Abstract
Background
Long COVID occurs at a lower frequency in children and adolescents than in adults. Morphologic and free-breathing phase-resolved functional low-field-strength MRI may help identify persistent pulmonary manifestations after SARS-CoV-2 infection.
Purpose
To characterize both morphologic and functional changes of lung parenchyma at low-field-strength MRI in children and adolescents with post–COVID-19 condition compared with healthy controls.
Materials and Methods
Between August and December 2021, a cross-sectional clinical trial using low-field-strength MRI was performed in children and adolescents from a single academic medical center. The primary outcome was the frequency of morphologic changes at MRI. Secondary outcomes included MRI-derived functional proton ventilation and perfusion parameters. Clinical symptoms, the duration from positive reverse transcriptase–polymerase chain reaction test result, and serologic parameters were compared with imaging results. Nonparametric tests for pairwise and corrected tests for groupwise comparisons were applied to assess differences in healthy controls, recovered participants, and those with long COVID.
Results
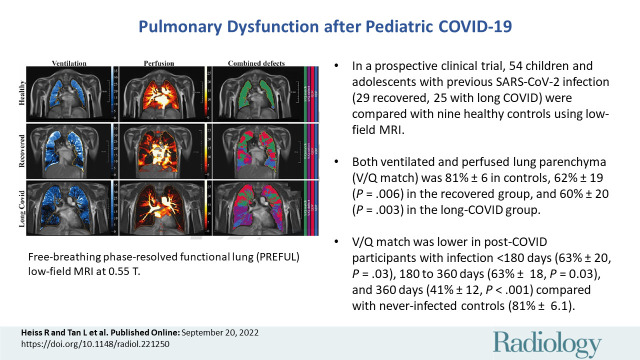
A total of 54 participants after COVID-19 infection (mean age, 11 years ± 3 [SD]; 30 boys [56%]) and nine healthy controls (mean age, 10 years ± 3; seven boys [78%]) were included: 29 (54%) in the COVID-19 group had recovered from infection and 25 (46%) were classified as having long COVID on the day of enrollment. Morphologic abnormality was identified in one recovered participant. Both ventilated and perfused lung parenchyma (ventilation-perfusion [V/Q] match) was higher in healthy controls (81% ± 6.1) compared with the recovered group (62% ± 19; P = .006) and the group with long COVID (60% ± 20; P = .003). V/Q match was lower in patients with time from COVID-19 infection to study participation of less than 180 days (63% ± 20; P = .03), 180–360 days (63% ± 18; P = .03), and 360 days (41% ± 12; P < .001) as compared with the never-infected healthy controls (81% ± 6.1).
Conclusion
Low-field-strength MRI showed persistent pulmonary dysfunction in children and adolescents who recovered from COVID-19 and those with long COVID.
Clinical trial registration no. NCT04990531
© RSNA, 2022
Supplemental material is available for this article.
See also the editorial by Paltiel in this issue.
Summary
Low-field-strength MRI showed persistent pulmonary dysfunction in children and adolescents who recovered from COVID-19 and those with long COVID.
Key Results
■ In a clinical trial, 54 children and adolescents with previous SARS-CoV-2 infection (29 recovered, 25 with long COVID) were compared with nine healthy controls with use of low-field-strength MRI.
■ Ventilated and perfused lung parenchyma (ventilation-perfusion [V/Q] match) was 81% ± 6.1 in healthy controls, 62% ± 19 (P = .006) in the recovered group, and 60% ± 20 (P = .003) in the group with long COVID.
■ V/Q match was lower in participants with time from COVID-19 infection until study participation of less than 180 days (63% ± 20; P = .03), 180–360 days (63% ± 18; P = .03), and 360 days (41% ± 12; P < .001) as compared with never-infected healthy controls (81% ± 6.1).
Introduction
SARS-CoV-2 has emerged as a global pandemic, causing more than 280 million documented infections and 5.4 million deaths through the end of 2021 (1). In comparison with adults, COVID-19 in children and adolescents has a milder course, with recovery within a few weeks (2). There is heterogeneity in the definition and inconsistency in reporting persistent symptoms, ranging from near 0% to 66% for several months after infection (3–8). These findings are further complicated by the fact that there are more objective findings of postacute sequelae and symptoms in younger patients (2,6).
Although there is an increasing understanding of the multiorgan damage caused by COVID-19 beyond the acute phase of infection (9), the nature, frequency, and definition of postacute sequelae in children and adolescents still remains unknown, with a discrepancy in the clinical appearance and objective findings (10). While a number of pediatric studies have lately prioritized research in mental health issues during the COVID-19 pandemic (11,12), other studies have raised concerns about ongoing disease manifestations, such as increased thrombotic state, microangiopathy, and inflammation (13,14).
As the lung is a primary target of the SARS-CoV-2 virus (15), CT has aided in the diagnosis of pulmonary manifestation of COVID-19 in adults (16). Even 3 months after infection, angiographic imaging of pulmonary microcirculation has revealed widespread microangiopathy in over 65% of patients (17). Such techniques using invasive procedures or ionization radiation are not feasible in children. They also seem to have limited diagnostic value, as lung parenchymal changes due to COVID-19 are less obvious and less pronounced in children (18,19). Therefore, there is an unmet clinical need to characterize pulmonary manifestations more precisely in children and adolescents after SARS-CoV-2 infection.
We used low-field-strength MRI for imaging the pediatric lung. At low field strength, this technique has improved the imaging quality near air-tissue interfaces without the need for ionizing radiation (20,21). The aim of the study was to characterize the morphologic and functional changes of lung parenchyma at low-field-strength MRI in children and adolescents with previous reverse transcriptase–polymerase chain reaction (RT-PCR)–positive SARS-CoV-2 infection compared with healthy controls.
Materials and Methods
Trial Design
This prospective study was approved by the local ethics committee (no. 21–206-B). All parents or guardians and participants (if appropriate) gave written informed consent to participate in the study.
