Abstract
Objective:
To examine the predictive value of neutrophil-to-lymphocyte ratio in localized prostate cancer for surgical pathology and recurrence in patients treated by radical prostatectomy
Patients and Methods:
We evaluated 1 258 patients treated by radical prostatectomy at San Raffaele Hospital between 2011 and 2017 and assessed the association between preoperative neutrophil-to-lymphocyte ratio and surgical pathology (advanced stage, grade group ≥4, nodal involvement, grade discordance between biopsy and surgical pathology) and biochemical recurrence.
Results:
The preoperative neutrophil-to-lymphocyte ratio was not significantly associated with advanced stage (≥T3), International Society of Urological Pathology (ISUP) grade (≥4) or discordance. At multivariable analysis, patients with higher neutrophil-to-lymphocyte ratio had lower risk of nodal involvement at final pathology (odds ratio [OR]: 0.77; 95% confidence interval [CI]: 0.64, 0.92; P=0.005). The preoperative level of neutrophil-to-lymphocyte ratio was associated with biochemical recurrence on univariate analysis (OR: 0.81, 95% CI: 0.68, 0.96; P=0.017). Such a relationship was not significant at multivariable analysis adjusting for tumor severity (OR: 0.93, 95% CI: 0.79, 1.09; P=0.4).
Conclusions:
Neutrophil-to-lymphocyte ratio does not have clinical utility for the prediction of adverse pathology and biochemical recurrence. Further research should focus on its value for predicting regional lymph node metastasis
Keywords: Localized prostate cancer, radical prostatectomy, neutrophil-to-lymphocyte ratio, nodal metastasis, biochemical recurrence
INTRODUCTION
It is widely acknowledged that inflammation is a hallmark of cancer,1 specifically, that inflammation influences the development and progression of malignancies.2 Cancer-related inflammation is mainly driven by cells of the innate immune system, mostly represented by neutrophils.3 However, there is also evidence that adaptive immune response, i.e., lymphocytes play a role in this process. For example, activation of peripheral lymphocytes is an essential step for establishing a chronic inflammation state that promotes cancer development.4 Moreover, aberrant accumulation of neutrophils has been observed in several malignancies such as colorectal, cervical, pancreatic and gastric cancer, being often associated with poor prognosis.5 Since the blood levels of such markers of systemic inflammation may reflect the tumor biology, increasing interest has been paid to whether they can be used to predict oncologic outcomes.
The neutrophil-to-lymphocyte ratio is a readily accessible marker of host inflammation. Recently, the clinical utility of this marker has been examined in a number of malignancies,6 including prostate cancer.7 Extensive literature shows that the neutrophil-to-lymphocyte ratio can predict outcome and treatment responses in metastatic prostate cancer.8-11 By contrast, evidence on the clinical utility of neutrophil-to-lymphocyte ratio in localized disease is somewhat less straightforward. For example, few studies observed that elevated neutrophil-to-lymphocyte ratio is related to high-grade cancer upon prostate biopsy12 and adverse pathologic features13,14 found in surgical specimens. Poor survival after radical prostatectomy has been observed for a preoperative neutrophil-to-lymphocyte ratio >1.76,15 and patients with a preoperative neutrophil-to-lymphocyte ratio >2.49416 and >517 had higher risk of biochemical recurrence. However, other studies have failed to replicate these findings.14,15,18 Moreover, the neutrophil-to-lymphocyte ratio is often categorized by splitting patients into arbitrary groups and comparing patients above and below the identified threshold. This approach reduces statistical power, and there is no widely accepted threshold used across studies. As a result, the prognostic reliability of neutrophil-to-lymphocyte ratio in surgically treated prostate cancer remains unclear.
Here we examine the association between preoperative levels of neutrophil-to-lymphocyte ratio, without using arbitrary thresholds, and both adverse pathology and biochemical recurrence after radical prostatectomy.
