Abstract
Purpose
To compare the effect of three commonly prescribed anti-inflammatory eye drops on corneal epithelial cells in vitro.
Methods
Three different lines of human corneal epithelial cells were tested: primary cells cultured from donor tissue, commercially available primary cells, and immortalized cells. Cells were seeded on 96-well plates and treated with the following eye drops: cyclosporine 0.05%, lifitegrast 5%, and tacrolimus 0.03% or 0.1%. Exposure times tested were 30 seconds, 1 minute, 2 minutes, 1 hour, 2 hours, 4 hours, and 24 hours. Brightfield images and viability assays were analyzed 48 to 72 hours after the initiation of treatments. At least five replicates were tested per drug and time exposure.
Results
Commercially obtained primary cells showed reduced viability following 1 hour with tacrolimus 0.1% (8%; P = 0.043%) and 4 hours with tacrolimus 0.03% (17%; P = 0.042%). Lifitegrast exposure reduced primary cell viability after 4 hours (10%; P = 0.042). Cell viability in primary cells was not deleteriously affected following exposure to cyclosporine for up to 4 hours. A similar trend was observed in both primary cells cultured from donor tissue and immortalized human corneal epithelial cells, demonstrating greater decreases in cell viability in tacrolimus compared to lifitegrast and cyclosporine. Light microscopy imaging for analysis of cell morphology and confluence supported the results.
Conclusions
Tacrolimus showed the highest impact on corneal epithelium survival in vitro, and cyclosporine proved the most protective.
Translational Relevance
Comparing anti-inflammatory eye drops on corneal epithelial cells in vitro may inform eye drop selection and development for clinical purposes.
Keywords: dry eye disease, corneal epithelial cells, anti-inflammatory, cyclosporine, tacrolimus, lifitegrast
Introduction
One common condition associated with inflammation of the ocular surface is dry eye disease (DED). Dry eye–associated inflammation can lead to a variety of symptoms, including ocular discomfort, visual disturbances, and red eye.1 The armamentarium of anti-inflammatory eye drops for the treatment of DED is wide and constantly evolving.2,3 The principal commercially available anti-inflammatory eye drops for the treatment of DED in the United States, cyclosporine (CsA) ophthalmic emulsion 0.05% and lifitegrast ophthalmic solution 5.0%, may be interchangeably prescribed. Tacrolimus has been well established in ophthalmology for the treatment of many conditions, including uveitis, corneal transplant rejection, and keratoconjunctivitis.4 For DED, treatment using tacrolimus initially started with off-label use of its skin ointment. Only more recently has its efficacy been explored in clinical trials and experimental studies and found to improve the status of the ocular surface in patients with Sjögren's syndrome–related DED.5,6
Studies comparing the effect of the above eye drops on corneal cells in vitro, however, are scarce.7 Although these drops have clinically been found to be protective and reduce corneal damage in DED patients,8–11 treatment-related discomfort such as instillation site burning and/or stinging sensation may limit patient adherence with treatment.5–7,12–17 Clinically, the mechanism underlying these reported adverse effects is not well understood, as these symptoms are not typically accompanied by corresponding changes from baseline on slit-lamp examination.18 Therefore, it is important to explore the impact of these drugs on corneal cells at a microscopic level to investigate potential causes such as epithelial cell toxicity.
The epithelial cytotoxic effect of eye drops such as benzalkonium chloride–preserved anti-glaucoma medications19,20 and natural tear substitutes21 has been thoroughly investigated utilizing cultured human ocular surface cells, as well as in vivo confocal microscopy.22,23 Studies comparatively analyzing the in vitro or in vivo effect of anti-inflammatory dry eye drops on human ocular surface cells, however, are limited. Our study aimed to evaluate and compare the effect of CsA, lifitegrast, and tacrolimus eye drops in an in vitro monolayer model of primary and immortalized human corneal epithelial (HCE) cells.
Materials and Methods
Donor Tissue
Human donor corneas, authorized for use for both clinical and research purposes by the decedents’ families, were obtained from the San Diego Eye Bank (San Diego, CA). Unutilized corneoscleral rims were collected after trephination of full-thickness grafts for transplant surgery. Tissue was then preserved in OptiSol-GS (Bausch & Lomb, Rochester, NY) at 4°C for up to 72 hours until processing. Eleven corneas from 10 donors were used for this study. Donors’ ages ranged from 48 to 78 years, with a median of 70.5 years.
