Abstract
Background and Objectives
To report the frequency of area postrema syndrome (APS) in glial fibrillary acidic protein-immunoglobulin G (GFAP-IgG)–positive patients and emphasize the importance of APS among the phenotypes in autoimmune GFAP astrocytopathy.
Methods
Eight GFAP-IgG–positive cases with APS were retrospectively identified during 2015–2021. The APS phenotypes were described. A literature review of 8 previously reported cases was also included in analysis.
Results
A total of 8 patients (11%) (1 woman, 7 men; mean age: 52.4 ± 18.4 years) presented with APS in a cohort of 74 GFAP-IgG–positive patients, 3 of whom (4%) had disease onset with APS. All patients had hiccups, and hiccups was the unique symptom of APS in 5 patients. The median time from disease onset to APS occurrence was 2 days (range 0–20), and the mean duration of APS episodes was 23.6 ± 11.4 days. No patient had isolated APS attack. All episodes were completely resolved with a mean duration of 9.3 ± 5.4 days after immunotherapy. APS manifestations of 8 cases in previous studies showed similar features with our cases. In total, coexisting aquaporin-4-IgG was only detected in one of the 16 cases.
Discussion
APS could be an early, but not isolated clinical manifestation of autoimmune GFAP astrocytopathy. Hiccups was the predominant symptom of APS in this disorder. APS attacks of autoimmune GFAP astrocytopathy have good response to immunotherapy.
Autoimmune glial fibrillary acidic protein (GFAP) astrocytopathy, first reported in 2016, is a steroid-responsive autoimmune disease.1 Immunoglobulin G (IgG) specific for GFAP in CSF is the hallmark of this disorder.2,3 The main clinical phenotype includes meningoencephalomyelitis.3 However, since autoimmune GFAP astrocytopathy is recently identified, the spectrum of its clinical and imaging phenotypes is still expanding.2,4-7
Area postrema syndrome (APS) is defined as episode of otherwise unexplained nausea, vomiting, and hiccups.8,9 It has been regarded as a core clinical manifestation of aquaporin-4 (AQP4)-IgG–seropositive neuromyelitis optica spectrum disorder (NMOSD), another classic autoimmune astrocytopathy.8 In 2020, a patient who had encephalitis onset with APS, however, with a final diagnosis as autoimmune GFAP astrocytopathy was reported.10 Thereafter, APS was increasingly reported as a noticeable manifestation of this disorder.11-17 However, the incidence and phenotypes of APS in autoimmune GFAP astrocytopathy have not been described comprehensively.
In this study, we reported a case series of GFAP-IgG–positive patients who presented with APS. The characteristics of APS, i.e., onset, duration, and immunotherapy response, were described. Furthermore, we identified 8 previously reported cases and summarized APS phenotypes, aiming to raise the awareness of these features in autoimmune GFAP astrocytopathy.
Methods
Participants
We identified a total of 74 GFAP-IgG–positive patients from Huashan Hospital Autoimmune Encephalitis Cohort during 2015–2021. Patients' clinical information was obtained from medical records and telephone interviews. APS was defined as an unexplained attack of nausea, vomiting, and hiccups (single or combined symptoms) persisting for at least 48 hours.9 Finally, 8 patients with symptoms of APS were included in this study. APS phenotypic data (symptomatic type of APS, onset and duration, and response to immunotherapy); demographics; clinical, immunologic, CSF, and MRI characteristics; and treatment and outcome of these patients were reviewed. We used the modified Rankin scale (mRS) to assess patient's disease severity and outcomes at the follow-up.