PATIENTS AND METHODS
We evaluated data from 1 258 patients who underwent radical prostatectomy at San Raffaele Hospital between 2011 and 2017 for clinically localized prostate cancer. An extended pelvic lymph-node dissection was performed in patients with a preoperative risk of nodal involvement greater than 5%.19 The preoperative neutrophil and lymphocyte counts were collected for all the patients, and the neutrophil-to-lymphocyte ratio was calculated by dividing the neutrophil count by the lymphocyte count. No patients had systemic inflammation at the time of blood collection, within one week before surgery. Biochemical recurrence was defined as a prostate-specific antigen (PSA) concentration of more than 0.2 ng/mL in two consecutive measurements. We excluded patients who were missing preoperative (n=194) and pathological data (n=47), leaving 1 017 patients eligible for analyses.
The primary endpoint of this study was to assess the relationship between the preoperative neutrophil-to-lymphocyte ratio and adverse pathologic features, namely advanced stage (≥T3), grade (≥8), nodal involvement, and discordance. Discordance was defined as diagnostic prostate biopsy showing different International Society of Urological Pathology (ISUP) grade when compared with surgical specimens. We also investigated the association between the neutrophil-to-lymphocyte ratio and biochemical recurrence after radical prostatectomy.
Our statistical analysis involved three steps. First, we divided our study cohort according to a value of neutrophil-to-lymphocyte ratio greater or smaller than 2. As there is no universally accepted value of abnormal neutrophil-to-lymphocyte ratio, we chose this threshold to be clinically straightforward and close to the median of distribution (2.1). Our threshold was chosen only for descriptive purposes; all subsequent analyses included the neutrophil-to-lymphocyte ratio as a continuous variable.
Second, we used univariate analysis to investigate the relationship between the preoperative neutrophil-to-lymphocyte ratio and advanced stage (≥T3), ISUP grade (≥4), nodal involvement, and discordance individually. The neutrophil-to-lymphocyte ratio was included as a non-linear term using restricted cubic splines with knots at quartiles. The analyses to predict each endpoint were then repeated in a multivariable setting accounting for age, preoperative PSA level, biopsy ISUP grade (1 vs. 2-3 vs. 4-5), and clinical T stage (T1 vs. T2 vs. T3). When the endpoint of interest was nodal involvement, the total number of nodes removed was included in the model.
Finally, we evaluated the association between preoperative neutrophil-to-lymphocyte ratio and biochemical recurrence. Since data on biochemical recurrence were available for only 648 (64%) patients, we investigated whether patients with available or missing biochemical recurrence data had similar disease characteristics using the Wilcoxon rank-sum and Chi-squared tests. The association between neutrophil-to-lymphocyte ratio and biochemical recurrence was examined using a multivariable Cox regression model. The model included the following covariates: age, preoperative PSA level, extraprostatic extension, seminal vesicle involvement, pathologic ISUP grade (1 vs. 2-3 vs. 4-5), positive surgical margins, and nodal status (pN0 vs. pN1 vs. pNx).
RESULTS
Table 1 describes the clinical and pathologic characteristics of our cohort stratified by the preoperative level of neutrophil-to-lymphocyte ratio. There were only small differences for patients above and below the identified threshold regarding clinical and pathologic features. We found significantly higher neutrophil-to-lymphocyte ratio in older men. At final pathology, patients with a neutrophil-to-lymphocyte ratio <2 had a higher rate of nodal involvement.
Table 1.
