Beatty Moody), R01AG034161 (Waldstein), and the National Institute on Aging’s Intramural Research Program ZIAG000513 (Evans). None of the authors for this article have any conflicts of interest pertaining to this work.
Glossary
- BMI
body mass index
- CES-D
Center for Epidemiologic Studies Depression scale
- HANDLS
Healthy Aging in Neighborhoods of Diversity across the Life Span
- IMT
intimal-medial thickness
- MRV
medical research vehicle
- SES
socioeconomic status
REFERENCES
- 1.Howard G, Cushman M, Kissela BM, Kleindorfer DO, McClure LA, Safford MM, Rhodes JD, Soliman EZ, Moy CS, Judd SE, Howard VJ, REasons for Geographic And Racial Differences in Stroke (REGARDS) Investigators. Traditional risk factors as the underlying cause of racial disparities in stroke: lessons from the half-full (empty?) glass. Stroke 2011;42:3369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berenson GS, Srinivasan SR, Bao W, Newman WP 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 1998; 338:1650–6. [DOI] [PubMed] [Google Scholar]
- 3.Grobbee DE, Bots ML. Carotid artery intima-media thickness as an indicator of generalized atherosclerosis. J Intern Med 1994;236:567–73. [DOI] [PubMed] [Google Scholar]
- 4.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med 1999;340:14–22. [DOI] [PubMed] [Google Scholar]
- 5.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 2007;115:459–67. [DOI] [PubMed] [Google Scholar]
- 6.Chambless LE, Folsom AR, Clegg LX, Sharrett AR, Shahar E, Nieto FJ, Rosamond WD, Evans G. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol 2000;151:478–87. [DOI] [PubMed] [Google Scholar]
- 7.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS, American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008;21:93–111; quiz 189–90. [DOI] [PubMed] [Google Scholar]
- 8.Krieger N Discrimination and health inequities. Int J Health Serv 2014;44:643–710. [DOI] [PubMed] [Google Scholar]
- 9.Lewis TT, Williams DR, Tamene M, Clark CR. Self-reported experiences of discrimination and cardiovascular disease. Curr Cardiovasc Risk Rep 2014;8:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark R, Anderson NB, Clark VR, Williams DR. Racism as a stressor for African Americans: a biopsychosocial model. Am Psychol 1999;54:805. [DOI] [PubMed] [Google Scholar]
- 11.Paradies Y A systematic review of empirical research on self-reported racism and health. Int J Epidemiol 2006;35:888–901. [DOI] [PubMed] [Google Scholar]
- 12.Paradies Y, Ben J, Denson N, Elias A, PriestA, Pieterse A, Gupta A, Kelaher M, Gee G. Racism as a determinant of health: a systematic review and meta-analysis. PLoS One 2015;10:e0138511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brondolo E, Love EE, Pencille M, Schoenthaler A, Ogedegbe G. Racism and hypertension: a review of the empirical evidence and implications for clinical practice. Am J Hypertens 2011;24:518–29. [DOI] [PubMed] [Google Scholar]
- 14.Dolezsar CM, McGrath JJ, Herzig AJM, Miller SB. Perceived racial discrimination and hypertension: a comprehensive systematic review. Health Psychol 2014;33:20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med 2005;61:1576–96. [DOI] [PubMed] [Google Scholar]
- 16.Kessler RC, Mickelson KD, Williams DR. The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. J Health Soc Behav 1999;40:208–30. [PubMed] [Google Scholar]
- 17.National Public Radio, Robert Wood Johnson Foundation, & Harvard TH Chan School of Public Health. Discrimination in America: final summary. 2010. January. In: Robert Wood Johnson Foundation [Internet]. New Jersey: Robert Wood Johnson Foundation; [about 21 screens]. Available at: https://www.rwjf.org/content/dam/farm/reports/surveys_and_polls/2018/rwjf443620. Accessed December 17, 2018. [Google Scholar]
- 18.Lewis TT, Lampert R, Charles D, Katz S. Expectations of racism and carotid intima-media thickness in African American women [published online March 4, 2019]. Psychosom Med 2019;81:759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson LM, Matthews KA, Derby CA, Bromberger JT, Thurston RC. The relationship between cumulative unfair treatment and intima media thickness and adventitial diameter: the moderating role of race in the Study of Women’s Health Across the Nation. Health Psychol 2016;35:313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Troxel WM, Matthews KA, Bromberger JT, Sutton-Tyrrell K. Chronic stress burden, discrimination, and subclinical carotid artery disease in African American and Caucasian women. Health Psychol 2003;22:300–9. [DOI] [PubMed] [Google Scholar]
- 21.The Jackson Heart Study. National Heart Lung, and Blood Institute. Available at: https://www.nhlbi.nih.gov/research/resources/obesity/population/jhs.htm. Accessed March 30, 2018.