With a sensitivity of 90%, specificity of 85%, precision of ±0.15, estimated prevalence of 0.3, confidence level of 95%, and dropout rate of 10%, the power calculation yielded 58 participants (including dropouts). The calculation for the number of participants needed for the control group comparison of two independent samples was derived from the preliminary data and was based on the expected perfusion deficit. In the healthy control group, the value was expected to be 1%, whereas in the group with COVID-19, the value was 16%. With a common (weighted) SD of 11.5, the group size of healthy controls was determined to be 10.
Between August and December 2021, we performed a cross-sectional investigator-initiated trial to investigate lung parenchymal changes in children and adolescents after SARS-CoV-2 infection (ClinicalTrials.gov identifier NCT04990531) at a single academic medical center. We enrolled consecutive patients with COVID-19 from a nationwide search. After assessment for clinical parameters, a blood sample was drawn, and all participants with COVID-19 and healthy controls underwent low-field-strength MRI. Clinical features during and after infection, the time period from positive RT-PCR test result, and laboratory parameters were compared with imaging results. Details are provided in the MRI Protocol and Statistical Analysis sections.
The coordinating clinical investigators were responsible for data collection and site monitoring. The first authors and corresponding author had constant access to the data and performed the statistical analysis as well as the creation of the first draft of the manuscript independent from commercial support.
Participants
In the group with COVID-19, inclusion criteria consisted of a required age of at least 5 years and below 18 years. Eligible patients were required to have a documented positive RT-PCR test result for SARS-CoV-2 regardless of the interval between positive testing and inclusion in the study. Exclusion criteria consisted of acute SARS-CoV-2 infection and a need for isolation or quarantine measures, pregnancy, any critical medical condition, the refusal to undergo MRI, and any contraindications to MRI (eg, electrical implants such as pacemakers or perfusion pumps).
In the healthy control group, inclusion criteria consisted of a required age of at least 5 years and below 18 years. Healthy controls were excluded when there was a previous SARS-CoV-2 infection confirmed by a positive RT-PCR or rapid antigen test result, any clinical or other suspicion of pulmonary disease, current respiratory infection or symptoms, pain resulting in respiratory limitation, acute SARS-CoV-2 infection and need for isolation or quarantine, pregnancy, critical condition, the refusal to undergo MRI, or general contraindications to MRI. In addition, volunteers were excluded if they had a positive test result for SARS-CoV-2 antibodies in their blood sample.
Definition of Long COVID
The definition of long COVID was based on the persistence of symptoms for a minimum of 12 weeks and at least one of the four following criteria (22,23): (a) symptoms that persisted from the acute COVID-19 phase or its treatment, (b) symptoms that resulted in a new health limitation, (c) new symptoms that occurred after the end of the acute phase but were understood to be a consequence of COVID-19, or (d) worsening of a preexisting underlying medical condition. This guideline reflects the current World Health Organization definition of long COVID (24).
Outcome Measures
The primary outcome was the determination of the frequency of morphologic changes of lung parenchyma at low-field-strength proton MRI. Secondary outcomes included functional lung changes comprising ventilation defects (whole-lung ventilation defect percentage [VDP]), perfusion defects (whole-lung perfusion defect percentage [QDP]), the match (ventilation-perfusion [V/Q] match), defect of both (whole-lung V/Q defect), laboratory assessments, and reported clinical symptoms.
Clinical Data and Blood Samples
Participants in the COVID-19 group were assessed for medical history, including symptoms during and after COVID-19 infection. Blood pressure and heart rate were measured in each individual. A blood sample was collected to assess blood count, interleukin 6, C-reactive protein, and antibodies against SARS-CoV-2 (spike protein and nucleocapsid antibodies, electrochemiluminescence immunoassay, Elecsys Anti-SARS-CoV-2 S and Anti-SARS-CoV-2 [Roche], respectively) (Appendix S1).
MRI Protocol
All participants underwent morphologic and functional low-field-strength MRI (0.55-T Magnetom Free.Max, Siemens Healthineers) for visualization of morphologic features and ventilation and perfusion of the lung (25–29). For all investigations, a standard body coil was used for free-breathing lung imaging. The final parameters were one two-dimensional central coronal section positioned at the middle of the lung hila: thickness, 15 mm; in-plane resolution, 1.7 × 1.7 mm; matrix, 128 × 128 (interpolated to 256 × 256); bandwidth, 1149 Hz per pixel; flip angle, 80°; repetition time/echo time, 292.8/1.6 msec; parallel imaging acceleration factor, 2; no partial Fourier; number of time points, 250; temporal resolution, 300 msec; and examination duration, 1 minute 15 seconds. For all MRI data and analyses, the evaluating radiologist (R.H., with 8 years of experience) was blinded to clinical features.
Morphologic lung imaging.—For morphologic lung assessment, images were acquired with a coronal and transversal turbo spin-echo sequence with periodically rotated overlapping parallel lines with enhanced reconstruction, or BLADE, readout and respiratory gating. The coronal image was acquired with a short–inversion time inversion-recovery preparation, T2 weighting (repetition time/echo time, 2500/74 msec), 1.5 × 1.5 mm in-plane resolution, 272 × 272 matrix, and 6-mm section thickness. The transversal image was proton density–weighted (repetition time/echo time, 2000/33 msec), with 1.3 × 1.3 mm in-plane resolution, 304 × 304 matrix, and 6-mm section thickness.
Functional lung assessment.—For free-breathing phase-resolved functional lung, or PREFUL, low-field-strength MRI, several parameters were calculated voxel-wise by using dedicated software (MR Lung version 2.0, Siemens Healthcare) after automatic registration to a midexpiration position and lung parenchyma segmentation (26).