Primary Cell Cultures
Primary HCE cells were either cultured from donor tissues or obtained from the American Type Culture Collection (PCS-700-010; ATCC, Manassas, VA). For HCE cell cultures from donor tissues, the corneoscleral donor tissue rims collected as described previously were washed in phosphate-buffered saline (PBS, 21-040-CM; Corning, Inc., Corning, NY) and subsequently incubated at 37°C for 1 hour in 1-mg/mL Dispase II (D4693; MilliporeSigma, Burlington, MA) dissolved in HyClone Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12, SH30023.01; Cytiva, Marlborough, MA). The epithelium was stripped off with gentle scraping using a surgical knife (ExactEtch; Cytosol Ophthalmics, Lenoir, NC) from the limbus to the center into PBS. The tissue was centrifuged at 1000 revolutions per minute for 5 minutes, and the cells were suspended in 1 mL corneal epithelial cell medium (PCS-700-030; ATCC) supplemented with corneal epithelial cell growth kit medium (PCS-700-040; ATCC), 33-µM Phenol red (P0290-100ML; MilliporeSigma), and 0.1× Antibiotic-Antimycotic (15240062; Thermo Fisher Scientific, Waltham, MA). Cells were plated on a 12-well plate and cultured at 37°C with 5% CO2 in a humidified incubator until 80% to 100% confluent; the medium was changed three times a week. Then, cells were transferred to a six-well plate and finally passaged onto a 96-well plate for experiments. For passage, cells were digested by TrypLE (12604013; Thermo Fisher Scientific) for 5 minutes. All plates were coated with FNC Coating Mix (Athena Enzyme Systems, Baltimore, MD). Cells obtained from ATCC were treated according to the manufacturer's instructions with Corneal Epithelial Cell Basal Medium (PCS-700-030; ATACC) and Corneal Epithelial Cell Growth Kit (PCS-700-040; ATCC). Experiments were conducted on passage 2 in a 96-well plate.
Immortalized Cell Cultures
Immortalized human corneal epithelial (iHCE) cells were previously described by Shalom-Feuerstein et al.24 Cells were generously provided by the Ruby Shalom-Feuerstein laboratory (Technion, Israel Institute of Technology, Haifa, Israel). Cell medium was prepared using DMEM/F12 (01-170-1A; Biological Industries, Beit-Haemek, Israel) with 5% FBS (04-001-1B; Biological Industries), 5-µg/mL insulin (I9278; MilliporeSigma), 0.5% dimethyl sulfoxide (D2650; MilliporeSigma), 10-ng/mL EGF (PHG0311L; Rhenium Research Laboratory Equipment, Modi'in-Maccabim-Re'ut, Israel), and 1% penicillin–streptomycin (03-031-1B; Biological Industries). Cells were cultured at 37°C with 5% CO2 in a humidified incubator.
In Vitro Treatments
The following anti-inflammatory drops were used for this study: CsA ophthalmic emulsion 0.05% (Restasis; Allergan, Irvine, CA), lifitegrast ophthalmic solution 5.0% (Xiidra; Shire, Lexington, MA), and tacrolimus 0.06% eye drops (Concept for Pharmacy, Kefar Saba, Israel) or 0.1% ophthalmic drops (San Diego Optimum Compounding Pharmacy, San Diego, CA). Tacrolimus 0.06% eye drops were further diluted in culture medium to prepare two testing drugs containing 0.03% and 0.1% tacrolimus, respectively.
When primary HCE cells obtained from ATCC and iHCE cells reached full confluence, cells were treated with 20% of the tested drug or 0.9% saline and 80% medium. The following drugs were tested: CsA 0.05%, lifitegrast 5.0%, and tacrolimus 0.03% or 0.1%. Treatment times tested for each drug were 30 seconds, 1 minute, 1 hour, and 4 hours. Following treatment, cells were rinsed twice with PBS and the medium was replaced. Brightfield images were captured in the center of each well using a 10× lens 72 hours after the initiation of treatments.
For validation and evaluation of time and concentration effect, the experiment was repeated with modifications on a separate line of primary cells cultured from human donor tissue as previously described. This time, cells were treated with 10% of the tested drug or balanced salt solution (BSS) and 90% medium. The treatments (CsA 0.05%, lifitegrast 5.0%, and tacrolimus 0.1%) were applied for 2 minutes, 1 hour, 2 hours, 4 hours, and 24 hours. The BSS group was compared to a no-treatment group.
Survival Assays
Cell survival was assessed using the XTT Cell proliferation kit (20-300-1000; Biological Industries) or CellTiter-Glo Luminescent Cell Viability Assay based on adenosine triphosphate quantification (G7570; Promega, Madison, WI). Assays were conducted 48 to 72 hours after applying the different treatments according to manufacturer's instructions. At least five replicates were performed for each tested drug or control at each time point.
Statistical Analysis
Statistical analysis was performed using JMP (SAS Institute, Cary, NC). At first, the non-parametric Kruskal–Wallis test was performed on a set of groups. Then, the Wilcoxon test with Steel adjustment was performed to obtain the statistical significance of individual groups compared to the control group. Comparisons between drugs were conducted using two-tailed Student's t-test statistical analysis (*P < 0.05, **P < 0.01).