Preoperative and pathological characteristics of the study cohort. Data are presented as medians with quartiles or frequencies with proportions. The P value indicates the statistical significance of the difference between the <2 and ≥2 neutrophil-to-lymphocyte groups.
| NLR <2 (n=440; 43%) |
NLR ≥2 (n=577; 57%) |
P Value | |
|---|---|---|---|
| Age, years | 64 (58, 68) | 65 (59, 71) | 0.001 |
| Total PSA, ng/mL | 6.4 (4.8, 9.3) | 6.3 (4.7, 8.9) | 0.3 |
| Neutrophils count, 109/L | 3.3 (2.7, 3.9) | 4.5 (3.8, 5.5) | <0.0001 |
| Lymphocytes count, 109/L | 2.3 (1.9, 2.7) | 1.5 (1.3, 1.9) | <0.0001 |
| Biopsy ISUP grade | |||
| 1 | 270 (61%) | 349 (60%) | 0.9 |
| 2-3 | 52 (12%) | 66 (11%) | |
| 4-5 | 118 (27%) | 162 (29%) | |
| Clinical T Stage | |||
| T1 | 316 (72%) | 375 (65%) | 0.048 |
| T2 | 59 (13%) | 86 (15%) | |
| T3 | 65 (15%) | 116 (20%) | |
| Extra-prostatic Extension | 162 (37%) | 230 (40%) | 0.3 |
| Seminal Vesicles Involvement | 80 (18%) | 101 (18%) | 0.8 |
| Positive Surgical Margins | 92 (21%) | 153 (27%) | 0.038 |
| Pathological ISUP grade | |||
| 1 | 118 (27%) | 156 (27%) | 0.9 |
| 2–3 | 210 (48%) | 267 (46%) | |
| 4–5 | 112 (25%) | 154 (27%) | |
| Nodal Status | |||
| pN0 | 294 (67%) | 387 (67%) | 0.046 |
| pN1 | 86 (20%) | 86 (15%) | |
| pNx | 60 (13%) | 104 (18%) | |
| Number of Nodes Removed | 20 (17, 28) | 23 (16, 30) | 0.3 |
| Number of Positive Nodes | 2 (1, 4) | 2 (1, 6) | 0.13 |
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; PSA, prostate-specific antigen; ISUP, International Society of Urological Pathology
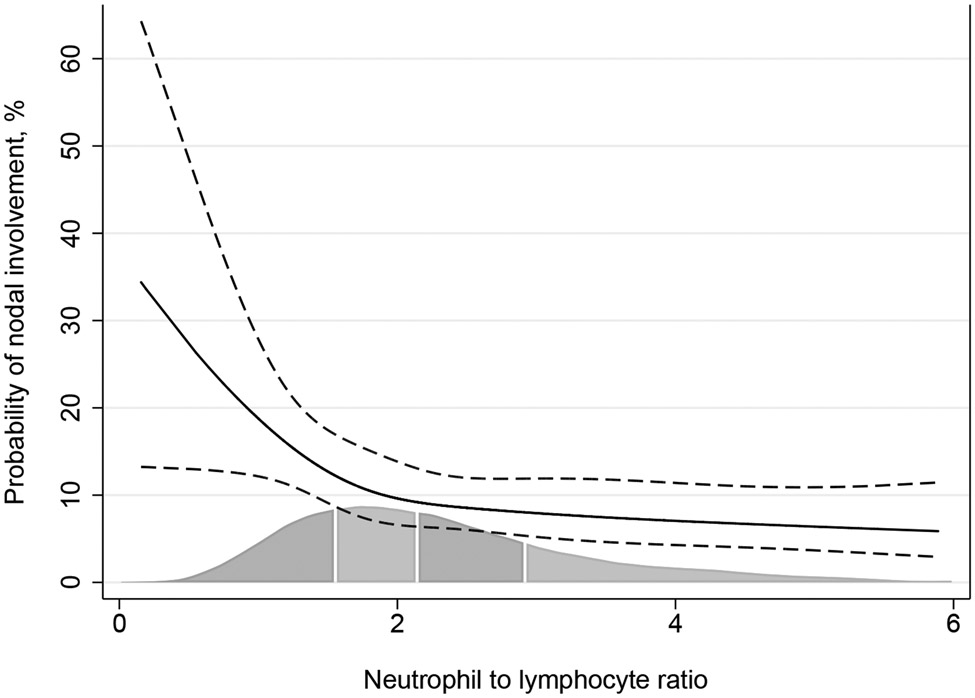
The univariate analysis assessing the relationship between the neutrophil-to-lymphocyte ratio and the risk of adverse pathologic features is shown in Table 2. Although we did not find any significant association between the neutrophil-to-lymphocyte ratio and the endpoints of interest, the odds ratio suggested a lower risk of nodal involvement with increasing preoperative neutrophil-to-lymphocyte ratio level (odds ratio [OR]: 0.88; 95% confidence interval [CI]: 0.76, 1.01; P=0.066), although this association did not meet conventional levels of statistical significance. At multivariable analysis, the relationship between neutrophil-to-lymphocyte ratio and nodal involvement was statistically significant, with a higher ratio associated with lower risk of nodal metastasis (OR: 0.77; 95% CI 0.64, 0.92; P=0.005). Figure 1 shows the relationship between preoperative neutrophil-to-lymphocyte ratio and the probability of nodal involvement at final pathology. The predicted probability of nodal involvement was calculated in patients with typical cancer severity, that is, obtained by setting variables to the mean value.