The following parameters were calculated: normalized perfusion (expressed as a
percentage) with respect to a full-blood signal region, determined as the
highest perfusion signal region in between the lungs and expected to reflect the
aorta or other available large vessel (27); regional ventilation (expressed as a percentage), calculated as
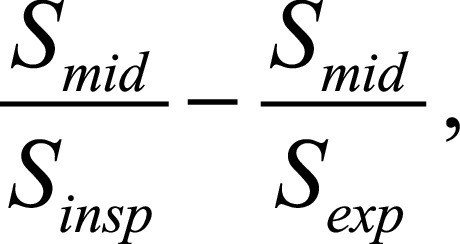 where
S is the signal value at end-inspiration
(insp), end-expiration (exp), and middle
position (mid) (30); and
flow-volume loop correlation, defined as the correlation of the flow-volume loop
(deduced from the ventilation reconstructed cycle) with respect to a healthy
region (largest connected region within the 80th and 90th ventilation
percentiles) (31). Based on those maps,
the percentage of defect areas (QDP, VDP) was calculated based on thresholds
optimized on a large sample (of 155 healthy volunteers and 95 patients with
various lung diseases scanned at 1.5 T with a fast low-angle shot sequence
[perfusion, 2%; fractional ventilation, 40% of the 90th percentile; and
flow-volume loop, 0.9]). The percentage of concurrent defect areas of perfusion
and ventilation metrics (V/Q defect and V/Q defect of the flow-volume loop) and
exclusive perfusion defects (ie, with no concurrent ventilation defect) was
derived, and vice versa (ie, exclusive ventilation defects with no concurrent
perfusion defect based on normalized perfusion and exclusive ventilation defects
based on flow-volume loop correlation). In addition, areas without defects on
both perfusion and ventilation maps (V/Q match and V/Q match based on
flow-volume loop) were calculated. An overview and explanation of all parameters
used is given in Table 1. PREFUL MRI
ventilation and perfusion measures were recently validated using V/Q SPECT and
dynamic contrast-enhanced MRI, as well as fluorine 19 and xenon 129
(129Xe) inhaled-gas MRI (27,32).
where
S is the signal value at end-inspiration
(insp), end-expiration (exp), and middle
position (mid) (30); and
flow-volume loop correlation, defined as the correlation of the flow-volume loop
(deduced from the ventilation reconstructed cycle) with respect to a healthy
region (largest connected region within the 80th and 90th ventilation
percentiles) (31). Based on those maps,
the percentage of defect areas (QDP, VDP) was calculated based on thresholds
optimized on a large sample (of 155 healthy volunteers and 95 patients with
various lung diseases scanned at 1.5 T with a fast low-angle shot sequence
[perfusion, 2%; fractional ventilation, 40% of the 90th percentile; and
flow-volume loop, 0.9]). The percentage of concurrent defect areas of perfusion
and ventilation metrics (V/Q defect and V/Q defect of the flow-volume loop) and
exclusive perfusion defects (ie, with no concurrent ventilation defect) was
derived, and vice versa (ie, exclusive ventilation defects with no concurrent
perfusion defect based on normalized perfusion and exclusive ventilation defects
based on flow-volume loop correlation). In addition, areas without defects on
both perfusion and ventilation maps (V/Q match and V/Q match based on
flow-volume loop) were calculated. An overview and explanation of all parameters
used is given in Table 1. PREFUL MRI
ventilation and perfusion measures were recently validated using V/Q SPECT and
dynamic contrast-enhanced MRI, as well as fluorine 19 and xenon 129
(129Xe) inhaled-gas MRI (27,32).
Table 1:
Functional Low-Field-Strength MRI Parameters
Statistical Analyses
Continuous variables are given as means with SDs and categorical variables as numbers with percentages. The occurrence of MRI changes is given as a percentage of the study sample. A nonparametric Mann-Whitney U test was used for pairwise comparisons. A nonparametric Kruskal-Wallis test with corrected Dunn test for post hoc comparisons between groups was used to assess differences in healthy controls, participants who recovered from COVID-19, and participants with long COVID. Adjusted P values are reported. Prism 9, version 9.3.1 (GraphPad Software) was used for all statistical analyses. P < .05 was considered to indicate statistically significant difference in all analyses.
Results
Participant Characteristics
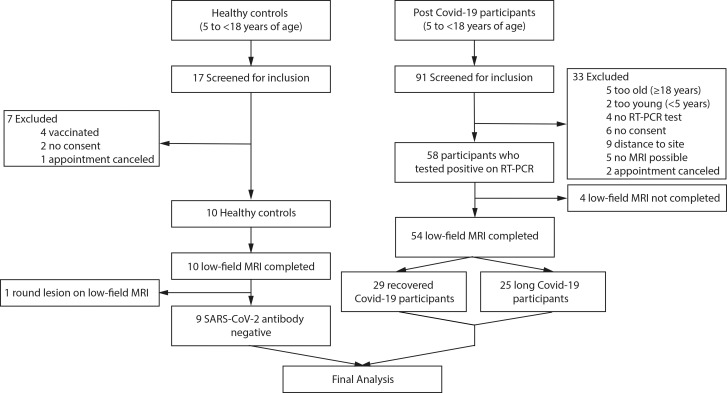
A total of 17 healthy controls and 91 pediatric patients after RT-PCR–positive SARS-CoV-2 infection were screened. Seven healthy controls and 33 patients with postacute COVID-19 were excluded before participation. One healthy control had a pulmonary nodule of unknown origin at morphologic MRI and was therefore excluded. Four patients with postacute COVID-19 were not able to complete low-field-strength MRI scanning and were therefore excluded. Overall, 54 participants with postacute COVID-19 and nine controls completed clinical, laboratory, and low-field-strength MRI assessments (Fig 1).
Figure 1:
Flowchart of the study. RT-PCR = reverse transcriptase–polymerase chain reaction.
