Abstract
A study in nonhuman primates reported that infusions of an antibody against α4β7 integrin, in combination with antiretroviral therapy, showed consistent, durable control of simian immunodeficiency virus (SIV) in rhesus macaques. The antibody used has pleiotropic effects, so we set out to gain insight into the underlying mechanism by comparing this treatment to treatment with non-neutralizing monoclonal antibodies against the SIV envelope glycoprotein that only block α4β7 binding to SIV Env but have no other host-directed effects. Similar to the initial study, we used an attenuated strain of SIV containing a stop codon in nef. The study used 30 macaques that all began antiretroviral therapy and then were divided into five groups to receive different antibody treatments. Unlike the published report, we found no sustained virologic control by these treatments in vivo.
A major focus of HIV therapeutic research is to develop treatments that result in “functional cures,” which are interventions capable of converting infected individuals into elite controllers. Such individuals would exhibit limited, controlled residual virus replication that neither contributes to excess morbidity or mortality, nor presents a transmission risk, all in the absence of ongoing combination antiretroviral therapy (ART) (1–3). Although ART has had a major impact on the disease worldwide, it is not universally available, it can have intolerable side effects, and it currently requires daily regimens (4). Thus, a short-term immunological intervention during drug-mediated suppression that leads to long-term control after cessation of therapy is highly attractive (5–7).
Such a therapy was suggested by the data of Byrareddy et al. (8), who reported long-term virologic control in nonhuman primates (NHPs) challenged with SIVmac239. In that study, short-term treatment with a primatized monoclonal antibody (mAb) binding the host-expressed integrin α4β7 (9), during and after ART, led to sustained control of viremia following all treatment cessation. Passive infusion of anti-α4β7 in NHPs (and humanized anti-α4β7 in humans) leads to significant redistribution of lymphocytes throughout the body (10), alters the activation potential of cells expressing the integrin, and interferes with SIV and HIV binding to the α4β7 integrin on target CD4 T cells (11), any of which might account for antiviral effects.
With the goal of discriminating these possibilities, we tested the same viral infection employed by Byrareddy et al. with an equivalent ART regimen, in combination with mAbs against the SIV envelope glycoprotein that also block virus binding to the integrin. SIV gp120, like HIV gp120, binds to α4β7 through contacts on the variable loop 2 (V2) region (12); the mAbs ITS09.01 and ITS12.01 bind to different regions of V2, each covering an α4β7 contact site (Fig. 1A) (13). These mAbs block the binding of the SIV Env to α4β7 to different extents, alone and in combination (Fig. 1, B and C), as does anti-α4β7 (14). However, unlike ITS103.01, the anti-V2 mAbs and the anti-α4β7 do not neutralize SIV mac239 in vitro (Fig. 1D).