Results
Concentration-Dependent Effect of Anti-Inflammatory Eye Drops on Primary HCE Cells
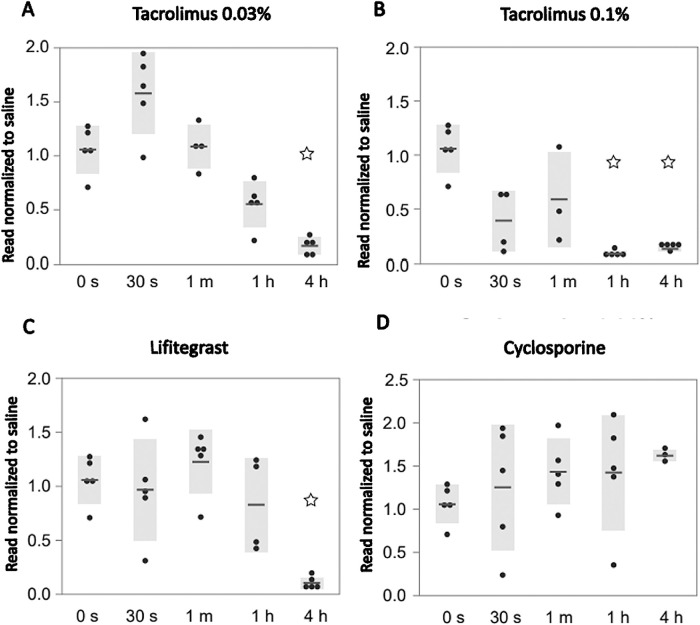
The cell viability analysis for primary HCE cells obtained from ATCC after treatment exposure is summarized in Figure 1. Cell survival, normalized to saline exposure, was significantly reduced after the 4-hour treatment with tacrolimus 0.03% (survival rate, 17%; SE = 3.59; n = 5; P = 0.042) (Fig. 1A) and after 1-hour treatment with tacrolimus 0.1% (8%; SE = 1.13; n = 5; P = 0.043) (Fig. 1B). Lifitegrast decreased cell viability after 4 hours (10%; SE = 2.21; n = 5; P = 0.042) (Fig. 1C). Cell viability on primary corneal epithelial cells was not altered following CsA treatment for up to 4 hours compared to saline (Fig. 1D).
Figure 1.
Effect of dry eye drugs on commercially obtained primary epithelial cell cultures. Primary epithelial cell cultures obtained from ATCC treated with (A) tacrolimus 0.03%, (B) tacrolimus 0.1%, (C) lifitegrast, and (D) cyclosporine for 30 seconds, 1 minute, 1 hour, and 4 hours. Cell survival was analyzed using the XTT assay after 72 hours and normalized to treatments with saline.
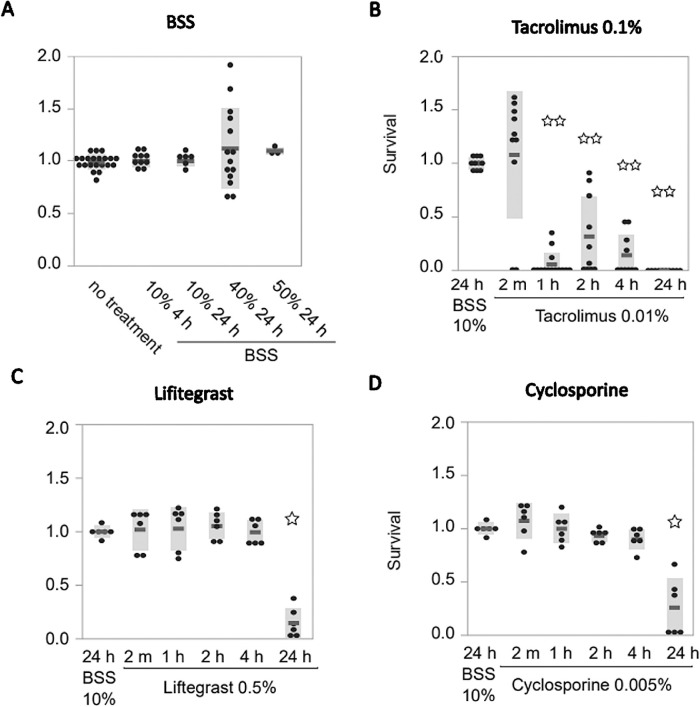
To validate these results in a second line of primary cells and for individual variations, primary HCE cells derived from at least three different donors were tested, this time with longer time exposures (up to 24 hours) and a lower concentration: 10% of each drug in 90% of regular growth medium (Fig. 2). We noticed a significant decrease in cell survival only after 24 hours with CsA treatment (26%; SE = 10.6; n = 6; P = 0.022) (Fig. 2D) and lifitegrast (15%; SE = 5.63; n = 6; P = 0.022) (Fig. 2C). Tacrolimus 0.1% showed significant decreases after 1 hour (5.7%; SE = 2.95; n = 14; P = 0.0002) (Fig. 2B). BSS exposure did not have a deleterious effect on cell survival compared to the no-treatment group (Fig. 2A).
Figure 2.
Effect of dry eye drugs on primary epithelial cell cultures from corneal transplant donor tissue. Testing eye drops in a separate primary cell line for validation using a lower concentration and longer treatment time (compare with Fig. 1). Primary epithelial cell cultures were treated with (A) BSS as control, (B) tacrolimus 0.1%, (C) lifitegrast, and (D) cyclosporine for 2 minutes, 1 hour, 2 hours, 4 hours, and 24 hours. Cell survival was analyzed using the CellTiter-Glo Luminescent Cell Viability Assay after 48 hours.