Detection of Autoantibodies
GFAPα-IgG in CSF is highly specific for autoimmune GFAP astrocytopathy.3 In addition, a combination of cell-based assays (CBA) and tissue-based assays (TBA) to detect autoantibodies is recommended.2,3 Therefore, GFAPα-IgG in patients' CSF and serum were detected using both CBA and TBA. For CBA, HEK293T cells were transfected with plasmids encoding human GFAPα (NM_002055.5) (Miao Ling Plasmid Sharing Platform, Wuhan, China). Twenty-four hours later, cells were fixed with 4% PFA for 5 minutes, permeabilized with 0.2% Triton X-100 for 10 minutes, and blocked with 10% normal goat serum for 30 minutes. Patient's samples (serum 1:100, CSF 1:2) were then applied and incubated overnight at 4°C. For TBA, CSF was incubated with a commercially available monkey cerebellum or hippocampus slide (Biosystems S.A., Barcelona, Spain) overnight at 4°C. Alexa Fluor 488 goat anti-human IgG (Invitrogen, Eugene, OR) was used as the secondary antibody to label autoantibodies. An astrocytic pattern of immunostaining compatible with previous studies was interpreted as positive.1,3,18 In addition, autoantibodies to AQP4, oligodendrocyte glycoprotein (MOG), and N-methyl-d-aspartate receptor (NMDAR) were detected with live CBA to examine possible coexisting autoimmunity.
Systematic Review of GFAP-IgG–Positive Cases With APS
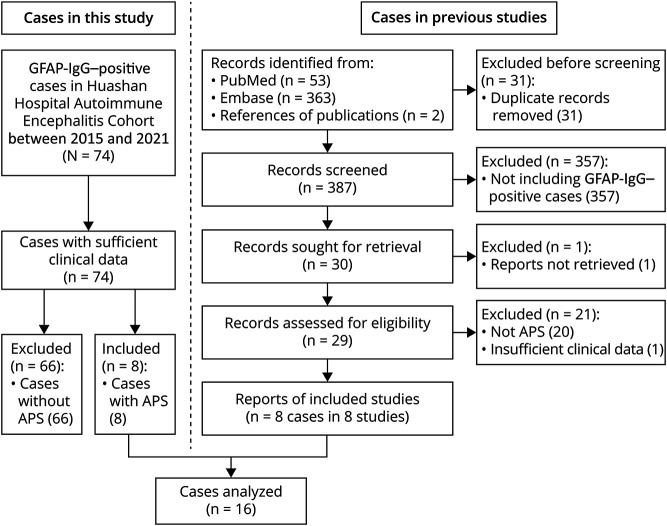
PubMed and Embase (search terms: [GFAP] and [“area postrema” or APS or hiccups or nausea or vomiting]; time frame: January, 2016, and March, 2022) were screened to identify published GFAP-IgG–positive cases with APS. We reviewed all previously reported cases with sufficient clinical data. Finally, we performed a pooled analysis on 16 (including 8 from our institute and 8 from the literature) patients' APS phenotypes (Figure 1).
Figure 1. Flowchart of All Included GFAP-IgG–Positive Cases.
APS = area postrema syndrome; GFAP = glial fibrillary acidic protein.
Statistical Analysis
Statistical analysis was performed with SPSS, version 20.0 (SPSS, Chicago, IL). The characteristics were reported as mean ± SD or median (range) for continuous variables and as frequencies and percentages for categorical variables. The Student t test, Mann-Whitney U test, and Fisher exact test were used, as appropriate. Statistical significance is considered if the 2-tailed p values were less than 0.05.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the institutional review board of Huashan Hospital, Fudan University. Written informed consent was obtained from each participant.
Data Availability
Anonymized data of this study are available on reasonable request.
Results
Incidence and Phenotypes of APS in GFAP-IgG–Positive Patients
We identified 8 patients (1 woman, 7 men; mean age: 52.4 ± 18.4 years) with APS attacks from 74 GFAP-IgG–positive patients (Figure 1). AQP4-IgG was negative in sera from all patients. One patient had coexisting autoantibodies to NMDAR and MOG. No underlying malignancy was found in these patients. No significant difference in the median mRS at disease nadir (disease severity) and at the last follow-up (outcome) was found between these patients and the other 66 patients without APS (both p > 0.05).
