Highlights
-
•
Lactobacillus metabolites exerted a dose, strain and cell line-dependent effect.
-
•
L. vaginalis and L.salivarius CFCs exhibited lowest IC50 on HeLa and SiHa cells.
-
•
Lactobacilli demonstrating inhibitory effect produced majorly l-lactic acid and H2O2.
-
•
Lactobacilli CFCs significantly restored E-cadherin and suppressed MMP9 levels.
-
•
Lactobacilli could be explored as biotherapeutics against cervical cancer.
Keywords: Lactobacillus, Cervical cancer, Probiotics, Human papillomavirus, HeLa & SiHa, E-cadherin & MMP9
Abstract
Cervical cancer is leading cause of cancer death in females worldwide. Vaginal lactobacilli colonizing cervical area are known to play an important role in maintaining cervical physiological conditions to ward away vaginal infections including bacterial vaginosis (BV) and cancer prevention. There are limited studies to study effect of Lactobacilli isolated from different sources on cervical cancer. The objective of the study was to investigate the potential of cell-free culture supernatants (CFCs) or metabolites of twelve well-characterized Lactobacillus species from different microenvironments for their anti-proliferative properties on HPV16 and HPV18 cervical cancer cells and to investigate the mechanisms of anti-proliferative and anti-metastatic activities. Lactobacillus metabolites exerted a dose, strain and cell line-dependent effect on cervical cells as demonstrated by 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay. The metabolites from vaginalis and L. salivarius exhibited the lowest half-maximal inhibitory concentration (IC50) on HeLa (131 and 167 ng/ml) respectively and SiHa (149 and 205 ng/ml) respectively. Lactobacilli demonstrating greater inhibitory effect produced majorly l-lactic acid and hydrogen peroxide (H2O2). Treatment with lactobacilli CFCs significantly upregulated E- cadherin levels in HeLa (p = 0.0451) and SiHa (p = 0.0051) cells and downregulated matrix metalloproteinase 9 (MMP9) levels in Hela cells (p = 0.0465) as measured by ELISA. Lactobacillus-derived metabolites could be explored as biotherapeutics for the control of HPV infections and cervical cancer.
Introduction
Cervical cancer is the fourth most common cancer in women worldwide with an estimated 604,000 new cases of cervical cancer reported globally in 2020, and approximately 342,000 deaths (Sung et al., 2021). Infection by high oncogenic risk- Human Papillomavirus (HPV) is the main attributable factor for cervical cancer development (Walboomers et al., 1999). Two HPV types, high-risk strains (16 and 18) cause 70 % of cervical cancers and pre-cancerous cervical lesions. The majority of the HPV infections and induced neoplastic lesions are transient and resolve spontaneously (Burd, 2013) indicating other environmental and host factors like cervicovaginal microflora may be involved in the progress of low grade squamous intraepithelial lesion (LSIL) to invasive cancer (Xie et al., 2020, Lin et al., 2022). Currently, the treatment used for cervical cancer includes chemotherapeutic interventions and radiation therapy which can result in cytotoxicity side effects to the host, recurrence and resistance to infection (Kuku et al., 2013). Hence, there is a need of enhancing the quality of life in cervical cancer patients using anticancer therapies with minimal side effects (Pourmollaei et al., 2020, Jahanshahi et al., 2020, Damani et al., 2021).
The healthy vaginal microenvironment is inhabited by lactobacilli which protect the vagina from reproductive and sexually transmitted infections. These beneficial microbes modify the cervicovaginal ecosystem by the production of antimicrobials, competition with pathogens and improving the epithelial barrier function (Graver and Wade, 2011, Spurbeck and Arvidson, 2011, Pramanick and Aranha, 2020 Dec 9). Depletion of these commensals leads to increased infectiousness by various pathogens like Gardnerella vaginalis, Prevotella, Trichomonas vaginalis, Neisseria gonorrhea and Chlamydia trachomatis (Spurbeck and Arvidson, 2011, Pramanick et al., 2022). The persistence of these infections could lead to the production of deleterious metabolites which increase the risk of oncogenic HPV and promote cervical cancer (Ho et al., 1995, Schiffman et al., 2016, Kwasniewski et al., 2018). Hence there is an indirect relationship between vaginal Lactobacillus and cervical cancer. This cycle of infection could be reversed with the restoration of the normal vaginal flora. However, the effect of lactobacilli has been demonstrated to be strain specific both in vitro (Pramanick and Aranha, 2020) and in vivo (Guo et al., 2012).
Studies have shown certain lactobacilli and their metabolites can inhibit the proliferation of cervical cancer cells and thus could play an important role in cancer prevention and treatment (Brotman et al., 2014, Yang et al., 2018, Maghsood et al., 2020). Nami et al., 2014a, Nami et al., 2014b reported that L. plantarum and L. acidophilus exhibited desirable probiotic properties and remarkable anticancer activity against human cancer cell lines, HeLa, MCF7, HT29 with no significant cytotoxic effects on normal cell line (HUVEC). Also, vaginal lactobacilli, including L. crispatus, L. rhamnosus and L. gasseri, have been shown to exert cytotoxic effects on cervical tumor cells (Nouri et al., 2016, Anton et al., 2018) but not on normal cells. Kim et al. (2015) stated that metabolites of L. casei had no significant effect on the growth of Ca Ski and HeLa cells. However, L. casei and L. paracasei strains isolated from human breast milk are shown to be effective against HeLa (Riaz Rajoka et al., 2018). Hence, the anticancer effects of different lactobacilli species from different environments on cervical cancer cell lines are still debated.
Earlier studies suggest that epithelial-mesenchymal transition (EMT) is involved in carcinogenesis, cancer progression and metastasis (Thiery, 2002). E-cadherin is the crucial factor involved in Epithelial-Mesenchymal Transition (EMT) and helps in cell connection. E-cadherin downregulation could be an important factor for cancer cell migration and metastasis (Ishiyama et al., 2010, Wang et al., 2017) and re-expression of this molecule is known to block invasiveness (Birchmeier and Behrens, 1994). In addition, clinical studies report Matrix metalloproteinase (MMP9) expression with progression of gynecological cancers. MMPs degrade various components of the ECM, including collagen, laminin, fibronectin, vitronectin, elastin and proteoglycans (Quintero-Fabián et al., 2019). Since MMPs play a critical role in cancer invasion, migration, metastasis and tumorigenesis, blocking tumor cell expression of MMPs can significantly reduce tumor invasion and metastasis in cervical cancers (Roomi et al., 2009). Studies have also shown the relationship between MMP and E-cadherin (Hsu et al., 2016, Gao et al., 2017).
The present study attempted to investigate the ability of CFCs from twelve different species of lactobacilli from different microenvironments to inhibit HPV 16 and HPV 18 infected human cervical cancer using HeLa and SiHa cell lines respectively and explore the mechanisms by which lactobacilli exerting their anti-proliferative and anti-metastatic activities. We also studied the secretion of total lactic acid, their isomers and hydrogen peroxide produced by Lactobacillus species in exerting the antiviral activity. We also explored the potential anti-metastatic effects of lactobacilli metabolites by measuring levels of the E-cadherin and MMP9 in treated cervical cells.
Materials and methods
Bacterial strains
Twelve standard Lactobacillus species obtained from the American Type Culture Collection (ATCC) described in Table 1 were used for the study.
Table 1.
