Abstract
Benzodiazepine receptor agonists and related medications, such as Z-drugs and dual orexin receptor antagonists (BZDs), have been associated with unintentional traumatic injury due to their central nervous system (CNS)-depressant effects. Drug-drug interactions (DDIs) may contribute to the known relationship between BZD use and unintentional traumatic injury, yet evidence is still lacking. We conducted high-throughput pharmacoepidemiologic screening using the self-controlled case series design in a large US commercial health insurance database to identify potentially clinically relevant DDI signals among new users of BZDs. We used conditional Poisson regression to estimate rate ratios (RRs) between each co-exposure (vs. not) and unintentional traumatic injury (primary outcome), typical hip fracture (secondary outcome), and motor vehicle crash (secondary outcome). We identified 48 potential DDI signals (1.1%, involving 39 unique co-dispensed drugs), i.e., with statistically significant elevated adjusted RRs for injury. Signals were strongest for DDI pairs involving zolpidem, lorazepam, temazepam, alprazolam, eszopiclone, triazolam, and clonazepam. We also identified four potential DDI signals for typical hip fracture, but none for motor vehicle crash. Many signals have biologically plausible explanations through additive or synergistic pharmacodynamic effects of co-dispensed antidepressants, opioids, or muscle relaxants on CNS depression, impaired psychomotor and cognitive function, and/or somnolence. While other signals that lack an obvious mechanism may represent true associations that place patients at risk of injury, it is also prudent to consider the roles of chance, reverse causation, and/or confounding by indication, which merit further exploration. Given the high-throughput nature of our investigation, findings should be interpreted as hypothesis generating.
Keywords: Benzodiazepines, drug interactions, injury, pharmacoepidemiology, population health, self-controlled case series
INTRODUCTION
More than one in eight US adults used a benzodiazepine (BZD) in the last year, with use most prevalent in persons aged 50–64 years followed by older adults.(Maust et al., 2019) BZD receptor agonists’ central nervous system (CNS)-depressant effects have been associated with unintentional traumatic injury, which is among the leading causes of mortality in all age groups.(Haagsma et al., 2016; National Center for Injury Prevention and Control, 2020) Medications with similar clinical effects such as Z-drugs (eszopiclone, zaleplon, zolpidem) and dual orexin receptor antagonists (i.e., suvorexant, lemborexant) may also increase injury risk.(Brandt and Leong, 2017; Haagsma et al., 2016) Among older adults, injuries resulting from motor vehicle crashes and falls are associated with increased mortality.(Hannan et al., 2004; National Center for Injury Prevention and Control, 2018) Drug-drug interactions (DDIs) may contribute to the known relationship between BZD use and unintentional traumatic injury, yet the underlying evidence base is severely lacking. The vast majority of DDI evidence comes from case reports or pharmacokinetic studies. The former provide a very low level of evidence; the latter provide data on surrogate measures that may lack clinical relevance. Prior population based DDI screening studies of unintentional traumatic injury among users of other CNS active drugs (e.g., opioids, antidepressants) have generated some signals among persons co-treated with BZDs.(7,8)
To generate robust real-world evidence on the role of BZD and related drug DDIs on hospital presentation for unintentional traumatic injury, either via an emergency department visit or inpatient hospitalization, we conducted high-throughput pharmacoepidemiologic screening of administrative healthcare data using an observational study design that eliminates confounding by static factors. Our hypothesis generating work aimed to provide an evidence-based list of BZD DDIs of potential clinical relevance to be confirmed or refuted in future etiologic studies.
METHODS
Overview.
