Abstract
Traditional Chinese medicine (TCM) is the key to unlock treasures of Chinese civilization. TCM and its compound play a beneficial role in medical activities to cure diseases, especially in major public health events such as novel coronavirus epidemics across the globe. The chemical composition in Chinese medicine formula is complex and diverse, but their effective substances resemble “mystery boxes”. Revealing their active ingredients and their mechanisms of action has become focal point and difficulty of research for herbalists. Although the existing research methods are numerous and constantly updated iteratively, there is remain a lack of prospective reviews. Hence, this paper provides a comprehensive account of existing new approaches and technologies based on previous studies with an in vitro to in vivo perspective. In addition, the bottlenecks of studies on Chinese medicine formula effective substances are also revealed. Especially, we look ahead to new perspectives, technologies and applications for its future development. This work reviews based on new perspectives to open horizons for the future research. Consequently, herbal compounding pharmaceutical substances study should carry on the essence of TCM while pursuing innovations in the field.
Keywords: Chinese medicine formula, Chemical components, Compound, Active ingredient, Mass Spectrometry, Disease Targets
Abbreviations: TCM, Traditional Chinese medicine; PK, Pharmacokinetic; PD, Pharmacodynamic; 2D, Two Dimensional; 3D, Three Dimensional; CDE, Center for Drug Evaluation; NMPA, National Medical Products Administration; R-strategy, Reduce strategy; HR-MS, High Resolution Mass Spectrometry; HPLC, High Performance Liquid Chromatography; UPLC, Ultra Performance Liquid Chromatography; Q-TOF/MS, Quadrupole time-of-flight mass spectrometry; GC-MS, Gas chromatography-mass spectrometry; ICPMS, inductively coupled plasma mass spectrometry; NL/PR, Neutral loss/precursor ion; OPLS-DA, Orthogonal partial least squares discriminant analysis; MD, Microdialysis; QqQ-MS, Triple quadruple mass spectrometry; MALDI MS, Matrix for surface-assisted laser desorption/ionization mass spectrometry; ADME, Absorption, Distribution, Metabolism, and Excretion; MSI, Mass spectrometry imaging; AFA DESI-MSI, Air flow-assisted desorption electrospray ionization mass spectrometry imaging; UF, Affinity ultrafiltration; MI, Molecular imprinting; QSAR, Quantitative structure-activity relationship; XO, Xanthine oxidase; COX-2, Cyclooxygenase 2; HTS, High Throughput Screening; AI, Artificial Intelligence; HUA, hyperuricemia
1. Introduction
As main form of clinical traditional Chinese medicine (TCM), Chinese medicine formulas are a group of herbs that have been reasonably formulated after the identification of therapeutic principles by doctors. Its carry valuable clinical experience of practitioners over the generations. With development and application of the concept by “principals, associates, adjuvants and messengers” and “seven emotions”, Chinese medicine formulas have enhanced efficacy and reduced toxicity compared with individual herbs (Tang and Duan, 2014, Tang et al., 2019). Active ingredients are foundation for explaining mechanism of Chinese medicine formula. From perspective of modern scientific, Chinese medicine formula is like a huge “mystery boxes”. Although it is experienced in human use and its clinical efficacy is clear, but what are its chemical components and potent substances? What are the overall dynamic changes with these components in body? Thus, how to use modern science to reveal substance in the “mystery boxes”, and characterize the complex substance-potency relationship has become a key point for inheritance, development and innovation of Chinese medicine formula (Yang, 2015, Xu et al., 2018b). With deepening understanding with TCM, many scientists have made a series of achievements in theoretical cognition, outstanding results and clinical translation of Chinese medicine formula.
For theoretical cognition, early scholars proposed the “shrapnel theory”, which is depending on synergistic action with multiple components and multiple targets in the pharmacodynamic substances in Chinese medicine formula (Xue and Lei, 1996). Luo constructed a complex substance research idea of Chinese medicine formula with “one combination, two basic clarifications, three chemical levels (formula, effective part and active ingredient) and four pharmacological levels (animal, tissue organ, cell and molecular)” (Luo and Wang, 1999). The research system of serum medicinal chemistry of TCM was established to provide the beginning for migratory components and metabolites in the blood (Wang, 2010, Qiu and Yao, 1999). Li proposed the theory of “equivalence component group” (Liu et al., 2014). It followed the content ratio of the original formula, evaluates the contribution of the “parts” to the “whole”, and located the “equivalent component groups” that could basically represent the medicinal effects of the original formula. Meanwhile, Cai’s team proposed the “efficacy theory” of TCM (Cai et al., 2015), in which the core substance effect is the summation of “efficacy form”; the same single target produces “superimposed effects” and multiple targets produce “synergistic effects”. In recent years, some scholars have also proposed the hypothesis of “multi-source homogeneity” for complex systems of TCM (Xiang et al., 2019), which means that the same active ingredient or (and) the same biological effect in a complex material system of TCM. Furthermore, there is also the “component structure” theory (Yan et al., 2015), which is depending on the holistic view of TCM and combining macroscopic and microscopic. Recently, the concept of “structural TCM” has been proposed (Qiao et al., 2021), which builds up the emerging direction from molecular interactions and aggregate conformation. It concretizes the aggregation and further elucidates active ingredients and their pharmacodynamic correlations.
In terms of research results, many scholars in China have made a series of highlighting progress over the years. As early as 2008, Chen’s team (Wang et al., 2008) was the first to elaborate the principle with “principals, associates, adjuvants and messengers” of the Chinese medicine formula, and to elucidate the multi-component and multi-target mechanism with action of compound Huang Dai tablets for the treatment of leukemia depending on molecular level. In order to solve the difficult problem of qualitative and quantitative analysis of complex components, Song’s study (Song et al., 2015) developed methods such as biocapture or mass spectrometry (MS) tracing to capture target binding components from TCM as equivalent component candidates. In 2015, SCIENCE featured a special issue on Shexiang Baoxin pill in its special issue on TCM, and established its “chemical fingerprint” from chemical composition to blood entry components, which provided an important basis for revealing its potent substances and molecular mechanisms (Yuan et al., 2018). In 2018, NATURE further reported the in vivo mechanism of action of cinnamaldehyde, one of the volatile blood-entering components in Shexiang Baoxin pill, from perspective of pharmacodynamic components (Liu et al., 2015). With the development of new technology, another research team (Liao et al., 2017) reported in PNAS an innovative way of “fishing” for target proteins with ingredients. And the study found that IMPDH2 is a key target for its anti-neuroinflammatory effect, thus clarifying that hematoxylin A in Chinese hematoxylin can induce the inactivation of key targets to inhibit inflammation. By observing the phenomenon of self-precipitation during the decoction of Chinese herbal compound, the scholars recently found that different active ingredients self-assemble into supramolecular structures during the decoction process under weak bonding induction and have better activity (or reduced toxicity) compared with the raw material monomer, which provides a new research strategy for new TCM (Li et al., 2019c, Wang et al., 2021b). The interaction between functional molecules in TCM and disease metabolism molecules has great advantages in discovery of functional components (Zhang et al., 2022, Wang et al., 2021c). During the novel coronavirus (COVID-19) outbreak, the research team (Dai et al., 2021, Lv et al., 2019) combined HTS2 technology, bioinformatics, and computer-aided drug design, finally found Lizhong Decoction, Liujunzi Decoction, Huanglian Detoxification Decoction, and Qingfei Detoxification Decoction could inhibit cytokine storm. The study provides a foundation for elucidating mechanism of TCM against novel coronaviruses.
For clinical applications of Chinese medicine formula, many new drug approvals have also gradually increased during recent years. According to data from the official website of the Center for Drug Evaluation (CDE) of National Medical Products Administration (NMPA), 11 new proprietary TCMs were approved for listing from 2019 to 2021. Including the total alkaloids of mulberry branch, Xiaoer Jingxing Zhike Granules, Shao Ma Zhijing Granules, Muscle Pain Relief Gel, etc. Among them, NMPA approved the listing of the total alkaloids of mulberry branch, known as “the first new TCM for diabetes approved for marketing in the past 10 years”, which has the advantages of clear composition, clear target and controllable quality. Hence, the development and application of Chinese medicine formula cannot be separated from the characterization of pharmacodynamic substances, and the pharmacodynamic substances is also an indispensable element in the quality aspect of new drug declaration.
In general, Chinese medicine formula is a necessary entry point for basic research on substances. Furthermore, the research on active ingredients of TCM is gradually changing from “single-army” to “group-army”, and the research on single compounds is changing to substance groups. With the development of innovative technologies, the understanding of the pharmacodynamic substances in Chinese medicine formula is gradually changing. The multidisciplinary cross-application with new technologies and methods has led to a more diversified study of potent substances, which is accompanied by many problems. Hence, this paper provides a comprehensive account of existing new approaches and technologies depending on previous studies with an in vitro to in vivo perspective. In addition, the bottlenecks of studies on Chinese medicine formula effective substances are also revealed. Especially, we look ahead to new perspectives, technologies and applications for its future development.
2. Strategies for pharmacodynamic substances study in Chinese medicine formula
2.1. The “R-strategy” for in vitro studies based on “chemical composition - active ingredient”
2.1.1. “Separation-analysis-attribution” for chemical composition
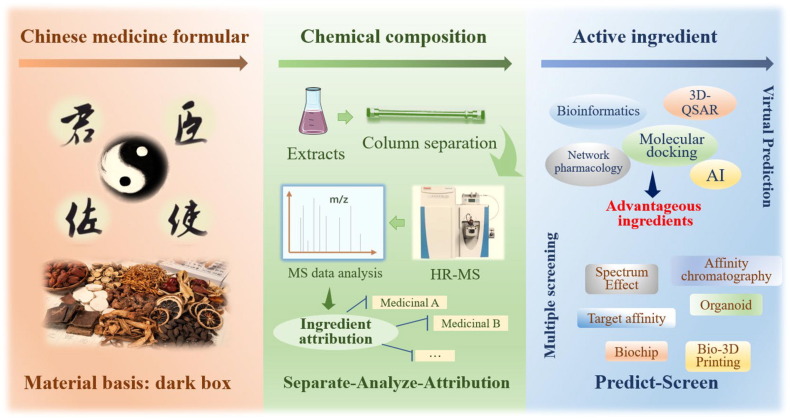
Chinese medicine formula consists of at least two and more herbs. The chemical composition of single herbs is rich in variety, and the ingredient types of compound prescriptions are numerous and complex. Most of them are secondary plant metabolites, so their chemical and active ingredients are transferable, and can be traced back to their origin. The first task for Chinese medicine formula is to characterize the chemical components in the “mysterious box”. With the modern analytical tools, the whole process of sample preparation, column chromatography separation, high resolution mass spectrometry (HR-MS) detection, and the establishment and retrieval of the composition database of herbs in formula is systematically studied and optimized. We use modern methodological systems and instrumentation platforms to conduct in-depth analysis of complex chemical profiles, such as Chinese medicine formula, qualitatively characterize and attribute chemical components to single herbs, and lay the material foundation for subsequent revelation of components and mechanisms (Fig. 1 ). Meanwhile, the characterization of chemical components is also an important step to unveil the Chinese medicine formula and to lay the foundation for TCM compounding theory.
Fig. 1.
Schematic diagram of the subtraction study of Chinese medicine formula ingredients in vitro.
2.1.2. Reducing the burden in complex ingredients: Virtual prediction and multivariate screening of active ingredients
The extract obtained by the traditional decoction covers both large and small molecules. It is a mixture that follows thermodynamics. The low content of most components, numerous congeners, large polarity span, and complex steric structure bring great challenges to the separation and structural identification for components of Chinese medicine formula. Under the complex chemical composition system, how to further reveal the active ingredients rapidly, precisely, and efficiently is the key problem. For recent years, with the development of research technology, especially the emerging discipline of “chemical biology of TCM”, the screening of active ingredients in complex substance systems has been rapidly growth (Zeng et al., 2019, Ai et al., 2018, Zhou et al., 2021). Firstly, computer-based virtual prediction strategies. Depending on the “ingredient-disease-gene-target”, many researchers have combined bioinformatics (network pharmacology, molecular docking), artificial Intelligence (AI) machine learning and other strategic ideas to firstly carry out virtual prediction of ingredients and initially narrow down the range of potential active ingredients (Zhou et al., 2021). Secondly, the development and application of multiple in vitro screening strategies. Active ingredients screening mainly based on spectroscopic relationships, bio chromatography, organs, 2D or 3D cells, organoids, tissue microarrays, bio-3D printed organs, etc (Fig. 1). Thirdly, the screening of target-acting components that depend on clear disease effects. Such as ultrafiltration affinity, click chemistry, biochip (gene and protein), cellular thermal shift analysis, magnetic target draping, etc. As research progresses, screening strategies for numerous active ingredients gradually tend to be trace, bionic, high-throughput, and rapid, focusing on efficient bioactive screening of trace amounts of TCM.
2.2. Molecular potency characterization strategy based on “in vivo component-pharmacological effect”
2.2.1. Revealing serum drug migration behaviors in Chinese medicine formula
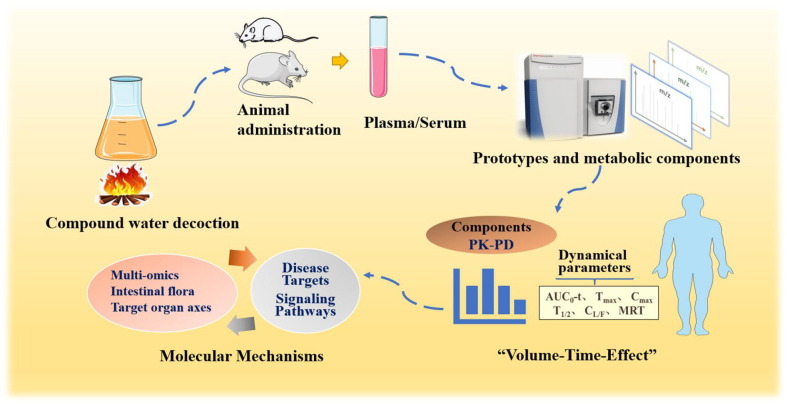
The prototypical and metabolic components of blood-entering are among the final substances for expression of the efficacy in TCM. Exposure behavior of complex components in vivo is a necessary way to elucidate efficacy substances. It excludes components that cannot be absorbed into blood, so serum medicinal chemistry further narrows the range of active ingredients. Serum medicinal chemistry is studied using drug-containing serum as a sample after administration to animals. Modern chemical analysis is used to comprehensively identify the blood-incorporated components of serum and to discover the active ingredients. It contains the pharmacokinetic characteristics of different components and metabolites (Wang, 2002). Serum medicinal chemistry studies mainly include (i) animal administration and collection of serum or plasma, (ii) preparation of serum or plasma samples, (iii) MS data acquisition using LC-MS, (iv) data processing and component identification, and (v) characterization of the metabolic behavior of components. In terms of technical means, serum medicinal chemistry is mainly divided into animal serum processing and analysis (Fig. 2 ). Firstly, multiple analysis techniques are used to improve the sensitivity of the assay while reducing the interference of endogenous impurities. Further studies have shown that serum medicinal chemistry has been combined with pharmacology, pharmacokinetics, Spectro pharmacology, network pharmacology, and prescription metabolomics to fully exploit the basic research of pharmacodynamic substances (Zhang et al., 2019e, Li et al., 2019b).