Lactobacillus strains used in this study.
| Species- Present nomenclature | Source |
|---|---|
| L. salivarius subsp. salivarius (ATCC® 11741™) | saliva |
| L. reuteri (ATCC® 23272™) | Feces, human |
| Limosilactobacillus fermentum (ATCC® 9338™) | feces. |
| Lactiplantibacillus plantarum (ATCC® 8014™) | Not stated |
| L. vaginalis (ATCC® 49540™) | Vagina of patient with trichomoniasis |
| L. johnsonii (ATCC® 33200™) | Human blood |
| L. delbreckii (ATCC® 7830™) | Emmenthal Cheese |
| L. acidophilus (ATCC® 314™) | Infant feces |
| Lactobacillus gasseri (ATCC® 19992™) | Feces |
| L. jensenii (ATCC® 25258™) | Human vaginal discharge |
| L. crispatus(ATCC® 53545™) | Human stool |
| L. rhamnosus (ATCC® 9595™) | Not stated |
Culture conditions and isolation
Bacterial species stored at −80 °C in De Man, Rogosa, and Sharpe (MRS) broth (Hi-Media) containing 20 % glycerol were streaked on lactobacilli MRS agar and incubated at 37 °C for 48 h anaerobically. Each isolated colony was observed for morphological characterization. Further, the cultures of all these isolates were gram stained and checked for gram reaction and cell morphology. These cultures were propagated in the MRS broth anaerobically at 37 °C for 48 h and growth was determined by measuring OD600 nm spectrophotometrically and pH checked using HiIndicator™ pH papers (Himedia).
Preparation of the CFCs from Lactobacillus cultures
Lactobacillus isolates were inoculated in MRS broth and incubated at 37 °C for 48 h under anaerobic conditions. The MRS broth cultures at the end of the exponential growth phase were centrifuged at 8000 rpm for 10 mins. Bacterial cells pellets were washed twice with sterile PBS and concentration was adjusted to 1 at OD600 nm. These cells were further conditioned with Dulbecco’s Modified Eagle medium (DMEM) (Gibco). These cultures were incubated in a shaker for 4 h followed by static overnight incubation at 37 °C. Further, the DMEM CFCs were obtained by centrifugation at 8000 rpm for 10 min at 4 °C followed by filter sterilization of cultures using 0.22 mm syringe filters (Axiva, India). The CFCs were stored at −80 °C till tested. Sterility checking was confirmed by inoculating aliquots of CFCs on MRS agar and incubating at 37 °C for 24 h.
Quantification of protein by Bradford Assay
Total protein quantitation was carried out by Bradford’s Protein Assay. Typically, BSA (1 mg/mL) was used as a standard and a calibration curve based on the concentration of BSA (ranging from, 0.3–4 µg/ml) was used to determine the unknown protein concentration of supernatants. In a 96 well microtitre plate 50 µl of the standard/sample was mixed with 50 µl of Bradford’s reagent. The plates were further incubated for 10-15mins and read at 595 nm using a microplate reader (Synergy H1, Biotek, USA). The experiment was performed in triplicates.
Cell culture and maintenance
Human cervical cancer cell lines HeLa (HPV 18+) (ATCC-CCL-2™) and SiHa (HPV 16+) (ATCC-HTB-35™) obtained from American Type Culture Collection (ATCC) were maintained as monolayer cultures at 37 °C under 5 % CO2in DMEM medium (Gibco) supplemented with 10 % heat inactivated fetal bovine serum (FBS) (Gibco) and 1 % penicillin/streptomycin mixture (Invitrogen).
Cell cytotoxicity 1 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide (MTT) assay
Cytotoxicity effects of Lactobacillus metabolites on cervical cancer cell lines were demonstrated by tetrazolium (MTT) assay (Mosmann, 1983). Here, 2 × 104cells in 96 well microplates were treated with the filtered CFCs of Lactobacillus species at different concentrations ranging from 10 % to 100 % (v/v). Untreated cells served as control to determine the background levels of cell death under the experimental conditions used. Plates were incubated at 37 °C under 5 % CO2 for 24 h. 10 µl of MTT solution (5 mg/mL in PBS) was added and incubated further for 4 h at 37 °C. 100 µl of DMSO was added and incubated for 30 mins. The absorbance was measured at 570 nm using a microplate reader. Cell viability and cytotoxicity were determined by formula:
Detection of lactic acid
Qualitative measurement
Lactic acid producers were screened by streaking Lactobacillus isolates on the sterile MRS agar plate having Bromo-cresol purple (BCP) (Himedia, India). On incubation at 37 °C for 48 h anaerobically, lactic acid producing bacteria showed the change in the colour of the media from purple to yellow.
Quantitative measurement
Lactic acid produced by lactobacilli in CFCs was estimated by using D-/l-Lactic Acid (D-/l-Lactate) (Rapid) test kit (Megazyme). Manufacturer’s instructions were followed to carry out the assay. The experiments were performed in duplicates.
Detection of hydrogen peroxide
Qualitative measurement of H2O2
Semi quantitative assay for isolates producing Hydrogen peroxide (H2O2) was assessed using method given by Pendharkar et al (2013) with slight modifications. Briefly Lactobacillus isolates were streaked onto MRS agar plate containing 3,3′,5,5′-tetramethylbenzidine (TMB) and horseradish peroxidase (HRP) (Sigma-Aldrich). Plates were incubated in anaerobic condition for 48 h and were exposed to air for variable time period. Isolates were scored as weak (>60 min), intermediate (15–60 min) and strong producing strains (<15 min); on the basis of the time required for the blue coloration to appear.
Quantitative estimation of, H2O2
The concentration of hydrogen peroxide was determined by measuring absorbance induced with the chromophore- o-dianisidine (Sigma, USA) described by Martín and Suárez (2010).
Enzyme linked immunosorbent assay for E-cadherin and MMP9
Human E-Cadherin ELISA Kit (cat. no. ab233611, Abcam), and Human MMP9 ELISA kit (cat. no. ab100610, Abcam) were used to detect E-cadherin and MMP-9 in the culture supernatants respectively. Samples were processed according to the manufacturer’s instructions. Untreated cells served as a control to determine the background levels of e-Cadherin and MMP under the experimental conditions used.
Statistical analysis
Data analysis was performed by Graph Pad Prism 8.4.2 software. Data are represented as means ± standard deviation (SD). Differences across groups were estimated by multiple comparisons of the data as calculated by One-way analysis of variance (ANOVA) with Kruskal-Wallis testfollowed by Dunn’s multiple comparison test for comparison between species. P value of <0.05 considered statistically significant. Correlation was carried out by using Spearman’s rank correlation.
Results
Colony characteristics of the standard Lactobacillus species
Cultural characteristics of twelve well characterized reference Lactobacillus species were studied by observing the well isolated colonies that appeared on the MRS agar plate. Further, gram staining was carried out for these Lactobacillus species. Colony characteristics of lactobacilli showed variation in size, shape, margin, elevation, opacity and consistency. Gram stained Lactobacillus colonies showed Gram-positive nature along with variable morphology characteristics like short, medium, long rods, to their appearance in chains and clusters.
