Abstract
The global distribution of the yellow fever mosquito Aedes aegypti is the subject of considerable attention because of its pivotal role as a biological vector of several high profile disease pathogens including dengue, chikungunya, yellow fever, and Zika viruses. There is also a lot of interest in the projected future species' distribution. However, less effort has been focused on its historical distribution, which has changed substantially over the past 100 years, especially in southern Europe where it was once widespread, but largely disappeared by the middle of the 20th century.
The present work utilises all available historical records of the distribution of Ae. aegypti in southern Europe, the Near East within the Mediterranean Basin and North Africa from the late 19th century until the 1960's to construct a spatial distribution model using matching historical climatic and demographic data.
The resulting model was then implemented using current climate and demographic data to assess the potential distribution of the vector in the present. The models were rerun with several different assumptions about the thresholds that determine habitat suitability for Ae. aegypti. The historical model matches the historical distributions well. When it is run with current climate values, the predicted present day distribution is somewhat broader than it used to be particularly in north-west France, North Africa and Turkey. Though it is beginning to reappear in the eastern Caucasus, this ‘potential’ distribution clearly does not match the actual distribution of the species, which suggests some other factors are responsible for its absence. Future distributions based on the historical model also do not match future distributions derived from models based only on present day vector distributions, which predict little or no presence in the Mediterranean Region. At the same time, the vector is widespread in the USA which is predicted to consolidate its range there in future. This contradiction and the implication for possible re-invasion of Europe are discussed.
Keywords: Vector-borne diseases, Historical distribution, Spatial modelling, Western Palaearctic region, Culicidae
Graphical abstract
Highlights
-
•
Aedes aegypti was historically widespread on the European continent but is now largely absent.
-
•
Spatial models of historical distribution, with matching contemporaneous data fit distribution well.
-
•
Historical model projected to 2015 matches USA distribution, & predicts wide presence in Europe.
-
•
Reasons for absence in Europe are discussed, but remain uncertain.
1. Introduction
The global distribution of the yellow fever mosquito Aedes (Stegomyia) aegypti (Linnaeus, 1762) is the subject of considerable attention because of its pivotal role as a biological vector of several high profile disease pathogens including dengue, chikungunya, yellow fever, and Zika viruses (Kraemer et al., 2015a; Schaffner and Mathis, 2014). Among the two recognised taxonomic forms, Ae. aegypti aegypti and Ae. aegypti formosus, the former (herein referred to as Ae. aegypti) is highly invasive and has become widespread, with potential to expand its distribution in the future (European Centre for Disease Prevention and Control, 2012; Kearney et al., 2009; Kraemer et al., 2015b). There is evidence that it originated from Africa where it became “domesticated”, i.e., switched to use human-generated water containers for larval development and humans as blood source (Powell et al., 2018). Its intercontinental spread around the tropical and subtropical world started with the rise of transatlantic shipping in the sixteenth century, which was followed by worldwide epidemics of diseases caused by pathogens transmitted by Ae. aegypti (Powell and Tabachnick, 2013). At the apogee of its distribution in Europe, during the early 20th century, Ae. aegypti had well established populations in the whole Mediterranean Basin (Christophers, 1960; Schaffner and Mathis, 2014). It was sometimes reported to be highly abundant (France: (Blanchard, 1917); Greece: (Blanc and Caminopetros, 1930); Italy: (La Face and Raffaele, 1928); Russia: (Marzinowsky, 1914); Portugal: (Sarmento and França, 1902)) and present over long periods in coastal and inland areas, far from Points of Entry (Spain: (Gil Collado, 1930); Greece: (Blanc and Caminopetros, 1930)). Since the other species suspected to contribute to dengue transmission Ae. (Fredwardsius) vittatus (Bigot, 1861) and Ae. (Stegomyia) cretinus Edwards, 1921 had only sporadic and patchy distributions, Ae. aegypti can reliably be incriminated as the responsible vector for significant epidemics of yellow fever in e.g. Spain, 1819-24, and dengue in e.g. Greece, 1927-28 (Hoffmann, 1931).
The mosquito almost disappeared from its western Palaearctic range by the 1960's, as a result of dedicated control campaigns (Marzinowsky, 1930) or possibly as a side effect of malaria vector control (Holstein, 1967). It is also suspected that the introduction of piped water to rural villages, and the consequent reduction in potential breeding sites (Blanc and Caminopetros, 1930; Holstein, 1967) contributed to the vector disappearance. No significant established population was reported between 1960 and 2000 but a few sporadic presence records exist (Schaffner and Mathis, 2014), and occurrence of remnant populations at some locations is suggested (Kotsakiozi et al., 2018). Aedes aegypti is now sporadically reported at Points of Entry (as defined by WHO International Health Regulation) like airports (Ibáñez-Justicia et al., 2020), or sea ports (Jeannin et al., 2019), but also imported used tyre depots (Scholte et al., 2010), or private hothouses (Kampen et al., 2016). Establishment of Ae. aegypti has recently been reported from Madeira (Almeida et al., 2007), southern Egypt (Abozeid et al., 2018) and the Caucasus (Yunicheva et al., 2008), and it has been spreading west along the Black Sea coast in Turkey (Akiner et al., 2016) and Crimea (Ganushkina et al., 2020) but has not yet established anywhere in the Mediterranean Basin.
Globally, particular features that have been associated with the presence of Ae. aegypti include urbanisation, socioeconomic factors, building design and construction features, the quality of water supply and management, and the quality of other public health infrastructure services (Jansen and Beebe, 2010). Overall, the geographical distribution of Ae. aegypti is not static, and appears to have undergone significant changes over time on several continents. In the Americas, large and coordinated mosquito eradication efforts were implemented following the 1947 resolution by member nations of the Pan American Health Organisation (PAHO). These resulted in a marked decline in Ae. aegypti populations in that part of the world and the subsequent successful eradication of the species in 19 countries of Central and South America by the 1960s (Schliessman and Calheiro, 1974). However, a suspension or reduction in control efforts after 1965, due to the costs of the programme activities and questions concerning the necessity or feasibility of eradication, was followed by the re-infestation by Ae. aegypti in most of these territories (Schliessman and Calheiro, 1974). In the USA, a Public Health Service programme to eradicate Ae. aegypti was initiated by the Communicable Disease Center (CDC) with funds appropriated by Congress in October 1963 (Morlan and Tinker, 1965). Subsequently the range of Ae. aegypti has retracted but elimination has not been achieved. The vector species is now extending its range again, in particular in south-central and south-western states (Hahn et al., 2017), not only in areas where the species was historically absent in the 1960's but also in Florida (Parker et al., 2019).
In south-east Asia, where the introduction of Ae. aegypti is considered more recent (late 19th century; Powell and Tabachnick, 2013), World War II resulted in an enormous increase of Ae. aegypti populations due to the destruction of cities, the need to house refugees, and the disruption of local public health and sanitation systems (Halstead, 2006). More recently, strong economic growth coupled with improved housing standards and vector control programmes have reduced Ae. aegypti populations in many countries (Halstead, 2006).