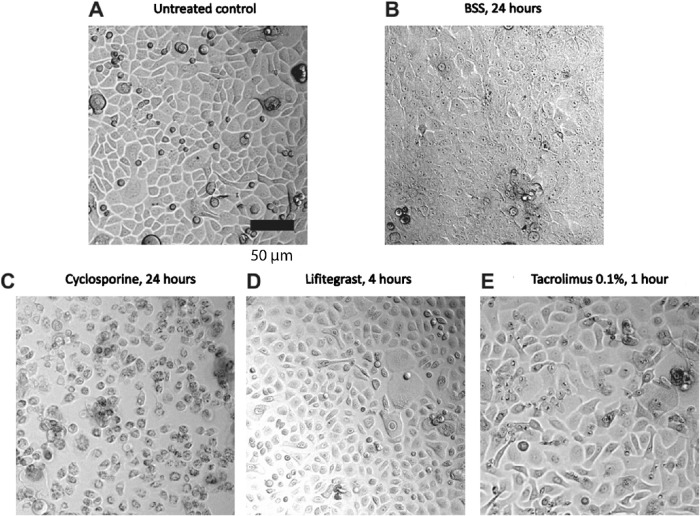
Representative pictures of primary corneal epithelial cells derived from donors at critical time points after the start of drug application are shown in Figure 3. In conditions where cell survival was significantly decreased (Figs. 3C, 3E), cells showed various morphological changes such as interrupted connections, cellular elongation, and enlargement. At earlier and less toxic stages (Fig. 3D), cell shrinkage can be observed. As controls, XTT assays performed on both primary and immortalized cell cultures showed no significant differences in cell survival between cells treated with PBS and those treated with saline (Supplementary Fig. S1).
Figure 3.
Representative pictures of cultured primary corneal epithelial cells at critical time points after the start of drug application. Phase contrast pictures of primary cells 24 hours after start of drug application with (A) untreated control, (B) BSS for 24 hours, (C) cyclosporine for 24 hours, (D) lifitegrast for 4 hours, and (E) tacrolimus for 1 hour. Note that BSS did not affect survival of cells but did alter transparency of the cells. Cells treated with 10% tested drug, 90% normal growth medium. Scale bar: 50 µm.
Effect of Anti-Inflammatory Eye Drops on iHCE Cells
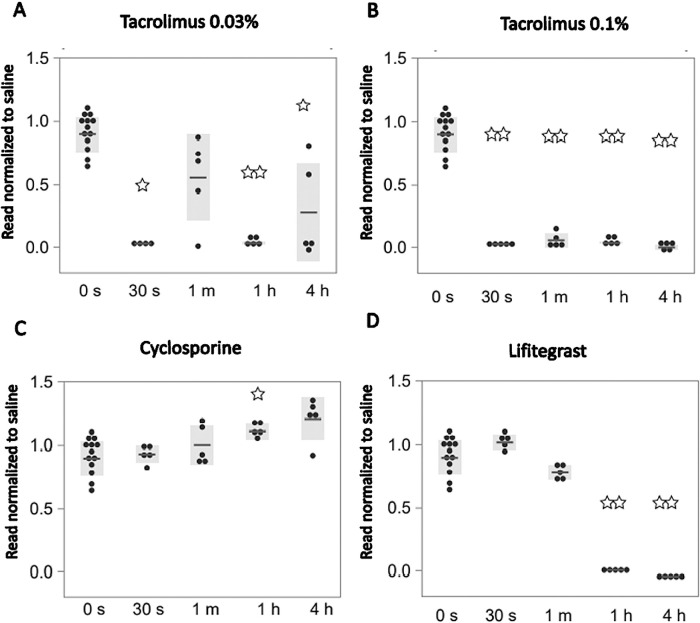
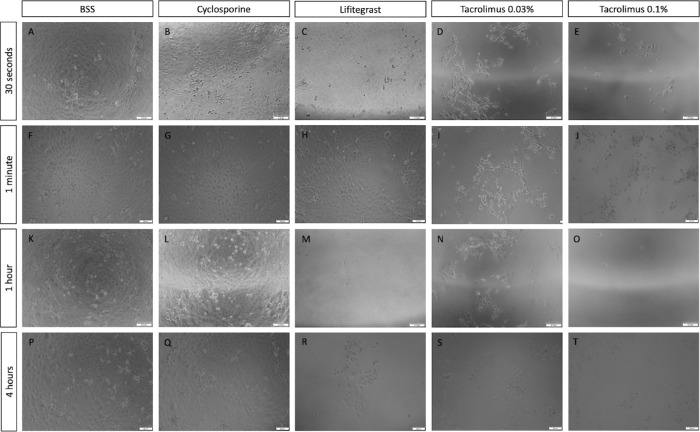
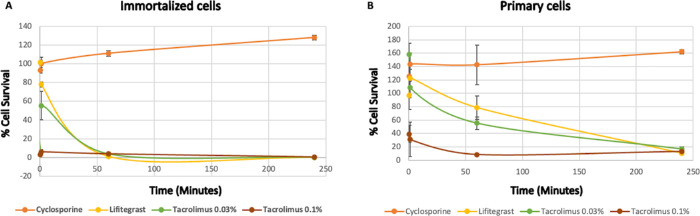
Results were further tested on another in vitro model of corneal epithelial cells. Comparison of treatments on immortalized epithelial cell cultures is summarized in Figure 4. Cell viability was significantly impaired on immortalized cells following 30-second treatment with tacrolimus 0.03% (3%; SE = 0.19; n = 4; P = 0.015%) (Fig. 4A) and tacrolimus 0.1% (3%; SE = 0.44; n = 5; P = 0.006%) (Fig. 4B). Of note, although significant decreases in cell viability were observed after 30 seconds, 1 hour, and 4 hours, no significant difference in cell viability was observed in the 1-minute treatment of tacrolimus 0.03% (Fig. 4A). Lifitegrast decreased cell viability after 1-hour treatment on immortalized cells (1%; SE = 0.39; n = 5; P = 0.006) (Fig. 4D). Cell viability was not altered following CsA treatment compared to saline, and a significantly higher viability was noted on immortalized cells treated for 1 hour (111%; SE = 2.79; n = 5; P = 0.016) (Fig. 4C). Gross morphology in vitro changes following different treatments and time exposures in immortalized cells are demonstrated by brightfield microscopy images (Fig. 5). Imaging of immortalized epithelial cells supported quantitative data from survival assays and demonstrated altered morphology, elongation of cells, and disruption to intercellular connections with decreased cell survival. A visualization and comparison of the overall results on cell survival in both primary and immortalized cell lines are shown in Figure 6.
