Key Points
Question
Is general anesthesia (GA) or conscious sedation (CS) superior in the treatment of patients with acute posterior circulation during endovascular therapy?
Findings
In this exploratory randomized clinical trial including 87 patients, the rates of favorable neurological outcomes were slightly lower in the CS group compared with the GA group but not significantly different.
Meaning
The findings show that CS was not superior to GA in patients with acute posterior circulation stroke; however, GA may be the better choice for these patients, considering the high rate of conversion from CS to GA.
This exploratory randomized clinical trial evaluates in patients with acute posterior circulation stroke during endovascular treatment with general anesthesia vs conscious sedation.
Abstract
Importance
No definitive conclusion can be made on the best choice of anesthesia for people with acute posterior circulation stroke during endovascular treatment. Only a few observational studies have focused on this topic in recent years, and they have differing conclusions.
Objective
To examine whether conscious sedation (CS) is a feasible alternative to general anesthesia (GA) during endovascular treatment in patients with acute posterior circulation stroke.
Design, Setting, and Participants
A randomized parallel-group exploratory trial with blinded end point evaluation (Choice of Anesthesia for Endovascular Treatment of Acute Ischemic Stroke [CANVAS II]) enrolled adult patients from March 2018 to June 2021 at 2 comprehensive care hospitals in China. Patients with acute posterior circulation stroke were enrolled, randomized, and monitored for 3 months. Of 210 patients admitted with acute ischemic posterior circulation stroke, 93 were recruited and 87 were included in the intention-to-treat (ITT) analysis after exclusions, 43 were assigned to GA and 44 to CS. All analyses were unadjusted or adjusted with the ITT principle.
Interventions
Participants were randomly assigned to CS or GA in a 1:1 ratio.
Main Outcomes and Measures
The primary end point was functional independence at 90 days evaluated with the modified Rankin Scale (mRS).
Results
A total of 87 participants were included in the ITT study (mean [SD] age, 62 [12] years; 16 [18.4%] female and 71 [81.6%] male). Of these, 43 were in the GA group and 44 in the CS group. The overall baseline median (IQR) National Institute of Health Stroke Scale (NIHSS) score was 15 (12-17). In the CS group, 13 people (29.5%) were ultimately transferred to GA. The CS group had a higher incidence of functional independence; however, no significant difference was found between the 2 groups (48.8% vs 54.5%; risk ratio, 0.89; 95% CI, 0.58-1.38; adjusted odds ratio [OR], 0.91; 95% CI, 0.37-2.22). However, GA performed better in successful reperfusion (mTICI 2b-3) under ITT analysis (95.3% vs 77.3%; adjusted OR, 5.86; 95% CI, 1.16-29.53).
Conclusion and Relevance
The findings in this study suggest that CS was not better than GA for the primary outcome of functional recovery and was perhaps worse for the secondary outcome of successful reperfusion.
Trial Registration
ClinicalTrials.gov Identifier: NCT03317535
Introduction
Stroke is a major cause of death and disability worldwide.1 Acute ischemic stroke (AIS) accounts for more than 70% of stroke cases, with a high rate of disability and mortality. Over the past 6 years, the efficacy and safety of endovascular therapy (EVT) in anterior AIS have been demonstrated by several influential randomized clinical trials (RCTs).2,3,4,5,6,7,8,9,10,11,12 However, endovascular treatment in the posterior circulation does not show consistent results for functional outcomes: the Endovascular Treatment Versus Standard Medical Treatment for Vertebrobasilar Artery Occlusion (BEST) and Basilar Artery International Cooperation Study (BASICS)13 trials reported no significant difference between EVT and medical therapy14; meanwhile, the Endovascular treatment for acute basilar artery occlusion (ATTENTION)15 and Basilar Artery Occlusion Chinese Endovascular (BAOCHE)16 trials presented at the European Stroke Organisation Conference 2022 found that EVT was better than medical therapy alone.
Anesthesia methods and management may play an essential role in functional outcomes. For anterior AIS, retrospective studies have shown that adverse effects of general anesthesia (GA) on clinical outcomes were more pronounced than those of conscious sedation (CS).17,18,19 However, RCTs and other retrospective analyses have indicated that functional outcomes with GA were better than or similar to those with CS or monitored anesthesia care.20,21,22,23,24,25,26,27 Regarding posterior AIS, only a few observational studies have focused on this issue: 2 preferred CS or monitored anesthesia care,28,29 2 found an association between GA and poor clinical outcomes,30,31 and 3 did not find substantial differences between GA and non-GA treatment, including local anesthesia, CS, and monitored anesthesia care.32,33,34 Due to the inconsistent results of posterior AIS and the lack of RCTs, we conducted what is to our knowledge the first RCT to compare functional outcomes in patients with acute posterior circulation stroke (PCS) undergoing EVT with either CS or GA.
