Abstract
Purpose:
The purpose of this integrative review was to establish the role of cardiac rhythm analysis (electrocardiogram; EKG) and echocardiogram in increasing clinical suspicion for and earlier diagnosis of cardiac amyloidosis.
Methods:
A literature review was conducted using PubMed and Scopus databases. Dates searched were from January 2017 to May 2021. Inclusion criteria included a diagnosis of cardiac amyloidosis, use of EKG, and echocardiogram participants 18 years and older. Articles were excluded if they were duplicates, had an irrelevant title, or were incomplete.
Results:
Results indicated neither EKG nor echocardiogram alone or in combination are sufficient for diagnosing cardiac amyloidosis. There is, however, a combination of findings that could potentially prove useful in “ruling in” cardiac amyloidosis and prompt further evaluation. Predominant findings in cardiac amyloidosis cohorts found on EKG showed low-voltage QRS complexes, a pseudo-infarct pattern in precordial leads, and an absence of left ventricular hypertrophy on EKG. There is no single echocardiogram finding specific to cardiac amyloidosis. Patients will generally present with thickened ventricular walls, and nearly all patients will display a preserved left ventricular ejection fraction until later stages of disease. Strain imaging, either via 2D or 3D transthoracic echocardiogram, is more useful in screening for or detecting cardiac amyloidosis and should be utilized in this instance. Findings in cardiac amyloidosis include decreased global longitudinal strain and relative apical sparing.
Conclusion:
Overall, EKG and echocardiogram are effective, feasible, and practical tools to increase clinical suspicion for cardiac amyloidosis for the purposes of early recognition and evaluation. These are useful only to “rule in” a diagnosis. Future studies are needed to validate these findings.
Amyloidosis refers to a family of diseases characterized by extracellular amyloid fibril deposition. Amyloid deposits result in significant dysfunction within the affected organs and soft tissues. The subtypes of amyloidosis most frequently encountered in clinical practice include light chain (AL), transthyretin mutated (ATTRm), and transthyretin wild-type (ATTRwt), previously referred to as senile amyloidosis (Stern & Kittleson, 2021).
Immunoglobulin light chain (AL) amyloidosis is the most common of these disorders. AL amyloidosis occurs secondary to the deposition of protein derived from immunoglobulin light chain fragments. AL amyloidosis deposits may affect any organ, and often involve the heart, kidney, gastrointestinal tissues, nerves, and soft tissue. Males and females are affected equally. It most commonly occurs in those between the ages of 40 to 80 (Gertz & Dispenzieri, 2020). Cardiac infiltration and staging remain the greatest prognostic indicators in AL amyloidosis (Milani et al., 2018)
ATTR amyloidosis includes two main subtypes: ATTRwt and ATTRm. In the case of ATTRwt, the transthyretin protein is non-mutated and gradually deposits amyloid proteins over a period of decades. Small protein deposits occur in soft tissues, which may lead to vascular issues and carpal tunnel syndrome. However, the primary pathologic feature of ATTRwt are the amyloid deposits that occur in the cardiac tissues. Prevalence is higher in males, and the most commonly affected age group is individuals between 65 and 95 years. ATTRm is the result of a transthyretin gene mutation that leads to more rapid deposition of amyloid protein, with certain mutations leading to corresponding patterns of organ involvement (Gertz & Dispenzieri, 2020).
The greatest morbidity and mortality occur in cardiac amyloidosis (CA), which is a progressive disorder and an often underdiagnosed cause of heart failure (Boldrini et al., 2020). Twenty-five percent of people with cardiac involvement of AL and ATTR die between 6 to 24 months of diagnosis, respectively (Gertz & Dispenzieri, 2020). Amyloid disorders frequently go undiagnosed and therefore are far more common than previously presumed (Grogan et al., 2017). The true global incidence of cardiac amyloidosis remains unknown, as there is a lack of published epidemiologic data, and the frequency differs between subtypes. A review by Ruberg and colleagues (2019) reports 10% to 15% of older adults had heart failure posthumously attributed to unrecognized amyloidosis. Another study revealed the mortality rate of amyloidosis within the United States has doubled over the past 30 years (Alexander et al., 2018).
