Abstract
Background and purpose
Diffusion tensor imaging (DTI) can detect microstructural changes of white matter in multiple sclerosis (MS) and might clarify mechanisms responsible for disability. Thus, we aimed to compare DTI metrics in relapsing-remitting MS patients (RRMS) with healthy controls (HCs), and explore the correlations between DTI metrics, total brain white matter (TBWM) and white matter lesion (WML) with clinical parameters compared to volumetric measures.
Material and methods
37 RRMS patients and 19 age/sex-matched HCs were included. All participants had clinical assessments, structural and diffusion scans on a 3T MRI. Volumetric and white matter DTI metrics; fractional anisotropy (FA), mean, radial and axial diffusivities (MD, RD and AD) were estimated and correlated with clinical parameters. The mean group differences were calculated using t-tests, and univariate correlations with Pearson correlation coefficients.
Results
Compared to HCs, statistically significant increases in MD (+3.6%), RD (+4.8%), AD (+2.7%) and a decrease in FA (−4.3%) for TBWM in RRMS was observed (p < .01). MD and RD in TBWM and AD in WML correlated moderately with disability status. Volumetric segmentation indicated a decrease in the total brain volume, GM and WM(−5%) with a reciprocal increase in CSF(+26%) in RRMS(p < .01). Importantly, DTI parameters showed a medium correlation with cognitive domains in contrast to white matter-related volumetric measurements in RRMS(Pearson correlation, p < .05).
Conclusions
Our study shows a correlation of DTI metrics with clinical symptoms of MS, in particular cognition. More generally, these findings indicated that DTI is a useful and unique technique for evaluating the clinical features of white matter disease and warrants further investigation into its clinical role.
Keywords: Multiple sclerosis, diffusion tensor imaging, volumetric MRI, white matter, clinical parameters
Introduction
Multiple sclerosis (MS) is one of the most common chronic inflammatory diseases among young adults characterised by demyelination and gradual axonal loss. 1 The clinical symptoms of MS include increased fatigue, blurred vision, bladder control issues, poor balance and coordination, cognitive deficits, poor muscle control and numbness or pain. 2 Depending on the type and extent of the disease, these symptoms may relapse and remit in phases and with varying intensity. 3 Diagnosis of MS is crucial in the early management of this disease which entails an array of clinical, laboratory and imaging workups. MRI plays a vital role in the diagnosis and management of MS as it recognises the focal structural abnormalities in the deep white and grey matter (WM, GM) regions. 4 Structural MRI involving T2/T2-FLAIR/T1 weighted sequences are the mainstay for assessing lesion burden and the presence of active lesions. However, the volume or number of these lesions cannot be accurately matched with clinical disability with this technique due to its inability to measure the change beyond the gross structural level. 5 The so-called clinic-radiological paradox makes this qualitative approach less specific to changes observed in total brain white matter (TBWM). 5 Recently, substantial advances in MRI technology in the detection and monitoring of MS were achieved. 6 A number of quantitative MRI measures (functional imaging, perfusion, diffusion imaging and spectroscopy) have been extensively utilised in research to predict disease progression and assess the efficacy of treatment. 7 One such advanced quantitative method is diffusion tensor imaging (DTI) which allows the evaluation of microstructural integrity of WM. 8
Diffusion tensor imaging is generally regarded as an extension of diffusion-weighted imaging (DWI) used in a routine clinical setting. 8 While DWI measures the magnitude of random motion of water molecules in the brain, DTI quantifies both magnitude and direction of this movement providing in vivo information of microstructural WM architecture of brain tissue as well as potential pathophysiological substrates of cognitive deficits in MS. The most common quantitative DTI parameters specific to MS are fractional anisotropy (FA; unitless), mean, radial and axial diffusivities (MD, RD, AD; in mm2/sec). 9 Being the main indicator, FA is a scalar quantity between 0 and 1 (0 represents perfect isotropic diffusivity, highest in compact WM tracts, decreased in the GM and near 0 in cerebrospinal fluid (CSF)). 10 A reduction in FA value can be reflective of tissue damage such as demyelination. 11 In a single white matter fibre bundle, AD can be regarded as an indicator of axonal integrity, 8 RD measures diffusion across the fibres and can be associated with myelin integrity. 8 MD is a scalar quantity used to measure overall diffusion (average diffusion) within a voxel, that may represent vasogenic oedema, as well as axonal and myelin loss. 8
Diffusion tensor imaging metrics have been employed in the evaluation of different tissue types as well as lesions in different subtypes of MS yielding mixed results. 8 Significantly increased diffusivity and decreased FA values have been noted in lesions and the TBWM of relapse-remitting MS (RRMS) patients and more in secondary progressive MS patients reflecting more axonal destruction in the latter. 12
The aim of this study was twofold. The first was to compare the DTI metrics in a group of RRMS patients with age and sex-matched healthy controls. The second was to identify and measure the correlations of clinical parameters with different DTI metrics in TBWM and white matter lesion (WML) as well as white matter-related volumetric measures.