Table 2.
Univariate and multivariable logistic regression to predict adverse pathological features. Adjustment for case mix based on age, preoperative PSA level, clinical stage, and biopsy grade. When nodal involvement was the endpoint of interest, the total number of nodes removed was included in the model.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Outcome | OR (95% CI) |
P Value | OR (95% CI) |
P Value |
| ≥T3 stage | 0.99 (0.92, 1.08) | 0.9 | 1.00 (0.91, 1.11) | 1 |
| ≥4 ISUP grade | Non-linear | 0.086 | Non-linear | 0.5 |
| Nodal involvement | Non-linear | 0.010 | Non-linear | 0.005 |
| Grade discordance | 0.97 (0.90, 1.05) | 0.5 | 0.94 (0.87, 1.02) | 0.2 |
Abbreviations: PSA, prostate-specific antigen; OR, odds ratio; CI, confidence interval
Figure 1.
Relationship between nodal involvement and preoperative neutrophil-to-lymphocyte ratio in patients with typical cancer severity. Solid line: predicted probability of node positive disease. Dashed lines: 95% confidence interval. Gray area represents the distribution of neutrophil-to-lymphocyte ratio.
We also assessed the relationship between neutrophil-to-lymphocyte ratio and the probability of biochemical recurrence after surgery. There were 119 cases of biochemical recurrences with a 3- and 5-year biochemical recurrence-free probability of 82% (95% CI: 79%, 85%) and 74% (95% CI: 69%, 79%), respectively. The number of patients who did not experience biochemical recurrence with available follow-up data at 3 and 5 years were 209 and 63, respectively. The median follow-up for patients without biochemical recurrence was 29 months (interquartile range: 13 – 48). Patients with available biochemical recurrence data were found to have significantly more aggressive disease. Table 3 describes our univariate and multivariable analyses. We found a significant relationship between neutrophil-to-lymphocyte ratio and biochemical recurrence at univariate (OR: 0.81; 95% CI 0.68, 0.96; P=0.017) but not multivariable analysis (OR: 0.93; 95% CI 0.79, 1.09; P=0.4).
Table 3.
Univariate and multivariable analyses to assess the relationship between neutrophil-to-lymphocyte ratio and biochemical recurrence after radical prostatectomy. Adjustment for case mix based on age, preoperative PSA level, pathological stage, pathological grade, nodal status, and margin status.
| Univariate | Multivariable | |||
|---|---|---|---|---|
| Outcome | HR (95% CI) |
P value | HR (95% CI) |
P value |
| Biochemical recurrence | 0.81 (0.68, 0.96) | 0.017 | 0.93 (0.79, 1.09) | 0.4 |
Abbreviations: PSA, prostate-specific antigen; HR, hazard ratio; CI, confidence interval
DISCUSSION
We found a significant association between neutrophil-to-lymphocyte ratio and nodal involvement in localized prostate cancer. Conversely, the preoperative level of neutrophil-to-lymphocyte ratio was not significantly associated with other pathologic characteristics nor with biochemical recurrence after surgery.