We used the self-controlled case series (SCCS) study design to conduct high-throughput screening of claims data from a large, commercial US health insurer. We sought to identify potentially clinically relevant DDI signals with BZD receptor agonists and related drugs (e.g., Z-drugs, dual orexin receptor antagonists; hereafter considered collectively as BZDs) resulting in emergency department presentation or inpatient hospitalization for unintentional traumatic injury. We defined unintentional traumatic injury as a fracture, dislocation, sprain, strain, intracranial injury, internal injury of thorax, abdomen, or pelvis, open wound, injury to blood vessels, crushing injury, injury to nerves or spinal cord, or certain traumatic complications and unspecified injuries—adapted from a diagnosis based definition used by the American College of Surgeons. We identified separate new-user cohorts of individuals dispensed distinct BZDs, within which we dichotomized observation time as co-exposed (vs. not, i.e., BZD alone) to each among hundreds of commonly dispensed oral medications; to avoid statistically unstable estimates, we excluded as study drugs those used in fewer than five BZD-treated patients. After adjusting for time-varying confounders that were assessed during each day of observation time (i.e., average daily benzodiazepine dose of categorized as quartiles (when feasible); follow-up month categorized as 1, 2, 3, 4, 5, 6, or ≥7; and ever having a prior traumatic injury of interest [binary]), we examined associations between each co-exposure (vs. not) and hospital presentation for: unintentional traumatic injury (primary outcome, hereafter considered injury); typical hip fracture (secondary outcome); and motor vehicle crash while the individual was driving (secondary outcome).(Leonard et al., 2021; Leonard et al., 2020)
We utilized an SCCS design because: its within-person nature (i.e., persons serves as their own ‘controls’) answers the question of why an outcome occurred when it did in a given individual; it is well suited to studying transient exposures in relation to an abrupt outcome; it eliminates static confounding factors within individual, a critical feature given the lack of randomization; it can adjust for time-varying confounders; it is computationally efficient, since analyses are limited to persons with an outcome; and there is substantial precedent for its use in DDI screening. Methodologic detail was adapted from our prior DDI screening work on opioids, antidepressants, muscle relaxants, antihyperglycemics, and hematological agents.(Leonard et al., 2021; Leonard et al., 2020; Leonard et al., 2019; Zhou et al., 2020)
Data Source.
We utilized enrollment information and healthcare claims (May 1, 2000–June 30, 2019) from Optum’s de-identified Clinformatics® Data Mart, a dataset with >71 million commercially insured and Medicare Advantage beneficiaries.
Statistical Analysis.
For each BZD for each outcome, we created an analytic file in which the person-day during an active BZD prescription was the unit of observation. The dependent variable was whether an injury occurred on the given observation day. Independent dichotomous variables were co-exposure to other medications and time-varying covariate status on the given observation day. The contrast of primary interest was the injury occurrence rate ratio (RR, i.e., rateco-exposed / rateBZDalone). We used conditional Poisson regression (xtpoisson with fe option, Stata v16) to estimate RRs and 95% confidence intervals (CIs). We used semi-Bayes shrinkage(Greenland and Poole, 1994; Steenland et al., 2000) to address multiple estimation and minimize false positive signals by increasing the validity of effect estimates and preserving nominal type-1 error; we avoided the traditional Bonferroni correction for multiple estimation as it may be overly conservative and result in high rates of false negatives. We considered findings as DDI signals if the semi-Bayes shrunk confounder adjusted RR exceeded 1.00 and 95% CI excluded 1.00. We compared our results with documentation in two drug interaction knowledgebases (IBM Micromedex, Wolters Kluwer Facts and Comparison eAnswers).
Institutional Review Board (IRB) Approval and Role of Funding.
The University of Pennsylvania’s IRB approved this research (protocol #831486). The National Institutes of Health had no input on the study’s conduct or interpretation.
RESULTS
Among 19 BZDs, we identified 97,564 new users (ranging from 3 [quazepam] to 24,406 [zolpidem]) who experienced an injury, contributing >18 million observation days. Users were predominantly white females, with median ages ranging from 49.5 years for clobazam to 71.5 years for lorazepam. Supplemental Table 1 summarizes data on confounder adjusted RRs for injury. Among 4,263 drug pairs examined, 48 (1.1%, consisting of BZD + one of 39 unique co-dispensed drugs) had statistically significant elevated adjusted RRs for injury after semi-Bayes shrinkage, and were therefore deemed potential DDI signals (Table 1, Figure 1). Signals included co-dispensed drugs in the following therapeutic classes: CNS (N = 21/48 signals), anti-infective (N = 7), endocrine and metabolic (N = 5), renal and genitourinary (N = 4), cardiovascular (N = 4), gastrointestinal (N = 3), respiratory (N = 2), hematological (N = 1), and antineoplastic (N = 1). Signals were strongest for the following pairs: zolpidem + minoxidil (RR 2.51, 95% CI 1.09–5.80); lorazepam + sulindac (2.50, 1.17–5.33); temazepam + phenobarbital (2.29, 1.11–4.73); alprazolam + eletriptan (2.27, 1.18–4.37); eszopiclone + megestrol (2.15, 1.11–4.19); temazepam + methadone (2.14, 1.16–3.94); triazolam + promethazine (2.09, 1.07–4.08); alprazolam + propranolol (2.08, 1.60–2.71); clonazepam + tegaserod (2.06, 1.04–4.11); and temazepam + rizatriptan (2.00, 1.07–3.73). Five (10.4%), two (4.1%), and four (8.3%) of the 48 signals are currently flagged as DDIs of concern by Micromedex only, Facts and Comparisons eAnswers only, and both knowledgebases, respectively.