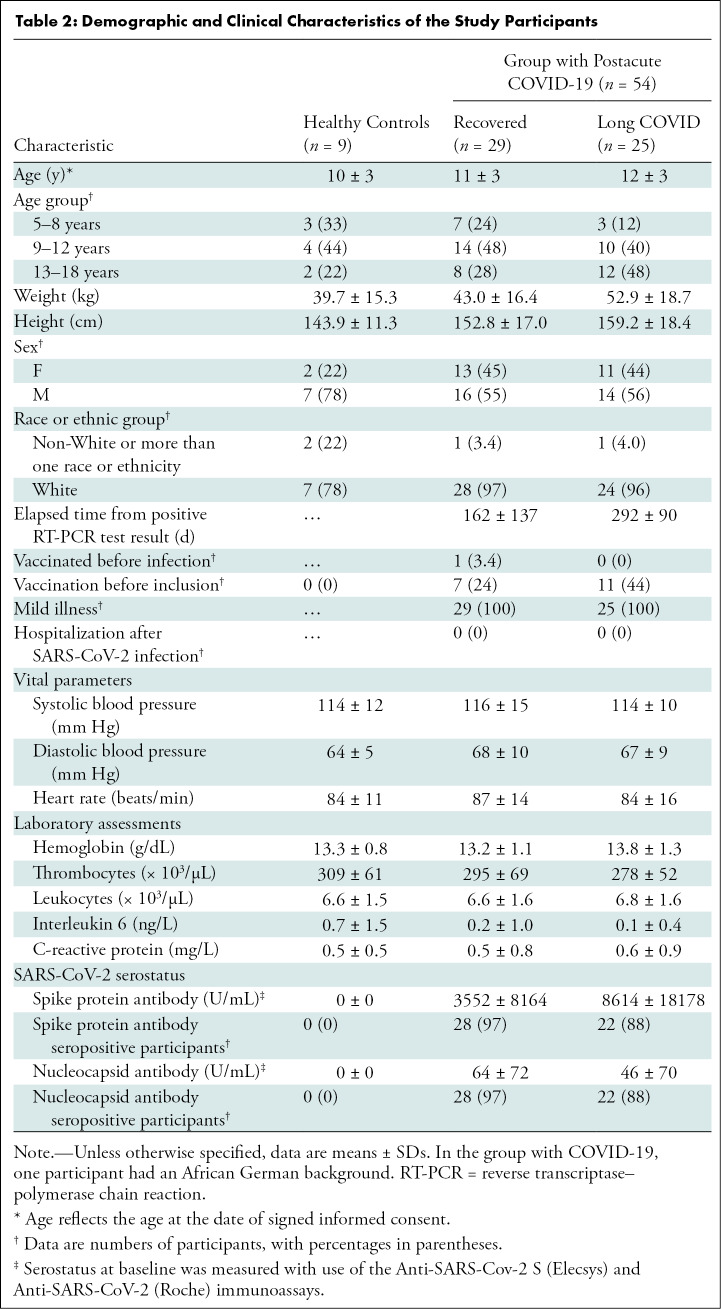
The characteristics of the participants were similar in both groups (Table 2). The mean age of participants with postacute COVID-19 was 12 years ± 3 (SD) (mean age of controls, 10 years ± 3), mean weight was 48 kg ± 18 (mean weight of controls, 40 kg ± 15), mean height was 156 cm ± 17 (mean height of controls, 144 cm ± 11), 30 of 54 participants with COVID-19 (56%) (78% of controls) were male, and 24 of 54 participants (44%) (22% of controls) were female. Of the 54 participants with RT-PCR–positive SARS-CoV-2 infection, 29 (54%) had recovered from infection, while 25 (46%) were classified as having long COVID. Sixteen participants reported shortness of breath (30%); 15, dyspnea (28%); six, impaired attention (11%); five, headache (9.3%); four, anosmia (7.4%); four, fatigue (7.4%); one, pneumonia (1.9%); one, ageusia (1.9%); and one, limb pain (1.9%) (Table S1). Preexisting conditions were found in two of nine healthy volunteers (22%), five of 29 recovered participants (17%), and 10 of 25 participants with long COVID-19 (40%) (Table S2). Detailed data on acute and postacute COVID-19 symptoms can be found in Table S1. Four participants with RT-PCR–positive SARS-CoV-2 infection did not show any symptoms during acute infection. None of the participants with COVID-19 required hospital admission during the primary infection period. The median interval between positive SARS-CoV-2 RT-PCR test and study participation was 222 days ± 134. There were no missing primary and/or secondary outcome data.
Table 2:
Demographic and Clinical Characteristics of the Study Participants
Primary Outcome
Of the 54 participants in the group with postacute COVID-19 and nine healthy controls scanned with low-field-strength MRI, only one participant in the recovered group showed any morphologic changes (linear atelectasis) (Fig S1).
Secondary Outcomes
When compared with healthy controls, participants with COVID-19 had larger ventilation defects (VDP, 13% ± 3.6 [SD] vs 23% ± 9.0; P < .001), larger but not statistically significant perfusion defects (QDP, 6.5% ± 5.0 vs 20% ± 19; P = .05), and larger combined defects (V/Q mismatch, 0.5 ± 0.8 vs 4.6 ± 5.7; P = .001) at functional low-field-strength MRI. V/Q match was lower in the group with COVID-19 (61% ± 19; P < .001) when compared with healthy controls (81% ± 6.1) (Fig S2).