Determination of growth
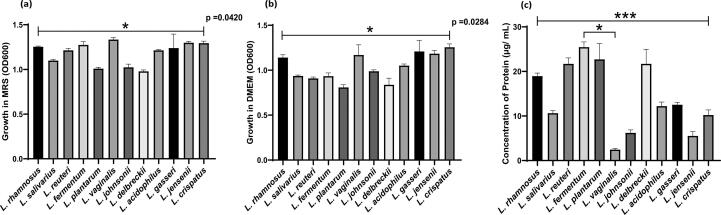
The mean growth of Lactobacillus cultures in MRS measured in 24 h was 1.2 ± 0.1247 (range 0.98–1.34) and in DMEM media was 1.04 ± 0.1532 (range 0.81–1.26) (Fig. 1a and b). Significant differences in optical density was observed between MRS and DMEM (p = 0.0317). Also significant differences were observed across species of the lactobacilli in MRS (p = 0.0420) and DMEM (p = 0.0284). L. crispatus showed maximum growth and L. plantarum showed minimum growth in DMEM (Fig. 1b).
Fig. 1.
The growth of different Lactobacillus species was measured at 600 nm in (a) MRS and (b) DMEM media (c) Protein concentration in DMEM CFCs determined by Bradford assay. Data are represented as mean ± SD, *p < 0.05, ***p < 0.001 indicating statistical significances after performing the Kruskal-Wallis test. The upper line indicates the Kruskal-Wallis test and downward line indicates Dunn’s multiple comparison test.
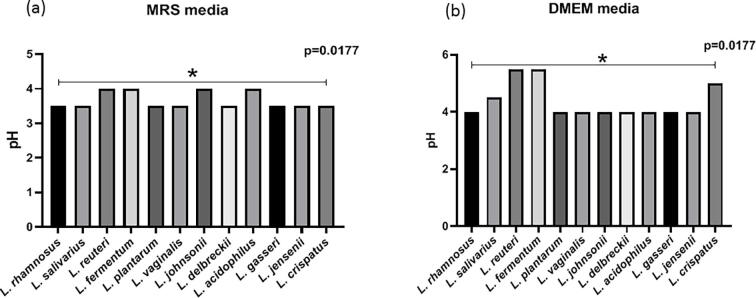
Determination of pH
Overall, all the Lactobacillus species reduced the pH of the MRS medium The mean pH of lactobacilli in MRS media was 3.67 ± 0.2 and in DMEM media was 4.37 ± 0.6 with significant difference (p = 0.0004). In MRS medium, all Lactobacillus isolates lowered pH to 3.5 except species of L. reuteri, L. fermentum, L. johnsonii and L. acidophilus, which lowered pH to 4. While in DMEM media, L. salivarius, L. crispatus, L. reuteri and L. fermentum isolates acidified media weakly. MRS and DMEM CFCs showed significant differences in pH among the species of the lactobacilli (p = 0.0177) (Supplementary Fig. 1).
Quantification of protein
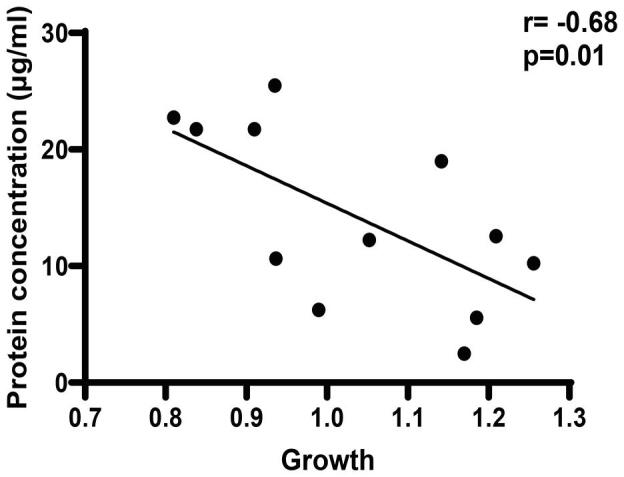
Concentration of protein in DMEM supernatants determined by Bradford Assay varies in different species of Lactobacillus (Fig. 1c). The protein concentration was observed in the range of 2.47–25.47 μg/mL. L. fermentum (25.48 ± 1.14) produced maximum amount of protein followed by L. plantarum (22.72 ± 1.06), L. reuteri (21.72 ± 1.32), and L. delbrueckii (21.72 ± 2.01) as compared to other Lactobacillus species. L. vaginalis produced less amount of protein (2.47 ± 0.25). Protein production by Lactobacillus species differed significantly among the isolates (p = 0.0005) while, significant difference was observed between the protein produced by L. fermentum and L. vaginalis (p = 0.0131). Significant negative correlation was observed between growth and protein concentration in DMEM (r = −0.68, p = 0.01) (Supplementary Fig. 2).
Antiproliferative effects of lactobacilli supernatants on cancer cells
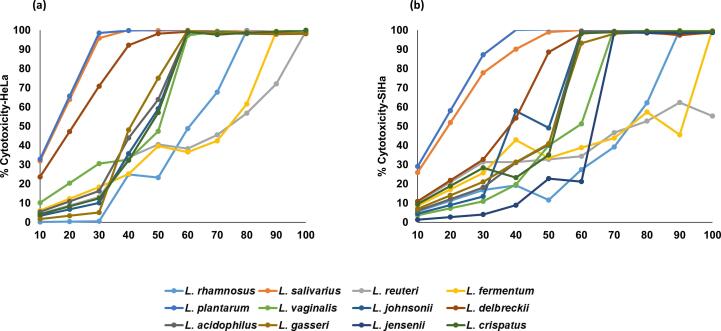
Dose dependent cytotoxicity of the CFCs was observed for both the cell lines. CFCs of the majority of the species were effective at <50 to 60 % on HeLa and SiHa respectively. L. rhamnosus, L. reuteri and L. fermentum required a higher volume of CFC on HeLa and SiHa to show the desired effect compared to other Lactobacillus species. L. plantarum and L. salivarius were potent against both cervical cancer cell lines at the concentration of 20 % (v/v) (Fig. 2). However, the metabolites from L. vaginalis and L. salivarius exhibited the lowest IC50 on HeLa (131 and 167 ng/ml) respectively and SiHa (149 and 205 ng/ml) respectively (Table 2). CFCs of lactobacilli were more active against HPV18 as compared to HPV16; however, no significant differences were observed.
Fig. 2.
Effect of cell free DMEM supernatants of Lactobacillus species (v/v) on (a) HPV18-HeLa and (b) HPV 16-SiHa cell lines in various concentrations ranging from 10 to 100 %. Cell proliferation was determined using the MTT colorimetric assay, measured at an optical density of 570 nm.
Table 2.