Finally in Australia, human behavioural changes in water storage practices (particularly a move from rainwater tanks to piped water supplies) has probably contributed to the regression of Ae. aegypti north into the warmer and more tropical regions (Jansen and Beebe, 2010).
Assessing and managing the risk for vector-borne diseases (VBDs) requires solid data about the presence and absence of the respective vectors or the likelihood of introduction, establishment, spread, and proliferation (Braks et al., 2011; Sedda et al., 2014). Since these data sets are incomplete at continental scale, much effort has been invested in building spatial models of current or future distributions, spread, and even abundance of many major vector species (Caminade et al., 2012; European Centre for Disease Prevention and Control, 2009; Kraemer et al., 2019; Liu-Helmersson et al., 2019; Wint et al., 2020a; Wint et al., 2020b). For Ae. aegypti in particular, the species occurs only at the eastern margin of continental Europe and thus available estimates of potential distributions within Europe are based on presence/absence data, or environment and climatic limits, of currently colonised areas outside Europe (Kraemer et al., 2015b; Rogers et al., 2006).
In this study, we re-evaluate the likelihood of establishment of Ae. aegypti in Europe and neighbouring areas by considering historical presence data for the western Palaearctic region. Based on historical distribution presence data retrieved from the literature and museum collections, matched with historical climate data sets, we use widely established spatial modelling techniques to identify the historical suitability in Europe and project this to current and future distributions in Europe and the USA where the species has persisted since the 18th century and continued to spread while it has disappeared from the Mediterranean Basin. It should be noted that this paper is not intended to investigate the drivers or their specific impact, thus models are essentially used to provide statistical pattern matching and not explanations of cause and effect.
2. Material and methods
2.1. Mosquito occurrence data
The Ae. aegypti historical (<1980) European distribution data collection was obtained through an online literature search in Ovid MEDLINE®, CAB direct, and Web of Science, without year and language restrictions applied to publication date.
Search terms included dengue, yellow fever and their vectors in countries of Europe, the Caucasus, Near East and northern Africa [title:(aegypti OR fasciata OR calopus OR argenteus) AND title:(dengue OR “yellow fever” OR distribution OR presence OR occurrence OR report OR spread OR dispersion OR introduction OR “risk map” OR model$ OR climat$ OR global$) AND title:(Mediterrane$ OR Europ$ OR Balkan OR Scandinavia$ OR Iberian OR Aland OR Albania OR Andorra OR Austria OR Belgium OR Benelux OR Bosnia OR Herzegovina OR Bulgaria OR Croatia OR Cyprus OR Czech OR Denmark OR Germany OR Spain OR Estonia OR Finland OR Faroe OR France OR Greece OR Hungary OR Ireland OR Eire OR Italy OR Kosovo OR Latvia OR Liechtenstein OR Lithuania OR Luxembourg OR Macedonia OR Malta OR Montenegro OR Netherlands OR Norway OR Poland OR Portugal OR Slovenia OR Romania OR “San Marino” OR Serbia OR Slovakia OR Switzerland OR Sweden OR “United Kingdom” OR “British Isles” OR “Great Britain” OR Wales OR England OR Scotland OR Turkey OR Yugoslavia OR Armenia OR Belarus OR Bielorussia OR Georgia OR Moldova OR Ukrain$ OR Ukrayina OR Russia$ OR USSR OR SSSR OR “Soviet Union” OR Azerba$ OR Azarba$ OR Turkmen$ OR Uzbek$ OR Kyrgyz$ OR Tajik$ OR Tadjik$ OR Kazak$)]. This search was first performed in the frame of a study on dengue in Europe mandated by WHO (Schaffner and Mathis, 2014) and completed by additional reference tracking. Some historical disease and vector occurrence reviews and maps were identified but their accuracy was often not sufficient for data extraction and therefore we tracked references to trace the original Ae. aegypti occurrence reports. Mosquito presence data were extracted when Ae. aegypti (or its synonyms Ae. argenteus, Culex calopus and Stegomyia fasciata) was mentioned as collected or observed at a location. Geographical coordinates (latitude/longitude) were then retrieved based on location name via Google Earth and associated to the date of observation when specified or to the date of publication. No obvious geographical bias in reporting was observed: some areas show substantial inland records (e.g. Greece, Spain, Turkey) whilst others show mainly/only coastal records, but in these cases inland areas can be considered less suitable for the mosquito, because of elevation or climate (e.g. France, the Balkan's Adriatic coast).
Modern (≥1980) mosquito distribution data for the western Palaearctic region have been collected routinely within the VectorNet project via online systematic literature search, reference tracking, and grey literature and unpublished dataset sharing (Braks et al., 2022). For both the historical and modern periods, mosquito introductions without evidence of establishment were excluded from the dataset. Modern records which could not be substantiated following information exchanges with the authors or with local colleagues were also excluded (Algeria (Aïssaoui and Boudjelida, 2017; Dahchar et al., 2017), Lebanon (Knio et al., 2005)). Modern vector distribution data for the USA was taken from the datasets described earlier (Kraemer et al., 2015a).
2.2. Modelling
The spatial distribution modelling was performed using both Random Forest and Boosted Regression Trees to model presence and absence, implemented through the VECMAP® Software Suite (AVIA-GIS, Belgium), to produce estimates of the probability of presence. Ten replicates of each method, with a 25 % holdback, were run, and the results averaged to produce an ensemble mean. These methods require approximately equal numbers of presence and absence points to be offered to each modelling run. The occurrence data did not include the requisite absence points, so these needed to be generated. There are a number of geostatistical ways absences can be generated, but we chose to infer absences based on environmental suitability, by assigning absences to areas within 6° north and south of the known vector presence records that can be defined as biologically unsuitable for the vector as described elsewhere (Schaffner et al., 2016). This buffer restricted the model to areas reasonably close to the training data.
A location was defined as unsuitable at elevations higher than 1300 m.a.s.l. (the highest presence record in the literature was 1290 m.a.s.l., in Turkey (Irfan and Vogel, 1927)), with precipitation of <300 mm per year, with a minimum temperature of <14 °C for >6 months per year, with a maximum temperature of >39 °C for 2 months or more, and finally with a human population density of <5 people per square kilometre (Kraemer et al., 2015a).
It should be noted that these environmental limits, especially the rainfall, may falsely exclude urban or desert areas where open water is artificially maintained. For the definition of presence, the 1910 decade was taken as the historical reference point, being the first decade with significant numbers of records, and to ensure that the ‘historical’ baseline was as far apart from the present as the data justify. We assumed that there was no new expansion after 1910 and that the range retraction did not start before 1940. Any locations with presence before that baseline was therefore assumed to still be present in 1910, and data presences for later dates were assumed to be present in 1910. This study therefore assumes that 1910 represents the maximum range, and further focusses on binary presence and absence rather than abundance.
Several models were implemented using (a) historical distributions from the western Palaearctic region with 1910 climate data, and projected forward to 2015 and 2050, and (b) modern (2015) distributions for the USA, with 2015 climate, hind casted with the 1910 climate, and projected to 2050. All model outputs were global.