Figure 4.
Effect of dry eye drugs on immortalized epithelial cell cultures. Immortalized epithelial cell cultures treated with (A) tacrolimus 0.03%, (B) tacrolimus 0.1%, (C) lifitegrast, and (D) cyclosporine for 30 seconds, 1 minute, 1 hour, and 4 hours. Cell survival was analyzed using the XTT assay after 72 hours and normalized to treatments with saline.
Figure 5.
Immortalized human corneal epithelial cells morphology following anti-inflammatory treatments. Brightfield images of iHCE cells 72 hours following treatments with saline (A, F, K, P), cyclosporine (B, G, L, Q), lifitegrast (C, H, M, R), tacrolimus 0.03% (D, I, N, S), and tacrolimus 0.1% (E, J, O, T). Treatment times: 30 seconds (A–E), 1 minute (F–J), 1 hour (K–O), and 4 hours (P–T). Cells treated with 20% tested drug, 80% normal growth medium. Scale bar: 200 µm.
Figure 6.
Comparison of eye drop effect on immortalized and commercially obtained primary epithelial cell cultures (a visualization of Figs. 1 and 4). Impact of cyclosporine, lifitegrast, and tacrolimus (0.03% and 0.1%) on the percentage of cell survival measured at 30 seconds, 1 minute, 1 hour, and 4 hours.
Discussion
Our study comparatively evaluated the in vitro effects of three common anti-inflammatory eye drops that are used in the treatment of DED. The results demonstrate a significant impact on cell viability of tacrolimus 0.1% and 0.03% in comparison to saline and BSS in models of both primary human corneal epithelial cells and immortalized human corneal epithelial cells. Due to tear dilution and rapid clearance of eye drops from the ocular surface within minutes,25 effects at shorter periods of exposure and lower concentrations most likely have higher clinical relevance in mimicking physiologic conditions of a single eye drop instillation. Longer time durations may have some utility for chronic eye drop users.
There are several potential explanations for why tacrolimus decreased cell viability at significantly shorter time durations than CsA or lifitegrast. Tacrolimus is known to be a powerful immunosuppressive and anti-inflammatory drug and is estimated to be 10 to 100 times more potent than CsA,26 despite having a similar molecular mechanism of action. The potency of tacrolimus for a variety of inflammatory diseases of the anterior segment could explain both its clinical efficacy and its increased toxicity. One theory is that transient toxicity may allow for better penetrance to the cornea, as a similar mechanism has been seen with other known corneal penetration-enhancer drugs such as ethylenediaminetetraacetic acid and preservatives such as benzalkonium chloride. These penetration enhancers are known to temporarily alter the corneal epithelial structure, which can cause mild irritation but has the advantage of allowing more drug to pass through the cornea.27
More research is needed to reconcile the epithelial effect of tacrolimus with its clinical efficacy and potential. In a 2018 report, the application of 0.03% or 0.1% tacrolimus ointment to rat eyes significantly delayed epithelial healing and induced apoptosis; of note, when the authors treated primary corneal epithelial cells to concentrations of 0% to 0.003% for 24 hours, they did not observe significantly decreased cell viability. These concentrations were significantly lower than those tested in our study (0.006%, 0.02%, and 0.01% after accounting for dilution). Thus, tacrolimus may be significantly more cytotoxic at high concentrations but more comparable to the other eye drops tested at lower concentrations. It is important to consider what concentrations would most accurately generalize to physiologic conditions in an in vitro study. Reports estimate that at most 5% of a drop penetrates the ocular structures, with the rest primarily draining through the nasolacrimal duct or being diluted by tears.28,29 Another study showed that Restasis had limited delivery of CsA to the cornea compared to alternative cationic emulsions.30 Our results do not negate the many studies supporting the efficacy and safety of tacrolimus as a treatment for DED but rather emphasize the importance of finding an optimum formulation that minimizes epithelial damage. Future research to evaluate the impact of concentration could test CsA and lifitegrast at different formulations and dilutions, in addition to tacrolimus. This could include 0.09% CsA drops (Cequa; Sun Pharmaceutical Industries, Princeton, NJ) or 2% compounded CsA drops.