Table 1.
Benzodiazepine drug interaction signals, given statistically significantly increased rates of hospital presentation for unintentional traumatic injury, by pharmacologic category of benzodiazepine, and by magnitude of association within therapeutic class of the co-administered drug
| Benzodiazepine | Co-administered drug | Co-administered drug therapeutic class | RR*, semi-Bayes shrunk | Lower bound of 95% CI** | Upper bound of 95% CI |
|---|---|---|---|---|---|
| Benzodiazepine receptor agonist, anxiolytic | |||||
| Alprazolam | Eletriptan | CNS | 2.27 | 1.18 | 4.37 |
| Vortioxetinea | 1.61 | 1.03 | 2.52 | ||
| Baclofenc | 1.24 | 1.01 | 1.50 | ||
| Tizanidinec | 1.19 | 1.04 | 1.36 | ||
| Bupropion | 1.16 | 1.01 | 1.34 | ||
| Gabapentinb | 1.14 | 1.04 | 1.25 | ||
| Propranolol | Cardiovascular | 2.08 | 1.60 | 2.71 | |
| Metolazone | Renal & Genitourinary | 1.86 | 1.33 | 2.61 | |
| Clarithromycina,b,c | Anti-infective | 1.49 | 1.01 | 2.19 | |
| Trimethoprimc | 1.19 | 1.02 | 1.40 | ||
| Sulfamethoxazolec | 1.18 | 1.01 | 1.39 | ||
| Apixaban | Hematological | 1.36 | 1.04 | 1.78 | |
| Clonazepam | Tegaserod | Gastrointestinal | 2.06 | 1.04 | 4.11 |
| Gemfibrozil | Cardiovascular | 1.64 | 1.06 | 2.53 | |
| Methylphenidate | CNS | 1.44 | 1.00 | 2.07 | |
| Amphetamine | 1.39 | 1.04 | 1.84 | ||
| Sumatriptan | 1.36 | 1.04 | 1.78 | ||
| Dextroamphetamine | 1.35 | 1.03 | 1.78 | ||
| Butalbitalb,c,d | 1.31 | 1.01 | 1.71 | ||
| Gabapentinb | 1.15 | 1.04 | 1.27 | ||
| Levothyroxine | Endocrine & Metabolic | 1.18 | 1.04 | 1.33 | |
| Furosemide | Renal & Genitourinary | 1.17 | 1.03 | 1.32 | |
| Diazepam | Benzonatate | Respiratory | 1.70 | 1.00 | 2.87 |
| Pregabalinb | CNS | 1.38 | 1.04 | 1.82 | |
| Bupropiona | 1.31 | 1.01 | 1.70 | ||
| Lorazepam | Sulindacc | CNS | 2.50 | 1.17 | 5.33 |
| Bupropion | 1.26 | 1.04 | 1.54 | ||
| Divalproex sodiuma,b | 1.23 | 1.00 | 1.51 | ||
| Sulfamethoxazolec | Anti-infective | 1.47 | 1.24 | 1.75 | |
| Trimethoprimc | 1.47 | 1.24 | 1.74 | ||
| Benzodiazepine receptor agonist, hypnotic | |||||
| Temazepam | Phenobarbitalb,d | CNS | 2.29 | 1.11 | 4.73 |
| Methadonea–e | 2.14 | 1.16 | 3.94 | ||
| Rizatriptan | 2.00 | 1.07 | 3.73 | ||
| Buspirone | 1.56 | 1.09 | 2.24 | ||
| Lovastatin | Cardiovascular | 1.47 | 1.01 | 2.14 | |
| Triazolam | Promethazine | Gastrointestinal | 2.09 | 1.07 | 4.08 |
| Nonbenzodiazepine GABA agonist hypnotic (Z-drug) | |||||
| Eszopiclone | Megestrol | Endocrine & Metabolic | 2.15 | 1.11 | 4.19 |
| Alendronate | 1.69 | 1.02 | 2.79 | ||
| Zaleplon | Metformin | Endocrine & Metabolic | 1.83 | 1.08 | 3.10 |
| Zolpidem | Minoxidil | Cardiovascular | 2.51 | 1.09 | 5.80 |
| Clarithromycina,b,c | Anti-infective | 1.63 | 1.14 | 2.33 | |
| Trimethoprimc | 1.19 | 1.02 | 1.38 | ||
| Methotrexate | Antineoplastic | 1.45 | 1.00 | 2.09 | |
| Rabeprazole | Gastrointestinal | 1.41 | 1.00 | 1.98 | |
| Spironolactone | Renal & Genitourinary | 1.22 | 1.01 | 1.47 | |
| Montelukast | Respiratory | 1.21 | 1.01 | 1.46 | |
| Nonbenzodiazepine hypnotic, miscellaneous | |||||
| Ramelteon | Furosemide | Renal & Genitourinary | 1.60 | 1.01 | 2.52 |
| Suvorexant | Metformin | Endocrine & Metabolic | 1.63 | 1.01 | 2.64 |
RR: rate ratio; CI: confidence interval; CNS: central nervous system; GABA: gamma-aminobutyric acid
RRs >2.00 are bolded to highlight 10 potential signals that may warrant particular attention in future etiologic work.