Methods
Trial Design and Patients
The double-center randomized parallel-group exploratory Choice of Anesthesia for Endovascular Treatment of Acute Ischemic Stroke in Posterior Circulation (CANVAS II) trial was conducted at Beijing Tiantan Hospital, Capital Medical University, and Baiyun Hospital, Guizhou Medical University, China, from March 2018 to June 2021 and was approved by the ethics committees of the hospitals (KY2017-074-02; No. 8 in 2020). Before randomization, written informed consent was obtained from all participants or their legal representatives. An independent data and security committee supervised the study. The trial protocol has been published and is available in Supplement 1. The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
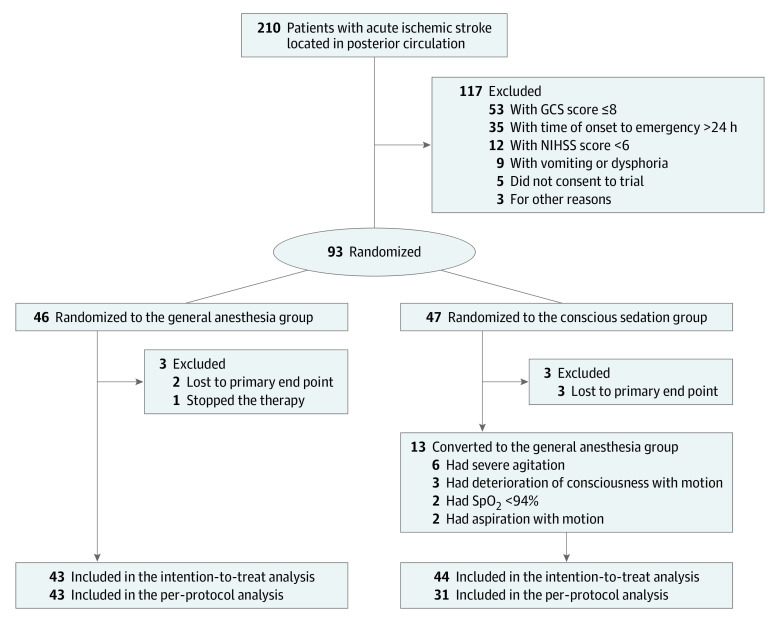
Eligible candidates were patients 18 years and older with acute PCS (basilar artery or vertebral artery) discovered by computed tomography angiography or magnetic resonance angiography whose condition was suitable for recanalization treatment35 with less than 24 hours from onset to primary treatment and whose modified Rankin Scale (mRS) score was 2 or lower before the stroke occurred. Exclusion criteria included unclear radiological images for identifying infarction and vessel occlusion, anterior circulation occlusion, intracranial hemorrhage, posterior circulation Acute Stroke Prognosis Early Computed Tomography score less than 6, pons-midbrain index score 3 or greater, severe agitation or seizures, loss of airway protective reflexes and/or vomiting on admission, intubation before EVT, unconsciousness, known allergy to anesthetics or analgesics, and refusal to participate on the part of the patient or their legal representative. Before recruitment, patients had to obtain agreement from the neuroradiologist and anesthesiologist that they were suitable for GA or CS. Reasons for failed recruitment were recorded in detail (Figure 1).
Figure 1. CONSORT Diagram.
Randomization and Masking
Enrolled participants were randomized in a 1:1 ratio for treatment with GA or CS. To balance the number of people entering the 2 groups, a computer-generated random block number table was used (blocks of 4). A designated staff member who was not involved in anesthesia management or follow-up performed the recruitment and generated the allocation randomization sequence. The staff implemented the allocation sequence through opaque, sealed, and stapled envelopes. The envelopes were numbered to ensure they were opened in the correct order. Because the neuroradiologist, anesthesiologist, and attending physicians participated in the whole treatment, the randomized result was unblinded to them. Eligible candidates and their medical agents were not blinded. The independent follow-up team was responsible for evaluating National Institutes of Health Stroke Scale (NIHSS) score at discharge, 30 days, and 90 days; mRS score at discharge, 30 days, and 90 days; mortality (up to 90 days), time-related outcomes (eg, length of assisted ventilation, stay in hospital, and stay in the neurological intensive) and any complications (up to 90 days, such as deep vein thrombosis and hemorrhagic transformation); the follow-up team was blinded to randomization.
Procedures and Intervention
We used protocols for standard anesthesia management during EVT (concomitant treatment) as described previously.36 The same anesthetics (propofol and remifentanil) were used with different regimes to test 2 ways of applying GA and CS. In the conscious sedation group, patients received propofol (0.3 to 0.5 mg/kg) and then continuous infusion of remifentanil (0.01 to 0.06 μg/kg/min) and propofol (1 to 2 mg/kg/h). In the general anesthesia group, patients received propofol (1 to 2 mg/kg) and remifentanil (0.2 to 0.8 μg/kg/min). Rocuronium (0.6 mg/kg) was used to achieve muscle relaxation. More specific anesthesia management information, such as oxygen saturation, carbon dioxide saturation, bispectral index values, and mechanical ventilation parameter settings, can be found in the protocol in Supplement 1, along with the conditions and reasons for conversion from CS to GA.
Outcome Measures
The primary end point was functional independence, defined as an mRS score of 2 or lower at 90 days (mRS range, 0 = no symptoms to 6 = death). The score was evaluated by blinded researchers with a recorded telephone call. Other out-of-hospital outcomes were assessed in the same way.
Secondary outcomes included changes in NIHSS score from baseline to 30 and 90 days after randomization; modified treatment in cerebral infarction (mTICI) score at baseline and after treatment; conversion rate; all-cause mortality and proportions of complications up to 90 days after randomization; and time-related outcomes, such as treatment time, length of stay in the hospital and intensive care unit, and time from onset to door. Adverse events, including hypotension, dysphoria or motion, pulmonary infection, deep vein thrombosis, and hemorrhagic transformation, were also recorded.
Statistical Analysis
According to a previous investigation in a small sample (Supplement 1), we assumed that the proportion of functional independence (mRs score ≤2) was 65% in the CS group and 35% in the GA group, with a target difference of 30%. Each group required 44 patients to achieve a power of 80% at a 2-tailed significance level of P < .05 with a dropout rate of 5%.29,36,37,38,39 We reported statistics with means and SDs or medians and IQRs as appropriate. Both intention-to-treat (ITT) and per-protocol (PP) analyses were performed.
Optimal methods, including the Cochran-Mantel-Haenszel test, χ2 test, t test, or Mann-Whitney U test, were selected to distinguish the differences between groups. Baseline NIHSS score, occlusion site, age, and intravenous tissue plasminogen activator use were included in the logistic regression model and modified Poisson regression to estimate the adjusted odds ratio (OR) and risk ratio (RR). The modified Poisson regression with robust (sandwich) estimation was an extra analysis based on the original protocol. We performed all statistical analyses with SPSS version 26.0 (IBM Corp).
Results
Patients and Baseline Characteristics
Of 210 patients admitted with acute ischemic PCS between March 2018 and March 2021, 93 were recruited and randomly assigned to the GA (46 [49.5%]) or CS group (47 [50.5%]); however, 6 of these patients were excluded from the trial due to missing data on primary end points or termination of therapy after randomization. Thus, 87 participants were included in the ITT study, 43 in the GA group and 44 in the CS group (mean [SD] age, 62 [12] years; 16 [18.4%] female and 71 [81.6%] male; baseline median [IQR] NIHSS score, 15 [12-17]). Seven patients in the GA group (16.3%) and 5 in the CS group (11.4%) received intravenous thrombolysis. In the ITT analysis, 13 patients who converted to anesthetic methods were reserved in the CS group. For the PP analysis, 43 patients were in the GA group, and 31 were in the CS group (Figure 1). The 2 groups were balanced in most baseline characteristics (Table 1; eTable 1 in Supplement 2). Although most of the time intervals were comparable, significant differences existed between the 2 groups in time from operating room door to puncture (median [IQR], 20 [15-30] vs 15 [10-22]; P = .003) and time from operating room door to reperfusion (median [IQR], 126 [96-140] vs 94 [75-135]; P = .02) (Table 1).