The question remains whether our findings of the transient effect of tacrolimus versus CsA, and lifitegrast directly correlates to a clinical comparison of the three formulations. Our findings support the hypothesis that tacrolimus drops may lead to more frequent or severe complaints of adverse effects such as ocular stinging or burning. However, head-to-head comparisons of tacrolimus, lifitegrast, and CsA in clinical trials as treatments for DED are limited. A 2021 comparison of tacrolimus and CsA for the treatment of DED demonstrated that the two had comparable efficacy, but the authors did not comment on the incidence of adverse effects.5 Rates of adverse events vary among reports, with one clinical trial of dry eye patients treated with 0.03% tacrolimus drops showing that virtually all patients complained of moderate burning sensation for approximately 30 minutes after instillation of eye drops.31 Hence, understanding epithelial toxicity and its relation to adverse effects has the potential to help improve patient adherence to these drugs.
Interestingly, in our study, CsA demonstrated a protective effect on the survival of immortalized epithelial cells in comparison to saline. This may be within expected lines, as ophthalmic emulsion of CsA has been previously been shown to induce a cytoprotective anti-apoptotic effect in vitro,10 and it corroborates well with the significant body of clinical evidence supporting the efficacy of CsA in patients with dry eye.32,33
There were several limitations to this study. First, we examined the effect of eye drops by measuring increases or decreases in cell viability. We intentionally quantified cell viability 3 days following treatments to test the end effect on cell survival, allowing the cells to either heal from any toxicity or execute the apoptotic program initiated by the drops. This experimental plan does not allow us to elucidate the molecular mechanisms leading to corneal epithelial cell death. Future experiments using gene expression profiling are needed to characterize the inflammatory markers and pathways responsible for these results. Furthermore, we applied the commercial eye drop emulsion to best model treatment in a real-life setting, but, as a result, we are unable to attribute the observed effects in this study to a single ingredient in the solution as marketed. Of note, as none of the eye drops used in this study contained preservatives, we did control for the presence of certain preservatives that are known toxins to the cornea.34 Last, in this study, we applied eye drop emulsions to cultures of monolayer corneal epithelial cells. Prior studies have demonstrated that the stratified corneal epithelium model20 more closely mimics the layered human corneal epithelium in vivo, including the ability to test its barrier properties. However, in testing drug toxicity, the monolayer model has showed non-inferiority and enables high multi-well testing capacity.35 Monolayer cells have furthermore been shown to be comparable in evaluation of cellular toxicity to both three-dimensional36 and in vivo models.37
Finally, some increased variability and unexpected findings were observed in our treatment groups. Lifitegrast and tacrolimus had more rapid-onset effects on cell viability in iHCE cells compared to primary cells. This may be due to differences in gene expression profile in iHCE cells that could lead to altered growth and heterogeneity.35 Still, similar overall trends were observed, which turns this into an advantage, as performing our study in multiple cell lines allowed us to validate and control for possible variation between cell lines and laboratories.38 Additionally, when immortalized cells were treated with tacrolimus 0.03% (Fig. 4), there was a significant decrease in cell survival at 30 seconds, 1 hour, and 4 hours of exposure but surprisingly not at 1 minute. This variability is most likely caused by the hydrophobic characteristics of tacrolimus, which does not make a homogeneous mixture as readily with the cell medium and thus could create different concentrations of the drug near the surface of the cells. This observation is relevant to the physiologic conditions of dry eye treatment, as surface irregularity may lead to altered concentration of the drug on different areas of the ocular surface.
Our findings on the effect of three anti-inflammatory eye drops on corneal epithelial cells in vitro have several translational implications. The sensitive detection of cytotoxicity and every effort to minimize it are of utmost importance, particularly because patients with more severe dry eye also simultaneously use eye drops more frequently, have reduced tear turnover rate, and have more susceptible corneal epithelium.39,40 Patients with more susceptible corneal epithelium may benefit from eye drops that have more cytoprotective properties and less cytotoxic properties toward corneal epithelial cells both in vitro and in vivo.
Conclusions
The impact on corneal epithelial cell culture viability was most pronounced in tacrolimus-containing eye drops and observed in CsA and lifitegrast at longer time durations at the concentrations tested. These findings may serve as a useful resource in the selection and development of anti-inflammatory eye drops for clinical purposes and provide further insights into the study of inflammatory and cell death pathways.
Supplementary Material
Acknowledgments
Supported by Research to Prevent Blindness and the National Institutes of Health (NIH) CORE Grant P30EY022589.