Fig. 2.
Schematic diagram of the pharmacodynamic characteristics of Chinese medicine formula components in vivo.
2.2.2. “Volume-time-effect” analysis based on pharmacokinetic-pharmacodynamic (PK-PD) markers
The traditional pharmacological theory includes the concepts of “drug properties go and keep”, “odor thickness”, “rise and fall”, “attribution”, etc. All of them cover the scientific connotation of pharmacokinetic and in vivo processes of drug components (Zhang et al., 2019a). However, for diseases not every ingredient in TCM can directly exert their medicinal effects. Despite the high content of some chemical components in TCM, their metabolism and elimination in body make it difficult to reach the concentration of efficacy due to their low bioavailability. On the contrary, there are also some components that undergo metabolic transformation in body, which enhances their activity (Yang, 2015). Hence, the components of compounded formulas are mainly able to be absorbed into the body circulation (prototype or metabolized), and these substances have perfect PK and tissue distribution, and then interact with tissue to exert pharmacodynamic effects (Fig. 2). PK-PD markers are components selected by a comprehensive evaluation of PK and PD characteristics of each component in TCM (Qiao et al., 2018, Hao et al., 2009). These components have exact pharmacological effects and suitable PK characteristics. PK-PD is a feasible method to reveal the in vivo transmission characteristics, potency trends, tissue targeting and their interactions of different medicinal flavors. Furthermore, PK-PD markers is important for the discovery of equivalent groups, the simplified substitution of original prescriptions, and the innovation of TCM.
2.3. Molecular mechanism elucidation of pharmacodynamic components by multidimensional strategy
The elucidation of molecular mechanisms of TCM further clarifies the in vivo action characteristics of pharmacodynamic components, which link disease targets and signaling pathways. Besides disease model and pharmacodynamic mechanism methods, many scholars have carried out in-depth exploration nearly years, such as the application of multi-omics, intestinal flora, target organ association axis and other multidimensional linkage strategies (Fig. 2). TCM formula metabolomics as biological language (Zhang et al., 2019b) that further expresses effectiveness of herbal medicinal substances and highlights the value of Chinese medicine formula. Modern research combines serum medicinal chemistry and metabolomics to establish a prescription efficacy bio evaluation system and discovered herbal medicinal substances. Furthermore, proteomics, transcriptomics, and genomics are used to reveal mechanism of TCM components from inside out. It covers the characteristics of holistic, complexity and dynamics, and is suitable for the pharmacodynamic substances under TCM theory (Jia, 2018). During recent years, intestinal flora has been found to play a beneficial role in improving the metabolic absorption, bioavailability and efficacy of active compounds. On the one hand, active ingredients inhibit the production of harmful bacteria and promote the growth of beneficial bacteria, and exerting medicinal effects (Chang et al., 2015, Boulangé et al., 2016, Quan et al., 2020). On the other hand, intestinal bacteria can produce variety of enzymes that are closely related to physiological processes. Active ingredients can regulate enzymes activity and interrupt disease process (Wu et al., 2019, Luck et al., 2019). Furthermore, target organ association axis can also elucidate components mechanism, such as intestine-kidney axis and intestine-brain axis studies can reflect the action characteristics of Chinese medicine formula.
3. Frontier approaches and technologies for pharmacodynamic substances study in Chinese medicine formula
3.1. Multi-method-based characterization of chemical components
3.1.1. One-dimensional LC-MS for qualitative or quantitative studies
In the past decades, the coupling of liquid chromatography and mass spectrometry has made a great contribution to the qualitative analysis of chemical constituents of TCM, and this has greatly improved the efficiency of analysis (Yu et al., 2021, Stavrianidi, 2020). The model of chemical composition has gradually matured and divided into steps such as (i) the establishment of chemical composition libraries, (ii) the establishment of LC-MS methods and data acquisition, and (iii) the identification and attribution of components depending on MS information. The specific research methods were also diversified. In Xiling Jiedu Capsule’s study (Gao et al., 2021), UPLC-quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF/MS) combined with compositional data mining used to identify the chemical components and 145 compounds were identified. Similarly, a combination of UPLC-Q-Orbitrap and UPLC-MS/MS were used to identification and determination of compounds in Tangshen formula (Wang et al., 2021d). In Guo’s study (Guo et al., 2021), they constructed a deep learning-assisted mass deficit filtering (MDF) to identify 183 components in QiangHuoShengShi decoction. Another research team using a “component knockout” strategy to identify and predict ginsenoside components in Xueshuantong capsule (Zuo et al., 2019). For the volatile components in Ermiao Wan, researcher also investigated using gas chromatography-mass spectrometry (GC–MS) (Huang et al., 2019).
3.1.2. Innovative 2D LC-MS for identification of ingredients in Chinese medicine formula
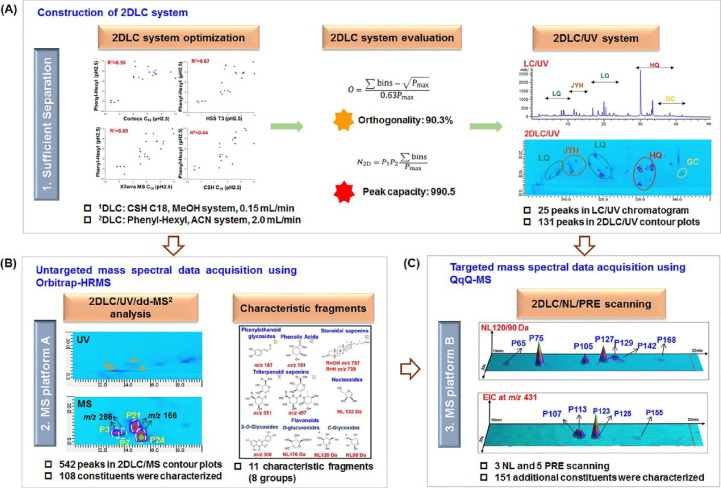
Currently, the chromatographic separation of TCMs and their compound extracts remains a great challenge. Problems such as complex composition, large span of polarity, multi co-flux components, and multi differentiating isomers still exist, and the application of traditional 1D LC-MS techniques is not able to solve such challenges. Giddings first introduced the concept of two-dimensional liquid chromatography (2DLC) (Giddings et al., 1984). 2DLC analysis starts with the separation of components in one chromatographic system, on top of which a different chromatographic system is used to fully separate each fraction again. 2DLC has become an attractive separation tool in the current research. Shang et al. (Shang et al., 2021) developed a strategy for analysis of overall chemical composition of a 2DLC combined with a dual MS platform (Orbitrap-MS and QqQ-MS) (Fig. 3 ). Under this platform, the optimized orthogonality was 90.3 %, and 542 peaks were detected in a TCM formula. In Wang’s experiment (Wang et al., 2017), a new approach for discovery and identification of unknown compounds in Erxian decoction using 2D MS was established. The strategy accelerating the efficiency of identification of unknown compounds in TCM. In addition, the similarly strategy was used in Erzhi wan (Fu et al., 2019a) and Dengzhan Shengmai (Sheng et al., 2017), which successfully identified a large amount of composition information. In the future, the technological enhancement of 2D LC-MS can effectively solve the tricky problems of co-flux and tautomerism (Feng et al., 2021).
Fig. 3.
Workflow of the 2DLC/dual-MS strategy (Shang et al., 2021). (A) Construction of the 2DLC/UV system; (B) untargeted mass spectral data acquisition using Orbitrap-HRMS; (C) targeted mass spectral data acquisition using QqQ-MS. Reprinted with permission from Ref. Shang et al., 2021. Copyright © 2021 Elsevier.
3.2. In vivo migration component pattern and process characterization
3.2.1. Identification of the prototype and metabolic components in blood
Among the complex chemical components of Chinese medicine formula, the chemical components that can be absorbed into blood and their metabolic components are often considered to be the effective substances of Chinese medicine formula. This paper summarizes the general idea of the identification of the prototype and metabolic components. After having obtained the prepared serum (or plasma) samples, the MS information of blank blood samples and drug-containing blood samples was collected using LC-MS. Firstly, the prototype components were found by chemical composition analysis. Secondly, the metabolic and prototype components were predicted and identified with corresponding data software, such as Metabolynx XS, UNIFI, Compound Discovery or OPLS-DA. Finally, metabolic pathways of metabolites in vivo including 1-phase metabolism and 2-phase metabolism were characterized. Under the strategy, many compound formulas have been studied for blood entry components, such as Xialian Capsule (Li et al., 2018), Wutou decoction (Cheng et al., 2021), Dan Zhi Tablet (Mi et al., 2019), Sanmiao Wan (Ma et al., 2021b), isochlorogenic acid A (Gong et al., 2020), Dahuang Zhechong pill (Ni et al., 2020), Smilacis Glabrae Rhizoma (Gegentana et al., 2020). In the current research, Zhang et al. (Zhang et al., 2019f) and Zhu et al. (Zhu et al., 2021) combined serum medicinal chemistry to establish an innovative herbal pharmacomics strategy capable to discover effective substances. However, there are still difficulties in the background deduction, which affects the accurate identification of target compounds. On this basis, Wu’s team (Chen et al., 2021, Zhu et al., 2020) established a multi-stage MS background deduction method and applied to the identification of blood-entering components of Lianhua Qingwen Capsules and Xiaoke wan, which greatly improved the analytical efficiency.
3.2.2. Metabolic behavior of complex components by microdialysis coupled with MS
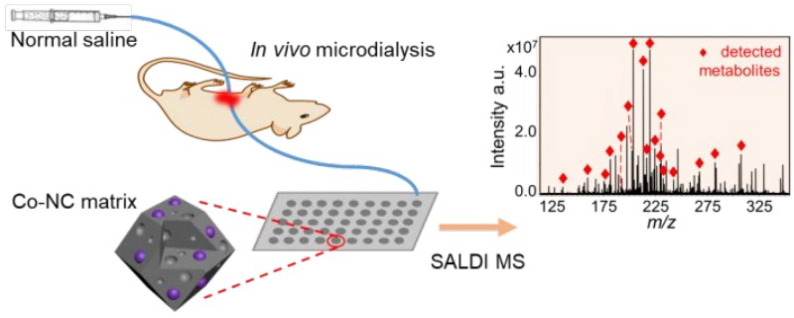
Microdialysis, consisting of dialysis membrane (probe) and microdialysis pump, is now widely used in pharmacology and physiology experiments. After administered, the probe is implanted in body to allow continuous sampling, and following Fick’s law of diffusion. Qualitative or quantitative characterization is performed in combination with LC or MS. Due to the advantages of minimally invasive, online, real-time, and microscopic techniques, microdialysis has been used for endogenous substance changes, in vivo and in vitro drug screening, drug metabolic, and PK of components (Wang et al., 2015). (i) For endogenous metabolic markers, Wang’s team (Wang et al., 2020a) depending on microdialysis combined UPLC-QqQ-MS for relevant active compounds and endogenous neuroactive substances, which helped in substance basis and mechanism of TCM. Similarly, microdialysis plays a key role in elucidating disease mechanisms (Xu et al., 2019, Xu et al., 2020, Mever et al., 2020, Su and Ho, 2019). (ii) For in vivo single drug studies, microdialysis being applied for PK monitoring and quantification of drugs in skin tissue (Voelkner et al. 2018) and plasma (Girondi et al., 2018, Wang et al., 2020c), among others. (iii) For complex component systems, investigators established the study of Ermiao wan metabolites in bile (Zhao et al., 2020) and Mahuang decoction in blood and brain (Wan et al., 2020). (iv) It was used for the evaluation of PK parameters of drugs, especially combining PD and PK (Wang et al., 2019, Guo et al., 2019). Although combination of microdialysis and MS offers the potential, the high salt hinders the sensitive and on-line detection of small molecule compounds in the physiological fluid. Therefore, Li’s team (Li et al., 2020) developed a Co-NC-assisted laser desorption/ionization ion (MALDI) source, which can be used for MALDI MS analysis even under high salt, ultimately improving the salt tolerance as a matrix for the first application in the online monitoring of liver metabolites in a rat model of liver injury (Fig. 4 ).
Fig. 4.
Metabolic behavior of complex components by microdialysis coupled with MS. Li’s team (Li et al., 2020) synthesized a cobalt-doped mesoporous carbon material (Co-NC) and developed a Co-NC-MALDI source. Reprinted with permission from Ref. Li et al., 2020. Copyright © 2020 American Chemical Society.
3.2.3. In vivo process studies of pharmacodynamic substances based on PK-PD
TCM is distinguished from chemical drugs. The in vivo processes of its complex components reflect the synergistic effects of each component. Thus, ADME processes helps to reveal the relationship between clinical use and efficacy. Besides traditional studies, modern studies include the interaction between active ingredients and metabolites, PK and PD correlation analysis, etc. (Sun et al., 2019, Wu et al., 2022). At present, PK has been widely used in the pharmacodynamic substances, formulation mechanisms, quality control studies, and drug delivery design, which comprehensively covers the features of modernization in TCM (Li et al., 2019d, Seo et al., 2022). HPLC and LC-MS/MS are commonly used for the components of drug ADME. The approach depending on multi-component drug concentration is commonly used, which uses mathematical functions and quantitative description to obtain PK parameters: area under curve (AUC0-t), time to peak (Tmax), peak concentration (Cmax), elimination half-life (T1/2), total clearance (CL/F) and mean retention time (MRT), etc. provide a basis for the development of dosing regimens (Zhong et al., 2020, Zhang et al., 2018).
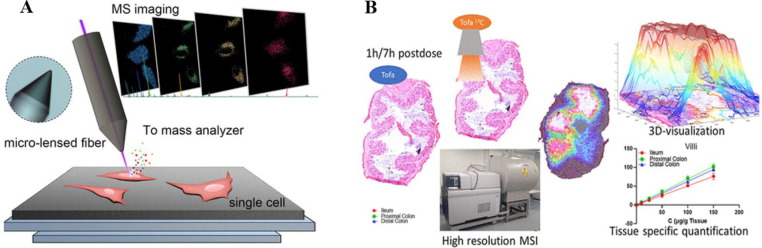
Mass spectrometry imaging (MSI) has been gradually applied to the in vivo distribution and PK study of TCM (Fig. 5 ). The in situ and fine visualization features of MSI have good applications in vivo distribution of drugs and mechanisms (Cho et al., 2017, Deng et al., 2021, Hale and Cooper, 2021). In the current study, there is a direct analysis of changes in the spatial catabolic profile of rat kidney after aristolochic acid I administration using airflow-assisted desorption electrospray mass spectrometry imaging (AFA DESI-MSI) (Wang et al., 2020d). Direct observation of lipid and metabolite distribution in whole-body tissue sections has also been performed using infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) (Stutts et al., 2020). The distribution of small molecule drugs in subcellular locations (Meng et al., 2020) and dynamic spatial changes of drug over a 24-hour period (Guo et al., 2022b). LC-MS/MS combined with DESI or MALDI-MSI quantification can be used to develop imaging effects in systemic tissues (Wei et al., 2021b, Beng et al., 2022) and quantitatively visualize (Huizing et al., 2021).