Protein concentrations of CFCs of different Lactobacillus species and the half maximal inhibitory concentrations (IC50) of lactobacilli CFCs on both HeLa and SiHa cells. Statistical significances were determined by using Kruskal-Wallis test.
| Lactobacillus species | Total Protein in DMEM CFCs (μg/mL) Mean ± SD |
IC50 concentration (V/V%) |
IC 50 Protein concentration (μg/mL) |
Significance P value |
||
|---|---|---|---|---|---|---|
| HeLa | SiHa | HeLa | SiHa | |||
| L. rhamnosus | 18.975 ± 0.66 | 61.488 | 75.00 | 1.167 ± 0.04 | 1.423 ± 0.05 | 0.100 |
| L. salivarius | 10.642 ± 0.56 | 15.651 | 19.27 | 0.167 ± 0.01 | 0.205 ± 0.01 | 0.100 |
| L. reuteri | 21.725 ± 1.32 | 76.889 | 80.00 | 1.670 ± 0.10 | 1.738 ± 0.11 | 0.400 |
| L. fermentum | 25.475 ± 1.14 | 75.000 | 80.00 | 1.911 ± 0.09 | 2.038 ± 0.09 | 0.200 |
| L. plantarum | 22.725 ± 1.06 | 15.227 | 17.20 | 0.346 ± 0.05 | 0.391 ± 0.06 | 0.400 |
| L. vaginalis | 2.475 ± 0.25 | 52.743 | 60.00 | 0.131 ± 0.01 | 0.149 ± 0.02 | 0.200 |
| L. johnsonii | 6.225 ± 0.66 | 42.301 | 50.00 | 0.263 ± 0.03 | 0.311 ± 0.03 | 0.200 |
| L. delbreckii | 21.725 ± 2.01 | 21.177 | 40.00 | 0.460 ± 0.07 | 0.869 ± 0.13 | 0.100 |
| L. acidophilus | 12.225 ± 0.90 | 45.568 | 52.00 | 0.557 ± 0.04 | 0.636 ± 0.05 | 0.200 |
| L. gasseri | 12.558 ± 0.52 | 41.554 | 51.50 | 0.522 ± 0.02 | 0.647 ± 0.03 | 0.100 |
| L. jensenii | 5.558 ± 0.95 | 53.000 | 60.00 | 0.295 ± 0.05 | 0.334 ± 0.06 | 0.400 |
| L. crispatus | 10.225 ± 1.14 | 50.000 | 55.00 | 0.511 ± 0.06 | 0.562 ± 0.06 | 0.400 |
| P value | 0.0005 | 0.0004 | 0.0003 | |||
Lactic acid production
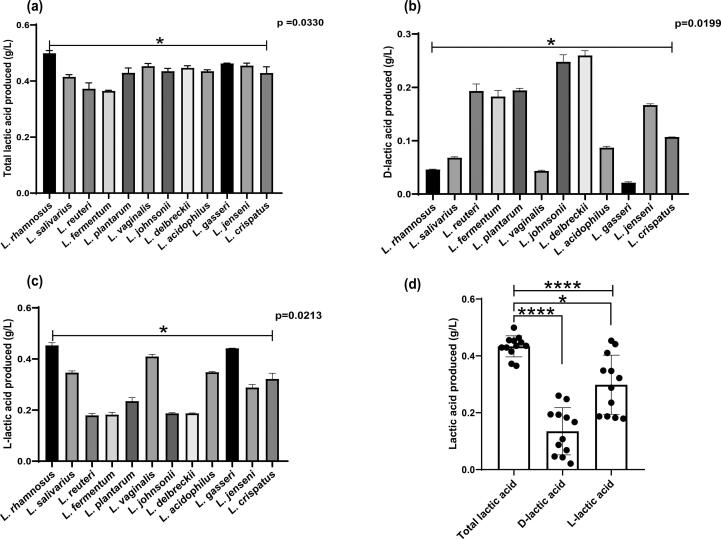
Lactic acid produced by Lactobacillus isolates was determined qualitatively on MRS-BCP agar. All lactobacilli evaluated were able to change the colour of bromocresol purple in MRS agar to yellow. The average total lactic acid in culture supernatants of all the isolates was 0.4 g/L. L. rhamnosus (0.499 g/L) produced maximum total lactic acid while L. fermentum (0.364 g/L) produced minimum total lactic acid (Fig. 3a). Notably, L. reuteri, L. fermentum, L. plantarum, L. johnsonii, and L. delbrueckii produced more d-isomer of lactic acid (Fig. 3b) whereas L. rhamnosus, L. gasseri, L. vaginalis, L. crispatus and L. salivarius produced mainly l-lactic acid (Fig. 3c). Significant differences were observed across the species of the lactobacilli, for total lactic acid (p = 0.0330), d-lactic acid (p = 0.0199), and l-lactic acid (p = 0.0213) and (Fig. 3a,b,c). Variations were observed in the production of total, D- and L- lactic acid (p = <0.0001) Quantification of the lactic acid demonstrated that, though both isomers were produced, L-lactic acid produced was more compared to D- lactic acid in the culture medium (Fig. 3 d). Thus, acidity in the medium was primarily contributed by total lactic acid and l-lactic acid was the major determinant.
Fig. 3.
Quantitative determination of (a) Total lactic acid, (b) d-lactic acid (c) l-lactic acid by different lactobacilli in DMEM CFCs after 24 h incubation at 37 °C were measured in duplicate using Lactate colorimetric kits. (d) Distribution of total lactic acid, D- and/L- isomers of lactic acid by lactobacilli in DMEM CFCs. Data are represented as mean ± SD, *P < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 showing statistical significances after using the Kruskal Wallis test. The upper line indicates the Kruskal Wallis test and downward line indicates Dunn’s multiple comparison test.
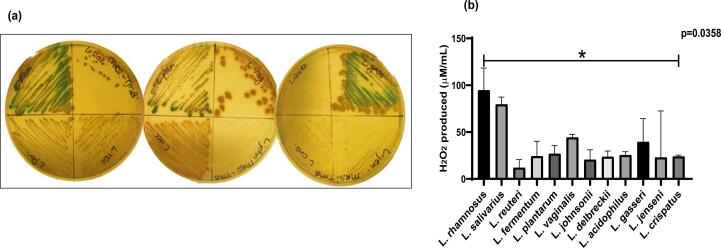
Hydrogen peroxide production
H2O2 producing lactobacilli were checked on MRS-TMB HRP agar qualitatively. L. rhamnosus, L. gasseri, L. plantarum, L. vaginalis, L. salivarius, were strong H2O2 producers.L. delbrueckii and L. fermentum were medium producers H2O2 (Fig. 4a). Significant differences were observed for H2O2 levels in the DMEM media by Lactobacillus species. Detectable concentration of H2O2 observed in the range of 12–94.67 µM/mL respectively. Of the 12 species of lactobacilli, L. rhamnosus (94.67 µM) and L. salivarius (80 µM) produced maximum H2O2 in DMEM. Significant differences were observed across the species of the lactobacilli (p = 0.0358) (Fig. 4b).
Fig. 4.
(a) Semi-quantitative H₂O₂ determination of Lactobacillus species on MRS-TMB and HRP agar media (b) Quantitative estimation of Hydrogen peroxide by Lactobacillus species in DMEM media using o-dianisidine colorimetric assay. Data represented as mean ± SD. Kruskal Wallis test was used for comparisons among species of lactobacilli *P < 0.05.
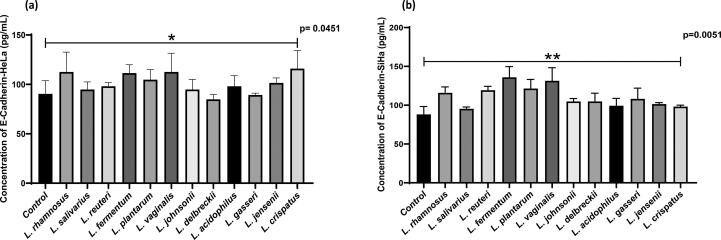
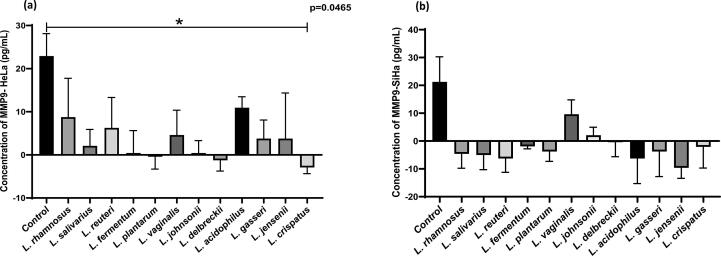
ELISA for measurement of E-Cadherin and MMP9
We explored the potential anticancer effects of lactobacilli by measuring levels of the E-cadherin and MMP9 using ELISA assays. As shown in Fig. 5(a) and (b), after 24 h, treatment with lactobacilli upregulated E- cadherin levels in HeLa (p = 0.0451) and SiHa (p = 0.0051) cells significantly when compared with their respective controls (untreated cells). Furthermore, Lactobacillus supernatants significantly downregulated MMP9 levels in Hela cells (p = 0.0465) (Fig. 6-a). No significant differences were observed in downregulation of expression of MMP9 in SiHa cells treated with Lactobacillus supernatants (Fig. 6-b).