Disclosure: R. Sella, None; Y. Cohen-Tayar, None; T. Noguchi, None; E.N. Finburgh, None; R.R. Lian, None; A.A. Abbas, None; D.F. Hakim, None; J.J. Bu, None; J. Zhao, None; P. Shaw, None; I. Bahar, None; N.A. Afshari, None
References
- 1. Akpek EK, Amescua G, Farid M, et al.. Dry eye syndrome preferred practice pattern. Ophthalmology. 2019; 126: P286–P334. [DOI] [PubMed] [Google Scholar]
- 2. Aragona P, Giannaccare G, Mencucci R, Rubino P, Cantera E, Rolando M. Modern approach to the treatment of dry eye, a complex multifactorial disease: a PICASSO board review. Br J Ophthalmol. 2021; 105: 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clayton JA. Dry eye. N Engl J Med. 2018; 378: 2212–2223. [DOI] [PubMed] [Google Scholar]
- 4. Zhai J, Gu J, Yuan J, Chen J.. Tacrolimus in the treatment of ocular diseases. BioDrugs. 2011; 25: 89–103. [DOI] [PubMed] [Google Scholar]
- 5. Moawad P, Shamma R, Hassanein D, Ragab G, El Zawahry O. Evaluation of the effect of topical tacrolimus 0.03% versus cyclosporine 0.05% in the treatment of dry eye secondary to Sjogren syndrome. Eur J Ophthalmol. 2021; 32: 673–679. [DOI] [PubMed] [Google Scholar]
- 6. Moscovici BK, Holzchuh R, Chiacchio BB, Santo RM, Shimazaki J, Hida RY.. Clinical treatment of dry eye using 0.03% tacrolimus eye drops. Cornea. 2012; 31: 945–949. [DOI] [PubMed] [Google Scholar]
- 7. Holland EJ, Darvish M, Nichols KK, Jones L, Karpecki PM.. Efficacy of topical ophthalmic drugs in the treatment of dry eye disease: a systematic literature review. Ocul Surf. 2019; 17: 412–423. [DOI] [PubMed] [Google Scholar]
- 8. Jin R, Li Y, Li L, Kim J, Yoon HJ, Yoon KC.. Comparative analysis of 0.1% cyclosporin A cationic emulsion and 0.05% cyclosporin A emulsion in murine dry eye cases with different severities. Exp Ther Med. 2021; 22: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alam J, de Souza RG, Yu Z, Stern ME, de Paiva CS, Pflugfelder SC. Calcineurin inhibitor voclosporin preserves corneal barrier and conjunctival goblet cells in experimental dry eye. J Ocul Pharmacol Ther. 2020; 36: 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hwang S-B, Park JH, Kang S-S, et al.. Protective effects of cyclosporine A emulsion versus cyclosporine A cationic emulsion against desiccation stress in human corneal epithelial cells. Cornea. 2020; 39: 508–513. [DOI] [PubMed] [Google Scholar]
- 11. Iaccheri B, Torroni G, Cagini C, et al.. Corneal confocal scanning laser microscopy in patients with dry eye disease treated with topical cyclosporine. Eye (Lond). 2017; 31: 788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen M, Gong L, Sun X, et al.. A comparison of cyclosporine 0.05% ophthalmic emulsion versus vehicle in Chinese patients with moderate to severe dry eye disease: an eight-week, multicenter, randomized, double-blind, parallel-group trial. J Ocul Pharmacol Ther. 2010; 26: 361–366. [DOI] [PubMed] [Google Scholar]
- 13. Baiza-Durán L, Medrano-Palafox J, Hernández-Quintela E, Lozano-Alcazar J.. A comparative clinical trial of the efficacy of two different aqueous solutions of cyclosporine for the treatment of moderate-to-severe dry eye syndrome. Br J Ophthalmol. 2010; 94: 1312–1315. [DOI] [PubMed] [Google Scholar]
- 14. Sall K, Stevenson OD, Mundorf TK, Reis BL, Group CPS.. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology. 2000; 107: 631–639. [DOI] [PubMed] [Google Scholar]
- 15. Atallah RT, Castanos MV, Najac R, Donnenfeld E.. Six months’ treatment with lifitegrast in patients with moderate-to-severe symptomatic dry eye: a retrospective chart review. Clin Ophthalmol. 2019; 13: 1033–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. White DE, Zhao Y, Ogundele A, et al.. Real-world treatment patterns of cyclosporine ophthalmic emulsion and lifitegrast ophthalmic solution among patients with dry eye. Clin Ophthalmol. 2019; 13: 2285–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sall K, Stevenson OD, Mundorf TK, Reis BL.. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000; 107: 631–639. [DOI] [PubMed] [Google Scholar]
- 18. Sheppard JD, Wirta DL, McLaurin E, et al.. A water-free 0.1% cyclosporine A solution for treatment of dry eye disease: results of the randomized phase 2B/3 ESSENCE study. Cornea. 2021; 40: 1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ammar DA, Kahook MY.. The effects of combination glaucoma medications on ocular surface epithelial cells. Adv Ther. 2009; 26: 970–975. [DOI] [PubMed] [Google Scholar]
- 20. Nakagawa S, Usui T, Yokoo S, et al.. Toxicity evaluation of antiglaucoma drugs using stratified human cultivated corneal epithelial sheets. Invest Ophthalmol Vis Sci. 2012; 53: 5154–5160. [DOI] [PubMed] [Google Scholar]