Projections and hind casts were made by running the base model with the covariates from the matching dates. VECMAP estimates the model parameters for the input sample points, stores these and then applies the models to the raster image covariates pixel by pixel. This means that a model can be prepared using the matching covariates, but then applied to covariates from a different time period. For this to be valid, covariates from the different periods must be compatible; it would not be appropriate to use e.g. remotely sensed temperature as contemporary covariates while using temperature based on weather station for future or past estimates.
Models were run with and without human population density as a covariate. As Ae. aegypti is highly anthropophilic, human density can be used as a proxy to the occurrence of suitable breeding sites with artificial watering. Models including human population density are therefore likely to be better predictors of the actual distribution, whilst the models omitting the human population should provide an indication of the climatic envelope limiting the vector's distributions. As the human population is largely concentrated in settlements, the models incorporating populations are likely to be patchy and often with such fine details that cannot easily be distinguished on continental-scale maps. Models without human population as a covariate may therefore be easier to read.
The output distribution models were evaluated using (a) area under the receiver operating characteristic (ROC) curve (AUC) using an online calculator (Eng, 2017), or (b) Cohen's Kappa. For both, a value of 0.8 or above indicates excellent agreement between sample and model. For Kappa, values of 0.4–0.6 indicate ‘moderate’ agreement.
2.3. Model covariates
The covariates used for spatial modelling were those for which we were able to obtain data for past, present and future periods, namely monthly minimum and maximum temperature, total monthly precipitation, monthly minimum relative humidity and human population. These climatic and demographic variables have successfully been used in modelling current and future distributions of Aedes species (Kraemer et al., 2019).
2.4. Climate data
Mean monthly temperature (minimum and maximum), precipitation (total) and relative humidity data at 0.0416667 degree resolution (approx. 5 km) for 2015 and 2050 were taken from the Worldclim or derived projected climate datasets (Kraemer et al., 2019).
Historic climate data for these parameters images were calculated for the years 1910 to 1960. The Climate Research Unit (CRU) at East Anglia have produced historic mean monthly world coverages at 0.5° resolution for these years (Harris et al., 2014). We decided to use the data from WorldClim 1.3 (Hijmans et al., 2005) as a template for pattern scaling the CRU datasets, and we used the data in the form of MetGrid files (Jones, 2019).
We constructed the mean 0.5° grids for rainfall, mean temperature and diurnal temperature range from the MetGrid files and subtracted them from the annual CRU data to create anomaly grids for each year. These anomalies were then interpolated to 2.5 min using a regular grid bicubic interpolation. Note that this was not a spline, as the derivatives were not constrained to equality at the edge of the kernel; however, tests on the data showed that the effect was not noticeable.
The interpolated anomalies added back to the 2.5 min data as tmax = tmean + diurnal range/2 and tmin = tmean - diurnal range/2; although historically mean temperature was calculated by many complicated formulae, the present WMO definition is (tmax+tmin)/2.
Neither MetGrid nor CRU data include relative humidity. The CRU data do include vapour pressure, but MetGrid and WorldClim do not; this means that there is no scaling template. Even more, the CRU images look to lack definition, which is not surprising, as they must have been constructed from sparse data.
The relative humidity images are therefore constructed from the dewpoint as follows:
where vd is the vapour pressure at the dewpoint, and vx is vapour pressure at ambient temperature. The dewpoint can be estimated according to Linacre (Linacre, 1977) from the mean temperature and diurnal range. We used this to derive the relative humidity coverages from the previously constructed tmax and tmin.
2.5. Human population data
Historic population data was taken from the Hyde historical population datasets (Klein Goldewijk, 2017a; Klein Goldewijk et al., 2011), and the future population density from PBL IMAGE datasets (Klein Goldewijk, 2017b), based on the Shared Socio-economic pathway SSP2 median assumption scenarios (van Vuuren et al., 2017).
3. Results
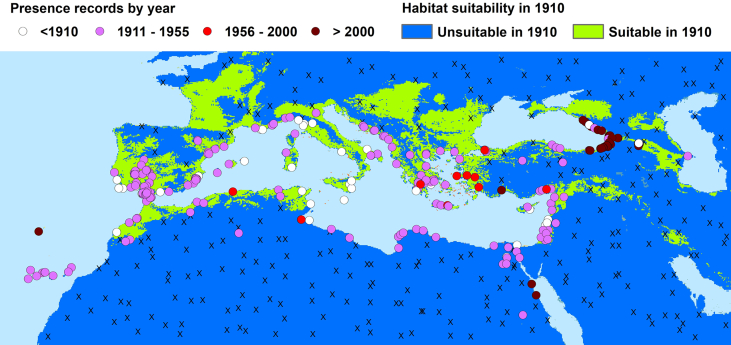
A total of 263 distribution data presence points of Ae. aegypti were gathered for the western Palaearctic region (WPR), distributed over 24 countries and comprising 213 historical and 50 modern data (Table S1, Figs. 1 and S1). They were extracted from 90 documents (containing original data), 3 museum entomological collections, 3 unpublished datasets and 1 personal communication; the detailed dataset with references can be downloaded from Figshare (Schaffner, 2022). The earliest occurrence record dates back to 1839 (Macquart: Canary Islands, as Culex calopus (Christophers, 1929)). The end of the ‘historical’ period is fixed at 1955, when the species was considered as disappeared in almost all countries of the WPR. Occurrences reported posterior to 1955 are considered ‘modern’ data. These included a few sporadic observations reported over the period 1955–2000, and populations established since 2001 which have been observed to breed over more than one season in previously-free areas. Our historical data set used for modelling includes all historical data we had at the time of the modelling work, by April 2021, from 1839 to 1955.
Fig. 1.
Reported occurrences of Aedes aegypti in the western Palaearctic region for different periods, with inferred absences and calculated suitability for the 1910 climate. Dots: presences, according to a period (colour); Crosses: Calculated absences for the historical period (up to 1955); Dots can overlap.
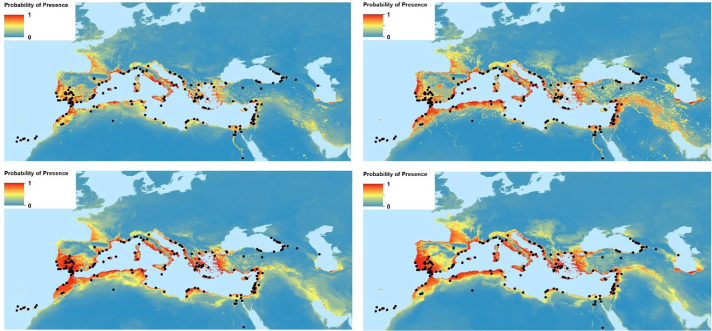
The models of both historical WPR (Fig. 2) and modern USA distributions are statistically very reliable, both with AUC >0.8 and kappa >0.5 without considering human population, and >0.7 including human population (Table 1). This indicates that the modelling process provides an accurate output for both sets of data, and that the parameters used are indeed appropriate to the task for both historical and modern time periods and both WPR and USA geographies.
Fig. 2.