Materials and methods
Participants
Thirty-seven clinically definite RRMS patients (20 treated with dimethyl fumarate, 17 treated with fingolimod), aged between 23 and 54 year (mean age, 39.04 ± 1.77 year), undergoing immunomodulatory therapy for a minimum of 6 month with no new clinical symptoms in the last 6 month, were included in the current study after providing written informed consent. 19 age and sex-matched healthy controls (HCs) (mean age, 38.15 ± 2.80 year) were selected from the local research register. The inclusion criteria for patients were 1) fulfilling RRMS diagnosis according to McDonalds’ criteria, and 2) Expanded Disability Status Scale (EDSS) ≤ 4.0. 13 The selection of HCs was based on 1) no prior/current psychiatric or neurological disorders and 2) not currently on any medications. All participants have also successfully completed an MRI safety checklist and committed to complete all components and sessions of the study (including imaging and neuropsychological evaluations). Approval to conduct this study was obtained from the human research ethics committee of the local healthcare district (HREC no required). The demographic details and clinical characteristics of both groups of RRMS and HCs are presented in Table 1.
Table 1.
Cross-sectional analysis of mean demographic scores and disease-related variables for RRMS (undergoing with two different types of disease-modifying therapies) and HCs groups at baseline.
| Characteristics | HCs (N = 19) | RRMS (N = 37) |
|---|---|---|
| Sex (% female) | 80% | 78% |
| Age | 35 ± 1.61 | 37 ± 1.21 |
| Disease duration (years) | — | 7 ± 1 |
| EDSS | — | 1.9 ± 0.15 |
| MSSS | — | 3.7 ± 0.3 |
| ARR | — | 1 ± 0.22 |
| tARCS | 90 ± 3.5 | 83 ± 2.7 |
| Memory | 90 ± 2.5 | 86 ± 3.8 |
| Fluency | 89 ± 4.3 | 79 ± 2.5* |
| Visuospatial | 99 ± 0.63 | 102 ± 0.69* |
| Language | 94 ± 4.12 | 85 ± 3.03 |
| Attention | 97 ± 2.1 | 94 ± 2.62 |
| SDMT | 64 ± 2.31 | 52 ± 1.75** |
| DASS-21 | 8 ± 1.62 | 28 ± 4.24** |
| Stress | 5 ± 1.2 | 13 ± 1.90** |
| Anxiety | 2 ± 0.52 | 8 ± 1.44** |
| Depression | 2 ± 0.39 | 8 ± 1.50** |
| MFIS | 12 ± 2.5 | 36 ± 3.03** |
| Physical fatigue | 5 ± 0.93 | 18 ± 1.50** |
| Cognitive fatigue | 7 ± 1.7 | 18 ± 1.65** |
Values are expressed in mean ± standard error of measurement.
HCs: healthy controls; MS: multiple sclerosis; EDSS: expanded disability status scale; MSSS: multiple sclerosis severity score; ARR: Annualised relapse rate; tARCS: total audio recorded cognitive screen; SDMT: Symbol Digit Modalities Test; DASS: depression, anxiety, stress scale; MFIS: modified fatigue impact scale.
*p-value ≤ .05, **p-value ≤ .01.
Clinical assessments
The clinical assessment consisted of a comprehensive neurological examination for disability status by their treating neurologist using EDSS. 14 MS Severity Score (MSSS) 15 was also calculated. The neuropsychological test for cognitive performance was assessed using total Audio Recorded Cognitive Screen (tARCS), 16 which measures executive functioning/attention, memory, language, verbal fluency and visuospatial functioning. Symbol Digit Modalities Test (SDMT) 17 was utilised for measuring information processing, speed and attention. Fatigue was assessed with the Modified Fatigue Impact Scale (MFIS). 18 All clinical assessments were carried out on the same day as the imaging session.
Imaging (structural and DTI)
The MRI data were obtained with a 3T Magnetom PRISMA system (Siemens Healthineers, Erlangen, Germany) equipped with a 64-channel receiver head coil.
The imaging protocol included three dimensional (3D) isotropic T1 weighted magnetisation-prepared rapid gradient-echo (T1-MPRAGE), 3D T2 weighted fluid-attenuated spin-echo sequence (T2-FLAIR) and diffusion-weighted sequence.
The 3D T1-MPRAGE sequence was used for anatomical reference with the following parameters: repetition time (TR): 2000 ms; echo time (TE): 3.5 ms, inversion time (TI): 1100 ms and flip angle of 7°. Field of view (FOV) was 256 mm2 with a voxel size of 1 mm3 and acquisition time of 5 min.
For assessment of lesion load, a 3D T2-FLAIR weighted sequence was used with TR/TE/TI = 5000/386/1800 ms, and a flip angle of 120°. FOV was 256 mm2 with a voxel size of 1 mm3 and an acquisition time of 6 min.
Axial diffusion-weighted MRI were obtained using a fat-suppressed single-shot echo-planar imaging (EPI) sequence. The MRI signal was sensitised to diffusion by application of a pair of bipolar trapezoidal gradient pulses with a duration of effective δ = 17.9 ms and effective diffusion time Δ = 31.9 ms in 64 equally and spherically distributed directions; TR/TE: 9400/69 ms; number of slices:70; slice thickness: 2 mm; no gap; FOV: 240 mm2 with a voxel size of 2 mm 3 ; and b-value of 3000 s/mm2. In addition, three non–diffusion-weighted volumes (b-value = 0 s/mm2 and AP/PA directions) were acquired for distortion correction. The total acquisition time was 10 min.