- 21. Geerling G, Daniels JT, Dart JK, Cree IA, Khaw PT.. Toxicity of natural tear substitutes in a fully defined culture model of human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2001; 42: 948–956. [PubMed] [Google Scholar]
- 22. Martone G, Frezzotti P, Tosi GM, et al.. An in vivo confocal microscopy analysis of effects of topical antiglaucoma therapy with preservative on corneal innervation and morphology. Am J Ophthalmol. 2009; 147: 725–735.e1. [DOI] [PubMed] [Google Scholar]
- 23. Giannaccare G, Buzzi M, Fresina M, Velati C, Versura P.. Efficacy of 2-month treatment with cord blood serum eye drops in ocular surface disease: an in vivo confocal microscopy study. Cornea. 2017; 36: 915–921. [DOI] [PubMed] [Google Scholar]
- 24. Shalom-Feuerstein R, Serror L, De La Forest Divonne S, et al.. Pluripotent stem cell model reveals essential roles for miR-450b-5p and miR-184 in embryonic corneal lineage specification. Stem Cells. 2012; 30: 898–909. [DOI] [PubMed] [Google Scholar]
- 25. Friedlaender MH, Breshears D, Amoozgar B, Sheardown H, Senchyna M.. The dilution of benzalkonium chloride (BAK) in the tear film. Adv Ther. 2006; 23: 835–841. [DOI] [PubMed] [Google Scholar]
- 26. Kino T, Hatanaka H, Miyata S, et al.. FK-506, a novel immunosuppressant isolated from a streptomyces II. Immunosuppressive effect of FK-506 in vitro. J Antibiot. 1987; 40: 1256–1265. [DOI] [PubMed] [Google Scholar]
- 27. Ibrahim MM, Maria DN, Wang X, Simpson RN, Hollingsworth T, Jablonski MM.. Enhanced corneal penetration of a poorly permeable drug using bioadhesive multiple microemulsion technology. Pharmaceutics. 2020; 12: 704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jünemann AG, Chorągiewicz T, Ozimek M, Grieb P, Rejdak R.. Drug bioavailability from topically applied ocular drops. Does drop size matter? Ophthalmol J. 2016; 1: 29–35. [Google Scholar]
- 29. Kumar S, Karki R, Meena M, Prakash T, Rajeswari T, Goli D.. Reduction in drop size of ophthalmic topical drop preparations and the impact of treatment. J Adv Pharm Technol Res. 2011; 2: 192–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Daull P, Lallemand F, Philips B, Lambert G, Buggage R, Garrigue J-S.. Distribution of cyclosporine A in ocular tissues after topical administration of cyclosporine A cationic emulsions to pigmented rabbits. Cornea. 2013; 32: 345–354. [DOI] [PubMed] [Google Scholar]
- 31. Moscovici BK, Holzchuh R, Sakassegawa-Naves FE, et al.. Treatment of Sjögren's syndrome dry eye using 0.03% tacrolimus eye drop: prospective double-blind randomized study. Cont Lens Anterior Eye. 2015; 38: 373–378. [DOI] [PubMed] [Google Scholar]
- 32. Leonardi A, Van Setten G, Amrane M, et al.. Efficacy and safety of 0.1% cyclosporine A cationic emulsion in the treatment of severe dry eye disease: a multicenter randomized trial. Eur J Ophthalmol. 2016; 26: 287–296. [DOI] [PubMed] [Google Scholar]
- 33. Mah F, Milner M, Yiu S, Donnenfeld E, Conway TM, Hollander DA.. PERSIST: physician's evaluation of Restasis® satisfaction in second trial of topical cyclosporine ophthalmic emulsion 0.05% for dry eye: a retrospective review. Clin Ophthalmol. 2012; 6: 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F.. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010; 29: 312–334. [DOI] [PubMed] [Google Scholar]
- 35. Rönkkö S, Vellonen K-S, Järvinen K, Toropainen E, Urtti A.. Human corneal cell culture models for drug toxicity studies. Drug Deliv Transl Res. 2016; 6: 660–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parnigotto PP, Bassani V, Montesi F, Conconi MT.. Bovine corneal stroma and epithelium reconstructed in vitro: characterisation and response to surfactants. Eye. 1998; 12: 304–310. [DOI] [PubMed] [Google Scholar]
- 37. Saarinen-Savolainen P, Järvinen T, Araki-Sasaki K, Watanabe H, Urtti A.. Evaluation of cytotoxicity of various ophthalmic drugs, eye drop excipients and cyclodextrins in an immortalized human corneal epithelial cell line. Pharm Res. 1998; 15: 1275–1280. [DOI] [PubMed] [Google Scholar]
- 38. Ohji M, SundarRaj N, Hassell JR, Thoft RA.. Basement membrane synthesis by human corneal epithelial cells in vitro. Invest Ophthalmol Vis Sci. 1994; 35: 479–485. [PubMed] [Google Scholar]
- 39. Sorbara L, Simpson T, Vaccari S, Jones L, Fonn D.. Tear turnover rate is reduced in patients with symptomatic dry eye. Cont Lens Anterior Eye. 2004; 27: 15–20. [DOI] [PubMed] [Google Scholar]
- 40. Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015; 112: 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.