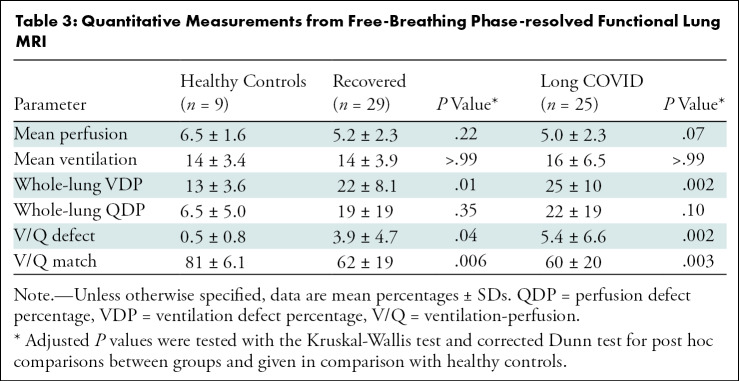
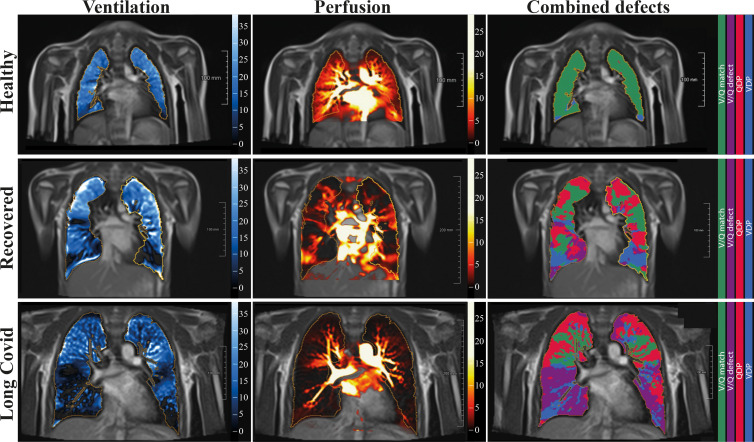
When separating the group with COVID-19 according to clinical characteristics, the overall VDP was lower in healthy controls (13% ± 3.6 [SD]) than in the recovered group (22% ± 8.1; P = .01) or the group with long COVID (25% ± 10; P = .002). Similarly, QDP was higher in the recovered group (19% ± 19; P = .35) and in the group with long COVID (22% ± 19; P = .10) as compared with healthy controls (6.5% ± 5.0), but the difference was not statistically significant. Combined V/Q defects were lower in healthy controls, with 0.5% ± 0.8 compared with 3.9% ± 4.7 (P = .04) in the recovered group and 5.4% ± 6.6 (P = .002) in the group with long COVID. Similarly, V/Q match was higher in healthy controls, with 81% ± 6.1 compared with 62% ± 19 (P = .006) in the recovered group and 60% ± 20 (P = .003) in the group with long COVID (Tables 3, S3; Fig S3). Representative functional low-field-strength MRI scans are shown in Figure 2; the corresponding morphologic images are shown in Figure S4. For a complete imaging list displaying combined V/Q defects, see Figures S5–S7).
Table 3:
Quantitative Measurements from Free-Breathing Phase-resolved Functional Lung MRI
Figure 2:
Free-breathing phase-resolved functional lung, or PREFUL, low-field-strength MRI at 0.55 T with calculated parameters at the axial plane after automatic registration to a midexpiration position and lung parenchyma segmentation. From left to right, representative color-coded images from functional lung MRI show ventilation defects (blue), perfusion defects (red), ventilation-perfusion (V/Q) match (green), and V/Q defects (purple) in a healthy control participant (upper row, 7-year-old boy), a participant who recovered from COVID-19 (middle row, 10-year-old boy), and a participant with long COVID (bottom row, 15-year-old boy). QDP = perfusion defect percentage, VDP = ventilation defect percentage.
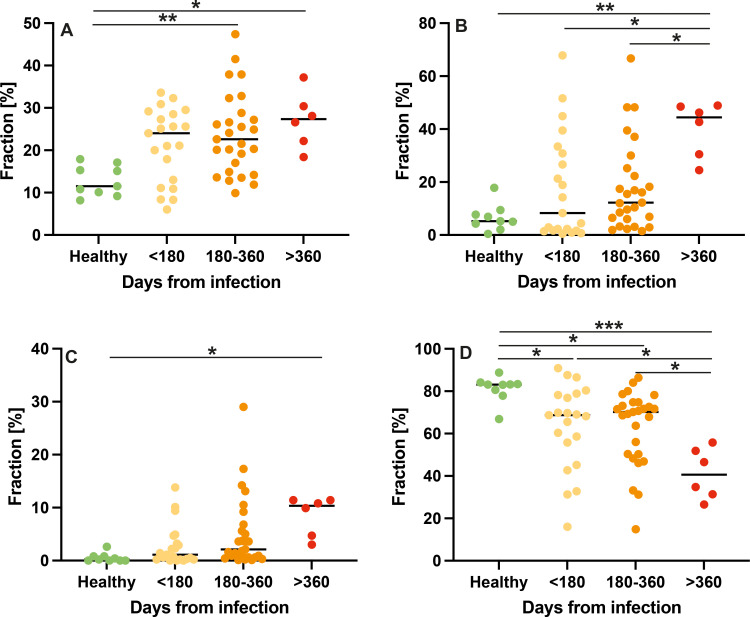
When participants were separated according to time from infection, an increase in ventilation, perfusion, and combined defects was found (Fig 3A–C). In turn, V/Q match was reduced in participants with long COVID with time from infection to study participation of less than 180 days (63% ± 20; P = .03), 180–360 days (63% ± 18; P = .03), and 360 days (41% ± 12; P < .001) as compared with never-infected healthy controls (81% ± 6.1) (Fig 3D).
Figure 3:
Dot plots show the comparison of low-field-strength MRI parameters with respect to the interval from first infection. The y-axis shows the fraction of defect or nondefect lung parenchyma on the automated measured axial plane. (A) Ventilation defects, (B) perfusion defects, (C) ventilation-perfusion (V/Q) defects, and (D) V/Q match in healthy controls and in participants at less than 180 days, 180–360 days, and more than 360 days after SARS-CoV-2 infection. Midlines indicate the means, and dots represent a single data point or measurement for one participant. * = P < .05, ** = P < .01, and *** = P < .001.
Laboratory Assessments
Of the 54 participants with postacute COVID-19, four showed negative spike protein and nucleocapsid antibody level at time of presentation (time range to RT-PCR–proven infection, 186–416 days). Two participants with postacute COVID-19 had reactive spike protein antibodies without reactive nucleocapsid antibodies (time range to RT-PCR–proven infection, 40–339 days). All nine healthy controls were confirmed with negative spike protein and nucleocapsid antibody levels.
Inflammation parameters, including C-reactive protein, interleukin 6, and blood counts, were not suggestive of a current infection on the day of study for any participant.