Fig. 5.
Effect of metabolites of Lactobacillus species on E-Cadherin levels in cervical cancer cell lines (a) HeLa and (b) SiHa. Data are represented as mean ± SD, *P < 0.05, ** p < 0.01 indicating statistical significances after performing the Kruskal-Wallis test.
Fig. 6.
Effect of CFCs of Lactobacillus species on MMP9 levels in cervical cancer cell lines (a) HeLa and (b) SiHa. Data are represented as mean ± SD, *P < 0.05, indicating statistical significances after performing the Kruskal-Wallis test.
Discussion
Cervical cancer, caused by HPV is the most common gynecologic cancer among all women. Vaginal dysbiosis has been associated with risk of HPV infection and cervical cancer (Brusselaers et al., 2019). Lactobacillus as probiotics have been explored as a prophylactic and therapeutic for urogenital infections (Ballini et al., 2015, Chee et al., 2020). Antitumor activity by lactobacilli have been demonstrated in breast cancer (Motevaseli et al., 2018), human myeloid leukemia (Tuo et al., 2015) and colon cancer (Yue et al., 2020). Recent studies by Palma et al. (2018) have shown the effectiveness of long-term usage of probiotics in preventing HPV infection; whereas Ou et al. (2019) reported the absence of positive effect on HPV. However, protection of women by lactobacilli against HPV infection and cervical cancer may be species specific (Nouri et al., 2016, Brotman et al., 2014).
Several investigators have demonstrated the antiproliferative activity of metabolites of one or two Lactobacillus species on cancer cell growth (Motevaseli et al., 2013, Nouri et al., 2016, Wang et al., 2017, Nami et al., 2014a, Nami et al., 2014bb; Kim et al., 2015). The effect of lactobacilli may be mediated by cells, or their secreted products (Maghsood et al., 2020). To our knowledge, this is the first study investigating the effect of twelve different Lactobacillus species, commonly described in the vaginal environment, and isolated from different sources for their antiproliferative activity on HPV 16 and HPV18 positive cervical cancer cells. HPV 16 and 18 are well-established precursors for cervical cancers, anogenital cancers, oropharyngeal and non-oropharyngeal squamous cell carcinomas (Kobayashi et al., 2018).
Distinctly, these CFCs exerted cytotoxic effects in concentration dependent manner, a similar concentration dependent effect observed by Tiptiri-Kourpeti et al. (2016) on colon carcinoma cells by L. casei. Though all species exerted antiproliferative effect on cervical cells, selective species were more efficient in their cytotoxicity. Our studies including that of others (Chuah et al., 2019, Happel et al., 2020, Pramanick and Aranha, 2020 Dec 9) have demonstrated different strains of the same species also vary in their functional properties, hence investigation of several species need to be undertaken to identify the promising probiotic for particular function. Studies have shown that probiotics isolated from different environments or from the same environmental niche have varying functional properties ((Bazireh et al., 2020, Kahraman et al., 2022). Our study further showed HeLa cells were more sensitive than SiHa cells; likewise, sensitivity of HeLa to chemotherapeutic agents has been observed by Xu et al. (2012). This differential sensitivity of cells to anticancer drugs could be correlated to p21Waf1/Cip1 levels (Funaoka et al., 1996) or due to differences in bacterial genome versatility and diversity among the strains (Truong et al., 2017, Sela et al., 2018). Whole genome sequencing of these isolates would further give a better insight of the differences in genomic diversity and validate the functional properties of different species.
Among the lactobacilli used in our study, L. plantarum and L. salivarius showed the maximum antiproliferative activity when crude supernatant was used for evaluating antiproliferative activity. The bioactivity is attributed due to the cumulative effects of lactic acid, hydrogen peroxide, bacteriocins, biosurfactants and exopolysaccharides in crude supernatant. CFCs from different Lactobacillus species showed variations in the amounts of protein in different species. When protein content was evaluated for antiproliferative activity it was observed that L. salivarius and L. vaginalis were effective at lower protein concentrations. Notably L. vaginalis is commonly seen in vagina whereas L. salivarius is seen in the oral, intestine and female reproductive tract. Interestingly, HPV is implicated in cervical, anal and oropharyngeal cancers (Schiffman et al., 2016). L. salivarius and L. vaginalis are the microbiota commonly identified in the vagina of healthy women and known to play a protective role against urogenital infections (Madhivanan et al., 2014, Pino et al., 2019, Pramanick and Aranha, 2020 Dec 9). So it would be interesting to evaluate the indigenous microbiota of healthy individuals for their protective activity against HPV cancers of oral and cervico-vaginal origin.
The antiproliferative activity of lactobacilli studied was independent of pH and protein. Study by Motevaseli et al. (2013) reported anticancer activity was found to be independent of pH. Witkin and Linhares (2016) stated that lactobacilli produce both isomers of lactic acid. Our results showed that, though both isomers were produced, levels of l-isomer of lactic acid was more than d-isomer of lactic acid. l-lactic acid has been found to be 17-fold more potent than d-lactic acid in inactivating HIVBa-L in vitro (Aldunate et al., 2013). Likewise, the L.isomer of Lactic acid appears to be more bioactive than d-lactic acid as most of the isolates exerting greater bioactivity were l-lactic acid producers. It would be useful to investigate the two isomers in pure form for their anti-proliferative activity on cervical cancer cells.
Hydrogen peroxide producing lactobacilli are critical in maintaining a healthy vaginal ecosystem. Earlier experimental studies showed that women with H2O2 producing lactobacilli were at lower risk of dysbiosis (Mitchell et al., 2015). Production of H2O2 differed within species of Lactobacillus; L. plantarum, L. vaginalis, and L. salivarius were characteristic in producing more H2O2 in MRS media and exerting anticancer activity.
E-cadherin is 12 kDa calcium dependent membrane glycoprotein, has notable physiological activities regarding the cell–cell adhesion, structural integrity and epithelial tissue polarity (Li et al., 2017). Previous research has demonstrated that invasion and metastasis are important for tumor progression which results in the reduction of E-cadherin in cancers (Kourtidis et al., 2017). Increasing evidences has shown that E-cadherin levels are usually downregulated in several human cancers, including oral squamous carcinoma, lung and skin cancer, as well as cervical cancer. Hence, depletion of E-cadherin levels has been considered as a cause of the poor prognosis (Dohadwala et al., 2006). Our study showed that Lactobacillus supernatants notably upregulated E-cadherin levels in cervical cancer cells, indicated that this effect may be a factor responsible for anticancer effects in cervical cancer cells.