Table 1. Baseline Demographic, Clinical, and Treatment Data.
| Characteristic | Patients, No. (%) | P value | |
|---|---|---|---|
| General anesthesia (n = 43) | Conscious sedation (n = 44) | ||
| Age, mean (SD), y | 64 (11) | 60 (13) | .07 |
| Female | 10 (23.3) | 6 (13.6) | .25 |
| Male | 33 (76.7) | 38 (86.4) | |
| BMI, mean (SD)a | 26 (3) | 26 (3) | .80 |
| Medical history | |||
| Hypertension | 32 (74.4) | 31 (70.5) | .68 |
| Atrial fibrillation | 7 (16.3) | 7 (15.9) | .96 |
| Coronary artery disease | 6 (14.0) | 7 (15.9) | .80 |
| Dyslipidemia | 17 (39.5) | 14 (31.8) | .45 |
| Diabetes | 13 (30.2) | 10 (22.7) | .43 |
| Transient ischemic attack | 5 (11.6) | 4 (9.1) | .97 |
| Past stroke | 9 (20.9) | 7 (15.9) | .55 |
| Smoking | 24 (55.8) | 29 (65.90) | .34 |
| Drinking | 11 (25.6) | 17 (38.6) | .19 |
| Etiology | |||
| Cardiogenic thrombosis | 8 (18.6) | 9 (20.5) | .83 |
| Atherosclerosis | 35 (81.4) | 35 (79.5) | |
| Drug use | |||
| Antiplatelet agent | 12 (27.9) | 12 (27.3) | .95 |
| Anticoagulant agent | 2 (4.7) | 3 (6.8) | .66 |
| Statin | 10 (23.3) | 10 (22.7) | .95 |
| Hypoglycemic agent | 9 (20.9) | 8 (18.2) | .75 |
| ASA class, median (IQR) | 3 (3-4) | 3 (3-4) | .90 |
| Premorbid modified Rankin Scale score, median (IQR) | 0 (0-1) | 0 (0-0) | .28 |
| Clinical status at admission | |||
| Modified Rankin Scale score, median (IQR) | 4 (4-5) | 4 (4-4) | .58 |
| NIHSS score, median (IQR) | 16 (12-21) | 15 (12-18) | .69 |
| Glasgow Coma Scale score, median (IQR) | 11 (10-12) | 12 (9-14) | .36 |
| Systolic blood pressure, mean (SD), mm Hg | 160 (21) | 161 (22) | .83 |
| Arterial pressure, mean (SD), mm Hg | 113 (14) | 113 (15) | .97 |
| Lesion location | |||
| Basilar artery | 9 (20.9) | 12 (27.3) | .72 |
| Vertebral artery V4 segment | 27 (62.8) | 24 (54.5) | |
| Basilar artery combined V4 | 7 (16.3) | 8 (18.2) | |
| IV-tPA pretreatment | 7 (16.3) | 5 (11.4) | .51 |
| Time interval, median (IQR), min | |||
| Onset to door | 210 (90-390) | 300 (151-450) | .14 |
| Onset to operating room | 400 (270-570) | 448 (313-716) | .39 |
| Onset to puncture | 415 (290-586) | 467 (330-731) | .45 |
| Onset to reperfusion | 524 (405-717) | 562 (416-819) | .63 |
| Door to puncture | 185 (150-255) | 151 (108-214) | .08 |
| Operating room to puncture | 20 (15-30) | 15 (10-22) | .003 |
| Door to reperfusion | 284 (240-337) | 237 (189-340) | .05 |
| Operating room to reperfusion | 125 (96-140) | 94 (75-135) | .02 |
| Puncture to reperfusion | 99 (68-118) | 81 (55-116) | .15 |
| Operating time | 125 (98-145) | 105 (71-135) | .07 |
Abbreviations: ASA, American Society of Anesthesiologists physical status classification; BMI, body mass index; IV-tPA, intravenous alteplase; NIHSS, National Institute of Health Stroke Scale.
Calculated as weight in kilograms divided by height in meters squared.
Primary Outcome
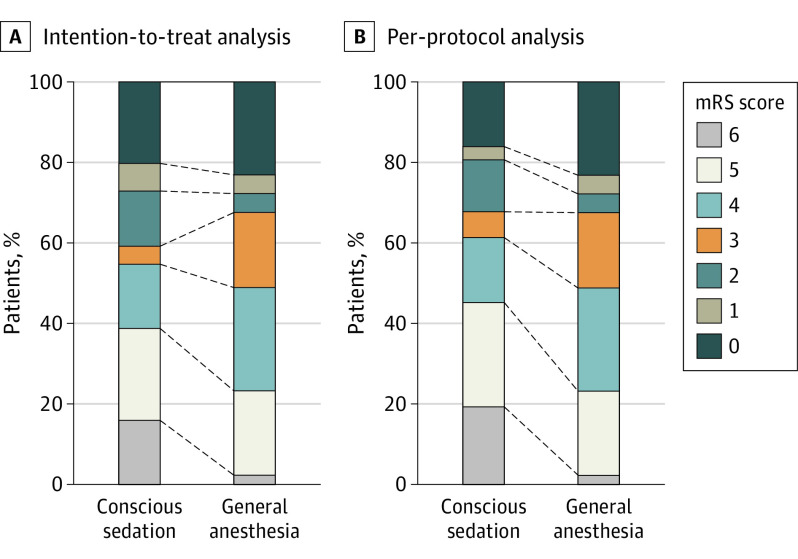
In the ITT analysis, the proportion of favorable neurological outcomes was higher in the CS group (Table 1; eTable 2 in Supplement 2); however, no significant difference was detected in the 2 arms (48.8% vs 54.5%; RR, 0.89; 95% CI, 0.58-1.38; adjusted OR, 0.91; 95% CI, 0.37-2.22). A similar result was found in the PP population between the GA and CS groups (48.8% vs 61.3%; RR, 0.93; 95% CI, 0.80-1.10; adjusted OR, 0.64; 95% CI, 0.23-1.80) (Table 2, eTable 2 in Supplement 2; Figure 2).