New targeted therapies for AL and ATTR amyloidosis have heightened the clinical importance of early recognition (Zhang et al., 2021). Targeted therapies are most effective when implemented in the early disease stage and poorer outcomes are a result of delayed diagnosis. Despite this revelation, patients will visit up to five specialists and experience a median 21.7-month delay from symptom onset to diagnosis (Bishop et al., 2018). These data illustrate a trend of missed opportunities for diagnoses in both outpatient and inpatient settings (Grogan et al., 2017). This delay in diagnosis has been attributed to the varied and often vague clinical presentation and presumed rarity of the disease.
Accurate detection of CA requires providers to demonstrate increasing clinical suspicion for disease processes that were once considered “rare.” This is exceedingly true considering the implementation of novel treatment therapies. Furthermore, clinicians are too often falsely reassured by absence of data previously considered paramount for diagnosis of CA. A pro–B-type natriuretic peptide (BNP) is a relatively inexpensive and easily obtained cardiac biomarker that can be useful in detecting CA in certain cases. Studies have shown that BNP may be a sensitive, but not specific biomarker for CA (Perfetto et al., 2016). However, traditional cardiac biomarkers do not always correlate to the degree of myocardial infiltration, especially in ATTR, which despite similar myocardial infiltration, presented with significantly lower serum BNP levels (Perfetto et al., 2016). Overall survival in cardiac amyloidosis has been shown to improve with early clinician suspicion and screening for at-risk patients (Bishop et al., 2018).
In recent years, the bulk of research has focused on emerging advanced imaging for accurate detection of CA, such as cardiac MRI, T1 mapping, and scintigraphy studies. While the aforementioned modalities represent an exciting advance in accurate diagnosis, cost and accessibility present considerable limitations. Additionally, these tests are often performed only after a person has been referred to a specialist, which limits their role as a screening method.
The roles of electrocardiogram (EKG) and echocardiogram in the evaluation of CA have increased in recent years as noninvasive, cost-effective methods compared with the traditional diagnostic gold standard of endomyocardial biopsy (Boldrini et al., 2020). Both EKG and echocardiogram are commonly performed, cost effective, and accessible in standard practice. Therefore, to increase clinician awareness, these techniques could prompt clinical suspicion for amyloid disease. This review aims to analyze the role of EKG and echocardiogram in the early detection of CA.
METHODS
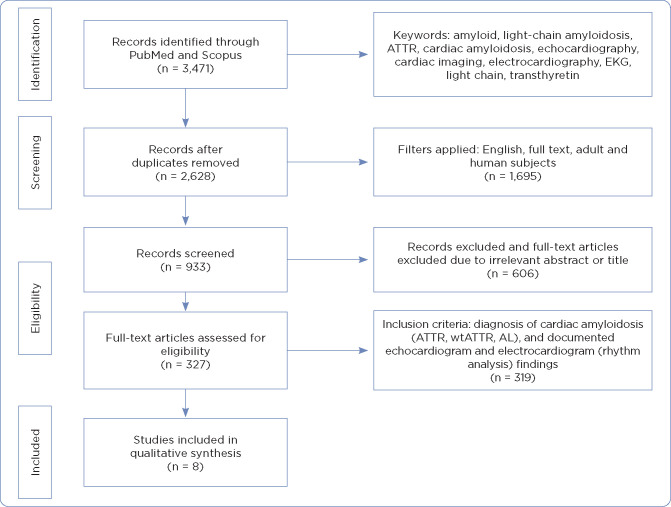
A literature search was conducted using PubMed and Scopus databases. Dates searched were from January 2017 to May 2021. The search included abstracts, titles, and full-text articles. Book chapters, letters, notes, and grey literature were excluded. The search was limited to English-only publications comprising only adult and human subjects. The search resulted in a total of 3,471 articles. After exclusion of duplicates, 2,628 articles were reviewed (Figure 1). Articles were reviewed for the following inclusion criteria: diagnosis of cardiac amyloidosis (ATTR, wtATTR, AL), participants 18 years and older, and documented echocardiogram and EKG (rhythm analysis) findings. Articles were excluded if they were duplicates or subjects were diagnosed with amyloidosis with absence of cardiac involvement. The primary author reviewed the remaining articles, excluding those with irrelevant subjects and titles. Final articles published with most recent relevant data were ultimately selected. A total of eight articles were selected based on inclusion and exclusion criteria.