Fig. 1. Activity of SIV Env-specific mAbs.
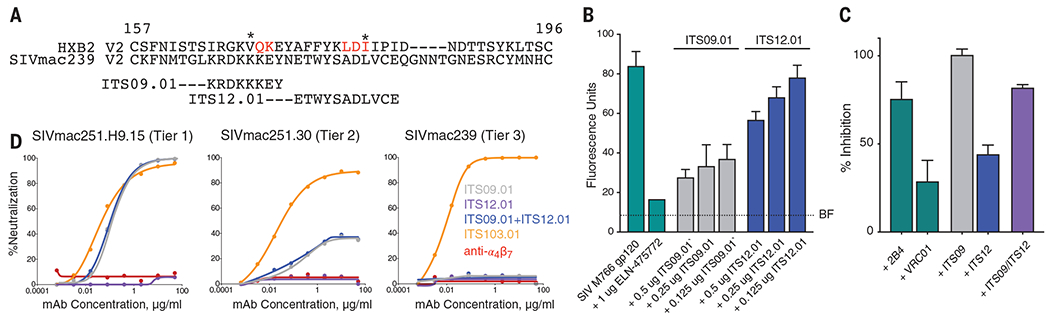
(A) Alignment of the amino acid sequences of the V2 domains of HIV HXB2 and SIVmac239. The regions of SIV V2 containing epitopes recognized by the SIV V2 mAbs ITS09.01 and ITS12.01 are indicated. Amino acids identified as part of the α4β7 binding site in HIV V2 are indicated in red. Asterisks denote the positions of V2 sieve residues in the RV144 vaccine trial. (B) Adhesion of α4β7-expressing RPMI8866 cells to SIVmac766 gp120 in the presence of decreasing concentrations of ITS09.01 and ITS12.01. Adhesion of cells to gp120 alone or in the presence of the α4β7 antagonist ELN-475722 is included. BF indicates background fluorescence in the absence of cells. Conditions are carried out in triplicate; error bars indicate SD. (C) Inhibition of α4β7 adhesion by the combination of IT12.01 and ITS09.01. The α4β7 mAb 2B4 is used as a positive specificity control (14), and VRC01, an antibody to HIV that does not bind SIV, is used as a negative nonspecific reagent control. For (B) and (C), results shown are representative of three independent experiments and indicate percentage inhibition relative to adhesion in the absence of any mAb. Conditions are run in triplicate and error bars indicate ±1 SD. (D) ITS09.01, ITS12.01, and anti-α4β7 do not neutralize the Tier 3 SIV mac239. ITS09.01 shows partial neutralization of the Tier 2 virus, and complete neutralization of the Tier 1 clone. By contrast, ITS103.01, which targets the CD4 binding site, completely neutralizes nearly all strains of SIV including SIVmac239. Single-letter abbreviations for the amino acid residues are as follows: A, Ala; C, Cys; D, Asp; E, Glu; F, Phe; G, Gly; H, His; I, Ile; K, Lys; L, Leu; M, Met; N, Asn; P, Pro; Q, Gln; R, Arg; S, Ser; T, Thr; V, Val; W, Trp; and Y, Tyr.
Our study used 30 animals; all were infected with the same dose, inoculation route, and lot of challenge virus stock used by Byrareddy et al. (8). We determined that this virus has a stop codon at position 93 in Nef, resulting in a Nef− phenotype with lower peak viral loads and attenuated pathogenesis compared to Nef-open SIVmac239 (15). In our animals, this mutation was largely repaired by week 2, and completely by week 5 (fig. S1).
At week 5, all 30 animals began daily ART therapy, with the substitution of the clinically used raltegravir in place of the functionally and efficaciously similar L-870812 as the integrase inhibitor. Because control of viremia was slower than reported by Byrareddy et al., the initial ART-only treatment period was extended by 6 weeks to attain stable virologic control. More rapid repair to a functional Nef in our animals could explain the slower virologic control by the ART regimen. Indeed, the Nef reversion kinetics and early virus–host dynamics most likely affect pathogenesis throughout (Fig. 2, B and C). The attenuated nature of the virus is also evident by the small CD4 T cell loss over a year (Fig. 2E), although acute loss of CD4 cells (Fig. 2D) was seen.