Causal contrast is concomitant use of benzodiazepine + co-administered drug vs. use of benzodiazepine alone.
p-values <0.05 for lower bounds equal to 1.00 after rounding
Drug interaction with impact on benzodiazepine documented in Facts & Comparisons eAnswers.
Drug interaction with impact on benzodiazepine documented in Micromedex.
Finding may be particularly affected by protopathic bias.
Drug interaction with impact on co-administered drug documented in Micromedex.
Drug interaction with impact on co-administered drug documented in Facts & Comparisons eAnswers.
Figure 1. Benzodiazepine + co-administered drug pairs and associations with unintentional traumatic injury.
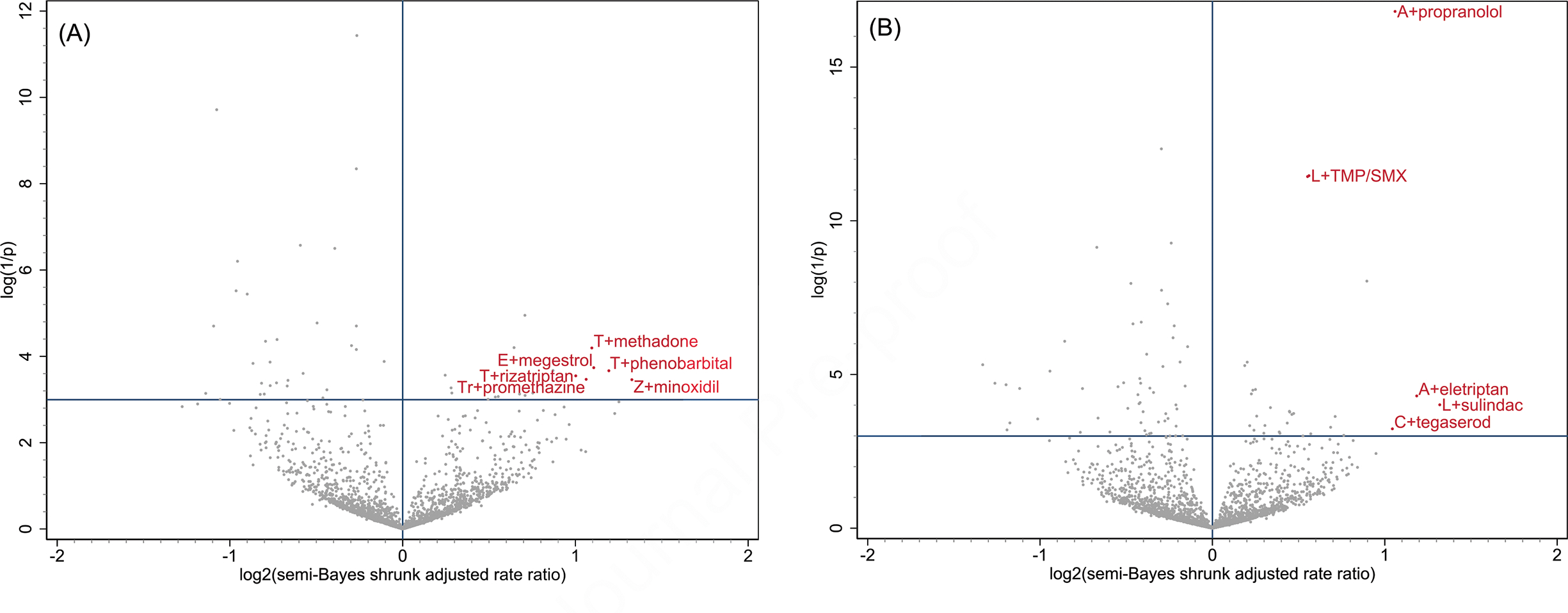
Panel (A) depicts associations for benzodiazepine and nonbenzodiazepine hypnotics: estazolam, eszopiclone [E], flurazepam, quazepam, ramelteon, suvorexant, tasimelteon, temazepam [T], triazolam [Tr], zaleplon, and zolpidem [Z]
Panel (B) depicts associations for benzodiazepine anxiolytics: alprazolam [A], chlordiazepoxide, clobazam, clonazepam [C], clorazepate, diazepam, lorazepam [L], and oxazepam
X-axes, representing the magnitude of association, are the log base 2 semi-Bayes shrunk adjusted rate ratio (RR) for benzodiazepine + co-administered drug vs. benzodiazepine alone. Y-axes, representing statistical significance, are the log (1/p-value) for the semi-Bayes shrunk adjusted RRs.