Figure 1.
PRISMA diagram. ATTR = transthyretin amyloidosis; EKG = electrocardiogram; wATTR = wild-type ATTR amyloidosis; AL = amyloid light chain amyloidosis. Adapted from Moher et al. (2009).
RESULTS
Sample Characteristics
A total of 1,751 subjects were included from the eight selected studies. All studies included cohorts with ATTR or AL amyloidosis confirmed via biopsy or advanced imaging. Three of the studies (Löfbacka et al., 2021; Pagourelias et al., 2017; Boldrini et al., 2020) included additional control groups with diagnoses such as hypertensive and hypertrophic cardiomyopathies and increased septal thickness in an effort to discriminate amyloid-related changes on echocardiogram. Three studies included both ATTR and AL types of CA, while four studies included only AL. Löfbacka and colleagues (2021) was the singular study that was exclusive to ATTR.
Electrocardiogram
Many of the studies (six of eight) provided EKG information, the majority obtained in the 3 months surrounding diagnosis. The hallmark findings of CA on EKG historically have been accepted as increased ventricular wall thickness plus low voltage (Sperry et al., 2016). The studies included in this review evaluated findings that could be useful as screening criteria for CA. The primary findings identified through this review include (1) pseudo-infarct pattern, (2) low-voltage QRS complexes, (3) minimal or absence of left ventricular (LV) hypertrophy as defined by the Sokolow-Lyon criteria, (4) atrial fibrillation, and (5) first-degree atrioventricular (AV) block.
Pseudo-Infarct Pattern. Four of the studies report on pseudo-infarct pattern, with encouraging results in reported in three. In a 2018 Malaysian-based retrospective study by Roslan and colleagues (2018), 13 patients with biopsy-proven AL CA were evaluated. Nearly 70% of their subjects demonstrated a pseudo-infarct pattern. Costache and colleagues (2019) performed a case series in a Romanian Hospital of six patients with a diagnosis of CA, confirmed by either bone scintigraphy or peripheral biopsy, to evaluate the role of both echocardiogram and scintigraphy in evaluating CA. All six subjects underwent evaluation via 12-lead EKG, and pseudo-infarct pattern in precordial leads was identified in all six subjects.
A 2017 retrospective cohort study of 100 patients in Belgium and published by Pagourelias and colleagues focused on echocardiogram parameters for distinguishing CA on echocardiogram but also described EKG changes. The study included three cohorts. Cohort one consisted of 40 patients with endomyocardial biopsy-confirmed CA. Cohorts two and three consisted of 40 patients with hypertrophic cardiomyopathy (HCM) and 20 patients with hypertensive cardiac changes, respectively. EKG findings within cohort one, CA-confirmed patients, included presence of Q waves, indicative of a pseudo-infarct pattern in 45% (18/40) as opposed to 37.5% (15/40) of the HCM cohort and 0% (0/20) of the hypertensive cohort.
In contrast, Zhang and colleagues (2021) conducted a retrospective cohort study in the United States. The study included 242 total subjects consisting of 113 patients with endomyocardial biopsy-confirmed CA compared with a control group (n = 129). EKG was performed on all the subjects within the CA group (n = 113). Of the 113 CA patients, only 27% displayed a pseudo-infarct pattern.
