Abstract
Taxanes plus carboplatin (TP) regimen may be an acceptable alternative adjuvant chemotherapy strategy in patients with triple-negative breast cancer (TNBC); however, the difference with the anthracycline-based regimen is yet to be clarified. Therefore, this study aimed to assess the difference between platinum-based and anthracycline-based regimens in prolonging the survival time in TNBC. Using exploratory landmark analysis, we found that the platinum-based TP regimen offers a longer disease-free survival (DFS) than the anthracycline-based regimen in TNBC patients with a DFS of > 4 years.
Keywords: Landmark analysis, Platinum-based, Anthracycline-based, Triple-negative breast cancer, Prognosis
To the Editor,
Breast cancer, as the most common cancer in females, threatens women’s health worldwide [1]. Triple-negative breast cancer (TNBC) is a solid malignancy with negative expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2), which accounts for 15%-20% of breast cancer [2]. The anthracycline-based regimen, such as epirubicin plus cyclophosphamide followed by docetaxel or paclitaxel (ECT) regimen, is considered a standard adjuvant chemotherapy regimen and improves survival outcomes of early TNBC [3, 4]. In our previous study, [5] adjuvant carboplatin plus docetaxel or paclitaxel (TP) showed non-inferiority for disease-free survival (DFS) and overall survival (OS) compared with ECT regimen in TNBC patients.
The platinum-based regimen is an effective alternative adjuvant chemotherapy regimen and is widely used in neoadjuvant chemotherapy for increased pCR rate in patients with TNBC, [4, 6] but it is still unclear whether a platinum-based regimen as adjuvant treatment in TNBC patients has a difference of survival benefit compared with an anthracycline-based regimen. Landmark analysis based on the DFS and OS time can minimize the immortal time bias induced by including events in the hazard model, [7, 8] and provide potential evidence of this difference. Thus, we excavated the landmark analysis aiming to investigate the role of platinum-based adjuvant settings in TNBC patients (ClinicalTrials.gov identifier NCT01150513). The final date of follow-up was January 20, 2021, with a median follow-up of 97.6 months. The Kaplan–Meier method and Breslow test were used to evaluate the prognostic value of early TNBC patients with 2-sided tests set at P < 0.05. The landmark analysis was performed using EmpowerStats software (version EmpowerR 2.2, X&Y Solutions, USA).
Based on our previous study, [5] we enrolled all TNBC patients treated with the TP regimen or the ECT regimen in the landmark analysis at 4 years. For TNBC patients with a DFS of > 4 years, a total of 125 (81.2%) patients in the ECT regimen and 127 (82.5%) patients in the TP regimen were analyzed in the study. TNBC patients (a DFS of > 4 years) were well balanced between the ECT regimen and TP regimen, and the detailed clinicopathological characteristics were seen in Table 1. As shown in Fig. 1, in TNBC patients with a DFS of ≤ 4 years, DFS (HR, 1.59; 95% CI, 0.86–2.95; P = 0.32; Fig. 1A) and OS (HR, 1.24; 95% CI, 0.61–2.54; P = 0.26; Fig. 1B) had no difference between the TP regimen and the ECT regimen. In TNBC patients with a DFS of > 4 years, the TP regimen had a longer DFS (HR, 0.28; 95% CI, 0.10–0.74; P = 0.01) (Fig. 1A) and was not associated with a better OS (HR, 0.39; 95% CI, 0.05–2.75; P = 0.4) (Fig. 1B) than the ECT regimen.
Table 1.