Data points in each upper right quadrant represent statistically significant elevated semi-Bayes shrunk adjusted RRs for the association between benzodiazepine + co-administered drug vs. benzodiazepine alone and unintentional traumatic injury (i.e., potential drug interaction signals). For ease of reading, we limited labeling to upper right quadrant data points with log base 2 semi-Bayes shrunk adjusted RR ≥1 or log (1/p value), for the semi-Bayes shrunk adjusted RR, ≥10. TMP/SMX: trimethoprim/sulfamethoxazole
In analyses of secondary outcomes, 17 and 16 BZDs under study provided >800,000 and >300,000 observation days for typical hip fracture and motor vehicle crash, respectively. Among 1,308 drug pairs examined, we identified the following four DDI signals for typical hip fracture, each with clonazepam: trimethoprim (RR 2.11, 1.07–4.16); cephalexin (2.10, 1.05–4.19); mirtazapine (2.10, 1.07–4.15); and sulfamethoxazole (2.03, 1.01–4.09). We identified no DDI signals among 564 drug pairs examined for motor vehicle crash.
DISCUSSION
In this pharmacoepidemiologic screening study using commercial health insurance data, we identified 48 potential BZD DDI signals associated with injury. The plurality (44%) of signals involved co-dispensed CNS-active drugs, an expected finding given their pharmacodynamic effects. Given the high-throughput nature of our investigation, findings should be interpreted as hypothesis generating and serve to focus limited resources for the conduct of future etiologic studies.
Many identified signals have biologically plausible underpinnings. Synergistic pharmacodynamic effects of co-dispensed antidepressants, opioids, muscle relaxants, and anticonvulsants/gabapentinoids on CNS depression, impaired psychomotor and cognitive function, and/or somnolence (as examples) may result in increased injury rates.(Bolton et al., 2008; Emeny et al., 2019; Gales and Menard, 1995; Granek et al., 1987; Kanner and Gidal, 2008; Leonard et al., 2021; Leonard et al., 2020; Leveille et al., 1994; Orriols et al., 2012; Rapoport et al., 2011; Ray et al., 1992; Ray et al., 1987; Spina and Leon, 2017; Zint et al., 2010) This potential mechanism supports our findings for alprazolam + vortioxetine, temazepam + methadone, alprazolam + tizanidine, and clonazepam + gabapentin (as examples) with injury, and clonazepam + mirtazapine with hip fracture. It is also noteworthy that these CNS-active co-dispensed drugs could have a direct pharmacodynamic effect on injury by themselves, leading to elevated RRs for their corresponding drug pairs. Additionally, hypotensive effects of co-dispensed antihypertensives (e.g., propranolol) and diuretics (e.g., furosemide) may also result in increased injury rates. Other plausible signals are supported by potential pharmacokinetic mechanisms. For example, our injury finding for alprazolam + clarithromycin may be explained by inhibition of alprazolam’s hepatic metabolism. Clarithromycin, a strong cytochrome P450 3A4 inhibitor,(Fohner et al., 2017) can increase alprazolam’s concentration and consequently its pharmacologic effect and result in excessive sedation.(Dresser et al., 2000; Whirl-Carrillo et al., 2021) Of note, future etiologic work that confirms or refutes these signals may provide evidence to refine the Beers Criteria from the American Geriatrics Society, given their current recommendation against the use of some but not all of these medications when used alone or in combination in older adults.(The 2019 American Geriatrics Society Beers Criteria® Update Expert Panel, 2019) Our lack of signaling for other expected pairs (e.g., temazepam + ziprasidone, RRinjury 1.84, 0.95–3.58) may be due to limited statistical precision and suggests that the semi-Bayes shrinkage method was appropriately conservative for use in this hypothesis generating context.