Fig. 5.
Application of mass spectrometry imaging (MSI) technology. (A) A nanoscale resolution mass spectrometry imaging technique using micro-lensed fiber as a laser desorption ion source is proposed. Combining this system with a time-of-flight mass spectrometer, it achieved the visualization of drug distribution inside a single cell at the resolution of 300 nm (Meng et al., 2020). Reprinted with permission from Ref. Meng et al., 2020. Copyright © 2020 Wiley‐VCH GmbH. (B) The work investigates the suitability of MSI in quantitatively visualizing intestinal transmural drug distribution. One- and 7-h postdose sections of the ileum, proximal- and distal-colon from rats that received an oral solution of Tofa were subjected to matrix-assisted laser desorption ionization (MALDI)-MSI. A dilution series of individual concentrations sprayed over an entire tissue section allowed for tissue type-specific quantitation (Huizing et al., 2021). Reprinted with permission from Ref. Huizing et al., 2021. Copyright © 2021 American Chemical Society.
3.3. New technology reveals key pharmacophore groups
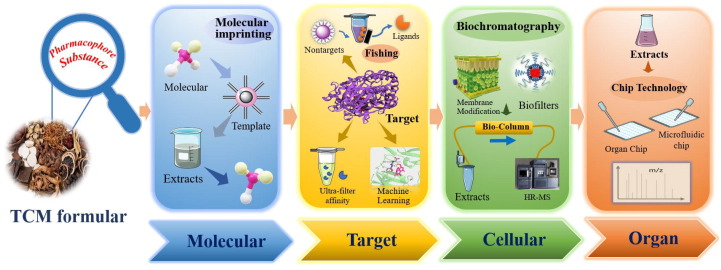
Most of the chemical components of TCM are in trace amounts, and the yields of most active compounds are basically at the milligram level, making it failure to meet the requirements of cellular or animal level. Thus, how to developed innovative techniques to reveal the multi-component pharmacophore has become a key problem. Bioactivity screening plays an important role in pharmacodynamic substances, and in vitro screening methods, affinity ultrafiltration, biochromatography, organ chip, microfluidic chip, molecular blotting, AI and other technologies have been developed and applied (Fig. 6 ). Due to the affinity between targets and components, they can extract active ingredients from complex natural products without tedious separation. Hence, these new approaches can reduce the time and cost of herbal medicinal pharmacodynamic substance discovery (Luan et al., 2020, Xu et al., 2021).
Fig. 6.
Schematic diagram of innovative technology to discover key efficacy components.
3.3.1. Target point fishing
Targets are the biological basis of drug action on cells and organisms. The most important feature of a Chinese medicine compound for disease treatment is that it contains multiple active ingredients that act synergistically through multiple targets. Thus, screening of potent substances depending on disease targets is a widely used approach. In brief, the target protein is combined with a stationary phase magnetic adsorption material to “fishing” the ligand compound from complex extract by using the magnetic adsorption physical properties of the material while maintaining protein activity. The pendant compounds are eluted by desorption. Ligands are then structurally characterized by LC-HRMS or directly. Compared with traditional one-to-one screening method, the target fishing strategy can quickly and efficiently identify active compounds from the complex system of TCM. It has gradually become a powerful tool for screening biologically active components. This strategy was used for neuraminidase immobilized magnetic beads to screen inhibitors from crude extracts of mockstrawberry (Luo et al., 2020) and α-glucosidase inhibitors in Epimedium (Shen et al., 2020) and amyloid β (Aβ) inhibitors in Scutellaria baicalensis (Guo et al., 2022a). The traditional fishing strategy with a single target needs to be further enhanced to match the multi-component and multi-target characteristics of TCM (Chen et al., 2020a). Chen et al. (2017) developed a new strategy for dual-target used microporous hollow fibers filled with target enzymes as decoys to “capture” ligands in the extracts, followed by identification of the ligands by LC-MS in Huangqin ginseng decoration. Although traditional fishing strategy is efficient and rapid, it also suffers from the problems of many false positives and weak specificity (Wubshet et al., 2019). To further improve the accuracy and sensitivity of the screening, researchers (Li et al., 2019a, Cheng et al., 2020) introduced a novel carrier material nickel ion (Ni2+) functionalized magnetic mesoporous silica microspheres (MMSM@PDA-Ni2+). This approach preserves the enzyme activity and spatial conformation with a mild immobilization strategy through the novel materials introduced, making it a powerful screening tool for the future.
3.3.2. Biological chromatography
Biochromatography is the combination of biologically active substances such as receptors, carrier proteins, cell membranes and carriers as stationary phases. It is a novel chromatographic technique that uses the specific binding of the stationary phase to compounds to screen drug candidates. The screened compounds can be bound to the stationary by hydrophobic forces, van der Waals forces, electrostatic interactions and binding sites of specific ligands (Gu et al., 2020). Cell membrane chromatography is a bioaffinity chromatographic using cell membranes as stationary phases. It has the dual function of chromatographic separation and active ingredient characterization. Gu et al., 2020 developed a new BMMC/CMC stationary phase modified with 3-mercaptopropyltrimethoxysilane to achieve covalent binding to cell membrane components. Lifetime of the CMC column was significantly increased from 3 days to 12 days. There was also the use of epidermal growth factor receptor HEK 293 cell membrane with magnetic nanoparticles was successfully assembled and applied (Bu et al., 2018). Wu’s team (Chen et al., 2021) prepared a bioactive column by achieving the covalent immobilization of ACE2 protein on silica stationary phase. In combination with C18 column, 2D LC-MS was used to screen eight active ingredients in LianhuaQingwen capsules that were exposed to humans and had ACE2-targeting effects. As an emerging technology, biocolumn is innovative in the screening of active ingredients in TCM, but its reusability is poor and its durability needs to be improved in the future.
3.3.3. Ultrafiltration affinity
Ultrafiltration is a semi-permeable membrane based on having a low molecular weight cut-off, which allows liquids and solutes below a specific molecular weight to pass through the membrane (Kok et al., 2020). Due to its operational simplicity, ultrafiltration has been shown to be a more rapid screening method compared to other methods (Fan et al., 2020). In most studies, herbal extracts are incubated with the target and retained in the ligand-receptor complex, while unbound compounds are separated by centrifugal force or pressure, and the ligand is released from the receptor by washing the membrane with an appropriate eluent. Finally, the active compounds are identified and analyzed (Liu et al., 2013, Li et al., 2014). In recent years, this technique has been widely used for screening and identification of active compounds in TCM. In α-glucosidase inhibitor screening, numerous investigators have obtained the inhibitors from Gymnema sylvestre (Chen and Guo, 2017), Guava leaves tea (Wang et al., 2018), Polygonum aviculare L. leaves (Cai et al., 2020), Cerasus humilis (Bge.) Sok. leaf (Li et al., 2022a) and other plants. Affinity ultrafiltration coupled with HPLC-MS was used to identified inhibitors, explore the structure–activity relationships. Moreover, investigators used nearly the same strategy to screen XO inhibitors such as galangal extract (Lin et al., 2018) and Lindera reflexa Hemsl (Fu et al., 2019b), and Zishen pill (Huai et al., 2019, Wang et al., 2020b) and Warburgia ugandensis (Xie et al., 2020) for cyclooxygenase 2 (COX-2) and 5-LOX inhibitors, Azadirachta indica screening for superoxide dismutase inhibitors (Fan et al., 2022), and herbal extracts screening for the identification of 5-HT2C receptor inhibitors (Zhang et al., 2020a), among others.
3.3.4. Molecular imprinting
Molecular imprinting (MI) has been developed for various applications in biology and chemistry with improved recognition selectivity and detection sensitivity. MI-SPE provides a powerful tool for active ingredient screening of complex matrices. It offers significant advantages such as ease of operation, high throughput, high selectivity and durability. The novel preparation strategies for molecularly imprinted polymers (MIPs) are divided into multi parts such as virtual imprinting, multitemplate imprinting, surface imprinting, which hold great promise for medicinal substance preparation studies (Arabi et al., 2020, Orowitz et al., 2020). Saeedeh Ansari (Ansari and Masoum, 2021) reviewed the sensor design, sensing mechanisms and properties of fluorescent sensors depending on molecular imprinting/carbon dot (MIP/CDs) for various target analytes during the past few years. Molecular blotting techniques combined with bioassays can be used to explore bioactivity studies from the perspective of herbal multicomponent isolation. Xie’s research (Xie et al., 2018) prepared calycosin molecularly imprinted polymers (calycosin-MIP), which selectively recognize flavonoid glycosidic elements. The calycin-MIPs material was used for the knockdown of flavonoid sapogenins in AR by a solid-phase extraction procedure. Fang et al. 2020 developed a multiphase extraction method using molecularly imprinted polymer (MIP)-coated ionic liquid-based silica (SiO2@IL@MIP) for the simultaneous extraction and separation of ephedrine, 10 drugs, and urine samples from Hemisphagnum. Ma’s research (Ma et al., 2019) synthesized a novel magnetic molecularly imprinted polymer-doped reticulated graphene oxide (Fe3O4@SiO2-GO@MIPs) for the selective identification and extraction of four flavonoids. For Chinese medicine formula, Gu’s team (Gu et al., 2015) directed the isolation of paeoniflorin and its structural analogues for the chemical composition, which is the main active ingredient in Gui Zhi Fu Ling capsule.
3.3.5. Organ chips
In 2015, NATURE reported on organ-on-a-chip technology (Reardon, 2015), where research on developing miniature models of human organs on a chip will become an alternative to animal models. Organ chips technology represents the latest advancement in this field of work, and it offers a promising approach to address the limitations of traditional preclinical research disease models. This emerging technology focuses on the use of organ-on-a-chip models for the evaluation of the active efficacy of components of therapeutic drugs. Organ chips can be a promising in vitro approach for complex component exposure in humans by combining human cell culture with dynamic microfluidics to improve physiological simulation. With the rapid development of nanoparticle research, new techniques are being developed for in vitro modeling and analysis of nanotherapies in human physiological systems (Kang et al., 2021, Osaki et al., 2018). Tissue culture model studies of the organism's skin, intestinal barrier, and liver can all be used for on-chip drug active ingredient screening and efficacy evaluation. Even the integration of organs on a chip into systemic tissues through microfluidic connections in the device, such as skin-liver and intestine-liver, constitutes a relevant study for advancing the PKs of drug components (Nitsche et al., 2022). In the last few years, significant progress has been made in the development of organ chip-based drug screening components and systems. To date, the functions of various organs and tissues, such as liver, kidney, lung and intestine, have been replicated as in vitro models (Yu et al., 2010, Mi et al., 2016). Microfluidic platforms in skin-on-a-chip mode reduce the amount of medium and the number of cells by 36-fold compared to traditional transwell medium (Abaci et al., 2015). All these platforms are simple to operate, allow the evaluation of drug parameters, and are powerful tools for in vitro screening of drugs (Wufuer et al., 2016). However, accurate prediction of drug efficacy and organ interactions remains difficult. This is because in conventional cell culture systems usually do not retain their original organ function and morphology. These issues may become a major obstacle to the wider application of organ chips in the future.
3.3.6. Artificial Intelligence
Advances in information technology have created a foundation for the advancement of the subject area and industry of TCM. With the in-depth research on screening for active pharmaceutical ingredients in multi systems, many artificial intelligence methods have been gradually developed and applied during the past few years, such as network pharmacology and molecular docking in bioinformatics, 3D-QSAR model, computer ADME prediction, and deep machine learning, etc. These technologies are often used to reposition or develop disease-specific pharmacodynamic components by chemical similarity. High-speed computation is used to correlate drug components with disease molecules and targets for virtual drug screening and in vivo mechanism of action prediction. Shenqi wan and galangal extracts have been studied using network pharmacology and molecular docking (Zhang et al., 2019d, Ou et al., 2020). Yong's team (Yong et al., 2018) developed a 3D-QSAR pharmacophore model for URAT1, and both aqueous and ethanolic extracts from ashwagandha were found to have inhibitory effects on URAT1 protein. Furthermore, the studies of XO inhibitors are prolific (Abdizadeh et al., 2020, Chen et al., 2020b, Sankar and K, L., Jeyachandran, S., Pandi, B., , 2021). For ADME prediction, it has been used in studies of rutin (Liu et al., 2021a), cardioplegia capsules (Guo et al., 2018), and Chrysoeriol (Liu et al., 2021b) to screen bioactive compounds and reveal PK properties. In terms of deep machine learning, it has been used to design drug targets and new drug discovery now commonly combine machine learning and deep learning algorithms to improve the efficiency and quality of the development output (Zeng et al., 2022, Li et al., 2022b, He et al., 2019). Similar strategies applied in classical Chinese herbal formulas (Yang et al., 2020), pediatric poria granules (Guo et al., 2020) and gentian violet (Wang et al., 2021a). Although there is a renewed interest in virtual screening for drug, the problem of diversity in the library of drug-like compounds and the problem of false positives in the output results should be resolved in the future.
3.4. Innovative technologies for active ingredient efficacy evaluation and mechanism revelation
The screening and activity verification of active ingredients can be performed at the molecular, cellular, tissue and animal levels. However, due to the complexity of the chemical composition of TCM and its compound, many potential active ingredients are present in small amounts, making it failure to screen and validate them at the animal level. For recent years, 3D cells, organoid, and 3D printing technologies have been gradually applied to drug activity screening and validation (Xu et al., 2018a, Luan et al., 2020, Organoids, 2021, Ihrie and Henske, 2022).
3.4.1. Multicellular 3D culture
Cell culture is an indispensable experimental technique in the drug development process. 3D cell culture has become a valuable tool. It is closer to the reality in vivo and helps bridge the gap between in vitro and in vivo models, reducing the number of animals used early in the experiment and the potential for errors. Compared to traditional 2D cells, 3D cells have a higher degree of cell-to-cell interaction and maintains a more ordered tissue processing of the tiny structures of organs in vivo (Barbosa et al., 2021). 3D cell culture has made many promising achievements since its introduction (Booij et al., 2019, Froehlich et al., 2016, Wei et al., 2021a). Current research achieved an effective anti-asthma herbal drug evaluation depending on 3D airway smooth muscle cell arrays and gel imaging system. Its reliability in vitro mimicry further reflects the advantages of easy operation, high throughput, non-invasive, real-time, and high sensitivity, providing a broad prospect for treatment of asthma diseases (Qin et al., 2021). According to Li’s reports (Li et al., 2013), proliferation and cytotoxicity to different concentrations of Ganoderma lucidum spores, Ginkgo biloba and Epimedium extracts was evaluated based on 3D cultures.