Clinicopathological features in triple-negative breast cancer patients with a DFS > 4 years (%)
| ECT arm (n = 125) | TP arm (n = 127) | P value | |
|---|---|---|---|
| Ages (median [IQR]) | 48 (42.0—53.0) | 48 (42.5—57.0) | 0.49 |
| Menopasusal status | 0.98 | ||
| Premenopausal | 75 (60.00) | 75 (59.06) | |
| Postmenopausal | 50 (40.00) | 52 (40.94) | |
| Histological type | 0.20 | ||
| Ductal | 117 (93.60) | 117 (92.13) | |
| Lobular | 4 (3.20) | 1 (0.79) | |
| Medullary | 3 (2.40) | 4 (3.15) | |
| Others | 1 (0.80) | 5 (3.94) | |
| Histological grade | 0.30 | ||
| Grade 2 | 35 (28.00) | 25 (19.69) | |
| Grade 3 | 78 (62.40) | 88 (69.29) | |
| Missing or unkown | 12 (9.60) | 14 (11.02) | |
| Ki67 | 0.54 | ||
| < 20% | 15 (12.00) | 13 (10.24) | |
| 20% ≤ to < 50% | 41 (32.80) | 43 (33.86) | |
| ≥ 50% | 65 (52.00) | 70 (55.12) | |
| Missing or unkown | 4 (3.20) | 1 (0.79) | |
| pT | 0.35 | ||
| pT1 | 67 (53.60) | 67 (52.76) | |
| pT2 | 56 (44.80) | 60 (47.24) | |
| pT3 | 2 (1.60) | 0 (0.00) | |
| pN | 0.42 | ||
| pN0 | 81 (64.80) | 93 (73.23) | |
| pN1 | 35 (28.00) | 29 (22.83) | |
| pN2 | 4 (3.20) | 3 (2.36) | |
| pN3 | 5 (4.00) | 2 (1.57) | |
| pTNM stage | 0.53 | ||
| stage 1 | 47 (37.60) | 50 (39.37) | |
| stage 2 | 69 (55.20) | 72 (56.69) | |
| stage 3 | 9 (7.20) | 5 (3.94) | |
| Intravascular invasion (%) | 0.27 | ||
| Yes | 99 (79.20) | 110 (86.61) | |
| No | 17 (13.60) | 10 (7.87) | |
| Missing or unkown | 9 (7.20) | 7 (5.51) | |
| PD-L1 status | 0.16 | ||
| negative | 69 (55.20) | 57 (44.88) | |
| postive | 24 (19.20) | 24 (18.90) | |
| Missing or unkown | 32 (25.60) | 46 (36.22) | |
| Surgery | 0.07 | ||
| Radical surgery | 94 (75.20) | 81 (63.78) | |
| Breast conserving | 31 (24.80) | 46 (36.22) |
DFS disease-free survival, ECT, docetaxel or paclitaxel followed by epirubicin plus cyclophosphamide, TP docetaxel or paclitaxel plus carboplatin
Fig. 1.
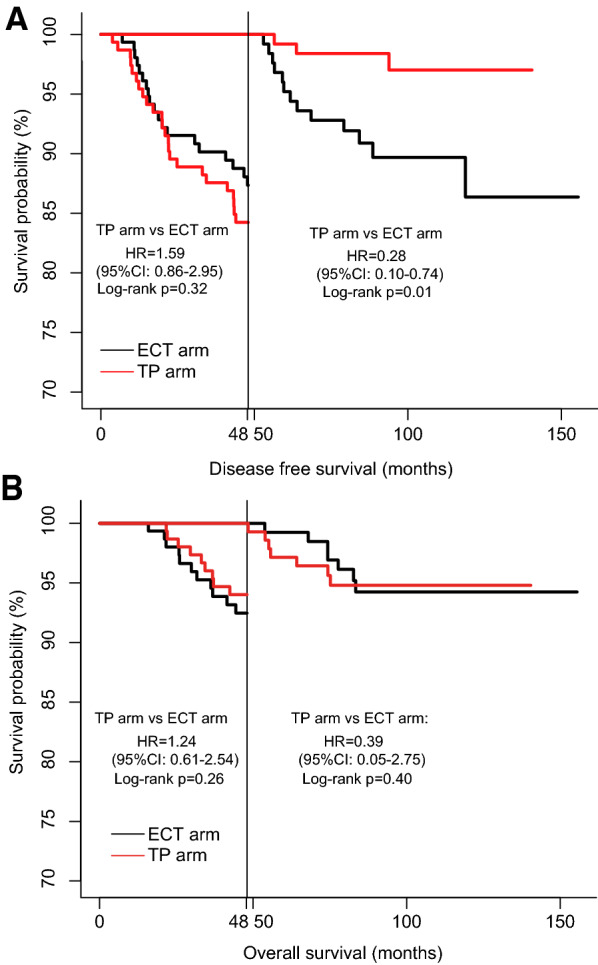
Landmark analysis plots showing the DFS and OS rates of different subgroups. (A) DFS of the subgroup with DFS of ≤ 4 years and DFS of > 4 years. (B) OS of the subgroup with a DFS of ≤ 4 years and DFS of > 4 years. TP, docetaxel or paclitaxel plus carboplatin; ECT, epirubicin and cyclophosphamide followed by docetaxel or paclitaxel; DFS, disease-free survival; OS, overall survival
This is the first landmark analysis assessing the difference in therapeutic effect between a platinum-based regimen and an anthracycline-based regimen as adjuvant treatment in TNBC patients, which showed that the TP regimen seems to have a longer DFS than the ECT regimen with a life expectancy of more than 4 years. The following reason may explain the differences. Firstly, the 5-year DFS rate of the TP regimen in our previous study (84.4%) was almost consistent with that in another study (86.5%) [4]. However, the results in our control group were inconsistent with that in the other study, probably because the ECT regimen administered in our control group was stronger than that of cyclophosphamide, epirubicin plus fluorouracil followed by the docetaxel regimen used in the previous study. Secondly, a recent study showed that high-dose anthracycline-based chemotherapy elicits a state of immunological dormancy and promotes resistance to chemotherapy in ER-negative BC patients (including those with TNBC) receiving adjuvant chemotherapy [9]. Based on the results of a previous study, anthracycline-based ECT regimen may evade chemotherapy by going senescence, leading to TNBC relapsed. Nevertheless, the TP regimen may be a potentially preferred adjuvant chemotherapy regimen for TNBC patients, especially, in whom, for some reason, the standard anthracycline-taxane regimen is not being used.