Data analysis, post-processing and segmentation
Structural volumetric and diffusion data were exported from Siemens Syngo in DICOM format. Total brain volume, in addition to WM, GM and ventricular CSF volumes were calculated using FSL SIENAX. 19
To identify the healthy control WM regions corresponding to the WML location in RRMS, an average lesion mask was generated and co-registered to the HCs T1-MPRAGE images for segmentation. Diffusion tensor imaging analyses were performed by icometrix (Leuven, Belgium) using MRtrix 3.0_RC2-61-g068b1398. 20 The icometrix DTI pipeline consisted of denoising, 19 Gibbs ringing artefact removal, 21 followed by bias field correction using Advanced Normalization Tools N4. 22 (ANTs 2.1.0). Subsequently, motion and eddy-current induced distortions were corrected for through affine registrations of all images to the first b = 0 image. Susceptibility-induced artefacts were corrected for by creating an artefact free template from the AP and PA phase-encoded b = 0 to which all images were non-rigidly registered, restricting deformation to the phase-encoding direction. 23 Finally, the corrected diffusion data were rigid transformed to T1 subject space using niftyreg
(http://cmictig.cs.ucl.ac.uk/wiki/index.php/NiftyReg) and data were processed with icobrain for WM and lesion segmentation. 24 After pre-processing, the DTI tensors were estimated using an iteratively reweighted linear least square fitting method implemented in Dipy 0.14.0. 25 to identify and reject outliers. Then, using the DTI tensor values, FA, MD, RD and AD were calculated.
Statistical analysis
The statistical analysis was performed using SPSS (IBM Corp. Released in 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp) software. Normality distribution was checked using Kolmogorov-Smirnov and Shapiro-Wilk tests. The difference in means between groups (i.e. RRMS vs HCs) for clinical variables and DTI metrics were calculated using the t-test (unpaired and unequal variance). Results are presented as mean ± standard error of measurement (Mean ± SEM). Univariate correlations between clinical variables and all MRI data were performed using the Pearson correlation coefficient (r). The threshold for p-value was set to 0.05 to indicate statistical significance.
Results
Demographics and clinical characteristics
Age and sex did not show any statistical difference between HCs and RRMS groups (p > .05). No significant difference was noted between the two groups in the scores for total ARCS (tARCS), memory, language and attention (p > 0.05). Poor performance was observed in RRMS for fluency, visuospatial function and SDMT (p ≤ 0.05). The RRMS group showed higher anxiety, depression and stress (p ≤ .01) compared with HCs, while RRMS fatigue scores were statistically higher (p ≤ 0.01). Detailed demographic characteristics and clinical details of both groups are shown in Table 1.
Morphological and DTI group differences
As can be seen in Table 2, there was a statistically significant increase in MD (+3.6%), RD (+4.8%) and AD (+2.7%) and a decrease in FA (−4.3%) for TBWM in RRMS patients compared to HCs (p ≤ 0.01). In WML, there was an even greater increase in MD, RD and AD by +12.2%, +13.1% and +7.4%, respectively, while FA was significantly reduced (−16.9%, p ≤ 0.01), in comparison to corresponding WM location in HCs. Volumetric segmentation indicated statistically significant differences in the total brain volume (TBV), GM and WM (−5%) with a reciprocal increase in CSF by (+26%) in RRMS patients compared with HCs (p ≤ 0.05).
Table 2.
Mean values of DTI parameters for the TBWM and WML segmentation for RRMS patients compared to HCs. MD, RD and AD are in 10−13 mm 2 /s, except FA which is unitless.
| Regions | DTI measure | HCs | RRMS | Difference % | p-value |
|---|---|---|---|---|---|
| Sample size | — | N = 19 | N = 37 | — | — |
| TBWM | MD | 0.527 ± 3.E−03 | 0.546 ± 3.E−03 | 3.6 | 9.E−05 |
| — | RD | 0.421 ± 4.E−03 | 0.441 ± 3.E−03 | 4.8 | 1.E−04 |
| — | AD | 0.739 ± 4.E−03 | 0.755 ± 4.E−03 | 2.7 | 7.E − 03 |
| — | FA | 0.356 ± 4.E−03 | 0.340 ± 3.E−03 | −4.3 | 5.E − 03 |
| WML | MD | 0.410 ± 4.E−03 | 0.471 ± 9.E−03 | 12.2 | 4.E − 05 |
| — | RD | 0.343 ± 5.E−03 | 0.397 ± 9.E−03 | 13.1 | 8.E − 05 |
| — | AD | 0.573 ± 5.E−03 | 0.610 ± 9.E−03 | 7.4 | 3.E − 03 |
| — | FA | 0.270 ± 6.E−03 | 0.231 ± 5.E−03 | −16.9 | 3.E − 05 |
Values are expressed in mean ± standard error of measurement.
HCs: healthy controls; RRMS: relapse-remitting multiple sclerosis; MD: mean diffusivity; RD: radial diffusivity; AD: axial diffusivity; FA: fractional anisotropy; TBWM: total brain white matter; WML: white matter lesion.