3.4.2. Organoid culture
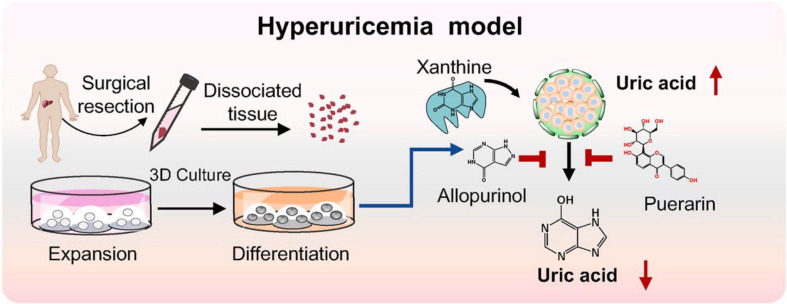
Organoids are 3D “microorgan models” prepared by self-organization of different types of stem cells that mimic the structure and function of the original organ (Fatehullah et al., 2016). This culture system consists of a self-renewing stem cell population that can differentiate into a variety of organ-specific cell types. It has a spatial organization similar to that of the corresponding organ and is able to replicate some of its functions, thus providing a highly physiologically relevant system (Lancaster and Knoblich, 2014). As several countries have developed strategic roadmaps to phase out animal experimentation for regulatory purposes, organoid culture is a must for preclinical and regulatory settings and will help to implement the 3Rs (reduce, refine, and replace) principles. For recent years, organoid research has focused on in vitro models of disease and organ reconstruction, which will open new avenues for drug screening and activity validation studies. Further studies have shown that organoid-derived glomeruli can maintain expression of markers in culture for 96 h and can be evaluated for efficacy (Hale et al., 2018). The 3D organoid culture for high throughput screening (HTS) was further scaled down and its stability in large-scale primary compound evaluation was demonstrated (Du et al., 2020, Li et al., 2022c). Organoids are also increasingly used for the treatment of metabolic diseases and the validation of drug activity. Recently, a 3D organoid culture system for mimicking hyperuricemia (HUA) in vitro was established using cultured human liver organoids. The preclinical application potential of this organoid model was verified by measuring the antihyperuricemic effect of the widely used allopurinol, as well as the reported bioactive substance puerarin from TCM (Hou et al., 2022) (Fig. 7 ). Currently, the effects of the matrix and vascular system have not been considered in the current organoid cultures (Zietek et al., 2021). Organoids will play a beneficial role in the study of disease mechanisms as an ideal model for the evaluation of active ingredients.
Fig. 7.
A 3D organoid culture system for mimicking hyperuricemia (HUA) in vitro was established using cultured human liver organoids. The organoid model was verified by measuring the antihyperuricemic effect of the widely used allopurinol, as well as the reported bioactive substance puerarin from TCM (Hou et al., 2022). Reprinted with permission from Ref. Hou et al., 2022. Copyright © 2021 Elsevier.
3.4.3. 3D Bio-Printing
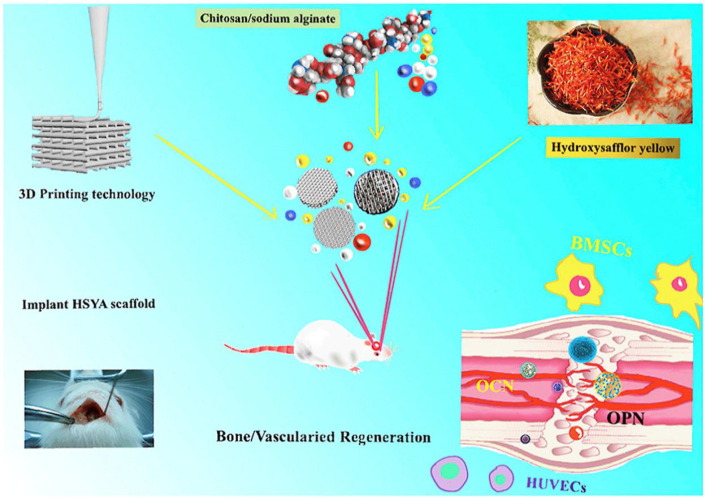
Three-dimensional (3D) animal-like organs derived from pluripotent stem cells or tissue stem cells have a good potential for studying disease mechanisms, and drug screening. However, the lack of precise architecture and large-scale tissue size becomes a key limiting factor for organoid technology. The emergence of organoid 3D bioprinting technology during the past few years has been able to address some of these technical barriers. For drug screening and drug delivery systems research, a state-of-the-art 3D printing technology consisting of cells, bio-ink and a printing strategy that meets the requirements (Rawal et al., 2021). There are three types of biological 3D printing strategies: inkjet printing (IBP), extrusion printing (EBP), and optical printing (LBP). 3D bioprinting has shown recent advances in the fabrication of cell-filled artificial tissues and organs (Zhang et al., 2019c). Paulina et al. (Bernal et al., 2022) molded gelatin hydrogels containing organoids into bioprints of centimeter-scale 3D structures in 20 s by optical tomography after printing. According to NATURE, Lutolf’s team designed an innovative 3D bioprinting (Brassard et al., 2021), which they termed bioprint-assisted tissue, to overcome the limitations of organoid. In the future, 3D bioprinting-based in vitro drug evolution approaches will fall into three categories: mini-tissues, monolithic organs, and tissue/organ constructs. 3D bioprinting is currently widely used in liver models, heart tissues, vascularized constructs, and cancer models to fully contribute to drug discovery, toxicology, and other preclinical studies in TCM research (Nie et al., 2020, Gao et al., 2021, Deng et al., 2020) (Fig. 8 ).
Fig. 8.
The novel hydroxy-safflower yellow A/scaffolds hold the substantial potential to be further developed as effective and safe bone tissue engineering biomaterials for bone regeneration by 3D bioprinting enhanced osteogenesis and angiogenesis (Deng et al., 2020). Reprinted with permission from Ref. Deng et al., 2020. Copyright © 2020 Elsevier.
4. Shortcomings of pharmacodynamic substances study in Chinese medicine formula
4.1. Neglecting the synergistic relationship of ingredients
From ancient times to now, the interpretation of the effectiveness connotation of Chinese medicine formula has always been inseparable from traditional approach. The “principals, associates, adjuvants and messengers” have always played an important role. The analytical idea of focusing on single components in the previous research strategy, some of the study results will show false positives, and it is difficult to not fully reflect the action characteristics of a compound composed of multiple single herbs. Therefore, most of the current studies did not start from the “whole” to find the correlation of the “parts”, and did not reveal the scientific connotation of “ruler, subject, adjuvant, and ambassador” of Chinese medicine formula through the synergistic effects between components. The scientific connotation of Chinese medicine formula has not been revealed through the synergistic effects among the components. To address this issue, the following techniques may be suitable for this scenario. Under the condition that the overall efficacy of the herbal medicine is definite, researchers should try to combine spectroscopic studies with component knockout strategies, where we can look at the multi-targeted action of the disease. This would allow for maximum characterization of the interactions between the components of TCM. Based on an isolation-analysis research strategy, we should focus less on the activity of single components and their single-target mechanisms of action and emphasized the synergistic effects between multiple components and compounds with multiple targets.
4.2. High connotation substances are ignored
In the current research, the high connotation substances in compound prescriptions are neglected. For example, there are few studies on inorganic substances. Inorganic elements play a part in the molecular action of the human body. TCMs are often accompanied by a series of complex physical and chemical changes during the process of water decoction, and most of their soup solutions present a mixed-phase system. Some of the compounded prescriptions form macromolecules or new substances during the water decoction process, and some of these substances can also reflect the medicinal effects of the compound. Although some researchers have conducted related studies, they are not yet systematic. In the future, we should use inorganic elemental analyses based on the usage of Chinese medicine to determine the transfer rate of these substances and to confirm their contribution to pharmacological efficacy through appropriate pharmacological characterisation. The self-assembly of new substances during the decoction process should be the focus of attention in combination with physical and chemical methods. Particularly, we should also focus on the generation of new substances in vivo to investigate more realistically the migration process in vivo. It is well known that concoction is a characteristic process step in TCM before clinical use. However, the substances in TCM after concoction are less well revealed. For most of the current TCM, the methods of preparation are diverse. As the method of preparation varies, it may have a greater effect on the efficacy of herbs. Most of the substances that are highly connoted after preparation are not known. For example, the toxicity of He Shou Wu is reduced and its potency is increased after nine steaming and nine drying processes. Therefore, it is important to standardize the method of preparation and to combine it with the actual clinical situation in order to maximize the effect of the preparation.
4.3. Weak research on macromolecules
Biological macromolecules (polysaccharides, proteins and nucleic acids) are a large class of components of biological active substances in TCM and have a wide range of biological activities. However, they have not been fully recognized compared with small molecule components. In current research reports, the analysis of chemical composition is mostly focused on small molecule compounds, while large molecules such as polysaccharides and proteins are neglected. As the traditional use of decoction, the medicinal effect of its macromolecules should not be ignored. Although the discovery and application of macromolecules have been gradually carried out during the past few years, the research such as polysaccharides and proteins involved in compounded is even more rare and the foundation is relatively weak. Compared with monosaccharides, compounded polysaccharides can combine the biological activities of each monosaccharide and play a synergistic effect. In most cases, polysaccharides are absorbed as oligosaccharides, which directly or indirectly affect intestinal flora to influence efficacy (Ma et al., 2021a), proteins are mostly absorbed as oligopeptides to exert their functions, miRNAs can be absorbed through the gastrointestinal tract and can act as intercellular inhibitory signaling molecules in target tissues (Zhang et al., 2020b). Hence, the study of biomolecules should be different from that of small molecules in terms of approach and strategy. Biomolecules focus on the integration of the whole and the parts. In terms of analytical methods, the development of qualitative and quantitative assays should be gradually optimized. For the characterization of pharmacological activity, the correlation between molecular weight and activity should be emphasized. Future research will tend to highlight the specificity of different TCMs.
4.4. Weak relevance of TCM theory to clinical practice
Chinese medicine formula arises from “evidence-law-formulation-agent” in TCM theory. Its key aspects depending on the appearance of disease, treatment methods, formulation principles, and Chinese medicine preparations, the process of diagnosing and using medicine contains the clinician’s way of thinking and method of using medicine based on solid TCM theory, which fully reflects the uniqueness thinking. The existing research model is only depending on chemical means and focusing on the characterization of single components. Some compounds are poorly water-soluble and failure to be absorbed, ignoring the actual drug use situation in TCM. And the in vivo processes and morphology of TCM compound drugs are ignored, and the clinical relevance is weak. Moreover, the composition-only thinking has been dominant in the process of TCM compounding research, resulting in most pharmacological researchers ignoring and neglecting the relationship between TCM and compounding pharmacological properties. Future research directions might consider biological studies to reflect the “intervention-response” mechanism of herbal medicine, to further approach the clinic to reflect the “herbal compounding-biological organism” interaction, and to be able to cut into the “four gases and five tastes”. In addition, the pharmacological basis of compounding can also be explained from the perspective of “elevation and sinking”, This allows the pharmacological theory to be conveyed in the formulation of the pharmacological basis of compound prescriptions.
5. Conclusions and perspective
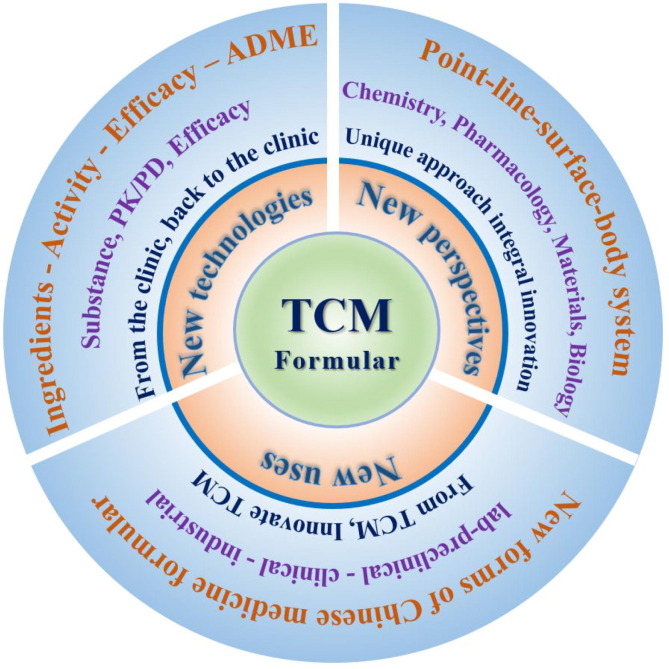
In the future, the discovery and application of the active ingredients of Chinese medicine formulas should be based on the “holistic view” and “TCM theory”. The TCM research on the material basis should be in line with the multi-component and multi-target action characteristics. It should reflect the holistic, systematic and dynamic characteristics of TCM in vivo and in vitro. To comprehensively reveal new perspectives, new technologies and new uses of the substance research of Chinese medicine formula (Fig. 9 ).
Fig. 9.
Schematic diagram of comprehensively reveal new perspectives, new technologies and new uses of the substance research of Chinese medicine formula.
5.1. Innovative ideas for clinical use
Although the existing research ideas of Chinese medicine formula are diverse, most of them are failure to ensure the accurate representation of the utility value of prescriptions in the research process. The research methods do not focus on the design and retention of the pharmacological perspective of prescriptions. A comprehensive research idea of the active ingredients of TCM should be established based on clinical usage and efficacy, with “substance-pharmacokinetic-efficacy” as the core. For now, depending on compounded active substances, the relationship between prescription fingerprint, metabolic fingerprint and efficacy target activity is an effective path to reveal the objective expression of the effectiveness of TCM. Furthermore, the compounded pharmacodynamic substances of TCM are dynamic. Whether it is a single chemical component or a complex substance system, its entry into the organism may undergo structural–functional changes under the metabolism of target organs, forming alternative or synergistic mechanisms. The research should be based on multi-level material levels such as TCM extracts, active parts and active ingredients. Integrate bioinformatics-molecular biology-specific target organ system to reveal the key manifest forms of TCM components. Establish characteristic animal disease models to carry out in vivo validation of key components and build pharmacodynamic basis of TCM components. In the future, there is a need to further innovate the idea of research on the pharmacodynamic substances of herbal compounds that are suitable for clinical use: “from clinic, back to clinic”.
5.2. The development and application of new methods
The development and application of new technology and new method is an important part of the continuation and deepening of the medicinal substance of the Chinese medicine formula. It is divided into two aspects: single technology innovation and integrated method innovation. (i) Independent technology alternative. In order to solve the problem of necking in the research of compounding potent substances of TCM, new instruments and technologies have appeared during the past few years to open new horizons. The development and application of various technologies such as multidimensional MS, 3D printing, organoid, biochip, AI, etc. have gradually solved the mystery of the medicinal effect substances of TCM under a single technology. (ii) Integrated method combination innovation. Traditional technologies and methods for compounding research of TCM are often developed and applied for a single target, for complex ingredient systems, TCM theories, clinical efficacy, etc. And the single technology often cannot support the systematic research of compound prescriptions. Hence, technological innovation integrating multiple methods is a new form of compounding pharmacodynamic substance basis research in the new era. In the future, we need to integrate the cross-disciplinary development of chemistry, pharmacology, analysis, materials, biology and other disciplines to gradually establish a “point-line surface-body” innovation system.