- 22.Cardarelli R, Cardarelli KM, Fulda KG, Espinoza A, Cage C, Vishwanatha J, Young R, Steele DN, Carroll J. Self-reported racial discrimination, response to unfair treatment, and coronary calcification in asymptomatic adults—the North Texas Healthy Heart Study. BMC Public Health 2010;10:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis TT, Everson-Rose SA, Powell LH, Matthews KA, Brown C, Karavolos K, Sutton-Tyrrell K, Jacobs E, Wesley D. Chronic exposure to everyday discrimination and coronary artery calcification in African-American women: the SWAN Heart Study. Psychosom Med 2006;68:362–8. [DOI] [PubMed] [Google Scholar]
- 24.Albert MA, Ravenell J, Glynn RJ, Khera A, Halevy N, de Lemos JA. Cardiovascular risk indicators and perceived race/ethnic discrimination in the Dallas Heart Study. Am Heart J 2008;156:1103–9. [DOI] [PubMed] [Google Scholar]
- 25.Everage NJ, Gjelsvik A, McGarvey ST, Linkletter CD, Loucks EB. Inverse associations between perceived racism and coronary artery calcification. Ann Epidemiol 2012;22:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill LK, Sherwood A, McNeilly M, Anderson NB, Blumenthal JA, Hinderliter AL. Impact of racial discrimination and hostility on adrenergic receptor responsiveness in African American adults. Psychosom Med 2018;80:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucas T, Wegner R, Pierce J, Lumley MA, Laurent HK, Granger DA. Perceived discrimination, racial identity, and multisystem stress response to social evaluative threat among African American men and women. Psychosom Med 2017;79:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beydoun MA, Poggi-Burke A, Zonderman AB, Rostant OS, Evans MK, Crews DC. Perceived discrimination and longitudinal change in kidney function among urban adults. Psychosom Med 2017;79:824–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beatty Moody DL, Chang Y, Brown C, Bromberger JT, Matthews KA. Everyday discrimination and metabolic syndrome incidence in a racially/ethnically diverse sample: Study Of Women’s Health Across the Nation. Psychosom Med 2018;80:114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krieger N Embodying inequality: a review of concepts, measures, and methods for studying health consequences of discrimination. Int J Health Serv 29:295–352. [DOI] [PubMed] [Google Scholar]
- 31.Udo T, Grilo CM. Cardiovascular disease and perceived weight, racial, and gender discrimination in US adults. J Psychosom Res 2017;100:83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Essed P Understanding Everyday Racism: An Interdisciplinary Theory. Vol 2. Amsterdam: Sage; 1991. [Google Scholar]
- 33.Shariff-Marco S, Breen N, Landrine H, Reeve BB, Krieger N, Gee GC, Williams DR, Mays VM, Ponce NA, Alegría M, Liu B, Willis G, Johnson TP. Measuring everyday racial/ethnic discrimination in health surveys: how best to ask the questions, in one or two stages, across multiple racial/ethnic groups? Du Bois Rev 2011;8:159–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown TN. Measuring self-perceived racial and ethnic discrimination in social surveys. Sociol Spectr 2001;21:377–92. [Google Scholar]
- 35.Chae DH, Takeuchi DT, Barbeau EM, Bennett GG, Lindsey J, Krieger N. Unfair treatment, racial/ethnic discrimination, ethnic identification, and smoking among Asian Americans in the national Latino and Asian American study. Am J Public Health 2008;98:485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowleg L The problem with the phrase women and minorities: intersectionality—an important theoretical framework for public health. Am J Public Health 2012; 102:1267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crenshaw KW. Mapping the margins: intersectionality, identity politics, and violence against women of color. Stanford Law Rev 1991;43:1241–99. [Google Scholar]