Clinical correlations
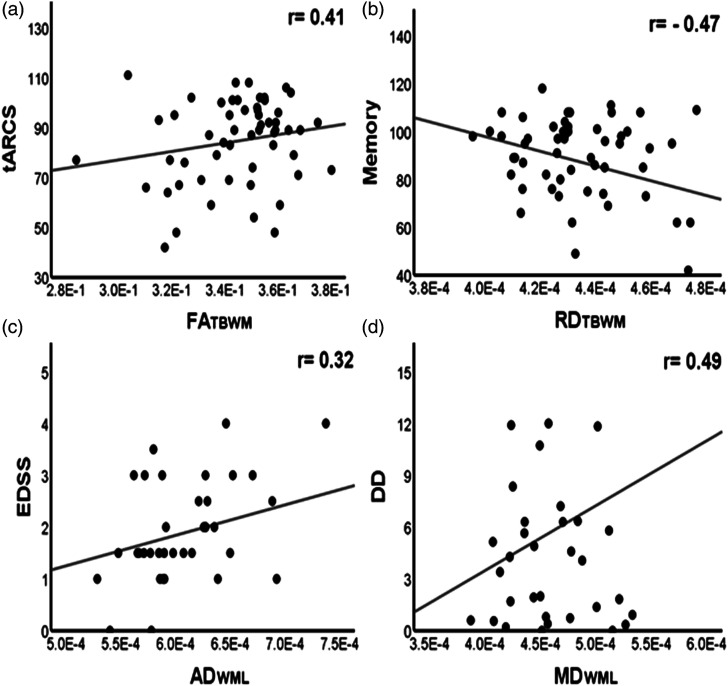
Relapsing-remitting MS patients (RRMS): The FATBWM has shown moderate positive correlations with tARCS, memory, fluency and attention (r = 0.41, 0.41, 0.35 and 0.41, p ≤ .05), while negative correlations were noted for MDTBWM with memory and fluency (r = −0.37 and −0.39, p ≤ .05). RDTBWM also was negatively correlated with tARCS, memory and fluency (|r| ≥ 0.43, p ≤ .01). Both EDSS and disease duration were positively correlated with MD/RDTBWM (r ≥ 0.37, p ≤ .05) and with AD/MDWML (r ≥ 0.32, p ≤ .05), while no clinical correlation with ADTBWM was detected. It can also be seen that total white matter volume (TWMV) correlated with fluency (r = 0.38; p ≤ .05) and with disease duration (r = −0.46; p ≤ .01). Additionally, TBV correlated with fluency (r = 0.33; p ≤ .05), and with EDSS and disease duration (r = −0.33 and −0.44; p ≤ 0.05). Importantly, more DTI parameters (16 metrics) with stronger correlations with clinical parameters are obtained than volume measurements (5 metrics). Statistically significant correlations between clinical parameters and DTI metrics for RRMS are listed in Table 3, and major correlations are shown in Figure 1.
Table 3.
Pearson’s correlation between DTI metrics and selected volumetrics with clinical parameters in RRMS (top) and healthy (bottom) groups. Only statistically significant correlations are listed.
| RRMS group DTI and volumetrics | |||||||
|---|---|---|---|---|---|---|---|
| tARCS | Memory | Fluency | Attention | EDSS | DD | ||
| TBWM | FA | 0.41* | 0.41* | 0.35* | 0.41* | —— | —— |
| MD | —— | −0.37* | −0.39* | —— | 0.38* | 0.44** | |
| RD | −0.43** | −0.47** | −0.45** | —— | 0.37* | 0.44** | |
| WML | AD | —— | —— | —— | —— | 0.32* | 0.39** |
| MD | —— | —— | —— | —— | —— | 0.49** | |
| TWMV | —— | —— | —— | 0.38* | —— | —— | −0.46** |
| TBV | —— | —— | —— | 0.33* | —— | −0.33* | −0.44** |
RRMS: relapse-remitting multiple sclerosis; DTI: diffusion tensor imaging; FA: fractional anisotropy; MD: mean diffusivity; RD: radial diffusivity; AD: axial diffusivity; WML: white matter lesion; TBWM: total brain white matter; tARCS: total audio recorded cognitive screen; EDSS: expanded disability status scale; DD: disease duration; TWMV: total white matter volume; TBV: total brain volume.
*p-value ≤ .05, **p-value ≤ .01.
Figure 1.
Scatter plots showing some significant correlations: (a) FATBWM with tARCS (r = 0.41), (b) RDTBWM with Memory (r = −0.47), (c) ADWML with EDSS (r = 0.32) and (d) MDWML with DD (r = 0.49). FA: fractional anisotropy; RD: radial diffusivity; AD: axial diffusivity; MD: mean diffusivity; TBWM: total brain white matter; WML: white matter lesion; tARCS: total audio recorded cognitive screen; EDSS: expanded disability status scale; DD: disease duration. FATBWM is unitless, while RDTBWM, ADWML and MDWML are in mm 2 /s.