5.3. Expanding the direction of “from TCM, innovative TCM”
In 2015, Youyou Tu was awarded the Nobel Prize in Physiology or Medicine for her discovery of artemisinin in the TCM. The great contribution of TCM has once again proved to the world the great charm and its active ingredients. In recent years, TCM has made great progress in development and innovation. Monomeric components such as ephedrine, artemisinin, indocyanine, arsenic trioxide, galanthamine, ginsenoside Rg3, etc., originate from TCM and innovated in TCM. Thus, depending on the research foundation of single herbs, the research direction of “drug, forming research, preclinical research, clinical evaluation, industrialization transformation” and application expansion should be established to cover the new compounded drugs depending on the pharmacological substances. Taking the value of clinical use as the guide, the Chinese medicine formula gradually develops to a clear material basis, clear mechanism of action, stable quality control and sufficient evidence-based medical evidence. It will gradually gain worldwide recognition and expand clinical application and industrial development. In particular, the development of ideas and methods of translational medicine has brought new opportunities and ideas for the uses of Chinese medicine formula.
Acknowledgements
This work was supported by National Natural Science Foundation of China (U20A20406), National Natural Science Foundation of China (82104475), China Postdoctoral Science Foundation (2021 M690475). The authors would like to thank the reviewers and also the authors of all references. The reviewer’s advice really makes the great improvement of this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abaci H.E., Gledhill K., Guo Z., Christiano A.M., Shuler M.L. Pumpless microfluidic platform for drug testing on human skin equivalents. Lab chip. 2015;15(3):882–888. doi: 10.1039/c4lc00999a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdizadeh R., Heidarian E., Hadizadeh F., Abdizadeh T. Investigation of pyrimidine analogues as xanthine oxidase inhibitors to treat of hyperuricemia and gout through combined QSAR techniques, molecular docking and molecular dynamics simulations. J. Taiwan Inst. Chem. Eng. 2020;113:72–100. doi: 10.1016/j.jtice.2020.08.028. [DOI] [Google Scholar]
- Ai X., Lu W., Zeng K., Li C., Jiang Y., Tu P. Microfluidic Coculture Device for Monitoring of Inflammation-Induced Myocardial Injury Dynamics. Anal Chem. 2018;90:4485–4494. doi: 10.1021/acs.analchem.7b04833. [DOI] [PubMed] [Google Scholar]
- Ansari S., Masoum S. Recent advances and future trends on molecularly imprinted polymer-based fluorescence sensors with luminescent carbon dots. Talanta. 2021;223(Pt 1) doi: 10.1016/j.talanta.2020.121411. [DOI] [PubMed] [Google Scholar]
- Arabi M., Ostovan A., Bagheri A.R., Guo X., Wang L., Li J., Wang X., Li B., Chen L. Strategies of molecular imprinting-based solid-phase extraction prior to chromatographic analysis. TrAC, Trends Anal. Chem. 2020;128 doi: 10.1016/j.trac.2020.115923. [DOI] [Google Scholar]
- Barbosa M., Xavier C., Pereira R.F., Petrikaitė V., Vasconcelos M.H. 3D Cell Culture Models as Recapitulators of the Tumor Microenvironment for the Screening of Anti-Cancer Drugs. Cancers. 2021;14:190. doi: 10.3390/cancers14010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beng H., Hu J., Zhang R., Huang Y., Chen X., Tan W. Quantitative DESI mass spectrometry imaging of lung distribution of inhaled drug. J Drug Deliv Sci Technol. 2022;66 doi: 10.1016/j.jddst.2021.102794. [DOI] [Google Scholar]
- Bernal P.N., Bouwmeester M., Madrid-Wolff J., Falandt M., Florczak S., Rodriguez N.G., Li Y., Größbacher G., Samsom R.A., van Wolferen M., van der Laan L., Delrot P., Loterie D., Malda J., Moser C., Spee B., Levato R. Volumetric Bioprinting of Organoids and Optically Tuned Hydrogels to Build Liver-Like Metabolic Biofactories. Adv Mater. 2022;34:e2110054. doi: 10.1002/adma.202110054. [DOI] [PubMed] [Google Scholar]
- Booij T.H., Price L.S., Danen E. 3D Cell-Based Assays for Drug Screens: Challenges in Imaging, Image Analysis, and High-Content Analysis. SLAS discovery. 2019;24:615–627. doi: 10.1177/2472555219830087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulangé C.L., Neves A.L., Chilloux J., Nicholson J.K., Dumas M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8:42. doi: 10.1186/s13073-016-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brassard JA., Nikolaev M, Hofer M, Lutolf MP. Recapitulating macro-scale tissue self-organization through organoid bioprinting. Nat Mater. 2021;20(1):22–29. doi: 10.1038/s41563-020-00803-5. [DOI] [PubMed] [Google Scholar]
- Bu Y., Hu Q., Ke R., Sui Y., Xie X., Wang S. Cell membrane camouflaged magnetic nanoparticles as a biomimetic drug discovery platform. Chem Commun. 2018;54:13427–13430. doi: 10.1039/c8cc08530g. [DOI] [PubMed] [Google Scholar]
- Cai S., Wang X., Shang M., Xu F., Liu G. “Efficacy Theory” may help to explain characteristic advantages of traditional Chinese medicines. China Journal of Chinese Materia Medica. 2015;40:3435–3443. doi: 10.4268/cjcmm20151720. [DOI] [PubMed] [Google Scholar]
- Cai Y., Wu L., Lin X., Hu X., Wang L. Phenolic profiles and screening of potential α-glucosidase inhibitors from Polygonum aviculare L. leaves using ultra-filtration combined with HPLC-ESI-qTOF-MS/MS and molecular docking analysis. Ind. Crops Prod. 2020;154 doi: 10.1016/j.indcrop.2020.112673. [DOI] [Google Scholar]
- Chang C.J., Lin C.S., Lu C.C., Martel J., Ko Y.F., Ojcius D.M., Tseng S.F., Wu T.R., Chen Y.Y., Young J.D., Lai H.C. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi: 10.1038/ncomms8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Gao Y., Wu F., Luo X., Ju X., Liu G. Computationally exploring novel xanthine oxidase inhibitors using docking-based 3D-QSAR, molecular dynamics, and virtual screening. New J. Chem. 2020;44:19276–19287. doi: 10.1039/D0NJ03221B. [DOI] [Google Scholar]
- Chen G., Guo M. Rapid Screening for α-Glucosidase Inhibitors from Gymnema sylvestre by Affinity Ultrafiltration-HPLC-MS. Front Pharmacol. 2017;8:228. doi: 10.3389/fphar.2017.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Wang X., Liu Y., Di X. Dual-target screening of bioactive components from traditional Chinese medicines by hollow fiber-based ligand fishing combined with liquid chromatography-mass spectrometry. J Pharm Biomed Anal. 2017;143:269–276. doi: 10.1016/j.jpba.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Chen X., Xue S., Lin Y., Luo J., Kong L. Immobilization of porcine pancreatic lipase onto a metal-organic framework, PPL@MOF: A new platform for efficient ligand discovery from natural herbs. Anal Chim Acta. 2020;1099:94–102. doi: 10.1016/j.aca.2019.11.042. [DOI] [PubMed] [Google Scholar]
- Chen X., Wu Y., Chen C., Gu Y., Zhu C., Wang S., Chen J., Zhang L., Lv L., Zhang G., Yuan Y., Chai Y., Zhu M., Wu C. Identifying potential anti-COVID-19 pharmacological components of traditional Chinese medicine Lianhuaqingwen capsule based on human exposure and ACE2 biochromatography screening. Acta pharm Sin B. 2021;11:222–236. doi: 10.1016/j.apsb.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Lu E., Fan M., Pi Z., Zheng Z., Liu S., Song F., Liu Z. A comprehensive strategy to clarify the pharmacodynamic constituents and mechanism of Wu-tou decoction based on the constituents migrating to blood and their in vivo process under pathological state. J Ethnopharmacol. 2021;275 doi: 10.1016/j.jep.2021.114172. [DOI] [PubMed] [Google Scholar]
- Cheng G., Pi Z., Zheng Z., Liu S., Liu Z., Song F. Magnetic nanoparticles-based lactate dehydrogenase microreactor as a drug discovery tool for rapid screening inhibitors from natural products. Talanta. 2020;209 doi: 10.1016/j.talanta.2019.120554. [DOI] [PubMed] [Google Scholar]
- Cho Y.L., Kim Y.P., Son J.G., Son M., Lee T.G. On-Chip Peptide Mass Spectrometry Imaging for Protein Kinase Inhibitor Screening. Anal chem. 2017;89:799–806. doi: 10.1021/acs.analchem.6b03557. [DOI] [PubMed] [Google Scholar]
- Dai Y., Qiang W., Gui Y., Tan X., Pei T., Lin K., Cai S., Sun L., Ning G., Wang J., Guo H., Sun Y., Cheng J., Xie L., Lan X., Wang D. A large-scale transcriptional study reveals inhibition of COVID-19 related cytokine storm by traditional Chinese medicines. Sci bulletin. 2021;66:884–888. doi: 10.1016/j.scib.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z., Chen J., Lin B., Li J., Wang H., Wang D., Pang L., Zeng X., Wang H., Zhang Y. A novel 3D printed bioactive scaffolds with enhanced osteogenic inspired by ancient Chinese medicine HYSA for bone repair. Exp Cell Res. 2020;394 doi: 10.1016/j.yexcr.2020.112139. [DOI] [PubMed] [Google Scholar]
- Deng Y., He M., Feng F., Feng X., Zhang Y., Zhang F. The distribution and changes of glycoalkaloids in potato tubers under different storage time based on MALDI-TOF mass spectrometry imaging. Talanta. 2021;221 doi: 10.1016/j.talanta.2020.121453. [DOI] [PubMed] [Google Scholar]
- Du Y., Li X., Niu Q., Mo X., Qui M., Ma T., Kuo C.J., Fu H. Development of a miniaturized 3D organoid culture platform for ultra-high-throughput screening. J Mol Cell Biol. 2020;12:630–643. doi: 10.1093/jmcb/mjaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M., Chen G., Sun B., Wu J., Li N., Sarker S.D., Nahar L., Guo M. Screening for natural inhibitors of human topoisomerases from medicinal plants with bio-affinity ultrafiltration and LC–MS. Phytochem Rev. 2020;19:1231–1261. doi: 10.1007/s11101-019-09635-x. [DOI] [Google Scholar]
- Fan M.X., Chen G.L., Guo M.Q. Potential Antioxidative Components in Azadirachta indica Revealed by Bio-Affinity Ultrafiltration with SOD and XOD. Antioxidants. 2022;11:658. doi: 10.3390/antiox11040658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L., Xie J., Lin L., Tian M., Row K.H. Multi-phase extraction of ephedrine from Pinellia ternata and herbal medicine using molecular imprinted polymer coated ionic liquid-based silica. Phytochem Anal. 2020;31:242–251. doi: 10.1002/pca.2888. [DOI] [PubMed] [Google Scholar]
- Fatehullah A., Tan S.H., Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18:246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- Feng K., Wang S., Han L., Qian Y., Li H., Li X., Jia L., Hu Y., Wang H., Liu M., Hu W., Guo D., Yang W. Configuration of the ion exchange chromatography, hydrophilic interaction chromatography, and reversed-phase chromatography as off-line three-dimensional chromatography coupled with high-resolution quadrupole-Orbitrap mass spectrometry for the multicomponent characterization of Uncaria sessilifructus. J Chromatogr A. 2021;1649 doi: 10.1016/j.chroma.2021.462237. [DOI] [PubMed] [Google Scholar]
- Froehlich K., Haeger J.D., Heger J., Pastuschek J., Photini S.M., Yan Y., Lupp A., Pfarrer C., Mrowka R., Schleußner E., Markert U.R., Schmidt A. Generation of Multicellular Breast Cancer Tumor Spheroids: Comparison of Different Protocols. J Mammary Gland Biol Neoplasia. 2016;21:89–98. doi: 10.1007/s10911-016-9359-2. [DOI] [PubMed] [Google Scholar]
- Fu L.L., Ding H., Han L.F., Jia L., Yang W.Z., Zhang C.X., Hu Y., Zuo T.T., Gao X.M., Guo D.A. Simultaneously targeted and untargeted multicomponent characterization of Erzhi Pill by offline two-dimensional liquid chromatography/quadrupole-Orbitrap mass spectrometry. J Chromatogr A. 2019;1584:87–96. doi: 10.1016/j.chroma.2018.11.024. [DOI] [PubMed] [Google Scholar]
- Fu Y., Yang J., Chen S., Sun X., Zhao P., Xie Z. Screening, and identification of the binding position, of xanthine oxidase inhibitors in the roots of Lindera reflexa Hemsl using ultrafiltration LC-MS combined with enzyme blocking. Biomed Chromatogr. 2019;33:e4577. doi: 10.1002/bmc.4577. [DOI] [PubMed] [Google Scholar]
- Gao G., Ahn M., Cho W.W., Kim B.S., Cho D.W. 3D Printing of Pharmaceutical Application: Drug Screening and Drug Delivery. Pharmaceutics. 2021;13(9):1373. doi: 10.3390/pharmaceutics13091373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegentana X.u., F., Li, F. C., Zhang, Y. F., Shen, S. J., Yang, P., Yang, X. X., Shang, M. Y., Liu, G. X., Li, Y. L., XuanWang, Cai, S. Q., Discovery of the active compounds of Smilacis Glabrae Rhizoma by utilizing the relationship between the individual differences in blood drug concentration and the pharmacological effect in rats. J Ethnopharmaco. 2020;258 doi: 10.1016/j.jep.2020.112886. [DOI] [PubMed] [Google Scholar]
- Giddings J.C. Two-dimensional separations: concept and promise. Anal chem. 1984;56 doi: 10.1021/ac00276a003. [DOI] [PubMed] [Google Scholar]