- 38.Lewis TT, Van Dyke ME. Discrimination and the health of African Americans: the potential importance of intersectionalities. Curr Dir Psychol Sci 2018;27:176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pantesco EJ, Leibel DK, Ashe JJ, Waldstein SR, Katzel LI, Liu HB, Weng N, Evans MK, Zonderman AB, Beatty Moody DL. Multiple forms of discrimination, social status, and telomere length: interactions within race. Psychoneuroendocrinology 2018;98:119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beatty Moody D, Leibel DK, Darden TM, Ashe JJ, Waldstein SR, Katzel LI, Liu HB, Weng N, Evans MK, Zonderman AB. Interpersonal-level discrimination indices, sociodemographic factors, and telomere length in African-Americans and whites. Biol Psychol 2018;141:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunte HE, Williams DR. The association between perceived discrimination and obesity in a population-based multiracial and multiethnic adult sample. Am J Public Health 2009;99:1285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du Bois WEB. (1897). Strivings of the Negro people. Atlantic Monthly Company. Available at: https://www.theatlantic.com/magazine/archive/1897/08/strivings-of-the-negro-people/305446/. Accessed December 29, 2018. [Google Scholar]
- 43.Banks KH. “Perceived” discrimination as an example of color-blind racial ideology’s influence on psychology. Am Psychol 2014;69:311–3. [DOI] [PubMed] [Google Scholar]
- 44.Major B, Gramzow RH, McCoy SK, Levin S, Schmader T, Sidanius J. Perceiving personal discrimination: the role of group status and legitimizing ideology. J Pers Soc Psychol 2002;82:269. [PubMed] [Google Scholar]
- 45.Wilkins CL, Kaiser CR. Racial progress as threat to the status hierarchy: implications for perceptions of anti-white bias. Psychol Sci 2014;25:439–46. [DOI] [PubMed] [Google Scholar]
- 46.Norton MI, Sommers SR. Whites see racism as a zero-sum game that they are now losing. Perspect Psychol Sci 2011;6:215–8. [DOI] [PubMed] [Google Scholar]
- 47.Richeson JA, Sommers SR. Toward a social psychology of race and race relations for the twenty-first century. Annu Rev Psychol 2016;67:439–63. [DOI] [PubMed] [Google Scholar]
- 48.National Urban League. State of Black America Report 2018. In: The State of Black America [Internet]. New York: I am Empowered; [about 12 screens]. Available at: http://soba.iamempowered.com/sites/soba.iamempowered.com/files/SOBA2018-Digital%20Inclusion%20Index.pdf. Accessed December 28, 2018. [Google Scholar]
- 49.Okhomina VI, Glover L, Taylor H, Sims M. Dimensions of and responses to perceived discrimination and subclinical disease among African-Americans in the Jackson Heart Study. J Racial Ethn Health Disparities 2018;5:1084–92. [DOI] [PubMed] [Google Scholar]
- 50.Glymour MM, Mujahid M, Wu Q, White K, Tchetgen Tchetgen EJ. Neighborhood disadvantage and self-assessed health, disability, and depressive symptoms: longitudinal results from the health and retirement study. Ann Epidemiol 2010;20:856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jonas BS, Mussolino ME. Symptoms of depression as a prospective risk factor for stroke. Psychosom Med 2000;62:463–71. [DOI] [PubMed] [Google Scholar]
- 52.Pizzi LT, Jutkowitz E, Frick KD, Suh DC, Prioli KM, Gitlin LN. Cost-effectiveness of a community-integrated home-based depression intervention in older African Americans. J Am Geriatr Soc 2014;62:2288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart JC, Janicki DL, Muldoon MF, Sutton-Tyrrell K, Kamarck TW. Negative emotions and 3-year progression of subclinical atherosclerosis. Arch Gen Psychiatry 2007;64:225–33. [DOI] [PubMed] [Google Scholar]
- 54.Williams DR, Gonzalez HM, Neighbors H, Nesse R, Abelson JM, Sweetman J, Jackson JS. Prevalence and distribution of major depressive disorder in African Americans, Caribbean blacks, and non-Hispanic whites. Arch Gen Psychiatry 2007;64:305–15. [DOI] [PubMed] [Google Scholar]
- 55.Knox S, Barnes A, Kiefe C, Lewis CE, Iribarren C, Matthews KA, Wong N, Whooley M. History of depression, race, and cardiovascular risk in cardia. Int J Behav Med 2006;13:44–50. [DOI] [PubMed] [Google Scholar]