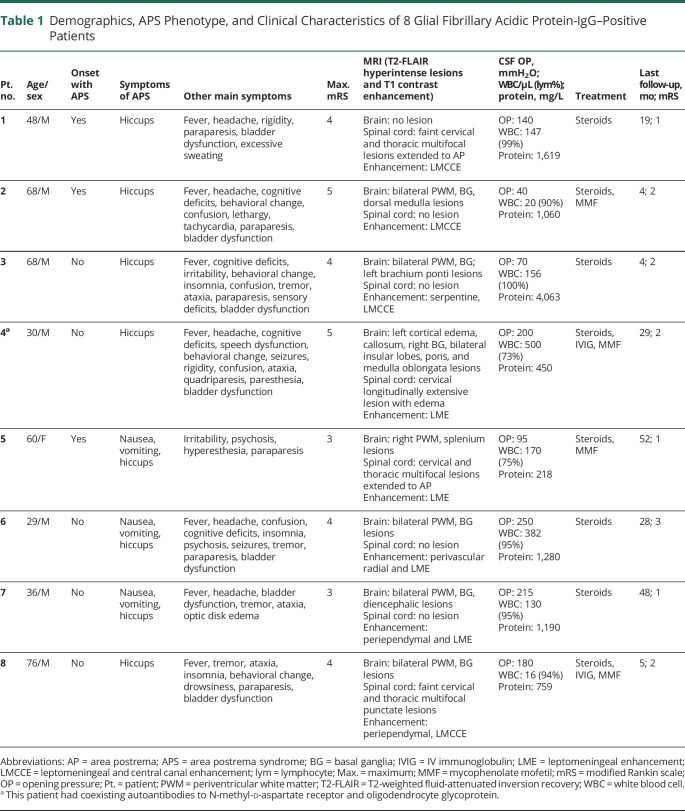
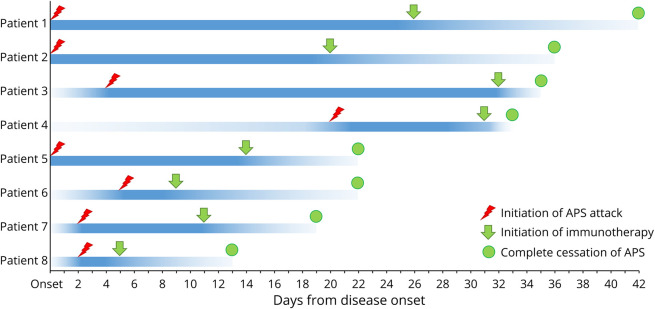
APS occurred as an inaugural symptom in 3 patients. All patients suffered from APS attacks within 1 month after the disease onset, 7 of whom had APS within 1 week after the disease onset. The median time from disease onset to APS occurrence was 2 days (range 0–20). The mean duration of APS episodes was 23.6 ± 11.4 days in our patients. All patients had hiccups, 5 of whom only presented with hiccups. Hiccups was also the last that could be resolved by immunotherapy among 3 symptoms of APS. No patient had isolated APS attack because all patients also presented with symptoms of meningoencephalomyelitis or encephalomyelitis during the whole clinical course. APS phenotypes and clinical features are presented in Table 1 and Figure 2.
Table 1.
Demographics, APS Phenotype, and Clinical Characteristics of 8 Glial Fibrillary Acidic Protein-IgG–Positive Patients
Figure 2. Time Points of APS Onset, Immunotherapy Initiation, and Cessation of APS in Each Glial Fibrillary Acidic Protein-IgG–Positive Case.
The x-axis indicates days from the onset of disease. Each row of the line on the y-axis represents a patient. Symbols of red lightning, green arrows, and dots represent APS onset, immunotherapy initiation, and cessation of APS, respectively. Note that APS occurred at the early stage of disease and was responsive to immunotherapy. APS = area postrema syndrome; Pt = patient.