- Girondi N.G., Barreto F., Pigatto M.C., Dalla Costa T. Sensitive analytical method to quantify clindamycin in plasma and microdialysate samples: Application in a preclinical pharmacokinetic study. J Pharm Biomed Anal. 2018;153:57–62. doi: 10.1016/j.jpba.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Gong K., Yang Y., Li K., Zhu L., Zhi X., Cai W. Identification of the metabolites of isochlorogenic acid A in rats by UHPLC-Q-Exactive Orbitrap MS. Pharma bio. 2020;58:992–998. doi: 10.1080/13880209.2020.1822421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Chen X., Wang Y., Liu Y., Zheng L., Li X., Wang R., Wang S., Li S., Chai Y., Su J., Yuan Y., Chen X. Development of 3-mercaptopropyltrimethoxysilane (MPTS)-modified bone marrow mononuclear cell membrane chromatography for screening anti-osteoporosis components from Scutellariae Radix. Acta Pharm Sin B. 2020;10:1856–1865. doi: 10.1016/j.apsb.2020.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu R., Li S., Ni F., Zhao Y., Cao L., Huang W., Wang Z., Xu X., Xiao W. Extraction of Paeoniflorin and Its Derivatives from Gui-Zhi Fu-Ling Capsule Using Molecular Imprinting Technology. World Science and Technology-Modernization of Traditional Chinese Medicine. 2015;5:1051–1055. doi: 10.11842/wst.2015.05.025. [DOI] [Google Scholar]
- Guo S., Mei S., Wang Q., Li X., Chen Y., He Q., Zhao Z. Determination of cantharidic acid in rat blood by microdialysis combined with UHPLC-MS/MS. Int J Mass Spectrom. 2019;440:20–26. doi: 10.1016/j.ijms.2019.03.001. [DOI] [Google Scholar]
- Guo S., Li K., Chen Y., Li B. Unraveling the drug distribution in brain enabled by MALDI MS imaging with laserassisted chemical transfer. Acta Pharm Sin B. 2022;12:2120–2126. doi: 10.1016/j.apsb.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F., Tang X., Zhang W., Wei J., Tang S., Wu H., Yang H. Exploration of the mechanism of traditional Chinese medicine by AI approach using unsupervised machine learning for cellular functional similarity of compounds in heterogeneous networks, XiaoErFuPi granules as an example. Pharmacol Res. 2020;160 doi: 10.1016/j.phrs.2020.105077. [DOI] [PubMed] [Google Scholar]
- Guo R., Zhang X., Su J., Xu H., Zhang Y., Zhang F., Li D., Zhang Y., Xiao X., Ma S., Yang H. Identifying potential quality markers of Xin-Su-Ning capsules acting on arrhythmia by integrating UHPLC-LTQ-Orbitrap, ADME prediction and network target analysis. Phytomedicine. 2018;44:117–128. doi: 10.1016/j.phymed.2018.01.019. [DOI] [PubMed] [Google Scholar]
- Guo J., Zhang L., Shang Y., Yang X., Li J., He J., Gao X., Chang Y.X. A strategy for intelligent chemical profiling-guided precise quantitation of multi-components in traditional Chinese medicine formulae-QiangHuoShengShi decoction. J Chromatogr A. 2021;1649 doi: 10.1016/j.chroma.2021.462178. [DOI] [PubMed] [Google Scholar]
- Guo M., Zhu F., Qiu W., Qiao G., Law B.Y.K., Yu L., Wu J., Tang Y., Yu C., Qin D., Zhou X., Wu A. High-throughput screening for amyloid-bbinding natural small-molecules based on the combinational use of biolayer interferometry and UHPLCLDAD-Q/TOF-MS/MS. Acta Pharm Sin B. 2022;10:1694–1708. doi: 10.1016/j.apsb.2021.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale O.J., Cooper H.J. Native Mass Spectrometry Imaging of Proteins and Protein Complexes by Nano-DESI. Anal chem. 2021;93:4619–4627. doi: 10.1021/acs.analchem.0c05277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale L.J., Howden S.E., Phipson B., Lonsdale A., Er P.X., Ghobrial I., Hosawi S., Wilson S., Lawlor K.T., Khan S., Oshlack A., Quinlan C., Lennon R., Little M.H. 3D organoid-derived human glomeruli for personalised podocyte disease modelling and drug screening. Nat Commun. 2018;9:5167. doi: 10.1038/s41467-018-07594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao H., Zheng C., Wang G. Thoughts and experimental exploration on pharmacokinetic study of herbal medicines with multiple-components and targets. Acta Pharmaceutica Sinica. 2009;44:270–275. doi: 10.3321/j.issn:0513-4870.2009.03.008. [DOI] [PubMed] [Google Scholar]
- He S., Zhang X., Lu S., Zhu T., Sun G., Sun X. A Computational Toxicology Approach to Screen the Hepatotoxic Ingredients in Traditional Chinese Medicines: Polygonum multiflorum Thunb as a Case Study. Biomolecules. 2019;9:577. doi: 10.3390/biom9100577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C., Hu Y., Jiang H., Xu Z., Sha W., Liu J., Ren J., Yao M. Establishment of a 3D hyperuricemia model based on cultured human liver organoids. Free Radic Biol Med. 2022;178:7–17. doi: 10.1016/j.freeradbiomed.2021.11.023. [DOI] [PubMed] [Google Scholar]
- Huai J., Zhao X., Wang S., Xie L., Li Y., Zhang T., Cheng C., Dai R. Characterization and screening of cyclooxygenase-2 inhibitors from Zi-shen pill by affinity ultrafiltration-ultra performance liquid chromatography mass spectrometry. J Ethnopharmacol. 2019;241 doi: 10.1016/j.jep.2019.111900. [DOI] [PubMed] [Google Scholar]
- Huang B., Hu X., Wang J., Li P., Chen J. Study on chemical constituents of herbal formula Er Miao Wan and GC-MS based metabolomics approach to evaluate its therapeutic effects on hyperuricemic rats. J Chromatogr B. 2019;1118–1119:101–108. doi: 10.1016/j.jchromb.2019.04.032. [DOI] [PubMed] [Google Scholar]
- Huizing L., McDuffie J., Cuyckens F., van Heerden M., Koudriakova T., Heeren R., Vreeken R.J. Quantitative Mass Spectrometry Imaging to Study Drug Distribution in the Intestine Following Oral Dosing. Anal Chem. 2021;93(4):2144–2151. doi: 10.1021/acs.analchem.0c03956. [DOI] [PubMed] [Google Scholar]
- Ihrie R.A., Henske E.P. Modeling tuberous sclerosis with organoids. Science. 2022;375:382–383. doi: 10.1126/science.abn6158. [DOI] [PubMed] [Google Scholar]
- Jia D. Chinese People's Liberation Army Naval Medical University; 2018. Mechanism Study of Active Compounds from Traditional Chinese Medicine Using Novel Target Identification Technologies Combined with Omic Techniques. [Google Scholar]
- Kang S., Park S.E., Huh D.D. Organ-on-a-chip technology for nanoparticle research. Nano converg. 2021;8:20. doi: 10.1186/s40580-021-00270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok B.P., Ghimire S., Kim W., Chatterjee S., Johns T., Kitamura S., Eberhardt J., Ogasawara D., Xu J., Sukiasyan A., Kim S.M., Godio C., Bittencourt J.M., Cameron M., Galmozzi A., Forli S., Wolan D.W., Cravatt B.F., Boger D.L., Saez E. Discovery of small-molecule enzyme activators by activity-based protein profiling. Nat Chem Biol. 2020;16:997–1005. doi: 10.1038/s41589-020-0555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M.A., Knoblich J.A. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- Li N., Li S., Li T., Yang H., Zhang Y., Zhao Z. Co-Incorporated Mesoporous Carbon Material-Assisted Laser Desorption/Ionization Ion Source as an Online Interface of In Vivo Microdialysis Coupled with Mass Spectrometry. Anal chem. 2020;92:5482–5491. doi: 10.1021/acs.analchem.0c00227. [DOI] [PubMed] [Google Scholar]
- Li S., Liu S., Liu Z., Pi Z., Song F., Jin Y. A target-group-change strategy based on the UPLC-Q-TOF-MSE method for the metabolites identification of Fufang-Xialian-Capsule in rat's plasma. J Chromatogr B. 2018;1085:42–53. doi: 10.1016/j.jchromb.2018.03.046. [DOI] [PubMed] [Google Scholar]
- Li Y., Meng Q., Yang M., Liu D., Hou X., Tang L., Wang X., Lyu Y., Chen X., Liu K., Yu A.M., Zuo Z., Bi H. Current trends in drug metabolism and pharmacokinetics. Acta Pharm Sin B. 2019;9:1113–1144. doi: 10.1016/j.apsb.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Wang P., Guo W., Huang X., Tian X., Wu G., Xu B., Li F., Yan C., Liang X.J., Lei H. Natural Berberine-Based Chinese Herb Medicine Assembled Nanostructures with Modified Antibacterial Application. ACS Nano. 2019;13:6770–6781. doi: 10.1021/acsnano.9b01346. [DOI] [PubMed] [Google Scholar]
- Li S., Wang R., Hu X., Li C., Wang L. Bio-affinity ultra-filtration combined with HPLC-ESI-qTOF-MS/MS for screening potential α-glucosidase inhibitors from Cerasus humilis (Bge.) Sok. leaf-tea and in silico analysis. Food chem. 2022;373(Pt:B), 131528. doi: 10.1016/j.foodchem.2021.131528. [DOI] [PubMed] [Google Scholar]
- Li D., Zang R., Yang S., Wang J., Wang X. Cell-based high-throughput proliferation and cytotoxicity assays for screening traditional Chinese herbal medicines. Process Biochem. 2013;48:517–524. doi: 10.1016/j.procbio.2013.02.005. [DOI] [Google Scholar]
- Li D., Xu L., Qi J., Yu B. Screening and analysis of cyclooxygenase-2 inhibitors from the complex matrix: A case study to illustrate the important effect of immobilized enzyme activity in magnetic ligand fishing. J Pharm Biomed Anal. 2019;175 doi: 10.1016/j.jpba.2019.112795. [DOI] [PubMed] [Google Scholar]
- Li Z., Xu H., Gong Y., Chen W., Zhan Y., Yu L., Sun Y., Li A., He S., Guan B., Wu Y., Xiong G., Fang D., He Y., Tang Q., Yao L., Hu Z., Mei H., He Z., Cai Z., Huang W. Patient-Derived Upper Tract Urothelial Carcinoma Organoids as a Platform for Drug Screening. Adv Sci. 2022;9:e2103999. doi: 10.1002/advs.202103999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Yao Y., Chen M., Ding H., Liang C., Lv L., Zhao H., Zhou G., Luo Z., Li Y., Zhang H. Comprehensive evaluation integrating omics strategy and machine learning algorithms for consistency of calculus bovis from different sources. Talanta. 2022;237 doi: 10.1016/j.talanta.2021.122873. [DOI] [PubMed] [Google Scholar]
- Li D.Q., Zhao J., Li S.P., Zhang Q.W. Discovery of xanthine oxidase inhibitors from a complex mixture using an online, restricted-access material coupled with column-switching liquid chromatography with a diode-array detection system. Anal Bioanal Chem. 2014;406:1975–1984. doi: 10.1007/s00216-013-7612-8. [DOI] [PubMed] [Google Scholar]
- Li F., Zhao Y., Lin R., Zhao M., Su J., Wen A., Xi M. Research Progress on the Effective Substances and the Mechanism of Traditional Chinese Medicine Compound. Chinese Pharmaceutical Journal. 2019;54:1037–1044. doi: 10.11669/cpj.2019.13.001. [DOI] [Google Scholar]
- Liao L.X., Song X.M., Wang L.C., Lv H.N., Chen J.F., Liu D., Fu G., Zhao M.B., Jiang Y., Zeng K.W., Tu P.F. Highly selective inhibition of IMPDH2 provides the basis of antineuroinflammation therapy. PNAS. 2017;114:E5986–E5994. doi: 10.1073/pnas.1706778114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Liu X., Zhao M. Screening of xanthine oxidase inhibitor from selected edible plants and hypouricemic effect of Rhizoma Alpiniae Officinarum extract on hyperuricemic rats. J. Funct. Foods. 2018;50:26–36. doi: 10.1016/j.jff.2018.09.024. [DOI] [Google Scholar]
- Liu X.Y., Chang Y.L., Wang X.H., Wang Y., Ren X.Y., Ma J.M., Yu A.X., Wei J., Fan Q.Q., Dong Y., Song R.L., Yao J.L., Shan D.J., She G.M. An integrated approach to uncover anti-tumor active materials of Curcumae Rhizoma-Sparganii Rhizoma based on spectrum-effect relationship, molecular docking, and ADME evaluation. J Ethnopharmacol. 2021;280 doi: 10.1016/j.jep.2021.114439. [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu S., Liu Z. Screening and determination of potential xanthine oxidase inhibitors from Radix Salviae Miltiorrhizae using ultrafiltration liquid chromatography-mass spectrometry. J Chromatogr B. 2013;923–924:48–53. doi: 10.1016/j.jchromb.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Liu Y., Han C., Lu T., Liu Y., Chen H., Yang C., Tu Y., Li Y. Investigation of the interaction between Chrysoeriol and xanthine oxidase using computational and in vitro approaches. Int J Biol Macromol. 2021;190:463–473. doi: 10.1016/j.ijbiomac.2021.08.231. [DOI] [PubMed] [Google Scholar]
- Liu R., Runyon R., Wang Y., Oliver S.G., Fan T., Zhang W. Deciphering ancient combinatorial formulas: The Shexiang Baoxin pill. Science. 2015;347:S40–S42. [Google Scholar]
- Liu P., Yang H., Long F., Hao H.P., Xu X., Liu Y., Shi X.W., Zhang D.D., Zheng H.C., Wen Q.Y., Li W.W., Ji H., Jiang X.J., Zhang B.L., Qi L.W., Li P. Bioactive equivalence of combinatorial components identified in screening of an herbal medicine. Pharm Res. 2014;31:1788–1800. doi: 10.1007/s11095-013-1283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan X., Zhang L.J., Li X.Q., Rahman K., Zhang H., Chen H.Z., Zhang W.D. Compound-based Chinese medicine formula: From discovery to compatibility mechanism. J Ethnopharmacol. 2020;254 doi: 10.1016/j.jep.2020.112687. [DOI] [PubMed] [Google Scholar]
- Luck H., Khan S., Kim J.H., Copeland J.K., Revelo X.S., Tsai S., Chakraborty M., Cheng K., Tao Chan Y., Nøhr M.K., Clemente-Casares X., Perry M.C., Ghazarian M., Lei H., Lin Y.H., Coburn B., Okrainec A., Jackson T., Poutanen S., Gaisano H., Winer D.A. Gut-associated IgA+ immune cells regulate obesity-related insulin resistance. Nat commu. 2019;10:3650. doi: 10.1038/s41467-019-11370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S., Guo L., Sheng C., Zhao Y., Chen L., Li C., Jiang Z., Tian H. Rapid identification and isolation of neuraminidase inhibitors from mockstrawberry (Duchesnea indica Andr.) based on ligand fishing combined with HR-ESI-Q-TOF-MS. Acta Pharm Sin B. 2020;10:1846–1855. doi: 10.1016/j.apsb.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G., Wang Y. Chemical research system of Chinese medicine compounding. World Science and Technology-Modernization of Chinese Medicine. 1999;1:16. [Google Scholar]