Table 2. Primary Outcomes, Secondary Outcomes, and Adverse Events.
| ITT analysis | PP analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | OR (95% CI) | No. (%) | OR (95% CI) | |||||||
| GA (n = 43) | CS (n = 44) | Unadjusted | P value | Adjusteda | GA (n = 43) | CS (n = 31) | Unadjusted | P value | Adjusteda | |
| mRS score 0-2 at 90 d | 21 (48.8) | 24 (54.5) | 0.80 (0.34-1.85) | NA | 0.91 (0.37-2.22) | 21 (48.8) | 19 (61.3) | 0.60 (0.24-1.54) | NA | 0.64 (0.23-1.80) |
| mRS score at 90 d | ||||||||||
| Excellent outcome (mRS 0-1) | 10 (23.3) | 17 (38.6) | 0.48 (0.19-1.22) | NA | 0.58 (0.22-1.53) | 10 (23.3) | 14 (45.2) | 0.37 (0.14-1.00) | NA | 0.43 (0.15-1.27) |
| Poor outcome (mRS ≥3) | 22 (51.2) | 20 (45.5) | 1.26 (0.54-2.92) | NA | 1.10 (.45-2.69) | 22 (51.2) | 12 (38.7) | 1.66 (0.65-4.24) | NA | 1.56 (0.55-4.38) |
| Successful reperfusion (mTICI 2b-3) | 41 (95.3) | 34 (77.3) | 6.03 (1.24-29.40) | NA | 5.86 (1.16-29.53) | 41 (95.3) | 24 (77.4) | 5.98 (1.15-31.14) | NA | 5.12 (0.94-27.84) |
| NIHSS score, median (IQR)b | ||||||||||
| At discharge | 5 (3-12) | 8 (2-16) | NA | .68 | NA | 5 (3-12) | 8 (2-16) | NA | .86 | NA |
| 30 d After therapy | 2 (1-6) | 2 (0-9) | NA | .60 | NA | 2 (1-6) | 2 (0-7) | NA | .45 | NA |
| 90 d After therapy | 1 (1-3) | 0 (0-5) | NA | .23 | NA | 1 (1-3) | 0 (0-2) | NA | .14 | NA |
| GCS, median (IQR)c | ||||||||||
| At discharge | 15 (12-15) | 15 (10-15) | NA | .95 | NA | 15 (12-15) | 15 (12-15) | NA | .76 | NA |
| 30 d After therapy | 15 (14-15) | 15 (13-15) | NA | .94 | NA | 15 (14-15) | 15 (14-15) | NA | .99 | NA |
| 90 d After therapy | 15 (15-15) | 15 (15-15) | NA | .48 | NA | 15 (15-15) | 15 (15-15) | NA | .50 | NA |
| Assisted ventilation | 31 (72.1) | 18 (40.9) | 0.27 (0.11-0.66) | NA | 0.26 (0.10-0.69) | 31 (72.1) | 5 (16.1) | 0.74 (0.02-0.24) | NA | 0.06 (0.02-0.23) |
| Length of assisted ventilation, median (IQR), d | 4 (2-7) | 7 (3-13) | NA | .23 | NA | 4 (2-7) | 7 (2-16) | NA | .53 | NA |
| Length of stay in hospital, median (IQR), d | 9 (6-17) | 11 (6-15) | NA | .87 | NA | 9 (6-17) | 12 (6-15) | NA | .73 | NA |
| Length of stay in the neurological intensive care unit, median (IQR), d | 8 (5-15) | 9 (4-15) | NA | .77 | NA | 8 (5-15) | 9 (4-14) | NA | .70 | NA |
| Mortality | ||||||||||
| At discharge | 5 (11.6) | 4 (9.1) | 1.32 (0.33-5.27) | NA | 1.36 (0.33-5.67) | 5 (11.6) | 1 (3.2) | 3.95 (0.44-35.62) | NA | 4.63 (0.49-43.61) |
| At 30 d | 9 (22.0) | 9 (21.4) | 1.03 (0.36-2.93) | NA | 1.05 (0.35-3.14) | 9 (22.0) | 5 (17.2) | 1.35 (0.40-4.55) | NA | 1.56 (0.42-5.80) |
| At 90 d | 10 (23.3) | 9 (20.5) | 1.18 (0.43-3.26) | NA | 1.18 (0.41-3.41) | 10 (23.3) | 5 (16.1) | 1.58 (0.48-5.18) | NA | 1.81 (0.51-6.44) |
| Adverse events during treatment | ||||||||||
| Hypotensiond | 23 (53.5) | 7 (15.9) | 0.17 (0.06-0.45) | NA | 0.17 (0.06-0.47) | 23 (53.5) | 3 (9.7) | 0.09 (0.03-0.35) | NA | 0.10 (0.03-0.40) |
| Dysphoria or motion | 1 (2.3) | 16 (36.4) | 24.00 (2.01-191.36) | NA | 26.10 (3.14-215.94) | 1 (2.3) | 5 (16.1) | 8.08 (0.89-73.04) | NA | 7.22 (0.73-70.95) |
| Complications after EVT | ||||||||||
| Pulmonary infection | 28 (65.1) | 20 (45.5) | 0.45 (0.19-1.06) | NA | 0.51 (0.21-1.25) | 28 (65.1) | 12 (38.7) | 0.34 (0.13-0.88) | NA | 0.44 (0.16-1.22) |
| Deep venous thrombosis | 3 (7.0) | 3 (6.8) | 0.98 (0.19-5.12) | NA | 1.06 (0.19-5.90) | 3 (7.0) | 2 (6.5) | 0.92 (0.14-5.86) | NA | 1.11 (0.16-7.72) |
| Hemorrhagic transformatione | 3 (7.0) | 2 (4.5) | 0.64 (0.10-4.00) | NA | 0.42 (0.05-3.48) | 3 (7.0) | 1 (3.2) | 0.44 (0.04-4.49) | NA | 0.35 (0.02-6.29) |
Abbreviations: CS, conscious sedation; EVT, endovascular therapy; GA, general anesthesia; GCS, Glasgow Coma Scale; ITT, intention-to-treat; mTICI, modified treatment in cerebral infarction; mRS, modified Rankin Scale; NA, not applicable; NIHSS, National Institute of Health Stroke Scale; OR, odds ratio; PP, per-protocol.