Healthy controls: Stronger correlations can be detected between DTI metrics and clinical parameters in HCs group, while no correlations between TWMV and TBV and clinical parameters were observed (Table 3).
Discussion
Although structural MRI has high sensitivity in the assessment of T2 lesion burden, it suffers from low sensitivity and pathologic specificity to diffuse changes in otherwise normal-appearing white matter required to evaluate the disease severity. While brain atrophy is found to be a progressive marker for disability, 26 several factors including biological/diurnal effects, disease-related oedema/inflammation, treatment-related psuedoatrophy and technical parameters can influence accurate brain volume measurements. 27 In this study, DTI metrics showed medium correlations with tARCS, memory and attention in contrast to white matter-related volumetric measurements in RRMS, indicative of TBWM microstructural changes not accessible using traditional MRI methods.
The major findings of this study demonstrate that there is a marked reduction of FA and significantly increased diffusivities in TBWM and WML of RRMS patients compared to HCs, all of which had moderate correlation with several clinical symptoms mainly cognition, information processing and memory. Our results agree with others suggesting that diffusion-based microstructural changes may reflect various disease processes in MS patients such as neurodegeneration, axonal loss and gliosis.28–30 This explains why studying diffusion properties of TBWM is essential due to its ability to provide information about major diffuse WM pathology information that cannot be obtained with conventional MRI. 28 TBWM in MS demonstrates microscopic inflammatory changes as well as chronic injury including the presence of diffuse astrocytic hyperplasia, patchy edema, perivascular infiltration, gliosis, microglial activation and the increased reaction of proteolytic enzymes.31,32
Using quantitative DTI metrics, earlier studies confirmed these microscopic changes within the TBWM of different MS subtypes.33–35 Inspite of investigating an RRMS population with limited disability (mean EDSS of less than 2.0 in this study), all four DTI metrics demonstrated statistically significant differences in TBWM compared to healthy controls.
Although we found a reduction in FA values in TBWM, which is non-specific for demyelination, we detected increases in other diffusivities, reflecting general white matter integrity loss. As expected, DTI metrics showed a similar trend with larger and more statistically significant group differences relative to HCs. In line with other studies, we also observed higher diffusivities and lower FA in WML than in TBWM which may represent a higher degree of destruction in the former.9,29 Since diffusion is influenced by free space, it is conceivable that its increase in WML may result from the expansion of the extracellular space due to vasogenic edema or axonal loss. 8 Due to the nature of the cohort studied (stable patients with a mean disease duration of 7 years), the majority of detected lesions were chronic based on T2-FLAIR, in which increased diffusivities could be due to tissue loss, gliosis as well as low-grade inflammatory processes. 9
Compared to conventional structural imaging techniques, quantitative DTI yielded more moderately correlated metrics to clinical parameters, agreeing with other research. 8 We found a moderate EDSS correlation with TBWM and WML diffusivities but not with FA, corroborating other studies, 36 and hinting at the utility of diffusivities in measuring neuroinflammation during the early stages of the disease. This is also in agreement with other works, which was explained by clinically invisible microscopic white matter disruption. 37 There are many plausible reasons for the lack of correlation between FATBWM/WML and EDSS in our study, which might include the non-specificity of FA in distinguishing between disease characteristics due to a range of different pathological processes . 8 The moderate correlation of FATBWM with some clinical parameters (∼0.4) could be due to the low EDSS cohort studied, reflecting functional, yet pathologically affected fibre tracts. Interestingly, Tóth et al. concluded that clinical disability in patients with long disease duration (mean: 14 years) is best defined by WM AD, whereas information processing by other WM DTI metrics (FA, MD and RD) which agrees with our work.38–40
The absence of any clinical correlations with ADTBWM might be indicative of the relatively preserved axonal structure in the RRMS cohort with a short disease duration, while at the same time RDTBWM, indicative of myelin integrity, was clinically correlated with tARCS, memory and fluency. AD and RD were suggested as two DTI metrics that can act as surrogates of axonal and myelin integrity, respectively, and might be able to provide unique pathological information, aid in therapy monitoring and therapy stratification. 41 This correlation, however, was questioned by others, and suggested use with care. 42 Larger change in RD than in AD, compared to HCs, might hint to less axonal than myelin damage. The moderate correlations of MDTBWM, RDTBWM, ADWML and MDWML with disease duration could be a sign of early disruption which then leads to subsequent atrophy. 8 The absence of correlation with FA, might also indicate that MD and RD are more sensitive measures than FA, especially at low EDSS as in our case. This agrees with findings that RRMS groups with short and moderate disease durations, exhibited no difference in FA values. 39 Similarly, others found only a mild to moderate correlation between disease duration and decreasing FA values in TBWM. 43 In contrast, Deppe et al. noted that reduced cerebral FA correlated with disability and disease duration in early-stage RRMS, 44 as opposed to studying TBWM in our study.