Low-Voltage QRS Complexes. Six of the studies reported on low-voltage QRS complexes. Costache and colleagues (2019) in the Roman case series identified low-voltage QRS complexes in all six subjects. In the Zhang and colleagues (2021) retrospective cohort study EKG of the CA group, low-voltage QRS complexes were found in 52% of limb leads and 21% in precordial leads. Similarly, Pradel and colleagues (2019) reported on a French cohort of 58 patients with 48% demonstrating low-voltage QRS complex on EKG. In this retrospective study, 71 total patients were divided into a control group (n = 21) and a group consisting of patients with confirmed AL amyloidosis (n = 58). EKG criteria for the control group (n = 21) were not available for comparison. Löfbacka and colleagues (2021) performed a Swedish cross-sectional study composed of 58 total subjects (N = 58) divided into cohorts with confirmed ATTR CA (n = 33) and a control group with no evidence of CA (n = 25). In the ATTR population, 43% demonstrated low-voltage QRS complexes as measured by the Sokolow-Lyon criteria on EKG compared with none in the control group.
In contrast, in the Pagourelias and colleagues (2017) cohort, only 7 of 40 patients showed low-voltage QRS complexes. In the 2018 Malaysian-based retrospective study by Roslan and colleagues, only 3 of the 13 patients (23%) had low-voltage QRS complexes.
LV Hypertrophy. LV hypertrophy was evaluated in four of the studies. In the Costache and colleagues (2019) case series, absence of LV hypertrophy was identified on EKG in all six subjects. In contrast, in the Pagourelias and colleagues (2017) study, only 5 of the 40 patients demonstrated LV hypertrophy on EKG. Similarly, Zhang and colleagues (2021) reported only 1% of the subjects with LV hypertrophy, and Roslan and colleagues (2018) reported only 1 of the of the 13 patients demonstrated LV hypertrophy on EKG.
Atrial Fibrillation. Atrial fibrillation was reported in five of the studies, with all reporting low incidence of occurrence. Costache and colleagues (2019) noted atrial fibrillation in 3 of the 6 patients. Zhang and colleagues (2021) reported atrial fibrillation in 24% of subjects. Similarly, Roslan and colleagues (2018) reported 15.4% (2/13), and Pradel and colleagues (2019) reported 14% (8/58). Pagourelias and colleagues (2017) noted only 4 out of 40 CA patients in atrial fibrillation.
AV Block. Only three of the studies reported on AV block as a finding in patients with confirmed CA. In the 2018 study by Roslan and colleagues, almost all the patients (12/13) displayed first- degree AV block. Zhang and colleagues (2021) noted first-degree AV block in 33% of subjects in the CA cohort. In contrast, Pagourelias and colleagues (2019) noted first-degree AV block in only 5 of 40 patients (12.5%).
Summary of EKG Findings. No single EKG finding emerged as a reliable indicator of CA. However, three of the studies (Costache et al., 2019; Pagourelias et al., 2017; Zhang et al., 2021) revealed a combination of EKG findings in the CA population in concurrence with historical parameters that might be useful indicators in screening for CA. For the most part, EKG showed low-voltage QRS complexes, a pseudo-infarct pattern in precordial leads, and an absence of LV hypertrophy on EKG.
Two-Dimensional Echocardiogram
Left Ventricular Ejection Fraction. Transthoracic echocardiogram (TTE) was utilized in all eight studies. Six of the studies focused primarily on two-dimensional (2D) TTE, while two of the studies utilized 3D TTE. The most frequently noted parameter of a standard TTE is the left ventricular ejection fraction (LVEF), which assesses systolic function of the left ventricle. Generally speaking, a low-normal LVEF is greater than 50%, while a normal LVEF is considered greater than 55% (Yancy et al., 2013). Historically, a primary echocardiogram feature of CA is a preserved ejection fraction. LVEF typically stays in low-normal to normal range until later stages of disease (Grogan et al., 2017). All eight of the studies reflected this (Table 1), as all the patients in early- to mid-stage of CA disease maintained a relatively normal LVEF.