Acknowledgements
Thanks to all enrolled patients and physicians who participated in the study.
Abbreviations
- TNBC
Triple-negative breast cancer
- ER
Estrogen receptor
- PR
Progesterone receptor
- HER2
Human epidermal growth factor receptor-2
- ECT
Epirubicin plus cyclophosphamide followed by docetaxel or paclitaxel regimen
- TP
Carboplatin plus docetaxel or paclitaxel
- DFS
Disease-free survival
- OS
Overall survival
Author contributions
Conception and design: PY and FZ. Acquisition and analysis of data and provided the clinical data: FZ, TW, XW, JY and PY. Writing, review, and/or revision of the manuscript: FZ, TW, XW, FD, JY and PY. Study supervision: PY. All authors read and approved the final manuscript.
Funding
This work was supported by the CSCO Pilot Oncology Research Fund (Y-2019AZMS-0377), National Natural Science Foundation of China (82172650).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines and was also approved by the ethics committees of Cancer Hospital, Chinese Academy of Medical Sciences.
Consent for publication
All authors declare that they have no conflict of interest.
Competing interests
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fangchao Zheng, Tong Wei and Xue Wang have equally contributed to this work.
References
- 1.Yi M, Li T, Niu M, Luo S, Chu Q, Wu K. Epidemiological trends of women's cancers from 1990 to 2019 at the global, regional, and national levels: a population-based study. Biomark Res. 2021;9(1):55. doi: 10.1186/s40364-021-00310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sukumar J, Gast K, Quiroga D, Lustberg M, Williams N. Triple-negative breast cancer: promising prognostic biomarkers currently in development. Expert Rev Anticancer Ther. 2021;21(2):135–148. doi: 10.1080/14737140.2021.1840984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nitz U, Gluz O, Clemens M, et al. West german study planb trial: adjuvant four cycles of epirubicin and cyclophosphamide plus docetaxel versus six cycles of docetaxel and cyclophosphamide in HER2-negative early breast cancer. J Clin Oncol. 2019;37(10):799–808. doi: 10.1200/JCO.18.00028. [DOI] [PubMed] [Google Scholar]
- 4.Yu KD, Ye FG, He M, et al. Effect of adjuvant paclitaxel and carboplatin on survival in women with triple-negative breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2020;6(9):1390–1396. doi: 10.1001/jamaoncol.2020.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng F, Du F, Wang W, et al. Updated efficacy of adjuvant epirubicin plus cyclophosphamide followed by taxanes versus carboplatin plus taxanes in early triple-negative breast cancer in phase 2 trial: 81-year median follow-up. Breast Cancer Res Treat. 2021 doi: 10.1007/s10549-021-06401-6. [DOI] [PubMed] [Google Scholar]
- 6.Peng Z, Wei J, Wang F, et al. Camrelizumab combined with chemotherapy followed by camrelizumab plus apatinib as first-line therapy for advanced gastric or gastroesophageal junction adenocarcinoma. Clin Cancer Res. 2021;27(11):3069–3078. doi: 10.1158/1078-0432.Ccr-20-4691. [DOI] [PubMed] [Google Scholar]
- 7.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27(22):3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rovers KP, Bakkers C, van Erning FN, et al. Adjuvant systemic chemotherapy vs active surveillance following up-front resection of isolated synchronous colorectal peritoneal metastases. JAMA Oncol. 2020;6(8):e202701. doi: 10.1001/jamaoncol.2020.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lan Q, Peyvandi S, Duffey N, et al. Type I interferon/IRF7 axis instigates chemotherapy-induced immunological dormancy in breast cancer. Oncogene. 2019;38(15):2814–2829. doi: 10.1038/s41388-018-0624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