Many studies have examined the relationship between neutrophil-to-lymphocyte ratio and the pathologic features of prostate cancer. In a typical study, the relationship between neutrophil-to-lymphocyte ratio and the endpoint of interest is investigated, comparing patients with low neutrophil-to-lymphocyte ratio to those with high neutrophil-to-lymphocyte ratio, and using the median value of neutrophil-to-lymphocyte ratio14,20 or a previously described cutoff21 to dichotomize the cohort. Consequently, the available evidence is heterogeneous and rarely comparable. For example, higher risk of upgrading at final pathology has been observed for patients with a baseline neutrophil-to-lymphocyte ratio >2.514 and >3.21 Yet, we cannot use these data to determine the general predictive value of neutrophil-to-lymphocyte ratio, especially for a patient that has, for instance, a preoperative neutrophil-to-lymphocyte ratio equal to 2.7. Moreover, applying the same methodology to our population, we found only small differences between patients above and below the median value of neutrophil-to-lymphocyte ratio (2.1). By contrast, the inclusion of neutrophil-to-lymphocyte ratio as a continuous variable reflects its true predictive value and allows for reproducibility and external validity.
We found that the preoperative level of neutrophil-to-lymphocyte ratio was not significantly associated with the pathological stage and grade of prostate cancer, which is in line with findings by other groups.18 By contrast, prior evidence indicated that the probability of adverse pathology was higher in the presence of a preoperative neutrophil-to-lymphocyte ratio >3;21 similarly, higher rates of ISUP grade 4–5 tumors were found in patients with a baseline neutrophil-to-lymphocyte ratio >2.6.20 However, some factors affecting the blood level of the biomarker may be hypothesized in a different population. For instance, since age correlates with the levels of neutrophil-to-lymphocyte ratio,22 the different results may be explained by the older age of our cohort (median: 65 years vs. 6121 and 5920 years, respectively). Also, since many foods modulate inflammation,23 a different diet between American patients20,21 and our European cohort may be responsible for altered levels of neutrophil-to-lymphocyte ratio and thus, this may explain the difference with our results. That said, we also believe that the difference between such papers and our series may be explained by the use of a different methodology (i.e. use of cutoff values) that makes the studies not directly comparable.
We found some evidence of an association between the neutrophil-to-lymphocyte ratio and the risk of nodal involvement in surgically treated prostate cancer. The few studies that addressed this point found a non-significant relationship.13,18 Conversely, other investigators found nodal involvement to be more likely for a neutrophil-to-lymphocyte ratio >3,21 in contrast with our findings. However, Ozsoy et al. 21 described a lower percentage of nodal involvement compared with our cohort (9% vs. 17%, respectively). In addition, the authors did not describe the indication for lymphadenectomy (i.e., risk threshold of nodal involvement) or the number of nodes removed. As these two factors may affect the prevalence of lymph node metastasis, it is plausible that the reported relationship between neutrophil-to-lymphocyte ratio and nodal involvement might have been influenced as well. For instance, a patient with a low neutrophil-to-lymphocyte ratio (indicating higher risk of nodal metastasis, according to our data) might not have received a lymphadenectomy or he might have received a limited excision, resulting in pathological staging of pNx or pN0. Conversely, a lower threshold or a greater number of lymph nodes removed would have increased the probability to spot a nodal metastasis in such patients. We are confident that the median number of nodes removed in our cohort (22) gives insight on the actual nodal burden of our population. However, our finding of a lower probability of nodal metastasis for higher preoperative neutrophil-to-lymphocyte ratio has never been described earlier; thus, additional evidence is needed to draw definitive conclusions on this topic.