Discussion
The purpose of the study was to characterize both morphologic and functional changes of lung parenchyma at low-field-strength MRI in children and adolescents with post–COVID-19 condition compared with healthy controls. A total of 54 children and adolescents with post–COVID-19 condition (29 recovered, 25 with long COVID) and nine healthy controls were included. Only one participant showed any morphologic abnormality (linear atelectasis). Using functional parameters, we found a reduction in ventilation-perfusion (V/Q) match from 81% ± 6.1 (SD) in healthy controls to 62% ± 19 (P = .006) in the recovered group and 60% ± 20 (P = .003) in the group with long COVID. Furthermore, V/Q match was lower in participants with COVID with time from infection to study participation of less than 180 days (63% ± 20; P = .03), 180–360 days (63% ± 19; P = .03), and 360 days (41% ± 12; P < .001) as compared with never-infected healthy controls (81% ± 6.1).
Similar imaging approaches to the one used in our study have already proven to be able to visualize pathologic changes in pulmonary hypertension, cystic fibrosis, and chronic obstructive pulmonary disease (27–29). Specifically for COVID-19, our findings correspond to observations in adults, where vascular (17) or structural abnormalities (33) persist in previously hospitalized adults. Another technique using inhaled gas contrast agents (hyperpolarized 129Xe MRI) to assess lung function has already been successfully applied in adults with post–COVID-19 condition. These studies showed normal findings at CT but also a limitation in pulmonary capillary gas diffusion (34,35). In a recent study of 34 adult patients with post–COVID-19 condition, of whom 22 had never been hospitalized, and six controls, a correlation of 129Xe MRI parameters with CT pulmonary vascular abnormalities was described (36). Interestingly, Trinkmann et al (37) were also able to demonstrate that one-half of mostly younger adult study patients had persistent symptoms and reduced lung function for more than 2 months after infection. These results may partly correspond to the high frequency of abnormal imaging findings in our study sample, wherein 28% of participants with post–COVID-19 condition still reported dyspnea and 30% reported shortness of breath.
The low-field-strength MRI (0.55 T) used in this study has advantages for the morphologic imaging of lung parenchyma when compared with 1.5-T and 3-T systems (20). In contrast to studies that are mostly based on surveys or self-reported outcomes, which suggest less severe COVID-19 infections and sequelae in younger patients, our study demonstrates that widespread functional lung alterations are indeed present in children and adolescents. This expands the understanding of pediatric postacute COVID-19 disease, particularly given the increased incidence of SARS-CoV-2 infection (1). A better estimate on the prevalence of pediatric post–COVID-19 lung disease is further complicated by the inconsistent, largely symptom-based definitions of long COVID disease (9) and their limited applicability to children.
The pathophysiologic abnormalities of acute and postacute COVID-19 partly originate from direct endothelial damage, local inflammation, and prothrombotic milieu (15,38,39). A proposed mechanism is the angiotensin-converting enzyme 2–mediated entry of SARS-CoV-2, which allows the virus to directly invade endothelial cells (15,40). This may explain manifestations such as pulmonary microangiopathy and widespread capillary microthrombi seen in autopsies of patients who died of COVID-19 (15) and fibrotic-like consolidations found at CT (33). Previously described (14) persistent signs of inflammatory processes could not be confirmed in our study. Because children develop a robust, cross-reactive, and sustained immune response after SARS-CoV-2 infection (41), the observed pulmonary dysfunction in our study is an unexpected finding.
Our study had several limitations. First, we did not compare our measurements with another reference standard, such as V/Q scintigraphy, spirometry, or body plethysmography. However, most of these modalities either use ionization radiation, are invasive, or require active cooperation. Second, the functional low-field-strength MRI in our study used free breathing at all intervals, which was feasible starting from 5 years of age in 93% of the pediatric group with postacute COVID-19 condition. Third, our study lacked longitudinal data. Fourth, we had a low number of healthy controls. Finally, selection bias exists, as families with children with acute or postacute symptomatic COVID-19 and higher disease burden might have been more likely to participate in our study.
In summary, we report persistent pulmonary dysfunction as visualized at low-field-strength MRI in both children and adolescents who recovered from COVID-19 and those with long COVID. The further course and outcome of the observed changes currently remains unclear. Our results warrant further surveillance of persistent pulmonary damage in children and adolescents after SARS-CoV-2 infection. Given the already existing diagnostic value of lung MRI (42) and the translatability of the technology, these imaging approaches can be rapidly adopted in clinical routine care.
Acknowledgments
Acknowledgments
We thank the Imaging Science Institute Erlangen for providing us with measurement time and technical support. Many thanks to Natalie Wenisch, Kristin Pribylla, and Annika Maischak for technical assistance during MRI and to the staff of the Center for Social Pediatrics at the University Hospital Erlangen during patient recruitment. The present work was performed in (partial) fulfillment of the requirements for obtaining the degree “Dr. med.” for L.T. and in (partial) fulfillment of the requirements for obtaining the degree “Dr. rer. biol. hum.” for A.P.R.
R.H. and L.T. contributed equally to this work.
A.L.W. and F.K. are co–senior authors.
A.P.R. supported by the Junior Project (J089) and Clinician Scientist Program from the Interdisciplinary Center for Clinical Research (IZKF) at the Friedrich-Alexander-Universität Erlangen-Nürnberg. This work received funding from the Bayerisches Staatsministerium für Wissenschaft und Kunst. The funding bodies had no role in the study design, collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Disclosures of conflicts of interest: R.H. Member of the Siemens Healthineers speakers bureau. L.T. No relevant relationships. S.S. No relevant relationships. A.P.R. No relevant relationships. F.E. No relevant relationships. D.M. No relevant relationships. A.B. No relevant relationships. J.V.C. No relevant relationships. A.V. Research grant from Siemens Healthineers. M.R. No relevant relationships. O.R. No relevant relationships. A.M.N. Research support from Siemens Healthineers. S.L. No relevant relationships. S.B. Lecture fees from Siemens Healthineers; research coordinator for the Bavarian Digital Health Initiative by the BayFor (Bavarian Research Alliance); research support to institution from Siemens Healthineers. M.S.M. Honoraria for lectures from Siemens Healthineers and Bayer Healthcare. M.U. Member of the Siemens Healthineers speakers bureau. M.M. No relevant relationships. R.T. No relevant relationships. J.W. Consulting fees from Ipsen, Hexal, and Novo Nordisk; honoraria for lectures from Pfizer, Merck Serono, and Novo Nordisk; advisory board membership fees from Ipsen, Hexal, and Novo Nordisk; treasurer and past president of the German Society for Pediatric Endocrinology and board member of patient advocacy group for people with short stature. A.L.W. Research grant from Bayerischen Wissenschaftsministeriums zur Corona-Forschung. F.K. Research grants from Sanofi Genzyme, Else Kröner-Fresenius-Stiftung, IMI J2 EU Horizon 2020, Deutsche Gesellschaft für Ultraschall in der Medizin, Interdisciplinary Center for Clinical Research, UK Erlangen, Bayerische Forschungsstiftung, and the Bavarian Ministry of Health; lecture fees from Sanofi Genzyme and Siemens Healthineers; travel support from iThera Medical; patent for device and method for analyzing optoacoustic data, optoacoustic system, and computer program.