Cognitive decline is one of the essential clinical features of disease progression in MS. 45 In line with other findings, 39 we found a significant correlation between FATBWM and RDTBWM with cognitive deficits measured by tARCS in RRMS patients. This is in concordance with work where cognitively preserved and cognitively impaired RRMS patients diverged due to their reduced FA values. 46 In our study, we noticed the delayed recall in tARCS of MS patients were lower compared to HCs, implying mild cognitive deficits, albeit statistically insignificant. Microscopic damage and disconnection (mainly interlobar, interhemispheric and long-distance brain disconnection) in many brain structures responsible for cognition (cingulum, uncinate fasciculus, corpus callosum, superior and medial cerebellar peduncles) have been identified in DTI studies of regional areas. 47 The increase of RDTBWM might be indicative of early myelin layers compromise in RRMS leading to inefficient signal transmission and impaired cognition. 48
Poor memory and information processing speed are the two most common deficits observed in MS patients. We noted a statistically significant and moderately negative correlation between memory/fluency and MD/RD in TBWM. We also observed a positive but non-statistically significant correlation between SDMT and FATBWM (r = 0.23, p = 0.16) agreeing with other work highlighting the possible role of DTI as a measure of axonal integrity and cognitive function in MS. 49
While our patients showed statistically significant fatigue scores, no correlation between the fatigue domains and DTI metrics in TBWM and WML was observed. The absence of correlation might be due to the near “normal” fatigue scores in the patient group in addition to short disease duration, as well as the known metabolic abnormalities leading to global functional impairment in MS patients. 50
Limitations
There are limitations in our study which need to be acknowledged. Our study is cross-sectional in nature with a relatively small sample size. The disease duration in our group is 7 year and patients were undergoing two different types of disease-modifying therapy (DMT). We did not include a treatment naïve group in our study to account for treatment effects, but all patients were stable on the same DMT during the study period. The DTI method used is a single compartment mono-exponential model which might bias approximation of DTI metrics. 51
Conclusion
The overall results in our study suggest that DTI is a sensitive tool in the evaluation of subtle and inconspicuous disease processes within the total brain white matter that are otherwise undetectable with structural MRI. Besides, DTI provides microstructural information about white matter lesions. Our study also highlights the importance of radial/axial/mean diffusivities where we identified significant correlations of DTI metrics with clinical symptoms in a stable, mildly disabled cohort, particularly cognition. Future studies should include longitudinal trials with larger samples involving various subtypes of MS using advanced diffusion processing methods.
Acknowledgements
The authors acknowledge the patients and healthy controls who participated in this study, Hunter Medical Research Institute Imaging Centre (HMRI-IC) of the University of Newcastle and Hunter Medical Research Institute. This study was supported by an independent grant provided by Novartis Pharmaceuticals Australia Pty Ltd A. Alshehri was supported by a PhD scholarship with annual grant support from Imam Abdulrahman Bin Faisal University (IAU) in Saudi Arabia and the Saudi Arabian Cultural Mission (SACM) in Australia.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AA: did not declare any competing interests. OA: did not declare any competing interests. JA: did not declare any competing interests. NG: did not declare any competing interests. TB: is an employee of icometrix. RL: did not declare any competing interests. SR: did not declare any competing interests. JLS: has accepted travel compensation from Novartis, Biogen and Merck Serono. Her institution receives the honoraria for talks and advisory board commitment and also clinic support from Bayer Health Care, Biogen Idec, CSL, Genzyme Sanofi, Merck Serono, Novartis and Teva.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Independent investigator-initiated grant provided by Novartis pharmaceuticals.
Author notes: Part of this work was presented at the 28th ISMRM & SMRT Annual Conference. Abstract #4338. On-line, 2020.
ORCID iDs
Abdulaziz Alshehri https://orcid.org/0000-0003-4893-223X
Saadallah Ramadan https://orcid.org/0000-0003-3874-7866
References
- 1.Criste G, Trapp B, Dutta R. Axonal loss in multiple sclerosis: causes and mechanisms. In: Goodin DS. (ed). Handbook of clinical neurology (vol 122): Multiple sclerosis and related disorders. Amsterdam: Elsevier, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Lassmann H. Multiple sclerosis pathology. Cold Spring Harb. Persp 2018; 8: a028936. 2018/01/24. DOI: 10.1101/cshperspect.a028936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kister I, Bacon TE, Chamot E, et al. Natural history of multiple sclerosis symptoms. Int Journal MS Care 2013; 15: 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filippi M, Rocca MA, Ciccarelli O, et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol 2016; 15: 292–303. 2016/01/30. DOI: 10.1016/s1474-4422(15)00393-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barkhof F. The clinico-radiological paradox in multiple sclerosis revisited. Curr Opinion Neurology 2002; 15: 239–245. [DOI] [PubMed] [Google Scholar]