Our results are interesting regarding prior evidence that immune cells might mostly affect metastasis spread rather than primary tumor aggressiveness.24 In this context, our results suggest that the relative contribution of lymphocytes to the process of metastasis may be greater than that of neutrophils. In other words, the lower risk of nodal involvement for increasing neutrophil-to-lymphocyte ratio may more likely result from a low count of lymphocytes than from a high count of neutrophils.
We also found that the preoperative level of neutrophil-to-lymphocyte ratio was not associated with the risk of biochemical recurrence after surgery at multivariable analysis. This finding is in line with prior data.14,18,21,25 On the contrary, higher risk of biochemical recurrence has been observed for patients with preoperative neutrophil-to-lymphocyte ratio greater than 2,13 2.516 and 5.17 Given the established relationship between inflammation and cancer, it seems reasonable that a marker of systemic immunity may reflect the prognosis of the tumor. However, the high variability of the available literature precludes definite conclusions. It is similarly plausible that an elevated neutrophil-to-lymphocyte ratio cannot predict biochemical recurrence after surgery even though a higher ratio reflects poor prognosis as our findings support.
Our study has some limitations. First, our cohort did not have complete recurrence data for all patients. As a tertiary center, our institution provides care to patients who may not return for follow-up or may be followed up at a different institution. The comparison between patients with available and missing recurrence data showed that the latter group had more aggressive disease. Such bias would operate in the opposite direction of our findings. Thus, we are confident that this limitation did not affect our results. We also have to acknowledge that, given the relatively short follow-up of our population, a different association between biochemical recurrence and neutrophil-to-lymphocyte ratio at longer follow-up cannot be excluded. Although this does not represent a substantial limitation of the present study, future investigations should test such interesting hypothesis. Second, since there is evidence that surgical experience affects the efficacy of radical prostatectomy,26,27 the inclusion of surgeons with different levels of experience may have affected our results. For example, a patient might have an aggressive tumor, as evidenced by an elevated neutrophil-to-lymphocyte ratio, and still have complete cancer removal if treated by a sufficiently experienced surgeon. As a result, the reliability of neutrophil-to-lymphocyte ratio for the prediction of biochemical recurrence would appear limited. A final limitation is that we were not able to examine the relationship between the preoperative neutrophil-to-lymphocyte ratio and more clinically relevant oncologic endpoints such as metastasis and survival. Biochemical recurrence invariably precedes stronger oncologic endpoints such as metastasis or survival and often triggers postoperative treatments that may be associated with side effects for patients. Accordingly, we believe that the association between neutrophil-to-lymphocyte ratio and biochemical recurrence is of clear clinical interest.
Our findings showed that the prognostic reliability of neutrophil-to-lymphocyte ratio in localized prostate cancer seems limited. Although associated with biochemical recurrence at univariate analysis, such relationship was not confirmed in our multivariable model. Thus, our results do not support the inclusion of the neutrophil-to-lymphocyte ratio into preoperative risk calculators.
From a clinical research standpoint, the lack of a universally accepted threshold for abnormal neutrophil-to-lymphocyte ratio is a limitation for comparative studies. The identification of a clinically meaningful value of neutrophil-to-lymphocyte ratio is of primary importance to allow for adequate, high-level evidence. However, the heterogeneity of the current literature28 makes such a scenario unlikely. In this regard, the inclusion of the neutrophil-to-lymphocyte ratio in predictive models as a continuous variable may allow for fair comparative reports. If replicated, our findings of an association between nodal involvement and the neutrophil-to-lymphocyte ratio suggests that such a biomarker may assist urologists in treatment decisions. To date, the prediction of nodal metastasis before radical prostatectomy is well established19,29. By contrast, the identification of patients with nodal recurrence after local treatment may still be implemented. For example, roughly 10% of patients treated by salvage lymph-node dissection have negative pathology.30 In this context, the neutrophil-to-lymphocyte ratio may improve the currently available imaging-based strategy. Although the literature is still limited, the use of such a biomarker may be of added value with low additional costs. We intend to examine these possibilities in future studies.