Western Palaearctic region historical 1910 (left) and 2015 (right) modelled suitability for Aedes aegypti with population (top) and without population (bottom) in the covariates. Black dots: recorded historical (up to 1955) presence points.
Table 1.
Accuracy metrics for the range of spatial models run. WPR: western Palaearctic region.
| Vector Location Records | Casta time period | Covariate set | AUC WPR 1910 points | Kappa WPR 1910 points | AUC USA 2015 points | Kappa USA 2015 points |
|---|---|---|---|---|---|---|
| WPR Historical | 1910 | With population | 0.998 | 0.956 | 0.941 | 0.593 |
| WPR Historical | 1910 | Without population | 0.994 | 0.924 | 0.815 | 0.435 |
| WPR Historical | 2015 | With population | 0.971 | 0.862 | 0.980 | 0.805 |
| WPR Historical | 2015 | Without population | 0.956 | 0.772 | 0.814 | 0.481 |
| WPR Historical | 2050 | With population | 0.964 | 0.800 | 0.981 | 0.873 |
| USA 2015 | 1910 | With population | 0.917 | 0.714 | 0.980 | 0.813 |
| USA 2015 | 1910 | Without population | 0.822 | 0.492 | 0.943 | 0.745 |
| USA 2015 | 2015 | With population | 0.936 | 0.741 | 0.999 | 0.965 |
| USA 2015 | 2015 | Without population | 0.828 | 0.512 | 0.996 | 0.745 |
Contemporary (now) cast, hind cast or forecast using covariates from specified time period.
The historical WPR model demonstrates that historically (1910) the region was climatically suitable for the vectors well beyond the sample locations (Fig. 2, left). The suitability projected to 2015 (Fig. 2, right) suggests that the potential distribution of the vector has expanded since 1910, with the warming of the climate (Fig. 2, right bottom) and the increase in human population and warming (Fig. 2, right top). Quite large areas of southwest France, Greece and western Turkey are shown to be suitable in 2015. For 2050 this expansion pattern of suitability is projected to continue (Fig. S2, bottom), further highlighting Turkey and Greece, and the Near East, North Africa, and western France. Climatic variability and extreme events may also affect the vector presence, but they are not reflected by the simple climatic levels used here.
Accuracy metrics (Table 1) also suggest that the extrapolated historical WPR model provides a reasonable to good fit to the current 2015 USA distributions (AUC >0.8, Kappa >0.44), and is also substantially better with population in the covariate suite than without. Perhaps somewhat less expected is that the USA model's prediction for historical WPR also appears to work reasonably well. The agreement between the hindcast and spatially extrapolated contemporary USA data and the historical WPR samples points is also close (Table 1, rows 7–10, column 4, AUC >0.8, with and without population in covariates).
4. Discussion
These results show that the available historical climatic and demographic correlates can be used to successfully produce spatial models of historical Ae. aegypti distributions. Forward projections of these models suggest that areas with conditions suitable for the vector to survive and prosper have expanded, and they will continue to expand in the future at least as far as 2050. Models built using historical WPR distribution data can be reliably extrapolated to predict current vector presence in the USA, and models built using current USA vector distribution data extrapolate well to predict the historical distribution in the WPR. These results suggests that the approach of Rogers and Hay (European Centre for Disease Prevention and Control, 2012), who inferred the suitability of European habitats to Aedes vectors based largely on vector samples from outside Europe, was reasonable. When projected to 2050, the predicted WPR suitability for the vector (Table 1 and Fig. S2) is shown to expand further, and it does not suggest that the future environment is any less suitable for the vector than it is now. This concurs to some extent with the findings of other authors who have projected the Aedes distributions into the future (Kraemer et al., 2019, Trájer, 2021 #457; Liu-Helmersson et al., 2019).
We are left wondering why Ae. aegypti has not filled this large niche predicted suitable by the models in the WPR for the current climate, whereas it continues to spread in the parts of the USA which, as we have shown, has similar habitat suitability. Since the 1950's, the only records of present-day established populations in the WPR are in Madeira, along the eastern Black Sea coast and in southern Egypt, while introductions occurred at Points of Entry. With regards to the huge surveillance effort implemented while targeting the invasive species Ae. (Stegomyia) albopictus (Skuse, 1894), the distribution of Ae. aegypti cannot be considered underestimated in the WPR (Wint et al., 2022, In prep.).
There are a number of potential explanations why the vector has failed to re-establish in continental western and southern Europe as discussed below:
-
a)
The models based on European historical distributions overestimate suitability. Yet the suitability inferred by models trained with very distinct North-American and European distributions are similar which suggests that the models are realistic.
-
b)
Conditions at the reported Points of Entry are unsuitable or introductions occur at the wrong time of the year. Whilst this might be the case, there likely were plenty of potential introductions in other suitable areas that have not specifically been monitored, and still did not yet result in the establishment of populations in the surrounding areas. Models suggest ports of southern Europe (e.g. Algeciras and Barcelona, Spain) to be suitable for Ae. aegypti establishment, high local densities and initial dispersal (Da Re et al., 2021; Trájer, 2021).
-
c)
The more-or-less universal presence of piped water in Europe reduces the availability of larval breeding sites. However, the USA has a similar omni-presence of piped water, and this does not prevent the mosquito from thriving. Furthermore, relationships between socioeconomic factors and the distribution and abundance of Ae. aegypti in mainland USA is found to be inconsistent (Holeva-Eklund et al., 2021). This also weakens the hypothesis that the development of piped water distributions was a major contributor to the disappearance of Ae. aegypti from Europe.
-
d)
There is sufficient mosquito control (this includes control of urban mosquito species by public agencies, by pest control companies or by citizen indoor spraying) at Points of Entry and in urbanised areas in Europe to prevent the establishment of Ae. aegypti after introduction. However, the establishment of other container-breeding invasive species (e.g. Ae. albopictus) throughout Europe could not be prevented. Most mosquito control efforts in Europe focus on floodplains rather than habitats suitable for Ae. aegypti. Furthermore, mosquito abatement programmes in the USA are arguably more extensive than in Europe and have been in place since the early 1900's, yet the vector continues to persist.
-
e)
There is interspecific competition between Ae. aegypti and other mosquito species, particularly Ae. albopictus and perhaps Culex (Culex) pipiens Linnaeus, 1758. Though there are suggestions that the two Aedes container-breeding species do compete in terms of larval habitat colonisation and that asymmetric satyrisation of Ae. aegypti females by Ae. albopictus males may prevent establishment or result in a displacement of Ae. aegypti, it is also suggested that locally variable climate-driven mortality of Ae. albopictus eggs and rapid evolution for resistance to cross-mating may mitigate a competitive superiority (Burford Reiskind et al., 2018; Lounibos et al., 2010). As observed, Ae. albopictus would not dominate under all conditions and thus not fully displace Ae. aegypti. Further, these two species do coexist in the USA and at other places such as Brazil (Braks et al., 2003; Lounibos et al., 2016) and the range of Ae. aegypti continues to change, with ongoing re-establishment in southern USA and even expansion in other counties (Hahn et al., 2017; Monaghan et al., 2019). Finally, given that none of the other known invasive species, in particular Ae. albopictus, appeared before the mid-1970's and none occupied the predicted areas of suitability until decades later, we cannot expect any impact of other invasive species on the re-establishment of Ae. aegypti until very recently. This leaves plenty of time for some re-establishment to have occurred in the Mediterranean Europe before Ae. albopictus spread there.