- 6.Filippi M, Rocca MA, De Stefano N, et al. Magnetic resonance techniques in multiple sclerosis. Arch Neurol 2011; 68: 1514–1520. DOI: 10.1001/archneurol.2011.914. [DOI] [PubMed] [Google Scholar]
- 7.Rovaris M, Rocca MA, Filippi M. Magnetic resonance-based techniques for the study and management of multiple sclerosis. Br Med Bull 2003; 65: 133–144. 2003/04/17. DOI: 10.1093/bmb/65.1.133. [DOI] [PubMed] [Google Scholar]
- 8.Sbardella E, Tona F, Petsas N, et al. DTI measurements in multiple sclerosis: evaluation of brain damage and clinical implications. Mult Sclerosis Int 2013; 2013: 671730. 2013/04/23. DOI: 10.1155/2013/671730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filippi M, Iannucci G, Cercignani M, et al. A quantitative study of water diffusion in multiple sclerosis lesions and normal-appearing white matter using echo-planar imaging. Arch Neurol 2000; 57: 1017–1021. DOI: 10.1001/archneur.57.7.1017. [DOI] [PubMed] [Google Scholar]
- 10.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 2006; 51: 527–539. [DOI] [PubMed] [Google Scholar]
- 11.Cercignani M, Giulietti G, Dowell NG, et al. Characterizing axonal myelination within the healthy population: a tract-by-tract mapping of effects of age and gender on the fiber g-ratio. Neurobiol Aging 2017; 49: 109–118. 2016/09/30. DOI: 10.1016/j.neurobiolaging.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inglese M, Bester M. Diffusion imaging in multiple sclerosis: research and clinical implications. NMR Biomed 2010; 23: 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. 1983/11/01. DOI: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 14.Meyer-Moock S, Feng Y-S, Maeurer M, et al. Systematic literature review and validity evaluation of the expanded disability status scale (EDSS) and the multiple sclerosis functional composite (MSFC) in patients with multiple sclerosis. BMC Neurology 2014; 14: 58. DOI: 10.1186/1471-2377-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roxburgh RHSR, Seaman SR, Masterman T, et al. Multiple sclerosis severity score: using disability and disease duration to rate disease severity. Neurology 2005; 64: 1144–1151. 2005/04/13. DOI: 10.1212/01.Wnl.0000156155.19270.F8. [DOI] [PubMed] [Google Scholar]
- 16.Lechner-Scott J, Kerr T, Spencer B, et al. The audio recorded cognitive screen (ARCS) in patients with multiple sclerosis: a practical tool for multiple sclerosis clinics. Mult Scler J 2010; 16: 1126–1133. 2010/07/14. DOI: 10.1177/1352458510374743. [DOI] [PubMed] [Google Scholar]
- 17.Benedict RH, DeLuca J, Phillips G, et al. Validity of the symbol digit modalities test as a cognition performance outcome measure for multiple sclerosis. Mult Scler J 2017; 23: 721–733. 2017/02/17. DOI: 10.1177/1352458517690821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson RD. Psychometric properties of the modified fatigue impact scale. Int J MS Care 2013; 15: 15–20. 2014/01/24. DOI: 10.7224/1537-2073.2012-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veraart J, Novikov DS, Christiaens D, et al. Denoising of diffusion MRI using random matrix theory. Neuroimage 2016; 142: 394–406. 2016/08/16. DOI: 10.1016/j.neuroimage.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tournier JD, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage 2007; 35: 1459–1472. 2007/03/24. DOI: 10.1016/j.neuroimage.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Kellner E, Dhital B, Kiselev VG, et al. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med 2016; 76: 1574–1581. 2016/01/09. DOI: 10.1002/mrm.26054. [DOI] [PubMed] [Google Scholar]
- 22.Tustison NJ, Avants BB, Cook PA, et al. N4ITK: improved N3 bias correction. IEEE Transac Medic Imag 2010; 29: 1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modat M, Cash DM, Daga P, et al. Global image registration using a symmetric block-matching approach. J Med Imaging 2014; 1: 024003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain S, Sima DM, Ribbens A, et al. Automatic segmentation and volumetry of multiple sclerosis brain lesions from MR images. NeuroImage: Clin 2015; 8: 367–375. DOI: 10.1016/j.nicl.2015.05.003 10.1016/j.nicl.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garyfallidis E, Brett M, Amirbekian B, et al. Dipy, a library for the analysis of diffusion MRI data. Front Neuroinformatics 2014; 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andravizou A, Dardiotis E, Artemiadis A, et al. Brain atrophy in multiple sclerosis: mechanisms, clinical relevance and treatment options. Autoimmun Highlights 2019; 10: 7. DOI: 10.1186/s13317-019-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beadnall HN, Wang C, Van Hecke W, et al. Comparing longitudinal brain atrophy measurement techniques in a real-world multiple sclerosis clinical practice cohort: towards clinical integration? Ther Adv Neurol Disord 2019; 12: 1756286418823462. DOI: 10.1177/1756286418823462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagiwara A, Kamagata K, Shimoji K, et al. White matter abnormalities in multiple sclerosis evaluated by quantitative synthetic MRI, diffusion tensor imaging, and neurite orientation dispersion and density imaging. AJNR Am J Neuror 2019; 40: 1642–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Werring DJ, Clark CA, Barker GJ, et al. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology 1999; 52: 1626–1632. [DOI] [PubMed] [Google Scholar]