We also identified signals that lack an obvious pharmacokinetic or pharmacodynamic mechanism (e.g., eszopiclone + megestrol). While these findings may represent true associations that place patients at risk of injury, it remains prudent to consider the roles of chance, reverse causation, and/or within-person confounding by indication. Confounding could partially explain signals for co-dispensed drugs that are indicated for the treatment of infection or pain, as these indications may be risk factors for injury. Reverse causation would occur if the co-dispensed drug was prescribed in response to an injury event. For example, anti-infectives may be prescribed for prophylaxis of infection after an injury.(Bratzler et al., 2013; Stevens et al., 2014) Therefore, it is unclear if our signals for lorazepam + sulindac and alprazolam + trimethoprim/sulfamethoxazole (as examples) are true associations or the influence of confounding by indication or reverse causation. An additional limitation is information bias, as prescription dispensings in an administrative claims database may not reflect drug actual periods of drug availability or consumption. Follow-up etiologic studies should consider bias analyses to quantify the impact of misclassification among other biases.(Funk and Landi, 2014)
Randomized trials are very rarely conducted to study clinical outcomes of potential DDIs; most of the existing evidence arises from case reports and/or pharmacokinetic studies assessing changes in drug concentrations. Given the theoretical likelihood of co-dispensed drugs affecting injury rates in BZD users, we conducted a population-based pharmacoepidemiologic screening study to identify potential BZD DDI signals. Our findings serve as an evidence-based list of potential BZD interactions resulting in hospital presentation for injury. Future etiologic studies should seek to confirm or refute these signals.
Supplementary Material
Sources of Funding
The United States National Institutes of Health supported this work (R01AG060975, R01DA048001, R01AG064589, and R01AG025152).
Footnotes
Potential Conflicts of Interest
Dr. Pham Nguyen receives support from Acadia Pharmaceuticals Inc., unrelated to this project. Dr. Leonard is an Executive Committee Member of and Dr. Hennessy founded and directs the University of Pennsylvania’s Center for Pharmacoepidemiology Research and Training. The Center receives funds from Pfizer and Sanofi to support pharmacoepidemiology education. Dr. Leonard recently received honoraria from the American College of Clinical Pharmacy Foundation, the University of Massachusetts, and the University of Florida. Dr. Leonard receives support for conference travel from John Wiley & Sons. Dr. Leonard is a Special Government Employee of the United States (US) Food and Drug Administration and consults for their Reagan-Udall Foundation. Dr. Leonard’s spouse is an employee of Merck; neither Dr. Leonard nor his spouse own stock in the company. Dr. Horn is coauthor and publisher of The Top 100 Drug Interactions: A Guide to Patient Management and a consultant to Urovant Sciences and Seegnal US. Dr. Bilker serves on multiple data safety monitoring boards for Genentech. Dr. Dublin has research funding from GlaxoSmithKline. Dr. Hennessy has consulted for multiple pharmaceutical companies. All other authors declared no competing interests for this work.
All authors read, revised, and approved the manuscript. We have not submitted or published this work elsewhere in any form. The authors confirm contributions to the submitted work as follows: study conception and design: CEL, SH, SES, TPPN, WBB, TAM, EKA, SPC, SD, DWO, DJW; data analysis: CMB; interpretation of results: CEL, TPPN; drafting of the manuscript: TPPN, SES.
Disclosures
Dr. Pham Nguyen receives support from Acadia Pharmaceuticals Inc., unrelated to this project. Dr. Leonard is an Executive Committee Member of and Dr. Hennessy founded and directs the University of Pennsylvania’s Center for Pharmacoepidemiology Research and Training. The Center receives funds from Pfizer and Sanofi to support pharmacoepidemiology education. Dr. Leonard recently received honoraria from the American College of Clinical Pharmacy Foundation, the University of Massachusetts, and the University of Florida. Dr. Leonard receives support for conference travel from John Wiley & Sons. Dr. Leonard is a Special Government Employee of the United States (US) Food and Drug Administration and consults for their Reagan-Udall Foundation. Dr. Leonard’s spouse is an employee of Merck; neither Dr. Leonard nor his spouse own stock in the company. Dr. Horn is coauthor and publisher of The Top 100 Drug Interactions: A Guide to Patient Management and a consultant to Urovant Sciences and Seegnal US. Dr. Bilker serves on multiple data safety monitoring boards for Genentech. Dr. Dublin has research funding from GlaxoSmithKline. Dr. Hennessy has consulted for multiple pharmaceutical companies. All other authors declared no competing interests for this work.