Matrix metalloproteinases (MMPs) are a family of zinc- and calcium-dependent proteolytic enzymes. These enzymes are normally involved in the breakdown of the extracellular matrix within the context of physiological tissue remodelling and angiogenesis which have been linked to the aggressiveness of gynaecological cancers such as cervical cancer (Liu et al., 2018) MMPs-mediated degradation of extracellular matrix is strongly implicated in the invasion and metastasis of malignant cells. The expression of MMPs is elevated in some carcinomas and accelerates tumor progression (Quintero-Fabián et al., 2019). We found that treatment of cervical cancer cells with lactobacilli supernatants downregulated MMP9 concentration when compared with control cells. Therefore, the results suggested that lactobacilli metabolites may have the potential to inhibit cell migration and invasion in cervical cancer cells via regulation of EMT-associated factors. However, this needs to be confirmed using suitable cell migrations assays. Besides, E-cadherin and MMP9 detection in samples of patient treated with lactobacilli would validate and strengthen our findings.
Thus, our study shows that Lactobacillus supernatants could be explored as anti-proliferative and anti-metastatic agents. Earlier studies have also confirmed that Lactobacillus secreted metabolites and cell components also have probiotic properties (Maghsood et al., 2020, Teame et al., 2020). Though there is considerable evidence supporting the potential role of probiotic live LAB cells in improving health being of individuals, it is a challenge to effectively deliver the probiotic in the formulation without compromising the viability of probiotics (Terpou et al., 2019). Hence targeting against infections and cancers through use of probiotic metabolites could be an option that could be further focused and explored.
Further work is required to study the other mechanisms through which lactobacilli exhibits anti-cancer effects on expression of E6 and E7 oncogenes, and cancer related-genes and pathways. Also, the different specific components of CFCs like exopolysaccharides, biosurfactants could be isolated and evaluated for their antitumor activity. Nonetheless, our study showed the potential of anti-cervical cancer activity exhibited by different Lactobacillus species on HPV 16 and 18 cervical cancer cells. Studies of the immunologic responses, in vivo effects in cancer models can be further explored.
Conclusion
Anticancer activity of the CFCs from different Lactobacillus species evaluated was species specific and cell line specific. These metabolites could be used in isolation or integrated as prebiotics with beneficial probiotic bacteria as an effective adjunct strategy for cervical cancer. Further the metabolites could be explored as prophylaxis to control vaginal dysbiosis and thus modulate the vaginal microbiota to prevent transition from neoplasia to cervical cancer.
Funding
This research received funding from the Department of Health Research [Grant No. R. 11012/12/2018-HR] for carrying out the work.
CRediT authorship contribution statement
Krupali Pawar: Investigation, Formal analysis, Writing – original draft. Clara Aranha: Conceptualization, Methodology, Supervision, Writing – review & editing, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We express our gratitude to the Director (ICMR-NIRRH) for constant guidance and encouragement. The laboratory is funded by grants from Indian Council of Medical Research (ICMR), Govt. of India (Grant No. R.11012/12/2018-HR). The manuscript bears the NIRRH ID-RA/1111/08-2021.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crtox.2022.100088.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Supplementary Fig. 2.
References
- Aldunate M., Tyssen D., Johnson A., Zakir T., Sonza S., Moench T., Cone R., Tachedjian G. Vaginal concentrations of lactic acid potently inactivate HIV. J. Antimicrob. Chemother. 2013;68(9):2015–2025. doi: 10.1093/jac/dkt156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton L., Sierra L.-J., DeVine A., Barila G., Heiser L., Brown A.G., Elovitz M.A. Common cervicovaginal microbial supernatants alter cervical epithelial function: mechanisms by which Lactobacillus crispatus contributes to cervical health. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballini A., Santacroce L., Cantore S., Bottalico L., Dipalma G., Vito D., Saini R., Inchingolo F. Probiotics improve urogenital health in women. Open Access Maced. J. Med. Sci. 2015;6(10):1845–1850. doi: 10.3889/oamjms.2018.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazireh H., Shariati P., Azimzadeh Jamalkandi S., Ahmadi A., Boroumand M.A. Isolation of novel probiotic lactobacillus and enterococcus strains from human salivary and fecal sources. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.597946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier W., Behrens J. Review Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198(1):11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Brotman R.M., Shardell M.D., Gajer P., Tracy J.K., Zenilman J.M., Ravel J., Gravitt P.E. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J. Infect. Dis. 2014;210(11):1723–1733. doi: 10.1093/infdis/jiu330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusselaers N., Shrestha S., van de Wijgert J., Verstraelen H. Vaginal dysbiosis and the risk of human papillomavirus and cervical cancer: systematic review and meta-analysis. Am J Obstet Gynecol. 2019;221(1):9–18.e8. doi: 10.1016/j.ajog.2018.12.011. [DOI] [PubMed] [Google Scholar]
- Burd E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2013;16(1):1–17. doi: 10.1128/cmr.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee W.J.Y., Chew S.Y., Than L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb Cell Fact. 2020;19:203. doi: 10.1186/s12934-020-01464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah L.O., Foo H.L., Loh T.C., Mohammed Alitheen N.B., Yeap S.K., Abdul Mutalib N.E., Abdul Rahim R., Yusoff K. Postbiotic metabolites produced by Lactobacillus plantarum strains exert selective cytotoxicity effects on cancer cells. BMC Complement. Altern. Med. 2019;19(1):114. doi: 10.1186/s12906-019-2528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damani M., Baxi K., Aranha C., Sawarkar S.P. Recent advances in herbal drug nanocarriers against cervical cancer. Critical Reviews™ in Therapeutic Drug Carrier Systems. 2021;38(1):37–78. doi: 10.1615/CritRevTherDrugCarrierSyst.2020034170. [DOI] [Google Scholar]
- Dohadwala, M., Yang, S. C., Luo, J., Sharma, S., Batra, R. K., Huang, M., Lin, Y., Goodglick, L., Krysan, K., Fishbein, M. C., Hong, L., Lai, C., Cameron, R. B., Gemmill, R. M., Drabkin, H. A., & Dubinett, S. M. (2006). Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin E(2) induces transcriptional repressors ZEB1 and snail in non-small cell lung cancer. Cancer Res., 66(10), 5338–5345. https://doi.org/10.1158/0008-5472.CAN-05-3635. [DOI] [PubMed]
- Funaoka K., Shindoh M., Yamashita T., Fujinaga K., Amemiya A., Totsukaa Y. High-risk HPV-CIN with the p21Waf1/Cip1 level. Cancer Lett. 1996;108(1):15–23. doi: 10.1016/s0304-3835(96)04362-5. [DOI] [PubMed] [Google Scholar]