Table 1. Evidence Summary.
| Author | Country of origin | Study design | Sample size | ATTR | AL | Findings | Limitations | |
|---|---|---|---|---|---|---|---|---|
| EKG | Echocardiogram | |||||||
| Löfbacka et al. (2021) | Sweden | Cross-sectional | N = 58 | + | – |
|
|
|
| Zhang et al. (2021) | United States | Retrospective cohort | N = 242 | + | + |
|
|
|
| Boldrini et al. (2020) | Multinational | Cross sectional | N = 1,187 | + | + |
|
|
|
| Costache et al. (2019) | Romania | Case series | N = 6 | + | + |
|
|
|
| Roslan et al. (2018) | Malaysia | Retrospective | N = 13 | – | + |
|
|
|
| Lei et al. (2021) | China | Retrospective cohort | N = 74 | – | + |
|
|
|
| Pradel et al. (2019) | France | Prospective cohort | N = 71 | – | + |
|
|
|
| Pagourelias et al. (2017) | Belgium | Retrospective cohort | N = 100 | + | + |
|
|
|
Note. ATTR = transthyretin amyloidosis; AL = amyloid light chain amyloidosis; CAD = coronary artery disease; TTE = transthoracic echocardiogram; RWT = relative wall thickness; GLS = global longitudinal strain; GAS = global area strain; CA = cardiac amyloidosis; EKG = electrocardiogram; LVEF = left ventricular ejection fraction; AF = atrial fibrillation; LVH = left ventricular hypertrophy; + = included in study; – = not included in study.
Ventricular Wall Thickness. Another hallmark TTE finding in CA is thickened ventricular walls. There are numerous ways to calculate and report thickened ventricular walls, with newer calculations having been recently proposed. In a multicenter Italian study, Boldrini and colleagues (2020) established an increased wall thickness (IWT) score to accurately identify CA in patients with cardiomyopathy. The IWT score is comprised of echocardiogram-derived parameters of relative wall thickness (RWT), E-wave to e-wave (E/e’), tricuspid annular plane systolic excursion (TAPSE), longitudinal strain (LS), and systolic apex-to-base ratio (SAB). This cross-sectional study included 1,187 total subjects divided into two cohorts consisting of subjects with systemic AL amyloidosis (n = 494) and increased LV wall thickness (IWT) without presence of CA (n = 978).
Despite employing a variety of scoring methods to calculate wall thickness (RWT, IWT, septal thickness, interventricular septum thickness at end-diastole), all eight of the articles noted that wall thickness is present in CA.
Strain Imaging. Myocardial deformation imaging provides further measurements of cardiac mechanics as they relate specifically to systolic function. Collectively, this is often referred to as strain imaging. In traditional 2D echocardiography, these strain measurements can be obtained via tissue Doppler imaging by pulse wave or reconstructed color strain and strain rate values or non-Doppler–derived via 2D speckle tracking echocardiogram (Fihn et al., 2012).
Another important deformation parameter in relation to CA is the presence of an apical sparing pattern. An apical sparing describes a pattern of opposing strain values within segments of the left ventricle. The presence of an apical sparing pattern in CA cohorts was noted by Löfbacka and colleagues (2021), Pagourelias and colleagues (2017), Boldrini and colleagues (2020), Zhang and colleagues (2021), and Lei and colleagues (2021). In the study conducted by Roslan and colleagues (2018), strain imaging was available for only 7 out of 13 patients, all of which demonstrated apical sparing pattern.
Three-Dimensional Echocardiogram
3D TTE with speckle tracking focuses on mechanics of left ventricle–deriving global strain values from measurements of LV end-systolic and end-diastolic volumes, LV mass, and LVEF. Two of the studies (Pradel et al., 2019; Lei et al. 2021) evaluated the role of 3D echocardiography with speckle tracking. Both studies focused solely on the diagnosis of AL amyloidosis. In a 2021 retrospective cohort study, Lei and colleagues (2021) reviewed and performed strain analysis on 74 patients with confirmed AL amyloidosis. The primary focus of the study was to evaluate 3D speckle tracking echocardiography and conventional 2D echocardiogram in combination with additional markers to aid diagnosis of CA.