-
f)
Aedes aegypti with a potential to invade and establish in Europe is phenotypically different from those established elsewhere. This seems unlikely as the invasive form of this vector was shown to be a single lineage (Brown et al., 2011; Powell and Tabachnick, 2013) which has spread globally, continues to invade in the USA where the habitat suitability is comparable to Europe. Also, there are substantial trade and travel movements from the USA to Europe, and the mosquito population which was historically widespread in Europe disappeared.
-
g)
There are not sufficient suitable breeding sites in Europe. This can be discounted as the environmental habitats are shown to be suitable, and other container breeding species are doing well in Europe.
-
h)
Even if successfully introduced, Ae. aegypti cannot spread. Given the rapid spread of Ae. albopictus in southern Europe, this seems unlikely. However, at more northern locations, e.g. Germany, Ae. aegypti is certainly unable to establish while Ae. albopictus is present thanks to its diapausing eggs that are adapted to more temperate climate (Lounibos et al., 2003). At such northern locations, e.g. Saint-Nazaire, France, or Swansea, UK, historical vector presence and yellow fever outbreaks were solely reported aboard ships or within a port city. Both vector populations and pathogen transmission did not persist to the next year (Aeger, 1902). This suggests that failure to overwinter may have prevented spread. The climate has, however, become more suitable for the mosquito since then, and although the winter temperature constraint still applies to the northern latitudes it is less but less of a constraint in the warmer Mediterranean region.
-
i)
During the modern period, Ae. aegypti has not been imported into Europe often enough or in sufficient numbers to establish. Propagule pressure has been considered to have favoured the mosquito's invasion success (Lounibos, 2002). However, this seems unlikely as it has not applied to the other invasive species and there is considerable trade and travel to Europe from countries that harbour Ae. aegypti (including the USA). Further, the vector has been recorded at EU Points of Entry, and has established itself in Madeira after a single founder introduction event via a modern ship or aircraft (Seixas et al., 2019). Models suggest that a small quantity of eggs (10−1000) have the potential to cause establishment (Da Re et al., 2021).
-
j)
Though there are substantial cargo and travel movements, they are of the wrong type to carry enough Ae. aegypti to establish new populations at points of introduction. A decrease of mosquito presence on ships was reported with the change from wood to iron hulls, which led to the reduction, if not disappearance of water in the ship hold (in particular the bilge water) (Chantemesse and Borel, 1905; Theobald, 1911). Several publications, however, point to the fact that modern marine cargo shipments are relatively efficient at spreading Ae. aegypti (Fonzi et al., 2015) and they obviously survive intercontinental flights (Ibáñez-Justicia et al., 2020). Indeed, recent genomic data from Indo-Pacific suggest the incursion pathways of Ae. aegypti into Australia were mostly linked to aerial routes from tourism hotspots (Schmidt et al., 2020).
-
k)
A combination of any or all of the above prevented the vector re-establishing in continental western and southern Europe.
5. Conclusions
In summary, most of the putative reasons why Ae. aegypti has not re-emerged in Europe appear to be contradicted by the evidence of it thriving in the USA in broadly similar climatic and economic conditions. The vector distributions in the US are likely to have been affected by control efforts and land use changes, and to some degree by interaction with other vectors (e.g. Ae. albopictus), but this has not resulted in the widespread and complete disappearance that occurred in Europe. Was it really development of piped water supply systems and vector control that eliminated Ae. aegypti in Europe in the 1950's? What has prevented the Ae. aegypti adapted to USA conditions from establishing in Europe? Perhaps the most likely single reason is that the vector requires significant numbers to establish, and these are only feasible from ships carrying large numbers of people, with sufficiently humid conditions, i.e. with standing water bodies allowing reproduction during the weeks of travel, and with enough containers to sustain large populations. Relatively few of these exist today in the era of air-conditioned cabins, watertight freight containers, and improved sanitation, and thus the number of opportunities for introductions may, as yet, have been insufficient for a population to establish.
In the authors' opinion, it remains largely unanswered why Ae. aegypti has not re-colonised southern Europe despite suitable conditions as revealed by our models and it seems likely it is a combination of all the factors mentioned in our discussion rather that any single reason. It behoves the scientific community to delve further into this question as it may uncover limiting factors that can be used to mitigate the spread of the yellow fever mosquito in a future with increasing global trade and expanding global presence of the vector providing more chances of establishment, and with warmer winters and more intense precipitation providing more suitable larval habitats.
CRediT authorship contribution statement
Neil Alexander: Validation, Formal analysis, Investigation, Visualisation. Peter Jones: Validation, Data curation, Writing – review & editing. Moritz Kraemer: Validation, Data curation, Writing – review & editing. Francis Schaffner: Conceptualisation, Methodology, Data acquisition and curation, Writing – original draft, Visualisation. William Wint: Conceptualisation, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Visualisation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Bettina Menne from the WHO Regional Office for Europe, Climate Change and Sustainable Development Program, and the German Federal Ministry for Environment, Nuclear Safety and Nature Protection who funded the collection of historical distribution data. Finalisation of the study was possible thanks to the VectorNet project, under specific contracts of EFSA (EFSA SC1) and ECDC (SC1 ECD.9918 And SC2 ECD.10468) implementing framework contract No ECDC/2019/020. VectorNet also provided access to the modern distribution dataset, which is assembled thanks to the contributions of VectorNet experts. The Swiss Federal Food Safety and Veterinary Office is highly acknowledged for support of the National Centre for Vector Entomology. We finally thank colleagues for their critical review and contribution to the discussion: Marieta Braks, Olivier Briet, Alexander Mathis, Jeffrey Powell, and Vincent Robert.
Editor: Scott Sheridan
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2022.157566.
Contributor Information
William Wint, Email: william.wint@zoo.ox.ac.uk.
Peter Jones, Email: p.jones@cgiar.org.
Moritz Kraemer, Email: moritz.kraemer@zoo.ox.ac.uk.
Neil Alexander, Email: neil.alexander@zoo.ox.ac.uk.
Francis Schaffner, Email: francis.schaffner@uzh.ch.