Brain and Spinal Cord MRI
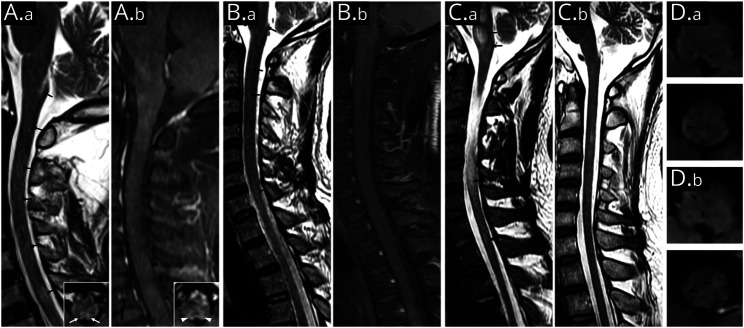
All patients' brain and spinal cord MRI scans were performed within 6 weeks of APS onset. The mean time interval from APS onset to MRI scan was 23.1 ± 6.9 days. Table 1 summarizes neuroimaging characteristics of patients. These images revealed that all patients had brain and/or spinal cord hyperintense lesions on T2-weighted fluid-attenuated inversion recovery (T2-FLAIR) sequences and enhancement of leptomeninges on T1-weighted postgadolinium sequences. Four patients had T2-FLAIR hyperintense lesions in the dorsal medulla, including the AP. Representative MRI is shown in Figure 3.
Figure 3. Area Postrema and Dorsal Medulla Lesions in Representative Glial Fibrillary Acidic Protein-IgG–Positive Patients.
Sagittal T2-weighted fluid-attenuated inversion recovery scan shows lesions in the area postrema and multifocal punctate lesions in medulla and cervical spinal cord (A.a [patient 5] and B.a [patient 1]; black arrows). Inset in (A.a) is the axial image (area postrema level), which shows bilateral lesions involving the dorsal medulla (white arrows). Sagittal T1-weighted MRI with gadolinium shows irregular patchy or linear enhancement in the medulla and cervical spinal cord (A.b and B.b; black arrowheads). Inset in (A.b) is the axial image (area postrema level), which shows hazy gadolinium enhancement of bilateral dorsal medulla (white arrowheads). T2-hyperintensity lesions in pons, medulla oblongata, and cervical longitudinally extensive lesions were noted at the initial presentation of patient 4 (C.a). Follow-up MRI at 6 months after immunotherapy reveals remission of most lesions (C.b). Axial images of patient 2 show scattered faint T2-hyperintensity lesions in dorsal medulla oblongata (D.a; black arrows) accompanied by patchy gadolinium enhancement (D.b; black arrowheads).
Treatment and Outcomes
In this study, 1 patient was initially misdiagnosed as gastroenteritis. The other 7 patients' initial clinical diagnosis was infectious encephalitis or meningitis. The median interval from onset to diagnosis of autoimmune disease or initiation of immunotherapy was 17 days (range 5–32). All patients were treated with first-line immunotherapy (IV corticosteroids, 8; IV immunoglobulin, 2); 4 were treated with second-line immunotherapy (all mycophenolate mofetil). All APS attacks were completely resolved after immunotherapy. The mean duration from treatment to complete remission of episodes was 9.3 ± 5.4 days. The median follow-up duration for our patients was 24 months (range 4–52). One patient (patient 5) experienced relapse 10 days after cessation of steroid. No episode of APS occurred during the relapse. Treatment and outcomes data are presented in Figure 2 and Table 1.