Statement of Integrity
Dr. Leonard had full access to study data and directed the analyses. He is responsible for the study’s integrity.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bolton JM, Metge C, Lix L, Prior H, Sareen J, Leslie WD, 2008. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol 28(4), 384–391. [DOI] [PubMed] [Google Scholar]
- Brandt J, Leong C, 2017. Benzodiazepines and Z-Drugs: An Updated Review of Major Adverse Outcomes Reported on in Epidemiologic Research. Drugs R D 17(4), 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, Fish DN, Napolitano LM, Sawyer RG, Slain D, Steinberg JP, Weinstein RA, American Society of Health-System, P., Infectious Diseases Society of, A., Surgical Infection, S., Society for Healthcare Epidemiology of, A., 2013. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt) 14(1), 73–156. [DOI] [PubMed] [Google Scholar]
- Dresser GK, Spence JD, Bailey DG, 2000. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet 38(1), 41–57. [DOI] [PubMed] [Google Scholar]
- Emeny RT, Chang CH, Skinner J, O’Malley AJ, Smith J, Chakraborti G, Rosen CJ, Morden NE, 2019. Association of Receiving Multiple, Concurrent Fracture-Associated Drugs With Hip Fracture Risk. JAMA Netw Open 2(11), e1915348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fohner AE, Sparreboom A, Altman RB, Klein TE, 2017. PharmGKB summary: Macrolide antibiotic pathway, pharmacokinetics/pharmacodynamics. Pharmacogenet Genomics 27(4), 164–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk MJ, Landi SN, 2014. Misclassification in administrative claims data: quantifying the impact on treatment effect estimates. Curr Epidemiol Rep 1(4), 175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gales BJ, Menard SM, 1995. Relationship between the administration of selected medications and falls in hospitalized elderly patients. Ann Pharmacother 29(4), 354–358. [DOI] [PubMed] [Google Scholar]
- Granek E, Baker SP, Abbey H, Robinson E, Myers AH, Samkoff JS, Klein LE, 1987. Medications and diagnoses in relation to falls in a long-term care facility. J Am Geriatr Soc 35(6), 503–511. [DOI] [PubMed] [Google Scholar]
- Greenland S, Poole C, 1994. Empirical-Bayes and semi-Bayes approaches to occupational and environmental hazard surveillance. Arch Environ Health 49(1), 9–16. [DOI] [PubMed] [Google Scholar]
- Haagsma JA, Graetz N, Bolliger I, Naghavi M, Higashi H, Mullany EC, Abera SF, Abraham JP, Adofo K, Alsharif U, Ameh EA, Ammar W, Antonio CA, Barrero LH, Bekele T, Bose D, Brazinova A, Catala-Lopez F, Dandona L, Dandona R, Dargan PI, De Leo D, Degenhardt L, Derrett S, Dharmaratne SD, Driscoll TR, Duan L, Petrovich Ermakov S, Farzadfar F, Feigin VL, Franklin RC, Gabbe B, Gosselin RA, Hafezi-Nejad N, Hamadeh RR, Hijar M, Hu G, Jayaraman SP, Jiang G, Khader YS, Khan EA, Krishnaswami S, Kulkarni C, Lecky FE, Leung R, Lunevicius R, Lyons RA, Majdan M, Mason-Jones AJ, Matzopoulos R, Meaney PA, Mekonnen W, Miller TR, Mock CN, Norman RE, Orozco R, Polinder S, Pourmalek F, Rahimi-Movaghar V, Refaat A, Rojas-Rueda D, Roy N, Schwebel DC, Shaheen A, Shahraz S, Skirbekk V, Soreide K, Soshnikov S, Stein DJ, Sykes BL, Tabb KM, Temesgen AM, Tenkorang EY, Theadom AM, Tran BX, Vasankari TJ, Vavilala MS, Vlassov VV, Woldeyohannes SM, Yip P, Yonemoto N, Younis MZ, Yu C, Murray CJ, Vos T, 2016. The global burden of injury: incidence, mortality, disability-adjusted life years and time trends from the Global Burden of Disease study 2013. Inj Prev 22(1), 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan EL, Waller CH, Farrell LS, Rosati C, 2004. Elderly trauma inpatients in New York state: 1994–1998. J Trauma 56(6), 1297–1304. [DOI] [PubMed] [Google Scholar]
- Kanner AM, Gidal BE, 2008. Pharmacodynamic and pharmacokinetic interactions of psychotropic drugs with antiepileptic drugs. Int Rev Neurobiol 83, 397–416. [DOI] [PubMed] [Google Scholar]