Adjusted odds ratios were estimated using a logistic regression model including age, baseline NIHSS score, occlusion site, and use of IV-tPA.
Missing data due to death or dropout. In ITT, data were missing for 4 patients in GA and 4 in CS at discharge, 9 in each group at 30 d, and 10 in GA and 9 in CS at 90 d. In PP, data were missing for 4 patients in GA and 1 in CS at discharge, 9 in GA and 5 in CS at 30 d, and 10 in GA and 5 in CS at 90 d.
Missing data due to death or dropout. In ITT, data were missing for 4 patients in GA and 4 in CS at discharge, 9 in each group at 30 d, and 10 in GA and 8 in CS at 90 d. In PP, data were missing for 4 patients in GA and 1 in CS at discharge, 9 in GA and 5 in CS at 30 d, and 10 in GA and 4 in CS at 90 d.
Defined as systolic blood pressure <120 mm Hg.
Includes symptomatic intracranial hemorrhage, defined as an increase in NIHSS score of >4 points within the first 36 h of stroke onset, and asymptomatic intracranial hemorrhage, defined as an increase in NIHSS score by ≤4 points within the first 36 h of stroke onset.
Figure 2. Neurological Outcomes by Modified Rankin Scale (mRS) Score.
Secondary Outcomes
The incidence of successful reperfusion (mTICI 2b-3) was 95.3% (41 of 43) for GA and 77.3% (34 of 44) for CS in the ITT analysis. The unadjusted difference was 6.03 (95% CI, 1.24-29.40). The adjusted odds ratio was 5.86 (95% CI, 1.16-29.53). The PP analysis reached the same conclusion (unadjusted OR, 5.98; 95% CI, 1.15-31.14). In the ITT and PP analyses, the CS group had a lower requirement for assisted ventilation than the GA group (ITT, 72.1% vs 40.9%; PP, 72.1% vs 16.1%). Significant differences were not found for the other secondary outcomes. Similar results were obtained in the modified Poisson regression. Additional information is shown in Table 2 and eTable 2 in Supplement 2.
Adverse Events
In the CS group, 13 patients (29.5%) changed their anesthesia methods, 6 due to severe agitation, 2 had oxygen saturation lower than 94%, 2 had aspiration and motion, and 3 experienced movements and deterioration of consciousness (Figure 1). The mortality rates at discharge, 30 days, and 90 days did not differ remarkably between the 2 groups in ITT or PP analysis. Similar results were obtained in the modified Poisson regression (eTable 2 in Supplement 2). However, hypotension during treatment was more prevalent in the GA group, similar to the ITT analysis and PP analysis results (ITT adjusted OR, 0.17; 95% CI, 0.06-0.47; PP adjusted OR, 0.10; 95% CI, 0.03-0.40). Moreover, dysphoria or motion was more frequent in the CS group in the ITT analysis (ITT adjusted OR, 26.10; 95% CI, 3.14-215.96). After adjustment, no significant differences were seen in the proportions of adverse events after EVT (eg, pulmonary infection, deep venous thrombosis, and hemorrhagic transformation) in the adjusted analysis (Table 2).
Supplementary Analyses
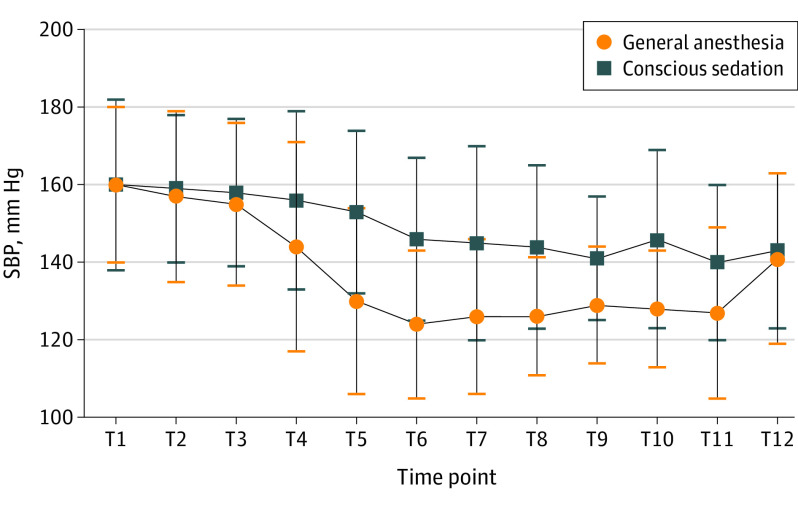
Significant group × time interaction effects (F, 4.327; P = .003) and main effect of time (F, 28.303; P < .001) were found for systolic blood pressure from groin puncture to therapy completion under ITT analysis. No differences were found at the starting point (door to sedation or induction) (Figure 3; eTable 3 in Supplement 2).
Figure 3. Perioperative Changes in Systolic Blood Pressure (SBP).
T1 indicates time in the door; T2, 10 minutes before sedation or induction; T3, sedation or induction; T4, groin puncture; T5, 10 minutes after sedation or induction; T6, 20 minutes after sedation or induction; T7, 30 minutes after sedation or induction; T8, 1 hour after sedation or induction; T9, 2 hours after sedation or induction; T10, reperfusion; T11, completion of therapy; T12, out of the room.
Discussion
To our knowledge, this trial is the first RCT to compare anesthetic management in patients with PCS during EVT. In this double-center study, 93 participants with acute PCS were randomly assigned to the GA or CS group. The 2 groups were not notably different in rates of 90-day favorable outcomes, mortality, successful reperfusion, operating room door-to-puncture time, operating room door-to-reperfusion time, assisted ventilation, intraoperative hypotension, or perioperative changes in systolic blood pressure.