CONCLUSIONS
Neutrophil-to-lymphocyte ratio does not have clinical utility for the prediction of adverse pathology and biochemical recurrence. Further research should focus on its value for predicting regional lymph node metastasis.
REFERENCES
- 1.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860-7(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A. Inflammation by remote control. Nature. 2005;435(7043):752-3(7043):752–753. doi: 10.1038/435752a. [DOI] [PubMed] [Google Scholar]
- 4.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7(5):411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Liang W, Ferrara N. The Complex Role of Neutrophils in Tumor Angiogenesis and Metastasis. Cancer Immunology Research. 2016;4(2):83–91. doi: 10.1158/2326-6066.CIR-15-0313. [DOI] [PubMed] [Google Scholar]
- 6.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. JNCI Journal of the National Cancer Institute. 2014;106(6). doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 7.Cao J, Zhu X, Zhao X, Li X-F, Xu R. Neutrophil-to-Lymphocyte Ratio Predicts PSA Response and Prognosis in Prostate Cancer: A Systematic Review and Meta-Analysis. Coleman WB, ed. PLoS ONE. 2016;11(7):e0158770–15. doi: 10.1371/journal.pone.0158770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawahara T, Yokomizo Y, Ito Y, et al. Pretreatment neutrophil-to-lymphocyte ratio predicts the prognosis in patients with metastatic prostate cancer. BMC Cancer. February 2016:1–6. doi: 10.1186/s12885-016-2134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu X, Gao X, Li X, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in prostate cancer: evidence from 16,266 patients. Scientific Reports. February 2016:1–7. doi: 10.1038/srep22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uemura K, Kawahara T, Yamashita D, et al. Neutrophil-to-Lymphocyte Ratio Predicts Prognosis in Castration-Resistant Prostate Cancer Patients Who Received Cabazitaxel Chemotherapy. BioMed Research International. August 2017:1–5. doi: 10.1155/2017/7538647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loubersac T, Nguile-Makao M, Pouliot F, Fradet V, Toren P. Neutrophil-to-lymphocyte Ratio as a Predictive Marker of Response to Abiraterone Acetate: A Retrospective Analysis of the COU302 Study. European Urology Oncology. January 2019:1–8. doi: 10.1016/j.euo.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Gokce MI, Hamidi N, Suer E, Tangal S, Huseynov A, Ibiş A. Evaluation of neutrophil-to-lymphocyte ratio prior to prostate biopsy to predict biopsy histology: Results of 1836 patients. CUAJ. 2015;9(11–12):761–765. doi: 10.5489/cuaj.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee H, Jeong SJ, Hong SK, Byun SS, Lee SE, Oh JJ. High preoperative neutrophil–lymphocyte ratio predicts biochemical recurrence in patients with localized prostate cancer after radical prostatectomy. World Journal of Urology. 2015;34(6):821–827. doi: 10.1007/s00345-015-1701-6. [DOI] [PubMed] [Google Scholar]
- 14.Gokce MI, Tangal S, Hamidi N, Suer E, Ibis MA, Beduk Y. Role of neutrophil-to-lymphocyte ratio in prediction of Gleason score upgrading and disease upstaging in low-risk prostate cancer patients eligible for active surveillance. CUAJ. 2016;10(11–12):383–385. doi: 10.5489/cuaj.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang WS, Cho KS, Kim KH, et al. Prognostic impact of preoperative neutrophil-to-lymphocyte ratio after radical prostatectomy in localized prostate cancer. Nature Publishing Group. 2016;19(3):298–304. doi: 10.1038/pcan.2016.20. [DOI] [PubMed] [Google Scholar]