Appendix A. Supplementary data
Supplementary material: Table S1, Figs. S1–S2
References
- Abozeid S., Elsayed A.K., Schaffner F., Samy A.M. Re-emergence of Aedes aegypti in Egypt. Lancet Infect. Dis. 2018;18:142–143. doi: 10.1016/S1473-3099(18)30018-5. [DOI] [PubMed] [Google Scholar]
- Aeger J.M. Yellow fever in France, Italy, Great Britain, and Austria, and bibliography of yellow fever in Europe. Bull. Yellow Fever Inst., US. 1902;8:25–35. [Google Scholar]
- Aïssaoui L., Boudjelida H. Diversity and distribution of culicinae fauna in Tebessa district (North-east of Algeria) Int. J. Mosq. Res. 2017;4:7–12. [Google Scholar]
- Akiner M.M., Demirci B., Babuadze G., Robert V., Schaffner F. Spread of the invasive mosquitoes Aedes aegypti and Aedes albopictus in the Black Sea region increases risk of chikungunya, dengue, and Zika outbreaks in Europe. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida A.P.G., Goncalves Y.M., Novo M.T., Sousa C.A., Melim M., Gracio A.J.S. Vector monitoring of Aedes aegypti in the autonomous region of Madeira, Portugal. Euro Surveill. 2007;12(E071115):6. doi: 10.2807/esw.12.46.03311-en. [DOI] [PubMed] [Google Scholar]
- Blanc G., Caminopetros J. Comment les fait épidémiologiques, en Grèce, montrent le rôle exclusif joué par le Stegomyia fasciata (Aedes aegypti) dans la transmission de la dengue. Arch. Inst. Pasteur Hell. 1930;2:277–294. [Google Scholar]
- Blanchard R. Le danger du paludisme et de la fièvre jaune en France; moyens de l'éviter. Bull. Acad. Med. 1917;77:657–669. [Google Scholar]
- Braks. M., Honório, N.A., Lourenço-De-Oliveira, R., Juliano, S.A., Lounibos, L.P., 2003. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Southeastern Brazil and Florida. J Med Entomol. 40, 785-794. doi:10.2807/esw.12.46.03311-en 10.1603/0022-2585-40.6.785. [DOI] [PubMed]
- Braks M., van der Giessen J., Kretzschmar M., van Pelt W., Scholte E.-J., Reusken C., Zeller H., van Bortel W., Sprong H. Towards an integrated approach in surveillance of vector-borne diseases in Europe. Parasit. Vectors. 2011;4:192. doi: 10.1186/1756-3305-4-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braks M., Schaffner F., Medlock J.M., Berriatúa E., Balenghien T., Mihalca A.D., Hendrickx G., Marsboom C., Van Bortel W., Smallegange R.C., Sprong H., Gossner C.M., Czwienczek E., Dhollander S., Briët O., Wint W. VectorNet: putting vectors on the map. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.809763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.E., McBride C.S., Johnson P., Ritchie S., Paupy C., Bossin H., Lutomiah J., Fernandez-Salas I., Ponlawat A., Cornel A.J., Black W.C., Gorrochotegui-Escalante N., Urdaneta-Marquez L., Sylla M., Slotman M., Murray K.O., Walker C., Powell J.R. Worldwide patterns of genetic differentiation imply multiple ‘domestications’ of Aedes aegypti, a major vector of human diseases. Proc. R. Soc. Lond. B Biol. Sci. 2011;278:2446–2454. doi: 10.1098/rspb.2010.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burford Reiskind M.O., Labadie P., Bargielowski I., Lounibos L.P., Reiskindm M.H. Rapid evolution and the genomic consequences of selection against interspecific mating. Mol. Ecol. 2018;27:3641–3654. doi: 10.1111/mec.14821. [DOI] [PubMed] [Google Scholar]
- Caminade C., Medlock J.M., Ducheyne E., McIntyre K.M., Leach S., Baylis M., Morse A.P. Suitability of european climate for the asian tiger mosquito Aedes albopictus: recent trends and future scenarios. J. R. Soc. Interface. 2012;9:2708–2717. doi: 10.1098/rsif.2012.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantemesse A., Borel F. Moustiques et fièvre jaune. Les Actual. Méd. 1905:5–95. [Google Scholar]
- Christophers S.R. Note on a collection of Anopheline and Culicine mosquitoes from Madeira and the Canary Islands. Indian J. Med. Res. 1929;17:518–529. [Google Scholar]
- Christophers S.R. Cambridge University Press; Cambridge: 1960. Aedes aegypti (L.) - the yellow fever mosquito. Its Life History, Bionomics and Structure. [Google Scholar]
- Da Re D., Montecino-Latorre D., Vanwambeke S.O., Marcantonio M. Will the yellow fever mosquito colonise Europe? Assessing the re-introduction of Aedes aegypti using a process-based population dynamical model. Ecol. Inform. 2021;61 doi: 10.1016/j.ecoinf.2020.101180. [DOI] [Google Scholar]
- Dahchar Z., Oudainia W., Bendali-Saoudi F., Soltani N. Inventory of culicidae of the wetland (of the west region of Annaba) J. Entomol. Zool. Stud. 2017;5:430–436. [Google Scholar]
- Eng J. Johns Hopkins University School of Medicine; Baltimore: 2017. ROC Analysis - Web-based Calculator for ROC Curves. [Google Scholar]
- European Centre for Disease Prevention and Control . ECDC; Stockholm: 2009. Development of Aedes albopictus risk maps. Technical Report. [Google Scholar]
- European Centre for Disease Prevention and Control . ECDC; Stockholm: 2012. The climatic suitability for dengue transmission in continental Europe. Technical Report. [Google Scholar]
- Fonzi E., Higa Y., Bertuso A.G., Futami K., Minakawa N. Human-mediated marine dispersal influences the population structure of Aedes aegypti in the Philippine archipelago. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganushkina L., Lukashev A., Patraman I., Razumeyko V., Shaikevich E. Detection of the invasive mosquito species Aedes (Stegomyia) aegypti and Aedes (Hulecoeteomyia) koreicus on the southern coast of the Crimean peninsula. J. Arthropod. Borne Dis. 2020;14:270–276. doi: 10.18502/jad.v14i3.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil Collado J. Datos actuales sobre la distribución geographica de los culicidos españoles. EOS - Rev. Esp. Entomol. 1930;6:329–347. [Google Scholar]
- Hahn M.B., Eisen L., McAllister J., Savage H.M., Mutebi J.-P., Eisen R.J. Updated reported distribution of Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in the United States, 1995–2016. J. Med. Entomol. 2017;54:1420–1424. doi: 10.1093/jme/tjx088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S.B. Dengue in the americas and Southeast Asia: do they differ? Rev. Panam. Salud Publica. 2006;20:407–415. doi: 10.1590/s1020-49892006001100007. [DOI] [PubMed] [Google Scholar]
- Harris I., Jones P.D., Osborn T.J., Lister D.H. Updated high-resolution grids of monthly climatic observations – the CRU TS3.10 dataset. Int. J. Climatol. 2014;34:623–642. doi: 10.1002/joc.3711. [DOI] [Google Scholar]