- Lv J., Jia Y., Li J., Kuai W., Li Y., Guo F., Xu X., Zhao Z., Lv J., Li Z. Gegen Qinlian decoction enhances the effect of PD-1 blockade in colorectal cancer with microsatellite stability by remodelling the gut microbiota and the tumour microenvironment. Cell Death Dis. 2019;10:415. doi: 10.1038/s41419-019-1638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Huang B., Tang W., Li P., Chen J. Characterization of chemical constituents and metabolites in rat plasma after oral administration of San Miao Wan by ultra-high performance liquid chromatography tandem Q-Exactive Orbitrap mass spectrometry. J Chromatogr B. 2021;1178 doi: 10.1016/j.jchromb.2021.122793. [DOI] [PubMed] [Google Scholar]
- Ma X., Lin H., He Y., She Y., Wang M., Abd El-Aty A.M., Afifi N.A., Han J., Zhou X., Wang J., Zhang J. Magnetic molecularly imprinted polymers doped with graphene oxide for the selective recognition and extraction of four flavonoids from Rhododendron species. J Chromatogr A. 2019;1598:39–48. doi: 10.1016/j.chroma.2019.03.053. [DOI] [PubMed] [Google Scholar]
- Ma P., Peng Y., Zhao L., Liu F., Li X. Differential effect of polysaccharide and nonpolysaccharide components in Sijunzi decoction on spleen deficiency syndrome and their mechanisms. Phytomedicine. 2021;93 doi: 10.1016/j.phymed.2021.153790. [DOI] [PubMed] [Google Scholar]
- Meng Y., Cheng X., Wang T., Hang W., Li X., Nie W., Liu R., Lin Z., Hang L., Yin Z., Zhang B., Yan X. Micro-Lensed Fiber Laser Desorption Mass Spectrometry Imaging Reveals Subcellular Distribution of Drugs within Single Cells. Angew Chem Int Ed Engl. 2020;59(41):17864–17871. doi: 10.1002/anie.202002151. [DOI] [PubMed] [Google Scholar]
- Mever M., Segers K., Drouin N., Guled F., Heyden Y.V., Eeckhaut A.V., Hankemeier T., Ramautar R. Direct profiling of endogenous metabolites in rat brain microdialysis samples by capillary electrophoresis-mass spectrometry with on-line preconcentration. Microchem. J. 2020;156 doi: 10.1016/j.microc.2020.104949. [DOI] [Google Scholar]
- Mi N., Cheng T., Li H., Yang P., Mu X., Wang X., Zu X., Qi X., Guo X., Ye J., Zhang W. Metabolite profiling of traditional Chinese medicine formula Dan Zhi Tablet: An integrated strategy based on UPLC-QTOF/MS combined with multivariate statistical analysis. J Pharm Biomed Anal. 2019;164:70–85. doi: 10.1016/j.jpba.2018.10.024. [DOI] [PubMed] [Google Scholar]
- Mi S., Du Z., Xu Y., Wu Z., Qian X., Zhang M., Sun W. Microfluidic co-culture system for cancer migratory analysis and anti-metastatic drugs screening. Sci Rep. 2016;6:35544. doi: 10.1038/srep35544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z.H., Wu L., Cao K.X., Zhang X.Q., Wang D.Y., Zeng Y.W., Liang L.L., Qiu X.D., Guo R.S., Cheng H.B., Chen Z.P. Investigation of the pharmacodynamic substances in dahuang zhechong pill that inhibit energy metabolism. J Ethnopharmacol. 2020;251 doi: 10.1016/j.jep.2019.112332. [DOI] [PubMed] [Google Scholar]
- Nie J., Gao Q., Fu J., He Y. Grafting of 3D Bioprinting to In Vitro Drug Screening: A Review. Adv Healthc Mater. 2020;9:e1901773. doi: 10.1002/adhm.201901773. [DOI] [PubMed] [Google Scholar]
- Nitsche K.S., Müller I., Malcomber S., Carmichael P.L., Bouwmeester H. Implementing organ-on-chip in a next-generation risk assessment of chemicals: a review. Arch toxicol. 2022;96:711–741. doi: 10.1007/s00204-022-03234-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organoids., Nat Biotechnol. 2021;39:1076. doi: 10.1038/s41587-021-01055-7. [DOI] [PubMed] [Google Scholar]
- Orowitz T.E., Ana Sombo P., Rahayu D., Hasanah A.N. Microsphere Polymers in Molecular Imprinting: Current and Future Perspectives. Molecules. 2020;25:3256. doi: 10.3390/molecules25143256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaki T., Sivathanu V., Kamm R.D. Vascularized microfluidic organ-chips for drug screening, disease models and tissue engineering. Curr Opin Biotechnol. 2018;52:116–123. doi: 10.1016/j.copbio.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou R., Lin L., Zhao M., Xie Z. Action mechanisms and interaction of two key xanthine oxidase inhibitors in galangal: Combination of in vitro and in silico molecular docking studies. Int J Biol Macromol. 2020;162:1526–1535. doi: 10.1016/j.ijbiomac.2020.07.297. [DOI] [PubMed] [Google Scholar]
- Qiao H., Di L., Ping Q., Hu L. Structural Chinese medicine: new research field on pharmacodynamic substance basis of traditional Chinese medicine. China Journal of Chinese Materia Medica. 2021;46:2443–2448. doi: 10.19540/j.cnki.cjcmm.20210129.601. [DOI] [PubMed] [Google Scholar]
- Qiao X., Wang Q., Wang S., Kuang Y., Li K., Song W., Ye M. A 42-Markers Pharmacokinetic Study Reveals Interactions of Berberine and Glycyrrhizic Acid in the Anti-diabetic Chinese Medicine Formula Gegen-Qinlian Decoction. Front Pharmacol. 2018;9:622. doi: 10.3389/fphar.2018.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Yuan Q., Zhang S., He C., Wei X., Liu M., Jiang N., Huang L., Zhuang L., Wang P. Biomimetic in vitro respiratory system using smooth muscle cells on ECIS chips for anti-asthma TCMs screening. Anal Chim Acta. 2021;1162 doi: 10.1016/j.aca.2021.338452. [DOI] [PubMed] [Google Scholar]
- Qiu F., Yao X. New ideas for in vivo direct material basis research of Chinese medicine. Pharmacology and clinics of Chinese materia medica. 1999;3:2–3. doi: 10.3969/j.issn.1001-859X.1999.03.001. [DOI] [Google Scholar]
- Quan L.H., Zhang C., Dong M., Jiang J., Xu H., Yan C., Liu X., Zhou H., Zhang H., Chen L., Zhong F.L., Luo Z.B., Lam S.M., Shui G., Li D., Jin W. Myristoleic acid produced by enterococci reduces obesity through brown adipose tissue activation. Gut. 2020;69:1239–1247. doi: 10.1136/gutjnl-2019-319114. [DOI] [PubMed] [Google Scholar]
- Rawal P., Tripathi D.M., Ramakrishna S., Kaur S. Prospects for 3D bioprinting of organoids. Bio-des. Manuf. 2021;4:627–640. doi: 10.1007/s42242-020-00124-1. [DOI] [Google Scholar]
- Reardon S. 'Organs-on-chips' go mainstream. Nature. 2015;523:266. doi: 10.1038/523266a. [DOI] [PubMed] [Google Scholar]
- Sankar M., K, L., Jeyachandran, S., Pandi, B., Screening of inhibitors as potential remedial against Ebolavirus infection: pharmacophore-based approach. J Biomol Struct Dyn. 2021;39:395–408. doi: 10.1080/07391102.2020.1715260. [DOI] [PubMed] [Google Scholar]
- Seo J.I., Yu J.S., Lee E.K., Park K.B., Yoo H.H. Molecular networking-guided strategy for the pharmacokinetic study of herbal medicines: Cudrania tricuspidata leaf extracts. Biomed Pharmacother. 2022;149 doi: 10.1016/j.biopha.2022.112895. [DOI] [PubMed] [Google Scholar]
- Shang Z., Xu L., Xiao Y., Du W., An R., Ye M., Qiao X. A global profiling strategy using comprehensive two-dimensional liquid chromatography coupled with dual-mass spectrometry platforms: Chemical analysis of a multi-herb Chinese medicine formula as a case study. J Chromatogr A. 2021;1642 doi: 10.1016/j.chroma.2021.462021. [DOI] [PubMed] [Google Scholar]
- Shen Y., Wang M., Zhou J., Chen Y., Wu M., Yang Z., Yang C., Xia G., Tam J.P., Zhou C., Yang H., Jia X. Construction of Fe3O4@α-glucosidase magnetic nanoparticles for ligand fishing of α-glucosidase inhibitors from a natural tonic Epimedii Folium. Int J Biol Macromol. 2020;165(Pt A):1361–1372. doi: 10.1016/j.ijbiomac.2020.10.018. [DOI] [PubMed] [Google Scholar]
- Sheng N., Zheng H., Xiao Y., Wang Z., Li M., Zhang J. Chiral separation and chemical profile of Dengzhan Shengmai by integrating comprehensive with multiple heart-cutting two-dimensional liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J Chromatogr A. 2017;1517:97–107. doi: 10.1016/j.chroma.2017.08.037. [DOI] [PubMed] [Google Scholar]
- Song H.P., Chen J., Hong J.Y., Hao H., Qi L.W., Lu J., Fu Y., Wu B., Yang H., Li P. A strategy for screening of high-quality enzyme inhibitors from herbal medicines based on ultrafiltration LC-MS and in silico molecular docking. Chem Commun. 2015;51:1494–1497. doi: 10.1039/c4cc08728c. [DOI] [PubMed] [Google Scholar]
- Stavrianidi A. A classification of liquid chromatography mass spectrometry techniques for evaluation of chemical composition and quality control of traditional medicines. J Chromatogr. A. 2020;1609 doi: 10.1016/j.chroma.2019.460501. [DOI] [PubMed] [Google Scholar]
- Stutts W.L., Knuth M.M., Ekelöf M., Mahapatra D., Kullman S.W., Muddiman D.C. Methods for Cryosectioning and Mass Spectrometry Imaging of Whole-Body Zebrafish. J Am Soc Mass Spectrom. 2020;31(4):768–772. doi: 10.1021/jasms.9b00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C.K., Ho C.C. Online profiling of living rat brain extracellular pH using a pH-Dependent solid phase extraction scheme coupled with microdialysis sampling and inductively coupled plasma mass spectrometry. Anal chim acta. 2019;1055:36–43. doi: 10.1016/j.aca.2018.12.020. [DOI] [PubMed] [Google Scholar]
- Sun S., Wang Y., Wu A., Ding Z., Liu X. Influence Factors of the Pharmacokinetics of Herbal Resourced Compounds in Clinical Practice. Evid Based Complement Alternat Med. 2019;2019:1983780. doi: 10.1155/2019/1983780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Duan J. Science Press; 2014. The Modern Research of Herb Pairs; p. 6. [Google Scholar]
- Tang Y., Shang E., Chen Y., Yue S., Shi X., Dong T., TSIM, W.K.K., Duan, J., Modern research approaches and strategies for compatibility effects and efficacy components of herbal pairs. Acta Pharm Sinica. 2019;54:1564–1573. doi: 10.16438/j.0513-4870.2019-0413. [DOI] [Google Scholar]
- Voelkner N., Voelkner A., Costa J., Sy S., Hermes J., Weitzel J., Morales S., Derendorf H. Dermal pharmacokinetics of pyrazinamide determined by microdialysis sampling in rats. Int J Antimicrob Agents. 2018;51:190–196. doi: 10.1016/j.ijantimicag.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Wan H., Pan L., Wang Y., Li C., Yu L., Zhou H., Wan H., He Y. Pharmacokinetics of seven major active components of Mahuang decoction in rat blood and brain by LC-MS/MS coupled to microdialysis sampling. Naunyn-Schmiedeberg's Arch. Pharmacol. 2020;393:1559–1571. doi: 10.1007/s00210-019-01786-0. [DOI] [PubMed] [Google Scholar]
- Wang X. Studies on Serum Pharmacochemistry of Traditional Chinese Medicine. World science and technology-modernization of traditional Chinese medicine. 2002;4:1–4. doi: 10.3969/j.issn.1674-3849.2002.02.001. [DOI] [Google Scholar]
- Wang P., Guo W., Huang G., Zhen J., Li Y., Li T., Zhao L., Yuan K., Tian X., Huang X., Feng Y., Lei H., Xu A. Berberine-Based Heterogeneous Linear Supramolecules Neutralized the Acute Nephrotoxicity of Aristolochic Acid by the Self-Assembly Strategy. ACS Appl. Mater. Interfaces. 2021;13:32729–32742. doi: 10.1021/acsami.1c06968. [DOI] [PubMed] [Google Scholar]
- Wang Z., He B., Liu Y., Huo M., Fu W., Yang C., Wei J., Abliz Z. In situ metabolomics in nephrotoxicity of aristolochic acids based on air flow-assisted desorption electrospray ionization mass spectrometry imaging. Acta Pharm Sin B. 2020;10:1083–1093. doi: 10.1016/j.apsb.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Huai J., Shang Y., Xie L., Cao X., Liao J., Zhang T., Dai R. Screening for natural inhibitors of 5-lipoxygenase from Zi-shen pill extract by affinity ultrafiltration coupled with ultra performance liquid chromatography-mass spectrometry. J Ethnopharmacol. 2020;254 doi: 10.1016/j.jep.2020.112733. [DOI] [PubMed] [Google Scholar]
- Wang L., Liu Y., Luo Y., Huang K., Wu Z. Quickly Screening for Potential α-Glucosidase Inhibitors from Guava Leaves Tea by Bioaffinity Ultrafiltration Coupled with HPLC-ESI-TOF/MS Method. J Agric Food Chem. 2018;66:1576–1582. doi: 10.1021/acs.jafc.7b05280. [DOI] [PubMed] [Google Scholar]
- Wang Y., Liu S., Wang R., Shi L., Liu Z., Liu Z. Study on the therapeutic material basis and effect of Acanthopanax senticosus (Rupr. et Maxim.) Harms leaves in the treatment of ischemic stroke by PK-PD analysis based on online microdialysis-LC-MS/MS method. Food funct. 2020;11:2005–2016. doi: 10.1039/c9fo02475a. [DOI] [PubMed] [Google Scholar]
- Wang N., Liu Y., Jia C., Gao C., Zheng T., Wu M., Zhang Q., Zhao X., Li Z., Chen J., Wu C. Machine learning enables discovery of Gentianine targeting TLR4/NF-κB pathway to repair ischemic stroke injury. Pharmacol Res. 2021;173 doi: 10.1016/j.phrs.2021.105913. [DOI] [PubMed] [Google Scholar]
- Wang T., Liu J., Luo X., Hu L., Lu H. Functional metabolomics innovates therapeutic discovery of traditional Chinese medicine derived functional compounds. Pharmacol Ther. 2021;224 doi: 10.1016/j.pharmthera.2021.107824. [DOI] [PubMed] [Google Scholar]
- Wang P., Wang L., Peng Z., Fu Z. Flow microdialysis sampling-chemiluminescent detection coupled with molecular docking for the investigation of binding behavior between salbutamol and DNA. Talanta. 2020;208 doi: 10.1016/j.talanta.2019.120367. [DOI] [PubMed] [Google Scholar]