Both Pradel and colleagues (2019) and Lei and colleagues (2021) concluded that patients with AL amyloidosis with cardiac involvement demonstrated significantly decreased global longitudinal strain (GLS). This is representative of impaired LV mechanics in CA. According to both studies, 3D TTE with speckle tracking provides an accurate assessment of cardiac function in amyloid disease, proving it useful in determining a diagnosis.
DISCUSSION
Both EKG and echocardiogram are often considered the first step towards a diagnosis of cardiac amyloidosis. Although not definitive or specific, results of both play a role in “ruling in” and considering the diagnosis. In this review, prominent EKG findings in the CA population include presence of a pseudo-infarct pattern, low-voltage QRS complexes, prolonged PR interval or first-degree AV block (type I) pattern, and the absence of LV hypertrophy per Sokolow-Lyon criteria on EKG. Atrial fibrillation or flutter were common rhythm disturbances noted.
Traditional echocardiogram findings include normal systolic function as evident by preserved LVEF until later stages of disease. The presence of thickened walls should increase the suspicion of CA despite a lack of other clinical findings. However, this finding alone is not specific for CA and is sensitive only when combined with EKG results. The combination of thickened ventricular walls and low-voltage QRS complexes is particularly useful when considering CA. Only one of the included studies (Löfbacka et al., 2021) challenged the classically taught “mismatch” of findings on echocardiogram with LV wall thickness combined with low-voltage QRS, as only 23% (n = 3) of patients displayed this pattern. However, this is the singular study that focused only on ATTR amyloidosis. It has been noted that the incidence of these findings is markedly decreased in ATTR compared with AL.
When available, strain imaging should be conducted, as it is particularly useful in assessing for CA. The primary clinical application for strain imaging is to evaluate LV function, most commonly via GLS (normal GLS is > 20%). Global longitudinal strain, that is, strain imaging, is particularly useful in recognizing clinical or subclinical LV dysfunction otherwise not detected in standard 2D echocardiogram. Findings of this review suggest that decreased GLS should prompt suspicion of cardiac amyloidosis.
Another deformation pattern commonly encountered in CA is the presence of apical sparing. Notably, all the patients included in the review by Roslan and colleagues (2018) were found to have apical sparing. This suggests apical sparing pattern is highly specific for CA, and strain imaging should be utilized when available. According to Pradel and colleagues (2019), in 2D echocardiogram, speckle tracking is arguably more useful in identifying LV dysfunction over tissue Doppler imaging. Limitations to GLS include interobserver and machine variability, image quality, and individual analysis competency.
Pradel and colleagues (2019) also noted 3D imaging revealed notable changes in patients with intermediate disease that were not discovered via conventional 2D TTE. Specifically, subtle changes to LV ejection fraction were more evident via 3D LV function assessment. 3D TTE is also more reproducible compared with 2D TTE, as it utilizes automatic wall motion tracking software (Pradel et al., 2019). Unfortunately, although accurate, 3D speckle tracking is more costly and specialized and is therefore less accessible.
Limitations
Limitations to this review include the lack of randomized control trials. The studies included are limited to retrospective and prospective studies, and case reviews. The sample size is another limitation, as five of the eight studies had samples fewer than 100 subjects. Additionally, nearly all the studies were performed at tertiary centers with departments specialized in recognizing CA, thus limiting generalizability to other less specialized settings.
CONCLUSION
Neither EKG nor echocardiogram alone or in combination are sufficient to diagnose CA. There are, however, a combination of findings that could potentially prove useful in “ruling in” CA and prompt further evaluation. Predominant findings in CA cohorts found on EKG showed low-voltage QRS complexes, a pseudo-infarct pattern in precordial leads, and an absence of LV hypertrophy on EKG.