- 56.Alegría M, Chatterji P, Wells K, Cao Z, Chen CN, Takeuchi D, Jackson J, Meng XL. Disparity in depression treatment among racial and ethnic minority populations in the United States. Psychiatr Serv 2008;59:1264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brody GH, Chen YF, Murry VM, Ge X, Simons RL, Gibbons FX, Gerrard M, Cutrona CE. Perceived discrimination and the adjustment of African American youths: a five-year longitudinal analysis with contextual moderation effects. Child Dev 2006;77:1170–89. [DOI] [PubMed] [Google Scholar]
- 58.Brown TN, Williams DR, Jackson JS, Neighbors HW, Torres M, Sellers SL, Brown KT. Being black and feeling blue: the mental health consequences of racial discrimination. Race Soc 2000;2:117–31. [Google Scholar]
- 59.English D, Lambert SF, Evans MK, Zonderman AB. Neighborhood racial composition, racial discrimination, and depressive symptoms in African Americans. Am J Community Psychol 2014;54:219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hurd NM, Varner FA, Caldwell CH, Zimmerman MA. Does perceived racial discrimination predict changes in psychological distress and substance use over time? An examination among black emerging adults. Dev Psychol 2014;50:1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schulz AJ, Gravlee CC, Williams DR, Israel BA, Mentz G, Rowe Z. Discrimination, symptoms of depression, and self-rated health among African American women in Detroit: results from a longitudinal analysis. Am J Public Health 2006;96:1265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hammen C Stress and depression. Annu Rev Clin Psychol 2005;1:293–319. [DOI] [PubMed] [Google Scholar]
- 63.Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis 1992;2:207–21. [PubMed] [Google Scholar]
- 64.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull 2004;130:355. [DOI] [PubMed] [Google Scholar]
- 65.Chae DH, Nuru-Jeter AM, Lincoln KD, Jacob Arriola KR. Racial discrimination, mood disorders, and cardiovascular disease among black Americans. Ann Epidemiol 2012;22:104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sims M, Diez-Roux AV, Dudley A, Gebreab S, Wyatt SB, Bruce MA, James SA, Robinson JC, Williams DR, Taylor HA. Perceived discrimination and hypertension among African Americans in the Jackson Heart Study. Am J Public Health 2012;102:S258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sims M, Diez-Roux AV, Gebreab SY, Brenner A, Dubbert P, Wyatt S, Bruce M, Hickson D, Payne T, Taylor H. Perceived discrimination is associated with health behaviours among African-Americans in the Jackson Heart Study. J Epidemiol Community Health 2016;70:187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beatty Moody DL, Taylor AD, Leibel DK, Al-Najjar E, Katzel LI, Davatzikos C, Gullapalli RP, Seliger SL, Kouo T, Erus G, Rosenberger WF, Evans MK, Zonderman AB, Waldstein SR. Lifetime discrimination burden, racial discrimination, and subclinical cerebrovascular disease among African Americans. Health Psychol 2019;38:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lewis TT, Cogburn CD, Williams DR. Self-reported experiences of discrimination and health: scientific advances, ongoing controversies, and emerging issues. Annu Rev Clin Psychol 2015;11:407–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Everson-Rose SA, Lutsey PL, Roetker NS, Lewis TT, Kershaw KN, Alonso A, Diez Roux AV. Perceived discrimination and incident cardiovascular events: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2015;182:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hickson DA, Lewis TT, Liu J, Mount DL, Younge SN, Jenkins WC, Sarpong DF, Williams DR. The associations of multiple dimensions of discrimination and abdominal fat in African American adults: the Jackson Heart Study. Ann Behav Med 2012;43:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB. Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS): overcoming barriers to implementing a longitudinal epidemiologic, urban study of health, race, and socioeconomic status. Ethn Dis 2010;20: 267–75. [PMC free article] [PubMed] [Google Scholar]