- Wang R., Yang J., Liu Y., Guan B., Feng Q., Wang Y., Lv S., Yang Z., Li Y. The Effect of Acupoint Application of Sinomenine for Rheumatoid Arthritis Measured by Microdialysis and UPLC-MS/MS. Evid Based Complement Alternat Med. 2019;2019:5135692. doi: 10.1155/2019/5135692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhang J., Pi Z., Zheng Z., Xing J., Song F., Liu S., Liu Z. Application of online microdialysis coupled with liquid chromatography-tandem mass spectrometry method in assessing neuroprotective effect of Rhizoma coptidis on diabetic rats. Anal Methods. 2015;7:45–52. doi: 10.1039/c4ay01809e. [DOI] [Google Scholar]
- Wang C., Zhang J., Wu C., Wang Z. A multiple-dimension liquid chromatography coupled with mass spectrometry data strategy for the rapid discovery and identification of unknown compounds from a Chinese herbal formula (Er-xian decoction) J Chromatogr A. 2017;1518:59–69. doi: 10.1016/j.chroma.2017.08.072. [DOI] [PubMed] [Google Scholar]
- Wang L., Zhou G.B., Liu P., Song J.H., Liang Y., Yan X.J., Xu F., Wang B.S., Mao J.H., Shen Z.X., Chen S.J., Chen Z. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. PNAS. 2008;105:4826–4831. doi: 10.1073/pnas.0712365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhou W., Wang Q., Zhang Y., Ling Y., Zhao T., Zhang H., Li P. A novel and comprehensive strategy for quality control in complex Chinese medicine formula using UHPLC-Q-Orbitrap HRMS and UHPLC-MS/MS combined with network pharmacology analysis: Take Tangshen formula as an example. J Chromatogr B. 2021;1183 doi: 10.1016/j.jchromb.2021.122889. [DOI] [PubMed] [Google Scholar]
- Wang X. Science Press; 2010. Serum medicinal chemistry of Chinese medicine. [Google Scholar]
- Wei W., Li Z., Li H., An Y., Qu H., Yao C., Zhang J., Li J., Zhang G., Shi Y., Guo D.A. Exploration of tissue distribution of ginsenoside Rg1 by LC-MS/MS and nanospray desorption electrospray ionization mass spectrometry. J Pharm Biomed Anal. 2021;198 doi: 10.1016/j.jpba.2021.113999. [DOI] [PubMed] [Google Scholar]
- Wei F., Zhang X., Cui P., Gou X., Wang S. Cell-based 3D bionic screening by mimicking the drug-receptor interaction environment in vivo. J Mater Chem B. 2021;9:683–693. doi: 10.1039/d0tb02661a. [DOI] [PubMed] [Google Scholar]
- Wu H., Cao Y., Wang J., Liu R., Sun Y., Zhang C., Sun Y. Pharmacokinetic and metabolic profiling studies of osmundacetone in rats by UPLC-MS/MS and UPLC-QE-Orbitrap-HRMS. Biomed Chromatogr. 2022;36:e5251. doi: 10.1002/bmc.5251. [DOI] [PubMed] [Google Scholar]
- Wu T.R., Lin C.S., Chang C.J., Lin T.L., Martel J., Ko Y.F., Ojcius D.M., Lu C.C., Young J.D., Lai H.C. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut. 2019;68:248–262. doi: 10.1136/gutjnl-2017-315458. [DOI] [PubMed] [Google Scholar]
- Wubshet S.G., Liu B., Kongstad K.T., Böcker U., Petersen M.J., Li T., Wang J., Staerk D. Combined magnetic ligand fishing and high-resolution inhibition profiling for identification of α-glucosidase inhibitory ligands: A new screening approach based on complementary inhibition and affinity profiles. Talanta. 2019;200:279–287. doi: 10.1016/j.talanta.2019.03.047. [DOI] [PubMed] [Google Scholar]
- Wufuer M., Lee G., Hur W., Jeon B., Kim B.J., Choi T.H., Lee S. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci. Rep. 2016;6:37471. doi: 10.1038/srep37471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang C., Li Y., Li K. Hypothesis on “one output multi-source” of traditional Chinese medicine complex system. Acta Pharm Sinica. 2019;54:801–807. doi: 10.16438/j.0513-4870.2018-1008. [DOI] [Google Scholar]
- Xie L., Xie J., Xu Y., Chen W. Discovery of anthocyanins from cranberry extract as pancreatic lipase inhibitors using a combined approach of ultrafiltration, molecular simulation and spectroscopy. Food Funct. 2020;11:8527–8536. doi: 10.1039/d0fo01262a. [DOI] [PubMed] [Google Scholar]
- Xie J., Xiong J., Ding L.S., Chen L., Zhou H., Liu L., Zhang Z.F., Hu X.M., Luo P., Qing L.S. A efficient method to identify cardioprotective components of Astragali Radix using a combination of molecularly imprinted polymers-based knockout extract and activity evaluation. J Chromatogr A. 2018;1576:10–18. doi: 10.1016/j.chroma.2018.09.027. [DOI] [PubMed] [Google Scholar]
- Xu Z., Fang L., Pan D. Regulate prescription of Chinese medicines. Nature. 2018;553:405. doi: 10.1038/d41586-018-01079-1. [DOI] [PubMed] [Google Scholar]
- Xu S., Li C., Zhou H., Yu L., Deng L., Zhu J., Wan H., He Y. A Study on Acetylglutamine Pharmacokinetics in Rat Blood and Brain Based on Liquid Chromatography-Tandem Mass Spectrometry and Microdialysis Technique. Front Pharmacol. 2020;11:508. doi: 10.3389/fphar.2020.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Lyu X., Yi M., Zhao W., Song Y., Wu K. Organoid technology and applications in cancer research. J Hematol Oncol. 2018;11:116. doi: 10.1186/s13045-018-0662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Sun L., Wang X., Zhu S., You J., Zhao X.E., Bai Y., Liu H. Integration of stable isotope labeling derivatization and magnetic dispersive solid phase extraction for measurement of neurosteroids by in vivo microdialysis and UHPLC-MS/MS. Talanta. 2019;199:97–106. doi: 10.1016/j.talanta.2019.02.011. [DOI] [PubMed] [Google Scholar]
- Xu Y., Wen G., Hu Y., Luo M., Dai D., Zhuang Y., Hall W. Multiple Attentional Pyramid Networks for Chinese Herbal Recognition. Pattern Recogn. 2021;110 doi: 10.1016/j.patcog.2020.107558. [DOI] [Google Scholar]
- Xue Y., Lei J. China Environmental Science Press; 1996. The theory of Chinese medicine compound sharpshooters - On modern research methods of Chinese medicine compounding. [Google Scholar]
- Yan H., Chen X., Zhang Z., Yu D., Jia X. Discussion about research ideas of Chinese materia medica based on Chinese materia medica components and “composition structure” theory. Chinese Traditional and Herbal Drugs. 2015;46:1103–1110. doi: 10.7501/j.issn.0253-2670.2015.08.001. [DOI] [Google Scholar]
- Yang X. Substance basis research on Chinese materia medica is one of key scientific problems of inheriting, development and innovation of Chinese materia medica. China Journal of Chinese Materia Medica. 2015;40:3429–3434. doi: 10.4268/cjcmm20151719. [DOI] [PubMed] [Google Scholar]
- Yang J., Tian S., Zhao J., Zhang W. Exploring the mechanism of TCM formulae in the treatment of different types of coronary heart disease by network pharmacology and machining learning. Pharmacol Res. 2020;159 doi: 10.1016/j.phrs.2020.105034. [DOI] [PubMed] [Google Scholar]
- Yong T., Xie Y., Chen S., Chen D., Su J., Jiao C., Hu H., Xiao C. Hypouricemic effect of Grifola frondosa on hyperuricemic mice and virtual screening of bioactives by 3D QSAR pharmacophore modeling. J Funct Foods. 2018;40:582–588. doi: 10.1016/j.jff.2017.11.049. [DOI] [Google Scholar]
- Yu L., Chen M.C., Cheung K.C. Droplet-based microfluidic system for multicellular tumor spheroid formation and anticancer drug testing. Lab chip. 2010;10(18):2424–2432. doi: 10.1039/c004590j. [DOI] [PubMed] [Google Scholar]
- Yu Y., Yao C., Guo D.A. Insight into chemical basis of traditional Chinese medicine based on the state-of-the-art techniques of liquid chromatography-mass spectrometry. Acta pharm Sin. B. 2021;11:1469–1492. doi: 10.1016/j.apsb.2021.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Han L., Fu P., Zeng H., Lv C., Chang W., Runyon R.S., Ishii M., Han L., Liu K., Fan T., Zhang W., Liu R. Cinnamaldehyde accelerates wound healing by promoting angiogenesis via up-regulation of PI3K and MAPK signaling pathways. Lab Invest. 2018;98:783–798. doi: 10.1038/s41374-018-0025-8. [DOI] [PubMed] [Google Scholar]
- Zeng K., Jiang Y., Wang J., Ye M., Li J., Ai X., Song Y., Han L., Liu K., Tu P. TCM chemical biology-emerging interdiscipline of “TCM chemistry” and “biology”. China Journal of Chinese Materia Medica. 2019;44:849–860. doi: 10.19540/j.cnki.cjcmm.20190222.011. [DOI] [PubMed] [Google Scholar]
- Zeng Z., Yao Y., Liu Z., Sun M. A deep-learning system bridging molecule structure and biomedical text with comprehension comparable to human professionals. Nature commun. 2022;13:862. doi: 10.1038/s41467-022-28494-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Bai G., Liu C. The concept, core theory and research methods of Chinese medicine quality markers. Acta Pharm. Sin. 2019;54:187–1186. doi: 10.16438/j.0513-4870.2018-0912. [DOI] [Google Scholar]
- Zhang W., Feng M., Miao Y., Zhang W., Ni Y. Review on the Application of Serum Pharmacochemistry of Traditional Chinese Medicine. Drug Eval. Res. 2019;42:1448–1453. doi: 10.7501/j.issn.1674-6376.2019.07.036. [DOI] [Google Scholar]
- Zhang B., Gao L., Ma L., Luo Y., Yang H., Cui Z. 3D Bioprinting: A Novel Avenue for Manufacturing Tissues and Organs. Engineering. 2019;5:777–794. doi: 10.1016/j.eng.2019.03.009. [DOI] [Google Scholar]
- Zhang J.Y., Hong C.L., Chen H.S., Zhou X.J., Zhang Y.J., Efferth T., Yang Y.X., Li C.Y. Target Identification of Active Constituents of Shen Qi Wan to Treat Kidney Yang Deficiency Using Computational Target Fishing and Network Pharmacology. Front Pharmacol. 2019;10:650. doi: 10.3389/fphar.2019.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A., Sun H., Yan G., Han Y., Zhao Q., Wang X. Chinmedomics: A Powerful Approach Integrating Metabolomics with Serum Pharmacochemistry to Evaluate the Efficacy of Traditional Chinese Medicine. Engineering. 2019;5:60–68. doi: 10.1016/j.eng.2018.11.008. [DOI] [Google Scholar]
- Zhang A., Sun H., Yan G., Han Y., Zhao Q., Wang X. Deciphering the Q-markers of nourishing: A Powerful Approach Integrating Metabolomics with Serum Pharmacochemistry to Evaluate the Efficacy of Traditional Chinese Medicine. Engineering. 2019;5:132–149. [Google Scholar]
- Zhang W.J., Wang S., Kang C.Z., Lv C.G., Zhou L., Huang L.Q., Guo L.P. Pharmacodynamic material basis of traditional Chinese medicine based on biomacromolecules: a review. Plant methods. 2020;16:26. doi: 10.1186/s13007-020-00571-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Wang Y., Yu M., Shang Y., Chang Y., Zhao H., Kang Y., Zhao L., Xu L., Zhao X., Difrancesco D., Baruscotti M., Wang Y. Discovery of Herbacetin as a Novel SGK1 Inhibitor to Alleviate Myocardial Hypertrophy. Adv Sci. 2022;9:e2101485. doi: 10.1002/advs.202101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Zhang Y., Li X., Zhang S., Zhu M., Du W., Xiao X. Research on Q-markers of Qiliqiangxin capsule for chronic heart failure treatment based on pharmacokinetics and pharmacodynamics association. Phytomedicine. 2018;44:220–230. doi: 10.1016/j.phymed.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Zhang B., Zhao S., Yang D., Wu Y., Xin Y., Cao H., Huang X.P., Cai X., Sun W., Ye N., Xu Y., Peng Y., Zhao S., Liu Z.J., Zhong G., Wang M.W., Shui W. A Novel G Protein-Biased and Subtype-Selective Agonist for a G Protein-Coupled Receptor Discovered from Screening Herbal Extracts. ACS Cent Sci. 2020;6:213–225. doi: 10.1021/acscentsci.9b01125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Li P., Wen X., Yang J. Study on the hepatobiliary behavior of Ermiao wan formula by microdialysis- LC-qTOF-MS. J Pharm Biomed Anal. 2020;189 doi: 10.1016/j.jpba.2020.113419. [DOI] [PubMed] [Google Scholar]
- Zhong C., Jiang C., Ni S., Wang Q., Cheng L., Wang H., Zhang Q., Liu W., Zhang J., Liu J., Wang M., Jin M., Shen P., Yao X., Wang G., Zhou F. Identification of bioactive anti-angiogenic components targeting tumor endothelial cells in Shenmai injection using multidimensional pharmacokinetics. Acta Pharm Sin B. 2020;10:1694–1708. doi: 10.1016/j.apsb.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Chen X., Chen X., Su C., Li L., Jiang Y., Zhang W., Guo S., Liu B. Construction of “2R network pharmacology” research method for effective components of traditional Chinese medicine based on “prediction of dominant components-screening of potential targets”. China Journal of Chinese Materia Medica. 2021;46:2363–2369. doi: 10.19540/j.cnki.cjcmm.20210222.201. [DOI] [PubMed] [Google Scholar]
- Zhu C., Cai T., Jin Y., Chen J., Liu G., Xu N., Shen R., Chen Y., Han L., Wang S., Wu C., Zhu M. Artificial intelligence and network pharmacology based investigation of pharmacological mechanism and substance basis of Xiaokewan in treating diabetes. Pharmacol Res. 2020;159 doi: 10.1016/j.phrs.2020.104935. [DOI] [PubMed] [Google Scholar]
- Zhu H., Chang W., Zhou C., Xu C., Su W., Gao F., Tan X., Lu S. Chemicalome and metabolome profiling of Chai-Gui Decoction using an integrated strategy based on UHPLC-Q-TOF-MS/MS analysis. J Chromatogr B. 2021;1185 doi: 10.1016/j.jchromb.2021.122979. [DOI] [PubMed] [Google Scholar]
- Zietek T., Boomgaarden W., Rath E. Drug Screening, Oral Bioavailability and Regulatory Aspects: A Need for Human Organoids. Pharmaceutics. 2021;13:1280. doi: 10.3390/pharmaceutics13081280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo T., Qian Y., Zhang C., Wei Y., Wang X., Wang H., Hu Y., Li W., Wu X., Yang W. Data-Dependent Acquisition and Database-Driven Efficient Peak Annotation for the Comprehensive Profiling and Characterization of the Multicomponents from Compound Xueshuantong Capsule by UHPLC/IM-QTOF-MS. Molecules. 2019;24:3431. doi: 10.3390/molecules24193431. [DOI] [PMC free article] [PubMed] [Google Scholar]