Abbreviations:
- QDP
- perfusion defect percentage
- RT-PCR
- reverse transcriptase–polymerase chain reaction
- VDP
- ventilation defect percentage
- V/Q
- ventilation-perfusion
References
- 1. WHO Health Emergency Dashboard . https://covid19.who.int. Accessed May 2022 .
- 2. Say D , Crawford N , McNab S , Wurzel D , Steer A , Tosif S . Post-acute COVID-19 outcomes in children with mild and asymptomatic disease . Lancet Child Adolesc Health 2021. ; 5 ( 6 ): e22 – e23 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Behnood SA , Shafran R , Bennett SD , et al . Persistent symptoms following SARS-CoV-2 infection amongst children and young people: a meta-analysis of controlled and uncontrolled studies . J Infect 2022. ; 84 ( 2 ): 158 – 170 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sterky E , Olsson-Åkefeldt S , Hertting O , et al . Persistent symptoms in Swedish children after hospitalisation due to COVID-19 . Acta Paediatr 2021. ; 110 ( 9 ): 2578 – 2580 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Radtke T , Ulyte A , Puhan MA , Kriemler S . Long-term symptoms after SARS-CoV-2 infection in children and adolescents . JAMA 2021. ; 326 ( 9 ): 869 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buonsenso D , Munblit D , De Rose C , et al . Preliminary evidence on long COVID in children . Acta Paediatr 2021. ; 110 ( 7 ): 2208 – 2211 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Osmanov IM , Spiridonova E , Bobkova P , et al . Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC global follow-up protocol: a prospective cohort study . Eur Respir J 2022. ; 59 ( 2 ): 2101341 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kikkenborg Berg S , Palm P , Nygaard U , et al . Long COVID symptoms in SARS-CoV-2-positive children aged 0-14 years and matched controls in Denmark (LongCOVIDKidsDK): a national, cross-sectional study . Lancet Child Adolesc Health 2022. ; 6 ( 9 ): 614 – 623 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nalbandian A , Sehgal K , Gupta A , et al . Post-acute COVID-19 syndrome . Nat Med 2021. ; 27 ( 4 ): 601 – 615 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zimmermann P , Pittet LF , Curtis N . How common is long COVID in children and adolescents? Pediatr Infect Dis J 2021. ; 40 ( 12 ): e482 – e487 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Molteni E , Sudre CH , Canas LS , et al . Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2 . Lancet Child Adolesc Health 2021. ; 5 ( 10 ): 708 – 718 . [Published correction appears in Lancet Child Adolesc Health. 2021 Aug 31.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zavala M , Ireland G , Amin-Chowdhury Z , Ramsay ME , Ladhani SN . Acute and persistent symptoms in children with polymerase chain reaction (PCR)-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection compared with test-negative children in England: active, prospective, national surveillance . Clin Infect Dis 2022. ; 75 ( 1 ): e191 – e200 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fogarty H , Townsend L , Morrin H , et al . Persistent endotheliopathy in the pathogenesis of long COVID syndrome . J Thromb Haemost 2021. ; 19 ( 10 ): 2546 – 2553 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buonsenso D , Di Giuda D , Sigfrid L , et al . Evidence of lung perfusion defects and ongoing inflammation in an adolescent with post-acute sequelae of SARS-CoV-2 infection . Lancet Child Adolesc Health 2021. ; 5 ( 9 ): 677 – 680 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ackermann M , Verleden SE , Kuehnel M , et al . Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19 . N Engl J Med 2020. ; 383 ( 2 ): 120 – 128 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ai T , Yang Z , Hou H , et al . Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases . Radiology 2020. ; 296 ( 2 ): E32 – E40 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Remy-Jardin M , Duthoit L , Perez T , et al . Assessment of pulmonary arterial circulation 3 months after hospitalization for SARS-CoV-2 pneumonia: dual-energy CT (DECT) angiographic study in 55 patients . EClinicalMedicine 2021. ; 34 : 100778 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shelmerdine SC , Lovrenski J , Caro-Domínguez P , Toso S ; Collaborators of the European Society of Paediatric Radiology Cardiothoracic Imaging Taskforce. Coronavirus disease 2019 (COVID-19) in children: a systematic review of imaging findings . Pediatr Radiol 2020. ; 50 ( 9 ): 1217 – 1230 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Steinberger S , Lin B , Bernheim A , et al . CT features of coronavirus disease (COVID-19) in 30 pediatric patients . AJR Am J Roentgenol 2020. ; 215 ( 6 ): 1303 – 1311 . [DOI] [PubMed] [Google Scholar]
- 20. Campbell-Washburn AE , Ramasawmy R , Restivo MC , et al . Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI . Radiology 2019. ; 293 ( 2 ): 384 – 393 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rashid S , Han F , Gao Y , et al . Cardiac balanced steady-state free precession MRI at 0.35 T: a comparison study with 1.5 T . Quant Imaging Med Surg 2018. ; 8 ( 7 ): 627 – 636 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koczulla A , Ankermann T , Behrends U , et al . S1-Leitlinie Long/ Post-COVID . AWMF online . https://www.awmf.org/uploads/tx_szleitlinien/020-027l_S1_Post_COVID_Long_COVID_2022-08.pdf. Published 2021. Accessed May 2022 .