Compared with anterior circulation stroke, occlusion in PCS mainly involves the brainstem.31 The brainstem controls many physiological functions that are essential for life, such as breathing, heartbeat, and blood pressure. AIS decreases blood perfusion in these critical areas, resulting in disorientation, diminished consciousness or coma, and disappearance of protective reflexes.28 Thus, intraoperative breathing and circulatory management, in which anesthesiologists play a vital role, must be emphasized.
The strengths of this study include the professional teams of neurointerventionists, anesthesiologists, nurses, and radiologists at 2 comprehensive stroke centers and the standard workflow and management of blood pressure, oxygen and carbon dioxide saturation, and glucose in both groups according to the Consensus Statement from the Society for Neuroscience in Anesthesiology & Critical Care.40 Compared with previous nonrandomized studies on PCS, this study balanced the basic features in the 2 groups without selection bias, reduced the potential influence of confounding factors. For example, we excluded patients with prehospital intubation because their condition is likely to be more severe with reduced likelihood of favorable outcome. Their inclusion could lead to overestimation of the harmful effects of GA. Few non-RCTs have excluded these patients.28,29,31,33 Moreover, nonrandomized studies tend to miss critical outcomes, such as hemodynamic instability and body movements and detailed symptomatic cerebral hemorrhage.
Similar to the Sedation vs Intubation for Endovascular Stroke Treatment (SIESTA),23 Anesthesia During Stroke (Anstroke),24 General or Local Anesthesia in Intra Arterial Therapy (GOLIATH),25 and General Anesthesia versus Sedation for Acute Stroke Treatment (GASS)27 trials in anterior circulation stroke, the CANVAS II trial showed no obvious difference in 90-day favorable outcomes and mortality in the 2 arms. Due to the different physiological characteristics, the evidence from anterior circulation stroke is not suitable for generalization to PCS. Two non-RCTs of PCS demonstrated that monitored anesthesia care or conscious sedation was as safe and effective as GA, with no difference in functional independence and mortality at 3 months.29,32 Compared with other studies showing poor results for GA,30,31,41 the 2 studies above either used matching methods to maintain comparable baseline characteristics or were registered RCTs with strict inclusion and exclusion criteria and standardized intraoperative management.29,32 Moreover, the incidence of successful reperfusion in the GA group was far higher than that in the BEST14 and BASICS13 trials (at 33% and 35.1%, respectively). This may come from comprehensive and standard management. Thus we suggest that GA is as safe as CS for patients with PCS based on current evidence.
Hemodynamic instability, such as intraoperative hypotension, is more common in general anesthesia and is associated with worse outcomes.42,43,44 Although hypotension in our trial was more common with GA than with CS (53.3% vs 15.9%), the trend of the mean pressure change in both groups was close to the target value of blood pressure management (systolic blood pressure, 140-180 mm Hg).40 A prospective cohort study of PCS also showed no difference in intraoperative hypotension between GA and CS.33 Similarly, the GASS trial27 reported that hemodynamics during treatment did not yield worse outcomes after EVT. Thus standard circulation management likely plays a vital role in minimizing poor outcomes caused by hemodynamic fluctuations.
Even though the GA group in the CANVAS II trial had a higher rate of ventilation, no difference was found in pulmonary infection. Previous studies have noted that hyperventilation and hypocapnia led to cerebral vasoconstriction, decreasing cerebral blood flow and adversely affecting the ischemic penumbra zone areas.24,38 To minimize this effect, the standard partial pressure of the carbon dioxide target (partial pressure 35 to 45 mm Hg) was reached by regulating ventilation.40 These findings reemphasize the importance of standardized intraoperative respiratory management during EVT.
In our study, the patients in the GA groups had a longer time from operating room door to puncture and from operating room door to reperfusion. The median difference between the 2 time intervals was 5 and 31 minutes, respectively. The time from onset to groin puncture was found to be associated with worse functional outcomes in a retrospective study of PCS41; however, we found no difference in this time interval between the 2 arms in our study. The median difference was 52 minutes, which is far longer than that reported by RCTs20,23,24,25,27 for anterior circulation stroke, ranging from 9 to 12 minutes. A retrospective observational study of PCS is consistent with our results.31 This time delay mainly comes from induction and intubation, and a professional team with a standard workflow could reduce the time delay as much as possible.
Furthermore, the rate of conversion from CS to GA in this trial was 29.5% (13 of 44), which is higher than that in previous studies. The conversion rate in RCTs is between 7% and 8% in the anterior circulation,24,27 compared with the rate in non-RCTs, which ranges from 3.0% to 13.3% in the posterior circulation.29,30,32,33,34 The reasons for conversion in the CANVAS II trial were severe agitation, oxygen saturation less than 94%, aspiration with motion, and deterioration of consciousness. The rapid progression of the disease is followed by fast declines in consciousness and respiratory and circulatory parameters, resulting in a high conversion rate in PCS. To ensure the safety of patients, we chose to change CS to GA. Because more neurointerventionists prefer GA over CS in patients with PCS during EVT, previous observational studies may have underestimated the high conversion rate in the CS group. Thus, individualized anesthesia management requires a multidisciplinary joint decision between the anesthesiologists, neurointerventionists, and neurologists based on the patient’s condition.
Our study shows that patients who received CS achieved similar rates of favorable outcomes as those who received GA without an increased risk of mortality, pulmonary infection, deep vein thrombosis, or hemorrhagic transformation (symptomatic or asymptomatic intracranial hemorrhage). These results suggest that CS may be an option for endovascular therapy in people with acute PCS.
Limitations
Certain limitations of this study need to be mentioned. First, CANVAS II was a double-center study conducted in China, and the results may not generalize to other populations. The interventionalists and the medical team are skilled after many years of integration and standardized procedures. Second, the small sample size may restrict the study power and subgroup analysis. However, this preliminary exploratory trial involving standard intraoperative management demonstrated safety and efficacy with CS for participants with acute PCS. Third, a diverse choice of drugs and different types of GA (total intravenous anesthetics, volatile anesthetics, or both) may influence the clinical outcomes.22 This trial deliberately used the same drugs (propofol and remifentanil) in 2 groups with different regimes to test 2 ways of applying GA and CS, with intubation being the key difference.
Conclusions
In this study, patients with acute PCS during EVT, CS was not better than GA for the primary outcome of functional recovery. Moreover, CS was perhaps worse than GA for the secondary outcome of successful reperfusion.