Fig. 2. Viral and T cell dynamics.
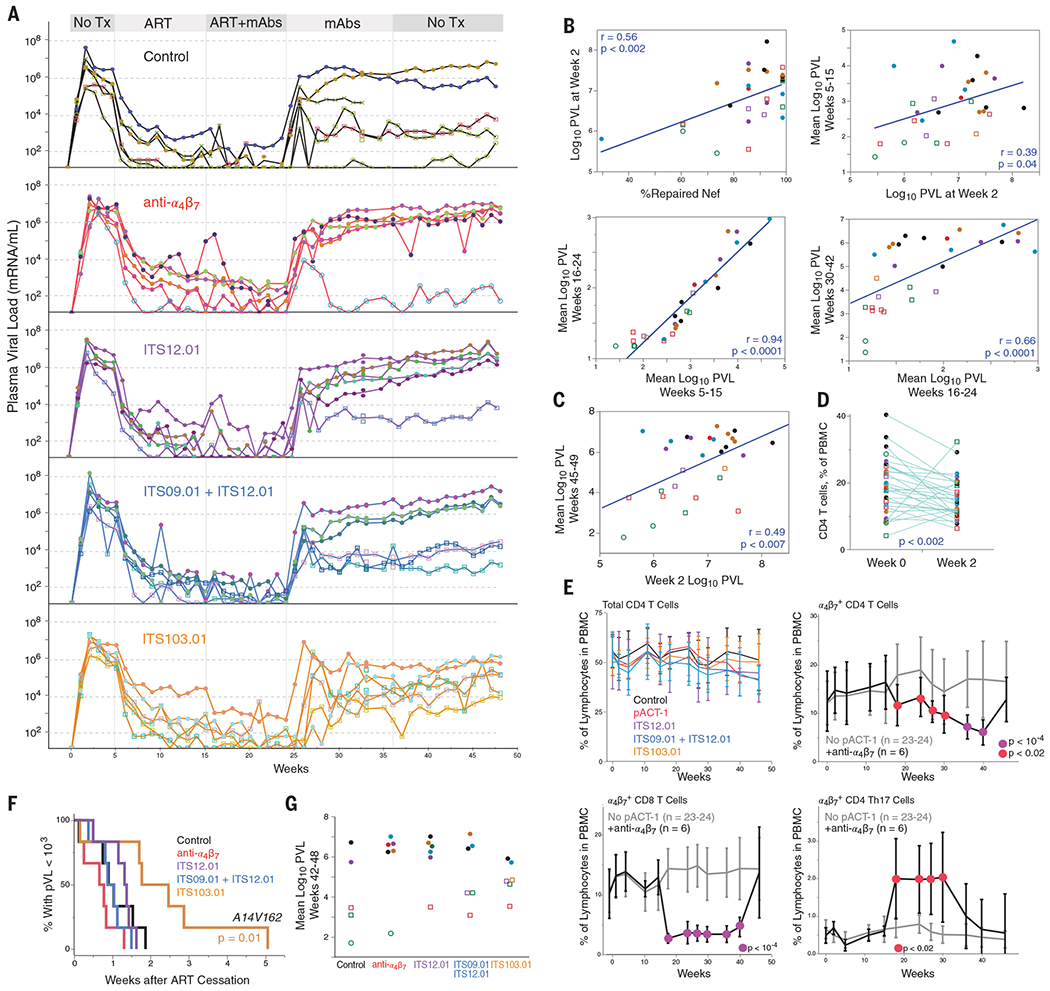
(A) Viremia in all 30 animals is shown by group. Symbols and colors are unique within each group only; the geometric mean pVL at weeks 42 to 48 is designated by open (circles, <103; squares, <104) or filled (>104) symbols. One animal in the control group was euthanized for causes unrelated to the study, designated by X symbols. (B) The fraction of viruses that have repaired Nef is correlated with peak pVL, measured at week 2. In turn, the pVL at each phase of the experiment strongly predicts subsequent pathogenesis. (C) The virus load at peak (week 2) is a strong predictor of the posttreatment set-point virus load, irrespective of treatment group (symbol colors as in other figures). P value is a linear least-squares regression after correcting for treatment group. (D) The loss of CD4 T cells during acute infection, measured as a fraction of PBMCs in the blood, averaged about 20%. P value is a paired Wilcoxon signed-rank test. (E) There was no major decline in the amounts of peripheral blood CD4 throughout the study. Anti-α4β7 altered the representation of α4β7-expressing cells in blood during treatment, compared to all other groups. Cells were identified as shown in fig. S4. Colored symbols indicate significance at each time point comparing anti-α4β7 to all others by Student’s t test. (F) Animals in the ITS103.01 group showed delayed viremia rebound (weeks to pVL over 103/ml). This was the only significant difference among groups (log-rank test). (G) The geometric mean pVL from weeks 42 to 48 for each animal.
At week 15, animals were assigned to five balanced treatment groups on the basis of sex, weight, peak plasma viral load (pVL), and week 5 pVL. The groups, all of which received ART, included (i) a control arm, in which animals received no antibody; (ii) anti-α4β7, in which animals received infusions of 50 mg of anti-α4β7 per kilogram of body weight every 3 weeks; (iii) ITS12.01, (iv) ITS09.01+ITS12.01, and (v) ITS103.01, in which animals received infusions of 20 mg/kg of each of the indicated antibodies every 3 weeks. ITS103.01 is a broadly SIV-neutralizing antibody used as a positive control for immune pressure. At week 24, ART was discontinued, and after week 36, mAb infusions were discontinued. Animals were followed through 48 weeks after infection. One animal in the control group was euthanized at week 33 because of temporomandibular joint ankylosis unrelated to the study.
None of the animals treated with anti-α4β7 generated substantive antidrug antibodies (table S1). Further, consistent with previous experiments with NHP mAbs against SIV (16), infusions of ITS09.01 and ITS12.01 showed no evidence of elicitation of antidrug activity, with a plasma half-life of 15.6 ± 2.0 days (fig. S2).