- Hijmans R.J., Cameron S.E., Parra J.L., Jones P.G., Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005;25:1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- Hoffmann J.M. The history of yellow fever and dengue in Europe. Ned. Tijdschr. Geneeskd. 1931;75:5384–5390. [In Dutch.] [Google Scholar]
- Holeva-Eklund W.M., Behrens T.K., Hepp C.M. Systematic review: the impact of socioeconomic factors on Aedes aegypti mosquito distribution in the mainland United States. Rev. Environ. Health. 2021;36:63–75. doi: 10.1515/reveh-2020-0028. [DOI] [PubMed] [Google Scholar]
- Holstein M. Dynamics of Aedes aegypti distribution, density and seasonal prevalence in the Mediterranean area. Bull. World Health Organ. 1967;36:541–543. [PMC free article] [PubMed] [Google Scholar]
- Ibáñez-Justicia A., Smitz N., den Hartog W., van de Vossenberg B., De Wolf K., Deblauwe I., Van Bortel W., Jacobs F., Vaux A.G.C., Medlock J.M., Stroo A. Detection of exotic mosquito species (Diptera: Culicidae) at international airports in Europe. Int. J. Environ. Res. Public Health. 2020;17:3450. doi: 10.3390/ijerph17103450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irfan J., Vogel R. Stechmückenfangplätze in Anatolien, 1926. Abhandlungen aus dem Gebiet der Auslandskunde, Band 26 - Reihe D. Medizin und Veterinärmedizin, Band 2. Vol. 26. 1927. pp. 286–292. [Google Scholar]
- Jansen C.C., Beebe N.W. The dengue vector Aedes aegypti: what comes next. Microbes Infect. 2010;12:272–279. doi: 10.1016/j.micinf.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Jeannin C., Perrin Y., Cornelie S., Ferreira O., Firmin Y., Garcia F., Gauchet J.D., Tounsi R., Lagneau C. IXth International EMCA Conference. Abstract Book. EMCA, La Rochelle, France. 2019. Surveillance and control of mosquitoes in the French Points of Entry under the International Health Regulation; p. 43. [Google Scholar]
- Jones P.G. Waen Associates Ltd; Dolgellau, Wales: 2019. Documentation for release of MarkSim Base Data from WorldClim 2.0. [Google Scholar]
- Kampen H., Jansen S., Schmidt-Chanasit J., Walther D. Indoor development of Aedes aegypti in Germany, 2016. Euro Surveill. 2016;21:30407. doi: 10.2807/1560-7917.ES.2016.21.47.30407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney M., Porter W.P., Williams C., Ritchie S., Hoffmann A.A. Integrating biophysical models and evolutionary theory to predict climatic impacts on species’ ranges: the dengue mosquito Aedes aegypti in Australia. Funct. Ecol. 2009;23:528–538. doi: 10.1111/j.1365-2435.2008.01538.x. [DOI] [Google Scholar]
- Klein Goldewijk K. Utrecht University; Utrecht: 2017. Anthropogenic Land-use Estimates for the Holocene; HYDE 3.2. DANS. [dataset] [Google Scholar]
- Klein Goldewijk K. Royal Netherlands Meteorological Institute; De Bilt: 2017. Pbl Image ssp Scenarios - Population Density (5 arcmin). Services KD. [dataset] [Google Scholar]
- Klein Goldewijk K., Beusen A., van Drecht G., de Vos M. The HYDE 3.1 spatially explicit database of human-induced global land-use change over the past 12,000 years. Glob. Ecol. Biogeogr. 2011;20:73–86. doi: 10.1111/j.1466-8238.2010.00587.x. [DOI] [Google Scholar]
- Knio K.M., Markarian N., Kassis A., Nuwayri-Salti N. A two-year survey on mosquitoes of Lebanon. Parasite. 2005;12:229–235. doi: 10.1051/parasite/2005123229. [DOI] [PubMed] [Google Scholar]
- Kotsakiozi P., Gloria-Soria A., Schaffner F., Robert V., Powell J.R. Aedes aegypti in the Black Sea: recent introduction or ancient remnant? Parasit. Vectors. 2018;11:396. doi: 10.1186/s13071-018-2933-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer M.U.G., Sinka M.E., Duda K.A., Mylne A., Shearer F.M., Brady O.J., Messina J.P., Barker C.M., Moore C.G., Carvalho R.G., Coelho G.E., Van Bortel W., Hendrickx G., Schaffner F., Wint G.R.W., Elyazar I.R.F., Teng H.-J., Hay S.I. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci. Data. 2015;2 doi: 10.1038/sdata.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer M.U.G., Sinka M.E., Duda K.A., Mylne A.Q.N., Shearer F.M., Barker C.M., Moore C.G., Carvalho R.G., Coelho G.E., Van Bortel W., Hendrickx G., Schaffner F., Elyazar I.R.F., Teng H.-J., Brady O.J., Messina J.P., Pigott D.M., Scott T.W., Smith D.L., Wint G.R.W., Golding N., Hay S.I. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. elife. 2015;4 doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer M.U.G., Reiner R.C., Brady O.J., Messina J.P., Gilbert M., Pigott D.M., Yi D., Johnson K., Earl L., Marczak L.B., Shirude S., Davis Weaver N., Bisanzio D., Perkins T.A., Lai S., Lu X., Jones P., Coelho G.E., Carvalho R.G., Van Bortel W., Marsboom C., Hendrickx G., Schaffner F., Moore C.G., Nax H.H., Bengtsson L., Wetter E., Tatem A.J., Brownstein J.S., Smith D.L., Lambrechts L., Cauchemez S., Linard C., Faria N.R., Pybus O.G., Scott T.W., Liu Q., Yu H., Wint G.R.W., Hay S.I., Golding N. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019;4:854–863. doi: 10.1038/s41564-019-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Face L., Raffaele G. Sulla presenza della Stegomyia fasciata nell’Italia meridionale e in Sicilia. Il Policlinico. 1928;35:2095. [Google Scholar]
- Linacre E.T. A simple formula for estimating evaporation rates in various climates, using temperature data alone. Agric. Meteorol. 1977;18:409–424. doi: 10.1016/0002-1571(77)90007-3. [DOI] [Google Scholar]
- Liu-Helmersson J., Rocklöv J., Sewe M., Brännström Å. Climate change may enable Aedes aegypti infestation in major european cities by 2100. Environ. Res. 2019;172:693–699. doi: 10.1016/j.envres.2019.02.026. [DOI] [PubMed] [Google Scholar]
- Lounibos L.P. Invasions by insect vectors of human disease. Annu. Rev. Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Lounibos L.P., Escher R.L., Lourenço-De-Oliveira R. Asymmetric evolution of photoperiodic diapause in temperate and tropical invasive populations of Aedes albopictus (Diptera: Culicidae) Ann. Entomol. Soc. Am. 2003;96:512–518. doi: 10.1603/0013-8746(2003)096[0512:aeopdi]2.0.co;2. [DOI] [Google Scholar]
- Lounibos L.P., O'Meara G.F., Juliano S.A., Nishimura N., Escher R.L., Reiskind M.H., Cutwa M., Greene K. Differential survivorship of invasive mosquito species in South Florida cemeteries: do site-specific microclimates explain patterns of coexistence and exclusion? Ann. Entomol. Soc. Am. 2010;103:757–770. doi: 10.1603/an09142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos L.P., Bargielowski I., Carrasquilla M.C., Nishimura N. Coexistence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in peninsular Florida two decades after competitive displacements. J. Med. Entomol. 2016;53:1385–1390. doi: 10.1093/jme/tjw122. [DOI] [PubMed] [Google Scholar]