Trial protocol
eTable1. Characteristics Supplement
eTable2. Univariate, multivariate regression and modified Poisson regression of important outcomes
eTable3. Perioperative change of systolic blood pressure
The CANVAS II Group nonauthor collaborators
Data sharing statement
References
- 1.GBD 2016 Stroke Collaborators . Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):439-458. doi: 10.1016/S1474-4422(19)30034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkhemer OA, Fransen PS, Beumer D, et al. ; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 3.Campbell BC, Mitchell PJ, Kleinig TJ, et al. ; EXTEND-IA Investigators . Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009-1018. doi: 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 5.Jovin TG, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296-2306. doi: 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 6.Bracard S, Ducrocq X, Mas JL, et al. ; THRACE investigators . Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15(11):1138-1147. doi: 10.1016/S1474-4422(16)30177-6 [DOI] [PubMed] [Google Scholar]
- 7.Muir KW, Ford GA, Messow CM, et al. ; PISTE Investigators . Endovascular therapy for acute ischaemic stroke: the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) randomised, controlled trial. J Neurol Neurosurg Psychiatry. 2017;88(1):38-44. doi: 10.1136/jnnp-2016-314117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martins SO, Mont’Alverne F, Rebello LC, et al. ; RESILIENT Investigators . Thrombectomy for stroke in the public health care system of Brazil. N Engl J Med. 2020;382(24):2316-2326. doi: 10.1056/NEJMoa2000120 [DOI] [PubMed] [Google Scholar]
- 10.LeCouffe NE, Kappelhof M, Treurniet KM, et al. ; MR CLEAN–NO IV Investigators . A randomized trial of intravenous alteplase before endovascular treatment for stroke. N Engl J Med. 2021;385(20):1833-1844. doi: 10.1056/NEJMoa2107727 [DOI] [PubMed] [Google Scholar]
- 11.Suzuki K, Matsumaru Y, Takeuchi M, et al. ; SKIP Study Investigators . Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: the SKIP randomized clinical trial. JAMA. 2021;325(3):244-253. doi: 10.1001/jama.2020.23522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zi W, Qiu Z, Li F, et al. ; DEVT Trial Investigators . Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA. 2021;325(3):234-243. doi: 10.1001/jama.2020.23523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langezaal LCM, van der Hoeven EJRJ, Mont’Alverne FJA, et al. ; BASICS Study Group . Endovascular therapy for stroke due to basilar-artery occlusion. N Engl J Med. 2021;384(20):1910-1920. doi: 10.1056/NEJMoa2030297 [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Dai Q, Ye R, et al. ; BEST Trial Investigators . Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol. 2020;19(2):115-122. doi: 10.1016/S1474-4422(19)30395-3 [DOI] [PubMed] [Google Scholar]
- 15.Tao C, Li R, Zhu Y, et al. Endovascular treatment for acute basilar artery occlusion: a multicenter randomized controlled trial (ATTENTION). Int J Stroke. 2022;17(7):815-819. doi: 10.1177/17474930221077164 [DOI] [PubMed] [Google Scholar]
- 16.Li C, Wu C, Wu L, et al. Basilar artery occlusion Chinese endovascular trial: protocol for a prospective randomized controlled study. Int J Stroke. 2022;17(6):694-697. doi: 10.1177/17474930211040923 [DOI] [PubMed] [Google Scholar]
- 17.Campbell BCV, van Zwam WH, Goyal M, et al. ; HERMES collaborators . Effect of general anaesthesia on functional outcome in patients with anterior circulation ischaemic stroke having endovascular thrombectomy versus standard care: a meta-analysis of individual patient data. Lancet Neurol. 2018;17(1):47-53. doi: 10.1016/S1474-4422(17)30407-6 [DOI] [PubMed] [Google Scholar]
- 18.McDonald JS, Brinjikji W, Rabinstein AA, Cloft HJ, Lanzino G, Kallmes DF. Conscious sedation versus general anaesthesia during mechanical thrombectomy for stroke: a propensity score analysis. J Neurointerv Surg. 2015;7(11):789-794. doi: 10.1136/neurintsurg-2014-011373 [DOI] [PubMed] [Google Scholar]
- 19.van den Berg LA, Koelman DL, Berkhemer OA, et al. ; MR CLEAN pretrial study group; Participating centers . Type of anesthesia and differences in clinical outcome after intra-arterial treatment for ischemic stroke. Stroke. 2015;46(5):1257-1262. doi: 10.1161/STROKEAHA.115.008699 [DOI] [PubMed] [Google Scholar]
- 20.Sun J, Liang F, Wu Y, et al. ; CANVAS Pilot Trial Investigators . Choice of anesthesia for endovascular treatment of acute ischemic stroke (CANVAS): results of the CANVAS pilot randomized controlled trial. J Neurosurg Anesthesiol. 2020;32(1):41-47. doi: 10.1097/ANA.0000000000000567 [DOI] [PubMed] [Google Scholar]
- 21.Campbell D, Diprose WK, Deng C, Barber PA. General anesthesia versus conscious sedation in endovascular thrombectomy for stroke: a meta-analysis of 4 randomized controlled trials. J Neurosurg Anesthesiol. 2021;33(1):21-27. doi: 10.1097/ANA.0000000000000646 [DOI] [PubMed] [Google Scholar]
- 22.Sivasankar C, Stiefel M, Miano TA, et al. Anesthetic variation and potential impact of anesthetics used during endovascular management of acute ischemic stroke. J Neurointerv Surg. 2016;8(11):1101-1106. doi: 10.1136/neurintsurg-2015-011998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schönenberger S, Uhlmann L, Hacke W, et al. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA. 2016;316(19):1986-1996. doi: 10.1001/jama.2016.16623 [DOI] [PubMed] [Google Scholar]
- 24.Löwhagen Hendén P, Rentzos A, Karlsson JE, et al. General anesthesia versus conscious sedation for endovascular treatment of acute ischemic stroke: the Anstroke trial (anesthesia during stroke). Stroke. 2017;48(6):1601-1607. doi: 10.1161/STROKEAHA.117.016554 [DOI] [PubMed] [Google Scholar]