There is no single echocardiogram finding specific to CA. Patients will generally present with thickened ventricular walls, and nearly all patients will display a preserved LVEF until later stages of disease. Strain imaging, either via 2D or 3D TTE, is more useful in screening for or detecting CA and should be utilized in this instance. Findings in CA include decreased GLS and relative apical sparing.
IMPLICATIONS FOR PRACTICE
Despite the fact that it has been considered a rare group of diseases, it is far more likely that the advanced practitioner will encounter systemic amyloidosis. As both EKG and echocardiogram are common practice, this information is applicable to providers in many settings, and are not specific to cardiology. Detection and outcomes are improved with collaboration. The advanced practitioner’s ability to recognize subtle changes in commonly performed screenings could result in prompt diagnoses and earlier intervention.
A provider should consider a diagnosis of CA if a patient undergoes an EKG that shows low-voltage QRS complexes, a pseudo-infarct pattern in precordial leads, and an absence of LV hypertrophy, or an echocardiogram that shows thickened ventricular walls with a preserved LVEF. Advanced practitioners are in a prime position in various clinical settings to recognize these changes and can make a profound impact on the mortality of CA.
Footnotes
The authors have no conflicts of interest to disclose.
References
- Alexander, K. M., Orav, J., Singh, A., Jacob, S. A., Menon, A., Padera, R. F.,…Dorbala, S. (2018). Geographic disparities in reported US amyloidosis mortality from 1979 to 2015: Potential underdetection of cardiac amyloidosis. JAMA Cardiology, 3(9), 865–870. 10.1001/jamacardio.2018.2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, E., Brown, E. E., Fajardo, J., Barouch, L. A., Judge, D. P., & Halushka, M. K. (2018). Seven factors predict a delayed diagnosis of cardiac amyloidosis. Amyloid, 25(3), 174–179. 10.1080/13506129.2018.1498782 [DOI] [PubMed] [Google Scholar]
- Boldrini, M., Cappelli, F., Chacko, L., Restrepo-Cordoba, M. A., Lopez-Sainz, A., Giannoni, A.,…Fontana, M. (2020). Multiparametric echocardiography scores for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging, 13(4), 909–920. 10.1016/j.jcmg.2019.10.011 [DOI] [PubMed] [Google Scholar]
- Costache, I. I., Buburuz, A. M., Crisu, D., Statescu, A. M., Ungureanu, C., & Aursulesei, V. (2019). The role of echocardiography and 99mTc-HDP scintigraphy in non-invasive diagnosis of cardiac amyloidosis: A case series and literature review. Medicine (Baltimore), 98(38), e17256. 10.1097/MD.0000000000017256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fihn, S. D., Gardin, J. M., Abrams, J., Berra, K., Blankenship, J. C., Dallas, A. P.,…Williams, S. V. (2012). 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation, 126(25), 3097–3137. 10.1161/CIR.0b013e3182776f83 [DOI] [PubMed] [Google Scholar]
- Gertz, M. A., & Dispenzieri, A. (2020). Systemic amyloidosis recognition, prognosis, and therapy: A systematic review. JAMA, 324(1), 79–89. 10.1001/jama.2020.5493 [DOI] [PubMed] [Google Scholar]