- Marzinowsky E.I. De l’existence de Stegomyia fasciata (St. calopus) en Russie. Bull. Soc. Pathol. Exot. 1914;7:590–593. [Google Scholar]
- Marzinowsky E.I. Sur la lutte contre la dengue. Bull. Soc. Pathol. Exot. 1930;23:797–803. [Google Scholar]
- Monaghan A.J., Eisen R.J., Eisen L., McAllister J., Savage H.M., Mutebi J.-P., Johansson M.A. Consensus and uncertainty in the geographic range of Aedes aegypti and Aedes albopictus in the contiguous United States: multi-model assessment and synthesis. PLoS Comput. Biol. 2019;15 doi: 10.1371/journal.pcbi.1007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlan H.B., Tinker M.E. Distribution of Aedes aegypti infestations in the United States. Am. J. Trop. Med. Hyg. 1965;14:892–899. doi: 10.4269/ajtmh.1965.14.892. [DOI] [PubMed] [Google Scholar]
- Parker C., Ramirez D., Connelly C.R. State-wide survey of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Florida. J. Vector Ecol. 2019;44:210–215. doi: 10.1111/jvec.12351. [DOI] [PubMed] [Google Scholar]
- Powell J.R., Tabachnick W.J. History of domestication and spread of Aedes aegypti - a review. Mem. Inst. Oswaldo Cruz. 2013;108:11–17. doi: 10.1590/0074-0276130395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J.R., Gloria-Soria A., Kotsakiozi P. Recent history of Aedes aegypti: vector genomics and epidemiology records. Bioscience. 2018;68:854–860. doi: 10.1093/biosci/biy119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D.J., Wilson A.J., Hay S.I., Graham A.J. In: Advances in Parasitology, Vol 62: Global Mapping of Infectious Diseases: Methods, Examples and Emerging Applications. Hay S.I., Graham A., Rogers D.J., editors. 2006. The global distribution of yellow fever and dengue; pp. 181–220. [DOI] [PubMed] [Google Scholar]
- Sarmento M., França C. Sur quelques culicides portugais. C R Hebdo Séances Mém. Soc. Biol. 1902;54:152–153. [Google Scholar]
- Schaffner F. Historical and modern distribution data of the yellow fever mosquito Aedes aegypti in the western Palaearctic region. Figshare. 2022 doi: 10.6084/m9.figshare.20343570. [Dataset] [DOI] [Google Scholar]
- Schaffner F., Mathis A. Dengue and dengue vectors in the WHO European region: past, present, and scenarios for the future. Lancet Infect. Dis. 2014;14:1271–1280. doi: 10.1016/S1473-3099(14)70834-5. [DOI] [PubMed] [Google Scholar]
- Schaffner F., Versteirt V., Van Bortel W., Zeller H., Wint W., Alexander N.S. VBORNET gap analysis: mosquito vector distribution models utilised to identify areas of potential species distribution in areas lacking records. Open Health Data. 2016;4 doi: 10.5334/ohd.27. [DOI] [Google Scholar]
- Schliessman D.J., Calheiro L.B. A review of the status of yellow fever and Aedes aegypti eradication programs in the Americas. Mosq. News. 1974;34:1–9. [Google Scholar]
- Schmidt T.L., Chung J., van Rooyen A.R., Sly A., Weeks A.R., Hoffmann A.A. Incursion pathways of the asian tiger mosquito (Aedes albopictus) into Australia contrast sharply with those of the yellow fever mosquito (Aedes aegypti) Pest Manag. Sci. 2020;76:4202–4209. doi: 10.1002/ps.5977. [DOI] [PubMed] [Google Scholar]
- Scholte E.J., Den Hartog W., Dik M., Schoelitsz B., Brooks M., Schaffner F., Foussadier R., Braks M., Beeuwkes J. Introduction and control of three invasive mosquito species in the Netherlands. Euro Surveill. 2010;15 pii=19710. [PubMed] [Google Scholar]
- Sedda L., Morley D.W., Braks M.A.H., De Simone L., Benz D., Rogers D.J. Risk assessment of vector-borne diseases for public health governance. Public Health. 2014;128:1049–1058. doi: 10.1016/j.puhe.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Seixas G., Salgueiro P., Bronzato-Badial A., Gonçalves Y., Reyes-Lugo M., Gordicho V., Ribolla P., Viveiros B., Silva A.C., Pinto J., Sousa C.A. Origin and expansion of the mosquito Aedes aegypti in Madeira Island (Portugal) Sci. Rep. 2019;9:2241. doi: 10.1038/s41598-018-38373-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theobald F.V. 1er Congrès International d’entomologie. Vol. 2. August 1910; Bruxelles: 1911. The distribution of the yellow fever mosquito (Stegomyia fasciata Fabricius) and general notes on its bionomics; pp. 145–170. [Google Scholar]
- Trájer A.J. Aedes aegypti in the Mediterranean container ports at the time of climate change: a time bomb on the mosquito vector map of Europe. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e07981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vuuren D.P., Stehfest E., Gernaat D.E.H.J., Doelman J.C., van den Berg M., Harmsen M., de Boer H.S., Bouwman L.F., Daioglou V., Edelenbosch O.Y., Girod B., Kram T., Lassaletta L., Lucas P.L., van Meijl H., Müller C., van Ruijven B.J., van der Sluis S., Tabeau A. Energy, land-use and greenhouse gas emissions trajectories under a green growth paradigm. Glob. Environ. Chang. 2017;42:237–250. doi: 10.1016/j.gloenvcha.2016.05.008. [DOI] [Google Scholar]
- Wint W., Petric D., Van Bortel W., Alexander N., Schaffner F. RVF vector spatial distribution models: vector abundance. EFSA Support Publ. 2020;17 doi: 10.2903/sp.efsa.2020.EN-1847. [DOI] [Google Scholar]
- Wint W., Van Bortel W., Schaffner F. RVF vector spatial distribution models: probability of presence. EFSA Support Publ. 2020;17 doi: 10.2903/sp.efsa.2020.EN-1800. [DOI] [Google Scholar]
- Wint W.G.R., Balenghien T., Berriatua E., Braks M., Marsboom C., Medlock J., Schaffner F., Van Bortel W., Alexander N., Alten B., Czweinczeck E., Dhollander S., Ducheyne E., Gossner C.M., Hansford K., Hendrickx G., Honrubia H., Matheussen T., Mihalca A., Richardson J., Sprong H., Versteirt V., Briet O. VectorNet: collaborative mapping of standardised distributions and surveillance for arthropod disease vectors in Europe and neighbouring countries. Euro Surveill. 2022;2022 doi: 10.2807/1560-7917.ES.2023.28.26.2200666. In preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunicheva Y.U., Ryabova T.E., Markovich N.Y., Bezzhonova O.V., Ganushkina L.A., Semenov V.B., Tarkhov G.A., Vasilenko L.E., Guzeeva T.M., Shevereva T.V., Sergiev V.P. First data on the presence of breeding populations of the Aedes aegypti L. mosquito in Greater Sochi and various cities of Abkhazia. Med. Parazitol. (Mosk) 2008;40:43. [In Russian.] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material: Table S1, Figs. S1–S2