- 25.Simonsen CZ, Yoo AJ, Sørensen LH, et al. Effect of general anesthesia and conscious sedation during endovascular therapy on infarct growth and clinical outcomes in acute ischemic stroke: a randomized clinical trial. JAMA Neurol. 2018;75(4):470-477. doi: 10.1001/jamaneurol.2017.4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren C, Xu G, Liu Y, Liu G, Wang J, Gao J. Effect of conscious sedation vs. general anesthesia on outcomes in patients undergoing mechanical thrombectomy for acute ischemic stroke: a prospective randomized clinical trial. Front Neurol. 2020;11:170. doi: 10.3389/fneur.2020.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurice A, Eugène F, Ronzière T, et al. ; GASS (General Anesthesia versus Sedation for Acute Stroke Treatment) Study Group and the French Society of Anesthesiologists (SFAR) Research Network . General anesthesia versus sedation, both with hemodynamic control, during intraarterial treatment for stroke: the GASS randomized trial. Anesthesiology. 2022;136(4):567-576. doi: 10.1097/ALN.0000000000004142 [DOI] [PubMed] [Google Scholar]
- 28.Weyland CS, Chen M, Potreck A, et al. Sedation mode during endovascular stroke treatment in the posterior circulation—is conscious sedation for eligible patients feasible? Front Neurol. 2021;12:711558. doi: 10.3389/fneur.2021.711558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jadhav AP, Bouslama M, Aghaebrahim A, et al. Monitored anesthesia care vs intubation for vertebrobasilar stroke endovascular therapy. JAMA Neurol. 2017;74(6):704-709. doi: 10.1001/jamaneurol.2017.0192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terceño M, Silva Y, Bashir S, et al. ; Catalan Stroke Code and Reperfusion Consortium (Cat-SCR) . Impact of general anesthesia on posterior circulation large vessel occlusions after endovascular thrombectomy. Int J Stroke. 2021;16(7):792-797. doi: 10.1177/1747493020976247 [DOI] [PubMed] [Google Scholar]
- 31.Du H, Tong X, Sun X, et al. Effect of anesthesia strategy during endovascular therapy on 90-day outcomes in acute basilar artery occlusion: a retrospective observational study. BMC Neurol. 2020;20(1):398. doi: 10.1186/s12883-020-01979-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li F, Wan J, Song J, et al. Impact of anesthetic strategy on outcomes for patients with acute basilar artery occlusion undergoing mechanical thrombectomy. J Neurointerv Surg. Published online November 3, 2021. doi: 10.1136/neurintsurg-2021-018000 [DOI] [PubMed] [Google Scholar]
- 33.Hu G, Shi Z, Li B, Shao W, Xu B. General anesthesia versus monitored anesthesia care during endovascular therapy for vertebrobasilar stroke. Am J Transl Res. 2021;13(3):1558-1567. [PMC free article] [PubMed] [Google Scholar]
- 34.Wu L, Jadhav AP, Chen J, et al. Local anesthesia vs general anesthesia during endovascular therapy for acute posterior circulation stroke. J Neurol Sci. 2020;416:117045. doi: 10.1016/j.jns.2020.117045 [DOI] [PubMed] [Google Scholar]
- 35.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 36.Liang F, Zhao Y, Yan X, et al. Choice of anaesthesia for endovascular treatment of acute ischaemic stroke at posterior circulation (CANVAS II): protocol for an exploratory randomised controlled study. BMJ Open. 2020;10(7):e036358. doi: 10.1136/bmjopen-2019-036358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mundiyanapurath S, Schönenberger S, Rosales ML, et al. Circulatory and respiratory parameters during acute endovascular stroke therapy in conscious sedation or general anesthesia. J Stroke Cerebrovasc Dis. 2015;24(6):1244-1249. doi: 10.1016/j.jstrokecerebrovasdis.2015.01.025 [DOI] [PubMed] [Google Scholar]
- 38.Takahashi CE, Brambrink AM, Aziz MF, et al. Association of intraprocedural blood pressure and end tidal carbon dioxide with outcome after acute stroke intervention. Neurocrit Care. 2014;20(2):202-208. doi: 10.1007/s12028-013-9921-3 [DOI] [PubMed] [Google Scholar]
- 39.Froehler MT, Fifi JT, Majid A, Bhatt A, Ouyang M, McDonagh DL. Anesthesia for endovascular treatment of acute ischemic stroke. Neurology. 2012;79(13)(suppl 1):S167-S173. doi: 10.1212/WNL.0b013e31826959c2 [DOI] [PubMed] [Google Scholar]
- 40.Sharma D, Rasmussen M, Han R, et al. Anesthetic management of endovascular treatment of acute ischemic stroke during COVID-19 pandemic: consensus statement from Society for Neuroscience in Anesthesiology & Critical Care (SNACC). J Neurosurg Anesthesiol. 2020;32(3):193-201. doi: 10.1097/ANA.0000000000000688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mokin M, Sonig A, Sananthan S, et al. Analysis of outcomes from the endovascular treatment of posterior circulation strokes in the modern era of stent retriever thrombectomy. Stroke. 2016;47:suppl 1. doi: 10.1161/str.47.suppl_1.wmp4 [DOI] [Google Scholar]
- 42.Brinjikji W, Murad MH, Rabinstein AA, Cloft HJ, Lanzino G, Kallmes DF. Conscious sedation versus general anesthesia during endovascular acute ischemic stroke treatment: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2015;36(3):525-529. doi: 10.3174/ajnr.A4159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schönenberger S, Hendén PL, Simonsen CZ, et al. Association of general anesthesia vs procedural sedation with functional outcome among patients with acute ischemic stroke undergoing thrombectomy: a systematic review and meta-analysis. JAMA. 2019;322(13):1283-1293. doi: 10.1001/jama.2019.11455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis MJ, Menon BK, Baghirzada LB, et al. ; Calgary Stroke Program . Anesthetic management and outcome in patients during endovascular therapy for acute stroke. Anesthesiology. 2012;116(2):396-405. doi: 10.1097/ALN.0b013e318242a5d2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTable1. Characteristics Supplement
eTable2. Univariate, multivariate regression and modified Poisson regression of important outcomes
eTable3. Perioperative change of systolic blood pressure
The CANVAS II Group nonauthor collaborators
Data sharing statement