We did not observe posttreatment control of plasma viremia in NHPs that received anti-α4β7 (Fig. 2A). There were sporadic instances of NHPs showing some posttreatment control of plasma viremia in most groups; in the anti-α4β7 treatment group, five out of six NHPs rebounded to a viremia in excess of 106 virions/ml. Once ART was stopped, rebound viremia was not delayed in any group except the group receiving the neutralizing mAb ITS103.01 (Fig. 2D). These data are consistent with the expectation that non-neutralizing mAbs would not affect viremia, and data that SHIV and HIV can escape from single neutralizing mAb monotherapy within 2 weeks (6, 17–19). Reflecting heterogeneous patterns of posttreatment viremia, there were no significant differences between groups in the distributions of viremia (Fig. 2G).
The lack of effect of anti-α4β7 on posttreatment viremia is in contrast to the results reported by Byrareddy et al. However, administration of this mAb to animals resulted in the expected impact on lymphocyte redistribution. For example (Fig. 2E), during therapy, there were decreases in the representation of α4β7-expressing CD4 and CD8 T cells from peripheral blood mononuclear cells (PBMCs), and a selective increase in α4β7-expressing T helper 17 (TH17) cells, consistent with a previous report (10). We also found no differences between groups in cell-associated viral load (SIV gag DNA, “CAVL”) from jejunal and rectal biopsies at any time point. As a whole, all animals had mucosal CAVL amounts consistent with corresponding pVL values independent of treatment (fig. S3).
Overall, we could not reproduce the clinically relevant findings of Byrareddy et al. Despite attaining therapeutic amounts of anti-α4β7, resulting in the expected impacts on lymphocyte distribution in vivo, there were no significant differences in long-term viral control in treated animals.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. P. Todd, A. Taylor, and D. Scorpio for veterinary and animal logistics support; M. Lewis and staff at BioQual, Inc. for expert animal assistance; D. Finzi for coordinating acquisition of reagents; F. Villinger for providing the virus challenge stock; members of the ImmunoTechnology Section for critical discussion and support; the Nonhuman Primate Immunogenicity Core (VRC) for assistance with specimen processing; the Flow Cytometry Core (VRC) for expert cytometry assistance; the Quantitative Molecular Diagnostics Core (ACVP/FNLCR) and Viral Evolution Core (VEC/FNLCR) for viral load measurements and viral sequence analysis; and J. Mascola, R. Koup, D. Douek, C. Dieffenbach, C. Lane, D. Barouch, and A. Fauci for support, advice, and critical feedback.
Funding:
This work was supported by the Intramural Research Programs of the Vaccine Research Center and the National Institute of Allergy and Infectious Diseases, National Institutes of Health; in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. HSN261200800001E; and by the NHP Reagent Resource grants OD010976 and AI126683. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of any trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
Competing interests: The authors declare no competing financial interests.
SUPPLEMENTARY MATERIALS
Data and materials availability:
Sequences are deposited in GenBank. All data are available in the manuscript or supplementary materials, or by request to M.R.
REFERENCES AND NOTES
- 1.Gao X et al. , Nat. Med 11, 1290–1292 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Allen TM et al. , J. Virol 75, 738–749 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goulder PJ et al. , AIDS Res. Hum. Retroviruses 12, 1691–1698 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Cihlar T, Fordyce M, Curr. Opin. Virol 18, 50–56 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Bar KJ et al. , N. Engl. J. Med 375, 2037–2050 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caskey M et al. , Nature 522, 487–491 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheid JF et al. , Nature 535, 556–560 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrareddy SN et al. , Science 354, 197–202 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazarovits AI et al. , J. Immunol 133, 1857–1862 (1984). [PubMed] [Google Scholar]
- 10.Calenda G et al. , J. Immunol 200, 810–820 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santangelo PJ et al. , Mucosal Immunol. 11, 932–946 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arthos J et al. , Nat. Immunol 9, 301–309 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Mason RD et al. , PLOS Pathog. 12, e1005537 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lertjuthaporn S et al. , PLOS Pathog. 14, e1007278 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fennessey CM et al. , Retrovirology 12, 49 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welles HC et al. , PLOS Pathog. 14, e1007395 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch RM et al. , Sci. Transl. Med 7, 319ra206 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Lynch RM et al. , J. Virol 89, 4201–4213 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tassaneetrithep B et al. , PLOS ONE 9, e108446 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequences are deposited in GenBank. All data are available in the manuscript or supplementary materials, or by request to M.R.