- Grogan, M., Dispenzieri, A., & Gertz, M. A. (2017). Light-chain cardiac amyloidosis: Strategies to promote early diagnosis and cardiac response. Heart, 103(14), 1065–1072. 10.1136/heartjnl-2016-310704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, C., Zhu, X., Hsi, D. H., Wang, J., Zuo, L., Ta, S.,…Liu, L. (2021). Predictors of cardiac involvement and survival in patients with primary systemic light-chain amyloidosis: roles of the clinical, chemical, and 3-D speckle tracking echocardiography parameters. BMC Cardiovasc Disorders, 21(1), 43. 10.1186/s12872-021-01856-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfbacka, V., Suhr, O. B., Pilebro, B., Wixner, J., Sundström, T., Lindmark, K.,…Lindqvist, P. (2021). Combining ECG and echocardiography to identify transthyretin cardiac amyloidosis in heart failure. Clinical Physiology and Functional Imaging, 41(5), 408–416. 10.1111/cpf.12715 [DOI] [PubMed] [Google Scholar]
- Milani, P., Dispenzieri, A., Scott, C. G., Gertz, M. A., Perlini, S., Mussinelli, R.,…Grogan, M. (2018). Independent prognostic value of stroke volume index in patients with immunoglobulin light chain amyloidosis. Circulation: Cardiovascular Imaging, 11(5), e006588. 10.1161/CIRCIMAGING.117.006588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagourelias, E. D., Mirea, O., Duchenne, J., Van Cleemput, J., Delforge, M., Bogaert, J.,…Voigt, J. U. (2017). Echo parameters for differential diagnosis in cardiac amyloidosis: A head-to-head comparison of deformation and nondeformation parameters. Circulation: Cardiovascular Imaging, 10(3), e005588. 10.1161/CIRCIMAGING.116.005588 [DOI] [PubMed] [Google Scholar]
- Perfetto, F., Bergesio, F., Grifoni, E., Fabbri, A., Ciuti, G., Frusconi, S.,…Cappelli, F. (2016). Different NT-proBNP circulating levels for different types of cardiac amyloidosis. Journal of Cardiovascular Medicine, 17(11), 810–817. 10.2459/JCM.0000000000000349 [DOI] [PubMed] [Google Scholar]
- Pradel, S., Magne, J., Jaccard, A., Fadel, B. M., Boulogne, C., Salemi, V. M. C.,…Mohty, D. (2019). Left ventricular assessment in patients with systemic light chain amyloidosis: A 3-dimensional speckle tracking transthoracic echocardiographic study. International Journal of Cardiovascular Imaging, 35(5), 845–854. 10.1007/s10554-018-01524-2 [DOI] [PubMed] [Google Scholar]
- Roslan, A., Kamsani, S. H., Nay, T. W., Tan, K. L., Hakim, N., Tan, A. M.,…Nuruddin, A. A. (2018). Echocardiographic and electrocardiographic presentations of patients with endomyocardial biopsy-proven cardiac amyloidosis. Medical Journal of Malaysia, 73(6), 388–392. https://pubmed.ncbi.nlm.nih.gov/30647209/ [PubMed] [Google Scholar]
- Ruberg, F. L., Grogan, M., Hanna, M., Kelly, J. W., & Maurer, M. S. (2019). Transthyretin amyloid cardiomyopathy: JACC State-of-the-Art Review. Journal of the American College of Cardiology, 73(22), 2872–2891. 10.1016/j.jacc.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry, B. W., Vranian, M. N., Hachamovitch, R., Joshi, H., McCarthy, M., Ikram, A., & Hanna, M. (2016). Are classic predictors of voltage valid in cardiac amyloidosis? A contemporary analysis of electrocardiographic findings. International Journal of Cardiology, 214, 477–481. 10.1016/j.ijcard.2016.04.030 [DOI] [PubMed] [Google Scholar]
- Stern, L. K., & Kittleson, M. M. (2021). Updates in cardiac amyloidosis diagnosis and treatment. Current Oncology Reports, 23(4), 47. 10.1007/s11912-021-01028-8 [DOI] [PubMed] [Google Scholar]
- Yancy, C. W., Jessup, M., Bozkurt, B., Butler, J., Casey, D. E., Drazner, M. H.,…Wilkoff, B. L. (2013). 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology, 62(16), e147–239. 10.1016/j.jacc.2013.05.019 [DOI] [PubMed] [Google Scholar]
- Zhang, K. W., Zhang, R., Deych, E., Stockerl-Goldstein, K. E., Gorcsan, J., & Lenihan, D. J. (2021). A multi-modal diagnostic model improves detection of cardiac amyloidosis among patients with diagnostic confirmation by cardiac biopsy. American Heart Journal, 232, 137–145. 10.1016/j.ahj.2020.11.006 [DOI] [PubMed] [Google Scholar]