- Gao, H., Lan, X., Li, S., & Xue, Y. (2017). Relationships of MMP-9, E-cadherin, and VEGF expression with clinicopathological features and response to chemosensitivity in gastric cancer. Tumor Biology. https://doi.org/10.1177/1010428317698368. [DOI] [PubMed]
- Graver M.A., Wade J.J. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann. Clin. Microbiol. Antimicrob. 2011;10:8. doi: 10.1186/1476-0711-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X.H., Zhao Z.D., Nam H.M., Kim J.M. Comparative evaluation of three Lactobacilli with strain-specific activities for rats when supplied in drinking water. Antonie Van Leeuwenhoek. 2012;102(4):561–568. doi: 10.1007/s10482-012-9751-x. [DOI] [PubMed] [Google Scholar]
- Happel A.-U., Kullin B., Gamieldien H., Wentzel N., Zauchenberger C.Z., Jaspan H.B., Dabee S., Barnabas S.L., Jaumdally S.Z., Dietrich J., Gray G., Bekker L.-G., Froissart R., Passmore J.-A., Lagenaur L.A. Exploring potential of vaginal Lactobacillus isolates from South African women for enhancing treatment for bacterial vaginosis. PLoS Pathog. 2020;16(6) doi: 10.1371/journal.ppat.1008559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho G.Y.F., Burk R.D., Klein S., Kadish A.S., Chang C.J., Palan P., Basu J., Tachezy R., Lewis R., Romney S. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. JNCI: J. Natl. Cancer Inst. 1995;87(18):1365–1371. doi: 10.1093/jnci/87.18.1365. [DOI] [PubMed] [Google Scholar]
- Hsu C.-C., Huang S.-F., Wang J.-S., Chu W.-K., Nien J.-E., Chen W.-S., Chow S.-E. Interplay of N-Cadherin and matrix metalloproteinase 9 enhances human nasopharyngeal carcinoma cell invasion. BMC Cancer. 2016;16(1) doi: 10.1186/s12885-016-2846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiyama N., Lee S.-H., Liu S., Li G.-Y., Smith M.J., Reichardt L.F., Ikura M. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell-cell adhesion. Cell. 2010;141(1):117–128. doi: 10.1016/j.cell.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M., Maleki Dana P., Badehnoosh B., Asemi Z., Hallajzadeh J., Mansournia M.A., Yousefi B., Moazzami B., Chaichian S. Anti-tumor activities of probiotics in cervical cancer. J. Ovarian Res. 2020;13(1) doi: 10.1186/s13048-020-00668-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahraman M., Karahan A.G., Terzioğlu M.E. Characterization of some microorganisms from human stool samples and determination of their effects on CT26 colorectal carcinoma cell line. Curr. Microbiol. 2022;79(8):225. doi: 10.1007/s00284-022-02915-4. [DOI] [PubMed] [Google Scholar]
- Kim S.-N., Lee W.M., Park K.S., Kim J.B., Han D.J., Bae J. The effect of Lactobacillus casei extract on cervical cancer cell lines. Współczesna Onkologia. 2015;4:306–312. doi: 10.5114/wo.2014.45292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Hisamatsu K., Suzui N., Hara A., Tomita H., Miyazaki T. A Review of HPV-related head and neck cancer. J. Clin. Med. 2018;7(9):241. doi: 10.3390/jcm7090241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtidis A., Lu R., Pence L.J., Anastasiadis P.Z. A central role for cadherin signaling in cancer. Exp. Cell Res. 2017;358:78–85. doi: 10.1016/j.yexcr.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuku S., Fragkos C., McCormack M., Forbes A. Radiation-induced bowel injury: the impact of radiotherapy on survivorship after treatment for gynaecological cancers. Br J Cancer. 2013;109(6):1504–1512. doi: 10.1038/bjc.2013.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwasniewski W., Wolun-Cholewa M., Kotarski J., Warchol W., Kuzma D., Kwasniewska A., Gozdzicka-Jozefiak A. Microbiota dysbiosis is associated with HPV-induced cervical carcinogenesis. Oncol. Lett. 2018;16:7035–7047. doi: 10.3892/ol.2018.9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z., Yin, S., Zhang, L., Liu, W., & Chen, B. (2017). Prognostic value of reduced E-cadherin expression in breast cancer: a meta-analysis. Oncotarget, 8(10), 16445–16455. https://doi.org/10.18632/oncotarget.14860. [DOI] [PMC free article] [PubMed]
- Lin W., Zhang Q., Chen Y., Dong B., Xue H., Lei H., Lu Y., Wei X., Sun P. Changes of the vaginal microbiota in HPV infection and cervical intraepithelial neoplasia: a cross-sectional analysis. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-06731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Li Y., Hu S., Chen Y., Gao L., Liu D., Guo H., Yang Y. Clinical significance of matrix metalloproteinase-2 in endometrial cancer: A systematic review and meta-analysis. Medicine. 2018;97(29):e10994. doi: 10.1097/MD.0000000000010994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhivanan P., Raphael E., Rumphs A., Krupp K., Ravi K., Srinivas V., Arun A., Reingold A.L., Klausner J.D., Riley L.W. Characterization of culturable vaginal Lactobacillus species among women with and without bacterial vaginosis from the United States and India: a cross-sectional study. J. Med. Microbiol. 2014;63(Pt 7):931–935. doi: 10.1099/jmm.0.073080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghsood F., Johari B., Rohani M., Madanchi H., Saltanatpour Z., Kadivar M. Anti-proliferative and anti-metastatic potential of high molecular weight secretory molecules from probiotic Lactobacillus reuteri cell-free supernatant against human colon cancer stem-like cells (HT29-ShE) Int. J. Pept. Res. Ther. 2020;26(4):2619–2631. [Google Scholar]
- Martín R., Suárez J.E. Biosynthesis and degradation of H2O2 by vaginal lactobacilli. Appl. Environ. Microbiol. 2010;76(2):400–405. doi: 10.1128/AEM.01631-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C., Fredricks D., Agnew K., Hitti J. Hydrogen peroxide-producing lactobacilli are associated with lower levels of vaginal interleukin-1β, independent of bacterial vaginosis. Sex. Transm. Dis. 2015;42(7):358–363. doi: 10.1097/OLQ.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Motevaseli E., Shirzad M., Akrami S.M., Mousavi A.S., Mirsalehian A., Modarressi M.H. Normal and tumour cervical cells respond differently to vaginal lactobacilli, independent of pH and lactate. J. Med. Microbiol. 2013;62:1065–1072. doi: 10.1099/jmm.0.057521-0. [DOI] [PubMed] [Google Scholar]
- Motevaseli E., Khorramizadeh M.R., Hadjati J., Bonab S.F., Eslami S., Ghafouri-Fard S. Investigation of antitumor effects of Lactobacillus crispatus in experimental model of breast cancer in BALB/c mice. Immunotherapy. 2018;10(2):119–129. doi: 10.2217/imt-2017-0088. [DOI] [PubMed] [Google Scholar]
- Nami Y., Abdullah N., Haghshenas B., Radiah D., Rosli R., Khosroushahi A.Y. Assessment of probiotic potential and anticancer activity of newly isolated vaginal bacterium Lactobacillus plantarum5BL. Microbiol. Immunol. 2014;58(9):492–502. doi: 10.1111/1348-0421.12175. [DOI] [PubMed] [Google Scholar]
- Nami Y., Abdullah N., Haghshenas B., Radiah D., Rosli R., Khosroushahi A.Y. Probiotic potential and biotherapeutic effects of newly isolated vaginal Lactobacillus acidophilus 36YL strain on cancer cells. Anaerobe. 2014;28:29–36. doi: 10.1016/j.anaerobe.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Nouri Z, Karami F, Neyazi N, et al. Dual Anti-Metastatic and Anti-Proliferative Activity Assessment of Two Probiotics on HeLa and HT-29 Cell Lines. Cell J. 2016;18(2):127-134. https://doi.org/10.22074/cellj.2016.4307. [DOI] [PMC free article] [PubMed]