Systematic Literature Review
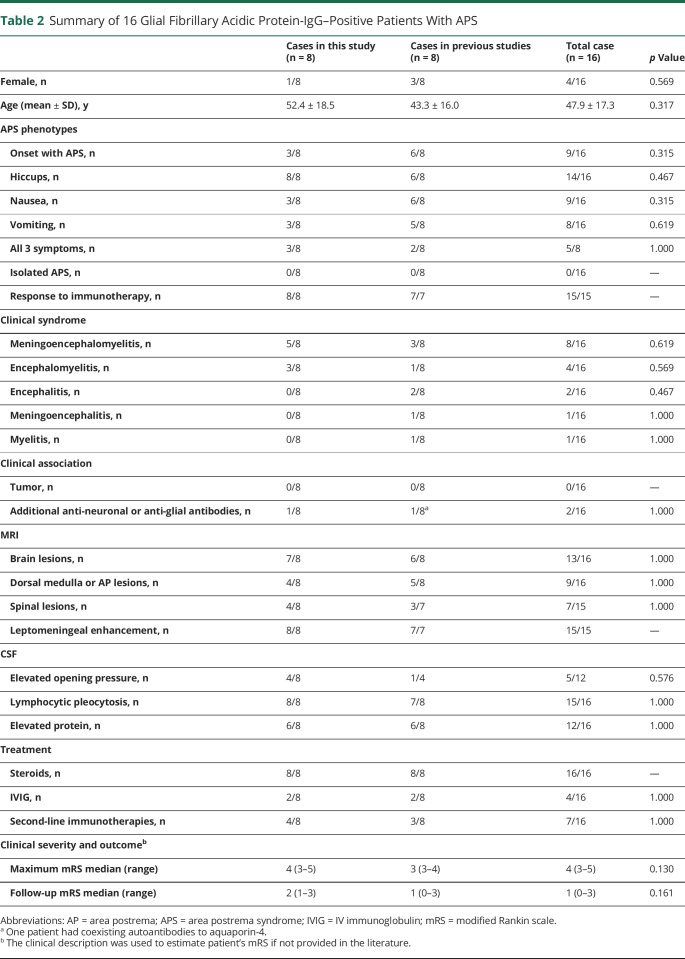
We identified 10 reported GFAP-IgG–positive cases with APS by an extensive literature review, while 8 of them had sufficient clinical data. Coexisting AQP4-IgG was reported in 1 patient. These 8 cases presented with similar APS phenotypes to our cases (Table 2). APS attacks were the inaugural symptoms in 9 of the pooled 16 cases. Hiccups was present in 14 of the 16 patients (88%). APS attacks in all cases showed a good response to immunotherapies, except that 1 published APS case spontaneously resolved before treatment. Isolated APS during the whole clinical course was not seen. Two patients in the literature had a relapsing course. APS was developed during the relapse of one patient. The other patient had APS during the first attack and relapse. Detailed demographics, APS phenotypes, and clinical characteristics of pooled cases are presented in Table 2.
Table 2.
Summary of 16 Glial Fibrillary Acidic Protein-IgG–Positive Patients With APS
Discussion
This study demonstrated that APS was present in 11% of a case series of autoimmune GFAP astrocytopathy. We also described the phenotypes of APS, thus expanding the clinical manifestations of this disorder. These findings supported that APS might not be a unique symptom of AQP4-IgG–seropositive NMOSD but could also be presented in autoimmune GFAP astrocytopathy.
Since 2018, several studies have noticed that APS could be a symptom of autoimmune GFAP astrocytopathy.10-18 However, the incidence of APS in this disorder is unknown. We found that 8 of the 74 patients (11%) with autoimmune GFAP astrocytopathy presented with APS and the frequency of APS as the onset symptom is 4%. They were slightly lower compared with patients with NMOSD (total incidence: 9%–44%; incidence as onset symptom: 7%–13%).9,19-24 Nevertheless, our study indicated that APS is not uncommon in autoimmune GFAP astrocytopathy and may fail to discriminate this disorder from AQP4-IgG–seropositive NMOSD.
Three features of APS in autoimmune GFAP astrocytopathy could be summarized as follows according to this study: (1) APS commonly appeared in the early phase of disease course, while a similar feature was found in AQP4-IgG–seropositive NMOSD.9,22,23 (2) Hiccups was the most common symptom of APS and the last symptom of APS relieved by immunotherapy in our patients. Therefore, hiccups, but not nausea or vomiting, could be the core component of APS in autoimmune GFAP astrocytopathy. By contrast, nausea and vomiting were more common in AQP4-IgG–seropositive NMOSD.4,20-23 (3) No patient in our case series only presented with isolated APS during the whole clinical course. However, APS could be the isolated symptom in AQP4-IgG–seropositive NMOSD, representing about 33% of the episodes of NMOSD.9,20 It is important that the phenotypes of APS demonstrated in previously reported cases were in line with our findings. In summary, these results suggested the combination of early APS and symptoms of meningoencephalomyelitis could be an important diagnostic clue of autoimmune GFAP astrocytopathy.