- 73.Williams DR, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol 1997;2:335–51. [DOI] [PubMed] [Google Scholar]
- 74.LaVeist TA, Rolley NC, Diala C. Prevalence and patterns of discrimination among U.S. health care consumers. Int J Health Serv 2003;33:331–44. [DOI] [PubMed] [Google Scholar]
- 75.Krieger N Racial and gender discrimination: risks factors for high blood pressure. Soc Sci Med 1990;30:1273–81. [DOI] [PubMed] [Google Scholar]
- 76.Evaluation of the MacArthur Foundation’s work in Mexico to reduce maternal mortality. MacArthur Foundation. Available at: https://www.macfound.org/press/publications/evaluation-macarthur-foundations-work-mexico-reducematernal-mortality-2002-2008/. Accessed March 30, 2018. [Google Scholar]
- 77.Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N YAcad Sci 2012;1186:69–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measur 1977;1:385–401. [Google Scholar]
- 79.Wendell CR, Waldstein SR, Evans MK, Zonderman AB. Distributions of subclinical cardiovascular disease in a socioeconomically and racially diverse sample. Stroke 2017;48:850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gary S Wilkinson’s WRAT-3: Wide Range Achievement Test Administration Manual. 3rd ed. Wilmington, DE: Wide Range Incorporated; 1993. [Google Scholar]
- 81.Introduction to Mediation, Moderation, and Conditional Process Analysis, Second Edition: A Regression-Based Approach. 2nd ed. New York, NY: Guilford; 2013. [Google Scholar]
- 82.Bonilla-Silva E More than prejudice: restatement, reflections, and new directions in critical race theory. Sociol Race Ethn 2015;1:73–87. [Google Scholar]
- 83.Jones MR, Diez-Roux AV, O’Neill MS, Guallar E, Sharrett AR, Post W, Kaufman JD, Navas-Acien A. Ambient air pollution and racial/ethnic differences in carotid intima-media thickness in the Multi-Ethnic Study of Atherosclerosis (MESA). J Epidemiol Community Health 2015;69:1191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manolio TA, Arnold AM, Post W, Bertoni AG, Schreiner PJ, Sacco RL, Saad MF, Detrano RL, Szklo M. Ethnic differences in the relationship of carotid atherosclerosis to coronary calcification: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2008;197:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Polak JF, Szklo M, O’Leary DH. Carotid intima-media thickness score, positive coronary artery calcium score, and incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2017;6. pii: e004612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Whitfield KE, Allaire JC, Belue R, Edwards CL. Are comparisons the answer to understanding behavioral aspects of aging in racial and ethnic groups? J Gerontol B Psychol Sci Soc Sci 2008;63:P301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whitfield KE, Baker-Thomas T. Individual differences in aging minorities. Int JAging Hum Dev 1999;48:73–9. [DOI] [PubMed] [Google Scholar]
- 88.Harrell SP. A multidimensional conceptualization of racism-related stress: implications for the well-being of people of color. Am J Orthopsychiatry 2000;70:42–57. [DOI] [PubMed] [Google Scholar]
- 89.Clark R, Gochett P. Interactive effects of perceived racism and coping responses predict a school-based assessment of blood pressure in black youth. Ann Behav Med 2006;32:1–9. [DOI] [PubMed] [Google Scholar]
- 90.Pascoe EA, Smart Richman L. Perceived discrimination and health: a meta-analytic review. Psychol Bull 2009;135:531–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: are view of neurobiological mechanisms and the integration of research from preclinical disease models. Stress 2009;12:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Everson SA, Roberts RE, Goldberg DE, Kaplan GA. Depressive symptoms and increased risk of stroke mortality over a 29-year period. Arch Intern Med 1998; 158:1133–8. [DOI] [PubMed] [Google Scholar]