- 30.Guo Y, Cai Y-Q, Cai Z-L, et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging 2002; 16: 172–178. [DOI] [PubMed] [Google Scholar]
- 31.Ludwin SK. The pathogenesis of multiple sclerosis. J Neuropathol Exp Neurol 2006; 65: 305–318. 2006/05/13. DOI: 10.1097/01.jnen.0000225024.12074.80. [DOI] [PubMed] [Google Scholar]
- 32.Moll NM, Rietsch AM, Thomas S, et al. Multiple sclerosis normal-appearing white matter: pathology-imaging correlations. Ann Neurology 2011; 70: 764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mezzapesa DM, Rocca MA, Falini A, et al. A preliminary diffusion tensor and magnetization transfer magnetic resonance imaging study of early-onset multiple sclerosis. Arch Neurol 2004; 61: 366–368. 2004/03/17. DOI: 10.1001/archneur.61.3.366. [DOI] [PubMed] [Google Scholar]
- 34.Cassol E, Ranjeva J-P, Ibarrola D, et al. Diffusion tensor imaging in multiple sclerosis: a tool for monitoring changes in normal-appearing white matter. Mult Scler J 2004; 10: 188–196. 2004/05/06. DOI: 10.1191/1352458504ms997oa. [DOI] [PubMed] [Google Scholar]
- 35.Gallo A, Rovaris M, Riva R, et al. Diffusion-tensor magnetic resonance imaging detects normal-appearing white matter damage unrelated to short-term disease activity in patients at the earliest clinical stage of multiple sclerosis. Arch Neurology 2005; 62: 803–808. [DOI] [PubMed] [Google Scholar]
- 36.Hannoun S, Bagory M, Durand-Dubief F, et al. Correlation of diffusion and metabolic alterations in different clinical forms of multiple sclerosis. PLoS One 2012; 7: e32525. 2012/04/06. DOI: 10.1371/journal.pone.0032525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asaf A, Evan S, Anat A. Injury to white matter tracts in relapsing-remitting multiple sclerosis: A possible therapeutic window within the first 5 years from onset using diffusion-tensor imaging tract-based spatial statistics. NeuroImage: Clin 2015; 8: 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tóth E, Faragó P, Király A, et al. The contribution of various MRI parameters to clinical and cognitive disability in multiple sclerosis. Front NeurologyOriginal Res 2019; 9: 1172. DOI: 10.3389/fneur.2018.01172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asaf A, Evan S, Anat A. Injury to white matter tracts in relapsing-remitting multiple sclerosis: a possible therapeutic window within the first 5 years from onset using diffusion-tensor imaging tract-based spatial statistics. NeuroImage: Clin 2015; 8: 261–266. DOI: 10.1016/j.nicl.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage 2010; 53: 1197–1207. 2010/07/06. DOI: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 41.Winklewski PJ, Sabisz A, Naumczyk P, et al. Understanding the physiopathology behind axial and radial diffusivity changes-what do we know? Front NeurologyMini Rev 2018; 9: 92. DOI: 10.3389/fneur.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage 2013; 73: 239–254. [DOI] [PubMed] [Google Scholar]
- 43.Kister I., Chamot E., Cutter G., et al. Increasing age at disability milestones among MS patients in the MSBase Registry. J Neurol Sci 2012; 318: 94–99. 2012/04/18. DOI: 10.1016/j.jns.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Deppe M, Tabelow K, Krämer J, et al. Evidence for early, non-lesional cerebellar damage in patients with multiple sclerosis: DTI measures correlate with disability, atrophy, and disease duration. Mult Scler J 2016; 22: 73–84. 2015/04/30. DOI: 10.1177/1352458515579439. [DOI] [PubMed] [Google Scholar]
- 45.Ferreira MLB. Cognitive deficits in multiple sclerosis: a systematic review. Arquivos de Neuro-Psiquiatria 2010; 68: 632–641. 2010/08/24. DOI: 10.1590/s0004-282x2010000400029. [DOI] [PubMed] [Google Scholar]
- 46.Hulst HE, Steenwijk MD, Versteeg A, et al. Cognitive impairment in MS: impact of white matter integrity, gray matter volume, and lesions. Neurology 2013; 80: 1025–1032. DOI: 10.1212/WNL.0b013e31828726cc. [DOI] [PubMed] [Google Scholar]
- 47.Rimkus CdM, Steenwijk MD, Barkhof F. Causes, effects and connectivity changes in MS-related cognitive decline. Demen Neuropsychologia 2016; 10: 2–11. 2016/01/01. DOI: 10.1590/s1980-57642016dn10100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahajan KR, Ontaneda D. The role of advanced magnetic resonance imaging techniques in multiple sclerosis clinical trials. Neurotherapeutics 2017; 14: 905–923. 2017/08/05. DOI: 10.1007/s13311-017-0561-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersen KW, Lasič S, Lundell H, et al. Disentangling white-matter damage from physiological fibre orientation dispersion in multiple sclerosis. Brain Commun 2020; 2: fcaa077. DOI: 10.1093/braincomms/fcaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Codella M, Assunta Rocca M, Colombo B, et al. A preliminary study of magnetization transfer and diffusion tensor MRI of multiple sclerosis patients with fatigue. J Neurol 2002; 249: 535–537. 2002/05/22. DOI: 10.1007/s004150200060. [DOI] [PubMed] [Google Scholar]
- 51.Hecke WV, Emsell L, Sunaert S. Diffusion Tensor Imaging. 1st ed., XVIII. New York: Springer-Verlag, 2016, p. 440. [Google Scholar]