- Leonard CE, Brensinger CM, Acton EK, Miano TA, Dawwas GK, Horn JR, Chung S, Bilker WB, Dublin S, Soprano SE, Pham Nguyen TP, Manis MM, Oslin DW, Wiebe DJ, Hennessy S, 2021. Population-Based Signals of Antidepressant Drug Interactions Associated With Unintentional Traumatic Injury. Clin Pharmacol Ther 110(2), 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CE, Brensinger CM, Pham Nguyen TP, Horn JR, Chung S, Bilker WB, Dublin S, Soprano SE, Dawwas GK, Oslin DW, Wiebe DJ, Hennessy S, 2020. Screening to identify signals of opioid drug interactions leading to unintentional traumatic injury. Biomed Pharmacother 130, 110531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CE, Zhou M, Brensinger CM, Bilker WB, Soprano SE, Pham Nguyen TP, Nam YH, Cohen JB, Hennessy S, 2019. Clopidogrel Drug Interactions and Serious Bleeding: Generating Real-World Evidence via Automated High-Throughput Pharmacoepidemiologic Screening. Clin Pharmacol Ther 106(5), 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveille SG, Buchner DM, Koepsell TD, McCloskey LW, Wolf ME, Wagner EH, 1994. Psychoactive medications and injurious motor vehicle collisions involving older drivers. Epidemiology 5(6), 591–598. [DOI] [PubMed] [Google Scholar]
- Maust DT, Lin LA, Blow FC, 2019. Benzodiazepine Use and Misuse Among Adults in the United States. Psychiatr Serv 70(2), 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Injury Prevention and Control, 2018. WISQARS: 10 leading causes of death, United States. https://www.cdc.gov/injury/wisqars/LeadingCauses.html. (Accessed 19 April 2022).
- National Center for Injury Prevention and Control, 2020. WISQARS™ — Web-based Injury Statistics Query and Reporting System. https://www.cdc.gov/injury/wisqars/index.html. (Accessed 19 April 2022).
- Orriols L, Queinec R, Philip P, Gadegbeku B, Delorme B, Moore N, Suissa S, Lagarde E, Group CR, 2012. Risk of injurious road traffic crash after prescription of antidepressants. J Clin Psychiatry 73(8), 1088–1094. [DOI] [PubMed] [Google Scholar]
- Rapoport MJ, Zagorski B, Seitz D, Herrmann N, Molnar F, Redelmeier DA, 2011. At-fault motor vehicle crash risk in elderly patients treated with antidepressants. Am J Geriatr Psychiatry 19(12), 998–1006. [DOI] [PubMed] [Google Scholar]
- Ray WA, Fought RL, Decker MD, 1992. Psychoactive drugs and the risk of injurious motor vehicle crashes in elderly drivers. Am J Epidemiol 136(7), 873–883. [DOI] [PubMed] [Google Scholar]
- Ray WA, Griffin MR, Schaffner W, Baugh DK, Melton LJ 3rd, 1987. Psychotropic drug use and the risk of hip fracture. N Engl J Med 316(7), 363–369. [DOI] [PubMed] [Google Scholar]
- Spina E, Leon J, 2017. Potentially Clinically Relevant Pharmacodynamic Interactions Between Antiepileptic Drugs and Psychotropic Drugs: An Update. Curr Pharm Des 23(37), 5625–5638. [DOI] [PubMed] [Google Scholar]
- Steenland K, Bray I, Greenland S, Boffetta P, 2000. Empirical Bayes adjustments for multiple results in hypothesis-generating or surveillance studies. Cancer Epidemiol Biomarkers Prev 9(9), 895–903. [PubMed] [Google Scholar]
- Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, Hirschmann JV, Kaplan SL, Montoya JG, Wade JC, Infectious Diseases Society of, A., 2014. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 59(2), e10–52. [DOI] [PubMed] [Google Scholar]
- The 2019 American Geriatrics Society Beers Criteria® Update Expert Panel, 2019. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 67(4), 674–694. [DOI] [PubMed] [Google Scholar]
- Whirl-Carrillo M, Huddart R, Gong L, Sangkuhl K, Thorn CF, Whaley R, Klein TE, 2021. An Evidence-Based Framework for Evaluating Pharmacogenomics Knowledge for Personalized Medicine. Clin Pharmacol Ther 110(3), 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Leonard CE, Brensinger CM, Bilker WB, Kimmel SE, Hecht TEH, Hennessy S, 2020. Pharmacoepidemiologic Screening of Potential Oral Anticoagulant Drug Interactions Leading to Thromboembolic Events. Clin Pharmacol Ther 108(2), 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Sturmer T, 2010. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf 19(12), 1248–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