In this study, GFAP-IgG was positive in all patients' CSF by CBA and TBA. All patients also presented with typical clinical, CSF, and MRI characteristics, indicative of autoimmune GFAP astrocytopathy. Since AQP4-IgG could coexist with GFAP-IgG,3,4 we confirmed that all patients were AQP4-IgG seronegative to exclude APS attributable to AQP4-IgG–related autoimmunity. In addition, APS in autoimmune GFAP astrocytopathy should be differentiated from several mimics. Nonspecific viral-like prodromal symptoms (i.e., headache, dizziness, nausea, and vomiting) are commonly presented in patients with autoimmune GFAP astrocytopathy. Hyponatremia is also frequently appeared in this disorder, leading to gastrointestinal symptoms. However, as mentioned above, hiccups, but not nausea or vomiting, was the prominent symptom of APS in patients with autoimmune GFAP astrocytopathy. It is more important that persistent APS could be rapidly resolved by immunotherapy in all patients, which strongly supported that APS in our cases was a specific symptom of GFAP-IgG–related autoimmunity.
The AP is located at the floor of the fourth ventricle.25 AP and its adjacent structures (e.g., nucleus tractus solitarius) are believed to constitute the emetic reflex center.25-27 AQP4 and GFAP are 2 important biomarkers of astrocytes. AQP4 is located at the cell surface, while GFAP is a cytoskeletal protein of astrocytes. Both proteins are enriched in the AP and responsible for supporting the interaction of astrocytes with neighboring neurons and maintenance of the blood-brain barrier.2,28 Histopathologic studies have shown that selective AQP4 reduction/loss and nonlytic astrocyte injury in the AP of AQP4-IgG–seropositive patients.22,28 These studies indicated that autoimmune reaction in the AP could be associated with APS. Since GFAP is an intracellular protein, GFAP-IgG seems unlikely to have a direct pathologic effect on astrocyte.3 Impairment of astrocytes might be mediated by T cells and other inflammatory components of the immune system.3,4,29 Given that both AQP4 and GFAP are pivotal in astrocytes, we might hypothesize that impairment of astrocytes in the AP or related neural network could cause clinical symptoms of APS in autoimmune GFAP astrocytopathy. Further pathologic studies of the AP and development of animal models are needed to elucidate the pathogenesis of astrocyte dysfunction and its association with APS in GFAP-IgG–related autoimmune astrocytopathy.
This study has several limitations. First, this was a single-center, retrospective study. We could not exclude the fact that APS attacks in a small proportion of patients might have been missed. In addition, we could not obtain some accurate features of APS (e.g., frequency and duration of the episode), which are important parameters to evaluate the burden of APS in patients.9 The incidence and phenotypes of APS, as well as the clinical relevance of APS reported in this study, still need to be validated in other independent studies. Second, not all patients had concurrent MRI during APS attacks, and some patients had received immunotherapies before MRI scans, which might exert an influence on radiologic confirmation of lesions in the AP. Finally, we observed some features of APS in autoimmune GFAP astrocytopathy, which might be different compared with that of AQP4-IgG–seropositive NMOSD. However, a direct comparison of the APS phenotype between these 2 autoimmune astrocytopathy was lacking in this study. In addition, whether APS could be considered a distinctive phenotype of GFAP-IgG–associated astrocytopathy needs further investigation.
Acknowledgment
The authors thank Dr. Mengfei Cai for his valuable suggestions and discussion.