- 23. Ceravolo MG , Arienti C , de Sire A , et al . Rehabilitation and COVID-19: the Cochrane Rehabilitation 2020 rapid living systematic review . Eur J Phys Rehabil Med 2020. ; 56 ( 5 ): 642 – 651 . [DOI] [PubMed] [Google Scholar]
- 24. Soriano JB , Murthy S , Marshall JC , Relan P , Diaz JV; . WHO Clinical Case Definition Working Group on Post-COVID-19 Condition . A clinical case definition of post-COVID-19 condition by a Delphi consensus . Lancet Infect Dis 2022. ; 22 ( 4 ): e102 – e107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heiss R , Grodzki DM , Horger W , Uder M , Nagel AM , Bickelhaupt S . High-performance low field MRI enables visualization of persistent pulmonary damage after COVID-19 . Magn Reson Imaging 2021. ; 76 : 49 – 51 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Voskrebenzev A , Gutberlet M , Klimeš F , et al . Feasibility of quantitative regional ventilation and perfusion mapping with phase-resolved functional lung (PREFUL) MRI in healthy volunteers and COPD, CTEPH, and CF patients . Magn Reson Med 2018. ; 79 ( 4 ): 2306 – 2314 . [DOI] [PubMed] [Google Scholar]
- 27. Behrendt L , Voskrebenzev A , Klimeš F , et al . Validation of automated perfusion-weighted phase-resolved functional lung (PREFUL)-MRI in patients with pulmonary diseases . J Magn Reson Imaging 2020. ; 52 ( 1 ): 103 – 114 . [DOI] [PubMed] [Google Scholar]
- 28. Glandorf J , Klimeš F , Voskrebenzev A , et al . Comparison of phase-resolved functional lung (PREFUL) MRI derived perfusion and ventilation parameters at 1.5T and 3T in healthy volunteers . PLoS One 2020. ; 15 ( 12 ): e0244638 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pöhler GH , Klimeš F , Behrendt L , et al . Repeatability of phase-resolved functional lung (PREFUL)-MRI ventilation and perfusion parameters in healthy subjects and COPD patients . J Magn Reson Imaging 2021. ; 53 ( 3 ): 915 – 927 . [DOI] [PubMed] [Google Scholar]
- 30. Klimeš F , Voskrebenzev A , Gutberlet M , et al . Free-breathing quantification of regional ventilation derived by phase-resolved functional lung (PREFUL) MRI . NMR Biomed 2019. ; 32 ( 6 ): e4088 . [DOI] [PubMed] [Google Scholar]
- 31. Moher Alsady T , Voskrebenzev A , Greer M , et al . MRI-derived regional flow-volume loop parameters detect early-stage chronic lung allograft dysfunction . J Magn Reson Imaging 2019. ; 50 ( 6 ): 1873 – 1882 . [DOI] [PubMed] [Google Scholar]
- 32. Kaireit TF , Kern A , Voskrebenzev A , et al . Flow volume loop and regional ventilation assessment using phase-resolved functional lung (PREFUL) MRI: comparison with 129xenon ventilation MRI and lung function testing . J Magn Reson Imaging 2021. ; 53 ( 4 ): 1092 – 1105 . [DOI] [PubMed] [Google Scholar]
- 33. Han X , Fan Y , Alwalid O , et al . Six-month follow-up chest CT findings after severe COVID-19 pneumonia . Radiology 2021. ; 299 ( 1 ): E177 – E186 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li H , Zhao X , Wang Y , et al . Damaged lung gas exchange function of discharged COVID-19 patients detected by hyperpolarized 129Xe MRI . Sci Adv 2021. ; 7 ( 1 ): eabc8180 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grist JT , Chen M , Collier GJ , et al . Hyperpolarized 129Xe MRI abnormalities in dyspneic patients 3 months after COVID-19 pneumonia: preliminary results . Radiology 2021. ; 301 ( 1 ): E353 – E360 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matheson AM , McIntosh MJ , Kooner HK , et al . Persistent 129Xe MRI pulmonary and CT vascular abnormalities in symptomatic individuals with post-acute COVID-19 syndrome . Radiology 2022. . 10.1148/radiol.220492. Published online June 28, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trinkmann F , Müller M , Reif A , et al . Residual symptoms and lower lung function in patients recovering from SARS-CoV-2 infection . Eur Respir J 2021. ; 57 ( 2 ): 2003002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Teuwen LA , Geldhof V , Pasut A , Carmeliet P . COVID-19: the vasculature unleashed . Nat Rev Immunol 2020. ; 20 ( 7 ): 389 – 391 . [Published correction appears in Nat Rev Immunol 2020;20(7):448.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Varga Z , Flammer AJ , Steiger P , et al . Endothelial cell infection and endotheliitis in COVID-19 . Lancet 2020. ; 395 ( 10234 ): 1417 – 1418 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McFadyen JD , Stevens H , Peter K . The emerging threat of (micro)thrombosis in COVID-19 and its therapeutic implications . Circ Res 2020. ; 127 ( 4 ): 571 – 587 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dowell AC , Butler MS , Jinks E , et al . Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection . Nat Immunol 2022. ; 23 ( 1 ): 40 – 49 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hirsch FW , Sorge I , Vogel-Claussen J , et al . The current status and further prospects for lung magnetic resonance imaging in pediatric radiology . Pediatr Radiol 2020. ; 50 ( 5 ): 734 – 749 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Muench P , Jochum S , Wenderoth V , et al . Development and validation of the Elecsys Anti-SARS-CoV-2 immunoassay as a highly specific tool for determining past exposure to SARS-CoV-2 . J Clin Microbiol 2020. ; 58 ( 10 ): e01694 – 20 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fact sheet, ElecsysT Anti-SARS-CoV-2 S . https://diagnostics.roche.com/content/dam/diagnostics/Blueprint/en/pdf/cps/Elecsys-Anti-SARS-CoV-2-S-factsheet-SEPT-2020-2.pdf. Accessed May 2022 .