- Ou Y.-C., Fu H.-C., Tseng C.-W., Wu C.-H., Tsai C.-C., Lin H. The influence of probiotics on genital high-risk human papilloma virus clearance and quality of cervical smear: a randomized placebo-controlled trial. BMC Women's Health. 2019;19(1) doi: 10.1186/s12905-019-0798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E., Recine N., Domenici L., Giorgini M., Pierangeli A., Panici P.B. Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: a promising solution against HPV-infection. BMC Infect. Dis. 2018;18(1):13. doi: 10.1186/s12879-017-2938-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendharkar S, Magopane T, Larsson PG, de Bruyn G, Gray GE, Hammarström L, & Marcotte H. Identification and characterisation of vaginal lactobacilli from South African women. BMC infectious diseases, 2013;13, 43.. https://doi.org/org/10.1186/1471-2334-13-43. [DOI] [PMC free article] [PubMed]
- Pino A., Bartolo E., Caggia C., Cianci A., Randazzo C.L. Detection of vaginal lactobacilli as probiotic candidates. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-40304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourmollaei S., Barzegari A., Farshbaf-Khalili A., Nouri M., Fattahi A., Shahnazi M., Dittrich R. Anticancer effect of bacteria on cervical cancer: Molecular aspects and therapeutic implications. Life Sci. 2020;246:117413. doi: 10.1016/j.lfs.2020.117413. [DOI] [PubMed] [Google Scholar]
- Pramanick R., Aranha C. Distinct functional traits of lactobacilli from women with asymptomatic bacterial vaginosis and normal microbiota. Microorganisms. 2020;8(12):1949. doi: 10.3390/microorganisms8121949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramanick R., Nathani N., Warke H., Mayadeo N., Aranha C. Vaginal dysbiotic microbiome in women with no symptoms of genital infections. Front. Cell. Infect. Microbiol. 2022;11 doi: 10.3389/fcimb.2021.760459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero-Fabián S., Arreola R., Becerril-Villanueva E., Torres-Romero J.C., Arana-Argáez V., Lara-Riegos J., Ramírez-Camacho M.A., Alvarez-Sánchez M.E. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019 doi: 10.3389/fonc.2019.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP Review Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002 Jun; 2(6):442-54. [DOI] [PubMed]
- Riaz Rajoka M.S., Zhao H., Lu Y., Lian Z., Li N.a., Hussain N., Shao D., Jin M., Li Q.i., Shi J. Anticancer potential against cervix cancer (HeLa) cell line of probiotic Lactobacillus casei and Lactobacillus paracasei strains isolated from human breast milk. Food Funct. 2018;9(5):2705–2715. doi: 10.1039/c8fo00547h. [DOI] [PubMed] [Google Scholar]
- Roomi M.W., Monterrey J.C., Kalinovsky T., Rath M., Niedzwiecki A. Patterns of MMP-2 and MMP-9 expression in human cancer cell lines. Oncol. Rep. 2009;21(5):1323–1333. doi: 10.3892/or_00000358. [DOI] [PubMed] [Google Scholar]
- Schiffman M., Doorbar J., Wentzensen N., de Sanjosé S., Fakhry C., Monk B.J., Stanley M.A., Franceschi S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primers. 2016;2(1) doi: 10.1038/nrdp.2016.86. [DOI] [PubMed] [Google Scholar]
- Sela U., Euler C.W., Correa da Rosa J., Fischetti V.A., Fowler V.G. Strains of bacterial species induce a greatly varied acute adaptive immune response: The contribution of the accessory genome. PLoS Pathog. 2018;14(1):e1006726. doi: 10.1371/journal.ppat.1006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurbeck R.R., Arvidson C.G. Lactobacilli at the front line of defense against vaginally acquired infections. Future Microbiol. 2011;6(5):567–582. doi: 10.2217/fmb.11.36. [DOI] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- T. Teame, A. Wang, M. Xie, Z. Zhang, Y. Yang, Q. Ding, C. Gao, R.E. Olsen, C. Ran, Z. Zhou, Paraprobiotics and Postbiotics of Probiotic Lactobacilli, Their Positive Effects on the Host and Action Mechanisms: A Review, Front. Nutr. 7. [DOI] [PMC free article] [PubMed]
- Terpou A., Papadaki A., Lappa I.K., Kachrimanidou V., Bosnea L.A., Kopsahelis N. Probiotics in food systems: significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients. 2019;11(7):1591. doi: 10.3390/nu11071591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiptiri-Kourpeti A., Spyridopoulou K., Santarmaki V., Aindelis G., Tompoulidou E., Lamprianidou E.E., Saxami G., Ypsilantis P., Lampri E.S., Simopoulos C., Kotsianidis I., Galanis A., Kourkoutas Y., Dimitrellou D., Chlichlia K., Nychas G.-J. Lactobacillus casei exerts anti-proliferative effects accompanied by apoptotic cell death and up-regulation of TRAIL in colon carcinoma cells. PLoS ONE. 2016;11(2) doi: 10.1371/journal.pone.0147960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong D.T., Tett A., Pasolli E., Huttenhower C., Segata N. Huttenhower C & Segata N Microbial strain-level population structure and genetic diversity from metagenomes. Genome Res. 2017;27(4):626–638. doi: 10.1101/gr.216242.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo Y., Jiang S., Qian F., Mu G., Liu P., Guo Y., Ma C. Short communication: Antiproliferative effect of 8 different Lactobacillus strains on K562 cells. J Dairy Sci. 2015;98(1):106–110. doi: 10.3168/jds.2014-8767. [DOI] [PubMed] [Google Scholar]
- Walboomers J.M.M., Jacobs M.V., Manos M.M., et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Wang KD, Xu DJ, Wang BY, Yan DH, Lv, Z., & Su, J.-R. Inhibitory Effect of Vaginal Lactobacillus Supernatants on Cervical Cancer Cells. Probiotics and Antimicrobial Proteins, 2017;10(2), 236–242. https://doi.org/1010.1007/s12602-017-9339-x. [DOI] [PubMed]
- Witkin S, & Linhares I. Why do lactobacilli dominate the human vaginal microbiota? BJOG: An International Journal of Obstetrics & Gynaecology, 2016; 124(4), 606–611. https://doi.org/1010.1111/1471-0528.14390. [DOI] [PubMed]
- Xie Y., Feng Y., Li W., Zhan F., Huang G., Hu H., Xiong Y., Tan B., Chen T. Revealing the disturbed vaginal micobiota caused by cervical cancer using high-throughput sequencing technology. Front Cell Infect Microbiol. 2020;7(10) doi: 10.3389/fcimb.2020.538336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Meng Q., Chong Y., Jiao Y., Zhao L., Rosen E.M., Fan S. Sanguinarine inhibits growth of human cervical cancer cells through the induction of apoptosis. Oncol. Rep. 2012;28:2264–2270. doi: 10.3892/or.2012.2024. [DOI] [PubMed] [Google Scholar]
- Yang X., Da M., Zhang W., Qi Q., Zhang C., Han S. Role of Lactobacillus in cervical cancer. Cancer Manage. Res. 2018;10:1219–1229. doi: 10.2147/cmar.s165228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y.-C., Yang B.-Y., Lu J., Zhang S.-W., Liu L., Nassar K., Xu X.-X., Pang X.-Y., Lv J.-P. Metabolite secretions of Lactobacillus plantarum YYC-3 may inhibit colon cancer cell metastasis by suppressing the VEGF-MMP2/9 signaling pathway. Microb. Cell Fact. 2020;19(1) doi: 10.1186/s12934-020-01466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]