Glossary
- AP
area postrema
- APS
area postrema syndrome
- AQP4
aquaporin-4
- CBA
cell-based assays
- TBA
tissue-based assays
- FLAIR
fluid-attenuated inversion recovery
- GFAP
glial fibrillary acidic protein
- IgG
immunoglobulin G
- MOG
oligodendrocyte glycoprotein
- mRS
modified Rankin scale
- NMDAR
N-methyl-d-aspartate receptor
- NMOSD
neuromyelitis optica spectrum disorder
Appendix. Authors
Study Funding
This study was supported by Clinical Research Plan of SHDC (No. SHDC2020CR2027B), National and Provincial Multidisciplinary Cooperation in Diagnosis and Treatment of Major Diseases Capacity Improvement Project (Shanghai Municipal Health Commission), Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01), Science and Technology Commission of Shanghai Municipality, Shanghai 2020 “Science and Technology Innovation Action Plan” experimental animal research project, topic: Animal model research, development and application (No. 201409001400), and State Key Laboratory of Genetic Engineering, Human Phenome Institute, Zhangjiang Fudan International Innovation Center, Fudan University. These funders played a role in the data collection.
Disclosure
B. Deng reports no disclosures relevant to the manuscript; J. Wang reports no disclosures relevant to the manuscript; H. Yu reports no disclosures relevant to the manuscript; L. Jin reports no disclosures relevant to the manuscript; Y. Qiu reports no disclosures relevant to the manuscript; X. Liu reports no disclosures relevant to the manuscript; P. Wang reports no disclosures relevant to the manuscript; X. Zhang reports no disclosures relevant to the manuscript; X. Chen reports no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosure.
References
- 1.Fang B, McKeon A, Hinson SR, et al. Autoimmune glial fibrillary acidic protein astrocytopathy: a novel meningoencephalomyelitis. JAMA Neurol. 2016;73(11):1297-1307. [DOI] [PubMed] [Google Scholar]
- 2.McKeon A, Benarroch EE. Glial fibrillary acid protein functions and involvement in disease. Neurology. 2018;90(20):925-930. [DOI] [PubMed] [Google Scholar]
- 3.Flanagan EP, Hinson SR, Lennon VA, et al. Glial fibrillary acidic protein immunoglobulin G as biomarker of autoimmune astrocytopathy: analysis of 102 patients. Ann Neurol. 2017;81(2):298-309. [DOI] [PubMed] [Google Scholar]
- 4.Gravier-Dumonceau A, Ameli R, Rogemond V, et al. Glial fibrillary acidic protein autoimmunity: a French cohort study. Neurology. 2022;98(6):e653–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul P, McKeon A, Pittock SJ, et al. GFAP IgG associated inflammatory polyneuropathy. J Neuroimmunology. 2020;343:577233. [DOI] [PubMed] [Google Scholar]
- 6.Héraud C, Capet N, Levraut M, et al. Glial fibrillary acidic protein (GFAP) astrocytopathy presenting as mild encephalopathy with reversible splenium lesion. Neurol Ther. 2022;11(1):499-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sechi E, Flanagan EP. Area postrema syndrome in autoimmune GFAP astrocytopathy. Mult Scler. 2020;26(2):255-256. [DOI] [PubMed] [Google Scholar]
- 8.Wingerchuk DM, Banwell B, Bennett JL, et al. , International Panel for NMO Diagnosis. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shosha E, Dubey D, Palace J, et al. Area postrema syndrome: frequency, criteria, and severity in AQP4-IgG-positive NMOSD. Neurology. 2018;91(17):e1642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciron J, Sourdrille F, Biotti D, et al. Area postrema syndrome: another feature of anti-GFAP encephalomyelitis. Mult Scler. 2020;26(2):253-255. [DOI] [PubMed] [Google Scholar]