- 93.Kamarck TW, Everson SA, Kaplan GA, Manuck SB, Jennings JR, Salonen R, Salonen JT. Exaggerated blood pressure responses during mental stress are associated with enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation 1997;96:3842–8. [DOI] [PubMed] [Google Scholar]
- 94.Matthews KA, Owens JF, Kuller LH, Sutton-Tyrrell K, Lassila HC, Wolfson SK. Stress-induced pulse pressure change predicts women’s carotid atherosclerosis. Stroke 1998;29:1525–30. [DOI] [PubMed] [Google Scholar]
- 95.Fluharty M, Taylor AE, Grabski M, Munafo MR. The association of cigarette smoking with depression and anxiety: a systematic review. Nicotine Tob Res 2017;19:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Forsyth J, Schoenthaler A, Chaplin WF, Ogedegbe G, Ravenell J. Perceived discrimination and medication adherence in black hypertensive patients: the role of stress and depression. Psychosom Med 2014;76:229–36. [DOI] [PubMed] [Google Scholar]
- 97.Bao YP, Han Y, Ma J, Wang RJ, Shi L, Wang TY, He J, Yue JL, Shi J, Tang XD, Lu L. Cooccurrence and bidirectional prediction of sleep disturbances and depression in older adults: meta-analysis and systematic review. Neurosci Biobehav Rev 2017;75:257–73. [DOI] [PubMed] [Google Scholar]
- 98.Lovato N, Gradisar M. A meta-analysis and model of the relationship between sleep and depression in adolescents: recommendations for future research and clinical practice. Sleep Med Rev 2014;18:521–9. [DOI] [PubMed] [Google Scholar]
- 99.Glover LM, Sims M, Winters K. Perceived discrimination and reported trust and satisfaction with providers in African Americans: the Jackson Heart Study. Ethn Dis 2017;27:209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kramer MR, Black NC, Matthews SA, James SA. The legacy of slavery and contemporary declines in heart disease mortality in the U.S. south. SSM Popul Health 2017;3:609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jacoby SF, Dong B, Beard JH, Wiebe DJ, Morrison CN. The enduring impact of historical and structural racism on urban violence in Philadelphia. Soc Sci Med 2018;199:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lukachko A, Hatzenbuehler ML, Keyes KM. Structural racism and myocardial infarction in the United States. Soc Sci Med 2014;103:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bor J, Venkataramani AS, Williams DR, Tsai AC. Police killings and their spill-over effects on the mental health of black Americans: a population-based, quasi-experimental study. Lancet 2018;392:302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging. Hormones (Athens) 2009;8:7–22. [DOI] [PubMed] [Google Scholar]
- 105.Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S. Socioeconomic status in health research: one size does not fit all. JAMA 2005;294:2879–88. [DOI] [PubMed] [Google Scholar]
- 106.Limdi NA, Howard VJ, Higginbotham J, Parton J, Safford MM, Howard G. US mortality: influence of race, geography and cardiovascular risk among participants in the population-based REGARDS cohort. J Racial Ethn Health Disparities 2016;3:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.National Public Radio, Robert Wood Johnson Foundation, & Harvard TH Chan School of Public Health. Discrimination in America: Experiences and Views of African Americans, 2017. Report. Available at: https://www.rwjf.org/content/dam/farm/reports/reports/2017/rwjf441128. Accessed April 26, 2018. [Google Scholar]
- 108.Davey A, Halverson CF Jr., Zonderman AB, Costa PT Jr. Change in depressive symptoms in the Baltimore Longitudinal Study of Aging. J Gerontol B Psychol Sci Soc Sci 2004;59:P270–7. [DOI] [PubMed] [Google Scholar]
- 109.National Health and Nutrition Examination Survey 2015–2016. Available at: https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/SMQ_I.htm#SMQ925. Accessed December 28, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.