- 16.Gazel E, Tastemur S, Acikgoz O, et al. Importance of Neutrophil/Lymphocyte Ratio in Prediction of PSA Recurrence after Radical Prostatectomy. Asian Pacific Journal of Cancer Prevention. 2015;16(5):1813–1816. doi: 10.7314/APJCP.2015.16.5.1813. [DOI] [PubMed] [Google Scholar]
- 17.Sharma V, Cockerill PA, Viers BR, et al. The association of preoperative neutrophil to lymphocyte ratio with oncologic outcomes following radical prostatectomy for prostate cancer. JURO. 2015;193(S):e55–e56. doi: 10.1016/j.juro.2015.02.252. [DOI] [Google Scholar]
- 18.Maeda Y, Kawahara T, Koizumi M, et al. Lack of an Association between Neutrophil-to-Lymphocyte Ratio and PSA Failure of Prostate Cancer Patients Who Underwent Radical Prostatectomy. BioMed Research International. April 2016:1–6. doi: 10.1155/2016/6197353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandaglia G, Fossati N, Zaffuto E, et al. Development and Internal Validation of a Novel Model to Identify the Candidates for Extended Pelvic Lymph Node Dissection in Prostate Cancer. European Urology. 2017;72(4):1–9. doi: 10.1016/j.eururo.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 20.Kwon YS, Han CS, Yu JW, et al. Neutrophil and Lymphocyte Counts as Clinical Markers for Stratifying Low-Risk Prostate Cancer. Clinical Genitourinary Cancer. 2016;14(1):e1–e8. doi: 10.1016/j.clgc.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Özsoy M, Moschini M, Fajkovic H, et al. Elevated preoperative neutrophil–lymphocyte ratio predicts upgrading at radical prostatectomy. Prostate Cancer and Prostatic Diseases. 2018;21(1):1–6. doi: 10.1038/s41391-017-0015-8. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Chen Q, Luo X, et al. Neutrophil-to-Lymphocyte Ratio Positively Correlates to Age in Healthy Population. J Clin Lab Anal. 2014;29(6):437–443. doi: 10.1002/jcla.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minihane AM, Vinoy S, Russell WR, et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 2015;114(07):999–1012. doi: 10.1017/S0007114515002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2007;27(1):11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 25.Zanaty M, Ajib K, Alnazari M. Prognostic utility of neutrophil-to-lymphocyte and platelets-to-lymphocyte ratio in predicting biochemical recurrence post robotic prostatectomy. July 2018:1–8. doi: 10.2217/bmm-2017-0321. [DOI] [PubMed] [Google Scholar]
- 26.Vickers AJ, Bianco FJ, Serio AM, et al. The Surgical Learning Curve for Prostate Cancer Control After Radical Prostatectomy. JNCI Journal of the National Cancer Institute. 2007;99(15):1171–1177. doi: 10.1093/jnci/djm060. [DOI] [PubMed] [Google Scholar]
- 27.Bravi CA, Tin A, Vertosick E, et al. The Impact of Experience on the Risk of Surgical Margins and Biochemical Recurrence after Robot-Assisted Radical Prostatectomy: A Learning Curve Study. JURO. 2019;202(1):108–113. doi: 10.1097/JU.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang L, Li X, Wang B, et al. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Localized and Advanced Prostate Cancer: A Systematic Review and Meta-Analysis. PLoS ONE. 2016;11(4):e0153981–13. doi: 10.1371/journal.pone.0153981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stabile A, Muttin F, Zamboni S, et al. Therapeutic approaches for lymph node involvement in prostate, bladder and kidney cancer. Expert Review of Anticancer Therapy. 2019;19(0):1–17. doi: 10.1080/14737140.2019.1659135. [DOI] [PubMed] [Google Scholar]
- 30.Fossati N, Suardi N, Gandaglia G, et al. Identifying the Optimal Candidate for Salvage Lymph Node Dissection for Nodal Recurrence of Prostate Cancer: Results from a Large, Multi-institutional Analysis. European Urology. October 2018:1–8. doi: 10.1016/j.eururo.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]