- 11.Lin H, Huang Y, Zeng H, et al. Overlapping clinical syndromes in patients with glial fibrillary acidic protein IgG. Neuroimmunomodulation. 2020;27(1):69-74. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Bhekharee AK, Zhang X. NMOSD or GFAP astrocytopathy? A case report. Mult Scler Relat Disord. 2020;43:102202. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez J, Romero R. A case of autoimmune glial fibrillary acidic protein astrocytopathy (GFAP) meningoencephalomyelitis responsive to infliximab (P1. 2-009). Neurology. 2019;92(15 Suppl):P1.2-009. [Google Scholar]
- 14.Adachi H, Shiomi Y, Kimura A, Shimohata T, Yoneda Y, Kageyama Y. A case of autoimmune glial fibrillary acidic protein (GFAP) astrocytopathy. Clin Neurol. 2021;61(6):401-404. [DOI] [PubMed] [Google Scholar]
- 15.Gao X, Tang Y, Yang GD, Wei W. Autoimmune glial fibrillary acidic protein astrocytopathy associated with area postrema syndrome: a case report. Front Neurol. 2021;12:803116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwami K, Nomura T, Seo S, et al. Autoimmune glial fibrillary acidic protein astrocytopathy presenting with area postrema syndrome-like symptoms without medulla oblongata lesions. Neuroimmunomodulation. 2022:1-6. doi: 10.1159/000524344. [DOI] [PubMed] [Google Scholar]
- 17.Koh PX, Tay KY, Yeo T, et al. Glial fibrillary acidic protein astrocytopathy in a patient with recent mRNA SARS-CoV-2 vaccination: coversheet. Neuroimmunol Rep. 2022;2:100053. [Google Scholar]
- 18.Long Y, Liang J, Xu H, et al. Autoimmune glial fibrillary acidic protein astrocytopathy in Chinese patients: a retrospective study. Eur J Neurol. 2018;25(3):477-483. [DOI] [PubMed] [Google Scholar]
- 19.Camara-Lemarroy CR, Burton JM. Area postrema syndrome: a short history of a pearl in demyelinating diseases. Mult Scler. 2019;25(3):325-329. [DOI] [PubMed] [Google Scholar]
- 20.Misu T, Fujihara K, Nakashima I, Sato S, Itoyama Y. Intractable hiccup and nausea with periaqueductal lesions in neuromyelitis optica. Neurology. 2005;65(9):1479-1482. [DOI] [PubMed] [Google Scholar]
- 21.Iorio R, Lucchinetti CF, Lennon VA, et al. Intractable nausea and vomiting from autoantibodies against a brain water channel. Clin Gastroenterol Hepatol. 2013;11(3):240-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apiwattanakul M, Popescu BF, Matiello M, et al. Intractable vomiting as the initial presentation of neuromyelitis optica. Ann Neurol. 2010;68(5):757-761. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi T, Miyazawa I, Misu T, et al. Intractable hiccup and nausea in neuromyelitis optica with anti-aquaporin-4 antibody: a herald of acute exacerbations. J Neurol Neurosurg Psychiatry. 2008;79(9):1075-1078. [DOI] [PubMed] [Google Scholar]
- 24.Long YM, Liang JY, Wu LZ, et al. Different phenotypes at onset in neuromyelitis optica spectrum disorder patients with aquaporin-4 autoimmunity. Front Neurol. 2017;8:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller AD, Leslie RA. The area postrema and vomiting. Front Neuroendocrinol. 1994;15(4):301-320. [DOI] [PubMed] [Google Scholar]
- 26.Borison HL. Area postrema: chemoreceptor circumventricular organ of the medulla oblongata. Prog Neurobiol. 1989;32(5):351-390. [DOI] [PubMed] [Google Scholar]
- 27.Han W, de Araujo IE. Nausea and the brain: the chemoreceptor trigger zone enters the molecular age. Neuron. 2021;109(3):391-393. [DOI] [PubMed] [Google Scholar]
- 28.Popescu BFG, Lennon VA, Parisi JE, et al. Neuromyelitis optica unique area postrema lesions: nausea, vomiting, and pathogenic implications. Neurology. 2011;76(14):1229-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan Z, Li H, Huang L, et al. CD8(+) T-cell predominance in autoimmune glial fibrillary acidic protein astrocytopathy. Eur J Neurol. 2021;28(6):2121-2125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data of this study are available on reasonable request.